Abstract
Antibiotic-resistant bacteria could be tackled by identifying trade-offs of evolution, such as high fitness costs, which may be harnessed to force reversion to susceptibility. A decline in antimicrobial resistance can occur through compensatory mutations or by genetic reversion to the wild-type allele, which reduce fitness costs associated with resistance. We analyse here the impact of antibiotic-free environments on declining ciprofloxacin resistance in eight nfxB defective clinical strains of Pseudomonas aeruginosa spanning varied clone types and ciprofloxacin resistance levels. Ciprofloxacin resistance declines in just 100 generations, which is mainly caused by newly acquired mutations in the genes encoding the overproduced efflux pump MexCD-OprJ and not by the reversion of nfxB mutations of the parental strains. The rapid reversion of ciprofloxacin resistance in P. aeruginosa suggests the potential for reusing this essential antibiotic and underlines the need to implement evolution-based approaches against nfxB defective resistant mutant strains.
Similar content being viewed by others
Introduction
The efficacy of antibiotics is severely compromised by the emergence and spread of antibiotic-resistant bacterial pathogens. Among them, Pseudomonas aeruginosa stands out1,2. This opportunistic pathogen causes nosocomial and chronic infections in people with cystic fibrosis (pwCF) or chronic obstructive pulmonary disease3,4,5. These infections are frequently treated with the fluoroquinolone ciprofloxacin6,7, but resistance is rapidly acquired by chromosomal mutations. The two principal resistance mechanisms are the acquisition of mutations in the ciprofloxacin target-encoding genes parCE (encoding DNA topoisomerase IV) and gyrAB (encoding DNA gyrase)8,9,10,11,12, and the acquisition of mutations in genes encoding the regulators of the efflux pumps that extrude this drug, leading to their overproduction6,10,13,14,15. Ciprofloxacin resistance of clinical P. aeruginosa isolates from patients with persistent airway infections10,16,17,18,19,20 is frequently due to the overproduction of the efflux pump MexCD-OprJ21. Overproduction of this efflux pump is mediated by the acquisition of loss-of-function mutations in the gene encoding its transcriptional repressor NfxB. In addition, mexCD-oprJ can be transiently overexpressed in the presence of inducer compounds, such as the antiseptic dequalinium chloride22, which temporally inactivates NfxB. Hence, the use of either dequalinium chloride or ciprofloxacin can lead to reduced quinolones efficacy because of a transient or stable overproduction, respectively, of the MexCD-OprJ efflux pump. However, it has also been described that the temporal or stable inactivation of NfxB, in clinical and laboratory strains of P. aeruginosa, not only causes ciprofloxacin resistance but also transient23 and stable24,25 collateral sensitivity (CS) to aminoglycosides18,20,21,23,26. This robust trade-off, by which the acquisition of resistance to one drug leads to the susceptibility to another25, has been described to emerge in clinical strains presenting, among others, mutations in nfxB18. In addition, it has been recently proved that the combinations ciprofloxacin-tobramycin or dequalinium chloride-tobramycin are effective in driving P. aeruginosa (antibiotic-resistant mutants to different drugs and varied clinical strains) to extinction20,23,26. However, the stability of both ciprofloxacin resistance and CS to tobramycin, associated with nfxB loss-of-function mutations, has not been determined in the absence of antibiotic selective pressure.
During periods of antibiotic restriction, it is possible that wild-type susceptible strains outcompete resistant ones and that compensatory evolution of fitness costs associated with resistance occurs27,28,29,30,31,32,33. However, restricting the use of antibiotics would be insufficient when resistance mutations do not trigger a physiological cost, since compensatory evolution is not expected to occur34. Furthermore, in those situations where compensatory evolution happens, it is more likely to be due to secondary mutations than to genetic reversions restoring the wild-type allele35. Therefore, there is uncertainty regarding the ability of periods of drug restriction to reverse antimicrobial resistance (AMR)36,37,38,39. However, rapid decline of resistance has been reported using Adaptive Laboratory Evolution (ALE) assays in antibiotic-free medium, with this decline being drug-specific and genetic background dependent40,41. The reason for the lineage-dependent decline is that fitness costs associated with a specific resistance mutation may differ in distinct genomic backgrounds42, due to epistatic and pleiotropic interactions42,43,44,45,46.
In this work, we analyse the possible decline or reversion of ciprofloxacin resistance in different clinical strains containing nfxB loss-of-function mutations, which are highly prevalent. We performed 15 days of ALE experiments in two different antibiotic-free environments: (I) environment absent of antibiotic (e. g. antibiotic restriction periods after the use of ciprofloxacin or ciprofloxacin-tobramycin therapies20,26) and (II) environment absent of antibiotic but containing dequalinium chloride (e.g., dequalinium chloride concentrations resulting from dequalinium chloride-tobramycin therapies23). We hypothesised that fitness costs of nfxB defective mutants could drive compensatory evolution in these antibiotic-free environments, causing a rapid decline of ciprofloxacin resistance (conceptual Fig. 1A). Having in mind that dequalinium chloride leads to the overproduction of the efflux pump in strains presenting a functional regulator, reversions to the wild-type allele seem less likely to be selected in its presence, although mutations in nfxB impeding its inhibition by dequalinium chloride could also be selected. In addition, and keeping in mind that each nfxB loss-of-function mutation may have a different effect on bacterial physiology -and on their associated fitness costs- depending on the starting genomic background, we hypothesised that the frequency and level of this resistance decline could be different in the eight nfxB defective clinical strains that were analysed.
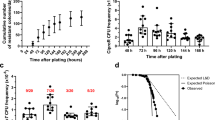
A Compensatory evolution in antibiotic-free environments may result in a fitness (F) improvement in nfxB ciprofloxacin-resistant mutants (RCIP) and, therefore, in the reversion or decline of ciprofloxacin resistance. This decline of resistance may occur in different antibiotic-free environments, such as those resulting from drug restriction periods (after the use of ciprofloxacin20,26) or those resulting from the presence of dequalinium chloride (after the use of transient CS-based therapies23. B Schematic representation of the experimental set-up of this work. C Ciprofloxacin (CIP) MICs (µg/mL) of populations evolved 15 days in antibiotic-free environments. CIP MICs of nfxB177 ciprofloxacin-resistant mutant, before and after 15 days of ALE assays on rich medium (LB) or on dequalinium chloride (DC) supplemented medium (10 µg/mL). Four replicate populations for each condition are represented. CIP resistance of the nfxB177 evolved populations declined after evolution in LB or in DC with respect to that of the nfxB177 mutant, reaching levels close to that of the PA14 wild-type strain (0.064 µg/mL, indicated with a dashed line). Statistical significance was computed by non-parametric Kruskal-Wallis (Rank Sums) test, followed by each-pair comparison test using the Wilcoxon method where * equals p = 0.0367. D Relative fitness of populations evolved 15 days in antibiotic-free environments. Growth curves of PA14 and of the nfxB177 ciprofloxacin-resistant mutant, before and after 15 days of ALE assays on rich medium (LB) or on DC supplemented medium (10 µg/mL), 4 replicate populations for each condition, were recorded in antibiotic-free medium. Statistical significance was computed by one-way ANOVA followed by Dunnett’s multiple comparison test where **** (p < 0.0001). The fitness of each population was represented as the area under the growth curve. Relative fitness of the nfxB177 mutant and of the nfxB177 populations was calculated with respect to the PA14 wild-type strain. OD600nm vs time measurements are included in Supplementary Data 2. Relative fitness is lower in the nfxB177 mutant than in its parental strain PA14. Fitness of the nfxB177 evolved populations improved after evolution in LB or in DC with respect to that of the nfxB177 mutant, presenting fitness levels close to or even higher than the PA14 strain. The mean ± SD of values from three replicates is represented.
