Abstract
The dopamine transporter (DAT) is linked to neuropsychiatric disorders including ADHD, Parkinson’s disease, and substance use disorders. Accordingly, DAT is the target of illicit drugs and clinically important medicines. However, the number and function of ligand binding sites in DAT is enigmatic due to conflicting data from available structures and molecular pharmacology. Herein, we design force sensors with DAT ligands and measure their interaction forces with wild-type and mutated DATs, from which two distinct populations of unbinding strengths and off-rates are detected. The high-force population is reduced by V152I and S422A mutations, or by substituting Na+ with K+ or NMDG+. In contrast, several modifications including mutation G386H, acetylation of K92 and K384, mutation K92A, mutation K384A, or protonation of H477 decrease the low-force population. The present data delineate the threshold of binding strength, which may account for certain ligand binding sites to be imperceptible in crystal or cryo-EM structures. Furthermore, the force spectra provide the information on the position and kinetic rates of a herein detected ligand binding site in DAT.
Similar content being viewed by others
Introduction
The monoamine transporters of dopamine (DAT), serotonin (SERT), and norepinephrine (NET) regulate neurotransmission by reuptake of released monoamines into presynaptic neurons1. Although these transporters serve as targets for psychostimulants such as cocaine and methamphetamine2, they also serve as important drug targets for the treatment of neuropsychiatric disorders such as Attention Deficit Hyperactivity Disorder (ADHD), anxiety, depression and sleep disorders. Understanding the binding modes of ligands is essential for the development of novel medications with selected pharmacological properties, targeting just one transporter rather than all three. Moreover, designing drugs to specifically bind to conformationally different states or allosteric sites has been a topic of great interest, in order to discover efficacious medications without concomitant side effects3,4,5,6.
The recent cryo-electron microscopy (cryo-EM) structures of human DAT (hDAT)7,8 showed binding to the central binding site (S1-site) for substrates and inhibitors, but no ligand was discovered to bind to the S2 binding site. Yet, computational simulations and biochemical experiments9,10,11,12,13,14,15,16 predicted two sites, with the secondary binding site located in the extracellular vestibule (S2-site). Residues within the respective sites in SERT have been probed by various functional and mutagenic approaches and supported the notion that for instance the allosteric action of antidepressants is evolutionarily conserved among SLC6 family members17,18. In addition to ligand binding to S1 and S2, the recent cryo-EM structure of hDAT19 in complex with the inhibitor MRS7292 showed a hitherto structurally uncharacterized allosteric modulatory site close to the extracellular vestibule, sequestered underneath the extracellular loop 4 and adjacent to transmembrane helix 1b.
In general, high binding affinity can be kinetically described as resulting from a fast association rate paired with a slow dissociation of the drug from the target protein, while from a thermodynamic perspective, non-conserved residues in the primary binding site (S1-site) are assumed to be responsible for ligand selectivity through reduced interaction energy between ligand and transporter20,21, which results in a faster dissociation rate. Unexpectedly, it was observed that the selectivity of some ligands, which discriminated between SERT and DAT, was determined by their differing association rates rather than by their - indistinguishable - dissociation rates22. This finding implies that a selectivity filter might exist in the entry pathway, which controls access to the central S1 site14,23,24. We previously developed a nano-pharmacological force sensing method25 and clearly observed two allosterically linked ligand binding sites in SERT by using force sensors functionalized with the antidepressant citalopram (CIT). Subsequently, the two binding sites of citalopram in SERT were reported by X-ray crystallography26. However, the X-ray crystal and cryo-EM structures of SERT with several other ligands reported only one binding site27,28. In order to resolve these contrasting findings and clarify ligand binding interactions at these transporters, we hypothesize that the identification of binding sites by X-ray or cryo-EM structures might be related to the binding strength between the ligand and the transporter.
To test this hypothesis, we use single molecule force spectroscopy based on atomic force microscopy (AFM) to detect the challenging weaker ligand (S2) binding site in DAT. We probe the binding pocket of DAT with selected DAT ligands to investigate the interaction forces and the underlying binding kinetics. To verify the central S1 site, we test mutation S422A and mutation V152I, which are located in the center of DAT. In order to explore the second binding site, we test the K92A, K384A, G386H, H477Y, and H477A mutations, acetylation of K92 and K384, and the protonation of H477, which are located in the extracellular vestibule. Interestingly, the concealed binding site described in our study shares a large interface with the recently reported allosteric modulatory site19.
Results and discussion
Conjugation of cocaine analogue MFZ2-12 to AFM tips
We conjugated ligands of DAT to the AFM cantilever tips by using a flexible polyethylene glycol (PEG) linker of sufficient length (e.g. 6 nm) to allow for an unconstrained recognition process. The tip chemistry was designed so that the density of the PEG linker on the cantilever surface was about 1000 molecules per square micron29, which corresponds to a distance of about 30 nm between two PEG molecules. As the radius of the AFM tips is about 10 nm, this design enables single molecular measurements with soft cellular surfaces. In support of the single PEG linker per AFM tip setup, we found that about fifty percent of the cantilever tips showed no binding events, which additionally indicates the low density of PEG molecules on the AFM tips. N-nor-MFZ2-12 (methyl (1 R,2S,3S,5S)-3-(3,4-dichlorophenyl)-8-azabicyclo[3.2.1]octane-2-carboxylate) was synthesized30 and covalently linked to the cantilever tip via its N-ethylamino derivative31 and an azido-PEG-NHS linker (Fig. 1a, Methods, Supplementary Fig. 1) employing click chemistry32. The successful linkage of this high affinity cocaine analogue to the tip was evidenced by specific force measurements (Supplementary Fig. 2) and recognition imaging (Supplementary Fig. 3). Force measurements were performed on live CHOK1 cells stably expressing human DAT fused with yellow fluorescence protein (YFP).
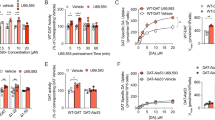
a The drug molecule MFZ 2-12, was covalently conjugated to the AFM cantilever tip via its N-ethylamino derivative and an azido-PEG-NHS linker (Supplementary Fig. 1). b The force measurements (Supplementary Fig. 2) revealed that, in the presence of 10 µM Zn2+, the binding activity of MFZ2-12 to DAT is significantly higher than that in the absence of Zn2+ (one-tailed t-test with equal variance assumed, n = 5 cells. The box range indicates the standard deviation, the small square in the box indicates the mean value, the full width bar indicates the median value, the whisker indicates the range of outlier with coefficient equals to 1). c Two populations of unbinding events were observed from repeatedly recorded force curves. Ten example curves are shown for each. The noise in the force curves originates from thermal fluctuation of the cantilever with a mean value of 0.6 nm (corresponding equation and calculation in the Methods section). d Statistic probability density functions (PDF) of the unbinding force show two peaks on cells expressing DAT, whereas the PDF on CHO cells without DAT has much lower binding probability (area under the curve) and a single peak different from those on DAT. All PDF measurements were conducted on at least two biological replicas with very similar results. e A mutation within the S1 site of DAT (S422A) abolished the second peak (measured in the presence of 10 µM Zn2+) in the force PDF. f Substitution of Na+ with Li+ reduced the population of stronger unbinding events of the wtDAT. Measurements in panel d, e, and f were performed at pulling speed of 6 µm/s. Within each panel, the same cantilever was used to compare different condition. For different panels, the cantilevers are different. Source data are provided as a Source Data file.
