Abstract
The efficiency of the oxygen reduction reaction (ORR) is limited by the scaling relationship in the conventional oxygen associative pathway. To break such limitations, we present an approach to effectively activate the oxygen dissociative pathway through co-confining single p-block (In, Sn, Pb) atoms and interstitial H atoms within Pd metallenes, leading to good ORR performance. PdPbHx metallenes exhibit a high mass activity of 1.36 A mg−1 at 0.95 V (vs. RHE), which is 46.9 times higher than that of the benchmark Pt/C. The minimal performance decay after 50,000 potential cycles confirms a good stability. In situ vibrational spectroscopy investigations and theoretical calculations highlight that interstitial H atoms facilitate the direct dissociation of O2 while single Pb atoms enhance O2 adsorption strength. The electroactive PdPbHx metallenes is attributed to the up-shifted Pd-4d orbitals induced by H and Pb atoms. This research supplies critical inspiration for developing highly efficient ORR electrocatalysts.
Similar content being viewed by others
Introduction
Fuel cells hold immense potential as a pristine and efficient energy technology, offering zero carbon emissions, elevated energy efficiency, and reduced operating temperatures1,2,3. However, the sluggish reaction kinetics of the cathodic oxygen reduction reaction (ORR) pose a significant obstacle in electrocatalysis, hindering the widespread adoption of commercial fuel cells4,5. This challenge is primarily ascribed to the multiple proton-coupled electron transfer steps and corresponding oxygen-containing intermediates involved in the ORR process6,7,8,9. Specifically, most electrocatalysts exhibit an oxygen-associative mechanism that involves the adsorption of three intermediates: superoxide (*OOH), hydroxyl (*OH), and oxygen atom (*O)10,11,12,13,14,15,16,17. These intermediates exhibit the scaling relationship of ΔG*OOH ≈ ΔG*OH + 3.2 eV and ΔG*O ≈ 2ΔG*OH8,18. Consequently, optimizing the binding energy of each intermediate independently becomes a great challenge, resulting in a high theoretical overpotential required to drive the ORR process and subsequently diminishing ORR activitys6,19,20. According to the well-established ORR free energy diagram, the rate-determining step (RDS) in ORR is either O2 → *OOH or *OH → H2O7. Considering that the*OH → H2O step is unavoidable during the ORR process, there is a critical need for a practical approach that bypasses *OOH formation and directly dissociates the adsorbed oxygen molecule (*O2) into two *O species to further enhance ORR performance21,22. Nevertheless, achieving this objective entails the development of an efficient electrocatalyst with robust O2 adsorption capability, which is a longstanding challenge that persists in the field.
Two-dimensional metallenes, distinguished by their large specific surface area, high electrical conductivity, and maximized atomic utilization, are promising candidates in the domain of ORR23,24,25,26,27. Moreover, their adjustable microscopic structures make them an ideal platform for regulating the reaction mechanisms28,29. Prior research has explored diverse strategies such as alloying30,31, defect engineering32,33, and surface strain25,34 to modulate the d-band center of active sites in metallenes, thereby controlling their binding energies with oxygen-containing intermediates and improving ORR performances. However, it should be noted that the strong attractive interactions between surface atoms in metallenes inherently generate a compressed strain34, which results in relatively low binding energy with *O2. This induces a significant challenge in triggering the oxygen dissociation pathway within metallenes, as it requires overcoming a moderate energy barrier.
Herein, we present a series of highly efficient catalysts of Pd-based metallenes for alkaline ORR achieved by co-confining single p-block (In, Sn, Pb) atoms in the sub-surface and interstitial H atoms within the lattice of Pd metallenes. Among them, PdPbHx metallenes exhibit a high mass activity of 1.36 A mg−1 at 0.95 V (vs. RHE), which is 46.9 times higher than that of commercial Pt/C and shows a competitive performance with previous works on Pd-based catalysts. Notably, the PdPbHx metallenes demonstrate negligible activity decay even after undergoing 50,000 potential cycles, exhibiting no observable changes in structure and composition. In situ attenuated total reflection Fourier transform infrared (ATR-FTIR) spectroscopy analysis reveals that interstitial H atoms induce the oxygen dissociative pathway, while the confined single Pb atoms further enhance the pathway by promoting stronger O2 adsorption. Density functional theory (DFT) calculations elucidate that the improved electroactivity of Pd sites is potentially attributed to the introduction of interstitial H and single Pb atoms, which facilitates the adsorption of crucial intermediates and accelerates the electron transfer to promote the direct dissociation of O2. Additionally, the improved p-d overlapping improves the selectivity of the oxygen dissociative pathway due to the decreased energy barriers, leading to high ORR performance.
Results
Morphology and structure characterizations
Building on the fact that p-block elements exhibit good miscibility with Pd and can form various solid solutions with Pd (Supplementary Fig. 1), a series of PdMHx (M= In, Sn, Pb) metallenes were synthesized using a seed-mediated method, as illustrated in Fig. 1a and Supplementary Fig. 2. In this process, Pd and M ions were co-reduced epitaxially on the surface of Pd metallene seeds to form PdM metallenes with a defect-rich structure. The Pd atoms located at the defect areas of PdM metallenes exhibit higher energy and increased activity, making them susceptible to oxidation and etching by residual air35. These Pd atoms are subsequently re-deposited on the surface and edges of the PdM metallenes, effectively burying the M atoms within the lattice. As the Pd atoms from the interior and edges of the defect areas migrate, the pores gradually expand. Meanwhile, hydrogen atoms generated in situ from the decomposition of N,N-dimethylformamide (DMF) permeate into the lattice of PdM during the etching process, resulting in the formation of PdMHx nanorings (Supplementary Figs. 2–8).
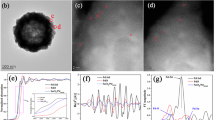
a Scheme of the synthesis process of PdMHx metallenes. b TEM image, (c) HAADF-STEM image, (d) AFM image and corresponding height profile, (e) EDS-mapping images, (f) atomic-resolution rainbow-colored HAADF-STEM image of PdPbHx metallenes. g The measured atomic intensity line scanning profile along the rectangles labeled in (f). Source data are provided as a Source Data file.
The morphology of the PdMHx metallenes was characterized using transmission electron microscopy (TEM) and high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM). As a typical structure, PdPbHx metallenes show the interconnected ring-like shape (Fig. 1b, c), with a thickness of 3.93 ± 0.2 nm by analyzing the atomic force microscopy (AFM) images and corresponding height profiles (Fig. 1d). The observed reduction in the hole size of the nanorings in AFM images relative to TEM images is predominantly a consequence of the tip effect inherent to AFM measurements36,37. Energy dispersive X-ray spectroscopy (EDS) elemental mapping demonstrated the uniform distribution of Pd and Pb elements in the PdPbHx metallenes, confirming their alloy feature (Fig. 1e). The Pd/Pb atomic ratio, determined based on EDS results, was found to be 91.3/8.7 (Supplementary Table 1), which was consistent with the data obtained from inductively coupled plasma atomic emission spectroscopy (ICP-AES, Supplementary Table 2). Furthermore, atomic-resolution HAADF-STEM images and corresponding intensity profiles (Fig. 1f, g) revealed that the atomic structure of the PdPbHx metallenes consisted of relatively bright spots (high peak intensity) and numerous relatively dark spots (low peak intensity). Given the higher atomic number of Pb (Z = 82) compared to Pd (Z = 46), the brighter spots were attributed to Pb atoms, while the darker spots were attributed to Pd atoms. Importantly, the Pb atoms were found to be atomically dispersed within the Pd lattice without any Pb-Pb bonds, indicating a single-atom alloy feature. Notably, the PdInHx and PdSnHx metallenes show a similar ring-like morphology and single-atom alloy structure to that of the PdPbHx metallenes (Supplementary Figs. 9 and 10), demonstrating the universality of the synthetic method.
