Abstract
Electrocatalytic nitrate reduction (NO3RR) is a promising route for sustainable ammonia synthesis under mild conditions. The widely studied Co-based catalysts undergo significant reconstruction due to nitrate oxidation and electric-field reduction during NO3RR, leading to activity degradation. To address this issue, we develop a Co6Ni4 heterostructured catalyst that consists of interlaced metallic Co and Ni domains. Operando X-ray absorption spectroscopy and other in-situ characterization techniques, in conjunction with theoretical calculations, demonstrate that Ni domains function as electron reservoir, which transfer electrons to Co and prevent the accumulation of high-valence Co. Besides, the abundant Co/Ni interfaces also facilitate the NO3RR process, thereby achieving a NH3 Faraday efficiency of 99.21%, a NH3 yield rate of 93.55 mg h-1 cm-2, and a NO3RR stability of 120 h. Our analyses delve into the underlying causes of the observed stability of metallic Co in Co6Ni4 and provide compelling evidence that the discrepancy between the adsorption quantity of NO3- on catalyst surface and the corresponding electron supply is a pivotal factor influencing the reconstruction process.
Similar content being viewed by others
Introduction
Ammonia (NH3) is considered as one of the most promising green energy carriers owing to its high energy density (13.6 MJ L−1), zero carbon emissions during combustion, mature transportation and storage schemes, as well as easy integration with hydrogen energy1,2,3. Because of the low bond energy (204 kJ mol−1) and the high water solubility of nitrate (NO3-)4,5, NH3 production by electrocatalytic NO3- reduction reaction (NO3RR) can proceed under mild conditions. Moreover, this process can easily be coupled with green electricity technology. Hence, it is considered as one of the technologies expected to replace the energy-intensive Haber process6,7. The recent development of plasma and microbubble technologies for the preparation of nitrates from air is anticipated to address the issue of raw material supply for NO3RR8,9.
Currently, it is imperative to develop electrocatalysts with high Faraday efficiency (FE), high yield rate of NH3, as well as high energy efficiency (EE) for the commercialization of NO3RR. Attaining a high NH3 yield rate requires high current density, but this usually is achieved at high overpotential, which may reduce the EE and promote the competing hydrogen evolution reaction (HER)10,11,12. In alkaline media, HER is suppressed and the NO3RR current throughput on most non-noble metals is enhanced, hence most studies on high NH3 yield rate are conducted in alkaline electrolyte13,14,15. NO3RR is a complex 8-electron reduction process. Typically, NO₂—a relatively stable intermediate—serves as a demarcation intermediate, allowing the cascade reaction to be divided into two steps: the reduction of NO3- to NO2- and the subsequent hydrogenation reduction of NO2-16,17.
Cobalt-based catalysts, such as Co-containing compounds18, alloys19, and single-atom catalysts20, have been extensively investigated due to their capacity to facilitate the conversion of NO3- to NO2- and NO2- to NH3 simultaneously. Among them, heterostructured catalysts have garnered significant attention from researchers owing to their advantages in regulating the electronic structure to improve the adsorption of intermediates21,22, and providing multiple active sites for the adsorption of complex intermediates, thereby facilitating the tandem progression of the NO3RR23,24. However, due to the strong oxidability of NO3- and the reducing property of the applied electric field, Co-based catalysts usually exhibit a violent reconstruction during the NO3RR process, and their reconstruction behavior appears to be controversial25,26. For example, research found that high-valence Co compounds were reduced to metallic Co, which was considered as the active sites27,28,29, and some studies also claimed that metallic Co can be transformed into Co(OH)2 during NO3RR, which can facilitate water splitting and thus the hydrogenation steps in NO3RR30,31. While the catalytic activity of Co(OH)2 towards NO3RR is significantly diminished in comparison to that of metallic Co32. In particular, it has been reported that the accumulation of high-valence Co would break the “three-step relay mechanism” and thus deteriorate catalytic performance33, but this issue still remains unresolved and is lack of systematic study. Most current studies on Co-based catalysts primarily emphasize performance improvement, with little exploration into the underlying causes of its reconstruction. A deep understanding of the reconstruction behavior, especially at industrial-level current densities, is essential for designing catalysts that meet industrial requirements, yet awaiting to be explored.
In this work, we prepared a series of CoxNi10-x (0 ≤ x ≤ 10) heterostructured catalysts consisting of small-size Ni and Co domains, among which the Co6Ni4 catalyst demonstrates noteworthy NO3RR activity and stability. It only requires − 0.276 V (vs. reversible hydrogen electrode, RHE) to attain the current density of 1 A cm−2, with a high FENH3 97.85%. Moreover, the catalyst displays the highest FENH3 of 99.21% with an NH3 yield of 93.55 mg h−1 cm−2 and demonstrates stability over 120 h. Operando X-ray absorption spectroscopy (XAS) and in situ Raman spectroscopy, combined with in situ electrochemical impedance spectroscopy (EIS) and density functional theory (DFT) calculations, prove that the mismatch between the amount of NO3- adsorbed on the catalyst surface and the electron supply is the key to the reconstruction. Furthermore, in situ attenuated total reflection-Fourier transform infrared spectrometer (ATR-FTIR) and other experimental techniques reveal that the Ni domains in the Co6Ni4 heterostructured catalyst influence the adsorption of NO3- and NO2-, alter the electronic structure of Co, and enhance the electron transport of the catalyst, thereby rendering Co in a stable electron-rich state. Consequently, the reconstruction of Co and the accumulation of Co(OH)2 are effectively inhibited, which endows the Co6Ni4 with stable NO3RR performance. Moreover, a substantial number of Co/Ni interfaces assures optimal electron transfer capability and superior NO3RR performance. Finally, the Co6Ni4 catalyst is employed as a bifunctional catalyst to achieve a steady output of NH3 for up to 240 h in a membrane electrode assembly system.
Results
Material characterization
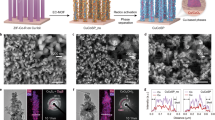
The Co6Ni4 heterostructured catalyst and control samples with single components (i.e., Ni catalyst and Co catalyst) were electrodeposited onto the carbon paper (CP). All the catalysts have a cauliflower-like morphology (Supplementary Fig. 1). The Co6Ni4 catalyst is composed of hexagonal close-packed (hcp) Co (PDF#05-0727) and face-centered cubic (fcc) Ni (PDF#04-0850), as illustrated by the X-ray diffraction (XRD) pattern (Supplementary Fig. 2). The transmission electron microscopy (TEM) image indicates that the particles of Co6Ni4 are composed of lamellar and dendritic structures (Fig. 1a). High-resolution transmission electron microscopy (HR-TEM) images show that Co6Ni4 is constituted of randomly interlaced Co and Ni nanodomains (Fig. 1b). The disordered atom arrangement and well-defined lattice fringes of the (100) plane manifest the Co domains in Co6Ni4 have both amorphous and hcp phases (Fig. 1c). The lattice spacing of fcc-Ni(111) was observed in Fig. 1d, confirming the high crystallinity of Ni domains. The selected area electron diffraction (SAED) also reveals the poor crystallinity of Co domains and high crystallinity of Ni domains in Co6Ni4. The high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) image and the corresponding Energy-dispersive X-ray spectroscopic (EDS) elemental mappings reveal a uniform distribution of Ni and Co elements on the submicron scale (Fig. 1e and Supplementary Fig. 3), and the atomic ratio of Co to Ni is 6:4 (Supplementary Fig. 4). However, the distribution of Ni and Co elements is uneven on the nanoscale, as evidenced by the EDS linear scanning analysis (Fig. 1e), indicating the formation of a heterostructure. Furthermore, the interlaced distribution of Co and Ni domains in Co6Ni4 was further confirmed by aberration-corrected (AC) HAADF-STEM images (Supplementary Fig. 5) and corresponding EDS elemental mapping (Fig. 1f). The aforementioned characterization techniques corroborate the assertion that Co6Ni4 is a heterostructured catalyst composed of hcp/amorphous Co domains and fcc Ni nanocrystalline domains. The control sample of Co catalyst also exhibits a mixed phase of hcp and amorphous, whereas, for the control sample of Ni catalyst, it exhibits an fcc structure with high crystallinity (Supplementary Figs. 1, 2, and 6).
a TEM image of Co6Ni4 and (b–d) corresponding HR-TEM images. The inset in (a) shows the SAED pattern. e TEM-EDS line-scan of the region marked with white arrows in the inset. f AC-HAADF-STEM image and the corresponding elemental mappings of Co6Ni4. g Co K-edge and i Ni K-edge XANES spectra of Co6Ni4 and the references. h, j The k3-weighted Fourier transform of the k-function from Co K-edge and Ni K-edge EXAFS, respectively. Source data for the figures (e, g–j) are provided as a Source Data file.
