Abstract
Discectomy is the current surgical procedure for lumbar intervertebral disc (IVD) herniation. Discectomy was performed to remove the IVD material and relieve the pain inflicted by nerve root compression and axonotoxic effects, such as inflammatory cytokines in the IVD material; however, defects within the IVD caused by discectomy may impair tissue healing and predispose patients to subsequent IVD degeneration. Given that viable cells with the capacity for IVD regeneration are scarce, discectomy alone is not conducive to tissue repair. Here, we report the use of an acellular, bioresorbable, ultra-purified alginate (UPAL) gel implantation system to prevent IVD degeneration after discectomy and demonstrate its feasibility and safety in phase 1/2, open-label, non-randomized clinical trials conducted at a double center. This study comprised two parts: a prospective study on UPAL gel implantation after discectomy in patients with lumbar disc herniation, and a subsequent prospective study on patients who underwent discectomy without UPAL implantation as a control group. The control group was recruited separately. The primary outcomes of this study were the feasibility and safety of UPAL implantation, and the secondary outcomes included physical function scores, self-report questionnaires (SRQs) evaluating pain and health-related quality of life and magnetic resonance imaging (MRI)-based measures of IVD tissues. The UPAL gel implantation demonstrated 100% feasibility and safety (n = 40). The physical function scores improved significantly postoperatively in both groups, with the UPAL group demonstrating greater improvements over time compared to the control group. The SRQ scores were significantly higher in the UPAL group than in the control group from the early postoperative period to 12 weeks. MRI revealed that the disc degeneration score was significantly lower in IVDs with UPAL implantation than in those that underwent discectomy alone. The findings of this study suggest that the UPAL gel is a novel therapeutic strategy after discectomy in cases of lumbar IVD herniation. Trial number: UMIN000034227, UMIN000042282.
Similar content being viewed by others
Introduction
The intervertebral disc (IVD) is the main soft tissue of the spine that supports the trunk and contributes to the load-bearing function and maintenance of trunk mobility, as it functions as a cushion connecting the vertebral bodies1,2,3. The IVD is composed of the inner structure of the nucleus pulposus (NP) and the external annulus fibrosus (AF). The NP is a gelatinous tissue containing 70–90% water and is rich in extracellular matrix with low cell density2,4. The IVDs have a limited regenerative capacity owing to poor nutrient supply, low oxygen tension, acidic pH, and a low capacity for cell division1,5,6,7,8.
Lumbar IVD herniation is one of the most common spinal disorders; it can cause severe low back and leg pain due to compression of the NP on the nerve root and axotoxic effects, such as inflammatory cytokines in the NP and has been reported to occur most often in adults between the ages of 20 and 50 years9. Discectomy is the standard surgical treatment to remove IVD material impinging on the nerve root and relieve pain10,11,12. However, IVD defects after discectomy are difficult to cure and have been reported to promote IVD degeneration, worsen clinical outcomes, and cause low back pain13,14,15.
Although the use of defect-filling soft biomaterials for IVD defects after discectomy has been attempted in fundamental and translational research6,16,17,18,19,20, there are no clinically available biomaterials because these soft materials, such as hydrogels, can be extruded from IVD defects by mechanical compressive loading under high intradiscal pressure2,21. Alginate, which has been used for the representative three-dimensional (3D) culture of IVD cells, can also promote the self-repairing ability of IVDs22,23. However, naturally occurring, commercial-grade alginates contain mitogenic and cytotoxic impurities that induce foreign body reactions24. To overcome the inevitable limitations in its clinical application, highly purified biocompatible alginates with reduced endotoxicity to <50 EU/mL (< 1/10,000 compared with commercially available laboratory alginate) have been developed with minimal risk of adverse effects25. Cell-free, bioabsorbable, ultra-purified alginate (UPAL) is used to fill IVD defects after discectomy and can be adapted to various defect shapes without covering or suturing the surface of the IVD by rapid gelation (< 5 min) with crosslinking via calcium ions to cover the surface26. Sheep cadaveric lumbar spines implanted with the UPAL gel showed sufficient biomechanical properties without material protruding from the IVD2,26,27. In addition, the UPAL gel optimizes the tissue repair environment of implanted IVDs after discectomy and promotes the self-repair capacity of the remaining tissue, leading to endogenous repair in transrational animal models26,27. Furthermore, the UPAL gel is biologically safe according to the International Organization for Standardization and Good Laboratory Practice standards26.
Therefore, as the next step, we conducted phase 1/2 clinical trials in which the UPAL gel was implanted into the IVD after discectomy in patients with lumbar IVD herniation28. The primary objective of these trials was to investigate the feasibility and safety of UPAL gel implantation. The secondary objective was to determine the clinical efficacy of this treatment from the perspective of pain suppression and/or IVD degeneration. These pilot studies were designed to generate a rationale for a subsequent phase 3 validation study with sufficient power.
This study was a first-in-human, single-arm, open-label, phase 1/2 trial conducted at a double center and comprised two parts: a prospective study of UPAL gel implantation after discectomy in patients with lumbar disc herniation, which was followed by a prospective study with a control group of patients with lumbar disc herniation who underwent discectomy without UPAL gel implantation to compare and evaluate the efficacy of the UPAL gel.
Results
UPAL gel implantation after discectomy group
Between November 2018 and September 2020, 41 patients with lumbar disc herniation were registered in the UPAL gel implantation group (Fig. 1). The participants were observed and evaluated 1, 4, 12, 24, 48, and 96 weeks after surgery (Supplementary Table 1). The allergy test results for sodium alginic acid at the time of enrollment were negative in all cases. Forty patients underwent UPAL implantation after discectomy, except for one patient in whom UPAL injection was discontinued due to intraoperative spinal fluid leakage. All 40 patients were followed up for 24 weeks after UPAL implantation, and no patients were excluded from the per protocol set (PPS). At 48 weeks postoperatively, 32 patients completed the follow-up, and eight did not complete the follow-up; four were lost to follow-up, two had recurrent herniation and underwent reoperation, and two did not attend the clinic but completed the self-report questionnaires (SRQs) alone. At 96 weeks postoperatively, 26 patients completed the follow-up; of the remaining six patients, four were lost to follow-up and two were unable to undergo magnetic resonance imaging (MRI) (one due to claustrophobia and one due to pregnancy; one pregnant patient was evaluated at 96 weeks postoperatively with means other than MRI, and MRI was performed at 133 weeks postoperatively).
Control cohort (discectomy only) group
Between December 2020 and March 2022, after all surgeries in the UPAL group were completed, 35 patients were enrolled in the control group (Fig. 1). The participants were observed and evaluated 1, 4, 12, 24, 48, and 96 weeks after surgery, as in the UPAL group, excluding the allergy test for sodium alginate and laboratory tests (Supplementary Table 1). Thirty-four patients underwent lumbar discectomy; one patient was excluded from the safety analysis set (SAF) on the post-enrollment screen due to severe pain that precluded a preoperative MRI scan. In four cases, T1ρ MRI was not available before surgery due to the replacement of MRI systems at the evaluation facility. For these four cases, all evaluation criteria except T1ρ were met and included in the evaluation. At 24 weeks postoperatively, 32 patients had completed the follow-up; two were excluded from the PPS, with one excluded due to deviation from the protocol at 1 week postoperatively and one because of hernia recurrence at 4 weeks postoperatively that required revision surgery. At 48 weeks after surgery, 29 patients completed the follow-up, one was lost to follow-up, and two were not seen and completed the questionnaires alone. At 96 weeks postoperatively, 24 patients were available for follow-up, three were lost to follow-up, one experienced hernia recurrence and underwent reoperation, and one completed a questionnaire alone (Fig. 1).
