Abstract
The hydroboration of alkynes is a textbook example of a syn-selective concerted addition reaction, while trans-selective additions of borane to alkynes remain to be developed. We herein report a transition metal-free anti-addition of pinacolborane to alkynes, facilitated by the counteranion effect. This work further develops Chan alkyne reduction by utilizing the borane instead of aluminohydride reagents, enabling the facile synthesis of valuable five-membered boracycles that constitute isosteric alternatives to bioactive butenolides and a versatile platform for abundant downstream transformations. The practical method is distinguished by excellent regioselectivity, a broad substrate scope, and high compatibility with a variety of functional groups. The exploration of trans-selective patterns affords not only a stereo-complementary approach to traditional organic synthesis, but also mandates a new perspective on the noncanonical trans-hydroboration mechanism. A combination of control experiments and computational studies at the DFT level of theory reveal the previously unrecognized role of the HMDS counteranion in a stepwise intermolecular hydrogen transfer process.
Similar content being viewed by others
Introduction
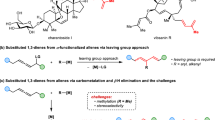
Boron-containing drug candidates have garnered increasing attention by virtue of their unique pharmacological properties and the growing prominence of boron neutron capture therapy (BNCT)1,2,3. As the example of 1,2-benzazaborine4,5,6,7,8 serving as an alternative to naphthalene shows, the bioisosteric substitution of carbon by boron in the ring has emerged as a compelling strategy for the design of boracyclic frameworks and the advancement of drug discovery (Fig. 1A). In the design and exploitation of boron-containing drugs, B(OH)2 often serves as a bioisosteric alternative to COOH because of the identical number of valence electrons, a comparable molecular geometry, and the ability to engage in hydrogen bond interactions with a biological receptor9. Analogously, oxaboroles, in their own right, emerge as promising pharmacophores present in fungicidal molecules10,11 comparable to butenolides that are prominent structural motifs featured in numerous antifungal natural products as well as drug molecules. Therefore, based on the concept of bioisosterism, we proposed that the five membered-ring hemiboronic esters represent potential bioisosteres of butenolides. Moreover, oxaboroles represent a potent scaffold for the stereospecific assembly of multi-substituted allylic alcohols, which constitute the core skeleton in many pharmaceuticals (Fig. 1B).
A Exploring boron as a bioisosteric replacement for carbon in the ring. B Polysubstituted allylic alcohol-containing drugs. C Synthesis of boracycles via alkyne hydroboration. D Trans-Selective Chan alkyne reduction. E Chloride counteranion influence on Ru-catalyzed trans-hydrometallation. F Counteranion-enabled trans-selective hydroboration of alkynes.
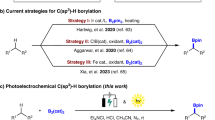
The classical hydroboration of alkynes has proven to be an exceptionally useful method for the stereospecific construction of linear alkenyl boronates due to its rigorous stereochemical course resulting from cis-addition of a B-H bond to the π-system12,13,14,15. During the past decade, although considerable efforts were dedicated to exploring the stereo-complementary anti-addition mode16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32, further expanding the potential of trans-hydroboration for the boracycle synthesis still remains underexplored (Fig. 1C)33,34. In this context, Chan alkyne reduction35 with trans-stereoselectivity shows the in-situ formation of cycloaluminum species before quenching (Fig. 1D, left). This successful way to challenge the canonical syn-selectivity consists in a quasi-intramolecular hydride delivery followed by trapping of the aluminum functionality from the rear. However, to date, there have been few reports on an analogous access to isolable oxaboroles by using benign boranes instead of aluminohydride reagents (Fig. 1D, right).
Metal counterions have proven capable of finely tuning reactivity and selectivity in metal catalysis through their synergistic interaction with substrates, steering intermediate formation36,37,38,39,40. Recently, ruthenium-catalyzed trans-hydrostannation as well as trans-hydrosilylation of propargyl alcohols have been developed that provide robust access to various α-functionalized trisubstituted allylic alcohols with exceptional levels of stereo- and regioselectivity (Fig. 1E, left)41,42,43,44,45. Assistance by the coordinating chloride ion unit of the [Cp*RuCl] catalyst able to engage in hydrogen bonding with an unprotected propargylic -OH group ensures this unorthodox reactivity46,47. However, this directing strategy cannot be extended to analogous trans-hydroboration reactions because the common borane reagents will instantly react with the hydroxy group. When suitably protected propargyl alcohol derivatives are used instead, without the hydrogen bonding direction, the E/Z- and regioisomer ratios in the ruthenium-catalyzed hydroboration proved to be quite variable and primarily dependent on steric factors of alkyne substrates (Fig. 1E, right)48. Hence, a broadly applicable method for the regiocontrolled trans-hydroboration of propargyl alcohols with further formation of valuable (E)-β-borocycles remains a highly desirable yet challenging task.
