Abstract
Despite the promising potential of the perfluoro-tert-butyl group in diverse fields such as magnetic resonance imaging, material science and drug design, incorporating this group into organic molecules is still a formidable task, primarily due to its bulky structure and unique fluorine effect. Herein, we describe a stable and scalable reagent for radical-type perfluoro-tert-butylation, which is synthesized in large scale from commercial perfluoro-tert-butanol and a designed benzothiazole hypervalent iodonium salt. Highly E-selective photo-driven C(sp2)–H functionalization of styrene derivatives is achieved in a triplet-triplet energy transfer halted manner, while thermally disfavored Z-products are also accessible by removing the energy antagonist. The application of this method is further demonstrated by late-stage functionalization and divergent synthesis of perfluoro-tert-butylated compounds.
Similar content being viewed by others
Introduction
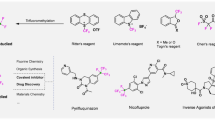
Over the past two decades, fluoroalkylation reactions have garnered extensive research interest1,2 owing to their promising applications in the fields of pharmaceuticals, agrochemicals, and functional materials3,4,5. Perfluoro-tert-butyl (PFtB) group, characterized by its exceptional structural bulkiness and robust electron-withdrawing capacity6,7, stands out as an extreme example within the realm of fluoroalkyl groups. More importantly, PFtB-containing compounds exhibit considerable promise in magnetic resonance imaging (MRI)8,9,10, material science11 and drug design12 (Fig. 1), which significantly amplifies their importance and potential applications. For example, PFtB labeling offers an efficient tool for monitoring RNA conformational behavior9 (Fig. 1, left one) and PERFECTA10 (Fig. 1, left two) is proven to possess excellent cellular compatibility, relaxation times and sensitivity for in vivo 19F-MRI applications. As a result, the effect of introducing PFtB into molecules is much more than introducing a super CF3 to simply alter the physical and biological properties.
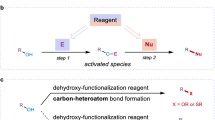
Despite significant value demonstrated by PFtB-containing compounds has been shown across various fields, synthetic methodologies for incorporating PFtB into organic molecules remain limited and challenging. Traditionally, indirect ways are employed to synthesize PFtB-containing compounds, which demand limited commercial PFtB motifs9,13,14,15, such as (CF3)3COH and (CF3)3CC ≡ CH, or pre-functionalized substrates16,17 (Fig. 2a). Though direct perfluoro-tert-butylation is highly appealing to chemists, it is still far from well-developed. In 1972, anion-type perfluoro-tert-butylation of organic compounds was firstly reported using highly toxic perfluoro-iso-butene18. Recently, Hu et al. modified this method to generate (CF3)3C− species elegantly19,20 (Fig. 2b), while methods to introduce PFtB into universal substrates were still unreported. Given the intrinsic properties of (CF3)3C−, namely its weak nucleophilicity, electron deficiency, substantial steric hindrance and instability, anion-type perfluoro-tert-butylation encounters significant constraints. These constraints manifest as incompatibility with transition metal catalysis and the necessity for highly reactive substrates, such as alkyl halides and arynes19,20 (Fig. 2b), making perfluoro-tert-butylation different from well-established trifluoromethylation.
Correspondingly, radical-type perfluoro-tert-butylation has been much less studied, probably due to the scarcity of practical reagents to provide (CF3)3C·, a notable electrophilic carbon-centered radical21,22. PFtB halides (Fig. 2c, left), sole known sources of (CF3)3C·, were proven to generate (CF3)3C· under conditions like photolysis. However, these halides suffer from poor synthetic economy, inherent toxicity and instability, making radical-type perfluoro-tert-butylation still undeveloped (Fig. 2c, right, only two reported cases23,24). Consequently, there is a pressing need to develop novel reagents and reaction pathways for radical-type perfluoro-tert-butylation.
Deoxygenative coupling of alcohols has recently attracted considerable attention25,26,27,28, primarily due to the broad accessibility of alcohols in comparison to other radical precursors. In this regard, developing reagents from (CF3)3COH, a bulk chemical renowned for its stability, cost-effectiveness and low toxicity, is highly desirable yet challenging because of the low reactivity29. For example, pKa value for (CF3)3CO–H, (CF3)2HCO–H, (CH3)3CO–H and (CH3)2HCO–H were 5.4, 9.3, 17.1 and 15.3, respectively30,31, indicating the weak nucleophilicity of (CF3)3CO−. As a result, (CF3)3COH is commonly used as non-nucleophilic protonic solvent and (CF3)3CO− is a widely used counter anion of ionic liquids.
Usually, strong electrophiles are employed to match the low reactivity of (CF3)3COH13,14,15, however, corresponding products of established methods are unable to release (CF3)3C·. In our proposal, an equivalent of aza-aryl cation could serve as a precursor to an aza-arene with a (CF3)3CO– group, in which the covalent linkage of (CF3)3CO– would further facilitate the production of (CF3)3C· (Fig. 3a).
