Abstract
The magnetic quantum phenomena triggered by electrons in carbon-based materials are challenging to decipher and exploit, thus sparking extensive research interest. Carbon quantum dots (CQDs), emerging candidates in nanomedicine, exhibit fascinating behaviors related to electron spin, relaxation, and migration. Herein, we report a magnetic edge state structure within nonmetallic CQDs that generates nitrogen hyperfine splitting at room temperature. Furthermore, a series of near-infrared (NIR) absorption bandgaps are produced based on spin–orbit coupling and dipole–dipole interactions, exhibiting potential in photothermal and thermoelectric catalysis. By modulating the surface ligands and solvent, relaxation rates are accelerated through spin averaging, which results in CQDs serving as desirable T1 contrast agents with the highest relaxivity for magnetic resonance imaging (MRI) and NIR-II cancer therapy agents. Combining these characteristics, we propose an MRI-guided approach to precision cancer therapy that offers a pathway for the rapid advancement of nanomedicine.
Similar content being viewed by others
Introduction
Spin averaging is a phenomenon in which electrons or atom relaxation behaviors are typically accelerated by regulating the intrinsic properties of materials or the external environment1,2. The relaxation rate often determines the quality of magnetic resonance imaging (MRI)3. There are currently no reports on improving the effectiveness of T1 contrast agents (CAs) or thermoelectric catalysis agents through spin averaging. In contrast, the challenging realization of hyperfine interaction (HFI) and zero-field splitting (ZFS) has attracted notable attention4,5. Carbon-based materials are hosts for spin and spin-valley qubits, which have weak spin-orbit coupling, leading to the challenging realization of HFI and ZFS at room temperature5,6,7,8. Although solid-state HFI and quantum coherence applications have been reported9, the instability of the structure in solution makes it difficult to achieve efficient spin averaging, HFI, and ZFS for biological applications10,11. Carbon quantum dots (CQDs), which are zero-dimensional and composed of hydrophobic carbon cores (in the insulating state) and hydrophilic shell surfaces, are considered to be time-reversal symmetric fermionic systems12,13,14,15. The possibility of preserving the HFI and ZFS exists when the time-reversal symmetry is broken, as the energy eigenstate that is at least doubly degenerate splits into different electronic states due to the influence of twist angles16,17,18, lattice distortions19,20,21 and surface ligands22,23,24. Currently, extensive research on CQD electron states has focused primarily on core states25,26,27, surface states26,28, and defect states29,30, with limited reports on magnetic edge states. Thus, manipulating the electron spin, relaxation, and migration behavior of magnetic edge states to achieve efficient spin averaging, HFI, and ZFS is an unexplored and challenging endeavor.
Precision cancer therapy should have high tumor targeting ability, potent tumor-killing efficacy, short treatment duration, and minimal side effects on the body31. Currently, nanomedicine provides ample opportunities for precision cancer therapy, with a focus on carbon-based nanomaterials that can target and treat tumors32,33. Nonmetallic CQDs, favored for their excellent biocompatibility and low cytotoxicity, have found widespread applications in the diagnosis and phototherapy of cancers34,35,36. The integration of therapeutic functionalities with MRI components is a promising goal for the clinical application of CQDs36. In the current landscape of reported MRI CAs, the toxicity of Gd-based CAs and the low relaxivity and stability of organic small molecule CAs like 2,2,6,6-tetramethylpiperidinyl-1-oxide (TEMPO) restrict their clinical utility. Therefore, it is imperative to develop high relaxivity carbon-based CAs, such as non-metallic CQDs, for commercial advancement37,38. However, achieving phototherapy in the second near-infrared (NIR-II) window or utilizing MRI with non-metallic CQDs is highly challenging37,38,39. Our research is dedicated to introducing the magnetic edge states of CQDs while achieving both MRI and NIR-II cancer therapy, which shows potential for precision cancer therapy.
In this work, we synthesized highly paramagnetic CQDs through a bottom-up approach by free radical polymerization. The magnetic edge states of the CQDs were stabilized by hydrogen bonds formed with surface ligands (e.g., –COOH, –C=O, –OH). These magnetic edge states, driven by spin–orbit coupling and dipole–dipole interactions, exhibit ZFS to generate distinct absorption bandgaps in the NIR region. Moreover, they exhibited rapid electron and hydrogen atom relaxation times in aqueous solutions, displaying excellent performance in MRI T1 imaging and NIR-II photothermal applications. The T1 relaxivity of CQDs reaches 270 mM–1 s–1, surpassing current Gd-based and non-metal organic CAs like TEMPO, thus offering higher commercial value. The temperature gradient during photothermal processes induces the migration of hot carriers. Due to the faster migration of hot electrons compared to hot holes, they have sufficient time to generate superoxide radicals, resulting in excellent thermoelectric catalytic performance. By utilizing protein corona formation, which is enriched in tumors with serum macromolecules, we achieved precision cancer therapy. This research holds promise for the construction of integrated diagnostic and therapeutic nanoplatforms.
Results
The structure and electron spin relaxation behavior of magnetic edge states
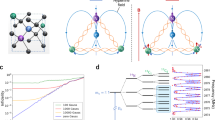
The Zeeman splitting of the magnetic edge state was obtained via electron paramagnetic resonance (EPR), whereby the spin levels were split by a magnetic field and transitions were induced by microwave absorption. A sharp derivative signature and a seven-line signal were generated, with the spin Hamiltonian defined as follows (Fig. 1a):
where the Zeeman term \({H}_{{{\rm{Z}}}}={\mu }_{{{\rm{B}}}}{B}_{{{\rm{g}}}}{S}_{i}\)(2) contains the effect of the magnetic field B on the ith spin Si via the Landé tensor g (with the Bohr magneton μB); \({H}_{{{\rm{Hy}}}}={\sum }_{{{\rm{i}}},{{\rm{n}}}}{S}_{{{\rm{i}}}}{A}_{{{\rm{in}}}}{I}_{{{\rm{n}}}}\) (3) defines the HFI between the electron spin Si and the spin of the nth nucleus In, mediated by the HFI of Ain; \({H}_{{{\rm{D}}}}={\sum }_{{{\rm{i}}}\ne {{\rm{j}}}}{S}_{{{\rm{i}}}}{D}_{{{\rm{ij}}}}{S}_{{{\rm{j}}}}\) (4) the dipolar coupling, with \({D}_{{{\rm{ij}}}}={g}_{{{\rm{i}}}}{g}_{{{\rm{j}}}}{\mu }_{0}{\mu }_{{{\rm{B}}}}^{2}/4\Pi {r}_{{{\rm{ij}}}}^{3}\) (5), which contains the vacuum permeability μ0 and the spin–spin distance rij; and \({H}_{{{\rm{Ex}}}}={\sum }_{{{\rm{i}}}\ne {{\rm{j}}}}{S}_{{{\rm{i}}}}{J}_{{{\rm{ij}}}}{S}_{{{\rm{j}}}}\) (6) represents the exchange term produced by the exchange coupling constant J. The g factors (e.g., 2.0045(1), 2.0015(6), 1.9986(14), 1.9986(18), 1.9957(14), 1.9928(6), and 1.9870(1)) indicate the spin density localized on the magnetic edge state. The intensities of the middle five splitting peaks follow a ratio of 1:2:3:2:1, which indicates the hyperfine splitting of two equivalent nitrogen nuclei. The overall EPR spectrum predominantly showed nitrogen hyperfine splitting with some hydrogen hyperfine splitting.
