Abstract
Fungal diversity influences both plant diversity and ecosystem functioning, but how fungi mediate the relationship between plant diversity and ecosystem multifunctionality is not as well understood. To address this knowledge gap, we manipulate plant species richness and soil fungal diversity (via fungicide addition) in 190 experimental plant communities and measure ten ecosystem functions to assess ecosystem multifunctionality. We find that reduced fungal diversity (via fungicide addition) decreases ecosystem multifunctionality, but only in plant communities with low species richness, indicating that soil fungal diversity can buffer the effects of plant diversity loss on ecosystem multifunctionality. Selection effects (i.e., superiority of dominant plant species) and phylogenetic clustering of the fungal community (i.e., functional redundancy) increase with plant species richness when fungicide is added, revealing potential mechanisms through which species-rich plant communities can mitigate the negative effects of reduced fungal diversity and maintain ecosystem multifunctionality. Our study emphasizes the importance of interactions between plant and fungal diversity for ecosystem multifunctionality, and highlights the need to conserve fungal diversity, especially in ecosystems with low plant diversity that are threatened by global change.
Similar content being viewed by others
Introduction
The relationship between plant diversity and ecosystem functioning has long been a focus of ecological research1,2,3,4. Plant diversity affects many ecosystem functions, such as primary productivity, nutrient cycling, and water conservation. When combined, the multiple functions are considered as ecosystem multifunctionality, which represents the capacity of a natural ecosystem to provide multiple simultaneous ecosystem functions, including their synergies and trade-offs5,6. Soil fungal diversity also contributes to ecosystem multifunctionality, supporting functions such as litter decomposition and carbon cycling7,8. Moreover, soil fungal diversity tends to be positively related to plant diversity and promotes nutrient uptake and carbon assimilation of host plants9, which in turn can mediate the relationship between plant diversity and ecosystem multifunctionality10,11. However, the increasing threat to fungal diversity posed by global change12,13 has raised concern about potential disruptions to the relationship between plant diversity and ecosystem multifunctionality mediated by impacts on fungal diversity14,15. Past studies have explored the direct relationships between fungal diversity and ecosystem multifunctionality16,17, or plant diversity and single ecosystem functions18,19, but how fungal diversity may mediate the plant diversity and ecosystem multifunctionality relationship has received little research attention.
Complementarity and selection effects are crucial mechanisms that are frequently invoked to explain positive relationships between diversity and ecosystem functioning20,21,22. Complementarity effects emerge when high diversity communities demonstrate increased ecosystem functioning via complementarity (breadth and partitioning) of resource use, abiotic facilitation, and positive plant-fungal feedbacks23. Selection effects occur when high diversity increases the likelihood that a species associated with high ecosystem functioning will occur in the community (i.e., positive selection effects)22,24. Fungal diversity may play a role in mediating these complementarity and selection effects. For example, reduced fungal diversity could increase host plant dependency on soil nutrient availability15 and intensify interspecific competition among plants25 due to there being fewer available distinct nutrient foraging strategies, thereby reducing complementarity effects26. However, despite reduced fungal diversity, high abundances of fungal taxa that directly affect plant growth (e.g., arbuscular mycorrhizal fungi) could still allow for strong specialized plant-fungal interactions, promoting complementarity effects and synergistic relationships among ecosystem functions27. Furthermore, reduced fungal diversity can impact plant species performance or composition, such as favoring species that do not strongly depend on fungal mutualists (i.e., non-mycorrhizal plant species), potentially altering selection effects28.
Relationships among individual ecosystem functions reflect synergies and trade-offs among multiple ecosystem functions and can also affect ecosystem multifunctionality29. Positive relationships might lead to simultaneous increases or decreases in functioning for multiple variables, whereas negative relationships might lead to compensatory increases in other functions when any one function declines, thereby maintaining the stability of multifunctionality. For example, communities with multiple positive relationships among ecosystem functions typically have high ecosystem multifunctionality but low resilience to species extinctions30. Both plant and fungal diversity may affect relationships among ecosystem functions. For example, plant diversity has been shown to promote positive relationships among ecosystem functions31, while fungal diversity might also promote positive relationships among ecosystem functions supported by fungal taxa, such as litter decomposition and nutrient cycling32. However, studies to date have primarily focused on these relationships in highly modified ecosystems or on the individual effects of plant or fungal diversity, while those that explore the effects of both plant and fungal diversity on relationships among ecosystem functions in natural ecosystems remain relatively scarce.
Reductions in fungal diversity not only change fungal community composition, but also phylogenetic structure, with potential implications for ecosystem functioning and multifunctionality. For example, strong phylogenetic clustering of fungi could indicate high functional redundancy of the fungal community (assuming phylogenetic conservatism, where closely related fungal taxa support similar ecological functions)33, which may in turn influence host plant growth34 and ecosystem functioning35. Functional redundancy may buffer against the negative effects of disturbance on fungal functioning36, but could also trade-off with a reduced ability of the fungal community to support a diverse set of ecosystem functions (i.e., complementarity)35. Therefore, the role of fungal phylogenetic structure in mediating the relationship between plant diversity and ecosystem multifunctionality also remains unclear.
Here, we show that soil fungi mediate the relationship between plant diversity and ecosystem multifunctionality by buffering against the loss of plant diversity. We use a greenhouse experiment where plant communities are grown along a gradient of species richness (1, 2, 4, 6, and 8 plant species) crossed with fungicide application in half of the communities to reduce fungal diversity prior to planting (Supplementary, Fig. S1). We assess ecosystem multifunctionality using both average value and multiple thresholds methods, based on measurements of primary productivity, floral abundance, crown interception of light, water and wind, water conservation, microbial biomass, litter decomposition, soil enzyme activity, and carbon, nitrogen, and phosphorus stocks. We use plant aboveground biomass data to quantify changes in complementarity and selection effects, mechanisms that underpin diversity-multifunctionality relationships. For each community, we also assess fungal community diversity, phylogenetic structure, and the relative abundance of fungal functional groups as potential drivers of ecosystem multifunctionality. We address the following research questions:
-
1.
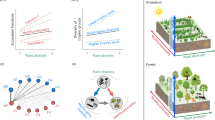
Does fungal diversity affect the relationship between plant diversity and ecosystem multifunctionality? We hypothesize that reduced fungal diversity (via fungicide addition) can decrease ecosystem multifunctionality and weaken the positive relationship between plant species richness and ecosystem multifunctionality (corresponding to scenario 8 in Fig. 1).
Fig. 1: Hypothesized relationships among plant species richness, fungal diversity, and ecosystem multifunctionality. a Green arrows represent the effects of plant species richness on fungal diversity and ecosystem multifunctionality. Brown arrows represent the effects of fungal diversity on plant species richness, ecosystem multifunctionality and the relationship between plant species richness and ecosystem multifunctionality. b The full set of possible linear changes in the relationship between plant species richness and ecosystem multifunctionality with fungicide addition (i.e., reduced fungal diversity), corresponding to the scenarios presented in panel (c). The yellow line represents the hypothesized relationship between plant species richness and ecosystem multifunctionality without any change in fungal diversity (i.e., control communities). The blue, red and brown lines represent how fungicide addition could increase, decrease or have no effect on ecosystem multifunctionality, respectively (represented by straight arrows oriented up and down). Curved arrows represent how changes in fungal diversity could also enhance or inhibit the strength of the relationship between plant species richness and ecosystem multifunctionality. c The range of potential impacts of fungicide addition on ecosystem multifunctionality and the relationship between plant species richness and ecosystem multifunctionality, corresponding to panel (b). Upward arrows represent an increase, downward arrows represent a decrease, and crosses represent no change. Specifically, we hypothesized that reduced fungal diversity (i.e., via the fungicide addition treatment) will decrease ecosystem multifunctionality and weaken the relationship between plant species richness and ecosystem multifunctionality, corresponding to scenario 8.
-
2.
Are the relationships among fungal diversity, plant diversity and ecosystem multifunctionality mediated by changes in complementarity and selection effects? We hypothesize that reduced fungal diversity (via fungicide addition) can decrease complementarity effects and increase selection effects, especially in plant communities with high species richness.
-
3.
Does fungal and plant diversity influence the relationships among ecosystem functions, with consequences for ecosystem multifunctionality? We hypothesize that reduced fungal diversity (via fungicide addition) can weaken correlations among ecosystem functions, decreasing ecosystem multifunctionality.
-
4.
Do changes in the phylogenetic structure of the fungal community mediate the relationship between plant diversity and ecosystem multifunctionality? We hypothesize that fungicide addition may induce phylogenetic clustering of fungi through selection for specific fungal taxa (e.g., those with fungicide resistance), leading to functional redundancy and lower ecosystem multifunctionality.