Participant characteristics
The baseline characteristics and surgical summaries of the 40 patients who underwent UPAL implantation (UPAL group) and the 34 who underwent discectomy (control group) are detailed in Table 1. The mean age at surgery was 36.3 (standard deviation (SD): 7.5) years in the UPAL group and 37.5 (SD: 8.0) years in the control group, and the sex ratio and body mass index were comparable between the two groups. Regarding disc herniation, most lumbar lesions were at the L4–L5 and L5–S1 levels, which were comparable between the two groups. The herniations were classified into four types based on surgical findings, most of which were extrusion types (subligamentous or transligamentous), with no significant differences between the two groups. The time from symptom onset to surgery was comparable between the groups. The mean operative time was 103.7 (SD: 31.3) min in the UPAL group and 91.9 (SD: 19.6) min in the control group. In the UPAL group, the operation time was approximately 10 min longer than that of the control group due to the time needed to inject the UPAL solution, followed by gelation (5 min). The mean amount of removed disc material during discectomy was comparable between the two groups, with an average of 0.5 (SD: 0.3) mL of UPAL solution being implanted into the disc in the UPAL group (Table 1).
Primary outcomes: Feasibility and safety
The primary outcomes of this phase 1/2 trial were the feasibility and safety of UPAL implantation following discectomy. Forty patients in the UPAL group underwent UPAL gel implantation after discectomy (Fig. 1). After a 5 × 5 mm incision was made into the AF, the disc material was removed, and the UPAL solution was injected into the disc. The average filling volume of the UPAL solution was 0.46 ± 0.26 mL. UPAL gelation was confirmed in all cases after the application of the CaCl2 solution, suggesting that the feasibility of UPAL gel implantation was 100%.
Table 2 summarizes the adverse events (AEs) collected in this study. No AEs associated with UPAL implantation occurred postoperatively. Preoperative allergy test results for UPAL were negative for all patients. Laboratory data were acceptable after hernia surgery, and no abnormal values were found (Supplementary Table 2). There was no recurrence of herniation, the most relevant and serious complication of disc herniation treatment, until 24 weeks after UPAL implantation and two cases between 24 and 48 weeks postoperatively. In the two cases with herniation recurrence, the findings during reoperation indicated normal hernia recurrence and ruled out the involvement of UPAL implantation. No recurrence of herniation was observed between 48 and 96 weeks postoperatively. In the control group, there was one case each of hernia recurrence and herpes zoster as serious complications 24 weeks after discectomy (Table 2). There was also one case of hernia recurrence between 48 and 96 weeks after surgery. In total, two cases of hernia recurrence were identified in both UPAL and control groups (Fig. 1 and Table 2).
Secondary outcomes
The secondary outcomes of this study included physical function scores, SRQs for evaluating pain and health-related quality of life, and MRI assessment of IVD tissues (Supplementary Table 1). Physical function scores consisted of the finger-to-floor distance (FFD) measurement, straight leg raise (SLR) test, Modified Schober’s test (MST) to assess lumbar spine range of motion, and Japanese Orthopedic Association (JOA) score to assess the severity of the functional and clinical conditions related to the lumbar spine. These assessments were performed for 96 weeks postoperatively to compare the results between the UPAL and control groups.
Physical function scores
Physical examination showed that FFD significantly decreased at 4 weeks after surgery compared to baseline (P < 0.01), and the SLR test and MST values also increased in both groups from baseline (P < 0.01 at 1 week postoperatively, P < 0.01 at 4 weeks postoperatively, respectively); these changes persisted until 96 weeks after surgery (Fig. 2A–C and Supplementary Table 3). Similarly, the JOA scores promptly improved after surgery in both groups (P < 0.01) (Fig. 2D and Supplementary Table 3). In a comparison of the groups, FFD was significantly smaller in the UPAL group at 12 weeks after surgery (mean difference [MD]: – 5.6, 95% confidence interval [CI]: – 11.1, – 0.2, P = 0.04). The SLR test value was significantly higher in the UPAL group than in the control group at 48 and 96 weeks after surgery (MD: 4.5, 95% CI: 0.9, 8.0, P = 0.02, and MD: 3.6, 95% CI: 0.5, 7.0, P = 0.02, respectively), and the MST value was significantly greater at 4 weeks after surgery (MD: 0.7, 95% CI: 0.1, 1.4; P = 0.03) (Fig. 2A–C and Supplementary Table 3). Furthermore, the JOA scores were significantly higher in the UPAL group than in the control group at 1 and 12 weeks after surgery (MD: 1.8, 95% CI: 0.6, 3.1, P < 0.01, and MD: 1.6, 95% CI: 0.1, 3.1, P = 0.01, respectively) (Fig. 2D and Supplementary Table 3).
A Finger-to-floor distance measurement. B Straight leg raise test. C Modified Schober’s test. D Japanese Orthopedic Association (JOA) score. These scores were assessed as the physical examination preoperatively and at 1, 4, 12, 24, 48, and 96 weeks after surgery in the UPAL implantation group (blue) and control group (orange). Box plots show the median (center line), 25th–75th percentiles (box edges), and range within 1.5 × interquartile range (whiskers); outliers are displayed as dots. *P < 0.05, **P < 0.01, [95% confidence interval (CI)] between the two groups; P-values were determined with an unpaired two-tailed t test or Mann-Whitney U test.
SRQs for the evaluation of pain and health-related quality of life
Regarding the SRQs for pain evaluation, the low back pain visual analog scale (VAS; 0–100 mm, the higher the score, the worse the pain) and leg pain VAS scores significantly decreased from baseline to immediately after surgery in both groups (P < 0.01, P < 0.01, respectively) and continued until 96 weeks postoperatively (Fig. 3A and B and Supplementary Table 4). The VAS scores were comparable between the two groups over the entire period (Fig. 3A and B and Supplementary Table 4). In addition, the Oswestry Disability Index evaluation (ODI; 0–100 points, the higher the score, the more severe the disability) was used to assess the activities of daily living disability due to low back pain and leg pain, and the Rolland–Morris disability questionnaire (RDQ; 0–24 points; higher scores indicate worse quality of life) was used to evaluate quality of life specific to low back pain; both values significantly decreased in each group from immediately after surgery to 96 weeks compared to baseline (P < 0.01, P < 0.05, respectively) (Fig. 3C and D and Supplementary Table 4). In addition, the ODI in the UPAL group was significantly lower than that in the control group at 4 weeks postoperatively (MD: 7.8, 95% CI: 1.0, 14.6, P = 0.02), and the RDQ value was significantly lower in the UPAL group at 4 weeks postoperatively (MD: 2.9, 95% CI: 0.4, 5.4, P = 0.01) (Fig. 3C and D and Supplementary Table 4). The results of the JOA Back Pain Evaluation Questionnaire (JOABPEQ; 0–100 points, with higher scores indicating better health status), a multidimensional assessment of health status associated with lumbar spine disorders, are shown in Fig. 3E and Supplementary Fig. 1. All five scores improved in both groups from 1 week to 96 weeks after surgery (P < 0.05 for all five scores after 1 week postoperatively, except gait disturbance and social life disturbance in the control group) (Fig. 3E and Supplementary Table 5). Furthermore, the scores of lumbar dysfunction at 12 weeks postoperatively (MD: 11.5, 95% CI: 0.9, 22.2; P < 0.01), gait disturbance at 4 weeks postoperatively (MD: 16.5, 95% CI: 4.3, 28.8; P < 0.01), and social life disturbance at 4 weeks postoperatively (MD: 11.4, 95% CI: 1.4, 21.5; P = 0.02) were significantly higher in the UPAL group than in the control group (Fig. 3E and Supplementary Table 5). The results of the 36-item Short Form Health Survey (SF-36), which indicates health-related quality of life, are shown in Fig. 3F and Supplementary Fig. 1. All parameters improved in both groups from the time of surgery to the time of final follow-up, except for the mental component summary (P < 0.05 for physical functioning and physical component summary from 1 week after surgery in both groups; P < 0.05 for bodily pain, general health, vitality, role emotional and mental health from 4 weeks postoperatively; P < 0.05 for social functioning and role/social component summary from 12 weeks postoperatively) (Fig. 3F and Supplementary Table 6). The physical functioning score at 1 week after surgery (MD: 9.1, 95% CI: 0.04, 18.1, P < 0.05), vitality score at 96 weeks after surgery (MD: 11.4, 95% CI: 0.6, 22.2, P = 0.04), and physical component summary score at 12 weeks after surgery (MD: 3.8, 95% CI: 0.3, 7.3, P = 0.01) were significantly higher in the UPAL group at each time point than in the control group (Fig. 3F and Supplementary Table 6). There were significant weak correlations with the injected UPAL volume in the SLR test; psychological disorders score on JOABPEQ at 24 weeks postoperatively; vitality on SF-36 at 1 week postoperatively; and general health, mental health, and mental component summary on SF-36 at 24 weeks postoperatively (Supplementary Table 7).
