Abstract
The cloning of disease resistance genes in wheat has been disproportionately slow, tedious and costly because of the large and complex genome. Wheat gene cloning projects in the late 1990s and early 2000s were multi-year endeavors, often spanning a decade or longer. The development of genomics-assisted gene cloning tools and speed breeding have significantly accelerated gene cloning in wheat over the past years. Here, we present an optimized high-throughput disease resistance gene cloning workflow that allows to identify causal genes in less than six months. As a proof-of-principle, we clone the stem rust resistance gene Sr6, which has been a historically relevant source of resistance to confine a major stem rust outbreak in North America in the mid-20th century. Sr6 encodes a CC-BED-domain-containing nucleotide-binding and leucine-rich repeat (NLR) immune receptor. The workflow provides a basis to tackle the systematic cloning of all the genetically described disease resistance genes by the wheat community, which will facilitate knowledge-guided deployment of resistance genes in wheat breeding.
Similar content being viewed by others
Introduction
About one-fifth of the global wheat production is lost to diseases and pests1. Breeding for durable disease resistance continuous to be a major endeavor and priority of most wheat improvement programs2,3. To date, around 460 disease resistance genes have been formally designated in wheat, 68 of which have been cloned using forward genetic approaches3,4,5,6,7,8,9,10,11,12,13,14. Bread wheat (Triticum aestivum) has an exceptionally large ( ~ 15 gigabases) and repeat-rich genome, which has hampered disease resistance gene cloning through traditional map-based cloning approaches in the past. The first disease resistance genes in wheat, for example, were cloned eight years after the cloning of the first rice disease resistance gene15. To accelerate gene cloning in wheat, multiple genomics-based protocols have been developed since 2016. Some of these protocols are based on reducing the complexity of the wheat genome through chromosome sorting16,17, target enrichment18,19, or focusing on the transcribed portion of the wheat genome5,20. Other rapid gene cloning protocols made use of the latest technological developments in long-read DNA sequencing21.
Many of the above tools deploy ethyl methanesulfonate (EMS), a chemical that induces random point mutations, to identify candidate genes. While working with bread wheat can be challenging due to its genome size, bread wheat is particularly amenable to EMS treatments. Because of the polyploidy, bread wheat tolerates mutation rates that are around six times higher compared to diploid plant species22. Furthermore, the vast majority of the EMS-induced loss-of-function mutants are found to carry mutations in the corresponding resistance genes and not in downstream signaling components20,23, probably because of the genetic redundancy.
Here, we report the optimized disease resistance gene cloning workflow for increasing speed and throughput. As a proof-of-concept, we show it can clone wheat stem rust resistance gene Sr6 in 179 days, requiring only three square meters of plant growth space.
Results
An optimized workflow for rapid disease resistance gene cloning
In order to clone the majority of the described wheat disease resistance genes in the foreseeable future, we need rapid and scalable gene cloning protocols. Here, we present an optimized disease resistance gene cloning workflow that combines EMS mutagenesis, speed breeding24, and genomics-assisted gene cloning tools. This combination maximizes speed and minimizes space and costs of gene cloning projects (Fig. 1, detailed description in the methods section).
Created in BioRender. Krattinger, S. (2025) https://BioRender.com/ot3naaq.
To test this workflow, we targeted a stem rust (Puccinia graminis f. sp. tritici; Pgt) resistance gene in bread wheat. The wheat cultivar WL711 and two near isogenic introgression lines, TA5602 and TA5605, in the genetic background of WL711 were immune to Pgt isolate H3 at the four-leaf stage25,26,27 (Fig. 2a), indicating that all three near isogenic lines likely carry a common stem rust resistance gene. Molecular marker analyses indicated that WL711 carries the stem rust resistance gene Sr628, whose molecular identity remains unknown. We performed EMS mutagenesis on TA5602 and TA5605. To reduce plant growth space, EMS-treated grains were sown at high density (15 grains per 64 cm2 well), at which most plants produced a single fertile tiller. In most cases, each spike therefore represents one M2 family. Individual spikes harboring the M2 grains were harvested and placed in soil without threshing. We performed stem rust inoculation on three-week-old M2 seedlings. Loss-of-resistance mutants (i.e., mutants with increased Pgt sporulation compared to the respective wild-type) were transferred to single pots. After ten days of recovery, these putative mutants were re-inoculated to confirm susceptibility to Pgt isolate H3. At the same time, leaf tissues were harvested for RNA sequencing (RNA-Seq). Using this workflow, we screened ~ 1200 and ~ 2800 M2 families in the TA5602 and TA5605 genetic background, respectively, using three square meters of growth space (Fig. 1). In total, 98 loss-of-resistance mutants, including 36 mutants in the TA5602 background and 62 in the TA5605 background, were identified (Fig. 2b and Supplementary Figs. 1 and 2). Ten mutants in the TA5602 background were selected for RNA-Seq. In addition, we produced isoform sequencing (Iso-Seq) data for the wild-type TA5602 parent. The transcriptome data were used for MutIsoSeq analysis20 by comparing the TA5602 Iso-Seq data to the RNA-Seq data of the ten selected mutants. Only one transcript, transcript/1805, was identified that carried an EMS-type point mutation in each of the 10 sequenced mutants (Supplementary Data 1). This transcript encodes a nucleotide-binding and leucine-rich repeat (NLR) protein with an N-terminal coiled coil (CC) domain followed by a zinc-finger BED domain (BED-NLR). All detected point mutations in transcript/1805 resulted in predicted amino acid substitutions or truncation of the BED-NLR (Fig. 2c, Supplementary Data 2). We amplified and Sanger-sequenced the corresponding genic region of transcript/1805 from the remaining 88 mutants and detected missense or nonsense mutations in all but one mutant. This one mutant in the TA5605 background might harbor second-site mutations or a mutation in the regulatory region. In total, we found 104 point mutations in the BED-NLR gene among the 97 mutants. Six mutants carried two or three point mutations. The majority (95.2%) of the point mutations were typical EMS-type (G/C to A/T) mutations, while the remaining mutations were A/T to T/A transversion mutations. In total, we identified 17 non-redundant premature stop codon mutations and 75 non-redundant amino acid substitutions (Fig. 2c and Supplementary Data 2). The entire workflow from initiating the mutagenesis to the identification of the BED-NLR gene was completed in 179 days.
