Abstract
Carbonaceous chondrites are amongst the most chemically primitive solid materials in the Solar System, yet many are depleted in moderately volatile elements. Here, we report enrichments in heavier zinc isotopes in heated carbonaceous chondrites compared to the typical ranges for chondritic meteorites. Our results indicate that impact-driven thermal metamorphism under low-pressure conditions led to partial sublimation of zinc. First-principles calculations support that zinc escapes from solids in the absence of melting, consistent with shock heating and rapid outgassing. The resulting solid residue is strongly enriched in heavier Zn isotopes with minimal recondensation. These findings link extreme isotopic signatures to collisional processing, revealing that asteroid-scale impacts can drive volatile loss from undifferentiated asteroids. These carbonaceous chondrites provide the first unequivocal evidence for purely kinetic Zn isotope fractionation during volatilization. Impact-induced volatilization drives volatile depletion in asteroidal parent bodies, with implications for the delivery and distribution of volatiles in early planetary systems.
Similar content being viewed by others
Introduction
Chondrites, which are primitive meteorites formed 2–3 million years after the Solar System’s formation1, are considered foundational building blocks for terrestrial planets2. The evolution of moderately volatile elements (MVE) in chondrites provides key insights into the origin and depletion of volatiles on terrestrial planets. Unlike terrestrial planets, which have undergone extensive melting and differentiation, chondrites retain their original composition with two primary constituents, i.e., chondrules and matrix1. Chondrules are silicate-rich spherules formed by transient heating events in the protoplanetary disk, embedded within a fine-grained matrix that accreted at lower temperatures, preserving presolar grains and volatile compounds3,4. Among carbonaceous chondrites (CC), except for the Ivuna-type (CI) chondrites and samples returned from Ryugu and Bennu, which closely mirror the solar photosphere in their non-volatile element composition, there is a noticeable variation in the depletion of MVE correlated with their condensation temperatures5,6,7,8,9.
An early protoplanetary disk is characterized by high temperatures (ranging from ~1000 to >2000 K) that could lead to dust vaporization and subsequent condensation, along with chondrule-forming events and giant impacts followed by magma oceans4,10. These processes likely facilitated the mobilization of MVE, including Zn. This is evidenced by the mass-dependent Zn isotopic fractionation (reported as δ66Zn relative to JMC-Lyon) observed between chondrite groups11. Previous studies have identified a negative correlation between δ66Zn and 1/[Zn] among different groups of carbonaceous chondrite12,13,14, opposite to the expected trend if evaporation controls Zn abundance and isotopic fractionation. This has been interpreted as reflecting the mixing between an isotopically light/volatile poor- and an isotopically heavy/volatile rich- reservoir. Similarly, magmatic iron meteorites, representing the cores of early-formed asteroids, display this same relationship15. The origin of the light Zn isotopic composition of chondrules was suggested to reflect the removal of isotopically heavy Zn from chondrules (or their precursors) via sulfide segregation13,16,17 or as a result of partial condensation processes18, where Zn-depleted chondrites exhibited enrichment in lighter Zn isotopes. Again, evaporation most likely played a limited role in the volatile depletion observed in CC parent bodies or their precursors.
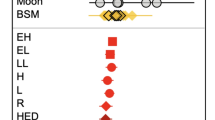
Despite these lines of evidence, several CC meteorites are highly depleted in volatile elements and display a trend of increasing δ66Zn values coupled with decreasing Zn content19. These CC meteorites experienced heating processes by thermal metamorphism after the accretion of their parent body20,21,22. During these processes, light Zn isotopes might be volatilized, leaving the residual solid phase enriched in heavy Zn isotopes19. Heavy Zn isotope enrichments related to evaporation have been observed in lunar mare basalts, martian meteorites, impact melt sheets, impact glasses and tektites, and nuclear detonation materials23,24,25,26,27,28,29,30,31,32,33,34. Previous studies indicate that, compared to theoretical predictions, actual evaporation is likely to generate a subdued isotopic fractionation35. A limited fractionation model for Zn isotopes was further proposed to explain various planetary evaporation events under impact-related conditions34. However, extremely high δ66Zn values reported in heated CC chondrites (up to 16.7‰; Fig. 1)19 exceed those predicted by these models.
To address this discrepancy and further explore the behavior of Zn during volatilization from a solid state, we analyzed the Zn isotopic compositions of eight heated CC meteorites from Antarctica, including heated CM chondrites and CY (Yamato-type) chondrites. These meteorites record extensive aqueous and thermal alteration, followed by relatively short-lived thermal metamorphism19,22,36. CY chondrites, a recently recognized group of CCs, are considered to potentially be thermally metamorphosed CI chondrites36,37,38,39, as documented to exhibit notably heavy O isotopic compositions similar to the CI field (Fig. S1). We also estimate the relative evaporation of Zn, Cd, Fe, and Mg from solid materials from first-principles calculations. Altogether, we demonstrate that evaporative loss by impacts significantly contributes to the volatile depletion in chondritic parent bodies.
