Abstract
The activity of the telencephalon is shaped by pallial and subpallial GABAergic neurons, two large populations produced in the embryonic ganglionic eminence. However, knowledge about the fate specification of neuron subtypes is limited, especially whether there is a common mechanism directing the fate choice of pallial versus subpallial populations remains unknown, largely because each population comprises numerous subtypes. Here, using sc-RNA sequencing combined with loss-of-function we profile ganglion eminence lineages and find that Foxg1 deletion causes the pallial population to adopt subpallial fates in mice. We delineate developmental trajectories and reveal FOXG1-driven transcriptional programs that specify neuron subtypes in each GE lineage and transcription factors that direct lineage bifurcation decisions. We uncover a common mechanism that drives pallial fate over subpallial fate across ganglion eminence lineages. Our study illuminates the control of production between pallial and subpallial populations and offers transcriptomic insights into the pathogenesis of GABAergic neuron-related disorders.
Similar content being viewed by others
Introduction
The proper functioning of the mammalian telencephalon requires neural networks of interconnected excitatory and GABAergic inhibitory neurons. GABAergic neurons exert powerful and tight control over neuronal activity and consequently modulate information processing1. Telencephalic GABAergic neurons comprise two large populations: i) pallial interneurons, which are widely spread throughout the cortex and the olfactory bulb (OB), that regulating cognition, emotions, and instinctive behaviors; and ii) subpallial projection neurons/interneurons, which are positioned in the striatum, the globus pallidus (GP), and the amygdala, that constructing the basal ganglia neural circuits to control the movement, reward, and fear memory1,2,3,4,5,6,7. Both populations in turn comprise diverse neuron subtypes that are generated in the ganglion eminence (GE), a transient ventral structure of the developing telencephalon8. Dysregulation of the specification of GABAergic neuron subtypes leads to many neuropsychiatric disorders, including epilepsy, autism spectrum disorder (ASD), schizophrenia, and mixed dyskinesia9,10. A decreased number of pallial cortical interneurons (CINs) has been observed in humans and recapitulated in several animal models of ASD and schizophrenia11,12. Abnormal striatal GABAergic interneuron (SIN) function is also reportedly related to the onset of ASD and hyperkinetic disorders13,14. However, knowledge about the fate control of GABAergic neuron subtypes during development and the pathogenesis of GABAergic neuron-linked neuropsychiatric disorders is limited.
Telencephalic GABAergic neurons are generated in an extremely complex manner in GE that is roughly divided into three neurogenic progenitor zones, the lateral (which is further divided into dorsal and ventral parts), medial and caudal ganglionic eminence zones (LGE, MGE, and CGE, respectively)15,16. The dorsal-lateral GE (dLGE) and CGE give rise to pallial OB interneurons (OBINs), PROX1high CINs, subpallial amygdala neurons and GP arkypallidal neurons3,17,18, while the ventral LGE (vLGE) mainly produces striatal projection neurons (SPN)19. The MGE is responsible for generating pallial LHX6high CINs and subpallial GP prototypical neurons and SINs4,20,21. The dLGE/CGE and MGE except the vLGE in turn are capable of generating both pallial and subpallial populations. Progresses have been made on mechanisms underlying the specification of distinct GABAergic neuron subtypes. Transcription factors (TF) NKX2-1 and LHX6 are known to specifically regulate LHX6high CIN and SIN fates in the MGE lineage22,23. In the dLGE/CGE lineage, PROX1 is reportedly required for PROX1highCIN identity, and ETV1 and NR2F2 are involved in the development of OBINs and amygdala neurons, respectively24,25,26. However, knowledge of neuron subtype specification is still limited, especially whether there exists a common mechanism directing the fate choice of pallial versus subpallial populations remains unknown.
FOXG1, a member of the Forkhead-box family of transcription factors, acts as a major spatiotemporal regulator and has been linked to a broad array of developmental processes ranging from telencephalic pattern formation and cell fate specification to neuronal circuit specialization27,28,29,30. Clinically, patients with FOXG1 mutations suffer from severe epilepsy, mixed dyskinesia, ASD-like social deficits, stereotypies, and schizophrenia31,32, strongly suggesting a possible role of FOXG1 in the development of GABAergic neurons. The constitutive disruption of Foxg1 impairs the dorsal-ventral patterning of the telencephalon, resulting in the loss of the GEs that generate GABAergic neurons33. Our previous studies have shown that embryonic disruption of Foxg1 in the subpallium results in the failed tangential migration of CINs, while postnatal deletion of Foxg1 in GABAergic neurons leads to abnormal distributions in the cortex of CINS28,34,35. Recently it is reported that the dynamic expression level of FOXG1 is required for the entrance into the cortex, and for the tangential migration as well as for cortical laminar positioning of CINs36. However, whether FOXG1 controls the fate choice of pallial versus subpallial GABAergic neurons remains unclear.
In this study, we show that conditional disruption of Foxg1 in the mouse GE causes the pallial GABAergic population, including OBINs and CINs, to adopt subpallial fates across the MGE and dLGE/CGE lineages. By combining single-cell RNA sequencing (scRNA-seq), Cleavage Under Targets and Tagmentation (CUT&Tag), and in utero electroporation (IUE) methods, we delineated the developmental trajectories of GABAergic neuron subtypes in the MGE and dLGE/CGE lineages and revealed FOXG1-driven transcriptional programs that guide their lineage bifurcation decisions and differentiation trajectories. We identified common determinants downstream of FOXG1 that control the fate choice of pallial versus subpallial populations across the GE lineages. Our study provides transcriptomic insight into the production between the pallial and subpallial GABAergic neurons and the pathogenesis of GABAergic neuron-related neuropsychiatric disorders.
Results
Conditional knockout of Foxg1 in the ganglion eminence drives GABAergic neurons toward a subpallial fate
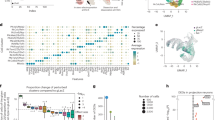
Given that the MGE and dLGE/CGE produce both pallial and subpallial GABAergic neurons (Fig. 1a), to explore the mechanisms underlying the fate control, we specifically disrupted Foxg1 in the GE by crossing Dlx5/6-Cre-ires-eGFP with Foxg1fl/fl. In control mice, EGFP-labeled GABAergic neurons were detected in both the pallium (cortex and OB) and the subpallium at E16.5; however, in Foxg1 cKO mice, the majority of EGFP+ neurons accumulated in the subpallium, with few EGFP+ neurons observed in the pallium (Fig. 1b). At E18.5, subpallial accumulation became even more pronounced (Supplementary Fig. 1a). To exclude the possibility that EGFP may be downregulated by loss of Foxg1, we next crossed Dlx5/6-Cre-ires-eGFP; Foxg1fl/fl to Ai9 reporter mice and found that at E18.5 many AI9+ cells populated in the cortex and the OB in the control mice, but few AI9+ cells were detected in the Foxg1 cKO cortex and the OB accompanied by the majority of AI9+ cells accumulated in the subpallium. The number of pallial AI9+ CINs and OBINs was significantly decreased in the Foxg1 cKO. This finding was coincident with that observed in Dlx5/6-Cre-ires-eGFP; Foxg1fl/fl mice (Fig. 1b). To determine whether this accumulation was a consequence of fate transformation, we used scRNA-seq to profile the developing subpallium at E16.5 (Supplementary Fig. 1b, c), a crucial time point during which GE derivatives are undergoing fate specification. Unsupervised clustering of cellular transcription profiles and uniform manifold approximation and projection (UMAP) dimensionality reduction revealed 4 known main cell populations based on previously reported marker genes, namely, progenitor cells and neurons derived from MGE, vLGE, and dLGE/CGE (Supplementary Fig. 1d, e, Supplementary Data 1).
a Schematic of the ganglion eminence derivatives and their corresponding pallial or subpallial fates. b Immunostaining for EGFP in control and Dlx5/6-Cre-IRES-EGFP;Foxg1fl/fl mice at E16.5, and immunostaining for AI9 in Dlx5/6-Cre-IRES-EGFP;Foxg1fl/fl;Ai9 and the control Dlx5/6-Cre-IRES-EGFP;Ai9 mice at E18.5 as well as quantitative analysis of AI9+ neurons in pallium and OB between two genotypes (n = 3 mice in each genotype). Data are presented as means ± SEM. Two-tailed unpaired t test; ****P < 0.0001; Pallium: ****P < 0.0001, OB: ****P < 0.0001. c Cell type identification in the control and Foxg1 cKO MGE lineages. UMAP plots of MGE cells. Color: cell identity. Bar plots showing the percentages of each MGE cell type in the two genotypes respectively. Blue box: pallial fate; orange box: subpallial fate. Immunostaining of anti-SST in control and Foxg1 cKO mice at E16.5. Arrows: subpallial SST+ interneurons; arrowheads: indicating pallial SST+ interneurons. d Cell type identification in the control and Foxg1 cKO dLGE/CGE lineages. UMAP plots of dLGE/CGE lineages. Color: cell identity. Bar plots showing the percentages of each dLGE/CGE cell type in the two genotypes respectively. Blue box: pallial fate, orange box: subpallial fate. Immunostaining of anti-EGFP with PAX6, anti-PROX1 and in situ hybridization of Nr2f2 at E16.5 brains. All the immunostaining and in situ hybridization experiments in (c, d) were repeated independently at least three times with similar results. Source data of (b–d) are provided as a Source Data file. MGE medial ganglionic eminence, dLGE dorsal-lateral ganglionic eminence, vLGE ventral-lateral ganglionic eminence, CGE caudal ganglionic eminence, OB olfactory bulb, GP Globus pallidum, CIN cortical interneuron, SIN striatal interneuron, OBIN olfactory bulb interneuron, SPN striatal projection neuron, UMAP uniform manifold approximation and projection.
To examine the effects of Foxg1 deletion in each GE lineage, we next isolated the MGE lineage based on the combination of Nkx2-1, Lhx6, and Lhx8 expression22,23 and the dLGE/CGE lineage based on Prox1 and Etv1 expression24,26 (Supplementary Fig. 1e) for detailed analysis. The vLGE lineage was excluded since vLGE produce subpallial striatal projection neurons (SPNs) (Fig. 1a). Two cluster stability metrics, reclassification statistics (SCCAF)37 and centroid-based reassignment (Reassign)38 were used to assess the clustering robustness of MGE and dLGE/CGE cells. The scores of SCCAF and Reassign under different resolutions were consistent (Supplementary Fig. 1f). MGE cells were annotated into 5 major categories, namely, progenitor cells, precursors, LHX6high CINs, SINs, and GP prototypic neurons (Fig. 1c, Supplementary Fig. 2a, Supplementary Data 1). The progenitor category further consisted of subclusters of G2/M phase cells (Cluster 12/11), and S phase cells (Cluster 8/7). The striatal interneuron category contained subclusters of Crabp1+ Htr3a+ SINs (Cluster 2) and Sst+Chodl+ (Cluster 1). The pallial LHX6highCIN category contains CXCR4highLHX6highCINs (Clusters 6/14) that represent the CINs undergoing tangential migration, and CXCR4lowLHX6highCINs (Cluster 4) representing CINs that have not initiated the tangential migration yet (Fig. 1c, Supplementary Fig. 2a, Supplementary Data 1). As expected, in Foxg1 cKO mice, the percentage of pallial LHX6high CINs among total MGE cells sharply decreased, accompanied by markedly increased percentages of subpallial SINs and GP prototypic neurons (Fig. 1c). Given that both LHX6high CINs and SINs contain SST+ interneurons39,40, we conducted anti-SST staining, and detected a subpallial accumulation of SST+ interneurons in Foxg1 cKO mice (Fig. 1c), supporting the observed changes in cell fates. SPNs are known to be generated in the vLGE and lack the expression of NKX2-1 and LHX619,41. We identified Cluster 3 as a new SPN subtype that expressed NKX2-1 (Fig. 1c), and exhibited a typical SPN molecular profile41 with high expression of TFs including Meis2, Foxp1, and Foxp2 (Supplementary Fig. 2b, Supplementary Data 1). We detected the distribution of NKX2-1+FOXP2+ and LHX6+FOXP1+ neurons in the striatum by immunostaining and Meis2 expression in control MGE by in situ hybridization (Supplementary Fig. 3a–c), which suggested that the MGE, not the vLGE, gave rise to NKX2-1+SPNs. We also crossed Nkx2-1-Cre with Ai9 reporter mice to trace the NKX2-1+ lineages. Double immunostaining of AI9 together with FOXP1 or FOXP2 detected small portions of AI9+FOXP1+ and AI9+FOXP2+ neurons in the striatum at E16.5 (Supplementary Fig. 3d), demonstrating that these neurons were SPNs derived from NKX2-1+ progenitors. The percentage of NKX2-1+ SPNs was slightly increased in Foxg1 cKO mice (Fig. 1c). To further cross-validate the clustering of the MGE cells, we accessed a published scRNA-seq dataset of human GW09 MGE cells8 containing 1,112 subpallial progenitors and 1,566 postmitotic MGE neurons to perform data integration with our control MGE cells and identified 3 GABAergic neuronal populations, including SINs, CINs, and subpallial GABAergic neurons (including GP prototypical neurons and SPNs) (Supplementary Fig. 3e, Supplementary Data 1), consistent with our MGE cell identities.