EPR spectra of CQDs (25 μg mL−1) in a DMSO and b aqueous solutions at 0 min, 10 min, 20 min and 30 min. Different time points reveal the variation in relaxation rates of magnetic edge state electrons. c Absorption spectra of CQDs in DMSO. d Absorption spectra of CQDs and ox-CQDs (25 μg mL−1) in aqueous solution. inset: IR thermal images of CQDs and ox-CQDs (1 mL, 250 μg mL−1) at different time points under 1064 nm laser irradiation (1 W cm−2). e Simulation structure of the CQDs and equatorial layers. a.u., arbitrary units. Source data are provided as a Source Data file.
The shape and linewidth of the EPR signal exclude magnetic impurities and are consistent with previous indications of delocalized spin states, providing conclusive evidence for the existence of edge spin states. The magnetic edge state electrons in dimethyl sulfoxide (DMSO) solution are influenced more notably by the surface ligands (e.g., –COOH, –C=O) than by the solvent effect. As a result, the electron spin state is influenced by the electron-withdrawing ability of the surface ligands, leading to the presence of multiple distinct g-factors. The decrease in the EPR signal due to the slow spin relaxation time of the electron led to a preference for electron spin–orbit coupling rather than lattice interactions. The oxidation of ligands and the disruption of surface hydrogen bonds reduced dipole–dipole interactions, resulting in a reduced population of spin electrons in the magnetic edge state.
The hyperfine structure disappeared from the EPR spectrum when water was used as the solvent, while the EPR intensity remained relatively unchanged (Fig. 1b). The broader linewidth of the Lorentzian peak in water suggested a shorter electron spin lifetime, indicating a faster interaction between the spin and lattice environment. When an external magnetic field was applied, the population in the higher-energy (<α|) state was always smaller than that in the lower-energy (<β|) state. Therefore, in water, the microwave energy absorbed by electrons in the <β| state quickly transfers to the <α| state to interact with the lattice environment and rapidly decays back to the <β| state (Fig. S1a). This process maintained the population of both energy levels and provided a stable EPR signal. Finally, the strong hydrogen bonding interaction between the solute and solvent, driven by the higher dielectric constant of water compared with DMSO solution, favored the cross-interaction between the singlet state (S = 0, 1[D···A+–]) and the triplet state (3[D···A+–]). As a result, the nuclear hyperfine nitrogen and hydrogen signals became indistinguishable. The observed single-line EPR signal arose from the exchange interactions (HEx) among spin-containing sites with strengths large enough to yield a random frequency modulation, averaging out the dipolar and Fermi contact interactions. Thus, electrons in aqueous solution exhibit faster T1 relaxation times, laying a solid foundation for subsequent MRI.
Then, we investigated the ZFS of the magnetic edge state through absorption spectroscopy. The decrease in the absorption intensity of the magnetic edge state electrons correlated with the decrease in the EPR signal, indicating that the absorption bandgaps at 720, 820, 920, and 1065 nm originated from the ZFS of the magnetic edge states (Fig. 1c and Fig. S1b-c). This coincided with the prediction that edge nitrogen atoms feature large flakes, i.e., equatorial layers, with low-lying S1 states (absorbing in the NIR region)40. Based on the number of g-factors (7), the unpaired electrons are likely situated between two nitrogen nuclei, splitting into four energy levels (±5/2, ±3/2, ±1/2, and 0). The CQDs exhibited a broad absorption band ranging from 900 to 1400 nm in aqueous solution (Fig. 1d), which was distinct from the four split peaks observed in DMSO solution. This indicates that the polarity and dielectric constant of the solvent can disrupt the time-reversal symmetry, leading to efficient ZFS.
The CQDs generated more photoexcited high-spin state electrons than did the oxidized CQDs (ox-CQDs), resulting in unpaired electrons in the magnetic edge state possessing higher reactivity (Fig. S2). The TEMPO radical does not deactivate the photoexcited state and therefore does not interact with the singlet state (1[D···A+–]). However, an approximately 67% reduction in the EPR signal of the TEMPO radical in the CQD aqueous solution was observed upon NIR-II light irradiation. This observed effect can be attributed to the radiationless quenching process facilitated by the rapid vibrational relaxation of the triplet state. This process occurs through an intersystem crossing from the excited triplet state (3[D···A+–]) to the ground-state vibrational manifold. The exchange interactions (J2) between 3[D···A+–], which undergoes rapid exchange (J1, S1), and the S = 1/2 TEMPO system (S2) play a crucial role in sustaining this process, as does the strength of the interaction (∝ −J2S1S2). Due to the faster vibrational relaxation of electrons exhibited by the interacting system (S1, S2) in an aqueous solution, most of the energy is released as heat. The CQDs exhibit a notable increase in photothermal temperature and photothermal stability, with a photothermal conversion efficiency (PCE) of 43.11%, which is higher than that of the ox-CQDs at 22.89%, which was attributed to the greater number of magnetic edge state excitons in the CQDs (Fig. S3). Based on the above results, we have inferred the structure of the magnetic edge states in CQDs and provided valuable insights for the development of magnetic edge states in other nanomaterials (Fig. 1e).
NIR-II photo-thermoelectric mechanism of magnetic edge states
We conducted transient absorption (TA) measurements to investigate the photophysical details of the CQDs in DMSO and water. The CQDs in DMSO exhibited a photobleaching (PB) signal at approximately 1050 nm, corresponding to the magnetic edge states, while the PB signal for the aqueous solution was observed in the range of 1000-1400 nm (Fig. 2a and b and Fig. S4). The decay process of the magnetic edge states at 1050 nm revealed that these features returned to the ground state in ~11.3 ps in DMSO, while the lifetime of the excited-state electrons was 2 ps in water, which was attributed to the rapid optical relaxation of electrons (Fig. 2c). These results elucidate the reasons for the excellent photothermal properties of the CQDs in water.
2D pseudoo-color maps of the TA spectra of CQDs in a DMSO with a pump wavelength of 700 nm (1 kHz, 100 fs) and b H2O with a pump wavelength of 550 nm (1 kHz, 100 fs). c Bleach signal kinetics of CQDs in DMSO collected at 1023 nm for λpump = 700 nm and H2O collected at 1150 nm for λpump = 550 nm. Dots are the experimental points, and the solid lines are the fittings. d EPR spectra of CQDs (25 μg mL−1) in DMSO solutions after 1064 nm laser irradiation for 0 min and 10 min. The signals of the six peaks in the spectra represent superoxide radicals, with DMPO acting as a radical scavenger in DMSO. e EPR spectra of CQDs (25 μg mL−1) in aqueous solution after 1064 nm irradiation for 0 min and 10 min. TEMPO was used as a photoexcited high spin state electron scavenger to measure photoexcited electron production based on the extent of signal reduction. f Schematic energy level diagram of magnetic edge-state electrons under NIR light excitation. μe, electron mobility. μh, hole mobility. Je, Current density. Source data are provided as a Source Data file.