Results
Decline of ciprofloxacin resistance in the PA14 isogenic loss-of-function nfxB177 mutant in antibiotic-free environments
In a previous work, we induced transient nfxB-mediated tobramycin CS in varied clinical isolates of P. aeruginosa by using dequalinium chloride. In addition, we drove these clinical isolates to extinction by combining tobramycin and dequalinium chloride23. Importantly, dequalinium chloride did not select for ciprofloxacin resistance after 15 days of evolution (a hundred bacterial generations)23. Something not addressed in said article is determining if the PA14 isogenic loss-of-function nfxB177 mutant (where the overproduction of the MexCD-OprJ efflux pump leads to ciprofloxacin resistance) may show a decline in ciprofloxacin resistance in the absence of antibiotic selective pressure, as a result of compensatory evolution. Therefore, in this work we performed ALE assays in antibiotic-free environments, with or without dequalinium chloride (see Methods; Fig. 1B). Four replicate populations of the PA14 isogenic loss-of-function nfxB177 mutant were evolved for 15 days, with daily sequential dilutions (1%) in test tubes (64 populations). We observed that the nfxB177 mutant reverted the ciprofloxacin MIC to the values of its parental strain PA14 after ALE in the absence of antibiotic (Wilcoxon test with FDR correction adj p < 0.01; Fig. 1C). We hypothesised that this loss-of-function mutation in nfxB could lead to a high fitness cost when grown in the absence of antibiotics and, therefore, a rapid compensatory evolution (and decline of AMR) could happen in just a hundred bacterial generations. Hence, we compared the fitness of the nfxB177 mutant strain and that of the eight nfxB177 populations obtained after 15 days of ALE in antibiotic-free environments (in the absence or presence of dequalinium chloride) with that of its parental strain PA14 (Fig. 1D). To do so, we estimated the fitness as the area under the growth curve recorded in antibiotic-free medium and analysed it for the nfxB177 mutant and of each nfxB177 population with respect to the PA14 parental strain. The nfxB177 mutant strain presented a reduced fitness relative to its parental strain, while the evolved populations in antibiotic-free medium reverted to the fitness level of the PA14 parental strain (One-way Anova followed by Dunnett’s multiple comparison test; p < 0.0001; Fig. 1D). Given these results, we hypothesised that the nfxB177 ciprofloxacin-resistant populations had acquired compensatory mutations during ALE, which would be responsible for the decline of ciprofloxacin resistance observed in antibiotic-free, non-selective environments. In addition, we hypothesised that this compensatory evolution, and the associated decline of ciprofloxacin resistance could also occur in highly prevalent clinical isolates presenting mutations in this gene (see below).
Decline of ciprofloxacin resistance in nfxB defective clinical strains of P. aeruginosa in antibiotic-free environments
To analyse changes in ciprofloxacin susceptibility associated with compensatory evolution in different nfxB defective mutants in antibiotic-free environments, four biological replicates of 8 clinical isolates were evolved in the absence of antibiotics (Fig. 1B). Strains from the Copenhagen CF Centre, Department of Clinical Microbiology, at Rigshospitalet, belong to six different clone types (defined as lineages which differ by >10,000 SNPs), harbour different nfxB mutations (4 frameshift and 4 amino acid substitutions including 2 stop codons) and ciprofloxacin resistance levels (0.75–1.5 µg/mL) (strains 258, 296, 357, 76, 316, 225, 398 and 79; Table 1)19, were evolved in the absence of antibiotic. We analysed two different antibiotic-free environments, with or without dequalinium chloride. These strains were evolved for 15 days (~100 bacterial generations), with daily sequential dilutions (1%) in test tubes (64 populations). MIC variations greater than 2-fold (determined using MIC test strips, allowing detection of small MIC changes) and with adjusted p values of <0.05 (Kruskal–Wallis test followed by each-pair Wilcoxon test with FDR correction) indicated biologically and statistically relevant variations of susceptibility, as previously described26,41. We observed a decline of ciprofloxacin MIC in 43 out of 64 populations (67%) evolved in antibiotic-free environments -in the absence or presence of dequalinium chloride- for 15 days (Figs. 2 and 3; Supplementary Table 1). Importantly, the MIC values of 42 of these evolved populations dropped down below the EUCAST clinical breakpoint (Fig. 3 and Supplementary Table 1). Specifically, a decline of ciprofloxacin resistance, from 3- to 16-fold, was observed in the clinical isolates 258, 296, 357, 76, 225 and 398 after ALE in the absence of dequalinium chloride (24 out of 32 populations) and in 258, 296, 76, 225 and 398 after ALE in the presence of dequalinium chloride (21 out of 32 populations). Interestingly, the highest resistance decline occurred in the clinical isolates 296 and 76 in both antibiotic-free environments. In the absence of dequalinium chloride, we observed a decline of up to 16-fold in the clinical isolate 296 and of up to 12-fold in the clinical isolate 76. In the presence of dequalinium chloride, we observed a decline of up to 16-fold in the isolate 76 and of up to 11-fold in the isolate 296. These results indicate that these two clinical isolates are the most likely to show significant decreases in ciprofloxacin resistance during periods of drug restriction. However, it is relevant to remark that ciprofloxacin resistance also declined in populations from the clinical isolates 258, 357, 225, and 398 (Fig. 3 and Supplementary Table 1), with AMR decline differing depending on the parental strain. These results suggest a strong influence of the genomic background on the compensatory evolution and on AMR decline that should not be underestimated.
Susceptibility to antibiotics from different structural families was analysed in populations (1 to 4) from 8 clinical isolates (64 populations) submitted to ALE assays in the absence of antibiotic (rich medium -LB- or rich medium supplemented with 10 µg/mL of dequalinium chloride -DC-) for 100 bacterial generations. Intensity of the colour is proportional to the log-transformed fold change of MIC of each replicate population (Rep) with respect to the MIC of the respective parental strain. Statistical significance was computed by non-parametric Kruskal–Wallis (Rank Sums) test followed by each-pair comparison test using the Wilcoxon method corrected by FDR. Only differences with FDR adjusted p-values of <0.05 were considered statistically significant. Changes of MICs above or below an increase or a decrease of 2-fold, respectively, were considered biologically relevant to classify a population as “resistant” (purple) or “susceptible” (yellow) with respect to its respective parental strain, as previously described26. Detailed information on the biological and statistical significance of the comparisons is presented in the Source Data. An important decline of ciprofloxacin MIC was observed in 43 out of 64 populations evolved in antibiotic-free environments (LB or DC), presenting 42 of these populations MIC values below the EUCAST clinical breakpoint (Fig. 3 and Supplementary Table 2). CIP ciprofloxacin, TOB tobramycin, IPM imipenem, CAZ ceftazidime, ATM aztreonam, FOF fosfomycin.
