Abstract
The rapid increase in atmospheric CO2 levels due to industrialization underscores the urgent need for innovative carbon valorization strategies. Photocatalytic CO2 reduction presents a sustainable solution; however, conventional systems suffer from inefficient charge separation and limited product applicability. Herein, a green and scalable tandem strategy is developed by integrating S-scheme photocatalysis with palladium-catalyzed carbonylation. A rationally designed CeO2/Bi2S3 heterojunction leverages its hierarchical structure, broad visible-light absorption, oxygen-vacancy-mediated charge dynamics, and the S-scheme charge transfer mechanism to achieve highly efficient photocatalytic CO2-to-CO conversion (14.05 mmol g−1, 98% selectivity). The generated CO is directly utilized in a subsequent carbonylation reaction under mild conditions, yielding high-value amides with near-quantitative CO utilization. This integrated approach eliminates the risks of CO handling and enhances economic viability, providing a direct and effective route for converting CO2 into fine chemicals. By bridging photocatalysis with industrial catalysis, this work advances sustainable carbon recycling technologies and opens avenues for the development of efficient CO2 conversion systems.
Similar content being viewed by others
Introduction
The escalating atmospheric concentration of carbon dioxide (CO2), driven largely by industrialization and fossil fuel consumption, has raised critical environmental concerns, notably global warming and ocean acidification. As a major contributor to the greenhouse effect, CO2 is a key driver of climate change, underscoring the urgent need for efficient strategies to capture and convert it into valuable products. Traditional carbon capture and storage technologies primarily focus on CO2 sequestration in underground reservoirs; however, these approaches offer limited economic incentives. In contrast, carbon capture and utilization present a promising alternative by converting CO2 into value-added chemicals and fuels, thereby integrating environmental sustainability with economic attractiveness1,2,3,4,5.
Among various CO2 conversion technologies, photocatalytic CO2 reduction has attracted considerable attention due to its ability to harness abundant solar energy for sustainable carbon recycling6,7,8,9,10. Compared with thermocatalytic and electrocatalytic processes, which require high temperatures or external electricity, photocatalysis operates under mild conditions using only sunlight as the energy source11,12,13,14,15, thus positioning it as a promising approach for large-scale CO2 conversion. However, single photocatalysts often suffer from intrinsic limitations such as inefficient charge separation, insufficient redox potential, and low solar-to-chemical conversion efficiency. Conventional systems typically exhibit rapid electron-hole recombination, leading to energy losses and poor catalytic performance. Furthermore, single-component catalysts struggle to simultaneously achieve strong light absorption and the high redox potentials needed for effective photoreactions, resulting in low product yields and limited practical applications.
To address these challenges, heterojunction photocatalysts have emerged as an effective strategy, with S-scheme heterojunctions gaining particular attention for their ability to enhance charge separation while preserving strong redox capabilities16. Unlike traditional type-II heterojunctions, which facilitate charge transfer at the expense of redox potential, S-scheme heterojunctions promote directional charge migration while retaining the system’s strongest oxidative and reductive potentials, thereby facilitating photochemical reactions and significantly improving photocatalytic efficiency (Fig. 1). Nevertheless, the photoconversion of CO2 to C2+ products remains challenging due to the facile desorption of C1 species (e.g., carbon monoxide (CO)) and the sluggish kinetics of C-C coupling17,18,19,20. As a result, CO2 photoreduction predominantly yields CO or syngas (CO and hydrogen (H2)), which require further processing for practical applications2,21,22,23,24,25. CO is highly flammable, poisonous, and difficult to store and transport, posing substantial safety risks. While syngas serves as a valuable industrial feedstock, its relatively low economic value, limited utilization rates, and the complexities of gas separation hinder its widespread adoption. These limitations have driven the development of tandem catalytic strategies to further convert CO2-derived CO into high-value chemicals26,27,28,29,30,31. On the other hand, CO is a crucial feedstock in organic carbonylation reactions, many of which are industrially established. Among various catalytic carbonylation methods, palladium (Pd)-catalyzed carbonylation reactions stand out for their ability to synthesize valuable pharmaceutical intermediates and fine chemicals via simple one-pot transformations32,33,34,35,36. However, conventional Pd-catalyzed carbonylation typically requires harsh conditions, including elevated temperatures and pressures, limiting energy efficiency and scalability. Therefore, designing an innovative approach that integrates photocatalytic CO2 reduction with Pd-catalyzed carbonylation under mild conditions is highly desirable. Such a strategy enables a cascade catalytic process for the production of high-value chemicals, enhancing the economic viability of CO2 photoconversion and mitigating the risks associated with CO handling37.
Cerium oxide (CeO2) has attracted considerable interest in photocatalysis due to its characteristic electronic structure, where the reversible redox transition between Ce4+ and Ce3+ generates oxygen vacancies that act as charge carrier trapping sites, improving charge separation efficiency38,39,40,41,42. Meanwhile, bismuth sulfide (Bi2S3), a layered semiconductor with a direct bandgap of 1.29 eV, exhibits excellent visible-light absorption43,44,45,46,47. Given that its conduction band (CB) is more negative than that of CeO2, Bi2S3 functions as an effective reduction photocatalyst in the construction of an S-scheme heterojunction with CeO2, thereby achieving efficient carrier separation and strong redox capability35,48,49,50,51. Based on this rationale, this study integrates a tandem strategy that combines CO2 photoreduction and Pd-catalyzed carbonylation. In this system, CO2 is photoreduced by a CeO2/Bi2S3 S-scheme heterojunction, and the in situ generated CO is directly employed in Pd-catalyzed carbonylation of aryl iodides under mild conditions. This tandem system not only improves the utilization efficiency of photocatalytic CO2 reduction products, but also circumvents the hazards of CO handling, offering a safe, green, and scalable strategy for converting CO2 into high-value pharmaceutical intermediates (Fig. 1). By integrating S-scheme photocatalysis with tandem catalysis, this study provides an efficient route for direct CO2 valorization, optimizing charge carrier utilization and eliminating intermediate processing inefficiencies. The strategic design of this catalytic system ensures high selectivity, enhanced conversion efficiency, and reduced energy consumption. This work advances next-generation carbon utilization technologies and offers valuable insights for industrial applications, supporting the global transition toward a circular carbon economy.