Outlined below (Fig. 1F), we disclose a stereo-complementary trans-selective addition of HBpin to alkynes promoted solely by hexamethyldisilazide (HMDS) anion. The reaction leverages the directing effect of readily available propargylic hydroxy groups and proceeds without any transition metal catalyst49,50. While previous studies on the alkali metal reagent-activated canonical syn-stereospecific addition of boranes to alkynes51,52,53 were mostly focused on demonstrating the efficacy of the alkali metal cations, particularly that of Li(I)54,55,56, the role of the counteranion has been largely overlooked or considered to be hardly relevant. A set of mechanistic control experiments in combination with DFT calculations suggest that a so-far unique anion-mediated stepwise trans-hydroboration process is operative. Distinct from the Chan’s alcohol-aluminum adduct undergoing an intramolecular B-H shift, a three component adduct intermediate involving the HMDS anion is generated and subjected to an intermolecular hydride transfer. The resulting oxaboroles are not only bioisosteric to butenolides, but also function as highly versatile building blocks for downstream functionalization to access a series of β-functionalized allylic alcohols.
Results
The tertiary propargyl alcohol 1 was selected as model compound, which was reacted with 2 equivalents of HBpin and an alkali metal reagent in THF as the solvent; this choice was made because such highly sterically hindered substrates, in protected form, tend to undergo cis-addition when subjected to Ru-catalyzed hydroboration48. As depicted in Table 1, a series of lithium reagents was examined first, with LiHMDS producing the desired product, that is the cyclic boronate 2, in 20% yield (entry 1-4). Other bases mainly induced the decomposition of 1 into phenylacetylene; this observation suggested that the [HMDS]- anion plays a pivotal role for the reaction. In line with this notion, NaH as well as NaNH2 proved unfruitful (entry 5, 6) whereas the use of NaHMDS or KHMDS both furnished 2 in similar, though modest, yield (entries 7 and 8). A significant improvement was reached by increasing the equivalents of base and extending the reaction time (entry 9-12). With 2 equivalents of NaHMDS, the yield was improved to 86% after 6 h (entry 11). Importantly, no side products formed by cis-addition were detected. Other borane reagents were evaluated as well. Simple BH3 performed well with 73% yield (entry 13), catecholborane (HBCat) only led to 35% yield (entry 14), whereas 9-BBN and BH3•SMe2 did not give the product (entry 15 and 16). Finally, 2 equivalent of NaHMDS with HBpin turned out to be best suited.
Substrate scope
With the optimized conditions established, we explored the substrate scope of this trans-hydroboration (Fig. 2). Tertiary propargyl alcohols bearing different functional groups reacted smoothly to form the corresponding oxaborole products in good to excellent yields. Propargyl alcohols with aryl substituents bearing electron-withdrawing groups tend to give higher yields, while electron-donating groups on the arene led to somewhat more moderate outcomes. Notably, a vast array of unsaturated functional groups was preserved under the reductive conditions, including -CN (7), azide (8), ester (9), -NO2 (17) and sulfonamide (19) groups. Halogen, -CF3, -OCF3 and heterocyclic substituents were tolerated as well (10-13, 26-28). Isolated carbon-carbon triple bonds without an adjacent hydroxy group were found to be inert, which is a particularly remarkable aspect of chemoselectivity (21, 22). This highlights the crucial role of the hydroxyl group’s negative inductive effect in propargyl alcohols, which increases the electrophilicity of the triple bond, enabling the reaction. Spirocyclic boronate products could be easily prepared from cyclic tertiary alcohols, whereby even a strained four-membered ring remained intact (31). In addition, a fused boracycle was accessible by virtue of intramolecular condensation of the boronate primarily formed with a neighboring benzylic hydroxy substituent (37). In addition to the tertiary alcohols, secondary and primary propargyl alcohols also performed well, delivering the desired products in generally good yields, while leaving the chiral center intact (38-48). Regioselective trans-addition to propargyl alcohols with conjugated enyne or diyne subunits turned out to be feasible (49-54); once again, the distal alkene and alkyne units were shown not to interfere. However, alkyl substituted propargyl alcohols are currently not suitable for this reaction. The molecular structure assigned to 24 was confirmed by single-crystal X-ray diffraction (CCDC No.: 2346237).
