Abstract
Subsoils below 30 cm store more than half of global soil carbon. Microbial carbon use efficiency (CUE) serves as a key indicator of microbial control over soil carbon turnover, but the general patterns and drivers of microbial CUE across soil depths remain poorly understood. Here, we report a decreasing trend in microbial CUE with increasing soil depths through large-scale soil sampling across 60 sites spanning tropical to boreal forests. Using multiple analytical and statistical approaches complemented by experiments, we further identify depth-dependent drivers of microbial CUE. In the topsoil (0–10 cm), microbial CUE is primarily regulated by microbial diversity, whereas in deep subsoil (70–100 cm), it is predominantly driven by soil physicochemical protections. Our findings underscore the need to incorporate depth-specific microbial CUE drivers into carbon cycle models for more accurate predictions of whole-soil carbon storage and its feedback to climate change.
Similar content being viewed by others
Introduction
As the largest organic carbon pool in terrestrial ecosystems, soil organic carbon (SOC) provides essential ecosystem services such as soil conservation and climate regulation1,2. SOC storage is a dynamic equilibrium between carbon inputs and outputs3, with soil microorganisms playing a crucial role in both sequestration and decomposition processes4,5,6. Microbial carbon use efficiency (CUE), defined as the proportion of carbon assimilated into microbial biomass as opposed to being respired7, plays a crucial role in mediating the flow of carbon through soil8,9,10,11,12,13,14. While microbial CUE has been extensively studied in surface soils15,16,17,18,19, its dynamics and underlying mechanisms in subsurface soils remain poorly understood14,20,21. This knowledge gap is critical, as subsoils deeper than 30 cm store more than 50% of global SOC22, and their response to climate change remains a major source of uncertainty in soil carbon dynamic predictions23,24.
Until now, there is no consensus on how microbial CUE changes with soil depth. Owing to the general decline in substrate availability and quality with increasing depth25,26, microorganisms need to allocate more energy to the mining of resources by exoenzymes, leading to an expected decrease in CUE20. However, experimental findings at the site level have reported varying trends, including decreases27, increases28, or even no change21 in CUE with depth, likely due to site-specific abiotic and biotic conditions and differences in CUE estimation methods. In addition, plant carbon input is generally lower in subsoil than in topsoil22, leading to a greater proportion of microbially-derived carbon to total carbon storage at greater depths29. Microbial processes (e.g., microbial CUE) are thus particularly important for soil carbon-climate feedbacks in subsoil21. Consequently, understanding the depth-related patterns of microbial CUE is essential for developing a comprehensive framework of soil carbon dynamics under climate change.
Increasing attention has been paid to the drivers of microbial CUE, whereas the interactions among different drivers across broad geographical scales, especially in subsoil, remain largely unexplored. Traditionally, environmental and substrate conditions are considered major drivers of CUE20,30. For example, rising temperatures typically reduce CUE31 due to increased metabolic energy demands30, and lower water availability is expected to decrease CUE by elevating osmoregulatory costs and limiting substrate supply to microbial cells7. Beyond environmental factors, substrate conditions also play a crucial role, with greater substrate availability or quality generally enhancing CUE32. Recent evidence further emphasizes the roles of physicochemical protection and microbial community structure in regulating soil carbon cycling33,34,35, both of which likely affect CUE by regulating substrate conditions. Stronger physicochemical protection, for instance, may lower CUE by diverting more substrate toward maintenance rather than growth36. Importantly, these factors (e.g., physicochemical protection, substrate availability and microbial community structure) do not act independently but interact to shape microbial CUE30,37,38,39. Environmental conditions can directly influence CUE via microbial metabolism40 and indirectly through their effects on physicochemical protection41, substrate availability42 and microbial community structure43.
In addition, owing to variations in environmental conditions2, substrates44, physicochemical protection45 and microorganisms46 across soil horizons, the dominant drivers of microbial CUE may change with depth. In topsoil, where microbial diversity and substrate availability are typically high47,48, these factors likely play a key role in driving CUE variations. In contrast, subsoil is generally characterized by stronger physical protection from soil aggregates and chemical protection by Fe/Al minerals26,49, suggesting that physicochemical protection may become a dominant factor regulating CUE at greater depths. However, most previous studies have primarily focused on topsoil9,50,51, leaving empirical evidence on the key determinants of CUE in subsoil largely lacking, particularly across broad geographic scales.
The overall aim of this study is to reveal the general patterns and drivers of microbial CUE with soil depth. We hypothesize that: (1) CUE is higher at greater soil depth, and (2) substrate availability and microbial diversity have a larger predictive power of CUE in surface soil while physicochemical protection factors are more important in deep soil. To test these hypotheses, we performed a large-scale study using soil samples from three horizons (topsoil: 0–10 cm, subsoil: 35–50 cm, and deep subsoil: 70–100 cm) collected across 60 forest sites in China (Fig. 1a). Microbial CUE was determined using the well-established substrate-independent 18O-H2O method7,21. To identify the key drivers of CUE, we assessed various abiotic and biotic factors, including those related to climate, plant biomass carbon, physicochemical protection, substrate availability, and microbial community structure. Additionally, we conducted manipulative experiments (i.e., aggregate disruption, microbial reciprocal transplantation, and glucose addition) to evaluate the direct effects of microbial community structure, aggregate protection, and substrate availability on CUE. We performed three types of statistical analyses (i.e., classification and regression tree, partial correlation, and structural equation modelling) to determine the relative importance of these factors at different soil horizons. We find that microbial CUE decreases with increasing soil depth, being primarily regulated by microbial diversity in the topsoil and by soil physicochemical protection in the deep subsoil.