The dLGE/CGE cells were annotated as 6 known main cell populations, namely, progenitor cells, precursors, PROX1high CINs, SP8high OBINs, GP arkypallidal neurons, and amygdala neurons (Fig. 1d, Supplementary Fig. 4a, Supplementary Data 1). Similar to that observed in MGE lineage, in the Foxg1 cKO dLGE/CGE lineage, the percentages of pallial PROX1high CINs and OBINs were obviously decreased. In contrast, the percentages of subpallial amygdala neurons and GP arkypallidal neurons were strongly increased (Fig. 1d). Immunostaining for PAX6 and PROX1 supported the decreases in OBINs and CINs, and in situ hybridization of Nr2f2 supported the increase in amygdala neurons in Foxg1 cKO mice (Fig. 1d). We identified Cluster 10 as a new OBIN type (Fig. 1d) with high expression of the TF Id3 and typical OBIN markers Etv1 and Id2 but lacking Sp8 (Supplementary Fig. 4b, Supplementary Data 1), a TF known to control OBIN identity18, suggesting that Id3 may regulate ID3high OBIN specification instead of Sp8. ID3high OBINs displayed a scattered distribution in both embryonic and adult OBs42 (Supplementary Fig. 4c), suggesting that ID3high OBIN is a new neuron type rather than a transient developing state. The percentage of ID3high OBINs was sharply decreased in Foxg1 cKO mice (Fig. 1d). Taken together, these findings indicate that loss of Foxg1 in the GE drives GABAergic neurons toward a subpallial fate.
Transformation of LHX6high CINs toward subpallial trajectories begins at the precursor stage in the Foxg1 cKO MGE lineage
To examine the effects of Foxg1 cKO on fate choice during the MGE lineage development process, we analyzed the developmental trajectories of MGE lineages by applying RNA velocity analysis43, an unbiased method that infers cell differentiation states and trajectories based on the ratio of unspliced RNA to spliced RNA. In the control mice, velocity streamlines mapped two main trajectories that reflected the pseudotime order of the MGE lineage: i) which developed from progenitor (Cluster 8, 11, 9) to precursor (Cluster 0) and toward the three well-known neuronal subtypes, LHX6high CINs (Clusters 4, 6, and 14), SINs (Clusters 2 and 1), GP prototypic neurons (Cluster 5); ii) which developed from progenitor (Cluster 8, 11, 9) and toward NKX2-1+SPNs (Cluster 3) (Fig. 2a). Note that LHX6high CINs, SINs and GP prototypic neurons shared a common precursor state, while NKX2-1+SPNs segregated earlier from progenitors (Fig. 2a). The developmental trajectories of the MGE lineages were validated by two more unbiased trajectory inference methods, CellRank44 and partition-based graph abstraction (PAGA)45 (Supplementary Fig. 5a, b). In the Foxg1 cKO MGE lineages, RNA velocity analysis showed that the orientation of the LHX6high CIN trajectory from the precursor stage shifted strongly toward the SINs and GP prototypic neurons, and the NKX2-1+ SPN trajectory did not change markedly (Fig. 2a).
a Trajectory analyses of the MGE lineage. Streamlines of RNA velocity indicate the forward developmental direction of MGE lineage, and the yellow dashed lines show the direction in which the pallial LHX6high CIN trajectory transformed towards the subpallial SIN and GP prototypic neuron trajectories in Foxg1 cKO mice. URD branching trees of the MGE lineage; roots are progenitors, tips are SINs, LHX6high CINs, GP prototypic neurons, and NKX2-1+ SPNs. Color: cell identity. b Double immunostaining of anti-NKX2-1 with LHX6 at E16.5, and quantitative analysis of LHX6+NKX2-1+ neurons among total LHX6+ neurons within the yellow-boxed regions (n = 4 mice in each genotype) showing an increased percentage in Foxg1 cKO mice. Data are presented as means ± SEM. Two-tailed unpaired t test; **P < 0.01, ***P < 0.001; Control vs. Foxg1 cKO: **P = 0.0012. Source data are provided as a Source Data file. c UMAP plots showing the subclusters of MGE precursors (Cluster 0) in control and Foxg1 cKO mice. d Box plots showing the expression levels of genes specific for pre-CINs and pre-SINs/GP prototypic neurons in two genotypes (in each genotype, GE cells from three mice were pooled together). The boxes indicate the median and quartiles, the whiskers indicate minimum and maximum values. Triangles in the box indicate mean expression values. Source data are provided as a Source Data file. e Pie plots showing a decreased percentage of Pre-CINs and an increased percentage of Pre-SINs/GP prototypic neurons among the Foxg1 cKO MGE precursors compared to that of the control mice.
To more intuitively show the entire process of cell differentiation from the initial progenitor stage to the terminal differentiated neuron stage and more convincingly illustrate pallial LHX6high CINs transformed toward the subpallial neuron fates after Foxg1 deletion, we further reconstructed a branching trajectory tree of MGE lineages based on the transcriptional similarity of pseudotime-ordered cells using the URD analysis46. Consistently, the URD trajectories showed that two developmental branches separated from MGE progenitors, one of which gave rise to NKX2-1+SPNs, while the other was initially shared by LHX6high CINs, SINs, and GP prototypic neurons before separating into three branches developing toward distinct pallial or subpallial fate (Fig. 2a). In the Foxg1 cKO, the URD tree exhibited a much shorter LHX6high CIN branch accompanied by much longer SIN and GP prototypic neuron branches compared to the control mice (Fig. 2a), suggesting that fewer LHX6high CINs but more SINs and GP prototypic neurons were differentiated during the developmental process of MGE lineages. These results demonstrate that pallial LHX6high CINs shifted strongly toward the subpallial neuron fates in the Foxg1 cKO MGE lineages.
It is known that in the MGE lineage, as development progresses, NKX2-1 expression is maintained in subpallial SINs and GP prototypic neurons but is downregulated in the pallial CIN trajectory, while LHX6 is upregulated in all three trajectories17,22,47. We thus examined NKX2-1 and LHX6, and found that in control mice, NKX2-1 was not detected in pallial LHX6+ CINs but was expressed in the majority of subpallial LHX6+ neurons. However, in Foxg1 cKO mice, the number of LHX6+ neurons expressing NKX2-1 in the subpallium significantly increased with few pallial LHX6+ CINs were observed (Fig. 2b). Taken together, these findings indicate that the trajectory of LHX6high CIN in Foxg1 cKO mice is transformed toward subpallial SIN and GP prototypic neuron fates in the MGE lineage.
Considering that Dlx5/6-cre medicated Foxg1 deletion initiated in dividing progenitors in the subventricular zone48, we reclustered common precursor Cluster 0 and found that in control mice, this cluster exhibited a subtype blueprint comprising pre-CINs, pre-SINs, and pre-GP prototypic neurons with a gradient of complementary expression of a set of TFs, suggesting skewing toward different fates (Fig. 2c, d, Supplementary Fig. 5c). Nkx2-1 and Lhx8 were highly expressed in pre-SINs and pre-GP prototypic neurons, in line with their known roles in specifying SINs and GP neurons17,22,47. Pre-CINs displayed high expression levels of Zeb2 and Cux2, consistent with their roles in regulating CIN development49,50 (Fig. 2c, d, Supplementary Fig. 5c). However, in Foxg1 cKO mice, Nkx2-1 was upregulated while Zeb2 and Cux2 were downregulated in pre-CINs. Lhx8 expression was particularly high in Foxg1 cKO pre-SINs and pre-GP prototypic neurons. Lhx6, which is required for all three neuron types, was upregulated in the entire precursor population of Foxg1 cKO (Fig. 2c, d, Supplementary Fig. 5c). The upregulation of Lhx6, a downstream target of NKX2-123, may be a consequence of Nkx2-1 upregulation in Foxg1 cKO mice, reflecting the increased subpallial fates. As expected, the percentage of pre-CINs among the total common precursors in Foxg1 cKO mice sharply decreased, and the percentage of pre-SINs/pre-GP prototypic neurons markedly increased in Foxg1 cKO mice (Fig. 2e). Overall, the transformation of LHX6high CINs towards subpallial trajectories of SINs and GP prototypic neurons begins at the precursor stage in the Foxg1 cKO MGE lineage.
FOXG1-driven transcriptional programs direct the fate choice of LHX6high CINs versus SINs/GP prototypic neurons in the MGE lineage
To explore the mechanism directing pallial versus subpallial fates in the MGE lineage, we first mapped the full developmental trajectories of neuron subtypes in the control MGE lineage by using dynamic RNA velocity analysis43,51 and identified the top 500 genes with the most dynamic expression along each trajectory (Fig. 3a, Supplementary Data 2). Nkx2-1 and Lhx8 were downregulated in the LHX6high CIN trajectory but maintained in the SIN and GP prototypic neuron trajectories; notably, Lhx8 was expressed at relatively higher levels in the GP prototypic neuron trajectory (Fig. 3b, Supplementary Fig. 6a), consistent with previous reports22,47. TFs, including Cux2 and Tcf4, and migration-related genes, including Epha5 and Erbb4, were upregulated in the LHX6high CIN trajectory, while in the SIN and GP prototypic neuron trajectories, were transiently upregulated in the precursor state and subsequently downregulated (Fig. 3b, Supplementary Fig. 6a). Dach1 and Pbx3 were upregulated in the SIN and GP prototypic neuron trajectories, respectively (Fig. 3b). Prox1 was downregulated in the LHX6high CIN trajectory but maintained both the SIN and GP prototypic neuron trajectories (Fig. 3b). In the cell type-specific state, previously known and new neuron subtype-specific genes (e.g., LHX6high CIN: Mef2c; SIN: Prokr2; GP prototypic neuron: Gbx1, Adarb2, Ncald) were identified here (Supplementary Fig. 6a). In the NKX2-1+ SPN trajectory, cells initially exhibited downregulation of E2f2 but later displayed sharp upregulation of a set of TFs required for SPN identity19,41, including Foxp1, Foxp2, and Meis2 (Supplementary Fig. 6b). Overall, we defined the specification of the control MGE lineage by the dynamic expression of genes to establish LHX6high CINs, SINs and GP prototypic neurons.
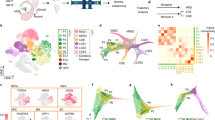
a Heatmap of the top 500 most dynamic gene cascades for the MGE lineage trajectories in the control mice. Cells of each trajectory are ordered in columns based on their pseudotime. Color of top bars: cell identity. Color bar: smoothened spliced mRNA counts. b Scatterplots showing the expression levels of representative genes along the developmental trajectories for LHX6high CINs, SINs, and GP prototypic neurons in the control mice. Dots: single cells. Vertical axis: normalized gene expression. Horizontal axis: cellular pseudotime of each trajectory. Color: cell identity. c Venn plot showing the number of overlapping genes among the top 500 pallial (LHX6high CIN) most dynamic genes, the top 500 subpallial (SIN/GP prototypic neuron) most dynamic genes, and the MGE DEGs. Bar charts showing the identification of the three category DEGs of MGE. Highlighted genes are direct targets of FOXG1 (refer to Supplementary Data 4, 5). d Gene programs of connected modules identified by NMF. The connections among the 8 gene modules of different developmental stages in the MGE lineage and the top 10 ranked TFs of each gene module are shown. Dots: NMF modules; Connections indicate the transcription similarity between two modules; Genes among the MGE DEGs are bold (refer to Supplementary Data 3). e Immunostaining against mCherry and LHX6 at E16.5 in WT mice (E12.5 IUE) and quantification analysis of the percentage of pallial or subpallial mCherry+LHX6+ neurons among total mCherry+LHX6+ neurons distributed in the entire pallial and subpallial regions in two groups (n = 4 mice in pCAGIG-mCherry group, n = 5 mice in pCAGIG-mSox4-cDNA-mCherry group). Data are presented as means ± SEM. Two-tailed unpaired t test; ***P < 0.001, ****P < 0.0001; Pallium: ****P < 0.0001, Subpallium: ***P = 0.0001. Source data are provided as a Source Data file. White arrows: pallial mCherry+LHX6+ neurons, yellow arrows: subpallial mCherry+LHX6+ neurons. DEGs differential expression genes, NMF non-negative matrix factorization; TFs transcription factors, IUE in utero electroporation, WT wild type.