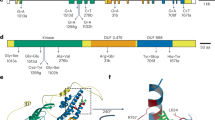
a Wheat stem rust infection phenotype of wheat lines WL711, TA5602, TA5605, Fielder, CDC Landmark, Thatcher, Avocet S and Julius. Images of leaves were taken 12 days after inoculation. Infection types were analyzed using a 0–4 scale according to the system of Stakman et al. 32: 0, no uredia; 1, small uredia; 2, small to medium-sized uredia; 3, medium-sized uredia; 4, large uredia; hypersensitive necrotic or chlorotic flecks of varying size present. Scale bar = 1 cm. The experiment was repeated independently twice with similar results. b Representative images showing the phenotype of ten loss-of-function TA5602 mutants. Images of stems were taken 12 days after inoculation. Scale bar = 1 cm. Spgt=Susceptible to Pgt. The experiment was repeated independently three times with similar results. c The gene structure of the BED-NLR. Exons are represented as rectangular bars, and the intron is shown as a black line between the exons. The predicted protein domains are indicated by different colors. Vertical bars represent 92 EMS-induced amino acid polymorphisms, upper bars = amino acid substitutions, lower bars = premature stop codon mutations. VIGS1 and VIGS2 indicate the positions of the two virus-induced gene silencing (VIGS) constructs. The sgRNA in red font indicates CRISPR/Cas9 targeting site. d Virus-induced gene silencing of BED-NLR resulted in susceptibility to Pgt isolate H3. Representative images showing the results of the VIGS experiment using barley stripe mosaic virus (BSMV). BSMV-γVIGS1 and BSMV-γVIGS2 = two silencing constructs targeting BED-NLR, BSMV-γGFP = control with a GFP silencing construct. Images were taken 12 days after inoculation. Scale bar = 1 cm. The experiment was repeated independently three times with similar results. e CRISPR/Cas9-mediated knock-out of BED-NLR in wheat cultivar Fielder resulted in susceptibility to Pgt isolate H3. The sgRNA was designed to target the coding sequence of the BED domain. Four mutant families (Mut1 – Mut4) carrying three types of deletions or insertions within the sgRNA region were susceptible to Pgt isolate H3. Images were taken 12 days after inoculation. Scale bar = 1 cm. The experiment was repeated independently twice with similar results.
BED-NLR corresponds to the wheat stem rust resistance gene Sr6
A BLAST search with the BED-NLR coding sequence (CDS) against publicly available bread wheat genomes found perfect full-length matches on the short arm of chromosome 2D in wheat cultivars Fielder (TraesFLD2D01G132300.1) and CDC Landmark (TraesLDM2D01G126500.1)21,29,30. Fielder and CDC Landmark were both resistant to Pgt isolate H3 (Fig. 2a). The stem rust resistance gene Sr6 is located on chromosome 2D. Because WL711 has been reported to carry Sr6, and the BED-NLR gene is located on chromosome 2D, we hypothesize that the identified BED-NLR gene is Sr6. Wheat cultivars Red Egyptian and McMurachy are historically relevant Sr6 donor lines. We amplified and Sanger-sequenced the full genomic region of the BED-NLR gene from WL711, Red Egyptian, McMurachy, and Selkirk (a derivative of McMurachy). All four wheat lines carry the same BED-NLR genomic sequence as TA5602 and TA5605. A Kompetitive Allele Specific PCR (KASP) marker derived from the BED-NLR showed complete linkage with disease resistance in an F2 population of 119 plants derived from a cross between TA5605 (infection type, IT = 0) and the susceptible wheat accession CItr 8860 (IT = 4)31,32. Three informative recombination events were found between the stem rust resistance in this population and the SSR marker Xcfd43 that was previously shown to be linked to Sr633 (Supplementary Fig. 3). We further validated the identity of the BED-NLR gene by virus-induced gene silencing (VIGS) and CRISPR/Cas9-mediated gene editing. Silencing of the BED-NLR in TA5605, Red Egyptian and McMurachy increased susceptibility to Pgt isolate H3 (Fig. 2d and Supplementary Figs. 4 and 5). Knock-out of the BED-NLR-encoding gene in wheat cultivar Fielder also resulted in susceptibility to the same Pgt isolate (Fig. 2e and Supplementary Fig. 6). Sr6-mediated resistance was reported to be temperature-sensitive34,35. We confirmed that the resistance to Pgt isolate H3 in TA5605, Red Egyptian, McMurachy, and Selkirk was highly effective at 20 °C, but ineffective or significantly compromised at 26 °C (Fig. 3). All four wheat lines were susceptible to the Sr6-virulent Pgt isolate PTKST36 at 20 °C (Fig. 3). Together, these data demonstrate that the cloned BED-NLR gene from TA5602/TA5605 is Sr6.