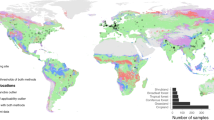
a The 60 soil sampling sites spanning forest ecosystems, with soil samples collected to a depth of one meter. Numbers in parentheses represent the number of sampling sites for each forest type. The geographic variation in CUE indicates values from the topsoil. The background map of China, made with National Geomatics Center of China (https://www.ngcc.cn), is public available. b, c, Box plots illustrating microbial CUE among soil horizons across all sites (n = 60; b) and in different forest types (n = 7, 25, 11, 9, and 8 for tropical, subtropical, warm-temperate, cool-temperate and boreal forests, respectively; (c). Horizontal lines inside the box indicate the median; box limits represent the upper and lower quartiles; and the whiskers are 1.5 times interquartile range. White dots denote mean values, while colored dots represent individual data points. Statistical analysis was performed using a two-sided, paired samples t-test. Topsoil: 0–10 cm, subsoil: 35–50 cm and deep subsoil: 70–100 cm.
Results
Reduced microbial CUE at greater soil depths
Microbial CUE exhibited substantial spatial variability across tropical to boreal forests. CUE values in the topsoil, subsoil and deep subsoil ranged from 0.10 to 0.68 (minimum to maximum), 0.07 to 0.39 and 0.05 to 0.34, respectively, across the 60 sampling sites (Supplementary Fig. 1). However, correlation analyses indicated that CUE was not significantly associated with mean annual temperature (MAT; Supplementary Fig. 2) or mean annual precipitation (MAP; Supplementary Fig. 3). Moreover, CUE showed a decreasing trend with soil depth, with mean values of 0.35 ± 0.15 in the topsoil, 0.21 ± 0.09 in the subsoil, and 0.16 ± 0.07 in the deep subsoil (P < 0.05, Fig. 1b). This decreasing trend was observed across most forest types, except for tropical and subtropical forests, where no differences were found between the subsoil and deep subsoil horizons (P > 0.05, Fig. 1c).
To examine the relationship between SOC and microbial CUE, we analyzed CUE–SOC correlations across different soil horizons and found depth-dependent variations. In the topsoil and subsoil, CUE showed a positive correlation with SOC, though the relationship was weaker in the subsoil than in the topsoil (P < 0.001, Fig. 2a–b). In contrast, a negative CUE–SOC relationship was observed in the deep subsoil (P < 0.05, Fig. 2c). These patterns remained consistent when CUE values were standardized across all soil horizons (Supplementary Fig. 4).
a–c Linear regressions depicting the relationship between SOC and CUE in the topsoil (0–10 cm, a), subsoil (35–50 cm, b) and deep subsoil (70–100 cm, c). The dots correspond to the values for the 60 sites across China’s forests (n = 60). Linear regression model with two-sided test was used for the statistical analysis, and relationships are denoted with solid lines and fit statistics (R2 and P values). The solid lines represent the significant fitted linear regression (P < 0.05), and the shading around the fitted lines represent the 95% confidence intervals around the predicted mean. Note that the x-axis scale varies among the panels due to overall shifts in microbial CUE across soil horizons.
Depth-dependent drivers of microbial CUE
To identify the key drivers of microbial CUE, we examined various biotic and abiotic factors, including climate variables (MAT and MAP), plant biomass carbon (aboveground (AGB) and belowground biomass carbon (BGB)), physicochemical protection (e.g., soil aggregates and mineral-associated organic matter (OC-MAOM)), substrate characteristics (e.g., carbon availability index (CAI) and SOC decomposability (DSOC)), and microbial properties (e.g., bacterial diversity and fungal diversity). Classification and regression tree (CART) analysis was firstly conducted to determine the factors most strongly correlated with CUE (Supplementary Figs. 5–6). When data from all three soil horizons were combined, the analysis revealed that soil horizon served as an automatic classification factor, grouping into two categories: topsoil, and subsoil with deep subsoil (Supplementary Fig. 5). In topsoil, bacterial diversity emerged as the strongest predictor of CUE, whereas in subsoil and deep subsoil, substrate availability and soil aggregates were the primary predictors, respectively. These findings were further supported by separate analyses for each soil horizon, which consistently identified bacterial diversity, substrate availability, and macroaggregates as the most influential factors for CUE in topsoil, subsoil, and deep subsoil, respectively (Supplementary Fig. 6).
Partial correlation analysis was further performed to determine the relationships of CUE with all individual factors when controlling other groups of factors that do not include this factor (Supplementary Figs. 7–9). In the topsoil, CUE was correlated with bacterial and fungal alpha diversity (indicated by Shannon index), regardless of whether other factors were controlled (P < 0.05, Supplementary Fig. 7). In the subsoil, CUE was correlated with substrate availability and quality (P < 0.05, Supplementary Fig. 8). However, in the deep subsoil, CUE was correlated with substrate properties and physicochemical protection variables irrespective of whether other factors were controlled (P < 0.05, Supplementary Fig. 9). These results indicate that microbial diversity was the dominant factor of CUE in the topsoil, whereas substrate properties and physicochemical protection variables were the dominant factors in deeper soil horizons.