MFZ2-12 binds to two sites in DAT
Zn2+ can bind to an endogenous Zn2+ binding site on DAT, which is comprised of residues located in the extracellular loop 2 and 4 (EL2 and EL4)19,33. Zn2+ binding has previously been shown to stabilize the outward-open conformation of DAT33,34. By this action Zn2+ affects substrate uptake and inhibitor binding. Our single-molecule force measurements showed that the binding activity (percentage of curves showing unbinding events) of MFZ2-12-linked AFM probe to DAT reached a maximum at 10 µM Zn2+ (Fig. 1b). Under these conditions, the unbinding force switched between two distinct ranges of strength during the continuous force measurements (Fig. 1c). From repeatedly recorded force data, we obtained the probability density function (PDF) of the unbinding forces (Fig. 1d). The experimental spread of the data in the force measurements reflects the stochastic nature of the force-induced unbinding process35,36. The PDF distributions are composed of the measured forces, each of which is represented by a Gaussian of unitary area with the width of force detection uncertainty. As such, they can be viewed as equivalents of continuous histograms. The maximum of the PDF reflects the most probable unbinding force of the bond and can be extracted with Gaussian fitting according to a standard procedure35. Likewise, two maxima in the force PDF were extracted by fitting with a multi-Gaussian function, from which the most probable unbinding forces of two sites were determined. Importantly, the uncertainty of determining the most probable unbinding forces (standard error of the peak position from Gaussian fitting) is very low, which is less than 1 pN. Thus, the force measurements have the capability to distinguish the unbinding forces arising from different sites or mutations for data shown here. Since the PDF in Fig. 1d showed two peaks in the distribution of unbinding force on DAT, it implied the existence of two binding sites in DAT for DAT inhibitor, MFZ2-12. On the contrary, force measurements by the same dopamine tip on untransfected CHO cells without DAT (green dash line in Fig. 1d) showed much lower binding activity (area under the PDF curve) and only a single peak in the force PDF, which is different from those on DAT. It is interesting to find that Zn2+ has no influence on the unbinding force from the S2 site (peak at 24 pN of red and blue curves in Fig. 1d). However, the absence of Zn2+ increases the unbinding force from the S1 site from 41 pN to 50 pN. Since the force measurements were performed in physiological conditions, the conformation of the transporter is dynamic. When the transporter attempts to change the conformation from outward-facing to the occluded state or the inward-facing state, it may clamp the tethered ligand, which blocks DAT from completing the transition to the inward-facing state. As a consequence, binding is tight, resulting in a stronger unbinding force. In the presence of 10 µM Zn2+, the probability of such a conformational transition became lower, leading to a more prominent peak near 40 pN. The cryo-EM structure19 suggests that Zn2+ increases the probability for the DAT to exist in the outward-open conformation, facilitating the binding of ligand, but also hinders DAT occlusion triggered by substrates bound to the S1. Without Zn2+, the binding probability at the S1 site becomes lower (smaller area under the 50 pN peak of the blue curve in Fig. 1d). However, once bound at the S1 site, the binding strength is higher, possibly due to the transporter moving to the inward-facing conformation, which binds the ligand more tightly.
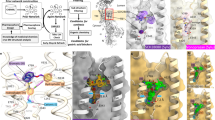
The primary substrate binding site S1 is located in the center of DAT, halfway through the membrane, while the secondary binding site S2 is predicted to be located along the path that the substrate takes to reach the S1 site (Fig. 2). To directly discriminate between a ligand’s binding to the S1 and S2 sites, we designed mutants that specifically affect binding to only one of the two substrate binding sites. The S1 exposed residues S422 (conserved between the human monoamine transporters) and V152 (V in NET and DAT, I in SERT) were shown to affect substrate binding37,38,39,40,41,42,43 (Fig. 2b).
The inhibitor cocaine is bound to the S1 and shown in space-filling mode in blue. The residues of the S1 in contact with cocaine are colored violet, the residues of the S2 are shown in yellow, the residues (V152, S422, G386, K384, and K92) tested by mutation are highlighted in red, the residues in contact with the inhibitor MRS7292 are shown in green. a Full transporter model with the scaffold domain shown in light salmon, the bundle domain in light blue. b Zoom into the substrate binding sites. The hydrophobic gate residue F320 (colored in orange) is prominently in contact with substrate in S1 and inhibitors bound to S2.
Force measurements were performed on DAT-S422A (Fig. 1e) and DAT-V152I (Supplementary Fig. 4). These two mutations are located in the S1 site of DAT39,40,44,45,46 and, accordingly, allow for assigning the two populations of unbinding events to the previously identified binding sites in other transporters of the LeuT fold. In the DAT-S422A and DAT-V152I, the unbinding events with lower force remained unchanged, while the unbinding events with higher force diminished. This suggests that the stronger unbinding events originated from binding to the S1 site. Substitution of Na+ to Li+ also reduced the population of the stronger unbinding events on wild type (wt) DAT (Fig. 1f), which indicates that the stronger unbinding events from the S1 site are sodium dependent and the remaining weaker unbinding events from the S2 site are sodium independent. For different panels, the slight variation of the peak position may result from varying spring constants of the cantilever tips or different pulling velocities. Within each panel, the same cantilever tip and the same pulling speed was used to compare wild type and mutants, or different ion compositions.
Due to the force-dependent bond survival probability, the unbinding force F increases with force loading rate r, the relation of which can be described by Bell-Evans’ theory35:
where kB is Boltzmann’s constant, T is temperature, koff is dissociation rate constant, and xβ is the width of the energy barrier. The measured data of unbinding force vs. force loading rate were fitted by applying Bell-Evans’ theory and the koff and xβ parameters were extracted for both substrate binding sites, respectively (Supplementary Fig. 5). To acquire the kinetic on-rate kon, the binding activity Pa was measured for various contact time tc between AFM tip and cell surface (Supplementary Fig. 6). The on-rate at the S1 and S2 sites was then determined respectively by fitting the data with the two-dimensional kinetic model47:
where mr is the surface density of DAT molecules in the cell membrane, Ka is the two-dimensional affinity, and t0 is the lag time. The surface density of DAT in the cell membrane was determined from the fluorescence intensity calibrated with standard samples of YFP (detailed method in Supplementary Information). In the presence of zinc ions, koff extracted from the force spectra (Supplementary Fig. 5) of the S1 and S2 sites were 0.12 s−1 and 0.7 s−1, respectively, indicating that association of the ligand to the S1 site is more stable than to the S2 site. The on-rates at the S1 and S2 sites (Supplementary Fig. 6) were determined as 4660 nm2/s and 2029 nm2/s, reflecting a faster association to the S1 site than to the S2 site. In the absence of zinc ions, koff derived from the force spectra (Supplementary Fig. 5) of S1 and S2 were 0.03 s−1 and 0.8 s−1, whereas the on-rates at S1 and S2 (Supplementary Fig. 6) were 208 nm2/s and 425 nm2/s, respectively. These results reveal that, in the absence of zinc, the binding of the ligand to the S1 site was decelerated, yet once bound, the complexation was significantly more stable. This suggests that the probability of DAT to dwell in the outward-open conformation is lower in the absence of zinc. The presence of 10 µM zinc increases the probability of DAT to be outward-open, in which the access path to the orthosteric ligand binding site S1 is open, thus increases the binding activity and the on-rates. In parallel this also increases accessibility to the S2, but binding to the S2 is in addition sensitive to the degree of vestibule opening, as S2 includes residues from the bundle and the scaffold domain. The S2 geometry in the presence of zinc might not be optimal for binding, thereby reducing the interactions between DAT and ligand and consequently affecting the stability of S2 bound ligands differently from S1 bound ligands. From the data of kinetic rates, the two-dimensional affinity (kon/koff) for the S1 site was calculated as 41112 nm2 and 7192 nm2, and 2979 nm2 and 520 nm2 for the S2 site in the presence and absence of zinc, respectively. Basically, zinc increases the affinity at both S1 and S2 by 5-6-fold, which is dominantly determined by the kinetic on-rate rather than by the kinetic off-rate; different from conventional intuitive conjecture.