Results and discussion
CO2 Photoreduction Performance and Reaction Mechanism over CeO2/Bi2S3 Heterojunctions
The CeO2/Bi2S3 heterojunctions are denoted as CBx, where “C” and “B” represent CeO2 and Bi2S3, respectively, and “x” indicates the molar ratio of Bi2S3 to CeO2. The photocatalytic CO2 reduction performance of CeO2, CBx, and Bi2S3 was assessed using an online closed gas circulation system (OLPCRS-2, Shanghai Boyi Scientific Instrument Co., Ltd.), equipped with a glass reaction cell and a gas chromatograph (GC-2030, Shimadzu Corp., Japan). Tris(2,2’-bipyridyl)ruthenium(II) chloride hexahydrate ([RuII(bpy)3]Cl2·6H2O) was employed as the molecular catalyst, while 1,3-dimethyl-2-phenyl-2,3-dihydro-1H-benzo[d]imidazole (BIH) was used as the hole scavenger. CO was identified as the major reduction product, accompanied by a small amount of H2. No other carbonaceous products, such as methane (CH4) or C1/C2 hydrocarbons, were detected, confirming the high selectivity of the CO2 photoreduction process. Both pure CeO2 and pristine Bi2S3 exhibit poor CO2 reduction activity due to rapid recombination of photogenerated electrons and holes (Fig. 2a, b). However, the formation of the CeO2/Bi2S3 heterojunction significantly enhances photocatalytic performance. The optimal sample (containing 1% Bi2S3, as identified in Supplementary Fig. 1) delivers CO and H2 yields of 14.05 and 0.38 mmol g–1, respectively, after 8 hours of irradiation, achieving an impressive CO selectivity of approximately 98%, outperforming many previously reported composite photocatalysts (Supplementary Fig. 2). To investigate the influence of light intensity, photocatalytic experiments were conducted under varying light fluxes (Supplementary Fig. 3). The CO2 reduction activity increases progressively with light intensity, showing accelerated enhancement up to 1.0 W cm–2, which is identified as the optimal condition and thus adopted for all subsequent experiments unless otherwise specified. Higher intensities were not explored due to the output limitations of the xenon lamp.
The production yields of a CO and b H2 over CeO2, CB1, and Bi2S3 during an 8-hour experiment conducted under UV-visible light irradiation. c Various control experiments over CB1 under different conditions: (I) without [RuII(bpy)3]Cl2·6H2O and BIH, (II) without [RuII(bpy)3]Cl2·6H2O, (III) without BIH, (IV) without the catalyst, and (V) under standard conditions. d Total ion chromatography and mass spectrum of the photocatalytic reduction products of 13CO2 on CB1. e Gibbs free energy diagram of H2 photoreduction over Bi and S sites. f Gibbs free energy diagram of CO2 photoreduction over a Bi2S3 (211) slab. The purple, yellow, blue, orange, and pink spheres represent Bi, S, C, O, and H atoms, respectively. The error bars (mean ± standard deviation) were obtained based on three independent photocatalytic experiments.
Comparative experiments demonstrate that the simultaneous presence of the molecular catalyst and hole scavenger substantially improves photocatalytic efficiency, ensuring an adequate supply of CO for downstream carbonylation reactions (Fig. 2c). The apparent quantum efficiency (AQE) of the CeO2/Bi2S3 composite for CO2 photoreduction was evaluated under monochromatic irradiation at 365, 380, 400, 420, 450, and 500 nm. The resulting AQE profile closely matches the absorption spectrum and reaches a maximum of 1.20% (Supplementary Fig. 4), confirming that light absorption predominantly drives photocatalytic activity. The recyclability of the CeO2/Bi2S3 nanohybrid was further validated through eight consecutive CO2 photoreduction cycles (totaling 64 h of operation), during which only a slight decline in CO and H2 production is observed (Supplementary Fig. 5), demonstrating excellent durability. To confirm the origin of the CO product, an isotopic tracer experiment using 13CO2 as the substitute source gas was conducted. The total ion chromatogram (Fig. 2d) displays peaks corresponding to O2/Ar, N2, and CO at retention times (RTs) of 1.422, 1.763, and 3.585 min, respectively. The CO peak predominantly exhibits a mass spectrum signal at m/z = 29 (13CO), accompanied by additional fragments at m/z = 13 and 16 (13C and O), as shown in the inset of Fig. 2d. These results unequivocally verify that the CO is derived from the CO2 reactant.
During CO2 photoreduction, H2 production via water (H2O) splitting competes with CO formation. To optimize CO selectivity, varying volumes of H2O were introduced into the system to evaluate their effects on gas production and CO selectivity. As illustrated in Supplementary Fig. 6, the addition of 50 μL of H2O results in high CO selectivity but low CO yield. Increasing the H2O volume to 200 μL improves CO production but also enhances H2 generation, reducing CO selectivity. An optimal performance is achieved at 100 μL of H2O, providing sufficient protons while suppressing excessive H2 formation. Density functional theory (DFT) calculations reveal that the H2 evolution barrier at both Bi and S sites in Bi2S3 poses a substantial energetic challenge (Fig. 2e), further contributing to the high CO selectivity observed.
In situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS), in combination with DFT simulations, was employed to elucidate the reaction mechanism of CO2 photoreduction. As shown in Supplementary Fig. 7, upon introducing CO2 into the system in the dark, characteristic signals of bidentate carbonate (b-CO32–, 1118 cm–1) and monodentate carbonate (m-CO32–, 1261 cm–1) appear, indicating CO2 chemisorption on the CeO2/Bi2S3 heterojunction. This observation is further corroborated by CO2 temperature-programmed desorption (CO2-TPD) results (Supplementary Fig. 8), which reveal stronger CO2 chemisorption on pure Bi2S3 and the CeO2/Bi2S3 heterojunction compared to pristine CeO2, highlighting the role of Bi2S3 as a favorable active site for CO2 adsorption and activation. Upon subsequent light irradiation, new adsorption bands corresponding to *COOH (1521 and 1602 cm–1), *COO (carboxyl, 852 and 1016 cm–1), and *C = O (carbonyl, 1698 cm–1) emerge, confirming the formation of key intermediates in the CO2-to-CO conversion pathway. Based on these findings, the proposed mechanism for CO2 photoreduction over the CeO2/Bi2S3 heterojunction is summarized as follows, with * denoting the active site22:
The Gibbs free energy diagram for CO2 photoreduction (Fig. 2f) identifies the formation of the *COOH intermediate as the rate-limiting step. Moreover, CO desorption from the catalyst surface occurs spontaneously, whereas further hydrogenation to CH4 requires overcoming a considerable energy barrier, thus explaining the system’s high selectivity for CO.