A, B Visual Analog Scale (VAS) for low back and leg pain (0–100 mm, the higher the score, the worse the pain). C Oswestry Disability Index (ODI; 0–100 points, the higher the score, the more severe the disability). D Roland Morris Disability Questionnaire (RDQ; 0–100 points, the higher score, the better the quality of life). E Lumbar dysfunction, gait disturbance, and social life disturbance of the Japanese Orthopedic Association Back Pain Evaluation Questionnaire (JOABPEQ; 0–100 points, with higher scores indicating better health status). F Physical functioning, vitality, and physical component summary of 36-item Short Form Health Survey (SF-36: 0–100 points, the higher score, the better the quality of life). These scores were examined preoperatively and at 1, 4, 12, 24, 48, and 96 weeks after surgery in the UPAL implantation group (blue) and control group (orange). Box plots show the median (center line), 25th–75th percentiles (box edges), and range within 1.5 × interquartile range (whiskers); outliers are displayed as dots. *P < 0.05, **P < 0.01, [95% confidence interval (CI)] between the two groups; P-values were determined with an unpaired two-tailed t test or Mann-Whitney U test.
MRI analysis
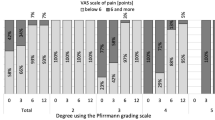
The results of MRI analysis are shown in Fig. 4. MRI T2-weighted sagittal images of representative cases showed that the discs implanted with the UPAL had a high signal intensity with a clear boundary between the NP and AF at 24 weeks postoperatively, and the same trend continued at 96 weeks. In contrast, the control group, which only underwent discectomy, showed a decrease in disc signal at 24 and 96 weeks postoperatively compared with preoperatively (Fig. 4A). The Pfirrmann disc degeneration grading score (five-point scale from 1 to 5; the higher the score, the more advanced the degeneration) in the UPAL group improved at 24 weeks postoperatively compared with preoperatively and was maintained until 96 weeks postoperatively. While the Pfirrmann score in the control group was higher postoperatively than preoperatively, the UPAL group had a significantly lower grade than the control group at 24 and 96 weeks (P < 0.01 and P < 0.01, respectively), and there was a significant difference between the two groups in the amount of change in grade compared with preoperatively at 24 and 96 weeks postoperatively (P < 0.01 and P < 0.01, respectively) (Fig. 4B and Supplementary Fig. 2A). Regarding the type of disc herniation, preoperative Pfirrmann grading in three types of disc herniation (subligamentous extrusion, transligamentous extrusion, and sequestration) in the UPAL group was equivalent (4.0, 3.5, 3.6, respectively, P = 0.39), and the amount of change at 24 and 96 weeks postoperatively were also comparable among these groups (24w: – 0.5, – 0.36, – 0.42, respectively. P = 0.93, 96w: – 0.5, – 0.38, – 0.29, respectively, P = 0.87). The disc height index (DHI), which was calculated as the ratio of IVD height to the proximal vertebral body height measured for the middle portion on mid-sagittal T2-weighted images, tended to decrease in both groups from preoperatively to 24 weeks postoperatively and 96 weeks postoperatively and was comparable between the two groups (preoperatively: 95% CI: – 0.04, 0.04, P > 0.99; 24 weeks postoperatively: – 0.04, 0.04, P = 0.97; 96 weeks postoperatively: – 0.05, 0.05; P = 0.97) (Fig. 4B and Supplementary Fig. 2A).
A Representative T2-weighted, mid-sagittal images of lumbar intervertebral discs (IVDs) in the UPAL gel implantation group and control group preoperatively and at 24 and 96 weeks after surgery. B Pfirrmann grading and the disc height index are shown. Box plots show the median (center line), 25th–75th percentiles (box edges), and range within 1.5 × interquartile range (whiskers); outliers are displayed as dots. **P < 0.01 between the two groups; P-values were determined with the Mann–Whitney U test. C, D T1ρ, T2*, and apparent diffusion coefficient (ADC) values derived from diffusion-weighted images of the IVDs are shown. T1ρ, T2*, and ADC values of lesional IVDs were normalized by the normal IVD values and evaluated for comparison. C Results of patients who underwent T1ρ, T2*, and ADC from preoperatively to 96 weeks postoperatively to evaluate within-group changes over time in each group. Data are presented as mean ± 95% confidence interval (CI), *P < 0.017 between each time point; P-values were determined with one-way analysis of variance with post-hoc tests using the Bonferroni paired t test. D Data obtained from MR images of all cases, including missing values, to compare the two groups at each time point. Box plots show the median (center line), 25th–75th percentiles (box edges), and range within 1.5 × interquartile range (whiskers); outliers are displayed as dots. *P < 0.05 between the two groups at each time point; P-values were determined with an unpaired two-tailed t test or Mann-Whitney U test. w, weeks.
For the quantitative MRI analysis of IVD, T1ρ, T2*, and apparent diffusion coefficient (ADC) values derived from diffusion-weighted images (DWIs) of lesional IVD were normalized by normal IVD values and evaluated for comparison (Fig. 4C and D and Supplementary Fig. 2C). These MRI sequences were selected because they have been preliminarily reported to have potential usefulness as objective and quantitative assessments of IVD degeneration29,30,31,32. T1ρ, T2*, and ADC in patients with MRI available from preoperatively to 96 weeks postoperatively are shown in Fig. 4C and Supplementary Fig. 2C to evaluate changes over time in each group. T1ρ and T2* values in both groups did not change significantly between preoperatively and postoperatively. The ADC values were significantly higher at 24 weeks postoperatively than preoperatively in the control group (P < 0.01) and lower at 96 weeks postoperatively in both groups than at 24 weeks postoperatively (UPAL, P = 0.01; control, P < 0.01). Data from the MR images of all cases, including missing values, were used to compare the two groups at each time point (Fig. 4D). T1ρ and T2* values were comparable between the two groups from preoperatively to 96 weeks postoperatively. The change in the T2* value from preoperatively to 24 weeks postoperatively was positive in the UPAL group and negative in the control group (95% CI: – 0.005, 0.4, P = 0.06). The ADC values were comparable between the two groups preoperatively and at 96 weeks postoperatively; however, the ADC value in the control group was significantly higher than that in the UPAL group at 24 weeks postoperatively (MD: 0.04, 95% CI: 0.004, 0.08, P = 0.04), and the change from preoperatively to 24 weeks postoperatively was also significantly higher in the control group (MD: 0.06, 95% CI: 0.02, 0.10; P < 0.01).
Discussion
The current study confirmed the feasibility and safety of UPAL gel implantation for a herniated NP after discectomy and demonstrated that UPAL gel implantation improves early postoperative pain and health-related quality of life compared with the control group. Furthermore, MRI results indicated that UPAL could prevent IVD degeneration after discectomy. This is the first report of a clinically applicable soft biomaterial that can easily and safely compensate for the cavity after discectomy, with the potential to suppress early postoperative pain and inhibit progressive IVD degeneration following discectomy.