TA5605 together with Sr6-positive, Sr26-positive, and Sr62-positive bread wheat lines and Thatcher were inoculated with Pgt isolate H3 and Ug99 isolate PTKST at different temperature conditions. Images were taken 9–12 days after inoculation, infection type and disease severity were analyzed using a 0–4 scale according to the system of Stakman et al. 32. Scale bar = 1 cm. The experiment was repeated independently twice with similar results.
Discussion
Here, we present an optimized workflow for the rapid identification of disease resistance genes. The main goal was to optimize speed and to minimize plant growth space. Time and required plant growth space are two of the most important factors that have limited the scalability of disease resistance gene cloning. EMS mutagenesis is a straightforward approach to cloning disease resistance genes in wheat. The genomic redundancy of hexaploid bread wheat has greatly facilitated EMS-mutagenesis-based gene cloning in comparison to diploid species. In bread wheat, EMS-mutagenesis results in an induced point mutation every ~ 34 kb on average22. It has been shown that EMS populations of ~ 1000 M2 families are sufficient to identify > 10 loss-of-function mutants, which is sufficient for most genomics-based gene cloning protocols17,18,20. EMS-based forward genetic screens for disease resistance genes are suitable for pathogens that allow the host plant to develop fertile tillers and seeds.
In our optimized workflow, we used compact planting during the generation and screening of EMS populations. Such compact planting has several advantages: First, the growth space required to produce and screen 1000 M2 plants is ~ 0.7 square meters. Second, compact planting results in most wheat plants producing a single fertile tiller, which means that each spike represents one independent M2 family. This approach reduces the necessity to keep track of individual mutant families. In the case of Sr6, we bulk-harvested all M1 spikes. Despite not keeping track of individual mutant families, the 98 identified loss-of-function mutants carried 93 non-redundant mutations.
The Sr6 protein contains an N-terminal CC domain and a zinc-finger BED domain, followed by the canonical NB-ARC and leucine-rich repeat (LRR) domains. Sr6 is a paralog of the wheat stripe rust resistance genes Yr5, Yr7, YrSP, and Pm6Sl, and an ortholog of the barley leaf rust resistance gene Rph15. Xa1 from rice is a homolog of Sr613,37,38,39. All of these genes encode BED-NLRs that share a common domain structure but confer resistance to different pathogens.
Sr6 is a historically relevant source of stem rust resistance. In 1950, the stem rust race 15B suddenly spread across North America, causing a severe stem rust epidemic. Although race 15B has been identified for decades prior to 1950, its occurrence was rare, and its sudden spread in 1950 might have been favored by a particularly aggressive variant of race 15B combined with favorable weather conditions. Most bread wheat varieties growing in Canada and the United States at that time were susceptible to race 15B. Race 15B thus posed a serious threat to North American wheat production. Wheat cultivar McMurachy became one of the sources of resistance to race 15B. McMurachy was derived from a single stem rust-free wheat plant that was discovered in a heavily infected wheat field in Manitoba and was named after the farmer who found it ref. 40. Subsequent genetic studies revealed that McMurachy and other sources of resistance to race 15B, including wheat lines Red Egyptian and Kenya 58, all carried the same resistance gene, which was designated Sr641,42.
History repeated itself. In the early 21st century, severe stem rust epidemics caused by Ug99 resulted in great wheat yield losses across East Africa, threatening global wheat production. The outbreak of Ug99 was mainly caused by Pgt races that overcame the widely deployed Sr31 stem rust resistance gene43. An atlas of cloned rust resistance genes3 comprising the majority of the described wheat rust resistance genes would provide a powerful resource to implement knowledge-guided approaches in wheat disease resistance breeding, which will limit the risk of sudden rust outbreaks such as the ones caused by 15B and Ug99.
Methods
Plant materials
Bread wheat accessions WL711, TA5602, and TA5605 were used for the identification of the Sr6 gene. TA5602 and TA5605 are backcross lines (BC3) in the genetic background of WL711. TA5602 is a wheat-Aegilops geniculata translocation line (BC3F6), carrying a small terminal segment from Ae. geniculate chromosome 5 Mg translocated to chromosome 5D of wheat. TA5602 carries the stripe rust resistance gene Yr40 and the leaf rust resistance gene Lr5726. TA5605 (BC3F11) carries the leaf rust resistance gene Lr58 (= Lr9) introgressed from Aegilops umbellulata20,25. Bread wheat lines WL711, TA5602, and TA5605 were obtained from the Wheat Genetics Resource Center (WGRC, https://www.k-state.edu/wgrc/genetic_resources/), CDC Landmark and Julius were obtained from the UK Germplasm Resources Unit (https://www.seedstor.ac.uk/), Red Egyptian and McMurachy were received from the International Maize and Wheat Improvement Center (CIMMYT, https://www.cimmyt.org/work/genetic-resources/). TA5605 was crossed to wheat accession CItr 8860 to derive an F2 mapping population.