Our attempt to synthesize the proposed reagent through typical approaches all failed (see Supplementary Information, SI, section 1), which promoted us to look for more suitable solution, which was, developing more reactive yet stable reagents to match the weak nucleophilicity of perfluoro-tert-butanol. Basing on Zhao’s work15 and our longstanding interest in hypervalent iodine chemistry32,33,34, we designed a class of highly reactive diaryliodonium salts as equivalents of aza-aryl cations (Fig. 3b, up). Probably due to the lack of proper synthetic methods35,36,37,38 and the highly reactive nature of these species, these aza-aryl cation equivalents were still unreported39.
After lots of failed attempts, we were pleased to find compound 2 could be obtained via a mild σ metathesis from commercially available PIFA and corresponding ArTMS. After highly selective substitution with (CF3)3CO− to generate compound 3, reagent 4 (RPFtB) could be synthesized in large scale (Fig. 3b, down). (The structures of 2a and 4 were confirmed undoubtedly by X-Ray diffraction analysis.) We found that RPFtB showed an irreversible reduction potential of −0.05 V in MeCN, versus SCE, indicating its plausible reduction under various conditions (SI, section 8).
In this article, we elucidate the utilization of RPFtB in the synthesis of PFtB-containing compounds via photocatalysis. Our findings reveal that single electron reduction of RPFtB efficiently releases (CF3)3C·, and this radical can be manipulated to participate in various catalytic cycles, leading to the production of diverse compounds. Concerning the difficulties illustrated by previous studies19,20,23,24, radical perfluoro-tert-butylation basing on RPFtB provides an opportunity to access these valuable products in a practical way.
Results
Reaction development
Photocatalysis provides chemists opportunities to utilize transient species, especially radicals, into organic synthesis. Among numerous successes in this area, olefin chemistry stands as one of the most established fields through photo-driven radical pathways, leading to products with great diversity. As one of the most basic and important cases, photo-driven Heck-type reaction (vinyl C(sp2)–H functionalization) has largely broadened the edge of the classical textbook reaction in both substrate capability and selectivity40. However, despite lots of successes have been made, developing photocatalytic methods for C(sp2)–H functionalization of electron-deficient radicals is still challenging due to the lack of mild way to generate these radicals and re-establishment of unsaturation ensuing radical addition41.
Considering PFtB substituted olefins’ value in further transformation and the lack of synthetic methods, we were motivated to develop a general strategy for selective radical C(sp2)–H perfluoro-tert-butylation. Styrene derivatives, which were challenging substrates for this photocatalytic reaction42,43,44 with regard to plausible polymerization, isomerization through triplet-triplet energy transfer (TTET)45,46 and dimerization47, were chosen as our prototypical substrates. Investigation of the reaction conditions (see SI section 6 for detailed optimizations) suggested the best conditions listed in Fig. 4. We identified fac-Irppy3 (E1/2 (M+/M*) = −1.73 V, versus SCE48) as an effective photocatalyst, together with CuTC as transition metal catalyst. For electron-deficient or electron neutral styrene derivatives, when the reaction was carried out in DCM under irradiation of blue LEDs (10 W and 425 nm), no additional base was needed to deliver corresponding E-alkene product in satisfying yield and selectivity (entry 1, 78%, E:Z > 20:1). In this case, the choice of base and solvent played a crucial role in determining E/Z-selectivity. The addition of several kinds of base would lead to a higher ratio of Z-isomer (entries 2 and 3) and similar phenomena was also found when using different solvents (entries 4 and 5). The initial valence and source of the copper catalyst would not influence the result, indicating both Cu(I) and Cu(II) catalyst were able to involve the catalytic cycle (entry 6). Conditions for electron-enriched styrene derivatives, which were easy to undergo acid-catalyzed polymerization, were studied by using p-Me substituted substrate. To our delight, when organic base DtBP (2,6-di-tert-butylpyridine) was added to adjusting the acidity, the yield significantly increased from 44% to 88% (entries 7 and 8). It was also surprising that, in comparison with entry 3, p-Me substituted product showed a lower tendency for photo-induced isomerization in the presence of base even in a longer time scale (entry 9), indicating that substitution effect played a crucial role in this isomerization. Change of photocatalyst would influence the yield and reaction would not take place under photocatalyst-free condition (entries 10 and 11). An obvious drop of yield was observed without copper catalyst (entry 12), indicating the importance of rate-matching in radical-polar crossover to compete with unproductive pathways.
With the optical conditions in hand, we next explored the scope and limitation of this radical perfluoro-tert-butylation (Fig. 5 and SI section 7). Previous studies on Ir-based photocatalyzed C(sp2)–H functionalization showed that E/Z-selectivity suffered from position effect and electron effect of the substitutions due to the incontrollable TTET process, and method like flow chemistry was utilized to obtain better selectivity42,44. Thus, we were motivated to search for a general solution to gain good yield and selectivity for substrates with broad structural diversity.
