Abstract
Calix[4]arenes display inherent chirality, with broad applications in synthetic and medicinal chemistry and in materials sciences. However, their use is hindered by their limited synthetic accessibility, primarily due to the lack of enantioselective methods for preparing chiral calix[4]arenes with an ABCC substitution pattern. Here, we address this challenge by presenting a simple, efficient, and metal-free protocol for organocatalytic desymmetrisation of prochiral diformylcalix[4]arenes. Through this highly effective and sustainable approach, we synthesize structurally unique products in gram-scale reactions. Accordingly, this method facilitates extensive post-functionalisations of the carbonyl groups, including for organocatalyst development. Furthermore, our experimental mechanistic studies demonstrate that desymmetrisation determines enantiocontrol in esterification reactions catalysed by N-heterocyclic carbenes. These findings underscore the broad potential of this method for providing versatile access to inherently chiral calix[4]arenes with an ABCC substitution pattern while offering a valuable platform for asymmetric molecular recognition and catalysis.
Similar content being viewed by others
Introduction
Chiral N-heterocyclic carbenes (NHCs)1 have recently emerged as a transformative class of nucleophilic organocatalysts2. The success of NHCs stems from their broad spectrum of activation modes, which enable stereocontrolled transformations under mild reaction conditions with excellent selectivity3,4. As such, NHCs have prompted significant advancements in synthetic chemistry, including in asymmetric synthesis5,6.
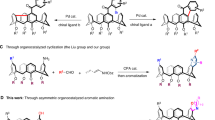
In asymmetric synthesis, NHC-mediated transformations have rapidly expanded into a major area of research thanks to the stability and accessibility of chiral carbene precursors. Case in point, NHC-catalysed desymmetrisation of prochiral or meso-compounds7,8 stands out as a highly promising platform for enantioselective transformations (Fig. 1A)9,10. More specifically, NHC-catalysed stereoselective oxidative esterification of prochiral aldehydes offers a versatile pathway to various products with central chirality, including dihydropyridines11, and non-carbon stereogenic centers12,13,14. In the last two years, though, research has increasingly focused on the development of axially chiral compounds15,16,17. In this context, carbene-catalysed desymmetric esterification has gained prominence as a key method for atroposelective synthesis, providing access to a wide range of biaryls18 and related ethers19,20,21,22,23. Concurrently, our group has developed a versatile approach to synthesizing planar chiral [2.2]paracyclophanes over NHCs24. Despite these advances25, to the best of our knowledge, NHC-mediated desymmetrisation has never been used to prepare inherently chiral products26.
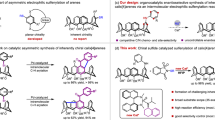
Among inherently chiral molecules, calix[4]arenes encompass the most studied class to date27. Their unique three-dimensional curved structure exemplifies a chiral macrocycle with a wide range of applications. For instance, calix[4]arenes enable chiral recognition as host-guest molecules, (bio)sensors28, drug/gene delivery systems, enzymatic assays, and smart materials29. Beyond these applications, inherently chiral calix[4]arenes have been successfully employed as catalysts and ligands30,31,32,33. However, synthesizing inherently chiral calix[4]arenes remains a major challenge34. Most synthetic approaches rely on the separation of racemic mixtures by chiral HPLC or chemical resolution through diastereomeric intermediates with chiral auxiliaries35,36. These methods are limited by their maximum yield of enantiopure products, which is only 50%.
Introducing chiral-directing groups, typically to the upper rim of the calix[4]arene, has provided some diastereocontrol during subsequent functionalisation (Fig. 1B)37,38,39,40. More recently, innovative strategies, such as metal-catalysed annulation41,42,43,44,45, Povarov reaction46,47, and sulfenylation48, have leveraged non-chiral directing groups that either interact with chiral reaction partners or generate active chiral species for enantioselective desymmetric functionalisation. But none of these methods yield calix[4]arenes derivatised at more than one phenolic unit. And while inherently chiral derivatives with two or more substituted phenolic units49 have facilitated the enantioselective preparation of inherently chiral calix[4]arenes with an ABCC substitution pattern (Fig. 1B)50, enzymatic desymmetrisation of prochiral alcohol resulted in less than 20% yield of the desired product. Therefore, no progress has been made in stereocontrolled access to calix[4]arenes with an ABCC aromatic ring substitution pattern since 199851.
