Abstract
Severe fever with thrombocytopenia syndrome (SFTS) is an emerging tick-borne disease caused by the SFTS virus (SFTSV), which has high mortality rates and poses a significant threat to public health. To identify potential therapeutic targets against SFTSV, we conduct genome-wide knockout screening, which identifies the previously known host factor CCR2, and reveals prolow-density lipoprotein receptor-related protein 1 (LRP1) as an entry factor for SFTSV. Knockdown or knockout of LRP1 significantly attenuate SFTSV infection in mouse embryonic fibroblasts (MEFs). Additionally, inhibition of LRP1 suppresses SFTSV pseudovirus infection in MEFs, suggesting its role in viral entry. The interaction between the SFTSV glycoprotein Gn and LRP1 via the CLI and CLII domains is revealed by co-IP and surface plasmon resonance (SPR). Moreover, LRP1 antagonists and neutralizing antibodies effectively attenuate SFTSV infection in MEFs. Administration of an LRP1-neutralizing antibody in a lethal male mouse model reduces the viral load, mitigates tissue damage, and improves survival. This study identifies LRP1 as a host entry receptor for SFTSV, providing a target for therapeutic strategy development.
Similar content being viewed by others
Introduction
Severe fever with thrombocytopenia syndrome virus (SFTSV), also known as Dabie Bandavirus, was first identified in Henan, China, in 20091. SFTSV is transmitted mainly through tick bites, but can also spread through contact with patients’ blood or body fluids2,3,4,5. Transmission from pets, such as cats or dogs, to humans has also been reported6,7. The number of human cases has been increasing annually, with the geographic range extending from East Asia to Southeast Asia8,9. SFTSV infection can lead to viral hemorrhagic fever, thrombocytopenia, and neurological and gastrointestinal symptoms, with mortality rates ranging from 10% to 30%10,11,12. However, there are currently no effective drugs or vaccines available13,14. The World Health Organization classified SFTSV as a priority pathogen that demands immediate attention in the 2017 annual review of diseases15.
SFTSV is an enveloped negative-sense RNA virus consisting of three segments (L, M, S) and belongs to the genus Bandavirus, family Phenuiviridae, and order Bunyavirales. The L segment encodes the RdRp protein, which is responsible for viral replication16. The M segment encodes the glycoprotein precursor and is subsequently cleaved into Gn and Gc, which bind to receptors to facilitate viral entry17. The S segment encodes the capsid protein NP and immunomodulator NS18,19,20. Although some reports have resolved the interaction between SFTSV and the host, few host factors are currently known21. The C-type lectin DC-SIGN has been reported to be a receptor for SFTSV Gn/Gc-driven entry into dendritic cells22. NMMHC-IIA and CCR2 can bind to Gn and promote virus binding and internalization23,24. Nevertheless, blocking or reducing the expression of certain proteins does not completely eliminate virus infection, suggesting that other entry factors may exist. To address this, we performed unbiased, genome-wide CRISPR screening and identified prolow-density lipoprotein receptor-related protein 1 (LRP1) as an entry factor for SFTSV.
In this study, we investigated the role of LRP1 in SFTSV infection both in vivo and in vitro. We first demonstrated the importance of LRP1 in SFTSV infection and its role in viral entry. Second, we revealed that LRP1 mediated SFTSV infection by binding to the glycoprotein Gn. Cellular and biochemical analyses demonstrated direct binding of Gn to the extracellular Cluster II (CLII) and Cluster I (CLI) domains of LRP1. Third, we demonstrated the therapeutic potential of inhibiting LRP1 in SFTSV infection through a neutralizing antibody, the LRP1 inhibitor LRPAP, and the Fc-LRP1-Cluster II protein. Finally, the successful validation of targeting LRP1 as a strategy for treating SFTS in a mouse model revealed its promising prospects for clinical application.
Results
CRISPR screening identifies LRP1 as a crucial host factor for SFTSV infection
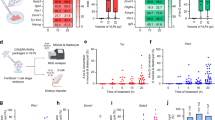
To identify host factors essential for cell survival in response to SFTSV, we performed genome-wide CRISPR screening with SFTSV (isolate AH001). Mouse embryonic fibroblast (MEF) cells were selected for screening because of their significant cytopathic effects following SFTSV infection. Propidium iodide (PI) staining revealed substantial cell death at 72 h post infection with SFTSV at a multiplicity of infection (MOI) of 1 (Supplementary Fig. 1a). Additionally, viral loads in MEFs increased rapidly after SFTSV infection, whereas cell viability decreased sharply (Supplementary Fig. 1b, c). MEFs stably expressing Cas9 were transduced with lentiviruses carrying a pooled population of 62,804 single guide RNAs (sgRNAs) targeting 20611 genes (Mouse-GeCKOv2 Library B), followed by puromycin treatment. High coverage and homogeneous KO library cells were obtained (Supplementary Fig. 1d–f). We used a two-round infection strategy to positively select host factors essential for SFTSV infection (Fig. 1a). Initially, we infected library cells with SFTSV at an MOI of 1. The surviving cells were collected 5 days postinfection and reseeded for 3 days to recover. A second infection was carried out at an MOI of 1, and the remaining cells were collected for DNA extraction and sequencing analysis 10 days post infection.
a Schematic representation of the genome-wide CRISPR screening used to identify host factors essential for SFTSV infection (constructed by Figdraw.). MEFs were modified with a CRISPR knockout library, and subjected to two rounds of SFTSV infection. The surviving cells were then collected for sequencing analysis. n = 2, with two biological replicates. b Volcano plot of the CRISPR screening results, with log2-fold change representing gene enrichment on the x-axis and the log10 robust rank aggregation (RRA) p-values on the y-axis. p values were calculated using MAGeCK software based on a two-sided test of the negative binomial distribution in positive selection. The top 8 membrane-related proteins, identified by the MAGeCK score, are labeled. c The top 8 membrane-related proteins from the genome-wide screening, ranked by beta score. d Percentage of cells infected with SFTSV (MOI of 1, 24 hpi) in the indicated knockout (KO) cell pools, as measured by immunostaining with a mouse anti-NP antibody. Relative infection levels were normalized to those of the nontargeting (NT) control group. Data are mean ± SD, n = 3 independent experiments. Intracellular viral RNA and protein levels in WT and LRP1-KD MEFs pretreated with siLrp1-1 (100 nM final concentration) or scrambled siRNA (100 nM final concentration) cells. RNA levels were measured by RT‒qPCR (e), and protein levels were assessed by Western blotting (f). Data are mean ± SD, n = 6 independent experiments. Two-tailed unpaired t test, ****P < 0.0001. g Viral titers in the supernatants of WT and LRP1-KD MEFs after infection with SFTSV (MOI of 1) were monitored over time with TCID50 assays. Data are mean ± SD, n = 3 independent experiments. Two-tailed Student’s t-test was used for comparisons between two groups. Source data are provided as a Source Data file.
A total of 224 genes were identified as candidate host factors that may facilitate SFTSV infection (log2-fold change [lfc] ≥ 2, p-value < 0.05, good sgRNA ≥ 1) (Supplementary Data 1). We generated a volcano plot illustrating the changes in each gene during positive selection, and the top eight enriched (lfc) membrane-related genes were highlighted: INSIG2, LRP1, CCR2, TMEM90B, RTN2, ISLR2, LMAN2, and TMEM243 (Fig. 1b). The maximum likelihood estimation (MLE) module in MAGeCK was used to estimate the relative screening effects (Fig. 1c). CCR2 (C-C motif chemokine receptor 2) was previously reported as a receptor for SFTSV24, confirming the validity of our screening. Among these identified proteins, INSIG2 and RTN2 are endoplasmic reticulum–related, and the remaining six cell surface membrane proteins were selected for subsequent validation. The most enriched sgRNAs in the CRISPR screen targeting these hit proteins were used to knock out genes in MEFs to construct knockout (KO) cell pools for LRP1, CCR2, LMAN2, TMEM90B, ISLR2, and TMEM243. The gene knockout efficiency was confirmed by RT‒qPCR (Supplementary Fig. 1g). KO cells for each hit protein were then infected with SFTSV at an MOI of 1, and the infection was detected by immunostaining. We found that knockout of LRP1, CCR2, and LMAN2 reduced viral infection, with LRP1 knockout resulting in the most pronounced effect (Fig. 1d).