The lifetimes of magnetic edge state electrons in different solvents also determine their distinct electronic behaviors. By investigating the EPR spectra under NIR light irradiation (Fig. 2d and e and Fig. S5), we found that the excited-state electrons at the magnetic edge reacted with surface-adsorbed oxygen to form superoxide radicals. Such rapid optical relaxation times generally make effective charge separation challenging (Fig. 2c). Thus, we attributed this to the difference in the migration speeds of the hot carriers. In the CQD system, temperature gradients can alter the Fermi-Dirac distribution, resulting in the equal generation of electrons and holes. When electrons diffuse from the hot end to the cold end, their migration is more pronounced, leading to more efficient charge separation. Consequently, this process afforded CQDs potent catalytic capabilities, laying the foundation for subsequent thermoelectric catalytic therapy (TCT). Additionally, the mechanistic schematic diagram illustrates that the excited-state electrons tend to return to the singlet state in DMSO, whereas they are more likely to interact with the lattice environment and undergo rapid vibrational relaxation in water (Fig. 2f and Fig. S10). This observation suggested that the excited-state electrons in water are more prone to sacrificing the singlet state to maintain the high-spin state. These results demonstrate that the manipulation of electron spin states and excited-state dynamics can be achieved by changing the solvent and temperature.
Structure and biosafety assessment of CQDs
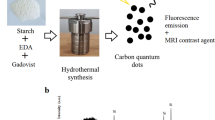
The CQDs were prepared from citric acid (1 g) in a mixture of formamide and formic acid by a solvothermal method based on free radical polymerization (Fig. 3a). The synthesis mass of CQDs is about 50 mg with synthesis yields of 5%. The formed CQDs possessed a highly conjugated core and abundant surface ligands, where spin electrons HFI with nitrogen nuclei were confined by intramolecular hydrogen bonding. The morphologies of the CQDs and ox-CQDs were characterized by transmission electron microscopy (TEM) and atomic force microscopy (AFM). As shown in Fig. 3b, the diameter of the CQDs was 2.43 ± 0.64 nm, which was similar to that of the ox-CQDs. Similar well-resolved lattice fringes with an interlayer spacing of 0.21 nm were observed in the high-resolution TEM (HRTEM) images, indicating graphite-like core structures in the CQDs. Dynamic light scattering analysis, as shown in Fig. S6, indicates that the size of the CQDs was approximately 5.3 nm in aqueous solution. Moreover, AFM images revealed that the CQDs and ox-CQDs had similar heights of ~2 nm (Fig. 3c and Fig. S7), corresponding to a spherical morphology.
a Schematic of the synthesis of CQDs. b TEM and HRTEM (top right corner inset) images of the CQDs. Bottom left corner inset: Particle size distribution chart, which contains histogram fitted to a Gaussian curve. Experiment independently repeated 3 times with similar results. c AFM images of the CQDs. Inset: height profile along the line. Experiment independently repeated 3 times with similar results. d FT-IR spectra of the CQDs and ox-CQDs. e, f Hydrogen NMR spectra of the CQDs and ox-CQDs in DMSO-d6. g Photographs of the CQD solid and solution. h PL imaging of the major mouse organs conducted at different time points before and after intravenous injection of 100 µL of CQD aqueous solution (100 µL, 1000 µg mL−1). (Ex, 589 nm laser; Em, 650 nm longpass [LP] optical filter). Scale bar: 5 mm. i Live/dead cell imaging assays of 4T1 cells treated with CQDs (500 μg mL−1) after 1064 nm laser irradiation (1 W cm−2) for 10 min. Viable cells (green) and dead cells (red) were stained with Calcein AM and propidium iodide (PI), respectively. Source data is provided as a Source Data file.
To investigate the rapid decrease in nitrogen hyperfine splitting structures in CQDs treated in DMSO solution, we performed Fourier transform infrared (FT-IR) spectroscopy, Hydrogen nuclear magnetic resonance (NMR), X-ray photoelectron spectroscopy (XPS) and Raman spectroscopy characterization. The FT-IR spectrum of the CQDs featured weaker vibration bands v (C=OOH) at 1700 cm−1 than the spectrum of the ox-CQDs, indicating that DMSO can oxidize hydroxyl or carbonyl groups to generate additional carboxyl groups (Fig. 3d). The in situ hydrogen NMR spectra of the CQDs and ox-CQDs in DMSO-d6 in different regions are shown in Fig. 3e. The proton signals at 2.5 and 3.3 ppm were attributed to DMSO and H2O, respectively, and the increase in H2O content was ascribed to the dehydration condensation of surface ligands. Moreover, the upfield shift in the active hydrogen at 7.70 ppm reflected the disruption of surface hydrogen bonding (Fig. 3f). The XPS spectra revealed the presence of C, N, and O (Fig. S8). The ox-CQDs exhibited a higher oxygen content (18.3%) than the CQDs (17.1%). The high-resolution C 1 s XPS spectrum revealed the presence of C=C (284.9 eV), C-N (286 eV), C-O (287.2 eV), C=N/C=O (288.4 eV) and O-C=O (290.4 eV) in the CQDs. The high-resolution O 1 s XPS spectrum revealed the presence of C=O (531 eV), C-O/O-H (531.8 eV) and O=C-O (533.6 eV). The C 1 s spectrum of the CQDs featured a higher C-O content (10.0%) than the ox-CQD (7.0%), and a lower C=N/C=O content (8.9%) than the ox-CQDs (13.8%). The O 1 s spectra showed that the CQDs exhibited a higher C-O/O-H content (8.3%) than the ox-CQD (5.8%), and a lower C=O content (7.1%) than the ox-CQDs (8.5%). These results indicated that the C-O/O-H on the surface of the CQDs were likely oxidized to C=O. We believe that the surface ligands of CQDs (e.g., -OH, -NH2) can form stable hydrogen bonds with -COOH and -C=O groups. Upon oxidation and dehydration by DMSO, these hydrogen bonds are disrupted, leading to the loss of magnetic edge-state electrons. In the Raman spectra of the CQDs and ox-CQDs powder, the peaks at 1360, 1480, 1588, and 1620 cm−1 were assigned to the D, A, G, and D’ bands and attributed to the disordered graphitic lattice, amorphous carbon in sp3 hybridization of the carbon polymorphs, and the breathing and stretching modes of the sp2-domains and edge defects, respectively (Fig. S9). After oxidation, the ratio of the intensities of the D and G bands increased from 1.14 to 1.45, indicating the reduced order of the graphite-like cores and the reorganization of surface ligands. The noticeably enhanced D’-band at 1645 cm−1 can be ascribed to the highly oxidized surface in the ox-CQDs, which is consistent with the XPS results. These results suggest that surface ligand fields can protect magnetic edge-state electrons. The hyperfine splitting structure is likely related to the interaction between unpaired electrons on the nitrogen nuclei and different intensities of ligand dipole moments. When the ligand field is disrupted, the high-reactivity unpaired electrons pair with other substances, resulting in a decreased intensity of their EPR signals.