The oxidation state of the prepared catalysts was examined by X-ray photoelectron spectroscopy (XPS) and X-ray absorption near-edge structure (XANES). The XPS survey spectra of Co6Ni4, Co, and Ni catalysts demonstrate that all of them contain O (Supplementary Fig. 7a, b, e, h), due to the inevitable oxidation in air34,35. In the Co 2p spectra, the peaks at 778.6 eV and 781.0 eV can be attributed to Co0 and Co2+, respectively36 (Supplementary Fig. 7c, f). In the Ni 2p spectra, the peaks at 852.9 eV and 855.8 eV can be assigned to Ni0 and Ni2+, respectively (Supplementary Fig. 7d, g). In the Co6Ni4 catalyst, the binding energy of Co0 exhibits a shift of 0.2 eV to lower energy compared to that in Co catalyst, while the binding energy of Ni0 displays a shift of 0.3 eV to higher energy compared to that in Ni catalyst, which suggests that there is an electronic interaction between Co and Ni in Co6Ni4 catalyst, resulting in the electron cloud center shifting towards Co37,38. The XANES spectra and extended X-ray absorption fine structure (EXAFS) are highly sensitive to the oxidation state39,40 and local environment41,42. In the Co K-edge XANES spectra (Fig. 1g), the absorption edge energy of Co6Ni4 is located below the Co foil, implying that the Co component in Co6Ni4 is in an electron-rich state. The Ni K-edge XANES spectrum of Co6Ni4 resembles that of Ni foil, but the absorption edge energy of Co6Ni4 is slightly higher than that of Ni foil (Fig. 1i), indicating the Ni domains in Co6Ni4 are in an electron-deficient state due to the shift of the electron cloud center towards Co. Similarly, the wavelet transforms (WTs) for the k3-weighted Co K-edge and Ni K-edge XAFS signals of Co6Ni4 catalyst show the characteristics of Co foil and Ni foil (Supplementary Fig. 8), respectively, confirming that the Co and Ni components in Co6Ni4 are predominantly in a metallic state. Moreover, in the Co K-edge EXAFS spectrum of Co6Ni4 catalyst, the main peak at 2.18 Å can be ascribed to Co-M (Co/Ni) coordination (Fig. 1h), while the peak intensity is obviously lower than that of Co foil, implying the lower atomic ordering of the Co component in Co6Ni443. Similarly, the main peak in the Ni K-edge EXAFS spectrum of Co6Ni4 catalyst (Fig. 1j), the main peak at 2.18 Å is assigned to Ni-M (Ni/Co) coordination and has almost the same intensity as that of Ni foil, indicating the high atomic ordering of Ni component in Co6Ni444. To summarize, the XAS results suggest that the Co component in Co6Ni4 is predominantly in the metallic state with poor atomic ordering, whereas the Ni component in Co6Ni4 is also mainly in the metallic state but with high atomic ordering. Both the XAS and XPS results imply the electronic shift between Ni and Co domains.
Electrocatalytic NO3RR performance
The electrocatalytic NO3RR performance of the prepared electrocatalysts was conducted using an H-type cell with a three-electrode system. All potentials are referenced to the RHE. Initially, the CP was measured and its reduction current response to NO3- was found to be minimal, thereby indicating that the CP did not exert discernible influence on the NO3RR performance evaluation of the catalysts (Supplementary Fig. 9)45. The UV-vis spectrophotometer was employed to quantitatively analyze the concentrations of NH3 and NO2- in the electrolyte after NO₃RR (Supplementary Fig. 10)46,47.
Linear sweep voltammetry (LSV) tests were performed in 1 M KOH with and without 0.3 M KNO3. Figure 2a demonstrates a significant enhancement in the current density of both catalysts upon adding KNO3, indicating the occurrence of the NO3RR process. The Co6Ni4 catalyst exhibits the most pronounced NO3RR response, reaching a current density of 1 A cm−2 at − 0.276 V. The FE and yield rate of NH3 were calculated by collecting the electrolyte after 1-hour chronoamperometric test and subsequently analyzing the product (Supplementary Fig. 11). Co6Ni4 catalyst exhibits an FENH3 greater than 90% across a wide potential range from − 0.176 V to − 0.576 V. At an applied potential of − 0.476 V, the FENH3 reaches its maximum value of 99.21%, accompanied by a high yield rate of 93.55 mg h−1 cm−2. Furthermore, the highest NH3 yield rate of 102.94 mg h−1 cm−2 was observed at − 0.576 V, accompanied by an FENH3 of 94.59% (Fig. 2b, c). The FE and yield rate of NH3 in Co and Ni catalysts exhibit a notable decline in comparison to that observed in Co6Ni4 catalyst, except for potentials of − 0.076 V and − 0.176 V, at which Co catalyst manifests greater FENH3 than Ni and Co6Ni4 catalysts. Subsequently, by-product analyses indicate that the FE of NO2- follows the order: Ni catalyst > Co6Ni4 catalyst > Co catalyst, and N2H4 was undetected (Supplementary Fig. 12a–c). The decreased FE of H2 observed for the synthesized catalysts suggests that the NO3RR dominates the reaction process (Supplementary Fig. 12d). Moreover, the half-cell EE of the catalysts was calculated by assuming that the overpotential of OER is zero. It suggests that when the applied potential is less than − 0.176 V, which corresponds to a larger reduction current of NO3-, Co6Ni4 catalyst exhibits the highest EE in comparison to Co and Ni catalysts (Fig. 2d). Conversely, when the applied potential is greater than − 0.176 V, Co exhibits the highest EE among the three catalysts, due to its higher FENH3. Additionally, the results of 40 consecutive cycles of operation demonstrated the stability of the NH3 production on Co6Ni4, which exhibited an average FENH3 of 95.83% and an average NH3 yield rate of 82.74 g h−1 cm−2 for a 120 h test (Fig. 2e and Supplementary Fig. 13). The structure and morphology of Co6Ni4 remained largely unchanged after the cycling, except for the formation of corrosion-induced pores on the surface (Supplementary Figs. 14–16).
a LSV curves (without iR compensation) of Co6Ni4, Co, and Ni catalysts for NO3RR and HER. b FE, c Yield rate, and d Energy efficiency of NH3 for Co6Ni4, Co, and Ni catalysts (potentials are not iR-corrected). The chronoamperometry tests were performed in various applied potentials for 1 h to evaluate the NO3RR performance of prepared catalysts. e Stability test of Co6Ni4 catalyst for 120 h continuous NO3RR at − 0.476 V (vs. RHE) (without iR compensation), during which the electrolyte was sampled for FE and yield calculation every 3 h, and then the cell chambers were filled with fresh electrolyte. The electrolyte for NO3RR and HER is 1 M KOH solution with and without 0.3 M KNO3. f 1H NMR spectra of the electrolytes after NO3RR using 0.1 M K14NO3 and K15NO3 as the nitrogen sources or without nitrate. g Comparison of FE and yield rate of NH3 produced by Co6Ni4 catalyst measured by UV-vis spectrophotometer and NMR. h Comparisons of NO3RR performances between Co6Ni4 and recent reported catalysts. The overpotential corresponding to the 1 A cm−2 is inversely proportional to the color intensity. The error bar represents the standard deviation of the data from three independent experiments. The source data for the above figures are provided as a Source Data file.
The 15N isotope labeling experiments were conducted to verify that the produced NH3 was derived from the feeding NO3- electrolyte (Fig. 2f). After electrolysis at − 0.376 V, triple coupling and doublet peaks corresponding to 14NH4+ and 15NH4+ were detected in the 1H nuclear magnetic resonance (NMR) spectra of the electrolytes containing 14NO3- and 15NO3-, respectively48. In contrast, the NH3 peak was absent in the NMR spectrum without adding nitrate into the electrolyte. Moreover, the discrepancy between the NMR-derived results and those calculated by the UV method is within 5%, indicating that the test is accurate (Fig. 2f and Supplementary Fig. 17)49. Furthermore, the NO3RR performance of Co6Ni4 was evaluated at varying NO3- concentrations, which shows that the reduction current of Co6Ni4 towards NO3RR increases with the increase of NO3- concentration (Supplementary Fig. 18a). This is because an elevated NO3- concentration can effectively mitigate the impact of mass transfer during NO3RR50. Upon the application of a potential of −0.376 V, the FENH3 of Co6Ni4 is observed to exceed 85% in the range of 0.1–0.5 M NO3-. It is further noted that the maximum FE and yield rate of NH3 is achieved at a NO3- concentration of 0.3 M (Supplementary Fig. 18b). The Co6Ni4 reduced the concentration of NO3- from 0.3 M to 0.05 M in a 10 h continuous NO3RR. It is also noted that the FENH3 decreases with the reduction of NO3- concentration. The production of NO2- is minimal throughout the process (Supplementary Fig. 18c). The above results demonstrate that Co6Ni4 exhibits superior NO3RR comprehensive properties, exceeding the majority of previously reported catalysts, as depicted in Fig. 2d and Supplementary Table 2.