Thus, to further validate the role of LRP1 in SFTSV infection, we used RNAi to knock down LRP1 in MEFs. The knockdown efficiency was then assessed by qPCR and Western blotting (Supplementary Fig. 1h, i). The siRNA with the highest knockdown efficiency (siLRP1-1) had no effect on cell viability (Supplementary Fig. 1j) and was used for subsequent experiments. Consistently, the knockdown of LRP1 reduced the intracellular levels of viral RNA and proteins (Fig. 1e, f). Additionally, the number of virions released into the supernatant also decreased (Fig. 1g). These findings indicate that LRP1 serves as a critical host factor regulating SFTSV infection.
LRP1 is required for SFTSV infection
Because LRP1 could not be completely knocked down with RNAi, we used CRISPR/Cas9 to knock out LRP1, using two sgRNAs to remove exon 1 and exon 2 of LRP1 to successfully generates several knockout (KO) monoclonal cell lines (Fig. 2a). As expected, compared with wild-type (WT) cells, all the LRP1 knockout monoclonal cells were resistant to SFTSV infection (Fig. 2a). We randomly selected two monoclonal cell lines from them for further validation: K3 and K7. The gene editing in these cell lines was verified by DNA sequencing (Supplementary Fig. 2a). The levels of intracellular viral RNA and viral proteins were significantly reduced in the LRP1-KO MEFs (Fig. 2b, c). Immunostaining of the SFTSV NP protein and titration of the SFTSV progeny virions in the cell culture supernatant further validated the inhibitory effect of LRP1 knockout on viral infection (Fig. 2d, e). LRP1 knockout mice are not viable, but the results of the CCK8 assays indicated that knocking out LRP1 did not affect cell viability (Supplementary Fig. 2b), thereby excluding interference with cell viability. Moreover, this effect of LRP1 was validated in HepG2, Vero, and THP-1 cells (Supplementary Fig. 2c, d). Collectively, these results demonstrate that deletion of LRP1 can effectively inhibit SFTSV infection.
a LRP1 was knocked out by simultaneously transducing two sgRNAs into MEFs, and monoclonal LRP1 KO cells (K2, K3, K5, K7, and K8) were isolated. Monoclonal cells were infected with SFTSV (MOI of 1, 48 hpi), and the level of the intracellular viral protein NS was assessed by western blotting. n = 2 independent experiments. Intracellular viral RNA and protein levels in WT and LRP1-KO MEFs. RNA levels were measured by RT‒qPCR (b), and protein levels were assessed by Western blotting (c). Data are mean ± SD, n = 6 independent experiments. Two-tailed unpaired t test, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. ns not significant (24 h: WT vs. K3, P = 0.0003; WT vs. K7, P < 0.0001; 48 h: WT vs. K3, P < 0.0001; WT vs. K7, P < 0.0001; 72 h: WT vs. K3, P < 0.0001; WT vs. K7, P < 0.0001). d SFTSV infection in WT and LRP1 KO cells (MOI of 1, 48 hpi) was detected by immunostaining. Nuclei were stained with DAPI, and the virus was stained with mouse anti-SFTSV NP. Scale bars, 200 μm. e Viral titers in the supernatant of WT and LRP1 KO MEF cells after infection with SFTSV (MOI of 1) were monitored over time with TCID50 assays. Data are mean ± SD, n = 3 independent experiments. Two-tailed Student’s t-test was used for comparisons between two groups. Source data are provided as a Source Data file.
LRP1 acts as an entry factor for SFTSV
As a cell surface receptor, LRP1 plays an important role in the endocytosis of numerous ligands25,26. Therefore, we speculated that LRP1 functions during the viral entry stage. To validate this, a pseudovirus with an SFTSV glycoprotein that can mimic the entry of SFTSV was used to infect LRP1 KO and WT MEFs (Supplementary Fig. 3a, b). LRP1 knockout inhibited SFTSV pseudovirus infection but had no effect on the VSV pseudovirus (Fig. 3a). To further confirm the role of LRP1 in viral entry, we blocked the LRP1 receptor with a neutralizing antibody at different time points: 4 hours before SFTSV inoculation, simultaneously with SFTSV inoculation, and 4 hours after SFTSV inoculation. We found that blocking LRP1 with neutralizing antibodies 4 hours after SFTSV inoculation had no inhibitory effects on viral infection (Fig. 3b), further confirming that LRP1 is a key factor in SFTSV entry. To better understand the role of LRP1 in viral entry, we evaluated the effect of LRP1 deletion on SFTSV binding and internalization (Fig. 3c). SFTSV binding and internalization were significantly lower in the LRP1-KO cells than in the WT cells (Fig. 3d, e). This effect was further supported by direct visualization of cell-bound virions by confocal microscopy (Fig. 3f). Immunostaining revealed an 80.8% reduction in the number of cell-bound SFTSV virions in the LRP1-KO cells (1.27 virions per cell) compared to the WT cells (6.62 virions per cell) at an MOI of 10. (Fig. 3g). At an MOI of 50, LRP1 KO cells (2.64 virions per cell) also presented a 71.2% reduction in viral adsorption compared to that in WT MEFs (9.17 virions per cell) (Supplementary Fig. 3c, d). These results suggest that LRP1 is a host factor for SFTSV entry.
a A SFTSV-glycoprotein pseudovirus was used to infect WT and LRP1 KO MEFs (MOI = 5, 48 h). VSV-glycoprotein pseudovirus was used as a positive control. n = 3 independent experiments. b An LRP1 neutralizing antibody (anti-LRP1; Invitrogen #MA1-27198) was administered at 30 µg/mL 4 h before, during, and 4 h after SFTSV infection (MOI of 1). The levels of intracellular intracellular viral RNA and protein were measured by RT‒qPCR and Western blotting 48 h post infection. Data are mean ± SD, n = 6 independent experiments. c Overview of the binding and internalization assay (constructed by Figdraw.). Effects of LRP1 deletion on the binding (d) and internalization (e) of SFTSV (MOI of 10) in MEFs. Relative viral RNA was measured by RT‒qPCR, and the data were normalized to the SFTSV S mRNA level in WT cells. Data are mean ± SD, n = 6 independent experiments. Two-tailed unpaired t test, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. ns not significant (binding: WT vs. Lrp1 KO, P < 0.0001; internalization: WT vs. Lrp1 KO, P < 0.0001) f, g Visualization of virion (MOI of 10) binding to cell membranes in WT and LRP1 KO MEFs by confocal microscopy (f). The cell membrane was defined by the membrane protein PDPN, and the virus was stained with a rabbit anti-SFTSV antibody. Scale bars, 20 µm. The number of virions that bound to the cell membrane was quantified and analyzed (g). Data are means ± SD, n = 138 cells examined over three independent experiments. Source data are provided as a Source Data file.
LRP1 binds to the SFTSV glycoprotein Gn directly via its CLI/CLII motif
To analyze the physical interaction between SFTSV and LRP1, SFTSV Gn and Gc were overexpressed in HEK293T cells. A co-IP assay revealed that only Gn interacted with endogenous LRP1, whereas Gc and the mCherry control did not (Fig. 4a). In LRP1, the extracellular domain is comprised of four discrete cysteine-rich complement-like repeat clusters (CLI, CLII, CLIII, and CLIV). These clusters are interspersed with multiple epidermal growth factor precursor repeats and YWTD β-propeller domains, and remain noncovalently associated with the transmembrane and cytoplasmic domains. These four cysteine-rich repeat clusters are responsible for ligand binding27. To determine which cluster is responsible for binding to Gn, we generated plasmids expressing each cluster fused with the transmembrane and cytoplasmic domains. Additionally, we created negative control plasmids containing only the transmembrane domain (TM) or the cytoplasmic domain (CT) (Fig. 4b). Co-IP assays revealed that Gn interacted with CLI and CLII but not with CLIII, CLIV, TM + CT, or CT alone (Fig. 4c, Supplementary Fig. 4a). To further demonstrate the direct interaction between LRP1 and Gn, we expressed the ectodomain of the SFTSV Gn protein (amino acids 20–453), which formed a dimer under nonreducing conditions (Supplementary Fig. 4b). Surface plasmon resonance (SPR) experiments were performed and demonstrated that Gn directly bound to CLII with high affinity (KD = 16.8 nM), but not to CLIII, CLIV or the control Fc (Fig. 4d, Supplementary Fig. 4c, d). On the basis of these findings, we successfully inhibited infection by both SFTSV (Fig. 4e) and the pseudovirus (Supplementary Fig. 4e) with recombinant LRP1-CLII-Fc protein, achieving an IC50 of 1.339 μg/mL for SFTSV (Fig. 4f). Viral titers in the supernatant were also reduced with the LRP1-CLII-Fc protein at a concentration of 4 μg/mL (Fig. 4g). We then reintroduced each LRP1 cluster into LRP1 KO MEFs and infected them with SFTSV (MOI of 1) for one day, after which the intracellular viral RNA and protein levels were measured (Fig. 4h, i). Viral titers in the supernatant were also measured by TCID50 assays (Fig. 4j). The reintroduction of TM + CT + CLI or TM + CT + CLII into LRP1 KO cells restored SFTSV infection. Furthermore, the roles of TM + CT + CLI and TM + CT + CLII were also validated by their overexpression in HeLa (Supplementary Fig. 4f–h) and 293 T (Supplementary Fig. 4i–k) cells. Consistently, only TM + CT + CLI and TM + CT + CLII, rather than the other LRP1 clusters, increased SFTSV infection. Together, these results demonstrate that LRP1 mediates SFTSV entry by directly binding to the viral glycoprotein Gn through its CLI and CLII motifs.