Results and discussion
Samples
We analyzed a set of heated carbonaceous chondrites, including heated CM [Pecora Escarpment (PCA) 02010 and PCA 02012], CY [Yamato (Y)−86789, Y-86720, Belgica (B)−7904, among which Y-86789 is paired with Y-86720], CV [Asuka (A)−881655], CR (Y-793495) and CO (Y-790992) meteorites36,40,41, to provide a diverse contextual framework. Our study primarily focuses on CM and CY samples, which display evidence of heating events, as indicated by their depleted volatile element abundances and water contents19,21,41,42,43. Two heated CM meteorites, PCA 02010 and PCA 02012, previously analyzed for their Zn isotopic compositions19, were reassessed using different sample fractions. The two meteorites underwent significant heating at temperatures >900 °C22,44, as indicated by their extremely heavy isotopic compositions of Zn, K, and Rb, resulting from evaporation from post-accretionary heating events19,45,46. The CM and CY samples were subjected to much shorter heating durations (ranging from hours to several years) compared to the prolonged thermal processing of CO and CV chondrites, which lasted millions of years47. The estimated temperature order of the other heated chondrites, based on open system heating severity, is 500 °C < A881655 < B-7904 < Y-86720 = Y-86789 (≥700 °C), with Y-86720 and Y-86789 specifically exhibiting thermal metamorphism above 700 °C21,48. Notably, B-7904 and, to a lesser extent, Y-86789 and Y-86720 differ from other CYs by clearly preserving chondrules or chondrule pseudomorphs39,47. Metamorphism has modified their mineralogy, with phyllosilicates recrystallizing into olivine and pyroxene47.
Zinc contents and isotopic compositions
In this study, the analyzed carbonaceous chondrites show a wide range of Zn concentrations from 1.77 to 162 µg/g, with δ66Zn values ranging from 0.07 to 24.2‰ (Fig. 1; Table S1). The CY chondrite samples exhibit lower Zn concentrations (31.0–162 µg/g) and higher δ66Zn values (1.36–24.2‰) compared to the CI chondrites, which have relatively high Zn contents of 309 ± 44 µg/g and homogeneous δ66Zn values of 0.46 ± 0.08‰12,13,14,49. Among the CYs, the paired CY samples display extremely high δ66Zn values (18.2 ± 0.04‰ and 24.2 ± 0.01‰), which represent the highest δ66Zn ever measured across all meteorites, including EL chondrites (a subgroup of enstatite chondrites known for heavy Zn isotope signatures)50. By comparison, B-7904, which is relatively rich in chondrules47, displays a markedly lower δ66Zn value, closer to the CI chondrite range. The heated CV, CR, and CO samples have δ66Zn of 0.27 ± 0.01‰, 0.07 ± 0.02‰, and 0.17 ± 0.02‰, respectively, aligning within the typical ranges previously reported for these groups (Fig. 2). The two heated CM chondrites display significant depletion in Zn contents (1.77–14.9 µg/g) and enrichment in heavy Zn isotopes (δ66Zn = 10.5–16.7‰). Two separate fragments of the CM chondrite PCA 02010 show slight differences in Zn contents (1.77 µg/g and 2.79 µg/g) and δ66Zn (10.5 ± 0.09‰ and 11.3 ± 0.01‰). The δ66Zn values measured in this study also diverge from the previously reported value of 5.42‰ for PCA 0201019. This suggests isotopic heterogeneity within the individual PCA 02010 chondrite aliquots. Sample PCA 02012 exhibits a Zn content of 14.9 µg/g and δ66Zn of 16.7‰, aligning with the earlier study19.
The ranges for various sub-groups of carbonaceous chondrites are after reference13,14. The colored arrows indicate the anticipated trends: one showing an increase in δ66Zn values due to evaporation, and the other showing a decrease in δ66Zn values resulting from incomplete condensation or sulfide separation.
Origin of elevated δ 66Zn in carbonaceous chondrites
This study demonstrates that the analyzed carbonaceous chondrites display a wide range of δ66Zn values, extending beyond the typical range previously reported for these meteorites (Fig. 1). The CO, CV, and CR samples analyzed here exhibit δ66Zn values consistent with those of their respective chondrite groups. In contrast, the paired CY (Y-86720 and Y-86789) and heated CM chondrites show exceptionally high δ66Zn values relative to both CI chondrites and the typical carbonaceous chondrite range. Notably, the chondrule-rich CY sample B-7904 exhibits a significantly lower δ66Zn value than other CYs, although it remains elevated relative to the broader carbonaceous chondrite trend (Fig. 1). Terrestrial contamination could influence isotopic compositions, however, no terrestrial samples, including continental crustal rocks (~0.28 ± 0.05‰)51, reach the elevated levels observed in the measured samples. Additionally, the chondritic Sr/Mg and Ba/Mg ratios and REE patterns of the samples, which contrast with the typical light-REE enrichment and negative Eu anomaly of the upper continental crust (Fig. S4), further exclude terrestrial contamination as a contributing factor.
