Abstract
The precise control over regio- and stereoselectivity from the same substrate represents a significant challenge in organic chemistry. Herein, a switchable organocatalytic enantioselective carbosulfenylation/sulfenolactonization of cyclohexa-1,4-dienes to access the chiral bicyclo[m.n.1] ring systems, which are the critical core skeleton of many important natural products and biologically active compounds, is achieved. By simply tuning the substituent of the sulfenylating agent, a series of synthetically challenging chiral bridged bicyclo[3.3.1]nonanes and 2-oxabicyclo[3.2.1]octanes bearing three consecutive stereocenters are obtained with good yields and excellent enantioselectivities (up to 94% yield and 97% ee). Furthermore, the initial investigation of the bicyclic derivative as a chiral ligand in metal catalysis is also conducted. Our findings offer a version of switchable divergent asymmetric synthesis in which different products can be controllably generated from an identical set of substrates by simply adjusting reaction parameters.
Similar content being viewed by others
Introduction
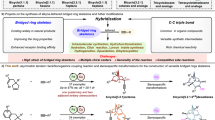
The densely substituted bicyclo[m.n.1] systems exist ubiquitously in numerous natural products and therapeutic agents with impressive biological and medicinal properties. Generally, bridged bicyclo[m.n.1] moieties possess structural diversity with multiple stereogenic centers incorporating quaternary carbon centers at the bridgehead (Fig. 1)1,2,3,4,5. For these reasons, the straightforward construction of such complex molecules remains challenging, especially in asymmetric manners6,7. In the realm of the synthesis of enantioenriched bicyclo[m.n.1] skeletons, using stoichiometric amounts of optically active substrates (chiral pool) and multistep syntheses was mainly investigated8,9,10,11,12. Until recently, several elegant catalytic asymmetric methods have been devised. Among them, strategies based on transition-metal or organo-catalyzed asymmetric Michael/aldol13,14,15, formal [m + n] annulation16,17,18,19,20, and desymmetrizing cyclizations21,22,23,24,25,26,27,28,29,30,31,32,33,34,35 were established (Fig. 2a, entries 1, 2). Despite these great advances, enantioselective synthesis of functionalized bicyclo[m.n.1] derivatives with high structural diversity from identical substrates are significantly less studied (Fig. 2a, entry 3). A formidable obstacle in this research field is the scarcity of efficiency to precise control regio- and stereoselectivity from the same substrate. For this reason, the development of strategies to explore the highly regioselective synthesis of complex molecules from identical materials is in great demand.
In particular, catalytic chemodivergent synthesis has emerged as a powerful synthetic tool because it allows access to different products from an identical set of substrates by tuning the reaction parameters (catalysts, reagents, additives, solvents, and other factors)36,37,38,39. Considering catalytic asymmetric halogenation/chalcogenation of alkenes enables the formation of valuable chiral heterocyclic backbones40,41,42, divergent cyclization strategies to surmount the regioselective bias of substrates have frequently been witnessed. For instance, Denmark43,44,45,46,47,48,49,50, Chen51,52,53,54, Zhao55,56,57,58,59 and others60,61 developed efficient approaches for divergent enantioselective cyclization using electrophilic sulfur (SAr) and trifluoromethylthiolation (SCF3) regents. The aforementioned successful examples realized divergent transformations via regioselective cyclization of the double bond with the same nucleophile, mostly controlled the divergent reactions by tuning the alkene substituents, length of the alkyl chain, and were generally limited to building monocyclic compounds. By comparison, starting materials equipped with two potential nucleophiles have the ability to create more structurally different types of products bearing more than two stereocenters, yet to the best of our knowledge, divergent enantioselective halogenation/chalcogenation of such alkenes to construct bridged bicyclo[m.n.1] system is surprisingly underexplored (Fig. 2a, entry 3). As a continuation of our ongoing effort in the catalytic enantioselective cyclization of alkenes62,63 and the powerfulness of the catalytic enantioselective desymmetrization reactions in the construction of all-carbon quaternary stereocenters64,65, we envisioned that a suitable dual-nucleophile system could be identified to deliver complex cyclic architectures with high structural diversity under readily modulated catalytic conditions. Herein, we report a switchable asymmetric regioselective sulfenocyclization of cyclohexa-1,4-dienes bearing two potential nucleophilic sites (aryl and carboxylic acid). By tuning the sulfenylating reagents, a series of functionalized bicyclo[3.3.1]nonane and 2-oxabicyclo[3.2.1]octane containing three new stereogenic centers, including an all-carbon quaternary bridgehead center, were produced in high diastereo- and enantioselectivity (Fig. 2b).