In this work, we develop a method for synthesizing inherently chiral calix[4]arene derivatives through oxidative NHC catalysis (Fig. 1C). Through our highly efficient protocol using prochiral calix[4]arenes, we prepare valuable synthon products containing carbonyl groups suitable for further functionalisation and development.
Results and discussion
Optimisation of the model reaction
Building on insights from our previous study24, we selected methanol as the initial substrate for esterification of the prochiral diformylcalix[4]arene 1a. Mixing 1a with excess methanol (Table 1, entry 1), an NHC precursor derived from l-valine (pre-C1), an oxidant (Kharasch reagent, 3,3’,5,5’-tetra-tert-butyldiphenoquinone, DQ), and a base (cesium carbonate) resulted in an inseparable (by standard column chromatography) mixture of an inherently chiral monoester and an achiral diester. However, this process lacked enantiocontrol (59:41 er), as shown by chiral HPLC analysis. Despite these initial challenges, we identified the optimal alcohol substrate and found that 2-naphthol (entry 2) promoted the separation of the products 3a/4a. Through this reaction, we obtained ester 3a in 58% yield, albeit with low optical purity (52:48 er).
Based on these proof-of-concept experiments, we set out to improve the efficiency and stereochemical outcomes by varying reaction conditions for the model reaction. To this end, we tested several NHC precursors, solvents, oxidants, bases, and other parameters (for full details, please refer to the Supplementary Information (SI) file). However, none of the numerous NHC precursors tested in this study improved the stereocontrol over catalysts with enantiodiscrimination governed by steric hindrance from the chiral backbone. For instance, a camphor-derived NHC precursor (pre-C2) resulted in a product with a low enantiomeric ratio (64:36 er). Nevertheless, enantioselectivity was significantly improved when using bifunctional NHCs (pre-C3,4) combining an NHC moiety with hydrogen-bond-donating52,53 (thio)urea functionality54,55,56,57,58,59,60. In both cases (entries 4 and 5), the corresponding ester 3a was isolated in good yields and excellent optical purity (98:2 er). Upon further optimisation, the reaction became less tolerant to changes in base and solvent. For example, substituting cesium carbonate with rubidium carbonate or using DMSO or MTBE as solvents reduced the enantiocontrol and yield whilst increasing the proportion of the diester (entries 6-8). Similarly, most alternative oxidants proved unsuitable, especially MnO2 (entry 9). With MnO2, the yield was only 24%, and the optical purity was slightly reduced. However, decreasing the amount of catalyst precursor to 10 mol% (entry 10) and increasing the base loading to 200 mol% (entry 11) improved the overall yield. As a result, the product was obtained in a high yield (84%) with an excellent enantiomeric excess (97.5:2.5 er).
Reaction scope
After optimising the reaction conditions, we investigated the scope of the desymmetrisation reaction using various lower-ring-substituted diformylcalix[4]arenes and substituted 2-naphthols (Fig. 2). When performed with the opposite enantiomeric form of the chiral catalyst (ent-pre-C3), the reaction yielded 80% of the opposite enantiomeric product (ent-3a) with excellent enantiopurity (98.5:1.5 er). Subsequently, we examined the influence of alkyl-group substitution on the lower ring of diformylcalix[4]arenes, focusing on their impact on reaction rate and stereochemical outcomes (Fig. 2A). The desymmetrisation reaction was well-tolerated by various alkyl chain substitutions. For instance, the tetraethyl derivative produced the desired product 3b in 73% yield, with excellent enantiomeric purity (97:3 er). These values matched the results of the less-polar tetraoctyl derivative. Then, we explored the scope of this method using various substituted naphthols (Fig. 2B). Naphthols bearing electron-donating groups (EDGs) at positions 7 or 8 slightly shortened the reaction times (typically ~10 min for EDG-substituted naphthols, in contrast to over 30 min for those with electron-withdrawing groups (EWGs)). Other than this difference in reaction time, no significant deviations were observed in yield (56–84%) or stereochemical outcomes (96:4-99:1 er). When we faced some difficulties in separating the major product 3 (usually for methoxy-substituted derivatives) from minor components (diester 4 or starting material 1a), we directly subjected the reaction mixture to sodium borohydride reduction, thereby yielding pure alcohols 5. Both 1-naphthol and its tetrahydro derivative produced the corresponding product in high yields and reasonable stereochemical outcomes. We also revisited the desymmetrisation of 1a using methanol (Fig. 2C), noting a significant decrease in the reaction rate. Product 5 v was isolated after reduction, albeit in moderate yield (44%, two steps) and with slightly lower optical purity (85:15 er).