Results
Fungicide reduced soil fungal diversity but not fungal abundance
The fungicide addition treatment significantly reduced soil fungal diversity by 21% compared to the control treatment (df = 1, F = 6.90, P = 0.030) but did not affect fungal abundance (based on the ITS rRNA copy number estimated from qPCR) (df = 1, F = 0.11, P = 0.775, Supplementary Fig. S2), allowing us to better assess how reduced fungal diversity mediates the relationship between plant species richness and ecosystem multifunctionality. The fungicide addition treatment reduced plant diversity at harvest relative to the control treatment, with this effect stronger in communities with higher sown plant species richness (plant richness × fungicide addition treatment: df = 1, β = -0.15, P < 0.001, Supplementary Fig. S3). The fungicide addition treatment also affected plant community composition at harvest, with impacts again strongest in communities with the highest sown plant species richness (Supplementary Fig. S4). Specifically, in communities with eight plant species, fungicide addition reduced the relative abundance of Fabaceae by 99% and Compositae by 78% compared to the control treatment but increased the relative abundance of Gramineae and Chenopodiaceae by 164% and 69%, respectively (all P < 0.023, Supplementary Fig. S4).
Fungicide influenced the positive relationship between plant species richness and ecosystem multifunctionality
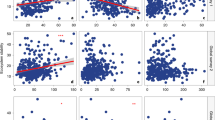
The positive relationship between plant species richness and ecosystem multifunctionality was more pronounced in communities where fungal diversity had been reduced through fungicide addition (plant species richness × fungicide addition treatment: df = 1, β = 0.009, P = 0.02, Fig. 2). Specifically, ecosystem multifunctionality was positively related to plant species richness in communities that received fungicide addition (R2 = 0.13, F = 15.93, P < 0.001), whereas no relationship was present in control communities (R2 < 0.01, F = 1.01, P = 0.317, Fig. 2). Moreover, in communities with only one or two plant species, fungicide addition reduced ecosystem multifunctionality by 10% (df = 42, t = -2.79, P = 0.029) and 11% (df = 22, t = -2.70, P = 0.029, Fig. 2), respectively, compared to control communities. However, no differences were observed in communities with higher plant species richness (all P > 0.26). Considering the multiple thresholds method, Diversity-Maximized Multifunctionality (Mmde, the number of functions achieving Tmde at the highest level of diversity) was higher in the fungicide addition treatment than in the control treatment, indicating stronger diversity-multifunctionality relationships (Supplementary Fig. S5). This result was consistent with the average value method (Fig. 2) and supported that the positive relationship between plant species richness and ecosystem multifunctionality was stronger in communities that received fungicide addition compared to control communities. Similar relationships were observed for some individual ecosystem functions, such as the positive relationships of phosphorus stocks and crown interception with plant species richness in communities that received fungicide addition (R2 = 0.16, F = 5.56, P = 0.032 and R2 = 0.33, F = 12.88, P = 0.001, respectively; full results for all individual ecosystem functions are presented in Supplementary Fig. S6 and Supplementary Table S3).
Orange triangles and blue circles represent control and fungicide addition treatment plant communities, respectively. A series of two-sided t-tests with False Discovery Rate correction were used to test whether ecosystem multifunctionality differed between fungicide addition and control treatment plant communities at each level of plant species richness (n = 32, 16, 24, 16, and 4 in control plant communities with 1, 2, 4, 6, and 8 species, respectively; and n = 32, 16, 24, 16, and 10 in fungicide addition plant communities with 1, 2, 4, 6, and 8 species, respectively). *0.01 < P < 0.05, none = P > 0.05. Solid and dashed lines represent significant (P < 0.05) and non-significant relationships based on linear mixed models (n = 92 in control plant communities and n = 98 in fungicide addition plant communities), respectively. Source data are provided as a Source Data file.
Fungicide promoted the diversity-ecosystem multifunctionality relationship through increased selection effects
The relationship between plant species richness and selection effects depended on the fungicide addition treatment (plant richness × fungicide addition treatment: df = 1, β = -19.31, P < 0.001, Fig. 3b). Specifically, selection effects were positively related to plant species richness in communities that received fungicide addition (R2 = 0.30, F = 50.17, P < 0.001, Fig. 4b), whereas no such relationship was observed in control communities (R2 < 0.01, F = 0.46, P = 0.617). The strength of the relationship between ecosystem multifunctionality and selection effects also depended on the fungicide addition treatment (df = 1, β < 0.001, P = 0.002, Fig. 3d). Ecosystem multifunctionality was positively related to selection effects in communities that received fungicide addition (R2 = 0.43, F = 50.17, P < 0.001, Fig. 3f) but not in control communities (R2 < 0.01, F = 0.46, P = 0.500). We also found that complementarity effects were 177% higher in control communities than those that received fungicide addition (df = 1, F = 8.30, P = 0.005, Fig. 3a), and that they were not related to plant species richness, regardless of the fungicide addition treatment (both R2 < 0.01, P > 0.81). The strength of the relationship between complementarity effects and ecosystem multifunctionality also depended on the fungicide addition treatment (df = 1, β < 0.001, P < 0.001, Fig. 3c), although ecosystem multifunctionality was positively related to complementarity effects in both fungicide addition (R2 = 0.24, F = 20.92, P < 0.001) and control treatment communities (R2 = 0.06, F = 1.26, P = 0.041).
a, b Relationships between plant species richness and mean ( ± 1 SE) complementarity and selection effects of plant communities. The inset box in panel (a) shows the mean ( ± 1 SE) complementarity effect of control and fungicide addition treatment plant communities across all levels of plant species richness by a One-way ANOVA (n = 92 in control communities and n = 98 in fungicide addition communities). *P = 0.005. c, d Relationships between complementarity and selection effects with mean ( ± 1 SE) ecosystem multifunctionality (average value method) of plant communities. Orange triangles and blue circles represent control and fungicide addition treatment plant communities, respectively. Solid and dashed lines represent significant (P < 0.05) and non-significant relationships based on linear mixed models (n = 92 in control plant communities and n = 98 in fungicide addition plant communities), respectively. Source data are provided as a Source Data file.
a, b Pairwise correlations between ecosystem functions in plant communities without (a) and with (b) fungicide addition. Significant pairwise Pearson’s correlation coefficients are shown, with the color gradient (darker red = stronger positive correlation, darker blue = stronger negative correlation) and square size (larger = stronger correlation) representing the correlation coefficient direction and magnitude (n = 92 in control communities and n = 98 in fungicide addition communities). *0.01 < P < 0.05; **0.001 < P < 0.01; ***P < 0.001. c Principal component analysis (PCA) of ecosystem functions (n = 190). Different symbol types represent the plant species richness of communities and symbol color represents the control (orange) and fungicide addition (blue) treatment plant communities. Points closer together in the plot represent communities with more similar ecosystem function composition and points and error bars represent the mean ± 1 SE for each treatment, respectively. The arrow length represents the relative importance of each ecosystem function to the principal components. d, e Relationships between PCA axis values and complementarity effect or selection effect. f, g Relationships between PC1 axis values and plant species richness or ecosystem multifunctionality (average value method). Orange triangles represent control treatment plant communities and blue circles represent fungicide addition treatment plant communities. Solid and dashed lines represent significant (P < 0.05) and non-significant relationships based on linear mixed models (n = 92 in control plant communities and n = 98 in fungicide addition plant communities), respectively. Source data are provided as a Source Data file.
Fungicide altered associations among ecosystem functions
When fungal diversity was high (i.e., in control communities), we observed fewer significant pairwise correlations among ecosystem functions than in fungicide addition treatment communities, indicating that soil fungi may mediate interdependence of ecosystem functions (Fig. 4a, b). Similarly, the influence of plant species richness on ecosystem functioning composition (based on a principal component analysis) depended on the fungicide treatment (plant species richness × fungicide addition treatment: df = 1, β = 1.89, P = 0.017, Fig. 4c). PC1 (interpreted as synergy among ecosystem functions) was positively related to plant species richness in fungicide addition communities (R2 = 0.14, F = 15.93, P < 0.001, Fig. 4f) and no relationship was observed in control communities (R2 < 0.01, F = 1.01, P = 0.317). Ecosystem multifunctionality had a strong positive relationship with PC1 in both fungicide addition (R2 = 0.61, F = 152.40, P < 0.001, Fig. 4g) and control communities (R2 = 0.25, F = 30.77, P < 0.001).
Fungicide maintained positive effects of plant species richness on soil fungal diversity and phylogenetic clustering
Both Shannon diversity and the nearest-taxon-index (NTI; high NTI represents strong phylogenetic clustering) of soil fungal communities at harvest were positively related to plant species richness, but only in communities that received fungicide addition (R2 = 0.03, F = 4.27, P = 0.041, Supplementary Fig. S7b, and R2 = 0.05, F = 5.71, P = 0.019, Fig. 5a, respectively). This result can be visualized by observing the phylogenetic clustering of treatment effects on fungal operational taxonomic units (OTUs; rings 3–5 in Supplementary Fig. S8a). Moreover, ecosystem multifunctionality was positively related to both Shannon diversity (R2 = 0.04, F = 4.79, P = 0.031, Supplementary Fig. S7b) and NTI of soil fungi (where high NTI represents strong phylogenetic clustering, R2 = 0.05, F = 7.71, P = 0.019, Fig. 5b).
a Relationship between the mean ( ± 1 SE) nearest-taxon-index (NTI; a measure where high values indicate strong phylogenetic clustering of the fungal community) of the soil fungal community and the species richness of plant communities, with and without fungicide addition. (b) Relationship between ecosystem multifunctionality (average value method) and NTI, with and without fungicide addition. Orange triangles and blue circles represent control and fungicide addition treatment plant communities, respectively. Solid and dashed lines represent significant (P < 0.05) and non-significant relationships based on linear mixed models (n = 92 in control plant communities and n = 98 in fungicide addition plant communities), respectively. Source data are provided as a Source Data file.