Hierarchical Architecture and Charge Separation Mechanism of CeO2/Bi2S3 Heterojunctions
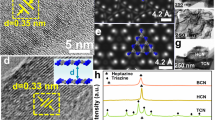
A comprehensive structural characterization and charge separation analysis were conducted to elucidate the superior photocatalytic performance of the CeO2/Bi2S3 heterojunctions in CO2 reduction. As depicted in Fig. 3a, CeO2 nanoparticles are synthesized using an oil bath method at a low temperature (75 °C), while Bi2S3 nanoflowers are obtained through a solvothermal process at 140 °C. Zeta potential measurements in ethanol solution reveal surface charges of − 19.61 mV for CeO2 and + 12.33 mV for Bi2S3 (Supplementary Fig. 9), enabling their spontaneous electrostatic self-assembly into a well-defined hierarchical heterostructure. The field emission scanning electron microscopy (FESEM) images (Fig. 3b, c) unveil the distinctive morphology of the composite: Bi2S3 forms uniform nanoflowers (~3 μm in diameter), offering a high-surface-area scaffold for CeO2 nanoparticles that are uniformly anchored on the Bi2S3 surface. This hierarchical nanoarchitecture maximizes interfacial contact and ensures intimate coupling between the two components, which is essential for efficient charge transfer and separation. In line with these structural features, nitrogen adsorption-desorption isotherms and pore size distribution curves (Supplementary Fig. 10) reveal that pure Bi2S3 exhibits the highest surface area and mesoporosity, whereas bare CeO2 shows the lowest. The CeO2/Bi2S3 heterojunction displays intermediate values, combining the porous characteristics of Bi2S3 with the structural robustness of CeO2. Consistently, CO2 adsorption isotherms (Supplementary Fig. 11) demonstrate that pristine Bi2S3 achieves the highest CO2 uptake, followed by the CeO2/Bi2S3 heterojunction and pure CeO2, in accordance with their respective surface areas. Energy-dispersive X-ray (EDX) analysis (inset in Fig. 3d) confirms the presence of Ce, O, Bi, and S elements, while elemental mappings (Fig. 3f) highlight the homogeneous distribution of CeO2 across the Bi2S3 nanoflowers. The high-resolution transmission electron microscopy (HRTEM) image of the CeO2/Bi2S3 heterojunction provides clear evidence of lattice fringes corresponding to CeO2 (111) and (200) planes (0.31 and 0.27 nm), as well as Bi2S3 (221) and (230) planes (0.28 and 0.31 nm) (Fig. 3e). The coherent lattice structure at the heterojunction interface corroborates the successful formation of CeO2/Bi2S3 heterostructures, providing a robust platform for effective charge carrier separation. The X-ray diffraction (XRD) patterns (Supplementary Fig. 12) verify the crystallinity of CeO2 (PDF#34-0394), while the characteristic peaks of Bi2S3 remain undetectable due to its low content in the hybrids. Moreover, the post-reaction XRD pattern (Supplementary Fig. 13) remains nearly identical to that of the fresh sample, indicating the excellent photostability of the CeO2/Bi2S3 heterojunction.
a Schematic diagram for the preparation of the CeO2/Bi2S3 heterojunctions (HMTA: hexamethylenetetramine, PVP: polyvinylpyrrolidone, EG: ethylene glycol). FESEM images of b pure CeO2 and c the CeO2/Bi2S3 heterostructure. d TEM image, EDX spectrum (inset) and e HRTEM image of the CeO2/Bi2S3 nanohybrid. f EDX elemental mappings of Ce, O, Bi and S elements in the CeO2/Bi2S3 composite. The high-resolution XPS data of g Ce 3d, h O 1s, i Bi 4f and S 2p of CeO2, Bi2S3 and the CeO2/Bi2S3 heterojunction.
UV-vis diffuse reflectance spectroscopy (DRS) was employed to explore the optoelectronic properties. The absorption spectrum of the heterostructure (CB1) demonstrates a significant enhancement in the visible light region relative to pure CeO2, primarily due to the superior light-harvesting capability of Bi2S3 (Supplementary Fig. 14). The estimated bandgaps of CeO2 and Bi2S3 are 3.22 and 1.29 eV, respectively (Supplementary Fig. 15), indicating the formation of a well-matched heterojunction. This hierarchical heterostructure effectively enhances light absorption and interfacial carrier transfer while suppressing the recombination of photogenerated carriers with strong redox potentials, ultimately improving photocatalytic efficiency for CO2 reduction. The synergistic interplay between CeO2 and Bi2S3, enabled by their intimate nanoscale contact and structural complementarity, holds promise for solar-driven catalytic applications.
X-ray photoelectron spectroscopy (XPS) analysis was performed to examine the surface chemical states and elemental composition of the CeO2/Bi2S3 heterojunction. As shown in Fig. 3g, the high-resolution Ce 3d spectra exhibit two distinct peaks located at 885.43 and 901.42 eV, corresponding to Ce 3d5/2 and 3d3/2 in CeO2, respectively. Deconvolution of the Ce 3d spectra further reveals multiple fitted peaks associated with different oxidation states of cerium, indicating the coexistence of Ce3+ and Ce4+ species (Supplementary Table 1). The presence of Ce3+ suggests a considerable amount of oxygen vacancies in the CeO2 lattice, which benefits charge carrier transport and surface reactivity. The O 1s spectra (Fig. 3h) consist of three distinct peaks associated with lattice oxygen (OL), surface hydroxyl oxygen (OOH), and adsorbed oxygen species (OH2O). The high proportion of surface OOH implies abundant oxygen vacancies and active sites that can facilitate interfacial charge interactions and photocatalytic reactions. In the Bi 4f core level (Fig. 3i), the peaks at 157.94 and 163.25 eV are assigned to Bi 4f7/2 and 4f5/2, respectively, confirming the exclusive presence of Bi3+ in Bi2S3. Meanwhile, the S 2p region displays two characteristic peaks at 160.72 and 161.90 eV, corresponding to S 2p3/2 and 2p1/2 of S2–, verifying the stoichiometric integrity of Bi2S3.
To gain deeper insights into the interfacial charge transfer mechanism within the CeO2/Bi2S3 heterojunction, comparative XPS analyses were performed on pure CeO2, bare Bi2S3, and CB1 under both dark and illuminated conditions. Notably, the Ce 3d and O 1s spectra of CB1 exhibit shifts to lower binding energies compared to those of pure CeO2 in the dark (Fig. 3g), suggesting increased electron density on CeO2 and indicating its role as the electron acceptor upon heterojunction formation. In contrast, the Bi 4f and S 2p spectra of CB1 (Fig. 3i) show positive shifts relative to pure Bi2S3, signaling decreased electron density around Bi and S atoms. These opposing shifts between CeO2 and Bi2S3 confirm the establishment of an interfacial electric field (IEF) directed from Bi2S3 to CeO2, which facilitates directional migration and spatial separation of charge carriers at the interface. Under light irradiation (CB1-Light), the binding energies of Ce 3d and O 1s shift positively, whereas those of Bi 4f and S 2p shift negatively, compared to the dark state (CB1-Dark). Such complementary shifts in binding energy indicate that photoexcited electrons transfer from CeO2 to Bi2S3, while photoholes accumulate in CeO2, consistent with the charge separation behavior driven by the IEF at the heterojunction. This charge transfer mechanism further validates the effective separation of photogenerated charge carriers, thereby enhancing the photocatalytic performance of the CeO2/Bi2S3 heterostructure. Moreover, a comparison of the XPS data between fresh and post-reaction CB1 samples reveals negligible changes in the Ce 3d, O 1s, Bi 4f, and S 2p peaks (Supplementary Fig. 16), confirming the chemical stability of the heterojunction during photocatalytic processes.