aGeneral reaction conditions: NaHMDS (0.4 mmol, 2 M in THF), propargyl alcohol (0.2 mmol), HBpin (0.4 mmol), THF (2 ml), room temperature, 6 h, then quenching with aqueous NH4Cl solution. bIsolated yield. cNaHMDS (0.24 mmol), HBpin (0.2 mmol), 0 °C, 12 h. A The scope of tertiary allylic alcohols. B The scope of secondary and primary allylic alcohols. C The scope of conjugated enyne and diene subsets.
The new methodology described herein represents a straightforward and productive approach to introduce the oxaborole motif into bioactive compounds, endowing the boron core unit with potential targeting ability (Figs. 3 and 4). Due to the good functional group compatibility, this five-membered boracycle was successfully embedded into compounds derived from natural products or drug molecules upon their tethering to an alkyne, namely linalool (55), piperonylacetone (56), ethinylestradiol (57), normuscone (58), nabumetone (59), chromanone (60) and watermelon ketone (61). Due to the good compatibility, the ester group could be adopted to tie borneol (62). Additionally, potential antifungal oxaboroles (63-67) were prepared to enrich the boron-based compound library. In general, an acidic environment may be more conducive to inhibiting fungal growth. Thus, they constitute potential bioisosteric equivalents of antifungal butenolide derivatives57,58. Two bora-butenolide isosterics of bioactive natural products (70 and 73) were synthesized by further semi-hydrogenation.
Derivatizations
To demonstrate the practicality of this NaHMDS-promoted trans-hydroboration, a gram-scale reaction was carried out, giving the target product in 93% yield. Even when carried out in air, a yield of 90% was obtained (Fig. 5A). A formyl group-bearing oxaborole building block was prepared (Fig. 5B, 75), enabling facile installation of the oxaborole skeleton onto drug molecules at a late stage (76 and 77). The oxaborole moiety may enhance the antimicrobial activity in cooperation with hydrazide-hydrazones (78 and 79). This oxaborole was then subjected to diverse downstream transformations (Fig. 5C). Protodeborylation of 11 led to the formal trans-hydrogenation product 80. A set of representative sp3-, sp2-, sp-hybridized carbon entities could be introduced at the β-position through Pd-catalyzed Suzuki-Miyaura cross coupling (81-83). Oxidative cleavage of C-B bond furnished the β-hydroxy ketone 84. The B-OH group of 11 was replaced by a mesityl substituent on treatment with the corresponding Grignard reagent (85). Furthermore, versatile oxa-spirocyclic scaffolds are well within reach. This asset was exemplified by the synthesis of spirocyclic ether and spirolactone derivatives from 32 through tandem cross coupling/annulation (86-89). The oxaborole 91 was successfully employed on the stereoselective construction of the Bervastation framework (Fig. 5D)59.
Mechanistic investigations
Next, a series of control experiments was conducted to gain insights into the reaction mechanism (Fig. 6). Specifically, the hydride on the double bond was unambiguously shown to derive from the borane reagent by treatment of the propargyl alcohol substrate with DBpin, which resulted in 95% deuterium incorporation (eq. 1). To exclude any particular influence of the sodium ion, excess 15-crown-5 ether was added to the mixture; under these conditions, the product was formed in a largely unchanged 85% yield (eq. 2). In contrast, the use of NaH instead of NaHMDS produced only a trace amount of product (eq. 3). When NaH (2 equiv.) was supplemented with a substoichiometric amount of NaHMDS (20 mol%), however, a 49% yield was obtained (eq. 4).
