Abstract
Plant diversity can alter soil carbon stocks, but the effects are difficult to predict due to the multitude of mechanisms involved. We propose that these mechanisms and their outcomes can be better understood by testing how plant diversity affects particulate organic matter (POM) and mineral-associated organic matter (MAOM) depending on whether MAOM storage is “saturated” and the total soil organic matter pool is limited by plant inputs. Such context-dependency of plant-diversity effects on POM, MAOM, and total soil organic matter helps explain inconsistencies in plant-diversity–soil-carbon relationships across studies. Further illumination of this context-dependency is required to better predict consequences of biodiversity losses and gains, and manage ecosystems as carbon sinks and nutrient stores.
Similar content being viewed by others
Introduction
Recent studies have indicated that the amount of organic carbon (C) stored in soils can be sensitive to plant diversity1,2,3,4,5. Thus, widespread biodiversity losses6,7 can be a threat to the diverse soil functions associated with soil organic C, and restoration or enhancement of plant diversity (e.g., diversified crop rotation and reforestation) could increase soil C storage and help mitigate climate change8,9,10,11. The effectiveness of such management strategies, however, is hampered by incomplete knowledge of the processes by which plant diversity affects soil C storage. It remains especially unclear how plant diversity relates to the C that is stored in particulate organic matter (POM) and mineral-associated organic matter (MAOM), which tend to have different sources, formation pathways, residence times, and responses to land management12,13,14,15,16,17,18. We offer two explanations for the existence and persistence of this knowledge gap: (i) to the best of our knowledge, few of the hundreds of experimental studies of plant diversity gradients have measured POM and MAOM19,20,21, and (ii) although conceptual frameworks of POM and MAOM have been developed and revised, none of these prior efforts have focused on the role of plant diversity.
Here, we consider how plant diversity affects the formation of POM and MAOM via the quantity of inputs of plant-derived C to soil22,23,24 and transformations of organic matter in soil, as mediated by the quality of organic matter inputs and the composition and metabolic activities of bacteria, fungi, and animals in soil25,26,27,28,29,30,31,32,33,34,35. For example, effects of plant diversity on plant litter quality36 and soil microclimate31 could interact to determine conditions for microbial growth and utilization of the litter37. This, in turn, could affect the decomposition or accumulation of POM and the formation of microbial residues in MAOM. Likewise, plant diversity-related changes in rhizodeposition28 could affect the generation of dissolved organic matter and formation of MAOM via direct sorption. Here, we briefly review and synthesize how these and various other processes link plant diversity and the two key sub-pools of soil organic matter (SOM), highlight research gaps, and provide new hypotheses and methodological recommendations for future studies.
Plant diversity affects particulate organic matter directly via the production of structural plant inputs and indirectly by mediating the translocation and transformation of those inputs
Particulate organic matter represents partly decomposed plant fragments that, if not occluded within aggregates38, have relatively short residence time in soil39 and can be quickly decomposed under certain environmental conditions13. Particulate organic matter can be translocated to the mineral soil by bioturbating fauna or directly released to the soil via root mortality40,41,42. Plant diversity can both directly and indirectly affect the processes that are relevant to the accumulation of POM in organic layers or the mineral soil, including the production, decomposition, translocation, and biotic transformation of structural plant compounds (i.e., of plant litter)43,44.
Greater plant diversity often enhances above- and/or belowground productivity across land uses3,43,45,46,47,48,49,50,51,52, which likely results in higher inputs of aboveground and belowground litter to the soil53,54,55,56. Belowground inputs could directly replenish POM pools as these inputs are released into the mineral soil57. In contrast, aboveground litter commonly decomposes prior to incorporation into the mineral soil. Mixtures of aboveground litter under diverse plant communities could decompose more slowly than the individual litters these mixtures are composed of, if: (i) phenolics are transported between litters and form resistant complexes with proteins58, which hinder biotic decomposition, (ii) decomposer communities that are adapted to decompose specific components of litter are impaired by the heterogeneity of litter mixtures59, or (iii) resource competition among fungi hinders decomposition of part of the litter60,61. Increased aboveground litter production and decelerated decomposition of that litter could result in the accumulation of POM in organic layers (e.g., in coniferous forests)62. POM may also accumulate more in diverse forest and grassland communities with higher abundance of bioturbators such as earthworms63,64,65,66 due to enhanced transfer of structural compounds to the mineral soil. However, aboveground litter mixtures can also decompose more quickly67 due to improved microclimatic conditions68, greater habitat diversity, substrate diversity, enzymatic capabilities36,60, and nutrient transfer via leaching or fungi58,69,70,71 among litters. Such increases in litter decomposition in more diverse plant communities may be more likely if litters with sufficiently different biochemical qualities (pertaining to C/N or lignin/N ratios) co-occur72. Likewise, experimental communities with more plant species have sometimes been reported to have higher community-weighted N contents (N content weighted according to the abundance of individual species in a community) in plant biomass, which could alleviate potential microbial N limitations and accelerate the decomposition of that biomass once senescent73,74,75. Such accelerated decomposition could, in turn, reduce the retention of structural litter compounds in soil and thus POM. Notably, whether litter mixtures decompose more quickly, including belowground mixtures54, or whether community-weighted N contents in plant biomass are higher in diverse communities, may vary with time and may strongly depend on the environmental context, such as soil texture, fertility, or moisture68,76,77, and the presence of certain plant functional groups, such as legumes66,78,79. There is persistent uncertainty about the relevance of litter decomposition studies (i.e., litter mass loss studies) for the formation and dynamics of SOM80,81,82, and biodiversity-mediated effects on litter composition (e.g., N content) may have opposing effects on the persistence of litter-derived organic matter over short and long time scales21,83,84. An understanding of the effects of plant diversity on POM dynamics thus requires more research on the links between rates of litter mass loss and retention of POM in organic and mineral soil layers (sensu Mueller et al.82).
Whether POM accumulates in the mineral soil does not only depend on the amount of structural plant inputs, but also on the capacity of the microbial community to further transform those inputs, which is reflected in individual or combined microbial traits such as C use efficiency or diversity25,26,85. Studies in grassland biodiversity experiments, forests, and diversified crop rotations reported positive effects of plant diversity on microbial biomass C28,63,86,87,88,89,90,91,92, microbial diversity1,90,93 (but see Prober et al. and Dasser et al.94,95), and microbial C use efficiency96,97 (but see Prommer et al.98) across climates and soil types, which indicates that the microbial community may often have higher capacity to degrade litter and POM under more vs. less diverse plant communities. This capacity could further be altered by plant-mediated differences in the abundance of saprophagous soil fauna29. Such animals can transform POM into feces, which are often more easily decomposable than the material the feces originate from99. However, the fact that many studies investigate plant diversity and productivity, litter decomposition, and SOM dynamics separately complicates predictions of how plant diversity affects the formation of POM. This is complicated further by the fact that plant diversity has not always been found to be related to productivity100.