Structural equation modeling (SEM) was also performed to assess the influencing pathways of climate, plant biomass carbon, substrate characteristics, physicochemical protection, and microbial properties on CUE for each soil horizon. In the topsoil and subsoil, only substrate characteristics and microbial properties exerted significant controls on CUE (P < 0.05, Fig. 3a–b). In contrast, in the deep subsoil, only physicochemical protection variables exhibited significant effects on CUE (P < 0.05, Fig. 3c). Among all tested factors, substrate characteristics and microbial properties had the highest prediction power of CUE variation in the topsoil and subsoil, whereas in the deep subsoil, physicochemical protection had the highest prediction power (Fig. 3d–f). These results further confirm that microbial CUE in the topsoil and subsoil is primarily regulated by substrate condition (i.e., availability and quality) and microbial diversity (i.e., bacterial and fungal diversity), whereas in the deep subsoil, it is predominantly governed by physicochemical protection.
a–c, Structural equation models illustrating the direct and indirect effects of various factor groups on CUE in the topsoil (a), subsoil (b) and deep subsoil (c). Pentagons represent the first principal component of the corresponding factor group, derived from principal component analysis, including climate, plant biomass, physicochemical protection, substrate and microbial properties. Black dashed and solid arrows indicate significant negative and positive relationships (P < 0.05), respectively, while gray arrows indicate nonsignificant relationships (P > 0.05). Numbers adjacent to the arrows represent standardized path coefficients. Statistical significance is based on maximum likelihood tests with n = 60 independent soil samples. Asterisks indicate significant relationships. For exact P values, see Supplementary Fig. 10. Standardized total effects (sum of direct plus indirect effects) of different factor groups on microbial CUE in the topsoil (d), subsoil (e) and deep subsoil (f), as obtained from the structural equation models. Plant BC, plant biomass carbon; MAT, mean annual temperature; MAP, mean annual precipitation; AGB, aboveground biomass carbon; BGB, belowground biomass carbon; Mac, macroaggregates; Mic, microaggregates; POC, content of SOC stored in the POM fraction; MAOC, content of SOC stored in the MAOM fraction; OC-Fe, content of SOC associated with Fe oxides; Bac_div, bacterial diversity; Fun_div, fungal diversity; CAI, carbon availability index; DSOC, decomposability of SOC.
The above statistical analyses (i.e., CART, partial correlation, and SEM) showed that microbial diversity primarily regulate microbial CUE in the topsoil, while physicochemical protection governs CUE in the deep subsoil. To further investigate the direct effects underlying these depth-associated variations, we performed microbial reciprocal transplantation, aggregate disruption, and glucose addition experiments. In both uncrushed and crushed (aggregate disruption) soils, inoculating the topsoil with deep subsoil microorganisms (away inoculum) reduced CUE compared to inoculating it with its own inoculum (P < 0.01, Supplementary Fig. 11a), introducing topsoil microorganisms into the deep subsoil had no effect (Supplementary Fig. 11a). When microorganisms were controlled (by inoculating with either their own or away inoculum), the removal of aggregate protection through crushing led to an increase in CUE in the deep subsoil (P < 0.05) but had no effect in the topsoil (Supplementary Fig. 11b). Additionally, glucose addition increased CUE in the deep subsoil (P < 0.01) but had no notable impact in the topsoil (Supplementary Fig. 12). These results collectively demonstrate that microorganisms primarily mediate CUE in the topsoil, whereas physicochemical protection is the key determinant in the deep subsoil.
Discussion
Based on large-scale soil sampling to a depth of 1 meter across tropical to boreal forests, this study provides empirical evidence of a depth-informed insight into CUE variations and drivers across broad geographic scales. As in previous cross-scale studies9,10,13,16, our results show that microbial CUE exhibited substantial spatial variability (Fig. 1a). However, CUE was independent of climatic factors such as MAT and MAP (Supplementary Figs. 2–3), which contrasts with earlier findings from forests in China9,16 but aligns with results from a recent global-scale synthesis18. Importantly, our results show that CUE decreases with increasing soil depth, and it is primarily regulated by microbial community structure in the topsoil, whereas in the deep subsoil, it is predominantly controlled by physicochemical protection. This knowledge is crucial for improving predictions of whole-soil carbon storage and its feedback to climate change.
The decreased CUE with soil depth can be explained by reduced substrate quality and availability20, lower microbial diversity46, and enhanced physicochemical protection33 at greater depths. First, the lower substrate quality and availability at greater depths could attenuate microbial CUE. As substrates serve as a direct energy source for microorganisms, their quality and availability may largely determine microbial CUE. Microorganisms require additional energy to decompose low-quality or scarce substrates, leading to lower CUE under these conditions14. Consistent with this, we observed a general decline in substrate quality and availability with increasing soil depth across a broad geographical scale (Supplementary Fig. 13), contributing to the reduction in CUE. Further supporting this, glucose addition increased CUE in the deep subsoil but had no effect in the topsoil (Supplementary Fig. 12), suggesting that limited substrate availability constrains CUE at greater depths. Second, as microorganisms regulate soil carbon decomposition and stabilization52,53, shifts in microbial diversity across soil horizons can influence CUE. Our results show a decline in bacterial and fungal diversity with depth (Supplementary Fig. 14), likely due to reduced soil resource availability (e.g., lower substrate levels; Supplementary Fig. 13). Given that higher microbial diversity enhances the efficient utilization of diverse soil substrates, a decline in diversity may contribute to reduced CUE. Finally, stronger physicochemical protection at greater depths (Supplementary Fig. 15) may further suppress CUE by restricting substrate accessibility and quality.