The work function (Φ) is a crucial parameter for determining the direction of electron transfer in semiconductors. Electrostatic potential calculations reveal that the Φ values of CeO2 (111) and Bi2S3 (211) are 6.10 and 4.75 eV, respectively (Fig. 4a, b), indicating a higher Fermi level (EF) in Bi2S3 compared to CeO2. This difference drives electron migration from Bi2S3 to CeO2 until their Fermi levels equilibrate at the interface. This observation is consistent with the XPS measurements and further underscores the role of the heterojunction in facilitating charge carrier separation. To further elucidate the photoinduced charge transfer mechanism in the CeO2/Bi2S3 heterojunction, the energy band structures of CeO2 and Bi2S3 were systematically examined. Ultraviolet photoelectron spectroscopy (UPS) measurements (Supplementary Fig. 17), in conjunction with bandgap estimations from DRS (Supplementary Fig. 15), reveal that the valence band maxima (VBM) of CeO2 and Bi2S3 are positioned at 2.59 and 0.42 V, respectively, while their conduction band minima (CBM) are calculated to be − 0.63 and − 0.87 V versus the standard hydrogen electrode (SHE, Supplementary Fig. 18). As previously discussed, the EF of CeO2 is situated at a lower energy than that of Bi2S3, facilitating electron migration from Bi2S3 to CeO2. As a result of charge redistribution, both an IEF and band bending develop at the interface upon heterojunction formation. Upon light irradiation, electrons in the VBs of both CeO2 and Bi2S3 are excited to their respective CBs. The coexistence of band bending, the IEF oriented from Bi2S3 to CeO2, and Coulombic interactions among photogenerated charge carriers collectively dictate the charge transfer dynamics. Notably, excited electrons in the CB of CeO2 transfer to the VB of Bi2S3, where they subsequently recombine with holes. In contrast, the photogenerated electrons in the CB of Bi2S3 and holes in the VB of CeO2, which possess the strongest redox capabilities in the heterojunction, remain spatially separated and actively participate in subsequent photocatalytic reactions. This directional charge transfer behavior confirms the formation of an S-scheme heterojunction between CeO2 and Bi2S3, as illustrated in Fig. 4c.
Calculated electrostatic potentials of a CeO2 (111) and b Bi2S3 (211) slabs. The orange, red, purple, and yellow spheres represent Ce, O, Bi, and S atoms, respectively. c Formation of CeO2/Bi2S3 S-scheme heterojunction and the proposed charge transfer and separation mechanism. CO2 RR and H2O OR denote the CO2 reduction reaction and H2O oxidation reaction, respectively. d EPR spectra of DMPO-•O2– and DMPO-•OH species measured in an aqueous suspension containing DMPO as spin trap and CeO2, Bi2S3 or CB1 as photocatalyst. e The TRPL spectra of CeO2, CB1 and Bi2S3 monitored at an emission wavelength of 465 nm under different conditions.
In situ irradiation XPS provides compelling evidence for this S-scheme charge transfer pathway in the CeO2/Bi2S3 heterojunction (as discussed above), resulting in enhanced charge separation efficiency, as further corroborated by photochemical analyses, electron paramagnetic resonance (EPR) spectroscopy, and time-resolved photoluminescence (TRPL) spectroscopy. As shown in Supplementary Fig. 19, CB1 consistently exhibits the highest photocurrent density in long-term photocurrent tests compared to pristine CeO2 and Bi2S3, indicating superior charge separation in the CeO2/Bi2S3 composite. Electrochemical impedance spectroscopy (EIS) results are in correspondence with the transient photocurrent data, as evidenced by the smaller semicircular radius in the Nyquist plot of CB1 relative to pure CeO2 and Bi2S3 (Supplementary Fig. 20), implying reduced charge-transfer resistance. The generation of reactive oxygen species (ROS) and charge separation behavior were further investigated by EPR spectroscopy using 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as a spin-trapping agent. As depicted in Fig. 4d, CB1 exhibits stronger EPR signals for both •\({{\rm{O}}}_{{2}^{-}} \) and •OH radicals compared to pristine Bi2S3 or CeO2, indicating enhanced ROS generation. This enhancement arises from the efficient accumulation of high-energy photoelectrons in the Bi2S3 CB and photoholes in the CeO2 VB, reinforcing the S-scheme mechanism. The pronounced EPR signal further supports prolonged carrier lifetime and effective spatial charge separation, both essential for improving photocatalytic performance23,52. TRPL decay profiles reveal that the average lifetime of the CB1 hybrid at 465 nm in an Ar atmosphere is shorter than those of pure CeO2 and Bi2S3 (Fig. 4e). Given that the fluorescence signal originates from both CeO2 and Bi2S3, this reduction suggests efficient bidirectional carrier migration within the heterojunction, which effectively suppresses charge recombination in CeO2 and Bi2S3 while facilitating carrier separation with strong redox potentials. Furthermore, in a CO2 atmosphere, the lifetime of CB1 is further reduced compared to CB1-Ar, attributed to the rapid consumption of photoexcited electrons in CO2 surface photoreduction. Notably, this effect becomes more pronounced upon the introduction of molecular catalysts and hole scavengers, which accelerate charge consumption. This observation underscores the pivotal role of these additives in enhancing photocatalytic efficiency through promoting charge separation and utilization. Together, these findings unambiguously confirm that the CeO2/Bi2S3 heterojunction follows an S-scheme charge transfer pathway, enabling efficient charge separation, suppressed recombination, and enhanced photocatalytic CO2 reduction activity.
Femtosecond transient absorption spectroscopy (fs-TAS) was employed to clarify the charge transfer dynamics within the CeO2/Bi2S3 S-scheme heterojunctions. Upon excitation at 340 nm, both pristine CeO2 and the CeO2/Bi2S3 composite (CB1) exhibit pronounced negative absorption features centered at approximately 450 nm, corresponding to the ground state bleaching (GSB) of CeO2. Concurrently, a significant positive absorption signal is observed at around 620 nm, attributed to the excited state absorption (ESA) of CeO2. In contrast, pure Bi2S3 shows a distinct negative peak at ~ 530 nm, indicative of its GSB (Fig. 5a–f). To further clarify the contributions of specific charge carriers, trapping agents were introduced (Supplementary Fig. 21). The addition of an electron-trapping agent (AgNO3) significantly attenuates both the CeO2 GSB at 450 nm and its associated ESA at 620 nm, confirming that these signals originate from photoelectrons in its CB. Similarly, the introduction of a hole-trapping agent (lactic acid, LA) reduces the 530 nm GSB feature of Bi2S3, thereby verifying its association with photogenerated holes.