Visually, the CQDs appeared as black solids and exhibited a purple‒red color when dissolved in water (Fig. 3g). Then, we simulated the morphology and optical behavior of the CQDs in mouse blood, and the CQDs (500 μg mL-1) were dissolved in mouse serum. It can be observed from the TEM images that the CQDs can form protein coronas with the macromolecules present in the serum, maintaining particle sizes of 30-80 nm (Fig. S10a). Therefore, we propose that the abundant surface ligands of CQDs not only play a protective role for magnetic edge-state electrons but also facilitate coupling with blood proteins (e.g., hemoglobin) through hydrogen bonding, resulting in increased particle size and enhanced tumor accumulation via endocytosis. The CQDs in the serum exhibited clear excitation-independent red emission centered at 620 nm with a photoluminescence quantum yield (PLQY) of 37.8% under 589 nm excitation (Fig. S10b–f). However, the CQD emission was centered at 640 nm with a PLQY of 6.2% in water, while it shifted to 670 nm with a PLQY of 56.0% in DMSO (Table S1). The PL quenching and rapid relaxation of electrons in water are closely related. Distinctions in the PL lifetimes of water, serum and DMSO are evident, indicating differences in the stabilities of the singlet state in water and DMSO due to their contrasting polarities or the distinct equilibrium between the singlet and triplet states in these environments (Fig. S10c). Notably, the nonradiative decay process was notably faster in water, consistent with the observed PL quenching effect. Exciton transfer between the CQDs and proteins appeared to suppress vibrational dissipation within the lattice, resulting in enhanced PL intensity and lifetime, similar to what was observed in DMSO solution. Due to the negative charge of CQDs, they exhibit excellent dispersion and luminescence in aqueous solution and serum-containing media. In high ionic environments, this electrostatic repulsion is canceled, leading to a minor reduction in luminescent intensity (Fig. S11).
The biosafety of the CQDs were evaluated in vitro and in vivo. The cytotoxicity of the CQDs at various concentrations was assessed in 4T1, B16, and ID8 cells using a CCK-8 assay. After incubating for 48 h, the CQDs did not reduce cell viability at concentrations up to 2000 μg mL−1, highlighting their low cytotoxicity (Fig. S12a). The cell fluorescence imaging capability of the CQDs was also assessed in 4T1 cells. Following incubation with the CQDs (500 µg mL−1) for approximately 2 h, gradually enhanced red emission was observed in the 4T1 cells, indicating successful cellular uptake of the CQDs (Fig. S12b). The cytotoxicity of the CQDs under NIR-II irradiation was assessed via a live/dead cell viability staining assay. Untreated 4T1 cells and non-irradiated cells incubated with CQDs at 1000 μg mL−1 showed green fluorescence, indicating cell viability. However, when subjected to irradiation with a 1064 nm laser at 1 W cm−2, the majority of 4T1 cells exhibited red fluorescence, indicating the efficient NIR-II cell-killing capability of the CQDs (Fig. 3i). These results establish a strong basis for further photothermal therapy (PTT) and TCT studies in mice.
We evaluated the biocompatibility and biodistribution of the CQDs in mice by in vivo red PL imaging (Fig. 3h and Fig. S13). Mice were intravenously injected with an aqueous solution of CQDs (100 µL, 1000 µg mL−1) via the tail vein. Over a period of 0 to 48 h postinjection, major organs, including the heart, lung, liver, spleen, and kidney, were excised for ex vivo red PL imaging to quantify the PL intensity. Before injection, the major organs did not exhibit red PL under 589 nm excitation. However, following the injection of the CQDs, red PL signals were detected in the liver and kidney. These signals were notably stronger than those in other organs at 1 h postinjection. This signal gradually decreased and completely disappeared after 48 h, indicating that the CQDs primarily accumulated in the liver and kidneys shortly after injection. To reduce toxicity and enhance tumor targeting, we speculate that the addition of 2-Iodylbenzoic acid can be utilized to modulate the spin defects or polarons and surface charges of CQDs. Our previous studies have demonstrated this strategy can enhance EPR intensity and reduce liver uptake of CQDs29,35. Then we investigated the pharmacokinetics of CQDs in living mice after intravenous injection, venous blood and urine samples at various time points were collected to measure the CQD contents. The blood half-life time of 109.65 min and the fluorescence of urine basically disappears within 48 hours indicated the safety of CQDs (Fig. S14). These findings suggest that the CQDs were rapidly excreted through the liver and kidney after intravenous administration. Besides, blood samples were collected from mice at 1, 15, and 60 days using 1000 μg/mL CQD aqueous solution before MRI and NIR-II cancer therapy. Biochemistry tests were conducted on these samples, with untreated CQD mice serving as controls. The results of these tests are presented in Fig. S15. It is noteworthy that all blood parameters (ALT, AST, ALP, BUN, CR, CK and LDH) showed no statistically significant differences compared to the control group. Taken together, these findings indicate that there was minimal to no significant inflammation or infection in mice treated with the synthesized CQDs as MRI CAs and photosensitizers. These results strongly support the safety and potential clinical applicability of CQDs.
Relaxometric properties of CQDs and MRI images
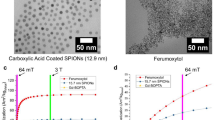
Variations in local T1 relaxation times play a crucial role in generating contrast in MR images. CAs can thus be used to distinguish between normal and abnormal tissues by adjusting the T1 value. The MRI signal intensity increased linearly with increasing concentration of the CQDs below 600 μg mL-1 (Fig. 4a and b). By employing linear fitting and plotting the relaxation rate (1/T1) against concentration (Fig. 4c), we determined the longitudinal relaxivity (r1) of the CQDs as 270 mM–1 s–1, which is potentially the highest among currently available and reported CAs. Comparing MRI in aqueous solutions, the aggregation effect of CQDs in serum slightly reduces the T1 relaxation capability of MRI, but it does not affect the imaging quality (Fig. S16). These results demonstrate the ultrafast spin relaxation characteristics of intrinsic electrons and atoms within the CQD system. The exceptional r1 value of the CQDs suggested their potential as a replacement for commercial Gd-based CAs (Fig. 4d), reducing the exposure to toxic Gd3+ compounds and offering a safer alternative for enhancing MRI images (Fig. S17). Moreover, we consider it as a notable non-metallic CA to replace TEMPO, owing to its low toxicity and nanostructured structure suitable for long-term biological applications41,42,43. Furthermore, it could potentially serve as a drug carrier for achieving stereotactic body radiation therapy.
aT1-weighted image of water and CQD aqueous solution (0, 100, 400, 500, and 600 μg mL−1) measured at 9.4 T. Scale bar: 2 mm. b Plot of T1-weighted MR signal intensity as a function of CQD concentration. (n = 3 for each concentration); the calculation was based on the mean intensity (mean ± SD) in different independent sample. cT1 relaxation time of the CQDs. d Relaxivities of CQDs, commercial Gd-based CAs, and other reported CAs45,46. Data represent 48 independent samples with no replicates. eT1-weighted MRI used to track the targeting ability of CQDs. The encircled area represents the tumor region. (Pre: pre-infection, Post: post-injection, IT: the intensity of the tumor, IN: the intensity of the near tissue). Source data are provided as a Source Data file.
To verify the potential of the CQDs as targeted CAs for tumor treatment, we evaluated the in vivo distribution and modal MRI capability in tumor-bearing mice models (Fig. 4e). Through observation, after 4 h of injection, MRI signal contrast (IT/IN) of the intensity at the tumor site (IT) and the near tissue (IN) in the CQDs treated mouse can reached a value higher than 5.5, which can last for a long observation time window in more than 12 h. The CQDs exhibited excellent tumor enrichment and maintained their intensity for up to 24 h, which can be attributed to the formation of large aggregates in the protein corona and the inherently high paramagnetic susceptibility of the CQDs. Furthermore, the CQDs were efficiently absorbed by organ tissues and rapidly metabolized in vitro, consistent with the in vivo metabolic results, further demonstrating their favorable biocompatibility. Overall, these findings provide promising prospects for tumor-targeted therapies.