Plant species richness and the fungicide addition treatment also influenced the relative abundance of fungal functional groups, orders and OTUs. In control treatment communities, the relative abundance of arbuscular mycorrhizal (AM) fungi was negatively related to plant species richness but no such relationship was observed in the fungicide addition treatment (plant species richness × fungicide addition treatment: β = 0.001, P = 0.032, Fig. 6a). This was likely because fungicide addition reduced the relative abundance of AM fungi by 92–97% across all levels of plant species richness (all P < 0.05, Fig. 6b). We also found that the relative abundance of saprophytic fungi was 89% lower in communities that received fungicide addition than in control communities (df = 91, t = 2.34, P = 0.022, Fig. 5d). The random forest analysis identified several fungal orders that may influence ecosystem multifunctionality (Supplementary Fig. S9) and which were affected by the fungicide addition and plant species richness treatments (Supplementary Fig. S10). Specifically, fungicide addition reduced the relative abundance of Zoopagales and Eurotiales, but plant species richness had positive relationships with relative abundance Microascales. Fungicide addition alleviated the negative effects of plant species richness on the relative abundance of Magnaporthales and Filobasidiales (all P < 0.047, Supplementary Fig. S10), which might involve in the degradation of organic matter.
The mean (±1 SE) relative abundance of (a) arbuscular mycorrhizal, (b) plant pathogenic and (c) wood saprophytic fungi in communities along a gradient of plant species richness, with and without fungicide addition. Inset text presents estimated effect sizes and statistical significance of the individual and interactive effects of fungicide and plant species richness on the relative abundance of fungal functional groups, based on linear mixed models (n = 190). Orange triangles and blue circles represent control and fungicide addition treatment plant communities, respectively. A series of two-side t-tests with False Discovery Rate correction were used to test whether relative abundance of fungal functional groups differed between fungicide and control treatments at each level of plant species richness (n = 32, 16, 24, 16, and 4 in control plant communities with 1, 2, 4, 6, and 8 species, respectively; and n = 32, 16, 24, 16, and 10 in fungicide addition plant communities with 1, 2, 4, 6, and 8 species, respectively). ***P < 0.001; **0.05 < P < 0.001; *P < 0.05; none P > 0.05. F fungicide treatment; P plant species richness; F × P interactions between fungicide treatment and plant species richness. Source data are provided as a Source Data file.
Discussion
In our experiment, fungicide addition (i.e., reduced fungal diversity) reduced ecosystem multifunctionality by 6%, but strengthened the positive relationship between plant species richness and ecosystem multifunctionality (Fig. 2a). This finding indicates that soil fungi support the maintenance of ecosystem multifunctionality in low diversity plant communities and the relationship between plant diversity and ecosystem multifunctionality. Fungicide addition also enhanced selection effects, especially in plant communities with high species richness (Fig. 3), which was potentially driven by dominance of non-mycorrhizal Suaeda plant species (i.e., Chenopodiaceae, average relative abundance of 34% across all treatments). Selection effects were positively related to ecosystem multifunctionality in the fungicide addition treatment (Fig. 3), indicating that high plant diversity can buffer against the loss of fungal diversity through the disproportionate contribution of high-functioning plant species. Fungicide addition promoted correlations among ecosystem functions, which had positive impacts on ecosystem multifunctionality (Fig. 4). Furthermore, fungicide addition (i.e., reduced fungal diversity) promoted a positive relationship between plant species richness and phylogenetic clustering, potentially contributing to higher ecosystem multifunctionality through more specialized plant-fungal interactions.
In contrast to our first hypothesis (scenario 8 in Fig. 1), we found that fungicide addition (i.e., reduced fungal diversity) strengthened the positive relationship between plant diversity and ecosystem multifunctionality, and this effect might be due to the strong negative effects of fungicide on ecosystem multifunctionality that were only observed in communities with low plant species richness (i.e., one or two plant species) (Fig. 2). This result corresponds to scenario 6 in Fig. 1. We posit that reduced fungal diversity directly affects ecosystem multifunctionality, such as through altered nitrogen stocks or litter decomposition rate, and that low diversity plant communities are less able to counteract this effect through the selection effects that occur in high diversity plant communites7. Taken together, we found that although plant communities with high sown species richness had similar ecosystem multifunctionality regardless of whether they were treated with fungicide vs. the control, they achieved this via different mechanisms (i.e., selection effects vs. fungal diversity, respectively). These key results demonstrate how both plant and fungal diversity contribute to ecosystem multifunctionality, highlighting the importance of maintaining biodiversity across a range of taxa and trophic levels37, especially in low diversity plant communities such as coastal wetlands with high soil salinity38 and agricultural fields under long-term cultivation19.
Importantly, we found that fungicide application reduced fungal diversity but not abundance, meaning that our findings can be attributed to changes in soil fungal diversity and not abundance. Although the effect of fungicide on fungal diversity was modest (i.e., a 21% reduction in fungal diversity; no reduction in fungal evenness, Supplementary Fig. S11), this still led to substantial impacts on ecosystem multifunctionality, further emphasizing the importance of fungal diversity. The unchanged fungal abundance could be explained by the 1-month period between carbendazim treatment and soil sample collection (which coincided with seed sowing), during which time fungal abundance could have recovered to pre-treatment levels without a concomitant recovery of fungal diversity. For example, the fungal necromass resulting from the fungicide treatment may have provided novel resources for saprophytic fungi such as Mortierella39, which increased in relative abundance with the fungicide addition treatment. Overall, we believe that the effect of fungicide on fungal diversity but not abundance further strengthens our conclusions regarding the roles of plant and fungal diversity in the maintenance of ecosystem multifunctionality.
In partial support of our second hypothesis, we found that fungicide addition (i.e., reduced fungal diversity) amplified the positive effects of plant species richness on selection effects (Fig. 4c), which were strong enough to overcome the reduced complementarity effects associated with reduced fungal diversity (Fig. 4b). One possible explanation for this result could be that fungicide addition reduced the relative abundance of arbuscular mycorrhizal fungi, favoring non-arbuscular mycorrhizal plant species, which were more likely to be present in plant communities with high species richness. This explanation is consistent with our results showing that the relative abundance of Chenopodiaceae (Suaeda glauca and S. salsa) increased with reduced fungal diversity, and that this effect was stronger in communities with high plant species richness (Supplementary Fig. S4). Suaeda spp. were the dominant species in our experimental plant communities, and the drivers of selection effects40. Suaeda spp. are non-mycorrhizal plants41, and this may help explain their increased dominance and the strong selection effects observed with fungicide addition, which also reduced the relative abundance of AM fungi (Supplementary Fig. S12). Our findings further reflect the dominance of Suaeda spp. in coastal wetlands of the Yellow River Delta42, where they support high primary productivity and nutrient cycling rates, among other ecosystem functions43. Our findings are consistent with other studies showing that dominant species play an important role in maintaining multiple ecosystem functions, and that these dominant species might contribute disproportionately to ecosystem multifunctionality through selection effects44,45. Therefore, reduced fungal diversity may promote dominant species and selection effects, especially at higher levels of sown plant species richness, offering a potential mechanism explaining why the positive relationship between plant species richness and ecosystem multifunctionality was more pronounced with reduced fungal diversity.
In contrast to our third hypothesis, the fungicide addition treatment promoted synergy among ecosystem functions (i.e., stronger positive and negative relationships), which resulted in an overall positive relationship with ecosystem multifunctionality (Fig. 4a, b, c, g). Furthermore, the PCA divided individual ecosystem functions into two groups, one related to plant traits such as productivity and crown interception, and the other to fungal-mediated functions such as litter decomposition and microbial biomass. These two groups exhibited a trade-off relationship, whereby the decline in one could be compensated by the other. This was consistent with the result that high plant diversity can offset reductions in ecosystem multifunctionality resulting from decreased fungal diversity (Fig. 2).
