Abstract
Reconstructing how prehistoric humans used the products obtained from large cetaceans is challenging, but key to understand the history of early human coastal adaptations. Here we report the multiproxy analysis (ZooMS, radiocarbon, stable isotopes) of worked objects made of whale bone, and unworked whale bone fragments, found at Upper Paleolithic sites (Magdalenian) around the Bay of Biscay. Taxonomic identification using ZooMS reveals at least five species of large whales, expanding the range of known taxa whose products were utilized by humans in this period. Radiocarbon places the use of whale products ca. 20–14 ka cal BP, with a maximum diffusion and diversity at 17.5–16 ka cal BP, making it the oldest evidence of whale-bone working to our knowledge. δ13C and δ15N stable isotope values reflect taxon-specific differences in foraging behavior. The diversity and chronology of these cetacean populations attest to the richness of the marine ecosystem of the Bay of Biscay in the late Paleolithic, broadening our understanding of coastal adaptations at that time.
Similar content being viewed by others
Introduction
Whales are the largest living animals on Earth and the current populations of many species are a mere fraction of their abundance in the past1. Before intensive whaling depleted the populations of most species, whales were a valuable source of food and other resources (e.g., oil, bone, baleen2). They were thus a key part of subsistence for many coastal human groups worldwide, including hunter-gatherers and Neolithic farmers, with acquisition methods that included scavenging freshly beached animals, opportunistic killing and organized whaling2,3,4,5,6,7,8. However, reconstructing the beginning of whale utilization is challenging because prehistoric coastal sites are an especially fragile part of the archeological record, many of them having been lost to marine erosion or flooded by the last marine transgressions9. In most cases, the only available evidence is indirect, in the form of materials of coastal origin transported by people into inland sites. In Europe, a number of sites attributed to the Middle and Upper Magdalenian culture (ca. 19-14 ka cal BP, or 19-14 millennia before present, at the end of MIS2) in southwestern France and in Atlantic Iberia have yielded an invaluable record of this type of evidence10: whale barnacles attesting to the transport of whale skin, blubber and meat11; unworked whale bones transported and processed at the habitation site12; worked whale teeth13; and, especially, more than 150 tools and projectile heads made of whale bone presumably of Atlantic origin, mostly found scattered from Asturias to the central part of the northern Pyrenean range14. This record, mostly identified within the last ten years, represents (to our knowledge) the oldest evidence of a regular utilization of whale products by humans, for dietary needs and raw materials10.
Understanding this Magdalenian utilization of whale products is of crucial importance, not only because it opens a window into early human interactions with whales, but more broadly because of the light it sheds onto the history of human coastal adaptations. Indeed, even though the earliest evidence of the use of coastal resources in Europe dates back to the Middle Paleolithic (older than 40 ka cal BP, and up to ca. 150 ka BP15,16,17), and similar evidence exists for the Early Upper Paleolithic18, the richness and diversity of evidence related to seashore exploitation increases dramatically in association with the Magdalenian culture. Despite a sea level rise of ca. 120 m since that period making the Magdalenian shoreline inaccessible to today’s archeologists, depictions of marine animals, remains of seals, dolphins, marine fish and birds, the use of marine mollusks as food and the use of their shells as raw material for ornaments, are all attested with unprecedented frequency and wide diffusion in the Middle and Upper Magdalenian, especially in Iberia and in the Pyrenees10,19,20. This combined evidence points to a stronger link with the seashore compared to the earlier part of the European Upper Paleolithic, suggesting the inception of a settled coastal economy in certain parts of Europe at this time. Understanding the role that whale products played in this process is thus key to the ongoing investigations into the existence, chronology and organization of hunter-gatherer early coastal adaptations, a subject that is today considered central in prehistoric research21,22,23. However, the information provided by the whale remains found in Magdalenian assemblages is limited for several reasons. First, it is unknown to which whale species they correspond. Indeed, these remains were identified only through visual macroscopic criteria and most of them are small, fragmented and/or worked, thus precluding any precise anatomical or taxonomic identification beyond the cetacean category. Furthermore, although these morphological criteria are robust, they have a margin of uncertainty, meaning that some of the remains are classified only as “likely” made of cetacean bone14,24. Since different whale species have different ecologies and feeding behavior, this generic (and sometimes uncertain) identification as cetacean gives only low resolution insight into the range of interactions that foragers could have had with these animals, and deprives us of the environmental information that the presence of certain particular whale species could indicate25. Finally, there is substantial uncertainty regarding the dating of these whale remains. Because of their typology, their stratigraphic context, and the characteristics of the archeological assemblages they were found in, they can be attributed to the Magdalenian period in the broad sense of the term. But since the majority are from ancient excavations with poor chrono-stratigraphic resolution, they can only be collectively ascribed to a broad time span of several millennia, precluding a precise reconstruction of the chronology, rhythm and evolution of the utilization of whale products.
Here we analyze a large sample of worked bone objects (n = 83) from 26 Magdalenian cave and rockshelter sites in the Cantabrian region and southwestern France, all visually identified as made of whale bone (Supplementary Data 1 and 2, Supplementary Figs. 1 through 64). We also analyze 90 unworked bone fragments from the single assemblage of Santa Catalina cave (Biscay), also ascribed to whale on a visual basis, found among the faunal remains of the site’s Upper Magdalenian occupation and showing traces of anthropic processing (notably percussion notches: Supplementary Figs. 65 through 90). We sampled these for taxonomic identification through collagen peptide mass fingerprinting (ZooMS, or Zooarcheology by Mass Spectrometry26,27). Thirty-seven of the worked objects and 31 of the unworked bone fragments were also sampled for radiocarbon dating using the compact AMS ECHoMICADAS, and for carbon and nitrogen stable isotope analysis (δ13C and δ15N).
In this work, we assess the range of whale taxa whose products were utilized by the Magdalenian foragers and the chronology of this utilization, showing that these groups availed themselves of the carcasses of at least five species starting ca. 20-19 ka cal BP, with a peak time ca. 17.5-16 ka cal BP. The results of the stable isotope analysis, placed in the context of patterns of isotopic niche partitioning among contemporary whales, contribute to reconstructing whales’ relative foraging behavior and past ocean ecology, showing that the distribution of the stable isotope ratios in Paleolithic whales is structured by taxa and overlaps to some extent with those of their modern counterparts, suggesting broadly similar feeding strategies and analogies with today’s arctic water communities. Even though the Paleolithic seashore itself is no longer accessible, and the range of taxa identified here might not reflect the full range of species present in the Bay of Biscay at that period, the analysis of these whale bones brought inland by the hunter-gatherers opens a unique window into whale ecology and the marine environments in the northeastern Atlantic at that period, and on the timing and nature of their utilization by human groups.
Results
Taxonomic identification using ZooMS
Of the 173 bone specimens (83 worked objects and 90 bone fragments) analyzed with ZooMS, all but four yielded a taxonomic identification, demonstrating the power of this approach to identify taxa on highly transformed and/or fragmented remains of late Pleistocene age. Of the 83 worked objects, 71 were confirmed as cetaceans, while 8 were identified as large terrestrial mammals and 4 did not yield a ZooMS identification, indicating that the macroscopic visual attribution was correct in 90% of the cases (71/79). The visual misidentification of 8 objects made of bone from large terrestrial mammals was due to their thoroughly porous aspect, normally a diagnostic feature of whale bones, but also present in some anatomical elements of certain terrestrial species (in this case mammoth, rhinoceros, reindeer and equids), and that can be misleading when dealing with small, fragmented objects. Of the 90 unworked bone fragments, the attribution as cetacean was confirmed for 60 bones (67%), with the other 30 being identified mostly as large land mammals, but also one seal. The higher error rate for Santa Catalina (33% vs. 10%) is a consequence of the fact that the visual selection of putative whale-bone fragments was more inclusive at this site (see “Methods” below).
Overall, ZooMS analyses of 131 cetacean specimens reveal the presence of at least six cetacean taxa in the northeastern Atlantic during the Magdalenian (Fig. 1 and Supplementary Data 3): fin whale, Balaenoptera physalus (n = 65); sperm whale, Physeter macrocephalus (n = 32); gray whale, Eschrichtius robustus (n = 11); blue whale, Balaenoptera musculus (n = 2); one species of porpoise (harbor porpoise or Dall’s porpoise, Phocoenidae, n = 1); and at least one species of Balaenid whale (Balaenidae), with 13 samples that can be attributed either to the North Atlantic right whale, Eubalaena glacialis, or to the bowhead whale, Balaena mysticetus (two species that are indistinguishable using ZooMS), both present in the North Atlantic. The remaining 7 samples yielded ZooMS spectra that could not be attributed to a precise cetacean taxon. Among the six cetacean taxa, only the sperm whale had previously been unambiguously documented in the Magdalenian record, through the presence of two carved teeth and several depictions on other portable objects from the Bay of Biscay region28. The other taxa—fin whale, gray whale, blue whale, right and/or bowhead whale and porpoise—had (to our knowledge) previously not been identified in this archeological context.
a worked objects; b unworked bone fragments. 1: blank, Tito Bustillo, sperm whale (#Hum1); 2: projectile point with massive base, Isturitz, blue whale (#15); 3: projectile point, Brassempouy, fin whale (#822); 4: possible foreshaft, Las Caldas, sperm whale (#982); 5: projectile point with massive base, Ermittia, gray whale (#968); 6: unidentified object, Saint-Michel, sperm whale (#799); 7–10: unworked fragments of fin whale bone, Santa Catalina (#SC B6 109 1085, SC B8 144 439, SC B8 124 1180, SC B6 135 24). Whale drawings courtesy of Uko Gorter. Black silhouettes from phylopic.org, CC0 1.0 license. Pictures by AL and JMP. See Supplementary Data 1 for source data.
Radiocarbon dating
Of the 37 worked objects sampled for radiocarbon dating, 5 failed as a result of poor collagen preservation, while the remaining 32 yielded results (Fig. 2, and see “Methods” below for calibration and caveats). The two earliest dates are from the Cantabrian sites of Rascaño and El Juyo, ca. 20.2-19.6 and 19.6-19 ka cal BP, respectively. The two artifacts were found among assemblages attributed to the Cantabrian Lower Magdalenian (Rascaño IVb and El Juyo 8), and our results confirm this archeological association.