The reaction shows excellent functional group tolerance and is compatible in late-stage functionalization. Reactions were done in 0.2 mmol scale using standard conditions unless otherwise noted. All the yields above were isolated yields for E-isomer. a 2 equiv. CaCO3 was added as base. b 2 equiv. DtBP was added as base.
Position effect was investigated by testing various halogen substitutions (6b-6f), which all gave excellent yields from 80% to 95% and high selectivities (E:Z > 20:1). Several electron-withdrawing substitutions, including halogens (6g-6j), an ester group (6k), a sulfone group (6l, CCDC2405100), an aldehyde group (6m), a cyano group (6n), perfluoroalkoxy groups (6o and 6p) and a BPin group (6q) could be accommodated, furnishing products with satisfying results. Simple styrene (6r) and 1-/2-naphthylethylene (6s and 6t) could also gave corresponding products, however, an obvious drop of selectivity occurred in the case of 1-/2-naphthylethylene. Substrates with a tert-butyl group (6u), methyl groups (6v, 6w and 6x), a phenoxy group (6y), a methoxy group (6z) and an acetoxy group (6aa) could be transformed with reasonable results in the presence of DtBP to prevent polymerization. Internal alkynyl group (6ab) and chloromethyl group (6ac) were compatible in the system to show that the mild condition might be used in sensitive molecules.
1,2-diazole (6ad), thiazole (6ae) and pyridine (6af) were also tolerated to give yields from 38% to 70% and E:Z > 20:1. Late-stage functionalization of natural products and drug molecules modified with a styrene handle, such as febuxostat intermediate (6ag), naproxen (6ah), estrone (6ai), oxaprozin (6aj), sulbactam (6ak) and indomethacin (6al), could be transformed efficiently, thus conforming the generality of our conditions.
To further test the ability of our photochemical synthetic platform, different kinds of substrates were examined under slightly modified conditions (Fig. 6 and SI section 7). Following extensive screening of various radical reaction partners, we were pleased to find that phenylacetylene derivatives could be transformed to deliver PFtB substituted alkynes in moderate to good yields (9a–g)49, which represented a rare example for C(sp)–H fluoroalkylation via radical mechanism50. Electron enriched aromatic compounds like acetanilide, benzothiophene, benzofuran and indole derivatives were also suitable partner in the catalytic cycle (10a–f). It was also notable that these perfluoro-tert-butylation reactions were highly regioselective (e.g., 10e), indicating that both electronic and steric effect played an important role. Other common motifs, like 1,1-disubstituted alkene (with aryl substitutions, 11a, with alkyl substitutions, 11b), pyridine-derived 1,3-diene (12)51,52 and silyl enol ether (13), were also reactive substrates under our conditions. However, unactivated arenes showed low conversions under our conditions and only strong electron-donating group could slightly promote the transformation to compete with unproductive pathways of PFtB radical. (SI, section 7)
Unsaturated compounds including alkynes and electron-enriched aromatic compounds are also good reaction partners under slightly modified conditions. Reactions were done in 0.2 mmol scale using standard conditions unless otherwise noted. All the yields above were isolated yields. a 2 equiv. Na2CO3 was added as base. b 2 equiv. DtBP was added as base. c DMF as solvent.
The usefulness of RPFtB in organic synthesis was also demonstrated in other radical reactions. As shown in Fig. 7, under simply modified conditions, diverse compounds could be synthesized using RPFtB. By removing the copper catalyst, the alkyl radical intermediates could react with simple hydrogen donor (14)42 or undergo intramolecular cyclization (15a, 15b)53 to obtain difunctionalized products. Also, the hypervalent organo-copper species could undergo C–N (16) or C–O (17) reductive elimination to form cyclic products54. These findings largely extended the applicability of our platform.
Mechanism study
To gain a deeper understanding of the reaction mechanism, TREPR experiments were conducted, and the key intermediate was directly characterized using our recently developed ultrawide single sideband phase-sensitive detection (U-PSD) time-resolved electron paramagnetic resonance (TREPR) technique55. The measurements were performed with a simplified reaction system consisting of RPFtB (10 mM) and Ir[dF(CF3)ppy]2(dtbbpy)PF6 (1 mM).
The reaction was triggered via photoinduced electron transfer between Ir(III)* and RPFtB with an excitation wavelength of 450 nm. To our delight, a radical intermediate with a lifespan of 400 μs was observed (see SI, Fig. S21). A transient EPR spectrum centered at g = 2.0028 was successfully acquired by integrating the signals between 1–10 µs after the laser pulse, showing a well-resolved splitting pattern (9 aF = 1.84 mT) (Fig. 8a), which aligned well with (CF3)3C· of literature reports22. Under reaction condition with a higher concentration (50 mM) of RPFtB, the expected carbon-centered radical generated via single-electron transfer from the Ir(III)* catalyst to RPFtB, was not directly observed. This suggested that the β-scission rate was extremely fast, which nearly occurred within a time window close to the instrumental resolution limit (40 ns), indicating that the rate constant must exceed 107 s−1 (see SI in detail, Fig. S22).
