Abstract
Wetlands play a vital role in contaminant cycling and uptake. Understanding how sulfate (SO42‒) influences the conversion of inorganic mercury (Hg(II)) to toxic methylmercury (MeHg) is critical for predicting wetland responses to land use and climate change. Here, we sampled surface and pore waters across SO42‒ gradients in three freshwater Everglades wetlands to assess linkages between SO42‒, MeHg, dissolved organic matter (DOM), and inorganic sulfide (S(‒II)). Increasing SO42‒ concentrations increase S(‒II) and DOM concentrations and DOM aromaticity. MeHg concentration show a unimodal response to surface water SO42‒, which reflect high Hg(II) methylation at low-to-intermediate SO42‒concentration (2-12 mg/L) and low Hg(II) methylation at higher SO42‒concentrations ( > 12 mg/L). MeHg concentrations in surface waters correlate positively with MeHg concentrations in prey fish. The coherent biogeochemical relationships between SO42‒ and MeHg concentrations and biologic uptake improve MeHg risk assessment for aquatic food webs and are globally relevant due to anthropogenic and climate-driven increases in SO42‒.
Similar content being viewed by others
Introduction
Mercury (Hg) is a global pollutant that, when converted to neurotoxic methylmercury (MeHg), poses severe risks to wildlife and humans1. Freshwater wetlands are important locations for the cycling of Hg between environmental compartments, with both source (e.g., atmospheric deposition)2 and sink processes (e.g., photo-reduction of inorganic divalent Hg (Hg(II)) to elemental Hg (Hg(0)))3,4 governing Hg(II) concentrations in wetland waters. The microbial conversion of Hg(II) to MeHg is a critical step in the Hg cycle responsive to hydrologic and biogeochemical perturbations due to internal (e.g., nutrient cycling, land and water management) and external factors (e.g., climate change variables)5, which can have cascading effects that influence MeHg risk to biota6. MeHg risk is defined as the potential for MeHg to be formed within or proximal to aquatic systems that results in efficient MeHg assimilation in the aquatic food web. For over three decades7,8, sulfate (SO42‒), a terminal electron acceptor for anaerobic microbial metabolism, has been identified as an important geochemical predictor of the potential of MeHg formation in freshwater wetlands9,10,11,12,13,14,15. However, due to the individual biogeochemical complexities of sulfur (S), organic carbon (C), and Hg in the environment, there is still uncertainty in the response between SO42‒ concentrations and MeHg formation in wetlands and food web uptake16,17,18, the locations where MeHg formation occurs (e.g., sediments19 versus water column versus periphyton)20,21, and the direct or indirect role of SO42‒ reducing bacteria in the conversion of Hg(II) to MeHg19,22. A revisitation of linkages between SO42‒ concentrations and the potential for MeHg formation and uptake in biota is needed. Although wetland MeHg concentrations reflect the balance between Hg(II) methylation3,4,19 and MeHg demethylation9,23, differences in MeHg concentration within and across boreal15 and subtropical wetlands9,24 are primarily attributed to differences in Hg(II) methylation. Freshwater environments globally are threatened by increasing SO42‒ concentrations due to expansion in agricultural sulfur (S) use (primarily as elemental S)25,26, climate-driven increases in mineral weathering that release SO42‒ in boreal and Arctic systems27,28, and sea-level rise that delivers marine SO42‒ to coastal wetlands29.
Sulfate (SO42‒) exerts primary and secondary controls in MeHg formation through a combination of redox-dependent geochemical and microbial processes. From a geochemical perspective, in freshwater wetlands with low SO42‒ (<1 mg/L), Hg(II) aqueous speciation is primarily governed by the binding of Hg(II) to thiol groups in dissolved organic matter (DOM)30. Hg(II) speciation can be drastically altered in wetlands with appreciable SO42‒ and labile organic carbon, as dissimilatory SO42‒ reduction produces inorganic sulfide (S(‒II)), which outcompetes DOM thiol groups for Hg(II), resulting in the formation of nano-particulate metacinnabar (nano-β-HgS)31. Further, microbially mediated dissimilatory SO42‒ reduction, which utilizes soil organic matter (SOM), can catalyze both the release of highly aromatic DOM from soil and sediment29,32,33 and the abiotic sulfurization of DOM, enriching the DOM in thiol groups34. Nascent nano-β-HgS particles are smaller and less crystalline when formed under environmental conditions (i.e., low Hg(II) to DOM)31,35 and in the presence of DOM of higher aromaticity36. From a microbial perspective, SO42‒ can have primary and secondary effects on microorganisms that convert Hg(II) to MeHg, which have the requisite hgcAB gene pair37. In pure culture, SO42‒ reducing bacteria are highly efficient at converting Hg(II) to MeHg38,39,40, with the most pronounced MeHg formation by heterotrophic bacteria existing in cultures with intermediate S(‒II) concentrations39, high DOM aromaticity40, and high DOM thiol content41. However, in nature, hgcAB+ organisms span diverse phylogenetic groups beyond SO42‒ reducing bacteria, including methanogenic archaea and fermentative organisms19,42. Notably, in two systems with environmental19 and manipulated22 gradients in SO42‒, the diversity and abundance of the hgcAB gene pair decreased with increasing SO42‒. The combination of SO42‒ effects on Hg(II) bioavailability for methylation and activity of the microbial community are understood to contribute to the nonlinear response observed between SO42‒ and MeHg8,9,11,12,13,14,15,43. Given the potential for fluctuations in SO42‒ concentrations in wetlands in response to land management (e.g., agricultural practices), water management (e.g., storm water treatment, phosphate mitigation via alum additions)44,45, or climate change processes27,28,29, as well as the uncertainties in the geochemical and microbial responses pertaining to MeHg formation, ecosystem-scale studies are needed to solidify linkages between SO42‒, MeHg formation, and subsequent MeHg uptake in aquatic food webs.
Here, field campaigns were conducted along hydrologic transects in three differing wetlands of the freshwater Florida Everglades, a managed wetland ecosystem with high atmospheric Hg deposition rates2,46. The wetland transects exhibited distinct horizontal geochemical gradients in SO42‒ spanning from low SO42‒ (<0.5 mg/L) wetlands that receive SO42‒ solely from the atmosphere to those highly enriched in SO42‒ (>65 mg/L) from agricultural runoff45,47. Across geochemical SO42‒ gradients, surface waters and pore waters were sampled for pertinent biogeochemical parameters, including filtered and particulate Hg(II) and MeHg (f.Hg(II), p.Hg(II), f.MeHg, p.MeHg), S species (SO42‒, S(‒II)), and dissolved organic carbon (DOC) concentration and DOM composition (specific ultraviolet absorbance at 254 nm (SUVA254)). We present data showing coherence between SO42‒ concentrations in wetlands, shifts in DOM composition, and MeHg formation across a comprehensive field study, which included the examination of MeHg concentrations across vertical gradients in wetlands (between surface and pore waters) and lateral gradients across wetlands. Lastly, relationships between MeHg concentrations in surface waters were compared to MeHg concentrations in prey fish (Gambusia holbrooki), an indicator species with a short life span (≤6 months) that reflects recent MeHg risk to the aquatic food web17,43,48. The findings advance a new ecosystem-scale conceptual model of the effects of SO42‒ on Hg risk to the aquatic food web and are discussed in context of parallel efforts that quantified the effects of SO42‒ on DOM thiol content34, the abundance and metabolic capabilities of hgcAB+ microorganisms19, downgradient impacts on MeHg formation and biouptake in Everglades National Park48, and strategies to mitigate the risk of Hg to wildlife and humans. This information will be vital to anticipate future effects of SO42− on Hg(II) methylation potential across subtropical wetlands in response to management actions, climate change, and international efforts to mitigate atmospheric Hg releases in the environment.