Plant diversity affects mineral-associated organic matter directly via the production of dissolved organic matter and indirectly by altering soil microbial traits and processes
Mineral-associated organic matter is bound to minerals or occluded within small microaggregates (˂50 µm18) and can persist in soil for centuries to millennia101. However, recent studies indicate that at least part of that pool can also cycle on shorter time scales102,103. Mineral-associated organic matter forms via interaction of reactive mineral surfaces with microbial residues12 and dissolved organic matter (e.g., root exudates or dissolved organic matter produced via depolymerization of plant biomolecules in organic layers or the mineral soil). Thus, plant diversity-mediated changes in the production and quality of root exudates and other dissolved organic matter, and alteration of factors influencing the formation of microbial residues (e.g., microbial traits as affected by faunal abundance, nutrient contents, and microclimatic conditions) have high potential to affect the formation of MAOM28,31,104.
Plant diversity could affect the quantity of dissolved organic matter indirectly, if dissolved organic matter is simply proportional to plant litter or biomass, but also directly, because some plant species release more dissolved organic matter (e.g., lignin monomers) from their decomposing litter or exude more C from their roots105,106,107. Plant diversity in temperate grasslands has also been shown to alter community-weighted root traits that are related to exudation rates, such as root length, density, or diameter105,108. Some studies harboring more diverse plant communities have indeed found higher quantities of dissolved organic matter in general109, and root exudates in particular, mostly in temperate grasslands1,28,92,110,111 but also in subtropical forests112. These root exudates, and dissolved organic matter in general, could extend to deeper soil layers likely due to increased rooting depth and density109,111 and/or increased number of preferential flow paths created through roots or macrofauna such as earthworms113. Such increased concentrations of dissolved organic matter can boost MAOM formation via direct sorption on mineral surfaces114. However, dissolved organic matter can also desorb existing organic matter from mineral surfaces115,116 and thus decrease MAOM-C or accelerate its turnover.
Higher inputs of dissolved vs. structural organic matter, e.g., via more complete litter decomposition117, and higher amounts of bioavailable compounds in dissolved organic matter109 under more diverse plant communities could also indirectly boost the formation of MAOM via microbial residues. That is, microorganisms can produce biomass, dissolved organic matter, and eventually residues more efficiently when growing on easily decomposable substrates, which is reflected in emergent microbial traits such as diversity or C use efficiency25,26,118. These traits can further be influenced by plant-diversity effects on nutrients, the microenvironment, and soil fauna. Lower leaching losses of nitrogen and higher contents of total and available phosphorus under diverse plant communities across climates and ecosystems119,120 could alleviate potential microbial nutrient limitations and enhance C use efficiency121. Plant diversity can also stabilize microenvironmental conditions: higher levels of shade by plant cover may result in a more uniform water distribution in the topsoil86,122, increased soil porosity may improve drainage33, or soil temperatures may be more constant due to elevated canopy shade and/or reduced soil thermal diffusivity with increased SOM31,123. Combined, these conditions could directly influence microbial growth, activity, or C use efficiency123,124 and alter the formation of microbial residues in MAOM16. These conditions could also indirectly affect microbial growth and activity via positive effects on the species richness and abundance of soil fauna such as arthropods29,125,126,127,128,129,130, protists129,131, and bacterial-feeding and omnivorous nematodes132,133,134. For example, selective feeding of soil fauna such as nematodes and protists on certain microbial groups can alter microbial community structure, increase microbial activity135, and may affect traits such as C use efficiency42. However, negative or neutral effects of plant diversity on the abundance and richness of soil fauna have been reported as well136,137,138,139,140, and multiple studies indicate higher relevance of plant identity or functional group than plant diversity in affecting the composition of soil fauna, at least in short-term studies141,142,143,144,145,146,147,148. The lack of quantitative linkages between plant diversity and MAOM in general, and between dissolved organic matter inputs and the direct sorption and microbial pathways of MAOM formation in particular, render sound statements on how plant diversity affects the formation of MAOM difficult. Long-term biodiversity experiments are scarce but urgently needed to test such relationships on adequate time scales.
Context specificity of plant-diversity effects on C storage in soil via POM and MAOM
The varied and complex interactions of processes by which plant diversity can influence POM and MAOM, as highlighted above, and the scarcity of empirical studies that mechanistically link POM and MAOM dynamics to plant diversity, make it difficult to generalize about the direction and magnitude of plant-diversity effects on these SOM pools and total SOM. There is some evidence, however, that certain plant diversity-mediated processes are more relevant and predictable in certain environmental contexts, and that progress in this field can be made by investigating plant diversity and SOM dynamics in light of these contexts. We specifically hypothesize that (i) net effects of plant diversity on C contents are the most positive, and result in accrual of both POM and MAOM, for soils whose C storage is limited primarily by plant inputs, that (ii) net effects of plant diversity on C contents are positive, but weaker and mediated largely through effects on POM, for soils closer to their capacity for MAOM formation, and that (iii) some links between plant diversity and SOM dynamics can weaken or offset positive effects on C contents. These hypotheses provide a basis for future research and help explain why the observed effects of plant diversity on soil C are not consistently positive and vary in strength across sites3.
Plant-diversity effects on soil C are the most positive in soils whose C storage is limited by plant inputs
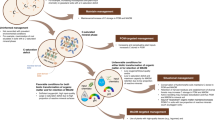
Soils that have low C contents and/or abundant reactive mineral surfaces have the potential to store large additional amounts of C, perhaps most persistently in MAOM, but also in POM13. However, this potential often remains untapped due to insufficient inputs of organic matter. Such soils include those newly developing, e.g., after glacier retreat149; soils under agricultural use150, where plant residues are often removed and nutrient retention is low; soils after disturbances, such as mining151,152; grasslands and forests recovering from intensive grazing and timber harvest153,154, respectively; or deeper soil layers150. Although C-saturation, which assumes a finite capacity of soils for C storage, is an evolving and debated concept155,156,157,158, global-scale studies suggest that the majority of soils may currently exist in a state characterized by SOM pools that are smaller than their potential and constrained by plant inputs150,159. More diverse plant communities in such environmental contexts could alleviate this deficiency of plant inputs by boosting plant productivity160, enhanced rooting depths111, and increased abundance of bioturbators. These factors would foster the processes involved in both POM (e.g., production of plant litter and transfer of this litter to the mineral soil) and MAOM formation (e.g., root exudation) and result in a net increase of C stored in these pools and overall soil C storage (Fig. 1).