After proving RPFtB’s ability to deliver (CF3)3C· under our photocatalytic condition, radical clock experiment was carried out to investigate the behavior of this electrophilic and sterically hindered radical. 18 was treated under our standard conditions (Fig. 8b) to afford cyclopropyl ring opened products 19 and 20, which indicated a free radical addition process21 without the formation of CuRf. Our initial optimization showed an obvious drop of yield under copper-free condition, which might be attributed to the incompatible rate of benzyl radical oxidation (by Ir(IV), E1/2 (M+/M) = +0.77 V)48, and unproductive pathways like radical polymerization or HAT (from the solvent) would be favored. When the benzyl radical was trapped by Cu(II) catalyst to form organocopper(III) intermediate56, β-H elimination would occur to deliver PFtB substituted olefins.
DFT calculations were done at the TPSS-D3/def2-TZVP//TPSS-D3/def2-SVP level to give insights into the selectivity of β-H elimination57. As shown in Fig. 8c, there was a 13.4 kcal/mol energy gap between two configurations of PFtB substituted organocopper species (blue lines) int 1 and int 2, which was caused by the steric repulsion. Similar energy gap for CF3 substitution was 5.6 kcal/mol, a much lower barrier for σ rotation process. The energy difference for concerted Z- and E- selective elimination was −3.3 kcal/mol (Gts2−Gint2−Gts1+Gint1) in the case of PFtB, however, −1.1 kcal/mol for CF3, indicating the higher steric repulsion in both int 2 and int 2’ would slightly enhance the Z-selective elimination. The energy provided by DFT suggested E-selective elimination was much favored due to the decisive σ rotation process and the Z-selectivity observed in our condition (Fig. 1, entry 2) was not caused by weak intramolecular interaction such as C–H···F hydrogen bond.
In order to show the details of E/Z isomerization, a mixture of 6a and 7a was treated with different conditions listed in Fig. 8d. When the olefin together with the photocatalyst was exposed to blue LEDs for 5 min, a fully conversion from 6a to 7a was detected and no loss of the olefin was observed. To prove the E-selectivity under no base additive conditions was not the result of acid-catalyzed thermo-isomerization via carbonium cation58, the mixture was treated with excess amount of HOTf (SI, Table S7). It was found that, the strong acid led to a pot of messy crude probably because of the cationic polymerization, which promoted us to find a more suitable explanation for the selectivity. We were pleased to find that, the side product MBTO played a crucial role in the isomerization. Serving as an internal base in the system, MBTO would quench the HOTf to form MBTO-HOTf, which efficiently suppressed the isomerization in a competitive SET process59. In the presence of 1.0 equiv. MBTO-HOTf and ca. 3 mol% photocatalyst, the E:Z ratio could be remained even in longer time scale (4 h, from 35:65 to 30:70) without the loss of yield. Similar effect could also be found in the presence of DtBP-HOTf, however, with much lower efficiency (5 min, from 35:65 to 18:82).
Basing on the mechanism studies, the selective radical C(sp2)–H perfluoro-tert-butylation of styrene derivatives is proposed to undergo the process described in Fig. 9. First, single electron transfer from the Ir(III)* catalyst to RPFtB can generate carbon radical intermediate I, which then undergoes fast C–O bond cleavage to release (CF3)3C· II. Radical addition of II to an unsaturated bond affords intermediate III, which can be quenched by a H-donor or an intramolecular aromatic ring. When intermediate III is trapped by Cu(II) to form organo-copper(III) intermediate IV, E-selective β-H elimination occurs to generate product V and Cu(I). Efficient triplet-triplet energy transfer of V with Ir(III)* will produce Z-product VI following an irreversible manner. However, one equivalent of MBTO-HOTf (or base-HOTf) is generated along the elimination, which efficiently suppresses the TTET process and leads to the observed E-selectivity.
Z-Selective C(sp2)–H perfluoro-tert-butylation of styrene derivatives
After fully studying the reaction mechanism, we were pleased to further test the efficiency of TTET-allowed selectivity-reversed reaction by removing the energy antagonist 8 (Fig. 10 and SI, section 8). Corresponding products, with a highly strained double bond caused by PFtB substitution, were not available by traditional methods.