Stem rust inoculations
Pgt isolates H327 and PTKST36 were used for inoculations. Isolates were propagated on stem rust seedlings of the wheat line ThatcherLr9. ThatcherLr9 is resistant to the leaf rust isolates commonly used in our lab and is thus used to prevent leaf rust contamination in our Pgt isolates. Freshly harvested urediniospores kept in a desiccator at 4 °C for no more than one month were used for inoculation experiments as needed. For the spray inoculation, urediniospores were suspended in FC-43 oil (3 M Fluorinert FC-43) or Isopar L (MAGICFX, MFX3086) and sprayed onto plants using a glass sprayer connected to a high-pressure air pipe. Inoculated plants were placed in an inoculation box equipped with a humidifier overnight and transferred to a growth room or a chamber with different temperature condonations as needed. Infected plants were evaluated, and leaves were scanned using an Epson Perfection V600 Photo scanner 9 to 12 days after inoculation.
Optimized causative gene identification workflow for wheat
The workflow consists of four steps (Fig. 1): mutagenesis (3 days; Day 1-3), generation of M2 population (87 days; Day 4-90), mutant screening (52 days; Day 91-142), and RNA sequencing and data analysis (37 days; Day 143–179).
Day 1: Around 1500 grains of wheat accession TA5602 and ~ 3000 grains of accession TA5605 were soaked in three one-liter flasks (1500 grains per flask) with 200 mL water at 4 °C for 16 h.
Day 2: Water was removed from flasks, and grains were washed three times using ~ 800 mL distilled water each. The flasks were then placed upside down on blotting papers to drain off water. Then, 200 mL of 0.6% EMS solution (Sigma-Aldrich, M0880) was added to each of the one-liter flasks, followed by an incubation on a shaker at 80 rpm at room temperature for 16 h. After incubation, the EMS solution was removed, and grains were washed with 500 mL distilled water per flask on a shaker at 80 rpm. Washing was performed three times for 45 min each. Grains were then transferred into mesh bags and washed under running tap water for 30 min. After the washing, grains were transferred into 55 × 28 cm black plastic trays with blotting paper at the bottom, which were later covered with plastic lids and kept at 4 °C for 36 h.
Day 3: 18-well trays (length: 8 cm, width: 8 cm, height: 8.5 cm per well) were filled with Stender soil. Each tray was moisturized with 3 liters of water containing 3 g of PLANTIFOL 20-20-20 fertilizer. Tweezers were used to press the treated grains about 1 cm deep into the soil of the 18-well trays, 15 grains per well.
During EMS-mutagenesis, a dose-response curve experiment testing different EMS concentrations is usually performed to determine the optimal EMS concentration that results in 50% normal plant growth. We have optimized and standardized EMS-mutagenesis and growing procedures, which resulted in a minimum 85% survival rate with a good mutation frequency. First, the germination rate of treated grains is critical for mutation frequency, as germinated grains have active growing meristems where heritable mutations are introduced. The grains used for EMS-treatment were harvested and dried in a 30 °C oven for one week, and subsequently stored in a seed storage cabinet (10 °C, 30% humidity) for up to one year. Before EMS treatment, we tested the germination activity by placing around 20 grains on a Petri dish with water-soaked blotting paper. The grains started to germinate after 24 h. We found that EMS treatment reduces the germination vigor of grains. During sowing, we thus just pressed the grains 1 cm deep into the soil and left them uncovered.
Day 4–82: The 18-well trays containing treated grains were placed in a greenhouse under speed-breeding conditions (22/2 h day/night, 21/18 °C), and M1 plants were grown without additional fertilizer until the spikes were dry.
Day 83–90: Individual spikes were harvested and put into big paper envelopes without threshing. Spikes were dried at 30 °C for 7 days to break dormancy. Under the dense planting condition, most of the M1 plants only produce a single spike, which means each harvested spike represents one M2 family.
Day 91–121: 18-well trays (length: 8 cm, width: 8 cm, height: 8.5 cm per well) were filled with Stender soil until 80% of the total volume. Two to four spikes were added to each well (depending on the spike size and grain number per spike) and covered with soil. Three liters of water containing 3 g of 20-20-20 fertilizer were added to each tray. After three weeks growing in speed-breeding conditions (22/2 h day/night, 21/18 °C), M2 plants were inoculated with 30 mg Pgt urediniospores per tray as described above.
Day 122–142: Putative loss-of-function M2 mutants were selected and transferred to round pots (D: 10 cm, H: 9 cm). Only one plant per M1 spike was transferred. To exclude seed contamination, we performed TA5602/TA5605-specific PCR amplifications. About 100 mg of leaf tissue was harvested from each mutant, then total DNA was extracted using the Maxwell RSC Plant DNA Kit (Promega, AS1490). PCR amplifications were performed in 25 μL reactions containing 1× GoTaq Green Master Mix (Promega, M7122), ∼ 100 ng genomic DNA, and 100 nM of the TA5602-specific primer pair 5Mg_9.4Mb-F/R44 (Supplementary Data 3). For TA5605, we used the Lr9-specific marker SCS55045 (Supplementary Data 3). Plants were led to recover for 10 days, then inoculated again as described above to validate their phenotypes. At the same time, 60-100 mg leaf tissue was harvested from each mutant and wild-type four days after inoculation. Total RNA samples were extracted from ten validated loss-of-function mutants and wild-type using the Maxwell RSC Plant RNA Kit (Promega, AS1500).