Conjugation of dopamine and HAMBA to AFM tips
In addition to evaluating MFZ2-12, a high affinity (IC50 = 1 nM) inhibitor of DAT30, we also aimed to investigate the force, which governs interactions between DAT and its cognate substrate(s). We examined dopamine and 4-(((2-hydroxyethyl)-amino)methyl)benzamide (HAMBA, Supplementary Fig. 7a), which we discovered to bind specifically to DAT. We note that this interaction was zinc dependent and was not seen on untransfected CHO cells or cells expressing SERT (Supplementary Fig. 7b–d). As with MFZ2-12, we observed two populations of unbinding events in the PDF obtained from repeated force curve measurements with the HAMBA-linked cantilever (Supplementary Fig. 7e). This suggests that there are two binding sites in DAT for HAMBA. To confirm these findings with the cognate substrate dopamine, we attempted to conjugate dopamine directly to the AFM cantilever tips. Although the conjugation can be achieved by using NHS-PEG-aldehyde or NHS-PEG-acetal linker, we found that the phenyl group in these linkers interfered with substrate-transporter interactions. To resolve this problem, we tested the NHS-PEG-maleimide linker. Maleimide48 is widely used for coupling of molecules with thiol (SH) or pyridyl-dithio-propionyl (PDP) groups. Herein, we developed an approach that is feasible to conjugate molecules with a primary amine group directly to maleimide linker (Methods, Supplementary Fig. 8a). This method paves a way to conjugate drug molecules to AFM tips or other surface/molecules.
Repeated force measurements with AFM tip functionalized with dopamine showed two populations of unbinding events for dopamine (Fig. 3b–e, red solid curves), further supporting the existence of two binding sites in DAT to which substrates also bind.
a The dopamine molecule was covalently conjugated to the AFM cantilever tip via NHS-PEG-maleimide linker (Supplementary Fig. 8a). Two populations of unbinding events, red solid lines, measured in the absence of Zn2+, (at scanning speed of 1.5 µm/s) were observed from repeatedly recorded force curves. Substitution of Na+ in the buffer by K+ (b) or NMDG (c) diminished the population of the stronger binding events (around 20 pN), indicating that the stronger binding events originate from the central S1 site, which is sodium dependent. Mutation within the S1 site of DAT (V152I) also reduced the population of stronger binding events (d) while the overall binding activity is preserved. Mutation in the extracellular vestibule (G386H) results in weaker binding (shift of the first force peak to the left) but does not affect the binding force at the S1 site e. Source data are provided as a Source Data file.
Sodium dependency of substrate binding
DAT is a member of the solute carrier 6 family (SLC6), which utilizes the energy contained in the pre-existing sodium gradient across the plasma membrane to support concentrating substrate uptake. Transporters of the LeuT fold have two Na+ binding sites (Na1 and Na2) located in close proximity to the central binding pocket S1. We replaced Na+ with K+ (Fig. 3b) or N-methyl-D-glucamine (NMDG, Fig. 3c) to assign the two populations of unbinding events to the S1 and the S2 site. In the absence of Na+, events with an unbinding force around 20 pN became infrequent with both ligands (Fig. 3b and c, blue dash curves). In contrast, the population of events with weaker unbinding forces was not altered. This finding is consistent with the interpretation that the unbinding events requiring stronger pulling force originated from binding to the central S1 site, because substrate/inhibitor binding to the S1 site is sodium-dependent, while the sodium binding sites are distant from S2. We verified this conclusion by experiments with two mutants, i.e. DAT-V152I and DAT-G386H: V152 and G386 are located in the S1 site and the extracellular vestibule, respectively. Similar to MFZ2-12, the interaction of dopamine with the S1 site of DAT was disrupted by the mutation V152I (Fig. 3d, blue dash curve). It is evident, however, that binding to the S2 site (the first peak in Fig. 3d) was not affected. To directly detect binding to S2, we generated mutants that would specifically disrupt these interactions (Fig. 2b). The DAT mutant G386H is equivalent to the SERT mutant G402H. While G386 is not in direct contact with any S2 binding ligand in homolog SERT structures, the much larger histidine residue would be able to reach the S2 site. Previous studies18,25 demonstrated that replacing glycine with histidine prevented the binding of CIT to the S2 site of SERT. Our experiments showed that dopamine was still able to bind to the S2 site of DAT-G386H, albeit with lower binding strength (as evidenced by the left-shift of the first peak in Fig. 3e).
Identification of the S2 site location in DAT
We next focused on three amino acid residues in the extracellular vestibule to obtain further information on the location of the S2 site in DAT: these were lysine residues DAT-K92 and DAT-K384 and histidine DAT-H477. The location of H477 in TM10 is illustrated in Fig. 2b. The corresponding residue of DAT-H477 in SERT is SERT-E494, which is involved in the binding of S-CIT in the secondary binding site in SERT26. The residue DAT-K92 is in TM1 and DAT-K384 is in EL4. Their equivalent in SERT are SERT-Q111 and SERT-K399. These two residues are closer to the extracellular side than SERT-E494 and they are not involved in the secondary binding site of S-CIT in SERT. However, in the computational docking model10, K92 of DAT forms a hydrogen bond with the catechol hydroxyls of dopamine; K384 forms a hydrogen bond with the protonated amine of dopamine; and H477 forms a π-π interaction with the aromatic ring of dopamine. Of note, the reported crystal structures and other experiments described different S2 sites for S-CIT binding to SERT14,17,26,49.
To examine the role of DAT-K92 and DAT-K384, we tested a distinct chemical method. The chemical modification of the lysines allows us to use the wildtype DAT and avoid complications after mutagenesis. We treated the cells with sulfo-NHS-acetate to target DAT-K92 and DAT-K384 (Fig. 4a, Methods). The rationale underlying this approach was as follows: sulfo-NHS-acetate cannot permeate through membranes and it is known to selectively acetylate the ε-NH2- group of lysines. The only lysine residues in DAT accessible from the extracellular side are DAT-K92 and DAT-K384. After acetylation of DAT-K92 and DAT-K384, the population of unbinding events with stronger force remained (blue dash line in Fig. 4b) but the population of weaker force was reduced. This implied that at least one of the two lysines was involved in the binding of dopamine. We calculated the on- and off-rate and showed them in Fig. 4d and e. The on- and off-rate at S1 site before treatment with sulfo-NHS-acetate (SNA) is 1876 ± 512 nm2s−1 and 0.11 ± 0.03 s−1 respectively (mean ± SD). After the treatment, the off-rate remains 0.12 ± 0.01 s−1, but the on-rate decreases significantly to 508 ± 99 nm2s−1 (p = 0.001). This indicates that the mutation at S2 site by chemical modification at K92 and K384 has little influence on the off-rate of the S1 site but has strong influence on the on-rate of the S1 site. This suggests that the S2 site of wide type DAT is helpful for dopamine to enter the deep central S1 site. To further identify the role of the two lysines, we generated DAT mutants with a single mutation K92A or K384A. From the force measurements (green and magenta thin curves in Fig. 4b), we found that both K92A and K384A diminished the population of weaker unbinding events, indicating that both lysines are involved in the S2 site.
a Cells expressing wtDAT were treated with 3 mM sulfo-NHS-acetate (SNA) for 30 min to acetylate K92 and K384 of DAT. After the treatment, the population of the weaker unbinding events decreased substantially (dashed blue curve in b, measured in the absence of Zn2+, at pulling speed of 6 µm/s). For further identification, DAT mutants with single mutation K92A or K384A were measured, both of which reduced the population of weaker unbinding events (green and magenta thin solid curves in b). c The pH value of the buffer was reduced from 7.4 to 5.5 to examine the role of H477 in supporting binding to the S2. Histidine is the only amino acid residue with a pKa value within this pH range and there is no histidine at S1 site (Supplementary Table 1). The drop in pH reduction has a markedly more pronounced effect on unbinding from the S2 site (the first peak around 12 pN of the red solid line in c, measured in the absence of Zn2+, at scanning speed of 1.5 µm/s) than from the S1 site (the second peak around 20 pN). d Kinetic on-rate at the S1 site of untreated wtDAT is significantly higher than those of DATs changed at K92, K384, or H477, indicating that the S2 site helps dopamine enter the S1 site. e Kinetic off-rate at the S2 site is much higher than that at the S1 site. (One-tailed t-test with equal variance assumed, n = 3–8 cells. The box range indicates the standard deviation, the small square in the box indicates the mean value, the full width bar indicates the median value, the whisker indicates the range of outlier with coefficient equals to 1). Source data are provided as a Source Data file.