Results
Reaction optimization
To validate whether the dual nucleophile system could implement the regioselective cyclization, cyclohexa-1,4-diene derivatives 1 were selected as model substrates employing sulfenylating reagent (1.2 equiv.) and chiral catalyst (10 mol%) in hexafluoroisopropanol (HFIP) at 0 °C. Initially, different ester groups were tested in the presence of readily accessible (R)-BINAP monosulfide (BINAP(S)) C1 and sulfenylating reagent S1 (Table 1, entries 1-5). Indeed, substrates 1 bearing methyl (Me), ethyl (Et), isopropyl (i-Pr), or phenyl (Ph) ester delivered corresponding bicyclo[3.3.1]nonane derivatives in moderate to high yields and ee values (Table 1, entries 1-4). Accordingly, tert-butyl (t-Bu) ester was proved to be the most promising substrate to enhance the ee value of product 2a to 90% (Table 1, entry 5). Next, several sulfenylating reagents with different activities were screened (see the Supplementary Information for more details). Replacing regent S1 with 5,5-dimethyl-3-(phenylthio)−2,4-imidazolidinedione S2 completely shut down the transformation (Table 1, entry 6). Evaluation of N-(arylthio)-succinimides revealed the bicyclo[3.3.1]nonane 2a was obtained with improved yield and ee value by using reagent S3 (Table 1, entry 7). To our delight, N-[(4-bromophenyl)]-succinimide S4 could further increase the yield of 2a to 92% with excellent enantioselectivity (95% ee) (Table 1, entry 8). When the sulfenylating agent S5 bearing diisopropyl group was used, product 2a was barely observed, but triggered the 5-endo sulfenolactonization product 3a in 17% yield and 60% ee, thus supporting our hypothesis on the enantioselective chemodivergent synthesis (Table 1, entry 9). Because previous reports revealed Lewis base catalysis plays a vital role in electrophilic alkene functionalization, a survey of various chiral Lewis base catalysts was conducted (Table 1, entries 10-13). For example, (R)-BINAP(S) derivative C2 gave 2a in excellent yield (94%) and enantioselectivity (94% ee) (Table 1, entry 10). In comparison, (R)-BINAP disulfide derivatives C3 and C4 generally delivered 2a in slightly lower ee values (Table 1, entries 11, 12). To our surprise, the chiral BINAM-based selenophosphoramide C5 developed by the Denmark group has been widely used for enantioselective sulfenocyclization of alkenes, yet showed rather sluggish for this transformation (Table 1, entry 13). In addition, (R)-BINAP, (R)-BINAP monoxide and dioxide were totally ineffective for this reaction, suggesting that the P = S moiety of the catalyst is a critical factor for achieving high reactivity and enantioselectivity (see the Supplementary Information for more details). Then, we next turned our attention to investigating the reaction conditions of switchable access to 2-oxabicyclo[3.2.1]octane 3a (Table 1, entries 14-19). To improve the activity and site-selectivity control for the 5-endo sulfenolactonization, the tunability of the carbonyl group and sulfenylating agents were exploited. Switching the ester moiety to more reactive carboxylic acid could specifically deliver the targeted 5-endo product 3a in 37% yield and 78% ee (Table 1, entry 14). On the other hand, replacing S5 with other sulfenylating agents such as S3 and S4 dramatically affected the enantioselectivity (Table 1, entries 15-16). After carefully screening the catalysts (see the Supplementary Information for more details), we identified that (R)-DM-BINAP monosulfide C2 could promote the sulfenolactonization of the carboxylic acid substrate with a good yield (54%) in 91% ee (Table 1, entry 17). However, (R)-BINAP disulfide derivatives C3 and C4 led to inferior results in terms of enantioselectivities (Table 1, entries 18, 19). Subsequently, the stoichiometry of S5 and reaction concentration were evaluated, yielding compound 3a at 69% yield and 91% ee (Table 1, entry 20).
Substrate scope
With the optimized conditions in hand, the generality of enantioselective 6-endo carbosulfenylation was assessed. As illustrated in Fig. 3, a broad range of substituted cyclohexa-1,4-dienes were first tested to produce optically pure bicyclo[3.3.1]nonanes. The starting materials bearing electron-donating groups (Me, OMe) on the different positions of the phenyl ring delivered the desired products 2b-2f in good yields (63-93%) with high enantioselectivities (80-95% ee). Naphthyl derivatives 1g and 1h proceeded smoothly to provide the corresponding bicyclo[3.3.1]nonanes in excellent ee values (94-96%). Pleasingly, N-Boc and N-Tosyl protected substrates could be successfully converted into products 2i and 2j in 91% and 92% ee, providing additional versatility for the products.
General reaction conditions for 6-endo carbosulfenylation: 1 (0.10 mmol), C1 (0.01 mmol), S4 (0.12 mmol) in HFIP (1.0 mL) at 0 °C for 24 h. General reaction conditions for 5-endo sulfenolactonization: 1 (0.10 mmol), C2 (0.01 mmol), S5 (0.15 mmol) in HFIP (0.5 mL) at 0 °C for 48 h. Isolated yields. The ee values were determined by HPLC analysis on a chiral stationary phase. a, S4 (0.25 mmol) was used.