Although some carbonaceous chondrites (e.g., PCA samples) exhibit evidence of terrestrial weathering21,52,53, terrestrial chemical weathering and alteration fail to account for the observed high δ66Zn, given the resistance of Zn isotopes to aqueous alterations. For instance, weathered igneous rocks display δ66Zn slightly lower or comparable to their unaltered igneous protoliths54,55. Furthermore, there is no correlation between fluid-mobile element concentrations (Pb/Th and Rb/Th) and δ66Zn (Fig. S3). Aqueous alteration may also have preceded thermal metamorphism in the precursors of many CC chondrites20,21. However, aqueous alteration does not exert a first-order effect on volatile element concentrations56. In addition, no significant enrichment of heavy Zn isotopes was observed in those previously reported CC chondrites with aqueous alteration imprints19, as well as the CR (Y-793495) and CO chondrites (Y-790992) in this study. The high δ66Zn values are intrinsic signatures of these meteorites.
Fractionation of stable isotopes in multi-isotope space (e.g., δ68Zn–δ66Zn) follows different mass-dependent laws that can be used to distinguish between kinetic and equilibrium fractionation mechanisms57,58. Equilibrium fractionation typically occurs when different substances reach thermodynamic equilibrium, whereas kinetic fractionation takes place under nonequilibrium, unidirectional processes (such as rapid evaporation or diffusion). In three-isotope plots, the slopes or curves corresponding to kinetic and equilibrium processes marginally differ. For Zn isotopes, theoretical calculations predict the equilibrium fractionation slope in δ68Zn–δ66Zn space to be ~1.942, compared to 1.971 for kinetic fractionation of vapor-phase Zn. Furthermore, for ZnS and ZnCl2 (common species involved in Zn loss during vaporization), the calculated kinetic fractionation slopes are 1.981 and 1.986, respectively, while the equilibrium fractionation slopes are 1.961 and 1.972. Detailed calculation methodologies are provided in the Supplementary Materials. The measured δ68Zn–δ66Zn mass-dependent slope for our samples is 1.985 ± 0.003 (Fig. S2), consistent, within analytical error, with theoretical predictions for kinetic evaporation. These CC chondrites offer the first evidence of purely kinetic Zn isotope fractionation occurring during volatilization.
Zinc isotopic fractionation mechanisms of carbonaceous chondrites
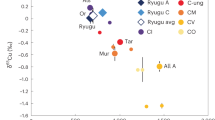
The increase in Mg/Zn ratios, coupled with decreasing δ66Zn has been observed across different groups of carbonaceous chondrites12,13. The results from samples analyzed in this study also follow this pattern (Fig. 2). The compositional gradient from CI-like to CR chondrites, the latter marking the endpoint of volatile depletion, is indicative of the accretionary history involving the mixing of chemically and isotopically distinct reservoirs12,18,37,46,59. The correlation of δ66Zn with stable Cr isotope anomalies ε54Cr13 supports the idea of mixing between isotopically heavy/volatile-rich matrix material and isotopically light/volatile-poor chondrule material, where either partial condensation following near-complete evaporation of Zn50,60 or sulfide segregation during chondrule formation13 leads to a predominance of light Zn isotopes in chondrules.
By contrast, in heated CM and CY samples, δ66Zn values are elevated and Zn depletion is pronounced. This indicates that evaporation processes control Zn isotopic fractionations. Under high ambient pressures or in effectively closed systems, Zn evaporation yields only minimal isotopic fractionation30,35,61. The presence of a confining gas allows vapor to recondense onto solids (a back-reaction)61,62, and limited Zn diffusion within the condensed phase further suppresses isotopic shifts23,63. As a result, in near-equilibrium evaporation at high temperatures, the fractionation factor (α) is approximately 163. Experiments and natural analogs support this trend. In a 1-bar furnace, an experimental study observed α = ~0.996 in the air (rising to ~0.9978 under more reducing conditions)63, whereas silicate melt glass from the Trinity nuclear test (formed at ~1 atm) recorded α = ~0.999–0.999528,30. Similar high fractionation factors (mostly ranging from 0.999 to 0.9999)34 are also inferred for lunar mare basalts, diogenites, tektites, and terrestrial impact glasses23,24,25,26,27,29,30,60,64 (Fig. 3). Such high fractionation factors are inconsistent with rapid unidirectional evaporation and instead indicate near-equilibrium or diffusion-limited volatilization conditions.