Electrocatalytic NO3RR mechanism of Co6Ni4
Given that Co-based catalysts typically exhibit violent restructuring during NO3RR, ex-situ XRD was initially employed to detect compositional alterations in Co6Ni4, Co, and Ni catalysts. It was found that a portion of the metallic Co present in the Co catalyst was oxidized to Co(OH)₂ under OCP conditions (Supplementary Fig. 19a). This observation suggests that the metallic Co will spontaneously react with NO3- in the electrolyte. As the applied reduction potential increases, the diffraction peak intensity of Co(OH)2 decreases, indicating that the reduction electric field counteracted the oxidation effect of NO3- on Co. When the reduction potential is set to − 0.176 V, the diffraction peak of Co(OH)2 is almost invisible, which indicates that the Co catalyst is in dynamic equilibrium between NO3- oxidation and electron reduction. Upon applying a negative potential of − 0.376 V, the diffraction peak of Co(OH)2 reappears and remains at a more negative potential. This indicates that the oxidation of NO3- to Co will be stronger than the reduction of electrons, with a negative shift of the applied potential. In comparison, the ex-situ XRD patterns of the Co6Ni4 and Ni catalysts did not exhibit obvious changes at varying test potentials (Supplementary Fig. 19b, c).
Since XRD can only detect the crystalline phase, and the ex-situ measurement may be unable to avoid the influence of environmental factors, in situ Raman spectroscopy is employed to observe the time-dependent alterations in catalyst composition throughout NO3RR. As illustrated in Fig. 3a, the Co catalyst exhibits the accumulation of Co(OH)2 at specific potential values during the NO3RR51,52, aligning with the findings of ex-situ XRD analysis. In contrast, the Co6Ni4 catalyst does not generate Co(OH)2 throughout the entire NO₃RR process (Fig. 3b). The ex-situ XRD patterns and in situ Raman spectra of the Ni catalyst in the NO3RR process demonstrate that there are no compositional alterations (Supplementary Figs. 19c and 20), implying that Ni component in Co6Ni4 circumvents the reconstruction of Co component.
In situ Raman spectra of a Co and b Co6Ni4 catalysts during NO3RR (potentials are not iR-corrected). Electrochemical operando XANES for Co K-edge of c Co and d Co6Ni4 catalysts (potentials are not iR-corrected). e The Co K-edge absorption energy from the operando XANES spectra of Co6Ni4 and Co catalysts (potentials are not iR-corrected). f Operando XANES for Ni K-edge of Co6Ni4 catalyst (potentials are not iR-corrected). g The Rct of prepared catalysts in NO3RR against applied potential (without iR compensation). h The comparison of the conductivity of Co6Ni4, Co, and Ni catalysts. The error bar represents the standard deviation of the data from three independent experiments. i The potential-dependent adsorption capacity of NO3- on Co6Ni4, Co, and Ni catalysts (potentials are not iR-corrected). Source data for the above figures are provided as a Source Data file.
Raman spectroscopy is a surface-sensitive technique which may have some limitations in characterizing the composition of materials. Hence, operando XAS was used to further investigate the reconstruction of the electrocatalysts under NO3RR conditions. The Co K-edge XANES curves of the Co catalyst demonstrate an obvious shift under varying applied potentials (Fig. 3c). Conversely, the Co K-edge XANES curves of Co6Ni4 exhibited minimal variation at different potentials (Fig. 3d). The variation in the Co absorption edge position in Co6Ni4 and Co catalysts during NO3RR is manifested in Fig. 3e. For the Co6Ni4 catalyst, the absorption edge energy of Co increases with the application of a reduction potential. Upon removing the applied potential, the absorption edge returns to a near-pristine state. The absorption edge energy is always below the Co foil, and the variation range is only 0.06 eV, indicating that the Co component in Co6Ni4 is in a highly stable electron-rich state. In the case of the Co catalyst, the air oxidation results in a slightly positive shift in the Co K-edge compared to that of the Co foil. Furthermore, the additional positive shift in absorption edge energy, both in the OCP state and upon applying a potential of − 0.076 V, suggests that some metallic Co has undergone oxidation. When the applied potential was − 0.276 V, the absorption edge energy exhibited a negative shift, indicating that the reconstructed high-valence Co was reduced. As the applied potential was further decreased, the absorption edge energy exhibited a positive shift, indicating the further oxidation of metallic Co. Once the applied potential was removed, the absorption edge energy retained a higher level. Throughout the entire testing process, the variation range of the absorption edge energy reached as high as 0.50 eV, and finally, Co remained in a higher valence, indicating that the Co catalyst underwent a significant reconstruction during NO3RR, accompanied by a considerable accumulation of high-valence Co. The Co K-edge EXAFS spectra also illustrate that the Co component in Co6Ni4 catalyst exhibits enhanced stability during NO3RR compared to the Co catalyst (Supplementary Fig. 21a, b). Moreover, the Ni component of Co6Ni4 is also in a highly stable state during NO3RR, which further corroborates that Ni can significantly impede Co reconstruction (Fig. 3f and Supplementary Fig. 21c).
To gain insight into the underlying mechanism of Ni inhibiting Co reconfiguration, in situ EIS (Supplementary Fig. 22) tests were conducted on the synthesized catalysts, and theoretical calculations were performed on the adsorption of NO3- by the catalyst at varying potentials (Supplementary Fig. 23). The Rct of the catalysts decreases as the potential becomes more negative53,54, and when the reduction potential exceeds -0.276 V, the Rct demonstrates minimal further decrease (Fig. 3g). Notably, for the Co catalyst, an increase in resistance is even observed at − 0.576 V. This can be attributed to the formation of hydroxides at sufficiently negative potentials. The conductivity of Co6Ni4 is also markedly higher than that of the Co catalyst, while the conductivity of the Ni catalyst exhibits a value between them (Fig. 3h), which is consistent with the results of in situ EIS. The electrochemical adsorption of NO3- onto the catalyst during NO3RR is enhanced by negatively shifting the applied potential according to the DFT calculations (Fig. 3i). Consequently, there is insufficient electron supply to the adsorbed NO3- on the Co catalyst surface when the applied potential is lower than − 0.376 V, leading to partial oxidation of Co into Co(OH)2. When the applied potential is between − 0.276 V and − 0.176 V, the electron migration capacity of the Co catalyst is sufficient to meet the demand for NO3- reduction, resulting in minimal Co(OH)2 generation. When the applied potential is higher than − 0.176 V, NO3- adsorbed on the surface of the Co catalyst cannot be reduced due to the weak reducing electric field, so part of the metallic Co is oxidized to Co(OH)2. In contrast, the Co6Ni4 heterostructured catalyst exhibits enhanced electron transport capacity, where electrons are continuously transferred from Ni domains to Co domains, rendering it in a stable electron-rich state. This facilitates rapid electron migration to the adsorption site for NO3- reduction, preventing Co oxidation.
To further elucidate the reason why the metallic Co component in Co6Ni4 can maintain its stability in tandem NO3RR, we investigated the adsorption of intermediates. CoxNiy (x and y represent the atomic ratios of Co and Ni) heterogeneous catalysts with different Ni and Co contents were synthesized to investigate the role of Co and Ni elements in NO3RR (Supplementary Figs. 24–26 and Table 1). It was observed that the reduction current of these catalysts for NO3RR increased in conjunction with the rise in Ni content. When the atomic ratio of Co to Ni is 6:4, the catalyst exhibits the most intense current response to NO3-. As the atomic ratio of Ni is further increased, the current intensity gradually declines (Fig. 4a). These findings imply that the incorporation of an optimal quantity of Ni into the electrocatalyst is beneficial for enhancing the NO3RR current (Fig. 4b). However, the introduction of an insufficient or excessive amount of Ni may impede the equilibrium between the reduction of NO3- to NO2- and the reduction of NO2- to NH3. The Co6Ni4 configuration exhibits the highest FENH3, which suggests that the tandem reaction proceeds most harmoniously (Supplementary Fig. 27a). Catalysts with increased Ni content will display increased FE of NO2-. This indicates that the NO2- adsorption onto Ni is poor, which leads to the accumulation of NO2- in the electrolyte. Conversely, catalysts with increased Co content will exhibit a reduced FE of NO2-. This indicates that the NO2- adsorption onto Co is strong, which can promote the further conversion of NO2- to NH₃ (Supplementary Fig. 27b). In addition, Co6Ni4 displays the largest double layer capacitance (Cdl) and the lowest electron transfer resistance (Rct) in NO3RR, which lends credence to its NO3RR performance (Fig. 4c and Supplementary Figs. 28, 29).
a LSV curves (without iR compensation) of NO3RR for Co, Co8Ni2, Co6Ni4, Co4Ni6, Co2Ni8, and Ni catalysts, which is red, purple, blue, orange, black and green, respectively. The shaded regions represent the error bars. b The FE of NH3 and NO2- for prepared catalysts at −0.276 V (without iR compensation). c The Cdl and Rct of synthesized catalysts, which is represented by blue histograms and red dots, respectively. d LSV-derived potentials and Tafel slopes for NO3RR, NO2RR using Co, Co6Ni4, Ni catalysts at the current density of 100 mA cm-2 (potentials are not iR-corrected). The error bar represents the standard deviation of the data from three independent experiments. e Adsorption energy of NO3- and NO2- on Co, Co6Ni4, and Ni catalysts. f The online DEMS spectra of Co6Ni4 under NO3RR condition. In situ ATR-FTIR spectra of (g) Co6Ni4, (h) Co, and (i) Ni catalysts measured under NO3RR condition (potentials are not iR-corrected). Source data for the above figures are provided as a Source Data file.