Day 143–179: RNA samples from ten loss-of-function mutants and TA5602 were sent for Illumina sequencing and PacBio isoform sequencing (Iso-Seq), respectively. Sequencing was performed as a service at Novogene. The TA5602 RNA sample was used for Iso-Seq. The Iso-Seq library was prepared using the SMRTbell Express Template Prep Kit 2.0 (Pacific Biosciences, 100-938-900) following the Iso-Seq protocol. One SMRT Cell 8 M (Pacific Biosciences, 101-389-001) was sequenced on a PacBio Sequel II system, which produced 11.4 Gb of HiFi reads. The RNA samples of the ten loss-of-function mutants were used for Illumina sequencing. The RNA samples were enriched using oligo(dT) beads and then used to prepare strand-specific cDNA libraries. Sequencing was done on an Illumina Novaseq 6000 system, which produced an average of 55 Gb paired-end reads (2 × 150 bp) for each sample. The MutIsoSeq pipeline20 was used to analyze data.
Sanger sequencing of BED-NLR from EMS mutants and Sr6-positive wheat lines
A BLAST search with the BED-NLR coding sequence (CDS) against the genome sequence of bread wheat cultivars Fielder found a match on chromosome arm 2DS30. The corresponding genomic sequence chr2D_Fielder: 67411603-67418585 was downloaded and aligned to the BED-NLR CDS using MUSCLE Alignment of Geneious Prime (Version 2020.2.4). Primers were designed based on the genomic sequence of Fielder. PCR amplifications were performed in 25 μL reactions containing 1 × GoTaq Green Master Mix (Promega, M7122), ∼ 100 ng genomic DNA, and 100 nM primers. Nested PCR46 primer pairs FL-F1/FL-R1 (for first round PCR) and FL-F2/FL-R2 (for second round PCR) were used to amplify a 5097-bp fragment from the EMS mutants and Sr6-positive wheat lines. The PCR products were subsequently Sanger-sequenced using primers FL-F2/F3/F4/F5/FL-R2/R3/R4 (Supplementary Data 3). Sanger sequencing files were aligned to the BED-NLR CDS and the Fielder genomic sequence to find polymorphisms using MUSCLE Alignment of Geneious Prime (Version 2020.2.4). Primer sequences are provided in Supplementary Data 3.
Virus-induced gene silencing
To develop specific virus-induced gene silencing (VIGS) probes targeting the BED-NLR, we first performed a BLAST search with the BED-NLR CDS against publicly available bread wheat genome assemblies21,29,30. BED-NLR sequence stretches with little homology outside the BED-NLR were selected as targets (Fig. 2c). Primers flanked by XbaI or ApaI were designed to amplify the two targets from the DNA of TA5605, which resulted in a 229-bp (VIGS1) and 244-bp (VIGS2) fragment (Supplementary Data 3). Fragments were cloned into the BSMV-γ (BSMV, barley stripe mosaic virus) vector by XbaI or ApaI digestion and ligation, resulting in constructs BSMV-γVIGS1 and BSMV-γVIGS2. These constructs, together with BSMV-γGFP21, BSMV-α, and BSMV-β, were transformed into Agrobacterium tumefaciens strain GV310147. Agrobacteria were cultured overnight at 28 °C in lysogeny broth with appropriate antibiotics. Cells were collected by centrifugation at 3,500 g for 10 min, then re-suspended using infiltration buffer (10 mM MgCl2, 10 mM MES pH 6.5 with KOH buffer and 150 mM Acetosyringone) and adjusted to OD600 = 0.7. Equal volumes of BSMV-α and BSMV-β were mixed with BSMV-γVIGS1, BSMV-γVIGS2, or BSMV-γGFP, respectively. After 3 h incubation at 28 °C, cultures were infiltrated into Nicotiana benthamiana leaves. Infiltrated leaves were harvested 5 days after infiltration and homogenized with PBS buffer (Gibco, 10010023) containing 1% celite (Thermo Fisher Scientific, 68855-54-9). The buffer containing viral particles was rub inoculated on seedlings of TA5605, Red Egyptian, and McMurachy at the two-leaf stage, maintained in a growth room at 16/8 h day/night at 20 °C. Twelve seedlings were used for each of the constructs. After two weeks, when viral symptoms were clearly visible, plants were spray-inoculated with Pgt isolate H3 as described above and kept in the same growth room (16/8 h day/night, 20 °C). The infected leaves were sampled at 48 hpi. Total mRNA was extracted and transcribed to cDNA using TransScript® Uni One-Step gDNA Removal and cDNA Synthesis SuperMix (Transgen, AU311). RT–qPCR was performed on a CFX96 Touch Deep Well Real-Time PCR Detection System (BioRad) using SYBR® Premix Ex TaqTM II (TaKaRa, RR820A). The gene-specific primers Sr6-VIGS-F/R were used to quantify the expression levels of the BED-NLR, and Actin-F/R was used as the endogenous control (Supplementary Data 3). The 2−ΔΔCT method was used to normalize and calibrate transcript values relative to the endogenous β-Actin expression level. To quantify the stem rust biomass from BSMV-infected leaf tissues, leaf samples were collected at 9 dpi. DNA was extracted using a CTAB DNA extraction method. DNA concentrations were measured on a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific). Then, DNA was diluted to 20 ng/µL and later used as a template for qPCR. The qPCR reactions were performed by using the rust-specific primer pair ITS1rustF10d/ITS1rustR3c48 (Supplementary Data 3) and the wheat-specific primer pair Actin-F/R on a CFX96 Touch Deep Well RealTime PCR Detection System (BioRad). The 2−ΔΔCT method was used to normalize and calibrate stem rust gene amplification values relative to the wheat endogenous Actin control48. Data represent the mean ± standard deviation (n = 3 independent biological replicates). Differences were assessed using one-way ANOVA. Infected plants were evaluated, and leaves were scanned using an Epson Perfection V600 Photo scanner 12 days after inoculation.