Dashed lines represent the Zn isotopic fractionation factors. In (A), the α values range from 0.9999 to 0.9990, indicating the limited Zn isotopic fractionation expected under high-P conditions associated with impact events. The data sources of reported materials are summarized in reference34. In (B), theoretical fractionation factors for evaporation into a high vacuum are displayed (0.9753 for elemental Zn, 0.9897 for ZnS, and 0.9927 for ZnCl2). The CI chondrites ([Zn] = 309 µg/g; δ66Zn = 0.46‰)12,14,49 are used to represent the starting material for CY chondrites. The initial material for CM chondrites is based on the water- and volatile-rich CM sample Lonewolf Nunataks 94101 (LON 94101; [Zn] = 144 µg/g; δ66Zn = 0.32‰)19. Small colored dots in the background represent results from a Monte Carlo simulation. The simulation follows the Rayleigh distillation model: δ66Zn = (1000 + δ66Zn0) × f α−1 – 1000, where δ66Zn0 is the initial Zn isotopic composition, α is the fractionation factor, and f is the evaporative loss ratio of Zn. The δ66Zn0 is assigned randomly following a normal distribution (mean ± σSD). The simulation incorporates isotopic fractionation under different scenarios characterized by α values of 0.9847 (±0.00005), 0.9897 (±0.00005), 0.9927 (±0.00005), and 0.9990 (±0.00005). Each scenario is simulated over 10,000 trials to ensure robust statistical outcomes. The f is randomly selected between 0 and 1 in each trial.
In contrast, under low-pressure or vacuum conditions, evaporation follows a kinetic regime that strongly fractionates Zn isotopes65. With negligible recondensation, lighter isotopes escape preferentially, and the fractionation factor is controlled by mass differences of the evaporating species. Theoretical α values in free evaporation are far below unity: on the order of 0.9753 for atomic Zn vapor, 0.9897 for ZnS, and 0.9927 for ZnCl2 (by taking the square root of the ratio of the mass numbers of the light and heavy isotopologues of the vaporizing species). This yields extremely heavy residual Zn isotope compositions (high δ66Zn) in the remaining solids, as observed in the most volatile-depleted meteorites19. Indeed, heated carbonaceous chondrites that lost large fractions of Zn and other volatiles are highly enriched in heavy Zn isotopes, indicating an open-system evaporation process. Using Monte Carlo simulations (modeling details in the caption of Fig. 3), we reproduce the elevated δ66Zn in B-7904 (and to a lesser extent in PCA 02010) with an evaporation fractionation factor of α = 0.9990 ± 0.00005 (SD) (Fig. 3), consistent with an impact-induced volatilization under elevated pressure (although still in the kinetic regime). Under a more plausible limited-fractionation scenario, the isotopic heterogeneity observed among individual PCA 02010 chondrite aliquots may be governed by back-reactions and limited Zn diffusion within the condensed phase. Notably, other thermally metamorphosed chondrites (e.g., PCA 02012, Y-86789, and Y-86720) plot along expected Rayleigh fractionation trajectories, reinforcing that open-system kinetic evaporation with minimal back-reaction governed their Zn loss. Therefore, the collective evidence points to extensive Zn evaporation at high temperatures and relatively low ambient pressure, with only limited recondensation. Such conditions would produce pronounced kinetic isotope effects and leave the residual solids substantially enriched in heavy Zn isotopes.
Evaporation of zinc from the solid phase
Kinetic Zn isotope fractionation during volatilization observed here is distinctly different from the more subdued or mixed processes involving partial recondensation or melt-phase interactions. By contrast, the exceptionally high δ66Zn values, along with the linear mass-dependent trend, demonstrate predominantly open-system kinetic evaporation. Thermal events responsible for heating CM chondrites occurred hundreds of millions (possibly even billions) of years after the formation of the Solar System, with a recent study suggesting an age of at least 3 Ga22. This timing rules out short-lived radionuclides as the heat source and instead points to collisions, which aligns with the collision ages associated with asteroid families22. Similarly, recent work suggests the CI parent body may have undergone collisional heating, producing partially dehydrated, “CY-like” materials that were then ejected38. A single impact could explain both the chemical similarities in non-volatile elements between CY and CI and their differences in water content and volatile inventories. Notably, these heated chondrites show no evidence of large-scale melting. Their mineral structures are consistent with solid-state metamorphism21,48. This raises the likelihood that Zn can be lost via evaporation from the solid state during impact heating and without extensive melting.
In this study, we performed first-principles calculations to evaluate the feasibility of Zn evaporation from a solid phase (Mg2SiO4) under vacuum and compared Zn loss with that of Cd, Mg, and Fe (Fig. 4). Our results indicate that both Zn and Cd can readily escape from the solid phase without melting, requiring less energy—and thus having a higher likelihood—to vaporize compared to Fe and Mg. Among these elements, Cd is lost most efficiently, followed by Zn, then Fe and Mg. An earlier study reported substantial Zn loss from CM2 chondrite (Murchison) under low-pressure (~10 Pa) heating between 400 and 1000 °C, most notably around 700 °C66. Taken together, these observations support that Zn can undergo significant vaporization from solid phases in the absence of melting. These findings also align well with observations in chondrites, where Cd generally exhibits stronger depletion relative to Zn, and Zn shows more pronounced depletion than less volatile elements41,66. In fact, carbonaceous chondrites are highly porous and volatile-rich. Compared with ordinary chondrites, they can reach higher post-shock temperatures under moderate shock pressures, and their threshold for partial melting is lower67. For instance, under shock pressures of ~20–30 GPa, local materials may be momentarily heated to ~600–800 °C52, which is still below the melting threshold, consistent with the observation that not all heated CM chondrites show melting features. The vapor pressure of Zn is relatively high under low-pressure regimes21,41,66. Once temperatures exceed ~600 °C in near-vacuum conditions (as on the surface of an asteroid), Zn becomes prone to sublimation.