A comparative investigation of NO2RR and NO3RR was undertaken to further understand the role of Co and Ni components in heterostructured catalysts. Upon changing the reduction object from NO3- to NO2-, the overpotential and Tafel slope of Co6Ni4 and Ni catalysts increase, while the overpotential and Tafel slope of Co catalyst decreases (Fig. 4d and Supplementary Fig. 30). This further substantiates the hypothesis that Ni facilitates the adsorption of NO3-, while Co can promote the adsorption of NO2-, and they jointly promote the tandem reaction55. The findings suggest that the reaction kinetics of NO2RR is superior to NO3RR on Co catalysts, while the reaction kinetics of NO3RR is higher than NO2RR on Ni catalysts (Supplementary Fig. 31). We also use DFT to calculate the adsorption energy of NO2- and NO3- on various catalysts. It is found that the NO2- adsorption energy of the Co catalyst is greater than that of the Ni catalyst, whereas the NO3- adsorption energy of the Ni catalyst is greater than that of the Co catalyst (Fig. 4e). As expected, the Co/Ni interfaces of Co6Ni4 exhibits the strongest adsorption towards both NO2- and NO3-. Moreover, the NO2- adsorption on the Co component of Co6Ni4 and the NO3- adsorption on the Ni component of Co6Ni4 both are enhanced compared to the Co catalyst and Ni catalyst, respectively, which are conducive to the cascade electroreduction of NO3-. Therefore, the abundant Co/Ni interfaces and the synergistic effect between Co and Ni greatly contribute to the exceptional performance of the Co6Ni4 catalyst.
In situ ATR-FTIR was employed to detect the reaction intermediates of NO3RR at different potentials, thereby elucidating the underlying reaction pathway. The peaks appearing at about 1180, 1292, 1347, 1403, 1450, and 1637 cm−1 can be attributed to the reaction intermediates of *NH2OH, *NO2, *NO3, *NO3, *NH4+, and *H2O, respectively56,57,58,59. The peak intensity of NO3RR intermediates for the prepared catalysts is in the order of Co6Ni4 catalyst > Ni catalyst > Co catalyst (Fig. 4g–i), which is consistent with the performance of NO3RR. The *NO3 absorption peak strength of the Ni catalyst exhibits a notable increase in comparison to that of the Co catalyst, thereby indicating that Ni catalysts has higher NO3- adsorption capacity. Moreover, the Co catalyst has an obvious *NH2OH absorption peak and a weaker *NO2 absorption peak; while the relative strength of *NH2OH and *NO2 absorption peaks of the Ni catalyst is opposite to that of Co catalyst. This indicates that NO2- accumulation is easier to occur on the Ni catalyst and its subsequent hydrogenation ability is insufficient. The aforementioned results demonstrate that Ni and Co components in Co6Ni4 contribute to the equilibrium of NO3- → NO2- and NO2- → NH3, respectively. In addition, online differential electrochemical mass spectrometry (DEMS) has detected the signals of NH2OH, NHOH, and NOH, which strongly manifests that the NO3RR on Co6Ni4 catalyst follows the hydroxylamine reaction path (Fig. 4f and Supplementary Fig. 32), thus corroborating the in situ ATR-FTIR results60,61.
Based on the aforementioned in situ characterizations, we performed DFT calculations to reinforce the reaction mechanism. By calculating the charge density, it is evident that electron shift occurs at the Co/Ni interface of Co6Ni4, supporting our XAS and XPS analyses. Due to the delocalized nature of metallic bonds, the interfacial electron clouds undergo dynamic rearrangement. This ultimately leads to a shift of the electron cloud center towards Co, rendering it in an electron-enriched state, while Ni becomes electron-deficient (Fig. 5a). The electron-deficient state of Ni can enhance the adsorption of NO3-, while the electron-rich state of Co can facilitate the adsorption of *H and improve the hydrogenation process while maintaining structural stability62,63. Furthermore, the alteration in Gibbs free energy during the NO3RR process on Co6Ni4, Co and Ni catalysts was calculated. These three catalysts have the same rate-determining step (RDS), i.e., *NO → *NOH. The energy barrier of RDS on Co6Ni4 is 0.63 eV, which is lower than that on Ni (0.68 eV) and Co (1.11 eV) (Fig. 5b and Supplementary Fig. 33). Moreover, compared with Co and Ni catalysts, Co6Ni4 catalyst displays the least disparity in charge density difference between *NO adsorption and *NOH adsorption, also suggesting the lowest RDS energy barrier (Fig. 5c)64. In addition, the d-band center of Co6Ni4 is situated closer to the Fermi level, which lends further support to its NO3RR performance (Fig. 5d)65.
a Charge density difference and averaged electron density discrepancy for Co6Ni4 heterostructure interfaces. b Free energy changes of various intermediates generated during NO3RR on synthesized catalysts. c The electron density mappings of *NO and *NOH on Co6Ni4, Co, and Ni catalysts. The yellow and cyan regions represent the accumulation and deficiency of electrons, respectively. d Projected density of state (pDOS) for the d orbitals of Co6Ni4, Co, and Ni. e The schematic NO3RR mechanism of Co6Ni4. The thickness of the black arrow represents the adsorption strength. Source data for the figures (a, b, d) are provided as a Source Data file.
To summarize, by enhancing electron transfer and regulating NO3- adsorption, the reconstruction of metallic Co domains in Co6Ni4 is effectively suppressed during NO3RR (Fig. 5e). Initially, Ni domains will spontaneously shift electron to Co domains, thereby rendering it electron-rich, which can rapidly reduce a small amount of NO3- adsorbed on the surface of the Co site. The crystalline Ni domains in Co6Ni4 significantly enhance the electron transfer rate, allowing electrons to be rapidly transferred to the Co region in the heterogeneous structure, reducing NO3- adsorbed on the surface under a strong reducing electric field. Secondly, substantial interfaces and Ni domains in the heterostructrued Co6Ni4 provide the primary active sites for NO3- adsorption, which prevents a considerable quantity of NO3- from being adsorbed on the Co site. As a joint result of the above mechanisms, the accumulation of Co(OH)2 has been effectively prevented when Co6Ni4 catalyzes NO3- reduction, thereby avoiding the rapid decline in catalytic activity and selectivity that would otherwise occur. The formation of the Co6Ni4 heterostructure regulates the electronic structure of Co and Ni as well as the tandem adsorption of NO3- and NO2-, thereby enabling the smooth proceeding of the tandem reaction of NO3- → NO2- and NO2- → NH3. Moreover, the numerous Co/Ni interfaces within Co6Ni4 facilitate electron transfer from Ni domains to Co domains, and meanwhile provide abundant active sites for reactant adsorption, thus assuring superior NO3RR performance.
Performance in a membrane electrode assembly reactor
The Co6Ni4 heterostructured catalyst exhibited better OER performance than commercial RuO2, particularly exhibiting stable operation at a current density of 250 mA cm-2 for 1200 h (Supplementary Fig. 34). Consequently, Co6Ni4 is employed as both the cathode and anode catalysts for the construction of a membrane electrode assembly (MEA) reactor utilizing 1 M KOH for the water oxidation process and 1 M KOH + 0.3 M KNO3 for the NH3 production.
At room temperature (25 ± 2 °C), this MEA reactor demonstrated a maximum FENH3 of 98.90% at 2.1 V and the FENH3 maintains above 90% across a broad voltage range from 1.9 V to 2.3 V (Fig. 6a, b and Supplementary Fig. 35). Notably, this reactor could operate stably for 240 h at a voltage of 2.2 V, with an average FE and yield rate of NH3 up to 98.05% and 22.82 mg h−1 cm−2, respectively (Fig. 6c). In addition, the absence of visible bubbles in the cathode chamber during the operation of the system also indicates that Co6Ni4 is highly efficient for the reduction of NO3- (Supplementary Movie 1).
a Polarization curve (without iR compensation) of the MEA reactor. b The FE and yield rate of NH3 at different voltages (without iR compensation). c The stability test and corresponding FENH3 and yieldNH3 of Co6Ni4 in the MEA system at 2.2 V (without iR compensation) for 10 cycles (24 h per cycle). Both the anode and cathode catalysts of the system are Co6Ni4. The error bar represents the standard deviation of the data from three independent experiments. The source data for the above figures are provided as a Source Data file.