Currently, there are no available clinical treatments that prevent or repair IVD degeneration; however, recent tissue engineering approaches have revealed the molecular cascade involved in IVD degeneration, and treatments and research aimed at regenerating or inhibiting IVD degeneration have been attempted2,33. Notably, cell- and biomaterial-based therapies have attracted attention as treatments for IVD degeneration. Although stem cell-based therapies present many challenges for clinical application, including immune rejection, host tissue engraftment, potential tumor formation, and pathogenic infection26,34,35,36,37, biomaterial-based therapies can be performed in a single-step process and have the great advantage of providing safe and stable treatment on demand2,26,38. Because patients with lumbar disc herniation, a major IVD disorder, are typically relatively young (< 45 years old) and disc cells are expected to remain in these individuals26,39,40, biomaterial-based IVD repair therapy is considered potentially useful. Biomaterials must support cell survival and induce differentiation of the remaining progenitor cells to achieve the essential and sustained suppression of disc degeneration. Soft biomaterials, such as alginate, collagen, fibrin, and hyaluronic acid, have been analyzed in in vivo experiments to determine the induction and activation mechanisms of residual disc cells2,26,28,41,42,43,44. In this study, we developed an UPAL gel alginate with excellent biocompatibility and safety and investigated its clinical application in patients with lumbar disc herniation.
This clinical trial achieved 100% feasibility with UPAL gel implantation after discectomy for patients with lumbar disc herniation; the UPAL solution was injected into the IVD defect after discectomy through a 16 G catheter, remained in the disc, and gelation on the surface of the AF was confirmed after applying the calcium solution in all cases. Similar to the UPAL implantation into the lumbar discs of large animals in previous studies8,26, the UPAL implantation technique can be performed successfully in human disc herniation surgery. No serious UPAL-related AEs were observed after UPAL implantation. In addition, preoperative allergy test results for UPAL were negative in all patients, and postoperative blood test data did not show any abnormal reactions. Although UPAL is a highly purified soft biomaterial with 1/10,000 the endotoxin content of conventional alginate acid and has been demonstrated to be safe in in vivo studies8,26, this study demonstrated that its use for human IVDs is safe and acceptable.
In this study, MRI scans showed that the Pfirrmann grade, which indicates disc degeneration, was significantly lower in UPAL-implanted IVDs than in discs that underwent discectomy alone, suggesting that the UPAL gel may be effective in regulating disc degeneration. Biomaterials for IVD repair must possess biomechanical and biological properties, including biocompatibility, support for cell survival, promotion of extracellular matrix production, suppression of inflammatory reactions, and inhibition of pathological fibrosis2,45. Because the IVD is a load-bearing tissue that supports the trunk of the body, biomechanical properties that prevent protrusion after implantation are required for the safety of material implantation into the IVD. UPAL injected into the IVD has been shown to have sufficient biomechanical properties to prevent protrusion through crosslinking with calcium ions on the disc surface2,26. The UPAL gel has the great advantage of eliminating the need for suturing of the fibrous ring because it adheres to the AF by electrostatic and chemical interactions characterized by diffusion and physisorption2,26. The biomechanical properties, such as stiffness and viscoelastic properties of the UPAL gel, are comparable to those of the NP, i.e., it does not have sufficient mechanical properties to maintain the IVD height after discectomy26. In addition, UPAL gel disappeared 24 weeks after implantation into the disc in sheep26. This indicates that the gel is gradually degraded by physiological ion replacement (divalent to monovalent ions), hydrolysis, or depletion by cell encapsulation26. A similar time sequence is expected in humans, although current MRI scans cannot evaluate the duration of gel degradation, because the moisture composition of the gel is similar to that of water. This study showed that the UPAL group had significantly lower IVD degeneration scores than the control group; however, clinical outcomes were similar between the two groups at 94 weeks after surgery. Although this study showed no disc height differences between the UPAL and control groups, preventing IVD degeneration after discectomy may lead to a significant difference between the groups in the longer follow-up. Because there is a discrepancy between MRI and clinical evaluations, further evaluation of IVD degeneration and clinical outcomes would be important. Therefore, we plan to perform follow-up clinical and MRI analyses 5 and 10 years postoperatively.
Regarding the expected biological mechanism of action for IVD repair by UPAL, a preclinical study reported that when UPAL gel was injected into the disc after NP aspiration, it inhibited disc tissue degeneration histologically, and the percentage of type II collagen-positive cells was significantly increased after UPAL gel implantation26. This is a fundamental aspect of NP cell function for extracellular matrix production26. In addition, the UPAL gel significantly increased the percentage of GD2+Tie2+ NP progenitor cells26. Previous basic studies suggested that progenitor cells produce aggregates expressing type II collagen and aggrecan, the clonal pluripotency of which leads to the emergence of mesenchymal lineages for tissue reconstruction26,46. These findings indicate that viable endogenous NP cells migrated to the post-discectomy wound filled with UPAL gel, recruiting additional residual NP progenitor cells. This would eventually promote endogenous IVD repair by stimulating extracellular matrix production26. In this study, we performed T1ρ, T2*, and DWI measurements for quantitative MRI evaluation and found significant postoperative changes on DWI. However, these results could be largely influenced by the discectomy procedure itself, which involves removing the NP, which is still experimental in the evaluation of IVDs, and further evidence is required in the future. For instance, we are considering texture analysis using T2-weighted images as an additional quantitative MRI technique to further validate the efficacy of UPAL gel in promoting disc repair. This method allows the assessment of local heterogeneity associated with disc repair using texture analysis, which captures the spatial distribution of gray-level intensity in voxels47,48 (Supplementary Fig. 3).
The current study demonstrated that the scores of SRQs for evaluating pain and health-related quality of life, including the ODI, RDQ, SF36, and JOABPEQ, were significantly higher in the UPAL implantation group than in the control group, which underwent discectomy alone in the early period up to 12 weeks after surgery. In addition, physical function scores, including lumbar range of motion and JOA scores, were significantly better in the UPAL group than in the control group during the early postoperative period, indicating that UPAL could play a significant role in regulating pain and improving quality of life during the early postoperative period after lumbar disc herniation surgery. A significant problem caused by IVD disorders is low back pain and the consequent significant reduction in quality of life; however, no biomaterial is clinically effective in controlling low back pain2,27. Proposed key factors in IVD-derived pain are intradiscal inflammation, indicated by tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), and sensory nerve ingrowth into the AF, which is triggered by nerve growth factor/high-affinity tyrosine kinase A (TrkA) signaling27,49,50,51,52. This clinical study lacks experimental data to support these hypothesized mechanisms because it was impossible to include biomarker data or histological evidence by consuming the local IVD tissue, which is invasive. However, UPAL gel implanted into the IVD suppressed TNF-α and IL-6 production, downregulated TrkA expression, and reduced nociceptive behaviors in a rat in vivo study27, suggesting that UPAL may have contributed to pain control in patients with lumbar disc herniation through these mechanisms.
Regarding cost-effectiveness, the sales price of the UPAL is expected to be less than twice that of an interbody cage. If IVD degeneration progresses, lumbar interbody fixation surgery using four pedicle screws with/without interbody cages will be necessary. The UPAL treatment will be both medically and economically beneficial in preventing degeneration and avoiding the need for reoperation.
The limitations of our study include the open-label and non-randomized nature of the trial and the small number of participants. As the first human study, the priority was to confirm the feasibility and safety of UPAL implantation; therefore, the clinical trial employed a sequential design, in which the UPAL group was treated first, followed by the control group. We designed this study to assess the feasibility and safety of this treatment, as well as to evaluate key study parameters, including preliminary efficacy measures for future larger trials, and to provide a benchmark for sample size calculations. This study was underpowered to analyze the effectiveness of the UPAL for categorical indicators, including sex and hernia type, which should be considered in the next phase of the study. Guidelines in translational research recommend that pilot studies be conducted to allow testing protocols to be evaluated under trial conditions before evaluation in a fully randomized controlled trial. As this study design could introduce time-related biases, such as changes in surgical technique or patient selection criteria over time, the study facilities were limited, and the patient selection criteria and surgical techniques for the UPAL and control groups were strictly standardized. This phase 1/2 study was sufficient to demonstrate the validity and possible benefits of conducting a subsequent phase 3 study53,54. Another point of debate is the clinical relevance of these results. Although several outcome parameters of SRQs and health-related quality of life showed significant differences favoring the UPAL group, some parameters showed small differences. This may be because the symptoms associated with herniated discs significantly improve after discectomy. For example, the minimum clinically important difference (MCID) for the SF-36 subscales has been reported as 10 points, and for the ODI, it has been reported as 8–12 points in the treatment of spinal disorders55,56,57. A future challenge is determining the MCID in this novel treatment. Finally, no multiple error rate correction was applied to the between-group tests, which increased the risk of false-positive findings.