In partial support of our fourth hypothesis, we found that reduced fungal diversity slightly strengthened the positive relationship between plant species richness and phylogenetic clustering of the fungal community (i.e., NTI), but that this was in turn positively related to ecosystem multifunctionality (Fig. 5). Although fungicide decreased fungal richness and diversity (Supplementary Fig. S7), plant species richness was positively related to phylogenetic clustering of the fungal community in the fungicide addition treatment, suggesting that plant species richness can act as a biotic filter on the fungal niche46. Recent studies found higher phylogenetic clustering might indicate fungal functional redundancy and therefore resilience to disturbance, such as warming, drought and nitrogen deposition40,47. Furthermore, increased plant species richness alleviated the negative impacts of fungicide on the relative abundance of some fungal taxa, such as Magnaporthales and Filobasidiales, which were identified as fungal orders that contributed strongly to ecosystem multifunctionality (Supplementary Fig. S10). Magnaporthales and Filobasidiales mainly include saprophytic fungi, which can promote litter decomposition and carbon cycling48. Both orders also include pathogenic fungi of gramineous plants49,50, potentially promoting selection effects associated with the dominant plant species by reducing the relative abundance of their competitors51.
Although many experiments have used fungicide to explore interactions between plant and fungal diversity52, the potential for direct influence of fungicide on ecosystem functioning should be considered. For example, fungicide may represent an additional carbon input that could directly affect ecosystem functioning. However, we found that carbon stocks were not affected by fungicide addition (Supplementary Fig. S6h), indicating that any impacts of fungicide were likely due to changes in fungal communities rather than altered carbon input. We also note that other biotic interactions, such as plant-bacterial and fungal-bacterial interactions, are likely to influence the relationship between plant diversity and ecosystem multifunctionality, and should be incorporate into future studies. Finally, the mean temperature (30 °C) in our greenhouse experiment was higher than generally encountered in the field, which could affect fungal diversity and composition14. However, all treatments were conducted in a consistent environment, meaning that temperature was not confounded with treatment effects. Despite this, the artificially controlled conditions of a greenhouse might limit our ability to extrapolate our results to the field. Different fungicides could also affect the outcome of the experiment through selective effects on fungal taxa53 (e.g., stronger impacts on arbuscular mycorrhizal and saprophytic fungi than plant pathogens, Fig. 6a-c). Thus, future research may aim to investigate how our findings generalize along abiotic gradients and other biotic interactions in controlled and field conditions.
We showed that the positive relationship between plant species richness and ecosystem multifunctionality was more pronounced in communities where fungal diversity had been reduced. Our findings demonstrate that soil fungi influence the relationship between plant diversity and ecosystem multifunctionality and buffer the effects of plant diversity loss on ecosystem multifunctionality. Selection effects (i.e., superiority of dominant species) increased with plant species richness when fungicide was added, indicating that species-rich plant communities can mitigate the negative effects of reduced fungal diversity to maintain ecosystem multifunctionality. Moreover, reduced fungal diversity promoted a positive relationship between plant species richness and phylogenetic clustering of fungal communities, which showed the importance of fungal functional redundancy in the maintenance of ecosystem multifunctionality and plant-fungal interactions. Ongoing global change poses a significant threat to both plant and fungal diversity, affecting ecosystem functions across a range of ecosystems54,55. Our results demonstrate that both plant and fungal diversity are key to the maintenance of ecosystem multifunctionality, and that ecosystem multifunctionality is especially sensitive to changes in fungal diversity in low diversity plant communities that lack the ability to compensate through strong section effects. A diverse fungal community can help to buffer the negative impacts of plant diversity loss on ecosystem multifunctionality, while plant diversity can in turn mitigate the negative impacts of fungal diversity loss by promoting phylogenetic clustering and functional redundancy of the fungal community. Our study highlights the urgent need for conservation efforts to safeguard both plant and fungal diversity, which are crucial to the maintenance of multifunctionality in the face of global environmental change, especially in fast processing ecosystems with low plant diversity.
Methods
Location and study species
The experiment was conducted in a greenhouse at Baimiao Experimental Station, Shandong University, China (36°23′10″ N, 120°36′44″ E). We chose two plant species from each of four families of plants that commonly co-occur with high species richness, abundance, and broad distributions in wetlands of the Yellow River Delta43. The four families were Gramineae (Eleusine indica (L.) Gaertn and Setaria viridis (L.) Beauv.); Chenopodiaceae (Suaeda glauca Bunge and Suaeda salsa (Linnaeus) Pallas); Asteraceae (Crepis bonii Gagnepain and Xanthium strumarium L.); and Fabaceae (Glycine soja Sieb. et Zucc. and Melilotus officinalis (L.) Pall.). These species were selected to represent different functional groups56 (i.e., grasses, non-mycorrhizal, herbs, and legumes, respectively) and we used two plant species within each family to reduce the potential for confounding effects of differences in phylogenetic distance with differences in species richness. For example, plant communities with two or more species always contained plant pairs from within the same family (Supplementary Fig. S1) so that any changes in ecosystem multifunctionality could be more closely associated with increased plant species richness rather than phylogenetic diversity. The seeds of each plant species were collected from the Yellow River Delta Field Experiment Station (37°51′9″ N, 118°48′53″ E) in October 2020 and stored in a -4 °C seed storage cabinet until the experiment began.
Experimental design
Plant communities were grown in planting boxes (52 cm long × 35 cm wide × 30 cm high) that contained a soil mixture comprising 18.5 kg of field soil from Shandong University Garden and 13.5 kg of peat growing medium (Pindstrup, Denmark). Coarse litter and stones were removed from the field soil with a 5 mm sieve. Before planting, seeds were surface sterilized with 3% hydrogen peroxide solution for 5 min (to reduce the influence of non-soil microbes) and then soaked in sterilized water for 20 min. Seeds were planted at a depth of 2.5 mm in March 2021. Based on germination rates that were measured before starting the experiment (following seed germination experiment), each plant community was sown at a rate calculated to produce ~1000 total sprouting seedlings, evenly distributed across the species in the community. The planted seed mass and estimated abundance of each plant species per plant community are presented in Supplementary Data 1.
About the seed germination experiment, based on the weight and size of their seeds, the eight plant species were divided into three seed types: small (Eleusine indica, Crepis bonii, Suaeda salsa, Setaria viridis), medium (Suaeda glauca, Melilotus officinalis, Glycine soja) and large (Xanthium strumarium). We sowed 1 g of seeds of each small seed type species, 5 g of seeds of each medium seed type species, and 25 g of seeds of large seed type species in individual planting boxes (52 cm long × 35 cm wide × 30 cm high) that contained a soil mixture comprising 18.5 kg of field soil from Shandong University Garden and 13.5 kg of peat growing medium (Pindstrup, Denmark). Coarse litter and stones were first removed from the field soil with a 5 mm sieve. Planting boxes were placed in the greenhouse and watered every 2 days. After 2 weeks, seedlings in each planting box were counted and the number of germinated seedlings per 1 g of seed was calculated for each species. This value was used to determine the mass of seeds sown and the estimated number of germinated seedlings expected in each plant community for the main experiment (Supplementary data 1).
The experimental communities were planted with five levels of plant species richness (1, 2, 4, 6, 8), such that there were eight different communities with plant species richness of one (i.e., one community per plant species), four different communities with plant species richness of two and six species, six different communities with plant species richness of four, and one community containing all eight plant species (Supplementary data 1and Supplementary Fig. S1). Each of these 23 different plant communities was replicated eight times and we randomly selected the different plant functional groups to be sown in communities with four or more plant species (but always keeping plant species from each functional group together as described above).
To investigate the impact of reduced soil fungal diversity on ecosystem multifunctionality, we mixed the broad-spectrum fungicide carbendazim with the soil of half the planting boxes 1 month before planting (i.e., four of the eight replicates of each plant community), at a 0.4% mass ratio. As a broad-spectrum fungicide, carbendazim strongly inhibits many fungal taxa by inhibiting cell division through the prevention of mitotic spindle formation during karyokinesis57. Before sowing seeds, we used high-throughput sequencing of soil samples to confirm that the carbendazim treatment had reduced soil fungal diversity but not abundance (Supplementary Fig. S2).
Plant communities were watered every day and soil moisture content maintained at ~75% saturated field capacity using a soil moisture sensor (Item-6466, Spectrum, Aurora, CO, United States). Any seedlings of plants that did not belong to the sown community were removed by hand. The daily mean temperature was 30 °C (10% more than field environment) and air humidity maintained at ~85% by ventilating fan throughout the experimental period. In previous research, we had observed that heterogeneous fungicide concentration (due to varying soil aggregate sizes) could inflict localized plant mortality, and that high diversity plant communities were especially at risk of local extinction and therefore lower plant species richness, because the lower relative abundance of each plant species increased the risk of random mortality. To mitigate this potential for random species loss in the eight species plant community, we planted six extra replicates of the fungicide addition treatment, thoroughly mixed the soil and fungicide, and waited 1 month before sowing seed. However, we observed no local extinction of plant species due to fungicide in the experiment and retained these six replicates in the statistical analysis to include all the available data. In total there were 190 plant communities (23 plant communities nested within 5 levels of species richness × 2 fungicide addition treatments × 4 replicates + 6 extra plant communities) (Supplementary Fig. S1).
