Abstract
Borylation chemistry plays a crucial role in the development of new synthetic methodologies. However, the reactivity of zinc-boryl species has not been fully explored, particularly in relation to diverse reaction pathways. Here we show that a zinc-boryl species is successfully synthesized from bis(catecholato)diboron, exhibiting amphiphilic reactivity. This compound acts as a nucleophilic boron anion with methyl iodide and as an electrophile with N,N’-dicyclohexylcarbodiimide, facilitating zinc-boron bond dissociation and generating zinc-carbon and zinc-nitrogen bonds while cleaving carbon-nitrogen double bonds. The enhanced reactivity is likely due to the stronger covalency of the zinc-boron bond. Additionally, the zinc-boryl compound promotes the catalytic diborylation of azobenzene, underscoring its versatility as a reactive intermediate. Density functional theory studies illuminate the electronic structure and reactivity of the zinc-boron bond, providing insights into potential applications in synthetic chemistry.
Similar content being viewed by others
Introduction
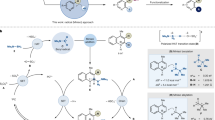
Organozinc compounds have become ubiquitous and versatile intermediates, finding widespread applications in synthetic chemistry1,2,3,4,5,6,7. Common zinc-carbon bonded reagents, such as ZnEt₂, serve as mild carbon-centered nucleophiles in organic synthesis8,9,10,11. Recently, there has been a growing interest in a new class of heteroatom-zinc species, where the heteroatom introduces unique reactivity to the Zn-heteroatom bond12,13,14,15. Boron, with its empty 2p orbital when it is three-coordinated, presents a particularly attractive option16,17,18. The Zn-B bonds present in zinc-boryl compounds can potentially provide access to nucleophilic boryl anions and unlock new reactivity modes. However, structurally characterized zinc-boryl species are extremely rare.
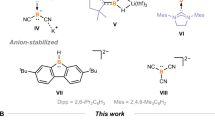
Their synthesis has generally relied on nitrogen-stabilized bulky boryl frameworks, all of which utilized lithium boryl compounds as precursors. Furthermore, these zinc-boryl reagents have been demonstrated to show limited reactivity with small electrophiles, likely due to the presence of bulky groups on the boryl ligand. These groups can also hinder the reactivity of the empty p orbital at the boron atom. On the other hand, Zinc-boryl compounds prepared from diboron esters (such as B2pin2, B2cat2) have been proposed as catalytically active species for various borylation reactions22,23,24,25,26. Therefore, it is of great interest to synthesize such a zinc-boryl ester complex and further investigate its synthetic applications. (Fig. 1a)19,20,21
In the literature, stable metal-boryl bonds can also be achieved by installing bulky ligands at the metal sites27. Based on this, NHC-carbene stabilized copper- and gold-boryl complexes have been successfully isolated28,29,30,31. Previously, attempts to isolate Zn-boryl complexes derived from diboron esters with NHC carbene ligands resulted in decomposed zinc powder and a minor product involving a Zn-inserted NHC ring product32. Moreover, the bulky β-diketiminate species proved to be an ideal ligand for stabilizing low-valent metals, highly reactive low-coordinate species, and metal-boryl complexes33,34,35,36,37,38. Inspired by these achievements, we envisioned preparing a stable Zn-B bond by introducing a β-diketiminate-based ligand at the zinc site, thus enhancing the reactivity of the Zn-B bond. During the review process of this manuscript, the Aldridge group reported a Zn-Bpin bonded compound, and its DMAP adduct has been fully characterized (Fig. 1b)39.
In this work, we successfully synthesized a zinc-boryl compound 1 starting from the common diboron ester, B2cat2 (Fig. 1c). Compound 1 demonstrates the ability to act as a nucleophilic Bcat anion, engaging productively with an electrophilic partner. Furthermore, this zinc-boryl species exhibited a rich diverse reactivity profile, undergoing transformations with N-containing unsaturated substrates such as carbodiimide, azo benzenes, and isonitriles. Notably, compound 1 was also found to be a competent catalyst in diboration processes. Besides, DFT computation studies were carried out to understand the reaction mechanism.