In conclusion, this phase 1/2 study of UPAL gel implantation in patients with lumbar disc herniation demonstrated its feasibility and safety in preventing IVD degeneration after discectomy. The present study suggests that the UPAL gel is a novel therapeutic strategy after discectomy in cases of lumbar IVD herniation. This pilot study generated a rationale for a subsequent phase 3 clinical trial.
Methods
Ethics
This study was approved by the ethics committees of Hokkaido University Hospital (approval numbers: H30-10, 018-0411, 019-0414) and Eniwa Hospital (approval number: dMD001-H1), as well as by the Pharmaceuticals and Medical Devices Agency (Japan). The study was monitored and approved by the Clinical Research and Medical Innovation Center in Hokkaido University Hospital. The publication of the deidentified, analytic dataset for this study has been approved by the Center. Written informed consent was obtained from all patients before enrollment. The consent included the subject’s authorization for use/disclosure of samples or data. The trial was conducted as per the Declaration of Helsinki and Good Clinical Practice guidelines. The results were not stratified by hospital, and each hospital had the authority to terminate the study in accordance with its policies and ethical considerations in terms of safety.
Study design
This study was a first-in-human, single-arm, open-label, phase 1/2 trial conducted at two centers. This study comprised two parts: phase I involved a prospective study on UPAL gel implantation after discectomy in patients with lumbar disc herniation, with patient recruitment from November 13, 2018 to September 1, 2020 (UPAL group), and phase II included a subsequent prospective study on patients with lumbar disc herniation who underwent discectomy without UPAL implantation as a control group to compare and evaluate the efficacy of the UPAL gel. The control study began in December 10, 2020, when all surgeries to implant the UPAL were completed, and patient recruitment continued until March 30, 2022. All studies were registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN000034227, https://center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000039018, Registered date: Sept. 21, 2018, UMIN000042282, https://center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000048247, Registered date: Oct. 29, 2020). Data were prospectively collected, recorded in electronic case report forms, and securely stored in a password-protected Electronic Data Capture system. Access was limited to authorized personnel.
Patients
The patient inclusion criteria and evaluation schedules were comparable in these studies. The subjects were healthy patients aged 20–49 years, with herniated NP, since lumbar disc herniation is most common in people aged in their 20 s to 40 s9. Eligible patients in this study were those with a symptomatic herniated NP who were unresponsive to nonoperative treatment and required nerve decompression and surgical excision of the herniated lumbar IVD fragments13,28. The inclusion criteria for the degree of pain were as follows: (1) persistent and predominant pain (score of > 40 on the 100 mm VAS, in which higher scores represent worse pain) which is unresponsive to 6 consecutive weeks of therapy (2) acute/uncontrolled leg pain, defined as a score of > 80 on the 100-mm VAS28. Based on the clinical signs, symptoms, and MRI findings, the patients were diagnosed with a lumbar IVD hernia. Furthermore, only patients who underwent single-level discectomy were eligible for the study28. The exclusion criteria were as follows: (1) psychiatric, infectious, or malignant diseases; (2) previous surgery at the same lumbar level; (3) cases with spondylolisthesis of grade ≥ 1 and those with local kyphosis involving the affected disc level on plain radiography of the lumbar spine in the flexion, neutral, or extension positions were excluded because they were not suitable for implantation into the disc due to segmental spinal instability28. The complete inclusion and exclusion criteria are described in detail in a previous study protocol28.
Candidates were recruited from local orthopedic hospitals. Patients with a symptomatic herniated NP indicated for surgical treatment were asked to complete a screening questionnaire to determine their eligibility. Those who met the inclusion criteria were contacted by the investigators to confirm their willingness to participate in the study and for baseline assessment and were assigned to the UPAL or control group depending on when they underwent surgery (the first 40 patients from November 2018 were allocated to the UPAL group, and subsequent cases from January 2021 onward were allocated to the control group)28. Patients selected for the study used prescribed medications during the study period, and the type of medication and dosage used were recorded at the baseline assessment28. However, for pain assessment, the use of opioid analgesics was discontinued during the evaluation on the day before and after surgery. The participants received 7000 Japanese yen as transportation expenses for each outpatient visit.
UPAL gel
The UPAL gel is produced and packaged at a GMP facility and is then shipped to medical institutions. The production process has been previously documented26, and standardized protocols are available to ensure consistent gel quality and purity. The main product standards are listed in Supplementary Table 8.
Procedures
All patients underwent open discectomy for lumbar IVD herniation, which was performed using standard or tubular retractors with or without an operating microscope or loupes13,28. To standardize the discectomy procedure, an AF incision of 5 × 5 mm was made using a No.15 surgical blade28. The size of the AF opening was measured in patients with transligamentous extrusion- or sequestration-type herniated discs. After the AF incision, as much disc material as possible was removed, rather than performing a limited discectomy. A manual, nonautomated approach was used for discectomy, and discectomy was performed at the discretion of the treating surgeon13. Although surgeon preference and skill might confound the outcomes, this was a limitation that was intentionally allowed to facilitate the recruitment of participants from the wider orthopedic community28,53.
After discectomy, the surgeon evaluated the feasibility of AF re-approximation according to the following stopping criteria before UPAL implantation: (1) the diameter of the AF incision for discectomy exceeded 5 mm; (2) some unsuitable reasons for UPAL gel implantation, such as spinal fluid leakage due to an accidental tear of the dura mater; and (3) the volume of saline delivered to the IVD defect exceeded 2 mL, indicating AF rupture28. If any of the stopping criteria were met, UPAL gel implantation was not performed after standard discectomy, and the patient was excluded from the study. None of the stopping criteria should be met for the procedure to proceed. If all of the abovementioned criteria were not met, a board-certified orthopedic specialist injected the UPAL gel into the IVD.
The UPAL gel was manufactured under the quality management system and provided by Mochida Pharmaceutical Co. (Mochida Pharma Co. Ltd., Tokyo, Japan). The specific UPAL implantation method involved delivering up to 2 mL of UPAL solution into the IVD defect using a syringe with a 16 G catheter until the level of the UPAL solution reached the level of the AF at the disc opening, and then 10 mL of 0.1 mol/L CaCl2 solution was added to the surface of the UPAL solution to gelate the alginate28. Five minutes after application of the CaCl2 solution, the surgical site was irrigated with saline solution, and UPAL gelation was confirmed.
UPAL gel implantation was performed based on the following stopping criteria: (1) the volume of UPAL solution injected into an IVD defect exceeded 2 mL; (2) the UPAL solution did not gel upon treatment with CaCl2; and (3) other reasons included an allergic reaction for which the treating surgeon determined the participant to be unsuitable28. If either of the abovementioned stopping criteria during UPAL gel implantation were met, the surgical site was irrigated with a full dose of normal saline, and an assessment of safety after the injection of the UPAL solution was performed for up to 24 weeks28. After surgery, patients were not restricted and could leave their beds based on their pain levels.
Participant follow-up
The participants were observed at 1, 4, 12, 24, 48, and 96 weeks after the surgery for assessment (Supplementary Table 1). Solicited local and systemic AEs, including pain in various parts of the body; fever; systemic disorders, such as cancer, heart disease, pneumonia, abnormal blood coagulation, and death; and complications associated with discectomy, such as wound infection, and recurrence of herniation, were evaluated by classifying them as serious AEs or serious adverse reactions according to the Pharmaceutical and Medical Device Act currently in effect in Japan58 and processed according to the Japanese guidelines for Good Clinical Practice13. Recurrence of herniation was specified as the presence of neurological clinical symptoms consistent with re-herniation at the same disc level where discectomy was performed, in which all extruded NP material was removed during the initial surgery and a new offending fragment, including the gel, was identified, or cases in which all NP material extruded at the initial surgery had not been removed13,28. Unsolicited AEs were recorded until 24 weeks after surgery, and serious AEs were recorded throughout the study.