CRISPR/Cas9-mediated knock-out of BED-NLR
To develop a specific single-guide RNA (sgRNA) targeting the BED-NLR, we first performed a BLAST search with the BED-NLR CDS against the assembly of wheat cultivar Fielder. The BED-NLR-specific DNA segment ‘ACATAAAAGATGGTAATGGG’ was selected to target the BED domain. Primer pair Sr6-CRSPR-F/R (Supplementary Data 3) containing the sgRNA sequence was first annealed and then ligated to the AarI (Thermo Fisher Scientific, ER1581) digested plasmid JD633 using T4 DNA ligase (NEB, M0202S). The resulting construct was used for Agrobacterium tumefaciens-mediated transformation of wheat cultivar Fielder49. The transgenic T0 plants were grown in a greenhouse under speed-breeding conditions (22/2 h day/night, 21/18 °C) to set T1 grains. Twelve T1 grains from four independent T0 plants, along with six grains from each control line-Fielder, TA5605, and Mugami-were sown and grown in a greenhouse under speed-breeding conditions (22/2 h day/night, 21/18 °C) until the five-leaf stage. Then, plants were inoculated with 30 mg urediniospores of Pgt isolate H3 per tray as described above. Infected plants were evaluated, and leaves were scanned using an Epson Perfection V600 Photo scanner 12 dai. Genomic DNA of T1 plants was extracted using a CTAB DNA extraction method. Primer pairs Sr6-extron3-F/R (Supplementary Data 3) were designed to amplify a 473 bp DNA segment containing the sgRNA target region. Amplicons were Sanger sequenced using primer pairs Sr6-extron3-F/R, and sequence reads were aligned to the BED-NLR CDS to identify CRISPR/Cas9-midiated edits. For Mut2, we found Sanger sequencing data from all susceptible plants to have heterozygous double peaks from the sgRNA target region. To confirm the BED-NLR edits from susceptible Mut2 plants, PCR amplicons were cloned into the pGEM®-T Easy vector (Promega, A1360) and subsequently transformed into E. coli strain DH5a. Then, ten single colonies were selected for PCR amplification and Sanger sequencing using primer pair M13-F/M13-R (Supplementary Data 3). Five colonies carried a 1 bp insertion and the remaining five colonies a 5 bp deletion, confirming that Mut2 carries two independent edits.
Generating, phenotyping and genotyping of an F2 population for Sr6/BED-NLR
TA5605 was crossed with stem rust wheat accession CItr 8860 (https://npgsweb.ars-grin.gov/gringlobal/search) to develop a segregating F2 population for Sr6/BED-NLR. Grains of the F2 population, together with the parental lines, were sown in 18-well trays filled with Stender soil (four grains per well). Each tray was given 3 liters of water containing 3 g of PLANTIFOL 20-20-20 fertilizer. Seedlings at the four-leaf stage were inoculated with Pgt isolate H3 as described above. DNA was extracted from seedlings using the Maxwell RSC Plant DNA Kit (Promega, AS1490). A 10 μL PCR reaction containing 5 μL of PowerUp SYBR Green Master Mix (Thermo Fisher Scientific, A25742), ∼50 ng genomic DNA, and 50 nM primers (Xcfd43-F/Xcfd43-R, Supplementary Data 3) was used to amplify and measure melting curves of SSR marker Xcfd43 on a QuantStudio 6 Flex Real-Time PCR machine as follows: hold stage at 95 °C for 10 min; PCR stage, 95 °C for 15 s, 57 °C for 20 s, 72 °C for 25 s, 40 cycles in total; melt curve stage, 95 °C for 25 s, 55 °C for 30 s, increase the temperature to 95 °C at a speed of 0.05 °C/s, fluorescence was measured during the temperature increase. To design diagnostic KASP primers for Sr6, we performed a BLAST search with the BED-NLR CDS against publicly available bread wheat genome assemblies21,29,30. Different alleles of Sr6 were aligned to the BED-NLR CDS using MUSCLE Alignment of Geneious Prime (Version 2020.2.4). KASP primers were designed to specifically amplify the Sr6 allele from TA5602. A 5 μL reaction containing 2.5 μL of KASP Master Mix (Low ROX KBS-1016-016), 0.07 μL of assay mix (Sr6-KASP-F/Sr6-KASP-R1/KASP-R2; Supplementary Data 3), and 2.5 μL (50 ng) of DNA was used for the KASP assay. PCR cycling was performed using an ABI QuantStudio 6 Flex Real-Time PCR machine as follows: pre-read at 30 °C for 60 s; hold stage at 94 °C for 15 min, followed by ten touchdown cycles (94 °C for 20 s; touchdown at 61 °C, decreasing by 0.6 °C per cycle for 60 s), followed by 29 additional cycles (94 °C for 20 s; 55 °C for 60 s). Plates were read at 30 °C for endpoint fluorescent measurement.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
PacBio HiFi reads and Illumina RNA-seq data of TA5602 and mutants were deposited in the European Nucleotide Archive (ENA) under accession PRJEB67960. The Sr6 genomic and coding sequences were deposited in NCBI GenBank under accession PP861160. Source data are provided in this paper.
References
Savary, S. et al. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 3, 430–439 (2019).
Wulff, B. B. H. & Krattinger, S. G. The long road to engineering durable disease resistance in wheat. Curr. Opin. Biotechnol. 73, 270–275 (2022).