Results and discussion
Chemistry of canals contributing to freshwater Everglades wetlands
Canals, which drain Lake Okeechobee and the Everglades agricultural area45, exert considerable control on the biogeochemistry of waters in the three water conservation areas studied (water conservation areas 2A and 3A (WCA-2A and WCA-3A), and the Arthur R. Marshall Loxahatchee National Wildlife Refuge (LOX)), and exhibit distinct chemistries (Tables S1–S3 and Fig. 1). The levee-6 (L-6) canal, which discharges into northwest WCA-2A, drains water with DOM of higher aromaticity (DOM SUVA254 = 3.48 ± 0.16 L/mg m; avg ± 1 standard deviation) and higher concentrations of DOC (33.1 ± 4.2 mg/L), SO42‒ (64.7 ± 32.4 mg/L), and chloride (Cl‒) (131 ± 37.4 mg/L) compared to the levee-28 (L-28) canal (DOM SUVA254 = 3.30 ± 0.34 L/mg m; DOC = 19.6 ± 2.9 mg/L; SO42‒ = 6.84 ± 3.65 mg/L; Cl‒ = 32.8 ± 18.0 mg/L), the latter draining into northwest WCA-3A (Fig. 1). The Palm Beach Canal, which is an outer canal along the northeast perimeter of LOX, contains water of lower DOM aromaticity (DOM SUVA254 = 2.63 ± 0.34 L/mg m) and intermediate DOC, SO42‒, and Cl‒ concentration compared to the L-6 and L-28 canals (DOC = 19.9 ± 12.5 mg/L; SO42‒ = 43.9 ± 40.6 mg/L; Cl‒ = 96.2 ± 58.7 mg/L). Of the four Hg fractions measured from the three canals over the study (f.Hg(II), f.MeHg, p.Hg(II), p.MeHg; n = 23), Hg was primarily present as f.Hg(II) across all sites (Table S3). Particulate fractions accounted for <25% of the total Hg in canal waters (Fig. S1). Of the filtered fractions (f.Hg(II) and f.MeHg), f.MeHg was only responsible for 9-11% and f.Hg(II) accounted for the remaining 89–91% of the THg. Further, concentrations of f.Hg(II) and f.MeHg were similar across the three canals, with mean values ranging from 0.67–0.74 ng/L and 0.07–0.10 ng/L, respectively. One outlier was observed of elevated f.MeHg (1.11 ng/L) from the L-28 canal (08/18/2014). Differences in the chemistry of canal waters are interpreted to reflect upgradient agricultural practices that enrich waters in inorganic anions (SO42‒, Cl‒) from agricultural practices45,47 and mobilize highly aromatic DOM from peat soils33, which has important implications on the biogeochemical cycling of Hg in downgradient wetlands.
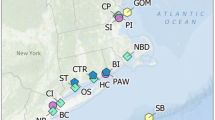
Locations of surface water and pore water collection across hydrologic flow paths in water conservation areas 2A and 3A, and a geochemical gradient in the Arthur R. Marshall Loxahatchee National Wildlife Refuge (LOX). Flow vectors are presented based on the Everglades Depth Estimation Network (EDEN) model (vectors shown for 11/17/2015)50.
Surface and pore water biogeochemistry across wetland transects
The chemistry of surface and pore waters across the three wetlands exhibits distinct lateral and vertical differences that reflect proximal canal inputs and biogeochemical transformations of organic C, S, and Hg that arise from these inputs. The highest spatial resolution sampling was conducted in August 2014 (Fig. 2) across WCA-2A (n = 16) and WCA-3A (n = 15), and December of 2015 in LOX (n = 5), with sampling events in other years conducted at a reduced number of sites or only including surface waters (Fig. S2 and Table S2)49. Surface water SO42‒ concentrations of wetlands were comparable to adjacent canals at the top of each transect (i.e., near km = 0) and exhibited a concentration hierarchy of WCA-2A > LOX > WCA-3A (Fig. 2a–c). With increased distance from canals, surface water SO42‒ concentrations consistently decreased (e.g., in Fig. 2, SO42‒ decreased from 65.8 to 50.6 mg/L, 22.5 to <0.5 mg/L, and 4.1 to <0.5 mg/L across transects in WCA-2A, LOX, and WCA-3A, respectively). Further, surface waters were sub-oxic to anoxic in all three wetlands, immediately downgradient of canal inputs (Fig. S3a–c), with dissolved oxygen concentrations generally increasing with distance from canal inputs49. The oxidation-reduction potential (ORP) of wetlands surface water and pore waters (Fig. S3d–f) reflected the degree of canal influence, with significantly lower ORP in pore waters of WCA-2A (‒271 ± 12.3 mV; average ± std) compared to WCA-3A (‒168.7 ± 18.4 mV) or the interior of LOX (LOX8, ‒25 mV) (Welch’s t-test; p < 0.001). At the most downgradient site in WCA-3A, which is proximal to the L-29 canal, notable increases in SO42‒ and Cl‒ concentrations were observed (Fig. 2b) due to canal backflow into WCA-3A, as supported by the Everglades depth estimation network (EDEN) flow model (Fig. 1)50. The Florida Everglades is naturally a low SO42‒ environment, with concentrations between 0.1–1.0 mg/L in regions unimpacted by agricultural S inputs45, and therefore SO42‒concentrations observed in interior WCA wetlands greatly exceeded background concentrations (up to ~70-fold) and were distributed across the entire range observed (≤0.5–72.0 mg/L; Fig. S4), creating vastly different water quality conditions across downgradient freshwater wetlands.
Biogeochemical constituents of (a–c) SO42‒ concentration, d–f inorganic sulfide (S(-II)) concentration, g–i the molar ratio of SO42‒ to chloride (SO42‒ / Cl‒), j–l dissolved organic carbon (DOC) concentration, and (m–o) DOM specific ultraviolet absorbance at 254 nm (SUVA254) in water conservation area 2A (WCA-2A) (August, 2014), WCA-3A (August, 2014), and the Arthur R. Marshall Loxahatchee National Wildlife Refuge (LOX) as a function of distance from canal inputs (November, 2015). For each transect, canal conditions are presented at the time of wetland sampling. Dashed lines are presented to guide the eye. Oxidation-reduction potential (ORP) and dissolved oxygen (O2) data were presented in Fig. S3.
Several lines of evidence support the prominence of dissimilatory SO42‒ reduction in wetlands downgradient of canal inputs, explaining the decrease in surface water SO42‒ concentration with increased distance from canals. First, in sediment pore waters, SO42‒ concentrations were significantly lower than corresponding surface waters (Mann–Whitney rank sum test, p < 0.001), by ≤60.5, ≤26.5, and ≤3.5 mg/L in WCA-2A, LOX, and WCA-3A, respectively (Fig. 2a–c). Second, concentrations of inorganic sulfide (S(‒II)), a byproduct of dissimilatory SO42‒ reduction, were highest in pore waters of WCA-2A (S(‒II) = 1.6–9.4 mg/L), intermediate at locations in LOX and WCA-3A nearest canals (S(‒II) ≤0.45 and ≤0.16 mg/L, respectively), and below detection limit at locations in LOX and WCA-3A with SO42‒ ≤ 0.5 mg/L (S(‒II) ≤0.01 mg/L) (Fig. 2d–f); this hierarchy matched the magnitude of negative ORP across the wetlands (Fig. S3d–f). Although pore water SO42‒ and S(‒II) concentrations varied with distance from the canal (Fig. 2d–f), particularly across WCA-2A, a significant positive correlation was observed between pore water SO42‒ and S(‒II) concentrations across all sites (Fig. S5; R2 = 0.64, p < 0.001; n = 60). Notably, S(‒II) concentrations were above the detection limit (S(‒II) >0.01 mg/L) in anoxic surface waters of WCA-2A (Fig. S6) but below the detection limit in surface waters from WCA-3A and LOX. Third, in wetland locations with evidence of SO42‒ reduction, we observed consistent decreases in the molar ratio of SO42‒ to Cl‒ with increased distance from canals (SO42‒/Cl‒; Fig. 2g–i), the latter being a conservative analyte inert to biogeochemical transformations. Concentrations of Cl‒ were largely uniform across the WCA-2A and WCA-3A (Fig. S7)49 and therefore decreases in the molar SO42‒/Cl‒ are interpreted to be primarily due to microbial SO42‒ reduction, consistent with previous observed increases in δ34S of SO42‒ due to reduction of isotopically light S47, the abundance of genes of SO42‒ reducing bacteria (e.g., dsrA)19, and landscape SO42‒ models51. These wetland transects document SO42‒ contamination of the WCAs, extending >10 km downgradient of active points of canal water release (WCA-2A, WCA-3A) or through intrusion from canals (LOX). Wetland SO42‒ concentrations governed the spatial extent of SO42‒ reducing conditions, with S(-II) accumulating in pore waters across all sites as a function of SO42‒ availability (Fig. S5) and extending to surface waters in WCA-2A (Fig. S6).