Results
Synthesis and characterization
The zinc amido compound 2 was chosen as the starting material12, as its reaction with B2cat2 generates stable B-N bonded products, which might be a driving force for the formation of the Zn-B bond. Indeed, treatment of compound 2 and B2cat2 at room temperature in toluene afforded the desired zinc boryl product 1 in a 53% isolated yield (Fig. 2a). The11B NMR of 1 shows a broad singlet at 39 ppm, indicating the presence of a three-coordinated boron moiety. Furthermore, the formation of the Zn-B bond was unambiguously confirmed by single-crystal X-ray analysis (Fig. 2b). The crystals of 1 were obtained from a hexane solution, exhibiting a dimeric structure. The zinc atom exhibits a Zn-O interaction distance of 2.325(1) Å, which is notably longer than typical Zn–O coordination bonds (generally <2.15 Å)40,41. This extended bond arises from a weak coordination interaction involving the oxygen atom of the Bcat group, resulting in the formation of a six-membered ring structure that stabilizes the dimeric assembly. In contrast, in toluene solution, it forms a monomer, as supported by single-crystal X-ray analysis (see Supplementary Fig. S51). However, both crystals obtained from the hexane or toluene solutions show the same signal in1H NMR (see Supplementary Fig. S55). The DOSY NMR results confirm that compound 1 exists as a monomer in the C6D6 solution (see Supplementary Fig. S55). The Zn-B bond length of compound 1 (2.055(1) Å) is close to that of N-heterocyclic boryl zinc species (2.052, 2.075 Å)19,20,21, but the distance is slightly longer than the Cu-boryl complexes(average 1.990 Å)19,28,42,43,44,45,46.
Metal boryl complexes serve as essential reactive intermediates or catalysts in the formation of various borylated reagents used in organic synthesis47,48,49,50,51,52. The reactivity of the metal-boron bond is crucial for the further transformation. To gain deeper insights into the Zn-B bond in compound 1, DFT computation studies were carried out (Fig. 3) at the BP86 level of theory (see Supplementary information). The computed molecule is a simplified model of 1 by substituting dipp groups with 2,6-dimethyl phenyl moieties. Figure 3 shows the natural localized molecular orbital (NLMO) calculated for the Zn(II)-boryl complex 1, describing the Zn-B sigma bonding interaction. For comparison, Fig. 3 also presented the corresponding NLMO calculated for the closely related Mg(II)-boryl species, [HC{(Me)CN(Dipp)}2Mg(DMAP)(Bpin). The NLMO plots clearly indicated that when compared to the Mg-B sigma bond, the Zn-B sigma bond contains a greater orbital contribution from the metal center. Furthermore, the Natural Bond Orbital (NBO) analysis revealed that the boron atom carries a charge of +0.478, which is higher than that of magnesium (+0.382) and lithium boryl species (+0.072)3,52,53. Meanwhile, the zinc metal center in compound 1 carries a charge of 0.950, indicating a more covalent character of the Zn-B bond compared with the known magnesium and lithium boryl species. To further support the covalent nature of the Zn–B bonding interaction in 1, we performed Quantum Theory of Atoms in Molecules (QTAIM) and Principal Interacting Orbital (PIO) analyses. Both the molecular graph from QTAIM and the PIO results indicate that the Zn–B bond exhibits covalent character. The results of these analyses are provided in the Supplementary Information.
Reactivity of the zinc-boryl compound
The reaction of compound 1 with 4-dimethylaminopyridine (DMAP) afforded a product, compound 3, where the DMAP unit was coordinated to the zinc atom, as confirmed by X-ray single-crystal analysis (Fig. 4a). This observation implies that the zinc metal center in compound 1 is more electrophilic compared to the boryl group, which is consistent with the DFT computational results discussed above.
a Reaction of compound 1 with DMAP; b Reaction of compound 1 with MeI; c Reaction of compound 1 with N,N’-dicyclohexylcarbodiimide (Molecular structure of compounds 6 and 5: thermal ellipsoids are set at the 30% probability level, hydrogen atoms are omitted and the side Dipp parts are set as sticks model for clarity).
The magnesium, lithium, and copper boryl compounds have demonstrated their reactivity of nucleophilic boryl anions in reactions with electrophiles33,45,53,54. The similar reactivity of zinc-boryl compound 1 was also examined (Fig. 4b). The reaction of compound 1 with MeI in C6D6 over 6 h resulted in a clear conversion, as indicated by a new 11B NMR signal at 35.2 ppm, suggesting the formation of the methyl-substituted boronic ester. The MeBcat could also be synthesized from Rh-Me complex with B2cat255, but our synthetic route indicates a clear nucleophilic Bcat- derived from the Zn-B σ−bond. Additionally, the zinc-I species 4 was identified by 1H NMR, which is consistent with the reported data (see Supplementary Fig. S31)56,57. High-resolution mass spectrometry (HRMS) further confirmed the identities of the generated compounds. This reaction demonstrated the nucleophilic activity of the Bcat- anion derived from compound 1.