We were glad to find that PFtB substituted styrene showed a complete conversion in 4 h, delivering corresponding thermally disfavored Z-isomer 7b in 70% yield. Substrates with electron-withdrawing groups were less likely to isomerize regarding the trend shown in 7b to 7e (slower rate and lower conversion). p-t-Bu and o-Me substitutions would not influence the conversion (7f and 7 g), while 2,4,6-trimethyl substrate showed much lower yield even in longer time scale (7h), probably owing to triplet energy difference caused by the weaker conjugation and the unignorable steric effect. 1-Naphthyl substrate, with expanded π system, was less effective in this transformation (7i). Substrates basing on thiazole and pyridine cores were also examined (7j and 7k), showing good to excellent conversion. Finally, oxaprozin derived substrate was tested (7l) to show orthogonality of isolated π system (an ester group and a diphenyloxazole) to this photoinduced isomerization.
Above results indicated that the Ir-catalyzed TTET process was widely feasible in our system and further highlighted the importance of our energy antagonist strategy in selectivity control, so that we could achieve this unusual tunable C(sp2)–H perfluoro-tert-butylation.
Discussion
In summary, we have developed a stable and scalable reagent for radical-type perfluoro-tert-butylation. The precursors of the reagent (hypervalent iodine(III) reagent and perfluoro-tert-butanol) are either commercially available or easy handling, making the synthesis of the reagent highly applicable. The reagent is proven to release (CF3)3C· under single electron reduction condition, and a diverse synthetic platform for perfluoro-tert-butylation of unsaturated compounds, such as alkenes, alkynes and electron enriched aromatic compounds, are built by photocatalysis. Notably, both E- and Z-selective C(sp2)–H perfluoro-tert-butylation of styrene derivatives could be achieved through a controllable TTET process, owing to the unique property of our reagent. Our work largely extends the diversity and applicability of PFtB-containing compounds and will gain broad interest in related research fields, such as hypervalent iodine chemistry, fluorine chemistry, photocatalysis and magnetic resonance imaging.
Methods
General procedure for the synthesis of 2b
To a 250 mL oven-dried flask with a magnetic stirring bar, PIFA (43.0 g, 100 mmol, 1.0 equiv.), anhydrous DCM (100 mL) were added. Then compound 1b (22.5 g, purity = 92%, ca. 100 mmol, 1.0 equiv.) was added dropwise to the mixture at room temperature. The mixture was allowed to stir at room temperature for another 2 h after the solid (PIFA) completely disappeared and the solution turned brown. 100 mL pre-cooled (−20 °C) pentane was added to the solution and the flask was cooled to −20 °C. The cooled mixture was then filtered and the white cake was quickly washed with pre-cooled pentane (3×30 mL). The cake was dried in vacuo providing compound 2b as an off-white solid (32.9 g, 73% isolated yield).
Note: The cake should be washed quickly because the brown impurity would lead to the decomposition to form black oil.
General procedure for the synthesis of 3
To a 250 mL oven-dried flask with a magnetic stirring bar, perfluoro-tert-butanol (19.6 g, 83 mmol, 1.14 equiv.), anhydrous THF (100 mL) were added under nitrogen atmosphere. Then the solution was cooled to 0 °C. Then n-BuLi (2.5 M in hexane, 31.2 mL, 78 mmol, 1.07 equiv.) was dropped into the mixture. The mixture was stirred at 0 °C for 10 min. Then compound 2b (32.9 g, 73 mmol, 1.0 equiv.) was added and the mixture was allowed to heat to 60 °C and stirred for 24 h. The solvent was removed under reduced pressure and the crude was purified by flash chromatography (petroleum ether to petroleum ether/ethyl acetate = 200/1) to provide compound 3 as a white solid (21.6 g, 80% isolated yield).
General procedure for the synthesis of 4 (RPFtB)
To a 250 mL oven-dried sealed tube with a magnetic stirring bar, compound 3 (18.5 g, 50 mmol, 1.0 equiv.), anhydrous DCM (50 mL), MeOTf (10.7 g, 65 mmol, 1.3 equiv.) were added under nitrogen atmosphere. Then the solution was heated to 80 °C and stirred for 18 h. The tube was cooled to room temperature and 30 mL pre-cooled Et2O (−20 °C) was added. The cooled mixture was then filtered and the white cake was washed with pre-cooled Et2O (3×30 mL). The cake was dried in vacuo providing compound 4 as a white solid (24.5 g, 92% isolated yield).
General procedure for the perfluoro-tert-butylation
Under an ambient atmosphere, in a 25 mL Schlenk tube equipped with a magnetic stirring bar, S.M. (starting material, 1.0 equiv.), 4 (1.2 equiv.), fac-Irppy3 (1 mol%) and CuTC (10 mol%) (and 2.0 equiv. of base if needed) were dissolved in degassed DCM (0.1 M). The tube was irradiated at blue LEDs (425 nm, 10 W), 25 °C for different time depending on the substrate. The solvent was removed under reduced pressure (15 °C to 35 °C) and the crude was purified by flash chromatography. No special work-up procedure was needed unless otherwise noted.
