Abstract
Microbial ecosystems are fundamental to planetary and human health, yet human activities are accelerating their loss. Disruptions to microbial communities undermine environmental stability, biodiversity, and health. Urgent action is required to preserve microbial diversity. The Microbiota Vault Initiative provides a global framework to safeguard microbiomes from human, animal, and environmental sources. It proactively archives microbial diversity for future needs, prioritizing depositor sovereignty, equitable collaboration, and ethical governance. By sharing limited information on deposits, the initiative fosters microbial conservation and collaboration between local and global researchers. It complements other efforts to ensure the resilience of microbiomes in an era of rapid environmental change.
Similar content being viewed by others
Introduction
Microbial ecosystems underpin life on Earth, yet they are increasingly imperiled by human activities, including unsustainable agricultural practices and overuse of antibiotics. These disturbances disrupt microbial networks essential for environmental stability, biodiversity, and animal and human health, contributing to the global rise of chronic diseases and ecosystem degradation. As future restoration efforts will depend on the microbial diversity preserved today, proactive measures are urgently required. In this Perspective, we focus on one such initiative, the Microbiota Vault Initiative (MVI), a not-for-profit non-governmental organization that we have developed to address the urgent need. Inspired by the Svalbard Global Seed Vault (SGSV), the MVI shares a vision of safeguarding diversity, although its goals and implementation differ substantially. Unlike the SGSV, which provides backup for existing seed banks with well-documented restoration outcomes, the MVI proactively archives microbiota across human, animal, and environmental domains in anticipation of future unknown needs in restoration efforts.
The MVI embodies an integrative One Health approach by safeguarding microbial biodiversity critical to human and animal health, plant resilience, and environmental functions such as soil fertility, nutrient cycling, climate regulation, and pathogen resistance. By fostering equitable collaboration, honoring diverse knowledge systems, and ensuring depositor sovereignty, the MVI provides a culturally attuned framework for mitigating microbial loss (Table 1). Coordinated action through this initiative offers a timely opportunity to protect health, biodiversity, and ecosystem resilience through the lens of One Health, underscoring the deep interdependence of microbial diversity, environmental integrity, and well-being across species.
There is broad recognition of the essential role that naturally evolved microbes play in ecosystems, including in humans1,2,3,4. Human activities have disrupted these evolutionary connections in host-associated microbiomes [humans1,2,3,4, animals5,6,7, and plants8,9,10] and have been associated with an alarming rise in chronic diseases such as allergic, autoimmune, and metabolic disorders4,11,12,13,14,15. The loss of microbial diversity extends to environmental ecosystems16,17,18, jeopardizing agricultural systems and environmental resilience.
The loss of crucial microbes in both human and environmental contexts represent a growing but often overlooked crisis. In human health, several key examples illustrate how modernization disrupts the coevolved microbial partnerships19,20. The decline in prevalence of Bifidobacterium longum subsp. infantis—a bacterium that evolved to digest human milk oligosaccharides and support infant immune development—in industrialized societies has been associated with increased risks of allergies, diabetes, and other immune conditions21,22. This decline, likely due to increased use of Cesarean sections, formula feeding, and antibiotics, has become so severe that some pediatricians now recommend probiotic supplementation23. Similarly, the decrease of Helicobacter pylori and Treponema succinifaciens24 from gut microbiomes in industrialized countries shows broader implications of urbanization. While H. pylori’s reduction has decreased stomach ulcers, its absence correlates with increased rates of allergic asthma and metabolic disorders20, highlighting the complex roles that these co-evolved microbes have in human health. In environmental systems, the thawing of Arctic permafrost threatens unique cold-adapted microorganisms like Methanoflorens stordalenmirensis, which helps regulate methane emissions25, and various Acidobacteria species crucial for carbon cycling. The loss of these environmental microbes creates a dangerous feedback loop: as climate change kills off permafrost microbes, their absence accelerates the release of greenhouse gases, further warming the planet26,27. These parallel losses in both human and environmental microbiomes demonstrate how changes caused by human activity can disrupt microbial ecosystems that evolved over millions of years, with cascading effects on both human and planetary health. Just as we strive to preserve endangered macro-organisms, urgent global efforts are needed to understand and preserve microbial diversity.