To explore further the reactivity of the Zn-B bond in compound 1, we conducted a series of reactions with unsaturated N-containing substrates. The reaction of compound 1 and N,N’-dicyclohexylcarbodiimide (DCC) has been explored. Based on previous literature reports33, we expected that the borylation might occur at the C atom, forming a ZnN2C four-member ring. To our surprise, the reaction of compound 1 and DCC gave a clean conversion to compound 5 at 85 °C (Fig. 4c). X-ray crystallographic analysis confirmed the structure of this new compound as a zinc imide complex. In this case, DCC acted as an equivalent of Cy-nitrene, which was inserted into the Zn-B bond. This indicates a C=N bond cleavage event facilitated by the Zn-Bcat compound 1 that displays unusual reactivity. To elucidate the mechanism behind the unique bond cleavage process, we conducted a control experiment. Upon heating the reaction at 60 °C, a new 11B signal at 26.8 ppm was observed, and the 1H NMR spectrum also showed the appearance of a new set of signals, suggesting the presence of a stable transition intermediate. X-ray single crystal analysis confirmed the structure of this species, compound 6, as a 1,2-addition compound involving the Zn-B bond, where the zinc atom coordinates with the central carbon atom and the oxygen atom (O1), forming a five-membered ring. And the Bcat group is connected to the terminal N-Cy moiety. Continued heating of 6 at 85 °C led to a smooth transformation into the final product 5, proving it to be a key intermediate in this transformation. Attempts to isolate any other side products from these reactions were not successful. Similar results regarding the cleavage of the C=N double bond leading to imido species have also been reported for low-valent main group metals58,59 and transition metal borylenes60,61. In contrast to its nucleophilic reactivity with MeI, compound 1 acts as an electrophile toward DCC, demonstrating amphiphilic character within the Zn-B bond. In these reactions, it is interesting to find out whether the related transformations have been initiated by a highly reactive nucleophilic or electrophilic boron moiety. To understand the reaction mechanism for the formation of compounds 5 and 6, DFT computational studies have been conducted, and the results are summarized in the next section.
The reactivity of compound 1 towards isonitrile was also investigated. When an equimolar amount of tBuNC was added to a toluene solution of compound 1, an immediate color change from colorless to dark green was observed11.B NMR analysis revealed the formation of a new species with a signal at 17.2 ppm, along with the presence of unreacted compound 1. Upon the addition of another equivalent of tBuNC, a complete conversion of compound 1 was observed. The product, compound 7, was successfully isolated (Fig. 5a). X-ray single crystal analysis revealed the formation of a C-C coupled moiety derived from tBuNC, coordinated with the Bcat unit to generate a four-membered C2NB heterocycle (Fig. 5c). The C-N bond lengths of 1.280(4) and 1.301(4) Å, along with the terminal C-C bond length of 1.495(4) Å, suggest the formation of two N=C double bonds and a C-C single bond in the isonitrile-coupled moiety. This interesting C-C coupling pathway that gives a C=N-B-C 4-membered ring product was also observed when excess isonitrile reacted with the above-mentioned manganese-carbonyl boryl and low valent aluminum complexes62,63. However, unlike the Mn (I) carbene complex, 7 does not show carbene character judging from the planar C-N-B-C ring and its Zn-C bond length of 1.96 Å, which is the average bond length of a Zn-C single bond3,64. Regarding the reaction pathway, though mechanistic study is still underway, it is believed that there is an insertion of tBuNC into the Zn-B bond, followed by an attack of second tBuNC at the C centre that cleaves C-B bond. This is in contrast to the previously reported Au-B(o-tol)2 complex30, where the isocyanide was transformed into an azaallenylgold compound, implying distinct reaction pathways in the two systems.
a Reaction of compound 1 with isocyanide; b Reaction of compound 1 with azobenzene; c Molecular structure of compound 7. (thermal ellipsoids are set at the 50% probability level, and the hydrogen atoms are omitted for clarity); d Molecular structure of compound 8. (thermal ellipsoids are set at the 30% probability level, hydrogen atoms are omitted and the side Dipp parts are set as sticks model for clarity).