The figure shows the ciprofloxacin MIC (µg/mL) of each starting genetic background at time zero (t0) and the corresponding MIC (µg/mL) value acquired in the populations after 15 days of ALE in antibiotic-free environments: rich medium -LB- (left panel) and rich medium supplemented with 10 µg/mL of dequalinium chloride -DC- (right panel). Each of the points represents a population, and the colours indicate different clinical isolates. The size of the points is proportional to the number of populations presenting the same respective value. Raw MIC values appear in Supplementary Table 1. Points below the diagonal line indicate cases with a decrease of ciprofloxacin resistance level after compensatory evolution. Points below the horizontal line indicate cases with a MIC below the ciprofloxacin resistance clinical breakpoint (0.5 µg/mL). Most of the populations (42 out of 64) showed a decline of resistance after 15 days of ALE in the presence or absence of dequalinium chloride. Importantly, all these populations presented a ciprofloxacin MIC below the clinical breakpoint, with the degree of decline being dependent on the genetic context.
As hypothesised, the decline in ciprofloxacin resistance in antibiotic-free environments occurred, possibly due to compensatory evolution of fitness costs associated with ciprofloxacin resistance. In addition, the presence of dequalinium chloride, which does not select for stable ciprofloxacin resistance mutations23, did not prevent this decline of resistance from occurring (Figs. 2 and 3, and Supplementary Table 1). Altogether, these results support that ciprofloxacin resistance of clinically relevant nfxB defective mutants can be rapidly compensated in the absence of selective pressure, reverting back to susceptibility (based on standard laboratory antibiotic susceptibility testing). In addition, these results support that the combinations of ciprofloxacin-tobramycin or dequalinium chloride-tobramycin that we previously proposed for treating P. aeruginosa infections20,23,26, could be followed by a period of antimicrobial restriction if nfxB loss-of-function mutants are selected. This strategy would allow a rapid decline of resistance towards ciprofloxacin, possibly avoiding the selection of resistance to other antibiotics, something that we study below.
Evolution of cross-resistance and collateral sensitivity in antibiotic-free environments
We analysed if the populations resulting from 15 days of ALE in antibiotic-free environments could also have variations in the level of resistance to antibiotics from different structural families (tobramycin, aztreonam, fosfomycin, ceftazidime, and imipenem). To do that, we measured MICs for these antibiotics in the 64 populations and in the original parental strains (Fig. 2 and Supplementary Table 1) and evaluated the biological (fold-change ± 2) and statistical significance (adjusted p value < 0.05) of such changes. Although we observed that compensatory evolution led to a decrease of ciprofloxacin resistance in 43 out of 64 populations evolved in antibiotic-free environments, susceptibility to tobramycin reverted in just 12 populations (5 and 7 populations evolved in antibiotic-free environments with or without dequalinium chloride). An increase of fosfomycin resistance was observed in all the populations belonging to the clinical isolate 76 evolved in the absence or presence of dequalinium chloride. Moreover, an increase in aztreonam, ceftazidime, and imipenem resistance was observed in all the populations belonging to this clinical isolate. We also observed a decrease in ceftazidime resistance in three populations belonging to the clinical isolate 225 evolved in the absence of dequalinium chloride (Fig. 2 and Supplementary Table 1). Finally, a general decrease in aztreonam resistance was observed in the populations belonging to the clinical isolate 357 evolved in the absence or presence of dequalinium chloride. These results reflect the difficulty in finding a robust pattern of susceptibility that emerges in different clinical strains in the absence of selective pressure. It also highlights the necessity of identifying prevalent resistance mutations (i.e. nfxB defective variations) that can be easily compensated, causing a robust decline of AMR (i.e. ciprofloxacin).
Decline of ciprofloxacin resistance is due to mutations in the operon encoding the MexCD-OprJ efflux pump
The DNA of the populations from the clinical isolates 258, 296, 357, 76, 225 and 398, which showed a decline of ciprofloxacin resistance in antibiotic-free environments, was subjected to whole-genome sequencing. Sequencing reads were then compared to the genomes of their parental strains (Table 1)19, to obtain insights into the underlying molecular causes of the decline of ciprofloxacin resistance in antibiotic-free environments, in the presence or absence of dequalinium chloride (Supplementary Table S2 and Supplementary Data 1). We identified genetic variations (SNPs and Indels) in frequencies that ranged from 100% to 10% of the reads. First, it was analysed whether the molecular reversion of resistance47, by the restoration of the mutated allele to a wild-type one35,40, or the acquisition of new genetic variations could explain the observed decline of ciprofloxacin resistance. Importantly, none of the original nfxB mutations present in the parental strains reverted back to the wild-type sequence, indicating that compensatory evolution by secondary mutations, and not genetic reversion, was causing the observed ciprofloxacin resistance decline. During the analysis, the region comprising the MexCD-OprJ efflux pump encoding genes was observed to be frequently mutated across the different genetic backgrounds (Supplementary Table S2). In particular, we detected genetic modifications (SNPs and small insertions and deletions) in the genes mexC, mexD and the intergenic region between nfxB and mexCD-oprJ, but no mutations were detected in oprJ (Fig. 4). Further, a deletion (1.4 kb) comprising the gene nfxB and most of mexC was detected to be acquired in populations from the clinical strain 296, both evolved in the absence or presence of dequalinium chloride (Supplementary Table S2) and a 7.3 kb deletion containing nfxB, mexC and part of the genes mexD and morA was detected in one population from the clinical strain 76. The identified mutations clearly explain the decline of ciprofloxacin resistance observed in these populations. These results indicate that the overexpression of mexCD-oprJ due to mutations in nfxB, and the associated ciprofloxacin resistance, is likely compensated in absence of antibiotics mainly by mutations disrupting the structural genes encoding the efflux pump system MexCD-OprJ.
The position of the genetic modifications detected in the mexCD-oprJ operon after compensatory evolution in antibiotic-free environments with or without dequalinium chloride is indicated in the figure with blue lines. The position of the original nfxB mutations present in each parental clinical strain is indicated with red lines. The coloured shapes next to each genetic modification indicate the genetic context—clinical strain—in which the corresponding mutation was fixed. The genetic variations detected in each replicate evolved population during the analysis and their frequencies are indicated in Supplementary Table S2.