Pseudocolor plots and transient absorption spectra recorded at the indicated delay times upon 340 nm excitation for a, d pure CeO2 in Ar, b, e CB1 in Ar, and c, f pristine Bi2S3 in Ar. Corresponding kinetic decay curves at 450 nm for pure CeO2 and CB1 in Ar within g 50 ps and h 7000 ps. i Corresponding kinetic decay curves at 530 nm of pure Bi2S3 and CB1 in Ar within 50 ps. Decay pathways of photogenerated charge carriers in j pure CeO2 and k the CeO2/Bi2S3 heterojunction.
For pristine CeO2, EPR measurement reveals the presence of oxygen vacancies (Supplementary Fig. 22), consistent with the XPS analysis. Under these conditions, the relaxation of photogenerated electrons in the CB, as reflected in the decay of the GSB signal, primarily proceeds via two processes: trapping by oxygen-vacancy-induced defect states (Process I) and recombination with photoholes in the VB (Process II). However, when CeO2 is coupled with Bi2S3, an additional relaxation channel emerges. Specifically, photoelectrons in the CeO2 CB transfer to Bi2S3 (Process III), where they recombine with the photoholes. This photoelectron transfer process is corroborated by ultrafast kinetic measurements extracted from the initial 50 ps window at 450 nm. Notably, CB1 displays a markedly faster decay than pristine CeO2 (Supplementary Table 2), providing compelling evidence for the characteristic photoelectron transfer dynamics of an S-scheme heterojunction (Fig. 5g). When extending the observation window to 7000 ps, both CeO2 and CB1 demonstrate a rebound in their transient kinetic traces. This interesting phenomenon arises from the backflow of photoelectrons that are initially trapped in defect states and subsequently re-enter the CB, resulting in the appearance of an ESA signal at 620 nm (Fig. 5h)53. Importantly, CB1 exhibits a nearly quenched ESA signal in this wavelength range, suggesting that shallow-trapped photoelectrons return to the CeO2 CB and then rapidly transfer to Bi2S3. This observation further substantiates the S-scheme charge transfer mechanism. Clearly, defect states in CeO2 play a pivotal role. Initially, these defects act as traps for photogenerated carriers; however, they subsequently release the carriers back into the CB, substantially extends carrier lifetimes. This process enhances charge transfer and separation efficiency between CeO2 and Bi2S3, which is critical for improved photocatalytic performance. On the other hand, complementary kinetic analysis at 530 nm reveals that the lifetime of photogenerated holes in the CB1 composite is significantly shorter than that in pristine Bi2S3 (Fig. 5i). This reduction is ascribable to partial neutralization of Bi2S3 holes by electrons transferred from CeO2, further validating the establishment of an S-scheme heterojunction between CeO2 and Bi2S3.
In conclusion, the comprehensive fs-TAS study, complemented by detailed kinetic and charge-trapping analyses, unequivocally demonstrates that the CeO2/Bi2S3 heterojunction operates via an S-scheme charge transfer mechanism. This configuration not only promotes efficient spatial separation of photoexcited carriers but also significantly reduces undesirable recombination pathways by facilitating rapid photoelectron transfer from CeO2 to Bi2S3. Moreover, the dynamic role of defect states in CeO2—initially trapping and subsequently releasing photogenerated carriers—effectively prolongs carrier lifetimes, further enhancing charge separation efficiency. These synergistic effects collectively contribute to the improved photocatalytic performance of the heterojunction, offering valuable insights for the design of next-generation photocatalysts with superior activity.
Design Concept and Photocatalytic Performance of Tandem Carbonylation for the Synthesis of High-Value Chemicals
Despite significant advancements in photocatalytic CO2 reduction, most studies have primarily focused on converting CO2 into CO, while the efficient downstream utilization of CO remains a key challenge. The designed CeO2/Bi2S3 S-scheme heterojunctions exhibit both high selectivity and efficiency for CO production; however, further transformation of CO—a low-value intermediate—into high-value fine chemicals via subsequent reactions is essential for achieving practical and efficient CO2 conversion. To address this challenge, a tandem catalytic system was developed, as illustrated in Fig. 6a, which seamlessly integrates CeO2/Bi2S3-based photocatalytic CO2 reduction with a Pd-catalyzed carbonylation reaction. This approach enables the direct synthesis of valuable organic products by leveraging CO as a reactive intermediate. A crucial factor in the success of this tandem system lies in the effective integration of two catalytic modules with distinct mechanisms. To this end, a two-chamber online setup was constructed (Supplementary Fig. 23), ensuring both operational connectivity and functional independence while maintaining excellent airtightness. In this configuration, the left chamber facilitates the photocatalytic reduction of CO2, enabling the continuous and sustainable CO generation. The produced CO then diffuses into the right chamber, where it undergoes aminocarbonylation reactions to yield amide products.
a Schematic of the tandem reaction. b Aminocarbonylation of three different substrates. c Product variation in three aminocarbonylation reactions over time. d CO conversion at 12 and 24 h. e Comparative experiments under different conditions. f GC-MS analysis of different mass-to-charge ratios (m/z) when using 13CO2 as the CO source in the tandem reaction for p-DET synthesis. The inset shows the standard mass spectrum of p-DET from the NIST library. The error bars (mean ± standard deviation) were obtained based on three independent photocatalytic experiments.
The aminocarbonylation of aryl iodides was carried out in 1,4-dioxane using triethylamine (Et3N) as the organic base and tetrakis(triphenylphosphine)palladium-(0) (Pd(pph3)4) as the catalyst. To examine the influence of molecular structure on reaction performance, three isomeric tolyl iodides were selected. Their aminocarbonylation reactions were initiated solely by xenon-lamp irradiation, which enabled both CO generation and Pd-catalyzed carbonylation (Fig. 6b), thus demonstrating the excellent compatibility of reaction conditions for the two processes. Reaction products were identified and quantified using gas chromatography-mass spectrometry (GC-MS), with standard calibration curves provided in Supplementary Fig. 24. The time-dependent evolution of diethyltoluamides (DETs) yield was systematically monitored over 24 h. As shown in Fig. 6c, prolonged reaction time results in a steady increase in product yield, with N,N-diethyl-p-toluamide achieving the highest yield of 0.81 mg L–1 at 24 h. Furthermore, CO utilization efficiency was assessed as a key performance indicator. Among the three selected substrates studied, the reaction yielding N,N-diethyl-p-toluamide exhibits the highest CO utilization rate at 12 h. Notably, after 24 h, CO utilization in all reactions approaches 100% (Fig. 6d), indicating highly efficient incorporation of CO into the target products. Based on these promising results, the effect of Pd(pph3)4 loading on the aminocarbonylation efficiency was further investigated. As depicted in Supplementary Fig. 25, increasing catalyst amounts improve the product yield; however, the enhancement rate diminishes at higher loadings, suggesting a non-linear relationship between Pd concentration and catalytic activity. Considering both reaction performance and cost efficiency, a moderate Pd(pph3)4 loading was selected for subsequent experiments.