Measurement of ecosystem multifunctionality
To assess ecosystem multifunctionality, we measured ten ecosystem functions: primary productivity, floral abundance, crown interception of light, water and wind, water conservation, microbial biomass, litter decomposition, soil enzyme activity, carbon stocks, nitrogen stocks, and phosphorus stocks. These variables reflect multiple types of ecosystem functions, including nutrient cycling, climate regulation, and soil properties and fertility, and are related to the concepts of “supporting” and “regulating” ecosystem services58. Detailed protocols for the measurement of each ecosystem function are described in the next section.
To minimize the influence of non-random selection of ecosystem functions, we used both the average value (Eq. 1) and multiple thresholds methods (Eq. 2) to quantify ecosystem multifunctionality via the multifunc package59. First, the average value method is simply the average value of standardized ecosystem functioning59 (Supplementary Table S1):
\({fi}\) is the measurement of a single ecosystem function i; \(r\) is a mathematical function that transforms \({fi}\) to be positive; \(g\) is the mathematical function to transform ecosystem function i to [0,1] by the min-max normalization method59; and \(F\) is the total number of ecosystem functions that were measured. High values of our ecosystem multifunctionality measure could be considered to represent productive and ‘fast’ systems, although we acknowledge that this may not always be a desirable outcome for many ecosystems6. However, our experimental system was based on the Yellow River Delta coastal wetlands, where high values of ‘fast’ ecosystem multifunctionality reflect the natural state of high productivity and rapid biomass and nutrients cycling rates60,61.
While the average value method offers a straightforward and interpretable metric for assessing ecosystem multifunctionality, it fails to account for potential trade-offs among these functions and assumes interchangeability of functionalities. To overcome these limitations, we also quantified multifunctionality using the multiple thresholds method. The multiple thresholds method captures the number of functions that simultaneously exceed different thresholds of the maximum observed value of each function and evaluates whether more (or fewer) functions are performing simultaneously at high (or low) levels, providing a more nuanced and comprehensive understanding of ecosystem multifunctionality59.
In other words, ecosystem multifunctionality using the multiple thresholds method was the number of ecosystem functions with standardized values greater than a given threshold (\({t}_{i}\)). This approach involved testing the effect of plant species richness on ecosystem multifunctionality across the full range of ecosystem multifunctionality thresholds between 1% and 99% (Supplementary Fig. S5), providing the following indices: Tmin (the lowest threshold where diversity-multifunctionality relationships become significant), Tmax (the highest threshold where diversity-multifunctionality relationships become significant) and Tmde (the threshold where the diversity-multifunctionality relationship is strongest). At the same time, Mmin, Mmax, and Mmde indicate the number of functions (i.e., multiple threshold multifunctionality) at the respective thresholds of Tmin, Tmax and Tmde, respectively. Thus, it can be inferred that biodiversity exhibits a strong positive association with multifunctionality if Tmin is low and the other five indices are high, and vice versa59. We found similar relationships between plant diversity and ecosystem multifunctionality using both the average value and multiple thresholds methods, and hence we present results from only the average value method in the main text to aid interpretability62.
Measurement of single ecosystem function
We measured ten single ecosystem functions, including primary productivity, floral abundance, crown interception, water conservation, microbial biomass, litter decomposition, soil enzyme activity, carbon stocks, nitrogen stocks and phosphorus stocks.
-
(1)
Primary productivity. Aboveground biomass of communities was harvested in November 2021, after 6 months of growth, and considered as primary productivity. All plants were cut along the soil surface and sorted according to species. Plant tissues were dried at 80 °C for 48 h before being weighed.
-
(2)
Floral abundance. Floral abundance is important to visiting insects, plant reproduction, maintenance of biodiversity, and intrinsic esthetic value63,64,65. The total number of plants that had flowered was counted at harvest to represent the floral abundance of each community66.
-
(3)
Crown interception. Crown interception represents the capability of a plant community to influence the microenvironment and allocation of resources67. The capability of the plant community to intercept wind, rain and light was used to assess the response of crown interception to reduced fungal diversity. To measure wind interception, we used a 30 cm diameter fan to blow air across the plant community at 4.2 m s-1, from 25 cm away and 50 cm above the ground. We then measured wind speed with an anemometer at 50 cm above the ground on the other side of the plant community. The proportional reduction in wind speed from 4.2 m s-1 was used as a measure of crown wind interception. To measure crown rain interception, we used a water sprayer to spray water evenly onto each plant community for 5 min from a height of 2 m above the ground. A 100 mL beaker was placed in the middle of the plant community to collect water during the spray period. The proportional reduction in the volume of water in the beaker compared with a control (i.e., the same spray treatment but with no plants; n = 6) was used as a measure of crown rain interception. Finally, to measure light interception, we measured the light level at 25 cm above the soil surface in the middle of the plant community using an illuminometer (TES-1339R, TES Electrical Electronic Corp.). The proportional reduction in light compared with the no plants control was used as a measure of crown light interception.
-
(4)
Water conservation. To assess water conservation, we measured changes in soil moisture content. Before measuring water conservation, we ceased watering the plant communities for 3 days and then provided sufficient water until soil moisture content was at 50% of saturated field capability (measured using a soil moisture meter, Item-6466, Spectrum, Aurora, CO, United States). Each plant community was then provided with 1 L of water and soil moisture content was measured again after 30 min. The proportional increase in soil moisture content compared with the no plants control was used as a measure of water conservation.
-
(5)
Microbial biomass. Microbial biomass was used to represent the overall response of soil microbes to our experimental treatments68. To measure microbial biomass and other soil characteristics described below (i.e., carbon cycling, nitrogen cycling, phosphorus cycling and soil enzyme activity), we collected 400 g of fresh soil from each community using the five-point sampling method (five 80 g samples collected from points in a quincunx [i.e., at the community center and halfway towards each of the four corners] and then pooled together for analysis). Of this soil, 200 g was stored in a -80 °C refrigerator and the other 200 g was placed on plastic sheets in a cool, ventilated room to air dry. Microbial biomass was quantified using 10 g of fresh soil following the arginine ammonification method69.
-
(6)
Litter decomposition. Leaf and wood decomposition rates were used to assess litter decomposition. We used standardized litters that were not closely related to the experimental plant species to assess decomposition of both foliar and woody plant material, providing a more comprehensive assessment of ecosystem functioning70. To measure these ecosystem functions, we buried nylon mesh bags (15 cm × 10 cm, mesh aperture of 1 mm) containing 5 g of green tea leaves (Camellia sinensis (Linnaeus) Kuntze) or 10 g of bamboo chips (Phyllostachys edulis (Carriere) J. Houzeau) into the soil at a depth of 5 cm before seeds were sown. The green tea leaves and bamboo chips were dried at 80 °C for 48 h and weighed before burial. At harvest, soil was washed off the litter surface with water, and the litter was again dried at 80 °C for 48 h and weighed. The proportional reduction in dried litter mass was used to represent the litter decomposition rate of the plant community.
-
(7)
Soil enzyme activity. N-acetyl-β-D-glucosaminidase (NAG), β-glucosidase, and phosphomonoesterase are three soil enzymes involved in nitrogen, carbon, and phosphorus cycling, and therefore are important for ecosystem functioning71. To assess N-acetyl-β-D-glucosaminidase activity, we measured the ability of 1 g of fresh soil to hydrolyze N-acetyl-β-D-glucosamine, following Parham and Deng72. To assess β-glucosidase activity, we measured the ability of 1 g of fresh soil to hydrolyze para-nitrophenyl-β-D-glucopyranoside, following Turner, Hopkins73. Finally, to assess phosphomonoesterase activity, we measured the ability of 1 g of fresh soil to hydrolyze β-nitrophenyl phosphate, following Lazcano, Gomez-Brandon74.
-
(8)
Carbon stocks. Soil organic carbon content was used to represent carbon stocks. We used 5 g of air-dried soil to measure soil organic carbon content with the potassium dichromate oxidation spectrophotometric method75.
-
(9)
Nitrogen stocks. Soil total nitrogen, nitrate, and ammonium content were used to represent nitrogen stocks. We used 1 g of air-dried soil to measure total nitrogen content with the Kjeldahl method76. To measure nitrate and ammonium concentrations, we first added 5 g of fresh soil to 2 mol L-1 KCl solution following a 1:5 v/v ratio, and the solution was centrifugated at 8000 g. The supernatant was used to determine nitrate and ammonium concentration using hydrazine sulfate reduction77 and Nessler’s reagent colorimetric method78, respectively.
-
(10)
Phosphorus stocks. Soil total phosphorus content was used to represent phosphorus stocks. We used 1 g of air-dried soil to measure total phosphorous content with the molybdenum antimony colorimetric method79.
Measurement of complementarity and selection effects
Complementarity effects occur when, due to resource partitioning and positive interactions, the net yield (i.e., biomass) in multispecies plant communities is on average higher than predicted based on the weighted average of the component species in monoculture communities. Selection effects occur when species with higher-than-average yields in monoculture dominate the biomass of multispecies plant communities and is measured by the covariance between the yield of species in monoculture communities and their change in relative yield in the mixture communities. We followed the method described by Loreau and Hector21
For a given plant community, \({Y}_{0}\) was the total observed yield; \({Y}_{E}\) was the total expected yield based on monoculture communities; \(\varDelta {RY}\) was the deviation from the expected relative yield; \(M\) was the yield of corresponding plant species in monoculture communities; and \(N\) was the number of plant species.