Discussion
The rationale behind the reconstruction of Co for NO3RR has been elucidated: the number of electrons required for the reduction of adsorbed NO3- and the number of electrons provided by the electric field are in disequilibrium. To resolve this problem, a Co6Ni4 heterostructured catalyst was constructed and proved effective in inhibiting metallic Co reconstruction for two reasons. Firstly, Ni modulates the electronic structure of Co, rendering it in an electron-rich state. The Ni nanocrystals markedly enhance the electron transport capacity of Co6Ni4, facilitating the expeditious transfer of electrons from the reducing electric field to the Co site during NO3RR, thereby sustaining its electron-rich state. Secondly, the Co/Ni interfaces and Ni domain exhibit a greater affinity towards NO3- adsorption compared with the Co domain, thus reducing the NO3- adsorption on Co sites. The rich Co/Ni interfaces further ensure the enhanced electron transfer and improved NO3RR performance. Furthermore, the preference adsorption of NO3- and NO2- onto different sites ensures that NO3RR can be conducted in a tandem configuration with greater efficiency, enabling Co6Ni4 to exhibit a Faradaic efficiency exceeding 90% over a wide potential range from − 0.176 V to − 0.576 V. Our findings on the reconstruction of Co-based catalysts are expected to shine light on the future research endeavors in developing industrial-grade, efficient, and stable electrocatalysts for the NO3RR, as well as to propel advancements in the application of ammonia energy.
Methods
Chemical reagents
Ammonium chloride (NH4Cl, 99%), potassium nitrite (KNO2, 97%), potassium nitrate-15N (K15NO3, 98 at.% 15N), salicylic acid (C7H6O3, 99%), sodium nitroferricyanide dihydrate (Na2[Fe(CN)5NO]·2H2O, 99%), sulfanilamide (C6H8N2O2S, 99%), N-(1-naphthyl) ethylenediamine dihydrochloride (C12H16Cl2N2, 99%), sulfamic acid (NH3SO3, 99%), p-dimethylaminobenzaldehyde (C9H11NO, 99%), nickel oxide (NiO, 99%), cobaltous oxide (CoO, 99%), maleic acid (C4H4O4, 99%), and ruthenium dioxide (RuO2, 99%) were purchased from Adamas-beta. Potassium nitrate (KNO3, ≥ 99%), phosphoric acid (H3PO4, 85%), hydrochloric acid (HCl, 36–38%), nitric acid (HNO3, 65-68%), sulfuric acid (H2SO4, 95–98%), and hydrogen peroxide (H2O2, ≥ 30%) were purchased from Sinopharm Chemical Reagent Co. Ltd (Shanghai, China). Potassium hydroxide (KOH, 85%), sodium citrate (C6H5Na3O7, 98%), sodium hypochlorite (NaClO, 6–14%), and Potassium sulfate (K2SO4, ≥ 99%) was purchased from General-reagent. Nickel chloride hexahydrate (NiCl2·6H2O, 98%) was obtained from Aladdin. Cobalt chloride hexahydrate (CoCl2·6H2O, ≥ 99%) was purchased from ThermoFisher Scientific. Toray Carbon Paper (CP, TGP-H-60), Nafion 211 membrane, Nafion 117 membrane, and membrane electrode device were obtained from the Sci materials hub. All reagents were used directly without further purification. The platinum electrode clamp, graphite rod electrode, and Hg/HgO reference electrode were purchased from Tianjin Aida Hengsheng Technology Development Co., Ltd.
Catalyst synthesis
Co6Ni4 and control samples (Co and Ni catalysts) were prepared on carbon paper (CP) via electrodeposition in a three-electrode system. CP were ultrasonicated in 5 M aqueous HNO3 solution, deionized water, and ethanol for 15 min consecutively, and then dried in an oven at 60 °C. The Ag/AgCl electrode filled with saturated KCl solution, a graphite rod, and the carbon paper with 5 × 5 mm geometric area were used as the reference, counter, and working electrodes, respectively. 0.404 g of CoCl2·6H2O, 0.309 g of NiCl2·6H2O, and 0.481 g of NH4Cl were dissolved in 30 mL ultrapure water under magnetic stirring. Electrodeposition experiments were conducted via amperometry at − 2.0 V (vs. reversible hydrogen electrode (RHE)) for 400 s. The obtained catalysts were rinsed with ethanol and dried in a vacuum oven at 60 °C. The preparation methods of Co and Ni catalysts were similar to the synthesis of Co6Ni4 except the metal precursor in the electrolyte is only 0.1 M CoCl2 or NiCl2. Co2Ni8, Co4Ni6, and Co8Ni2 were synthesized by controlling the volume ratios of 0.1 M CoCl2 and 0.1 M NiCl2 to 5:25, 11:19, and 23:7, respectively.
Material characterization
X-ray diffraction (XRD) was measured with a Bruker D8 Advance powder diffractometer, utilizing Cu Kα radiation (operating at 40 kV, 40 mA). Scanning electron microscopy (SEM) was conducted using a Zeiss GeminiSEM 300 system equipped with an OXFORD Xplore energy-dispersive X-ray spectroscopic (EDS) apparatus. The transmission electron microscopy (TEM), high-resolution TEM (HRTEM), high-angle annular dark-field scanning TEM (HAADF-STEM), selected area electron diffraction (SAED), and EDS were performed on an FEI Tecnai G2 F20 system using copper grids. X-ray photoelectron spectroscopy (XPS) was carried out on an ESCALAB 250Xi system (ThermoFisher Scientific), using a monochromatic Al Kα X-ray radiation source. X-ray absorption spectra (XAS) data at the Co and Ni K-edges were obtained at the National Synchrotron Radiation Research Center (NSRRC) in Taiwan. The element fractions of the samples were quantified by inductively coupled plasma mass spectrometry (ICP-MS) via the Agilent 5110(OES) system. The nuclear magnetic resonance (NMR) tests were carried out on a Bruker AV600. Online-differential electrochemical mass spectrometry (DEMS) was performed using a QAS 100 DEMS system provided by Linglu Instruments (Shanghai) Co., Ltd. Fourier transform infrared spectrometer (FTIR) spectroscopy was carried out with a Thermo-Fisher Scientific Nicolet iS50 FTIR spectrometer. The Raman spectra were obtained on a Labian HR-Evolution Raman microscope under excitation with 532 nm laser light. The H2 was quantitatively analyzed via gas chromatography (GC-8860, Agilent).
Electrochemical measurements
All electrochemical tests were carried out using a Corrtest CS310M electrochemical workstation in a typical H-type cell (50 mL + 50 mL) at ambient temperature (25 ± 2 °C). The cathode chamber and anode chamber were separated by a Nafion 211 proton exchange membrane. The Nafion 211 membrane (2 * 2 cm2, 25.4 μm) was successively treated in 3 wt.% H2O2, 1 M H2SO4 and DI-water at 80 °C for 1 h, and finally rinsed with DI-water. The prepared catalysts on carbon paper, Hg/HgO electrode filled with 1 M KOH, and graphite rod served as the working, reference, and counter electrodes, respectively. The mass of the carbon paper was measured using an electronic balance before and after electrodeposition to determine the catalyst loading. The mass loading of the catalysts was about 5 mg cm−2, and the geometric area of the working electrode exposed to the electrolyte was controlled at 0.25 cm2. Unless otherwise stated, the catholyte and anolyte were 1 M KOH mixed with 0.3 M KNO3 (R = 1.49 ± 0.22 Ω, pH = 13.8 ± 0.1) and 1 M KOH, and their volume was both 30 mL. The nitrate reduction reaction (NO3RR) was measured at a stirring rate of 500 rpm in the cathodic chamber. All the measured potentials (vs. Hg/HgO) were converted to the RHE scale without iR compensation (Supplementary Fig. 36). The calibration was conducted by employing a platinum sheet as the working electrode, a Hg/HgO electrode as the reference, and a graphite rod as the counter electrode within a hydrogen-saturated 1 M KOH solution. Cyclic voltammetry was conducted at a scanning rate of 1 mV s−1, with the calibration reference determined as the average potential corresponding to the zero-current intercept. Linear sweep voltammetry (LSV) was performed across the range from 0.3 V to − 0.576 V (vs. RHE) at a scan rate of 10 mV s−1. Before that, the catalysts would be activated by testing CV at 10 mV s−1 for many cycles until reaching a relatively stable state. Chronoamperometry (CA) was conducted at a constant potential for 1 h to detect the product. After the NO3RR reaction was stopped, the electrolyte was promptly transferred to a refrigerator (2–6 °C) for storage and ensure quantitative analysis of the reaction products was completed within 72 h. The catalyst was not activated prior to the stability test, and the electrolyte was replaced every 3 h during the testing process. Electrochemical impedance spectroscopy (EIS) was carried out at a measuring range of 0.01–105 Hz. Tafel curves were built from Eq. (1):
where η, a, and b referred to overpotential, electron transfer coefficient, and Tafel slope, respectively. The linear relationship of potentials against current density was fitted to obtain the Tafel slope. The electrochemical double-layer capacitance (Cdl) was obtained by performing cyclic voltammetry (CV) at different scan rates of 20, 40, 60, 80, and 100 mV s−1 in the non-Faradaic electrochemical range.
The hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) test methods are analogous to those employed in NO3RR except that the electrolyte is 1 M KOH and has a different test potential. The RuO2 catalyst ink was prepared by adding 5 mg RuO2 to a mixed solution comprising 950 μL isopropanol and 50 μL Nafion solution. The mixture was then ultrasonicated for 1 h to achieve a homogeneous dispersion. Then, 200 μL of the ink was dropped onto the CP electrode with an area of 1 cm2 and dried under infrared light. The mass loadings of RuO2 on the electrode were 1 mg cm−2.