Canal contributions also had a marked influence on the DOC concentration and DOM composition within wetlands. Across wetland gradients, surface water DOC concentrations and DOM SUVA254, a proxy for aromatic C content52, were consistent between neighboring canals and adjacent wetland sites (Fig. 2j–o). In wetland pore waters, however, DOC concentrations were significantly higher compared to surface waters (Mann–Whitney rank sum test, p < 0.001, n = 70), particularly in regions with active SO42‒ reduction near canal inputs (Fig. 2j–l), and DOM in pore waters was of modestly higher aromaticity (DOM SUVA254; Welch’s t-test, p < 0.001, n = 70; Fig. 2m–o). Higher DOC concentration and greater DOM aromaticity in wetland pore waters near canal inputs compared to surface waters are interpreted to be the result of SO42‒stimulating degradation of peat by SO42‒ reducing bacteria33 that release DOM of higher aromaticity (e.g., phenolic groups)29,32. Further, the anoxic (Fig. S3a–c), sulfidic conditions that prevail due to SO42‒ inputs are known to prevent enzymatic degradation of aromatic DOM53 and promote the sulfurization of DOM, the latter enriching DOM in thiol functional groups proportional to SO42‒ inputs to wetlands34. With increased distance from canals in WCA-2A and WCA-3A, surface water DOC concentrations decreased (e.g., from 37.0 to 32.7 and 23.1 to 15.0 mg/L, respectively) and DOM SUVA254 decreased (e.g., from 3.7 to 3.1 and 3.9 to 2.4 L/mg m, respectively) (Fig. 2m, n), likely the result of the photochemical transformation of DOM54 and autochthonous DOM production in wetland surface waters55. The decadic absorption coefficient of DOM at 254 nm (α254), which is a bulk DOM metric that scales positively with DOC concentrations and DOM SUVA254, showed similar trends across the three wetlands with higher DOM α254 near canal inputs and in wetlands with higher SO42‒49.
The effects of SO42‒ on DOM quantity and composition were observed across all wetland surface waters over the seven sampling events of this study (Fig. 3a–b), with significant positive correlations between SO42‒ and DOC concentration (R2 = 0.52, p < 0.001; n = 92) and SO42‒ concentration and DOM SUVA254 (R2 = 0.30, p < 0.001; n = 92). These relationships are interpreted to arise from upgradient and within-wetland processes resulting from the biogeochemical cascade due to SO42‒ effects on DOM mobilization from peat29,32,33. Greater variability is observed between correlations of DOC concentration and DOM SUVA254 and SO42‒ concentration at lower SO42‒ concentrations (Fig. 3a, b), which we interpret to be the result of canal waters with intermediate SO42‒ concentrations stimulating aromatic DOM release and SO42‒ reduction that depletes available SO42‒ (Fig. S5). In summary, canal water SO42- inputs lower the wetland redox status and stimulate S(‒II) production and the release of aromatic DOM across distinct lateral and vertical geochemical gradients within all three Everglades wetlands, and subsequently these biogeochemical changes govern the geochemical nature of Hg(II)31,36 and bioavailability for methylation39,40.
Semi-log scatter plots between the concentration of sulfate and (a) dissolved organic carbon (DOC) concentration, (b) DOM specific ultraviolet absorbance at 254 nm (SUVA254), (c) filtered inorganic divalent Hg (f.Hg(II)) concentration, and (d) filtered methylmercury (f.MeHg) concentration. In subplots a and b, dashed gray lines and dotted blank lines present the linear regression and 95% confidence intervals between SO42‒ and DOC concentration and DOM SUVA254. In subplots c and d, dashed gray lines and dotted black lines present the unimodal equations and 95% confidence intervals of the fit, respectively (Eq. 1, S1; Fig. S9).
Mercury speciation and biological uptake across wetland transects
The lateral and vertical spatial distribution of Hg fractions was tightly coupled to the biogeochemical cascade linked to canal SO42‒ inputs. Across all wetlands, particulate Hg fractions were measured (p.Hg(II), p.MeHg)49 and were a minor fraction (accounted for ≤10% of the total Hg) (Fig. S1), consistent with previous observations4, and thus the analyses in this study focused on f.Hg(II) and f.MeHg. Concentrations of f.Hg(II) in wetland surface waters were consistent with those of adjacent canals (Fig. 4a–c) and showed modest variability from up-to-downgradient along wetland transects and between surface and pore waters. Modestly higher f.Hg(II) concentrations were observed in pore waters with elevated DOC concentration, but f.Hg(II) concentration did not linearly correlate to DOC concentration (R2 = 0.06; n = 112), consistent with previous observations in the Everglades4. Rather, f.Hg(II) concentration exhibited a nonlinear relationship to SO42‒ concentration, where f.Hg(II) concentrations were low at SO42‒<1.0 mg/L, increased between 1 and 10 mg/L SO42‒, and decreased at SO42‒ >10 mg/L (Fig. 3c). There was considerable variability in the relationship between f.Hg(II) and SO42‒ concentration, and thus the fit of data was not of statistical significance (p = 0.09; SI Section S1). We interpret the relationship to be from SO42‒ (1) increasing f.Hg(II) concentration at low-to-intermediate SO42‒ concentration, due to increases in DOC concentration and DOM SUVA254 (Fig. 3a, b) that enhance complexation of Hg(II)30, and (2) decreasing f.Hg(II) concentration at high SO42‒ concentration through S(‒II) scavenging f.Hg(II) and forming nano-β-HgS(s)31,36 that aggregate35. Although additional source and sink processes also influence f.Hg(II) concentrations in Everglades wetlands, including rainfall delivery of f.Hg(II)2,46, photo-reduction of Hg(II) to Hg(0)3,4, and partitioning of Hg(II) to peat43, SO42‒ exhibits notable control on the concentration of f.Hg(II), which can limit MeHg formation in wetlands.
Concentrations of filtered (a–c) inorganic Hg (f. Hg(II)), d–f methylmercury (f. MeHg), and (g–i) the percentage of total Hg as MeHg (%MeHg) in water conservation area 2A (WCA-2A), WCA-3A, and the Arthur R. Marshall Loxahatchee National Wildlife Refuge (LOX) as a function of distance from canal inputs (August 2014). For each transect, canal conditions are presented. Dashed lines are presented to guide the eye.
The concentration of f.MeHg and percentage of total Hg as MeHg (% MeHg) showed dramatic differences between the three wetlands, with distinct vertical trends between surface and pore waters and lateral trends across wetlands. The %MeHg term is used to normalize f.MeHg concentration data to the total Hg concentration at a given location, as f.Hg(II) concentrations varied by an order of magnitude within and between the studied wetlands (e.g., Fig. 4a–c and S8a, b) and is the precursor for f.MeHg9,56. Canals draining into WCAs typically had f.MeHg concentrations and %MeHg of 0.10 ng/L and 10%, respectively (Table S3 and Fig. S8), with rare instances of higher concentrations (e.g., L-28 canal during August 2014; Fig. 4e)49. In WCA-2A, which had the highest SO42‒ loading, f.MeHg concentrations and %MeHg were low in both surface and pore waters at the first 4 wetland locations downgradient of the L-6 canal input (0.01–0.04 ng/L and 1.1–3.4 %MeHg, respectively; Fig. 4d and Fig. S8). Further downgradient, average f.MeHg concentrations and %MeHg were higher in surface waters (0.27 ± 0.05 ng/L and 16.4 ± 2.3 %MeHg, respectively; ±1 standard deviation) compared to pore waters (0.06 ± 0.04 ng/L and 4.5 ± 2.8 %MeHg, respectively) or the L-6 canal (Mann–Whitney rank sum test, p < 0.001, n = 14). In WCA-3A, which had lower SO42‒ loading than WCA-2A, f.MeHg concentrations and %MeHg were highest near the L-28 canal input, were greater in pore waters compared to surface waters, and decreased monotonically with distance from the canal (Fig. 4e, h and Fig. S8d, f) consistent with decreases in surface water SO42‒ (Fig. 2b). The highest f.MeHg and %MeHg levels observed across all three wetlands were in pore waters (≤5.0 ng/L and ≤84.5%, respectively) and surface waters (≤2.5 ng/L and ≤60%, respectively) of WCA-3A near the L-28 canal input, which is consistent with other studies on MeHg spatial distribution across the system4,43,51. In LOX, which had the widest range in SO42‒ concentrations, f.MeHg and %MeHg were low adjacent to the canal (≤0.09 ng/L and ≤13.8%, respectively) (Fig. 4f, i), increased to a maximum approximately 4 km from the canal (≤1.50 ng/L and ≤47%, respectively), and declined in the interior of the wetland (≤0.07 ng/L and ≤6.2%, respectively) were SO42‒ concentrations were <1 mg/L; similar behavior was observed in both surface and pore waters f.MeHg concentration across the LOX wetland SO42‒ gradient.