a Overview of mechanistic links between plant diversity and POM and MAOM. Increases in plant diversity (from left to right) also increase: plant productivity (indicated by more leaves and deeper roots), diversity of litter species and dissolved organic matter/root exudates (indicated by the various colors of leaves and drops), and abundance and diversity of soil fauna and microorganisms. In turn, these changes could increase bioturbation rates and production of feces, grazing of soil fauna on microorganisms, direct sorption (or desorption) of dissolved organic matter on (from) mineral surfaces, and microbial C use efficiency (or other microbial traits), all of which can influence both accumulation and/or decomposition of POM and MAOM. High plant diversity can also affect the microclimate, such as via increased shading, maintenance of soil temperature and moisture (indicated in circles aboveground), or elevated nutrient abundance and availability, which can favor microbial activity/biomass production and the microbial pathway of MAOM formation and decomposition of POM. Arrows indicate processes; “+” and “−“ indicate expected intensification/weakening of processes. Nutrients are indicated by capital “N” and “P.” “+/−” after POM and MAOM indicates that increases in plant diversity can have both positive and negative effects on these pools, and we hypothesize that the direction of plant-diversity effects is context-dependent, as highlighted in panel b. b Relevance of links between increased plant diversity and POM, MAOM, and total SOM in different environmental contexts, including recommendations on how to test these hypotheses; ++, +, o, and − indicate strong and moderate increases, no change, and decreases, respectively. Created in BioRender. Eisenhauer, N. (2025) https://BioRender.com/1hwb0e8.
Plant-diversity effects on soil C are positive but weaker in soils closer to their capacity for MAOM formation
Soils with higher C contents and a mineral phase closer to their theoretical C saturation threshold likely have lower capacity to store additional amounts of C in MAOM150. Such soils include those under many “natural,” older forests and, to a lesser extent, grasslands150. Plant diversity-mediated processes that foster MAOM formation (such as favorable conditions for microbial growth or increased quantity and quality of dissolved organic matter/root exudates) will thus be less relevant in such contexts, but plant-diversity effects on soil C can still be positive, albeit weaker, when retention of C in POM is fostered due to (i) increased plant productivity and plant litter production and/or (ii) reduced decomposition and mineralization of that litter or POM in mineral soil (e.g., due to antagonistic effects of litter mixtures on decomposition or conditions that hamper microbial activity in the mineral soil, such as low oxygen availability or pH). Losses in plant diversity and reduced plant inputs could have more profound effects on soil C in such ecosystems than gains in plant diversity. This may specifically be true in soils that are more reliant on POM, such as many coniferous forests in both mineral soil and organic horizons161, as POM in these soils can be more quickly lost upon disturbance than MAOM161.
Some links between plant diversity and SOM dynamics can weaken or offset positive effects on C contents
Net positive effects of plant diversity in the ecosystem contexts highlighted above could be weakened (or even offset) by potential negative links between plant diversity and POM and MAOM. For example, plant-diversity effects that stabilize microenvironmental conditions and render them more favorable for microbial activity may increase C mineralization and offset some of the positive effects that are mediated by increased C inputs. This negative link may be the most relevant in soils with a mineral phase closer to its C saturation threshold [hypothesis ii)], where potential gains in C due to increased structural plant inputs can be offset by accelerated decomposition of POM, or in ecosystems in which climate is a strong limiting factor for microbial processes and in which SOM contains high amounts of POM, such as in colder climates and/or permafrost soils162,163. Likewise, increased inputs of dissolved organic matter do not necessarily lead to net formation of MAOM if desorbing existing SOM from mineral surfaces116. This has potentially high relevance in soils with a mineral phase closer to its theoretical C saturation threshold [hypothesis ii)]. Finally, interactions among different litter types that result in synergistic effects on litter decomposition could increase C mineralization and result in smaller positive or neutral to negative net effects of plant diversity on POM (and/or MAOM) stocks, which may specifically be relevant in ecosystems that store large amounts of C in organic layers, such as coniferous forests161.
Synergy and outlook
Plant diversity can be a potent regulator of the dynamics of POM and MAOM via various interrelated pathways (Fig. 1) and thus has a sustained impact on C storage in soil. However, the mechanistic links between these pathways and the quantity and dynamics of POM and MAOM have hardly been explored. We thus emphasize the need to directly link plant diversity and the processes highlighted here to POM and MAOM dynamics. This includes: (i) disentangling the effects of above- and belowground inputs in diversity-induced gradients of plant productivity, e.g., by excluding aboveground inputs in one set of plots164,165,166; (ii) testing the role of decomposition of aboveground and belowground litter in diverse litter mixtures with different qualities in the formation of POM and MAOM (perhaps using isotopically labeled litter167), which is often studied separately77; (iii) determining net effects of increased quantities of dissolved organic matter and root exudates, including their molecular composition, on MAOM formation under plant-diversity gradients, e.g., using 13C pulse labeling168; and (iv) establishing soil fauna inventories and exploring changes in microbial community composition and traits, such as C use efficiency, as affected by plant diversity97,169. Individual and combined effects of two or more mechanisms could be systematically investigated in mesocosm or Ecotron experiments170,171 and cross-institutional field experiments100,172, respectively, employing standardized soil fractionation schemes12,173, microbial methods such as phospholipid fatty-acid and DNA extractions, multi omics174, and isotopic and spectroscopic techniques such as 13C/15N labeling and Fourier-transform infrared or nuclear magnetic resonance spectroscopy.
We believe that mechanistically studying the links between plant diversity and POM and MAOM will help better predict the consequences of gains and losses in plant diversity on C and nutrient storage in different ecosystems and eventually inform related soil management strategies. However, it will be important to study these links in different environmental contexts [as per our hypotheses (i)–(iii) highlighted above; Fig. 1], which likely determine whether plant diversity has positive, neutral, or negative effects on POM and MAOM, and overall C storage. To optimally test this context-dependency of plant diversity effects requires multi-site, distributed field experiments175, or mesocosm experiments that span climates or soil types (e.g., with low and high organic matter contents). We specifically call for studies (i) along C-saturation gradients, including gradients in soil texture, mineralogy, and depth, (ii) along gradients of soil parameters that affect microbial traits, such as pH or oxygen availability176, and (iii) in the presence and absence of certain key plant functional groups, such as N-fixing plants and trees with divergent leaf or mycorrhizal traits (e.g., needle-leaves vs. broadleaves; associations with arbuscular vs ectomycorrhizal fungi). This also necessitates that such experiments have to be performed over longer periods of time, e.g., for several years, with repeated samplings or isotopic tracers.