While much microbial biodiversity and mechanisms underlying sustaining natural ecosystems and health remain to be discovered, viewed through the lens of One Health, it is our obligation to future generations to preserve this microbial diversity28. The concept of preserving Earth’s microbial heritage draws parallels to seed banks, which safeguard plant genetic diversity. Similarly, the MVI is a non-profit organization that operates as a public charity (www.microbiotavault.org). Its primary aim is to support local collections to preserve microbial biodiversity globally, including environmental, plant, animal, and human-associated microbiota.
The microbiota vault initiative: a framework for safeguarding microbial ecosystems
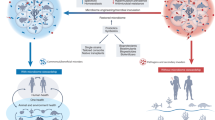
The goal of the MVI is to foster an inclusive, global effort to preserve microbial specimens while fostering extramural research. It is intentionally non-commercial and based on an equitable framework that is designed to address the urgent need for microbial preservation amidst global biodiversity loss. Inspired by the Svalbard Global Seed Vault (www.croptrust.org), the initiative prioritizes microbial biodiversity preservation, fostering the creation of local collections and offering secure cryogenic storage of microbiomes from diverse human and environmental sources (Fig. 1).
The Microbiota Vault educates scientists and promotes the creation of Local Collections for preservation and research. These local collections can request that the Microbiota Vault preserve a safe backup of their specimens, maintaining all rights over their deposits; they have the option to request sequencing for open access under the license of their choice, and the MV will publish on its webpage a database of the deposited specimens, including metadata, links to sequences, and contact information about depositors. Those in the global community interested in doing research can then contact the depositor from the Local Working Collection. For the use of data derived from the Microbiota Vault, explicit acknowledgments to the specific Local Working Collection and to the Microbiota Vault will be required.
The MVI acts at the request and on behalf of sovereign depositors, and worldwide local collections, to preserve backup copies of specimens of human, animal, plant, or environmental origin. These samples are not accessible to anyone other than the depositors, or their registered agent. Additionally, upon request by the depositing collections, the Microbiota Vault may sequence microbial DNA (or RNA), depositing in open-access databases under the Creative Commons license of their choice (https://creativecommons.org/licenses/). This helps depositors define the terms of their collaborations29 and foster global research connections, leading to global capacity strengthening in microbiome research. The MVI does not engage in discovery research, therapeutic development, patenting, profit generation, or the assertion of material or intellectual property rights over its collections. Instead, it focuses on building a collaborative, multidisciplinary network. Through initiatives such as the annual Global Microbiome Network Symposia (GloMiNe), the MVI brings together researchers, public health professionals, legal experts, anthropologists, sociologists, and other stakeholders to engage in dialogue around microbiome science, research ethics, standardization, and equitable access and benefit-sharing.
Equity, ethics, education, and global collaboration
The MVI is committed to educating and preserving microbial diversity while ensuring adequate representation of all people. The initiative fully supports multilateral efforts within the Global Biodiversity Framework towards equitable sharing of benefits from the use of biological materials or their data30,31. The initiative is deeply committed to equity and ethical engagement with local communities, including Indigenous peoples who hold and protect critical microbiome knowledge and biodiversity29,32. The MVI acknowledges that decisions regarding the common good and the future of traditional communities should be made by the communities themselves. Its framework aims to support the development of equitable and inclusive approaches to sovereignty and governance in microbiome research.
As a non-profit organization, the MVI operates under its bylaws and global policies to maximize inclusion, providing fiduciary oversight and educating prospective depositors on ethical research practices, access and benefit-sharing requirements under the Nagoya Protocol, and standardized methods for metadata design.