Across all wetland surface waters sampled over the seven field campaigns, a nonlinear unimodal trend was observed between surface water SO42‒ and f.MeHg concentrations (Fig. 3d). A nonlinear, 3-parameter equation was used to fit field data, defined in Eq. 1,
where \(a\), \(b\), and \({x}_{0}\) were 11.9, 1.30, and 27.6, respectively (R2 = 0.41, p < 0.001; n = 93). A maximum in f.MeHg concentration was observed at a SO42‒ concentration between 4 and 6 mg/L, and lower f.MeHg concentrations were observed at lower and higher SO42‒ concentrations. There was good agreement between observed and predicted f.MeHg concentrations at observed f.MeHg concentrations ≥0.1 ng/L (Fig. S9a) with no systematic difference in residual f.MeHg concentrations (i.e., the difference between observed and predicted f.MeHg concentrations) across the range of SO42‒ concentration (Fig. S9b), indicating the unimodal fit was robust across the conditions of the three wetlands. At both high (>30 mg/L) and low SO42‒ concentration (<1 mg/L), which corresponded to low f.MeHg concentration (<0.1 ng/L), a modest discrepancy was noted between observed and predicted f.MeHg concentrations (Fig. S9a). Across all wetlands, the %MeHg was significantly correlated (R2 = 0.77, p < 0.001; n = 168) to the concentration of f.MeHg in water (Fig. S10), which has been observed in the Everglades9,19 and other systems10,15 to correspond with faster measured rates of MeHg production. Thus, wetlands with higher aqueous f.MeHg and %MeHg are interpreted to reflect recent MeHg production, as MeHg that is produced is susceptible to sink processes (e.g., photo-demethylation57 and adsorption to peat)58.
Vertical and lateral spatial trends in f.MeHg concentration and %MeHg in response to SO42‒ reflect a combination of (1) the redox state of wetland surface and pore waters, (2) geochemical processes governing Hg(II) aqueous speciation31,36 and bioavailability39,40,41,59, and (3) the microbial communities with the pre-requisite hgcAB gene pair for methylation37. Below we detail the observations from low to high SO42‒ concentration across the three wetlands and interpret environmental f.MeHg concentrations to reflect the balance between Hg(II) methylation3,4,19 and MeHg demethylation9,23, with differences primarily being driven by differences in Hg(II) methylation9,15,24. At SO42‒ concentrations <1 mg/L, observed in the middle of WCA-3A and LOX, f.MeHg concentrations were low in surface and pore waters, interpreted to be a combination of the absence of S(‒II) and higher redox state of the water column and wetland sediments (Fig. S3), lower f.Hg(II) concentration, DOC concentration, and DOM SUVA254 (Fig. 3a–c), and lower DOM thiol content34; all of these conditions decrease the bioavailability of Hg(II) to methylation39,40,41. Although higher hgcAB gene abundance was observed in peat of low SO42‒ Everglades wetlands19, the low ambient MeHg observed under these conditions is attributed to high wetland redox state and low Hg(II) bioavailability. The modest disagreement between observed and predicted f.MeHg at SO42‒ concentrations <1 mg/L (Fig. S9a) simply reflects the low MeHg formation under low, ambient levels of SO42‒ that are not well fit using a 3-parameter unimodal function. At intermediate SO42‒ concentrations (2–6 mg/L), such as in LOX and the upgradient portions of WCA-3A (Fig. 4e, f), f.MeHg concentrations were elevated in sulfidic pore waters compared to oxygenated surface waters, which is interpreted to reflect enhanced MeHg formation in surficial wetland sediments and advection and diffusion to surface waters3. The highest f.MeHg concentration and %MeHg were observed under these conditions (Figs. 3d, 4e), where microbial hgcAB gene abundance is elevated in sediments19 and sulfidic pore waters have high DOC concentration and DOM of more aromatic and thiol group content34, which promote Hg(II) methylation39,40,41 likely as nano-β-HgS(s)31,36. At locations of high SO42‒ concentrations (>12 mg/L) in WCA-2A downgradient from canals (Fig. 4d, g), f.MeHg concentration and %MeHg were notably higher in surface waters compared to pore waters. This observation cannot be explained by MeHg formation in sediments and advective or diffusive flux60,61, but may be attributed to MeHg formation by periphyton associated with emergent and submerged aquatic vegetation in the water column20,21. The shallow wetlands studied here are unlikely to support bulk water column methylation observed in deeper, thermally stratified waters where suspended particulate materials accumulate62,63. Lastly, at locations of high SO42‒ concentration in WCA-2A near the canal, f.MeHg concentration was low in both surface and pore waters (Fig. 4d, g), which is attributed to high S(‒II) conditions in surface and pore waters that decrease the concentration and bioavailability of f.Hg(II) (Fig. 3c) via the formation31,36 and aggregation35 of nano-β-HgS(s) and the low microbial abundance of communities with the hgcAB genes19. Despite the elevated concentrations of highly aromatic, sulfurized DOM34 under these sulfidic conditions, crystalline nano-β-HgS(s)36 will form and dramatically decrease f.Hg(II) concentrations in the wetland pore and surface waters (Figs. 3c, 4a) and contributes to decreased MeHg formation64. Despite many inferential studies that concluded direct links between SO42‒ loading, the activity of SO42‒ reducing bacteria, and MeHg production3,4,9,21,45, a recent microbial metagenomic analysis of sediments across these same sites did not observe hgcAB+ organisms with the genes for dissimilatory SO42‒ reduction19. Rather, MeHg concentration and experimental methylation rates were governed by synergy between the hgcAB abundance of other microbial clades and DOM composition (SUVA254)19. We interpret that SO42‒ inputs directly alter Hg(II) geochemistry and bioavailability and indirectly alter the microbial metabolism, which stimulates MeHg formation at low-to-intermediate SO42‒ concentrations (2–12 mg/L) by SO42‒ reducing bacteria consuming fermentation products and/or stimulating methanogenic activity through syntrophy19.
The field data underlying these biogeochemical relationships span multiple years (2012–2019) and seasons (May and December) (Table S2) and leverage geochemical34, hydrological, and microbial insights19, providing a comprehensive dataset to develop a framework for MeHg production and risk. Taken together, a highly consistent trend is observed across all wetland surface waters between concentrations of SO42‒, the DOM α254 (which scales positively with increases in DOC concentrations and DOM SUVA254), and f.MeHg concentration (Fig. 5). We interpret the spread in f.MeHg at a given SO42‒ concentration (Figs. 3d, 5) to be the effects of seasonality on MeHg formation, as noted previously4,17, with higher observed MeHg formation in the spring and summer months (Fig. S11) due to higher rates of microbial metabolism and regular rainfall delivering Hg(II)3 to wetlands. The unimodal relationship here between SO42‒ and f.MeHg concentration (Fig. 3d, 5) is consistent with the conceptual model first proposed by Gilmour and Henry (1994)8 but incorporates recent innovations in the understanding of Hg(II) nano-scale geochemistry and the complexities of microbial Hg(II) methylation, which provides mechanistic insights that explain MeHg formation across the greater Everglades16 and in response to increases43 and decreases in SO42‒ loading51. We assert that the clear coherence in the relationship between SO42‒ and f.MeHg concentration here is a product of the study design, with field campaigns that sampled lateral hydrologic flow paths from canal sources downgradient across wetlands, resolved vertical gradients between wetland surface waters and sediment pore waters, and coupled routine biogeochemical data (e.g., Figs. 2–5) with parallel efforts on SO42‒ effects on DOM composition34 and abundance and metabolic capabilities of hgcAB+ microorganisms19. Although the assertion of SO42‒ is a master variable on MeHg formation in the Everglades is not in agreement with conclusions from state environmental reports65,66, which may be due to differences in study design or approaches used for biogeochemical characterization, the observed findings are congruent with studies on the effects of SO42‒ on MeHg formation in diverse wetlands9,10,11,12,13,14,15.