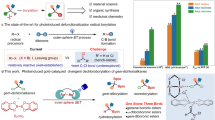
Additionally, compound 1 was found to react with azobenzene, forming a new zinc-imide product 8 (Fig. 5b). The structure of compound 8 was confirmed by X-ray single-crystal analysis, which revealed the formation of Zn-N and B-N bonds (Fig. 5d). The N-N bond length of 1.434(3) Å is within the expected range of a N-N single bond. The diborylation of azobenzene is a known transformation; however, it typically requires either stoichiometric amounts of highly electrophilic boron reagents or noble metal catalysts65,66,67. Based on the formation of 8, we hypothesized that compound 1 might promote the catalytic diborylation of azobenzenes, driven by the regeneration of a strong B-N bond. To test this hypothesis, we investigated the reaction of compound 8 with B2cat2, which led to the regeneration of compound 1, suggesting the establishment of a catalytic cycle. After screening various reaction conditions (see Supplementary information), we achieved the diboration of azobenzene with an isolated yield of 82% at 60 °C in toluene, using 10 mol% of catalyst 1. We evaluated substrates with substituents exhibiting different electronic effects in this system (Fig. 6). Complex 1 proved effective in catalyzing the diborylation of substrates containing electron-withdrawing groups. In contrast, it did not facilitate the reaction with substrates bearing electron-donating groups. The catalytic efficiency of the Zn-boryl complex for the diborylation of azobenzene and substrates with electron-withdrawing groups is comparable to that achieved with precious metal catalysts68, and this was accomplished without the addition of a base in the catalytic system to fully utilize the boron source69.
DFT computational studies
To shed light on the reactivity of the Zn-B bond, DFT computations were conducted on the reaction of compound 1 with DCC shown in Fig. 7 at ωB97X-D level in toluene (see Supplementary information). We selected this reaction for DFT studies because the formation of 6 clearly signifies the potential electrophilic nature of the boryl ligand, allowing us to distinguish a Zn-B bond and an Mg-/Li-B bond. The formation of the final thermodynamically more stable product 5 occurs through two steps (Fig. 7). In the first step, the Zn-B bond reacts with one of the C=N bonds in DCC to yield the zincate-borylation intermediate INT1 (a model for compound 5). This step proceeds through transition structure TS1 with an overall barrier of 22.4 kcal/mol. In the second step, the nitrogen atom bonded to the boryl group further coordinates with the Zn metal center, resulting in the cleavage of the Zn-C bond and the release of Cy-NC through the transition structure TS2. The second step requires overcoming a barrier of 26.5 kcal/mol, which is higher than that of the first step. This elucidates the experimental observation of 6 (INT1 in Fig. 7) as a product at a lower temperature. These findings contrast sharply with the reaction of Mg-Bpin species with carbodiimide, where the boryl group attaches to the electrophilic carbon center in the final product[33]. Consequently, we also examined the hypothetical reaction pathway in which the Bcat acts as a nucleophile, attacking the electrophilic carbon atom of DCC. However, such a reaction mechanism needs to overcome a much higher energy barrier of 32.3 kcal/mol (see Supplementary information). Based on these results, we can conclude that a unique concerted reactivity of the Zn-B bond contributes to such an interesting isonitrile elimination result observed in the reaction with DCC.
In summary, we employed a straightforward metathesis strategy to synthesize a Zn-boryl complex from a Zn imide complex and B2Cat2. This Zn-Bcat complex exhibits a unique Zn-boron bond, displaying significant differences from Zn-boryl compounds derived from lithium-boryl precursors. The enhanced covalent character boosts its reactivity towards unsaturated nitrogen-containing bonds, enabling it to exhibit behavior akin to highly reactive diborane(4) species67,70,71. The Zn-Bcat complex can act as a typical nucleophile, reacting with CH3I to form C-B bonds. Additionally, it activates carbodiimide (DCC), facilitating the addition of C=N bonds and cleaving inert C=N double bonds through a unique concerted reactivity, leading to the generation of imide Zn complexes. It also activates NC triple bonds in isonitrile, converting them to double bonds while facilitating C-C single-bond coupling. Furthermore, the N=N double bond of azobenzene can be inserted into the Zn-B bond, resulting in borylated N-N bonded species, and it further functions as a catalyst in the diborylation of azobenzene. DFT computational results confirm the enhanced covalent character of the Zn-B bond, indicating that its reaction pathway differs from that of known nucleophilic boryl reagents. Ongoing studies in our laboratory focus on exploring the reactivity of zinc-boron bonded species with various unsaturated substrates and their catalytic applications in organic synthesis.
Methods
Synthesis of 1
Compound 2 (58.3 mg, 0.1 mmol) was dissolved in 2 mL of toluene in a 4 mL vial. One equivalent of bis(catecholato)diboron (B₂cat₂, 23.7 mg, 0.1 mmol) was then added as a solid. The solution was stirred overnight, during which the color changed from pale yellow to chartreuse. The solvent was removed under vacuum, and the residue was extracted with hexane (2 mL, three times) and filtered through Celite. The pure product was isolated as single crystals from a frozen concentrated hexane solution (31.9 mg, 53%).