a HEK293T cells were transfected with Strep-tagged Gn, Gc, and mCherry for 48 h. Cells were lysed in 1% NP40, and immunoprecipitation was performed with anti-Strep-tag agarose beads, followed by Western blotting with rabbit anti-LRP1 (CST #64099) and mouse anti-strep (Abbkine #ABT2230) antibodies. n = 3 independent experiments. b LRP1 contains four ligand-binding clusters—CLI, CLII, CLIII, and CLIV—along with transmembrane and cytoplasmic domains. Reconstituted LRP1 plasmids were generated with HA tags as indicated. c HEK293T cells were cotransfected with plasmids encoding various truncated LRP1 proteins and Strep-tagged Gn for 48 h. Immunoprecipitation was performed using anti-Strep-tag agarose beads, followed by Western blotting analysis with the indicated antibodies. n = 3 independent experiments. d Surface plasmon resonance (SPR) experiments of LRP-CLII-Fc binding to SFTSV Gn. A purified SFTSV Gn ectodomain was immobilized on the chip to achieve a 1000 RU response. LRP1 CLII-Fc or IgG1-Fc (negative control) were tested at serial concentrations (0, 1.95, 3.9, 7.8125, 15.625 and 31.25 nM) in HBS buffer. n = 2 independent experiments. e–g LRP1-CLII competitively inhibits SFTSV infection. Different concentrations of LRP1-CLII were added to MEFs 4 h before infection with SFTSV (MOI of 1). Viral RNA levels were measured by RT‒qPCR 24 h post infection (e). n = 3 independent experiments. The IC50 value was calculated on the basis of the inhibition rates (f). Viral titers in the supernatant were also measured with TCID50 assays (g). The dashed lines indicate the limit of detection. n = 3 independent experiments. h–j Various truncated LRP1 proteins were reintroduced into LRP1-KO MEFs to rescue SFTSV infection. The cells were infected for 24 h, and the intracellular viral RNA and protein levels were measured by RT‒qPCR (h) and Western blotting (i). Viral titers were measured by TCID50 assays (j). The dashed lines indicate the limit of detection. n = 3 independent experiments. Data are all mean ± SD. Two-tailed Student’s t-tests were used for comparisons. Source data are provided as a Source Data file.
Targeting LRP1 blocks SFTSV infection both in vitro and in vivo
On the basis of the important role of LRP1 in SFTSV infection, we proposed a therapeutic strategy in which LRP1 was targeted for the prophylactic treatment of SFTS. We used a human anti-LRP1 neutralizing antibody anti-LRP1 and the competitive inhibitor LRPAP to test the therapeutic potential of targeting LRP1. We pretreated MEFs with the anti-LRP1 antibody or LRPAP 4 hours before SFTSV inoculation and assessed the intracellular levels of viral RNA one day postinfection. The anti-LRP1 antibody (Supplementary Fig. 5a–c) and LRPAP (Supplementary Fig. 5d–f) inhibited SFTSV infection in a dose-dependent manner, as shown by reductions in intracellular viral RNA and viral titers in the supernatant. The IC50 for the anti-LRP1 antibody was 0.4278 μg/mL, while that for LRPAP was 1.366 μg/mL (Supplementary Fig. 5b, e). Both the anti-LRP1 antibody and LRPAP significantly inhibited pseudovirus infection at the indicated concentrations (Supplementary Fig. 5g). We selected the anti-LRP1 neutralizing antibody, which exhibited a better inhibitory effect, for in vivo experiments. A previously reported lethal mouse model for SFTSV was used28. In this model, mitomycin C was administered daily via intraperitoneal injection at a dose of 0.03 mg per mouse three days before infection and 0.003 mg per mouse continued for three days after infection (Fig. 5a). Thirty micrograms of anti-LRP1 antibody per mouse was intraperitoneally administered 4 hours before intraperitoneal inoculation with 105 TCID50 of SFTSV. Satisfactorily, administration of the anti-LRP1 antibody significantly improved the survival of the mice (Fig. 5b), and a more rapid recovery of body weight and temperature was observed (Fig. 5c, d). In the control group, SFTSV infection resulted in a mortality rate of 56.7% (17 of 30), whereas the anti-LRP1 neutralizing antibody treatment group had a reduced mortality rate of 16% (4 of 25) (Fig. 5b).
a Flowchart of the mouse experiment. Ten-week-old C57BL/6 J mice were administered immunosuppressive mitomycin C for 3 days (0.03 mg/mouse) before infection. An anti-LRP1 (Invitrogen #MA1-27198) was administered intraperitoneally (30 μg/mouse), followed by intraperitoneal administration of 105 TCID50 of SFTSV 4 h later. A low dose of mitomycin C (0.003 mg/mouse) was administered for the remaining 3 days. For sample collection, independent experiments were performed, and a subset of the mice in each group was euthanized on day 6 post infection. Serum, liver, spleen, lung, heart, and kidney tissues were collected for further pathogenesis analysis. b Survival of mitomycin C–pretreated C57BL/6 J mice infected with SFTSV in the absence (n = 30) or presence (n = 25) of the anti-LRP1 antibody and mock control mice (n = 11). n = 2 independent experiments. Survival curves were generated and analyzed by the log-rank (Mantel‒Cox) test, p = 0.0003. c, d Daily monitoring of body weight (c) and body temperature (d) in the mice infected with SFTSV in the absence (n = 12) or presence (n = 12) of the anti-LRP1 antibody and mock control mice (n = 8). n = 2 independent experiments. e–g. Protective effects of anti-LRP1 on SFTSV-induced pathogenesis. Tissue viral RNA levels were quantified by RT‒qPCR (n = 6) (e). Immunohistochemical staining of SFTSV virus (f) and H&E analysis (g) of viral pathogenesis in the liver, spleen, and lung. The dashed black circles indicate damaged liver tissues. The black arrows indicate neutrophil infiltration. The black circles indicate the widening of the pulmonary interstitium. SFTSV was detected with a rabbit anti-SFTSV antibody. Scale bars, 120 μm.
The viral load was significantly reduced in the serum, liver, spleen, heart, lungs, and kidney of the mice treated with the anti-LRP1 antibody (Fig. 5e), and the reduced SFTSV infection was further verified by immunohistochemical staining in the spleen, liver, lung, heart and kidney tissues (Fig. 5f, Supplementary Fig. 5h). To analyze tissue damage, we performed hematoxylin and eosin (H&E) staining of the liver, spleen, lungs, heart, and kidneys of the mice from each group. The H&E staining results revealed significant liver damage in the virus-infected group (mitomycin C + SFTSV, MitoC + SFTSV), characterized by disruption of the liver lobular architecture, loss of hepatic cords, swelling and rounding of hepatocytes, and damage to parenchymal cells, with the presence of small foci of necrosis (Fig. 5f, indicated by dashed black circles). Additionally, neutrophil infiltration was observed in the liver (Fig. 5g, indicated by black arrows). In contrast, no such tissue damage was observed in the liver samples of the control group (mitomycin C, MitoC) or the antibody-protected group (mitomycin C + SFTSV + anti-LRP1, Anti-LRP1). In the spleen, the virus-infected group (MitoC + SFTSV) presented significant atrophy of the white pulp, blurring of lymphoid follicular structures, and a marked reduction in lymphocyte density, accompanied by red pulp expansion, indicating severe tissue damage caused by viral infection. The spleens of mice in the antibody-protected group (Anti-LRP1) exhibited mild damage, with tissue integrity similar to that of the control group (MitoC). Compared with the antibody-treated group (Anti-LRP1) and the control group (MitoC), the virus-infected group (MitoC + SFTSV) presented widening of the pulmonary interstitium (Fig. 5g, indicated by black circles) in the lungs. No tissue damage was observed in the heart or kidneys, probably due to low virus loads in these tissues. (Supplementary Fig. 5i). These in vivo experiments indicate that LRP1 is crucial in SFTSV infection and pathogenesis in mice, highlighting the promising potential of targeting LRP1 for clinical applications in SFTS.