With multiple electron-donating substituents in the ortho, meta and para position of the phenyl ring have less effect on the yields and enantioselectivities (2k-2o, 62-90% yields, 93–97% ee). Noteworthily, the absolute configuration of 2k and 2l were unambiguously confirmed as 5 R, 9S, 11 R by X-ray single crystal diffraction analysis. In addition, weakly deactivating halogen (fluoro, chloro, bromo) substituted substrates were also applicable to furnish the anticipated compounds 2p-2r in good yields (67-82%) and ee values (72-78%). Substrates bearing heteroaromatic rings, including thiophene, furan, and indole moieties, were also reactive under these conditions, yielding exclusive products 2s–2u in high yields (79–86%) with excellent enantioselectivities (90–95%). Notably, the furan moiety underwent further sulfenylation at the α-position, affording the final product 2t with an aryl sulfide segment. Replacing the ester group with a phenyl group was also evaluated in the catalytic system, yielding the corresponding 2v in 67% yield with a significantly reduced enantioselectivity (55% ee).
a Gram-scale reactions. b Further transformation of chiral bridged bicyclo[3.3.1]nonane. Reaction conditions: a) H2O2 (1.8 equiv.), HFIP, 25 oC, 18 h, 62%, 4:1 dr, 94% ee. b) m-CPBA (6.0 equiv.), CH2Cl2, 42%, 94% ee. c) m-CPBA (2.5 equiv.), CH2Cl2, 60%, 94% ee. d) Mg, MeOH, 25 oC, 24 h, 99%, 94% ee. e) (i) n-BuLi (1.2 equiv.), Et2O (0.1 M), 0 oC; (ii) ICH2Cl (3.0 equiv.), i-PrMgCl (3.0 equiv.), THF, −78 oC, 45 min then −60 oC, 12 h. 25% for two steps, 96% ee. c Evaluation of 4’ as potential ligand in asymmetric catalysis.
Next, the feasibility of switchable divergent synthesis of 2-oxabicyclo[3.2.1]octane was further explored (Fig. 3). Notably, the structure of 3a was assigned as 1S, 5S, 8 R by X-ray crystallographic analysis. Electron-donating substituents (Me, OMe) on the phenyl ring had no significant effect on the reaction, furnishing the desired products 3b-3e in moderate to good yields (55-75%) with high enantioselectivities (84-90% ee). Multiply-substituted starting materials were also well-tolerated to deliver 3f-3h in up to 68% yield and 93% ee value. Sterically demanding 1- and 2-naphthyl derivatives reacted well to give products 3i and 3j in moderate to good yields (63% and 51%) and excellent ee values (93% and 94%). Correspondingly, the starting materials bearing halophenyl also led to 3k-3m with high enantioselectivities (85-94% ee), albeit relatively low yields were observed (39-48%). In the presence of the strong electron-withdrawing group (CF3), the reaction proceeded well with reasonable control of the enantioselectivity (3n, 86% ee). It is important to stress that the phenethyl-substituted product 3o was also isolated in 35% yield and 78% ee. To enhance the yields of certain low-yielding reactions, such as 3g, 3k, and 3o, we investigated the addition of one equivalent of acid, including MsOH, TfOH, TFA, and BF3·OEt2, as an additive. However, this strategy completely inhibited the reaction and instead resulted in the decarboxylation and aromatization of the starting materials.
Synthetic transformation of the products and synthetic utility
Based on the versatility of phenylthiol moiety, several additional transformations of chiral bridged bicyclo[3.3.1]nonane were conducted (Fig. 4). Notably, a gram-scale synthesis of 2a and 3a was achieved with decent yield while maintaining enantioselectivity (Fig. 4a). As shown in Fig. 4b, starting from 2a with 94% ee value, the phenylthiol moiety could be oxidized using hydrogen peroxide (H2O2) to deliver the sulfoxide 4 with 4:1 dr and 94% ee (confirmed by X-ray crystallographic analysis). On the other hand, oxidation of 2a in the presence of 6.0 equiv. meta-chloroperoxybenzoic acid (m-CPBA) led to epoxide 5 in 42% yield and 94% ee. Switching to 2.5 equiv. m-CPBA could selectively oxidize the phenylthiol moiety to sulfone and afforded product 6 in 60% yield. Additionally, reductive desulfonylation of 6 occurred effectively to establish bicyclo[3.3.1]nonane 7 in excellent yield and ee value. Upon the strong leaving group ability of the sulfonyl group, the sulfone 6 was transformed into the terminal olefin 8 in 96% ee after two steps. To evaluate the synthetic utility of the resulting bicyclo[3.3.1]nonane derivative, the isolated sulfoxide isomer 4’ (94% ee) was employed as an efficient chiral sulfoxide alkene ligand (Fig. 4c). From commercially available [Rh(coe)2Cl]2, the desired [(4’)RhOH]2 catalyst was prepared in situ under mild conditions. Encouragingly, the [(4’)RhOH]2-catalyzed asymmetric 1,4-addition of phenylboronic acid to cyclic enone 9 proceeded smoothly to furnish the product 10 in 46% yield and 81% ee, indicating the chiral sulfoxides could be utilized as promising ligands in metal-catalyzed asymmetric reactions66,67.