Data availability
Crystallographic data for compound 2a, 4 and 6l have been deposited at the Cambridge Crystallographic Data Centre under deposition number no. CCDC2405101 (compound 2a), CCDC2405099 (compound 4) and CCDC2405100 (compound 6l). Copies of the crystal data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif. All these data are available in the main text and supplementary files, or from the corresponding author upon request. Source data are provided with this paper.
References
Ni, C. & Hu, J. The unique fluorine effects in organic reactions: recent facts and insights into fluoroalkylations. Chem. Soc. Rev. 45, 5441–5454 (2016).
Qing, F.-L., Liu, X.-Y., Ma, J.-A., Shen, Q., Song, Q. & Tang, P. A fruitful decade of organofluorine chemistry: new reagents and reactions. CCS Chem 4, 2518–2549 (2022).
Purser, S., Moore, P. R., Swallow, S. & Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 37, 320–330 (2008).
Muller, K., Faeh, C. & Diederich, F. Fluorine in pharmaceuticals: looking beyond intuition. Science 317, 1881–1886 (2007).
O’Hagan, D. Understanding organofluorine chemistry. An introduction to the C−F bond. Chem. Soc. Rev. 37, 308–319 (2008).
Hansch, C., Leo, A. & Taft, R. W. A survey of Hamm substituent constants and resonance and field parameters. Chem. Rev. 91, 165–195 (1991).
Andreades, S. Fluorocarbanions. Rates of base-catalyzed hydrogen-deuterium exchange, isotope effects and acidity of monohydrofluorocarbons. J. Am. Chem. Soc. 86, 2003–2010 (1964).
Wu, T. et al. Perfluoro-tert-butanol: a cornerstone for high performance fluorine-19 magnetic resonance imaging. Chem. Commun. 57, 7743–7757 (2021).
Kiviniemi, A. & Virta, P. Characterization of RNA invasion by 19F NMR spectroscopy. J. Am. Chem. Soc. 132, 8560–8562 (2010).
Tirotta, I. et al. A superfluorinated molecular probe for highly sensitive in vivo 19F-MRI. J. Am. Chem. Soc. 136, 8524–8527 (2014).
Lagow, R. J., Shimp, L. A. & Clark. L. C., Jr. Fluorinated tetramethylpentane blood substitutes. US4187252, February 5, 1980.
Otomo. S. Fluorinated alkyl benzenes as corrosion and rust inhibitors. EP569884, November 18, 1993.
Takada, N., Abe, T. & Sekiya, A. Preparation and physicochemical properties of F-tert-butyl alkyl ethers. J. Fluor. Chem. 92, 167–171 (1998).
Michelena, O., Padro, D., Carrillo-Carrión, C., del Pino, P., Blanco, J., Arnaiz, B., Parak, W. J. & Carril, M. Novel fluorinated ligands for gold nanoparticle labelling with applications in 19F-MRI. Chem. Commun. 53, 2447–2450 (2017).
Meng, H., Wen, L., Xu, Z., Li, Y., Hao, J. & Zhao, Y. Nonafluoro-tert-butoxylation of diaryliodonium salts. Org. Lett. 21, 5206–5210 (2019).
Yagupolskii, Y. L. & Petko, K. I. N-perfluoro-tert-butylazoles. J. Fluor. Chem. 176, 9–13 (2015).
Wang, L., Feng, Z., Luo, Z., Guo, Z., Wang, J. & Yi, W. Direct synthesis of N-perfluoro-tert-butyl secondary amines from N-trifluoromethyl secondary amines. Chem. Sci. https://doi.org/10.1039/D4SC06335J (2024).
Delyagina, N. I., Pervova, E. Y. & Knunyants, I. L. Reaction of perfluoro-tert-butyl carbanion with olefins, containing an activated double bond, and with three-membered heterocycles and various halo derivatives. Russ. Chem. Bull. 21, 326–329 (1972).
Wang, Q. et al. Fluorination triggers fluoroalkylation: nucleophilic perfluoro-tert-butylation with 1,1-dibromo-2,2-bis(trifluoromethyl)ethylene (DBBF) and CsF. Angew. Chem. Int. Ed. 60, 27318–27323 (2021).
Wei, Z., Wen, L., Zhu, K., Wang, Q., Zhao, Y. & Hu, J. Regioselective aromatic perfluoro-tert-butylation using perfluoro-tert-butyl phenyl sulfone and arynes. J. Am. Chem. Soc. 144, 22281–22288 (2022).
Avila, D. V., Ingold, K. U., Lusztyk, J., Dolbier, W. R. & Pan, H. Q. Perfluoro-tert-butyl, a reactive, neutral, electrophilic carbon-centered radical par excellence. Tetrahedron 52, 12351–12356 (1996).
Lloyd, R. V. & Rogers, M. T. Electron-spin resonance study of some perfluoroalkyl radicals. J. Am. Chem. Soc. 95, 1512–1515 (1973).