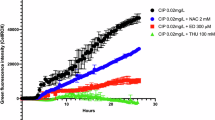
To further confirm that mutations in genes encoding the efflux pump, such as mexC, are responsible for decline of ciprofloxacin resistance and compensation of fitness costs, we resort to the simplest genetic context, the PA14 isogenic loss-of-function nfxB177 mutant. We isolated a clone from the nfxB177 population 2 evolved in the presence of dequalinium chloride, which only acquired a single mutation in mexC (Supplementary Table S2 and Supplementary Data 1). We measured ciprofloxacin resistance levels in the nfxB177mexC mutant and in the PA14 and nfxB177 strains. Ciprofloxacin resistance level of the double mutant declined from 2 µg/mL in nfxB177 to 0.094 µg/mL, the level of resistance of the PA14 susceptible wild-type strain. In addition, we analysed whether this decline of ciprofloxacin resistance associated with a mutation in mexC would also be responsible for compensation of fitness cost. For that, we compared the fitness of nfxB177mexC and nfxB177 mutants with respect to that of the PA14 wild-type (Fig. 5A). The acquisition of the mexC mutation restores fitness of nfxB177 mutant to levels of the wild-type strain. These results indicate that mutations in the gene mexC, encoding the efflux pump, can reduce levels of resistance to ciprofloxacin and fitness cost associated with the overproduction of the efflux pump. To go further and check whether this double mutant can really outcompete nfxB177 after 15 days of evolution, we performed competition assays between both strains during this period. We observed that the nfxB177 mutant, which overproduces the efflux pump, is outcompeted by both the wild-type strain and the nfxB177mexC double mutant (Fig. 5B, C). Fitness compensation can occur by reversion to the wild-type allele or because of mutations in different gene(s). Here we see that the second event is the cause of fitness recovery in the evolved strains. The reason for the selection of secondary compensatory mutations in mexC instead of reversion of original nfxB mutations to the wild-type sequence may be that the second event is less frequent to occur, as expected40,41, and/or that the nfxB177mexC mutant outcompetes the wild-type strain. Competition assays between both strains were performed, showing that the nfxB177mexC double mutant do not outcompete the wild-type strain after 15 days of evolution (Fig. 5D). Hence the cause of selecting secondary compensatory mutations in mexC is that the mutational space to inactivate mexC is larger than the one required to restore the wild-type allele in the nfxB177 mutant.
A Relative fitness of PA14 (orange), nfxB177 (dark blue), and nfxB177mexC (light blue) isogenic strains. The fitness of each population was measured as the area under the growth curve recorded in LB antibiotic-free medium. Relative fitness of the strains was calculated relative to the PA14 wild-type parental strain. The mean ± SD of values from three replicates is represented. Statistical significance was computed by one-way ANOVA followed by Dunnett’s multiple comparison test, where **** (p < 0.0001). Relative fitness is lower in the nfxB177 mutant than in its parental strain PA14, and a secondary compensatory mutation in mexC revert the fitness to the wild-type levels. B, C, D Competition among PA14 (orange), nfxB177 (dark blue), and nfxB177mexC (light blue) isogenic strains. An equal amount of bacteria from strain PA14 and nfxB177 (B), nfxB177 and nfxB177mexC (C), or PA14 and nfxB177mexC (D) were cultured in LB antibiotic-free medium for 15 days with daily 1/100 dilutions in triplicates. CFUs/mL of each strain at 0, 3, 5, 7, 11, and 15 days of competition were measured and are represented as the mean ± SD of values from the three biological replicates. PA14 and the compensated nfxB177mexC outcompete nfxB177, while nfxB177mexC does not outcompete the PA14 wild-type strain.
Finally, and in addition to the mentioned mutations acquired in the region comprising the MexCD-OprJ efflux pump encoding genes, strains accumulated mutations in a large variety of genes in a clone type-specific manner (Supplementary Data 1). Genes targeted by mutations included flagella, pili, phenazines, and pyoverdine encoding genes underlining evolution to the specific ALE conditions. We did not find specific genetic variations that could clearly explain the cross-resistance and CS patterns of some of the evolved clinical strains (Supplementary Data 1). However, we cannot exclude that changes at the transcriptional level of genes encoding AMR determinants, or indirect effects caused by stress response or membrane restructuring, could be associated with susceptibility changes.
Relative fitness of nfxB defective clinical strain populations after ALE in antibiotic-free environments
Next, we analysed whether the observed decline of ciprofloxacin resistance, mainly associated with mutations in genes encoding the MexCD-OprJ efflux pump, was due to the compensation of fitness cost associated with nfxB mutations. For that, we compared the fitness of the populations from the clinical isolates 258, 296, 357 and 76, where a robust decline of ciprofloxacin resistance occurred in all the replicate populations, at least in one of the antibiotic-free environments, compared to their parental strains (Fig. 6A). The highest fitness improvement was observed in isolate 76 in both environments (One-way Anova followed by Dunnett’s multiple comparison test; p < 0.05; Fig. 6A and Supplementary Table 3), leading to a marked decline of ciprofloxacin resistance in these populations (up to 12- and 16-fold in the absence and presence of dequalinium chloride, respectively) (Figs. 6A and 3; Supplementary Tables 1 and 3). Importantly, the clinical strain 76 is the one originally presenting the highest fitness cost (Fig. 6B and Supplementary Table 4). However, we observed that the clinical strain 296, which presented a subtle initial fitness cost (Fig. 6B and Supplementary Table 4), also had a significant decline of resistance in antibiotic-free environments (up to 16- and 11-fold in the absence and presence of dequalinium chloride, respectively) (Figs. 6A and 3; Supplementary Tables 1 and 3) but, in this case, fitness was not improved (Fig. 6A and Supplementary Tables 3). This suggests that the compensatory evolution of fitness costs associated with resistance is strongly influenced by the set of mutations initially present in each parental strain. In other words, historical contingency not only restricts the evolution of resistance but also constrains compensatory evolution.
A Relative fitness of populations evolved after 15 days in antibiotic-free environments (rich medium -LB- or rich medium supplemented with dequalinium chloride -DC-). The fitness of each population was measured as the area under the growth curve. Relative fitness of the populations was calculated relative to their parental strains. The area below the curve of the clinical strains showing a robust decline of ciprofloxacin resistance in at least one of the environments (258, 296, 357 and 76), before and after antibiotic-free ALE, were recorded in antibiotic-free medium. Statistical significance was computed by one-way ANOVA followed by Dunnett’s multiple comparison test where * (p < 0.05), **** (p < 0.0001). Each population was used as a biological replicate for statistical analysis (n = 4). The populations belonging to the clinical strain 76 presented an increased fitness after evolution in both antibiotic-free environments. The centre of the box plot is denoted by the median, a horizontal line dividing the box into two equal halves. The bounds of the box are defined by the lower quartile (25th percentile) and the upper quartile (75th percentile). The upper and lower boundaries of the whiskers are the maximum and minimum values of the dataset, respectively. Raw data appear in Supplementary Table 3 and OD600nm vs time measurements are included in Supplementary Data 2. B Relative fitness of nfxB defective clinical strains used in this study. Growth curves of four different clinical strains (258, 296, 357 and 76) were recorded in antibiotic-free medium. The fitness of each isolate was measured as the area under the growth curve. Relative fitness of each clinical strain was calculated with respect to the clinical strain presenting the highest fitness (strain 357). Statistical significance was computed by one-way ANOVA followed by Dunnett’s multiple comparison test where ** (p = 0.0014), **** (p < 0.0001). Relative fitness is importantly lower in the isolate 76, which is one of the isolates where compensatory evolution of fitness cost clearly occurs. Raw data appears in Supplementary Table 4. The mean ± SD of values from three replicates is represented.