Mechanistic investigations
To gain insight into the reaction mechanism, we conducted the mechanistic studies through 31P NMR measurement of the catalytically active species generated from the reaction participants (Fig. 5)68. The original two peaks at −0.47 and +40.30 ppm of (R)-BINAP(S) belong to phosphine (III) and phosphine sulfide (V) moieties (Fig. 5a (i)). After 1.0 equiv. sulfenylating agent S4 was reacted with (R)-BINAP(S), the signal of phosphine (III) disappeared, and a new signal was observed at +46.08 ppm, which suggested that the formation of an unsymmetrical and electron-deficient phosphorus species (Fig. 5a (ii)). When the amount of S4 was increased to 6.0 equiv., the signal of the P = S (V) group vanished, and a broad, weak peak at +40.48 ppm was newly observed. We postulate that P = S (V) group act as a soft Lewis base that could interact with excess S4 to form the putative P = S+―SAr1 as active chemical species (Fig. 5a (iii)). Interestingly, when (R)-BINAP(S) was treated with excess reagent S4 and substrate 1a (Fig. 5a (iv)), the 31P NMR spectroscopic property exactly corresponds with the unsymmetrical phosphorus compound in Fig. 5a (ii). Considering the above results showed (R)-BINAP could not catalyze the reaction, while (R)-BINAP monosulfide efficiently promote the transformation, we speculate the moiety P = S+―SAr1 species in Fig. 5a (iii) serve as the actual +SAr1 transfer agent, not the P+―SAr1 group of the species in Fig. 5a (ii). To confirm the validity of the catalytically active species, an ESI-HRMS analysis of the mixture of (R)-BINAP(S) with excess reagent S4 was performed. As illustrated in Fig. 5b, the transient species in Fig. 5a (ii) was successfully captured. However, the intermediate Fig. 5a (iii) was not detected might be due to its instability in methanol.
Density Functional Theory (DFT) calculations were conducted on the transition states of electrophilic addition to further elucidate the origins of enantioselectivity (see the Supplementary Data 1). As shown in Fig. 6, the calculated free energy of transition state TS1-R is 2.4 kcal/mol higher than that of TS1-S for the cyclohexa-1,4-diene substrate bearing a tert-butyl group, which aligns with the observed high enantioselectivity. Structural analysis indicates that steric repulsion between cyclohexa-1,4-diene and (R)-BINAP(S) C1 is less pronounced in TS1-S than in TS1-R, as evidenced by the closest hydrogen-to-hydrogen (H···H) distance of 2.52 Å in TS1-S compared to 2.34 Å in TS1-R. Furthermore, Fig. 6 shows that when the ester moiety is replaced with a more reactive carboxylic acid, the calculated free energy of TS2-R is 1.6 kcal/mol lower than that of TS2-S. Structural analysis further suggests that steric repulsion between cyclohexa-1,4-diene and (R)-BINAP(S) C2 is reduced in TS2-R relative to TS2-S, with an H···H distance of 2.65 Å in TS2-R versus 2.55 Å in TS2-S. These findings indicate that steric repulsion plays a crucial role in determining the enantioselectivity of these reactions.
Based on these studies and previous reports, a possible catalytic cycle for this reaction is proposed in Fig. 7. HFIP may act as a hydrogen-bonding donor to activate sulfenylating agent S4 or S5 to react with (R)-BINAP(S) C1 or C2 to generate chiral sulfenylating complex I. The sulfenocyclization of cyclohexadienes then proceeds in a stepwise manner. The sulfenylating complex I undergoes electrophilic addition to the double bond of cyclohexa-1,4-dienes via transition states TS1-S and TS2-R, respectively, leading to the formation of chiral thiiranium ions INT1 and INT2. The electrophilic addition steps (TS1-S and TS2-R) are identified as the enantioselectivity-determining steps and are also proposed to be crucial for chemoselectivity. Subsequently, 6-endo carbosulfenylation or 5-endo sulfenolactonization selectively occurs. The switchable cyclization diverges into the corresponding chiral bicyclo[3.3.1]nonane 2a or 2-oxabicyclo[3.2.1]octane 3a, simultaneously releasing species II and regenerating complex I for the next catalytic reaction cycle.