Discussion
Since the first isolation in 2009, the number of annual SFTSV infections has increased sharply in recent years29, which has attracted ongoing public attention. SFTSV displays broad tropism and can infect humans, ticks, and wild animals. Elucidating the host factors mediating viral infection is vital for understanding the emergence, transmission, and infection of this virus. Since SFTSV can infect a wide array of cell types—including epithelial cells, plasmablasts, macrophages, and fibroblasts1,30,31—different receptors may facilitate infection in distinct cell types.
Previous studies have shown that DC-SIGN, a C-type lectin receptor, is considered a potential entry factor for SFTSV, Rift Valley fever virus (RVFV), and Uukuniemi Virus (UUKV)22. DC-SIGN is exclusively expressed on dendritic cells and macrophages and mediates nonspecific binding via viral glycoproteins. Additionally, nonmuscle myosin heavy chain IIA (NMMHC IIA) has been identified as an attachment factor for SFTSV, promoting viral infection through endocytosis and phagocytosis23. CCR2, which is highly expressed in immune cells, has also been reported to play a pivotal role in SFTSV entry24. However, considering the broad cell and tissue tropism of this virus, which extends beyond immune cells, it is likely that multiple receptors specific to particular cell types, contribute to SFTSV entry.
Genome-wide CRISPR screening is a powerful strategy for investigating virus-host interactions. Two groups have used this method to identify the cellular host factors required for SFTSV infection, but their results differ because of different screening strategies24,32. In this study, we utilized SFTSV-induced cytopathy, a commonly used approach for studying virus‒host interactions, in MEFs as a screening outcome. We identified several hit genes, including LRP1, TMEM243, LMAN2, TMEM90b, ISLR2, and the previously reported CCR2. Knocking out these genes revealed that LRP1 depletion produced the greatest reduction in SFTSV infection (Fig. 1d).
We further validated various aspects of LRP1. First, LRP1 knockdown or knockout impaired infection by SFTSV and pseudovirus in diverse cell types. Second, LRP1 knockout in susceptible cells reduced LRP1 binding and internalization. Third, co-ip and in vitro biochemical assays revealed that SFTSV Gn directly interacted with Cluster I (LRP1-CLI) and Cluster II (LRP1-CLII) within the extracellular domain of LRP1. Fourth, LRP1-neutralizing antibodies or soluble LRP1-CLII-Fc fusion proteins dose-dependently inhibited SFTSV and pseudovirus infection in various cell types. Reconstitution experiments demonstrated that LRP1-CLI and LRP1-CLII were essential for SFTSV infection. Finally, the administration of an LRP1-neutralizing antibody significantly reduced SFTSV infection and pathogenesis in mice. Collectively, these findings establish LRP1 as an entry receptor for SFTSV across a broad range of cell types from mice to humans.
LRP1, a transmembrane receptor within the low-density lipoprotein receptor (LDLR) family, mediates and regulates the endocytosis of over 50 distinct ligands33. Members of the LRP family have been documented as entry receptors for a variety of viruses. For example, LDLRAD3 serves as the receptor for venezuelan equine encephalitis virus (VEEV), whereas VLDLR mediates the entry of eastern equine encephalitis virus (EEEV), crimean-congo hemorrhagic fever virus (CCHFV), and several alphaviruses34,35,36,37. In addition, LRP6 functions as the receptor for canine distemper virus (CDV), whereas LRP1 has been identified as the receptor for RVFV and oropouche orthobunyavirus (OROV)38,39,40. These findings indicate that LRPs may serve as evolutionarily conserved genes for pathogen interactions. Notably, RVFV, OROV, and SFTSV bind to the CLII extracellular domain of LRP1, suggesting structural similarities among their Gn proteins and positioning LRP1 as a potential panbunyaviral receptor. Given the pressing need to develop broad-spectrum inhibitors against emerging viruses, these observations highlight the potential of LRP1 as a panbunyaviral target.
Despite these findings, there are several limitations to this study. First, a complete LRP1 knockout (KO) mouse is not viable, because of the lethal effect of LRP1 deletion in the mouse embryonic germline. A conditional knockout of LRP1 in specific tissues and cells may be possible41. Conditional deletion of LRP1 in the spleen or liver may help further elucidate the role of LRP1 in SFTSV infection. Second, several host factors involved in SFTSV entry have been reported, including DC-SIGN, NMMHC-IIA, CCR2, and LRP1 as identified in this study22,23,24. It remains unclear which receptor plays the dominant role in SFTSV infection. Furthermore, it remains elusive whether these receptors act synergistically or complementarily during infection.
As SFTSV infection progresses, a range of symptoms, including leukopenia, thrombocytopenia, increased vascular permeability, and eventually systemic hemorrhage, can manifest. These clinical signs correspond to viral invasion of multiple organs and cell types at different points in the disease course. Recent reports have identified several immune cells as targets in fatal SFTSV infection. Specifically, antibody-producing B-lineage plasmablasts and macrophages in secondary lymphoid organs are deemed key targets at the terminal stage of lethal infection30,42. Moreover, intermediate monocytes and IFN-I-inducible plasmablasts have also been recognized as major targets for SFTSV43. Infection of these immune cells by SFTSV can induce systemic immunopathology and dysregulation, likely contributing to fatal outcomes. However, considering that the median age of the SFTSV patients is 61 years44, it is plausible that a weakened immune response in these individuals allows the virus to establish infection in nonimmune organs or cells, which ultimately develops into viremia and an extensive immune cell infection, culminating in death. This scenario diverges from the alternative proposition that the virus initially infects immune cells, causing immune dysfunction and subsequent viremia leading to systemic infection. In light of the complexity and stealth of viral infections, the cell types and receptors responsible for the initial establishment of SFTSV infection warrant further investigation.
In summary, our study identified LRP1 as a critical entry factor for SFTSV, advancing our understanding of SFTSV–host interactions and pathogenesis. We demonstrated that the genetic knockout of LRP1 or the use of an LRP1-neutralizing antibody inhibited SFTSV infection across multiple cell types, and a mouse model further underscored the importance of LRP1 in SFTS pathogenesis. These findings suggest that LRP1 represents a promising therapeutic target, offering hope for addressing the current lack of specific antiviral treatments and the high regional incidence and mortality of SFTS.
Methods
Cells and viruses
MEF, HEK293T, Vero E6, HeLa, Huh-7, and HepG2 cells were cultured at 37 °C and 5% CO2 in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin. THP-1 (ATCC) cells were cultured in RPMI 1640 medium supplemented with 10% FBS and 1% penicillin–streptomycin. All cell lines were confirmed to be free of mycoplasma.
SFTSV (AH001) was isolated from a patient with severe fever with thrombocytopenia syndrome (SFTS) and propagated in Vero E6 cells. The virus was sequenced, and the sequences were deposited in BankIt under accession numbers PQ619853, PQ619852, and PQ619854.
Genome-wide knockout screening and data analysis
The Mouse-GeCKOv2 Library B (Addgene), comprised of 62,804 sgRNAs targeting 20,611 genes, was originally generated by the Zhang laboratory45 and obtained from Azenta. To prepare the virus library, HEK293T cells (in 15-cm dishes) were transfected with 20 μg of library plasmid together with 12 μg of psPAX2 and 8 μg of pMD2.G. After 8 h, the medium was changed to DMEM containing 10% FBS. The supernatant was collected 48 h post transfection, centrifuged at 3000 × g and 4 °C for 10 min, and then filtered through a 0.22 μm filter. The aliquots were stored at −80 °C. The virus titer was quantified with the Lenti-X qRT‒PCR Titration Kit (Takara).
To perform the screen, MEFs stably expressing Cas9 were seeded in 15-cm dishes at 6.5 × 106 cells per dish. In total, 1.3 × 108 cells were infected with the sgRNA lentivirus library at an MOI of 0.3 to achieve library coverage >60046. Forty-eight hours after infection, the cells were reseeded and selected with 2 μg/ml puromycin for 2 days. The cells were then reseeded and allowed to recover in DMEM containing 6% FBS (without puromycin) for 2 days. Next, 2.2 × 108 untreated library cells were collected as the control sample, and another 2.2 × 108 library cells were infected with SFTSV at an MOI of 1 as the screen sample. A plate of MEF-Cas9 cells without the sgRNA library was used as an infection control. Five days postinfection, when the MEF-Cas9 cells (infected control) had detached due to the severe cytopathic effect (CPE), the remaining library cells in the screen sample were reseeded and allowed to recover for 3 days. These reseeded library cells were then rechallenged with SFTSV at an MOI of 1, and the surviving cells were collected 10 days post-infection for DNA extraction47. Two parallel screens were conducted.