In vivo NIR-II PTT and TCT
The feasibility of utilizing the CQDs for tumor PTT and TCT in the NIR-II region was further investigated. Twenty mice bearing 4T1 tumors were randomly divided into four groups. When the tumor volume reached ~70 mm3, the mice were intravenously injected with 100 μL of phosphate-buffered saline (PBS) or the CQDs and subsequently kept in darkness or subjected to 1064 nm laser irradiation at 1 W cm−2 for 10 min. The groups consisted of mice injected with PBS without laser irradiation (G1), mice injected with PBS with laser irradiation (G2), mice injected with the CQDs (100 μL, 1000 μg mL−1) without laser irradiation (G3), and mice injected with the CQDs followed by laser irradiation (G4).
The tumor-accumulating capability of the CQDs was confirmed by evaluating the red PL intensity from excised tumors at various time intervals after intravenous injection of a 1000 μg mL−1 CQD aqueous solution. Enhanced red PL signals from the tumor were detectable at 1 to 12 h. post-injection, disappearing after 24 h (Fig. S18). Therefore, 3 h after CQD injection was chosen as the time point for photothermal tumor ablation. Under 1064 nm laser irradiation at 1 W cm−2, thermographic images were collected using an IR thermal mapping apparatus to monitor temperature changes at the tumor sites (Fig. 5a). The temperature of the tumor region in G4 rapidly increased to 50 °C within 10 min of laser irradiation, while the temperature increase in G2 was much lower than in G2, and the change in temperature was negligible. Due to the concurrent presence of TCT, complete tumor eradication can be easily achieved at lower temperatures. Hematoxylin and eosin (H&E)-stained sections of the tumors revealed notable destruction and collapse of tumor cell nuclei following PTT and TCT (Fig. 5b). The mice in G4 had significantly improved survival rates compared with those in G1–G3. Moreover, the tumors in G4 completely disappeared after phototherapy, with no recurrence for more than 90 days (Fig. 5c–e and Fig. S19 to S22). The body weights of the mice were maintained during tumor treatment (Fig. S23). Afterward, all the mice were sacrificed to collect their internal organs, including the heart, liver, spleen, lung, and kidney. H&E staining was subsequently performed to evaluate any local or systemic inflammatory responses induced by CQD ingestion (Fig. 5f and Fig. S24). No discernible damage was observed in the organs of the mice treated with G4 after day 90, showcasing similar tissue morphology similar to that of healthy mice, which verified the excellent biocompatibility of the CQDs. These results demonstrated the excellent NIR-II photothermal and thermoelectric catalytic capacity of the CQDs under 1064 nm laser irradiation, positioning them as promising agents for clinical tumor PTT and TCT.
a IR thermal images of mice intravenously injected with 100 μL of PBS and CQD aqueous solution (1000 μg mL−1) at different time points under 1064 nm laser irradiation (1 W cm−2) at the tumor sites. b H&E staining of the tumor tissues before and after PTT and TCT. The experiment was independently repeated 3 times with similar results. c Growth curves of 4T1 tumors from mice after different treatments (n = 5 for each group); the calculation was based on the mean tumor size (mean ± SD) for each mouse. ****p < 0.0001, ns not significant, two-tailed paired t-test. d Photographs of G1-G3 on day 16 and G4 on day 90. e Survival rates of G1-G4 mice. P < 0.01: significant. Kaplan–Meier analysis, log-rank test. f H&E staining of major organs in G4 mice after 90 days compared with those in healthy mice. The experiment was independently repeated 3 times with similar results. (G1: intravenous injection of PBS, G2: intravenous injection of PBS + 1064 nm laser irradiation, G3: intravenous injection of the CQDs, G4: intravenous injection of the CQDs + 1064 nm laser irradiation). Source data is provided as a Source Data file.
Discussion
Herein, we synthesized highly paramagnetic CQDs using a bottom-up approach based on radical polymerization. The EPR and TA spectra revealed magnetic and optical properties, including the presence of a fast spin and optical relaxation time of unpaired electrons between two edge nitrogen nuclei at room temperature. By manipulating the electron relaxation and migration behavior of the magnetic edge states, the CQDs exhibited notable MRI T1 imaging ability and NIR-II photo-thermoelectric catalytic properties in aqueous environments. Furthermore, we discovered that coupling CQDs with protein macromolecules led to the formation of protein coronas, which enabled the passive enrichment of CQDs in tumor tissues. Finally, we developed a precise MRI-guided approach for NIR-II cancer phototherapy through a combination of these three characteristics. This advancement holds great promise for the progression of nanomedicine and spintronics.
In the maturing landscape of quantum technology, this study serves as a valuable reference for carbon-based materials striving to achieve efficient spin averaging, HFI, and ZFS. Manipulating the relaxation behavior of electrons or hydrogen atoms in nonmetallic CQDs is often challenging, impeding the realization of efficient magnetic applications like MRI37,38. Furthermore, their electron mobility lags behind that of most traditional metallic semiconductor materials, making it challenging to achieve thermoelectric or piezoelectric conversions44. Nevertheless, our findings demonstrated that by adjusting the intrinsic structure, ligands, and external environment of CQDs, it is feasible to achieve electron relaxation and migration properties superior to those of metal-based nanomaterials, thus presenting compelling evidence for prospective quantum and biological applications of carbon-based materials.
Methods
Materials
Citric acid, formamide, formic acid, ethanol, and DMSO were used. Water was deionized and purified. All reagents had at least analytical grade purity and were used as received without further purification. All animal experiments were approved by the University of Macau Animal Ethics Committee (protocol no. UMARE-015-2019). According to the guidelines, mice were sacrificed when the largest tumor size limit was 1.5 cm (at the largest dimension), 1500 mm3 (for a subcutaneous tumor model) or a weight loss of 20% (for an orthotopic tumor model). All mice were maintained under specific pathogen-free conditions in the University of Macau animal facility. This study was not sex/gender-specific. Although breast cancer is generally diagnosed among females, males can develop it.
Sample preparation
For the CQDs, 1 g of citric acid was placed in a Teflon autoclave with a mixture of formamide and formic acid and then subjected to a constant temperature of over 180 °C for a few hours. The resulting dark brown solution was combined with ethanol and centrifuged to eliminate any residual impurities. The resulting precipitate was redissolved in water and dialyzed against deionized water using 5000 Da dialysis bags for 24 h (5000 g mol−1 was selected as the minimum molecular weight for calculating the minimum relaxation rate). The solution in the dialysis bags was collected and freeze-dried to obtain the CQDs. From a 1 g citric acid reaction, only about 50 mg of CQDs can be obtained, hence our rough estimate of the synthesis yield at approximately 5%. Ox-CQDs: ox-CQDs were prepared by dispersing 100 mg of the CQDs in 10 mL of DMSO and stirring the mixture at room temperature for 30 min. The resulting dark brown solution was mixed with 30 mL of ethanol, stirred for 1 min, and centrifuged at 8000 × g min−1 for 10 min to remove any residual impurities. The precipitate was redissolved in ethanol, and the purification process was conducted twice. The last precipitate was freeze-dried to obtain the black product of the ox-CQDs. After DMSO oxidation and purification, 50 mg of CQDs can produce about 20 mg of ox-CQDs. The synthesis yield was calculated to be 20 mg / 50 mg × 100% = 40%.