Three-dimensional scatter plot between the surface water concentration of SO42‒, DOM absorbance at 254 nm (DOM α254), and concentration of filtered methylmercury (f.MeHg) in surface water across water conservation area 2A (WCA-2A), WCA-3A, and the Arthur R. Marshall Loxahatchee National Wildlife Refuge (LOX).
The concentrations of f.MeHg in wetland surface waters significantly correlated with the concentration of MeHg in gambusia (Fig. 6; R2 = 0.68; p < 0.001; n = 33), a resident fish species17. The total Hg concentration of Gambusia was primarily MeHg (91.2 ± 14.1%, n = 33)49. Provided that these resident prey fish live relatively short lives (≤6 months) and feed on a combination of periphyton and zooplankton17, the data provide strong support that SO42‒ promotes MeHg formation in surficial sediments and compartments of the water column resulting in enhanced risk of MeHg bioaccumulation at the base of the aquatic food web. Figure 7 synthesizes a framework on the association between SO42‒, DOM, and the potential for MeHg formation in proximity to aquatic food webs, which dictates MeHg risk. We further conclude that water concentrations of f.MeHg may be a good proxy to predict food web risk in the Everglades due to the strong relationship observed here and within Everglades National Park48. The highest gambusia MeHg concentrations were observed at locations with intermediate SO42‒ concentrations, with fish MeHg concentrations exceeding 1000 ng/g dry weight, similar to that reported previously across the WCAs67 and greater Everglades ecosystem16,68. Taken together, this study shows coherence in the underlying hydrologic and biogeochemical processes that govern MeHg risk in the freshwater Everglades (Fig. 7), which support the use of SO42‒ concentration to model risk across the freshwater ecosystem51 and to forecast the influence of water management decisions on MeHg formation and food web bioaccumulation in Everglades National Park48.
Scatter plot between concentration of filtered methylmercury (f.MeHg) in surface water and concentration of MeHg of gambusia across water conservation area 2A (WCA-2A), WCA-3A, and the Arthur R. Marshall Loxahatchee National Wildlife Refuge (LOX). The dashed gray line is the linear fit to the data, and dotted black lines correspond to the 95% confidence intervals of the fit.
Low risk is observed at high (~>15 mg/L) and low (<1 mg/L) SO42‒ that facilitate unfavorable conditions for MeHg formation, due to geochemical bioavailability of Hg(II), wetland redox status, and hgcAB abundance of microbial community19. High risk is observed at intermediate SO42‒ (2–12 mg/L) that facilitates a suitable redox status, Hg(II) bioavailability (due to DOM aromaticity36,40 and thiol content)34,41, and hgcAB abundance19 that collectively promote MeHg formation in proximity to the aquatic food web.
Strategies to mitigate MeHg risk in wetlands
Wetlands globally are being affected by increases in SO42‒ due to a variety of new pressures, which will influence Hg cycling. Agricultural use of S-containing fertilizers has increased 200% in the last 30 years in the United States25, and climate change processes are increasing SO42‒ concentrations across the globe. For example, enhanced mineral weathering in the Arctic has increased SO42‒ concentrations in the Mackenzie River by 45% in the last 60 years28, and sea-level rise is threatening coastal wetlands with marine SO42‒ that will result in the contraction of freshwater ecosystems29. The comprehensive framework presented here (Fig. 7) for the Florida Everglades support that efforts to decrease SO42‒ concentrations from agricultural sources have promise to decrease aqueous and biological MeHg concentrations in wetlands (Figs. 5, 6), offering a local strategy to mitigate Hg contamination. Local reductions in SO42‒ concentration or load may yield a relatively fast response, as previous work in the Everglades demonstrated that declines in SO42‒ could elicit fast declines (i.e., ≤1–2 years) in MeHg concentrations51. Reducing agricultural S could be achieved as part of a sustainable strategy to manage S use44, akin to nitrogen and phosphorus, which would also be expected to decrease wetland conditions of DOC concentrations, DOM aromaticity, and DOM thiol content32,33,34, all of which decrease MeHg formation19,40,41 and increase photo-demethylation of MeHg in surface waters57. Other local management strategies, including minimizing the drying and re-wetting of Everglades soils69, may have beneficial outcomes by reducing the inadvertent oxidation of legacy S in peat to SO42‒45. Changes in water delivery and water quality as part of local management and restoration activities may be effective in mitigating Hg methylation and other potential biogeochemical responses, such as DOM release, related to elevated SO42- concentrations in the Everglades as well as other subtropical wetland systems.
Beyond local management options to mitigate Hg, decreases in atmospheric deposition of Hg stemming from national and international efforts are expected to potentially reduce the formation43 and food web uptake of MeHg70, but the timeline of a response on the landscape is uncertain. Across the conterminous US, the atmospheric deposition of Hg has declines over the last two decades due to decreased domestic emissions, but atmospheric deposition in south Florida has remained consistently elevated (1999–2023)46 due to effective Hg oxidation in the troposphere in this region2 and the air masses in southern Florida being distinct from the rest of the conterminous US. For these reasons, total Hg concentrations in water have been observed to be rather static in Everglades National Park (2008–2018)48. Declines in atmospheric deposition in South Florida are expected to occur in response to global reductions in Hg emissions, via the implementation of the United Nations’ Minamata Convention on Mercury and reductions in carbon emissions that also decrease Hg releases5. Current scenario projections of global reductions71 suggest that decreases in atmospheric Hg deposition will be modest over the coming three decades, with considerable uncertainty based on Hg reduction scenarios. However, despite the projected decreases in atmospheric Hg deposition, other geochemical and ecological factors may exert disproportionate control over ecosystem responses and lead to a range of potential outcomes that do not have a 1:1 response with declining Hg emissions and deposition72,73. Natural resource managers are tasked with balancing short- and long-term strategies to decrease MeHg formation and uptake in the aquatic food web, with SO42‒ reductions offering an attractive local approach that has other positive benefits on ecosystem health44,45.
Methods
Field sampling locations
Field sites were selected to follow existing hydrologic and geochemical gradients across three freshwater, sawgrass-dominated wetlands of the Florida Everglades: water conservation areas 2A and 3A (WCA-2A, WCA-3A), and the Arthur R. Marshall Loxahatchee National Wildlife Refuge (LOX) (Fig. 1 and Supplementary Information (SI) Table S1). WCA-2A and WCA-3A receive high and intermediate concentrations of sulfate from canal inputs that drain upgradient agricultural lands, respectively, and generally exhibit decreasing sulfate concentrations from north to south34,45. Surface water flow vectors typically observed across WCA-2A and 3A are shown in Fig. 1, based on the Everglades Depth Estimation Network (EDEN) Surface-Water Interpolation Model Version 350. The WCA-2A transect consisted of 16 sites over 14.5 km within the wetland that followed water flow south from the levee-6 (L-6) canal inputs to the middle of WCA-2A; one additional site of the L-6 canal immediately upgradient of the wetland was sampled to characterize the water discharging into WCA-2A from the control structure. The WCA-3A transect consisted of 15 sites over 39.7 km, starting at the terminus of the levee-28 (L-28) canal and extending south to the intersection of WCA-3A and the L-29 canal. Due to lack of access to the L-28 canal within WCA-3A, the L-28 canal was sampled at the junction with highway I-75 to assess the composition of water entering WCA-3A (Fig. 1). LOX, in contrast to WCA-2A and WCA-3A, generally exhibits lower sulfate concentrations due to the interior regions being primarily rainfall-driven and hydrologically isolated from canal inputs by a perimeter canal34,45. However, contributions of canal-derived constituents (e.g., SO42‒, chloride (Cl‒)) intrude across the levees and penetrate the interior of LOX45,74. Five locations were sampled in LOX from the interior (LOX8) to the levee near the L-40 canal (LOX-136) (Fig. 1), which spanned 12.5 km. Sites across the three WCAs were previously evaluated to quantify the effects of SO42‒ on DOM reduced sulfur content and speciation34 and microbial metagenomic sequencing for the Hg methylation genes (hgcAB+)19.