A cornerstone of the MVI outreach efforts is the annual GloMiNe symposium. These events bring together scientists from diverse disciplines—microbiology, anthropology, ethics, public health, and bioinformatics—to share knowledge, discuss microbial biodiversity conservation, and strengthen local research capacity. Over four years, these symposia have fostered interdisciplinary collaboration and contributed to the establishment of a global microbiome network (Table 2).
In addition to symposia, the Microbiota Vault supports capacity strengthening through mentoring for scientists from low- and middle-income countries, who thanks to collaborating with the Digital Sequence Information Scientific Network (DSI) are offered travel grants to their meetings, further advancing global equity in microbiome research. As part of its global advocacy efforts, the MVI has engaged with United Nations delegations, coordinating educational screenings to highlight the importance of the microbiome, and presented at the UN COP24 conference in Cali, Colombia.
The microbiota vault launch phase
The MVI has just concluded its Launch Phase, refining a model for the interaction between a globally coordinated fundraising initiative and locally operated biobanks. Backup collections are deposited with a contractual legal framework in which Deposit Agreements are signed between depositors and the Microbiota Vault. During this initial phase, Local Working Collections from Benin, Brazil, Ethiopia, Ghana, Laos, Thailand, and Switzerland deposited 1204 human fecal specimens and 190 fermented food samples. These deposits required navigating complex local and global regulations for exporting samples to Switzerland, where they are housed securely in cryo-storage (−80 °C) at the Institute of Medical Microbiology, University of Zurich.
The MVI Launch Phase also enabled the development of standardized protocols for sample collection, preservation, transport, metadata development, and data annotation. For metadata, the Microbiota Vault has adopted minimum information about any (x) sequence (MIxS) standards, a modular framework developed by the international scientific community, which offers flexibility to accommodate additional sample types such as environmental or wildlife specimens33. Best practices emerging from this phase include robust traceability of samples, equitable biobanking frameworks, standardized metadata, harmonized storage protocols, advanced biobanking systems, and quality control adhering to the recommendations of the Swiss Biobanking Platform (https://swissbiobanking.ch/).
Future perspective
The MVI’s Launch Phase focused on human-associated microbiomes and fermented food microbes. To support broader ecological goals, it will expand to include environmental microbiota from threatened ecosystems. This aligns with a One Health framework by preserving microbes vital to environmental and planetary health, and by providing a framework for fostering research and strengthening local capacities. While reintroducing preserved microbes remains speculative, and functional restoration from frozen specimens is unproven, preservation offers a valuable opportunity for future research. Indeed, the application of “microbiome therapy” in humans34, animals35,36, and environmental ecosystems37,38, is promising. Stored samples may one day support fundamental microbiological research, ecosystem restoration, or biotechnological advances.
Around the world, other groups of scientists have independently recognized the need to conserve microbial communities through specimen preservation. Among them, the Global Microbiome Conservancy stands out as a research-driven effort actively collecting and studying human microbial samples from diverse geographic regions. While most other initiatives are also research-oriented (Table 3), they align closely with the Microbiota Vault Initiative (MVI) and offer complementary strengths that create opportunities for future collaboration. By partnering with and receiving specimens from local microbial collections, the MVI and similar efforts help safeguard regional ecosystems while advancing education, capacity strengthening, and inclusive governance. In particular, the MVI empowers local stakeholders through depositor sovereignty and strict standards for metadata and preservation protocols.
References
Blaser, M. J. The theory of disappearing microbiota and the epidemics of chronic diseases. Nat. Rev. Immunol. 17, 461–463 (2017).
Azad, M. B., Bridgman, S. L., Becker, A. B. & Kozyrskyj, A. L. Infant antibiotic exposure and the development of childhood overweight and central adiposity. Int. J. Obes. 38, 1290 (2014).
Bach, J. F. Infections and autoimmune diseases. J. Autoimmun. 25, 74–80 (2005).
Zimmermann, P., Messina, N., Mohn, W. W., Finlay, B. B. & Curtis, N. Association between the intestinal microbiota and allergic sensitization, eczema, and asthma: a systematic review. J. Allergy Clin. Immunol. 143, 467–485 (2019).