Structural and electronic properties
Figure 2a presents the X-ray diffraction (XRD) results, which show that the peaks of PdPbHx metallenes shift to a lower angle compared to Pd metallenes. This shift is in line with the standard PdHx phase (PDF#87-0637) with the H content of 0.32 (Supplementary Fig. 11), confirming the formation of metal hydride. In addition, we have plotted a standard curve of the relationship between the lattice constant and Pb content within fcc-PdxPb1-x alloys, demonstrating that the XRD peak shift of PdPbHx is mainly due to the incorporation of interstitial hydrogen atoms instead of Pb atoms (Supplementary Fig. 12). H solid-state nuclear magnetic resonance (ssNMR) was conducted to further investigate the presence of interstitial H atoms. Figure 2b shows a new peak at 26.1 ppm in the 1H solid state nuclear magnetic resonance (ssNMR) spectrum of PdPbHx metallenes, indicating the formation of the Pd-H bonds38,39. The interaction between Pd and H atoms was also confirmed by electron energy loss spectroscopy (EELS) (Fig. 2c) and X-ray photoelectron spectroscopy (XPS) results (Supplementary Fig. 13), which demonstrate that PdPbHx metallenes exhibit a lower EELS peak energy and a narrower valence band compared to Pd metallenes. These observations are consistent with the behavior observed in palladium hydride systems40,41.
a XRD patterns, (b) 1H ssNMR spectra, (c) EELS spectra of PdPbHx metallenes and Pd metallenes. d Pd K-edge XANES, (e) EXAFS spectra and (f) WT contour maps of PdPbHx metallenes and standard samples of Pd foil and PdO. g Pb L3-edge XANES, (h) EXAFS spectra and (i) WT contour maps of PdPbHx metallenes and standard samples of Pb foil and PbO. Source data are provided as a Source Data file.
X-ray absorption spectroscopy (XAS) was employed to study the coordination environments and chemical states of Pd and Pb atoms in PdPbHx metallenes. Figure 2d displays the X-ray absorption near-edge structure (XANES) spectra of Pd K-edge in PdPbHx metallenes, in which the adsorption edge in PdPbHx metallenes is close to that in the Pd foil, demonstrating that the Pd atoms in PdPbHx metallenes are mainly in the metallic state. The conclusion was consistent with XPS results (Supplementary Fig. 14). The coordination situation of Pd atoms in PdPbHx metallenes was further investigated by Fourier transform extended X-ray absorption fine structure (FT-EXAFS) and wavelet transform EXAFS (WT-EXAFS) (Fig. 2e, f and Supplementary Fig. 15). Figure 2e displays that the EXAFS at the Pd K-edge spectra of PdPbHx metallenes is the same as that of Pd foil, meaning the crystal structure remains unchanged after doping. The Pd-Pd/Pb bond length in PdPbHx metallenes was measured to be 2.82 Å (Fig. 2e, f and Supplementary Table 3), which is 0.08 Å longer than that in Pd foil (2.74 Å) due to the existence of interstitial H atoms24. XANES spectra of Pb L3-edge indicate that Pb atoms in PdPbHx metallenes are primarily in the metallic state (Fig. 2g). The FT-EXAFS and WT-EXAFS results (Fig. 2h, i, Supplementary Fig. 16) show that the Pb component in the PdPbHx metallenes only exhibits one peak of Pb-Pd bond, indicating that Pb atoms are atomically dispersed in the Pd lattice without aggregations. Additionally, the EXAFS fitting results of PdPbHx metallenes reveal a coordination number of approximately 9.1 for the Pb-Pd bond, slightly higher than that for the Pd-Pd bond with a value of 7.2 (Supplementary Table 3). This suggests that Pb atoms are mainly located in the interior area with a large coordination number. Conversely, XAS analysis of PdPb metallenes indicates that Pb atoms are distributed throughout the entire structure, including both the internal and exterior regions. (Supplementary Figs. 17–19). These inferences are further supported by the galvanic replacement experiment, where Cu was used to replace surface Pb atoms (Supplementary Table 4)23,33. XPS results (Supplementary Fig. 20) confirm that Pb atoms are confined within the internal region of PdPbHx metallenes, whereas Pb atoms are located outside PdPb metallenes rather than being confined solely within the internal region.
Evaluation of ORR performance
The electrocatalytic performance of PdPbHx metallenes for ORR was evaluated using a rotating disk electrode (RDE) measurement and compared to commercial Pt/C as the benchmark. Before the test, PdPbHx metallenes, PdHx metallenes (a comparison sample without the Pb element, Supplementary Fig. 21), and PdPb metallenes were deposited onto the carbon black support. Cyclic voltammetry (CV) curves were measured for these catalysts in a N2-saturated 0.1 M KOH solution at a scan rate of 50 mV s−1. The electrochemical active surface areas (ECSA), which provide an estimate of the available active sites for the ORR process, were calculated based on the integral calculation of the reduction peak areas of Pd-oxides. Figure 3a shows that PdPbHx metallenes/C exhibits the highest ECSA value and the most positive Pd-O peak potential compared to the other three catalysts (Supplementary Table 5, Supplementary Figs. 22 and 23). This observation indicates that PdPbHx metallenes possess a significant number of active sites, primarily located on steps and ledges within the nanoring structure42,43,44. Additionally, the CV curves of all samples at a scan rate of 10 mV s−1 showed both PdPbHx and PdPb exhibited lower current densities at 1.3 V during the positive sweep compared to PdHx (Supplementary Fig. 24), indicating that the introduction of Pb can reduce the oxidation degree at high potentials45. Furthermore, the ORR polarization curves were measured in an O2-saturated 0.1 M KOH solution at a scan rate of 10 mV s−1. As shown in Fig. 3b, the half-wave potential (E1/2) in PdPbHx metallenes/C is 0.958 V, which is much more positive than that in PdHx metallenes/C (0.910 V), PdPb metallenes/C (0.907 V), and commercial Pt/C (0.845 V). Noting that the LSV curves in Fig. 3b are the positive scan from 0.3 V to 1.1 V (Step I), which is higher to that observed during the negative scan from 1.1 V to 0.3 V (Step II) (Supplementary Fig. 25). This difference can be attributed to the reaction of Pd/Pt with OH− at high potentials, forming inactive Pd/Pt-based oxides46. However, the LSV curves (Step III) revert to the one observed in Step I, indicating that the Pd/Pt-based oxides may be electrochemically reduced back to their original active metal state under these test conditions. Additionally, quasi in situ XPS results show that the area ratio of Pd2+/Pd2++Pd0 always keeps below 0.25 across the whole testing potential (Supplementary Fig. 26), indicating the Pd in PdPbHx metallenes remained predominantly in its metallic state throughout the ORR process. The Tafel slope of PdPbHx metallenes/C is 59.8 mV dec−1, which is much lower than that of PdHx metallenes/C with 68.5 mV dec−1, PdPb metallenes/C with 88.9 mV dec−1, and commercial Pt/C with 101.3 mV dec−1 (Fig. 3c), suggesting the faster ORR reaction kinetics on PdPbHx metallenes/C among the four samples10. By applying the Koutecky-Levich equation to analyze polarization curves, the catalytic kinetic currents were calculated. These values are subsequently normalized using both the metal loading mass and ECSA to determine the mass activity (MA) and specific activity (SA) (Fig. 3d). Since the universally chosen potential (0.9 V) is too close to the diffusion limit region to provide reliable dynamic current density due to the fast kinetic of PdPbHx metallenes/C (Supplementary Fig. 27), we evaluated the activity at 0.95 V to eliminate the O2 diffusion limit23,47. As shown in Fig. 3d, at 0.95 V, PdPbHx metallenes/C exhibited outstanding MA (1.36 A mg−1Pd) and SA (1.59 mA cm−2), which are 46.9-fold and 31.9-fold enhancements compared to commercial Pt/C, respectively. Optimization experiments for ORR performance with varying Pb doping amounts revealed that the synthesized PdPbHx catalyst with a Pb atomic ratio of 8.7% exhibited better performance (Supplementary Fig. 28). Additionally, the ORR performance of PdPbHx metallenes was evaluated in acidic electrolytes, also showing much better activity compared to commercial Pt/C (Supplementary Fig. 29 and Supplementary Table 6). These results indicate the superior electrocatalytic activity of PdPbHx metallenes/C for the ORR compared to the other catalysts studied. Notably, when alloyed single atoms are changed into In and Sn, these hydride bimetallenes materials also exhibit good ORR performance (Supplementary Figs. 30, 31), highlighting the versatility of this strategy for enhancing ORR activity through the co-confinement of single p-block and interstitial H atoms in Pd-based metallenes.
a CV curves recorded in N2-saturated 0.1 M KOH solutions at 25 °C with a scan rate of 50 mV s−1. b ORR polarization curves recorded in O2-saturated 0.1 M KOH electrolyte at 25 °C, with a rotation rate of 1600 rpm and a positive scan rate of 10 mV s−1. c Corresponding Tafel plots. d The comparisons in mass activity and specific activity at 0.95 V. The error bars represent the standard deviation derived from three independent experimental measurements. e Durability performance of LSV evolutions of PdPbHx metallenes/C. f The changes in mass activities and specific activities of PdPbHx metallenes/C catalyst before and after the durability test for various potential-scanning cycles. Noting that the area value used for current density normalization (mA cm−2) was 0.196 cm2 corresponding to the glassy carbon substrate support area. The solution resistance (Rs = 40 ± 5 Ω) was obtained from EIS measurements. g Schematic illustration of ZABs. h Discharge and power density curves, i Galvanostatic discharge curves at current densities from 10 to 100 mA cm−2 of PdPbHx metallenes/C and commercial Pt/C. Source data are provided as a Source Data file.