1H NMR (400 MHz, C6D6) δ (ppm) = 7.09 (m, 6H, Ar-H), 6.82 (m, 4H, OCCHCH, cat-Ph), 6.55 (m, 4H, OCCHCH, cat-Ph), 5.09 (s, 1H, NC(CH3)CH), 3.34 (sept, J = 6.6 Hz, 4H, CH(CH3)2), 1.73 (s, 6H, NC(CH3)CH), 1.44 (d, J = 6.8 Hz, 12H, CH(CH3)2), 1.20 (d, J = 6.9 Hz, 12H, CH(CH3)2).13C{1H} NMR (101 MHz, C6D6) δ (ppm) = 167.6 (NC(CH3)CH), 148.4 (cat-Ph, OCCHCH), 144.7 (nac-Ph, N-C), 141.6 (nac-Ph, Ortho-C), 126.2 (nac-Ph, Meta-C), 123.8 (nac-Ph, Para-C), 121.8 (cat-Ph, OCCHCH), 112.1 (cat-Ph, OCCHCH), 96.2 (NC(CH3)CH), 28.5 (CH(CH3)2), 24.6 (CH(CH3)2), 23.4 (CH(CH3)2), 23.4 (NC(CH3)CH).11B NMR (96 MHz, C6D6) δ (ppm) = 39.7 (W1/2 = 589 Hz)HRMS: simulated (C35H45BN2O2Zn+H)+ = 601.2944, found = 601.2972 Element analysis (C76H104B2N4O4Zn2, 2 M+hexane) = C 70.76%, H 8.13%, N 4.34%; found C 70.33%, H 8.09%, N 4.28%.
Large-scale synthesis: Compound 2 (583 mg, 1 mmol) was dissolved in 12 mL of toluene in a 25 mL Schlenk tube. Bis(catecholato)diboron (B₂cat₂, 260 mg, 1.1 mmol) was then added as a solid. The solution was stirred overnight, during which the color changed from pale yellow to chartreuse. The solvent was removed under vacuum, and the residue was extracted with hexane (8 mL, three times) and filtered through Celite. The product was isolated as a white powder from a frozen concentrated hexane solution and further washed with cold hexane (2 mL, twice) to obtain pure products (294.3 mg, 49%).
Data availability
All the details of the NMR spectrum, crystal tables, and computational details were included in the supplementary information. Deposition numbers 2385052 (for 6), 2385048 (for 8), 2385050 (for 3), 2418166 (for 5), 2385054 (for 1•hexane), 2417677(for 1•toluene), and 2417391 (for 7) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/datarequest/cif. Source Data are provided with this manuscript. All data are available from the corresponding author upon request.
References
Knochel, P. & Singer, R. D. Preparation and reactions of polyfunctional organozinc reagents in organic synthesis. Chem. Rev. 93, 2117–2188 (1993).
Pu, L. & Yu, H.-B. Catalytic asymmetric organozinc additions to carbonyl compounds. Chem. Rev. 101, 757–824 (2001).
Rappoport, Z. & Marek, I. The Chemistry of Organozinc Compounds: R-Zn (John Wiley & Sons, 2007).
Bollermann, T., Gemel, C. & Fischer, R. A. Organozinc ligands in transition metal chemistry. Coord. Chem. Rev. 256, 537–555 (2012).
Erdik, E. Organozinc Reagents in Organic Synthesis (CRC Press, 2020).
Eckert, P., Sharif, S. & Organ, M. G. Salt to taste: the critical roles played by inorganic salts in organozinc formation and in the negishi reaction. Angew. Chem. Int. Ed. 60, 12224–12241 (2021).
Wei, B. & Knochel, P. Recent advances in cross-couplings of functionalized organozinc reagents. Synthesis 54, 246–254 (2022).
Feringa, B. L., Naasz, R., Imbos, R. & Arnold, L. A. Modern Organocopper Chemistry. Ch. 7 (Wiley-VCH, Weinhim, 2002).
Li, Y., Junge, K. & Beller, M. Zinc Catalysis. Ch. 2 (Wiley-VCH, Weinhim, 2015).
Lei, A., Huang, Z. & Liu, D. Zinc Catalysis. Ch. 11 (Wiley-VCH, Weinhim, 2015).
Mondal, B. & Roy, U. K. Making and breaking of Zn–C bonds in the cases of allyl and propargyl organozincs. Tetrahedron 90, 132169 (2021).
Chamberlain, B. M. et al. Polymerization of lactide with zinc and magnesium β-diiminate complexes: stereocontrol and mechanism. J. Am. Chem. Soc. 123, 3229–3238 (2001).
Cheng, M. et al. Single-site β-diiminate zinc catalysts for the alternating copolymerization of CO2 and epoxides: catalyst synthesis and unprecedented polymerization activity. J. Am. Chem. Soc. 123, 8738–8749 (2001).