Characterization
TEM images were captured using an FEI Tecnai-G2-F30 electron microscope with an acceleration voltage of 200 kV. AFM measurements were performed using a BRUKER ICON instrument. Raman spectra were acquired using a Horiba LABHRev-UV Raman spectrometer. Ultraviolet–visible absorption spectra were recorded on a JASCO V-770 spectrophotometer, and PL spectra were obtained using an Ocean Optics QE Pro spectrofluorometer. Femtosecond TA spectra of various samples were collected using an Ultrafast Systems HELIOS TA spectrometer. The laser source utilized was the Coherent Astrella-1K-F Ultrafast Ti: Sapphire Amplifier (800 nm, 1 kHz, <100 fs). The broadband probe pulses were generated by focusing a small portion of the fundamental 800 nm laser pulses into an Al2O3 plate. The 550 nm and 700 nm pump pulses were generated by a Light Conversion TOPAS-C optical parametric amplifier. Electron paramagnetic resonance (EPR) spectroscopy was conducted on a Magnettech ESR5000 (Bruker) instrument. The light source for these measurements was lasers, the NIR-I source was a 690 nm laser (1 W cm−2), and the NIR-II source was a 1064 nm laser (1 W cm−2). XPS analyses were conducted by an ESCALAB 250Xi spectrometer (Thermo Fisher Scientific, USA) with the monochromatic X-ray source (Al Kα hν = 1486.7 eV) in an ultrahigh-vacuum (UHV) chamber (base pressure, 5.0 × 10−10 mbar). Nuclear magnetic resonance (NMR) spectra were collected on a Bruker DSX400) spectrometer operating at 400 MHz (1H). Dynamic light scattering (DLS) spectra were analyzed on a Zetasizer Nano ZS analyzer (Malvern Instruments, Malvern, UK). 9.4 T magnetic resonance imaging (MRI) system (Bruker, USA) was used to evaluate the T1-weighted MRI intensity, and relaxation scaning mode was also conducted to assess the imaging capability. The relative intensity of MRI was calculated with the ROI method.
Dynamic light scattering (DLS) test
Eight hundred and fifty microliter of the sample solution was introduced into a DTS1070 folded capillary cell (Malvern, Worcestershire, U.K.), and the size was measured using a Zetasizer Nano ZS analyzer (Malvern Instruments, Malvern, UK) at 25.0 °C and a backscatter detector (173°)
Electron paramagnetic resonance (EPR) test
DMPO was used as a spin trap agent to measure the production of O2-• in DMSO. CQDs and ox-CQDs were dissolved in DMSO at a concentration of 25 μg mL−1, then 1% DMPO was added and mixed well. The mixture was sucked up by a capillary tube, sealed with clay and irradiated under NIR-II light (1 W cm-2). At different time intervals, the EPR spectroscopy was applied to detect the EPR signals. The EPR signal of DMPO alone at different time intervals was also measured under the same conditions as control. TEMPO was used as a spin-trap agent to measure the production of triplet-state electrons in aqueous solution. The specific procedures are similar. The signal of spin electrons was also measured by EPR spectroscopy. CQDs and ox-CQDs were dissolved in DMSO and aqueous solution at a concentration of 25 μg mL−1. The solution was sucked up by a capillary tube, sealed with clay and irradiated under NIR-II light (1 W cm-2). At different time intervals, the EPR spectroscopy was applied to detect the EPR signals.
Nuclear magnetic resonance (NMR) test
CQDs and ox-CQDs were dissolved in DMSO-d6 at the concentration of 1000 μg mL−1, the solution was encapsulated in NMR tubes. Then the solutions were sonicated for 5 min in NMR tubes before they were analyzed for NMR spectroscopy. After a time interval of 1 hour, repeat the above test.
Cell lines and culture
B16 murine melanoma cells, ID8 murine ovarian cancer, and 4T1 murine breast cancer cells were purchased from ATCC. Mouse breast cancer 4T1 and mouse melanoma cancer B16 cells were cultured in Roswell Park Memorial Institute 1640 medium (GIBCO), supplemented with 10% fetal bovine serum (GIBCO) and 100 U mL−1 penicillin and 100 µg mL−1 streptomycin (GIBCO). Mouse ovarian cancer ID8 cells were maintained in Dulbecco’s modified eagle medium (GIBCO), supplemented with 10% fetal bovine serum (GIBCO) and 100 U mL−1 penicillin and 100 µg mL−1 streptomycin (GIBCO). All cells were cultured in a humidified incubator at 37 °C with 5% CO2.
Cell viability assay (CCK-8 assay)
The CCK-8 assay was also conducted to evaluate the cytotoxicity of the CQDs and the ox-CQDs to 4T1, ID8, and B16 cells. First, ~5000 cells were seeded in 96-well plates and incubated for 12 h. Subsequently, the medium was replaced with 100 μl of fresh medium containing varying concentrations of the CQDs. After 48 h of incubation, the cells were treated with CCK-8 reagent for 3 h before the absorbance at 450 nm was measured using a Tecan microplate reader (Thermo Scientific).
Calcein AM and PI assay
To evaluate the impact of the CQDs on 4T1 cells, approximately 1 × 105 4T1 cells were cultured overnight in glass-bottom dishes. Subsequently, the 4T1 cells were subjected to different conditions: incubation without the CQDs, incubation with the CQDs (500 µg mL−1) for 1 h, irradiation with a 1064 nm laser for 10 min, and staining with calcein acetoxymethyl ester (calcein AM) at 37 °C for 30 min. After being washed thrice with PBS solution, the cells were observed using a fluorescence microscope (Olympus IX73 inverted microscope) with a 20× objective lens at 470 nm excitation and a 500 nm LP filter.
Cell imaging
4T1 cells were seeded in six-well plates and incubated for 24 h. Afterward, the cells were incubated with the CQDs (500 µg mL−1) for 0 min, 30 min, 60 min and 120 min. Following the removal of the culture medium, the cells were washed three times with PBS before PL imaging. Live cells were imaged using an Olympus IX73 inverted microscope equipped with a 40× objective lens at 587 nm excitation and a 610 nm LP filter.
In vivo pharmacokinetics
Five-week-old female BALB/c mice were used for pharmacokinetics studies with three mice for each experiment group. All mice were intravenously injected with CQDs at a dose of 20 mg kg−1. The blood was collected at needed time points using a capillary tube from the retro-orbital sinus. The concentration of CQDs in blood was estimated by the fluorescent intensity using an InGaAs array detector. The excitation laser was a 589 nm laser diode at a power density of ~ 100 mW cm−2, and emission was collected with 650-nm long-pass filter. The percentage of the CQD in blood was calculated as
where Ft is the fluorescent intensity of collected blood as measured with a 650-nm long-pass filter, Fcontrol is the fluorescent intensity of control blood, Finjected is the fluorescent intensity of the injected CQD (mixed with blood), Mt is the mass of the collected blood.