Energy difference as a function of distance to the surface for several divalent cations evaporating from the two, M1 (A) and M2 (B), sites of a slab of olivine placed in a vacuum, as obtained from first-principles calculations (details in Supplementary Materials). The plateau at high distances represents the complete evaporation state. The first 2 Å of displacement in each simulation is omitted because the energy differences in that region primarily reflect surface rearrangement structures rather than actual dissociation energies. The calculations show that Cd and Zn have lower ΔE values than Mg and Fe, indicating that Cd and Zn are more readily lost from the solid during evaporation. At the M2 site, ΔE for both Cd and Zn is extremely low, suggesting these elements can rapidly evaporate from the solid under high-temperature conditions.
Although the high temperatures generated by impact shocks may last only milliseconds to seconds, this interval can still suffice for Zn volatilization. The evaporation rate at these temperatures is rapid, and shock-induced fracturing accelerates outgassing. Moreover, sulfide (strong affinity of Zn for sulfide phases such as troilite and sphalerite) decomposition above ~500 °C liberates sulfur gases that carry Zn and form Zn oxide or metal vapor (Fig. 3). Zinc does not require complete lattice-scale diffusion to be lost; instead, thermal decomposition and the creation of fracture surfaces can directly transfer Zn into pore spaces or thin melt layers, where it then evaporates. In an open system (e.g., near the surface where ejecta are dispersed), newly formed Zn vapor readily diffuses into space68. Consequently, recondensation of light Zn isotopes back into the rock is minimal, leading to an enrichment of heavy Zn isotopes in the residue. In our samples, pronounced heavy Zn isotopic compositions indicate that Zn vapor did not fully recondense in situ. Nevertheless, δ66Zn in PCA 02010 and B-7904 is notably heavy but not as extreme as in other samples, possibly because evaporation was partly restricted, allowing some vapor to be trapped or re-equilibrated. Alternatively, slightly higher local pressures (e.g., from abundant volatiles released at shallow burial depth) may have partially suppressed isotopic fractionation, resulting in more moderate δ66Zn.
Impact-driven volatile depletion in asteroid parent bodies
The investigated carbonaceous chondrites provide the first unequivocal evidence of purely kinetic Zn isotope fractionation during volatilization from a solid state. By inducing sublimation, impacts facilitate evaporation in near-surface regions, even in the absence of melting, thereby driving extensive depletion of MVE such as Zn. Crucially, these collisions produce open-system conditions conducive to strong volatile escape. By comparison, internal heating processes (e.g., radioactive decay of 26Al or 60Fe) operate over much longer timescales (104–106 years) and tend to occur in more closed-system environments, enabling volatiles to be redistributed or sequestered internally. As impact-driven sublimation is effectively unconfined, the vapor rapidly escapes into space rather than recondensing locally. On a larger scale, the frequent high-energy impacts characteristic of the early Solar System would progressively strip volatiles from asteroidal surfaces (particularly given low gravity and an open escape path), eventually altering both the surface composition and deeper differentiation pathways. Notably, our findings reveal that even undifferentiated bodies likely show MVE depletion through purely kinetic, solid-state evaporation, emphasizing impacts as a key driver of volatile loss and heavy-isotope enrichment. Therefore, if volatile-poor, MVE-depleted chondritic material (resulting from collisional processing) contributed significantly to a growing planet’s mass, the resulting bulk volatile budget might mask the expected signals from fully differentiated planetesimals. In other words, rather than MVE depletion being solely attributed to differentiation and core-mantle segregation processes, collisional history must also be considered as a fundamental mechanism that alters the initial volatile inventory.
Overall, collisional heating is capable of inducing extreme isotopic shifts and permanently depleting near-surface volatiles on parent bodies. Future models of planetary assembly should thus include the possibility that even undifferentiated chondritic precursors underwent substantial impact-driven volatilization, potentially imprinting the volatile inventories and isotopic compositions of terrestrial planets.
Methods
Major and trace elements
The elemental content of samples was analyzed using an Agilent 7900 Quadrupole Inductively Coupled Plasma Mass Spectrometry instrument at the Institut de Physique du Globe de Paris (IPGP), France. The sample was introduced into a Scott spray chamber through a MicroMist nebulizer at an uptake rate of 0.2 mL/min. Polyatomic interferences were mitigated by analyzing atomic masses from 23 (Na) to 75 (As) in a collision-reaction cell, utilizing helium gas at a flow rate of 5 mL/min. Internal standards, including Sc, In, and Re, were added to the sample solutions to correct for any signal drift and matrix effects. A mixture of certified standards was measured across a range of concentrations to convert count measurements into solution concentrations.