Despite our best efforts, though, we failed to extend this method to other nucleophiles, such as amines, ureas, and azides, with significant conversion of the starting material. So to comprehensively explore the potential of our method, we introduced various phenols as examples of typical aromatic alcohols (Fig. 3). The desymmetrisation of phenol yielded the expected product 6a with the highest level of enantiopurity among all examples discussed so far (99.5:0.5 er). Furthermore, introducing both EDGs and EWGs at the para position of phenol (Fig. 3A) generated the corresponding products 6b-i in high-to-excellent (57-86%) yields whilst maintaining exceptional enantiocontrol (94:6-99.5:0.5 er). The position of the bromine as a substituent had no significant effect on the reaction efficiency or stereochemical outcomes. Given this functional group tolerance, we tested this method for late-stage modification of structurally diverse alcohols derived from natural and bioactive molecules (Fig. 3B). These desymmetrisation reactions proceeded in good-to-high (55-86%) yields, consistently producing esters with high levels of enantiopurity, except for ortho-substituted phenols with bulky substituents. The propofol-derived product 6o was obtained in a significantly lower reaction rate (without full conversion within 24 hrs) in a virtually racemic form. Broadly speaking, low-soluble (in DCM) starting phenols slightly lengthened the reaction. For instance, the ester derived from estrone (6r) was isolated in excellent (84%) yield as a single diastereomer (20:1 dr). With ezetimibe, which contains secondary alcohol, we demonstrated the chemoselectivity of our method to phenols. With ezetimibe, we exclusively isolated ester 6s in high (86%) yield, virtually matching the yield of the O-protected derivative (5t).
Reaction mechanism
We investigated the reaction mechanism and the origin of stereocontrol by combining experimental observations with DFT calculations. In line with several literature reports61, we proposed a possible catalytic pathway (Fig. 4). Briefly, carbene I is formed by base deprotonation of the corresponding azolium salt (pre-C3). Then, the nucleophilic carbene attacks (step A) the aldehyde carbon, yielding a tetrahedral intermediate II, which is proposed to be non-covalently stabilized by hydrogen bond formation with thiourea unit of carbene. This intermediate undergoes a 1,2-C-to-O proton shift (step B), generating a Breslow intermediate (III). In the presence of an oxidant (DQ), the Breslow intermediate is irreversibly oxidized (step C) into an acyl azolium intermediate (IV). The resulting acyl azolium is electrophilic at the carbonyl carbon and thus undergoes acyl substitution (step D) with alcohol 2 (or alkoxide). The final acyl substitution regenerates the carbene back to the catalytic cycle, yielding ester 3 or 6.
Focusing more on the formation of the Breslow intermediate (III), which we proposed to be crucial for enantiodiscrimination, we performed mechanistic and computational studies (Fig. 5). At the outset, we conducted desymmetrisation reactions of diformylcalix[4]arene 1a using deuterated 2-naphthols (d1 or d8) under optimised conditions (Fig. 5A). In both cases, deuterium was not incorporated into the aldehydic group of the products, as shown by 2H NMR. Although the reversible formation of the Breslow intermediate should induce aldehyde deuteration62. On the other hand, we accept the limitations of this experiment, such as the use of a lower amount of naphthol in comparison to diformyl derivative 1. So, we also investigated the kinetic isotope effect63,64 (KIE) of the desymmetrization reaction by introducing two parallel reactions (for more information, please refer to SI) and by performing an intremolecular competition reaction (Fig. 5B) with 1a-d2 or 1a as starting materials. In both experiments, we assessed secondary kinetic isotope effects (KIE ~ 1.5), which could be caused by rehybridization during 1,2-C-to-O proton shift. This finding indicates that proton shift (step B) is most likely the product-determining step.
Supporting our experimental mechanistic study, computational analysis provided insights into the initial enantiodiscrimination step. First, density functional theory (DFT) calculations revealed that the most stable conformer of the catalyst adopts a syn-anti conformation of the thiourea unit. In line with a previously reported conformational analysis65, we corroborated our DFT calculations with ROESY NMR experiments of pre-C3. These experiments revealed distinct conformations of the precursors in non-polar (dichloromethane (DCM)) and polar (DMSO) solvents, with a significant drop in enantioselectivity when performing desymmetrization in DMSO (Table 1, entry 7), highlighting carbene conformation as one of the potential determinants of enantiocontrol. We also proposed that the nucleophilic attack of carbene (process A) was the enantiodiscrimination step, so we further elucidated this step by calculating the energy of carbene-aldehyde adducts and energies of their transition states. Briefly, computations revealed only minor energetic preference (Fig. 5C) and low energetical barriers, suggesting a reversible initial nucleophilic attack by the carbene (for more details, please refer to the Supplementary Data 1).