We believe that further research along the lines of our hypotheses above [(i)–(iii); Fig. 1] is essential in gaining knowledge necessary to effectively guide biodiversity restoration efforts and management practices that aim at establishing or maintaining soils as C sinks and nutrient stores. Only if we close the remaining knowledge gaps can we comprehend the consequences of biodiversity loss for SOM dynamics and the related ecosystem functions and implement appropriate countermeasures (e.g., restoration).
References
Lange, M. et al. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 6, 6707 (2015).
Steinbeiss, S. et al. Plant diversity positively affects short-term soil carbon storage in experimental grasslands. Glob. Chang. Biol. 14, 2937–2949 (2008).
Chen, S. et al. Plant diversity enhances productivity and soil carbon storage. Proc. Natl Acad. Sci. USA 115, 4027–4032 (2018).
Chen, X. et al. Tree diversity increases decadal forest soil carbon and nitrogen accrual. Nature 618, 94–101 (2023).
Chen, X. et al. Effects of plant diversity on soil carbon in diverse ecosystems: a global meta-analysis. Biol. Rev. 95, 167–183 (2020).
Eichenberg, D. et al. Widespread decline in Central European plant diversity across six decades. Glob. Chang. Biol. 27, 1097–1110 (2021).
Jandt, U. et al. More losses than gains during one century of plant biodiversity change in Germany. Nature 611, 512–518 (2022).
Weisser, W. W. et al. Biodiversity effects on ecosystem functioning in a 15-year grassland experiment: patterns, mechanisms, and open questions. Basic Appl. Ecol. 23, 1–73 (2017).
Bai, Y. & Cotrufo, M. F. Grassland soil carbon sequestration: current understanding, challenges. Solutions 608, 603–608 (2022).
Wooliver, R., Kivlin, S. N. & Jagadamma, S. Links among crop diversification, microbial diversity, and soil organic carbon: mini review and case studies. Front. Microbiol. 13, 854247 (2022).
Eisenhauer, N. et al. A belowground perspective on the nexus between biodiversity change, climate change, and human well-being. J. Sustain. Agric. Environ. 3, 1–12 (2024).
Lavallee, J. M., Soong, J. L. & Cotrufo, M. F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Chang. Biol. 26, 261–273 (2020).
Angst, G. et al. Unlocking complex soil systems as carbon sinks: multi-pool management as the key. Nat. Commun. 14, 2967 (2023).
Mueller, C. W. et al. Initial differentiation of vertical soil organic matter distribution and composition under juvenile beech (Fagus sylvatica L.) trees. Plant Soil 323, 111–123 (2009).
Bol, R., Poirier, N., Balesdent, J. & Gleixner, G. Molecular turnover time of soil organic matter in particle-size fractions of an arable soil. Rapid Commun. Mass Spectrom. 23, 2551–2558 (2009).
Liang, C., Schimel, J. P. & Jastrow, J. D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2, 1–6 (2017).
Totsche, K. U. et al. Microaggregates in soils. J. Plant Nutr. Soil Sci. 181, 1–33 (2018).
Chenu, C. & Plante, A. T. F. T. Clay-sized organo-mineral complexes in a cultivation chronosequence: revisiting the concept of the ‘primary organo-mineral complex’. Eur. J. Soil Sci. 57, 596–607 (2006).
Tong, H. et al. Crop rotational diversity alters the composition of stabilized soil organic matter compounds in soil physical fractions. Can. J. Soil Sci. 103, 213–233 (2023).
Jia, Y. et al. Plant and microbial pathways driving plant diversity effects on soil carbon accumulation in subtropical forest. Soil Biol. Biochem. 161, 108375 (2021).
Sun, T. et al. General reversal of N- decomposition relationship during long- term decomposition in boreal and temperate forests Tao. Proc. Natl Acad. Sci. USA 121, e2401398121 (2024).
Castellano, M. J., Mueller, K. E., Olk, D. C., Sawyer, J. E. & Six, J. Integrating plant litter quality, soil organic matter stabilization, and the carbon saturation concept. Glob. Chang. Biol. 21, 3200–3209 (2015).
Cotrufo, M. F., Wallenstein, M. D., Boot, C. M., Denef, K. & Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter?. Glob. Chang. Biol. 19, 988–995 (2013).
Craig, M. E. et al. Fast-decaying plant litter enhances soil carbon in temperate forests but not through microbial physiological traits. Nat. Commun. 13, 1–10 (2022).
Angst, G. et al. Stabilized microbial necromass in soil is more strongly coupled with microbial diversity than the bioavailability of plant inputs. Soil Biol. Biochem. 190, 109323 (2024).
Sokol, N. W. et al. Life and death in the soil microbiome: how ecological processes influence biogeochemistry. Nat. Rev. Microbiol. 20, 415–430 (2022).
Chen, X., Chen, H. Y. H., Searle, E. B., Chen, C. & Reich, P. B. Negative to positive shifts in diversity effects on soil nitrogen over time. Nat. Sustain. 4, 225–232 (2021).
Eisenhauer, N. et al. Root biomass and exudates link plant diversity with soil bacterial and fungal biomass. Scientific Reports 7, 44641 (2017).
Zhang, Y., Peng, S., Chen, X. & Chen, H. Y. H. Plant diversity increases the abundance and diversity of soil fauna: a meta-analysis. Geoderma 411, 115694 (2022).
Tilman, D., Wedin, D. & Knops, J. Productivity and sustainability influeced by biodiversity in grassland ecosystems. Nature 379, 718–720 (1996).
Huang, Y. et al. Enhanced stability of grassland soil temperature by plant diversity. Nat. Geosci. 17, 44–50 (2023).
Joly, F.-X. et al. Tree species diversity affects decomposition through modified micro-environmental conditions across European forests. N. Phytol. 214, 1281–1293 (2017).
Fischer, C. et al. Plant species richness and functional groups have different effects on soil water content in a decade-long grassland experiment. J. Ecol. 107, 127–141 (2019).
Leimer, S., Oelmann, Y., Wirth, C. & Wilcke, W. Time matters for plant diversity effects on nitrate leaching from temperate grassland. Agric. Ecosyst. Environ. 211, 155–163 (2015).
Mueller, K. E., Hobbie, S. E., Tilman, D. & Reich, P. B. Effects of plant diversity, N fertilization, and elevated carbon dioxide on grassland soil N cycling in a long-term experiment. Glob. Chang. Biol. 19, 1249–1261 (2013).
Beugnon, R. et al. Tree diversity effects on litter decomposition are mediated by litterfall and microbial processes. Oikos 2023, e09751 (2023).