All data with error values (standard deviation) were obtained from three independent experimental trials.
Calculation of the Faradaic efficiency, yield rate, and half-cell energy efficiency
The Faradaic efficiency (FE) was calculated as follows (2, 3):
where n is the number of electrons involved in the respective reaction (8 for NO3- to NH3, 2 for NO3- to NO2-, 6 for NO2- to NH3, and 2 for HER), F is the Faraday constant (96485 C mol−1), C (mol L−1) is the concentration of products in the electrolytes, V (L) is the volume of the electrolyte, m (mol) is the amount of generated H2, and Q (C) is the total charge used for electrochemical reaction.
The yield rate was obtained using the Eq. (4):
Where C (mol L−1) is the concentration of products in the electrolytes, V (L) is the volume of the electrolyte, M (g mol-1)is the molar mass of the products in the electrolytes, t (s) is the electrolysis time, and s (0.25 cm2) is the geometric area of the electrode.
The half-cell energy efficiency (EE) was calculated by the following Eq. (5):
In the equation, Eθ represents the equilibrium potential of NO3- electroreduction to NH3 (0.69 V vs. RHE, alkaline condition), 1.23 V is the equilibrium potential of oxygen evolution reaction (the overpotential of the OER is zero), FE is for NH3, E (vs. RHE) is the applied potential.
Three separate experiments were conducted to test FE and yield, and the error bar represents the standard deviation.
Quantification of nitrogen constituents
All solid reagents are stored in a vacuum oven at 100 °C for 2 h to remove moisture before being prepared into a standard solution. The electrolyte after the reaction is diluted to the detection limit of the UV-vis spectrophotometer before adding the Chromogenic Agent.
Detection of NH3
The indophenol blue method was employed to determine the concentrations of NH3. Three distinct coloring agents were initially prepared: a) 1.0 M KOH solution containing 5 wt% sodium citrate and 5 wt% salicylic acid; b) 0.05 M NaClO solution; and c) 1 wt% sodium nitroferricyanide solution. A predetermined volume of electrolyte was extracted and diluted to 2 mL with ultrapure water. Subsequently, 2 mL of solution a, 1 mL of solution b, and 0.2 mL of solution c were added to the aforementioned electrolyte. The resulting mixture was kept at room temperature (25 ± 2 °C) for 2 h and then analyzed using a UV-vis spectrophotometer. The concentration of NH3 was determined by measuring the absorbance at approximately 655 nm. A calibration curve was constructed using a series of standard NH4Cl solutions.
Detection of NO2 -
To determine NO2- concentrations, the Griess method was employed. A chromogenic reagent was formulated by dissolving 0.4 g p-amino benzenesulfonamide and 0.02 g N-(1-Naphthyl) ethylenediamine dihydrochloride in a mixture of 5 mL ultrapure water and 1 mL phosphoric acid (ρ = 1.70 g mL−1). Then, 0.1 mL of this chromogenic reagent was added to 5 mL of diluted electrolyte. After stirring for 20 min, UV-vis absorbance was measured in the 400–700 nm range. The NO2- concentration was quantified using the absorbance at approximately 540 nm. A calibration curve was established using a range of standard KNO2 solutions.
Detection of NO3 -
The concentration of NO3- was quantified using the following methodology: A 2 mL sample of electrolyte was combined with 1.5 mL of 2 M H2SO4. Subsequently, 750 μL of a 2 wt.% sulfamic acid solution was introduced to the mixture. The resulting solution was analyzed using a UV-vis spectrophotometer, and the absorption intensity at approximately 210 nm was measured. To establish a reference, a calibration curve was constructed using various standard KNO3 solutions.
Detection of N2H4
The quantity of N₂H₄ was ascertained through the implementation of the Watt and Chrisp methodology. A color reagent was typically prepared by combining 10 mL of HCl, 100 mL of ethanol, and 2 g of 4-(dimethylamino)benzaldehyde. Subsequently, 2.5 mL of the diluted electrolyte was mixed with 1 mL of the as-prepared color reagent. The mixture was then incubated in the dark for 10 min prior to the UV-vis absorption tests. The absorbance at ~460 nm was used to quantify the concentration of N2H4.
NMR measurements
Determination of NH3
The concentration of NH3 was quantitatively verified by 1H nuclear magnetic resonance (NMR, 600 MHz) using DMSO-d6 as solvent and maleic acid as the internal standard. The NMR calibration curve was obtained using a series of standard solutions, which consists of a determined concentration of NH4Cl aqueous solution (1 mL), 20 mM maleic acid (1 mL), 0.5 M H2SO4 (1 mL), and DI-water (0.4 mL). 0.5 mL of the above mixed liquid was evenly mixed with 0.5 mL DMSO-d6 for the NMR test. The peak area ratio between NH4+ and maleic acid was calculated for calibration curves. Using Co6Ni4 as NO3RR catalyst, the potential of -0.376 V was maintained in a solution composed of 0.3 M NO3- and 1 M KOH for 1 h, and the electrolyte was taken to be tested. The pH of the as-obtained electrolyte was adjusted to 2.0 before NMR measurement by the same method.
15N isotope-labeling experiment
Isotope-labeling experiments were conducted to trace the source of ammonia during electrochemical nitrate reduction. Quantification of 14NH4+ and 15NH4+ was performed using a 600 MHz 1H NMR spectrometer. K14NO3 and K15NO3 were used as the nitrogen sources for 14NO3- and 15NO3-, respectively. The method of obtaining and configuring the test solution is the same as the calibration of NH3, except that the concentration of NO3- in the electrolyte is 0.1 M.
Detection of overflow NH3
We designed a device to detect NH3 that may escape during NO3RR. 1 M HCl solution is used as a trapping agent and a hose is used to connect the reagent bottle containing the trapping agent to the sealed NO3RR chamber to absorb ammonia that may escape during the reaction (Supplementary Fig. 37a). After the electrocatalytic reaction, the ammonia content in the trapping agent was detected by UV-vis spectrophotometer, and the results showed that no NH3 overflow (Supplementary Fig. 37b). The setup of the electrochemical procedure and the detection method of NH3 are consistent with the above.
Electrochemical in situ FTIR spectroscopy
In situ FTIR was performed in an electrolytic cell (Supplementary Fig. 38a) using an electrolyte consisting of 1 M KOH and 0.3 M KNO3 under potentiostatic programs for Co6Ni4 and control samples. The potential range was from 0.274 V to − 0.576 V (vs. RHE), with spectra collected at 50 mV intervals. A background spectrum was acquired at 0.3 V vs. RHE and used to normalize all spectra obtained at different potentials.
Electrochemical in situ Raman spectroscopy experiments
The measurements were conducted via LSV in a custom-designed cell (Supplementary Fig. 38b), with a scan rate of 1 mV s−1, and a potential range from 0.024 V to − 0.576 V (vs. RHE). Raman spectroscopy tests were performed at 0.1 V intervals during the LSV sweep.
Operando XAS tests
The operando XAS tests of Co K-edge and Ni K-edge were performed at the Taiwan Photon Source (TPS) 44 A quick-scanning beamline at the NSRRC with a homemade operando XAS instrument (Supplementary Fig. 38c). The data was collected by applying a constant voltage (− 0.076 V, − 0.276 V, and − 0.476 V) on the catalyst for 10 min in a mixed electrolyte compromise with 1 M KOH and 0.3 M KNO3. All experiments were carried out under ambient conditions.
Online DMES measurements
Online DEMS measurements were conducted using a mass spectrometer and an electrochemical workstation (Linglu QAS100). The working electrode was Co6Ni4, the counter electrode was a carbon rod, and the reference electrode was Hg/HgO. The electrolyte solution comprised 1.0 M KOH and 0.3 M KNO3. Chronoamperometry was conducted at − 0.376 V for the acquisition of mass signals.
Membrane electrode device performance test
The two-electrode system was constructed using 1 cm2 carbon paper electrodeposited with Co6Ni4 as the cathode and anode. The ion exchange membrane was Nafion 117 (2 * 2 cm2, 183 μm), and the pretreatment method was the same as that of Nafion 211. The catholyte and anolyte were 1 M KOH, added with 0.3 M KNO3 and 1 M KOH. Each reservoir contained 0.6 L of catholyte and anolyte, which were circulated in both chambers using a peristaltic pump (Kamoer KXP100-GB). The flow rates for the cathodic chamber and anodic chamber were 20 mL min−1 and 15 mL min−1, respectively. To minimize the effect of KNO3 consumption on current density, the electrolyte is replaced every 24 h.