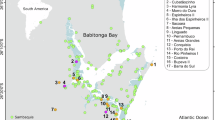
All circles correspond to worked objects except site 8, which is the assemblage of unworked fragments of whale bone from Santa Catalina. The size of the circles is proportional to the numbers of remains per site, except in Santa Catalina, where all the unworked remains are considered as a single one so as not to overwrite the representation. Bathymetric data are from Natural Earth (www.naturalearthdata.com). The Sables des Landes region appears in yellow. 1: La Paloma, 2: Las Caldas, 3: La Viña, 4: Tito Bustillo, 5: El Pendo, 6: El Juyo, 7: El Rascaño, 8: Santa Catalina, 9: Iruroin, 10: Ermittia, 11: Urtiaga, 12: Isturitz, 13: Bourrouilla, 14: Duruthy, 15: Brassempouy, 16: Saint-Michel, 17: Tuc d’Audoubert, 18: Mas d’Azil, 19: La Vache, 20: Courbet, 21: La Madeleine, 22: Andernach. Maps by AL. See Supplementary Data 1 for source data.
Only four worked objects, from three sites in the western Pyrenees and Cantabria (Duruthy, Isturitz and El Pendo), yielded dates in the 19-17.5 ka cal BP time range. The chronological distribution of the dates then shows a sharp rise to the 17.5-16 ka cal BP time range, with 26 objects from 12 sites (from west to east, 2 from La Paloma, 1 from Las Caldas, 3 from Ermittia, 1 from Urtiaga, 1 from Iruroin, 5 from Isturitz, 1 from Bourrouilla, 2 from Duruthy, 3 from Brassempouy, 2 from Saint-Michel, 3 from Mas d’Azil and 2 from La Vache), accompanied by a wide geographic extension from Asturias (Las Caldas) to the central Pyrenees (La Vache, Le Mas d’Azil) and a maximal diversity of taxa used. The distribution of the dates ends abruptly after ca. 16 ka cal BP.
At Santa Catalina, from the 31 fragments of unworked whale bone sampled for radiocarbon dating, all but one yielded reliable results (Fig. 2). The two fragments attributed to the North Atlantic right whale or bowhead whale (one of which was dated three times) yielded identical 14C dates and might come from a single individual that died ca. 16.5-16 ka cal BP. The 28 other dates are all from fragments of fin whale bone and, with one exception, cluster between ca. 16-14.5 ka cal BP (thus after the 17.5-16 ka cal BP time range that yielded most of the dates on worked objects). The number of acquisition events represented by these 27 dates remains undetermined, and the degree of fragmentation of the bones precludes the calculation of an anatomical Minimum Number of Individuals; but the distribution of the dates appears compatible with a minimum of two individuals, one ca. 15.5 and one ca. 15 ka cal BP. A single date ca. 13.6–13.2 ka cal BP is the only possible evidence of a later episode of bone acquisition on a fin whale individual.
Stable isotope analysis
Carbon (δ13C) and nitrogen (δ15N) stable isotope ratios were successfully measured in 55 whale bone samples and ranged from −16.8‰ to −11.6‰ in δ13C ratios and from 9.1‰ to 17.5‰ in δ15N ratios (Fig. 3). In total, 8 samples failed collagen extraction, 2 samples failed IRMS analysis and for 7 samples the atomic C:N ratio was too high (ranging between 3.77 and 6.28) or the N peak was below 700 mV (Supplementary Data 1).
The distribution of the stable isotope ratios is structured by taxa: fin whale samples are clustered at low δ15N values and (with one exception) intermediate values of δ13C; the single blue whale sample is nested among the fin whales; right/bowhead whale samples show low δ13C ratios and intermediate values of δ15N; gray whales display high δ13C ratios and intermediate values of δ15N; and sperm whales show elevated values both of δ15N values and δ13C (Table 1). The one sample where ZooMS failed to distinguish between right/bowhead and fin whales—due to the overall poor quality of the spectra and specifically the absence of a clear peptide marker at P2_ɑ2 292—is nested within the fin whale samples, strongly suggesting it might have corresponded to a fin whale (Fig. 3). We found no evidence that these patterns of stable isotopic signatures across taxa are driven by differences in the radiocarbon age of samples: there was no significant relationship between conventional 14C age and the δ13C values (see Methods below, and Supplementary Fig. 98).
A comparison between the stable isotopic signatures of Paleolithic whale samples with modern counterparts shows in most cases an overlap (at least partial) between the two. The large majority of modern samples used for this comparison originate from the same oceanic basin (Atlantic) as our samples, except for gray whale (Pacific) (Supplementary Data 4). Still, on average we found higher δ13C and δ15N ratios in ancient fin whales, higher δ15N ratios in ancient sperm whales, and higher δ13C ratios in bowhead/right whales and in gray whales when compared with modern whales (Supplementary Discussion, and Supplementary Figs. 99 through 102).
Discussion
Whale bone chronological and geographical distribution
A previous study of whale-bone objects from the Cantabrian coast suggested that, according to contextual evidence (both archeological and radiocarbon), the utilization of whale bone might have appeared in this region and in this archeological context ca. 18–17.5 ka cal BP14. Our results confirm this archeological association and the earlier evidence of whale-bone working the Cantabrian region, and push back the chronology of its inception by at least one millennium (with the caveat of radiocarbon dating uncertainty; see Methods below). The whale bones used for tool manufacture do not necessarily come from fresh carcasses, but they are unlikely to have been more than a few decades old, given the hazards of bone preservation in the open air on the seashore29, and given that the low density of cetacean bones30 suggests that they will be particularly susceptible to density-mediated attrition31. Accordingly, at our level of chronological resolution, the date of the material is essentially the same as the date of the bone working activity. Therefore, according to our results, the manufacturing of implements made of whale bone began ca. 20-19 ka cal BP on the southern shores of the Bay of Biscay. Since the visual identification of whale-bone objects from more ancient (Gravettian) archeological contexts remains uncertain32,33, these 20-19 ka cal BP dates represent to our knowledge the oldest evidence for the working of whale bone by hunter-gatherers. They place the inception of this industry at the beginning of the Late Glacial, when the sea level began to rise again after the LGM lowstand34. Between ca. 17.5 and 16 ka cal BP, the amount of archeological evidence increases sharply and the geographic distribution of the objects expands, encompassing an area between Asturias and the central Pyrenees, perhaps with an extension to the north and east of the Aquitaine Basin (given their archeological context, the objects found at La Madeleine, Dordogne, and Courbet, Tarn, might indeed date to the same period35,36,37). This period, which archeologically corresponds to the Middle Magdalenian in the Cantabrian region and the Late Middle Magdalenian in southwest France38, thus appears as the peak time for the production and diffusion of objects made of whale bone. No chronological trend appears regarding the type of objects manufactured or the taxa used, and this period in general can be considered as representing the maximum diffusion and diversity of this whale-bone industry. This is in accordance with other categories of archeological evidence showing the existence of active exchange/diffusion networks between the Cantabrian coast and the central Pyrenees at that time (lithic raw materials, art, and types of tools14).
Most of the objects made of whale bone are weapon elements (projectile points and foreshafts) typologically similar to the antler points that make up a large part of the Magdalenian hunting equipment39. From what can be observed on the finished objects, and on the few pieces of manufacturing waste documented37, the manufacturing techniques used to work whale bone into objects do not differ from those used for working antler. The choice of using whale bone to make one part of the weapon tips might be linked to the dimensions of the raw material, that make it possible to manufacture very long implements, and perhaps the specific mechanical properties of whale bone as compared to antler and to terrestrial bone14,24,40.
The chronological distribution of the whale-bone objects ends abruptly after ca. 16 ka cal BP. The only worked object that might be attributed to a later date is the whale-bone foreshaft from the Upper Magdalenian site of Andernach, in Rhineland—an object that was found to be made of gray whale bone in this study, and that can be attributed to ca. 16–15.5 ka cal BP according to the AMS dates from the context in which it was discovered40. Compared to the rest of the samples, this object is thus both the most geographically outlying and the most recent. Only tentative explanations can be put forth to explain this rarefaction. The period ca. 16–14 ka cal BP corresponds archeologically to the Upper Magdalenian—a cultural phase that, like the preceding one, yielded evidence for the exploitation of a number of coastal resources (such as mollusks10). The acquisition of fin whale carcasses at Santa Catalina after 16 ka cal BP, and the presence of a foreshaft made of gray whale bone at Andernach with contextual dates younger than 16 ka cal BP, both show that hunter-gatherer groups still had access to whale bone at that period. Therefore, the nearly complete lack of evidence for whale-bone working after 16 ka cal BP is not linked to an abandonment of coastal resources, or to a lack of suitable raw material or of technical skill. It could thus be either a purely cultural and technical choice (a disregard for whale bone as a raw material for tool making), or a question of archeological visibility linked to the interruption of the whale-bone diffusion networks. Indeed, since the whale-bone objects were probably manufactured on the seashore14,24, all objects of this type found at inland sites are imports. If the exchange and circulation dynamics that are responsible for the diffusion of these objects ceased to function, the whale-bone objects likely remained at the coastal sites, which are now submerged. There is currently no way to discriminate between those two hypotheses.
Cetacean diversity and ecology
The taxa identified in this study provide a picture of the rich biodiversity of cetaceans in the northeastern Atlantic during the Magdalenian period that may have been accessible to hunter-gatherers. With its highly productive waters and complex bathymetry, the Bay of Biscay remains today an area of high diversity and abundance of cetacean species41, but it may have been even more so in the past, given that multiple species were subsequently subject to intensive exploitation1. In addition, the climate in the Bay of Biscay was substantially cooler during the Magdalenian period, with sea ice likely present at least seasonally42, which means that the cetacean community may have more closely resembled that of today’s arctic waters. Given the role large cetaceans play in marine ecosystem functionality, these differences in cetacean composition and abundance may have translated into non-negligible effects on local primary productivity, with cascading effects across the broader ecosystem43,44,45.
Fin whales, sperm whales, blue whales and harbor porpoises are still present in the Bay of Biscay today41. Given their wide distribution, including cold waters46, it is not surprising that they were also present during the Magdalenian period, but they may have been more abundant prior to human exploitation1. Our results show that at least one balaenid species was found in the Bay of Biscay at the time, although the imprecision of the ZooMS ID does not allow for a distinction between right and bowhead whales; DNA analysis would be required to verify the species identities for these specimens (analyses which fell outside the scope of this study). Both species were heavily exploited in the western North Atlantic, with right whales now extirpated47 and bowheads nearly so48. Right whales were present in the Bay of Biscay until relatively recent times, where a coastal calving ground formed the basis of a Basque whaling industry from the 11th–18th centuries47. In the cooler Magdalenian waters42, it is more likely that right whales, if present, were using the area as summer feeding grounds. The sea-ice bound bowhead whales could have been a more likely species, with stable isotope data also providing some support in this direction (Supplementary Fig. 101). It is not impossible that both species could have been present, in different seasons.