Physical examination and SRQs for evaluating pain and health-related quality of life were administered 1 day before surgery (baseline) and at each visit up to 96 weeks after surgery. MRI examinations were conducted before surgery (baseline) and at 24 and 96 weeks postoperatively (Supplementary Table 1). In addition to the usual laboratory tests performed during surgery, including blood count, liver function profile, renal function profile, electrolyte analysis, and inflammatory response analysis, the UPAL group also underwent tests for the analysis of immune markers, such as IgA, IgE, and IgG, which were performed 1 day before surgery (baseline) and at each visit up to 24 weeks after surgery (Supplementary Table 1).
Outcomes
The primary outcomes of this study were the feasibility and safety of UPAL implantation after discectomy28. The feasibility of UPAL implantation was determined based on whether the UPAL implantation was completed. Based on the aforementioned preoperative and intraoperative criteria, the completion of UPAL gel implantation was determined when the surface of the UPAL injected into the IVD was gelatinized. The safety endpoints included AEs that could not be ruled out as being associated with UPAL.
The secondary outcomes of this study were as follows: (1) physical function score; (2) SRQ scores; and (3) MRI-based measures of the morphological and compositional qualities of IVD tissues (Supplementary Table 1)28.
Physical examinations to evaluate physical function included FFD measurement, SLR test, MST, and JOA score. FFD was measured as the shortest distance (cm) from the floor to the fingertips when the patient stood and bent forward with the arms extended without bending the knees59,60. The SLR test measured the maximum angle (degrees) between the horizontal plane and the lower extremity with the knee in passive extension and the lower extremity elevated59. MST was performed to evaluate the range of motion of the lumbar spine in the following steps: three marks (one on the lumbosacral joint, one on the spinous process 10 cm above the first mark, and one on the spinous process 5 cm below the first mark) were placed with the patient in the standing position, and the distance between the lower and upper marks at full flexion of the body minus the distance at full extension was measured (cm)61. The JOA score was used to assess the severity of the functional and clinical conditions associated with lumbar disc herniation; the JOA score ranged from 0 to 29, with higher scores indicating better functional status61.
SRQs used to evaluate pain and health-related quality of life included the VAS for back and leg pain, ODI, RDQ, JOABPEQ, and SF-3628. Using a 100-point VAS (0–100 mm, the higher the score, the worse the pain), low back pain and leg pain on the more painful side were assessed. Disabilities in activities of daily living due to lower back and leg pain were assessed using the ODI (0–100 points, the higher the score, the more severe the disability)62. Quality of life specific to low back pain was assessed using the RDQ (0–24 points; higher scores indicated worse quality of life)63. A multidimensional assessment of health status, including pain-related disability, lumbar spine dysfunction, gait dysfunction, social disability, and psychological disability associated with lumbar spine disorders, was performed using the JOABPEQ (0–100 points, with higher scores indicating better health status)64. Health-related quality of life was evaluated using the SF-36 (0–100 points; higher score, better quality of life)65.
In lumbar spine MRI examinations, conventional T2-weighted images were obtained using a turbo spin-echo sequence. Next, T1ρ, T2*, and DWIs in the sagittal plane were taken using a 3D gradient-echo or an echo planar-imaging pulse sequence to objectively and quantitatively assess IVD degeneration29,30,31,32. MRI was performed using a 3.0 Tesla scanner. The Pfirrmann grading system (5-point scale from 1 to 5, the higher the score, the more advanced the degeneration) was used to evaluate IVD degeneration on the MR images66. Two board-certified orthopedic surgeons with > 10 years of experience evaluated the treated lumbar IVDs on mid-sagittal T2-weighted images according to the Pfirrmann grading system66. The intervertebral DHI was calculated as the ratio of the IVD height to the proximal vertebral body height measured for the middle portion on mid-sagittal T2-weighted images67,68. For quantitative evaluation, the T1ρ, T2*, and ADC values derived from the DWIs of the IVD were evaluated. The 3D gradient-echo images with different spin lock and echo times were fitted on a pixel-by-pixel basis to generate T1ρ and T2* relaxation time maps, respectively29. Regions of interest (ROIs) for the IVDs were semi-automatically placed on mid-sagittal T2-weighted images using a workstation (Jim8, Xinapse Systems Ltd., Essex, UK) to include the entire IVD while ensuring that nearby structures were not included32. The ROIs were then superimposed onto the corresponding T1ρ, T2*, and ADC maps for measurement32. A radiological technologist confirmed the accuracy of the ROI position32. T1ρ, T2*, and ADC values obtained from treated discs were calculated as a percentage of the normal disc (L2/3) value.
Statistical analyses
Regarding sample size calculation, there are no published data on the quantitative interactions between biomaterials and IVD tissue after discectomy; therefore, it was not possible to estimate effect sizes at this time, and a preliminary sample size could not be calculated for this pilot study28. We targeted 40 participants for this study, as we anticipated that this sample size would be achieved within a reasonable time frame, given the study recruitment rate within our community47. In addition, a sample size of 40 subjects is considered sufficient for an exploratory clinical trial with feasibility and safety as primary endpoints because it has a 95% probability of detecting a difference in one or more AEs, representing an incidence rate of 7.5%28. However, with limitations in detecting between-group differences in clinical assessments, such as scoring, this study aimed to provide a basis for determining useful assessment parameters and setting the number of patients to be included in larger validation studies.
In each study, in the UPAL group, SAF was defined as the group of enrolled patients who underwent UPAL implantation (regardless of whether the implantation was complete or incomplete), and in the control group, SAF was defined as the group of enrolled patients who underwent discectomy. The PPS included the patients who underwent complete UPAL implantation among the enrolled patients in the UPAL group and the patients who underwent discectomy in the control group.
Feasibility was evaluated as the proportion of patients who underwent complete UPAL gel implantation among those who underwent discectomy followed by UPAL solution injection28. Safety was evaluated according to the incidence of AEs that were definitely related to UPAL in all patients who underwent UPAL implantation following discectomy28.
Analyses of efficacy, a secondary endpoint, were performed for the PPS that complied with the study protocol. Data are expressed as mean ± standard deviation and frequency (percentage), as appropriate. For continuous metrics, data normality was assessed using the D’Agostino–Pearson omnibus test. The analysis of between-group differences for continuous variables was evaluated at different time points during follow-up using the unpaired t test; age at surgery, body mass index, time from onset to surgery, operation time, and removed disc material were evaluated upon enrollment or during operation. FFD, SLR test, Modified Schober’s test, JOA score, VAS, ODI, RDQ, JOABPEQ, and SF-36 were evaluated preoperatively and postoperatively at weeks 1, 4, 12, 24, 48, and 96. MRI assessment (DHI, T1ρ, T2*, and ADC) was performed preoperatively and at 24 and 96 weeks postoperatively. The Mann-Whitney U test was performed to test for differences between the groups for data that were not normally distributed. For the analysis of intergroup differences in ordinal categorical variables (Pfirrmann disc degeneration grading score), the Mann–Whitney U test was performed preoperatively and at 24 and 96 weeks postoperatively. The Wilcoxon signed-rank test was performed to analyze within-group differences in FFD, SLR test, Modified Schober’s test, JOA score, VAS, ODI, RDQ, JOABPEQ, and SF-36 from baseline to postoperative weeks 1, 4, 12, 24, 48, and 96 for each group. The Chi-squared test was performed to analyze sex, disc herniation level, herniation type, and current smoking status between the two groups. For analysis of within-group changes in MRI assessment (T1ρ, T2*, and ADC), a repeated analysis of variance was used to evaluate the change over time at three time points (preoperatively, 24 weeks postoperatively, and 96 weeks postoperatively) for each group. In this analysis, “time point” (preoperatively, 24 weeks postoperatively, and 96 weeks postoperatively) was incorporated into the model as a factor. When significant differences were detected among the time points within each group, a post-hoc test with Bonferroni correction was performed to conduct paired comparisons among the three time points. Bonferroni post hoc analyses were used to set a significance level of 0.017 (dividing 0.05 by 3). The difference in treatment effects between the groups at each follow-up time point was summarized by calculating the mean along with a 95% CI. All 95% CIs were calculated using Student’s t-distribution. Spearman’s rank correlation test was performed to analyze the correlation between the injected UPAL volume and various clinical scores. A two-tailed probability level of 0.05 was considered significant. No multiple error rate correction was applied for the between-group tests, despite the number of time points, because this was an exploratory phase 1/2 trial. Data analysis and curation was conducted using Microsoft Excel (2021, ver 2502, Build 16.0.18526.20168 64 bit), BellCurve for Excel (ver 4.07), and SAS (ver 9.4).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
No data for this study have been deposited elsewhere. All data that support the finding of this study are available in this article and are provided from the corresponding author upon request. Source data are provided in this paper.