Computational methods
DFT calculations
Our simulation was conducted using the Vienna Ab Initio Simulation Package (VASP), which is renowned for its reliability and precision (Supplementary Data 1)66,67. The projector augmented wave (PAW) pseudopotentials, along with the Perdew−Burke−Ernzerhof (PBE) formulation of the generalized gradient approximation (GGA) for exchange-correlation energy, were employed68,69,70,71. The plane wave energy cutoff was precisely set at 500 eV, and a 5 × 5 × 1 k-point mesh was employed using the Monkhorst-Pack method72. Moreover, a 15 Å vacuum layer was introduced between adjacent units in the thickness direction to prevent interference among adsorbed intermediates. The convergence criteria for each atom were set with an energy threshold of less than 10-6 eV and a force threshold of 0.001 eV/Å73. To adequately account for weak van der Waals interactions, the empirical dispersion-corrected DFT-D3 method was applied within the Grimme scheme framework74. VASPsol incorporated implicit solvation where the solvent parameters were those of water75. In addition, charge transfers were determined using the Bader analysis code developed by Henkelman and colleagues76,77. Investigating the dynamic evolution mechanisms of catalysts is crucial, so we employ ab initio molecular dynamics methods to deepen our research. The simulations were configured with a Nosé-Hoover thermostat within the NVT ensemble at a temperature of 300 K78. It is worth noting that the POSCAR files for all model structures are available in the corresponding supplementary materials.
The Gibbs free energy changes (ΔG) calculation
The calculation of Gibbs free energy was performed within the electrochemical framework developed by Nørskov et al. In an alkaline medium, the reaction process proceeds through the following steps:
where * represents the adsorption site.
The equation to calculate the Gibbs free energy change of the multistage NO3RR process is as follows (15)79:
where ΔE is the electronic energy difference directly obtained from DFT calculations, ΔZPE is the zero-point energy difference, T is the room temperature (298.15 K), and ΔS is the entropy change. ΔGU = −e U, where U is the applied electrode potential. ΔGpH = kBT × ln 10 × pH, where kB is the Boltzmann constant, and the pH value is set to 14.
The surface energy calculation. The surface energies (σ) of different facets were calculated by (16):
where Esub is the total energy of the slab model for the surface. Ebulk represents the average energy of one atom in the bulk, and n is the number of atoms in the slab model. A is the area of the surface (1 eV Å–2 = 16.02 J m–2).
Data availability
All data are available from the corresponding authors on request. Source data are provided in this paper.
References
Rosca, V., Duca, M., de Groot, M. T. & Koper, M. T. M. Nitrogen cycle electrocatalysis. Chem. Rev. 109, 2209–2244 (2009).
Li, S., Fu, X., Nørskov, J. K. & Chorkendorff, I. Towards sustainable metal-mediated ammonia electrosynthesis. Nat. Energy 9, 1344–1349 (2024).
Gao, R. et al. A bifunctional catalyst for green ammonia synthesis from ubiquitous air and water. Adv. Mater. 35, 2303455 (2023).
Zhang, G. et al. Ammonia recovery from nitrate-rich wastewater using a membrane-free electrochemical system. Nat. Sustain. 7, 1251–1263 (2024).
Chen, G.-F. et al. Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper–molecular solid catalyst. Nat. Energy 5, 605–613 (2020).
Xu, H., Ma, Y., Chen, J., Zhang, W.-x & Yang, J. Electrocatalytic reduction of nitrate – a step towards a sustainable nitrogen cycle. Chem. Soc. Rev. 51, 2710–2758 (2022).
van Langevelde, P. H., Katsounaros, I. & Koper, M. T. M. Electrocatalytic nitrate reduction for sustainable ammonia production. Joule 5, 290–294 (2021).
Yang, K., Han, S., Wang, Y., Zhang, B. & Yu, Y. Sustainable production and in-place utilization of a liquid nitrogenous fertilizer. Joule 7, 1948–1955 (2023).
Bose, S., Mofidfar, M. & Zare, R. N. Direct conversion of N2 and air to nitric acid in gas–water microbubbles. J. Am. Chem. Soc. 146, 27964–27971 (2024).
Chen, F.-Y. et al. Electrochemical nitrate reduction to ammonia with cation shuttling in a solid electrolyte reactor. Nat. Catal. 7, 1032–1043 (2024).
Lv, J. et al. Promoting the OH cycle on an activated dynamic interface for electrocatalytic ammonia synthesis. Nat. Commun. 15, 6675 (2024).
Shi, X. et al. Synergistic effect of Ni/Ni(OH)2 core-shell catalyst boosts tandem nitrate reduction for ampere-level ammonia production. Angew. Chem. Int. Ed. 63, e202406750 (2024).
Li, Y. et al. Improved nitrate-to-ammonia electrocatalysis through hydrogen poisoning effects. Angew. Chem. Int. Ed. 63, e202411068 (2024).
Chen, F.-Y. et al. Efficient conversion of low-concentration nitrate sources into ammonia on a Ru-dispersed Cu nanowire electrocatalyst. Nat. Nanotechnol. 17, 759–767 (2022).
Murphy, E. et al. Synergizing Fe2O3 nanoparticles on single atom Fe-N-C for nitrate reduction to ammonia at industrial current densities. Adv. Mater. 36, 2401133 (2024).
Han, S. et al. Ultralow overpotential nitrate reduction to ammonia via a three-step relay mechanism. Nat. Catal. 6, 402–414 (2023).
Liu, Y. et al. Efficient tandem electroreduction of nitrate into ammonia through coupling Cu single atoms with adjacent Co3O4. Nat. Commun. 15, 3619 (2024).
Zhang, Y. et al. Regioselective doping into atomically aligned core–shell structures for electrocatalytic reduction of nitrate to ammonia. Adv. Energy Mater. 14, 2401834 (2024).
Kang, X. et al. Self-supported catalytic electrode of CoW/Co-Foam achieves efficient ammonia synthesis at ampere-level current density. Adv. Energy Mater. 14, 2303321 (2024).
Ni, J. et al. Atomic Co─P catalytic pair drives efficient electrochemical nitrate reduction to ammonia. Adv. Energy Mater. 14, 2400065 (2024).
Liu, D. X. et al. Gram‐level NH3 electrosynthesis via NOx reduction on a Cu activated Co electrode. Angew. Chem. Int. Ed. 63, https://doi.org/10.1002/anie.202315238 (2023).
Li, Y. et al. The synergistic catalysis effect on electrochemical nitrate reduction at the dual-function active sites of the heterostructure. Energy Environ. Sci. 17, 4582–4593 (2024).
Qiao, L. et al. In situ reconstructed Cu/β-Co(OH)2 tandem catalyst for enhanced nitrate electroreduction to ammonia in ampere-level. Adv. Energy Mater. 14, 2402805 (2024).
Xu, Y.-Z., Abbott, D. F., Dürr, R. N., Huan, T. N. & Mougel, V. A bio-inspired dendritic MoOx electrocatalyst for efficient electrochemical nitrate reduction to ammonia. Adv. Energy Mater. 14, 2402294 (2024).
Jang, W. et al. Homogeneously mixed Cu–Co bimetallic catalyst derived from hydroxy double salt for industrial-level high-rate nitrate-to-ammonia electrosynthesis. J. Am. Chem. Soc. 146, 27417–27428 (2024).
Fang, J.-Y. et al. Ampere-level current density ammonia electrochemical synthesis using CuCo nanosheets simulating nitrite reductase bifunctional nature. Nat. Commun. 13, 7899 (2022).
Zhou, X. et al. Dynamically restructuring nanoporous Cu–Co electrocatalyst for efficient nitrate electroreduction to ammonia. ACS Catal 14, 12251–12259 (2024).
Yang, K. et al. Unveiling the reaction mechanism of nitrate reduction to ammonia over cobalt-based electrocatalysts. J. Am. Chem. Soc. 146, 12976–12983 (2024).
Liang, S., Teng, X., Xu, H., Chen, L. & Shi, J. H* Species regulation by Mn-Co(OH)2 for efficient nitrate electro-reduction in neutral solution. Angew. Chem. Int. Ed. 63, e202400206 (2024).
Ye, S. et al. Elucidating the activity, mechanism and application of selective electrosynthesis of ammonia from nitrate on cobalt phosphide. Energy Environ. Sci. 15, 760–770 (2022).
Zhu, W. et al. Weakened d–p orbital hybridization in in situ reconstructed Ru/β-Co(OH)2 heterointerfaces for accelerated ammonia electrosynthesis from nitrates. Energy Environ. Sci. 16, 2483–2493 (2023).
Deng, X., Yang, Y., Wang, L., Fu, X.-Z. & Luo, J.-L. Metallic Co nanoarray catalyzes selective NH3 production from electrochemical nitrate reduction at current densities exceeding 2 A cm−2. Adv. Sci. 8, 2004523 (2021).
Chen, X., Cheng, Y., Zhang, B., Zhou, J. & He, S. Gradient-concentration RuCo electrocatalyst for efficient and stable electroreduction of nitrate into ammonia. Nat. Commun. 15, 6278 (2024).
Yu, W. et al. Laser-controlled tandem catalytic sites of CuNi alloys with ampere-level electrocatalytic nitrate-to-ammonia reduction activities for Zn–nitrate batteries. Energy Environ. Sci. 16, 2991–3001 (2023).