Subsequently, we experimentally examined the origin of stereocontrol (Fig. 5D). Conducted with a reduced amount of oxidant (50 mol%), the model reaction yielded 3a with excellent enantiocontrol (98:2 er). This result indicated that desymmetrisation determines stereocontrol, as confirmed by kinetically resolving rac-3a, which yielded enantioenriched 3a (91:9 er), albeit with a low selectivity factor (2.9). Based on these findings, not only dialdehydes but also those calix[4]arenes, emerge as excellent candidates for further elaboration in desymmetrization processes.
Synthetic utilisation of the chiral product
To evaluate the practicality of our desymmetrisation method and synthetic utility of chiral product 3a, we conducted a gram-scale reaction of 1a under optimised conditions (Fig. 6A). In this gram-scale reaction, we isolated the inherently chiral product 3a in 79% yield, with an excellent stereochemical outcome (99:1 er). Post-functionalisation increased molecular complexity through modifications of the aldehydic and ester group (Fig. 5B). As expected, Wittig olefination produced α,β-unsaturated ester 8 in high yield (86%), retaining optical purity from aldehyde 3a. Unsurprisingly, NHC-mediated oxidative esterification with excess methanol afforded diester product 9 in high yield without changing optical purity. Similar outcomes were observed in borohydride reduction, and oxidative condition also led to the expected products with high yields and preserved stereochemical integrity, but Bayer-Viliger oxidation produced formate 10 in a slightly lower yield (43%). By Pinnick oxidation, the corresponding carboxylic acid 11 was formed in 83% yield with retained optical purity. Lastly, the ester group of derivative 3a was hydrolysed under excess of lithium hydroxide, producing acid 12 in high yield. This compound was suitable for X-ray crystallographic analysis, enabling us to confirm its structure and absolute configuration.
The synthetic utility of the inherently chiral calix[4]arene synthon was further demonstrated through the development of an organocatalyst that combines the inherent chirality of calix[4]arene with a centrally chiral catalyst to enhance its properties (Fig. 6B). In this context, catalysts incorporating a centrally chiral secondary amine with calix[4]arene can promote reactions in water. It is hypothesized that a hydrophobic and a hydrophilic region are generated through the formation of hydrogen bonds between the functionalized calix[4]arene catalyst and interfacial water molecules66. To develop these catalysts, we modified a secondary amine-based chiral catalyst by introducing a carboxylic function to the upper ring of calix[4]arene (Fig. 6C). This additional carboxylic unit could facilitate aldol-type reactions, where carboxylic acids are often used as additives. As the starting chiral synthon, carboxylic acid 12 was converted by EDCI-mediated amine coupling to the desired product 13 in high yield (76%). But to avoid having to purify polar intermediates, such as free acid, we prepared 14 via NHC-mediated oxidative esterification in high yield. After deprotection, the proposed organocatalyst 15 was isolated and tested in an aldol reaction conducted in water, yielding a highly enantioenriched aldol product (18).
Additionally, we highlighted the synthetic potential of our desymmetrization method by applying this approach to chiral recognition studies. Firstly, we applied a chiral carboxylic acid 12 as a key chiral synthon in the synthesis of compound 24 (Fig. 6D), which was introduced as a chiral solvating agent for mandelic acid67. By adapting this follow-up transformation (EDCI-mediated coupling followed by Baeyer-Villiger oxidation), we obtained formate 23 in high yields in both steps (68 and 72%). In the final step, the reduction of both amide and formate groups provided versatile access to target compound 24. Additionally, we tested and confirmed aldehydes 3a and 12 as potential chiral solvating agents for amino alcohols via imine formation (for more details, please refer to the Supporting Information file).
In summary, our straightforward method for enantioselective desymmetrisation of diformylcalix[4]arenes provides versatile access to unique, inherently chiral calix[4]arenes with ABCC substitution patterns68. This operationally simple and highly effective strategy shows excellent functional group tolerance, enabling post-functionalisation of natural and bioactive compounds. Furthermore, the feasibility of gram-scale desymmetrisation, the utility of the resulting valuable synthon, and its broad synthetic applications underscore the significance of this method. Complemented by DFT calculations, comprehensive experimental mechanistic studies demonstrate that desymmetrisation determines enantiocontrol in esterification reactions catalysed by N-heterocyclic carbenes, reinforcing the potential of diformyl derivatives as valuable starting materials for further elaboration. Moving forward, ongoing research in our laboratories will focus on the synthesis of inherently chiral molecules via organocatalytic reactions and their diverse applications.