More importantly, our results reveal depth-dependent changes in controls of microbial CUE through a comprehensive assessment of its relationships with climate, plant carbon input, substrate characteristics, physicochemical protection, and microbial properties, supported by manipulative experiments. In the topsoil, microbial CUE was primarily mediated by microorganisms. A recent study by Domeignoz-Horta et al.7 reported a positive microbial diversity–CUE relationship using a model soil at 60% water holding capacity. Using soils from diverse environments, this study also revealed that higher bacterial or fungal diversity was associated with a higher CUE. This may be because greater microbial diversity enhances the efficient utilization of various carbon substrates7, thereby increasing CUE. However, the influence of microbial community structure on CUE was significant in the topsoil but not in deeper soil layers, likely due to the naturally lower microbial diversity at greater depths (Supplementary Fig. 14), which limits its effect on CUE. This was further confirmed by the microbial reciprocal transplant experiment: inoculating the topsoil with low-diversity microbial communities decreased CUE, whereas inoculating the deep subsoil with high-diversity communities had no effect. Moreover, despite the strong correlations between CUE and substrate in the topsoil (Fig. 3a), the glucose addition experiment showed no direct causal effect of substrate availability on CUE at this depth (Supplementary Fig. 12), suggesting that the observed associations may be mediated by other factors. These depth-dependent microbial influences on CUE imply that soil management strategies promoting microbial diversity may be more effective in modifying microbial processes (e.g., microbial CUE) in surface soils than in carbon-poor deep horizons, where such interventions may have limited impact.
Our results further indicate that physicochemical protection plays a dominant role in regulating microbial CUE in deep subsoil. Microaggregates and Fe-rich minerals can restrict oxygen diffusion54, limiting microbial activity55, while simultaneously occluding SOC within aggregate interiors and/or by binding it to Fe minerals, creating spatial disconnection between substrates and enzymes56. In response to these processes, microorganisms may allocate more energy toward SOC decomposition, leading to lower CUE under stronger physicochemical protection. In addition, the degree of protection intensifies with decreasing aggregate size and increasing Fe mineral content. Our results showed a higher physicochemical protection in deep subsoil than in topsoil (Supplementary Fig. 15), which may explain why this mechanism predominantly mediates CUE in deep subsoil but not in topsoil. This was further supported by aggregate disruption experiments, where the removal of aggregate protection promoted microbial CUE in deep subsoil but had no impact in topsoil. Physicochemical protection has long been recognized as a key factor in SOC persistence, especially in deeper soil layers45. Our results underscore the critical role of physicochemical protection in soil carbon cycling by regulating microbial CUE, particularly in deep subsoil, emphasizing the need to incorporate physicochemical protection into models for more accurate predictions of soil carbon dynamics under changing environmental conditions.
Although our study provides empirical evidence for the depth-dependent drivers of soil microbial CUE, several limitations should be acknowledged. First, short-term incubation experiments were employed to assess the direct effects of physicochemical protection and microbial community composition on CUE. Despite a 3-week preincubation designed to reduce potential disturbance effects, the disruption of soil aggregates may have caused a transient increase in soil respiration, potentially influencing short-term CUE estimates. Future studies involving longer-term incubations, in both laboratory and field settings, with controlled aggregate disruption, are warranted to improve our understanding of these processes. Second, while the observed microbial respiration indicates the activity and viability of the transferred microbial communities to some extent, additional direct evidence—such as measurements of enzyme activity—would more robustly confirm microbial viability. Nevertheless, these limitations do not undermine our principal conclusion that microbial CUE and its drivers are depth-dependent.
In conclusion, based on large-scale soil sampling and substrate-independent 18O-H2O measurements of microbial CUE, this study provides empirical evidence for depth-dependent changes in microbial CUE and underlying mechanisms (Fig. 4). Our findings reveal a decreasing trend in CUE with increasing soil depth, which is related to reduced substrate quality and availability, lower microbial diversity and/or enhanced physicochemical protection at greater depths. Moreover, our findings reveal that in the topsoil, CUE is primarily influenced by microbial community structure, whereas in deep soil layers, physicochemical protection plays a dominant role. These findings suggest that ecosystem management strategies (e.g., organic farming and forestation) that increase microbial diversity can enhance microbial CUE in carbon-rich topsoil but may not be applicable to carbon-poor deep subsoil. In contrast, implementing ecosystem management strategies that increase carbon substrate availability in deeper soil layers could enhance microbial CUE. By elucidating these depth-specific mechanisms, our study provides a foundation for prioritizing future research on microbial processes in subsoil to improve predictions of whole-soil carbon storage and its feedback to climate change.
Microbial CUE declines with increasing soil depth (shown on the right), driven by reduced microbial diversity and substrate availability or enhanced physicochemical protection (shown on the left). In the topsoil, microbial CUE is primarily regulated by microbial diversity, where higher microbial diversity leads to higher CUE. In contrast, in deep subsoil, CUE is predominantly controlled by physicochemical protection, with stronger protection resulting in lower CUE. The relative importance of climate, plant biomass carbon, pH, substrate, microbial diversity and physicochemical protection in shaping microbial CUE in the topsoil or deep subsoil is shown on the bottom; the length of each rectangular bar indicates the relative importance for the corresponding factor, based on structural equation modeling results presented in Fig. 3. R, microbial respiration; G, microbial growth; plant BC, plant biomass carbon.
Methods
Study area and soil sampling
Soils were collected from 60 representative sites across China’s forest ecosystems, covering a broad range of geographical and climatic conditions (Fig. 1a) These sites span latitudes from 18.26 to 50.43°N and longitudes from 81.02 to 129.47°E, with MAT ranging from −2.2 to 25.0 °C and MAP varying from 98 to 1873 mm. The study area encompasses major global forest biomes, including tropical (7 sites), subtropical (25 sites), warm-temperate (11 sites), cool-temperate (9 sites) and boreal (8 sites) forests. In this study, sampling sites with a MAT below 5.0 °C were classified as boreal forests, following the criteria used in previous studies57. Our previous studies have demonstrated large variability in soil physicochemical and microbial properties across these forests57,58, providing an ideal platform to investigate the general patterns and regulatory mechanisms of microbial CUE with soil depth.