Discussion
In summary, we have developed a switchable organocatalytic enantioselective desymmetrizing sulfenocyclization of cyclohexa-1,4-dienes. The readily accessible (R)-BINAP monosulfide derivatives were proved as reliable catalysts in this transformation, which provided access to various architecturally challenging chiral bridged bicyclic skeletons bearing three consecutive stereocenters with an all-carbon quaternary bridgehead stereocenter. By manipulating the nucleophilic component of the cyclohexa-1,4-diene substrates, the regioselective transformation was controlled by tuning the sulfenylating agents, affording bicyclo[3.3.1]nonanes or 2-oxabicyclo[3.2.1]octanes in good yields and good to excellent enantioselectivities. Further derivatizations and application as the chiral ligand of the cyclic systems lead to a series of synthetically useful and valuable molecules without loss of stereochemical integrity. Additional applications of this protocol and mechanistic details are currently under investigation by our group.
Methods
General procedure for the asymmetric carbosulfenylation
To a flame-dried vial was added (R)-BINAP(S) C1 (6.5 mg, 0.01 mmol), sulfur agent S4 (34 mg, 0.12 mmol, 1.2 equiv.) and HFIP (0.5 mL), the mixture was stirred at 0 oC for 5 min., then the solution of the ester substrate (0.10 mmol) in HFIP (0.5 mL) was added. The reaction was stirred at 0 oC for 24 h. Upon completion, the reaction mixture was diluted with CH2Cl2 (10 mL) and the volatiles were removed by rotary evaporation to afford crude product. The residue was purified by flash column chromatography (petro ether/EtOAc = 100/1) to afford the product 2.
General procedure for the asymmetric sulfenolactonization
To a flame-dried vial was added C2 (7.7 mg, 0.01 mmol), sulfur agent S5 (43.7 mg, 0.15 mmol, 1.5 equiv.) and HFIP (0.25 mL), the mixture was stirred at 0 oC for 5 min., then the solution of the carboxylic acid substrate (0.10 mmol) in HFIP (0.25 mL) was added. The reaction was stirred at 0 oC for 48 h. Upon completion, the reaction mixture was diluted with CH2Cl2 (10 mL) and the volatiles were removed by rotary evaporation to afford crude product. The residue was purified by flash column chromatography (petro ether/EtOAc = 50/1) to afford the product 3.
Data availability
The crystallographic data generated in this study have been deposited in the Cambridge Crystallographic Data Center, with the deposition numbers 2207250 (for 2k), 2207262 (for 2 l), 2207265 (for 3a), and 2207278 (for 4), respectively. The data can be obtained free of charge from the CCDC via www.ccdc.cam.ac.uk/getstructures. All Cartesian coordinates are available as separate Supplementary Data 1. All other data generated in this manuscript are available within the Article and its Supplementary Information. Data supporting the findings of this manuscript are also available from the corresponding author upon request.
References
Nicolaou, K. C. & Montagnon, T. Molecules that Changed the World (Wiley-VCH, 2008).
Presset, M., Coquerel, Y. & Rodriguez, J. Syntheses and applications of functionalized bicyclo[3.2.1]octanes: Thirteen years of progress. Chem. Rev. 113, 525–595 (2013).
Mak, J. Y. W., Pouwer, R. H. & Williams, C. M. Natural products with anti-bredt and bridgehead double bonds. Angew. Chem. Int. Ed. 53, 13664–13688 (2014).
Liu, J., Liu, X., Wu, J. & Li, C.-C. Total synthesis of natural products containing a bridgehead double bond. Chem 6, 579–615 (2020).
Gao, K., Hu, J. & Ding, H. Tetracyclic diterpenoid synthesis facilitated by ODI-cascade approaches to bicyclo[3.2.1]octane skeletons. Acc. Chem. Res. 54, 875–889 (2021).
Zhao, W. Novel syntheses of bridge-containing organic compounds. Chem. Rev. 110, 1706–1745 (2010).
Min, L., Liu, X. & Li, C.-C. Total synthesis of natural products with bridged bicyclo[m.n.1] ring systems via type II [5 + 2] cycloaddition. Acc. Chem. Res. 53, 703–718 (2020).
Nicolaou, K. C., Carenzi, G. E. A. & Jeso, V. Construction of highly functionalized medium-sized rings: Synthesis of hyperforin and perforatumone model systems. Angew. Chem. Int. Ed. 44, 3895–3899 (2005).
Mei, G., Liu, X., Qiao, C., Chen, W. & Li, C.-C. Type II intramolecular [5+2] cycloaddition: Facile synthesis of highly functionalized bridged ring systems. Angew. Chem. Int. Ed. 54, 1754–1758 (2015).
Tobal, I. E., Roncero, A. M., Garrido, N. M., Marcos, I. S. & Díez, D. Organocatalyzed synthesis of [3.2.1] bicyclooctanes. Molecules 23, 1039 (2018).