To clarify the essential conditions for successful carbonylation, control experiments were conducted under varying conditions (Fig. 6e). The results indicate that both CO presence and photothermal activation via xenon-lamp irradiation are necessary and sufficient to drive the reaction, demonstrating the feasibility of aminocarbonylation under exceptionally mild conditions. Temperature monitoring reveals a gradual increase from ~20 °C to a stable plateau at ~50 °C during the aminocarbonylation reaction (Supplementary Fig. 26), confirming the system’s mild photothermal nature. In contrast, a control experiment conducted under purely thermal conditions at ~50 °C results in significantly lower N,N-diethyl-p-toluamide yields compared to the photocatalytic system (Supplementary Fig. 27), thereby underscoring the essential role of photoinduced effects beyond simple heating in driving the transformation. In addition, to trace the carbon source of the final product’s carbonyl group, a 13C-labeled isotope experiment was performed. Compared with the standard mass spectrum from the National Institute of Standards and Technology (NIST) Mass Spectral library, mass spectrometric analysis of the labeled p-DET verifies that the carbonyl carbon originates from the upstream 13CO2 reduction process (Fig. 6f), unambiguously demonstrating the direct conversion of CO2 into high-value fine chemicals via this tandem strategy.
Overall, the successful implementation of this tandem photocatalytic-carbonylation system showcases a promising approach for converting CO2 into industrially relevant organic compounds under mild conditions, with in situ CO generation driven solely by light. The efficient utilization of CO, coupled with the scalable and environmentally benign nature of the reaction setup, offers a compelling strategy for future advancements in photocatalytic CO2 conversion and fine chemical synthesis.
In summary, this study proposes a synergistic tandem strategy that integrates S-scheme photocatalysis with Pd-catalyzed aminocarbonylation for efficient CO2 valorization. A hierarchical CeO2/Bi2S3 heterojunction was rationally designed and constructed via a facile electrostatic self-assembly method, exhibiting improved light absorption, promoted charge carrier separation, and enhanced redox capability, as evidenced by complementary DFT calculations, in situ irradiated XPS, and ultrafast spectroscopy. Consequently, an impressive CO2-to-CO conversion yield of 14.05 mmol g−1 with 98% selectivity was achieved. Importantly, the tandem system enabled the direct utilization of photogenerated CO in Pd-catalyzed carbonylation reactions under ambient conditions, yielding industrially relevant amides with nearly complete CO incorporation. Isotopic tracing and mechanistic analyses further validated the seamless integration of the two distinct photocatalytic steps. In addition to mitigating the risks associated with CO storage, this tandem approach significantly enhances the economic viability of CO2-derived products. By coupling light-driven CO2 reduction with established carbonylation chemistry, this work provides a scalable and sustainable pathway for sustainable carbon utilization, bridging fundamental photocatalysis and practical chemical manufacturing. Future research will focus on catalyst stability optimization and substrate scope expansion to broaden industrial applicability.
Methods
Chemicals
The chemicals used in this study were of analytical reagent (AR) grade and were obtained from reputable commercial suppliers. Cerium nitrate hexahydrate (Ce(NO3)3·6H2O, 99.9%), hexamethylenetetramine (HMTA), bismuth nitrate pentahydrate (Bi(NO3)3·H2O, 99.9%), 4-iodotoluene (C7H7I, 98%), and 3-iodotoluene (C7H7I, 99%) were purchased from Macklin. Polyvinylpyrrolidone (PVP, 98%) and tris(2,2’-bipyridyl)ruthenium(II) chloride hexahydrate ([RuII(bpy)3]Cl2·6H2O, 98%) were sourced from Shanghai Aladdin biochemical technology Co., Ltd. In addition, 2-iodotoluene (C7H7I, 98%), triethylamine (99.5%, extra dry, molecular sieves, water ≤50 ppm), diethylamine (C4H11N, 99%), and tetrakis(triphenylphosphine)palladium (Pd[P(C6H5)3]4, Pd(pph3)4, 99%) were obtained from Energy Chemical.1,4-dioxane (C4H8O2, 99%) and ethylene glycol (EG, 99%) were provided by Sinopharm Chemical Reagent Co., Ltd. All reagents were used as received without further purification.
Synthesis of pure CeO2
Typically, 0.52 g of Ce(NO3)3·6H2O and 0.67 g of HMTA were dissolved in a mixed solvent of 30 mL of ethanol and 30 mL of deionized water. The solution was stirred thoroughly in an oil bath at 75 °C for 12 h. After centrifugation and drying, the resulting CeO2 nanoparticles—with a yield of approximately 95%—were finely milled into a homogeneous powder and subsequently ultrasonicated in ethanol for 2 hours to ensure uniform dispersion.
Synthesis of pure Bi2S3
In a typical procedure, 0.60 g of Bi(NO3)3·H2O, 0.30 g of thiourea, and 0.10 g of PVP were sequentially added to 40 mL of EG. The mixture was sonicated until completely dissolved, then transferred to a reaction vessel and heated at 140 °C for 8 h. The precipitate was collected by centrifugation, washed, and dried to obtain Bi2S3 with a recovery yield exceeding 95%.
Synthesis of CeO2/Bi2S3 heterojunctions
CeO2/Bi2S3 heterojunctions were synthesized via electrostatic self-assembly in ethanol. Predetermined amounts of CeO2 and Bi2S3 were dispersed in ethanol according to target Bi2S3-to-CeO2 molar ratios of 0.5%, 1%, and 2%. The mixture was stirred thoroughly to ensure uniform dispersion and assembly, followed by drying to yield the heterojunction composites with a recovery of over 95%.
Photocatalytic CO2 reduction
The CO2 photoreduction reaction was conducted in an online gas-closed system equipped with a gas circulated pump (OLPCRS-2, Shanghai Boyi Scientific Instrument Co., Ltd.). In a typical experiment, 10 mg of photocatalyst, 2 mM of [RuII(bpy)3]Cl2·6H2O, 10 mM of BIH, 30 mL of acetonitrile, and 100 μL of H2O were added to a Pyrex glass reactor connected to the left chamber of the online system. The system was first evacuated using a vacuum pump and subsequently injected with ~60 kPa of high-purity CO2 (99.999%). After achieving adsorption equilibrium, a 300 W xenon lamp (PLS-SXE300 + , Beijing Perfectlight, China) was used as the light source. The reaction system was maintained at 8 °C using a circulating water bath. The resulting gaseous products were analyzed using a gas chromatograph (GC-2030, Shimadzu Corp., Japan) equipped with a barrier discharge ionization detector (BID) and a capillary column (Carboxen 1010 PLOT Capillary, 60 m × 0.53 mm).