To assess the electrochemical durability of PdPbHx metallenes/C, the accelerated stability test was conducted in an O2-saturated 0.1 M KOH solution between 0.6 and 1.0 V versus RHE at a scan rate of 200 mV s−1. Remarkably, after 50,000 cycles, the half-wave potential (E1/2) of PdPbHx metallenes/C exhibited a slight decrease of 8 mV, indicating good stability (Fig. 3e). ECSAs measured before and after 50,000 potential cycles remain at a high value without evident changes (Supplementary Fig. 32). The MA and SA of PdPbHx metallenes/C also remained at 95% and 96% of their initial values after 50,000 cycles, respectively (Fig. 3f). In contrast, the ORR activity of PdHx metallenes/C, PdPb metallenes/C, and commercial Pt/C decreased notably after 50,000 cycles (Supplementary Fig. 33). The long-term chrono-amperometry measurements were conducted at 0.6 V vs. RHE for PdPbHx metallenes/C and commercial Pt/C (Supplementary Fig. 34). The results showed that the PdPbHx metallenes/C maintains approximately 90.6% of its initial current density after testing for 30,000 s, whereas commercial Pt/C only can maintain 33.7%, demonstrating the extraordinary durability of PdPbHx metallenes compared to commercial Pt/C. Furthermore, the morphology, single atom alloy feature, and hydride nature of PdPbHx metallenes were retained after the accelerated stability measurement, as confirmed by imaging techniques (Supplementary Figs. 35–37, Table 1 and 2). The ORR performance of PdPbHx metallenes, including the mass activity and long-term stability, surpasses those of the recently reported Pd-based ORR catalysts under alkaline media (Supplementary Table 7). Simultaneously, the half-wave potential and mass activity at 0.95 V vs. RHE of PdPbHx metallenes also surpass those of most reported high-activity M-N-C materials. (Supplementary Table 8). In comparison, PdHx metallenes/C displayed noticeable morphology changes and substantial aggregation after 50,000 cycles (Supplementary Fig. 38). This enhanced durability of PdPbHx metallenes can be attributed to the unique nanoring structure, which exhibits a stronger binding force with the carbon support. The corresponding stability of the electrocatalysts has also been verified by molecular dynamics (MD) simulations at room temperature, where both PdPb and PdHx show much reduced stability due to the strong structural distortions (Supplementary Fig. 39). In comparison, the PdPbHx is much more stable. Importantly, the synthesis of PdPbHx metallenes can be easily scaled up to produce larger quantities without significant changes in crystal structures and catalytic performances, supporting the practical applicability of PdPbHx for future applications (Supplementary Fig. 40). To further validate the good ORR performances of PdPbHx metallenes, practical Zn-air batteries (ZABs) and H2-O2 AEMFC tests were conducted with fuel cell devices containing PdPbHx metallenes/C and commercial Pt/C as a cathode catalyst material. For ZABs performance, compared to the commercial Pt/C, PdPbHx metallenes/C exhibit higher open-circuit voltage (1.55 V), power density (254.1 mW cm−2) and specific capacity (794.1 mAh per gram Zn) (Fig. 3g, h and Supplementary Fig. 41). We also examined the rate capability of our PdPbHx metallenes/C at different current densities, further demonstrating the potential of PdPbHx metallenes/C as air cathodes for practical applications (Fig. 3i). The H2-O2 fuel cell utilizing PdPbHx metallenes/C achieved a peak power density of 722 mW cm−2 at a loading of 0.3 mg cm−2, surpassing the Pt/C-based systems (589 mW cm−2) (Supplementary Fig. 42).
Mechanistic studies via in situ ATR-FTIR spectroscopy
The four-electron ORR process involves two fundamental pathways as shown in Fig. 4a and summarized in Supplementary Table 948,49. The first pathway is the oxygen associative mechanism, where *OOH intermediates are formed through the protonation of *O-O. The second pathway is the oxygen dissociative mechanism, where *O-O* directly dissociates into two *OH species, followed by continuous protonation. Consequently, different ORR mechanisms can be distinguished by specific reaction intermediates: *O-O* species indicate the dissociative mechanism while the *OOH intermediate represents the associative mechanism. In situ ATR-FTIR spectroscopy was then performed to identify reaction intermediates and investigate the ORR mechanism on different metallene-based catalysts (Supplementary Fig. 43). In the wavenumber range of 1050–1200 cm−1, a peak around 1175 cm−1 corresponding to the *O-O* species was observed on PdPbHx metallenes/C and PdHx metallenes/C (Fig. 4b and c)50, while no obvious adsorption peak is observed in this region on Pd metallenes/C and PdPb metallene/C (Fig. 4d and Supplementary Fig. 44). The results indicate the dissociative pathway is exclusively occurred on the hydride metallenes, highlighting the unique role of interstitial H atoms in inducing the oxygen dissociation pathway. Notably, the peak around 1300 cm−1 corresponds to the O-O stretching mode of *OOH intermediate51,52 is observed on all metallene-based catalysts, indicating the presence of the oxygen associative pathways during the ORR process (Fig. 4e–g). To further elucidate the influence of doped Pb atoms, the wavenumber of the O2 adsorption peak (within the range of approximately 1430–1470 cm−1)52,53 and the integral area ratio of the *O-O*/*O2 peak was systematically analyzed for both PdPbHx metallenes/C and PdHx metallenes/C. As displayed in Fig. 4h, the wavenumber of the *O2 peak in PdPbHx metallenes/C is significantly lower than that in PdHx metallenes/C under all applied potentials, suggesting a stronger O2 adsorption energy on the former catalyst. Furthermore, the integral area ratio of the *O-O*/*O2 peak in PdPbHx metallenes/C is higher compared to that in PdHx metallenes/C during the entire ORR process (Fig. 4i). In summary, Pb atoms in PdPbHx metallenes enhance the adsorption strength of O2 molecules on the catalyst surface and facilitate the activation process of *O2 to *O-O*, thereby promoting the oxygen dissociative process and improving ORR performances in PdPbHx metallenes/C.
a The schematic diagram of the O2 associative pathway and the O2 dissociative pathway. In situ ATR-IR spectra under applied potentials in O2-saturated 0.1 M KOH in the wavenumber range (1050–1200 cm−1) of (b) PdPbHx metallenes/C, (c) PdHx metallenes/C, (d) Pd metallenes/C, and in the wavenumber range (1250–1550 cm−1) of (e) PdPbHx metallenes/C, (f) PdHx metallenes/C, (g) Pd metallenes/C. h The statistic of *O2 peak position of PdPbHx metallenes/C and PdHx metallenes/C under different potentials, obtained from (e, f). i The area ratio of *O-O*/*O2 peak of PdPbHx metallenes/C and PdHx metallenes/C, obtained from the integral of *O-O*/*O2 peak area in (b, e) and (c, f). Source data are provided as a Source Data file.