Eisenhauer, N. et al. Plant diversity effects on soil food webs are stronger than those of elevated CO2 and N deposition in a long-term grassland experiment. Proc. Natl Acad. Sci. USA 110, 6889–6894 (2013).
Mueller, C. W. & Koegel-Knabner, I. Soil organic carbon stocks, distribution, and composition affected by historic land use changes on adjacent sites. Biol. Fertil. Soils 45, 347–359 (2009).
von Lützow, M. et al. SOM fractionation methods: Relevance to functional pools and to stabilization mechanisms. Soil Biol. Biochem. 39, 2183–2207 (2007).
Sokol, N. W., Kuebbing, S. E., Karlsen-Ayala, E. & Bradford, M. A. Evidence for the primacy of living root inputs, not root or shoot litter, in forming soil organic carbon. N. Phytol. 221, 233–246 (2019).
Frouz, J. Effects of soil macro- and mesofauna on litter decomposition and soil organic matter stabilization. Geoderma 332, 161–172 (2018).
Angst, G. et al. Conceptualizing soil fauna effects on labile and stabilized soil organic matter. Nat. Commun. 15, 5005 (2024).
Cardinale, B. J. et al. Impacts of plant diversity on biomass production increase through time because of species complementarity. Proc. Natl Acad. Sci. USA 104, 18123–18128 (2007).
Ebeling, A. et al. Plant diversity impacts decomposition and herbivory via changes in aboveground arthropods. PLoS ONE 9, e106529 (2014).
Yang, Y., Tilman, D., Furey, G. & Lehman, C. Soil carbon sequestration accelerated by restoration of grassland biodiversity. Nat. Commun. 10, 1–7 (2019).
Liang, J. et al. Positive biodiversity-productivity relationship predominant in global forests. Science 354, aaf8957 (2016).
Tilman, D., Wedin, D. & Knops, J. Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature 379, 718–720 (1996).
Li, C. et al. Crop diversity for yield increase. PLoS ONE 4, 1–6 (2009).
van Ruijven, J. & Berendse, F. Diversity–productivity relationships: Initial effects, long-term patterns, and underlying mechanisms. Proc. Natl Acad. Sci. USA 102, 695–700 (2005).
Van Ruijven, J. & Berendse, F. Long-term persistence of a positive plant diversity–productivity relationship in the absence of legumes. Oikos 118, 101–106 (2009).
Wang, G. et al. Soil microbiome mediates positive plant diversity-productivity relationships in late successional grassland species. Ecol. Lett. 22, 1221–1232 (2019).
van Ruijven, J., Ampt, E., Francioli, D. & Mommer, L. Do soil-borne fungal pathogens mediate plant diversity–productivity relationships? Evidence and future opportunities. J. Ecol. 108, 1810–1821 (2020).
Cong, W.-F. et al. Plant species richness promotes soil carbon and nitrogen stocks in grasslands without legumes. J. Ecol. 102, 1163–1170 (2014).
Chen, H. et al. Plant species richness negatively affects root decomposition in grasslands. J. Ecol. 105, 209–218 (2017).
Cá, J., Lustosa Filho, J. F., da Silva, N. R., de Castro, C. R. T. & de Oliveira, T. S. C. N stocks in silvopastoral systems with high and low tree diversity: Evidence from a twenty-two year old field study. Sci. Total Environ. 833, 155298 (2022).
Peng, S. et al. Species mixtures enhance fine root biomass but inhibit root decay under throughfall manipulation in young natural boreal forests. Sci. Total Environ. 955, 176952 (2024).
Sokol, N. W. & Bradford, M. A. Microbial formation of stable soil carbon is more efficient from belowground than aboveground input. Nat. Geosci. 12, 46–53 (2019).
Gessner, M. O. et al. Diversity meets decomposition. Trends Ecol. Evol. 25, 372–380 (2010).
Smith, V. C. & Bradford, M. A. Do non-additive effects on decomposition in litter-mix experiments result from differences in resource quality between litters?. Oikos 102, 235–242 (2003).
Hättenschwiler, S., Tiunov, A. V. & Scheu, S. Biodiversity and litter decomposition in terrestrial ecosystems. Annu. Rev. Ecol. Evol. Syst. 36, 191–218 (2005).
Xiong, S. & Nilsson, C. The effects of plant litter on vegetation: a meta-analysis. J. Ecol. 87, 984–994 (1999).
Wan, X. et al. Functional identity drives tree species richness-induced increases in litterfall production and forest floor mass in young tree communities. N. Phytol. 240, 1003–1014 (2023).
Baldwin-Kordick, R. et al. Comprehensive impacts of diversified cropping on soil health and sustainability. Agroecol. Sustain. Food Syst. 46, 331–363 (2022).
Cesarz, S., Fahrenholz, N., Migge-Kleian, S., Platner, C. & Schaefer, M. Earthworm communities in relation to tree diversity in a deciduous forest. Eur. J. Soil Biol. 43, S61-S67 (2007).
Spehn, E. M., Joshi, J., Schmid, B., Alphei, J. & Körner, C. Plant diversity effects on soil heterotrophic activity in experimental grassland ecosystems. Plant Soil 224, 217–230 (2000).
Milcu, A., Partsch, S., Scherber, C., Weisser, W. W. & Scheu, S. Earthworms and legumes control litter decomposition in a plant diversity gradient. Ecology 89, 1872–1882 (2008).
Xiao, W., Chen, C., Chen, X., Huang, Z. & Chen, H. Y. H. Functional and phylogenetic diversity promote litter decomposition across terrestrial ecosystems. Glob. Ecol. Biogeogr. 29, 2261–2272 (2020).
Makkonen, M., Berg, M. P., van Logtestijn, R. S. P., van Hal, J. R. & Aerts, R. Do physical plant litter traits explain non-additivity in litter mixtures? A test of the improved microenvironmental conditions theory. Oikos 122, 987–997 (2013).
Cuchietti, A., Marcotti, E., Gurvich, D. E., Cingolani, A. M. & Pérez Harguindeguy, N. Leaf litter mixtures and neighbour effects: low-nitrogen and high-lignin species increase decomposition rate of high-nitrogen and low-lignin neighbours. Appl. Soil Ecol. 82, 44–51 (2014).
Berglund, S. L., Ågren, G. I. & Ågren, G. I. When will litter mixtures decompose faster or slower than individual litters? A model for two litters. Oikos 121, 1112–1120 (2012).
Handa, I. T. et al. Consequences of biodiversity loss for litter decomposition across biomes. Nature 509, 218–221 (2014).
Liu, J. et al. Synergistic effects: a common theme in mixed-species litter decomposition. N. Phytol. 227, 757–765 (2020).