All available structures suggest that there is no histidine residue located in the S1 site of DAT (Supplementary Table 1). However, the structure of hDAT (Fig. 2 and Supplementary Table 2) suggested that H477 was part of the vestibular S2 site and the corresponding residue in SERT (E494) is in direct contact with S-CIT bound to the S2-site (PDB-ID 5I73)26. Serotonin is also bound to this second binding site in SERT. We tried verifying this conjecture by generating two DAT mutants (i.e. H477A and H477Y). Unfortunately, these mutated transporters did not reach the plasma membrane as evidenced by the lack of uptake and surface fluorescence. As an alternative approach to interrogate the involvement of H477, we changed the pH of the buffer solution from 7.4 to 5.5. In these experiments we omitted Zn2+ to simplify the interpretation of possible outcomes. Any protonation-induced change in the recorded unbinding force would help determine any involvement of H477 in ligand binding to the S2 site, because (i) histidine is the only amino acid residue with a pKa value in the tested pH range and (ii) there is no other histidine in the S1 site or the outer vestibule. In fact, lowering the pH eliminated the first peak in the force PDF (Fig. 4c), whereas the second peak remained. These results indicate that H477 is involved in substrate binding to the vestibular S2 site. As shown in Fig. 4d and e, the off-rate at S1 site measured in pH5.5 changes to 0.29 ± 0.10 s−1, and the on-rate decreases significantly to 1016 ± 131 nm2s−1 (p = 0.005). This indicates that the change at the S2 site by protonating H477 at pH5.5 has slightly weakened the interactions with the S1 site and has a strong influence on the on-rate of the S1 site. The overall reduction of the affinity of the dopamine at the S1 site with lower pH is in agreement with the decrease of uptake by the DAT50. The reduction of the on-rate of the S1 site by the change at H477 supports the notion that the S2 site of the wildtype DAT is helpful for dopamine to enter the deep central S1 site.
While the role of the S2 binding site remains uncertain51,52, data has accumulated that indicates it could be associated with the on-rate of substrates and thus with the selectivity between SERT and DAT for ligands (e.g. desipramine and methylphenidate), as their affinity is associated with the on rates rather than by their almost indistinguishable dissociation rates15,22,41. This finding implies that a selectivity filter may exist in the entry pathway, which controls access to the central S1 site. The affinity to the S2 could play such a role, as it is of lower affinity and could serve as a ligand recruitment site to increase the local concentration of the substrate, similar to recruitment and temporary binding site for Na+ ions53,54. From the force measurements between dopamine and DAT, we found that after the mutation of the S2 site the kinetic on-rate at the S1 site was significantly reduced (Fig. 4d), indicating that the S2 site in DAT helps the entry and uptake of dopamine. It’s interesting to note that treatment with sulfo-NHS-acetate reduced the on-rate more than other treatment or mutation. This is possibly due to the two protruding acetate groups in the entry pathway in addition to the loss of the S2 binding site. On the contrary, chemical treatment or mutation at the lysines did not change the kinetic off-rate at the S1 site (Fig. 4e). However, reducing the pH to 5.5 slightly increased the off-rate of the S1 site (Fig. 4e), indicating that the lower pH slightly weakened the binding of dopamine in DAT.
Why do crystal structures capture ligand at S2 site in SERT but not in DAT?
In SERT, binding of S-CIT to the S1 site of SERT was shown to enhance binding of S-CIT to the S2 site25. To examine, whether or not the S1 site of DAT has a positive allosteric effect on the S2 site, we pre-filled the S1 site with dopamine (Supplementary Fig. 8e-h) or MFZ2-12 (Supplementary Fig. 9) by adding dopamine or MFZ2-12, respectively, into the bath solution. For an AFM tip carrying dopamine and in the presence of Zn2+, the unbinding force at the S2 site was 15.9 pN (without dopamine in solution, Supplementary Fig. 8e, red solid line) and 13.6 pN (with dopamine in solution, Supplementary Fig. 8e, purple dashed line). In the absence of Zn2+, the unbinding force at S2 was 12.3 pN (without dopamine, Supplementary Fig. 8f, red solid line) and 11.4 pN (with dopamine, Supplementary Fig. 8f, purple dashed line). For an AFM tip carrying MFZ2-12, the binding to the S1 site can be blocked by 50 µM MFZ2-12 in the solution, thus inducing the reduction of the overall binding activity (Supplementary Fig. 9a,b). In the presence of Zn2+, the unbinding force at the S2 site was 20.6 pN (absence of MFZ2-12, Supplementary Fig. 9c, red solid line) and 21.0 pN (presence of MFZ2-12, Supplementary Fig. 9c, purple dashed line). In the absence of Zn2+, the unbinding force at S2 was 25.6 pN (without MFZ2-12, Supplementary Fig. 9d, red solid line) and 23.0 pN (with MFZ2-12, Supplementary Fig. 9d, purple dashed line). All these data provide evidence that the addition of MFZ2-12 or dopamine to the bath solution did not increase the binding strength at the S2 site and, therefore, did not cause positive allosteric effects. This finding is strikingly different to SERT, where addition of S-CIT leads to force increase of up to 4.5 pN25. The difference in the effect between SERT and DAT most likely stems from the difference in off kinetics of the S2-bound ligand in response to allosteric structural changes induced by S1-bound ligand18. In SERT, the S1 ligand S-CIT might be partially hindering S-CIT to unbind from the S2 site, whereby S-CIT in S1 induces sufficient SERT closure to partially obstruct the pathway of S2 unbinding49. While the S1 ligands in DAT might also induce conformational changes in the outer vestibule, neither S1-bound ligands (dopamine and MFZ2-12) have such an effect.
Building on the data obtained for force measurements on SERT and DAT (Supplementary Fig. 10), a threshold of binding strength (between 10.1 and 13.4 pN, at force loading rate of 50 pN/s) could be estimated, which determines whether a ligand binding site can be detected by X-ray crystallography. It is reasonable to postulate a universal threshold of binding strength (affinity) for ligand binding to become visible in X-ray crystallography or cryo-EM structures, not just in neurotransmitter transporters. In contrast, the reason for AFM to have a much higher sensitivity for measuring low affinity binding is its unique single-molecule binding-event detection property, whereas both cryo-EM and X-ray crystallography average over a large number of particles. With saturating ligand concentration in the buffer the occupancy at the S2 site might be close to 100% in aqueous conditions at room temperature. While high ligand concentration will drive equilibrium to the bound state at the macroscopic level, but at the single molecule level, dissociation is a first order rate that only depends on the complex stabilizing forces, which are ligand concentration independent. As binding energy and thus binding forces are low for weakly bound ligands, these might delocalize or even detach. This effect may well result in averaging out leading to invisibility in the structure. Therefore, the findings in this work may provide a route to detect those weaker binding sites missed by X-ray crystallography or cryo-EM, which nevertheless are pivotal for ligand selectivity, dynamics, and function.
Information about location and binding properties of weaker secondary binding sites will be of immediate value for designing bitopic or bivalent molecules that include a primary pharmacophore, a secondary pharmacophore, and a linker of specified length and functionality between them55. The SERT ligand Lu AF60097 is a prime example of this approach, as it is able to bridge the two serotonin binding sites in SERT. The synergism of bivalent or bitopic ligands will certainly lead to higher affinity, improved selectivity, reduced side effects and optimized efficacy as already evidenced by these type of molecules for G-protein coupled receptors55. Indeed, we surmise the recent cryo-EM structure of vilazodone may also be revealing a bitopic binding mode that contributes to exceptionally high binding affinity at SERT6. We show here the potential of AFM for detecting a “hidden” binding site for DAT, but the principle is universal. We expect that our approach will be applicable to other membrane proteins, including transporters, ion channels, and receptors.