Theoretical calculations
Detailed theoretical investigations regarding the electronic structures and reaction trends for ORR were carried out to explore the outstanding ORR performances of PdPbHx metallenes. For demonstrating the electroactivity differences, the distributions of bonding and anti-bonding orbitals near the Fermi level (EF) for PdPb, PdHx, and PdPbHx surfaces were first compared (Fig. 5a–c, Supplementary Fig. 45). For the (111) surface of the PdPb, the bonding orbitals only partially distribute on the surface while the anti-bonding orbitals are slightly more dominant, which supplies limited electroactive sites to guarantee efficient electron transfer during the ORR (Fig. 5a). For PdHx, although the bonding orbitals become more evident on unsaturated Pd sites of the edge, the overall anti-bonding distributions are still dominant on the surface (Fig. 5b). With the introduction of Pb, the electronic distributions of PdPbHx are evidently optimized, where the surface sites are mainly dominated by the bonding orbitals, offering a highly electroactive surface to enable efficient adsorption of oxygen and accelerate electron transfer (Fig. 5c). The projected partial density of states (PDOS) is further compared to understand the electronic structures of different electrocatalysts. For the PdPb surface, the Pd-4d orbitals exhibit a relatively broad distribution with continuous peaks, covering from EV-6.0 eV to EV (EV denotes 0 eV) (Fig. 5d). For Pb sites, the 6 s orbitals show limited contributions, which are located far from the EF. In contrast, the Pb-6p orbitals cross the EF to increase the electron density, promoting the electron transfer of the electrocatalysts. Similar to the electronic distributions, the PDOS of PdHx are different due to the electronic modulations induced by the formation of the hydride system (Fig. 5e). Notably, Pd-4d orbitals become sharper when compared to that of PdPb, leading to the overall upshifting band to the EF with enhanced electroactivity. Meanwhile, the H-1s orbitals are well overlapping with the Pd-4d orbitals, supporting the stable structures of PdHx. For the PdPbHx, the Pd-4d orbitals become more concentrated and closer to the EF, which results in a slightly increased valence state induced by the electronic modulations of introduced Pb (Fig. 5f). Compared to PdPb, the Pb-6s orbitals remain similar while the 6p orbitals also become more concentrated near EF, which benefits the p-d overlapping with Pd-4d orbitals to enhance electron transfer and exchange efficiency. In addition, the H-1s orbitals are not strongly disturbed by the Pb, which guarantees the stability of the structures. In addition, we have included two additional compositions of 4.5% and 18% for Pb and supplied H contents of 0.2 and 0.4 as comparison samples. Both electronic structures and reaction trends have also been demonstrated. Compared to PdPbHx, we notice that the higher Pb and H concentrations will slightly downshift the d orbitals of Pd, which demonstrates that Pb and H compositions also influence electron transfer (Supplementary Fig. 46a–d). From the site-dependent PDOS, we notice that edge Pd atoms demonstrate higher 4 d orbitals than the Pd sites in PdPb (Fig. 5g). In particular, the nearby H atoms significantly improve the electroactivity of Pd sites, which benefits the direct dissociations of O2. For the Pb sites, the Pb-6s,6p orbitals are strongly different from the bulk metal, which are attributed to the isolated states of Pb atoms in PdPbHx (Fig. 5h). Moreover, the surface and edge Pb sites display increased electron density since both 6 s and 6p orbitals shift closer to the EF, which not only improves the overlapping with Pd-4d orbitals but also facilitates the electron transfer to enhance the adsorption of O2. Then, we have summarized the d-band and p-band centers in Pd and Pb sites, respectively (Fig. 5i). From PdPb to PdPbHx, the d-band center has gradually upshifted from EV-2.05 eV to EV-1.81 eV, revealing the increased electroactivity of Pd for stronger adsorption of O2. In contrast, the downshifted p-band center in PdPbHx is ascribed to the contributions of Pb-6p orbitals becoming higher near the EF, which promotes the electron transfer from PdPbHx to the O2 and thus enhances the adsorption of O2. For the introduction of Pb, we notice that further decrease or increase in the Pb compositions results in the decreases of d-band and p-band centers, indicating that a suitable Pb composition is critical to guarantee high electroactivity of Pd sites (Supplementary Fig. 46e, f). In comparison, the increasing H contents lead to the downshifted electroactivity of d-band and p-band centers towards decreasing electroactivity.
The electronic distributions of bonding and anti-bonding orbitals near the Fermi level of (a) PdPb, (b) PdHx, and (c) PdPbHx. Olive balls = Pd, Grey balls= Pb, and White balls = H. Blue isosurface = bonding orbitals, and green isosurface = anti-bonding orbitals. The PDOSs of (d) PdPb, (e) PdHx, and (f) PdPbHx. The site-dependent PDOSs of (g) Pd-4d and (h) Pb-6s, 6p of PdPbHx. i The d-band and p-band center evolutions of Pd and Pb in different electrocatalysts. j The comparison of adsorption energies for O2. k The reaction energy trends of ORR through the dissociative pathway for PdPbHx metallenes, and PdHx metallenes. l The comparison of ΔG of *O2 → *OOH and *O2 → 2*O reaction steps for PdPbHx metallenes, PdHx metallenes and PdPb metallenes. Source data are provided as a Source Data file.
As shown in Fig. 5j and Supplementary Fig. 47, the PdPbHx displays a lower adsorption energy of O2 than PdPb and PdHx, indicating the stronger adsorption ability with O2, which can promote the oxygen dissociative pathway. The increases in Pb and H contents in PdPbHx have been found to enhance the adsorption of O2 for the ORR (Supplementary Fig. 46g). The reaction energy change of the ORR dissociative process is further investigated under both equilibrium (U = 1.23 V) and limiting potential (UL). As shown in Fig. 5k, under the equilibrium potential of 1.23 V, both PdHx and PdPbHx demonstrate a spontaneous tendency for the generation of 2*O, supporting the high selectivity towards the dissociative pathways. Specifically, PdPbHx shows a smaller (RDS) energy barrier for the rate-determining step of *OH desorption, being 0.49 eV, compared to 0.56 eV of PdHx. This demonstrates that the introduction of Pb atoms further promotes the proceeding of the oxygen dissociative pathway by reducing the dissociation barriers and desorption of *OH during the ORR. Meanwhile, the PdPb, Pd, and Pt(111) all meet large energy barriers at the initial conversion from O2 to 2*O, indicating the oxygen dissociative pathway was difficult to occur (Supplementary Fig. 48). For both associative and dissociative mechanisms, it is worth noting that the chemisorption of O2 is the chemical step without any electron transfer involved, where the energy costs of O2 dissociation cannot be alleviated by introducing the electrochemical potential46. Based on the computational hydrogen electrode (CHE) model, the corresponding UL of ORR under the dissociative mechanism is evaluated to be 0.98 V and 0.95 V for PdPbHx metallenes and PdHx metallenes, respectively. The high ORR performance of PdPbHx metallenes is ascribed to the fast dissociation step, while the high barrier of the dissociation in PdPb metallenes significantly limits the kinetics of the ORR. The bulk Pt and Pd metallene have significantly higher RDS barriers for the dissociative ORR mechanisms compared to PdPbHx (Supplementary Fig. 48). The dissociation barrier of O2 further confirms that PdPbHx exhibits the smallest kinetic barrier of 0.08 eV to guarantee the efficient dissociative ORR (Supplementary Fig. 49a). In order to more intuitively compare the difference of ORR pathways on three catalysts, the ΔG changes of the associative pathways are demonstrated (Supplementary Fig. 49b). The first protonation from chemisorbed *O2 to *OOH is the potential limiting step for PdPbHx metallenes, PdHx metallenes, and PdPb metallenes. In contrast, the Pd and Pt(111) exhibit the last desorption step of *OH as the potential limiting step. For both PdPbHx and PdHx, the limiting potential of the dissociative pathway is much higher than that of the associative pathway, supporting the domination of the dissociative pathway during the ORR (Supplementary Fig. 49c). In comparison, the limiting potential for PdPb, Pd, and Pt(111) display a converse trend, where the associative pathways are more feasible with higher limiting potentials.