Bessler, H. et al. Nitrogen uptake by grassland communities: Contribution of N2 fixation, facilitation, complementarity, and species dominance. Plant Soil 358, 301–322 (2012).
Reich, P. B. et al. Impacts of biodiversity loss escalate through time as redundancy fades. Science 336, 589–592 (2012).
Chen, X. & Chen, H. Y. H. Plant mixture balances terrestrial ecosystem C:N:P stoichiometry. Nat. Commun. 12, 1–9 (2021).
Porre, R. J., van der Werf, W., De Deyn, G. B., Stomph, T. J. & Hoffland, E. Is litter decomposition enhanced in species mixtures? A meta-analysis. Soil Biol. Biochem. 145, 107791 (2020).
Angst, G. et al. Soil texture affects the coupling of litter decomposition and soil organic matter formation. Soil Biol. Biochem. 159, 108302 (2021).
Fornara, D. A., Tilman, D. & Hobbie, S. E. Linkages between plant functional composition, fine root processes and potential soil N mineralization rates. J. Ecol. 97, 48–56 (2009).
Spehn, E. M. et al. The role of legumes as a component of biodiversity in a cross-European study of grassland biomass nitrogen. Oikos 98, 205–218 (2002).
Prescott, C. E. Do rates of litter decomposition tell us anything we really need to know? Ecol. Manag. 220, 66–74 (2005).
Prescott, C. E. Litter decomposition: What controls it and how can we alter it to sequester more carbon in forest soils?. Biogeochemistry 101, 133–149 (2010).
Mueller, K. E. et al. Effects of litter traits, soil biota, and soil chemistry on soil carbon stocks at a common garden with 14 tree species. Biogeochemistry 123, 313–327 (2015).
Berg, È. Litter decomposition and organic matter turnover in northern forest soils. Forest Ecology and Management 133, 13–22 (2000).
Berg, B. et al. Factors influencing limit values for pine needle litter decomposition: a synthesis for boreal and temperate pine forest systems. Biogeochemistry 100, 57–73 (2010).
Whalen, E. D., Grandy, A. S., Geyer, K. M., Morrison, E. W. & Frey, S. D. Microbial trait multifunctionality drives soil organic matter formation potential. Nat. Commun. 15, 10209 (2024).
Lange, M. et al. Biotic and abiotic properties mediating plant diversity effects on soil microbial communities in an experimental grassland. PLoS ONE 9, e96182 (2014).
Thakur, M. P. et al. Plant diversity drives soil microbial biomass carbon in grasslands irrespective of global environmental change factors. Glob. Chang. Biol. 21, 4076–4085 (2015).
McDaniel, M. D., Tiemann, L. K. & Grandy, A. S. Does agricultural crop diversity enhance soil microbial biomass and organic matter dynamics? A meta-analysis. Ecol. Appl. 24, 560–570 (2014).
Chen, C., Chen, H. Y. H., Chen, X. & Huang, Z. Meta-analysis shows positive effects of plant diversity on microbial biomass and respiration. Nat. Commun. 10, 1332 (2019).
Tedersoo, L. et al. Tree diversity and species identity effects on soil fungi, protists and animals are context dependent. ISME J. 10, 346–362 (2015).
Tiemann, L. K., Grandy, A. S., Atkinson, E. E., Marin-Spiotta, E. & McDaniel, M. D. Crop rotational diversity enhances belowground communities and functions in an agroecosystem. Ecol. Lett. 18, 761–771 (2015).
Steinauer, K., Chatzinotas, A. & Eisenhauer, N. Root exudate cocktails: the link between plant diversity and soil microorganisms?. Ecol. Evol. 6, 7387–7396 (2016).
Loranger-Merciris, G., Barthes, L., Gastine, A. & Leadley, P. Rapid effects of plant species diversity and identity on soil microbial communities in experimental grassland ecosystems. Soil Biol. Biochem. 38, 2336–2343 (2006).
Prober, S. M. et al. Plant diversity predicts beta but not alpha diversity of soil microbes across grasslands worldwide. Ecol. Lett. 18, 85–95 (2015).
Dassen, S. et al. Differential responses of soil bacteria, fungi, archaea and protists to plant species richness and plant functional group identity. Mol. Ecol. 26, 4085–4098 (2017).
Eisenhauer, N., Reich, P. B. & Scheu, S. Increasing plant diversity effects on productivity with time due to delayed soil biota effects on plants. Basic Appl. Ecol. 13, 571–578 (2012).
Duan, P. et al. Tree species diversity increases soil microbial carbon use efficiency in a subtropical forest. Glob. Chang. Biol. 29, 7131–7144 (2023).
Prommer, J. et al. Increased microbial growth, biomass, and turnover drive soil organic carbon accumulation at higher plant diversity. Glob. Chang. Biol. 26, 669–681 (2020).
Joly, F.-X. et al. Detritivore conversion of litter into faeces accelerates organic matter turnover. Commun. Biol. 3, 660 (2020).
Spohn, M. et al. The positive effect of plant diversity on soil carbon depends on climate. Nat. Commun. 14, 6624 (2023).
Schmidt, M. W. I. et al. Persistence of soil organic matter as an ecosystem property. Nature 478, 49–56 (2011).
Jilling, A. et al. Rapid and distinct responses of particulate and mineral-associated organic nitrogen to conservation tillage and cover crops. Geoderma 359, 114001 (2020).
Jilling, A. et al. Minerals in the rhizosphere: overlooked mediators of soil nitrogen availability to plants and microbes. Biogeochemistry 139, 103–122 (2018).
Eisenhauer, N. et al. Plant diversity effects on soil microorganisms support the singular hypothesis. Ecology 91, 485–496 (2010).
Williams, A. et al. Root functional traits explain root exudation rate and composition across a range of grassland species. J. Ecol. 110, 21–33 (2022).
Smith, D. J. et al. Dissolved organic carbon characteristics are associated with changes in soil microbiome under different plant species. Appl. Soil Ecol. 196, 105313 (2024).
Scheibe, A. & Gleixner, G. Influence of litter diversity on dissolved organic matter release and soil carbon formation in a mixed beech forest. PLoS ONE 9, 1–21 (2014).
Peng, S. & Chen, H. Y. H. Global responses of fine root biomass and traits to plant species mixtures in terrestrial ecosystems. Glob. Ecol. Biogeogr. 30, 289–304 (2021).
Lange, M. et al. Plant diversity enhances production and downward transport of biodegradable dissolved organic matter. J. Ecol. 109, 1284–1297 (2021).