Discussion
Available information supports that reversion of resistance after periods of restriction in the use of an antibiotic is not as common as expected36,37,38,39. In fact, restriction on the use of an antibiotic in a specific geographic area does not always cause a reversion of resistance48. An important factor restricting this phenomenon is the antibiotic used to treat an infection36,38,40,49,50, since it determines the possible resistance mechanisms selected. In this sense, the fitness cost associated with AMR mutations strongly relies on the cellular functions altered31,51. It also relies on the environment52, something that may also affect compensatory evolution. In addition, CF airway environments are highly heterogeneous and show an extensive diversity, where different genotypes and resistance mechanisms may coexist53,54. Therefore, pre-existing resistant populations may follow different evolutionary landscapes in a CF environment, not always leading to compensatory evolution (and decline in AMR). This suggests that reversion of AMR is not a black-or-white issue and that the identification of unstable (reversible) mechanisms of resistance is of relevance for the design of evolution-based strategies to tackle AMR55.
For AMR mutations to be unstable when selective pressure ceases, fitness costs should be high or, at least, easily compensated by the acquisition of secondary mutations that reduce resistance levels56,57,58. While many studies have focused on the effect of fitness costs in the emergence of AMR, there are limited studies analysing the stability of AMR (and of their associated trade-offs, as CS). The extent to which compensatory evolution is contingent on the starting genetic background, on initial fitness costs, or even on the environmental conditions, has not been deeply analysed. In fact, some authors raised the question of the extent to which the initial fitness cost associated with the acquisition of resistance in a mutant could affect compensatory evolution40. A previous work described that the decline of resistance is partly associated with compensatory evolution of fitness costs, but that there is not a perfect correlation between the degree of compensation of fitness costs and the level of resistance decline41. Our results show that compensatory evolution of fitness costs associated with AMR is strongly contingent on the set of mutations originally present in each parental strain and that it is more important than the initial fitness cost in constraining compensatory evolution. Crucially, although decline of resistance is dependent on historical contingency, we observed a robust decline of ciprofloxacin resistance (below the EUCAST clinical breakpoint) after just 100 bacterial generations in 66% of the populations evolved in antibiotic-free environments. Although a deeper analysis will be required to know the extent to which these results are generalisable and if it is possible to apply this therapeutic strategy, this work shows the most robust and fast decline of resistance identified to date in clinical strains presenting prevalent mutations.
As we hypothesised, decline of ciprofloxacin resistance in antibiotic-free environments, in the presence or absence of dequalinium chloride, occurred. From a genetic point of view, a decline of AMR can occur by a molecular47 or phenotypic reversion of resistance35,40. In the first case, it is associated with the restoration of the wild-type allele. In the second, it is due to the acquisition of new genetic variations. Here, by whole-genome sequencing of all the populations presenting a decline of ciprofloxacin resistance, we identified compensatory mutations responsible for this phenotype. We found no reversion of original nfxB mutations to the wild-type sequence. In fact, compensatory mutations in the genes encoding the MexCD-OprJ efflux pump, such as mexC, whose expression is regulated by NfxB, seem to be responsible for the decline of ciprofloxacin resistance observed in these clinical strains. It has been previously stated that reversion to the wild-type allele occurs less frequently than compensatory mutations in secondary genes40,41, since the mutational space to inactivate a secondary gene is larger than the one required to restore the wild-type allele in a mutant30. In addition, secondary compensatory mutations may also be selected because their fitness is higher than that of the wild-type. Here we show that the reason for the selection of secondary compensatory mutations in mexC, instead of reverting the original nfxB mutation to the wild-type allele, does not rely on a higher fitness of the first. Hence, our results support that mutational space that allows mexC inactivation is larger than that leading to the wild-type allele in the nfxB177 mutant.
Altogether, these results suggest that ciprofloxacin resistance of clinically relevant nfxB defective mutants may be rapidly compensated in the absence of selective pressure, reverting back to susceptibility. This finding indicates that ciprofloxacin could potentially be reused in a short time after its restriction. However, it is important to remark that ciprofloxacin resistance is not only associated with nfxB loss-of-function mutations but, depending on the starting parental strain, can also be due to mutations in mexS and/or in gyrA/B20,26. Therefore, our results should be taken with caution, and a deeper analysis will be required to know the extent to which it is possible to apply this therapeutic strategy, based on exploiting the rapid decline of ciprofloxacin resistance, regardless of the ciprofloxacin pre-existing mutants causing the infection. This will be particularly relevant for persistent bacterial infections, because different resistant mutants can co-exist in a patient. It is also important to highlight that our results should not be generalised to other antibiotics. For that, new ALE assays would be required to detect prevalent resistance mutations that can be easily compensated, causing a robust decline of AMR during periods of drug restriction55.
Further, CS has been explored as another trade-off potentially exploitable to tackle AMR. To be exploited, CS must be robust and emerge in different genetic backgrounds20,26,59, since infections caused by P. aeruginosa in pwCF may contain different pre-existing resistant mutants54,60. In addition, an important requirement is that CS must be stable when antibiotic therapy ceases. The stability of CS, which has practically not been studied to date, is relevant for the implementation of evolutionary therapies based on the alternation or combination of drugs showing CS. We observed that compensatory evolution of fitness costs associated with ciprofloxacin resistance in antibiotic-free environments did not generally alter pre-existing CS to tobramycin. Therefore, it seems reasonable to propose implementation of periods of drug restriction after the application of ciprofloxacin and a switch back to ciprofloxacin, in particular when resistance to this antibiotic is due to loss-of-function mutations in nfxB. However, since preservation of CS to tobramycin was not robust, it is uncertain if the combination ciprofloxacin-tobramycin, which we previously proposed for the treatment of P. aeruginosa infections20,23,26, would be effective after a period of ciprofloxacin use restriction.
Finally, the application of cycling policies has been unsuccessful in some reports61,62. One of the reasons may be that these programs are chosen blindly, considering that any AMR mutation can be costly and assuming that AMR will disappear under exposure to the subsequent antibiotic63. Our purely evolutionary results allow us to inform clinicians about the possibility of using periods of restriction after the use of ciprofloxacin, especially if mutations in nfxB have been previously selected, since it renders decline of AMR. This may not be the case for other antibiotics, which indicates that, when deciding an antibiotic therapy for persistent bacterial infections, priority should be given to those drugs for which drug restriction periods are a real possibility. This approach could enhance the likelihood of using the same antibiotic later, even if the initial treatment fails and resistance develops, thus preserving its efficacy. We believe that the time has come to translate evolutionary knowledge into practical therapeutic strategies.
Methods
Growth conditions and antibiotic susceptibility tests
Bacteria were grown in Lysogeny Broth (LB) (Lenox, Pronadisa) at 37 °C and shaking of 250 rpm, in glass tubes. Antibiotic susceptibility was determined for ciprofloxacin, tobramycin, imipenem, ceftazidime, aztreonam, and fosfomycin at 37 °C, in Mueller-Hinton (MH) agar, using E-test strips (MIC Test Strip, Liofilchem®).