In summary, the experiments herein document the physical evidence for the existence of the secondary S2 binding site in DAT when accessed in a physiological context with DAT inhibitor or substrates. The approach utilized single molecule force spectroscopy and thus allowed for the extraction of kinetic on and off rate constants at the transient binding site which have not been reported by other methods. The quantitative force measurements between various ligands and transporters delineate the threshold of binding strength, which may account for why the second ligand binding site in DAT is not detected in X-ray crystal or cryoEM structures, to date. However, as has been shown in a recently published cryo-EM structure of hDAT19, the allosteric binding site described therein interfaces to a good extent with the concealed binding site described in our work. These observations show that DAT can support multiple binding modes of ligands, which result in a continuum of substrate, partial substrate, atypical inhibitor and canonical inhibitor. The physical evidence and identification of elaborated transient binding sites would be useful for deciphering mechanisms fundamental to DAT function in health and disease and ultimately provide rational drug design opportunities for improved therapeutic effectiveness.
Methods
Functionalization of AFM cantilever tips with cocaine analogue MFZ2-12
The cocaine analogue MFZ2-1230 and its derivative MFZ2-12-ethyl-NH2 (8-(2-Amino-ethyl)-3β-(3,4-dichloro-phenyl)-8-aza bicycle[3.2.1]octane-2β-carboxylic acid methyl ester) were prepared as described30,31. An alkyne functional group was added to MFZ2-12-ethyl-NH2 by using the previously described method25 which yielded MFZ2-12-pentyneamide (Supplementary Fig. 1).
AFM cantilever tips (Bruker, MSCT) were washed in chloroform (VWR Chemicals, 22711.324) (3× 5 min), dried, treated with ozone plasma for 15 min, washed again in chloroform (3 × 5 min), and dried in the air. The cleaned cantilevers were amino-functionalized56 using the gas phase method for reaction with APTES ((3-Aminopropyl)triethoxysilane) (Sigma-Aldrich, 440140). Afterwards, the cantilevers were pegylated by incubation for 1 h in 0.25 mL chloroform containing 1 mg azido-PEG-NHS (prepared as described25) and 5 µL trimethylamine (Sigma-Aldrich, 90335), resulting in acylation of the surface-bound amino groups (Supplementary Fig. 1). The cantilevers were then washed with chloroform (×3) and dried. Subsequently the alkyne-modified MFZ2-12 was coupled to the azido-terminated PEG via co-catalyst-accelerated copper(I)-catalyzed azide-alkyne cycloaddition. The highly water-soluble bathophenanthrolinedisulfonic acid was chosen as the co-catalyst32 which works best at a pH of 8.5 and may be used at concentrations equal to, or up to twice the concentration of Cu(I). Co-catalyst-accelerated copper(I)-catalyzed azide-alkyne cycloaddition is a particularly favorable reaction mechanism that allows small molecule tip-coupling at a concentration of 18 µM32. The reaction was carried out as follows in an argon-flooded polytetrafluoroethylene (PTFE) reaction-chamber57. Tris (tris-(hydroxymethyl)-aminomethane) in water (600 µL 0.5 M; pH 8.5 adjusted with HCl), 290 µL DMSO (dimethyl sulfoxide), CuSO4, (2.5 µL 100 mM), bathophenantroleinedisulfonic acid disodium salt trihydrate (25 µL 10 mM), MFZ2-12-pentyneamide (50 µL 6.5 mM in DMSO), and ascorbic acid (20 µL 1 M) were mixed in a reaction chamber, under Argon, and degassed by a gentle stream of Argon bubbling through the solution via a Pasteur pipette for 1-2 min. Finally, NaOH (10 µL 2 M) was added to readjust the pH to 8.5. Thus, the final concentrations of Tris, CuSO4, co-catalyst, MFZ2-12-pentyneamide, ascorbic acid and NaOH were 0.3 M, 0.25 mM, 0.25 mM, 0.3 mM, 20 mM, and 20 mM, respectively. The cantilevers were incubated in this solution for 17 h with continuous protection from oxygen by gentle perfusion of the chamber with Argon. The cantilevers were washed with phosphate-buffered saline (PBS, ×3) and stored in PBS under Argon atmosphere at 4 °C.
Generation of 4-(((2-hydroxyethyl)amino)-methyl)benzamide (HAMBA) on AFM tips
The AFM cantilever tips were cleaned and treated with APTES as described above. The cantilever tips were then pegylated by incubation for 1 h in 0.25 mL chloroform containing 1 mg NHS-PEG18-acetal and 5 µL triethylamine in a PTFE container, resulting in acylation of the surface-bound amino groups (Supplementary Fig. 8a). The cantilever tips were washed with chloroform (×3), dried in the air, and treated with citric acid (1% in water, pH~2.2) for 10 min to convert the acetal to aldehyde. After washing (×3) in Millipore water, the tips were treated in a solution containing 925 µL concentrated phosphate buffer (300 mM NaH2PO4, 1 mM EDTA, pH 7.5 adjusted with NaOH), 50 µL 1 M ethanolamine (pH 9.6), and 25 µL 1 M NaCNBH3 (16 mg NaCNBH3 in 250 µL 10 mM NaOH) for 10 min. The tips were then washed in concentrated phosphate buffer (×3) and stored in concentrated phosphate buffer at 4 °C. The NaCNBH3 solution was treated in one liter of water containing 3 g of Na2CO3 with agitation for one h before disposal.
Functionalization of AFM cantilever tips with dopamine
The conjugation of dopamine to the AFM tips is based on the reaction between its primary amine and maleimide48. At first, the AFM cantilever tips were cleaned and treated with APTES as described above. The tips were then pegylated by incubation for 1 h in 0.25 mL chloroform containing 1 mg NHS-PEG27-maleimide and 5 µL triethylamine in a PTFE container, resulting in acylation of the surface-bound amino groups (Supplementary Fig. 9a). The tips were washed with chloroform (×3), dried in the air, and then put into 200 µL DMSO solution containing 100 mM dopamine hydrochloride and 150 mM Hünig base (Diisopropylethylamine). After incubation for 3 h, the tips were washed in DMSO (×2), washed in chloroform (×3), dried in the air, stored under Argon in a small dish with parafilm at 4 °C.
Cells
CHOK1 cells were transfected with a construct of human DAT, DAT mutant S422A, V152I, G386H, K92A, K384A respectively, fused with yellow fluorescence protein (YFP) to yield CHOK1-YFP-hDAT-wt, CHOK1-YFP-hDAT-S422A, CHOK1-YFP-hDAT-V152I, CHOK1-YFP-hDAT-G386H, CHOK1-YFP-hDAT-K92A, and CHOK1-YFP-hDAT-K384A cells. The addition of YFP to the amino terminus of DAT neither alters its substrate nor its inhibitor pharmacology in CHOK1 cells (data not shown). Cells of CHOK1-YFP-hDAT-wt, CHOK1-YFP-hDAT-S422A, CHOK1-YFP-hDAT-K92A, and CHOK1-YFP-hDAT-K384A were grown on Petri dishes using 1:1 mixture of DMEM and HAM’s F12 (Gibco, 11039-021) containing 10% FBS (Gibco, 1600-044), 500 unit/mL penicillin and 100 µg/mL streptomycin (Thermo Fisher Scientific, 15140122). To keep the selection pressure sufficiently high, 1 mg/mL G418 (PAA Laboratories GmbH, Geneticin) was added to the medium. Cells of CHOK1-YFP-hDAT-V152I and CHOK1-YFP-hDAT-G386H were grown on Petri dishes using DMEM-high glucose (Sigma, D5796) containing 10% FBS Gold Plus (Bio&Sell, FBS.GP.0500), 500 unit/mL penicillin and 100 µg/mL streptomycin (Thermo Fisher Scientific, 15140122). To keep the selection pressure sufficiently high, 160 µg/mL Hygromycin B Gold (InvivoGen, ant-hg-1) was added to the medium.