The case of gray whales is more specific. Gray whales today have a strictly North Pacific distribution: they do not seem to have survived in the North Atlantic after the eighteenth century, having likely disappeared due to whaling despite the circumstances of their disappearance remaining poorly understood7,49. Previous evidence of their past presence in the North Atlantic dates either to the Holocene (10 to 0.25 ka cal BP) or to the middle of the Marine Isotope Stage 3 (MIS3), before 40 ka cal BP; with the absence of gray whales in the intervening period attributed to a drastic reduction or even extirpation of Atlantic gray whale50,51,52. Furthermore, molecular analyses revealed little genetic continuity between the late Pleistocene and the Holocene populations: most Atlantic Holocene specimens analyzed by Alter et al.50 are genetically closer to Holocene Pacific gray whales than to Pleistocene Atlantic specimens. This led these authors to suggest that the majority of the Atlantic Holocene population were the result of a second colonization event from the Pacific, after warming temperatures, sea-level rise, and decreases in sea ice permitted passage through the Bering Strait50. The 11 artifacts made of bones from gray whales presented here are evidence of the presence of this species in Atlantic waters at the end of MIS2, thus supporting a continuity between the Pleistocene and the Holocene populations.
The stable isotope composition of marine vertebrates is controlled by their diet and habitat preferences53,54 and thus the stable isotope dataset presented in this study sheds light onto past whale ecology. In general, the stable isotopic signatures of Paleolithic whales overlap to some extent with those of their modern counterparts, suggesting broadly similar feeding strategies, while existing differences can be ascribed to variation in stable isotope baseline values, feeding ground locations and possibly trophic level (Supplementary Discussion). As in modern whales, ancient fin whales and blue whale samples have relatively low nitrogen stable isotope ratios in comparison with the other whale samples, consistent with these species’ reliance on krill55,56, whereas gray whales display higher δ13C ratios, characteristic for their feeding behavior in benthic environments57, while sperm whales (the only toothed whale species in this dataset) show the most elevated δ15N values, reflecting a diet at a higher trophic level including large squid58 (Fig. 3). The fact that bowhead/right whales (which feed mostly on copepods) have higher δ15N values than fin whales (again, also found in modern whales) is less straightforward to explain, but likely explained by feeding in different water masses, highlighting the complexity of interpreting isotopic signatures (Supplementary Discussion). This said, in most cases the overlap in stable isotope signatures between modern and ancient whale samples is only partial, with ancient whales tending to exhibit higher δ15N and/or δ13C values. These differences may reflect a shift in whale feeding preferences (e.g., if modern whales now target prey at lower trophic levels or warmer waters), large scale temporal changes in the stable isotope baseline59, environmental change, or a combination. Indeed, previous studies found rapid changes in whale feeding behavior (and in corresponding stable isotope signatures) as a result of environmental changes: for example, Jory et al.60 found that a reduction in biomass of artic krill coincided with a dietary niche widening in fin whales in Canadian waters, with 60% of individuals changing their foraging strategy from specialist to generalist feeders in order to reduce intraspecific competition. Given the magnitude of the environmental change from the Late Pleistocene to the present, the changes in whale foraging strategies may well have been even stronger.
The stable isotope analysis complements the ZooMS results in showing a clear differentiation between whale species, and furthermore allowed us to identify as likely fin whale one specimen for which identification through ZooMS was imprecise (Fig. 3). It also suggests a higher likelihood that the Balaenidae samples correspond to bowhead whales rather than right whales (Supplementary Fig. 101) although this is less certain. While the arctic conditions in the region would favor the bowhead hypothesis, the region could similarly have functioned as a northerly feeding ground for right whales. These results highlight the added value of combining multiple analytical methods to maximize the information obtained from archeological records of human-whale interactions2,53.
Whale products acquisition
With the exception of the object from Andernach, all our specimens come from karstic environments (cave and rockshelter sites) with good preservation of osseous material, including small elements. The relative abundance of the different whale taxa in our sample is thus unlikely to be affected by taphonomic factors such as differential preservation of bone tissues. However, these proportions cannot be used to infer the relative frequency of the whale taxa in the past, because they are biased by the anthropic choices of the Magdalenian hunter-gatherers and by our sampling strategy. For example, the scarcity of the harbor porpoise remains might reflect a lower value of the bones of this smaller species for tool manufacture. Conversely, the large proportion of fin whales in our sample is biased by our decision to systematically analyze the whale bones from Santa Catalina, where this taxon dominates.
The taxonomic composition of the whale-bone assemblage gives nonetheless an indication of the way hunter-gatherers acquired the whale resources. The worked objects and unworked bone fragments we analyzed are mainly large cetaceans that predominantly forage offshore or in areas with deep water close to shore like the Bay of Biscay—namely fin whale, sperm whale and blue whale. It is extremely unlikely that these species would have been accessible to hunter-gatherers from the European Pleistocene other than through passive acquisition methods, such as the opportunistic acquisition of natural strandings or drift whales. In addition, the list of identified taxa also includes species whose ecology brings them substantially closer to the coast, namely gray whales, right/bowhead whales and harbor porpoises46. Bowhead whales and gray whales are the large cetaceans with the longest recorded history of active whaling, going back at least three millennia in arctic ecosystems53,61. However, there is no evidence that European Pleistocene hunter-gatherers had the necessary technologies for hunting these species, such as seafaring62, or multibarbed points that could have been used as harpoons heads (barbed points appear in the local archeological record only after 16 ka cal BP39). Overall, then, the archeological evidence points towards an opportunistic acquisition of whale resources in the Bay of Biscay during the Magdalenian period, unlike in more recent periods where this same region became an area of active whaling7,47.
Whales were likely familiar to coastal communities, and as such may well have played an important role in the Magdalenian cultural world. The Bay of Biscay’s highly productive waters make it a cetacean hotspot today41, and it was likely already the case during the Pleistocene. Along the Spanish coast in particular, water depth increases dramatically just a few kilometers from the coastline, bringing species that are typically found offshore, such as fin whales63, unusually close. These species could have been observed from high points along the coastline spouting at a distance, and irregularly closer to shore as dead or moribund individuals. The more coastal right/bowhead whales, gray whales and harbor porpoise were likely even better known. Gray whales in particular are the most coastal of the whale species46, spending summer at low-depth high-latitude feeding grounds, then migrate hugging the coastline to warmer lower-latitude calving areas in sheltered low-depth lagoons. Whereas in today’s climate the Bay of Biscay and the Mediterranean would more likely correspond to calving grounds, it is possible that during the cooler Upper Paleolithic climate they would have corresponded to feeding grounds49,64—habitat reconstructions indicate that adequate shallow shelf habitat was available there during the last glaciation50. In any case, they would have been conspicuously present at predictable seasons, and they are likely to have been part of the range of animals whose ecology would have been well known to Paleolithic hunter-gatherers.
Whale products utilization and transportation
The whale remains analyzed in this study attest to two different utilization behaviors. The first one is the use of whale bones as raw material for the manufacture of implements; as suggested in previous studies, the choice of this raw material might have been motivated by the large dimensions of these bones, enabling the manufacture of longer implements, and maybe their specific mechanical properties14,24. The two main types of objects manufactured are projectile points and foreshafts, both relating to hunting equipment. Thirty-three projectile points were analyzed with ZooMS, and the results show that the range of species used for their manufacture is varied, with a relative majority of sperm whale (13 of the 31 points whose species could be identified, i.e., 42%; vs. 3-23% for each of the 5 other species identified). For the 11 foreshafts analyzed, the dominance of sperm whale is even more pronounced (8 of 11: 73%). It is uncertain whether this higher frequency compared to the other taxa reflects the relative abundance of sperm whales among stranded individuals or if it testifies to a preference for this species on the part of the Magdalenian carvers. The second hypothesis might be supported by the fact that, among the large cetaceans present in our sample, sperm whales are the only toothed species, displaying a highly characteristic, long, straight, toothed jawbone that may have been considered as a particularly desirable block of raw material. An interest in sperm whale teeth in the Magdalenian is also evidenced by the two carved specimens found at Las Caldas and Mas d’Azil10. Nevertheless, since the precise anatomical parts used for tool-making are so far indeterminate, this supposition remains speculative.
The second utilization behavior, evidenced at Santa Catalina, is the transportation of unworked whale bones to habitation sites. The bones were found in the form of fragments, most of which are too small and fragmented to be identified anatomically; still, some of them can be attributed to ribs and to vertebrae (cf. fragments of vertebral disks), evidencing the presence of portions from the trunk, and several display percussion notches indicating anthropic breakage carried out at or outside the site. The transportation of these elements requires explanation, as the species involved (fin whale and right/bowhead whale) are large, and the site was by then 4-5 km from the coast and 70 m up a steep cliff65. Indeed, bones of large whales are bulky elements, usually abandoned on the spot where the whale is processed rather than carried as riders attached to the meat and blubber. Their use as implements at the site (ribs for building huts, vertebrae as stools or anvils, etc.) is implausible given their degree of fragmentation, and evidence for their use as raw material for toolmaking is very scarce. Only one fragment shows traces of scraping, and most of the elements are very spongy, while the whale-bone objects known from other sites show a denser osseous tissue indicating the use of denser parts of the skeleton. An alternative explanation is their use as bone fuel for hearths. The use of the bones of terrestrial mammals as fuel is frequently documented in Paleolithic contexts66, and activities linked to fire, including bone fuel, are intensive in level III of Santa Catalina (presence of charcoal, hearth-like structures, etc.67). However, at Santa Catalina the whale bones we analyzed are not specifically affected by burning, nor concentrated near the hearths; if the collecting of whale bone at this site has to do with fire, it could only be as a reserve for later use. Alternatively, since living animal whale bones are very rich in fat, these large spongy bones might have been brought to collect the oil by letting them drip, or by crushing them2. This hypothesis would explain the fragmentation of the bones. It is consistent with the strong interest of Paleolithic groups for fat (e.g., the nearly systematic breakage of the bones for marrow68), and with the fact that bone grease rendering is evidenced in the level III of Santa Catalina on the bones of ungulates69.
Finally, although our archeological sample includes only bones and bone objects, their presence shows that hunter-gatherers also had access to other whale resources2. Whale skin, blubber and meat found numerous dietary and technical uses among populations of the past, and the transportation of (at least) whale skin to the habitat was already evidenced among European hunter-gatherers through the discovery of right whale barnacles at the sites of Las Caldas, Nerja and Cueva Victoria11. Furthermore, the identification of several species of mysticetes in our sample proves that Magdalenian hunter-gatherers also had access to baleen plates. The baleen of right and bowhead whales in particular have long been appreciated as a strong and flexible material with many practical uses in several populations2. In this case, the osseous material acts as a proxy for other resources with more rapid rates of degradation.