Adaptive laboratory evolution experiments
Independent bacterial populations from stock cultures of 8 different ciprofloxacin-resistant nfxB defective clinical strains of P. aeruginosa from the Copenhagen CF Centre, Department of Clinical Microbiology at Rigshospitalet, Denmark (Table 1)19 -4 replicates of each-, were subjected to ALE in the absence of antibiotic or in the presence of 10 μg/mL of dequalinium chloride during 100 bacterial generations, resulting in 64 bacterial populations. Cultures were grown at 37 °C and 250 rpm for fifteen days in independent glass tubes to avoid cross-contamination. Every day, the cultures were diluted (1/100), adding 10 µl of bacteria in 1 mL of fresh LB, either containing or lacking 10 μg/mL of dequalinium chloride. Each condition was maintained during the entire ALE. At the end of the experimental evolution, all the replicate populations were preserved at −80 °C. In addition, MICs of the antibiotic to which the clinical isolates were initially resistant (ciprofloxacin), as well as to other and varied antibiotics, were determined at 37 °C in MH agar, using E-test strips.
Growth measurements
Growth curves were obtained by inoculating overnight cultures, in triplicate, to a final OD600 nm of 0.01 in LB in a 96-well microtiter plate (NunclonTM Delta Surface). OD600 nm of the bacterial cultures was measured every 30 min during 20 h at 37 °C using a Spark 10 M Plate Reader (Tecan). Fitness (W) was determined as the area under the growth curve recorded in antibiotic-free medium. Fitness cost of each clinical strain population was calculated with respect to its parental strain as previously stated40, using the equation: 1- (Wmutant/Wparental strain) and was expressed as percentage.
Whole-genome sequencing and analysis
The extraction of the genomic DNAs of the 8 parental strains and the 64 evolved populations, as well as DNA quality analysis was performed by the Translational Genomics Unit (Instituto Ramón y Cajal de Investigación Sanitaria-Hospital Ramón y Cajal from Madrid). Genomic DNA of each population was extracted by ChemagicTM DNA Bacterial Kit H96 (CMG-799 Chemagic™) using the equipment Chemagic™ 360/MSMI (PerkinElmer). The quantity and quality of the DNAs were evaluated using QUBIT 1x dsDNA BR 500rx (Thermofisher, Cat. Q33266) and QUBIT 1x dsDNA HS 500rx (Thermofisher, Cat. Q33231), by Qubit 2.0 Fluorometer Invitrogen (Thermofisher) and Agilent 4150 TapeStation System. Libraries construction and Whole Genome Sequencing were performed by Illumina ADN Prep Kit (Illumina). The sequencing was done using an Illumina SP300 (SP Reagent kit 2×150 nt reads) on the NovaSeq6000 system (Illumina Technology) in Healthincode (Valencia, Spain). Coverage was higher than 150x for all samples.
Sequencing reads were trimmed, and low-quality reads and potential contamination from adapters were removed using Trimmomatic (v 0.35) tool (Bolger et al., 2014). Genomic analysis was conducted by BacDist (Migle Gabrielaite and Maanmi, 2020) to identify genomic variants relative to PAO1 reference genome (NCBI: NC_002516.2) in the clinical strains or PA14 to identify genomic variants in the strain nfxB177 (NCBI: NC_008463). BacDist filtered mutations to only retain variants with a mapping quality of at least 50, a minimum coverage of 10, and a minimum fraction of 50% of reads supporting the variant. To evaluate the presence of populations with reads below the 50% of reads thresholds, manual curation was applied after inspection using IGV. A minimum coverage of 10, and a minimum fraction of 50% of reads supporting the variant were maintained as cut-offs for the analysis. Manual inspection was only applied to nfxB, mexC, mexD and oprJ genes and the intergenic region between nfxB and mexC. The list of identified mutations is present in Supplementary Table S2.
Competition experiments
Overnight bacterial cultures from nfxB177mexC, nfxB177 and the parental strain PA14 were normalised to an OD600nm of 4.0 and then mixed in a 1:1 (nfxB177mexC: nfxB177, PA14: nfxB177 and PA14: nfxB177mexC) ratio, in triplicate. Cultures were grown at 37 °C and 250 rpm for 15 days. Every day the cultures were diluted (1/100) in fresh LB. The determination of colony-forming units of nfxB177mexC and nfxB177 in each competing population was carried out by plating dilutions of the populations containing these strains in LB and LB supplemented with 0.1 µg/mL of ciprofloxacin. The determination of colony-forming units of PA14 in each competing population was carried out by plating dilutions of the populations containing this strain in LB and LB supplemented with 0.2 µg/mL of tobramycin.
Statistical analyses
All data were checked for normality using the Shapiro-Wilk and Anderson-Darling tests. For MIC data, which did not pass the normality test, differences between strains, evolution (LB or DC) and antibiotics were computed using the non-parametric Kruskal-Wallis (Rank Sums) test, followed by each-pair comparison test using the Wilcoxon method. Data were further corrected for multiple comparisons using the FDR (False Discovery Rate) method. Data from the independently evolved populations were used as biological replicates. Only differences in MICs with adjusted p Values of <0.05 and MIC differences relative to the starting strain with a fold-change of > ±2 were considered statistically (p < 0.05) and biologically (fold-change > ±2) significant. Statistical analyses were conducted using JMP Pro 17 version 17.2.0. Fitness data were analysed by parametric one-way ANOVA followed by Dunnett’s multiple comparison test. Three independent biological replicates were used for each experiment. Statistical analyses were conducted using GraphPad Prism 10 version 10.4.0.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Whole genome sequencing data of the evolved populations is publicly available through the SRA number PRJNA1158267. Data needed to evaluate the conclusions of this work are present in the manuscript, Supplementary Materials and Supplementary Data. Source data are provided with this paper.
References
Santajit, S. & Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. Biomed. Res Int 2016, 2475067 (2016).
Rello, J. et al. A global priority list of the TOp TEn resistant Microorganisms (TOTEM) study at intensive care: a prioritization exercise based on multi-criteria decision analysis. Eur. J. Clin. Microbiol. Infect. Dis. 38, 319–323 (2019).
Martinez-Solano, L., Macia, M. D., Fajardo, A., Oliver, A. & Martinez, J. L. Chronic Pseudomonas aeruginosa infection in chronic obstructive pulmonary disease. Clin. Infect. Dis. 47, 1526–1533 (2008).
Talwalkar, J. S. & Murray, T. S. The Approach to Pseudomonas aeruginosa in Cystic Fibrosis. Clin. Chest Med. 37, 69–81 (2016).
Tummler, B., Wiehlmann, L., Klockgether, J. & Cramer, N. Advances in understanding Pseudomonas. F1000Prime Rep. 6, 9 (2014).
Rehman, A., Patrick, W. M. & Lamont, I. L. Mechanisms of ciprofloxacin resistance in Pseudomonas aeruginosa: new approaches to an old problem. J. Med. Microbiol. 68, 1–10 (2019).