To investigate variations in microbial CUE and the underlying mechanisms across soil horizons, soils were collected from three distinct horizons: topsoil (0–10 cm), subsoil (35–50 cm) and deep subsoil (70–100 cm). Sampling from discontinuous horizons minimized interactions between adjacent soil horizons. At each site, soils were obtained from three random locations (each separated by more than 20 m). After manually removing litter and organic layers, soils from each horizon were collected, transported to the laboratory with ice bags, and homogenized into a composite sample per horizon per site. Fresh samples were sieved (2 mm) under field-moist conditions, with visible roots and rocks being removed. Each composite sample was then divided into three subsamples for different analyses: (1) air-dried for SOC, pH and physicochemical protection analyses, (2) stored at 4 °C for microbial CUE, CAI and DSOC measurements, (3) preserved at −80 °C for microbial community quantification.
Microbial CUE assessments
Microbial CUE was determined using a substrate-independent 18O-H2O method7,21. Briefly, six sets of 400 mg of fresh soil samples were placed into 2 ml screw-cap vials. In three sets, 18O-labeled water (99 atom% 18O) was added to achieve an enrichment of 20 atom% 18O and 60% of water holding capacity, while the other three sets received the same volume of non-labeled water as a natural abundance control. The vials were then transferred to 40 ml headspace bottles, sealed, and flushed with CO2-free air. After 24 h of incubation at 20 °C in darkness, a 5 ml gas sample was extracted from each bottle to measure CO2 concentration using a gas chromatograph (Agilent 6890, Agilent Corp.), allowing determination microbial respiration rates. The vials were subsequently frozen at −80 °C until DNA extraction. Total DNA was extracted and quantified using the FastDNA™ SPIN Kit for Soil (MP Biomedicals) following the manufacturer’s instructions. To remove water, the remaining DNA extract was dried in a silver capsule at 60 °C for 6 h, and the total oxygen content and 18O abundance were determined using IRMS-TC/EA (Thermo Scientific). Additionally, microbial biomass carbon (MBC), which was used to convert the amount of newly produced DNA into MBC, was measured using the CH3Cl fumigation extraction method59; to do so, six sets of 5 g of soil samples were weighed into 20 mL scintillation vials, with three sets undergoing fumigation and three serving as controls. Finally, microbial CUE was calculated as follows21,51:
where CUE represents microbial carbon use efficiency, Crespiration denotes the carbon allocated to microbial respiration, and Cgrowth refers to the carbon allocated to microbial biomass production, which was determined by measuring 18O-labeled DNA production:
where fDNA represents the conversion factor for newly produced DNA calculated as the ratio of soil MBC to soil DNA content, Ototal denotes the total oxygen content (μg), at%excess refers to the excess 18O atom percentage in the labeled sample compared to the control, at%final represents the18O atom percentage in soil water, w is the dry soil weight (g) and t is the incubation time (h).
Although the substrate-independent 18O-H2O method has been widely used to measure microbial CUE7,37, it requires lab incubation, which is typically conducted under aerobic conditions, as in this study. This can result in higher oxygen availability than in the natural soil environment, potentially influencing soil carbon cycling at different horizons. However, it is unlikely to alter the relative ranking of microbial CUE, except in cases where deep subsoils are consistently more anaerobic or aerobic than surface soils, a pattern not generally observed across our 60 upland forest sites. Since all soil samples were collected above the water table (gravimetric water content <36%), aerobic rather than anaerobic soil carbon processes would dominate across all horizons. Additionally, we assessed the potential effect of oxygen levels on microbial CUE by adjusting O2 concentrations to 5%, 10%, and 20% in deep subsoils from 15 sites and found no significant impact on CUE (Supplementary Fig. 16). Thus, we conclude that elevated oxygen availability during lab-based CUE measurements in deep subsoils does not affect our overall findings.
Assessing the direct influence of aggregate protection, substrate availability, and microbial communities on microbial CUE
To further investigate the direct effects of microbial communities and aggregate protection on microbial CUE in the topsoil and deep subsoil, we conducted an independent manipulative experiment combining aggregate disruption with microbial reciprocal transplantation. This experiment utilized soils from 15 of the 60 sites, with microbial inocula and both crushed and uncrushed subsamples prepared for analysis. Microbial inocula were obtained by suspending 1 g of dry-weight-equivalent fresh soil in 100 ml of sterilized deionized water, shaking for 0.5 h at 150 rpm, and filtering through a Whatman GF/C filter33. For aggregate disruption, soils were air-dried, and a portion was crushed using a ball mill to remove aggregate protection. Both crushed and uncrushed soil samples were sterilized via γ-irradiation60. The sterilized soils from both topsoil and deep subsoil were then inoculated with either their own microbial inoculum (own) or that from the corresponding opposite horizon (away), adding 0.5 ml of inoculum to 2.5 g of sterilized soil. After a 3-week preincubation to minimize the possible disturbances (e.g. soil crushing and packing) and activate microorganisms, microbial CUE was then measured as described above. To assess the direct impact of aggregate protection, we compared CUE values between crushed and uncrushed soils inoculated with the same microbial inoculum. To determine the direct influence of microbial communities, we compared CUE between soils receiving their own inoculum versus those receiving away inoculum for both crushed and uncrushed samples.
To directly assess the effects of substrate availability on microbial CUE, we conducted a glucose addition experiment using topsoil and deep subsoil samples from the previously selected 15 sites. In the treatment group, glucose was added to fresh soil at a concentration of 5 mg C g−¹ soil, while in the control group, an equal amount of deionized water was added. Microbial CUE was then measured as described earlier, and the CUE values were compared between the glucose-treated and control samples to evaluate the impact of substrate availability.