These results underline the crucial role of the [HMDS]- anion. Further insights were gained by 11B-NMR spectroscopy, which revealed that HBpin is instantly transformed into [HMDS-Bpin] (δ = 25.6 ppm)60,61 and BH4- (quint, δ = −43.4 ppm) upon addition of NaHMDS (Fig. 6a); a small amount of the adduct derived from the [HMDS]- ion and HBpin was observed as well (δ = 8.3 ppm) (see Figure S1 in the Supplementary Information). In contrast, when LiHMDS was added to HBpin in a 1:1 ratio, only a trace of HMDS-Bpin was detected in the 11B-NMR spectrum, indicating that LiHMDS is ineffective in activating HBpin under standard conditions as compared to NaHMDS (see Figure S2 in the Supplementary Information). Moreover, the addition of KHMDS to HBpin in a 1:1 ratio resulted in the formation of a precipitate, showing that the intermediates with potassium counterion are not well soluble; this heterogenous system may hinder the reaction, finally leading to a low yield. 11B-NMR titration experiments indicated that 1 equivalent of NaHMDS consumes approximately 2.8 equivalents of HBpin (Fig. 6b). When NaHMDS (0.2 mmol) was mixed with 3 equiv. HBpin in 0.5 ml THF, a mass of white precipitates was rapidly formed, which consists of insoluble NaBH4 (Fig. 6c). When a mixture of NaHMDS and HBpin (1:1) was stirred for 30 min to ensure complete disappearance of HBpin before the propargyl alcohol was added, the reaction still proceeded well and furnished the product in 70% yield (eq. 5). In this case, a transient adduct formed by reaction of the alkoxide with [HMDS-Bpin] was detected by online 11B-NMR (δ = 4.6 ppm, Fig. 6e). When 1 equivalent of HBpin was added to the mixture of equation 5, the yield of 11 was increased to 95% (eq. 6); in contrast, addition of soluble LiBH4 did not improve the yield (eq. 7). These results suggest that [HMDS-HBpin]- or a related Na[B-H] species is the actual reducing agent delivering the hydride to the activated substrate, not [BH4]-. The product resulting from this trans-hydroboration reaction has a signal in the range of 11–13 ppm (Fig. 6f), which suggests that the species primarily formed is a tetrahedral boron-ate complex62 prior to work-up.
On the basis of these experimental results, a stepwise trans-hydroboration mechanism is proposed that is triggered by NaHMDS (Fig. 7). Initially, the propargyl alkoxide A is generated at the cost of 1 equivalent of NaHMDS. The remaining NaHMDS promotes the conversion of HBpin into Na[HMDS-HBpin], which may undergo disproportionation to form [HMDS-Bpin], NaBH4 and other boron-ate complexes. The propargyl alkoxide A then adds to [HMDS-Bpin] to form the boron-ate complex B, which is reduced to give intermediate C via intermolecular hydride delivery. Due to the size of the HMDS ligand, the cis-configured isomer C’ is disfavored on steric grounds. Subsequent boron capture with dissociation of an N- or O-ligand (path A and B) leads to the corresponding boron-ate complexes D and E, respectively, which are hydrolyzed upon aqueous work-up to yield the final oxaborole product 2.
Computational studies
To gain insight into whether pathway A or B is operative and to elucidate the reaction mechanism of the trans-hydroboration process in more detail, we performed DFT calculations for the model reaction (Fig. 8). Based on the aforementioned experimental results, we took the propargyl alkoxide (A) as the starting point. The reaction initiates with the coupling between A and the [HMDS-Bpin] species, generating a boron-ate complex B through the transition state TS1, with an energy barrier (ΔG‡) of 7.9 kcal/mol. Subsequently, Na[B-H] attacks the alkyne group of B to complete an intermolecular hydride transfer via TS2 (ΔG‡ = 18.5 kcal/mol), generating intermediate C. The reaction pathway diverges at C, yielding the target product 2 through intermediate D (path A) or intermediate E (path B). Despite the negative charge of intermediate C, charge population analysis shows that the boron atom itself is actually positively charged due to electron withdrawal by the adjacent electronegative N- and O- ligands (see Figure S3 in the Supplementary Information). In path A, C first overcomes a transition state TS3A-1 to break the B-N bond, forming intermediate INT3A. The negatively charged carbon atom bonded to the phenyl group in C then attacks the boron atom, passing through the second transition state TS3A-2. This leads to the formation of the cyclized intermediate D with simultaneous removal of NaHMDS. The final oxaborole product 2 is then obtained upon hydrolysis of D. Path A exhibits an overall energy barrier of 11.9 kcal/mol (G(TS3A-1)-G(C)), and the product formation is exothermic by 27.7 kcal/mol (G(D)-G(C)). Conversely, path B involves breaking of the B-O bond through a single transition state TS3B and simultaneous formation of another cyclized intermediate E with a substantially higher ΔG‡ value of 22.8 kcal/mol (G(TS3B)-G(C)) and ΔG value of 12.7 kcal/mol (G(E)-G(C)). Thus, path A is both kinetically and thermodynamically more favorable than path B. On the other hand, intermediate C can convert to its cis-configured isomer C’ with a free energy change of −1.1 kcal/mol. However, due to the steric hindrance exerted by the TMS group, the formation of the cyclized cis-intermediate is infeasible, with a free energy barrier as high as 37.2 kcal/mol (see Figure S4 in the Supplementary Information). Therefore, it can be concluded that the trans-hydroboration reaction predominantly follows path A, which is more favorable compared to the cis-hydroboration reaction via path A’. In addition, an intramolecular hydride shift without the assistance of HMDS anion was calculated (Fig. 8, left), revealing a higher energy barrier that hinders this process. (ΔG‡ = 24.6 kcal/mol). Thus, the critical role of the [HMDS]- anion is attributable to its electronegative nitrogen atom and the steric hindrance of the TMS groups, which together facilitate the B-H transfer, B-C bond formation and cyclization, and impose the observed stereoselective course. We have also explored the mechanism of a possible direct hydroboration reaction between sodium alkoxide A and Na[HBpin-HMDS] (Na[B-H]). However, DFT calculations suggest that such a mechanism is energetically unfeasible (see Figure S5 in the Supplementary Information).