To examine the effects of Foxg1 cKO on transcriptional programs directing the fate choice, we identified 4729 MGE differential expression genes (DEGs) between genotypes (Supplementary Fig. 7a, Supplementary Data 3). Gene Ontology (GO) analysis revealed roles for FOXG1 associated with the terms cell morphogenesis, cell fate commitment, neuron differentiation, and neuron migration (Supplementary Fig. 7b). The MGE DEGs were then compared with the top 500 most dynamic genes in SINs/GP prototypic neurons and LHX6high CINs and obtained three category DEGs (referred to as three category DEGs of MGE): i) 83 DEGs specific to pallial fate, ii)158 DEGs specific to subpallial fate, iii)107 DEGs required for both fates (referred to as shared DEGs) (Fig. 3c, Supplementary Data 4). Among the 83 pallial DEGs, Cux2 and Mef2c, which are known to be required for CIN identity in the MGE lineage1,50, were downregulated, consistent with the transformation of LHX6high CINs toward subpallial trajectories in Foxg1 cKO mice. Among the 158 subpallial DEGs, Prox1, which is known to be required for CGE-derived CINs26, was found upregulated in the MGE lineage in this study, demonstrating its involvement in the development of the MGE lineage. Among the 107 shared DEGs, St18, previously reported to direct MGE progenitors to adopt GP prototypic neuron identity at the expense of CINs52, was upregulated here, in line with the increased number of prototypic GP neurons and decreased number of LHX6high CINs in our Foxg1 cKO mice. We also detected downregulation of a set of migration-related genes including Erbb4, Epha4, Robo1, Nrp1, and Nav3, consistent with their previously reported roles34,36 (Fig. 3c). Notably, we identified several TFs whose roles in the MGE lineage were previously unidentified, including Ncald, Hivep3, Nfix, Dach1, Pbx3, Csrnp3, Nfib, Runx1t1, Tcf4 and Zbtb20, whose downregulation or upregulation led to fate transformation in Foxg1 cKO mice (Fig. 3c).
To identify genes directly targeted by FOXG1, we conducted the CUT&Tag experiment using the E16.5 control and Foxg1 cKO GE cells and identified 3350 direct targets of FOXG1 (Supplementary Fig. 7c, Supplementary Data 5), which we subsequently compared with the three category DEGs of MGE. Notably, 116 genes (approximately 33% of the three category DEGs of MGE) were direct targets of FOXG1 (highlighted in Fig. 3c, Supplementary Data 4). To find groups of genes (gene modules) that are coexpressed within cell populations at each developmental stage, we applied non-negative matrix factorization (NMF)53 to the control MGE lineages. This approach produced modules of covarying genes and described cell identities in terms of module expression, which is more robust than individual gene expression measurements. Moreover, cell fate specification processes can also be robustly described by the joint activity of gene modules. We defined 8 gene modules and linked modules from consecutive stages to define ‘gene programs’ corresponding to different trajectories of the MGE lineage (Fig. 3d, Supplementary Fig. 8a). To emphasize the functions of crucial genes in the gene modules for different MGE trajectory development, we analyzed the top 10 ranked TFs of each gene module and identified a total of 56 candidate TFs that direct the lineage bifurcation decisions of LHX6high CINs, SINs, GP prototypic neurons and NKX2-1 SPNs (Fig. 3d). Notably, 48 of the 56 TFs approximately 86%, including Tcf4, Dlx2, and Zeb2 (bold in Fig. 3d), were found in the MGE DEGs that were directly/indirectly regulated by FOXG1.
To validate the roles of candidate TFs identified in the key gene module that direct the fate choice of pallial versus subpallial GABAergic neurons, we selected Sox4 as a representative TF (highlighted in Fig. 3d) and overexpressed Sox4 in the wild-type subpallium at E12.5. The results showed that the percentage of pallial mCherry+LHX6+ CINs among total mCherry+LHX6+ neurons distributed in the entire pallial and subpallial regions was significantly increased when examined at E16.5 after Sox4 overexpression, with the percentage of mCherry+LHX6+ subpallial neurons was dramatically reduced (Fig. 3e), coincident with our findings that Sox4 was drastically downregulated in the Foxg1 cKO mice accompanied by the decrease of LHX6+ CINs. These results demonstrate that Sox4 facilitates the pallial fate of GABAergic neurons in MGE lineages. Overall, we identified FOXG1-driven transcriptional programs that direct LHX6high CINs versus SINs/GP prototypic neurons in the MGE lineage.
ID3high OBINs transformed toward GP arkypallidal neurons and SP8high OBINs/PROX1high CINs transformed toward amygdala neurons in dLGE/CGE lineages
To examine the effects of Foxg1 cKO on fate choice in the dLGE/CGE lineages we next compared the developmental trajectories of ID3high OBINs, SP8high OBINs, PROX1high CINs, GP arkypallidal neurons and amygdala neurons between genotypes. The CGE is a caudal extension of the dLGE, and many genes are coexpressed in these two regions16,54, making it difficult to separate the dLGE and CGE lineages. We found that in the control dLGE/CGE lineages, Foxp2 and Sp8 exhibited complementary expression patterns (Fig. 4a). Foxp2 was highly expressed in a lineage that started from progenitor (Cluster 18) and passed through precursor (Clusters 14 and 3) to ID3high OBINs (Cluster 10) and GP arkypallidal neurons (Cluster 4), while Sp8 was highly expressed in the lineage from progenitor (Cluster 0) and passed through precursor (Clusters 13 and 8) to SP8high OBINs (Cluster 2), PROX1high CINs (Clusters 1 and 19), and amygdala neurons (Clusters 11 and 16) (Fig. 4a). We next performed double immunostaining of SP8 and FOXP2 at E16.5 and showed that SP8 and FOXP2 were not co-expressed (Fig. 4a), demonstrating the complementary expression pattern of SP8 and FOXP2 in the dLGE/CGE cells. CellRank and PAGA further confirmed that dLGE/CGE consisted of the FOXP2highSP8low and FOXP2lowSP8high two lineages (Supplementary Fig. 9a, b). The branching specification trees exhibited that ID3high OBINs and GP arkypallidal neurons derived from the FOXP2highSP8low lineage, while the FOXP2lowSP8high lineage diverged into SP8high OBINs, PROX1high CINs, and amygdala neurons (Fig. 4b).
a Streamlines of RNA velocity mapped the trajectories of the dLGE/CGE lineage in the control mice; the blue dashed line highlights the two separate dLGE/CGE lineages, including FOXP2highSP8low and FOXP2lowSP8high lineages. UMAP plots showing the expression patterns of Foxp2 and Sp8; the black dashed lines highlight the FOXP2highSP8low and FOXP2lowSP8high lineages. Co-immunostaining against SP8 and FOXP2 at E16.5 in wild type mice (the experiment was repeated independently three times with similar results). b URD branching trees of the FOXP2highSP8low lineage and FOXP2lowSP8high lineage in the control mice. Roots are progenitors, tips are ID3high OBINs, GP arkypallidal neurons, SP8high OBINs, PROX1high PINs, and amygdala neurons. Color: cell identity. c Streamlines of RNA velocity mapped the trajectories of the dLGE/CGE lineage in Foxg1 cKO mice. The yellow dashed lines showing the pallial trajectories transformed toward subpallial trajectories. d URD branching trees of the FOXP2highSP8low lineage and FOXP2lowSP8high lineage in the Foxg1 cKO mice. e Volcano plot showing the dLGE/CGE DEGs between the two genotypes. Two-tailed unpaired t test with Benjamini–Hochberg-corrected p value. The tested genes of adjusted p value < 0.05 and absolute logFC more than 0.322 were differentially expressed. f Bubble plot showing GO terms enriched in the dLGE/CGE DEGs (Hypergeometric test, Benjamini and Hochberg correction, adjusted p values < 0.05).
In the Foxg1 cKO FOXP2highSP8low lineage, velocity streamlines showed that the orientation of the ID3high OBIN trajectory shifted markedly toward the GP arkypallidal neurons (Fig. 4c). A shorter ID3high OBIN branch, together with a longer GP arkypallidal neuron branch, was also identified in the URD tree (Fig. 4d), suggesting that fewer ID3high OBINs but more GP arkypallidal neurons were differentiated during the developmental process of FOXP2highSP8low lineage after Foxg1 deletion. In the Foxg1 cKO FOXP2lowSP8high lineage, velocity streamlines showed that the SP8high OBINs and PROX1high CINs shifted toward the amygdala neurons (Fig. 4c). Similarly, shorter SP8high OBIN and PROX1high CIN branches with a longer amygdala neuron branch were identified in the URD tree (Fig. 4d), suggesting that fewer SP8high OBINs and PROX1high CINs but more amygdala neurons were differentiated during the developmental process of FOXP2lowSP8high lineage after Foxg1 deletion. Thus, in the absence of Foxg1, ID3high OBINs transformed toward GP arkypallidal neurons in the FOXP2highSP8low lineage, while SP8high OBINs/PROX1high CINs transformed toward amygdala neurons in the FOXP2lowSP8high lineage. We next identified 3550 dLGE/CGE DEGs between genotypes (Fig. 4e, Supplementary Data 6). GO analysis revealed roles for FOXG1 associated with the terms neurogenesis, forebrain development, cell migration, and cell fate commitment (Fig. 4f). These findings support that FOXG1 and downstream transcriptional programs direct the fate choice in the dLGE/CGE lineages.
Characterizing unique transcriptional programs specifying each neuron type in the FOXP2highSP8low and FOXP2lowSP8high dLGE/CGE lineages
To explore transcriptional mechanisms directing pallial versus subpallial fates in the dLGE/CGE lineages, we first mapped developmental trajectories of neuron subtypes in the control FOXP2highSP8low and FOXP2lowSP8high dLGE/CGE lineages, and identified top 500 most dynamic genes of each neuron subtype along their developmental progression (Supplementary Fig. 10a, b, Supplementary Data 7). The trajectories for both ID3high OBINs and GP arkypallidal neurons in the FOXP2highSP8low lineage showed downregulation of Dach1, St18 and Ccnd2 but upregulation of Foxp2 and Runx1t1 (Fig. 5a, Supplementary Fig. 10c). Zfhx3 and Meis2 were strongly upregulated in the precursor state and subsequently downregulated in both trajectories (Fig. 5a, Supplementary Fig. 10c). Note that Id2 and Hivep3 were upregulated in the ID3high OBIN trajectory but were downregulated after transient upregulation in the precursor state of the GP arkypallidal neuron trajectory (Supplementary Fig. 10c). In contrast, Tshz2 was upregulated in the GP arkypallidal neuron trajectory but was downregulated after transient upregulation in the precursor state of the ID3high OBIN trajectory (Fig. 5a). At the cell-type-specific state, neuron subtype-specific genes were involved (e.g., ID3high OBIN: Tcf4, Erbb4, Cux2, Epha5, Plxna4; GP arkypallidal neuron: Prox1) (Fig. 5a, Supplementary Fig. 10c).
a, b Scatterplots showing the expression levels of representative genes along the developmental trajectories of the FOXP2highSP8low lineage and FOXP2lowSP8high lineage in the control mice. Dots: single cells. Vertical axis: normalized gene expression. Horizontal axis: cellular pseudotime of each trajectory. Color: cell identity. c Venn plot showing overlapping genes among the top 500 most dynamic genes of ID3high OBINs, the top 500 most dynamic genes of GP arkypallidal neurons, and the dLGE/CGE DEGs, and the identification of the three category DEGs of FOXP2highSP8low lineage. d Venn plot showing overlapping genes among the top 500 most dynamic genes of SP8high OBINs/LHX6high CINs, the top 500 most dynamic genes of amygdala neurons, and the dLGE/CGE DEGs, and the identification of the three category DEGs of FOXP2lowSP8high lineage. e Gene programs of connected modules identified by NMF. The connections among the 5 gene modules of different developmental stages in the FOXP2highSP8low lineage and the top 10 ranked TFs of each gene module are shown. Dots: NMF modules; Connections indicate the transcription similarity between two modules; Genes among the dLGE/CGE DEGs are bold (refer to Supplementary Data 6). f Gene programs of connected modules identified by NMF. The connections among the 8 gene modules of different developmental stages in the FOXP2lowSP8high lineage and the top 10 ranked TFs of each gene module are shown. Genes among the dLGE/CGE DEGs are bold (refer to Supplementary Data 6). g Immunostaining against mCherry and PROX1 at E16.5 in wild type mice (E12.5 IUE) and quantification analysis of the percentage of pallial or subpallial mCherry+PROX1+ neurons among total mCherry+PROX1+ neurons that distributed in the entire pallial and subpallial regions in two groups (n = 5 mice in each group). Data are presented as means ± SEM. Two-tailed unpaired t test; **P < 0.01, ***P < 0.001, ****P < 0.0001; Pallium: ****P < 0.0001, Subpallium: **P = 0.0011. Source data are provided as a Source Data file. White arrows: pallial mCherry+PROX1+ neurons, yellow irregular circle: subpallial mCherry+PROX1+ neurons.
In the FOXP2lowSP8high lineage, all the SP8high OBIN, PROX1high CIN and amygdala neuron trajectories showed upregulation of Sp8, Tcf4, Runx1t1, and Csrnp3 and downregulation of Lig1 and Mcm5 (Fig. 5b, Supplementary Fig. 10d). Meis2 and Pbx1 were transiently upregulated in the precursor state and maintained in the SP8high OBIN trajectory but were subsequently downregulated in the PROX1high CIN and amygdala neuron trajectories (Supplementary Fig. 10d). Nr2f1 was upregulated in both the PROX1high CIN and amygdala neuron trajectories (Fig. 5b). St18 was expressed at a relatively higher level in the PROX1high CIN and amygdala neuron trajectories than in the SP8high OBIN trajectory (Supplementary Fig. 10d). At later points, the cell-type-specific programs involved neuron subtype-specific genes (e.g., SP8high OBINs: Tshz1; PROX1high CINs: Epha5, Nfix, Prox1, and Htr3a; amygdala neurons: Wls) (Fig. 5b, Supplementary Fig. 10d). Thus, we defined the specification of FOXP2highSP8low and FOXP2lowSP8high lineages according to the dynamic expression of genes along their developmental trajectories to establish the identity of each neuron subtype.