The gRNAs were amplified by a two-step PCR method with NEBNext® Ultra™ II Q5® Master Mix (New England Biolabs, NEB).
In the first step, twenty 100 μl PCR reactions (each containing 1.5 μg of genomic DNA) were performed with the forward primer NGS-F1(5′-CTCTTTCCCTACACG ACGCTCTTCCGATCTCTTGTGGAAAGGACGAAACA-3′) and the reverse primer NGS-R1 (5′-GTGACTGGAGTTCAGACGTGTGCTCTTCC GATCTCCGACTCGGTGCCACTTTTTCAA-3′).
The first-step PCR conditions were as follows: initial denaturation at 98 °C for 30 s; followed by 25 cycles of 98 °C for 10 s, 65 °C for 45 s, and 72 °C for 30 s; and a final extension at 72 °C for 2 minutes. Products from all first-step PCRs were pooled and used as the template for the second-step PCRs. For the second step, twenty 100 μl PCRs (each containing 6 μl of the pooled first-step PCR product) were performed using different primers each with unique barcodes (underlined) for each sample. Specifically:
-
Control sample 1: forward primer NGS-F2-index1 (5′-AATGATACGGCGACCACCGAGATCTACACAGCGCTAGACACTCTTTCCCTACACG-3′) and reverse primer NGS-R2-index1 (5′-CAAGCAGAAGACGGCATACGAGATAACCGCGGGTGACTGGAGTTC-3′)
-
Control sample 2: forward primer NGS-F2-index2 (5′-AATGATACGGCGACCACCGAGATCTACACGATATCGAACACTCTTTCCCTACACG-3′) and reverse primer NGS-R2-index2 (5′-CAAGCAGAAGACGGCATACGAGATGGTTATAAGTGACTGGAGTTC-3′)
-
Screen sample 1: forward primer NGS-F2-index3 (5′-AATGATACGGCGACCACCGAGATCTACACCGCAGACGACACTCTTTCCCTACACG-3′) and reverse primer NGS-R2-index3 (5′-CAAGCAGAAGACGGCATACGAGATCCAAGTCCGTGACTGGAGTTC-3′)
-
Screen sample 2: forward primer NGS-F2-index4 (5′-AATGATACGGCGACCACCGAGATCTACACTATGAGTAACACTCTTTCCCTACACG-3′) and reverse primer NGS-R2-index4 (5′-CAAGCAGAAGACGGCATACGAGATTTGGACTTGTGACTGGAGTTC-3′)
The second-step PCR conditions were as follows: initial denaturation at 98 °C for 30 s; followed by 13 cycles of 98 °C for 10 s, 65 °C for 45 s, and 72 °C for 30 s; and a final extension at 72 °C for 2 minutes. The resulting ~247-bp PCR products were subjected to electrophoresis on a 1.5% agarose gel, excised, and purified. Finally, the purified amplicons were sequenced on a NovaSeq 6000 platform (Illumina).
The quality of the paired-end FASTQ files obtained from sequencing was checked with FastQC (v0.11.9). The MAGeCK (v0.5) workflow was then used for sgRNA quality control and quantification. First, sgRNAs were quantified with the MAGeCK count command and read counts from different samples were median-normalized to adjust for library size and read count distribution effects. Next, on the basis of a negative binomial (NB) model, the MAGeCK test command was used to determine whether there was a significant difference in sgRNA abundance between the virus-infected and mock control groups. sgRNAs were ranked according to P values from the NB model. The fold change values for each sgRNA were mapped to their corresponding genes in MAGeCK, facilitating the determination of selection patterns at the gene level. In the design matrix of MAGeCK, samples are divided into experimental and control groups on the basis of treatment. The maximum-likelihood estimation (MLE) algorithm was then applied to estimate the beta effect size.
Propidium iodide (PI) staining
A total of 5 × 105 MEFs per well were seeded in 12-well plates. At 12 h post seeding, the cells were infected with SFTSV at an MOI of 1. Propidium iodide (PI) (Sangon) staining was performed at 0 (mock), 24, 48, and 72 h post infection. To do this, a final concentration of 2 μg/ml PI was added to the cells, which were subsequently incubated at 37 °C for 15 min. After being washed twice with PBS, the cells were photographed to document PI staining.
RNA interference
A total of 1.5 × 105 MEFs were seeded in 6-well plates. Twelve hours later, a 100 nM final concentration of small interfering RNA (siRNA, GenePharma) targeting lrp1 (GeneID: 16971) was transfected into each well with jetPRIME (Polyplus). The siRNA sequences used were as follows:
-
Lrp1-siRNA1: 5′-CCACCUGCUAUGAGUUUAATT-3′
-
Lrp1-siRNA2: 5′-GCCCAUUGGAUGAGUUUCATT-3′
-
Lrp1-siRNA3: 5′-GCGUGGUGUUCUGGUAUAATT-3′
A 21-mer nontargeting siRNA was included as a negative control:
-
control-siRNA: 5′- UUCUCCGAACGUGUCACGUTT -3′
The knockdown efficiency was measured by RT‒qPCR and Western blotting. One day after siRNA transfection, the MEFs were infected with SFTSV at an MOI of 1. Viral RNA and protein were assessed at the indicated time points by RT‒qPCR, Western blotting, and the TCID50 assays.
RNA extraction and RT‒qPCR
Total RNA was extracted with RNAiso Easy (Takara) following the manufacturer’s instructions, and reverse transcription was performed with Evo M-MLV RT Premix (Accurate Biotechnology #AG11706). Quantitative real-time PCR (RT‒qPCR) was conducted with SYBR® Green Premix Pro Taq HS (AG11701, Accurate Biotechnology) on a Roche LightCycler® 96.
-
Lrp1-Fwd: 5′-CAGCCGCAGTTGCATTCATT-3′
-
Lrp1-Rev: 5′-GGCTTGCACAAGGTGTTGTT-3′
-
SFTSV-S -Fwd: 5′-TCCCCAATTCTGGATGTGGC-3′
-
SFTSV-S -Rev: 5′-AGAGGCTTACGCAATGAGGA -3′
-
GAPDH-Fwd: 5′-GTGTTCCTACCCCCAATGTG-3′
-
GAPDH-Rev: 5′-GGTCCTCAGTGTAGCCCAAG-3′
-
Ccr2-Fwd: 5′-GGGAATGAGTAACTGTGTGATTG-3′
-
Ccr2-Rev: 5′-ACAACCCAACCGAGACCTCT-3′
-
Lman2-Fwd: 5′-GTTCCAGTTCCATGCCGCTC-3′
-
Lman2-Rev: 5′-GTGGTTCCAGATCGAGCCCT-3′
-
Tmem90b-Fwd: 5′-CACAGTAAGATCAGTGATGC-3′
-
Tmem90b -Rev: 5′-GCACTGGCCACTAGCCTGTGGC-3′
-
Tmem243-Fwd: 5′-CACTGATCAGTGCTTTTGTG-3′
-
Tmem243-Rev: 5′-AAGTATGCAGGCAGTAATAC-3′
-
Islr2 -Fwd: 5′-CTAGGAGTGGTCAGAGCGTG-3′
-
Islr2 -Rev: 5′ GACAGACTAAGCGTGGTCACG-3′
Absolute quantification of SFTSV RNA in mouse samples was performed by RT–qPCR as previously reported48. Briefly, the T7 promoter was tagged to a 480 bp L fragment by PCR and subsequently subjected to RNA in vitro transcription (NEB) and purification (NEB). The L RNA fragment was quantified by a Nanodrop and serially diluted to obtain RNA standards. The RT–qPCR standard curve was carried out simultaneously with the experiment RNA samples to determine the absolute quantity of SFTSV RNA copies. The following primers were used for absolute quantification:
-
L-probe: HEX-CAATGACAGACGCCTTCCATGGTAATAGGG-BHQ1
-
Forward (Fwd): 5′-AGTCTAGGTCATCTGATCCATTTAG-3′
-
Reverse (Rev): 5′-TGTCTCTGTCTTTATGTAAGTT-3′
Western blotting
The cells were lysed in RIPA lysis buffer (Biosharp) on ice for 20 min, and the lysates were subjected to centrifugation. Proteins were separated by SDS–PAGE and transferred onto a polyvinylidene difluoride (PVDF) membrane. All the membranes were blocked with 5% (w/v) nonfat dry milk in TBST for 1 h at room temperature. Primary antibodies against the following proteins were used: SFTSV NS (rabbit anti-NS, a gift from Professor Mifang Liang, Chinese Center for Disease Control and Prevention), SFTSV Gn (mouse anti-Gn, self-prepared), SFTSV Gc (mouse anti-Gc, self-prepared), GAPDH (rabbit anti-GAPDH, Proteintech #10494-1-AP; mouse anti-GAPDH, Proteintech#60004-1-Ig;), LRP1(rabbit anti-LRP1, CST #64099), Strep-tag (mouse anti-Strep-tag, Abbkine #ABT2230), HA (rabbit anti-HA, Proteintech #51064-2-AP). After incubation with the primary antibody overnight at 4 °C, the membranes were washed with TBST and then incubated for 1 h at room temperature with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibody (Apexbio).