Mochalina, E. P., Dyatkin, B. L., Galakhov, I. V. & Knunyant, I. L. Preparation of some compounds containing a perfluoro-tetrbutyl group. Dokl. Akad. Nauk SSSR 169, 1346–1349 (1966).
Yajima, T., Yamaguchi, K., Hirokane, R. & Nogami, E. Photoinduced radical hydroperfluoroalkylation and the synthesis of fluorinated amino acids and peptides. J. Fluor. Chem. 150, 1–7 (2013).
Dong, Z. & MacMillan, D. W. C. Metallaphotoredox-enabled deoxygenative arylation of alcohols. Nature 598, 451–456 (2021).
Sakai, H. A. & MacMillan, D. W. C. Nontraditional fragment couplings of alcohols and carboxylic acids: C(sp3)–C(sp3) cross-coupling via radical sorting. J. Am. Chem. Soc. 144, 6185–6192 (2022).
Intermaggio, N. E., Millet, A., Davis, D. L. & MacMillan, D. W. C. Deoxytrifluoromethylation of alcohols. J. Am. Chem. Soc. 144, 11961–11968 (2022).
Lin, Q., Ma, G. & Gong, H. Ni-catalyzed formal cross-electrophile coupling of alcohols with aryl halides. ACS Catal 11, 14102–14109 (2021).
Zheng, Y.-X., Gao, Y., Xiong, P. & Xu, H.-C. Unlocking HFIP for fluoroalkylation with molecular photoelectrocatalysis. Angew. Chem. Int. Ed. https://doi.org/10.1002/anie.202423241 (2025).
Dyatkin, B. L., Mochalina, E. P. & Knunyants, I. L. The acidic properties of fluorine-containing alcohols, hydroxylamines and oximes. Tetrahedron 21, 2991–2995 (1965).
Abraham, M. H., Grellier, P. L., Prior, D. V., Duce, P. P., Morris, J. J. & Taylor, P. J. Hydrogen bonding. Part 7. A scale of solute hydrogen-bond acidity based on log K values for complexation in tetrachloromethane. J. Chem. Soc., Perkin Trans. 2, 699–711 (1989).
Wu, Y., Jiang, Y., Wang, F., Wang, B. & Chen, C. Direct electrophilic and radical isoperfluoropropylation with i-C3F7-iodine(III) reagent (PFPI reagent). Commun. Chem. 6, 177 (2023).
Ge, C., Wang, B., Jiang, Y. & Chen, C. Diverse reactivity of the gem-difluorovinyl iodonium salt for direct incorporation of the difluoroethylene group into N- and O-nucleophiles. Commun. Chem. 5, 167 (2022).
Sheng, J. Y., Wang, Y., Su, X., He, R. & Chen, C. Copper-catalyzed [2+2+2] modular synthesis of multisubstituted pyridines: alkenylation of nitriles with vinyliodonium salts. Angew. Chem. Int. Ed. 56, 4824–4828 (2017).
Bielawski, M., Zhu, M. & Olofsson, B. Efficient and general one-pot synthesis of diaryliodonium triflates: optimization, scope and limitations. Adv. Synth. Catal. 349, 2610–2618 (2007).
Phipps, R. J., Grimster, N. P. & Gaunt, M. J. Cu(II)-catalyzed direct and site-selective arylation of indoles under mild conditions. J. Am. Chem. Soc. 130, 8172–8174 (2008).
Allen, A. E. & MacMillan, D. W. C. Enantioselective alpha-arylation of aldehydes via the productive merger of iodonium salts and organocatalysis. J. Am. Chem. Soc. 133, 4260–4263 (2011).
Toh, Q. Y., McNally, A., Vera, S., Erdmann, N. & Gaunt, M. J. Organocatalytic C–H bond arylation of aldehydes to bis-heteroaryl ketones. J. Am. Chem. Soc. 135, 3772–3775 (2013).
Bielawski, M., Malmgren, J., Pardo, L. M., Wikmark, Y. & Olofsson, B. One-pot synthesis and applications of N-heteroaryl iodonium salts. ChemistryOpen 3, 19–22 (2014).
Wang, G.-Z., Shang, R., Cheng, W.-M. & Fu, Y. Irradiation-induced Heck reaction of unactivated alkyl halides at room temperature. J. Am. Chem. Soc. 139, 18307–18312 (2017).
Bellotti, P., Huang, H. M., Faber, T. & Glorius, F. Photocatalytic late-stage C–H functionalization. Chem. Rev. 123, 4288–4289 (2023).
Straathof, N. J. W., Cramer, S. E., Hessel, V. & Noël, T. Practical photocatalytic trifluoromethylation and hydrotrifluoromethylation of styrenes in batch and flow. Angew. Chem. Int. Ed. 55, 15549–15553 (2016).