Hafeez, A. N. et al. Creation and judicious application of a wheat resistance gene atlas. Mol. Plant 14, 1053–1070 (2021).
Yu, G. et al. The wheat stem rust resistance gene Sr43 encodes an unusual protein kinase. Nat. Genet. 55, 921–926 (2023).
Ni, F. et al. Sequencing trait-associated mutations to clone wheat rust-resistance gene YrNAM. Nat. Commun. 14, 4353 (2023).
Dibley, K., Jost, M., McIntosh, R., Lagudah, E. & Zhang, P. The wheat stripe rust resistance gene YrNAM is Yr10. Nat. Commun. 15, 3291 (2024).
Li, H. et al. Cloning of the wheat leaf rust resistance gene Lr47 introgressed from Aegilops speltoides. Nat. Commun. 14, 6072 (2023).
Lu, C. et al. Wheat Pm55 alleles exhibit distinct interactions with an inhibitor to cause different powdery mildew resistance. Nat. Commun. 15, 503 (2024).
Li, H. et al. Wheat powdery mildew resistance gene Pm13 encodes a mixed lineage kinase domain-like protein. Nat. Commun. 15, 2449 (2024).
He, H. et al. A kinase fusion protein from Aegilops longissima confers resistance to wheat powdery mildew. Nat. Commun. 15, 6512 (2024).
Li, M. et al. A membrane associated tandem kinase from wild emmer wheat confers broad-spectrum resistance to powdery mildew. Nat. Commun. 15, 3124 (2024).
Zhao, Y. et al. Pm57 from Aegilops searsii encodes a tandem kinase protein and confers wheat powdery mildew resistance. Nat. Commun. 15, 4796 (2024).
Ma, C. et al. An Aegilops longissima NLR protein with integrated CC-BED module mediates resistance to wheat powdery mildew. Nat. Commun. 15, 8281 (2024).
Sharma, D. et al. A single NLR gene confers resistance to leaf and stripe rust in wheat. Nat. Commun. 15, 9925 (2024).
Keller, B., Wicker, T. & Krattinger, S. G. Advances in wheat and pathogen genomics: implications for disease control. Annu Rev. Phytopathol. 56, 67–87 (2018).
Thind, A. K. et al. Rapid cloning of genes in hexaploid wheat using cultivar-specific long-range chromosome assembly. Nat. Biotechnol. 35, 793–796 (2017).
Sánchez-Martín, J. et al. Rapid gene isolation in barley and wheat by mutant chromosome sequencing. Genome Biol. 17, 221 (2016).
Steuernagel, B. et al. Rapid cloning of disease-resistance genes in plants using mutagenesis and sequence capture. Nat. Biotechnol. 34, 652–655 (2016).
Arora, S. et al. Resistance gene cloning from a wild crop relative by sequence capture and association genetics. Nat. Biotechnol. 37, 139–143 (2019).
Wang, Y. et al. An unusual tandem kinase fusion protein confers leaf rust resistance in wheat. Nat. Genet. 55, 914–920 (2023).
Athiyannan, N. et al. Long-read genome sequencing of bread wheat facilitates disease resistance gene cloning. Nat. Genet. 54, 227–231 (2022).
Uauy, C., Wulff, B. B. H. & Dubcovsky, J. Combining traditional mutagenesis with new high-throughput sequencing and genome editing to reveal hidden variation in polyploid wheat. Annu. Rev. Genet. 51, 435–454 (2017).
He, H. et al. Large-scale mutational analysis of wheat powdery mildew resistance gene Pm21. Front. Plant Sci. 13, 988641 (2022).
Watson, A. et al. Speed breeding is a powerful tool to accelerate crop research and breeding. Nat. Plants 4, 23–29 (2018).
Kuraparthy, V. et al. A cryptic wheat–Aegilops triuncialis translocation with leaf rust resistance gene Lr58. Crop. Sci. 47, 1995–2003 (2007).
Kuraparthy, V. et al. Characterization and mapping of cryptic alien introgression from Aegilops geniculata with new leaf rust and stripe rust resistance genes Lr57 and Yr40 in wheat. Theor. Appl. Genet. 114, 1379–1389 (2007).
Jafary, H., Szabo, L. J. & Niks, R. E. Innate nonhost immunity in barley to different heterologous rust fungi is controlled by sets of resistance genes with different and overlapping specificities. Mol. Plant-Microbe Interact. 19, 1270–1279 (2006).
Ejaz, M. et al. Genetic variation for markers linked to stem rust resistance genes in Pakistani wheat varieties. Crop Sci. 52, 2638–2648 (2012).
Walkowiak, S. et al. Multiple wheat genomes reveal global variation in modern breeding. Nature 588, 277–283 (2020).
Sato, K. et al. Chromosome-scale genome assembly of the transformation-amenable common wheat cultivar ‘Fielder. DNA Res. 28, dsab008 (2021).
Green, G. J. Identification of physiologic races of Puccinia graminis f. sp. tritici in Canada. Can. J. Plant Pathol. 3, 33–39 (1981).
Stakman, E. C., Stewart, D. M. & Loegering, W. Q. Identification of Physiologic Races of Puccinia Graminis Var. Tritici. (1962).
Tsilo, T. J., Chao, S., Jin, Y. & Anderson, J. A. Identification and validation of SSR markers linked to the stem rust resistance gene Sr6 on the short arm of chromosome 2D in wheat. Theor. Appl. Genet. 118, 515–524 (2009).