CRISPR/Cas9 knockout
Single guide RNAs (sgRNAs) targeting Lrp1 (Gene ID: 16971) were designed with chopchop49 and cloned and inserted into a lenti-CRISPRv2 plasmid (Addgen#52961). Lentivirus was collected from the supernatant of HEK293T cells transfected with lenti-CRISPRv2, psPAX2 (Addgen #12260) and pMD2.G (Addgen #12259). LRP1 knockout (KO) clones were generated by lentiviral transduction with two guides, selected with 2 μg/mL puromycin for 2 days, and further recovered for 5‒10 days without puromycin. The resulting LRP1 KO pool was seeded in 96-well plates at a density of 1 cell per well to obtain single-cell clones. Each clone was validated by Western blot or Sanger sequencing.
To confirm the gene editing events, genomic DNA was extracted with the Qiagen DNeasy Blood & Tissue Kit (Qiagen) and used as a template for amplifying an approximately 400–800 bp region surrounding the PAM site. The following sgRNAs were used in this study:
-
Lrp1-sgRNA1: 5′-CGAAGCTCACAGCCCCATCT-3′
-
Lrp1-sgRNA2: 5′-GGTTATCAAGGGTAACATGT-3′
-
Lrp1-sgRNA3: 5′- ACAAGAAGTAACAGGACCAG -3′
-
Lrp1-sgRNA4: 5′- TCTGATTACACCACTTATTG -3′
Lrp1-sgRNA1 and Lrp1-sgRNA4 were also used to delete exon 1 and exon 2 of Lrp1 in human (Gene ID: 4035) and African green monkey (Gene ID:103238555) cells with a single or double nucleotide (underlined) modification in the Lrp1-sgRNA4 sequence:
-
Human Lrp1-sgRNA4: 5′-TCCGATTACACCACTTACTG-3′
-
African green monkey Lrp1-sgRNA4: 5′-TCTGATTACACCACTTACTG-3′
TCID50 assay
Monolayer Vero cells were seeded in 96-well plates and incubated overnight before virus infection. The virus samples were serially diluted from 1:10 to 1:108 in DMEM. Each dilution was used to infect Vero cells in quintuplicate and then incubated at 37 °C with 5% CO2 for 3 days. After incubation, the cells were fixed with 4% (w/v) paraformaldehyde and subjected to immunostaining with a primary mouse anti-SFTSV NP antibody (1:600, self-made) and a secondary goat anti-mouse IgG-FITC antibody (Invitrogen #F-2761). SFTSV NP-positive cells were counted under a fluorescence microscope, and viral titers were calculated according to the Reed ‒ Muench method as previously decribed50,51.
Immunostaining assay
Monolayer cells were fixed with 4% (w/v) paraformaldehyde for 30 min, followed by three additional PBS washes. The cells were then permeabilized with 0.5% Triton X-100 for 10 min, washed with three additional PBS washes, and blocked with 5% (w/v) bovine serum albumin (BSA) for 1 h at room temperature. The cells were subsequently incubated with primary antibody overnight at 4 °C, followed by three additional PBS washes. Next, the samples were incubated with the secondary antibody for 1 h at room temperature, stained with DAPI, and washed three more times. Finally, the samples were mounted and observed with the THUNDER Imager 3D assay (Leica) or confocal microscopy (Zeiss). The primary antibodies used for immunostaining included mouse anti-SFTSV NP (1:600, self-prepared), rabbit anti-SFTSV (1:200, a gift from Professor Mifang Liang, Chinese Center for Disease Control and Prevention), Syrian hamster anti-PDPN (1:200, Invitrogen #MA5-16113). The secondary antibodies used for immunostaining included the following: goat anti-mouse IgG-FITC antibody (Invitrogen #F-2761), goat anti-rabbit IgG 594 (1:200, Bioss #BB07081455), and goat anti-Syrian hamster IgG 488 (1:200, Invitrogen #A78958).
Virion binding assay
To visualize virus binding, WT, and LRP1 KO MEFs were incubated with SFTSV in precooled DMEM at an MOI of 50 or an MOI of 10 for 1 h at 4 °C. The cells were subjected to three washes with prechilled phosphate-buffered saline (PBS) containing 2% BSA. The cells were subsequently fixed with 4% paraformaldehyde and labeled with rabbit anti-SFTSV (1:200) and Syrian hamster anti-PDPN (1:200, Invitrogen #MA5-16113) antibodies for subsequent microscopy. The numbers of DAPI-stained cells and adsorbed virions from three independent views belonging to three independent wells were quantified automatically with ImageJ software.
Pseudotyped virus production and infection
SFTSV glycoprotein–pseudotyped viruses were generated as previously described52.
Briefly, the SFTSV M protein (YP_006504094.1) was human codon-optimized and cloned and inserted into pcDNA3.1 between the BamHI and XhoI restriction sites, resulting in the pcDNA3.1-SFTSV-G plasmid, which expresses the SFTSV glycoprotein. To produce the pseudotyped virus, HEK293T cells were cotransfected with pLenti-CMV-GFP, psPAX2, and pcDNA3.1-SFTSV-G. The pseudoviruses were harvested 48 h post transfection and titrated with the Lenti-X qRT‒PCR Titration Kit (Takara). pMD2.G, which encodes the vesicular stomatitis virus glycoprotein (VSV-G), was used as a positive control.
For pseudovirus infection, WT MEFs and LRP1 KO MEFs were infected with the SFTSV pseudoviruses at an MOI of 5 for 48 h. The GFP intensity in the infected cells was then measured by microscopy. For the neutralization assay, cells were pretreated with the corresponding neutralizing antibody or inhibitor before being subjected to SFTSV pseudovirus infection, as described above.
SFTSV binding and internalization assays
The cells were seeded in 12-well plates 12 h before infection. For the binding assay, the cells were incubated with SFTSV at an MOI of 10 in precooled DMEM for 1 h at 4 °C. After incubation, the cells were washed three times with precooled PBS supplemented with 2% bovine serum albumin. The washed cells were then collected for RNA extraction, and the relative level of bound virions was quantified by RT‒qPCR. For the internalization assay, following the binding step, the cells were further incubated with prewarmed DMEM containing 2% FBS and 50 mM NH4Cl at 37 °C for 3 h. The cells were then washed again with PBS and collected for RNA extraction. The relative level of internalized virions was subsequently quantified by RT‒qPCR.
Neutralization assays
MEFs were pretreated with serially diluted concentrations of anti-LRP1 antibody (Invitrogen #MA1-27198), LRP1-CLII-Fc (RD #2368-L2), and LRPAP (Sino Biological #50281-M08H) or with a negative IgG control (Proteintech #66002-1-Ig). After 4 h, the cells were infected with SFTSV at an MOI of 1. The cells were harvested 24 h post infection and analyzed for intracellular viral RNA levels using RT‒qPCR. The viral titer in the supernatant was measured with TCID50.
Coimmunoprecipitation
HEK293T cells were cotransfected with Strep-tagged SFTSV Gn and HA-tagged truncated LRP1 for 48 h and were subsequently lysed in 1% NP-40 supplemented with an EDTA-free protease inhibitor cocktail (Apexbio #K1007), 0.1 mM PMSF, and 5 mM NaF. Protein concentrations were measured by a BCA assay. A total of 600 μg of whole cell lysate was incubated with 30 μL of Strep-Tactin® Sepharose® resin (Iba#2-1201-010) or anti-HA nanobody agarose beads (AlpaLifeBio #KTSM1305) overnight at 4 °C. Subsequent wash steps were performed following the manufacturer’s instructions. The beads were subsequently eluted with 2× protein loading buffer (0.1 M Tris, pH 6.8, 4% SDS, 20% glycerol, 10% β-mercaptoethanol, 0.2% bromophenol blue) and denatured for 10 minutes at 95 °C. The eluate was further assessed by Western blotting.