Biochemistry tests
During the collection of the data about in vivo biomechanical parameters, blood samples were also collected carefully from the central artery of each mouse’s ear using a 5-gauge needle. This procedure was repeated on days 0, 1, 15 and 60 days, with 3–5 ml of blood being collected each time. EDTA vacuum tubes were used for collection, and the blood was continuously agitated to prevent coagulation. The blood samples were then stored at 4 °C for further processing. Meanwhile, 1.5–2 ml of the serum was separated from the whole blood by centrifuging at 3000 rpm for 15 min and stored frozen at −20 °C for subsequent analysis. The blood samples were utilized for routine blood tests using Rayto Chemray 420, providing valuable insights into the mouse’ hematological status. While the serum samples were specifically earmarked using Mindray BC-2800vet for liver and kidney biochemistry function tests, enabling a comprehensive assessment of the mice’ physiological responses (n = 3 per group).
In vivo tumor imaging
In vivo tumor imaging of the CQDs was conducted using 6- to 8-week-old female BALB/c mice. A total of 100 μL of CQD aqueous solution (1000 µg mL−1) was intravenously injected into 4T1 tumor-bearing mice at various time points (0, 1, 3, 6, 12, 24, and 48 h), after which the mice were sacrificed to retrieve the tumors. MRI used a 9.4-T magnetic field micro-MR scanner (Bruker) to record T1-weighted MR images from the mice with the following parameters: repetition time (TR), 1000 ms; echo time (TE), 8.5 ms; flip angle, 180°; number of excitations, 4; matrix size, 256 × 256; field of view (FOV), 4 × 4 cm2; slice number, 13; and slice thickness, 1 mm. Similarly, CQD accumulation in the tumor region was assessed via MRI at various time points (0, 1, 4, 12, and 24 h) without harming the mice. A ChemDocTM MP Imaging System (Bio-Rad Laboratories, Inc.) was used to capture images under 589 nm excitation with a 650/50 nm emission filter. Throughout the experiments, none of the animals exhibited any indications of acute toxicological effects.
In vivo metabolism study
For in vivo organ metabolism imaging of the CQD aqueous solution (1000 µg mL−1), 6–8-week-old female BALB/c mice were intravenously injected (via the tail vein) at different time points (0, 1, 3, 6, 12, 24, and 48 h) before being sacrificed to obtain organ images. Similarly, the mice injected with CQDs were placed in metabolic cages, and urine samples were collected at the corresponding time points. Images were obtained using a ChemDocTM MP Imaging System (Bio-Rad Laboratories, Inc.) with 589 nm excitation light and a 650/50 nm emission filter. No animals exhibited any signs of acute toxicological responses during the experiments.
Photothermal effect measurements
Photothermal effect data were obtained using UNT-T323 digital thermometers with a K-type thermocouple. A volume of 1 mL of the CQD aqueous solution (500 µg mL−1) was introduced into a quartz cuvette, which was then irradiated with a 1064 nm laser at a power density of 1 W cm−2 for 10 min. Pure water was used as a negative control. The thermocouple probe was connected to the digital thermometer and inserted into the CQD aqueous solution perpendicular to the light path. The temperature of the CQD aqueous solution was recorded at 30 s intervals using a digital thermometer. The change in temperature of the CQD aqueous solution as a function of time was monitored under 1064 nm laser irradiation until room temperature was reached. According to the obtained data, the PCE was calculated to be ~43%.
In vivo tumor PTT and TCT
A tumor-bearing mouse model was established by subcutaneously injecting 4T1 cells (5 × 105 per mouse) into the upper dorsal region of female BALB/c mice. Once the tumor volume reached ~70 mm3, the mice were randomly divided into four groups and intravenously injected with 100 μL of PBS or CQDs (1000 µg mL−1) (n = 5 per group) with and without a 1064 nm laser (1 W cm−2) irradiation for 10 min. The tumor volume was recorded every two days and calculated using the formula V = ((tumor length) × (tumor width)2)/2. Mice were sacrificed on day 16 after the initial drug treatment.
Histopathological evaluation
For histological analysis, the organs (heart, liver, spleen, lung, and kidney) were fixed in 10% formalin and then embedded in paraffin. Slices of these organs from the mice were stained with Hematoxylin & Eosin (H&E) and Immunohistochemistry (IHC). The histological sections were imaged by an optical microscope.
Statistical analysis
The results in all the experiments were presented as mean ± standard deviation (s.d.) (n ≥ 3). Statistical calculation of experimental data was analyzed using the log-rank test and a two-tailed Student’s t-test after normality and equal variance tests. Article drawing was performed using Origin 2021 or GraphPad Prism 8.0.1 software.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information. Extra data are available from the corresponding authors upon request. Source data are provided with this paper.
References
Witzel, W. M., Carroll, M. S., Cywinski, L. & Das Sarma, S. Quantum decoherence of the central spin in a sparse system of dipolar coupled spins. Phys. Rev. B 86, 035452 (2012).
Serrate, D., Yoshida, Y., Moro-Lagares, M., Kubetzka, A. & Wiesendanger, R. Spin-sensitive shape asymmetry of adatoms on noncollinear magnetic substrates. Phys. Rev. B 93, 125424 (2016).
Weiskopf, N., Edwards, L. J., Helms, G., Mohammadi, S. & Kirilina, E. Quantitative magnetic resonance imaging of brain anatomy and in vivo histology. Nat. Rev. Phys. 3, 570–588 (2021).
Gao, X. et al. Nuclear spin polarization and control in hexagonal boron nitride. Nat. Mater. 21, 1024–1028 (2022).
Banszerus, L. et al. Spin-valley coupling in single-electron bilayer graphene quantum dots. Nat. Commun. 12, 5250 (2021).
Kurzmann, A. et al. Kondo effect and spin-orbit coupling in graphene quantum dots. Nat. Commun. 12, 6004 (2021).
Banszerus, L. et al. Particle-hole symmetry protects spin-valley blockade in graphene quantum dots. Nature 618, 1–6 (2023).
Konschuh, S., Gmitra, M., Kochan, D. & Fabian, J. Theory of spin-orbit coupling in bilayer graphene. Phys. Rev. B 85, 115423 (2012).
Slota, M. et al. Magnetic edge states and coherent manipulation of graphene nanoribbons. Nature 557, 691–695 (2018).
Bepete, G. et al. Surfactant-free single-layer graphene in water. Nat. Chem. 9, 347–352 (2017).
Zhang, W. H. et al. Graphene oxide membranes with stable porous structure for ultrafast water transport. Nat. Nanotechnol. 16, 337–343 (2021).
Ge, Z. H. et al. Giant orbital magnetic moments and paramagnetic shift in artificial relativistic atoms and molecules. Nat. Nanotechnol. 18, 250–256 (2023).
Gutiérrez, C., Brown, L., Kim, C. J., Park, J. & Pasupathy, A. N. Klein tunnelling and electron trapping in nanometer-scale graphene quantum dots. Nat. Phys. 12, 1069–1075 (2016).
Lee, J. et al. Imaging electrostatically confined Dirac fermions in graphene quantum dots. Nat. Phys. 12, 1032–1036 (2016).
Freitag, N. M. et al. Large tunable valley splitting in edge-free graphene quantum dots on boron nitride. Nat. Nanotechnol. 13, 392–397 (2018).
Lin, J. X. Z. et al. Zero-field superconducting diode effect in small-twist-angle trilayer graphene. Nat. Phys. 18, 1221–1227 (2022).
Kuiri, M. et al. Spontaneous time-reversal symmetry breaking in twisted double bilayer graphene. Nat. Commun. 13, 6468 (2022).
Bhowmik, S. et al. Broken-symmetry states at half-integer band fillings in twisted bilayer graphene. Nat. Phys. 18, 639–643 (2022).