For AFM measurements, the density of the cells was about 10–30% coverage of the dish surface. The growth medium was exchanged to a physiological HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer containing 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, and 10 mM HEPES (pH 7.4 with NaOH). For buffer without sodium ion, NaCl was replaced by KCl, LiCl, or NMDG respectively. For buffer with pH 5.5, HEPES was replaced by MES (2-(N-Morpholino)ethanesulfonic acid).
For acetylation of amino acid residues K92 and K384 of DAT, cells were at first washed (×1) with HEPES buffer (with Na+, pH 7.4) and 1 mL buffer was left in the Petri dish (diameter 35 mm). Then, 900 μL of strong HEPES buffer (140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 100 mM HEPES, pH 8.0) was added to the dish. Subsequently, 100 μL of 60 mM sulfo-NHS-acetate in 1 mM NaH2PO4 (pH 6.0 before adding sulfo-NHS-acetate) was applied in the dish. The final concentration of sulfo-NHS-acetate is 3 mM. After 30 min, the cells were washed with normal HEPES buffer (×3).
Before force-distance curve measurements, the cells were examined by fluorescence microscope. A GFP filter set and an objective lens with magnification of 10× or 20× were used to detect the expression level of DAT. Cells with very low expression were excluded from force measurements. Optimal cells for force measurements were those flat, smooth, and middle-sized cells with homogeneous distribution of DAT. Usually cells at the edge of a cluster were used, to ensure that there was no other cell underneath the cantilever arms. Before transfer from the fluorescence microscope to the AFM, a mark point was made with a pen on the outside wall of the dish, so that the dish could be mounted in the AFM with the same orientation. With the optical microscope of the AFM, it is possible to find the selected cells.
AFM measurements
All force-distance curves were recorded at room temperature by using a PicoPlus 5500 AFM setup (Agilent Technologies, Chandler, AZ, USA) on living cells with the assistance of a CCD camera for localization of the cantilever tip on selected cells. The optical system of the AFM was focused on the cantilever tip, while the sample plate with the Petri dish was moved upwards by the step motor. Before the cells on the dish reached the focus, the piezo tube of the AFM was started to scan in the z-axis with a scanning range of 3 µm and at a scanning frequency of 1 Hz. Meanwhile, the sample plate was moved upwards by the step motor using manual control with 1 µm per step. Due to the resistance of the liquid, a gap between the approaching curve and the retraction curve appeared, when the AFM tip was close to the sample surface. About 2 µm before the AFM tip touched the sample surface, the approaching curve was no longer parallel to the retraction curve. With this signal, the movement of the step motor was stopped. Further approaching was accomplished by gradually changing the voltage on the piezo tube. With this approaching method, the indentation force of the first contact between the AFM tip and the sample surface can be controlled lower than 30 pN.
The functionalized cantilever (pegylated with MFZ2-12, HAMBA, or dopamine) with a nominal spring constant of 0.01 N/m was moved downward to the cell surface (Supplementary Fig. 2) and moved upward after the deflection of the cantilever reached the force limit. The deflection (z) of the cantilever was monitored by a laser beam on the cantilever surface and plotted versus the Z-position of the scanner, from which the force (F) can be determined according to Hook’s law (F = k z, with k being the cantilever spring constant). When the tip tethered molecule bound to DAT on the cell surface, a pulling force developed during the upward movement of the cantilever causing the cantilever to bend downwards. At a critical force, i.e. the unbinding force, the tip tethered molecule detached from DAT, and the cantilever jumped back to its neutral position. Dynamic force spectroscopy measurements were performed by varying the force loading rate which is the product of the pulling velocity multiplied by the effective spring constant. For this, the sweep range was fixed at 3000 nm, but the sweep rate varied from 0.25 to 2 Hz. For each cellular position and sweep rate, 100–200 force-distance cycles with 2000 data points per cycle and typical force limit of about 30 pN were performed. The spring constants of the cantilevers were determined using the thermal noise method58. Thereby, the thermal fluctuation q of the cantilever was calculated from the following equation: <q2 > = kBT/k, where kB is Boltzmann constant, T is the temperature, and k is the spring constant of the cantilever. For the cantilevers used for the force measurements, the nominal spring constant was 10 pN/nm, which resulted in a thermal noise of about 0.6 nm at room temperature.
Topographical image of living cells was acquired using a PicoPlus 5500 AFM (Agilent Technologies Inc. Chandler, AZ, USA) at contact mode with non-functionalized AFM cantilever (Bruker, MSCT) with a nominal spring constant of 0.01 N/m. The image was acquired in 1:1 mixture of cell culture medium and HEPES buffer at a scanning speed of 13.7 µm/s with pixel number of 256 × 256.
The recognition imaging59,60 was carried out using a PicoPlus 6000 AFM (Agilent Technologies Inc. Chandler, AZ, USA) with a PicoTREC module at acoustic AC (AAC) mode. The AFM cantilever (Bruker, MSCT) with a nominal spring constant of 0.03 N/m was functionalized with MFZ2-12. Images were recorded at a cantilever oscillation frequency of 3.2 kHz and with a scanning speed of 3 µm/s. The image size is 1.5 µm × 1.5 µm with pixel number of 128 × 128. Cells for recognition imaging were fixed at 37 °C for one day with 1% formaldehyde in HEPES buffer containing 10 µM Zn2+. The free aldehyde groups were inactivated by incubating the cells in 1 mL HEPES buffer containing 50 µL 1 M ethanolamine (pH 9.6) for 10 min. The cells were then washed with HEPES buffer for 5 times and the recognition images were acquired in HEPES buffer containing 10 µM Zn2+. The oscillation signal of the cantilever was split into the upper and lower parts in the PicoTREC module (Supplementary Fig. 3a). The upper part of the oscillation signal was used to construct the recognition image. When the tethered molecule on the cantilever tip bound with DAT in the cell membrane, the upper part of the cantilever oscillation was reduced, resulting in a dark spot in the recognition image.
Force data analysis
The unbinding event was identified by local maximum analysis using a signal-to-noise threshold of 2. The binding activity was calculated as the fraction of curves showing unbinding events. For example, if 150 curves from 1000 measured curves show unbinding events, the binding activity is 15%. One-tailed Student t-test was performed for statistical analysis. The unbinding force and effective spring constant (slope before rupture) were determined from force curves showing unbinding events. The force loading rate (r) of every individual curve was calculated by multiplying the effective spring constant with the pulling speed.
The probability density function (PDF) of the unbinding force was constructed61 from every unbinding event on the same cell at the same pulling speed. For each unbinding force value, a Gaussian of unitary area with its center representing the unbinding force and the width (standard deviation) reflecting its measuring uncertainty (square root of the variance of the noise in the force curve) was computed. All Gaussians from one experimental setting were accordingly summed up and normalized with its binding activity to yield the experimental PDF of unbinding force. The maximum of the PDF reflects the most probable unbinding force of the bond and can be easily extracted with Gaussian fitting according to a standard procedure in the literature35. Likewise, two maxima in the force PDF were extracted by fitting with a multi-Gaussian function, from which the most probable unbinding forces of two sites were determined. Importantly, the uncertainty of determining the most probable unbinding forces (standard error of the peak position from Gaussian fitting, which is proportional to the square root of the sum of the squared residuals and inversely proportional to the square root of the number of data points, detailed formula in ref. 62.) is very low, which is less than 1 pN for all peaks in this manuscript. Thus, the force measurements easily have the capability to clearly distinguish the unbinding forces arising from different sites or mutations for all data shown in this manuscript.