References
Suzuki, H. et al. Injection of ultra-purified stem cells with Sodium alginate reduces discogenic pain in a rat model. Cells 12, 505 (2023).
Yamada, K., Iwasaki, N. & Sudo, H. Biomaterials and cell-based regenerative therapies for intervertebral disc degeneration with a focus on biological and biomechanical functional repair: Targeting treatments for disc herniation. Cells 11, 602 (2022).
Frith, J. E. et al. An injectable hydrogel incorporating mesenchymal precursor cells and pentosan polysulphate for intervertebral disc regeneration. Biomaterials 34, 9430–9440 (2013).
Urban, J. P. & Roberts, S. Degeneration of the intervertebral disc. Arthritis Res. Ther. 5, 120–130 (2003).
Sudo, H. & Minami, A. Caspase 3 as a therapeutic target for regulation of intervertebral disc degeneration in rabbits. Arthritis Rheum. 63, 1648–1657 (2011).
Woiciechowsky, C. et al. Regeneration of nucleus pulposus tissue in an ovine intervertebral disc degeneration model by cell-free resorbable polymer scaffolds. J. Tissue Eng. Regen. Med. 8, 811–820 (2014).
Lee, C. H. et al. Protein-releasing polymeric scaffolds induce fibrochondrocytic differentiation of endogenous cells for knee meniscus regeneration in sheep. Sci. Transl. Med. 6, 266ra171 (2014).
Ukeba, D. et al. Combination of ultra-purified stem cells with an in situ-forming bioresorbable gel enhances intervertebral disc regeneration. EBioMedicine 76, 103845 (2022).
Battie, M. C. & Videman, T. Lumbar disc degeneration: epidemiology and genetics. J. Bone Jt. Surg. Am. 88, 3–9 (2006).
Sudo, H. et al. Protocol for treating lumbar spinal canal stenosis with a combination of ultrapurified, allogenic bone marrow-derived mesenchymal stem cells and in situ-forming gel: a multicentre, prospective, double-blind randomised controlled trial. BMJ Open 13, e065476 (2023).
Katz, J. N. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J. Bone Jt. Surg. Am. 88, 21–24 (2006).
Sloan, S. R. Jr. et al. Combined nucleus pulposus augmentation and annulus fibrosus repair prevents acute intervertebral disc degeneration after discectomy. Sci. Transl. Med. 12, eaay2380 (2020).
Bailey, A., Araghi, A., Blumenthal, S. & Huffmon, G. V. Prospective, multicenter, randomized, controlled study of anular repair in lumbar discectomy: two-year follow-up. Spine 38, 1161–1169 (2013).
McGirt, M. J. et al. A prospective cohort study of close interval computed tomography and magnetic resonance imaging after primary lumbar discectomy: factors associated with recurrent disc herniation and disc height loss. Spine 34, 2044–2051 (2009).
Barth, M., Diepers, M., Weiss, C. & Thome, C. Two-year outcome after lumbar microdiscectomy versus microscopic sequestrectomy: part 2: radiographic evaluation and correlation with clinical outcome. Spine 33, 273–279 (2008).
Endres, M. et al. Intervertebral disc regeneration after implantation of a cell-free bioresorbable implant in a rabbit disc degeneration model. Biomaterials 31, 5836–5841 (2010).
Bowles, R. D., Williams, R. M., Zipfel, W. R. & Bonassar, L. J. Self-assembly of aligned tissue-engineered annulus fibrosus and intervertebral disc composite via collagen gel contraction. Tissue Eng. Part A 16, 1339–1348 (2010).
Malhotra, N. R. et al. An injectable nucleus pulposus implant restores compressive range of motion in the ovine disc. Spine 37, E1099–E1105 (2012).
Gullbrand, S. E. et al. Translation of an injectable triple-interpenetrating-network hydrogel for intervertebral disc regeneration in a goat model. Acta Biomater. 60, 201–209 (2017).
Likhitpanichkul, M. et al. Fibrin-genipin adhesive hydrogel for annulus fibrosus repair: performance evaluation with large animal organ culture, in situ biomechanics, and in vivo degradation tests. Eur. Cell Mater. 28, 25–37 (2014).
Zeng, Y. et al. Injectable microcryogels reinforced alginate encapsulation of mesenchymal stromal cells for leak-proof delivery and alleviation of canine disc degeneration. Biomaterials 59, 53–65 (2015).
Mizuno, H. et al. Tissue-engineered composites of anulus fibrosus and nucleus pulposus for intervertebral disc replacement. Spine 29, 1290–1297 (2004).
Risbud, M. V. et al. Differentiation of mesenchymal stem cells towards a nucleus pulposus-like phenotype in vitro: implications for cell-based transplantation therapy. Spine 29, 2627–2632 (2004).
Zimmermann, H. et al. Towards a medically approved technology for alginate-based microcapsules allowing long-term immunoisolated transplantation. J. Mater. Sci. Mater. Med. 16, 491–501 (2005).
Ukeba, D. et al. Bone marrow aspirate concentrate combined with ultra-purified alginate bioresorbable gel enhances intervertebral disc repair in a canine model: A preclinical proof-of-concept study. Cells 13, 987 (2024).
Tsujimoto, T. et al. An acellular bioresorbable ultra-purified alginate gel promotes intervertebral disc repair: A preclinical proof-of-concept study. EBioMedicine 37, 521–534 (2018).
Ura, K. et al. Ultra-purified alginate gel implantation decreases inflammatory cytokine levels, prevents intervertebral disc degeneration, and reduces acute pain after discectomy. Sci. Rep. 11, 638 (2021).
Yamada, K. et al. Exploratory clinical trial on the safety and capability of dMD-001 in lumbar disc herniation: Study protocol for a first-in-human pilot study. Contemp. Clin. Trials Commun. 23, 100805 (2021).
Zhang, X. et al. Comparison of T1rho and T2* Relaxation Mapping in Patients with Different Grades of Disc Degeneration at 3T MR. Med. Sci. Monit. 21, 1934–1941 (2015).
Cui, Y. Z., Yang, X. H., Liu, P. F., Wang, B. & Chen, W. J. Preliminary study on diagnosis of lumbar disc degeneration with magnetic resonance T1p, T2 mapping and DWI quantitative detection technologies. Eur. Rev. Med. Pharm. Sci. 20, 3344–3350 (2016).
Pandit, P., Talbott, J. F., Pedoia, V., Dillon, W. & Majumdar, S. T1rho and T2 -based characterization of regional variations in intervertebral discs to detect early degenerative changes. J. Orthop. Res. 34, 1373–1381 (2016).
Hamaguchi, H. et al. Quantitative assessment of intervertebral disc composition by MRI: Sensitivity to diurnal variation. Tomography 9, 1029–1040 (2023).
Priyadarshani, P., Li, Y. & Yao, L. Advances in biological therapy for nucleus pulposus regeneration. Osteoarthr. Cartil. 24, 206–212 (2016).
Embree, M. C. et al. Exploiting endogenous fibrocartilage stem cells to regenerate cartilage and repair joint injury. Nat. Commun. 7, 13073 (2016).