Discussion
We report a transition metal-free, counteranion-enabled trans-hydroboration of alkynes, which features mild reaction conditions, a broad substrate scope, a remarkable functional group tolerance, and excellent regioselectivity. This transformation is readily scalable and enables a modular and robust access to the oxaborole scaffold. Because of the growing importance of boron-containing drugs, the relevance of the method was illustrated by applications to bioactive molecules as well as by the construction of bioisosteres of butenolides. Moreover, the oxaborole products constitute a flexible platform for the assembly of various trisubstituted alkenes and oxa-spirocycles. Experimental and computational studies at the DFT level of theory suggest that the reaction proceeds by an anion-mediated stepwise anti-addition process, whereby the [HMDS]- counterion accounts for the generation of the active hydride species as well as the formation of the reducible boron-ate intermediate.
Methods
General procedure via 2 equiv. HBpin
An oven-dried Schlenk tube equipped with a magnetic stir bar was charged with propargyl alcohol (0.2 mmol), anhydrous THF (2 mL) and NaHMDS (2.0 M THF solution, 0.4 mmol) at 0 °C under N2 atmosphere. The mixture was stirred for 10 min at 0 °C, and then HBpin (0.4 mmol, 58 μL) was introduced. Subsequently, the reaction vessel was sealed with a Teflon-lined screw cap and the mixture warmed to room temperature. After continuously stirring for 6 h, the reaction was quenched with aqueous NH4Cl solution (10 mL), followed by extraction with EtOAc (3 × 10 mL). The combined organic layer was dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The residue was purified by flash chromatography on silica gel to afford the desired product.
General procedure via 1 equiv. HBpin
An oven-dried Schlenk tube equipped with a magnetic stir bar was charged with propargyl alcohol (0.2 mmol), anhydrous THF (2 mL) and NaHMDS (2.0 M THF solution, 0.24 mmol) at 0 °C under N2 atmosphere. The mixture was stirred for 10 min at 0 °C, then HBpin (0.2 mmol, 29 μL) was introduced. Subsequently, the reaction vessel was sealed with a Teflon-lined and kept in an ice bath. After continuously stirring for 12 h at 0 °C, the reaction was quenched with aqueous NH4Cl solution (10 mL), followed by extraction with EtOAc (3 × 10 mL). The combined organic layer was dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The residue was purified by flash chromatography on silica gel to afford the desired product.
Data availability
The X-ray crystallographic coordinates for structures reported in this study have been deposited in the Cambridge Crystallographic Data Centre under accession (CCDC), under deposition numbers 2346237. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. All other data that support the findings of this paper, including starting material preparation, experimental procedures, compound characterizations, NMR spectra of new compounds, and DFT calculations, are available within the paper and its Supplementary Information. All data are also available from the corresponding author upon request. Source data are provided with this paper.
References
Fernandes, G. F. S., Denny, W. A. & Santos, J. L. D. Boron in drug design: recent advances in the development of new therapeutic agents. Eur. J. Med. Chem. 179, 791–804 (2019).
Song, S. et al. Recent developments in the medicinal chemistry of single boron atom-containing compounds. Acta Pharm. Sin. B. 11, 3035–3059 (2021).
Silva, M. P., Saraiva, L., Pinto, M. & Sousa, E. M. Boronic acids and their derivatives in medicinal chemistry: synthesis and biological applications. Molecules 25, 4323 (2020).