Zinc isotopes
Zinc isotopic measurements were conducted at IPGP using a Thermo Scientific Neptune Plus Multi-Collector Inductively-Coupled-Plasma Mass-Spectrometer. Approximately 35 mg of homogenized bulk powder from each sample (derived from a larger homogenized portion) was dissolved in a 1:3 mixture of concentrated HNO3 (500 μL) and HF (1.5 mL) and heated at 120 °C for ~48 h. The acids were then evaporated, and the residues were processed with a 3:1 mixture of concentrated HCl (3 mL) and HNO3 (1 mL) to dissolve any residual fluoride complexes. Samples were then evaporated to dryness and re-dissolved in 1.5 mol/L HBr (1 mL) for chemical purification. Procedures of sample pretreatment, chemical purification, and analytical methods are detailed in prior publications17. Isotope ratios were determined using the standard-sample-standard bracketing method with JMC-Lyon as the Zn isotope standard, expressed in the δxZn notation:
where X = 66 and 68. The precision and reproducibility of chemical separation and isotopic analyses were monitored using terrestrial rock standard BHVO-2 and BCR-2, yielding δ66Zn values of 0.29 ± 0.06‰ (2 SD, n = 4) and 0.25 ± 0.03‰ (2 SD, n = 3), respectively, aligning with published values11. The analytical results for Zn isotopes and bulk major and trace elements are detailed in Tables S1, S2.
Data availability
All data are available in the Supplementary Materials.
References
Scott, E. R. D. & Krot, A. N. Chondrites and Their Components. vol. 1 (Elsevier, 2014).
Alexander, C. M. O. D., Boss, A. P. & Carlson, R. W. The early evolution of the inner solar system: a meteoritic perspective. Science 293, 64–68 (2001).
Galy, A., Young, E. D., Ash, R. D. & Keith O’Nions, R. The formation of chondrules at high gas pressures in the solar nebula. Science 290, 1751–1753 (2000).
Alexander, C. M. O., Grossman, J. N., Ebel, D. S. & Ciesla, F. J. The formation conditions of chondrules and chondrites. Science 320, 1617–1619 (2008).
Lodders, K. & Fegley, B. Jr. Chemistry of the Solar System. Vol. 476 (Royal Society and Chemistry Publication, 2010).
Palme, H., Lodders, K. & Jones, A. Solar system abundances of the elements. In: Andrew M. Davis (ed.) Planets, Asteriods, Comets and The Solar System2nd edn, Vol. 2, pp. 15–36 (Elsevier, 2014).
Braukmüller, N., Wombacher, F., Funk, C. & Münker, C. Earth’s volatile element depletion pattern inherited from a carbonaceous chondrite-like source. Nat. Geosci. 12, 564–568 (2019).
Yokoyama, T. et al. Samples returned from the asteroid Ryugu are similar to Ivuna-type carbonaceous meteorites. Science 379, eabn7850 (2022).
Lauretta, D. S. et al. Asteroid (101955) Bennu in the laboratory: properties of the sample collected by OSIRIS-REx. Meteorit. Planet. Sci. 59, 2453–2486 (2024).
Scott, E. R. D. Chondrites and the protoplanetary disk. Annu. Rev. Earth Planet. Sci. 35, 577–620 (2007).
Moynier, F., Vance, D., Fujii, T. & Savage, P. The isotope geochemistry of zinc and copper. Rev. Mineral. Geochem. 82, 543–600 (2017).
Luck, J.-M., Othman, D. B. & Albarède, F. Zn and Cu isotopic variations in chondrites and iron meteorites: early solar nebula reservoirs and parent-body processes. Geochim. Cosmochim. Acta 69, 5351–5363 (2005).
Pringle, E. A., Moynier, F., Beck, P., Paniello, R. & Hezel, D. C. The origin of volatile element depletion in early solar system material: clues from Zn isotopes in chondrules. Earth Planet. Sci. Lett. 468, 62–71 (2017).
Paquet, M. et al. Contribution of Ryugu-like material to Earth’s volatile inventory by Cu and Zn isotopic analysis. Nat. Astron. 7, 182–189 (2023).
Bishop, M. C. et al. The Cu isotopic composition of iron meteorites. Meteorit. Planet. Sci. 47, 268–276 (2012).
Moynier, F., Blichert-Toft, J., Telouk, P., Luck, J.-M. & Albarède, F. Comparative stable isotope geochemistry of Ni, Cu, Zn, and Fe in chondrites and iron meteorites. Geochim. Cosmochim. Acta 71, 4365–4379 (2007).
van Kooten, E. & Moynier, F. Zinc isotope analyses of singularly small samples ( < 5 ng Zn): investigating chondrule-matrix complementarity in Leoville. Geochim. Cosmochim. Acta 261, 248–268 (2019).
Albarède, F. Volatile accretion history of the terrestrial planets and dynamic implications. Nature 461, 1227–1233 (2009).
Mahan, B., Moynier, F., Beck, P., Pringle, E. A. & Siebert, J. A history of violence: Insights into post-accretionary heating in carbonaceous chondrites from volatile element abundances, Zn isotopes and water contents. Geochim. Cosmochim. Acta 220, 19–35 (2018).