To compare the selectivity of different ORR pathways, the energy difference of O2 → 2*O and *O2 → *OOH were summarized for PdPb, PdHx and PdPbHx (Fig. 5l). For PdPb, the ΔG of *O2 → *OOH was 0.10 eV while the ΔG of *O2 → 2*O was 0.81 eV, demonstrating that oxygen was difficult to dissociate on PdPb. Different from PdPb, the ΔG of *O2 → 2*O of PdHx and PdPbHx were −0.17 eV and −0.87 eV, which is far below their ΔG of *O2 → *OOH (0.55 eV and 0.64 eV, respectively). The huge energy difference illustrates that PdHx and PdPbHx prefer to occur in the oxygen dissociative pathway, proving that the oxygen was more easily dissociated on PdPbHx. In addition, the RDS barrier comparisons further reveal the ORR mechanism preference. It was found that PdPbHx exhibits the smallest overall RDS barriers, supporting its superior ORR performances (Supplementary Fig. 49d). For both PdPbHx and PdHx, the smaller difference of RDS barriers between dissociative and associative mechanisms lead to the co-existence of both ORR mechanisms as observed by experimental characterizations in Fig. 4. On the other hand, associative mechanisms strongly prevail in PdPb, Pd metallene, and Pt (111) owing to their significantly lower RDS barriers. With the modulations of Pb and H compositions, the corresponding ΔG of *O2 → 2*O and *O2 → *OOH are also affected (Supplementary Fig. 46h–i). The Pb introduction is able to strengthen the adsorption of O2 to accelerate the dissociative mechanism, where a suitable composition optimizes the RDS barrier to reach superior ORR. The H introduction modulates the reaction trends of dissociative mechanisms, while its impact on associative mechanisms is limited. It is essential to optimize both Pb and H contents to ensure the highest electroactivity of the surface Pd sites during electrocatalysis. Additionally, the oxygen coverage on PdPbHx also affects the ORR trends (Supplementary Fig. 50a, b). Higher oxygen coverage results in higher energy barriers for direct dissociation mechanisms and favors the associative mechanisms (Supplementary Fig. 50c, d). As the oxygen coverage gradually increases, the surface electroactivity is weakened, especially for the adsorption of O2, which limits the direct dissociation of adsorbed O2, leading to increasing energy barriers for the dissociative mechanisms. These are attributed to the electronic modulations on the Pd sites by the O coverage (Supplementary Fig. 50e, f)54. For Pd-4d orbitals, we notice the downshifting trend with the oxygen coverage increases, which will reduce the overall electroactivity and binding strengths. The broad O-2p orbitals cover from EV-6.0 eV to EV + 2.0 eV, which will overlap with Pb-6p and Pd-4d orbitals to further modulate the surface electroactivity towards ORR. The decreasing d-band centers of Pd lead to the reduced adsorption strengths of O2 to promote the associative mechanism. These results indicate that the dissociative mechanism dominates the ORR at low oxygen coverage for PdPbHx while the associative mechanism becomes more feasible at high oxygen coverage.
Discussion
In summary, the synthesis of PdMHx (M = In, Sn, Pb) metallenes, with the inclusion of single p-block atoms and interstitial H atoms in the lattice of Pd metallenes, has led to the development of a highly efficient alkaline ORR catalyst. Among them, PdPbHx metallenes demonstrated a high MA of 1.36 A mg−1 under 0.95 V. This value is 46.9 times higher than that of commercial Pt/C and surpasses all previously Pd-based reported ORR catalysts. Additionally, the PdPbHx metallenes exhibited good durability, with no significant reduction in activity even after 50,000 potential-scanning cycles. The in situ ATR-FTIR spectra revealed that the presence of interstitial H atoms triggered the oxygen dissociative pathway, while the inclusion of Pb atoms enhanced the initial adsorption capacity of O2 on Pd metallenes, promoting the oxygen dissociative pathway. DFT calculations further demonstrated that the incorporation of hydride systems is able to optimize the electronic structures of PdPbHx, and the confined single Pb atoms facilitated electron transfer through p-d orbital coupling. This resulted in enhanced O2 adsorption and subsequent dissociation to *O with lower energy barriers when compared to the conventional conversion to *OOH, providing a more efficient pathway for the ORR. Our findings highlight the successful regulation of the ORR pathway through co-doping metal and non-metal atoms, offering valuable insights for the design of highly efficient electrocatalysts.
Methods
Materials
Palladium acetylacetonate (Pd(acac)2, 99%) was purchased from Sigma-Aldrich (USA). Plumbum acetylacetonate (Pb(acac)2, 99%), Indium acetylacetonate (In (acac)3, 99%) and Tin acetylacetonate (Sn(acac)2, 99%) were purchased from Aladdin (China). Commercial Pt/C catalyst (20 wt. %) was obtained from Johnson Mattkey (UK). Polyvinylpyrrolidone (PVP MW = 40000, K30) was obtained from Aladdin Co., Ltd. (China). Hexadecyl trimethyl ammonium bromide (CTAB, 99%) were obtained from Shanghai Macklin Biochemical Co., Ltd. (China). N,N-dimethylformamide (DMF, AR), potassium hydroxide (KOH, AR), isopropanol (CH3CH2CH2OH) and ethanol (CH3CH2OH, AR) were all purchased from Sinopharm Chemical Reagent Co., Ltd. (China). Carbon monoxide (ultra-purity) was purchased from Xin’guang Gas Co., Ltd. (Changchun, China). Nafion-ethanol solution was obtained from Adamas-beta Chemical (Switzerland). Milli-Q deionized water (DI water, 18.2 MΩ cm−1) was used in all experiments. All reagents were used as received without further purification.
Synthesis of PdMHx metallenes
The typical synthetic method of PdPbHx metallenes: Pd metallenes to be used as the seed were synthesized by using a modified protocol previously reported work55. First step: 50 mg Pd(acac)2, 160 mg PVP, and 185 mg CTAB were added to the mixed solution with 10 mL DMF and 2 mL water with magnetic stirring. The resulting homogeneous yellow solution as transferred into a Schlenk flask, and charged with CO gas. Then the flask was immediately shifted into an oil bath that had been pre-heated to a temperature of 40 °C and held for 12 h. Second step: 20 mg Pd(acac)2 and 10 mg Pb(acac)2 were added to the above solution. After the solution is ultrasonically mixed, and charged with CO gas. Then the flask was immediately shifted into an oil bath that had been pre-heated to a temperature of 40 °C and held for 12 h again to obtain PdPb metallenes via epitaxial growth. The obtained PdPb metallenes were separated by centrifugation and washed three times with ethanol. Third step: PdPb metallenes were dispersed in the 15 mL DMF and transferred into a 25 mL Teflon-lined stainless steel autoclave. The autoclave was heated at 160 °C for 4 h. The final product was collected by centrifugation (4695 g, 10 min) and washed three times with ethanol. The PdInHx and PdSnHx metallenes were synthesized via a similar procedure, except that In(acac)3 (10.0 mg) and Sn(acac)2 (10.0 mg) were utilized instead, respectively.
Synthesis of PdHx metallenes
The synthesis step was the same as PdPbHx metallenes except that Pb(acac)2 was removed from the second step.
Synthesis of PdPb metallenes
The synthesis step was the same as PdPbHx metallenes except the third step was removed.
Material characterizations
Transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) were performed on a JEOL JEM-2100F transmission electron microscope (Japan). Scanning transmission electron microscopy (STEM) images and corresponding energy-disperse X-ray spectroscopy (EDS) mapping, and electron-energy loss spectroscopy (EELS) were obtained by a JEOL ARM200CF (Japan). For atomic force microscopy (AFM) characterization, samples were deposited onto mica wafers and analyzed in tapping mode under ambient air conditions via the ScanAsyst (Dimension Icon, Veeco Instruments/Bruker, Germany). X-ray photoelectron spectroscopy (XPS) data were collected using a Thermo Fisher Scientific ESCALAB-250 instrument (USA) with a monochromatic Al-Kα radiation source (1486.6 eV) and a hemispherical detector (0.1 eV energy resolution). Inductively coupled plasma atomic emission spectroscopy (ICP-AES) measurements and X-ray diffraction (XRD) patterns were conducted using an ELAN 9000/DRC system and a Bragg-Brentano diffractometer (D8-tools, Germany), respectively. X-ray absorption spectra of the Pd K-edge and Pb L3-edge were collected in both transmission (for Pd foil, Pb foil, PdO, and PbO) and fluorescence (for the sample) mode at Shanghai Synchrotron Radiation Facility (SSRF) on beamline BL14W1.