Characterization of the soil fungal community
To characterize the soil fungal community, 25 g of soil from 5–10 cm below the soil surface was collected at harvest using a spade (sterilized in 75% ethanol) and following the five-point sampling method (five 5 g samples collected from points in a quincunx [i.e., at the community center and halfway towards each of the four corners] and then pooled for analysis). To assess the effect of fungicide addition on soil fungal diversity prior to planting, a 5 g soil sample was obtained by combining soil samples from the replicate planting boxes for each of the ten experimental treatment combinations (i.e., control or fungicide addition crossed with each level of plant species richness). Soil samples were stored at -80 °C until DNA extraction for ITS high-throughput sequencing to characterize the soil fungal community diversity and composition. Total soil DNA was extracted using MagPure Soil DNA LQ Extraction Kits (Magen, China). NanoDrop and agarose gel were used to confirm the presence of DNA and measure its concentration. Genome template DNA, barcoded primers, and Tks Gflex DNA Polymerase (Takara) were used for PCR amplification. The ITS I variable region was amplified with the universal primers ITS1F and ITS2 (Supplementary Table S4). Gel electrophoresis was used to visualize amplicon quality. The PCR products were purified with Agencourt AMPure XP beads (Beckman Coulter Co., USA) and quantified using the Qubit dsDNA assay kit. The DNA concentrations were then adjusted for sequencing, which was performed with two paired end read cycles of 250 base pairs each on an Illumina NovaSeq6000 (Illumina Inc., San Diego, CA) at the Oebiotechnology Company (Shanghai, China). Paired end reads were preprocessed using Trimmomatic software 1 to detect and trim ambiguous bases (N). After trimming, paired end reads were assembled using FLASH software80. Reads with ambiguous, homologous sequences or below 200 bp were removed from analyses. Reads with 75% of bases above Q20 were retained using QIIME software (version 1.8.0)81. Clean reads were subjected to primer sequences removal and clustering to generate operational taxonomic units (OTUs) using VSEARCH software with 97% similarity cutoff82. The representative read of each OTU was selected using QIIME. All reads were annotated and blasted against the UNITE database (confidence threshold of 70%)83.
To assess the effects of fungicide on fungal abundance prior to planting, we used qPCR (quantitative polymerase chain reaction) to estimate soil fungal abundance following Rousk, Bååth84 and using the same soil samples and primers from the original extraction and amplification. Fungal gene copy numbers were generated using a regression equation for each assay that related the cycle threshold (Ct) value to the known number of copies in the standards.
The ITS high-throughput sequencing data were used to construct a fungal phylogenetic tree using ‘constrained topology search’ in FastTree v2.1.11 in the Galaxy pipeline (http://ieg3.rccc.ou.edu:8081/)85. To characterize fungal phylogenetic structure, we quantified the mean-nearest-taxon-distance (MNTD) and nearest-taxon-index (NTI)86 for the soil fungi sampled from each plant community via iCAMP package87. MNTD was calculated as the phylogenetic distance between each operational taxonomic unit (OTU) and its closest relative in the sample. NTI was quantified as the number of standard deviations that observed MNTD was from the mean of the null distribution (999 randomizations). For a single community, NTI > +2 indicates that fungal OTUs were more closely related than would be expected by chance (i.e., strong phylogenetic clustering). Conversely, NTI < -2 indicates that fungal OTUs were more distantly related than would be expected by chance (i.e., phylogenetic overdispersion). Because of phylogenetic conservatism (where closely related taxa share similar ecological niches and functions33), phylogenetic clustering indicates potential functional redundancy of the fungal community, which could enhance resilience of fungal functions to disturbance but reduce complementarity effects.
\(f{i}_{k}\) was the relative abundance of OTU \(i\) in fungal community \(k\), \({n}_{k}\) was the number of OTUs in \(k\), and \(\min \left({\varDelta i}_{k}{j}_{k}\right)\) was the minimum phylogenetic distance between OTU \(i\) and all other OTUs \(j\) that are also part of fungal community \(k\) and the phylogenetic tree. \({MN}{{TD}}_{{obs}}\) and \({{MNTD}}_{{null}}\) were the phylogenetic distances for the observed community and one randomized community, respectively.
ITS sequences were further assigned into functional groups based on the FungalTraits database88. We parsed fungal OTUs into trophic modes based on genus information and divided fungal taxa into different functional groups by primary lifestyle as identified by the FungalTraits database and following Pölme, Abarenkov88.
Statistical analysis
To test how plant species richness and the fungicide addition treatment influenced ecosystem multifunctionality (Hypothesis 1), we used a linear mixed model with ecosystem multifunctionality (average value method) as the response variable, plant species richness (1, 2, 4, 6, and 8 plant species as a continuous variable), the fungicide addition treatment (addition, control), and their interaction as explanatory variables, and plant community (i.e., plant community composition) as a random factor [ecosystem multifunctionality ~ plant species richness × fungicide addition treatment + (1|plant community), LMM1] via lme489 and car90 package. We present the response variables, explanatory variables, sample sizes, and other model parameters for all models in Supplementary Table S2. If a significant plant species richness × fungicide addition treatment interaction was detected, multiple t-tests with False Discovery Rate correction were used to test whether ecosystem multifunctionality differed between the fungicide addition and control treatments for each level of plant species richness.
To test how plant species richness and the fungicide addition treatment influenced complementarity and selection effects, we used multiple linear mixed models containing complementarity and selection effects as response variables, and following the same general structure as LMM1 above (Hypothesis 2, Supplementary Table S2). Linear mixed models with similar structure were also used to test the relationships between ecosystem multifunctionality and complementarity or selection effects in the control and fungicide addition treatment plant communities.
To explore the effects of plant species richness and the fungicide addition treatment on the interrelationships among multiple ecosystem functions, a principal components analysis (PCA) was used to reduce multiple ecosystem functions to fewer dimensions by accounting for their collinearity (Hypothesis 3) via FactoMineR package91. High values on the resulting PC1 axis were interpreted as representing synergy among ecosystem functions92. To statistically test the individual and interactive effects of plant species richness and the fungicide addition treatment on the composition of ecosystem functioning, we used permutational analysis of variance (PERMANOVA) based on Bray-Curtis distances in ecosystem functioning between plant communities. To explore the effects of plant species richness and fungal diversity on the synergy among functions, we used the linear mixed models with the PC1 value as the as response variables, and following the same general structure as LMM1 (Supplementary Table S2). To explore the effects of PC1 values on ecosystem multifunctionality in the control and fungicide addition treatment, we also used the linear mixed models with ecosystem multifunctionality as response variables (Supplementary Table S2).
To test how relationships between plant species richness and fungal diversity were influenced by the fungicide addition treatment, we used linear mixed models with plant diversity at harvest, fungal Chao1 richness estimate and Shannon diversity index93 as response variables, and following the same general structure as LMM1 (Supplementary Table S2) via Rmisc94 and vegan95 package. To test how plant species richness and the fungicide addition treatment influenced the phylogenetic structure of soil fungal commnities (Hypothesis 4), we used linear mixed models with nearest-taxon-index (NTI) as the response variable, following the same general structure as LMM1 (Supplementary Table S2). We also used linear mixed models with similar structure to test the relationship between NTI and ecosystem multifunctionality in the control and fungicide addition treatment plant communities. Linear mixed models with similar structure were also used to test the relationships between ecosystem multifunctionality and the Chao1 fungal richness estimate and Shannon diversity index in the control and fungicide treated plant communities. To explore the effects of plant species richness and the fungicide addition treatment on the relative abundance of plant species, fungal taxa and fungal functional groups (i.e., arbuscular mycorrhizal, pathogenic and saprophytic fungi), we used linear mixed models with the same structure as LMM1 (Supplementary Table S2). To test the potential influence of individual fungal taxa on ecosystem multifunctionality, we used a random forest analysis with ecosystem multifunctionality as the response variable and the relative abundance of different fungal taxonomic taxa as explanatory variables via rfPermute package96. Percentage increases in the MSE (mean squared error) associated with the inclusion of each taxon were used to estimate the importance of these predictors, with higher MSE percentages implying more influential predictors97.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All raw sequencing data have been deposited in the NCBI GenBank Short Read Archive under accession number PRJNA1090839. Raw data of single ecosystem functions and ecosystem multifunctionality are available from the Figshare Database: https://doi.org/10.6084/m9.figshare.25460713. Source data are provided with this paper.
Code availability
All code for the current analyses can be found at Zenodo, https://doi.org/10.5281/zenodo.15256374.
References
Hooper, D. U. et al. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 75, 3–35 (2005).
Tilman, D., Isbell, F. & Cowles, J. M. Biodiversity and ecosystem functioning. Annu. Rev. Ecol. Evol. Syst. 45, 471–493 (2014).
van der Plas, F. Biodiversity and ecosystem functioning in naturally assembled communities. Biol. Rev. 94, 1220–1245 (2019).