Climate and vegetation properties
Climate variables, including MAT and MAP, were obtained from the WorldClim database (www.worldclim.org). Carbon input variables, specifically plant AGB and BGB, were extracted from 300 m resolution maps developed by Spawn et al.61. Data extraction was performed using ESRI ArcMap (version 10.3) software.
Soil and microbial property analyses
To investigate the factors driving microbial CUE at different soil horizons across China’s forests, data on physicochemical protection, substrate availability, substrate quality and microbial properties were analyzed.
Physicochemical protection properties
Physicochemical protection of SOC was deconstructed into physical protection, mediated by soil aggregates, and chemical protection, governed by minerals. Soil aggregation was assessed using the wet sieving technique62, quantifying microaggregates (0.25–0.053 mm) and macroaggregates (>0.25 mm), with SOC proportions in these fractions analyzed via an elemental analyzer (Multi EA 4000, Analytik Jena, Germany) to determine aggregate protection. A higher SOC proportion in microaggregates indicates stronger physical protection34. In addition, chemical protection was quantified by measuring Fe/Al oxide content and organo-mineral associations. The SOC stored in mineral-associated organic matter (OC-MAOM) and Fe oxides (OC-Fe) was measured following established protocols63. Organo-mineral associations were determined via fractionation techniques64, while organically complexed oxyhydroxides (Fep + Alp)65 and poorly crystallized oxyhydroxides (Feo + Alo)66 were determined using an inductively coupled plasma optical emission spectrometer (iCAP 6300, Thermo Fisher Scientific).
Substrate availability and quality
Substrate availability and quality were assessed using two key metrics: CAI and SOC decomposability (DSOC). CAI was determined as the ratio of basal respiration to substrate-induced respiration rate67. To measure this, a 60 g L−1 glucose solution was added to 5 g dry weight of fresh soil to determine substrate-induced respiration (glucose-added treatment), while the same amount of deionized water was added to a separate sample to measure basal respiration (ambient-substrate treatment). Substrate quality was evaluated through DSOC, calculated as the ratio of respiration rate at 20 °C to SOC content, providing an indicator of SOC decomposability.
Microbial properties
Microbial diversity was assessed using high-throughput sequencing technique. DNA was extracted with a PowerSoil DNA Kit (Qiagen, Carlsbad, USA) following the manufacturer’s protocol. The V4 region of the 16S rRNA gene was amplified for bacterial analysis, while the nuclear ribosomal internal transcribed spacer 2 (ITS2) region was amplified for fungal analysis. Detailed polymerase chain reaction conditions and data processing methods are available in Li et al.58 and the Supplementary Text in the supplementary information. Bacterial and fungal diversity was indicated by alpha diversity, which was quantified using the Shannon index.
Statistical analyses
A paired samples t-test was used to evaluate differences in microbial CUE between soil horizons using SPSS Statistics 23 (IBM). CART analysis was used to assess the impact of explanatory variables on CUE variation across all soil horizons and at each horizon. This non-parametric technique sequentially partitions a dataset consisting of a response variable and multiple potential predictor variables68. CART is particularly effective for analyzing the relative importance of predictors in explaining response variable variation, handling nonlinear relationships and high-order interactions68,69. The analysis was performed using the ‘rpart’ and ‘rpart.plot’ packages in R (version 4.0.3). Given the strong connections and inter-correlations among variables, partial correlation analysis was also conducted to examine the relationships of CUE with each variable tested using the ‘tidyverse’ and ‘ppcor’ packages in R (version 4.0.3). Additionally, SEM was employed to partition the indirect and direct effects of climate, carbon inputs, physicochemical protection, substrate and microbial properties on CUE. Principal component analysis was performed to generate a multivariate functional index prior to SEM analyses because of the strong correlations among variables within each category34,37. The first principal component was introduced as a new variable to represent the combined group properties in subsequent SEM analysis. The SEM was constructed using AMOS 21.0 (Amos Development Corporation, Chicago, IL).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Supplementary Information is available online. The sequence data generated in this study have been deposited in the National Omics Data Encyclopedia (NODE) under project accession number OEP00000792. The data supporting the main findings of this study are deposited in the Zenodo database (https://zenodo.org/records/15340227).
References
Paustian, K. et al. Climate-smart soils. Nature 532, 49–57 (2016).
Balesdent, J. et al. Atmosphere–soil carbon transfer as a function of soil depth. Nature 559, 599–602 (2018).
Smith, P. & Fang, C. A warm response by soils. Nature 464, 499–500 (2010).
Zhou, J. et al. Microbial mediation of carbon-cycle feedbacks to climate warming. Nat. Clim. Change 2, 106–110 (2012).
García-Palacios, P. et al. Evidence for large microbial-mediated losses of soil carbon under anthropogenic warming. Nat. Rev. Earth Environ. 2, 507–517 (2021).
Wieder, W. R., Bonan, G. B. & Allison, S. D. Global soil carbon projections are improved by modelling microbial processes. Nat. Clim. Change 3, 909–912 (2013).
Domeignoz-Horta, L. A. et al. Microbial diversity drives carbon use efficiency in a model soil. Nat. Commun. 11, 3684 (2020).
Fang, L. Multifaceted links between microbial carbon use efficiency and soil organic carbon sequestration. Glob. Change Biol. 31, e70045 (2025).
Wang, C. et al. Large-scale importance of microbial carbon use efficiency and necromass to soil organic carbon. Glob. Change Biol. 27, 2039–2048 (2021).