This study provided a chronological and taxonomic characterization of Late Paleolithic whale-bone industry. Its inception on the southern shores of the Bay of Biscay takes place within a more general trend towards the intensification of the exploitation of coastal resources at that period and in that region. The acquisition of whale products seems limited to the opportunistic utilization of beached or drift whales, but likely included the use of many resources besides bones—including baleen, the availability of which is now demonstrated, and whale oil, as possibly evidenced by the presence of unworked and fragmented bones at Santa Catalina. For this reason, stranding events certainly affected the groups’ mobility patterns by acting as attractive spots. In this perspective, although whales might not have been the primary impulse for the increased interest in the seashore during the Magdalenian, their presence undoubtedly contributed to this evolution and strengthened it, as was suggested for other regions70. Furthermore, the taxonomic diversity and chronological depth of the cetacean populations identified in this study—both previously undocumented for this region at this time period—attest to the richness of the marine and coastal ecosystem of the Bay of Biscay at the end of the Paleolithic, showing how much it represented a favorable milieu for human settlement.
Methods
Sample selection, photogrammetry and sampling
The archeological specimens analyzed in this study are curated in 10 museums in France, 7 museums in Spain, 1 museum in the United Kingdom, 1 museum in Germany, and 3 curatorial repositories in France, under the responsibility of the Ministry of Culture, for collections from sites with excavations in progress. The location of each analyzed specimen can be found in Supplementary Data 1. Permission to access, study, borrow or export (when relevant), and sample the specimens was obtained from each institution, curator and/or excavation director following each local procedure (see detailed list in Acknowledgements section).
A total of 185 worked objects visually identified as made of whale bone are known from 31 Magdalenian sites (Supplementary Data 2: 165 objects from previous studies14,24,40,71, plus 20 recently discovered through excavations or the reassessment of ancient collections). Minimally-invasive ZooMS analysis was tested on a subset of the samples, but proved to be unsuccessful (see below). In order to maintain the morphological integrity of the worked bone assemblage, a destructive ZooMS approach was applied to 83 of the 185 objects (i.e., 45%), with samples selected to ensure a representative selection of sites (n = 26) and of the objects’ typology (these 83 objects include 3 ZooMS results previously published36,37). In addition, at Santa Catalina, 41 unworked bone fragments had previously (i.e., before this study) been attributed to cetaceans on a visual basis12. In this study, the bone assemblage of Santa Catalina was reassessed and sampled to include all fragments that could potentially be whale bone, even if the morphological identification remained somewhat uncertain. A total of 90 unworked bone fragments from Santa Catalina (the 41 previously identified + 49 new specimens) were subjected to ZooMS analysis, mostly from archeological level III.
Half of the elements whose identification as whale bone was confirmed by ZooMS were subject to additional sampling for radiocarbon dating and stable isotope analysis: 37 of the 71 worked objects (52%), and 31 of the 60 bones from Santa Catalina (52%). The single object identified as made of porpoise bone through ZooMS analysis was a thin fragment of point, and permission was not granted to sample material sufficient for both ZooMS and 14C dating.
Before any sampling, photogrammetry was used to produce a 3D model of each worked object and each unworked bone fragment in its entirety. This was done for conservation reasons, in order to preserve a digital copy of the specimens before they were morphologically altered by the sampling. Each bone selected for sampling was photographed using a Nikon reflex with 40 or 60 mm lens, a portable light tent with different lights to provide diffuse light, and a turntable with markers for metric projection and angular notations. The Adobe® Photoshop® software was used for post-processing (color adjustment and background masking) and the Agisoft® Metashape® software was used for the photogrammetric process. For each artifact, both a dense cloud (for accuracy studies) and a textured mesh (for exports and measurements) were produced.
Depending on the shape of each element, sampling was done either: by cutting a piece from one of the extremities of the bone (using either a Stanley® mini hacksaw or a Dremel® rotary tool); by drilling with a Proxxon® Colt 2 pocket drill; or by coring using homemade diamond-coated core drills, 4–6 mm in diameter, mounted on a Moviluty® Minyflex® rotary tool. On average, 75.7 mg of bone was extracted from each specimen for ZooMS, with no significant difference between the worked objects and the unworked bone fragments. For radiocarbon dating only, the average mass of the sample was 95.3 mg on the worked objects, and 340.6 mg on the unworked fragments from Santa Catalina.
ZooMS
ZooMS was first developed as a destructive analytical technique, requiring ~10–30 mg of bone or bone powder for analysis26. This project aimed to limit the destructive testing of unique, Magdalenian modified bone objects in order to preserve their morphology. As a result, for the bone objects, a minimally-invasive collagen sampling method was attempted which had been developed for analysis of historic parchments72, and which had previously proved successful on ca. 500 years old modified bone objects from Quebec, Canada73. The 90 unworked cetacean bone samples, and 83 modified bone objects, also underwent a destructive ZooMS protocol to achieve accurate taxonomic identifications (see results above). The bone samples were analyzed using the destructive ZooMS methods listed below, based on a modified protocol as described in Buckley et al.26.
Minimally-invasive ZooMS
The bone objects were gently rubbed with a clean piece of PVC eraser; collagen is transferred from the object to the eraser crumbs through the triboelectric effect72. The collected eraser crumbs from each sample were incubated for four hours at 37 °C in 75 µL of 50 mM ammonium bicarbonate solution (NH4HCO3, pH 8.0, AmBic) and 0.4 µg of trypsin. Trypsin activity was terminated using 1 µL of 5% TFA solution, and peptides were purified using C18 ZipTip® pipette tips and eluted with 50 µL of conditioning solution (50% acetonitrile, 0.1% TFA). 1 µL of eluted peptides was then spotted onto a Bruker ground steel target plate, to which 1 µL of matrix (α-cyano-4-hydroxycinnamic acid) was added. Each sample was spotted in triplicate along with calibration standards, and run on a Bruker Ultraflex III MALDI-ToF-MS. Triplicate spectra were averaged and analyzed using mMass software74 and compared to a database of known collagen peptide masses (Supplementary Data 3)75,76,77,78.
The minimally-invasive sampling technique proved unsuccessful for the majority of the tested samples. This was likely due to a combination of factors, including cetacean bone morphology and sample contamination. Due to the physical requirements of cetaceans, their bones often take on one of two forms depending on the element—either very dense and highly mineralized, or very porous and friable. Both bone conditions are problematic for minimally-invasive sampling methods as they result in reduced availability of surface collagen. Other studies applying the minimally-invasive eraser method also found limited success compared with destructive approaches73,79,80; as a result, a destructive and/or double extraction was performed on the samples.
Destructive ZooMS
Subsamples of bone or bone powder, sometimes several per specimen and ranging from 20 to 70 mg, were demineralized in 250 µL of 0.6 M HCl (4 °C). The acid was removed and samples were rinsed three times in 200 µL of 50 mM AmBic. Samples were incubated in 100 µL of AmBic for one hour at 65 °C to gelatinize the collagen; then 0.4 µg of trypsin was added to 50 µL of supernatant and incubated overnight at 37 °C. Trypsin activity was terminated using 1 µL of 5% TFA solution, and peptides were purified using C18 ZipTip® pipette tips and eluted with 50 µL of conditioning solution. MALDI-TOF-MS was conducted as described above.
Destructive ZooMS double extraction
Several samples analyzed using the minimally-invasive sampling method returned results that suggested sample contamination, likely as a result of a consolidant that had been added to the specimens post-excavation. This contamination particularly affected specimens from the Saint-Périer excavations (during the 1930’s) at the site of Isturitz. While there is no mention in the reports for these excavations of the artifacts having been conserved with any type of consolidant, both minimally-invasive and destructive analyses of a number of these specimens consistently returned collagen identifications of cattle (Bos genus). Upon further inspection of the spectra, peptide markers that appeared to match those of various cetaceans could also be observed, however at a much lower intensity than the cattle fingerprint (Supplementary Fig. 91). This mixed signal in the collagen spectra led us to believe that consolidant contamination was a likely issue, as collagen-based glues made from cattle (and other animals) were known to have been used in conservation practices during the early 1900’s.
A double extraction method was developed in an attempt to remove or reduce the signal from the contaminating consolidant (Supplementary Fig. 91)81. In this method, bone or bone powder was immersed in 250 µL of 50 mM ammonium bicarbonate solution (NH4HCO3, pH 8.0, AmBic) and placed on a shaker at room temperature for 3 h. Samples were then centrifuged briefly then incubated at 65 °C for one hour to gelatinize. After incubation, the samples were centrifuged for one minute and the AmBic was transferred to a new eppendorf as the first extraction. Subsequently, 250 µL of 0.6 M HCl (4 °C) was added to each of the original eppendorfs containing the bone chips/powder, then vortexed and left to demineralize at room temperature for four hours. The acid was removed and samples were rinsed three times in 200 µL of 50 mM AmBic. 100 µL of AmBic was then added to the samples, followed by incubation for one hour at 65 °C to gelatinize the collagen for the second extraction. Both the first and second extraction underwent digestion, purification and MALDI-TOF-MS analysis as described above. While this modified protocol resulted in lower peptide recovery, it enabled us to enhance the collagen signal from the actual ‘host’ animal (in most cases, cetacean collagen peptide markers) while also confirming our suspicions regarding the use of a cattle-based consolidant (Supplementary Fig. 91).
With the exception of 7 samples (Hum-1 to Hum-18) for which collagen extraction was carried out at the EvoAdapta facility and MALDI-TOF-MS analysis at the University of York, all samples were analyzed for ZooMS at the Institute of Environmental Science and Technology (ICTA-UAB) of the Universitat Autònoma de Barcelona.
Bone collagen extraction protocol
All glassware was cleaned using the following procedure: submerged in Decon 90 at 90 °C and cooled overnight, washed three times with DI water, submerged overnight in 10% HCl, washed three times with Milli-Q. After drying, the glassware was wrapped in aluminum foil to prevent dust contamination during storage and finally baked out in the oven at 450 °C for 5 h.