Water collection and analyses
Wetland and canal locations were sampled approximately once annually from 2012 to 2019, spanning from May to December. Table S2 summarizes the sampling date ranges, cumulative precipitation and wet Hg deposition data (on an annual basis and the 3 months prior to sampling) from local stations maintained by the National Atmospheric Deposition Program46, and air temperature data during sampling. The cumulative annual precipitation and wet Hg deposition over the years of the study were within the range typically observed at these sites, based on available data records (1997–2023; Table S2). In total, 189 and 23 discrete water samples were collected from wetlands (WCA-2A, n = 99; WCA-3A, n = 78; LOX, n = 12)49 and canals75, respectively, across the study period during seven field campaigns. The density of samples across the three wetlands aimed to (1) span the complete range of SO42‒ concentrations of the freshwater Florida Everglades45 with comparable density as a function of SO42‒ concentration and (2) have higher sample density at the low-to-intermediate concentration range of SO42‒ (≤0.5–12 mg/L) (Fig. S4). At times, transects were sampled in a truncated design (Table S2), where some of the sites shown in Fig. 1 were skipped to best meet the abovementioned sampling goals.
At all sites, surface water was collected in 2 L polyethylene terephthalate (PETE) bottles at the air-water interface and stored in coolers on wet ice until processing the same day. For wetland sites only, pore water was collected 10 cm below the sediment-water interface at a rate of 100 mL min−1 using a Teflon sipper connected to Teflon tubing and a peristaltic pump. The sipper was repositioned laterally by ~0.5 m every 10 min to not deplete sediment pore water. First, pore water temperature, conductivity (Orion four-cell conductivity electrode), pH (Orion ROSS Ultra™ electrode), dissolved oxygen (DO) concentration (Orion RDO optical probe), and oxidation-reduction potential (ORP; Orion ORP Triode electrode) were measured using a flow-through cell (Geotech; 40 mL dead volume) and multi-parameter meters (Orion Star™ A329, Beckman Coulter pHi 410)56. Next, pore waters were in-line filtered during collection (QFF, 0.7 μm pore size, pre-combusted at 550 °C, Whatman™) for the following analyses: sulfide (high-density polyethylene (HDPE) bottles, preserved with 50% volume/volume (v/v) sulfide antioxidant buffer), inorganic anions (HDPE bottles, no preservation, stored at 4°C), major cations anions (HDPE bottles, no preservation, stored at 4 °C), DOC concentration and DOM ultraviolet and visible (UV-vis) light absorption (pre-baked amber borosilicate glass vials at 450 °C for 4.5 h, no preservation, stored at 4 °C), and filtered total Hg (f.THg) and filtered MeHg (f.MeHg) (acid-cleaned Teflon bottles, preserved with 1% trace-metal grade hydrochloric acid). Pore water particulate total Hg (p.THg) and particulate MeHg (p.MeHg) concentrations were not measured due to potential artifacts during sample collection. Surface waters were filtered within 8 h of collection by vacuum filtration through a QFF for the identical set of analyses as pore water samples detailed above; QFF filters were frozen (−20 °C) for quantification of p.THg and p.MeHg concentrations. Field replicates of wetland surface waters and pore waters (n = 8) were sampled in 5% of total samples49. The average relative percent deviations of field replicates for concentrations of f.THg, f.MeHg, DOC, and SO42‒ were 5.5, 4.2, 0.6, and 2.8%, respectively.
Complete details on the analysis methods and limits of quantification are provided in an associated ScienceBase data release49. Sulfide was quantified within 12 h of sample collection by an ion-selective electrode. Major inorganic anions (Cl‒, nitrate (NO3‒), SO42‒) were quantified by ion chromatography76. DOC concentration was quantified by persulfate oxidation (OI Analytical, model 700)77. UV-vis absorption spectra were measured from 190 to 800 nm, and decadic absorbance values were converted to absorption coefficients as
where \({\alpha }_{\lambda }\) is the decadic absorption coefficient (cm−1), \({A}_{\lambda }\) is the absorbance, and \(l\) is the path length (cm). The DOM specific ultraviolet absorbance at 254 nm (SUVA254), a proxy for DOM aromaticity52 that indicates DOM source and reactivity to Hg(II)19,36,40, was calculated as:
by dividing the \({\alpha }_{254}\) (m−1) by DOC concentration (mg L−1). Mercury measurements were made at the US Geological Survey Mercury Research Laboratory (Madison, WI). THg measurements (f.THg, p.THg) were made by BrCl oxidation (0.2 M; 1% v/v for f.THg and 5% for p.THg), stannous chloride reduction, dual amalgamation, and cold vapor atomic fluorescence spectroscopy (CVAFS) detection following a modified version of US Environmental Protection Agency (EPA) method 1631, Revision E (Brooks-Rand TDM-II)78. Filtered and particulate MeHg were analyzed using a modified version of the US EPA method 163079 with isotope dilution. Approximately 20 pg of isotopically-enriched Me199Hg and 1 mL of 1.6 M copper SO42‒ solution were added to 40 mL aliquots for MeHg analysis. Ambient MeHg was determined by relating the ratio of added Me199Hg to Me202Hg after distillation, aqueous phase ethylation, trapping on Tenax (Buchem B.V.), isothermal gas chromatography separation, and detection by inductively coupled plasma mass spectrometry (iCAP, Thermo Scientific) using an automated MeHg analyzer (MERX-M, Brooks-Rand). All field process blanks (n = 15) and quality assurance and quality control data are provided in the SI (Section S2; Fig. S12). The concentration of filtered Hg(II) (f.Hg(II)) was calculated as:
In cases where the concentration of f.MeHg was below the daily detection limit (n = 14 instances of 189 wetland and canal samples)49, the daily detection limit was used in the calculation of f.Hg(II). Dissolved gaseous Hg, reported at <0.04 ng L−1 in the Everglades3, was assumed to have minimal influence on calculated f.Hg(II) values.
Fish collection and analyses
Gambusia holbrooki (termed gambusia) were collected from wetland sites using a 30 cm diameter Teflon sieve. Gambusia was selected because they have a short life span (≤6 months), feed on a mixture of periphyton and zooplankton, and reflect MeHg availability to the proximal aquatic food web in the recent past17,43,48. A composite sample was collected for each location, often consisting of 20–30 individual fish. Samples were frozen on-site (‒80 °C), lyophilized, and homogenized via ball mill. Biological samples were first digested in 4.5 M Omnitrace nitric acid prior to biological MeHg analysis80. After the completion of MeHg analysis, samples were oxidized with bromine monochloride (BrCl, 10% v/v) and analyzed for total Hg by CVAFS using US EPA Method 1631, Revision E78.
Statistical analyses
Statistical comparisons between variables were assessed using linear regression analysis and paired t-tests (SigmaPlot v.14.5); p values <0.05 were considered statistically significant. For paired t-tests, a normality test (Shapiro–Wilk) was first performed. Data that passed the normality test were analyzed by a Welch’s t-test, where equal variances are not assumed, and those that did not pass the normality tests were analyzed by the Mann–Whitney rank sum test (suitable for non-parametric data).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All water and fish data in this study have been deposited in the USGS ScienceBase data release product (https://doi.org/10.5066/P976EGIX).
References
UN Environment. Global Mercury Assessment 2018 (UN Environment Programme Chemicals and Health Branch, 2019).
Coburn, S. et al. Mercury oxidation from bromine chemistry in the free troposphere over the southeastern US. Atmos. Chem. Phys. 16, 3743–3760 (2016).
Krabbenhoft, D. P., Hurley, J. P., Olson, M. L. & Cleckner, L. B. Diel variability of mercury phase and species distributions in the Florida Everglades. Biogeochemistry 40, 311–325 (1998).
Hurley, J. P. et al. System controls on the aqueous distribution of mercury in the northern Florida Everglades. Biogeochemistry 40, 293–310 (1998).
Sonke, J. E. et al. Global change effects on biogeochemical mercury cycling. Ambio 52, 853–876 (2023).
Eagles-Smith, C. A. et al. Modulators of mercury risk to wildlife and humans in the context of rapid global change. Ambio 47, 170–197 (2018).