Electrochemical measurements
The as-prepared Pd-based metallenes are stored in an ethanol solution, with the concentration of Pd determined by ICP-AES to be approximately 1.0 mgPd/mL. For catalytic evaluation, 5 mL of metallene solution (containing 5 mg of Pd) and 45 mg Ketjen carbon were added in 45 mL ethanol and then sonicated for 20 min to achieve a homogeneous dispersion. Subsequently, the sonicated mixture is subjected to an additional minute of oscillation. The ultrasonic and oscillating processes were repeated three times to ensure the catalyst was fully loaded on the Ketjen carbon. Then the solution was centrifuged and poured off the supernatant, dried in a 60 °C vacuum drying oven for 12 h. For the preparation of the electrochemical test catalyst ink, 1 mg of the as-synthesized PdPbHₓ metallenes/C powder was dispersed into a 1 mL mixture consisting of 750 μL deionized water, 245 μL isopropanol, and 5 μL of 5 wt% Nafion solution. This mixture underwent 30 min of sonication in an ice-water bath to form a homogeneous dispersion. The resulting uniform catalyst ink was then utilized for fabricating the working electrode. As a comparative benchmark, the commercial 20 wt% Pt/C catalyst from Johnson-Matthey was employed.
Electrochemical measurements were conducted using a three-electrode configuration on an Autolab electrochemical workstation. The working electrode consisted of a catalyst-coated glassy carbon electrode (GCE, 5 mm diameter) mounted on a rotator, while a platinum sheet (1.0 cm2) served as the counter electrode and a Hg/HgO electrode acted as the reference electrode. The preparation method of 0.1 M KOH electrolyte is as follows: dissolving 2.8 g of KOH in 500 mL of deionized water, followed by ultrasonication to ensure a uniform solution. The 0.1 M KOH electrolyte was freshly prepared before electrochemical measurements and stored in a sealed container at 25 °C. The calibration potential was approximately equal to the calculated value based on the Nernst equation (the measured pH value of 0.1 M KOH solution was 13.3 ± 0.3 under the actual ORR testing process). All the potentials were converted to the potentials referring to the RHE. Prior to catalyst deposition, the glassy carbon electrode was sequentially polished with alumina polishing slurries, rinsed thoroughly with deionized water, and left to air-dry naturally. Subsequently, 10 μL of the ink was dropped on a GCE to form a smooth thin film, yielding a Pd loading of 1.0 μg (5.1 μg cm−2). For Commercial Pt/C, the Pt loading of 2.0 μg (10.2 μg cm−2) were added to the GCE to obtain a similar limited diffusion current with other catalysts. The ECSA (m2·g−1PGMs) of the catalysts was calculated according to the equation:
where Q represents the surface charge for oxygen desorption, q is the charge required for the reduction of a PdO monolayer (424 μC cm−2) and m is the Pd loading amount. For ECSA, the cyclic voltammogram (CV) was obtained in N2-saturated 0.1 M KOH with a scan rate of 50 mV s−1. ORR polarization curves were measured in an O2-saturated 0.1 M KOH electrolyte, employing a scan rate of 10 mV s⁻¹ and a rotation speed of 1,600 rpm. iR-correction has been applied to the recorded ORR curves before determining the kinetic current (Ik) based on the Koutecký-Levich equation:
where Id is the limiting current density, and I is the measured current density at 0.9/0.95 V versus RHE. For each catalyst, the specific activity and mass activity were derived by normalizing the kinetic current against the corresponding electrochemical active surface area (ECSA) and the metal (platinum group metals, PGMs) mass, respectively. All activities were obtained by averaging at least three patches of materials and corresponding performances.
In the accelerated durability tests, a graphite rod was indeed used as the counter electrode, which could prevent Pt from the counter electrode dissolving and redeposition on the working electrode during the long hours of the test. The accelerated durability tests were conducted by cycling between 0.6 V and 1.0 V versus RHE at 200 mV s−1 for 50,000 cycles. For comparison, the ORR performance of the commercial Pt/C was also measured through similar procedures.
EIS measurements were conducted at the open-circuit potential (OCP) by applying a sinusoidal AC perturbation with a frequency range from 100 kHz to 10 mHz and an amplitude of 10 mV. Prior to the measurement, the OCP was stabilized with a fluctuation of less than 1 mV/min. All recorded potentials were corrected for ohmic drop using iR compensation (Rs = 40 ± 5 Ω in 0.1 M KOH).
Zn-air battery test
The anode and cathode have used a polished zinc foil (0.5 mm thickness) and a catalyst-coated hydrophobic carbon paper (Spring-P2), respectively. The electrolyte uses a solution composed of 0.2 M Zn(AC)2 and 6 M KOH. The catalyst ink was prepared by ultrasonically mixing 3 mg catalysts and 1 mg Vulcan XC 72 carbon in 600 μL dispersion solution (volume ratio of water/isopropanol/Nafion =44.2/55.2/0.6). To fabricate the cathode, the catalyst ink was then deposited onto the carbon paper via drop-casting, with a catalyst loading of 1.0 mg cm−2 (0.1 mgPd or Pt per cm2). By using the LAND CT3002A instrument, the discharge polarization curve was recorded.
Membrane electrode assembly (MEA) fabrication and fuel cell tests
The catalyst at the cathode and anode is 20 wt% PdPbHx/C or commercial 20 wt% Pt/C with a metal loading of 0.3 mgPd/Pt cm−2 and commercial 60 wt% PtRu/C (Shanghai Hesen Electric Co., Ltd) with a metal loading of 0.6 mg PtRu mgPtRu cm−2, respectively. The cathode ink was obtained by mixing the catalyst powder with water, isopropanol (volume ratio of 1:10), and 30 wt% home-made ionomer56 (mionomer/(mionomer+ mcatalyst) = 30 wt%). The anode slurry was also prepared using the same method mentioned above, except that the amount of ionomer is 23 wt% (mionomer/(mionomer+ mcatalyst) = 23 wt%).
A catalyst-coated membrane (CCM, ~4 cm2) was prepared by sequentially spraying cathodic and anodic slurry onto each side of the commercial membrane (Alkymer® W-25 alkaline membrane. Thickness: 25 μm). Afterwards, the CCM was immersed in 1 M NaOH aq. at 60 °C for 24 h (to promote the ion exchanging Cl− with OH− in alkaline conditions, ensuring the membrane’s suitability for alkaline environments), followed by washing 3 times with deionized water, and then MEA was fabricated by sandwiching the CCM between two carbon papers (Sunrise Power Co., Ltd.) at 25 °C and a gauge pressure of 0.2 MPa for 2 min.
The single cell performance was conducted on a home-made test system at 80 °C by feeding O2/H2 (100% relative humidity, RH) with a 400/400 mL min−1 flow rate at the cathode and anode. A durability test was performed at 80 °C and 100 mA cm−2.
XAFS data analysis
The collected XAFS data underwent systematic processing in Athena software (version 0.9.26), involving background subtraction, pre-edge linear correction, and post-edge linear calibration. Subsequent Fourier transform fitting analysis was conducted using Artemis (version 0.9.26). For Pd fitting, the parameters were configured as: k³-weighting, k-range from 3 to ~12 Å⁻¹, and R-range from 1 to ~3 Å; while for Pb fitting, the k-range was adjusted to 2 to ~10 Å⁻¹ (with the R-range maintained at 1 to ~3 Å). Four critical parameters- coordination number (CN), bond length (R), Debye-Waller factor (σ²), and E₀ shift (ΔE₀)- were optimized without imposing fixed constraints or correlation limitations. For Wavelet Transform (WT) analysis, the χ(k) data extracted from Athena was imported into the Hama Fortran code. The specific parameters for this analysis were defined as: R-range of 1–4 Å; k-range of 0– ~ 13 Å⁻¹ (for Pd) and 0– ~ 10 Å⁻¹ (for Pb); k-weight value of 2; and a Morlet mother wavelet with κ = 10 and σ = 1, which facilitated comprehensive distribution characterization.
XRD data refinement
The XRD data were refined using the Rietveld method with the HighScore software to optimize the reliability and structural parameters.