Protein expression and purification
The ectodomain of the SFTSV Gn glycoprotein (amino acids 20–453, YP_006504094) was prokaryotically codon-optimized and cloned and inserted into the pET-30a (+) vector between the NdeI and BamHI restriction sites to obtain the expression plasmid pET-30a-Gn-his. The expression plasmid was subsequently transformed into BL21(DE3) Escherichia coli cells. A single colony was cultured in 4 mL of Luria broth (LB) media at 37 °C until an OD600 of 0.6 was reached, which was then inoculated into 200 mL of LB media supplemented with 1 mM isopropyl-β-D-thiogalactoside (IPTG) to induce protein expression at 18 °C for 14 h. The cells were then collected and resuspended in 30 mL of lysis buffer (50 mM Tris-HCl, 500 mM NaCl(pH 7.5), 5% glycerol [v/v], 1 mM TCEP, 0.5 mM PMSF, 0.25 mg/mL lysozyme) and sonicated on ice for 10 minutes. The lysate was subsequently centrifuged at 10,000 × g and 4 °C for 30 min. The inclusion bodies in the pellet were isolated with 20 mL of cold binding/wash buffer (8 M urea, 20 mM Tris-HCl, 500 mM NaCl and 5 mM imidazole, pH 8.0). The Gn-his protein was purified with Ni-NTA Sepharose Resin (Sangon #C600791) and eluted with 3 mL of elution buffer (20 mM Tris-HCl, 500 mM NaCl, and 500 mM imidazole, pH 8.0). The Gn-his protein was further refolded by dialysis with a gradually decreasing urea concentration gradient. The refolded protein was subsequently concentrated with a centrifugal filter (Amicon #30 kDa). The protein was further purified with size-exclusion columns, and Coomassie blue staining was performed to determine its purity.
SPR assay
Surface plasmon resonance (SPR) assays were conducted with the GE Healthcare Biacore T200. The purified ectodomain of SFTSV Gn was immobilized on the CM5 chip at a concentration of 20 μg/mL in HBS buffer. Recombinant human LRP1 CLII-Fc (RD #2368-L2), LRP1 CLIII-Fc (RD #4824-L3), LRP1 CLIV-Fc (RD #5395-L4) or recombinant human IgG1 Fc (RD #110-HG-100) was loaded at serial concentrations (0, 1.95, 3.9, 7.8125, 15.625 and 31.25 nM) in HBS buffer. The experiments were conducted with the following settings: contact time of 180 s, flow rate of 30 μL/min, and dissociation time of 300 s. Data were baseline-corrected by subtracting the buffer-only controls.
Mouse experiments
Ten-week-old male C57BL6J mice were used in this study. To render the mice susceptible to SFTSV infection, mitomycin C (0.03 mg/mouse) were administered intraperitoneally 3 days before infection. Thirty micrograms /mouse of anti-LRP1 antibody (Invitrogen #MA1-27198) or a negative control IgG (Proteintech #66002-1-Ig) was intraperitoneally administered 4 h before the challenge. SFTSV was intraperitoneally inoculated at 105 TCID50 per mouse, and mitomycin C (0.003 mg/mouse) was intraperitoneally administered for three days following infection28. Body weight and temperature were monitored daily. The mice were euthanized when they exhibited no response to touch stimulation. Independent experiments were performed to collect tissue samples. A subset of mice was marked at the beginning and the mice were euthanized six days postinfection. Serum, liver, spleen, lung, heart, and kidney samples were collected for further pathological analysis.
The Animal Ethics Committee of the Anhui Medical University approved the research (LLSL20242191). The mice were housed under controlled environmental conditions with a 12-h light/dark cycle and observed daily. Ambient temperature was maintained at 22–25 °C and relative humidity ranged between 40% and 80% throughout the experimental period. The animal experiments were conducted in the BSL-3 animal facility of the Second Military Medical University.
H&E and immunohistochemical staining
The collected tissue samples were immersed in 4% paraformaldehyde and fixed at room temperature for 24 h to preserve tissue architecture. The tissues were subsequently dehydrated through a series of alcohol gradients (50%, 70%, 80%, 95%, and 100%) followed by clearing in xylene. The dehydrated tissues were then embedded in paraffin and sectioned into 4–5 µm thin slices with a microtome, which were subsequently mounted onto glass slides.
For H&E staining, the slices were dewaxed and the sections were stained with hematoxylin for 5–10 minutes to render the nuclei blue, followed by differentiation and bluing steps. Next, the sections were counterstained with eosin for 10–30 seconds, which imparted a pink hue to the cytoplasm. Finally, the sections were dehydrated through an alcohol gradient, cleared in xylene, and mounted with a coverslip.
For immunohistochemical staining, tissue sections were dewaxed and subjected to antigen retrieval by immersion in citrate buffer (pH 6.0), heated in a microwave for 10–15 min until boiling, and then allowed to cool to room temperature. After three washes with PBS, endogenous peroxidase activity was blocked with an endogenous peroxidase blocker (Beyotimet #P0100B) to reduce background interference. The sections were subsequently washed three times with PBS and blocked with 5% BSA for 1 h at room temperature to prevent nonspecific binding. A primary antibody (rabbit anti-SFTSV, 1:200) was applied, and the samples were incubated overnight at 4 °C. The next day, the slides were returned to room temperature for 40 min, washed three times with PBS, and then incubated with a secondary antibody for 1 h at room temperature. Following three additional PBS washes, a DAB substrate was used for color development for 30–60 s, which resulted in a brown precipitate. The sections were counterstained with hematoxylin for 2–5 min, blueing was achieved with lithium carbonate, and finally, the slides were cleared and mounted with coverslips.
Statistical analysis
All experiments were performed with n ≥ 3 biological replicates (as indicated). All the statistical analyses were performed with GraphPad Prism 8 software. Two-tailed unpaired Student’s t-test was used to determine the significance of variability between two groups. Survival curves were generated by the log-rank (Mantel‒Cox) test. The data were presented as means ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All the data generated in this study are included in the Source Data file, which accompanies this paper. The sequencing data generated in this study have been deposited in the NCBI Sequence Read Archive. The raw NGS data of the SFTSV virus AH001 can be accessed through project PRJNA1190154. The raw NGS data of CRISPR screening conducted in this study can be accessed through project PRJNA1157541. Source data are provided with this paper.
Code availability
The code used in this study is openly available on GitHub and can be accessed at https://github.com/Vinnish-A/SFTSV and a snapshot of the code is provided on Zenodo (https://doi.org/10.5281/zenodo.15072556).
References
Yu, X. J. et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 364, 1523–1532 (2011).
Liu, Y. et al. Person-to-person transmission of severe fever with thrombocytopenia syndrome virus. Vector Borne Zoonotic Dis. 12, 156–160 (2012).
Wu, Y. X. et al. Human-to-human transmission of severe fever with thrombocytopenia syndrome virus through potential ocular exposure to infectious blood. Int. J. Infect. Dis. 123, 80–83 (2022).
Zhang, X. et al. Rapid spread of severe fever with thrombocytopenia syndrome virus by parthenogenetic asian longhorned ticks. Emerg. Infect. Dis. 28, 363–372 (2022).
Zhou, C. M. et al. Oral and ocular transmission of severe fever with thrombocytopenia syndrome virus. Infect. Med. 1, 2–6 (2022).
Chung, J. K. et al. Severe fever with thrombocytopenia syndrome associated with manual de-ticking of domestic dogs. Vector Borne Zoonotic Dis. 20, 285–294 (2020).
Yamanaka, A. et al. Direct transmission of severe fever with thrombocytopenia syndrome virus from domestic cat to veterinary personnel. Emerg. Infect. Dis. 26, 2994–2998 (2020).
Yoshikawa, T. et al. Phylogenetic and geographic relationships of severe fever with thrombocytopenia syndrome virus in China, South Korea, and Japan. J. Infect. Dis. 212, 889–898 (2015).
Tran, X. C. et al. Endemic severe fever with thrombocytopenia syndrome, Vietnam. Emerg. Infect. Dis. 25, 1029–1031 (2019).
Yu, X. J. Risk factors for death in severe fever with thrombocytopenia syndrome. Lancet Infect. Dis. 18, 1056–1057 (2018).
He, F., Zheng, X. & Zhang, Z. Clinical features of severe fever with thrombocytopenia syndrome and analysis of risk factors for mortality. BMC Infect. Dis. 21, 1253 (2021).