Gilmour, C. C., Henry, E. A. & Mitchell, R. Sulfate stimulation of mercury methylation in freshwater sediments. Environ. Sci. Technol. 26, 2281–2287 (1992).
Gilmour, C. C. & Henry, E. A. Mercury methylation in aquatic systems affected by acid deposition. Environ. Pollut. 71, 131–169 (1991).
Gilmour, C. C. et al. Methylmercury concentrations and production rates across a trophic gradient in the northern Everglades. Biogeochemistry 40, 327–345 (1998).
Mitchell, C. P. J., Branfireun, B. A. & Kolka, R. K. Assessing sulfate and carbon controls on net methylmercury production in peatlands: an in situ mesocosm approach. Appl. Geochem. 23, 503–518 (2008).
St. Louis, V. L. et al. Production and loss of methylmercury and loss of total mercury from boreal forest catchments containing different types of wetlands. Environ. Sci. Technol. 30, 2719–2729 (1996).
Coleman Wasik, J. K. et al. The effects of hydrologic fluctuation and sulfate regeneration on mercury cycling in an experimental peatland. J. Geophys. Res. Biogeosci. 120, 1697–1715 (2015).
Branfireun, B. A., Roulet, N. T., Kelly, Carol, A. & Rudd, J. W. M. In situ sulphate stimulation of mercury methylation in a boreal peatland: toward a link between acid rain and methylmercury contamination in remote environments. Glob. Biogeochem. Cycles 13, 743–750 (1999).
Jeremiason, J. D. et al. Sulfate addition increases methylmercury production in an experimental wetland. Environ. Sci. Technol. 40, 3800–3806 (2006).
Tjerngren, I., Meili, M., Björn, E. & Skyllberg, U. Eight boreal wetlands as sources and sinks for methyl mercury in relation to soil acidity, C/N ratio, and small-scale flooding. Environ. Sci. Technol. 46, 8052–8060 (2012).
Pollman, C. D. Mercury cycling in aquatic ecosystems and trophic state-related variables — implications from structural equation modeling. Sci. Total Environ. 499, 62–73 (2014).
Cleckner, L. B., Garrison, P. J., Hurley, J. P., Olson, M. L. & Krabbenhoft, D. P. Trophic transfer of methyl mercury in the northern Florida Everglades. Biogeochemistry 40, 347–361 (1998).
Willacker, J. J., Eagles-Smith, C. A. & Ackerman, J. T. Mercury bioaccumulation in estuarine fishes: novel insights from sulfur stable isotopes. Environ. Sci. Technol. 51, 2131–2139 (2017).
Peterson, B. D. et al. Environmental formation of methylmercury is controlled by synergy of inorganic mercury bioavailability and microbial mercury-methylation capacity. Environ. Microbiol. 25, 1409–1423 (2023).
Hee-Sung, B., Dierberg, F. E. & Andrew, O. Periphyton and flocculent materials are important ecological compartments supporting abundant and diverse mercury methylator assemblages in the Florida Everglades. Appl. Environ. Microbiol. 85, e00156–19 (2019).
Cleckner, L. B., Gilmour, C. C., Hurley, J. P. & Krabbenhoft, D. P. Mercury methylation in periphyton of the Florida Everglades. Limnol. Oceanogr. 44, 1815–1825 (1999).
Jones, D. S. et al. Diverse communities of HgcAB+ microorganisms methylate mercury in freshwater sediments subjected to experimental sulfate loading. Environ. Sci. Technol. 54, 14265–14274 (2020).
Marvin-DiPasquale, M. C. & Oremland, R. S. Bacterial methylmercury degradation in Florida Everglades peat sediment. Environ. Sci. Technol. 32, 2556–2563 (1998).
Marvin-DiPasquale, M. et al. Methyl-mercury degradation pathways: a comparison among three mercury-impacted ecosystems. Environ. Sci. Technol. 34, 4908–4916 (2000).
Hinckley, E.-L. S., Crawford, J. T., Fakhraei, H. & Driscoll, C. T. A shift in sulfur-cycle manipulation from atmospheric emissions to agricultural additions. Nat. Geosci. 13, 597–604 (2020).
Lamers, L. P. M., Tomassen, H. B. M. & Roelofs, J. G. M. Sulfate-induced eutrophication and phytotoxicity in freshwater wetlands. Environ. Sci. Technol. 32, 199–205 (1998).
O’Donnell, J. A. et al. Metal mobilization from thawing permafrost to aquatic ecosystems Is driving rusting of arctic streams. Commun. Earth Environ. 5, 268 (2024).
Walsh, E. V., Hilton, R. G., Tank, S. E. & Amos, E. Temperature sensitivity of the mineral permafrost feedback at the continental scale. Sci. Adv. 10, eadq4893 (2024).
Cook, B. A., Peterson, B. D., Ogorek, J. M., Janssen, S. E. & Poulin, B. A. Simulated sea level rise in coastal peat soils stimulates mercury methylation. ACS Earth Space Chem. 8, 1784–1796 (2024).
Haitzer, M., Aiken, G. R. & Ryan, J. N. Binding of mercury(II) to dissolved organic matter: the role of the mercury-to-DOM concentration ratio. Environ. Sci. Technol. 36, 3564–3570 (2002).
Gerbig, C. A., Kim, C. S., Stegemeier, J. P., Ryan, J. N. & Aiken, G. R. Formation of nanocolloidal metacinnabar in mercury-DOM-sulfide systems. Environ. Sci. Technol. 45, 9180–9187 (2011).
Luek, J. L., Thompson, K. E., Larsen, R. K., Heyes, A. & Gonsior, M. Sulfate reduction in sediments produces high levels of chromophoric dissolved organic matter. Sci. Rep. 7, 8829 (2017).
Aiken, G. R., Gilmour, C. C., Krabbenhoft, D. P. & Orem, W. Dissolved organic matter in the Florida Everglades: implications for ecosystem restoration. Crit. Rev. Environ. Sci. Technol. 41, 217–248 (2011).
Poulin, B. A. et al. Spatial dependence of reduced sulfur in Everglades dissolved organic matter controlled by sulfate enrichment. Environ. Sci. Technol. 51, 3630–3639 (2017).
Deonarine, A. & Hsu-Kim, H. Precipitation of mercuric sulfide nanoparticles in NOM-containing water: implications for the natural environment. Environ. Sci. Technol. 43, 2368–2373 (2009).
Poulin, B. A. et al. Effects of sulfide concentration and dissolved organic matter characteristics on the structure of nanocolloidal metacinnabar. Environ. Sci. Technol. 51, 13133–13142 (2017).
Parks, J. M. et al. The genetic basis for bacterial mercury methylation. Science 339, 1332–1335 (2013).
Gilmour, C. C. et al. Mercury methylation by novel microorganisms from new environments. Environ. Sci. Technol. 47, 11810–11820 (2013).
Graham, A. M., Aiken, G. R. & Gilmour, C. C. Dissolved organic matter enhances microbial mercury methylation under sulfidic conditions. Environ. Sci. Technol. 46, 2715–2723 (2012).
Graham, A. M., Aiken, G. R. & Gilmour, C. C. Effect of dissolved organic matter source and character on microbial Hg methylation in Hg-S-DOM solutions. Environ. Sci. Technol. 47, 5746–5754 (2013).
Graham, A. M. et al. Sulfurization of dissolved organic matter increases Hg–sulfide–dissolved organic matter bioavailability to a Hg-methylating bacterium. Environ. Sci. Technol. 51, 9080–9088 (2017).
Jones, D. S. et al. Molecular evidence for novel mercury methylating microorganisms in sulfate-impacted lakes. ISME J. 13, 1695–1675 (2019).
Gilmour, C., Krabbenhoft, D. P., Orem, W., Aiken, G. R., & Roden, E. Appendix 3B-2: status report on ACME studies on the control of mercury methylation and bioaccumulation in the Everglades. In 2007 South Florida Environmental Report 1–39 (2007).
Gerson, J. R. & Hinckley, E.-L. S. It is time to develop sustainable management of agricultural sulfur. Earths Future 11, e2023EF003723 (2023).
Orem, W. et al. Sulfur in the South Florida ecosystem: distribution, sources, biogeochemistry, impacts, and management for restoration. Crit. Rev. Environ. Sci. Technol. 41, 249–288 (2011).