Hsieh, K. et al. Spontaneous Time-reversal Symmetry Breaking At Individual Grain Boundaries In Graphene. Phys. Rev. Lett. 126, 206803 (2021).
Gutiérrez, C. et al. Imaging chiral symmetry breaking from Kekule bond order in graphene. Nat. Phys. 12, 950–958 (2016).
Han, W., Kawakami, R. K., Gmitra, M. & Fabian, J. Graphene spintronics. Nat. Nanotechnol. 9, 794–807 (2014).
de la Torre, B. et al. Noncovalent control of spin-state in metal-organic complex by positioning on N-doped graphene. Nat. Commun. 9, 2831 (2018).
Baltic, R. et al. Superlattice of single atom magnets on graphene. Nano Lett. 16, 7610–7615 (2016).
Seyler, K. L. et al. Ligand-field helical luminescence in a 2D ferromagnetic insulator. Nat. Phys. 14, 277–281 (2018).
Xia, C. L., Zhu, S. J., Feng, T. L., Yang, M. X. & Yang, B. Evolution and synthesis of carbon dots: from carbon dots to carbonized polymer dots. Adv. Sci. 6, 1901316 (2019).
Zhang, B. H. et al. Assignment of core and surface states in multicolor-emissive carbon dots. Small 19, 2204158 (2023).
Shamsipur, M., Barati, A., Taherpour, A. A. & Jamshidi, M. Resolving the multiple emission centers in carbon dots: from fluorophore molecular states to aromatic domain states and carbon-core states. J. Phys. Chem. Lett. 9, 4189–4198 (2018).
Ding, H., Yu, S. B., Wei, J. S. & Xiong, H. M. Full-color light-emitting carbon dots with a surface-state-controlled luminescence mechanism. Acs Nano 10, 484–491 (2016).
Zhang, T. S. et al. Constructing oxygen-related defects in carbon nanodots with Janus optical properties: noninvasive nir fluorescent imaging and effective photocatalytic therapy. Adv. Mater. 35, 2302705 (2023).
Shi, W. Y. et al. Time-dependent phosphorescence color of carbon dots in binary salt matrices through activations by structural confinement and defects for dynamic information encryption. Angew. Chem. Int Ed. 62, e202303063 (2023).
Li, Z., Zou, J. H. & Chen, X. Y. In response to precision medicine: current subcellular targeting strategies for cancer therapy. Adv. Mater. 35, 2209529 (2023).
Geng, B. J. et al. Near-infrared phosphorescent carbon dots for sonodynamic precision tumor therapy. Nat. Commun. 13, 5735 (2022).
Cheng, Q. S. et al. Photocatalytic carbon dots-triggered pyroptosis for whole cancer cell vaccines. Adv. Mater. 36, 2408685 (2024).
Zhang, T. S., Wu, J., Tang, Z. K. & Qu, S. N. Tuning the photothermal properties of carbon dots in the deep-red to near-infrared wavelength regions for tumor therapy. Mater. Chem. Front 7, 2359–2372 (2023).
Zhang, T. S. et al., Polaron engineering promotes NIR-II absorption of carbon quantum dots for bioimaging and cancer therapy. Sci. Adv. 10, eadn7896 (2024).
Dordevic, L., Arcudi, F., Cacioppo, M. & Prato, M. A multifunctional chemical toolbox to engineer carbon dots for biomedical and energy applications. Nat. Nanotechnol. 17, 112–130 (2022).
Wang, H. et al. Paramagnetic properties of metal-free boron-doped graphene quantum dots and their application for safe magnetic resonance imaging. Adv Mater 29, https://doi.org/10.1002/adma. 201605416. (2016).
Zhang, J. et al. Carbon dots as a new class of Diamagnetic Chemical Exchange Saturation Transfer (diaCEST) MRI contrast agents. Angew. Chem. Int Ed. 58, 9871–9875 (2019).
Wang, Q. C. et al. Combination of efficient red fluorescence and high photothermal conversion in the second near-infrared window from carbon dots through photoinduced sodium-doping approach. Adv. Funct. Mater. 34, 2402976 (2024).
Zdrazil, L. et al. Magnetic Polaron states in photoluminescent carbon dots enable hydrogen peroxide photoproduction. Small 19, 2206587 (2023).
Akakuru, O. U. et al. Metal-free Organo-theranostic nanosystem with high nitroxide stability and loading for image-guided targeted tumor therapy. Acs Nano 15, 3079–3097 (2021).
Huang, L. X. et al. Organic radical contrast agents based on polyacetylenes containing 2,2,6,6-Tetramethylpiperidine 1-Oxyl (TEMPO): Targeted Magnetic Resonance (MR)/Optical bimodal imaging of folate receptor expressing HeLa tumors in vitro and in vivo. Macromol. Biosci. 15, 788–798 (2015).
Lu, X. D. et al. Glucose functionalized carbon quantum dot containing organic radical for optical/MR dual-modality bioimaging. Mat. Sci. Eng. C. -Mater. 82, 190–196 (2018).
Liu, Y. H. et al. Advances in carbon dots: from the perspective of traditional quantum dots. Mater. Chem. Front. 4, 1586–1613 (2020).
Li, Y. Q. et al. Enhancing the magnetic relaxivity of MRI contrast agents via the localized superacid microenvironment of graphene quantum dots. Biomaterials 250, 120056 (2020).
Shang, L. et al. Synergistic effect of oxygen- and nitrogen-containing groups in graphene quantum dots: red emitted dual-mode magnetic resonance imaging contrast agents with high relaxivity. Acs Appl. Mater. Interfaces. 14, 39885–39895 (2022).
Acknowledgements
This work was supported by the Science and Technology Development Fund of Macau SAR (0128/2020/A3, 0131/2020/A3, 0007/2021/AKP, 006/2022/ALC, and 0139/2022/A3). Funding from the University of Macau (MYRG2020-00164-IAPME). The Research and Development Grant for Chair Professor Fund from the University of Macau (CPG2020-00026-IAPME, CPG2021-00034-IAPME, CPG2022-00013-IAPME, CPG2025-00034-IAPME, SRG2023-00025-IAPME). The Shenzhen-Hong Kong-Macao Science and Technology Innovation Project (Category C) SGDX20210823103803021. Guangdong-Hong Kong-Macao Joint Laboratory for Photonic-Thermal-Electrical Energy Materials and Devices (EF010/IAPME-TZK/2020/GDSTC).
Author information
Authors and Affiliations
Contributions
Z. T. planned and supervised the project. T.Z., Z.T., H.S., and C.D. designed the overall studies, participated in the analysis of the results, and wrote the whole paper. T.Z. prepared the materials, carried out the spectroscopic measurements, and drafted the manuscript. H.C. assisted with the magnetic resonance imaging of mice. B.W. assisted in the measurement and analysis of transient absorption spectra. Q. C., Q. W., and B. Z., assisted with fluorescence imaging and phototherapy in vitro and in vivo. All the authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Kai Bao, Hak Soo Choi, and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, T., Cheng, Q., Cheng, H. et al. Manipulation of magnetic edge states in carbon quantum dots for magnetic resonance imaging and NIR-II photo-thermoelectric therapy. Nat Commun 16, 5867 (2025). https://doi.org/10.1038/s41467-025-60951-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-60951-7