Nakamura, T. Post-hydration thermal metamorphism of carbonaceous chondrites. J. Mineral. Petrol. Sci. 100, 260–272 (2005).
Tonui, E. et al. Petrographic, chemical and spectroscopic evidence for thermal metamorphism in carbonaceous chondrites I: CI and CM chondrites. Geochim. Cosmochim. Acta 126, 284–306 (2014).
Amsellem, E., Moynier, F., Mahan, B. & Beck, P. Timing of thermal metamorphism in CM chondrites: implications for Ryugu and Bennu future sample return. Icarus 339, 113593 (2020).
Moynier, F. et al. Isotopic fractionation of zinc in tektites. Earth Planet. Sci. Lett. 277, 482–489 (2009).
Herzog, G. F., Moynier, F., Albarède, F. & Berezhnoy, A. A. Isotopic and elemental abundances of copper and zinc in lunar samples, Zagami, Pele’s hairs, and a terrestrial basalt. Geochim. Cosmochim. Acta 73, 5884–5904 (2009).
Paniello, R. C., Day, J. M. D. & Moynier, F. Zinc isotopic evidence for the origin of the Moon. Nature 490, 376–379 (2012).
Kato, C., Moynier, F., Valdes, M. C., Dhaliwal, J. K. & Day, J. M. D. Extensive volatile loss during formation and differentiation of the Moon. Nat. Commun. 6, 7617 (2015).
Rodovská, Z. et al. Implications for behavior of volatile elements during impacts—zinc and copper systematics in sediments from the Ries impact structure and central European tektites. Meteorit. Planet. Sci. 52, 2178–2192 (2017).
Day, J. M. D., Moynier, F., Meshik, A. P., Pradivtseva, O. V. & Petit, D. R. Evaporative fractionation of zinc during the first nuclear detonation. Sci. Adv. 3, e1602668 (2017).
Day, J. M. D., van Kooten, E. M. M. E., Hofmann, B. A. & Moynier, F. Mare basalt meteorites, magnesian-suite rocks and KREEP reveal loss of zinc during and after lunar formation. Earth Planet. Sci. Lett. 531, 115998 (2020).
Wimpenny, J. et al. Experimental determination of Zn isotope fractionation during evaporative loss at extreme temperatures. Geochim. Cosmochim. Acta 259, 391–411 (2019).
Kamber, B. S. & Schoenberg, R. Evaporative loss of moderately volatile metals from the superheated 1849 Ma Sudbury impact melt sheet inferred from stable Zn isotopes. Earth Planet. Sci. Lett. 544, 116356 (2020).
Mathur, R. et al. Fingerprinting the Cretaceous-Paleogene boundary impact with Zn isotopes. Nat. Commun. 12, 4128 (2021).
Paquet, M., Sossi, P. A. & Moynier, F. Origin and abundances of volatiles on Mars from the zinc isotopic composition of Martian meteorites. Earth Planet. Sci. Lett. 611, 118126 (2023).
Long, Z.-Y. et al. Constraining the evaporative loss of zinc during impact processes using terrestrial impact glasses. Earth Planet. Sci. Lett. 646, 118979 (2024).
Day, J. M. D. & Moynier, F. Evaporative fractionation of volatile stable isotopes and their bearing on the origin of the Moon. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 372, 20130259 (2014).
King, A. J. et al. The Yamato-type (CY) carbonaceous chondrite group: analogues for the surface of asteroid Ryugu? Geochemistry 79, 125531 (2019).
Clayton, R. N. & Mayeda, T. K. Oxygen isotope studies of carbonaceous chondrites. Geochim. Cosmochim. Acta 63, 2089–2104 (1999).
Zhu, K. et al. CY1 chondrites produced by impact-dehydration of the CI chondrite parent body. Astrophys. J. Lett. 984, L54 (2025).
Schrader, D. L. et al. Reassessing the proposed “CY chondrites”: evidence for multiple meteorite types and parent bodies from Cr-Ti-H-C-N isotopes and bulk elemental compositions. Geochim. Cosmochim. Acta 390, 24–37 (2025).
Bell, M. S., Zolensky, M. E. & Yang, S. V. Evidence for thermal alteration in the Asuka 881655 chondrite. Meteorit. Planet Sci. 33, A13 (1998).
Wang, M. & Lipschutz, M. E. Thermally metamorphosed carbonaceous chondrites from data for thermally mobile trace elements. Meteorit. Planet. Sci. 33, 1297–1302 (1998).
Nakato, A. et al. PCA 02012: A unique thermally metamorphosed carbonaceous chondrite. Lunar Planet Sci. 44, A2708 (2013).
Garenne, A. et al. The abundance and stability of “water” in type 1 and 2 carbonaceous chondrites (CI, CM and CR). Geochim. Cosmochim. Acta 137, 93–112 (2014).