National Atmospheric Deposition Network (NADP). Annual Mercury Deposition Network Maps, 1998–2023. NADP Program Office, Wisconsin State Laboratory of Hygiene. https://nadp.slh.wisc.edu/networks/mercury-deposition-network/ (2024).
Bates, A. L., Orem, W. H., Harvey, J. W. & Spiker, E. C. Tracing sources of sulfur in the Florida Everglades. J. Environ. Qual. 31, 287–299 (2002).
Janssen, S. E. et al. Decadal trends of mercury cycling and bioaccumulation within Everglades National Park. Sci. Total Environ. 838, 156031 (2022).
Tate, M. T. et al. Chemical characterization of water, sediments, and fish from water conservation areas and canals of the Florida Everglades (USA), 2012 to 2019. U.S. Geological Survey Data Release. https://doi.org/10.5066/P976EGIX (2023).
Haider, S. et al. The Everglades depth estimationnetwork (EDEN) surface-water interpolation model, version 3. https://doi.org/10.3133/sir20205083 (2020).
Orem, W. H. et al. Ecosystem-scale modeling and field observations of sulfate and methylmercury distributions in the Florida Everglades: responses to reductions in sulfate loading. Aquat. Geochem. 26, 191–220 (2020).
Weishaar, J. L. et al. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 37, 4702–4708 (2003).
Freeman, C., Ostle, N. & Kang, H. An enzymic “latch” on a global carbon store: a shortage of oxygen locks up carbon in peatlands by restraining a single enzymes. Nature 409, 149 (2001).
Poulin, B. A. Selective photochemical oxidation of reduced dissolved organic sulfur to inorganic sulfate. Environ. Sci. Technol. Lett. 10, 499–505 (2023).
Lu, X. Q., Maie, N., Hanna, J. V., Childers, D. L. & Jaffé, R. Molecular characterization of dissolved organic matter in freshwater wetlands of the Florida Everglades. Water Res. 37, 2599–2606 (2003).
Poulin, B. A. et al. Geochemical factors controlling dissolved elemental mercury and methylmercury formation in Alaskan wetlands of varying trophic status. Environ. Sci. Technol. 53, 6203–6213 (2019).
Black, F. J., Poulin, B. A. & Flegal, A. R. Factors controlling the abiotic photo-degradation of monomethylmercury in surface waters. Geochim. Cosmochim. Acta 84, 492–507 (2012).
McCarter, C. P. R. et al. Long-term experimental manipulation of atmospheric sulfate deposition to a peatland: response of methylmercury and related solute export in streamwater. Environ. Sci. Technol. 56, 17615–17625 (2022).
Gilmour, C. C., Bullock, A. L., McBurney, A., Podar, M., & Elias, D. A. Robust mercury methylation across diverse methanogenic Archaea. mBio 9 (2018).
Choe, K.-Y. et al. Sediment-water exchange of total mercury and monomethyl mercury in the San Francisco Bay-Delta. Limnol. Oceanogr. 49, 1512–1527 (2004).
Gill, G. A. et al. Sediment−water fluxes of mercury in Lavaca Bay, Texas. Environ. Sci. Technol. 33, 663–669 (1999).
Capo, E. et al. Expression levels of HgcAB genes and mercury availability jointly explain methylmercury formation in stratified brackish waters. Environ. Sci. Technol. 56, 13119–13130 (2022).
Peterson, B. D. et al. Metabolically diverse microorganisms mediate methylmercury formation under nitrate-reducing conditions in a dynamic hydroelectric reservoir. ISME J. 17, 1705–1718 (2023).
Zhang, T. et al. Methylation of mercury by bacteria exposed to dissolved, nanoparticulate, and microparticulate mercuric sulfides. Environ. Sci. Technol. 46, 6950–6958 (2012).
Jerauld, M., Dierberg, F. E., DeBusk, W. F., & DeBusk, T. A. Appendix 3B-1: evaluation of factors influencing methylmercury accumulation in South Florida marshes. In 2015 South Florida Environmental Report, South Florida Water Management District (2015).
Julian II, P. et al. Chapter 3B: mercury and sulfur environmental assessment for the Everglades. In 2015 South Florida Environmental Report, South Florida Water Management District (2015).
Gabriel, M. C., Howard, N. & Osborne, T. Z. Fish mercury and surface water sulfate relationships in the Everglades protection area. Environ. Manag. 53, 583–593 (2014).
Kalla, P., Cyterski, M., Scheidt, D. & Minucci, J. Spatiotemporal effects of interacting water quality constituents on mercury in a common prey fish in a large, perturbed, subtropical wetland. Sci. Total Environ. 792, 148321 (2021).
Gilmour, C. C., Krabbenhoft, D. P., Orem, W., & Aiken, G. Influence of drying and rewetting on mercury and sulfur cycling in Everglades and STA soils. In 2004 Everglades Consolidated Report, Appendix 2B-1: 1–42 (2004).
Blanchfield, P. J. et al. Experimental evidence for recovery of mercury-contaminated fish populations. Nature 601, 74–78 (2022).
Zhang, Y. et al. Global health effects of future atmospheric mercury emissions. Nat. Commun. 12, 3035 (2021).
Wang, F. et al. How closely do mercury trends in fish and other aquatic wildlife track those in the atmosphere? – implications for evaluating the effectiveness of the Minamata convention. Sci. Total Environ. 674, 58–70 (2019).
Brigham, M. E. et al. Lacustrine responses to decreasing wet mercury deposition rates—results from a case study in Northern Minnesota. Environ. Sci. Technol. 48, 6115–6123 (2014).
Wang, H. et al. Surface water sulfate dynamics in the northern Florida Everglades. J. Environ. Qual. 38, 734–741 (2009).
Varonka, M. S. et al. Chemistry data from southern Florida canals collected in support of the aquatic cycling of mercury in the Everglades project. U.S. Geological Survey Data Release. https://doi.org/10.5066/P9J7OY4T (2020).
Brinton, T. I., Antweiler, R. C., & Taylor, H. E. Method for the Determination of Dissolved Chloride, Nitrate, and Sulfate in Natural Water using Ion Chromatography. U.S. Geological Survey Open File Report (95-426A) (U.S. Geological Survey, 1996).
Aiken, G. R. Chloride interference in the analysis of dissolved organic carbon by the wet oxidation method. Environ. Sci. Technol. 26, 2435–2439 (1992).
U.S. Environmental Protection Agency (US EPA). Method 1631, Revision E: Mercury in Water by Oxidation, Purge and Trap, and Cold Vapor Atomic Fluorescence Spectrometry Agency (US EPA, 2002).
U.S. Environmental Protection Agency (US EPA). Method 1630: Methyl Mercury in Water by Distillation, Aqueous Ethylation, Purge and Trap, and CVAFS. (US EPA, 2001).
Hammerschmidt, C. R., Fitzgerald, W. F., Lamborg, C. H., Balcom, P. H. & Tseng, C. M. Biogeochemical cycling of methylmercury in lakes and tundra watersheds of Arctic Alaska. Environ. Sci. Technol. 40, 1204–1211 (2006).
Acknowledgements
Financial support was provided to B.A.P., S.E.J., G.R.A. and D.P.K. by the US Geological Survey Greater Everglades Priority Ecosystems Science (GEPES) Program. We thank Eric Swain (USGS) for preparing vector flows from EDEN, Laura Flucke (USGS) for assistance with the GIS map, and Chris Eckley (US EPA) for helpful suggestions on the manuscript. We also thank the staff of the US Geological Survey Mercury Research Laboratory for field and analytical support. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the US Government.
Author information
Authors and Affiliations
Contributions
B.A.P. contributed conceptualization, investigation, formal analysis, and writing—original draft. M.T.T. contributed conceptualization, methodology, investigation, formal analysis, data curation, and writing— review and editing. S.E.J. contributed formal analysis and writing—review and editing. G.R.A. contributed conceptualization and investigation. D.P.K. contributed conceptualization, investigation, project administration, and writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Poulin, B.A., Tate, M.T., Janssen, S.E. et al. A comprehensive sulfate and DOM framework to assess methylmercury formation and risk in subtropical wetlands. Nat Commun 16, 4253 (2025). https://doi.org/10.1038/s41467-025-59581-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-59581-w