Photocatalytic Pd-catalyzed carbonylation reaction
Typically, 218 mg of 3-iodotoluene (or 2-iodotoluene/4-iodotoluene), ~70 mg of Pd(pph3)4, 206 μL of diethylamine, 278 μL of triethylamine and 10 mL of 1,4-dioxane were added to another Pyrex glass reactor connected to the right chamber of the online system. After evacuating the chamber, ~40 kPa of mixed gas from the left-side CO2 reduction chamber was introduced. Illumination was provided by a 300 W xenon lamp (PLS-SXE300 + , Beijing Perfectlight, China). The liquid-phase products were analyzed using gas chromatography-mass spectrometry (8890 GC System, 5977B GC/MSD, Agilent Technologies, USA) equipped with an HP-5 column (Agilent Technologies, USA).
Isotope-labeling experiments
The isotope-labeling experiments were carried out using 13CO2 (isotope purity: 99%, chemical purity: 99.9%) as the carbon source. Both gaseous and liquid products were analyzed by gas chromatography-mass spectrometry (8890 GC System, 5977B GC/MSD, Agilent Technologies, USA) equipped with HP-MOLESIEVE and HP-5 columns for detection of CO2 reduction and carbonylation products. Helium was used as the carrier gas. The injector and electron ionization (EI) source temperatures were set to 150 and 200 °C, respectively.
Data availability
The source data underlying Figs. 2a–c, e, f, 3g–i, 4a, b, d, e, 5a–i, 6c–e, and Supplementary Figs. 1–22, 24, 25, 27 are provided as a Source Data file, which is available in figshare with the identifier https://doi.org/10.6084/m9.figshare.29163971 and in the Source Data file. All data are available from the corresponding author on request. Source data are provided in this paper.
References
Zhang, X. et al. Enhancing photocatalytic H2O2 production with Au co-catalysts through electronic structure modification. Nat. Commun. 15, 3212 (2024).
Xu, F. et al. Unique S-scheme heterojunctions in self-assembled TiO2/CsPbBr3 hybrids for CO2 photoreduction. Nat. Commun. 11, 4613 (2020).
Wang, L. et al. S-scheme heterojunction photocatalysts for CO2 reduction. Matter 5, 4187–4211 (2022).
Mateo, D. et al. Titanium-perovskite-supported RuO2 nanoparticles for photocatalytic CO2 methanation. Joule 3, 1949–1962 (2019).
Dhakshinamoorthy, A. et al. Metal–organic framework (MOF) compounds: Photocatalysts for redox reactions and solar fuel production. Angew. Chem. Int. Ed. 55, 5414–5445 (2016).
Zhu, B. et al. Construction of 2D S-scheme heterojunction photocatalyst. Adv. Mater. 36, 2310600 (2023).
Fu, J. et al. Product selectivity of photocatalytic CO2 reduction reactions. Mater. Today 32, 222–243 (2020).
Jia, G. et al. Asymmetric atomic dual-sites for photocatalytic CO2 reduction. Adv. Mater. 36, 2403153 (2024).
Li, L. et al. Micro-alkaline environment enables CO2 electroreduction to multicarbons. Natl. Sci. Rev. 10, 230 (2023).
Son, H.-J. et al. Inorganometallic photocatalyst for CO2 reduction. Acc. Chem. Res. 54, 4530–4544 (2021).
Navalón, S. et al. Metal–organic frameworks as photocatalysts for solar-driven overall water splitting. Chem. Rev. 123, 445–490 (2023).
Yu, H. et al. Visible-light photochemical reduction of CO2 to CO coupled to hydrocarbon dehydrogenation. Angew. Chem. Int. Ed. 59, 6219–6223 (2020).
Fu, S. et al. Feeding carbonylation with CO2 via the synergy of single-site/nanocluster catalysts in a photosensitizing MOF. J. Am. Chem. Soc. 143, 20792–20801 (2021).
Kong, Y. et al. Electrochemical synthesis of organonitrogen compounds from N-integrated CO2 reduction reaction. Acta Phys. Chim. Sin. 40, 2307049 (2024).
Sastre, F. et al. Complete photocatalytic reduction of CO2 to methane by H2 under solar light irradiation. J. Am. Chem. Soc. 136, 6798–6801 (2014).
Zhang, L. et al. Charge-transfer dynamics in S-scheme photocatalyst. Nat. Rev. Chem. 9, 328–342 (2025).
Österbacka, N. et al. Spontaneous oxygen vacancy ionisation enhances water oxidation on BiVO4. ACS Energy Lett. 9, 153–158 (2023).
Li, S. et al. Constructing Cd0.5Zn0.5S/Bi2WO6 S-scheme heterojunction for boosted photocatalytic antibiotic oxidation and Cr(VI) reduction. Adv. Powder Mater. 2, 100073 (2023).
Ji, Q. et al. The role of oxygen vacancies of ABO3 perovskite oxides in the oxygen reduction reaction. Energy Environ. Sci. 13, 1408–1428 (2020).
Huang, K. et al. Effective photocatalytic hydrogen evolution by Ti3C2-modified CdS synergized with N-doped C-coated Cu2O in S-scheme heterojunctions. Chin. J. Struct. Chem. 42, 100204 (2023).
He, Y. et al. Selective conversion of CO2 to CH4 enhanced by WO3/In2O3 S-scheme heterojunction photocatalysts with efficient CO2 activation. J. Mater. Chem. A 11, 14860–14869 (2023).
Hu, P. et al. Nonmetal plasmon-induced carrier backflow and prolonged lifetime for CO2 photoreduction. ACS Catal. 14, 15025–15035 (2024).
Deng, X. et al. Ultrafast electron transfer at the In2O3/Nb2O5 S-scheme interface for CO2 photoreduction. Nat. Commun. 15, 4807 (2024).
Hu, P. et al. Highly selective photoconversion of CO2 to CH4 over SnO2/Cs3Bi2Br9 heterojunctions assisted by S-scheme charge separation. ACS Catal. 13, 12623–12633 (2023).
Meng, K. et al. Plasmonic near-infrared-response S-scheme ZnO/CuInS2 photocatalyst for H2O2 production coupled with glycerin oxidation. Adv. Mater. 36, 2406460 (2024).
Dhakshinamoorthy, A. et al. Selective gas-phase hydrogenation of CO2 to methanol catalysed by metal-organic frameworks. Angew. Chem. Int. Ed. 63, e202311241 (2024).
Qiu, S. et al. Efficient synthesis of phthalimides via cobalt-catalysed C(sp2)−H carbonylation of benzoyl hydrazides with carbon monoxide. Adv. Synth. Catal. 360, 3271–3276 (2018).