Quasi in situ XPS measurement
The PdPbHx sample was dropped onto three carbon papers, each with a Pd loading of 1 mg. These carbon papers were then subjected to chronoamperometry (i-t) tests at constant potentials of 0.7 V, 0.9 V, and 1.1 V for 200 s, respectively. Upon completion of the tests, the carbon papers were promptly transferred into centrifuge tubes and sealed under an argon atmosphere to prevent oxidation. Subsequently, XPS characterization was conducted to analyze the chemical states of the samples.
In situ ATR-FTIR measurement
Surface-enhanced infrared absorption spectroscopy (SEIRAS) in attenuated total reflection (ATR) configuration was adopted for the measurements. Electrochemical ATR-SEIRAS characterization was performed using a Thermo Nicolet 8700 spectrometer, which was outfitted with a liquid nitrogen-cooled MCT detector. During the testing process, 0.1 M KOH was used as the electrolyte, which, due to the low alkalinity, will not cause the gold film to detach from the prism. Additionally, to avoid any impact from multiple tests on the gold film, we only performed one oxygen reduction test at full voltage on each gold film. 30 μL of the catalyst ink was applied onto the gold-film working electrode and left to air-dry. Subsequently, the ink-coated silicon column- functioning as the prism with dimensions of 10 mm radius and 25 mm height- was integrated into a custom-built spectro-electrochemical cell to serve as the working electrode. The Hg/HgO was used as the reference electrode, which was introduced near the working electrode via a Luggin capillary while the Pt mesh (1 cm × 1 cm) served as the counter electrode. All spectra were normalized using the differential reflectance formula:
where Es and ER represent the sample and reference spectra, respectively. For the spectra results, all the spectral resolutions were 4 cm−1. The reference spectrum was recorded at 0 mV and the sample spectra were collected at the potentials from 1000 mV to 1600 mV (vs RHE) with the successive potential step of 100 mV.
Calculation setup
In this work, all the density functional theory (DFT) calculations are carried out by the CASTEP package to investigate the electronic structures and reaction trends of PdPb, PdHx, and PdPbHx for ORR57. In particular, we have selected the generalized gradient approximation (GGA) and Perdew-Burke-Ernzerhof (PBE) functionals to supply accurate descriptions of the exchange-correlation interactions58,59,60. We have selected the ultrasoft pseudopotentials with ultrafine quality for the plane-wave cutoff energy, which has been set to 380 eV by default. For further increases of the cutoff energy to 400 eV, the total energy of PdPbHx test model only shifts a very minor scale of around 11 meV, which justifies the use of this cutoff energy setting. The Broyden-Fletcher-Goldfarb-Shannon algorithm with the selection of coarse k-point has been applied for the energy minimization processes in this work61. The PdPb surface has been cleaved from the (111) surface of Pd with a four-layer thickness in a 5 × 5 × 1 supercell, where 9% Pd atoms are replaced by the Pb atoms. For PdHx, the metallene structure has been cleaved from the (111) surface of PdH with a three-layer thickness, where x is close to 0.3. The PdPbHx is further constructed by replacing 9% Pd atoms with Pb atoms, which matches the experimental characterizations. The Pd metallene has been cleaved from the Pd(111) surface with four-layer thicknesses in a 5 × 5 × 1 supercell, where the PdPb metallene is constructed by replacing 9% Pd atoms with the Pb atoms. The bulk Pt surface is cleaved from the Pt(111) surface with an eight-layer thickness in a 5 × 5 × 1 supercell. The structures are supplied in Supplementary Data 1. For all the surfaces, 20 Å vacuum space has been introduced in the z-axis to guarantee complete relaxation.
To achieve sufficient geometry optimizations, a series of strict convergence criteria are applied, which include that the Hellmann-Feynman forces should be converged to less than 0.001 eV/Å, the total energy difference should be converged to smaller than 5 × 10−5 eV/atom, and the maximum displacement for each atom should be smaller than 0.005 Å. In this work, we have performed DFT calculations based on the computational hydrogen electrode (CHE) model proposed by Nørskov et al. 18 The equilibrium potential on a reversible hydrogen electrode (RHE) is set to be the reference of applied potential. When an electrode potential U is applied, the chemical potential of the electron will be reduced by eU, which has been considered in all states involving an electron in the electrode. The limiting potential UL is then determined when all the reaction steps become spontaneous. The free energy (∆G) is calculated based on
where the ∆ZPE and ∆S are the changes of the zero-point energy and entropy, respectively at T = 298.15 K and 1 atm. The MD simulations have been carried out under 298 K and NVT mode in PdPbHx, PdHx, and PdPb metallene for 5 ps, where each step is 1 fs with a total of 5000 simulation steps (Supplementary Data 1).
Data availability
The data generated in this study are provided in the Supplementary Information and Source Data file. Source data are provided in this paper. Source data are provided with this paper.
References
Debe, M. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 486, 43–51 (2012).
Lin, F., Li, M., Zeng, L., Luo, M. & Guo, S. Intermetallic nanocrystals for fuel-cells-based electrocatalysis. Chem. Rev. 123, 12507–12593 (2023).
Gasteiger, H. et al. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B-Environ. 56, 9–35 (2005).
Tian, X., Lu, X., Xia, B. & Lou, X. Advanced electrocatalysts for the oxygen reduction reaction in energy conversion technologies. Joule 4, 45–68 (2020).
Wang, Q., Kaushik, S., Xiao, X. & Xu, Q. Sustainable zinc-air battery chemistry: advances, challenges and prospects. Chem. Soc. Rev. 52, 6139–6190 (2023).
Seh, Z. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, eaad4998 (2017).
Kattel, S. & Wang, G. Reaction pathway for oxygen reduction on FeN4 embedded graphene. J. Phys. Chem. C. 5, 452–456 (2014).
Kulkarni, A., Siahrostami, S., Patel, A. & Nørskov, J. Understanding catalytic activity trends in the oxygen reduction reaction. Chem. Rev. 118, 2302–2312 (2018).
Liang, J. et al. Gas-balancing adsorption strategy towards noble-metal-based nanowire electrocatalysts. Nat. Catal. 7, 719–732 (2024).
Sun, K. et al. Co (CN)3 catalysts with well-defined coordination structure for the oxygen reduction reaction. Nat. Catal. 6, 1164–1173 (2023).
Shao, R. et al. Promoting ordering degree of intermetallic fuel cell catalysts by low-melting-point metal doping. Nat. Commun. 14, 5896 (2023).
Huang, L. et al. An integrated platinum-nanocarbon electrocatalyst for efficient oxygen reduction. Nat. Commun. 13, 7270 (2022).
Bu, L. et al. Three-dimensional porous platinum-tellurium-rhodium surface/interface achieve remarkable practical fuel cell catalysis. Energy Environ. Sci. 15, 3877–3890 (2022).
Wu, Q. et al. Ultra-dense carbon defects as highly active sites for oxygen reduction catalysis. Chem 8, 2715–2733 (2022).
Xiao, F. et al. Atomically dispersed Pt and Fe sites and Pt-Fe nanoparticles for durable proton exchange membrane fuel cells. Nat. Catal. 5, 503–512 (2022).
Koper, M. Theory of multiple proton-electron transfer reactions and its implications for electrocatalysis. Chem. Sci. 4, 2710–2723 (2013).
Li, H. et al. Analysis of the limitations in the oxygen reduction activity of transition metal oxide surfaces. Nat. Catal. 4, 463–468 (2021).
Nørskov, J. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Zhang, N. et al. High-density planar-like Fe2N6 structure catalyzes efficient oxygen reduction. Matter 3, 509–521 (2020).
Su, H. et al. Dynamic evolution of solid-liquid electrochemical interfaces over single-atom active sites. J. Am. Chem. Soc. 142, 12306–12313 (2020).
Liu, M. et al. Avoiding sabatier’s limitation on spatially correlated Pt-Mn atomic pair sites for oxygen electroreduction. J. Am. Chem. Soc. 145, 25252–25263 (2023).
Zhou, W. et al. Regulating the scaling relationship for high catalytic kinetics and selectivity of the oxygen reduction reaction. Nat. Commun. 13, 6414 (2022).
Luo, M. et al. PdMo bimetallene for oxygen reduction catalysis. Nature 574, 81–85 (2019).