The bone collagen extraction protocol based on Longin82 followed the following steps: Bone samples were first demineralized in 0.2 M HCl for several days, with mechanical and visual checks. The acid was renewed several times during this step. The samples were then rinsed three times with Milli-Q, submerged for 20 min in 0.1 M NaOH (with new NaOH being added for another 20 min if discoloration appeared), before being rinsed again three times with Milli-Q, submerged into 0.1 M HCl for 10 min, then rinsed again three times with Milli-Q. In the next steps, the samples were gelatinized in weak (pH 3) HCl at 90 °C until dissolution, filtered with glass filter units (mesh size 10–20 μm), frozen with liquid nitrogen and lyophilized in clean (baked out) vials.
Radiocarbon dating
As anomalous peaks were observed in FTIR-ATR spectra of several samples, most likely indicating the presence of glue or consolidant, the XAD resin approach was applied to eliminate this type of contamination. This approach was developed by Stafford et al.83,84,85, is also described in van der Sluis et al.86. Lyophilized collagen samples were dissolved in 1 mL of (sub boiling distilled) 6 M HCl in 10 mL borosilicate tubes with PTFE lines caps before being hydrolyzed at 110 °C for 24 h. The hydrolysate was then passed through pre-conditioned XAD columns fitted with a filter frit at the bottom, filled with ±100 μl (circa 1 cm) of XAD 2 resin slurry and covered with the top filter frit. The latter was pushed down in order to remove air bubbles. The columns were then washed with 20 mL of 1 M HCl and preconditioned with 10 mL of 6 M HCl. After the sample hydrolysate had passed through, the column was washed with 1 bed volume of 6 M HCl in order to collect any amino acids in the void space and added to the collected sample. Samples were dried in small open beakers on the hotplate in the fume hood and were rinsed with Milli-Q to remove any leftover HCl by evaporation. The samples were then transferred in 200 μl (7-8 drops) of Milli-Q to combustion tubes using glass Pasteur pipettes, frozen and lyophilized.
Samples were connected to the CO2 extraction line in the radiocarbon laboratory of the Muséum national d’Histoire naturelle. After adding 900 mbar pure O2, samples were combusted at 900 °C for a duration of 10 to 20 minutes in the presence of a baked out silver strip (10 mg) to remove contaminants, cleaned on the CO2 extraction line (water trap, NOx oven fitted with copper and silver fiber wool), then volume calculated. The vacuum on the line reached 10-6 mbars. The CO2 gas sample was transferred to a fully-automated H2 reduction line using iron as a catalyst, where the vacuum reached 10-7 mbars.
Samples were run alongside standards of oxalic acid and phthalic acid. Graphite targets were pressed and analyzed on the same day with the ECHo-MICADAS at the Laboratoire des Sciences du Climat et de l’Environnement (LSCE) in Gif-sur-Yvette, France. Data reduction was performed using the BATS software (version 47)87. The first scans were discarded to eliminate possible contamination of the target with ambient atmosphere between target pressing and AMS measurement. Radiocarbon ages were calculated from F14C88, which is corrected for background and isotopic fractionation. Measurement parameters such as 12C current and 13CH current were monitored during 14C measurement. Time, current and isobar corrections were made before validation. For each individual run, normalization, correction for fractionation and background corrections were applied by measuring the oxalic acid II NIST standard, its 13C/12C ratio and the chemical blanks. The standard deviation of the blanks is generally less than 10% but an overestimated 30% is imposed to the blank value in order to take into account a potential variability of the contaminant which could be added during the sample preparation.
Six samples (Hum4, Hum5, Hum8, Hum9, Hum14 and Hum18) were chemically pretreated and prepared at the Higham Lab, University of Vienna, Austria. Sample pretreatment for these samples follows collagen extraction outlined in Brock et al.89. Three samples (Hum9, Hum14 and Hum18) were combusted offline, graphitized using an AGE graphitization unit, and measured at the VERA (Vienna Environmental Research Accelerator) AMS facility, University of Vienna. For details on aspects of target preparation and AMS measurement see Steier et al.90 and Golser & Kutschera91. Three other samples (Hum4, Hum5 and Hum8) were measured at the Keck AMS facility, University of California at Irvine. For details on aspects of target preparation and AMS measurement see Santos et al.92 and Beverly et al.93. These six samples have R-numbers and VERA or UCIAMS numbers instead of MUSE-numbers.
Collagen quality control
As diagenetic alteration can affect stable isotope values as well as radiocarbon ages, only results from good quality collagen with an atomic C:N ratio between 2.9 and 3.6 ought to be used94. However, studies by van Klinken95 on archeological bones and Guiry and Szpak96 on modern bones have suggested that values outside the 3.0-3.3 range for mammals may result in distorted isotopic compositions due to contamination by non-collagenous carbon. We compared the values for whale species that agree with different atomic C:N ratio ranges (2.9–3.3 and 3.3–3.6) (Supplementary Fig. 96). In these figures there is considerable overlap between samples with an atomic C:N ratio up to 3.3 and 3.6, only the gray whale samples show lower carbon stable isotope values (circa 1‰), although this could also be due to the small sample size. We believe most of the variation visible here is due to individual variation between the whales, rather than diagenetic alteration. Whales are exceptionally large (marine) mammals, thus resulting in a larger variation in stable isotope ratios in their bone collagen due to their longevity and slow bone turnover time. Samples with atomic C:N ratios between 2.9–3.6 were included in the discussion of the stable isotopes results. Additionally, stable isotope analysis was done on collagen, while 14C dating was performed on collagen after XAD resin treatment, which would have removed any, if present, remaining contamination. When examining the 14C ages against atomic C:N ratios from a single site (Santa Catalina) (Supplementary Fig. 97), there is no evidence of a correlation that might indicate contamination.
Calibration and reservoir correction
Dating of marine animals needs to take into account the marine reservoir effect: given that oceans are depleted in 14C in relation to the atmosphere, conventional radiocarbon dates for marine organisms appear older than for coeval terrestrial individuals97. Some studies use sample pairing between coeval terrestrial and marine taxa to aid interpretations of reservoir ages at archeological sites98. This was not possible in our case, because our samples are all from Pleistocene cave and rockshelter sites with complex stratigraphy (in which each excavated layer is the result of an accumulation of multiple occupation episodes) and most of our samples are from ancient excavations with poor stratigraphic resolution. We thus relied on the correction for the reservoir effect implemented for marine samples in OxCal 4.4, which is based on the Marine20 calibration curve99.
In addition, dating of marine organisms can be further refined by taking into account the fact that the marine reservoir effect varies across oceanic masses, being for example stronger (i.e., leading to seemingly older organisms) at higher latitudes and in deeper waters100. This deviation from the standard (globally averaged) marine reservoir effect is expressed as a DeltaR term. In the case of our whale specimens, however, it is not the coastal waters adjacent to the archeological sites that matter, but the water mass (or masses) where whales fed. The whale species identified in this dataset are very diverse in terms of their feeding ground locations and feeding depths. For example, previous studies estimated DeltaR values: between −168 and 504 ± 60 years for sperm whales (that feed in deep waters but can be found in either high latitudes or sub-tropical seas101); at 24 ± 58 years for bowhead whales (that forage in polar/sub-polar waters close to the sea surface102); and about 350 years for a fin whale (a species that forages at depths up to 400 meters and migrates vast distances103). Intra-specific variability in DeltaR values renders it difficult to obtain meaningful values per species. We have not found published estimates of DeltaR for gray whales, but the foraging strategy of this species (which feeds on invertebrates found in shallow sedimentary environments and migrates over very long distances) renders it particularly susceptible to intraspecific variation in DeltaR, especially as it will be dependent on the type of sediment (molluscs feeding on calcareous sediments appear over 2000 years older than suspension-feeding ones104).
In addition to these difficulties, DeltaR values published in the literature from modern (Holocene) whales need to be approached with caution when applied to Pleistocene samples. Indeed, the spatial variation in the reservoir effect of water masses likely varied over time, in response to changes in ocean circulation. The foraging grounds of whales are also likely to have been different, given that in the Late Pleistocene, the study region was substantially cooler. For these reasons, we have opted for not attempting to apply a species-specific DeltaR based on information from modern whales, and instead use a single DeltaR as suitable as possible to the period and region of our samples.
Choosing the most suitable reservoir correction required careful consideration of various parameters. Foote et al.105 used a DeltaR = 0 to correct for the reservoir effect in bowhead whales during the Pleistocene. However, this no longer seems suitable since the update of the Marine20 calibration curve, as there are large fluctuations in pre-Holocene reservoir ages97. Monge Soares et al.106 published a DeltaR = −117 ± 70 yr for the Late Pleistocene based on three samples pairs (shells and bones/charred wood) from the Cantabrian coast. Mangerud et al.107 showed that there is little difference between the DeltaR from whales and mollusks found at the same location. However, since the update of the Marine20 calibration curve, the DeltaR from Monge Soares et al. needs updating as well. Monge Soares et al. used three paired 14C ages for the calculation of their Late Pleistocene DeltaR (terrestrial 14C ages: 15 656 ± 75, 19 710 ± 120 and 23 040 ± 50 yr BP). Considering the ages of our samples, we decided it would be most suitable to use the two youngest paired 14C ages for an updated DeltaR, because the third sample pair was considerably older. These two paired Late Pleistocene 14C dates from Monge Soares et al. were used in the online application108 to calculate updated DeltaR values. These new DeltaR values are statistically the same (0.037 (χ2:0.05 = 3.84)). The weighted mean of these DeltaR and standard deviation are calculated according to: http://calib.org/marine/AverageDeltaR.html, which gives a new Delta R of -448 ± 39 yr. The calibration was done in OxCal 4.4 (Supplementary Figs. 92 through 95, and Supplementary Code 1)99.
Additionally, using all North Atlantic whales (various species) in the Marine Reservoir Correction Database we get a DeltaR of −168 ± 53, compared with the DeltaR of −117 ± 70 from Monge Soares et al. for the Holocene. This means that the latter slightly overestimate the reservoir age, making samples slightly younger. This may not be the case for the Late Pleistocene samples but it does strengthen our idea that the material used for paired dating in this region by Monge Soares et al. is not that far off from whales themselves. However, the DeltaR used here is not optimal for two species in this dataset, namely sperm whales due to their large variation in ΔR (between –168 and 504 ( ± 60) 14C yr101) and gray whales due to feeding in shallow waters where they might ingest calcareous sediment with different 14C ages104.
Heaton et al.100 propose a specific calibration of marine samples from polar regions, essentially calculating a range between a minimum and maximum DeltaR. The minimum is based on the Marine20 curve and the maximum is based on the latitude of the sample and the Large Scale Geostrophic Ocean General Circulation Model (LSG OGCM), which is suggested to use for samples from outside ∼40°S–40°N during glacial periods. Our samples are found between of 43–44 °N, although the whales could of course have been feeding at higher latitudes. However, we do not have the exact location.