Su, W. et al. Copper-catalysed asymmetric hydroboration of alkenes with 1,2-benzazaborines to access chiral naphthalene isosteres. Nat. Chem. 16, 1312–1319 (2024).
Wang, H. et al. Enantio- and regioselective [2 + 2 + 2] cycloaddition of BN-diynes for construction of C-B axial chirality. Chem. 10, 317–329 (2024).
Zhang, X. et al. N-heterocycle-editing to access fused-BN-heterocycles via ring-opening/C-H borylation/reductive C-B bond formation. Angew. Chem. Int. Ed. 63, e202318613 (2024).
Lyu, H. et al. Modular synthesis of 1,2-azaborines via ring-opening BN-isostere benzannulation. Nat. Chem. 16, 269–276 (2024).
Choi, S. & Dong, G. Rapid and modular access to multifunctionalized 1,2-azaborines via palladium/norbornene cooperative catalysis. J. Am. Chem. Soc. 146, 9512–9518 (2024).
Xiao, Y.-C., Yu, J.-L., Dai, Q.-Q., Li, G. & Li, G.-B. Targeting metalloenzymes by boron-containing metal-binding pharmacophores. J. Med. Chem. 64, 17706–17727 (2021).
Campbell, R. et al. Design, synthesis, and antifungal activity of 3-substituted-2(5h)-oxaboroles. ACS Med. Chem. Lett. 15, 349–354 (2024).
Stierli, D., Titulaer, R. & Rajan, R. Novel microbiocides. WO 2014/147009 A1 (Sept. 25, 2014).
Brown, H. C. Hydroboration (Benjamin, W. A., New York, 1962).
Matteson, D. S. Stereodirected synthesis with organoboranes (Springer, Berlin, 1995).
Hua, R. Addition reactions with unsaturated hydrocarbons (Wiley, Hoboken, 2022).
Geier, S. J., Vogels, C. M., Melanson, J. A. & Westcott, S. A. The transition metal-catalysed hydroboration reaction. Chem. Soc. Rev. 51, 8877–8922 (2022).
Altarejos, J., Valero, A., Manzano, R. & Carreras, J. Synthesis of tri- and tetrasubstituted alkenyl boronates from alkynes. Eur. J. Org. Chem. 30, e202200521 (2022).
Rej, S., Das, A. & Panda, T. K. Overview of regioselective and stereoselective catalytic hydroboration of alkynes. Adv. Synth. Catal. 363, 4818–4848 (2021).
Fürstner, A. How to break the law: trans-hydroboration and gem-hydroboration of alkynes. Isr. J. Chem. 63, e202300004 (2023).
Ohmura, T., Yamamoto, Y. & Miyaura, N. Rhodium- or iridium-catalyzed trans-hydroboration of terminal alkynes, giving (Z)-1-alkenylboron compounds. J. Am. Chem. Soc. 122, 4990–4991 (2000).
Cid, J., Carbó, J. J. & Fernández, E. Catalytic non-conventional trans-hydroboration: a theoretical and experimental perspective. Chem. Eur. J. 18, 1512–1521 (2012).
Gunanathan, C., Hölscher, M., Pan, F. & Leitner, W. Ruthenium catalyzed hydroboration of terminal alkynes to Z-vinylboronates. J. Am. Chem. Soc. 134, 14349–14352 (2012).
Obligacion, J. V., Neely, J. M., Yazdani, A. N., Pappas, I. & Chirik, P. J. Cobalt catalyzed Z-selective hydroboration of terminal alkynes and elucidation of the origin of selectivity. J. Am. Chem. Soc. 137, 5855–5858 (2015).
Gorgas, N. et al. Stable, yet highly reactive nonclassical iron(ii) poly-hydride pincer complexes: Z-selective dimerization and hydroboration of terminal alkynes. J. Am. Chem. Soc. 139, 8130–8133 (2017).
McGough, J. S., Butler, S. M., Cade, I. A. & Ingleson, M. J. Highly selective catalytic trans-hydroboration of alkynes mediated by borenium cations and B(C6F5)3. Chem. Sci. 7, 3384–3389 (2016).
Jang, W. J., Lee, W. L., Moon, J. H., Lee, J. Y. & Yun, J. Copper-catalyzed trans-hydroboration of terminal aryl alkynes: stereo-divergent synthesis of alkenylboron compounds. Org. Lett. 18, 1390–1393 (2016).