Methods
Representative procedure
The vial (4 ml) was charged with calix[4]arene 1 (0.06 mmol, 1.2 equiv.), pre-C3 (3.3 mg, 0.005 mmol, 0.1 equiv.), DQ (24.5 mg, 0.06 mmol, 1.2 equiv.), Cs2CO3 (32.6 mg, 0.10 mmol, 2.0 equiv.), and the corresponding alcohol 2 (0.05 mmol, 1.0 equiv.) and dissolved in DCM (1.0 ml) at room temperature (~20 °C). At this temperature, the reaction mixture was stirred for the indicated time. Once the alcohol 2 was no longer detected by thin-layer chromatography (TLC), the reaction mixture was directly loaded to the silica gel column chromatography, and the product was eluted by hexane/EtOAc mixtures.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and Supplementary Information file. Data supporting the findings of this manuscript are also available from the corresponding author upon request. The primary NMR data generated in this study have been deposited in the Figshare repository under accession code (https://doi.org/10.6084/m9.figshare.28105094)69. Cartesian coordinates are available as Supplementary Data 1. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number CCDC 2404192. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif, or by emailing da-ta_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
References
Hopkinson, M. M., Richter, C., Schedler, M. & Glorius, F. An overview of N-heterocyclic carbenes. Nature 510, 485–496 (2014).
Flanigan, D. M., Romanov-Michailidis, F., White, N. A. & Rovis, T. Organocatalytic reactions enabled by N-heterocyclic carbenes. Chem. Rev. 115, 9307–9387 (2015).
Liu, Y., Wang, Y., Wu, X. & Chi, Y. R. Exploring molecular complexity by N-heterocyclic carbene organocatalysis: new activation and reaction diversity. Chem. Rec. 23, e202200219 (2023).
Chen, X., Wang, H., Jin, Z. & Chi, Y. R. N-heterocyclic carbene organocatalysis: activation modes and typical reactive intermediates. Chin. J. Chem. 38, 1167–1202 (2020).
Shee, S., Ghosh, A. & Biju, A. T. Asymmetric Organocatalysis: New Strategies, Catalysts, and Opportunities, Vol. 10, 768 (Wiley, 2022).
MacMillan, D. W. C. The advent and development of organocatalysis. Nature 455, 304–308 (2008).
Nájera, C., Foubelo, F., Sansano, J. M. & Yus, M. Enantioselective desymmetrization reactions in asymmetric catalysis. Tetrahedron 106, 132629 (2022).
Borissov, A. et al. Organocatalytic enantioselective desymmetrisation. Chem. Soc. Rev. 45, 5474–5540 (2016).
Zhang, Y., Cai, H., Gan, X. & Jin, Z. N-heterocyclic carbene-catalyzed enantioselective (Dynamic) kinetic resolutions and desymmetrizations. Sci. China Chem. 67, 482–511 (2024).
Lu, S., Poh, S. B., Ong, J. Y. & Zhao, Y. N-Heterocyclic Carbenes in Organocatalysis Ch. 10, 287-308 (John Wiley & Sons, Ltd, 2019)
Di Carmine, G. et al. Enantioselective desymmetrization of 1,4-dihydropyridines by oxidative NHC catalysis. Chem. Eur. J. 25, 7469–7474 (2019).
Liu, J. et al. Carbene-catalyzed and pnictogen bond-assisted access to PIII-stereogenic compounds. Angew. Chem. Int. Ed. 63, e202404477 (2024).
Liu, H., Zhou, H., Chen, X. & Xu, J. N-heterocyclic carbene-catalyzed desymmetrization of siladials to access silicon-stereogenic organosilanes. J. Org. Chem. 87, 16127–16137 (2022).
Liu, J., Zhou, M., Deng, R., Zheng, P. & Chi, Y. R. Chalcogen bond-guided conformational isomerization enables catalytic dynamic kinetic resolution of sulfoxides. Nat. Commun. 13, 4793 (2022).
Kotwal, N. & Chauhan, P. Evolution in the asymmetric synthesis of biaryl ethers and related atropisomers. Chem. Commun. 60, 6837–6846 (2024).