Tao, F. et al. Microbial carbon use efficiency promotes global soil carbon storage. Nature 618, 981–985 (2023).
Malik, A. A. et al. Land use driven change in soil pH affects microbial carbon cycling processes. Nat. Commun. 9, 3591 (2018).
Yang, Y. et al. Unlocking mechanisms for soil organic matter accumulation: carbon use efficiency and microbial necromass as the keys. Glob. Change Biol. 31, e70033 (2025).
Pei, J., Fang, C., Li, B., Nie, M. & Li, J. Aridity-driven change in microbial carbon use efficiency and Its linkage to soil carbon storage. Glob. Change Biol. 30, e17565 (2024).
He, X. et al. Emerging multiscale insights on microbial carbon use efficiency in the land carbon cycle. Nat. Commun. 15, 8010 (2024).
Manzoni, S. et al. Reviews and syntheses: Carbon use efficiency from organisms to ecosystems – definitions, theories, and empirical evidence. Biogeosciences 15, 5929–5949 (2018).
Ren, C. et al. Thermal sensitivity of soil microbial carbon use efficiency across forest biomes. Nat. Commun. 15, 6269 (2024).
Li, L. et al. Asymmetric winter warming reduces microbial carbon use efficiency and growth more than symmetric year-round warming in alpine soils. Proc. Natl. Acad. Sci. USA. 121, e2401523121 (2024).
Hu, J. et al. Microbial carbon use efficiency and growth rates in soil: global patterns and drivers. Glob. Change Biol. 31, e70036 (2025).
Yu, K. et al. Nonlinear microbial thermal response and its implications for abrupt soil organic carbon responses to warming. Nat. Commun. 16, 2763 (2025).
Zhang, Q. et al. Whole-soil-profile warming does not change microbial carbon use efficiency in surface and deep soils. Proc. Natl. Acad. Sci. USA120, e2302190120 (2023).
Spohn, M., Klaus, K., Wanek, W. & Richter, A. Microbial carbon use efficiency and biomass turnover times depending on soil depth–implications for carbon cycling. Soil Biol. Biochem. 96, 74–81 (2016).
Jobbágy, E. G. & Jackson, R. B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 10, 423–436 (2000).
Bradford, M. A. et al. Managing uncertainty in soil carbon feedbacks to climate change. Nat. Clim. Change 6, 751–758 (2016).
Wang, M. et al. Global soil profiles indicate depth-dependent soil carbon losses under a warmer climate. Nat. Commun. 13, 5514 (2022).
Li, J. et al. Rising temperature may trigger deep soil carbon loss across forest ecosystems. Adv. Sci. 7, 2001242 (2020).
Fontaine, S. et al. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 450, 277–280 (2007).
Takriti, M. et al. Soil organic matter quality exerts a stronger control than stoichiometry on microbial substrate use efficiency along a latitudinal transect. Soil Biol. Biochem. 121, 212–220 (2018).
Li, J., Pei, J., Dijkstra, F. A., Nie, M. & Pendall, E. Microbial carbon use efficiency, biomass residence time and temperature sensitivity across ecosystems and soil depths. Soil Biol. Biochem. 154, 108117 (2021).
Ni, X. et al. A quantitative assessment of amino sugars in soil profiles. Soil Biol. Biochem. 143, 107762 (2020).
Manzoni, S., Taylor, P., Richter, A., Porporato, A. & Ågren, G. I. Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. N. Phytol. 196, 79–91 (2012).
Zhang, Q., Qin, W., Feng, J. & Zhu, B. Responses of soil microbial carbon use efficiency to warming: Review and prospects. Soil Ecol. Lett. 4, 307–318 (2022).
Saifuddin, M., Bhatnagar, J. M., Segrè, D. & Finzi, A. C. Microbial carbon use efficiency predicted from genome-scale metabolic models. Nat. Commun. 10, 3568 (2019).
Qin, S. et al. Temperature sensitivity of SOM decomposition governed by aggregate protection and microbial communities. Sci. Adv. 5, eaau1218 (2019).
Chen, L. et al. Regulation of priming effect by soil organic matter stability over a broad geographic scale. Nat. Commun. 10, 5112 (2019).
Doetterl, S. et al. Soil carbon storage controlled by interactions between geochemistry and climate. Nat. Geosci. 8, 780–783 (2015).
Feng, X. et al. Nitrogen input enhances microbial carbon use efficiency by altering plant–microbe–mineral interactions. Glob. Change Biol. 28, 4845–4860 (2022).
Duan, P. et al. Tree species diversity increases soil microbial carbon use efficiency in a subtropical forest. Glob. Change Biol. 29, 7131–7144 (2023).
Domeignoz-Horta, L. A. et al. Plant diversity drives positive microbial associations in the rhizosphere enhancing carbon use efficiency in agricultural soils. Nat. Commun. 15, 8065 (2024).
Shi, J. et al. Soil organic carbon increases with decreasing microbial carbon use efficiency during vegetation restoration. Glob. Change Biol. 30, e17616 (2024).
Dove, N. C., Torn, M. S., Hart, S. C. & Taş, N. Metabolic capabilities mute positive response to direct and indirect impacts of warming throughout the soil profile. Nat. Commun. 12, 2089 (2021).
Mao, X. et al. Climate-induced shifts in composition and protection regulate temperature sensitivity of carbon decomposition through soil profile. Soil Biol. Biochem. 172, 108743 (2022).
Zosso, C. U., Ofiti, N. O. E., Torn, M. S., Wiesenberg, G. L. B. & Schmidt, M. W. I. Rapid loss of complex polymers and pyrogenic carbon in subsoils under whole-soil warming. Nat. Geosci. 16, 344–348 (2023).