Zhou, Y. et al. Boosting electrocatalytic nitrate reduction to ammonia via promoting water dissociation. ACS Catal. 13, 10846–10854 (2023).
Yan, Q. et al. Enhancing compatibility of two-step tandem catalytic nitrate reduction to ammonia over P-Cu/Co(OH)2. Adv. Mater. 36, 2408680 (2024).
Wang, Y. et al. Highly selective NH3 synthesis from N2 on electron-rich Bi0 in a pressurized electrolyzer. Proc. Natl. Acad. Sci. USA 120, e2305604120 (2023).
Xu, Y. et al. Ultralow-content Pd in-situ incorporation mediated hierarchical defects in corner-etched Cu2O octahedra for enhanced electrocatalytic nitrate reduction to ammonia. Appl. Catal. B Environ. 306, 121094 (2022).
Agrestini, S. et al. Nature of the magnetism of iridium in the double perovskite Sr2CoIrO6. Phys. Rev. B 100, 014443 (2019).
Zhang, J. et al. Atomic-thick metastable phase RhMo nanosheets for hydrogen oxidation catalysis. Nat. Commun. 14, 1761 (2023).
Guan, D. et al. Utilizing ion leaching effects for achieving high oxygen-evolving performance on hybrid nanocomposite with self-optimized behaviors. Nat. Commun. 11, 3376 (2020).
Liu, H. et al. Insight into the role of metal–oxygen bond and O 2p hole in high-voltage cathode LiNixMn2–xO4. J. Phys. Chem. C 121, 16079–16087 (2017).
Stern, E. A., Sayers, D. E. & Lytle, F. W. Extended x-ray-absorption fine-structure technique. III. Determination of physical parameters. Phys. Rev. B 11, 4836–4846 (1975).
Su, H. et al. Dynamic evolution of solid–liquid electrochemical interfaces over single-atom active sites. J. Am. Chem. Soc. 142, 12306–12313 (2020).
Jiang, Z. et al. Molecular electrocatalysts for rapid and selective reduction of nitrogenous waste to ammonia. Energy Environ. Sci. 16, 2239–2246 (2023).
Feng, C. et al. Sub-nanometer-scale Cu9S5 enables efficiently electrochemical nitrate reduction to ammonia. Adv. Energy Mater. 15, 2403354 (2025).
Gao, Q. et al. Breaking adsorption-energy scaling limitations of electrocatalytic nitrate reduction on intermetallic CuPd nanocubes by machine-learned insights. Nat. Commun. 13, 2338 (2022).
Zhang, Z. et al. Graphdiyne enabled nitrogen vacancy formation in copper nitride for efficient ammonia synthesis. J. Am. Chem. Soc. 146, 14898–14904 (2024).
Dai, J. et al. Spin polarized Fe1−Ti pairs for highly efficient electroreduction nitrate to ammonia. Nat. Commun. 15, 88 (2024).
Ren, Z., Shi, K. & Feng, X. Elucidating the intrinsic activity and selectivity of Cu for nitrate electroreduction. ACS Energy Lett 8, 3658–3665 (2023).
Ren, Z., Shi, K., Meng, Z., Willis, M. D. & Feng, X. Complete single-pass conversion of dilute nitrate to ammonia using Cu/Co(OH)2 tandem electrocatalyst. ACS Energy Lett. 9, 3849–3858 (2024).
Fan, K. et al. Active hydrogen boosts electrochemical nitrate reduction to ammonia. Nat. Commun. 13, 7958 (2022).
Fan, Z. et al. Interfacial electronic interactions promoted activation for nitrate electroreduction to ammonia over Ag-modified Co3O4. Angew. Chem. Int. Ed. 63, e202410356 (2024).
Li, Y. et al. Hydrogen spillover mechanism at the metal–metal interface in electrocatalytic hydrogenation. Angew. Chem. Int. Ed. 63, e202407810 (2024).
Fu, Y. et al. Enhancing electrochemical nitrate reduction to ammonia over Cu nanosheets via facet tandem catalysis. Angew. Chem. Int. Ed. 62, e202303327 (2023).
Qi, S. et al. Ultrathin high-entropy Fe-based spinel oxide nanosheets with metalloid band structures for efficient nitrate reduction toward ammonia. Adv. Mater. 36, 2403958 (2024).
Hu, Q. et al. Ammonia electrosynthesis from nitrate using a ruthenium–copper cocatalyst system: A full concentration range study. J. Am. Chem. Soc. 146, 668–676 (2024).
Wang, F., Shang, S., Sun, Z., Yang, X. & Chu, K. P-block antimony–copper single-atom alloys for selective nitrite electroreduction to ammonia. ACS Nano 18, 13141–13149 (2024).
Liao, W. et al. Sustainable conversion of alkaline nitrate to ammonia at activities greater than 2 A cm−2. Nat. Commun. 15, 1264 (2024).
Ma, C. et al. Screening of intermetallic compounds based on intermediate adsorption equilibrium for electrocatalytic nitrate reduction to ammonia. J. Am. Chem. Soc. 146, 20069–20079 (2024).
Guan, J., Cai, L., Li, W., Zhou, H. & Huang, Y. Boosting nitrate electroreduction to ammonia on atomic Ru-Co pair sites in hollow spinels. Appl. Catal. B Environ. 358, 124387 (2024).
Xiao, Y. et al. Strained Au skin on mesoporous intermetallic AuCu3 nanocoral for electrocatalytic conversion of nitrate to ammonia across a wide concentration range. Angew. Chem. Int. Ed. 63, e202408758 (2024).
Chang, Z. et al. Dual-site W-O-CoP catalysts for active and selective nitrate conversion to ammonia in a broad concentration window. Adv. Mater. 35, 2304508 (2023).
Yang, W. et al. Triple regulations via Fe redox boosting nitrate reduction to ammonia at industrial current densities. Angew. Chem. Int. Ed. 64, e202415300 (2024).
Huang, T. et al. Coordination environment-tailored electronic structure of single atomic copper sites for efficient electrochemical nitrate reduction toward ammonia. Energy Environ. Sci. 17, 8360–8367 (2024).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Li, X. et al. Phthalocyanine-supported single-atom catalysts as a promising bifunctional electrocatalyst for ORR/OER: A computational study. ChemPhysMater 1, 237–245 (2022).
Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 27, 1787–1799 (2006).
Mathew, K., Sundararaman, R., Letchworth-Weaver, K., Arias, T. A. & Hennig, R. G. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways. J. Chem. Phys. 140, 084106 (2014).
Bader, R. F. W. A quantum theory of molecular structure and its applications. Chem. Rev. 91, 893–928 (1991).
Henkelman, G., Arnaldsson, A. & Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 36, 354–360 (2006).
Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 81, 511–519 (1984).
Zhang, B. et al. Defect-induced triple synergistic modulation in copper for superior electrochemical ammonia production across broad nitrate concentrations. Nat. Commun. 15, 2816 (2024).
Acknowledgements
This work was funded by the National Natural Science Foundation of China (22405191, H.C.), Natural Science Foundation of Shanghai Municipality (24ZR1468100, H.C.) and the Fundamental Research Funds for the Central Universities to H.C. X.Y. acknowledges support from the National Natural Science Foundation of China (52302302, X.Y.). W.H. acknowledges the support from the National Science and Technology Council (NSTC) in Taiwan (NSTC 113-2628-E-011-004-MY3, W.H.) and National Taiwan University of Science and Technology & Bandung Institute of Technology Joint Research Program (ITB-NTUST-2024-01, W.H.). We acknowledge support from the Max Planck-POSTECH-Hsinchu Center for Complex Phase Materials. We acknowledge support from the Xiaomi Young Talents Program. We thank Taiwan Light Source (TLS) (beamline 17A1) for the allocation of synchrotron beam time under Proposal No. 2024-2-027-1.
Author information
Authors and Affiliations
Contributions
H.C. and J.M. initiated and oversaw the research project. The experimental design was developed by H.C. and X.S. Experimental execution and data interpretation were carried out by X.S., W.H., M.X., P.Z., H.W., J.W., and H.Z., X.Y., X.S., and J.R. conducted theoretical modeling and computational analysis. X.S., W.H., Q.W., M.Y., C.P., and Z.H. contributed to operando/in situ characterization and subsequent analytical processes. X.S., H.C., W.H., X.Y., Z.H., and P.Z. collaboratively participated in experimental optimization and scientific discussions. The manuscript was primarily written by X.S., H.C., X.Y., W.H., and Z.H., and all authors critically evaluating results and revising the text.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shi, X., Huang, WH., Rong, J. et al. Revealing and modulating catalyst reconstruction for highly efficient electrosynthesis of ammonia. Nat Commun 16, 6161 (2025). https://doi.org/10.1038/s41467-025-61075-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-61075-8
This article is cited by
-
Beyond Haber-Bosch: emerging pathways for sustainable ammonia synthesis
ENGINEERING Chemical Engineering (2026)
-
Electrocatalytic nitrate to ammonia conversion: from mechanistic insights to catalyst engineering for practical applications
Catal (2025)