Chisholm, M. H., Gallucci, J. & Phomphrai, K. Coordination chemistry and reactivity of monomeric alkoxides and amides of magnesium and zinc supported by the diiminato ligand CH(CMeNC6H3-2,6-iPr2)2. A Comparative Study. Inorg. Chem. 41, 2785–2794 (2002).
Nagashima, Y., Yukimori, D., Wang, C. & Uchiyama, M. In situ generation of silylzinc by Si-B bond activation enabling silylzincation and silaboration of terminal alkynes. Angew. Chem. Int. Ed. 57, 8053–8057 (2018).
McNaught, A. D. & Wilkinson, A. Compendium of Chemical Terminology (Blackwell Science Oxford, 1997).
Stephan, D. W. The broadening reach of frustrated Lewis pair chemistry. Science 354, aaf7229 (2016).
Légaré, M.-A., Pranckevicius, C. & Braunschweig, H. Metallomimetic chemistry of boron. Chem. Rev. 119, 8231–8261 (2019).
Kajiwara, T., Terabayashi, T., Yamashita, M. & Nozaki, K. Syntheses, structures, and reactivities of borylcopper and -zinc compounds: 1,4-silaboration of an alpha,beta-unsaturated ketone to form a gamma-siloxyallylborane. Angew. Chem. Int. Ed. 47, 6606–6610(2008).
Campos, J. & Aldridge, S. Catalytic borylation using an air-stable zinc boryl reagent: systematic access to elusive acylboranes. Angew. Chem. Int. Ed. 54, 14159–14163 (2015).
Lu, W., Hu, H., Li, Y., Ganguly, R. & Kinjo, R. Isolation of 1,2,4,3-triazaborol-3-yl-metal (Li, Mg, Al, Au, Zn, Sb, Bi) derivatives and reactivity toward CO and isonitriles. J. Am. Chem. Soc. 138, 6650–6661 (2016).
Nagashima, Y., Takita, R., Yoshida, K., Hirano, K. & Uchiyama, M. Design, generation, and synthetic application of borylzincate: borylation of aryl halides and borylzincation of benzynes/terminal alkyne. J. Am. Chem. Soc. 135, 18730–18733 (2013).
Bose, S. K. et al. Zinc-catalyzed dual C-X and C-H borylation of aryl halides. Angew. Chem. Int. Ed. 54, 11843–11847 (2015).
Zhang, B., Zou, Y., Wang, L. & Zhang, H. Zinc catalysed C3-H borylation of indoles and 1,1-diboration of terminal alkynes. Chem. Commun. 57, 11185–11188 (2021).
Li, Y. et al. Zinc complexes with an ethylene-bridged Bis(β-diketiminate) ligand: syntheses, structures, and applications as catalysts in the borylation of aryl iodides. Organometallics 40, 482–489 (2021).
Grundy, M. E., Yuan, K., Nichol, G. S. & Ingleson, M. J. Zinc catalysed electrophilic C-H borylation of heteroarenes. Chem. Sci. 12, 8190–8198 (2021).
Kaur, U., Saha, K., Gayen, S. & Ghosh, S. Contemporary developments in transition metal boryl complexes: An overview. Coord. Chem. Rev. 446, 214106 (2021).
Laitar, D. S., Müller, P. & Sadighi, J. P. Efficient homogeneous catalysis in the reduction of CO2 to CO. J. Am. Chem. Soc. 127, 17196–17197 (2005).
Zinser, C. M. et al. Synthesis and reactivity of [Au(NHC)(Bpin)] complexes. Chem. Commun. 55, 6799–6802 (2019).
Suzuki, A., Guo, X., Lin, Z. & Yamashita, M. Nucleophilic reactivity of the gold atom in a diarylborylgold(i) complex toward polar multiple bonds. Chem. Sci. 12, 917–928 (2020).
Suzuki, A., Wu, L., Lin, Z. & Yamashita, M. Isomerization of a cis-(2-Borylalkenyl)Gold Complex via a Retro-1,2-Metalate Shift: Cleavage of a C-C/C-Si Bond trans to a C-Au Bond. Angew. Chem. Int. Ed. 60, 21007–21013 (2021).
Bose, S. K., Fucke, K., Liu, L., Steel, P. G. & Marder, T. B. Zinc-catalyzed borylation of primary, secondary and tertiary alkyl halides with alkoxy diboron reagents at room temperature. Angew. Chem. Int. Ed. 53, 1799–1803 (2014).
Pécharman, A.-F. et al. Easy access to nucleophilic boron through diborane to magnesium boryl metathesis. Nat. Commun. 8, 15022 (2017).
McWilliams, S. F. et al. Coupling dinitrogen and hydrocarbons through aryl migration. Nature 584, 221–226 (2020).