Yamamoto, K., Mohara, Y., Mutoh, Y. & Saito, S. Ruthenium-catalyzed (Z)-selective hydroboration of terminal alkynes with naphthalene-1,8-diaminatoborane. J. Am. Chem. Soc. 141, 17042–17047 (2019).
Sundararaju, B. & Fürstner, A. A trans-selective hydroboration of internal alkynes. Angew. Chem. Int. Ed. 52, 14050–14054 (2013).
Xu, S., Zhang, Y., Li, B. & Liu, S.-Y. Site-selective and stereoselective trans-hydroboration of 1,3-enynes catalyzed by 1,4-azaborine-based phosphine–Pd complex. J. Am. Chem. Soc. 138, 14566–14569 (2016).
Grams, R. J., Fritzemeier, R. G., Slebodnick, C. & Santos, W. L. trans-hydroboration of propiolamides: access to primary and secondary (E)-β-borylacrylamides. Org. Lett. 21, 6795–6799 (2019).
Shimoi, M., Watanabe, T., Maeda, K., Curran, D. P. & Taniguchi, T. Radical trans-hydroboration of alkynes with N-heterocyclic carbene boranes. Angew. Chem. Int. Ed. 57, 9485–9490 (2018).
Nagao, K., Yamazaki, A., Ohmiya, H. & Sawamura, M. Phosphine-catalyzed anti-hydroboration of internal alkynes. Org. Lett. 20, 1861–1865 (2018).
Yuan, K. et al. Pyridyl directed catalyst-free trans-hydroboration of internal alkynes. Org. Lett. 18, 720–723 (2016).
Wang, Q., Motika, S. E., Akhmedov, N. G., Petersen, J. L. & Shi, X. Synthesis of cyclic amine boranes through triazole-gold(i)-catalyzed alkyne hydroboration. Angew. Chem. Int. Ed. 53, 5418–5422 (2014).
Motika, S. E., Wang, Q., Akhmedov, N. G., Wojtas, L. & Shi, X. Regioselective amine–borane cyclization: towards the synthesis of 1,2-BN-3-cyclohexene by copper-assisted triazole/gold catalysis. Angew. Chem. Int. Ed. 55, 11582–11586 (2016).
Chan, K.-K., Cohen, N., De Noble, J. P., Specian, A. C. Jr. & Saucy, G. J. Org. Chem. 41, 3497–3505 (1976).
Jia, M. & Bandini, M. Counterion effects in homogeneous gold catalysis. ACS Catal. 5, 1638–1652 (2015).
Roediger, S., Saux, E. L., Boehm, P. & Morandi, B. Coupling of unactivated alkyl electrophiles using frustrated ion pairs. Nature 636, 108–114 (2024).
Farney, E. P. et al. Discovery and elucidation of counteranion dependence in photoredox catalysis. J. Am. Chem. Soc. 141, 6385–6391 (2019).
Das, S. et al. Asymmetric counteranion-directed photoredox catalysis. Science 379, 494–499 (2023).
Kaster, S. H. M. et al. Palladium-catalyzed cross-coupling of alcohols with olefins by positional tuning of a counteranion. Science 385, 1067–1076 (2024).
Rummelt, S. M. & Fürstner, A. Ruthenium-catalyzed trans-selective hydrostannation of alkynes. Angew. Chem. Int. Ed. 53, 3626–3630 (2014).
Fürstner, A. trans-hydrogenation, gem-hydrogenation, and trans-hydrometalation of alkynes: an interim report on an unorthodox reactivity paradigm. J. Am. Chem. Soc. 141, 11–24 (2019).
Frihed, T. G. & Fürstner, A. Progress in the trans-reduction and trans-hydrometalation of internal alkynes. applications to natural product synthesis. Bull. Chem. Soc. Jpn 89, 135–160 (2016).
Huwyler, N., Radkowski, K., Rummelt, S. M. & Fürstner, A. Two enabling strategies for the stereoselective conversion of internal alkynes into trisubstituted alkenes. Chem. Eur. J. 23, 12412–12419 (2017).
Fürstner, A. Lessons from natural product total synthesis: macrocyclization and postcyclization strategies. Acc. Chem. Res. 54, 861–874 (2021).
Rummelt, S. M., Radkowski, K., Roşca, D.-A. & Fürstner, A. Inter-ligand interactions dictate the regioselectivity of trans-hydrometalations and related reactions catalyzed by [Cp*RuCl]. hydrogen bonding to a chloride ligand as a steering principle in catalysis. J. Am. Chem. Soc. 137, 5506–5519 (2015).