We state that our results provide evidence of whale bone working from 20ka BP, which is based on two fin whales. If the absolute max DeltaR would be applied, these 2 fin whales would be between 2000-1500-1000 years younger (Fig. 3 in Heaton et al.100, although there is a substantial variation between 71 °N, 88 °N and 53 degrees °N, and again we do not know where these animals were feeding). It would be more realistic to assume sea ice most likely covered a large part of the North Atlantic waters, extending much further south than it does today. If so, most whales would not have been feeding too far north due to sea ice coverage (unlikely reaching the 71 and 88 °N, except for beluga’s and bowheads). Additionally, modern fin whales feed in various locations, have summer and winter feeding grounds, and can also adapt their feeding strategy to minimize interspecific competition, suggesting that the maximum value would not be a realistic assumption. Combining all of these assumptions, it is plausible that the maximum DeltaR was ~800 yr, although its value remains extremely uncertain.
Given that the results we present here reconstruct the chronology of whale acquisition using very broad time slices of 1.5 millennia (Fig. 2), it is unlikely that our conclusions are significantly affected by an uncertainty on DeltaR in the range of a few centuries. It does however mean that caution is needed when interpreting individual radiocarbon dating results. These can be improved in the future as more information becomes available regarding suitable corrections for the marine reservoir effect.
Isotope ratio mass spectrometry
Bone collagen samples (320–380 μg) were weighed into tin capsules (5 × 8 mm) and analyzed with a Thermo Scientific EA Flash 2000 coupled to a Delta V Advantage isotopic mass spectrometer in the Service de Spectrométrie de Masse Isotopique (SSMIM) in the Muséum national d’Histoire naturelle, Paris, France. Isotopic values of all samples were measured relative to the laboratory standard alanine, which has a reproducibility of 0.3 wt% for N and 0.6 wt% for C. The δ13C and δ15N values are reported relative to the VPDB and AIR, respectively. Three primary standards were used to calibrate the Alanine for δ15N air: IAEA USGS-25 (ammonium sulfate), IAEA N1 (ammonium sulfate) and IAEA N2 (ammonium sulfate), while one primary standard (from IAEA) was used to calibrate the Alanine for δ13C V-PDB: IAEA 600 (caffeine). Analytical precision is ± 0.2‰ for both δ13C and δ15N values. Five samples were run in duplicate and the difference fell between 0-0.3‰ for δ13C ratios, and 0–0.34‰ for δ15N ratios.
Subsamples (0.2–0.3 mg) of extracted bone collagen from six samples (Hum4, Hum5, Hum8, Hum9, Hum14 and Hum18) were taken for the analysis of isotope ratios of carbon and nitrogen by elemental analyzer-isotope ratio mass spectrometry (EA-IRMS; Thermo Scientific EA-Isolink with a Flash 2000 coupled to a Delta V Advantage isotope ratio mass spectrometer) in the Silver Laboratory (Large-Instrument Facility for Advanced Isotope Research) at the Center of Microbiology and Environmental Systems Science of the University of Vienna. Stable isotope values of all samples are measured relative to the laboratory standard alanine, which has a reproducibility of 0.07 wt% for N and 0.1 wt% for C. δ13C and δ15N values are reported relative to the VPDB and AIR standard, respectively, and are measured with an analytical precision of ± 0.1‰ for both δ13C and δ15N values. Acceptable stable isotope ratios have an atomic C:N ratio that falls between 2.9–3.694.
The difference in δ13C ratios between the balaenidae, fin and gray whales suggests these whales were feeding in different bodies of water, which in turn may have affected their radiocarbon ages. To investigate this, we plotted the conventional 14C age against the δ13C ratio from these whale bones (Supplementary Fig. 98). We found no correlation between these two variables (R2 = 8 × 10−5). The ages of the three whale groups studied broadly overlap: balaenidae (right/bowhead whales; n = 8) are dated from 16–16.5ka to 17.1–17.8ka cal BP; fin whales (n = 31) are dated from 13.2–13.5ka to 19.5–20ka cal BP; gray whales (n = 4) are dated from 16.3–16.9ka to 17.4–18ka cal BP.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data needed to support the conclusions of the paper are presented in the paper, the Supplementary Information, Supplementary Data, and Supplementary Code 1. The location of each analyzed specimen can be found in Supplementary Data 1. Source Data for Figs. 1 and 2, and for Supplementary Figs. 92 through 95, can be found in Supplementary Data 1. Source Data for Fig. 3 and for Supplementary Figs. 96 through 102 can be found in Supplementary Data 4. Collagen peptide mass spectra have been publicly deposited in the Nakala Repository under following link: https://doi.org/10.34847/nkl.dbbfl6fw.
Code availability
Code used in OxCal 4.4 can be found in Supplementary Code 1.
Change history
21 August 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41467-025-63273-w
References
Christensen, L. B. Marine mammal populations: reconstructing historical abundances at the global scale. Fish. Cent. Res. Rep. 14, 1–161 (2006).
Charpentier, A. et al. What’s in a whale bone? combining new analytical methods, ecology and history to shed light on ancient human-whale interactions. Quat. Sci. Rev. 284, 107470 (2022).
Smith, A. B., Kinahan, J. The invisible whale. World Archaeol. 16, 89–97 (1984).
Gusinde, M. et al. Los Indios de Tierra del Fuego: los Yamana, Buenos Aires, CAEA. (1986).
Turner, L. M. et al. An Aleutian Ethnography, Fairbanks, University of Alaska Press (2008).
Lee, S. M. et al. Chasseurs de baleines: la frise de Bangudae, Corée du Sud, Paris, Errance. (2011).
van den Hurk, Y. et al. The prelude to industrial whaling: identifying the targets of ancient European whaling using zooarchaeology and collagen mass-peptide fingerprinting. R. Soc. Open Sci. 10, 230741 (2023).
Nabais, M., Soares, R. & van den Hurk, Y. The Zooarchaeology of ancient whaling practices in Portugal: A review and a new Roman Republican contribution at Castelo Velho de Safara. PLoS ONE 19, e0310215 (2024).
Erlandson, J. M. The archaeology of aquatic adaptations: paradigms for a new millennium. J. Archaeological Res. 9, 287–350 (2001).
Pétillon, J.-M. et al. Life on the shore of the Bay of Biscay in the Late Upper Paleolithic: towards a new paradigm. In: Dupont, C., Marchand, G. (eds.), Archéologie des chasseurs-cueilleurs maritimes. De la fonction des habitats à l’organisation de l’espace littoral, Paris, Société préhistorique française, 23–36 (2016).
Álvarez-Fernández, E. et al. Mise à jour des recherches sur les chasseurs-cueilleurs côtiers dans le sud-est de la péninsule ibérique durant le Magdalénien: les restes archéofauniques d’origine marine. In: Costamagno, S., Boudadi-Maligne, M., Daujeard, C., Fernandez, P., Stoetzel, E. (eds.), Sociétés humaines et environnements dans la zone circumméditerranéenne du Pléistocène au début de l’Holocène, Les Eyzies-de-Tayac-Sireuil, Musée national de Préhistoire (Paléo hors-série), 50–63 (2022).
Castaños, P. et al. Estudio de los macromamíferos del yacimiento de Santa Catalina. In: Berganza E., Arribas J.L. (eds.), La Cueva de Santa Catalina (Lekeitio): La intervención arqueológica. Restos vegetales, animales y humanos, Bilbao, Kobie serie BAI 4, 331–360 (2014).
Corchón, M. S., Álvarez-Fernández, E. Nuevas evidencias de restos de mamíferos marinos en el Magdaleniense: los datos de La Cueva de Las Caldas (Asturias, España). Munibe 59, 47–66 (2008).
Lefebvre, A. et al. Interconnected Magdalenian societies as revealed by the circulation of whale bone artefacts in the Pyreneo-Cantabrian region. Quat. Sci. Rev. 251, 106692 (2021).
Cortés-Sánchez, M. et al. Earliest known use of marine resources by Neanderthals. PLoS ONE 6, e24026 (2011).
Gutiérrez-Zugasti, I. et al. A chrono-cultural reassessment of the levels VI–XIV from El Cuco rock-shelter: a new sequence for the Late Middle Paleolithic in the Cantabrian region (northern Iberia). Quat. Intel. 474, 44–55 (2018).
Zilhão, J. et al. Last Interglacial Iberian Neandertals as fisher-hunter-gatherers. Science 367, eaaz7943 (2020).
Álvarez-Fernández, E. Marine resource exploitation during the Middle and Early Upper Paleolithic in. Europe: Overv. available Evid. P@lethnology 7, 188–205 (2015).
Fano, M. A., Gutiérrez-Zugasti, I., Álvarez-Fernández, E., Fernández-García, R. Late Glacial and Postglacial use of marine resources in the Bay of Biscay, North Spain. In: Shell energy: Mollusc shells as coastal resources, Bailey, G. N., Hardy, K., Camara, A. eds., Oxbow Books, Oxford, 155–166 (2013).
Aura, J. E. et al. Palaeolithic - Epipalaeolithic Seapeople of the Southern Iberian coast (Spain): an overview. In: Dupont, C., Marchand, G. (eds.), Archéologie des chasseurs-cueilleurs maritimes. De la fonction des habitats à l’organisation de l’espace littoral, Paris, Société préhistorique française, 69–92 (2016).
Bicho, N., Haws, J. A., Davis, L. G. (eds). Trekking the Shore. Changing Coastlines and the Antiquity of Coastal Settlement, New York, Springer, (2011).
Stewart, K., Cunnane, S., Tattersall, I. eds. 2014. The role of freshwater and marine resources in the evolution of the human diet, brain and behavior. Journal of Human Evolution 77, 1–216.
Dupont, C., Marchand, G. (eds). Archéologie des chasseurs-cueilleurs maritimes. De la fonction des habitats à l’organisation de l’espace littoral, Paris, Société préhistorique française, 425 (2016).
Pétillon, J.-M. Circulation of whale-bone artifacts in the northern Pyrenees during the Late Upper Paleolithic. J. Hum. Evolution 65, 525–543 (2013).
Rodrigues, A. S. L., Horwitz, L. K., Monsarrat, S. & Charpentier, A. Ancient whale exploitation in the Mediterranean: species matters. Antiquity 90, 928–938 (2016).
Buckley, M., Collins, M., Thomas-Oates, J. & Wilson, J. C. Species identification by analysis of bone collagen using matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 23, 3843–3854 (2009).