Li, H. et al. Oxidative stability matters: A case study of palladium hydride nanosheets for alkaline fuel cells. J. Am. Chem. Soc. 144, 8106–8114 (2022).
Tao, L. et al. Precise synthetic control of exclusive ligand effect boosts oxygen reduction catalysis. Nat. Commun. 14, 6893 (2023).
Xie, L. et al. Modulating the bader charge transfer in single p-block atoms doped Pd metallene for enhanced oxygen reduction electrocatalysis. Angew. Chem. Int. Ed. 63, e202407658 (2024).
Lin, F. et al. Local coordination regulation through tuning atomic-scale cavities of Pd metallene toward efficient oxygen reduction electrocatalysis. Adv. Mater. 34, 2202084 (2022).
Qiu, Y. et al. Atomically dispersed CrOx on Pd metallene for CO-resistant methanol oxidation. Nano lett. 23, 9555–9562 (2023).
Wu, G. et al. In-plane strain engineering in ultrathin noble metal nanosheets boosts the intrinsic electrocatalytic hydrogen evolution activity. Nat. Commun. 13, 4200 (2022).
Xie, M. et al. Intermetallic single-atom alloy In-Pd bimetallene for neutral electrosynthesis of ammonia from nitrate. J. Am. Chem. Soc. 145, 13957–13967 (2023).
Lv, F. et al. A highly efficient atomically thin curved PdIr bimetallene electrocatalyst. Natl Sci. Rev. 8, nwab019 (2021).
Yu, H. et al. Defect-rich porous palladium metallene for enhanced alkaline oxygen reduction electrocatalysis. Angew. Chem. Int. Ed. 60, 12027–12031 (2021).
Huang, S. et al. Sublayer stable Fe dopant in porous Pd metallene boosts oxygen reduction reaction. ACS Nano 16, 522–532 (2022).
Wang, L. et al. Tunable intrinsic strain in two-dimensional transition metal electrocatalysts. Science 363, 870–874 (2019).
Wang, Y. et al. A universal synthesis of ultrathin Pd-based nanorings for efficient ethanol electrooxidation. Mater. Horiz. 10, 1416–1424 (2023).
Bukharaev, A., Berdunov, N., Ovchinnikov, D. & Salikhov, K. Three-dimensional probe and surface reconstruction for atomic force microscopy using a deconvolution algorithm. Scanning Microsc. Int 12, 225–234 (1998).
Markiewicz, P. & Cynthia, M. Atomic force microscopy probe tip visualization and improvement of images using a simple deconvolution procedure. Langmuir 10, 5–7 (1994).
Fan, J. et al. Ligand-confined two-dimensional rhodium hydride boosts hydrogen evolution. Matter 6, 1–12 (2023).
Hanneken, J., Baker, D., Conradi, M. & Eastman, J. NMR study of the nanocrystalline palladium-hydrogen system. J. Alloy Compd. 330, 714–717 (2022).
Baldi, A., Narayan, T., Koh, A. & Dionne, J. In situ detection of hydrogen-induced phase transitions in individual palladium nanocrystals. Nat. Mater. 13, 1143–1148 (2014).
Zhao, Z. et al. Synthesis of stable shape-controlled catalytically active β-palladium hydride. J. Am. Chem. Soc. 137, 15672–15675 (2015).
Fan, X. et al. Surface-enriched single-Bi-atoms tailoring of Pt nanorings for direct methanol fuel cells with ultralow-Pt-loading. Adv. Mater. 36, 2313179 (2024).
Li, M. et al. Programmable synthesis of high-entropy nanoalloys for efficient ethanol oxidation reaction. ACS Nano 17, 13659–13671 (2023).
Sun, Y. et al. Ultrathin PtPd-Based nanorings with abundant step atoms enhance oxygen catalysis. Adv. Mater. 30, 1802136 (2018).
Xu, R. et al. Improving the ORR performance by enhancing the Pt oxidation resistance. J. Catal. 416, 311–321 (2022).
Rahul, R. et al. The role of surface oxygenated-species and adsorbed hydrogen in the oxygen reduction reaction (ORR) mechanism and product selectivity on Pd-based catalysts in acid media. Phys. Chem. Chem. Phys. 17, 15146–15155 (2015).
Chen, C. et al. Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science 343, 1339–1343 (2014).
Zhang, T. & Anderson, A. Oxygen reduction on platinum electrodes in base: Theoretical study. Electrochim. Acta 53, 982–989 (2007).
Anderson, A., Roques, J. & Mukerjee, S. Activation energies for oxygen reduction on platinum alloys: theory and experiment. J. Phys. Chem. B. 109, 1198–1203 (2005).
Lin, C. et al. In-situ reconstructed Ru atom array on α-MnO2 with enhanced performance for acidic water oxidation. Nat. Catal. 4, 1012–1023 (2021).
Wang, T. et al. Enhancing oxygen reduction electrocatalysis by tuning interfacial hydrogen bonds. Nat. Catal. 4, 753–762 (2021).
Nayak, S., McPherson, I. & Vincent, K. Adsorbed intermediates in oxygen reduction on platinum nanoparticles observed by in situ IR spectroscopy. Angew. Chem. Int. Ed. 57, 12855–12858 (2018).
Li, H. Scalable neutral H2O2 electrosynthesis by platinum diphosphide nanocrystals by regulating oxygen reduction reaction pathways. Nat. Commun. 11, 3928 (2020).
Gomez-Marin, A., Rizo, R. & Feliu, J. Oxygen reduction reaction at Pt single crystals: a critical overview. Catal. Sci. Technol. 4, 1685–1698 (2014).
Huang, X. et al. Freestanding palladium nanosheets with plasmonic and catalytic properties. Nat. Nanotechnol. 6, 28–32 (2011).
Gong, S. et al. Completely methylene-free side chain enables significant microphase separation at medium IECs for fuel-cell anion exchange membranes. ACS Appl. Mater. Interfaces 16, 27741–27749 (2024).
Clark, S. et al. First principles methods using CASTEP. Z. Fur Kristallographie 220, 567–570 (2005).
Perdew, J. et al. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 46, 6671–6687 (1992).
Perdew, J., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Hasnip, P. & Pickard, C. Electronic energy minimisation with ultrasoft pseudopotentials. Comput. Phys. Commun. 174, 24–29 (2006).
Head, J. & Zerner, M. A broyden-fletcher-goldfarb-shanno optimization procedure for molecular geometries. Chem. Phys. Lett. 122, 264–270 (1985).
Acknowledgements
J. Fan and X. Cui acknowledged the support of the National Natural Science Foundation of China (12034002 (X.C.), 22279044 (X.C.), and 22402064 (J.F.)). B.H. acknowledged the support of the National Key R&D Program of China (2021YFA1501101), Research Grant Council of Hong Kong (15304023, 15304724, C1003-23Y), National Natural Science Foundation of China/Research Grant Council of Hong Kong Joint Research Scheme (N_PolyU502/21), the National Natural Science Foundation of China/Research Grants Council of Hong Kong Collaborative Research Scheme (CRS_PolyU504/22), the Shenzhen Fundamental Research Scheme-General Program (JCYJ20220531090807017), and the Natural Science Foundation of Guangdong Province (2023A1515012219).
Author information
Authors and Affiliations
Contributions
J.F., B.H. and X.C. supervised the execution of the overall project. Y.Q. designed and performed the synthetic and electrochemical experiments, characterized the catalyst, and analyzed the data. M.S. and B.H. conducted the DFT calculations. J.W. assisted with the idea design of catalysts. S.W. assisted with the data analysis. H.H. and X.Z. assisted with the electrochemical ORR tests. D.J. assisted with the calculations of the ORR mechanism. S.X. assisted with the drawing of the schematic diagram. D.W. assisted with the ssNMR tests. X.G. and Wei Z. performed the atomic-resolution HAADF-STEM tests. C.C. and Y. S. performed the alkaline H2-O2 fuel cell test. Weitao Z. assisted with the paper writing. The results of the manuscript were discussed by all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Zhao Cai and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qiu, Y., Sun, M., Wu, J. et al. Boosting oxygen reduction performances in Pd-based metallenes by co-confining interstitial H and p-block single atoms. Nat Commun 16, 5262 (2025). https://doi.org/10.1038/s41467-025-60400-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-60400-5