Tsai, Y.-C. The chemistry of univalent metal β-diketiminates. Coord. Chem. Rev. 256, 722–758 (2012).
Pécharman, A. F., Hill, M. S., McMullin, C. L. & Mahon, M. F. Magnesium Boryl Reactivity with 9-BBN and Ph3B: Rational B− B′ Bond Formation and Diborane Isomerization. Angew. Chem. Int. Ed. 56, 16363–16366 (2017).
Pécharman, A.-F., Hill, M. S. & Mahon, M. F. Diborane heterolysis: breaking and making B–B bonds at magnesium. Dalton Trans. 47, 7300–7305 (2018).
Pécharman, A.-F., Hill, M. S., McMullin, C. L. & Mahon, M. F. BO2]− as a Synthon for the Generation of Boron-Centered Carbamate and Carboxylate Isosteres. Angew. Chem. Int. Ed. 59, 13628–13632(2020).
Griffin, L. P. & Aldridge, S. Zinc borylation and reduction by a diborane(4) species via B–O bond formation. Chem. Sci. 15, 19577–19582 (2024).
Li, B., Weinert, H. M., Wölper, C. & Schulz, S. Reactions of Zinc hydride with silylenes: from oxidative addition to ligand exchange reactions. Organometallics 42, 457–464 (2023).
Hughes, J. W. J. et al. NacNac-zinc-pyridonate mediated ε-caprolactone ROP. Dalton Trans. 52, 17767–17775 (2023).
Drescher, W. & Kleeberg, C. Terminal versus bridging boryl coordination in N-heterocyclic carbene copper(I) boryl complexes: syntheses, structures, and dynamic behavior. Inorg. Chem. 58, 8215–8229 (2019).
Charman, R. S. C. et al. Acyclic boryl complexes of copper(I). Chem. Eur. J. 30, e202302704 (2024).
Babula, D. J. et al. Ring-expanded N-heterocyclic copper(I) boryl complexes: the structures of [(6-Dipp)CuBcat], [(6-Dipp)CuBneop], and [(6-Dipp)CuBhex]. Eur. J. Inorg. Chem. 26, e202300043 (2023).
Semba, K., Shinomiya, M., Fujihara, T., Terao, J. & Tsuji, Y. Highly selective copper-catalyzed hydroboration of allenes and 1,3-dienes. Chem. Eur. J. 19, 7125–7132 (2013).
Okuno, Y., Yamashita, M. & Nozaki, K. Borylcyanocuprate in a one-pot carboboration by a sequential reaction with an electron-deficient alkyne and an organic carbon electrophile. Angew. Chem. Int. Ed. 50, 920–923 (2011).
Irvine, G. J. et al. Transition metal−boryl compounds: synthesis, reactivity, and structure. Chem. Rev. 98, 2685–2722 (1998).
Saha, K., Roy, D. K., Dewhurst, R. D., Ghosh, S. & Braunschweig, H. Recent advances in the synthesis and reactivity of transition metal σ-borane/borate complexes. Acc. Chem. Res. 54, 1260–1273 (2021).
Braunschweig, H., Dewhurst, R. D. & Schneider, A. Electron-precise coordination modes of boron-centered ligands. Chem. Rev. 110, 3924–3957 (2010).
Guo, X., Yang, T., Sheong, F. K. & Lin, Z. Beyond the nucleophilic role of metal–boryl complexes in borylation reactions. ACS Catal. 11, 5061–5068 (2021).
Guo, X. & Lin, Z. Boryls, their compounds and reactivity: a structure and bonding perspective. Chem. Sci. 15, 3060–3070 (2024).
Segawa, Y., Yamashita, M. & Nozaki, K. Boryllithium: isolation, characterization, and reactivity as a boryl anion. Science 314, 113–115 (2006).
Segawa, Y., Suzuki, Y., Yamashita, M. & Nozaki, K. Chemistry of boryllithium: synthesis, structure, and reactivity. J. Am. Chem. Soc. 130, 16069–16079 (2008).
Laitar, D. S., Tsui, E. Y. & Sadighi, J. P. Catalytic diboration of aldehydes via insertion into the copper−boron bond. J. Am. Chem. Soc. 128, 11036–11037 (2006).
Dai, C. et al. Synthesis and Characterization of Rhodium(I) Boryl and Rhodium(III) Tris(Boryl) Compounds: Molecular Structures of [(PMe3)4Rh(B(cat))] and fac-[(PMe3)3Rh(B(cat))3] (cat = 1,2-O2C6H4). Inorg. Chem. 36, 272–273 (1997).