Wu, J. et al. From ketones, amines, and carbon monoxide to 4-quinolones: Palladium-catalyzed oxidative carbonylation. Org. Lett. 19, 6432–6435 (2017).
Mei, G. et al. Tandem electro-thermo-catalysis for the oxidative aminocarbonylation of arylboronic acids to amides from CO2 and water. Angew. Chem. Int. Ed. 63, e202314708 (2023).
Xia, Y.-S. et al. Tandem utilization of CO2 photoreduction products for the carbonylation of aryl iodides. Nat. Commun. 13, 2964 (2022).
Omae, I. Transition metal-catalyzed cyclocarbonylation in organic synthesis. Coord. Chem. Rev. 255, 139–160 (2011).
Lu, B. et al. Switchable radical carbonylation by philicity regulation. J. Am. Chem. Soc. 144, 14923–14935 (2022).
Xia, Y.-S. et al. A triple tandem reaction for the upcycling of products from poorly selective CO2 photoreduction systems. Nat. Synth. 3, 406–418 (2024).
Monticelli, S. et al. Unlocking full and fast conversion in photocatalytic carbon dioxide reduction for applications in radio-carbonylation. Nat. Commun. 14, 4451 (2023).
Moss, M. et al. Zirconocene-mediated carbonylative coupling of grignard reagents. Angew. Chem. Int. Ed. 55, 10017–10021 (2016).
González-Liste, P. J. et al. Catalytic rearrangement of aldoximes to primary amides in environmentally friendly media under thermal and microwave heating: Another application of the bis(allyl)-Ruthenium(IV) dimer [{RuCl(μ-Cl)(η3:η3-C10H16)}2]. ACS Sustain. Chem. Eng. 3, 3004–3011 (2015).
Huang, B. et al. Chemically bonded BiVO4/Bi19Cl3S27 heterojunction with fast hole extraction dynamics for continuous CO2 photoreduction. Adv. Powder Mater. 3, 100140 (2024).
Yan, H. et al. Highly efficient CeO2-supported noble-metal catalysts: From single atoms to nanoclusters. Chem. Catal. 2, 1594–1623 (2022).
Zhang, Q. et al. Oxygen vacancies in Co3O4 promote CO2 photoreduction. Appl. Catal. B 300, 120729 (2022).
Tian, J. et al. Oxygen vacancy mediated bismuth-based photocatalysts. Adv. Powder Mater. 3, 100201 (2024).
Sha Li, X. W., Cao, M., Lu, J., Qiu, L. & Yan, X. Engineering the interface and interaction structure on highly cokeresistant Ni/CeO2-Al2O3 catalyst for dry reforming of methane. Chin. J. Struct. Chem. 41, 2212007–2212014 (2022).
Teng, Y., Liu Hua, Z. X. & Zhiliang, J. CeO2 Particles anchored to Ni2P nanoplate for efficient photocatalytic hydrogen evolution. Chin. J. Struct. Chem. 41, 2201047–2201053 (2022).
Zhai, R. et al. Electron gathering at violet phosphorene-Ag interface for photoreduction of CO2 to ethylene. Appl. Catal. B 361, 124603 (2025).
Chen, X. et al. Modulating charge separation via in situ hydrothermal assembly of low content Bi2S3 into UiO-66 for efficient photothermocatalytic CO2 reduction. Appl. Catal. B 270, 118915 (2020).
Liu, Z. et al. Large-scale synthesis of ultralong Bi2S3 nanoribbons via a solvothermal process. Adv. Mater. 15, 936–940 (2003).
Xu, X. et al. Rational design of S-scheme CeO2/Bi2MoO6 microsphere heterojunction for efficient photocatalytic CO2 reduction. Acta Phys. Chim. Sin. 40, 2309031 (2024).
Li, M. et al. Regulating the electron–hole separation to promote selective oxidation of biomass using ZnS@Bi2S3 nanosheet catalyst. Appl. Catal. B 292, 120180 (2021).
Peng, J.-B. et al. The chemistry of CO: Carbonylation. Chem 5, 526–552 (2019).
Cheng, L.-J. & Mankad, N. P. Copper-catalyzed carbonylative coupling of alkyl halides. Acc. Chem. Res. 54, 2261–2274 (2021).
Friis, S. D. et al. The development and application of two-chamber reactors and carbon monoxide precursors for safe carbonylation reactions. Acc. Chem. Res. 49, 594–605 (2016).
Hermange, P. et al. Ex SituGeneration of stoichiometric and substoichiometric 12CO and 13CO and its efficient incorporation in palladium catalyzed aminocarbonylations. J. Am. Chem. Soc. 133, 6061–6071 (2011).
Gu, M. et al. Unveiling charge carrier dynamics at organic–inorganic S-scheme heterojunction interfaces: Insights from advanced EPR. Adv. Mater. 37, 2414803 (2025).
Xu, F. et al. Prolonging charge carrier lifetime via intraband defect levels in S-scheme heterojunctions for artificial photosynthesis. Angew. Chem. Int. Ed. 64, e202414672 (2025).
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2022YFE0115900 (J.Y.)), the National Natural Science Foundation of China (22378371 (F.X.), 52003213 (F.X.), 22361142704 (J.Y.), 22238009 (J.Y.), U24A2071 (J.Y.), and 22261142666 (J.Y.)), the Natural Science Foundation of Hubei Province of China (2025AFD020 (F.X.), 2022CFA001 (F.X.)), and the Fundamental Research Funds for the Central Universities, China University of Geosciences (Wuhan) (No. CUG22061 (J.Y.)). Partial support of the Iran National Science Foundation (Grant Number 4021464 (J.Y.)) was acknowledged. We also thanked the Faculty of Materials Science and Chemistry, China University of Geosciences (CUG), Wuhan, for its TEM facilities and the data analysis of Dr. Mingxing Gong.
Author information
Authors and Affiliations
Contributions
F.X., J.Y., and H.G. conceived and designed the experiments. F.Z. and X.D. carried out the synthesis of the materials, the photocatalytic test, and the characterizations of the materials. F.Z., X.D., C.A., and J.J.Z. performed the ultrafast TA measurements. F.X., F.Z., X.D., and J.F.Z. analyzed all the results. F.Z. and X.D. wrote the manuscript. F.X. conducted the DFT calculations, contributed to data analysis, and revised the manuscript. F.X., J.Y., and H.G. supervised the project. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Chao Zeng, Lan-Gui Xie and the other anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, F., Zhao, F., Deng, X. et al. Integrating S-scheme photocatalysis with tandem carbonylation: A green and scalable strategy for CO2 valorization. Nat Commun 16, 6882 (2025). https://doi.org/10.1038/s41467-025-60961-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-60961-5
This article is cited by
-
Advanced CeO2-Based Heterojunctions: Aspects of Structure–Function Relationships for Sustainable CO2 Conversion and H2 Production
Transactions of Tianjin University (2025)