Huey, D. J., Hu, J. C. & Athanasiou, K. A. Unlike bone, cartilage regeneration remains elusive. Science 338, 917–921 (2012).
Waskow, C. Maintaining what is already there: Strategies to rectify HSC transplantation dilemmas. Cell Stem Cell 17, 258–259 (2015).
Sicari, B. M. et al. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci. Transl. Med. 6, 234ra258 (2014).
Abbushi, A. et al. Regeneration of intervertebral disc tissue by resorbable cell-free polyglycolic acid-based implants in a rabbit model of disc degeneration. Spine 33, 1527–1532 (2008).
Andersson, G. B. Epidemiological features of chronic low-back pain. Lancet 354, 581–585 (1999).
Seki, S. et al. A functional SNP in CILP, encoding cartilage intermediate layer protein, is associated with susceptibility to lumbar disc disease. Nat. Genet 37, 607–612 (2005).
Takeoka, Y. et al. Reduced nucleotomy-induced intervertebral disc disruption through spontaneous spheroid formation by the Low Adhesive Scaffold Collagen (LASCol). Biomaterials 235, 119781 (2020).
Buser, Z. et al. Biological and biomechanical effects of fibrin injection into porcine intervertebral discs. Spine 36, E1201–E1209 (2011).
Yin, W., Pauza, K., Olan, W. J., Doerzbacher, J. F. & Thorne, K. J. Intradiscal injection of fibrin sealant for the treatment of symptomatic lumbar internal disc disruption: results of a prospective multicenter pilot study with 24-month follow-up. Pain. Med. 15, 16–31 (2014).
Kazezian, Z., Sakai, D. & Pandit, A. Hyaluronic acid microgels modulate inflammation and key matrix molecules toward a regenerative signature in the injured annulus fibrosus. Adv. Biosyst. 1, e1700077 (2017).
Huang, Y. C., Hu, Y., Li, Z. & Luk, K. D. K. Biomaterials for intervertebral disc regeneration: Current status and looming challenges. J. Tissue Eng. Regen. Med. 12, 2188–2202 (2018).
Sakai, D. et al. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat. Commun. 3, 1264 (2012).
van Griethuysen, J. J. M. et al. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 77, e104–e107 (2017).
Behdenna, A. et al. pyComBat, a Python tool for batch effects correction in high-throughput molecular data using empirical Bayes methods. BMC Bioinforma. 24, 459 (2023).
Mohd Isa, I. L. et al. Implantation of hyaluronic acid hydrogel prevents the pain phenotype in a rat model of intervertebral disc injury. Sci. Adv. 4, eaaq0597 (2018).
Liu, Y. et al. Follistatin-like protein 1 promotes inflammatory reactions in nucleus pulposus cells by interacting with the MAPK and NFkappaB signaling pathways. Oncotarget 8, 43023–43034 (2017).
Ohtori, S. et al. Tumor necrosis factor-alpha-immunoreactive cells in nucleus pulposus in adolescent patients with lumbar disc herniation. Spine 38, 459–462 (2013).
Burke, J. G. et al. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J. Bone Jt. Surg. Br. 84, 196–201 (2002).
Freitag, J. et al. Adipose derived mesenchymal stem cell therapy in the treatment of isolated knee chondral lesions: design of a randomised controlled pilot study comparing arthroscopic microfracture versus arthroscopic microfracture combined with postoperative mesenchymal stem cell injections. BMJ Open 5, e009332 (2015).
Moore, C. G., Carter, R. E., Nietert, P. J. & Stewart, P. W. Recommendations for planning pilot studies in clinical and translational research. Clin. Transl. Sci. 4, 332–337 (2011).
Whitmore, R. G. et al. Predictive value of 3-month lumbar discectomy outcomes in the NeuroPoint-SD Registry. J. Neurosurg. Spine 23, 459–466 (2015).
Hägg, O., Fritzell, P. & Nordwall, A. Swedish Lumbar Spine Study Group. The clinical importance of changes in outcome scores after treatment for chronic low back pain. Eur. Spine J. 12, 12–20 (2003).
Mannion, A. F., Junge, A., Grob, D., Dvorak, J. & Fairbank, J. C. T. Development of a German version of the Oswestry Disability Index. Part 2: sensitivity to change after spinal surgery. Eur. Spine J. 15, 66–73 (2006).
Mumme, M. et al. Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: an observational first-in-human trial. Lancet 388, 1985–1994 (2016).
Rao, R. et al. Clinical comparative study: efficacy and tolerability of tolperisone and thiocolchicoside in acute low back pain and spinal muscle spasticity. Asian Spine J. 6, 115–122 (2012).
Fukui, M. et al. JOA back pain evaluation questionnaire: initial report. J. Orthop. Sci. 12, 443–450 (2007).
Yasuda, T. et al. Clinical and imaging characteristics in patients undergoing surgery for lumbar epidural lipomatosis. BMC Musculoskelet. Disord. 19, 66 (2018).
Fairbank, J. C. & Pynsent, P. B. The oswestry disability index. Spine 25, 2940–2952 (2000).
Roland, M. & Morris, R. A study of the natural history of back pain. Part I: development of a reliable and sensitive measure of disability in low-back pain. Spine 8, 141–144 (1983).
Kasai, Y. et al. Verification of the sensitivity of functional scores for treatment results - Substantial clinical benefit thresholds for the Japanese Orthopaedic Association Back Pain Evaluation Questionnaire (JOABPEQ). J. Orthop. Sci. 22, 665–669 (2017).
Ware, J. E. Jr. & Sherbourne, C. D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30, 473–483 (1992).
Pfirrmann, C. W., Metzdorf, A., Zanetti, M., Hodler, J. & Boos, N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 26, 1873–1878 (2001).
Jarman, J. P. et al. Intervertebral disc height loss demonstrates the threshold of major pathological changes during degeneration. Eur. Spine J. 24, 1944–1950 (2015).
Huang, W., Chang, Z., Song, R., Zhou, K. & Yu, X. Non-fusion procedure using PEEK rod systems for lumbar degenerative diseases: clinical experience with a 2-year follow-up. BMC Musculoskelet. Disord. 17, 53 (2016).
Acknowledgements
The authors would like to thank all the participating patients and their families. We would like to thank Mitsuko Isaji, Satoshi Shimizu, and Yusuke Nakagawa for preparing the materials. We would also like to acknowledge support from Dr. Kazufumi Okada for performing statistical work. Funding was provided by Grant-in-Aid for the Ministry of Education, Culture, Sports, Science, and Technology of Japan (24H00668), Japan, “Project of Translational and Clinical Research Core Centers” from Japan Agency for Medical Research and Development, AMED (JP20lm0203045h0003), Japan, and the Mochida Pharmaceutical Co., Ltd. The funding sources had no involvement in study design; collection, analysis, and interpretation of data; writing of the report; or decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
H.S. is the principal investigator. H.S. designed the pilot study and filed funding applications. K.Y., K.M., T.I., K.K.T., N.Y., T.M., O.S., N.S., N.I., and H.S. designed and drafted the study protocol. K.Y., T.H., T.K., T.O., T.E., D.U., H.T., Y.A., Y.I., and H.S. screened, enrolled, and treated the patients. M.Y. I. provided statistical expertise to support the study methodology. K.K.T. conducted the MRI analysis. K.Y., K.K.T., and H.S. analyzed the results. K.Y., N.I., and H.S. contributed to discussions throughout the study. K.Y. and H.S. prepared and edited the manuscript. All authors contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Patents pertaining to this work have been filed (inventors: H.S. and N.I.). The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Ganjun Feng, Georg Duda, and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yamada, K., Hyakumachi, T., Kokabu, T. et al. Acellular, bioresorbable, ultra-purified alginate gel implantation for intervertebral disc herniation: Phase 1/2, open-label, non-randomized clinical trials. Nat Commun 16, 4285 (2025). https://doi.org/10.1038/s41467-025-59715-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-59715-0