Guo, X. et al. Climate warming leads to divergent succession of grassland microbial communities. Nat. Clim. Change 8, 813–818 (2018).
Chen, L. et al. Determinants of carbon release from the active layer and permafrost deposits on the Tibetan Plateau. Nat. Commun. 7, 13046 (2016).
Rumpel, C. & Kögel-Knabner, I. Deep soil organic matter—a key but poorly understood component of terrestrial C cycle. Plant Soil 338, 143–158 (2011).
Fierer, N., Schimel, J. P. & Holden, P. A. Variations in microbial community composition through two soil depth profiles. Soil Biol. Biochem. 35, 167–176 (2003).
Crowther, T. W. et al. The global soil community and its influence on biogeochemistry. Science 365, eaav0550 (2019).
Bahram, M. et al. Structure and function of the global topsoil microbiome. Nature 560, 233–237 (2018).
Schmidt, M. W. I. et al. Persistence of soil organic matter as an ecosystem property. Nature 478, 49–56 (2011).
He, P., Zhang, Y., Shen, Q., Ling, N. & Nan, Z. Microbial carbon use efficiency in different ecosystems: A meta-analysis based on a biogeochemical equilibrium model. Glob. Change Biol. 29, 4758–4774 (2023).
Zheng, Q. et al. Growth explains microbial carbon use efficiency across soils differing in land use and geology. Soil Biol. Biochem. 128, 45–55 (2019).
Cavicchioli, R. et al. Scientists’ warning to humanity: microorganisms and climate change. Nat. Rev. Microbiol. 17, 569–586 (2019).
Qin, S., Zhang, D., Wei, B. & Yang, Y. Dual roles of microbes in mediating soil carbon dynamics in response to warming. Nat. Commun. 15, 6439 (2024).
Dungait, J. A. J., Hopkins, D. W., Gregory, A. S. & Whitmore, A. P. Soil organic matter turnover is governed by accessibility not recalcitrance. Glob. Change Biol. 18, 1781–1796 (2012).
Blagodatskaya, Е et al. Oxygen and substrate availability interactively control the temperature sensitivity of CO2 and N2O emission from soil. Biol. Fertil. Soils 50, 775–783 (2014).
Six, J., Conant, R. T., Paul, E. A. & Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 241, 155–176 (2002).
Li, J. et al. Biogeographic variation in temperature sensitivity of decomposition in forest soils. Glob. Change Biol. 26, 1873–1885 (2020).
Li, J. et al. Key microorganisms mediate soil carbon-climate feedbacks in forest ecosystems. Sci. Bull. 66, 2036–2044 (2021).
Vance, E. D., Brookes, P. C. & Jenkinson, D. S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 19, 703–707 (1987).
McNamara, N., Black, H., Beresford, N. & Parekh, N. Effects of acute gamma irradiation on chemical, physical and biological properties of soils. Appl. Soil Ecol. 24, 117–132 (2003).
Spawn, S. A., Sullivan, C. C., Lark, T. J. & Gibbs, H. K. Harmonized global maps of above and belowground biomass carbon density in the year 2010. Sci. Data 7, 112 (2020).
Six, J., Elliott, E., Paustian, K. & Doran, J. Aggregation and soil organic matter accumulation in cultivated and native grassland soils. Soil Sci. Soc. Am. J. 62, 1367–1377 (1998).
Qin, S. et al. Temperature sensitivity of permafrost carbon release mediated by mineral and microbial properties. Sci. Adv. 7, eabe3596 (2021).
Lavallee, J. M., Soong, J. L. & Cotrufo, M. F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Change Biol. 26, 261–273 (2020).
Gentsch, N. et al. Properties and bioavailability of particulate and mineral-associated organic matter in A rctic permafrost soils, L ower K olyma R egion, R ussia. Eur. J. Soil Sci. 66, 722–734 (2015).
Gentsch, N. et al. Temperature response of permafrost soil carbon is attenuated by mineral protection. Glob. Change Biol. 24, 3401–3415 (2018).
Gershenson, A., Bader, N. E. & Cheng, W. Effects of substrate availability on the temperature sensitivity of soil organic matter decomposition. Glob. Change Biol. 15, 176–183 (2009).
De’ath, G. & Fabricius, K. E. Classification and regression trees: a powerful yet simple technique for ecological data analysis. Ecology 81, 3178–3192 (2000).
Cleveland, C. C. et al. Relationships among net primary productivity, nutrients and climate in tropical rain forest: a pan-tropical analysis. Ecol. Lett. 14, 939–947 (2011).
Acknowledgements
We thank Professor Joshua Schimel for insightful comments during the manuscript preparation, Ting Zhu, Jianjun Xu and Siyuan Xu for the help with data analysis, and Zihan Li for help with the illustration of the conceptual diagram. This work was supported by the National Natural Science Foundation of China (32430065 and 32471831) to M.N. and J.L., the Science and Technology Plan Project of Shanghai (23DZ1202700) to J.L., and the Natural Science Foundation of Shanghai (23ZR1404400) to J.L.
Author information
Authors and Affiliations
Contributions
J.L. designed the research. J.P. conducted the overall experiment and performed the overall analysis with the assistance from J.L. J.P. and J.L. wrote the original draft, and Y.L., M.R., P.S., W.G., B.L., C.F., and M.N. contributed to reviewing and editing. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Wolfgang Wanek, Zhenghu Zhou and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pei, J., Li, J., Luo, Y. et al. Patterns and drivers of soil microbial carbon use efficiency across soil depths in forest ecosystems. Nat Commun 16, 5218 (2025). https://doi.org/10.1038/s41467-025-60594-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-60594-8