Moon, J. et al. Recent advances in catalytic desymmetrization for the synthesis of axially chiral biaryls. ChemCatChem 16, e202400690 (2024).
Wang, Y. B. & Tan, B. Construction of axially chiral compounds via asymmetric organocatalysis. Acc. Chem. Res. 51, 534–547 (2018).
Wu, Y., Li, M., Sun, J., Zheng, G. & Zhang, Q. Synthesis of axially chiral aldehydes by N-heterocyclic-carbene-catalyzed desymmetrization followed by kinetic resolution. Angew. Chem. Int. Ed. 61, e202117340 (2022).
Zhou, B. A., Li, X. N., Zhang, C. L., Wang, Z. X. & Ye, S. Enantioselective synthesis of axially chiral diaryl ethers via NHC catalyzed desymmetrization and following resolution. Angew. Chem. Int. Ed. 63, e202314228 (2024).
Wu, Y. et al. Synthesis of axially chiral diaryl ethers via NHC-catalyzed atroposelective esterification. Chem. Sci. 15, 4564–4570 (2024).
Li, L. et al. Atroposelective synthesis of axially chiral diaryl ethers by N-heterocyclic-carbene-catalyzed sequentially desymmetric/kinetic resolution process. J. Org. Chem. 89, 4067–4073 (2024).
Liu, Y. et al. Carbene-catalyzed atroposelective construction of chiral diaryl ethers. J. Org. Chem. 89, 7630–7643 (2024).
Shee, S., Shree Ranganathappa, S., Gadhave, M. S., Gogoi, R. & Biju, A. T. Enantioselective synthesis of C−O axially chiral diaryl ethers by NHC-catalyzed atroposelective desymmetrization. Angew. Chem. Int. Ed. 62, e202311709 (2023).
Dočekal, V., Koucký, F., Císařová, I. & Veselý, J. Organocatalytic desymmetrization provides access to planar chiral [2.2]. Paracyclophanes. Nat. Commun. 15, 3090 (2024).
Zhang, M., Yang, X., Peng, X., Li, X. & Jin, Z. Asymmetric construction of axial and planar chirality with N-heterocyclic carbene (NHC) organocatalysis. Sci. China Chem. https://doi.org/10.1007/s11426-024-2363-4 (2024).
Tang, M. & Yang, X. Catalytic enantioselective synthesis of inherently chiral molecules: recent advances. Eur. J. Org. Chem. 26, e202300738 (2023).
Arnott, G. E. Inherently chiral calixarenes: synthesis and applications. Chem. Eur. J. 24, 1744–1754 (2018).
Nimse, S. B. & Kim, T. Biological applications of functionalized calixarenes. Chem. Soc. Rev. 42, 366–386 (2013).
Kumar, R. et al. Revisiting fluorescent calixarenes: from molecular sensors to smart materials. Chem. Rev. 119, 9657–9721 (2019).
Wang, C., Xu, L., Jia, Z. & Loh, T. P. Recent applications of macrocycles in supramolecular catalysis. Chin. Chem. Lett. 35, 109075 (2024).
Shirakawa, S. & Shimizu, S. Designed Molecular Space in Material Science and Catalysis, 51–68 (Springer, 2018).
Durmaz, M., Halay, E. & Bozkurt, S. Recent applications of chiral calixarenes in asymmetric catalysis. Beilstein J. Org. Chem. 14, 1389–1412 (2018).
Khiri, N. et al. Enantioselective hydrogenation catalysis aided by a σ-bonded calix[4]Arene to a p-chirogenic aminophosphane phosphinite rhodium complex. Organometallics 29, 3622–3631 (2010).
Li, S. Y., Xu, Y. W., Liu, J. M. & Su, C. Y. Inherently chiral calixarenes: synthesis, optical resolution, chiral recognition and asymmetric catalysis. Int. J. Mol. Sci. 12, 429–455 (2011).
Shirakawa, S., Kimura, T., Murata, S. I. & Shimizu, S. Synthesis and resolution of a multifunctional inherently chiral calix[4]arene with an abcd substitution pattern at the wide rim: the effect of a multifunctional structure in the organocatalyst on enantioselectivity in asymmetric reactions. J. Org. Chem. 74, 1288–1296 (2009).
Xu, Z. X., Zhang, C., Yang, Y., Chen, C. F. & Huang, Z. T. Effective nonenzymatic kinetic resolution of racemic m-nitro-substituted inherently chiral aminocalix[4]. Arenes Org. Lett. 10, 477–479 (2008).