Collins, M. et al. ZooMS: the collagen barcode and fingerprints. Spectrosc. Eur. 22, 6–10 (2010).
Pétillon, J.-M. et al. Échos de l’océan: phoques et baleines en Europe au Paléolithique récent. In: Cattelain, P., Gillard, M., Smolderen A. (eds.), Disparus? Les mammifères au temps de Cro-Magnon en Europe, Treignes, Cedarc, 335–354 (2018).
Christensen, M. et al. L’industrie osseuse des chasseurs-cueilleurs: le cas des nomades marins de Patagonie et Terre de Feu, Punta Arenas, Universidad de Magallanes. (2016).
Gray, N. M., Kainec, K., Madar, S., Tomko, L. & Wolfe, S. Sink or swim? bone density as a mechanism for buoyancy control in early Cetaceans. Anat. Rec. 290, 638–653 (2007).
Lyman, R. L. et al. Bone density and bone attrition. In: Pokines, J. T., L’Abbe, E. N., Symes, S. A. (eds.), Manual of forensic taphonomy, Boca Raton, CRC Press (2021).
Müller, K. & Reiche, I. Differentiation of archaeological ivory and bone materials by micro- PIXE/PIGE with emphasis on two Upper Palaeolithic key sites: Abri Pataud and Isturitz, France. J. Archaeological Sci. 38, 3234–3243 (2011).
Reiche, I., Müller, K., Staude, A., Goebbels, J. & Riesenmeier, H. Synchrotron radiation and laboratory micro X-ray computed tomography—useful tools for the material identification of prehistoric objects made of ivory, bone or antler. J. Anal. At. Spectrom. 26, 1802–1812 (2011).
Waelbroeck, C. et al. Sea-level and deepwater temperature changes derived from benthic foraminifera isotopic records. Quat. Sci. Rev. 21, 295–305 (2002).
Pétillon, J.-M., Chauvière, F.-X. Les mammifères marins. In: Cretin, C., Madelaine, S. (eds.), Animaux rares, gibiers inattendus: reflets de la biodiversité, Les Eyzies-de-Tayac - Sireuil, Musée national de préhistoire, 92–96 (2019).
Pétillon, J.-M. et al. A gray whale in Magdalenian perigord. Species identification of a bone projectile point from La Madeleine (Dordogne, France) using collagen fingerprinting. Paleo 30, 230–242 (2019).
Lucas, C. et al. The Magdalenian osseous industry from Courbet cave (Penne, Tarn, France) in the British Museum collections. Bull. de. la Soci.été pr.éhistorique française 120, 135–160 (2023).
Langlais, M. et al. Des segments chronoculturels au modèle archéo-stratigraphique du Magdalénien dans le Sud-Ouest français (21 000-16 000 cal. BP). In: Straus, L. G., Langlais, M. eds. Magdalenian chrono-stratigraphic correlations and cultural connections between Cantabrian Spain and Southwest France and beyond, Paris, Société préhistorique française, 109–135 (2020).
Pétillon, J.-M. Technological evolution of hunting implements among Pleistocene hunter-gatherers: osseous projectile points in the Middle and Upper Magdalenian (19–14 ky cal BP). Quat. Int. 414, 108–134 (2016).
Langley, M. C. & Street, M. Long range inland-coastal networks during the Late Magdalenian: evidence for individual acquisition of marine resources at Andernach-Martinsberg. Ger. Cent. Rhineland. J. Hum. Evolution 64, 457–465 (2013).
Laran, S. et al. Seasonal distribution and abundance of cetaceans within French waters - Part II: The Bay of Biscay and the English Channel. Deep Sea Res. Part II: Topical Stud. Oceanogr. 141, 31–40 (2017).
Westley, K. & Dix, J. The solutrean atlantic hypothesis: a view from the ocean. J. North Atl. 1, 85–98 (2008).
Roman, J. et al. Whales as marine ecosystem engineers. Front. Ecol. Environ. 12, 377–385 (2014).
Doughty, C. E. et al. Global nutrient transport in a world of giants. Proc. Natl Acad. Sci. 113, 868–873 (2016).
Gilbert, L., Jeanniard-du-Dot, T., Authier, M., Chouvelon, T. & Spitz, J. Composition of cetacean communities worldwide shapes their contribution to ocean nutrient cycling. Nat. Commun. 14, 5823 (2023).
Wilson, D. E., Mittermeier, R. A. Handbook of the Mammals of the World: Sea Mammals, Barcelona, Lynx Edicions, 4, 614 (2014).
Reeves, R. R., Smith, T. M., Josephson, E. A. Near-annihilation of a species: right whaling in the North Atlantic. In: Kraus, S. D., Rolland, R. M. (eds.), The Urban Whale: North Atlantic Right Whales at the Crossroads, Harvard, Harvard University Press, 39–74 (2007).
Allen, R. C. & Keay, I. Bowhead Whales in the Eastern Arctic, 1611–1911: population reconstruction with historical whaling records. Environ. Hist. 12, 89–113 (2006).
Rodrigues, A. S. L. et al. Forgotten Mediterranean calving grounds of grey and North Atlantic right whales: evidence from Roman archaeological records. Proc. R. Soc. B 285, 20180961 (2018).
Alter, S. E. et al. Climate impacts on transocean dispersal and habitat in gray whales from the Pleistocene to 2100. Mol. Ecol. 24, 1510–1522 (2015).
Hufthammer, A. K., Arntsen, L., Kitchener, A. C. & Buckley, M. Grey whale (Eschrichtius robustus) in Norwegian waters 2000 years ago. Palaeogeogr., Palaeoclimatol., Palaeoecol. 495, 42–47 (2018).
Garrison, E. G., Morgan, G. S., Mcgrath, K., Speller, C. & Cherkinsky, A. Recent dating of extinct Atlantic gray whale fossils (Eschrichtius robustus), Georgia Bight and Florida, western Atlantic Ocean. PeerJ 7, e6381 (2019).
Buss, D. L. et al. Archaeological evidence of resource utilisation of the great whales over the past two millennia: a systematic review protocol. PLoS ONE 18, e0295604 (2023).
García-Vernet, R., Borrell, A., Víkingsson, G., Halldórsson, S. D. & Aguilar, A. Ecological niche partitioning between baleen whales inhabiting Icelandic waters. Prog. Oceanogr. 199, 102690 (2021).
Borrell, A., Abad-Oliva, N., Gómez-Campos, E., Giménez, J. & Aguilar, A. Discrimination of stable isotopes in fin whale tissues and application to diet assessment in cetaceans. Rapid Commun. Mass Spectrom. 26, 1596–1602 (2012).
Blevins, C. et al. Sex- and age-specific migratory strategies of blue whales in the northeast Pacific. Ocean. Front. Mar. Sci. 9, 944918 (2022).
Caraveo-Patiño, J., Hobson, K. A. & Soto, L. A. Feeding ecology of gray whales inferred from stable-carbon and nitrogen isotopic analysis of baleen plates. Hydrobiologia 586, 17–25 (2007).
Ruiz-Cooley, R. I., Gendron, D., Aguíñiga, S., Mesnick, S. & Carriquiry, J. D. Trophic relationships between sperm whales and jumbo squid using stable isotopes of C and N. Mar. Ecol. Prog. Ser. 277, 275–283 (2004).
Reade, H. et al. Nitrogen palaeo-isoscapes: changing spatial gradients of faunal δ 15 N in late Pleistocene and early Holocene Europe. PLoS ONE 18, e0268607 (2023).
Jory, C. et al. Individual and population dietary specialization decline in fin whales during a period of ecosystem shift. Sci. Rep. 11, 17181 (2021).
Seersholm, F. V. et al. DNA evidence of bowhead whale exploitation by Greenlandic Paleo-Inuit 4000 years ago. Nat. Commun. 7, 13389 (2016).
Philippe, M. Un état des connaissances sur la navigation préhistorique en Europe atlantique. Bull. de. la Soci.été pr.éhistorique française 115, 567–597 (2018).
MacLeod, K. et al. Distribution and abundance of fin whales and other baleen whales in the European Atlantic. https://doi.org/10.13140/RG.2.2.19767.52649 (2009).
Speller, C. et al. Barcoding the largest animals on Earth: Ongoing challenges and molecular solutions in the taxonomic identification of ancient Cetaceans. Philos. Trans. R. Soc. Lond., Ser. B, Biol. Sci. 371, 1702 (2016).
Berganza E., Arribas J. L. El entorno físico de las ocupaciones de Santa Catalina. In: Berganza E., Arribas J.L. (eds.), La Cueva de Santa Catalina (Lekeitio): La intervención arqueológica. Restos vegetales, animales y humanos, Bilbao, Kobie serie BAI 4, 367–377 (2014).
Costamagno, S., Théry-Parisot, I., Castel, J.-C., Brugal, J.-P. Combustible ou non? analyse factorielle et modèles explicatifs sur des ossements brûlés paléolithiques. In: Théry-Parisot, I., Costamagno, S., Henry, A. (eds.), Gestion des combustibles au Paléolithique et au Mésolithique: nouveaux outils, nouvelles interprétations, Oxford, Archaeopress, 69–84 (2009).
Berganza E., Arribas J. L. Estructuras de combustión. In: Berganza E., Arribas J.L. (eds.), La Cueva de Santa Catalina (Lekeitio): La intervención arqueológica. Restos vegetales, animales y humanos, Bilbao, Kobie serie BAI 4, 33–48 (2014).
Costamagno, S., Rigaud, J.-P. L’exploitation de la graisse au Paléolithique. In: Costamagno, S. (ed.), Histoire de l’alimentation humaine: entre choix et contraintes, Paris, CTHS, 134–152 (2014).
Berganza, E., Arribas, J. L. Síntesis interpretativa. In: Berganza E., Arribas J.L. (eds.), La cueva de Santa Catalina (Lekeitio, Bizkaia). Industrias líticas y óseas, colorantes y arte mobiliar, Bilbao, Kobie serie BAI 10, 267–285.(2022).
Hussain, S. T. et al. Was the Late Glacial human occupation of northernmost Europe facilitated by whales? New data and perspectives on lithic technology and the paleoecology of the Vendsyssel area, Northern Jutland, Denmark. J. Isl. Coast. Archaeol. 1–29 https://doi.org/10.1080/15564894.2023.2277727 (2024).
Poplin, F. Le bison en os de baleine d’Isturitz (Pyrénées-Atlantiques). Arch.éologie des. Pyrénées Occidentales et. des. Landes 32, 27–28 (2020).
Fiddyment, S. et al. Animal origin of 13th-century uterine vellum revealed using noninvasive peptide fingerprinting. Proc. Natl Acad. Sci. 112, 15066–15071 (2015).