FOXG1-driven transcriptional programs direct the fate choice of ID3high OBINs versus GP arkypallidal neurons and SP8high OBINs/PROX1high CINs versus amygdala neurons
To investigate the effects of Foxg1 cKO on the transcriptional programs directing the fate choice in the FOXP2highSP8low lineage, we compared the dLGE/CGE DEGs with the top 500 most dynamic genes in ID3high OBINs and GP arkypallidal neurons and identified three category DEGs (referred to as three category DEGs of FOXP2highSP8low dLGE/CGE): i) 111 DEGs specific to ID3high OBINs, ii) 113 DEGs specific to GP arkypallidal neurons, iii) 119 DEGs shared by the two neuron types (Fig. 5c, Supplementary Data 8). We detected TFs with known roles in specifying OBIN and/or GP arkypallidal neuron17,48,55,56, including Ascl1, Npas1, Nr2f2, Tshz1, Gsx2, Nfix, and Meis2, together with a set of migration-related genes, including Epha4 which has been reported to regulate the CIN migration in our previous study34, whose downregulation or upregulation leading to the transformation of ID3high OBINs toward GP arkypallidal neurons in the Foxg1 cKO FOXP2highSP8low lineage (Fig. 5c, Supplementary Fig. 11a). We also identified TFs with previously unexplored roles in the development of the FOXP2highSP8lowdLGE/CGE lineage, including Tcf4, St18, Dach1, Hivep3, Prox1, Runx1t1, Zfhx3, Ncald, and Csrnp3 (Fig. 5c, Supplementary Fig. 11a) that controlled the fate choice of neuron subtypes. Notably, 116 DEGs (approximately 34% of the three category DEGs of FOXP2highSP8lowdLGE/CGE) were identified as FOXG1 direct target genes after compared with CUT&Tag data (highlighted in Supplementary Fig. 11a, Supplementary Data 8).
To identify FOXG1-driven transcriptional programs controlling fate choice in the FOXP2lowSP8high dLGE/CGE lineage, dLGE/CGE DEGs were overlapped with the top 500 most dynamic genes of SP8high OBINs/PROX1high CINs and amygdala neurons and obtained three category DEGs of FOXP2lowSP8high dLGE/CGE (Fig. 5d, Supplementary Data 9). TFs including Tshz1 and Npas3 were required for pallial fate and Etv1, Prox1, Nr2f2, and Npas1 were involved in both fates in the FOXP2lowSP8high dLGE/CGE lineage (Fig. 5d, Supplementary Fig. 11b), consistent with their previously reported roles25,26,48,56,57. Migration-related genes, including Nrp1, Epha4, and Slit1, which have been reported to regulate the interneuron migration in our previous study34, were also involved in both fates in the FOXP2lowSP8high dLGE/CGE lineage. Moreover, we identified TFs including St18, Npas1, Nfix, Sox5, Dach1, Csrnp3, Tcf4, Runx1t1, Zfhx3, Basp1, and Klf7 and a set of migration-related genes, whose roles in the development of the FOXP2lowSP8high dLGE/CGE lineage were previously unexplored (Fig. 5d, Supplementary Fig. 11b). We isolated 107 FOXG1 direct target genes in the FOXP2low SP8high lineage after compared with CUT&Tag data (highlighted in Supplementary Fig. 11b, Supplementary Data 9).
We next explored gene modules of the control FOXP2highSP8low and FOXP2lowSP8high lineages by NMF analysis. We defined 5 gene modules in the FOXP2highSP8low lineage (Fig. 5e, Supplementary Fig. 12a), identified the top 10 ranked TFs of each gene module and obtained a total of 34 candidate TFs that direct the lineage bifurcation decisions of ID3high OBINs and GP arkypallidal neurons (Fig. 5e). Approximately 88% of the 34 TFs including Meis2, Myt1l, Csrnp3, Prox1, and Tcf4 were regulated by FOXG1 (bold in Fig. 5e). Meis2 has been reported to promote the identity of subpallial GABAergic projection neurons and inhibit the fate of pallial interneurons58, coincidentally, in our Foxg1 cKO mice Meis2 was sharply upregulated in the subpallium accompanied by the increase of subpallial GABAergic neurons, demonstrating Meis2 functions as a direct target of FOXG1 to control the fate choice of pallial OBINs versus subpallial GP arkypallidal neurons.
We defined 8 NMF gene modules in the FOXP2lowSP8high lineage (Fig. 5f, Supplementary Fig. 12b) and identified a total of 46 candidate TFs from the top 10 ranked TFs that direct the lineage bifurcation decisions of SP8high OBINs, PROX1high CINs and amygdala neurons (Fig. 5f). Thirty-nine among the 46 TFs, including Etv1, Foxp2, St18, Prox1, Nr2f2, Nfix, Tcf4, and Meis2 were regulated by FOXG1 (bold in Fig. 5f). We chose Hmgb1 as a representative TF (highlighted in Fig. 5f) in the key gene module that directs the fate choice of pallial CINs/OBINs versus subpallial amygdala GABAergic neurons (Fig. 5f) and overexpressed Hmgb1 in wild-type subpallium at E12.5 by IUE. The results showed that the percentage of pallial mCherry+PROX1+ CINs among total mCherry+PROX1+ neurons distributed in the entire pallial and subpallial regions was remarkably increased with the percentage of mCherry+PROX1+ subpallial neurons was significantly reduced at E16.5 (Fig. 5g), consistent with the downregulation of Hmgb1 accompanied by the reduction of PROX1+ CINs in our Foxg1 cKO mice. These results demonstrate that Hmgb1 facilitates the pallial fate of GABAergic neurons in the FOXP2lowSP8high dLGE/CGE lineage. Overall, these findings revealed that FOXG1-driven transcriptional programs direct the fate choice of ID3high OBINs versus GP arkypallidal neurons in the FOXP2highSP8low lineage and SP8high OBINs/PROX1high CINs versus amygdala neurons in the FOXP2lowSP8high lineage.
Identifying common molecular determinants to control pallial versus subpallial fate across MGE and dLGE/CGE lineages
Given that loss of Foxg1 driven pallial GABAergic neurons to transform toward subpallial fates in all MGE and dLGE/CGE lineages, there should exist a common mechanism that controls the fate choice. We then compared the union set of the three category DEGs of the MGE lineage with that of the FOXP2highSP8low and FOXP2lowSP8high lineages to explore common molecular determinants that direct fate choice. Ultimately, we identified 40 overlapping genes, including TFs, migration-related genes and non-coding RNA, with upregulation or downregulation in Foxg1 cKO MGE and dLGE/CGE to contribute to fate choice of pallial versus subpallial GABAergic neurons (Fig. 6a, b, Supplementary Data 10). Combined with the CUT&Tag results, we identified that 19 genes of the 40 overlapping genes were direct targets of FOXG1 (highlighted in Fig. 6b and bold in Supplementary Data 10). The binding signal of FOXG1 was significantly enriched at the promoter or the intron regions of the 19 genes in the wild-type GE, while the binding signals of FOXG1 were decreased in Foxg1 cKO GE (Fig. 7b, Supplementary Fig. 13a). It has been shown that impaired development of GABAergic neurons is associated with neurodevelopmental disorders10, noteworthy we found that 32 of the 40 common molecular determinants were reported to be risk genes of neuropsychiatric disorders including ASD, Schizophrenia, Epilepsy, Bipolar disorder, Attention deficit hyperactivity disorder, and Movement disorders59,60,61,62,63 (Supplementary Data 10). Thus, our results provide evidence that the 40 common molecular determinants driven by FOXG1 contribute to the pathogenesis by controlling the fate of GABAergic neurons.
a Venn plot showing the counts of overlapping genes among the union set of the three category DEGs in the MGE lineage with that in the FOXP2highSP8low and FOXP2lowSP8high lineages. b Identification of the 40 overlapping genes driven by FOXG1 to control the fate of pallial versus subpallial GABAergic neurons across the MGE and dLGE/CGE lineages. “┫” indicates the repression of subpallial GABAergic neuron fate by FOXG1. The arrow indicates the activation of pallial GABAergic neuron fate by FOXG1. Highlighted genes are direct targets of FOXG1. c Triple immunostaining for FOXG1, NKX2-1, and LHX6 at E13.5, E16.5 and E18.5 wild type mice. Quantification of FOXG1 expression levels by measuring fluorescence intensity in NKX2-1highLHX6- progenitors positioned in the MGE ventricular zone (white-boxed region1) at E13.5, in NKX2-1lowLHX6high pre-CINs (white-boxed region2, 5, 8) and NKX2-1highLHX6high pre-SINs/GP prototypical neurons positioned in the MGE subventricular zone (white-boxed region2, 5, 8), in NKX2-1highLHX6high SINs/GP prototypic neurons located in the subpallium (white-boxed region3, 6, 9), and in NKX2-1-LHX6high CINs dispersed in the cortex (white-boxed region4, 7, 10) at E13.5, E16.5, E18.5 (n = 3 mice per time point). Data are presented as means ± SEM. One-way ANOVA with Tukey post hoc test; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; NKX2-1highLHX6- MGE progenitors vs. NKX2-1low/-LHX6high pre-CINs (E13.5: **P = 0.0093); NKX2-1highLHX6- MGE progenitors vs. NKX2-1highLHX6high pre-SINs/GP prototypical neurons (E13.5: **P = 0.003); NKX2-1low/-LHX6high pre-CINs vs. NKX2-1highLHX6high pre-SINs/GP prototypical neurons (E13.5: ****P < 0.0001; E16.5: **P = 0.0043; E18.5: *P = 0.0458); NKX2-1highLHX6high SINs/GP prototypical neurons vs. NKX2-1-LHX6high CINs (E13.5: ****P < 0.0001; E16.5: **P = 0.0014; E18.5: ***P = 0.0004). Source data are provided as a Source Data file. Blue arrows: low levels of FOXG1 in NKX2-1highLHX6high pre-SINs/GP prototypical neurons and NKX2-1highLHX6high SINs/GP prototypical neurons, yellow arrows: high levels of FOXG1 in NKX2-1highLHX6- MGE progenitors, NKX2-1lowLHX6high pre-CINs, and NKX2-1-LHX6high CINs.
a Immunostaining of anti-PROX1, anti-ST18, in situ hybridization of Erbb4 at E16.5 and in situ hybridization of Tcf4 at E15.5 (from Allen Brain Atlas, http://developingmouse.brain-map.org/experiment/show/100051538) showing high expression in the MGE and the dLGE/CGE (arrows) but low expression in the vLGE (arrowheads) in control mice (the experiments were repeated independently at least three times with similar results). While this unique expression pattern of PROX1 and ST18 was disrupted in Foxg1 cKO mice (yellow arrows). b Genome browser view of FOXG1 CUT&Tag data at the Tcf4 locus in the wild type GE cells (WT-Anti FOXG1: treated with FOXG1 antibody, WT-Negative Control: without FOXG1 antibody) and Foxg1 cKO GE cells (treated with FOXG1 antibody). Shadow showing FOXG1-binding position on the Tcf4 locus. Bar plots showing the activation of FOXG1 at the Tcf4 promoter site detected by luciferase assay (n = 3 replicates of the luciferase assay). Data are presented as means ± SEM. Two-tailed unpaired t test; ***P < 0.001, ****P < 0.0001; pCAG-FOXG1 vs. pCAG: ***P = 0.0009. Source data are provided as a Source Data file. c Double immunostaining of anti-mCherry with PROX1 at E16.5 and quantification analysis of the number of mCherry+PROX1+ CINs in Foxg1 cKO cortex at E16.5 (n = 3 mice in pCAGIG-mCherry group, n = 4 mice in pCAGIG-mTcf4-cDNA-mCherry group), showing that Tcf4 restoration in the Foxg1 cKO CGE rescued the pallial CIN fate. Arrows: mCherry and PROX1 co-expressed pallial CINs. Data are presented as means ± SEM. Two-tailed unpaired t test; ***P < 0.001, ****P < 0.0001; pCAGIG-mTcf4-cDNA-mCherry vs. pCAGIG-mCherry: ***P = 0.0003. Source data are provided as a Source Data file.