Castell, D. C., Lesotho, N., Nikolayenko, V. I. & Arnott, G. E. Inherently chiral calix[4]arenes: a chiral sulfoxide as an ortholithiation director. Eur. J. Org. Chem. 2017, 4328–4333 (2017).
Herbert, S. A. & Arnott, G. E. Synthesis of inherently chiral calix[4]arenes: stereocontrol through ligand choice. Org. Lett. 12, 4600–4603 (2010).
Herbert, S. A. & Arnott, G. E. An asymmetric ortholithiation approach to inherently chiral calix[4]. Arenes Org. Lett. 11, 4986–4989 (2009).
Xu, Z. X., Zhang, C., Zheng, Q. Y., Chen, C. F. & Huang, Z. T. A new approach to enantiopure inherently chiral calix[4]arenes: determination of their absolute configurations. Org. Lett. 9, 4447–4450 (2007).
Qian, P. F. et al. Asymmetric synthesis of chiral calix[4]arenes with both inherent and axial chirality via cobalt-catalyzed enantioselective intermolecular C−H annulation. Angew. Chem. Int. Ed. 63, e202412459 (2024).
Li, T. et al. Simultaneous construction of inherent and axial chirality by cobalt-catalyzed enantioselective C-H activation of calix[4]. Arenes Nat. Commun. 15, 7673 (2024).
Giovanardi, G., Scarica, G., Pirovano, V., Secchi, A. & Cera, G. Gold(I)-catalysed hydroarylations of alkynes for the synthesis of inherently chiral calix[4]arenes. Org. Biomol. Chem. 21, 4072–4083 (2023).
Zhang, Y. Z., Xu, M. M., Si, X. G., Hou, J. L. & Cai, Q. Enantioselective synthesis of inherently chiral calix[4]arenes via palladium-catalyzed asymmetric intramolecular C-H arylations. J. Am. Chem. Soc. 144, 22858–22864 (2022).
Zhang, X., Tong, S., Zhu, J. & Wang, M. X. Inherently chiral calixarenes by a catalytic enantioselective desymmetrizing cross-dehydrogenative coupling. Chem. Sci. 14, 827–832 (2022).
Jiang, Y. K. et al. Organocatalytic enantioselective synthesis of inherently chiral calix[4]arenes. Angew. Chem. Int. Ed. 63, e202407752 (2024).
Yu, S. et al. Catalytic enantioselective synthesis of inherently chiral calix[4]arenes via sequential Povarov reaction and aromatizations. Angew. Chem. Int. Ed. 63, e202410628 (2024).
Zhang, X. Y. et al. Enantioselective synthesis of inherently chiral sulfur-containing calix[4]arenes via chiral sulfide catalyzed desymmetrizing aromatic sulfenylation. Nat. Commun. 15, 9929 (2024).
Simões, J. B., Leite da Silva, D., Fernandes, S. A. & de Fátima, Â. Calix[n]arenes in action: recent applications in organocatalysis. Eur. J. Org. Chem. 2022, e202200532 (2022).
Browne, J. K., McKervey, M. A., Pitarch, M., Russell, J. A. & Millership, J. S. Enzymatic Synthesis of nonracemic inherently chiral calix[4]arenes by lipase-catalysed transesterification. Tetrahedron Lett. 39, 1787–1790 (1998).
For the very recent review on this topic, seeQin, W. & Cera, G. Enantioselective catalytic synthesis of inherently chiral calixarenes. Chem. Rec. e202400237. https://doi.org/10.1002/tcr.202400237 (2025).
Wang, H., Chi, Y. R. & Huang, X. Enantioselective dual catalysis of N-heterocyclic carbene and hydrogen-bond donor organocatalysts. Eur. J. Org. Chem. 2022, e202200548 (2022).
Chen, X. Y., Gao, Z. H. & Ye, S. Bifunctional N-heterocyclic carbenes derived from l-pyroglutamic acid and their applications in enantioselective organocatalysis. Acc. Chem. Res. 53, 690–702 (2020).
Zhao, Y., Zhang, Y. & Huang, Y. Enantioselective relay coupling of perfluoroalkyl and vinylogous ketyl radicals. Angew. Chem. Int. Ed. 63, e202409566 (2024).
Cai, Y. et al. Amide C–N bonds activation by a new variant of bifunctional N-heterocyclic carbene. Nat. Commun. 15, 496 (2024).
Li, E., Liao, X., Guo, F., Huang, Y. & Chen, J. N-heterocyclic carbene-catalyzed asymmetric SN2 alkylation via noncovalent activation. Org. Lett. 26, 7479–7483 (2024).