Bager, F., Madsen, M., Christensen, J. & Aarestrup, F. M. Avoparcin used as a growth promoter is associated with the occurrence of vancomycin-resistant Enterococcus faecium on Danish poultry and pig farms. Prev. Vet. Med. 31, 95–112 (1997).
Burgos, J. M., Ellington, B. A. & Varela, M. F. Presence of multidrug-resistant enteric bacteria in dairy farm topsoil. J. Dairy Sci. 88, 1391–1398 (2005).
Cho, I. et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 488, 621–626 (2012).
Berendsen, R. L., Pieterse, C. M. & Bakker, P. A. The rhizosphere microbiome and plant health. Trends Plant Sci. 17, 478–486 (2012).
Bulgarelli, D., Schlaeppi, K., Spaepen, S., Ver Loren van Themaat, E. & Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu Rev. Plant Biol. 64, 807–838 (2013).
Qu, Q. et al. Rhizosphere microbiome assembly and its impact on plant growth. J. Agric Food Chem. 68, 5024–5038 (2020).
Arrieta, M. C. et al. Associations between infant fungal and bacterial dysbiosis and childhood atopic wheeze in a nonindustrialized setting. J. Allergy Clin. Immunol. 142, 424–434.e410 (2018).
Arrieta, M. C. et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 7, 307ra152 (2015).
Russell, S. L. et al. Perinatal antibiotic treatment affects murine microbiota, immune responses and allergic asthma. Gut Microbes 4, 158–164 (2013).
Russell, S. L. et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 13, 440–447 (2012).
Keeney, K. M., Yurist-Doutsch, S., Arrieta, M. C. & Finlay, B. B. Effects of antibiotics on human microbiota and subsequent disease. Annu Rev. Microbiol 68, 217–235 (2014).
Peixoto, R. S. et al. Harnessing the microbiome to prevent global biodiversity loss. Nat. Microbiol 7, 1726–1735 (2022).
Fierer, N. et al. Reconstructing the microbial diversity and function of pre-agricultural tallgrass prairie soils in the United States. Science 342, 621–624 (2013).
Singh, B. K., Yan, Z. Z., Whittaker, M., Vargas, R. & Abdelfattah, A. Soil microbiomes must be explicitly included in one health policy. Nat. Microbiol 8, 1367–1372 (2023).
Sonnenburg, E. D. & Sonnenburg, J. L. The ancestral and industrialized gut microbiota and implications for human health. Nat. Rev. Microbiol 17, 383–390 (2019).
Blaser, M. J. & Falkow, S. What are the consequences of the disappearing human microbiota?. Nat. Rev. Microbiol. 7, 887–894 (2009).
Henrick, B. M. et al. Bifidobacteria-mediated immune system imprinting early in life. Cell 184, 3884–3898.e3811 (2021).
Huda, M. N. et al. Stool microbiota and vaccine responses of infants. Pediatrics 134, e362–e372 (2014).
Duar, R. M., Henrick, B. M., Casaburi, G. & Frese, S. A. Integrating the ecosystem services framework to define dysbiosis of the breastfed infant gut: the role of B. infantis and human milk oligosaccharides. Front Nutr. 7, 33 (2020).
Carter, M. M. et al. Ultra-deep sequencing of Hadza hunter-gatherers recovers vanishing gut microbes. Cell 186, 3111–3124.e13 (2023).
Fu, Q. et al. Genome sequence of a 45,000-year-old modern human from western Siberia. Nature 514, 445–449 (2014).
Woodcroft, B. J. et al. Genome-centric view of carbon processing in thawing permafrost. Nature 560, 49–54 (2018).
Li, Y. et al. Genomic insights into redox-driven microbial processes for carbon decomposition in thawing Arctic soils and permafrost. mSphere 9, e0025924 (2024).
Dominguez-Bello MG, K. R., Gilbert, J. & Blaser, M. J. Preserving microbial diversity: Microbiota from humans of all cultures are needed to ensure the health of future generations. Science 362, 33–34 (2018).