Since the expression levels of FOXG1 are critical for neuron subtype specification30, we chose MGE linages as an example and performed immunostaining against FOXG1 at E13.5 and quantified the expression levels of FOXG1 by fluorescence intensity. In the MGE subventricular zone we detected a higher level of FOXG1 in pallial NKX2-1lowLHX6high pre-CINs and a lower level of FOXG1 in subpallial NKX2-1highLHX6high pre-SINs/GP prototypic neurons when compared to that in NKX2-1highLHX6- progenitors positioned in the MGE ventricular zone (Fig. 6c). Note the expression level of FOXG1 was also higher in pallial NKX2-1-LHX6high CINs dispersed in the cortex than that in subpallial NKX2-1highLHX6high SINs/GP prototypic neurons located in the striatum and the globus pallidus (Fig. 6c). Similarly, at E16.5 and E18.5, a higher level of FOXG1 was detected in pre-NKX2-1-LHX6high CIN when compared to that in the pre- NKX2-1highLHX6high SINs/GP prototypic neurons in the MGE subventricular zone, and a higher level of FOXG1 in NKX2-1-LHX6high CINs positioned in the cortex than that in NKX2-1highLHX6high SINs/GP prototypic neurons. These findings demonstrate that higher levels of FOXG1 facilitate the pallial fate of GABAergic neurons, and lower levels of FOXG1 are required for the subpallial fate (Fig. 6c).
We found that Tcf4, Prox1, St18, and Erbb4 all exhibited a unique expression pattern, with high expression in the MGE and dLGE/CGE, which give rise to both pallial and subpallial neuron subtypes, but low expression in the vLGE, which produce only subpallial neurons (Fig. 7a). Note the unique expression pattern was disrupted in Foxg1 cKO when examined by immunostaining of anti-PROX1 or ST18 (Fig. 7a), suggesting the fine expression levels of these genes are critical for the fate choice of pallial versus subpallial neurons. Cux2, Hivep3, and Runx1t1 were expressed at relatively higher levels in the embryonic pallium than in the subpallium (Supplementary Fig. 14a). In contrast, Dach1 exhibited relatively higher expression in the subpallium (Supplementary Fig. 14a). The unique expression patterns of these genes and their upregulation/downregulation coincided with the fate transformations observed in our Foxg1 cKO mice, providing further evidence for their roles in directing fate choice.
Given that Tcf4 is involved in the development of CIN subtypes and is linked to ASD, epilepsy, and schizophrenia60,61, we selected Tcf4 as a representative gene to verify its role in directing pallial versus subpallial fate as a downstream target of FOXG1. CUT&Tag analysis showed that the binding signals of FOXG1 were notably enriched at the promoter region of Tcf4 in wild-type GE, but the binding signal was remarkably reduced in the Foxg1 cKO GE (Fig. 7b). We performed a luciferase assay and detected the direct activation of Tcf4 by FOXG1 (Fig. 7b). We then restored Tcf4 by IUE at E13.5 in the Foxg1 cKO CGE. At E16.5, mCherry+ CINs were not detected in the Foxg1 cKO cortex, while in Tcf4-restored Foxg1 cKO mice mCherry+ CINs were detected in the cortex and expressed PROX1, a common marker for CGE-derived CINs (Fig. 7c). Quantification analysis showed that the number of PROX1+mCherry+ CINs was significantly increased in Foxg1 cKO cortex after Tcf4 restoration (Fig. 7c), demonstrating the role of Tcf4 in fate selection. Thus, we provide evidence that common regulators driven by FOXG1 control the fate choices of pallial versus subpallial GABAergic neurons across the MGE and dLGE/CGE lineages.
Discussion
In sum, we revealed FOXG1-driven transcriptional programs that control the developmental trajectories of GABAergic neuron subtypes and identified common molecular determinants that direct the fate choice of pallial versus subpallial neuron fates across the MGE and dLGE/CGE lineages. This study elucidates the mechanisms underlying the production between pallial and subpallial GABAergic populations.
We identified St18 as a common molecular determinant of pallial versus subpallial fate, in agreement with previous reports that St18 promotes GP prototypic neuron identity at the expense of CINs52. It has been reported that Nfix is involved in the development of OBINs, and Zfhx3 is required for SPN identity55,64. Here we revealed that Nfix and Zfhx3 direct the specification of pallial versus subpallial fates in the MGE lineage and the FOXP2lowSP8high dLGE/CGE lineage respectively. Dach1 and Pbx3 have been found to be highly expressed in SPNs with their roles unexplored41,65. In this study, we showed that Dach1 controls the fate choice of ID3highOBINs versus GP arkypallidal neurons, and Pbx3 controls the fate choice of LHX6high CINs versus SIN/GP prototypic neurons. We also identified a set of TFs that were not previously implicated in this process but contribute to specifying pallial versus subpallial fates in each distinct lineage, such as Ncald and Runx1t1 in the MGE lineage; Hivep3 in the FOXP2highSP8lowdLGE/CGE lineage; and Csrnp3 and Runx1t1 in the FOXP2lowSP8high dLGE/CGE lineage. Our study thus deepens the understanding of the specification of neuron subtypes in the MGE and dLGE/CGE lineages. Future studies exploring the roles of these TFs in distinct lineages could provide more insights into the TF regulatory network controlling GABAergic neuron development.
Previous studies have shown that the dorsal and intermediate domains of the MGE mainly produce pallial cortical interneurons, whereas the ventrocaudal domain specializes in the production of GP projection neurons4,16. It is also known that progenitors in the MGE ventricular zone divide to generate intermediate progenitors that are committed to specific fates, with GP projection neurons generated earlier than interneurons49,66. In this study, we found that in MGE-lineage GP prototypic neurons, SINs and pallial LHX6high CINs originate from a common progenitor pool, and subsequently skew toward different fates. Similarly, the developmental trajectories of GP arkypallidal neurons and ID3high OBINs share a common precursor state in the FOXP2highSP8low dLGE/CGE lineage, and amygdala neurons, PROX1high CINs, and SP8high OBINs also originate from a common progenitor pool in the FOXP2lowSP8high dLGE/CGE lineage. These results offer insights into the spatiotemporal control of mechanisms underlying the diversification of GABAergic neurons. Due to limitations in anatomical sampling locations, several GABAergic neuron subtypes, such as preoptic area-derived CINs and MGE-produced amygdala neurons, were not investigated. Future studies of the entire ventral telencephalon could provide more insights into the development of these neuron types.
The CGE is considered a posterior extension of dLGE, rather than a distinct structure, and the two domains share many molecular markers16,54 that make it difficult to clearly distinguish the dLGE lineage from the CGE lineage. Here, we found that FOXP2 and SP8 display complementary expression patterns; thus, dLGE/CGE can be divided into FOXP2highSP8low and FOXP2lowSP8high lineages. We further revealed that ID3high OBINs and GP arkypallidal neurons were derived from the FOXP2highSP8low lineage, while SP8high OBINs, PROX1high CINs, and amygdala neurons were derived from the FOXP2lowSP8high dLGE/CGE lineage. Our findings help to elucidate the development of dLGE/CGE lineages.
It has been considered that SPNs originate from the vLGE19. Here, we identified a population of NKX2-1+ SPNs derived from the MGE that displayed a typical SPN molecular profile. We also found that NKX2-1+ SPNs segregated earlier from the other three neuron types in MGE developmental trajectories and further revealed transcriptional programs specifying NKX2-1+ SPNs. SP8 is considered to specify OBINs18. In this study, we identified an OBIN type in the FOXP2highSP8low dLGE/CGE lineage that expresses high levels of Id3 but lacks Sp8. The role of Id3 in the development of ID3high OBINs should be explored in future work. The present data therefore expand our understanding of the development of SPNs and OBINs. Future studies investigating the function of NKX2-1+SPN and ID3high OBIN in neuronal circuits would be particularly interesting.
FOXG1 functions extensively in the telencephalic development in a context-, lineage- and levels-dependent manner. Previously it was reported that FOXG1 expression is evident in postmitotic MGE-derived CINs, and then is downregulated when CINs tangentially migrate to the cortical plate but is upregulated in the radial migration inside the cortical plate36. In this study, we have found that when progenitors differentiate towards pallial GABAergic neurons FOXG1 needs to be upregulated, while when progenitors differentiate towards subpallial GABAergic neurons FOXG1 needs to be downregulated. Thus, higher levels of FOXG1 facilitate the pallial fate, and lower levels of FOXG1 are required for the subpallial fate. These findings expand our understanding of the regulatory mechanisms of FOXG1 in the development of GABAergic CINs and projection neurons.
Many neuropsychiatric disorders are caused by impaired GABAergic neuron development10. FOXG1 mutations cause ASD, schizophrenia, mixed dyskinesia, and severe epilepsy31. We found that many of the identified fate determinants driven by FOXG1 are known as risk genes for neurodevelopmental disorders. For example, of the common determinants across the MGE and dLGE/CGE lineages, Tcf4 is linked to ASD, Bipolar disorder, and schizophrenia, Erbb4 is linked to epilepsy, schizophrenia, and anxiety, and Ina is reported to be linked to dystonia59,60,61. Among the MGE lineage DEGs, Adgrl3 mutation leads to attention deficit hyperactivity disorder, and Tubb4a is related to dystonia; and of the dLGE/CGE lineage DEGs, Ank3 is reportedly linked to bipolar disorder67,68,69. Thus, our study offers new transcriptomic insights into the pathogenesis underlying FOXG1-related disorders as well as GABAergic neuron-related neuropsychiatric disorders. The analyses in this study were performed at E16.5, a key time point at which GABAergic neurons undergo postmitotic fate specification. The transcriptional programs identified at this developmental stage represent the most important fate determinants orchestrating GABAergic neuron specification. Given that gene expression during development is dynamic, regulators involved in fate specification at other time points remain to be explored in the future.
Methods
All animal studies were approved by the Southeast University Institutional Animal Care and Use Committee (#20211014001, Nanjing, China) and were performed by institutional and national guidelines.
Mice
ROSA-Ai9-tdTomato (stock no. 007905) and Nkx2-1-Cre (stock no. 008661) mice were purchased from the Jackson Laboratory. The Foxg1fl/fl mice were generated as previously described70. To obtain the Foxg1 conditional knockout mice, crossed Dlx5/6-Cre-IRES-EGFP48 with Foxg1fl/fl mice in this study. The Dlx5/6-Cre-IRES-EGFP; Foxg1fl/fl mice are referred to as Foxg1 cKO, and the Foxg1fl/fl mice are referred to as control. The day when detected the vaginal plug was assigned as E0.5. Unless noted otherwise, all experiments were performed using mice maintained on a CD1 background. The animals used for experiments include both male and female mice. All mice had free access to food and water, and were housed in the institutional animal care facility with a 12-hour light-dark schedule. The environmental temperature was maintained at 24°C, and the relative humidity was set at 40%. Unless noted otherwise, at the completion of all animal experiments, the mice were euthanized by CO2 inhalation.
Immunostaining and in situ hybridization analyses
Mice at E14.5, E16.5, and E18.5 were perfused with 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS), and brains were then dissected out, postfixed overnight at 4 °C, dehydrated in 30% sucrose in PBS, and sectioned on a cryostat (Leica, CM 3050S). For the immunostaining, in short, sections were permeabilized in PBS containing 0.1% Triton X-100 for 30 min, blocked with 10% calf serum for 2 h at room temperature (RT), and incubated in primary antibody diluted in blocking solution for 12–16 h at 4 °C. Sections were then incubated in secondary antibody for 3 h at RT. Immunostaining images were acquired with a confocal microscope (FLUOVIEW FV1000, Olympus), Adobe Illustrator, and Photoshop (Adobe). Antibodies for immunofluorescence are listed in Supplementary Data 11.
For in situ hybridization, digoxigenin (DIG) UTP (uridine 5′-triphosphate)–labeled RNA probes were used. Briefly, probes were obtained by PCR amplification (primers for PCR amplification are listed in Supplementary Data 12). Brain sections were hybridized with RNA probes at 65 °C 12–16 h, incubated with anti-digoxigenin-alkaline phosphatase antibody for 12–16 h at 4 °C, and then subjected to color development.
Tissue processing for scRNA-seq
E16.5 ganglion eminences (GEs) from Foxg1 cKO and control mice were dissected (in each genotype, GE cells from three mice were pooled together) and immediately submerged in/rinsed with fresh ice-cold Hanks’ balanced salt solution (Thermo Fisher, 12175-095). GEs were subsequently dissected and dissociated into a single-cell suspension using the Papain Cell Dissociation Kit (Miltenyi Biotec, catalog no. 130-092-628). In brief, subpallium tissues were dissociated in papain reagent containing 0.1% DNaseI at 37 °C with a shaking speed of 50 rpm for about 10 min. After re-adding the digestion solution, a second collection was performed following an additional 20 min of digestion to increase cell yield and viability. Cell suspensions were filtered using a 40 μm nylon cell strainer and red blood cells were removed by 1× Red Blood Cell Lysis Solution. Dissociated cells were washed with 1× DPBS containing 2% FBS. Cells were stained with 0.4% Trypan blue (Thermo Fisher, Cat. no. 15250061) to check the viability of Countess® II Automated Cell Counter (Thermo Fisher). The Chromium droplet-based sequencing platform (10x Genomics) was used to generate scRNA-seq libraries, following the manufacturer’s instructions. The cDNA libraries were purified, quantified using an Agilent 2100 Bioanalyzer, and sequenced on an Illumina Nova seq 6000.
scRNA-seq pre-processing, quality control, and initial analysis
Raw sequencing data of two samples, control and Foxg1 cKO, were first processed by Cell Ranger v5.0.0 pipeline for alignment, quality filtering, and UMI counting. The filtered cell-by-gene matrices from the two batches were subsequently integrated for initial analysis, using SCANPY v1.9.2 pipeline. To ensure the high quality of the integrated dataset, we established specific thresholds and excluded cells meeting certain criteria: those with over 10% of reads mapping to mitochondrial genes, fewer than 750 genes, or more than 5000 genes were removed. Additionally, genes expressed by fewer than 3 cells were filtered out from the dataset. Standard processing for integrated count matrix included normalization of each cell by total counts over all genes using SCANPY function “pp.normalize_total” (target_sum = 10,000; other parameters = default), and log+1-transformation (SCANPY function “pp.log1p”)71 of the results.