McGrath, K. et al. Identifying archaeological bone via non-destructive ZooMS and the materiality of symbolic expression: Examples from Iroquoian bone points. Sci. Rep. 9, 11027 (2019).
Strohalm, M., Hassman, M., Košata, B. & Kodíček, M. mMass data miner: an open source alternative for mass spectrometric data analysis. Rapid Commun. Mass Spectrom. 22, 905–908 (2008).
Buckley, M. & Collins, M. Collagen survival and its use for species identification in Holocene-lower Pleistocene bone fragments from British archaeological and paleontological sites. Antiqua 1, e1–e7 (2011).
Kirby, D. P., Buckley, M., Promise, E., Trauger, S. A. & Holdcraft, T. R. Identification of collagen-based materials in cultural heritage. Analyst 138, 4849–4858 (2013).
Buckley, M. et al. Species identification of archaeological marine mammals using collagen fingerprinting. J. Archaeological Sci. 41, 631–641 (2014).
Welker, F. et al. Ancient proteins resolve the evolutionary history of Darwin’s South American ungulates. Nature 522, 81–84 (2015).
Coutu, A. N. et al. Palaeoproteomics confirm earliest domesticated sheep in southern Africa ca. 2000 BP. Sci. Rep. 11, 6631 (2021).
Evans, Z., Paskulin, L., Rahemtulla, F. & Speller, C. F. A comparison of minimally-invasive sampling techniques for ZooMS analysis of bone artifacts. J. Archaeological Sci.: Rep. 47, 103738 (2023).
van der Sluis, L. G. et al. Identification and tentative removal of collagen glue in Palaeolithic worked bone objects: implications for ZooMS and radiocarbon dating. Sci. Rep. 13, 22119 (2023).
Longin, R. New method of collagen extraction for radiocarbon dating. Nature 231, 241–242 (1971).
Stafford, T. W. Jr, Duhamel, R. C., Haynes, C. V. Jr & Brendel, K. Isolation of proline and hydroxyproIine from fossil bone. Life Sci. 31, 931–938 (1982).
Stafford, T. W. Jr, Jull, A. J. T., Brendel, K., Duhamel, R. C. & Donahue, D. Study of bone radiocarbon dating accuracy at the University of Arizona NSF accelerator facility for radioisotope analysis. Radiocarbon 29, 24–44 (1987).
Stafford, T. W. Jr., Brendel, K. & Duhamel, R. C. Radiocarbon, 13C and 15N analysis of fossil bone: removal of humates with XAD-2 resin. Geochimica et. Cosmochima Acta 52, 2257–2267 (1988).
van der Sluis, L. G., Zazzo, A., Tombret, O., Thil, F. & Pétillon, J.-M. Testing the use of XAD resin to remove synthetic contamination from archaeological bone prior to radiocarbon dating. Radiocarbon 65, 1160–1175 (2023).
Wacker, L., Christl, M. & Synal, H.-A. Bats: A new tool for AMS data reduction. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. 268, 976–979 (2010).
Reimer, P. J. et al. INTCAL04 Terrestrial. Radiocarb. Age Calibration, 0-26 Cal. KYR BP, Radiocarb. 46, 1029–1058 (2004).
Brock et al. Current pretreatment methods for AMS radiocarbon dating at the Oxford radiocarbon accelerator unit (ORAU). Radiocarbon 52, 103–112 (2010).
Steier, P., Liebl, J., Kutschera, W., Wild, E. & Golser, R. Preparation methods of μg carbon samples for 14C Measurements. Radiocarbon 59, 803–814 (2017).
Golser, R. & Kutschera, W. Twenty years of VERA: toward a universal facility for accelerator mass spectrometry. Nucl. Phys. N. 27, 29–34 (2017).
Santos, G. M. et al. AMS 14 C sample preparation at the KCCAMS/UCI facility: status report and performance of small samples. Radiocarbon 49, 255–269 (2007).
Beverly, R. K. et al. The keck carbon cycle AMS laboratory, University of California, Irvine: status report. Radiocarbon 52, 301–309 (2010).
DeNiro, M. J. Postmortem preservation and alteration of in vivo bone collagen isotope ratios in relation to palaeodietary reconstruction. Nature 317, 806–809 (1985).
van Klinken, G. J. Bone collagen quality indicators for palaeodietary and radiocarbon measurements. J. Archaeological Sci. 26, 687–695 (1999).
Guiry, E. J. & Szpak, P. Quality control for modern bone collagen stable carbon and nitrogen isotope measurements. Methods Ecol. Evolution 11, 1049–1060 (2020).
Heaton, T. J. et al. Marine20 – The marine radiocarbon age calibration curve (0-55,000 cal BP). Radiocarbon 62, 779–820 (2020).
Dury, J. P. et al. Species-specific reservoir effect estimates: a case study of archaeological marine samples from the Bering Strait. Holocene 32, 1209–1221 (2022).
Bronk Ramsey, C. Bayesian analysis of radiocarbon dates. Radiocarbon 51, 337–360 (2009).
Heaton, T. J. et al. Marine radiocarbon calibration in polar regions: a simple approximate approach using Marine20. Radiocarbon 65, 848–875 (2023).
Beck, L. et al. Marine reservoir effect of spermaceti, a wax obtained from the head of the sperm whale: a first estimation from museum specimens. Radiocarbon 64, 1607–1616 (2022).
Pieńkowski, A., Coulthard, R. D. & Furze, M. F. A. Revised marine reservoir offset (Δ R) values for molluscs and marine mammals from Arctic North America. Boreas 52, 145–167 (2022).
Birkenmajer, K. & Olsson, U. Radiocarbon dating of whale bones from the 17th century whaling sites at Gashamna, Hornsund, South Spitsbergen. Bulletin of the Polish Academy of Sciences. Earth Sci. 46, 109–132 (1998).
England, J., Dyke, A. S., Coulthard, R. D., McNeely, R. & Aitken, A. The exaggerated radiocarbon age of deposit-feeding molluscs in calcareous environments. Boreas 42, 362–373 (2013).
Foote, A. D. et al. Ancient DNA reveals that bowhead whale lineages survived Late Pleistocene climate change and habitat shifts. Nat. Commun. 4, 1677 (2013).
Monge Soares, A. M. et al. Marine radiocarbon reservoir effect in Late Pleistocene and early Holocene coastal waters off Northern Iberia. Radiocarbon 58, 869–883 (2016).
Mangerud, J., Bondevik, S., Gulliksen, S., Hufthammer, A. K. & Høisæter, T. Marine 14C reservoir ages for 19th century whales and molluscs from the North Atlantic. Quat. Sci. Rev. 25, 3228–3245 (2006).
Reimer, R. & Reimer, P. J. An online application for ΔR calculation. Radiocarbon 59, 1623–1627 (2017).
Acknowledgements
We thank the people and institutions that granted access and sampling permissions for the material studied: I. Alonso Garcia and M. A. Pedregal Montes (Museo arqueológico de Asturias); F. Bοn, L. Bruxelles, M. Jarry and C. Pallier; J. Cook and C. Lucas (British Museum); J. Darricau (site d’Isturitz) and C. Normand; L. Ducamp (Maison de la dame, Brassempouy); P. Fatás Monforte (Museo nacional y centro de investigación de Altamira); G. Fleury (Muséum d’histoire naturelle de Toulouse); A. Fort, R. Nespoulet and M. Lebon (Musée de l’Homme); N. Fourment (Musée national de Préhistoire); S. Fraile Gracia (Museo nacional de ciencias naturales); E. Galán (Museo arqueológico nacional); I. García Camino (Arkeologi museoa in Bilbao); D. Haro-Gabay (abbaye d’Arthous); the musée de Lespugue; V. Merlin-Anglade (Musée d’art et d’archéologie du Périgord); R. Ontañón (Museo de Prehistoria y Arqueología de Cantabria); J. Primault; L. Rodriguez (Musée de Borda); C. Saint-Martin and P. Alard (musée du Mas d’Azil); S. San Jose Santamarta (Gordailua center); C. Schwab (Musée d’archéologie nationale); A. Simonet; M. Street (RGZM) and C. Langley; and É. Tartar. Thanks to L. Agudo Pérez and A. Cicero Cabañas for their help with the ZooMS analyses, to C. Merlet for his help with the material from Duruthy, to P. Reimer for her help on calculating the new DeltaR for the reservoir correction, and to D. Fiorillo (SSMIM, Service de Spectrométrie de Masse Isotopique du Muséum) for analyzing samples on the IRMS. We thank the York Center for Excellence in Mass Spectrometry (University of York) and the Laboratori de Proteòmica CSIC (Universitat Autònoma de Barcelona), for allowing access to their MALDI-TOF-MS. This work contributes to the ICTA-UAB María de Maeztu Program for Units of Excellence of the Spanish Ministry of Science and Innovation (CEX2019-000940-M), and EarlyFoods (SGR-Cat 2021, 00527). This study was funded by projects HumAntler (PCI2021-122053-2 B) (A.L.), Whalebone (HORIZON-MSCA-2021-PF-01-101059605) (A.L.) and PaleoCet (ANR-18-CE27-0018) (J.-M.P., A.Z.). This work is dedicated to É. Campmas and G. Marchand.
Author information
Authors and Affiliations
Contributions
Project conception, funding acquisition, project administration: J.-M.P., A.L., and A.Z. Identification and sampling of the archeological material: J.-M.P., A.L., F.-X.C., and J.T. ZooMS methodology and analysis: K.McG., C.S., and L.T.I. Radiocarbon and stable isotopes methodology and analysis: L.G.S., A.Z., F.T., and O.T. Photogrammetry and 3D models: F.B. Historical literature analysis: A.C., A.S.L.R. Excavation and scientific responsibility of archeological sites, access to archeological collections and samples provision: E.A.F., E.B., F.-X.C., M.D., E.D.M., C.H., A.B.M.-A, and M.R.V. Writing: J.-M.P., A.C., A.L., K.McG., A.S.L.R., C.S., L.G.S., and A.Z. All authors revised and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Danielle Buss, Ana Valenzuela-Toro, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
McGrath, K., van der Sluis, L.G., Lefebvre, A. et al. Late Paleolithic whale bone tools reveal human and whale ecology in the Bay of Biscay. Nat Commun 16, 4646 (2025). https://doi.org/10.1038/s41467-025-59486-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-59486-8
This article is cited by
-
Molecular and zooarchaeological identification of 5000 year old whale-bone harpoons in coastal Brazil
Nature Communications (2026)