Yan, P. et al. The essential role of biodiversity in the key axes of ecosystem function. Glob. Change Biol. 29, 4569–4585 (2023).
Maestre, F. T. et al. Plant species richness and ecosystem multifunctionality in global drylands. Science 335, 214–218 (2012).
Manning, P. et al. Redefining ecosystem multifunctionality. Nat. Ecol. Evol. 2, 427–436 (2018).
Wagg, C., Schlaeppi, K., Banerjee, S., Kuramae, E. E. & van der Heijden, M. G. A. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 10, 4841 (2019).
Li, J. et al. Fungal richness contributes to multifunctionality in boreal forest soil. Soil Biol. Biochem. 136, 107526 (2019).
van der Heijden, M. G. A., Martin, F. M., Selosse, M. A. & Sanders, I. R. Mycorrhizal ecology and evolution: the past, the present, and the future. N. Phytol. 205, 1406–1423 (2015).
Hazard, C., Kruitbos, L., Davidson, H., Taylor, A. F. S. & Johnson, D. Contrasting effects of intra- and interspecific identity and richness of ectomycorrhizal fungi on host plants, nutrient retention and multifunctionality. N. Phytol. 213, 852–863 (2017).
Ma, X. C. et al. Global negative effects of nutrient enrichment on arbuscular mycorrhizal fungi, plant diversity and ecosystem multifunctionality. N. Phytol. 229, 2957–2969 (2021).
Maestre, F. T. et al. Increasing aridity reduces soil microbial diversity and abundance in global drylands. Proc. Natl Acad. Sci. USA 112, 15684–15689 (2015).
Christiansen, C. T. et al. Enhanced summer warming reduces fungal decomposer diversity and litter mass loss more strongly in dry than in wet tundra. Glob. Change Biol. 23, 406–420 (2017).
Wu, L. W. et al. Reduction of microbial diversity in grassland soil is driven by long-term climate warming. Nat. Microbiol. 7, 1054–1062 (2022).
Fei, S. L. et al. Coupling of plant and mycorrhizal fungal diversity: its occurrence, relevance, and possible implications under global change. N. Phytol. 234, 1960–1966 (2022).
Wang, C., Guo, L. & Shen, R. F. Rare microbial communities drive ecosystem multifunctionality in acidic soils of southern China. Appl. Soil Ecol. 189, 104895 (2023).
Sasaki, T. et al. Plant and microbial community composition jointly determine moorland multifunctionality. J. Ecol. 110, 2507–2521 (2022).
Schnitzer, S. A. et al. Soil microbes drive the classic plant diversity-productivity pattern. Ecology 92, 296–303 (2011).
Jiao, S., Lu, Y. H. & Wei, G. H. Soil multitrophic network complexity enhances the link between biodiversity and multifunctionality in agricultural systems. Glob. Change Biol. 28, 140–153 (2022).
Wang, S. et al. How complementarity and selection affect the relationship between ecosystem functioning and stability. Ecology 102, e03347 (2021).
Loreau, M. & Hector, A. Partitioning selection and complementarity in biodiversity experiments. Nature 412, 72–76 (2001).
Tilman, D. et al. Diversity and productivity in a long-term grassland experiment. Science 294, 843–845 (2001).
Barry, K. E. et al. The future of complementarity: disentangling causes from consequences. Trends Ecol. Evol. 34, 167–180 (2019).
Tilman, D., Lehman, C. L. & Thomson, K. T. Plant diversity and ecosystem productivity: theoretical considerations. Proc. Natl. Acad. Sci. USA 94, 1857–1861 (1997).
Tedersoo, L., Bahram, M. & Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 367, eaba1223 (2020).
Wang, G. Z. et al. Soil microbiome mediates positive plant diversity-productivity relationships in late successional grassland species. Ecol. Lett. 22, 1221–1232 (2019).
Wagg, C. et al. Complementarity in both plant and mycorrhizal fungal communities are not necessarily increased by diversity in the other. J. Ecol. 103, 1233–1244 (2015).
Jia, P. et al. Plant diversity enhances the reclamation of degraded lands by stimulating plant-soil feedbacks. J. Appl. Ecol. 57, 1258–1270 (2020).
Fiedler, S. et al. Global change shifts trade-offs among ecosystem functions in woodlands restored for multifunctionality. J. Appl. Ecol. 58, 1705–1717 (2021).
Heilpern, S. A., Anujan, K., Osuri, A. & Naeem, S. Positive correlations in species functional contributions drive the response of multifunctionality to biodiversity loss. P. R. Soc. B. Biol. Sci. 287, 20192501 (2020).
Argens, L. et al. Relationships between ecosystem functions vary among years and plots and are driven by plant species richness. Oikos 2024, e10096 (2024).
Matsuoka, S., Suzuki, Y., Hobara, S. & Osono, T. Fungal succession and decomposition of composted aquatic plants applied to soil. Fungal Ecol. 35, 34–41 (2018).
Louca, S. et al. Function and functional redundancy in microbial systems. Nat. Ecol. Evol. 2, 936–943 (2018).
Montesinos-Navarro, A., Segarra-Moragues, J. G., Valiente-Banuet, A. & Verdú, M. Fungal phylogenetic diversity drives plant facilitation. Oecologia 181, 533–541 (2016).
Maherali, H. & Klironomos, J. N. Influence of phylogeny on fungal community assembly and ecosystem functioning. Science 316, 1746–1748 (2007).
Wohl, D. L., Arora, S. & Gladstone, J. R. Functional redundancy supports biodiversity and ecosystem function in a closed and constant environment. Ecology 85, 1534–1540 (2004).
Luo, Y. H. et al. Multitrophic diversity and biotic associations influence subalpine forest ecosystem multifunctionality. Ecology 103, e3745 (2022).
Li, J. et al. Fungi drive soil multifunctionality in the coastal salt marsh ecosystem. Sci. Total Environ. 818, 151673 (2022).
Maillard, F., Kennedy, P. G., Adamczyk, B., Heinonsalo, J. & Buée, M. Root presence modifies the long-term decomposition dynamics of fungal necromass and the associated microbial communities in a boreal forest. Mol. Ecol. 30, 1921–1935 (2021).
Xu, Z. W. et al. Plant community diversity alters the response of ecosystem multifunctionality to multiple global change factors. Glob. Change Biol. 30, e17182 (2024).
Wang, F. Y., Liu, R. J., Lin, X. G. & Zhou, J. M. Arbuscular mycorrhizal status of wild plants in saline-alkaline soils of the Yellow River Delta. Mycorrhiza 14, 133–137 (2004).
Zhang, K. et al. Self-organized mud cracking amplifies the resilience of an iconic “Red Beach” salt marsh. Sci. Adv. 9, eabq3520 (2023).
Yi, S. et al. Biodiversity, environmental context and structural attributes as drivers of aboveground biomass in shrublands at the middle and lower reaches of the Yellow River basin. Sci. Total Environ. 774, 145198 (2021).
Lisner, A., Konecna, M., Blazek, P. & Leps, J. Community biomass is driven by dominants and their characteristics - The insight from a field biodiversity experiment with realistic species loss scenario. J. Ecol. 111, 240–250 (2023).
Chen, J. W. et al. Direct and indirect effects of dominant plants on ecosystem multifunctionality. Front. Plant Sci. 14, 1117903 (2023).
Frew, A., Heuck, M. K. & Aguilar-Trigueros, C. A. Host filtering, not competitive exclusion, may be the main driver of arbuscular mycorrhizal fungal community assembly under high phosphorus. Funct. Ecol. 37, 1856–1869 (2023).
Bruno, D., Gutiérrez-Cánovas, C., Sánchez-Fernández, D., Velasco, J. & Nilsson, C. Impacts of environmental filters on functional redundancy in riparian vegetation. J. Appl. Ecol. 53, 846–855 (2016).
Feng, J. W., Liu, W. T., Chen, J. J. & Zhang, C. L. Biogeography and ecology of Magnaporthales: a case study. Front Microbiol. 12, 654380 (2021).
Spanu, P. D. The genomics of obligate (and Nonobligate) biotrophs. Annu. Rev. Phytopathol. 50, 91–109 (2012).
Sesma, A. & Osbourn, A. E. The rice leaf blast pathogen undergoes developmental processes typical of root-infecting fungi. Nature 431, 582–586 (2004).
Hantsch, L., Braun, U., Scherer-Lorenzen, M. & Bruelheide, H. Species richness and species identity effects on occurrence of foliar fungal pathogens in a tree diversity experiment. Ecosphere 4, 81 (2013).
Pichon, N. A. et al. Nitrogen availability and plant functional composition modify biodiversity-multifunctionality relationships. Ecol. Lett. 27, e14361 (2024).
Welc, M., Ravnskov, S., Kieliszewska-Rokicka, B. & Larsen, J. Suppression of other soil microorganisms by mycelium of arbuscular mycorrhizal fungi in root-free soil. Soil Biol. Biochem. 42, 1534–1540 (2010).