Li, L. et al. Metal-free alkene carbooxygenation following tandem intramolecular alkoxylation/Claisen rearrangement: stereocontrolled access to bridged [4.2.1] lactones. Chem. Sci. 10, 3123–3129 (2019).
Shen, X., Thach, D. Q., Ting, C. P. & Maimone, T. J. Annulative methods in the synthesis of complex meroterpene natural products. Acc. Chem. Res. 54, 583–594 (2021).
Rueping, M., Kuenkel, A., Tato, F. & Bats, J. W. Asymmetric organocatalytic domino Michael/aldol reactions: Enantioselective synthesis of chiral cycloheptanones, tetrahydrochromenones, and polyfunctionalized bicyclo [3.2.1]octanes. Angew. Chem. Int. Ed. 48, 3699–3702 (2009).
Ramachary, D. B., Anif Pasha, M. & Thirupathi, G. Organocatalytic asymmetric formal [3+2] cycloaddition as a versatile platform to access methanobenzo[7]annulenes. Angew. Chem. Int. Ed. 56, 12930–12934 (2017).
Lefranc, A., Gremaud, L. & Alexakis, A. Construction of bicyclo[3.2.1]octanes with four stereogenic centers by organocatalytic domino Michael/aldol reaction. Org. Lett. 16, 5242–5245 (2014).
Ahrendt, K. A., Borths, C. J. & MacMillan, D. W. C. New strategies for organic catalysis: The first highly enantioselective organocatalytic Diels-Alder reaction. J. Am. Chem. Soc. 122, 4243–4244 (2000).
Northrup, A. B. & MacMillan, D. W. C. The first general enantioselective catalytic Diels-Alder reaction with simple α,β-unsaturated ketones. J. Am. Chem. Soc. 124, 2458–2460 (2002).
Cao, C.-L., Sun, X.-L., Kang, Y.-B. & Tang, Y. Enantioselective formal [3+3] annulation for the direct construction of bicyclic skeletons with four stereogenic centers. Org. Lett. 9, 4151–4154 (2007).
Ishida, K., Kusama, H. & Iwasawa, N. Enantioselective preparation of 8-oxabicyclo[3.2.1]octane derivatives via asymmetric [3+2]-cycloaddition of platinum-containing carbonyl ylides with vinyl ethers. J. Am. Chem. Soc. 132, 8842–8843 (2010).
Zhou, Z. et al. Switchable regioselectivity in amine-catalysed asymmetric cycloadditions. Nat. Chem. 9, 590–594 (2017).
Gammack Yamagata, A. D. et al. Enantioselective desymmetrization of prochiral cyclohexanones by organocatalytic intramolecular Michael additions to α,β‐unsaturated esters. Angew. Chem. Int. Ed. 54, 4899–4903 (2015).
Zhu, C., Wang, D., Zhao, Y., Sun, W.-Y. & Shi, Z. Enantioselective palladium-catalyzed intramolecular α-arylative desymmetrization of 1,3-diketones. J. Am. Chem. Soc. 139, 16486–16489 (2017).
Manzano, R., Datta, S., Paton, R. S. & Dixon, D. J. Enantioselective silver and amine co‐catalyzed desymmetrizing cycloisomerization of alkyne‐linked cyclohexanones. Angew. Chem. Int. Ed. 56, 5834–5838 (2017).
Knowe, M. T., Danneman, M. W., Sun, S., Pink, M. & Johnston, J. N. Biomimetic desymmetrization of a carboxylic acid. J. Am. Chem. Soc. 140, 1998–2001 (2018).
Souillart, L. & Cramer, N. Highly enantioselective rhodium(I)-catalyzed carbonyl carboacylations initiated by C-C bond activation. Angew. Chem. Int. Ed. 53, 9640–9644 (2014).
Vicens, L., Bietti, M. & Costas, M. General access to modified α-amino acids by bioinspired stereoselective γ-C−H bond lactonization. Angew. Chem. Int. Ed. 60, 4740–4746 (2021).
Wu, H., Wang, Q. & Zhu, J. Copper-catalyzed enantioselective arylative desymmetrization of prochiral cyclopentenes with diaryliodonium salts. Angew. Chem. Int. Ed. 57, 2721–2725 (2018).
Li, S. et al. A concise access to bridged [2,2,1] bicyclic lactones with a quaternary stereocenter via stereospecific hydroformylation. Nat. Commun. 12, 5279 (2021).
Yuan, Z. et al. Palladium-catalyzed asymmetric intramolecular reductive Heck desymmetrization of cyclopentenes: access to chiral bicyclo[3.2.1]octanes. Angew. Chem. Int. Ed. 58, 2884–2888 (2019).
Yuan, Z. et al. Constructing chiral bicyclo[3.2.1]octanes via palladium-catalyzed asymmetric tandem Heck/carbonylation desymmetrization of cyclopentenes. Nat. Commun. 11, 2544 (2020).