Blake, M. P., Kaltsoyannis, N. & Mountford, P. Synthesis, molecular and electronic structure, and reactions of a Zn–Hg–Zn bonded complex. Chem. Commun. 51, 5743–5746 (2015).
Vedani, L., Gnägi-Lux, M., Dénès, F. & Renaud, P. Intramolecular asymmetric cyclopropanation using air-stable alkylboronic esters. Synlett 34, 2232–2238 (2023).
Boronski, J. T. et al. Inducing nucleophilic reactivity at beryllium with an aluminyl ligand. J. Am. Chem. Soc. 145, 4408–4413 (2023).
Boronski, J. T. et al. On the nature and limits of alkaline earth–triel bonding. Chem. Sci. 15, 15377–15384 (2024).
Matler, A. et al. Reactivity of terminal iron borylenes and Bis(borylenes) with carbodiimides: cycloaddition, metathesis, insertion and C−H activation pathways. Eur. J. Inorg. Chem. 2021, 4619–4631(2021).
Nutz, M. et al. Synthesis and trapping of iminoboranes by M=B/C=N bond metathesis. Angew. Chem. Int. Ed. 56, 7975–7979 (2017).
Braunschweig, H. et al. Interactions of isonitriles with metal–boron bonds: insertions, coupling, ring formation, and liberation of monovalent boron. Chem. Eur. J. 22, 11736–11744 (2016).
Zhang, C. et al. Evidence for carbene intermediates in isocyanide homologation by aluminium(I). Angew. Chem. Int. Ed. 62, e202307352 (2023).
Haynes, W. M., Lide, D. R. & Bruno, T. J. CRC Handbook of Chemistry and Physics 95th edn (CRC Press, Boca Raton, 2014).
Braunschweig, H. & Kupfer, T. Stoichiometric and homogeneous-catalytic diboration of the NN double bond of azobenzene. J. Am. Chem. Soc. 130, 4242–4243 (2008).
Beagan, D. M., Carta, V. & Caulton, K. G. A reagent for heteroatom borylation, including iron mediated reductive deoxygenation of nitrate yielding a dinitrosyl complex. Dalton Trans. 49, 1681–1687(2020).
Katsuma, Y., Wu, L., Lin, Z., Akiyama, S. & Yamashita, M. Reactivity of highly lewis-acidic diborane(4) toward C≡N and N=N Bonds: uncatalyzed addition and N=N bond-cleavage reactions. Angew. Chem. Int. Ed. 58, 317–321 (2019).
Ansell, M. B., Kostakis, G. E., Braunschweig, H., Navarro, O. & Spencer, J. Synthesis of functionalized hydrazines: facile homogeneous (N-heterocyclic carbene)-palladium(0)-catalyzed diboration and silaboration of azobenzenes. Adv. Synth. Catal. 358, 3765–3769 (2016).
Wang, G. et al. Homolytic cleavage of a B−B bond by the cooperative catalysis of two Lewis bases: computational design and experimental verification. Angew. Chem. Int. Ed. 55, 5985–5989(2016).
Prieschl, D. et al. Synthesis of complex boron–nitrogen heterocycles comprising borylated triazenes and tetrazenes under mild conditions. J. Am. Chem. Soc. 142, 1065–1076 (2020).
Katsuma, Y., Tsukahara, N., Wu, L., Lin, Z. & Yamashita, M. Reaction of B(2) (o-tol)(4) with CO and isocyanides: cleavage of the C identical withO triple bond and direct C-H borylations. Angew. Chem. Int. Ed. 57, 6109–6114 (2018).
Acknowledgements
This work was supported by grants from the Research Grants Council of The Hong Kong Special Administration Region (Project Nos. CityU 21310922, 11306523 and HKUST 16302222, 16301823) the National Natural Science Foundation of China (Project No 22301253), and a start-up fund from the City University of Hong Kong (Project No 9610578).
Author information
Authors and Affiliations
Contributions
G.X. performed all the experimental work. H.T.C. conducted the DFT calculations. Z. Lin supervised the computational study. S.L. and T.Y.W. prepared some of the starting materials. L.Z. and Q.Z. conducted the X-ray single-crystal characterization. Z. Lu conceived the ideas and designed and directed the research. All the authors revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare the following competing interest(s): A patent application has been filed (applicant: City University of Hong Kong; name of inventor(s): Z. Lu, G.X.; application number 63/728, 912; specific aspect of manuscript covered in patent application, Multi-Functional Zinc-Boryl Reagent for Efficient Synthesis and Catalysis). The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, G., Chan, H.T., Li, S. et al. A zinc boryl compound unlocks diverse reactivity pathways beyond nucleophilic borylation. Nat Commun 16, 6349 (2025). https://doi.org/10.1038/s41467-025-61062-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-61062-z