Unbinding events within the mean force ± standard deviation of the Gaussian fit were used to create an unbinding force vs. force loading rate plot for each peak in the force PDF respectively, to show dynamic aspects of the bonds. The Bell-Evans theory35 was employed to fit the width of the energy barrier xB and the dissociation rate constant koff for the obtained data.
To acquire the kinetic on-rate kon, the measured data of binding activity Pa vesus contact time tc were fitted with the two-dimensional kinetic model47,63: Pa = 1 – exp(- mr Ka (1 – exp(- kon (tc - t0) / Ka))), where mr is the surface density of DAT molecules in the cell membrane, Ka is the two-dimensional affinity, and t0 is the lag time.
Determination of the surface density of DAT molecules in the cell membrane
Lyophilized recombinant yellow fluorescence protein (YFP) powder (BIO-Vision, 4998-100) was reconstituted in water. A droplet of YFP solution was placed on the surface of cell culture dish (Greiner Bio-One, 627 160). A piece of thin glass was pressed on the YFP droplet. After measuring the area (A) of the YFP droplet, the surface density (d) of YFP molecules can be calculated as d = V·C·Av/A, where V is the volume of the YFP droplet, C is the Molar concentration, and Av is Avogadro’s constant. The fluorescence signal was measured in the middle of the droplet. A series of fluorescence signal corresponding to different surface density of YFP were measured. The relation between the fluorescence signal and the surface density of YFP is a linear function up to 300,000 molecules/µm2. Therefore, by measuring the fluorescence signal of the cells expressing DAT-YFP, the surface density of DAT in the cell membrane can be obtained. For each cell, we used and averaged the fluorescence signal at four positions in the thin membrane regions to minimize the influence of the molecules in the cytosol. This value is further divided by two, since the fluorescence signal is contributed by both the cell membrane in contact with the cell culture dish and the cell membrane in contact with the buffer solution.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data supporting the findings of this study are available within the paper. Source data are provided as a Source Data file. Additional information is available from the authors upon request. Source data are provided with this paper.
References
Kristensen, A. S. et al. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol. Rev. 63, 585–640 (2011).
Sitte, H. H. & Freissmuth, M. Amphetamines, new psychoactive drugs and the monoamine transporter cycle. Trends Pharmacol. Sci. 36, 41–50 (2015).
Newman, A. H., Ku, T., Jordan, C. J., Bonifazi, A. & Xi, Z.-X. New drugs, old targets: tweaking the dopamine system to treat psychostimulant use disorders. Annu. Rev. Pharmacol. Toxicol. 61, 609–628 (2021).
Tanda, G., Hersey, M., Hempel, B., Xi, Z.-X. & Newman, A. H. Modafinil and its structural analogs as atypical dopamine uptake inhibitors and potential medications for psychostimulant use disorder. Curr. Opin. Pharmacol. 56, 13–21 (2021).
Plenge, P. et al. The mechanism of a high-affinity allosteric inhibitor of the serotonin transporter. Nat. Commun. 11, 1–12 (2020).
Plenge, P. et al. The antidepressant drug vilazodone is an allosteric inhibitor of the serotonin transporter. Nat. Commun. 12, 1–12 (2021).
Nielsen, J. C. et al. Structure of the human dopamine transporter in complex with cocaine. Nature 632, 678–685 (2024).
Li, Y. et al. Dopamine reuptake and inhibitory mechanisms in human dopamine transporter. Nature 632, 686–694 (2024).
Huang, X., Gu, H. H. & Zhan, C.-G. Mechanism for cocaine blocking the transport of dopamine: insights from molecular modeling and dynamics simulations. J. Phys. Chem. B 113, 15057–15066 (2009).
Schmitt, K. C., Mamidyala, S., Biswas, S., Dutta, A. K. & Reith, M. E. Bivalent phenethylamines as novel dopamine transporter inhibitors: evidence for multiple substrate‐binding sites in a single transporter. J. Neurochem. 112, 1605–1618 (2010).
Shan, J., Javitch, J. A., Shi, L. & Weinstein, H. The substrate-driven transition to an inward-facing conformation in the functional mechanism of the dopamine transporter. PLoS ONE 6, e16350 (2011).
Gabrielsen, M., Sylte, I., Dahl, S. G. & Ravna, A. W. A short update on the structure of drug binding sites on neurotransmitter transporters. BMC Res. Notes 4, 559 (2011).
Merchant, B. A. & Madura, J. D. Insights from molecular dynamics: the binding site of cocaine in the dopamine transporter and permeation pathways of substrates in the leucine and dopamine transporters. J. Mol. Graph. Model. 38, 1–12 (2012).
Andersen, J. et al. Interrogating the molecular basis for substrate recognition in serotonin and dopamine transporters with high-affinity substrate-based bivalent ligands. ACS Chem. Neurosci. 7, 1406–1417 (2016).
Zhen, J. & Reith, M. E. Impact of disruption of secondary binding site S2 on dopamine transporter function. J. Neurochem. 138, 694–699 (2016).
Sun, W.-L. et al. Allosteric modulatory effects of SRI-20041 and SRI-30827 on cocaine and HIV-1 Tat protein binding to human dopamine transporter. Sci. Rep. 7, 3694 (2017).
Plenge, P. et al. Steric hindrance mutagenesis in the conserved extracellular vestibule impedes allosteric binding of antidepressants to the serotonin transporter. J. Biol. Chem. 287, 39316–39326 (2012).
Niello, M., Gradisch, R., Loland, C. J., Stockner, T. & Sitte, H. H. Allosteric modulation of neurotransmitter transporters as a therapeutic strategy. Trends Pharmacol. Sci. 41, 446–463 (2020).
Srivastava, D. K. et al. Structure of the human dopamine transporter and mechanisms of inhibition. Nature 632, 672–677 (2024).
Andersen, J. et al. Molecular determinants for selective recognition of antidepressants in the human serotonin and norepinephrine transporters. Proc. Natl Acad. Sci. 108, 12137–12142 (2011).
Severinsen, K. et al. Binding of mazindol and analogs to the human serotonin and dopamine transporters. Mol. Pharmacol. 85, 208–217 (2014).
Hasenhuetl, P. S. et al. Ligand selectivity among the dopamine and serotonin transporters specified by the forward binding reaction. Mol. Pharmacol. 88, 12–18 (2015).
Andersen, J. et al. Molecular basis for selective serotonin re-uptake inhibition by the antidepressant agent fluoxetine (Prozac). Mol. Pharmacol. 85, 703–714 (2014).
Andersen, J., Ringsted, K. B., Bang-Andersen, B., Strømgaard, K. & Kristensen, A. S. Binding site residues control inhibitor selectivity in the human norepinephrine transporter but not in the human dopamine transporter. Sci. Rep. 5, 15650 (2015).
Zhu, R. et al. Nanopharmacological force sensing to reveal allosteric coupling in transporter binding sites. Angew. Chem. Int. Ed. 55, 1719–1722 (2016).
Coleman, J. A., Green, E. M. & Gouaux, E. X-ray structures and mechanism of the human serotonin transporter. Nature 532, 334–339 (2016).
Coleman, J. A. & Gouaux, E. Structural basis for recognition of diverse antidepressants by the human serotonin transporter. Nat. Struct. Mol. Biol. 25, 170 (2018).
Coleman, J. A. et al. Serotonin transporter–ibogaine complexes illuminate mechanisms of inhibition and transport. Nature 569, 141 (2019).
Hinterdorfer, P., Baumgartner, W., Gruber, H. J., Schilcher, K. & Schindler, H. Detection and localization of individual antibody-antigen recognition events by atomic force microscopy. Proc. Natl Acad. Sci. 93, 3477–3481 (1996).
Newman, A. H., Zou, M.-F., Ferrer, J. V. & Javitch, J. A. 3 H] MFZ 2-12: A Novel radioligand for the dopamine transporter. Bioorg. Med. Chem. Lett. 11, 1659–1661 (2001).