Hou, S.-H. et al. Enantioselective type II cycloaddition of alkynes via C-C activation of cyclobutanones: Rapid and asymmetric construction of [3.3.1] bridged bicycles. J. Am. Chem. Soc. 142, 13180–13189 (2020).
Liu, R.-R. et al. Palladium/L-proline-catalyzed enantioselective α-arylative desymmetrization of cyclohexanones. J. Am. Chem. Soc. 138, 5198–5201 (2016).
Kučera, R. et al. Enantioselective total synthesis of (−)-himalensine A via a palladium and 4-hydroxyproline co-catalyzed desymmetrization of vinyl-bromide-tethered cyclohexanones. J. Am. Chem. Soc. 145, 5422–5430 (2023).
Shiomi, S. et al. A new organocatalytic desymmetrization reaction enables the enantioselective total synthesis of madangamine E. J. Am. Chem. Soc. 144, 1407–1415 (2022).
Zhou, W. et al. A bridged backbone strategy enables collective synthesis of strychnan alkaloids. Nat. Chem. 15, 1074–1082 (2023).
Lin, L. & Feng, X. Catalytic strategies for diastereodivergent synthesis. Chem. Eur. J. 23, 6464–6482 (2017).
Zhan, G., Du, W. & Chen, Y.-C. Switchable divergent asymmetric synthesis via organocatalysis. Chem. Soc. Rev. 46, 1675–1692 (2017).
Beletskaya, I. P., Nájera, C. & Yus, M. Chemodivergent reactions. Chem. Soc. Rev. 49, 7101–7166 (2020).
Beletskaya, I. P., Nájera, C. & Yus, M. Stereodivergent catalysis. Chem. Rev. 118, 5080–5200 (2018).
Denmark, S. E., Kuester, W. E. & Burk, M. T. Catalytic, asymmetric halofunctionalization of alkenes-a critical perspective. Angew. Chem. Int. Ed. 51, 10938–10953 (2012).
Matviitsuk, A., Panger, J. L. & Denmark, S. E. Catalytic, enantioselective sulfenofunctionalization of alkenes: Development and recent advances. Angew. Chem. Int. Ed. 59, 19796–19819 (2020).
Liu, S., Zhang, B.-Q., Xiao, W.-Y., Li, Y.-L. & Deng, J. Recent advances in catalytic asymmetric syntheses of functionalized heterocycles via halogenation/chalcogenation of carbon-carbon unsaturated bonds. Adv. Synth. Catal. 364, 3974–4005 (2022).
Denmark, S. E., Kornfilt, D. J. P. & Vogler, T. Catalytic asymmetric thiofunctionalization of unactivated alkenes. J. Am. Chem. Soc. 133, 15308–15311 (2011).
Denmark, S. E. & Chi, H. M. Catalytic, enantioselective, intramolecular carbosulfenylation of olefins. Mechanistic aspects: A remarkable case of negative catalysis. J. Am. Chem. Soc. 136, 3655–3663 (2014).
Denmark, S. E. & Chi, H. M. Lewis base catalyzed, enantioselective, intramolecular sulfenoamination of olefins. J. Am. Chem. Soc. 136, 8915–8918 (2014).
Denmark, S. E. & Jaunet, A. Catalytic, enantioselective, intramolecular carbosulfenylation of olefins. J. Am. Chem. Soc. 135, 6419–6422 (2013).
Denmark, S. E. & Jaunet, A. Catalytic, enantioselective, intramolecular carbosulfenylation of olefins. Preparative and stereochemical aspects. J. Org. Chem. 79, 140–171 (2014).
Denmark, S. E., Hartmann, E., Kornfilt, D. J. P. & Wang, H. Mechanistic, crystallographic, and computational studies on the catalytic, enantioselective sulfenofunctionalization of alkenes. Nat. Chem. 6, 1056–1064 (2014).
Denmark, S. E. & Chi, H. M. Catalytic, enantioselective, intramolecular sulfenoamination of alkenes with anilines. J. Org. Chem. 82, 3826–3843 (2017).
Denmark, S. E. & Kornfilt, D. J. P. Catalytic, enantioselective, intramolecular sulfenofunctionalization of alkenes with phenols. J. Org. Chem. 82, 3192–3222 (2017).
Xie, Y.-Y. et al. Lewis base/Brønsted acid co-catalyzed enantioselective sulfenylation/semipinacol rearrangement of di- and trisubstituted allylic alcohols. Angew. Chem. Int. Ed. 58, 12491–12496 (2019).
Luo, H.-Y. et al. Chiral selenide/achiral sulfonic acid co-catalyzed atroposelective sulfenylation of biaryl phenols via a desymmetrization/kinetic resolution sequence. J. Am. Chem. Soc. 144, 2943–2952 (2022).