Gao, Y. Y., Zhang, C. L., Jin, M. L., Gao, Z. H. & Ye, S. Bifunctional NHC-catalyzed remote enantioselective mannich-type reaction of 5-(Chloromethyl)furfural via trienolate intermediates. Angew. Chem. Int. Ed. 62, e202301126 (2023).
Li, E., Chen, J. & Huang, Y. Enantioselective seleno-michael addition reactions catalyzed by a chiral bifunctional N-heterocyclic carbene with noncovalent activation. Angew. Chem. Int. Ed. 61, e202202040 (2022).
Guo, F., Chen, J. & Huang, Y. A bifunctional N-heterocyclic carbene as a noncovalent organocatalyst for enantioselective Aza-Michael addition reactions. ACS Catal. 11, 6316–6324 (2021).
Liang, Z. Q., Gao, Z. H., Jia, W. Q. & Song, Y. Bifunctional N-heterocyclic carbene catalyzed [3+4] annulation of enals and aurones of enals and aurones. Chem. Eur. J. 21, 1868–1872 (2015).
De Risi, C. et al. Oxidative N-heterocyclic carbene catalysis. Chem. Eur. J. 29, e202202467 (2023).
Geng, H. et al. Practical synthesis of C1 deuterated aldehydes enabled by NHC catalysis. Nat. Catal. 2, 1071–1077 (2019).
Simmons, E. M. & Hartwig, J. F. On the interpretation of deuterium kinetic isotope effects in C-H bond functionalizations by transition-metal complexes. Angew. Chem. Int. Ed. 51, 3066–3072 (2012).
Wessels, A., Klussmann, M., Breugst, M., Schlörer, N. E. & Berkessel, A. Formation of Breslow Intermediates from N-heterocyclic carbenes and aldehydes involves autocatalysis by the Breslow intermediate, and a hemiacetal. Angew. Chem. Int. Ed. 61, e202117682 (2022).
Sandler, I., Larik, F. A., Mallo, N., Beves, J. E. & Ho, J. Anion binding affinity: acidity versus conformational effects. J. Org. Chem. 85, 8074–8084 (2020).
Li, Z.-Y. et al. Highly enantioselective aldol reactions catalyzed by reusable upper rim-functionalized calix[4]arene-based l-proline organocatalyst in aqueous conditions. Tetrahedron 73, 78–85 (2017).
Shirakawa, S., Moriyama, A. & Shimizu, S. Design of a novel inherently chiral calix[4]arene for chiral molecular recognition. Org. Lett. 9, 3117–3119 (2007).
Dočekal, V., Lóška, L., Císařová, I. & Veselý, J. Desymmetric esterification catalysed by bifunctional chiral N-heterocyclic carbenes provides access to inherently chiral calix[4]arenes. ChemRxiv https://doi.org/10.26434/chemrxiv-2024-6bs5m (2024).
Dočekal, V., Lóška, L., Císařová, I. & Veselý, J. Desymmetric esterification catalysed by bifunctional chiral N-heterocyclic carbenes provides access to inherently chiral calix[4]arenes. Figshare https://doi.org/10.6084/m9.figshare.28105094.
Acknowledgements
The authors gratefully acknowledge the Czech Science Foundation (24-12575S, J.V.) and Charles University Research Centre program (UNCE/24/SCI/010, V.D.) for financial support. Computational resources were provided by the e-INFRA CZ project (ID:90254), supported by the Ministry of Education, Youth and Sports of the Czech Republic. Furthermore, the authors thank Dr. Carlos V. Melo (Charles University) for editing the manuscript. The authors also thank Dr. Simona Petrželová and Filip Koucký for measuring select NMR experiments and Dr. Štícha and Dr. Urban (all of whom from Charles University) for MS and IR analysis.
Author information
Authors and Affiliations
Contributions
V.D. designed the project conceived the study, and performed the synthesis. L.L. performed the synthesis. A.K. performed DFT calculations. I.C. performed X-ray analysis. J.V. supervised the project. V.D. and J.V. wrote the manuscript. All authors have approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Alexey Salin, and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dočekal, V., Lóška, L., Kurčina, A. et al. Desymmetric esterification catalysed by bifunctional chiral N-heterocyclic carbenes provides access to inherently chiral calix[4]arenes. Nat Commun 16, 4443 (2025). https://doi.org/10.1038/s41467-025-59781-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-59781-4