Hoiby, N. Recent advances in the treatment of Pseudomonas aeruginosa infections in cystic fibrosis. BMC Med. 9, 32 (2011).
Lee, J. K., Lee, Y. S., Park, Y. K. & Kim, B. S. Alterations in the GyrA and GyrB subunits of topoisomerase II and the ParC and ParE subunits of topoisomerase IV in ciprofloxacin-resistant clinical isolates of Pseudomonas aeruginosa. Int J. Antimicrob. Agents 25, 290–295 (2005).
Feng, X. et al. Mutations in gyrB play an important role in ciprofloxacin-resistant Pseudomonas aeruginosa. Infect. Drug Resist. 12, 261–272 (2019).
Higgins, P. G., Fluit, A. C., Milatovic, D., Verhoef, J. & Schmitz, F. J. Mutations in GyrA, ParC, MexR and NfxB in clinical isolates of Pseudomonas aeruginosa. Int J. Antimicrob. Agents 21, 409–413 (2003).
Pasca, M. R. et al. Evaluation of fluoroquinolone resistance mechanisms in Pseudomonas aeruginosa multidrug resistance clinical isolates. Micro. Drug Resist 18, 23–32 (2012).
Bruchmann, S., Dotsch, A., Nouri, B., Chaberny, I. F. & Haussler, S. Quantitative contributions of target alteration and decreased drug accumulation to Pseudomonas aeruginosa fluoroquinolone resistance. Antimicrob. Agents Chemother. 57, 1361–1368 (2013).
Kohler, T. et al. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol. Microbiol. 23, 345–354 (1997).
Masuda, N. et al. Substrate specificities of MexAB-OprM, MexCD-OprJ, and MexXY-oprM efflux pumps in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44, 3322–3327 (2000).
Xu, C. et al. Mechanisms for development of ciprofloxacin resistance in a clinical isolate of Pseudomonas aeruginosa. Front. Microbiol. 11, 598291 (2020).
Jalal, S., Ciofu, O., Hoiby, N., Gotoh, N. & Wretlind, B. Molecular mechanisms of fluoroquinolone resistance in Pseudomonas aeruginosa isolates from cystic fibrosis patients. Antimicrob. Agents Chemother. 44, 710–712 (2000).
Jeannot, K. et al. Resistance and virulence of Pseudomonas aeruginosa clinical strains overproducing the MexCD-OprJ efflux pump. Antimicrob. Agents Chemother. 52, 2455–2462 (2008).
Imamovic, L. et al. Drug-driven phenotypic convergence supports rational treatment strategies of chronic infections. Cell 172, 121–134.e114 (2018).
Marvig, R. L., Sommer, L. M., Molin, S. & Johansen, H. K. Convergent evolution and adaptation of Pseudomonas aeruginosa within patients with cystic fibrosis. Nat. Genet. 47, 57–64 (2015).
Hernando-Amado, S. et al. Rapid phenotypic convergence towards collateral sensitivity in clinical isolates of Pseudomonas aeruginosa presenting different genomic backgrounds. Microbiol. Spectr. 11, e0227622 (2022).
Mulet, X. et al. Antagonistic interactions of Pseudomonas aeruginosa antibiotic resistance mechanisms in planktonic but not biofilm growth. Antimicrob. Agents Chemother. 55, 4560–4568 (2011).
Laborda, P., Alcalde-Rico, M., Blanco, P., Martínez, J. L. & Hernando-Amado, S. Novel inducers of the expression of multidrug efflux pumps that trigger Pseudomonas aeruginosa transient antibiotic resistance. Antimicrob. Agents Chemother. 63, https://doi.org/10.1128/aac.01095-19 (2019).
Hernando-Amado, S., Laborda, P. & Martinez, J. L. Tackling antibiotic resistance by inducing transient and robust collateral sensitivity. Nat. Commun. 14, 1723 (2023).
Pal, C., Papp, B. & Lazar, V. Collateral sensitivity of antibiotic-resistant microbes. Trends Microbiol. 23, 401–407 (2015).
Szybalski, W. & Bryson, V. Genetic studies on microbial cross resistance to toxic agents. I. Cross resistance of Escherichia coli to fifteen antibiotics. J. Bacteriol. 64, 489–499 (1952).
Hernando-Amado, S., Laborda, P., Valverde, J. R. & Martinez, J. L. Mutational background influences P. aeruginosa ciprofloxacin resistance evolution but preserves collateral sensitivity robustness. Proc. Natl. Acad. Sci. USA 119, e2109370119 (2022).
Andersson, D. I. & Levin, B. R. The biological cost of antibiotic resistance. Curr. Opin. Microbiol. 2, 489–493 (1999).
Bjorkman, J. & Andersson, D. I. The cost of antibiotic resistance from a bacterial perspective. Drug Resist. Update 3, 237–245 (2000).
Andersson, D. I. The biological cost of mutational antibiotic resistance: any practical conclusions?. Curr. Opin. Microbiol. 9, 461–465 (2006).
Martinez, J. L. & Baquero, F. Mutation frequencies and antibiotic resistance. Antimicrob. Agents Chemother. 44, 1771–1777 (2000).
Baquero, F., Alvarez-Ortega, C. & Martinez, J. L. Ecology and evolution of antibiotic resistance. Environ. Microbiol. Rep. 1, 469–476 (2009).
Tian, Z. X., Yi, X. X., Cho, A., O’Gara, F. & Wang, Y. P. CpxR activates MexAB-OprM efflux pump expression and enhances antibiotic resistance in both laboratory and clinical nalb-type isolates of Pseudomonas aeruginosa. PLoS Pathog. 12, e1005932 (2016).
Balasubramanian, D. et al. The regulatory repertoire of Pseudomonas aeruginosa AmpC ss-lactamase regulator AmpR includes virulence genes. PLoS ONE 7, e34067 (2012).
Wasels, F. et al. Fluoroquinolone resistance does not impose a cost on the fitness of Clostridium difficile in vitro. Antimicrob. Agents Chemother. 59, 1794–1796 (2015).
Levin, B. R., Perrot, V. & Walker, N. Compensatory mutations, antibiotic resistance and the population genetics of adaptive evolution in bacteria. Genetics 154, 985–997 (2000).
Andersson, D. I. & Hughes, D. Antibiotic resistance and its cost: is it possible to reverse resistance? Nat. Rev. Microbiol. 8, 260–271 (2010).
Schrag, S. J., Perrot, V. & Levin, B. R. Adaptation to the fitness costs of antibiotic resistance in Escherichia coli. Proc. Biol. Sci. 264, 1287–1291 (1997).
Enne, V. I., Livermore, D. M., Stephens, P. & Hall, L. M. Persistence of sulphonamide resistance in Escherichia coli in the UK despite national prescribing restriction. Lancet 357, 1325–1328 (2001).
Brolund, A., Sundqvist, M., Kahlmeter, G. & Grape, M. Molecular characterisation of trimethoprim resistance in Escherichia coli and Klebsiella pneumoniae during a two year intervention on trimethoprim use. PLoS ONE 5, e9233 (2010).