Luo, H.-Y. et al. Lewis base/Brønsted acid co-catalyzed asymmetric thiolation of alkenes with acid-controlled divergent regioselectivity. Chem. Eur. J. 25, 15411–15418 (2019).
Luo, H. Y. et al. Lewis base-catalyzed asymmetric sulfenylation of alkenes: Construction of sulfenylated lactones and application to the formal syntheses of (-)-nicotlactone B and (-)-galbacin. Chem. Comm. 55, 9367–9370 (2019).
Luo, J., Liu, Y. & Zhao, X. Chiral selenide-catalyzed enantioselective construction of saturated trifluoromethylthiolated azaheterocycles. Org. Lett. 19, 3434–3437 (2017).
Liao, L. & Zhao, X. Indane-based chiral aryl chalcogenide catalysts: Development and applications in asymmetric electrophilic reactions. Acc. Chem. Res. 55, 2439–2453 (2022).
Jiang, Q. & Zhao, X. Chiral bifunctional chalcogenide-catalyzed enantioselective electrophilic thiofunctionalization of alkenes. Chin. J. Org. Chem. 41, 443–454 (2021).
Liang, Y. et al. Enantioselective construction of axially chiral amino sulfide vinyl arenes by chiral sulfide-catalyzed electrophilic carbothiolation of alkynes. Angew. Chem. Int. Ed. 59, 4959–4964 (2020).
Luo, J., Cao, Q., Cao, X. & Zhao, X. Selenide-catalyzed enantioselective synthesis of trifluoromethylthiolated tetrahydronaphthalenes by merging desymmetrization and trifluoromethylthiolation. Nat. Commun. 9, 527 (2018).
Li, L., Li, Z., Huang, D., Wang, H. & Shi, Y. Chiral phosphoric acid catalyzed enantioselective sulfamination of amino-alkenes. RSC Adv. 3, 4523–4525 (2013).
Kesavan, A. & Anbarasan, P. Catalytic enantioselective oxysulfenylation of o-vinylanilides. Chem. Commun. 58, 282–285 (2022).
Yu, S.-N., Li, Y.-L. & Deng, J. Enantioselective synthesis of 2-bromomethyl indolines via BINAP(S)-catalyzed bromoaminocyclization of allyl aniline. Adv. Synth. Catal. 359, 2499–2508 (2017).
Long, H.-J. et al. Asymmetric bromoaminocyclization and desymmetrization of cyclohexa-1,4-dienes through anion phase-transfer catalysis. Org. Lett. 23, 8153–8157 (2021).
Zeng, X.-P., Cao, Z.-Y., Wang, Y.-H., Zhou, F. & Zhou, J. Catalytic enantioselective desymmetrization reactions to all-carbon quaternary stereocenters. Chem. Rev. 116, 7330–7396 (2016).
Petersen, K. S. Nonenzymatic enantioselective synthesis of all-carbon quaternary centers through desymmetrization. Tetrahedron Lett. 56, 6523–6535 (2015).
Trost, B. M. & Rao, M. Development of chiral sulfoxide ligands for asymmetric catalysis. Angew. Chem. Int. Ed. 54, 5026–5043 (2015).
Otocka, S., Kwiatkowska, M., Madalińska, L. & Kiełbasiński, P. Chiral organosulfur ligands/catalysts with a stereogenic sulfur atom: Applications in asymmetric synthesis. Chem. Rev. 117, 4147–4181 (2017).
Yamashita, K. et al. Mechanistic details of asymmetric bromocyclization with BINAP monoxide: Identification of chiral proton-bridged bisphosphine oxide complex and its application to parallel kinetic resolution. J. Am. Chem. Soc. 144, 3913–3924 (2022).
Acknowledgements
This work is funded by the National Natural Science Foundation of China 21871034 (J.D.) and 22301025 (Y.L.), Chongqing Municipal Science and Technology Bureau (CSTB2022NSCQ-MSX1013, J.D.) and the Science and Technology Department of Shaanxi Province (grant no. 2021JLM-31, J.D.). We thank Mr. Xiangnan Gong (CQU) for providing X-ray crystallographic analysis.
Author information
Authors and Affiliations
Contributions
J.D. designed and directed the project. B.-Q.Z. and L.C. performed the optimization studies, substrate scope analysis and mechanistic studies. W.-Y.X. conducted some of the synthetic experiments and collect some experimental data. J.D., Y.-L.L., and Y.L. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Yan-Kai Liu, Xiaodong Xiong, and the other, anonymous, reviewer for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, BQ., Chen, L., Xiao, WY. et al. Switchable organocatalytic enantioselective sulfenocyclization of cyclohexadienes enabling chemodivergent access to chiral bicyclo[m.n.1] ring systems. Nat Commun 16, 4705 (2025). https://doi.org/10.1038/s41467-025-59918-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-59918-5