Rillig, M. C. et al. The role of multiple global change factors in driving soil functions and microbial biodiversity. Science 366, 886–890 (2019).
de Gea, A. B., Hautier, Y. & Geisen, S. Interactive effects of global change drivers as determinants of the link between soil biodiversity and ecosystem functioning. Glob. Change Biol. 29, 296–307 (2023).
Semenova, G. & Maarel, V. E. Plant functional types - a strategic perspective. J. Veg. Sci. 11, 917–922 (2000).
Zhou, T. R., Guo, T., Wang, Y., Wang, A. D. & Zhang, M. Y. Carbendazim: Ecological risks, toxicities, degradation pathways and potential risks to human health. Chemosphere 314, 137723 (2023).
Garland, G. et al. A closer look at the functions behind ecosystem multifunctionality: a review. J. Ecol. 109, 600–613 (2021).
Byrnes, J. E. K. et al. Investigating the relationship between biodiversity and ecosystem multifunctionality: challenges and solutions. Methods Ecol. Evol. 5, 111–124 (2014).
Wang, Y. X., Liu, G. H. & Zhao, Z. H. Spatial heterogeneity of soil fertility in coastal zones: a case study of the Yellow River Delta, China. J. Soils Sediment. 21, 1826–1839 (2021).
Cao Q. X. et al. Effects of vegetation restoration age on Soil C: N: P stoichiometry in Yellow River Delta coastal wetland of China. Chin. Geogr. Sci. 34, 1045−1059 (2024).
Hu, W. G. et al. Aridity-driven shift in biodiversity-soil multifunctionality relationships. Nat. Commun. 12, 5350 (2021).
Ren, P. et al. Forest edges increase pollinator network robustness to extinction with declining area. Nat. Ecol. Evol. 7, 393–404 (2023).
Su, J., Zhao, Y., Xu, F. & Bai, Y. Multiple global changes drive grassland productivity and stability: a meta‐analysis. J. Ecol. 110, 2850–2869 (2022).
Gao, E. L., Ma, H., Yang, T., Kaiser-Bunbury, C. N. & Zhao, Z. G. Meadow transformations alter above- and below-ground ecological networks and ecosystem multifunctionality. Funct. Ecol. 37, 1703–1716 (2023).
Zirbel, C. R., Grman, E., Bassett, T. & Brudvig, L. A. Landscape context explains ecosystem multifunctionality in restored grasslands better than plant diversity. Ecology 100, e02634 (2019).
Williams, L. J., Paquette, A., Cavender-Bares, J., Messier, C. & Reich, P. B. Spatial complementarity in tree crowns explains overyielding in species mixtures. Nat. Ecol. Evol. 1, 63 (2017).
Zhang, T. A., Chen, H. Y. H. & Ruan, H. H. Global negative effects of nitrogen deposition on soil microbes. ISME J. 12, 1817–1825 (2018).
Alef, K. & Kleiner, D. Arginine ammonification, a simple method to estimate microbial activity potentials in soils. Soil Biol. Biochem. 18, 233–235 (1986).
Getaneh, S. et al. Impact of tree litter identity, litter diversity and habitat quality on litter decomposition rates in tropical moist evergreen forest. Ecosyst 9, 100023 (2022).
Mori, T., Aoyagi, R., Kitayama, K. & Mo, J. M. Does the ratio of beta-1,4-glucosidase to beta-1,4-N-acetylglucosaminidase indicate the relative resource allocation of soil microbes to C and N acquisition? Soil Biol. Biochem. 160, 108363 (2021).
Parham, J. A. & Deng, S. P. Detection, quantification and characterization of beta-glucosaminidase activity in soil. Soil Biol. Biochem. 32, 1183–1190 (2000).
Turner, B. L., Hopkins, D. W., Haygarth, P. M. & Ostle, N. Beta-glucosidase activity in pasture soils. Appl. Soil Ecol. 20, 157–162 (2002).
Lazcano, C., Gomez-Brandon, M., Revilla, P. & Dominguez, J. Short-term effects of organic and inorganic fertilizers on soil microbial community structure and function. Biol. Fert. Soils 49, 723–733 (2013).
Gillman, G. P., Sinclair, D. F. & Beech, T. A. Recovery of organic-carbon by the Walkley and black procedure in highly weathered soils. Commun. Soil Sci. Plan 17, 885–892 (1986).
Iskurt, C., Aliyev, E., Gengec, E., Kobya, M. & Khataee, A. Electrochemical oxidation of pretreated landfill leachate nanofiltration concentrate in terms of pollutants removal and formation of by-products. Chemosphere 307, 135954 (2022).
Downes, M. T. Improved hydrazine reduction method for automated-determination of low nitrate levels in freshwater. Water Res. 12, 673–675 (1978).
Song, H., Li, Z., Du, B., Wang, G. & Ding, Y. Bacterial communities in sediments of the shallow Lake Dongping in China. J. Appl. Microbiol. 112, 79–89 (2012).
Xu, Z., Guo, X., S. Caplan, J., Li, M. & Guo, W. Novel plant-soil feedbacks drive adaption of invasive plants to soil legacies of native plants under nitrogen deposition. Plant Soil 467, 47–65 (2021).
Reyon, D. et al. FLASH assembly of TALENs for high-throughput genome editing. Nat. Biotechnol. 30, 460–465 (2012).
Caporaso, J. G. et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336 (2010).
Rognes, T., Flouri, T., Nichols, B., Quince, C. & Mahe, F. VSEARCH: a versatile open source tool for metagenomics. Peerj 4, e2584 (2016).
Nilsson, R. H. et al. The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 47, 259–264 (2019).
Rousk, J. et al. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 4, 1340–1351 (2010).
Tan, G. et al. Current methods for automated filtering of multiple sequence alignments frequently worsen single-gene phylogenetic inference. Syst. Biol. 64, 778–791 (2015).
Webb, C. O., Ackerly, D. D., McPeek, M. A. & Donoghue, M. J. Phylogenies and community ecology. Annu. Rev. Ecol. Syst. 33, 475–505 (2002).
Ning, D. L. et al. A quantitative framework reveals ecological drivers of grassland microbial community assembly in response to warming. Nat. Commun. 11, 4717 (2020).
Pölme, S. et al. FungalTraits: A user friendly traits database of fungi and fungus-like stramenopiles. Fungal Divers 107, 129–132 (2021).
Bates, D., Mächler, M., Bolker, B. M. & Walker, S. C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Fox J. An R Companion to Applied Regression 3rd Edition, Vol. 608 (SAGE Publications, 2019).
Josse, Le. S. & Husson, J. F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 25, 1–18 (2008).
Raudsepp-Hearne, C., Peterson, G. D. & Bennett, E. M. Ecosystem service bundles for analyzing tradeoffs in diverse landscapes. Proc. Natl. Acad. Sci. USA 107, 5242–5247 (2010).
Gihring, T. M., Green, S. J. & Schadt, C. W. Massively parallel rRNA gene sequencing exacerbates the potential for biased community diversity comparisons due to variable library sizes. Environ. Microbiol. 14, 285–290 (2012).
Hope R. M. Rmisc: Ryan Miscellaneous. R Package Version 151. https://CRAN.R-project.org/package=Rmisc (2022).
Oksanen, J. et al. Vegan: Community Ecology Package. R Package Version 26−10. https://CRAN.R-project.org/package=vegan (2025).
Archer E. rfPermute: Estimate Permutation p-Values for Random Forest Importance Metrics. R Package Version 252. https://CRAN.R-project.org/package=rfPermute (2023).
Jiao, S. et al. Soil microbiomes with distinct assemblies through vertical soil profiles drive the cycling of multiple nutrients in reforested ecosystems. Microbiome 6, 146 (2018).
Acknowledgements
We thank the farmers for the management of Baimiao Experimental Station. We also thank Aiyuan Gao, Huijia Song, Wenhao Cui and Xinyue Liu for their help with the greenhouse experiment. This work was supported by the National Natural Science Foundation of China [Nos. U22A20558 (W.G.); 31970347 (W.G.); 32271588 (X.G.)] and China Postdoctoral Research Foundation [GCZ20230072 (Z.X.); 2024M750077 (Z.X.)]. Besides, authors gratefully acknowledge financial support for national exchanges from China Scholarship Council.
Author information
Authors and Affiliations
Contributions
Z.W. Xu, X. Guo, and W.H. Guo conceived the study; Z.W. Xu, Y Hu, and J.F Wang performed greenhouse experiments; Z.W. Xu performed data analysis. Z.W. Xu, M.Y. Li, and X. Guo wrote the initial manuscript. W.J. Allen, L.W. Wu, and Z.W. Xu edited the manuscript. All authors commented on drafts of the manuscript and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Warshi Dandeniya, Peter Manning and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, Z., Guo, X., Allen, W.J. et al. Soil fungi influence the relationship between plant diversity and ecosystem multifunctionality. Nat Commun 16, 5521 (2025). https://doi.org/10.1038/s41467-025-60661-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-60661-0