Pérès, G. et al. Mechanisms linking plant community properties to soil aggregate stability in an experimental grassland plant diversity gradient. Plant Soil 373, 285–299 (2013).
Mueller, K. E., Tilman, D., Fornara, D. A. & Hobbie, S. E. Root depth distribution and the diversity–productivity relationship in a long-term grassland experiment. Ecology 94, 787–793 (2013).
Liu, C. et al. Standing fine root mass and production in four Chinese subtropical forests along a succession and species diversity gradient. Plant Soil 376, 445–459 (2014).
Fischer, C. et al. Plant species diversity affects infiltration capacity in an experimental grassland through changes in soil properties. Plant Soil 397, 1–16 (2015).
Cotrufo, M. F., Haddix, M. L., Kroeger, M. E. & Stewart, C. E. The role of plant input physical-chemical properties, and microbial and soil chemical diversity on the formation of particulate and mineral-associated organic matter. Soil Biol. Biochem. 168, 108648 (2022).
Chari, N. R. & Taylor, B. N. Soil organic matter formation and loss are mediated by root exudates in a temperate forest. Nat. Geosci. 15, 1011–1016 (2022).
Keiluweit, M. et al. Mineral protection of soil carbon counteracted by root exudates. Nat. Clim. Chang. 5, 588–595 (2015).
Don, A. & Kalbitz, K. Amounts and degradability of dissolved organic carbon from foliar litter at different decomposition stages. Soil Biol. Biochem. 37, 2171–2179 (2005).
Tao, F. et al. Microbial carbon use efficiency promotes global soil carbon storage. Nature 618, 981–985 (2023).
Chen, X., Chen, H. Y. H. & Chang, S. X. Meta-analysis shows that plant mixtures increase soil phosphorus availability and plant productivity in diverse ecosystems. Nat. Ecol. Evol. 6, 1112–1121 (2022).
Liu, X. et al. Plant diversity and species turnover co-regulate soil nitrogen and phosphorus availability in Dinghushan forests, southern China. Plant Soil 464, 257–272 (2021).
Spohn, M. et al. Soil microbial carbon use efficiency and biomass turnover in a long-term fertilization experiment in a temperate grassland. Soil Biol. Biochem. 97, 168–175 (2016).
Renwick, L. L. R. et al. Long-term crop rotation diversification enhances maize drought resistance through soil organic matter. Environ. Res. Lett. 16, 84067 (2021).
De Frenne, P. et al. Forest microclimates and climate change: Importance, drivers and future research agenda. Glob. Chang. Biol. 27, 2279–2297 (2021).
Domeignoz-Horta, L. A. et al. Plant diversity drives positive microbial associations in the rhizosphere enhancing carbon use efficiency in agricultural soils. Nat. Commun. 15, 8065 (2024).
Haddad, N. M. et al. Plant species loss decreases arthropod diversity and shifts trophic structure. Ecol. Lett. 12, 1029–1039 (2009).
Li, Y. et al. Multitrophic arthropod diversity mediates tree diversity effects on primary productivity. Nat. Ecol. Evol. 7, 832–840 (2023).
Eisenhauer, N., Cesarz, S., Koller, R., Worm, K. & Reich, P. B. Global change belowground: impacts of elevated CO2, nitrogen, and summer drought on soil food webs and biodiversity. Glob. Chang. Biol. 18, 435–447 (2012).
Siemann, E. Experimental tests of effects of plant productivity and diversity on grassland arthropod diversity. Ecology 79, 2057–2070 (1998).
Scherber, C. et al. Bottom-up effects of plant diversity on multitrophic interactions in a biodiversity experiment. Nature 468, 553–556 (2010).
Chauvat, M., Titsch, D., Zaytsev, A. S. & Wolters, V. Changes in soil faunal assemblages during conversion from pure to mixed forest stands. Ecol. Manag. 262, 317–324 (2011).
Ledeganck, P., Nijs, I. & Beyens, L. Plant functional group diversity promotes soil protist diversity. Protist 154, 239–249 (2003).
Dietrich, P., Cesarz, S., Liu, T., Roscher, C. & Eisenhauer, N. Effects of plant species diversity on nematode community composition and diversity in a long-term biodiversity experiment. Oecologia 197, 297–311 (2021).
Cortois, R. et al. Possible mechanisms underlying abundance and diversity responses of nematode communities to plant diversity. Ecosphere 8, e01719 (2017).
Eisenhauer, N. et al. Plant diversity surpasses plant functional groups and plant productivity as driver of soil biota in the long term. PLoS ONE 6, e16055 (2011).
Mielke, L. et al. Nematode grazing increases the allocation of plant-derived carbon to soil bacteria and saprophytic fungi, and activates bacterial species of the rhizosphere. Pedobiologia 90, 150787 (2022).
Korboulewsky, N., Perez, G. & Chauvat, M. How tree diversity affects soil fauna diversity: a review. Soil Biol. Biochem. 94, 94–106 (2016).
De Deyn, G. B., Raaijmakers, C. E., Van Ruijven, J., Berendse, F. & Van Der Putten, W. H. Plant species identity and diversity effects on different trophic levels of nematodes in the soil food web. Oikos 106, 576–586 (2004).
Achury, R. et al. Plant diversity and functional identity alter ant occurrence and activity in experimental grasslands. Ecosphere 13, e4252 (2022).
Gastine, A., Scherer-Lorenzen, M. & Leadley, P. W. No consistent effects of plant diversity on root biomass, soil biota and soil abiotic conditions in temperate grassland communities. Appl. Soil Ecol. 24, 101–111 (2003).
Hasegawa, M. et al. The effects of mixed broad-leaved trees on the collembolan community in larch plantations of central Japan. Appl. Soil Ecol. 83, 125–132 (2014).
Salamon, J.-A., Schaefer, M., Alphei, J., Schmid, B. & Scheu, S. Effects of plant diversity on Collembola in an experimental grassland ecosystem. Oikos 106, 51–60 (2004).
Cesarz, S. et al. Tree species diversity versus tree species identity: Driving forces in structuring forest food webs as indicated by soil nematodes. Soil Biol. Biochem. 62, 36–45 (2013).
Viketoft, M. et al. Long-term effects of plant diversity and composition on soil nematode communities in model grasslands. Ecology 90, 90–99 (2009).
Perner, J. et al. Effects of plant diversity, plant productivity and habitat parameters on arthropod abundance in montane European grasslands. Ecography 28, 429–442 (2005).
Laossi, K.-R. et al. Effects of plant diversity on plant biomass production and soil macrofauna in Amazonian pastures. Pedobiologia 51, 397–407 (2008).
Eisenhauer, N. et al. Plant community impacts on the structure of earthworm communities depend on season and change with time. Soil Biol. Biochem. 41, 2430–2443 (2009).