Li, J. C., Zhao, J., Li, H., Fang, L. Q. & Liu, W. Epidemiology, clinical characteristics, and treatment of severe fever with thrombocytopenia syndrome. Infect. Med. 1, 40–49 (2022).
Kim, D., Lai, C. J., Cha, I., Jung, J. U. Current progress of severe fever with thrombocytopenia syndrome virus (SFTSV) vaccine development. Viruses 16, 128 (2024).
Takayama-Ito, M. & Saijo, M. Antiviral drugs against severe fever with thrombocytopenia syndrome virus infection. Front Microbiol 11, 150 (2020).
World Health Organization. Annual review of diseases prioritized under the Research and Development Blueprint. WHO Meeting Report (WHO, 2017).
Williams, H. M. et al. Structural insights into viral genome replication by the severe fever with thrombocytopenia syndrome virus L protein. Nucleic Acids Res. 51, 1424–1442 (2023).
Plegge, T., Hofmann-Winkler, H., Spiegel, M. & Pöhlmann, S. Evidence that processing of the severe fever with thrombocytopenia syndrome virus Gn/Gc polyprotein is critical for viral infectivity and requires an internal Gc signal peptide. PLoS ONE 11, e0166013 (2016).
Zhou, H. et al. The nucleoprotein of severe fever with thrombocytopenia syndrome virus processes a stable hexameric ring to facilitate RNA encapsidation. Protein Cell 4, 445–455 (2013).
Liu, S. et al. The severe fever with thrombocytopenia syndrome virus NSs protein interacts with CDK1 To induce G(2) cell cycle arrest and positively regulate viral replication. J. Virol. 94, e01575-19 (2020).
Yang, T., Huang, H., Jiang, L., Li, J. Overview of the immunological mechanism underlying severe fever with thrombocytopenia syndrome (Review). Int. J. Mol. Med. 50, 118 (2022).
Cao, J. et al. Severe fever with thrombocytopenia syndrome virus (SFTSV)-host interactome screen identifies viral nucleoprotein-associated host factors as potential antiviral targets. Comput Struct. Biotechnol. J. 19, 5568–5577 (2021).
Hofmann, H. et al. Severe fever with thrombocytopenia virus glycoproteins are targeted by neutralizing antibodies and can use DC-SIGN as a receptor for pH-dependent entry into human and animal cell lines. J. Virol. 87, 4384–4394 (2013).
Sun, Y. et al. Nonmuscle myosin heavy chain IIA is a critical factor contributing to the efficiency of early infection of severe fever with thrombocytopenia syndrome virus. J. Virol. 88, 237–248 (2014).
Zhang, L. et al. CCR2 is a host entry receptor for severe fever with thrombocytopenia syndrome virus. Sci. Adv. 9, eadg6856 (2023).
Herz, J. & Bock, H. H. Lipoprotein receptors in the nervous system. Annu. Rev. Biochem. 71, 405–434 (2002).
Herz, J. & Chen, Y. Reelin, lipoprotein receptors and synaptic plasticity. Nat. Rev. Neurosci. 7, 850–859 (2006).
Obermoeller-McCormick, L. M. et al. Dissection of receptor folding and ligand-binding property with functional minireceptors of LDL receptor-related protein. J. Cell Sci. 114, 899–908 (2001).
Jin, C. et al. Pathogenesis of emerging severe fever with thrombocytopenia syndrome virus in C57/BL6 mouse model. Proc. Natl Acad. Sci. USA 109, 10053–10058 (2012).
Yue, Y. et al. Epidemic characteristics of fatal cases of severe fever with thrombocytopenia syndrome in China from 2010 to 2023. J. Tropical Dis. Parasitol. 5, 257–261 (2024).
Yamaoka, S., Weisend, C. & Ebihara, H. Identifying target cells for a tick-borne virus that causes fatal hemorrhagic fever. J. Clin. Investig. 130, 598–600 (2020).
Liu, Y. et al. The pathogenesis of severe fever with thrombocytopenia syndrome virus infection in alpha/beta interferon knockout mice: insights into the pathologic mechanisms of a new viral hemorrhagic fever. J. Virol. 88, 1781–1786 (2014).
Liu, T. et al. SNX11 identified as an essential host factor for SFTS virus infection by CRISPR knockout screening. Virol. Sin. 34, 508–520 (2019).
Sagare, A. P., Deane, R. & Zlokovic, B. V. Low-density lipoprotein receptor-related protein 1: a physiological Aβ homeostatic mechanism with multiple therapeutic opportunities. Pharmacol, Ther. 136, 94–105 (2012).
Ma, H. et al. LDLRAD3 is a receptor for Venezuelan equine encephalitis virus. Nature 588, 308–314 (2020).
Adams, L. J. et al. Structural and functional basis of VLDLR usage by Eastern equine encephalitis virus. Cell 187, 360–374.e319 (2024).
Monteil, V. M. et al. Crimean-Congo haemorrhagic fever virus uses LDLR to bind and enter host cells. Nat. Microbiol 9, 1499–1512 (2024).
Clark, L. E. et al. VLDLR and ApoER2 are receptors for multiple alphaviruses. Nature 602, 475–480 (2022).
Gradauskaite, V. et al. LRP6 is a functional receptor for attenuated canine distemper virus. mBio 14, e0311422 (2023).
Ganaie, S. S. et al. Lrp1 is a host entry factor for Rift Valley fever virus. Cell 184, 5163–5178.e5124 (2021).
Schwarz, M. M. et al. Oropouche orthobunyavirus infection is mediated by the cellular host factor Lrp1. Proc. Natl Acad. Sci. USA 119, e2204706119 (2022).
Schwarz, M. M. et al. Lrp1 is essential for lethal Rift Valley fever hepatic disease in mice. Sci. Adv. 9, eadh2264 (2023).
Suzuki, T. et al. Severe fever with thrombocytopenia syndrome virus targets B cells in lethal human infections. J. Clin. Invest 130, 799–812 (2020).
Li, H. et al. Single-cell landscape of peripheral immune responses to fatal SFTS. Cell Rep. 37, 110039 (2021).
Liu, K. et al. A national assessment of the epidemiology of severe fever with thrombocytopenia syndrome, China. Sci. Rep. 5, 9679 (2015).
Joung, J. et al. Genome-scale CRISPR-Cas9 knockout and transcriptional activation screening. Nat. Protoc. 12, 828–863 (2017).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
Baggen, J. et al. Genome-wide CRISPR screening identifies TMEM106B as a proviral host factor for SARS-CoV-2. Nat. Genet 53, 435–444 (2021).
Zhu, Y. et al. Dual-gene detection in a single-tube system based on CRISPR-Cas12a/Cas13a for severe fever thrombocytopenia syndrome virus. Front. Microbiol. 13, 977382 (2022).
Labun, K. et al. CHOPCHOP v3: expanding the CRISPR web toolbox beyond genome editing. Nucleic Acids Res 47, W171–w174 (2019).
Wang, W. et al. The cap-snatching SFTSV endonuclease domain is an antiviral target. Cell Rep. 30, 153–163.e155 (2020).
Ning, Y. J. et al. Heartland virus NSs protein disrupts host defenses by blocking the TBK1 kinase-IRF3 transcription factor interaction and signaling required for interferon induction. J. Biol. Chem. 292, 16722–16733 (2017).
Du, S. et al. Cryo-EM structure of severe fever with thrombocytopenia syndrome virus. Nat. Commun. 14, 6333 (2023).
Acknowledgements
This work was supported in part by National Key Research and Development Program of China (2023YFC2606300) (G.X.), National Natural Science Foundation of China (81772203 (Y.L.), 82302524 (C.Z.)), Natural Science Foundation of Anhui Provincial (2308085Y49) (G.X.), Provincial-level Key R&D Program of Anhui Provincial (2022i01020022) (Y.L.), Cultivation Program of Outstanding scholar in University of Anhui Provincial (gxbjZD2020058) (Y.L.), Scientific Research Improvement Program of Anhui Medical University(2020xkjT003) (Y.L.).
Author information
Authors and Affiliations
Contributions
This project was designed and directed by G.X. and Y.L. C.X. and C.Z. conducted the major experiments. Bioinformatics analysis was performed by Z.X., Y.W., W.L., X.L., Y.Z., J.M., and S.Y. contributed some experimental data. The manuscript was written by C.X. and reviewed by C.Z., Y.D., G.X., and Y.L. All authors reviewed and approved the final draft.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xing, C., Zhang, C., Xu, Z. et al. Genome-wide CRISPR screening identifies LRP1 as an entry factor for SFTSV. Nat Commun 16, 4036 (2025). https://doi.org/10.1038/s41467-025-59305-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-59305-0