Forsyth, F. R. Interaction of temperature and light on the seedling reaction of McMurachy wheat to race 15B of stem rust. Can. J. Bot. 34, 745–749 (1956).
Mayama, S., Daly, J. M., Rehfeld, D. W. & Daly, C. R. Hypersensitive response of near-isogenic wheat carrying the temperature-sensitive Sr6 allele for resistance to stem rust. Physiol. Plant Pathol. 7, 35–47 (1975).
Terefe, T., Pretorius, Z. A., Visser, B. & Boshoff, W. H. P. First report of Puccinia graminis f. sp. tritici race PTKSK, a variant of wheat stem rust race Ug99, in South Africa. Plant Dis. 103, 1421–1421 (2019).
Marchal, C. et al. BED-domain-containing immune receptors confer diverse resistance spectra to yellow rust. Nat. Plants 4, 662–668 (2018).
Yoshimura, S. et al. Expression of Xa1, a bacterial blight-resistance gene in rice, is induced by bacterial inoculation. Proc. Natl. Acad. Sci. USA 95, 1663–1668 (1998).
Chen, C. et al. BED domain-containing NLR from wild barley confers resistance to leaf rust. Plant Biotechnol. J. 19, 1206–1215 (2021).
Stakman, E. C. & Rodenhiser, H. A. Race 15B of wheat stem rust-what it is and what it means. Adv. Agron. 10, 143–165 (1959).
Knott, D. R. & Anderson, R. G. The inheritance of rust resistance.: I. the inheritance of stem rust resistance in ten varieties of common wheat. Can. J. Agric. Sci. 36, 174–195 (1956).
Kolmer J. Early Research on the Genetics of Puccinia Graminis and Stem Rust Resistance in Wheat in Canada and the United States. (2001).
Wanyera, R., Kinyua, M., Jin, Y. & Singh, R. The spread of stem rust caused by Puccinia graminis f. sp. tritici, with virulence on Sr31 in wheat in Eastern Africa. Plant Dis. 90, 113–113 (2006).
Steadham, J. et al. An approach for high-resolution genetic mapping of distant wild relatives of bread wheat: example of fine mapping of Lr57 and Yr40 genes. Theor. Appl. Genet. 134, 2671–2686 (2021).
Gupta, S. K., Charpe, A., Koul, S., Prabhu, K. V. & Haq, Q. M. R. Development and validation of molecular markers linked to an Aegilops umbellulata–derived leaf-rust-resistance gene, Lr9, for marker-assisted selection in bread wheat. Genome 48, 823–830 (2005).
Green, M. R. & Sambrook, J. Nested polymerase chain reaction (PCR). Cold Spring Harb. Protoc. 2019, pdb. prot095182 (2019).
Yuan, C. et al. A high throughput barley stripe mosaic virus vector for virus induced gene silencing in monocots and dicots. PLoS ONE 6, e26468 (2011).
Barnes, C. W. & Szabo, L. J. Detection and identification of four common rust pathogens of cereals and grasses using real-time polymerase chain reaction. Phytopathology® 97, 717–727 (2007).
Hayta, S., Smedley, M. A., Clarke, M., Forner, M. & Harwood, W. A. An efficient Agrobacterium-mediated transformation protocol for hexaploid and tetraploid wheat. Curr. Protoc. 1, e58 (2021).
Acknowledgements
We thank Prof. Willem Boshoff from the University of the Free State, South Africa, for providing Pgt isolate PTKST. We are grateful to Elisabet Poquet Faig (KAUST) and Lingli Zou (KAUST) for greenhouse assistance. We thank Prof. Robert McIntosh (University of Sydney) for critical feedback on our manuscript. We thank Prof. Li Wan from CEMPS and Prof. Huagang He from Jiangsu University for insightful discussions. This research used the Ibex cluster managed by the Supercomputing Core Laboratory at King Abdullah University of Science and Technology (KAUST) in Thuwal, Saudi Arabia. We thank the KAUST Bioscience Core Lab for sequencing support and the KAUST Plant Growth Core Lab for greenhouse support. This publication is based upon work supported by the National Key Research and Development Program of China (2024YFD1201600) to Y.W., KAUST awards ORFS-CRG10-2021-4735 to B.B.H.W., and ORFS-CRG10-2021-4712 to S.G.K.
Author information
Authors and Affiliations
Contributions
Y.W., B.B.H.W., and S.G.K. designed the research. Y.W. performed EMS mutagenesis, mutant screening, MutIsoSeq data analysis, and generation and characterization of the mapping population. Y.W., L.Z., Y.O., and J.L. performed Sanger sequencing of mutants and VIGS experiments. K.Z. performed wheat transformation. X.W. and F.A. characterized CRISPR/Cas9 knock-out mutants. Y.W. and N.A. performed rust inoculations. Y.W. and S.G.K. drafted the manuscript with input from all co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Y., Wang, X., Zhang, L. et al. An optimized disease resistance gene cloning workflow for wheat. Nat Commun 16, 4904 (2025). https://doi.org/10.1038/s41467-025-60033-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-60033-8
This article is cited by
-
Genome-wide dissection of lesion mimic mutations in wheat: identification and functional characterization of candidate genes
Theoretical and Applied Genetics (2025)
-
Development and application of duplex and triplex assays for simultaneous detection of resistance genes to leaf rust, Fusarium head blight, powdery mildew, Septoria tritici blotch, eyspot, stem rust and yellow rust in wheat
Journal of Applied Genetics (2025)