To distinguish cell populations from the subpallium population, Nkx2-1- or Lhx6- or Lhx8-expressing cells (>0.5 the log normalized count data) were considered MGE lineage (1775 cells from control; 2850 cells from Foxg1 cKO), whereas Nkx2-1- or Lhx6- or Lhx8-negative (<0.5 the log normalized count data) and Prox1- or Etv1-expressing cells (>0.5 the log normalized count data) were labeled as dLGE/CGE lineages (1956 cells from control; 2120 cells from Foxg1 cKO).
scRNA-seq clustering and cell-type annotation
The analysis of clustering and cell-type annotation was performed on the integrated GE cells, the integrated MGE cells, and the integrated dLGE/CGE cells separately. SCTransform normalization algorithm (https://github.com/saketkc/pySCTransform) was implemented for each raw cell-by-gene matrix, and 5000 highly variable genes were reserved for clustering72. We further performed principal component analysis (SCANPY function “pp.pca”) and retained the significant principle components (MGE: 50 PCs; dLGE/CGE: 40 PCs). To align batches, we identified each cell’s top k-nearest-neighbors in each batch (BBKNN) separately73, and produced a batch-corrected graph using SCANPY function external.pp.bbknn (neighbors_within_batch = 3; other parameters = default). With batch-corrected graphs, we performed Uniform Manifold Approximation and Projection analysis (SCANPY function “tl.umap”), and clustered the cells using the Leiden algorithm74,75 (SCANPY function “tl.leiden”). To annotate these cell populations, we performed a comprehensive survey of existing literature, and collected a set of reliable marker genes. In detail, we generated UMAP plots of expression maps for these marker genes, and annotated these clusters associated with their markers.
Cluster stability measurements
We calculated two statistical metrics, reclassification statistics (SCCAF)37 and centroid-based reassignment (Reassign)38, to measure the reliability of each cluster for each annotation, using the scTriangulate framework (v0.13.0)38. We generated five clustering label sets (number of MGE clusters: 10, 13, 15, 17, 20; number of dLGE/CGE clusters: 17, 19, 21, 23, 26) using the Leiden algorithm. Following the standard tutorial of the scTriangulate framework (see https://sctriangulate.readthedocs.io/en/latest/tutorial.html), we computed the metrics for these label sets.
Data integration
To support the analysis of cell identity assignments, we accessed the recently published dataset of developing human ganglionic eminence cells8 to perform data integration with the mouse MGE cells. To map the GW9 human progenitor and MGE postmitotic cells to mouse E16.5 MGE cells, raw counts of the two datasets were first normalized using the scanpy functions separately, including “pp.normalize_total” (target_sum = 10,000) and “pp.log1p”. Then, we calculated the top 50 PCA components and built a KNN graph (function: pp.neighbors; parameters: n_neighbors = 6) for two datasets separately. Second, we aligned the gene features by intersecting the expressed genes in the two datasets. Finally, we used the scanpy function “tl.ingest” to map E16.5 mouse MGE postmitotic cells to GW9 human MGE postmitotic cells.
Differential gene expression analysis and functional annotation GO term analysis
To identify differentially expressed genes between two genotypes for the MGE lineage and the dLGE/CGE lineage, we used differential expression testing (two-tailed unpaired t-test) with “tl.rank_genes_groups” function, which also returned logarithmic fold-changes (logFC) and Benjamini–Hochberg-corrected p values of tested genes. The tested genes of adjusted p value less than 0.05 and absolute logFC more than 0.322 were differentially expressed.
Gene ontology (GO) enrichment analysis was performed with ClusterProfiler v4.10.0 software76. For the two GO enrichment analyses, the MGE DEGs and the dLGE/CGE DEGs were used as foreground genes, while all genes of the MGE dataset and all genes of the CGE dataset were used as background genes respectively. Functional enrichment was determined using hypergeometric test (adjusted p value < 0.5; Benjamini and Hochberg correction; ont = “BP”) for the input gene sets. We manually selected GO terms associated with cellular fate specification for inspection.
Constructing single-cell developmental trajectories and identifying putative dynamic genes
RNA velocity analysis exploits relative abundance of unspliced and spliced mRNAs to estimate the rates of gene splicing and degradation, enabling us to infer single-cell developmental trajectories and detect putative dynamic genes51. The analysis was performed for the MGE lineage and the dLGE/CGE lineage separately on two genotypes using scVelo v0.2.5 software51.
First, loom-format files containing spliced and unspliced mRNA counts were generated separately from the BAM files outputted by Cell Ranger, using velocyto v0.17.17 (command: velocyto run10x) software43. Then, the loom files were merged into the processed count matrices of MGE lineage and dLGE/CGE lineage separately, and we computed the moments of spliced/unspliced abundances (scVelo function “pp.moments”) for velocity estimation. To infer the trajectories, RNA velocities for control and Foxg1 cKO data were separately computed using “scvelo.tl.velocity” function based on the steady model, and velocity graphs were constructed with “scvelo.tl.velocity_graph”. Finally, the velocity features of cells were projected onto UMAP embeddings to display their putative developmental trajectories (scVelo functions “pl.velocity_embedding_stream”).
To identify dynamic genes, we ran the likelihood-based dynamical model, which generalized RNA velocity to systems with transient cell states (scVelo functions “tl.recover_dynamics” and “tl.velocity”) using standard workflow and default parameters. The dynamic genes were characterized by high likelihood of cellular unspliced and spliced abundances. To display the dynamic genes, we recovered the latent time (pseudotime) of the underlying cellular processes (“tl.latent_time”), and the top 500 likelihood-ranked genes were selected to demonstrate their expression dynamics along pseudotime (pl.heatmap).
Trajectory analysis using CellRank and Partition-based graph abstraction (PAGA)
To complement the results of trajectory analysis, we further applied the CellRank44 and Partition-based graph abstraction (PAGA)45 to map the trajectory for the MGE cells and dLGE/CGE cells, respectively.
To generate the PAGA graph, we first utilized the scanpy framework by denoising the data counts using “tl.draw_graph” with default parameters. Then we built the KNN graph (function: pp.neighbors; parameters: n_neighbors = 20, use_rep = ’X_diffmap’) with the diffusion map and generated the PAGA graph using function “tl.paga”.
To recover the directed differentiation trajectory, we used the CellRank framework to compute directed transition probabilities based on the KNN graph and pseudotime. We calculated the diffusion pseudotime (DPT)77 and the Palantir pseudotime78 accordingly. The pseudotime was generated using the Palantir python package (version: 1.3.3) with functions including palantir.utils.run_diffusion_maps (parameters: n_components = 40), palantir.utils.determine_multiscale_space (n_eigs = 3), and palantir.core.run_palantir (start_cell: progenitor cells for MGE cells; progenitor cells for dLGE/CGE cells). Secondly, to compute a directed cell-cell transition matrix, we used “kernels.PseudotimeKernel” (parameters: time_key = “palantir_pseudotime”) and “compute_transition_matrix” (parameters: threshold_scheme = “soft”, b = 36, nu = 4) in the CellRank python package (version: 2.0.6). Finally, we plotted the streamlines of cell-cell transition in umap embeddings (“plot_projection” function).
Construction and analysis of branching transcriptional trajectories using URD
We constructed the branching trajectory trees of MGE, FOXP2highSP8low dLGE/CGE, and FOXP2lowSP8high dLGE/CGE lineages separately using URD v1.1.1 software46 on two genotypes. First, we created URD objects (“CreateURD”) containing scRNA-seq data with a standard pipeline including pre-processing, normalization, and dimension reduction. Then we generated a diffusion map using “calcDM” function from URD with knn = 50 and sigma.use = 16. We assigned the progenitors as the root (cluster8 for the MGE lineages, cluster18 for the FOXP2highSP8low dLGE/CGE lineages, and cluster5 for the FOXP2lowSP8high dLGE/CGE lineages).
To calculate pseudotime, we ran “flood” simulations (“floodPseudotime” function, with parameters n = 50, and minimum.cells.flooded = 2), and processed the simulations into a pseudotime. After that, LHX6high PINs (cluster14), SINs (cluster1), GP prototypic neurons (cluster5), NKX2-1+ SPNs (cluster3) were defined as tips for the MGE lineage tree; ID3highOBINs (cluster10), GP arkypallidal neurons (cluster4) were defined as tips for the FOXP2highSP8low dLGE/CGE lineage tree; and SP8highOBINs (cluster2), PROX1highPINs (cluster1), amygdala neurons (cluster16) were defined as tips for the FOXP2lowSP8high dLGE/CGE lineage tree.
To find the developmental trajectories, we first calculated transition probabilities between cells using “pseudotimeWeightTransitionMatrix” and “pseudotimeDetermineLogistic” functions with parameters optimal.cells.forward = 20 and max.cells.back = 20. Then we conducted random walks from the tips back to the root, using “simulateRandomWalkFromTips” function based on the biased transition probabilities. The simulated random walks were further converted into visitation frequencies, using “processRandomWalks”. Finally, branching trajectory trees were built using “buildTree” function with divergence.method = ”preference”, cells.per.pseudotime.bin = 10, bins.per.pseudotime.window = 5, p.thresh = 0.001.
NMF modules and connected programs
Non-negative matrix factorization53 (NMF) can identify NMF gene modules (metagenes, significant genes within cell populations) from scRNA-seq data. We applied the NMF analysis (https://github.com/YiqunW/NMF) (Farrell et al., 2018) on the log+1-normalized read counts of MGE, FOXP2highSP8low dLGE/CGE and FOXP2lowSP8high dLGE/CGE lineages separately in control data, and detected NMF gene modules within specified developmental stages. First, we divided a lineage into several developmental stages, and detected NMF gene modules within each stage by executing the “run_nmf.py”. The script required an assigned K value (number of putative gene modules) and the following parameters: -rep 5 -scl “median” -miter 10000 -perm True -run_perm True -tol 1e-6 -a 0 -init “nndsvd”. Various K values (from 1 to 10) were used to determine the optimal number of gene modules for each stage. The principle was to obtain low reconstruction error and high stability scores, and to produce the reasonable biological meanings for generated gene modules. Finally, modules were manually examined based on their top-ranked genes and marker genes associated with cell clusters. The stages and K values for the lineages were as follows: MGE lineages: progenitors (stage 1, K = 1), precursors (stage 2, K = 1), MGE-derived neurons (stage 3, K = 4), SST+ interneurons (stage 4, K = 2); FOXP2highSP8lowdLGE/CGE lineages: progenitors (stage 1, K = 1), precursors1 (stage 2, K = 1), precursors2 (stage 3, K = 1), FOXP2highSP8low dLGE/CGE-derived neurons (stage 4, K = 2); FOXP2lowSP8highdLGE/CGE lineages: Progenitors (stage 1, K = 1), precursors1 (stage 2, K = 1), precursors2 (stage 3, K = 2), FOXP2lowSP8high dLGE/CGE-derived neurons1 (stage 4, K = 3), FOXP2lowSP8high dLGE/CGE-derived neurons2 (stage 5, K = 1);
To define ‘gene programs’ representing different trajectories of MGE and dLGE/CGE lineages, we linked modules from consecutive stages. The top 400 genes in each module were used to calculate the overlapping scores between pairs of gene modules in adjacent stages, and to generate continuous links and avoid potential disconnections due to sparsity of sampling and sequencing, module connections between two stages apart were also taken into account. To decide the final connections between modules, we connected each module to its most overlapped module in the previous two stages. Gene programs (modules and links) for each lineage were annotated on the basis of the cell-typing marker genes within the top-ranked genes. The top 10 gene weight-ranked TFs in each module were selected to demonstrate their differentially expressed between two genotypes.
Annotation of genes using existing datasets
Integration of TF annotations with four databases, TRRUST2, TFdb, GO, and a mouse TF_lists from the cisTarget resources website (https://resources.aertslab.org/cistarget/). TRRUST2 (www.grnpedia.org/trrust/) is a reference database that uncovered human and mouse transcriptional regulatory relationships. TFdb (http://gerg.gsc.riken.jp/TFdb/) is a database to retrieve mouse TF genes and related genes. In the GO database, TFs are labeled GO:0003700, “DNA binding TF activity”.