Halewood, M., Bagley, M. A., Wyss, M. & Scholz, A. H. New benefit-sharing principles for digital sequence information. Science 382, 520–522 (2023).
Scholz, A. H. et al. Multilateral benefit-sharing from digital sequence information will support both science and biodiversity conservation. Nat. Commun. 13, 1086 (2022).
United Nations Environment Program. UN Biodiversity Conference (COP 15): Decisions adopted by the Conference of the Parties to the Convention on Biological Diversity, https://www.cbd.int/doc/decisions/cop-15/cop-15-dec-09-en.pdf (2022).
Dominguez-Bello, M. G. et al. Ethics of exploring the microbiome of native peoples. Nat. Microbiol 1, 16097 (2016).
Yilmaz, P. et al. Minimum information about a marker gene sequence (MIMARKS) and minimum information about any (x) sequence (MIxS) specifications. Nat. Biotechnol. 29, 415–420 (2011).
Shtossel, O. et al. Recipient-independent, high-accuracy FMT-response prediction and optimization in mice and humans. Microbiome 11, 181 (2023).
Feng, H. et al. Fecal virus transplantation has more moderate effect than fecal microbiota transplantation on changing gut microbial structure in broiler chickens. Poult. Sci. 103, 103282 (2024).
Ma, Z. et al. Fecal microbiota transplantation improves chicken growth performance by balancing jejunal Th17/Treg cells. Microbiome 11, 137 (2023).
Sheth, R. U., Cabral, V., Chen, S. P. & Wang, H. H. Manipulating Bacterial Communities by in situ Microbiome Engineering. Trends Genet 32, 189–200 (2016).
Duarte Rosado, J. G. et al. Coral probiotics induce tissue-specific and putative beneficial microbiome restructuring in a coral-dwelling fish. ISME Commun. 5, ycaf052 (2025).
Acknowledgements
We acknowledge funding from the Gebert Rüf Foundation, Seerave Foundation, Amy P. Goldberg Foundation, Oak Foundation, Rockefeller Foundation, Pacific Star Foundation, Rutgers University, New England Biolabs, and the University of Zurich. For part of the work of the Launch Phase, equipment of the Genetic Diversity Centre of ETH Zurich was used. We thank Daniela Vargas-Robles and Joel Colombo for their work on the figure.
Author information
Authors and Affiliations
Contributions
M.G.D.-B. and M.F. conceived the Microbiota Vault Initiative. D.S., M.F., A.E., P.V. and N.A.B. contributed to infrastructure development and coordination of the Launch Phase. All authors contributed to the writing and critical revision of the manuscript. All authors approved the final version.
Corresponding author
Ethics declarations
Competing interests
M.G.D.-B., D.S., M.F., A.E., P.V., N.A.B., A.L., C.H., P.Z., A.M., S.S., A.L., S.W.P., J.Z., R.T., C.S., D.A.T., Y.Y.L., V.B., Y.T., M.H., A.K., R.K., J.A.G. and M.J.B. are founders of the initiative and/or participants in the Launch Phase. R.K. is a co-founder of Biota, Inc., and Micronoma, holds equity; he receives income as a consultant or as a scientific advisory board member for BiomeSense, GenCirq, DayTwo, Cybele, Biota, and Micronoma. The University of California, San Diego has reviewed and approved these arrangements in accordance with its conflict of interest policies.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dominguez-Bello, M.G., Steiger, D., Fankhauser, M. et al. The microbiota vault initiative: safeguarding Earth’s microbial heritage for future generations. Nat Commun 16, 5373 (2025). https://doi.org/10.1038/s41467-025-61008-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-61008-5
This article is cited by
-
Gifting future scientists the past through well-preserved specimens of modern microbial ecosystems
Nature Communications (2025)
-
Launching the IUCN Microbial Conservation Specialist Group as a global safeguard for microbial biodiversity
Nature Microbiology (2025)