Schwarz, B. et al. Non-significant tree diversity but significant identity effects on earthworm communities in three tree diversity experiments. Eur. J. Soil Biol. 67, 17–26 (2015).
Milcu, A., Partsch, S., Langel, R. & Scheu, S. The response of decomposers (earthworms, springtails and microorganisms) to variations in species and functional group diversity of plants. Oikos 112, 513–524 (2006).
Dümig, A., Smittenberg, R. & Kögel-Knabner, I. Concurrent evolution of organic and mineral components during initial soil development after retreat of the Damma glacier, Switzerland. Geoderma 163, 83–94 (2011).
Georgiou, K. et al. Global stocks and capacity of mineral-associated soil organic carbon. Nat. Commun. 13, 3797 (2022).
West, T. O. & Six, J. Considering the influence of sequestration duration and carbon saturation on estimates of soil carbon capacity. Clim. Change 80, 25–41 (2007).
Frouz, J. Effects of soil development time and litter quality on soil carbon sequestration: assessing soil carbon saturation with a field transplant experiment along a post-mining chronosequence. L. Degrad. Dev. 672, 664–672 (2017).
Qu, Q. et al. Belowground C sequestrations response to grazing exclusion in global grasslands: Dynamics and mechanisms. Agric. Ecosyst. Environ. 360, 108771 (2024).
Johnson, K., Scatena, F. N. & Pan, Y. Short- and long-term responses of total soil organic carbon to harvesting in a northern hardwood forest. Ecol. Manag. 259, 1262–1267 (2010).
Poeplau, C., Dechow, R., Begill, N. & Don, A. Towards an ecosystem capacity to stabilise organic carbon in soils. Glob. Chang. Biol. 30, e17453 (2024).
Begill, N., Don, A. & Poeplau, C. No detectable upper limit of mineral-associated organic carbon in temperate agricultural soils. Glob. Chang. Biol. 29, 4662–4669 (2023).
Cotrufo, M. F., Lavallee, J. M., Six, J. & Lugato, E. The robust concept of mineral-associated organic matter saturation: A letter to Begill et al., 2023. Glob. Chang. Biol. 29, 5986–5987 (2023).
Breure, T. S. et al. Revisiting the soil carbon saturation concept to inform a risk index in European agricultural soils. Nat. Commun. 16, 2538 (2025).
Hansen, P. M. et al. Distinct, direct and climate-mediated environmental controls on global particulate and mineral-associated organic carbon storage. Glob. Chang. Biol. 30, e17080 (2024).
Isbell, F. et al. Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature 526, 574–577 (2015).
Lugato, E., Lavallee, J. M., Haddix, M. L., Panagos, P. & Francesca Cotrufo, M. Different climate sensitivity of particulate and mineral-associated soil organic matter. Nat. Geosci. 14, 295–300 (2021).
Prater, I. et al. From fibrous plant residues to mineral-associated organic carbon–the fate of organic matter in Arctic permafrost soils. Biogeosciences 17, 3367–3383 (2020).
Mueller, C. W. et al. Large amounts of labile organic carbon in permafrost soils of northern Alaska. Glob. Chang. Biol. 21, 2804–2817 (2015).
Sayer, E. J. et al. Altered litter inputs modify carbon and nitrogen storage in soil organic matter in a lowland tropical forest. Biogeochemistry 156, 115–130 (2021).
Man, M. et al. Twenty years of litter manipulation reveals that above-ground litter quantity and quality controls soil organic matter molecular composition. Biogeochemistry 159, 393–411 (2022).
Pierson, D. et al. Mineral stabilization of soil carbon is suppressed by live roots, outweighing influences from litter quality or quantity. Biogeochemistry 154, 433–449 (2021).
Haddix, M. L., Paul, E. A. & Cotrufo, M. F. Dual, differential isotope labeling shows the preferential movement of labile plant constituents into mineral-bonded soil organic matter. Glob. Chang. Biol. 22, 2301–2312 (2016).
Wang, R. et al. A novel 13C pulse-labelling method to quantify the contribution of rhizodeposits to soil respiration in a grassland exposed to drought and nitrogen addition. N. Phytol. 230, 857–866 (2021).
Domeignoz-Horta, L. A. et al. Microbial diversity drives carbon use efficiency in a model soil. Nat. Commun. 11, 3684 (2020).
Eisenhauer, N. et al. Ecosystem consequences of invertebrate decline. Curr. Biol. 33, 4538–4547.e5 (2023).
Roy, J. et al. Ecotrons: powerful and versatile ecosystem analysers for ecology, agronomy and environmental science. Glob. Chang. Biol. 27, 1387–1407 (2021).
Schädler, M. et al. Investigating the consequences of climate change under different land-use regimes: a novel experimental infrastructure. Ecosphere 10, e02635 (2019).
Just, C. et al. A simple approach to isolate slow and fast cycling organic carbon fractions in Central European soils—importance of dispersion method. Front. Soil Sci. 1, e02635 (2021).
White, R. A. et al. The state of rhizospheric science in the era of multi-omics: A practical guide to omics technologies. Rhizosphere 3, 212–221 (2017).
Fraser, L. H. et al. Coordinated distributed experiments: an emerging tool for testing global hypotheses in ecology and environmental science. Front. Ecol. Environ. 11, 147–155 (2013).
Rousk, J. et al. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 4, 1340–1351 (2010).
Acknowledgements
We gratefully acknowledge the support of iDiv, which is funded by the German Research Foundation (DFG—FZT 118, 202548816), as well as the Jena Experiment (FOR 5000). G.A. acknowledges support by the Czech Academy of Sciences (LQ200962401 Soil fauna—the neglected driver of soil carbon formation and stability, to G.A.), N.E. acknowledges support by the DFG (Ei 862/29-1; Ei 862/31-1), and M.L. is funded by the Zwillenberg-Tietz Foundation (2023-03) and acknowledges support by the DFG (La 4685/1). K.E.M. was supported as a Mercator Fellow via DFG grant FOR 5000. We thank Gabriele Rada for tweaking (Fig. 1).
Author information
Authors and Affiliations
Contributions
Š.A. and G.A. conceived of the idea and designed the conceptual framework of the review. Š.A. and G.A. performed the literature review and wrote the first manuscript draft. K.E.M., M.L., and N.E. critically commented on the paper and added and/or revised content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Angst, Š., Angst, G., Mueller, K.E. et al. Un(der)explored links between plant diversity and particulate and mineral-associated organic matter in soil. Nat Commun 16, 5548 (2025). https://doi.org/10.1038/s41467-025-60712-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-60712-6