Cha, J. H. et al. Rhodamine-labeled 2β-carbomethoxy-3β-(3, 4-dichlorophenyl) tropane analogues as high-affinity fluorescent probes for the dopamine transporter. J. Med. Chem. 48, 7513–7516 (2005).
Lewis, W. G., Magallon, F. G., Fokin, V. V. & Finn, M. Discovery and characterization of catalysts for azide− alkyne cycloaddition by fluorescence quenching. J. Am. Chem. Soc. 126, 9152–9153 (2004).
Stockner, T. et al. Mutational analysis of the high-affinity zinc binding site validates a refined human dopamine transporter homology model. PLoS Comput. Biol. 9, e1002909 (2013).
Norregaard, L., Frederiksen, D., Nielsen, E. Ø. & Gether, U. Delineation of an endogenous zinc‐binding site in the human dopamine transporter. EMBO J. 17, 4266–4273 (1998).
Evans, E. Looking inside molecular bonds at biological interfaces with dynamic force spectroscopy. Biophys. Chem. 82, 83–97 (1999).
Merkel, R., Nassoy, P., Leung, A., Ritchie, K. & Evans, E. Energy landscapes of receptor-ligand bonds explored with dynamic force spectroscopy. Nature 397, 50 (1999).
Torres, G. E., Gainetdinov, R. R. & Caron, M. G. Plasma membrane monoamine transporters: structure, regulation and function. Nat. Rev. Neurosci. 4, 13–25 (2003).
Seddik, A. et al. Combined simulation and mutation studies to elucidate selectivity of unsubstituted amphetamine‐like cathinones at the dopamine transporter. Mol. Inform. 36, 1600094 (2017).
Beuming, T. et al. The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nat. Neurosci. 11, 780–789 (2008).
Lee, S.-H. et al. Importance of valine at position 152 for the substrate transport and 2β-carbomethoxy-3β-(4-fluorophenyl) tropane binding of dopamine transporter. Mol. Pharmacol. 57, 883–889 (2000).
Niello, M. et al. Persistent binding at dopamine transporters determines sustained psychostimulant effects. Proc. Natl Acad. Sci. 120, e2114204120 (2023).
Bisgaard, H. et al. The binding sites for benztropines and dopamine in the dopamine transporter overlap. Neuropharmacology 60, 182–190 (2011).
Felts, B. et al. The two Na+ sites in the human serotonin transporter play distinct roles in the ion coupling and electrogenicity of transport. J. Biol. Chem. 289, 1825–1840 (2014).
Penmatsa, A., Wang, K. H. & Gouaux, E. X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature 503, 85–90 (2013).
Wang, K. H., Penmatsa, A. & Gouaux, E. Neurotransmitter and psychostimulant recognition by the dopamine transporter. Nature 521, 322–327 (2015).
Penmatsa, A., Wang, K. H. & Gouaux, E. X-ray structures of Drosophila dopamine transporter in complex with nisoxetine and reboxetine. Nat. Struct. Mol. Biol. 22, 506–508 (2015).
Huang, J. et al. The kinetics of two-dimensional TCR and pMHC interactions determine T-cell responsiveness. Nature 464, 932 (2010).
Sharpless, N. E. & Flavin, M. The reactions of amines and amino acids with maleimides. Structure of the reaction products deduced from infrared and nuclear magnetic resonance spectroscopy. Biochemistry 5, 2963–2971 (1966).
Zhu, R. et al. Allosterically linked binding sites in serotonin transporter revealed by single molecule force spectroscopy. Front. Mol. Biosci. 7, 99 (2020).
Berfield, J. L., Wang, L. C. & Reith, M. E. Which form of dopamine is the substrate for the human dopamine transporter: the cationic or the uncharged species? J. Biol. Chem. 274, 4876–4882 (1999).
Piscitelli, C. L., Krishnamurthy, H. & Gouaux, E. Neurotransmitter/sodium symporter orthologue LeuT has a single high-affinity substrate site. Nature 468, 1129–1132 (2010).
Shi, L., Quick, M., Zhao, Y., Weinstein, H. & Javitch, J. A. The mechanism of a neurotransmitter: sodium symporter—inward release of Na+ and substrate is triggered by substrate in a second binding site. Mol. Cell 30, 667–677 (2008).
Szöllősi, D. & Stockner, T. Investigating the mechanism of sodium binding to SERT using direct simulations. Front. Cell. Neurosci. 15, 673782 (2021).
Gradisch, R. et al. Occlusion of the human serotonin transporter is mediated by serotonin-induced conformational changes in the bundle domain. J. Biol. Chem. 298, 101613 (2022).
Newman, A. H., Battiti, F. O. & Bonifazi, A. Portoghese medicinal chemistry lectureship: designing bivalent or bitopic molecules for G-protein coupled receptors. The whole is greater than the sum of its parts. J. Med. Chem. 63, 1779–1797 (2019).
Riener, C. K. et al. Simple test system for single molecule recognition force microscopy. Anal. Chim. Acta 479, 59–75 (2003).
Zhu, R., Gruber, H. J. & Hinterdorfer, P. in Nanoscale Imaging 19–33 (Springer, 2018).
Hutter, J. L. & Bechhoefer, J. Calibration of atomic‐force microscope tips. Rev. Sci. Instrum. 64, 1868–1873 (1993).
Stroh, C. M. et al. Simultaneous topography and recognition imaging using force microscopy. Biophys. J. 87, 1981–1990 (2004).
Stroh, C. et al. Single-molecule recognition imaging microscopy. Proc. Natl Acad. Sci. Usa. 101, 12503–12507 (2004).
Baumgartner, W., Hinterdorfer, P. & Schindler, H. Data analysis of interaction forces measured with the atomic force microscope. Ultramicroscopy 82, 85–95 (2000).
Richter, P. Estimating errors in least-squares fitting. The Telecommunications and Data Acquisition Report. Work of the US Gov. Public Use Permitted. Document ID: 19960009481 (1995).
Rico, F., Oshima, A., Hinterdorfer, P., Fujiyoshi, Y. & Scheuring, S. Two-dimensional kinetics of inter-connexin interactions from single-molecule force spectroscopy. J. Mol. Biol. 412, 72–79 (2011).
Acknowledgements
We thank C. J. Loland, J. Zhen, and I. Bahar for discussion, M. Waser, P. Pollheimer, M. Zou, V. Kumar, D. Sinwel, T. Haselgrübler, Y. Mayr, M. Kastner, M. Leitner, H. Seferovic, C. Rankl, and G. Kada for technical support, N. Müller for NMR facilities. This research is supported by Austrian Science Fund Grants SFB35 (F35 to P.H., M.F., H.H.S., T.S.), P32017 to T.S., P33955 and P35589 to H.H.S. and P31599-B27 (to R.Z., W.S.), the National Institute on Drug Abuse-Intramural Research Program, Z1A 000389 (to A.H.N.), WWTF grants CS15-033 (to H.H.S.) and LSC17-026 (to M.F.), and endowment from G. H. Glock (to M.F.).
Author information
Authors and Affiliations
Contributions
P.H., H.H.S., A.H.N., M.F., H.J.G., T.S., W.S., and R.Z. designed the experiments. A.H.N., H.J.G., L.W., and A.E. prepared compounds and crosslinkers. M.H., O.K., J.B., N.K., K.S., and J.A.K. prepared mutants and cell lines. R.Z., A.He., A.S.F., M.B., P.T., A.Ha., J.G., and S.H.S. measured and analyzed data. R.Z., P.H., H.H.S., A.H.N., M.F., T.S., and W.S. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhu, R., Sandtner, W., Stockner, T. et al. Revealing the location and dynamics of a concealed binding site in the dopamine transporter. Nat Commun 16, 4197 (2025). https://doi.org/10.1038/s41467-025-59511-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-59511-w