Honeker, R., Garza-Sanchez, R. A., Hopkinson, M. N. & Glorius, F. Visible-light-promoted trifluoromethylthiolation of styrenes by dual photoredox/halide catalysis. Chem. Eur. J. 22, 4395–4399 (2016).
Wei, X.-J., Boon, W., Hessel, V. & Noël, T. Visible-light photocatalytic decarboxylation of α,β-unsaturated carboxylic acids: facile access to stereoselective difluoromethylated styrenes in batch and flow. ACS Catal 7, 7136–7140 (2017).
Nevesely, T., Wienhold, M., Molloy, J. J. & Gilmour, R. Advances in the E→Z isomerization of alkenes using small molecule photocatalysts. Chem. Rev. 122, 2650–2694 (2022).
Yang, X.-J., Lin, J.-H. & Xiao, J.-C. BrCF2CN for photocatalytic cyanodifluoromethylation. Nat. Commun. 16, 445 (2025).
Chen, S., Zou, S. & Xi, C. Photocatalyzed 2:2 coupling of styrene and BrCF2CO2Me: a facile synthesis of bis-difluoroacetylated hexestrol derivatives. Chin. J. Org. Chem. 43, 1157–1167 (2023).
Flamigni, L., Barbieri, A., Sabatini, C., Ventura, B. & Barigelletti, F. Photochemistry and photophysics of coordination compounds: iridium. Top. Curr. Chem. 281, 143–203 (2007).
Chu, L. & Qing, F.-L. Copper-mediated aerobic oxidative trifluoromethylation of terminal alkynes with Me3SiCF3. J. Am. Chem. Soc. 132, 7262–7263 (2010).
Wu, Y., Jiang, Y., Wang, F., Wang, B. & Chen, C. Light promoted metal-free regio- and stereoselective isoperfluoropropylation of unactivated alkenes with an i-C3F7-iodine (III) reagent. Org. Chem. Front. 11, 370–379 (2024).
Cao, H., Cheng, Q. & Studer, A. Radical and ionic meta-C–H functionalization of pyridines, quinolines, and isoquinolines. Science 378, 779–785 (2022).
Haring, M., Balanna, K., Cheng, Q., Lammert, J. & Studer, A. Formal meta-C–H-fluorination of pyridines and isoquinolines through dearomatized oxazinopyridine intermediates. J. Am. Chem. Soc. 146, 30758–30763 (2024).
Melder, J. J. et al. Rudolph, M. & Hashmi, A. S. K. Easy access to functionalized indolines and tetrahydroquinolines via a photochemical cascade cyclization reaction. J. Am. Chem. Soc. 146, 14521–14527 (2024).
Coric, I., Müller, S. & List, B. Kinetic resolution of homoaldols via catalytic asymmetric transacetalization. J. Am. Chem. Soc. 132, 17370–17373 (2010).
Zhang, S., Zhou, S., Qi, J., Jiao, L. & Guo, X. Time-resolved electron paramagnetic resonance spectrometer based on ultrawide single-sideband phase-sensitive detection. Rev. Sci. Instrum. 94, 084101 (2023).
Wang, F., Wang, D., Mu, X., Chen, P. & Liu, G. Copper-catalyzed intermolecular trifluoromethylarylation of alkenes: mutual activation of arylboronic acid and CF3+ reagent. J. Am. Chem. Soc. 136, 10202–10205 (2014).
Xu, J. et al. Copper-catalyzed trifluoromethylation of terminal alkenes through allylic C–H bond activation. J. Am. Chem. Soc. 133, 15300–15303 (2011).
Hu, X.-S., He, J.-X., Zhang, Y., Zhou, J. & Yu, J.-S. Highly stereoselective positional isomerization of styrenes via acid-catalyzed carbocation mechanism. Chin. J. Chem. 39, 2227–2233 (2021).
Huang, Z., Qin, J., Hu, Y., Zhu, S. & Chu, L. Radical alkylcyanation of 1,6-enynes with isonitriles as bifunctional reagents. Org. Lett. 26, 10763–10768 (2024).
Acknowledgements
This work was supported by the National Natural Science Foundation (NNSF) of China (No. 22071129, received by C.C.). R.Z. thanks Mr. Ruiyang Gao (Fudan University) for helpful discussions.
Author information
Authors and Affiliations
Contributions
R.Z. and Y.L. performed the synthesis and DFT calculations. S.Z. performed the EPR study. R.Z. and S.Z. wrote the manuscript, Y.L., Y.W., X.C., F.W. and Y.J. discussed. X.G. and C.C. guided the project.
Corresponding authors
Ethics declarations
Competing interests
R.Z. and C.C. may benefit from a patent relevant to this work (CN119143694A). The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Chengshuo Shen and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, R., Zhou, S., Li, Y. et al. Selective radical-type perfluoro-tert-butylation of unsaturated compounds with a stable and scalable reagent. Nat Commun 16, 4458 (2025). https://doi.org/10.1038/s41467-025-59772-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-59772-5