CUT&Tag and identification of FOXG1-binding sites
The CUT&Tag assay79 was performed using E16.5 GEs dissected from wild-type and the Foxg1 cKO mice, and a FOXG1 rabbit polyclonal antibody. The cells isolated from the wild-type GEs were evenly divided into three samples, the two samples treated with FOXG1 antibody (listed in Supplementary Data 11) were referred to as replicate1 and replicate2 respectively, while the sample without FOXG1 antibody was referred to as negative control. The cells isolated from the Foxg1 cKO GEs (referred to as “Foxg1 cKO”) were also treated with FOXG1 antibody. Then, 1 × 105 cells were washed with 500 μl of wash buffer (Vazyme, TD903) [10× wash buffer (−), 50× protease inhibitor cocktail, ddH2O] and centrifuged at 600 × g for 5 min at RT. The cell pellets were resuspended in 100 μl of wash buffer. Concanavalin A-coated magnetic beads were washed twice with binding buffer (Vazyme, TD903) (10× binding buffer, ddH2O). Next, 10 μl of activated beads were added and incubated at RT for 10 min. Bead-bound cells were resuspended in 50 μl of antibody buffer (antibody buffer, 5% digitonin). Then, 1 μg of primary antibody (rabbit polyclonal anti-FOXG1 antibody) or nothing was added and incubated at 4° overnight. The primary antibody was removed using a magnet stand. Secondary antibody (1 μg, goat anti-rabbit; Vazyme, Ab207-01) was diluted in 50 μl of Dig-wash buffer [10× wash buffer (−), 50× protease inhibitor cocktail, ddH2O, 5% digitonin] and cells were incubated at RT for 1 h. Cells were washed three times with Dig-wash buffer to remove unbound antibodies. The Hyperactive pA/G-Transposon (2 µM; 2 µl) was diluted in 98 μl of Dig300 buffer [10× Dig-300 buffer (−), 5% digitonin, 50× protease inhibitor cocktail, ddH2O]. The cells were incubated with Dilute 100 μl of the pA/G-Tnp transposons at RT for 1 h. The cells were washed three times with Dig-300 buffer. Then, the cells were resuspended in 50 μl of diluted TTBL (5× Trueprep Tagment Buffer L, Dig300 buffer) and incubated at 37 °C for 1 h. Add 5 μl of Proteinase K, 100 μl of Buffer L/B, and 20 μl of DNA Extract Beads to each sample, fully mix by vortexing, and incubate at 55 °C for 10 min. For library amplification, 15 μl Fragmented DNA after purification was mixed with 25 μl 2× CAM (CUT&Tag Amplification Mix), and 5 μl of uniquely barcoded i5 and i7 primers from TruePrep Index Kit V2 for Illumina (Vazyme). A total of 50 μl of the sample was placed in a thermocycler and subjected to the following program: 72 °C for 3 min; 95 °C for 3 min; 11 cycles of 98 °C for 10 s, 60 °C for 5 s; 72 °C for 1 min and hold at 4 °C. To purify the PCR products, 100 μl VAHTS DNA Clean Beads (Vazyme #N411) were added and incubated at RT for 10 min. The libraries were washed twice with 80% ethanol and eluted in 22 μl of ddH2O.
The libraries were sequenced on an Illumina NovaSeq platform, and 150-bp paired-end reads were generated. Raw fastq reads were trimmed by Cutadapt to trim adapter and low-quality sequence, and then high-quality sequencing data (clean reads) were obtained. We utilized Bowtie2 to align clean reads with mouse genome (mm10), followed by a quality assessment of the alignment results to accomplish secondary data quality control. Model-based Analysis of ChIP-Seq (MACS2) was used for peak calling. Peak significance was assessed using a dynamic Poisson model to calculate p-values, followed by Benjamini–Hochberg correction to control the false discovery rate (FDR) at 5%. Based on the peak calling results from individual samples, we used the MAnorm to perform a differential analysis of peaks across three samples, aiming to identify sample-specific peaks and shared peaks within three groups. Bigwig file, which is used for the display of dense and continuous data as a graph, was generated by the bamCoverage command in deepTools. And, Integrative Genomics Viewer (IGV) was used for visualization.
Binding site of FOXG1 at Tcf4 loci was identified by CUT&Tag, and the conservation of the consensus binding site (TRTTTAY) for FOXG1 at each putative regulatory locus was further evaluated across species at the UCSC database (http://genome.ucsc.edu/) as previously reported70. A bioinformatics analysis using the UCSC Genome Browser identified the apparent conservation of a putative FOXG1 motif binding site, where was the highest peak within the promoter of Tcf4. A 4500-base pair (bp) fragment containing the binding site for FOXG1 was cloned into a luciferase reporter plasmid and then transfected into N2A cells together with an expression plasmid for FOXG1.
Luciferase assay
The overexpression vector of pCAG-Foxg1 (mouse, NM_008241) was customized from SYNBIO TECH (www.synbio-tech.com; Beijing, China). Reporter vector of pGL3-Tcf4-Pro (chr18:69340147-69344646) was homemade, in which the promoter fragment containing FOXG1-binding site of Tcf4 amplified by PCR (primers listed in Supplementary Data 12) was subcloned into the pGL3 basic vector between 5′ Xho I and 3′ Bgl II. CE Design V1.04 was used for primer design (Vazyme, Nanjing, China). Homologous recombination was performed with the ClonExpress II One Step Cloning Kit (Vazyme, catalog no. C112-01).
Neuro2a cells (RRID: CVCL0470, the cell bank of the Chinese Academy of Science, Shanghai, China) were cultured in Dulbecco’s modified Eagle’s medium/F12 (Thermo Fisher, catalog no. 11330032) containing 10% fetal bovine serum (Thermo Fisher, catalog no. 10099141 C), penicillin (100 U/ml), and streptomycin (100 μg/ml). A total of 1 × 105 cells were seeded for transfection. The transfection complex was prepared including pCAG vector (control) or overexpression vector pCAG-FOXG1 (500 ng per cell), reporter vector (500 ng per cell), pRL-SV40 plasmid containing Renilla luciferase gene (50 ng per cell), and Lipofectamine 3000 (1.5 μl per cell) (Thermo Fisher Scientific, catalog no. L3000015). For normalization of luciferase activity, the pRL-SV40 plasmid encoding Renilla luciferase was used as an internal control reporter and was used to combination with any experimental reporter vector to cotransfect mammalian cells. Cells were harvested 24 h after transfection. Firefly and Renilla luciferase activities were measured using a Dual Luciferase Reporter Assay System (Promega, E1910). The ratio of firefly luciferase readouts and Renilla luciferase readouts was calculated to represent the activity of the reporter vector. Three replicate wells were made for each transfection. The experiment was repeated three times, and results were shown as the means ± SEM.
In utero electroporation
To restore Tcf4 in Foxg1 cKO GEs, plasmids of pCAGIG-Tcf4-cDNA (1 μg/μl) were injected directly into the lateral ventricles of E13.5 Foxg1 cKO embryos. pCAG-mCherry (1 μg/μl) was used as the control reporter vector. pCAGIG-Tcf4-cDNA plasmid (Tcf4-cDNA, NM_001083967) was customized from the You Bio Company (www.youbio.cn/; ChangSha, China).
To overexpress Sox4 or Hmgb1 in WT subpallium, plasmids of pCAGIG-Sox4-cDNA (2 μg/μl) or pCAGIG-Hmgb1-cDNA (2 μg/μl) were injected directly into the lateral ventricles of E12.5 WT embryos. pCAG-mCherry (2 μg/μl) was used as the control reporter vector. pCAGIG-Sox4-cDNA plasmid (Sox4-cDNA, NM_009238) and pCAGIG-Hmgb1-cDNA plasmid (Hmgb1-cDNA, NM_010439) were customized from the You Bio Company (www.youbio.cn/; ChangSha, China).
Electroporation was performed using Tweezertrodes (diameter, 5 mm; BTX, Holliston, MA, USA) with five pulses (34 V for E13.5 embryos, 38 V for E12.5 embryos) for 50-ms duration and 950-ms intervals using a square wave pulse generator (ECM830, BTX). The uterus was then returned to the abdominal cavity, and the inner skin and outer skin were sutured. The surgically manipulated pregnant mice were put on an electric heating plate (50 °C) until waking up. Progenies were euthanized at E16.5 for analysis.
Quantifications and statistical analysis
Quantification of immunostaining analysis. The confocal images were acquired by FluoView FV1000 confocal microscopy (Olympus) with 20× objective lens. A minimum of five successive coronal sections were selected. Quantitative analyses of neurons were consistently performed in the same area by direct comparisons of brain sections. Three to six brains of each genotype from different litters were used. The experiments of cell counting and quantification of fluorescence intensity were all cross-quantified blindly (i.e., the investigator was unaware of the genotypes). All results are shown as means ± SEM, except indicated otherwise. GraphPad Prism 8 software was used for statistical analyses. Two-tailed unpaired Student’s t test was used for two-group comparisons in cell counting, one-way analysis of variance (ANOVA) with Tukey post hoc test was used for multiple-group comparisons in quantification of fluorescence intensity. The following convention was used: *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, not significant.
In Fig. 1b, to measure Ai9+ neuron number at E18.5 in two genotypes, a box of 800 μm by 400 μm was overlaid in pallium and a box of 300 μm by 300 μm was overlaid in OB.
In Fig. 2b, to measure LHX6+NKX2-1+ neurons and LHX6+ neurons at E16.5 in control and Foxg1 cKO, a box of 800 μm by 500 μm was overlaid.
In Fig. 3e, to quantify the percentage of pallial or subpallial mCherry+LHX6+ neurons among total mCherry+LHX6+ neurons distributed in the entire pallial and subpallial regions in two groups, all mCherry+LHX6+ cells distributed in the entire pallial and subpallial regions were counted, and pallial or subpallial mCherry+LHX6+ neurons were counted respectively.
In Fig. 5g, to quantify the percentage of pallial or subpallial mCherry+PROX1+ neurons among total mCherry+PROX1+ neurons distributed in the entire pallial and subpallial regions in two groups, all mCherry+PROX1+ cells distributed in the entire pallial and subpallial regions were counted, and pallial or subpallial mCherry+PROX1+ neurons were counted respectively.
In Fig. 6c, for quantification of fluorescence intensity, sections were imaged with the same confocal acquisition parameters at the same level for each of the individual protein assessed with immunostaining. The mean fluorescence intensities were analyzed using FV10-ASW4.2 software, with the same settings. The mean fluorescence intensities of FOXG1 in NKX2-1highLHX6- progenitors in the MGE VZ were measured at E13.5 and in NKX2-1lowLHX6high pre-CINs and NKX2-1highLHX6high pre-SINs/GP prototypic neurons positioned in the MGE SVZ, NKX2-1-LHX6high CINs dispersed in the cortex, and NKX2-1highLHX6high SINs/GP prototypic neurons located in the striatum and the globus pallidus were measured at E13.5, E16.5, E18.5.
In Fig. 7b, the data of the luciferase assay were analyzed with two tailed unpaired Student’s t test for two-group comparisons.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The scRNA-seq and CUT&Tag data generated in this study have been deposited in the Gene Expression Omnibus (GEO) repository and are publicly available now. The accession number of the scRNA-seq data set is GSE270101 of CUT&Tag data sets are GSE270103 and GSE283968. The published scRNA-seq data referenced in our manuscript was accessed from the GEO database with accession code GSE135827. GSE270101; GSE270103; GSE283968; GSE135827. Source Data are provided with this paper. Additional microscopy raw data from this study are available from the corresponding author (Chunjie Zhao, zhaocj@seu.edu.cn) upon request. They are not deposited to a public database due to their large size and size limitations of online depositories. Source data are provided with this paper.
References
Kepecs, A. & Fishell, G. Interneuron cell types are fit to function. Nature 505, 318–326 (2014).
Wichterle, H., Turnbull, D. H., Nery, S., Fishell, G. & Alvarez-Buylla, A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development 128, 3759–3771 (2001).
Nery, S., Fishell, G. & Corbin, J. G. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat. Neurosci. 5, 1279–1287 (2002).
Flandin, P., Kimura, S. & Rubenstein, J. L. The progenitor zone of the ventral medial ganglionic eminence requires Nkx2-1 to generate most of the globus pallidus but few neocortical interneurons. J. Neurosci. 30, 2812–2823 (2010).
Kreitzer, A. C. & Malenka, R. C. Striatal plasticity and basal ganglia circuit function. Neuron 60, 543–554 (2008).
Tan, Z. et al. An ErbB4-positive neuronal network in the olfactory bulb for olfaction. J. Neurosci. 42, 6518–6535 (2022).
Foster, N. N. et al. The mouse cortico-basal ganglia-thalamic network. Nature 598, 188–194 (2021).
Shi, Y. et al. Mouse and human share conserved transcriptional programs for interneuron development. Science 374, eabj6641 (2021).
Wang, N. et al. Neuronal targets for reducing mutant huntingtin expression to ameliorate disease in a mouse model of Huntington’s disease. Nat. Med. 20, 536–541 (2014).