Roşca, D.-A. et al. Ruthenium-catalyzed alkyne trans-hydrometalation: mechanistic insights and preparative implications. J. Am. Chem. Soc. 139, 2443–2455 (2017).
Longobardi, L. E. & Fürstner, A. trans-hydroboration of propargyl alcohol derivatives and related substrates. Chem. Eur. J. 23, 12412–12419 (2017).
Nagashima, Y., Hirano, K., Takita, R. & Uchiyama, M. trans-diborylation of alkynes: pseudo-intramolecular strategy utilizing a propargylic alcohol unit. J. Am. Chem. Soc. 136, 8532–8535 (2014).
Nogami, M. et al. Transition metal-free trans-selective alkynylboration of alkynes. J. Am. Chem. Soc. 139, 12358–12361 (2017).
Magre, M., Szewczyk, M. & Rueping, M. s‑Block metal catalysts for the hydroboration of unsaturated bonds. Chem. Rev. 122, 8261–8312 (2022).
Bage, A. D., Hunt, T. A. & Thomas, S. P. Hidden boron catalysis: nucleophile-promoted decomposition of HBpin. Org. Lett. 22, 4107–4112 (2020).
Bage, A. D., Nicholson, K., Hunt, T. A., Langer, T. & Thomas, S. P. The hidden role of boranes and borohydrides in hydroboration catalysis. ACS Catal. 10, 13479–13486 (2020).
Bisai, M. K., Yadav, S., Das, T., Vanka, K. & Sen, S. S. Lithium compounds as single site catalysts for hydroboration of alkenes and alkynes. Chem. Commun. 55, 11711–11714 (2019).
Wang, Z.-C., Wang, M., Gao, J., Shi, S.-L. & Xu, Y. nBuLi-promoted anti-markovnikov selective hydroboration of unactivated alkenes and internal alkynes. Org. Chem. Front. 6, 2949–2953 (2019).
Liu, J. et al. Hexamethyldisilazane lithium (LiHMDS)-promoted hydroboration of alkynes and alkenes with pinacolborane. J. Org. Chem. 87, 3442–3452 (2022).
Lu, A. et al. Small changes result in large differences: discovery of (-)-incrustoporin derivatives as novel antiviral and antifungal agents. J. Agric. Food Chem. 62, 8799–8807 (2014).
Braun, M., Hohmann, A., Rahematpura, J., Bühne, C. & Grimme, S. Synthesis and determination of the absolute configuration of fugomycin and desoxyfugomycin: cd spectroscopy and fungicidal activity of butenolides. Chem. Eur. J. 10, 4584–4593 (2004).
Pang, Y. et al. A highly efficient dimeric manganese-catalyzed selective hydroarylation of internal alkynes. Angew. Chem. Int. Ed. 59, 12789–12794 (2020).
Shintani, R., Fujie, R., Takeda, M. & Nozaki, K. Silylative cyclopropanation of allyl phosphates with silylboronates. Angew. Chem. Int. Ed. 53, 6546–6549 (2014).
Gu, Y., Shen, Y., Zarate, C. & Martin, R. A mild and direct site-selective sp2 C–H silylation of (poly)azines. J. Am. Chem. Soc. 141, 127–132 (2019).
Jin, H. & Fürstner, A. Regioselective trans-carboboration of propargyl alcohols. Org. Lett. 21, 3446–3450 (2019).
Acknowledgements
H.J. are grateful for financial support from Natural Science Foundation of China (Grant No. 22201136), Science Foundation of Jiangsu Province (Grant No. BK20220463), Jiangsu Specially Appointed Professor Plan, Nanjing University of Chinese Medicine (Grant No. XPT22201136 and ZYXPY2024-005). J.H. acknowledges Natural Science Foundation of China (Grant No. 22101130) as well as the support from Xiaomi foundation. All theoretical calculations were performed at the High-Performance Computing Center (HPCC) of Nanjing University.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
H.J. conceived the work and designed the experiments. Y.-W.L. conducted the experiments. Y.L. completed density functional theory (DFT) calculations. Y.Z., M.Z., M.-E.R. and P.H. assisted the work. J.H. directed DFT calculations. A.F. and H.J. discussed and co-wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Tsuyoshi Taniguchi and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, YW., Liu, Y., Zheng, Y. et al. Expedient access to bora-butenolide bioisosteres by counteranion-mediated trans-hydroboration of alkynes. Nat Commun 16, 4897 (2025). https://doi.org/10.1038/s41467-025-60052-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-60052-5