Dunai, A. et al. Rapid decline of bacterial drug-resistance in an antibiotic-free environment through phenotypic reversion. Elife 8 https://doi.org/10.7554/eLife.47088 (2019).
Hernando-Amado, S., Laborda, P., Valverde, J. R. & Martinez, J. L. Rapid decline of ceftazidime resistance in antibiotic-free and sublethal environments is contingent on genetic background. Mol. Biol. Evol. 39, https://doi.org/10.1093/molbev/msac049 (2022).
Vogwill, T., Kojadinovic, M. & MacLean, R. C. Epistasis between antibiotic resistance mutations and genetic background shape the fitness effect of resistance across species of Pseudomonas. Proc. Biol. Sci. 283, https://doi.org/10.1098/rspb.2016.0151 (2016).
Hernando-Amado, S., Sanz-Garcia, F. & Martinez, J. L. Antibiotic resistance evolution is contingent on the quorum-sensing response in Pseudomonas aeruginosa. Mol. Biol. Evol. 36, 2238–2251 (2019).
Trindade, S. et al. Positive epistasis drives the acquisition of multidrug resistance. PLoS Genet. 5, e1000578 (2009).
Salverda, M. L. et al. Initial mutations direct alternative pathways of protein evolution. PLoS Genet. 7, e1001321 (2011).
Kryazhimskiy, S., Rice, D. P., Jerison, E. R. & Desai, M. M. Microbial evolution. Global epistasis makes adaptation predictable despite sequence-level stochasticity. Science 344, 1519–1522 (2014).
Durao, P., Balbontin, R. & Gordo, I. Evolutionary mechanisms shaping the maintenance of antibiotic resistance. Trends Microbiol. 26, 677–691 (2018).
Sundqvist, M. et al. Little evidence for reversibility of trimethoprim resistance after a drastic reduction in trimethoprim use. J. Antimicrob. Chemother. 65, 350–360 (2010).
Seppala, H. et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N. Engl. J. Med 337, 441–446 (1997).
Gottesman, B. S., Carmeli, Y., Shitrit, P. & Chowers, M. Impact of quinolone restriction on resistance patterns of Escherichia coli isolated from urine by culture in a community setting. Clin. Infect. Dis. 49, 869–875 (2009).
Melnyk, A. H., Wong, A. & Kassen, R. The fitness costs of antibiotic resistance mutations. Evol. Appl. 8, 273–283 (2015).
Laborda, P., Martinez, J. L. & Hernando-Amado, S. Evolution of habitat-dependent antibiotic resistance in Pseudomonas aeruginosa. Microbiol. Spectr. e0024722 (2022).
Markussen, T. et al. Environmental heterogeneity drives within-host diversification and evolution of Pseudomonas aeruginosa. mBio 5, e01592–01514 (2014).
Lopez-Causape, C. et al. Evolution of the Pseudomonas aeruginosa mutational resistome in an international Cystic Fibrosis clone. Sci. Rep. 7, 5555 (2017).
Sanz-Garcia, F. et al. Translating eco-evolutionary biology into therapy to tackle antibiotic resistance. Nat. Rev. Microbiol. 21, 671–685 (2023).
Marcusson, L. L., Frimodt-Moller, N. & Hughes, D. Interplay in the selection of fluoroquinolone resistance and bacterial fitness. PLoS Pathog. 5, e1000541 (2009).
Nagaev, I., Bjorkman, J., Andersson, D. I. & Hughes, D. Biological cost and compensatory evolution in fusidic acid-resistant Staphylococcus aureus. Mol. Microbiol. 40, 433–439 (2001).
Bjorkman, J., Nagaev, I., Berg, O. G., Hughes, D. & Andersson, D. I. Effects of environment on compensatory mutations to ameliorate costs of antibiotic resistance. Science 287, 1479–1482 (2000).
Hernando-Amado, S., Sanz-García, F. & Martínez, J. L. Rapid and robust evolution of collateral sensitivity in Pseudomonas aeruginosa antibiotic-resistant mutants. Sci. Adv. 6, eaba5493 (2020).
Diaz Caballero, J. et al. Mixed strain pathogen populations accelerate the evolution of antibiotic resistance in patients. Nat. Commun. 14, 4083 (2023).
van Duijn, P. J. et al. The effects of antibiotic cycling and mixing on antibiotic resistance in intensive care units: a cluster-randomised crossover trial. Lancet Infect. Dis. 18, 401–409 (2018).
Schuetz, P. & Beardmore, R. E. Antibiotic strategies in critical care: back to square one?. Lancet Infect. Dis. 18, 360–361 (2018).
Nichol, D., Bonomo, R. A. & Scott, J. G. It’s too soon to pull the plug on antibiotic cycling. Lancet Infect. Dis. 18, 493 (2018).
Acknowledgements
We thank our colleague and friend Teresa Coque (Instituto Ramón y Cajal de Investigación Sanitaria-Hospital Ramón y Cajal, Madrid) for advising and helping us contacting the Translational Genomics Unit (Instituto Ramón y Cajal de Investigación Sanitaria-Hospital Ramón y Cajal, Madrid). We also thank Leticia Olavarrieta, from the Translational Genomics Unit, for the extraction of the genomic samples. Oihane Irazoqui from The Novo Nordisk Foundation Center for Biosustainability, the Technical University of Denmark, is thanked for her support and advice with whole genome sequencing and bioinformatics analysis. Trinidad Cuesta from CNB is thanked for her technical support in the competition experiments performed during the review process. Work was supported by MICIU/AEI/10.13039/501100011033 and FEDER (UE), grant PID2023-149913OA-I00 to SHA and by a Novo Nordisk Foundation (NNF) Challenge grant NNF19OC0056411 to HKJ. PL is the recipient of an ERS/EU RESPIRE4 Marie Skłodowska-Curie Postdoctoral Research Fellowship (Ref. nr: R4202305-01047; this project has received funding from the European Respiratory Society and the European Union’s H2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 847462). The Novo Nordisk Foundation is also acknowledged for supporting the NNF Center for Biosustainability (NNF18OC0052776) and the MICIU/AEI/ 10.13039/501100011033 is also acknowledged for supporting the National Center for Biotechnology through the “Severo Ochoa” grant CEX2023-001386-S.
Author information
Authors and Affiliations
Contributions
SHA designed the study and performed the experimental work. PL and RLR performed the bioinformatics analysis of the whole genome sequence data. JLM, PL, RLR, HKJ and SM participated in the design of the study. All authors participated in writing the manuscript and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Tomoya Maeda and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hernando-Amado, S., Laborda, P., La Rosa, R. et al. Ciprofloxacin resistance rapidly declines in nfxB defective clinical strains of Pseudomonas aeruginosa. Nat Commun 16, 4992 (2025). https://doi.org/10.1038/s41467-025-60330-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-60330-2
This article is cited by
-
Dissecting pOXA-48 fitness effects in clinical Enterobacterales using plasmid-wide CRISPRi screens
Nature Communications (2025)