Marín, O. Interneuron dysfunction in psychiatric disorders. Nat. Rev. Neurosci. 13, 107–120 (2012).
Peñagarikano, O. et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell 147, 235–246 (2011).
Dong, J. et al. NRG1 knockdown rescues PV interneuron GABAergic maturation deficits and schizophrenia behaviors in fetal growth restriction mice. Cell Death Discov. 8, 476 (2022).
Kalanithi, P. S. et al. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc. Natl Acad. Sci. USA 102, 13307–13312 (2005).
Knowles, R., Dehorter, N. & Ellender, T. From Progenitors to Progeny: Shaping Striatal Circuit Development and Function. J. Neurosci. 41, 9483–9502 (2021).
Bulfone, A. et al. Spatially restricted expression of Dlx-1, Dlx-2 (Tes-1), Gbx-2, and Wnt-3 in the embryonic day 12.5 mouse forebrain defines potential transverse and longitudinal segmental boundaries. J. Neurosci. 13, 3155–3172 (1993).
Flames, N. et al. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J. Neurosci. 27, 9682–9695 (2007).
Nóbrega-Pereira, S. et al. Origin and molecular specification of globus pallidus neurons. J. Neurosci. 30, 2824–2834 (2010).
Li, J. et al. Transcription factors Sp8 and Sp9 coordinately regulate olfactory bulb interneuron development. Cereb. Cortex 28, 3278–3294 (2018).
Su, Z. et al. Dlx1/2-dependent expression of Meis2 promotes neuronal fate determination in the mammalian striatum. Development 149, dev200035 (2022).
Sultan, K. T. et al. Clonally related GABAergic interneurons do not randomly disperse but frequently form local clusters in the forebrain. Neuron 92, 31–44 (2016).
Marin, O., Anderson, S. A. & Rubenstein, J. L. Origin and molecular specification of striatal interneurons. J. Neurosci. 20, 6063–6076 (2000).
Flandin, P. et al. Lhx6 and Lhx8 coordinately induce neuronal expression of Shh that controls the generation of interneuron progenitors. Neuron 70, 939–950 (2011).
Sandberg, M. et al. Transcriptional networks controlled by NKX2-1 in the development of forebrain GABAergic neurons. Neuron 91, 1260–1275 (2016).
Allen, Z. J. 2nd, Waclaw, R. R., Colbert, M. C. & Campbell, K. Molecular identity of olfactory bulb interneurons: transcriptional codes of periglomerular neuron subtypes. J. Mol. Histol. 38, 517–525 (2007).
Tang, K., Rubenstein, J. L., Tsai, S. Y. & Tsai, M. J. COUP-TFII controls amygdala patterning by regulating neuropilin expression. Development 139, 1630–1639 (2012).
Miyoshi, G. et al. Prox1 regulates the subtype-specific development of caudal ganglionic eminence-derived GABAergic cortical interneurons. J. Neurosci. 35, 12869–12889 (2015).
Kumamoto, T. et al. Foxg1 coordinates the switch from nonradially to radially migrating glutamatergic subtypes in the neocortex through spatiotemporal repression. Cell Rep. 3, 931–945 (2013).
Shen, W. et al. Foxg1 regulates the postnatal development of cortical interneurons. Cereb. Cortex 29, 1547–1560 (2019).
Cargnin, F. et al. FOXG1 orchestrates neocortical organization and cortico-cortical connections. Neuron 100, 1083–1096.e1085 (2018).
Liu, J. et al. FOXG1 sequentially orchestrates subtype specification of postmitotic cortical projection neurons. Sci. Adv. 8, eabh3568 (2022).
Kortüm, F. et al. The core FOXG1 syndrome phenotype consists of postnatal microcephaly, severe mental retardation, absent language, dyskinesia, and corpus callosum hypogenesis. J. Med Genet. 48, 396–406 (2011).
Craig, C. P. et al. Diagnosis of FOXG1 syndrome caused by recurrent balanced chromosomal rearrangements: case study and literature review. Mol. Cytogenet 13, 40 (2020).
Xuan, S. et al. Winged helix transcription factor BF-1 is essential for the development of the cerebral hemispheres. Neuron 14, 1141–1152 (1995).
Yang, Y. et al. Impaired interneuron development after Foxg1 disruption. Cereb. Cortex 27, 793–808 (2017).
Chen, D. et al. Loss of Foxg1 impairs the development of cortical SST-interneurons leading to abnormal emotional and social behaviors. Cereb. Cortex 29, 3666–3682 (2019).
Miyoshi, G. et al. Developmental trajectories of GABAergic cortical interneurons are sequentially modulated by dynamic FoxG1 expression levels. Proc. Natl Acad. Sci. USA 121, e2317783121 (2024).
Miao, Z. et al. Putative cell type discovery from single-cell gene expression data. Nat. Methods 17, 621–628 (2020).
Li, G. et al. Decision level integration of unimodal and multimodal single cell data with scTriangulate. Nat. Commun. 14, 406 (2023).
Fisher, J. et al. Cortical somatostatin long-range projection neurons and interneurons exhibit divergent developmental trajectories. Neuron 112, 558–573.e558 (2024).
Muñoz-Manchado, A. B. et al. Diversity of Interneurons in the Dorsal Striatum Revealed by Single-Cell RNA Sequencing and PatchSeq. Cell Rep. 24, 2179–2190.e2177 (2018).
Anderson, A. G., Kulkarni, A., Harper, M. & Konopka, G. Single-cell analysis of Foxp1-driven mechanisms essential for striatal development. Cell Rep. 30, 3051–3066.e3057 (2020).
Lein, E. S. et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176 (2007).
La Manno, G. et al. RNA velocity of single cells. Nature 560, 494–498 (2018).
Weiler, P., Lange, M., Klein, M., Pe’er, D. & Theis, F. CellRank 2: unified fate mapping in multiview single-cell data. Nat. Methods 21, 1196–1205 (2024).
Wolf, F. A. et al. PAGA: graph abstraction reconciles clustering with trajectory inference through a topology preserving map of single cells. Genome Biol. 20, 59 (2019).
Farrell, J. A., et al. Single-cell reconstruction of developmental trajectories during zebrafish embryogenesis. Science 360, eaar3131 (2018).
Nóbrega-Pereira, S. et al. Postmitotic Nkx2-1 controls the migration of telencephalic interneurons by direct repression of guidance receptors. Neuron 59, 733–745 (2008).
Stenman, J., Toresson, H. & Campbell, K. Identification of two distinct progenitor populations in the lateral ganglionic eminence: implications for striatal and olfactory bulb neurogenesis. J. Neurosci. 23, 167–174 (2003).
McKinsey Gabriel, L. et al. Dlx1&2-dependent expression of Zfhx1b (Sip1, Zeb2) regulates the fate switch between cortical and striatal interneurons. Neuron 77, 83–98 (2013).
Cobos, I., Long, J. E., Thwin, M. T. & Rubenstein, J. L. Cellular patterns of transcription factor expression in developing cortical interneurons. Cereb. Cortex 16, i82–i88 (2006).
Bergen, V., Lange, M., Peidli, S., Wolf, F. A. & Theis, F. J. Generalizing RNA velocity to transient cell states through dynamical modeling. Nat. Biotechnol. 38, 1408–1414 (2020).
Nunnelly, L. F. et al. St18 specifies globus pallidus projection neuron identity in MGE lineage. Nat. Commun. 13, 7735 (2022).
Brunet, J. P., Tamayo, P., Golub, T. R. & Mesirov, J. P. Metagenes and molecular pattern discovery using matrix factorization. Proc. Natl Acad. Sci. USA 101, 4164–4169 (2004).
Ma, T. et al. A subpopulation of dorsal lateral/caudal ganglionic eminence-derived neocortical interneurons expresses the transcription factor Sp8. Cereb. Cortex 22, 2120–2130 (2012).
Heng, Y. H. et al. NFIX Regulates Proliferation and Migration Within the Murine SVZ Neurogenic Niche. Cereb. Cortex 25, 3758–3778 (2015).
Ragancokova, D. et al. TSHZ1-dependent gene regulation is essential for olfactory bulb development and olfaction. J. Clin. Invest. 132, 1214–1227 (2022).
Stanco, A. et al. NPAS1 represses the generation of specific subtypes of cortical interneurons. Neuron 84, 940–953 (2014).
Dvoretskova, E. et al. Spatial enhancer activation influences inhibitory neuron identity during mouse embryonic development. Nat. Neurosci. 27, 862–872 (2024).
Yang, J. M. et al. erbb4 deficits in chandelier cells of the medial prefrontal cortex confer cognitive dysfunctions: implications for schizophrenia. Cereb. Cortex 29, 4334–4346 (2019).
Li, H. et al. Disruption of TCF4 regulatory networks leads to abnormal cortical development and mental disabilities. Mol. Psychiatry 24, 1235–1246 (2019).
Chen, H. Y. et al. Psychiatric risk gene transcription factor 4 (TCF4) regulates the density and connectivity of distinct inhibitory interneuron subtypes. Mol. Psychiatry 28, 4679–4692 (2023).
Ikeda, M. et al. A genome-wide association study identifies two novel susceptibility loci and trans population polygenicity associated with bipolar disorder. Mol. Psychiatry 23, 639–647 (2018).
Zhou, X. et al. Integrating de novo and inherited variants in 42,607 autism cases identifies mutations in new moderate-risk genes. Nat. Genet. 54, 1305–1319 (2022).
Zhang, Z. et al. Zfhx3 is required for the differentiation of late born D1-type medium spiny neurons. Exp. Neurol. 322, 113055 (2019).
Castiglioni, V. et al. Dynamic and cell-specific DACH1 expression in human neocortical and striatal development. Cereb. Cortex 29, 2115–2124 (2019).
Lim, L., Mi, D., Llorca, A. & Marín, O. Development and functional diversification of cortical interneurons. Neuron 100, 294–313 (2018).
Bruxel, E. M. et al. Meta-analysis and systematic review of ADGRL3 (LPHN3) polymorphisms in ADHD susceptibility. Mol. Psychiatry 26, 2277–2285 (2021).
Krajka, V. et al. H-ABC- and dystonia-causing TUBB4A mutations show distinct pathogenic effects. Sci. Adv. 8, eabj9229 (2022).
Zhang, C., Xiao, X., Li, T. & Li, M. Translational genomics and beyond in bipolar disorder. Mol. Psychiatry 26, 186–202 (2021).
Tian, C. et al. Foxg1 has an essential role in postnatal development of the dentate gyrus. J. Neurosci. 32, 2931–2949 (2012).
Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19, 15 (2018).
Hafemeister, C. & Satija, R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 20, 296 (2019).
Polański, K. et al. BBKNN: fast batch alignment of single cell transcriptomes. Bioinformatics 36, 964–965 (2020).
McInnes, L., Healy, J., Saul, N., & Großberger, L. UMAP: Uniform Manifold Approximation and Projection. J. Open Source Softw. 3, 861 (2018).
Traag, V. A., Waltman, L. & van Eck, N. J. From Louvain to Leiden: guaranteeing well-connected communities. Sci. Rep. 9, 5233 (2019).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics 16, 284–287 (2012).
Haghverdi, L., Büttner, M., Wolf, F. A., Buettner, F. & Theis, F. J. Diffusion pseudotime robustly reconstructs lineage branching. Nat. Methods 13, 845–848 (2016).
Setty, M. et al. Characterization of cell fate probabilities in single-cell data with Palantir. Nat. Biotechnol. 37, 451–460 (2019).
Kaya-Okur, H. S., Janssens, D. H., Henikoff, J. G., Ahmad, K. & Henikoff, S. Efficient low-cost chromatin profiling with CUT&Tag. Nat. Protoc. 15, 3264–3283 (2020).
Acknowledgements
We thank Z. Yang at Fudan University for the gift of SP8 antibody. We thank Y. Wei and L. Liu for assistance with laboratory and animal care and other members of the laboratory for discussions. Funding: This work was supported by grants 31930045 from the National Natural Science Foundation of China, STI2030-Major Projects 2021ZD0202300 from the Ministry of Science and Technology of China, and 32470872 from the National Natural Science Foundation of China (to C.Z.); “the Fundamental Research Funds for the Central Universities” (2242023K40004) (to X.Z.). This work was also supported by a New Cornerstone investigator program and a SANS investigator program awarded to H.P., who is also a SANS Senior Investigator and a New Cornerstone Investigator.
Author information
Authors and Affiliations
Contributions
Conceptualization, C.Z. and H.P.; supervision, C.Z. and H.P.; methodology, C.Z., H.P., J.S. and L.S.; investigation, L.S., F.X., S.D., F.H., B.Z., P.Z., P.J and X.Z.; scRNA-seq analysis and visualization, L.S. and F.X.; writing—original draft, C.Z. and L.S.; writing—review and editing: C.Z. and all coauthors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Edmund Au and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sun, L., Xiong, F., Huang, F. et al. Transcriptomic insights into fate choice of pallial versus subpallial GABAergic neurons. Nat Commun 16, 5032 (2025). https://doi.org/10.1038/s41467-025-60338-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-60338-8