Alexander, C. M. O., Howard, K. T., Bowden, R. & Fogel, M. L. The classification of CM and CR chondrites using bulk H, C and N abundances and isotopic compositions. Geochim. Cosmochim. Acta 123, 244–260 (2013).
Hu, Y., Moynier, F. & Yang, X. Volatile-depletion processing of the building blocks of Earth and Mars as recorded by potassium isotopes. Earth Planet. Sci. Lett. 620, 118319 (2023).
Pringle, E. A. & Moynier, F. Rubidium isotopic composition of the Earth, meteorites, and the Moon: Evidence for the origin of volatile loss during planetary accretion. Earth Planet. Sci. Lett. 473, 62–70 (2017).
Suttle, M. D. et al. The mineralogy and alteration history of the Yamato-type (CY) carbonaceous chondrites. Geochim. Cosmochim. Acta 361, 245–264 (2023).
Naraoka, H. et al. A chemical sequence of macromolecular organic matter in the CM chondrites. Meteorit. Planet. Sci. 39, 401–406 (2004).
Barrat, J. A. et al. Geochemistry of CI chondrites: Major and trace elements, and Cu and Zn isotopes. Geochim. Cosmochim. Acta 83, 79–92 (2012).
Moynier, F. et al. Nature of volatile depletion and genetic relationships in enstatite chondrites and aubrites inferred from Zn isotopes. Geochim. Cosmochim. Acta 75, 297–307 (2011).
Chen, H., Savage, P. S., Teng, F.-Z., Helz, R. T. & Moynier, F. Zinc isotope fractionation during magmatic differentiation and the isotopic composition of the bulk Earth. Earth Planet. Sci. Lett. 369–370, 34–42 (2013).
King, A. J., Schofield, P. F. & Russell, S. S. Thermal alteration of CM carbonaceous chondrites: Mineralogical changes and metamorphic temperatures. Geochim. Cosmochim. Acta 298, 167–190 (2021).
Hanna, R. D. et al. Distinguishing relative aqueous alteration and heating among CM chondrites with IR spectroscopy. Icarus 346, 113760 (2020).
Suhr, N. et al. Elemental and isotopic behaviour of Zn in Deccan basalt weathering profiles: Chemical weathering from bedrock to laterite and links to Zn deficiency in tropical soils. Sci. Total Environ. 619–620, 1451–1463 (2018).
Little, S. H. et al. Cu and Zn isotope fractionation during extreme chemical weathering. Geochim. Cosmochim. Acta 263, 85–107 (2019).
Rubin, A. E., Trigo-Rodríguez, J. M., Huber, H. & Wasson, J. T. Progressive aqueous alteration of CM carbonaceous chondrites. Geochim. Cosmochim. Acta 71, 2361–2382 (2007).
Young, E. D., Galy, A. & Nagahara, H. Kinetic and equilibrium mass-dependent isotope fractionation laws in nature and their geochemical and cosmochemical significance. Geochim. Cosmochim. Acta 66, 1095–1104 (2002).
Young, E. D. & Galy, A. The Isotope Geochemistry and Cosmochemistry of Magnesium. Rev. Mineral. Geochem. 55, 197–230 (2004).
Luck, J. M., Othman, D. B., Barrat, J. A. & Albarède, F. Coupled 63Cu and 16 O excesses in chondrites. Geochim. Cosmochim. Acta 67, 143–151 (2003).
Fang, L. et al. The origin of 4-Vesta’s volatile depletion revealed by the zinc isotopic composition of diogenites. Sci. Adv. 10, eadl1007 (2024).
Bourdon, B. & Fitoussi, C. Isotope fractionation during condensation and evaporation during planet formation processes. ACS Earth Space Chem. 4, 1408–1423 (2020).
Alexander, C. M. O. et al. The lack of potassium-isotopic fractionation in Bishunpur chondrules. Meteorit. Planet. Sci. 35, 859–868 (2000).
Sossi, P. A. et al. An experimentally-determined general formalism for evaporation and isotope fractionation of Cu and Zn from silicate melts between 1300 and 1500 °C and 1 bar. Geochim. Cosmochim. Acta 288, 316–340 (2020).
Jiang, Y. et al. Implications of K, Cu and Zn isotopes for the formation of tektites. Geochim. Cosmochim. Acta 259, 170–187 (2019).
Langmuir, I. The evaporation, condensation and reflection of molecules and the mechanism of adsorption. Phys. Rev. 8, 149–176 (1916).
Matza, S. D. & Lipschutz, M. E. Thermal metamorphism of primitive meteorites—VII. Mineralogy-petrology of heated Murchison (C2) and alteration of C30 and other chondrites. Geochim. Cosmochim. Acta 42, 1655–1667 (1978).
Scott, E. R. D., Keil, K. & Stöffler, D. Shock metamorphism of carbonaceous chondrites. Geochim. Cosmochim. Acta 56, 4281–4293 (1992).
Sossi, P. A. & Fegley, B. Thermodynamics of element volatility and its application to planetary processes. In: High Temperature Gas-Solid Reactions in Earth and Planetary Processes (eds. King, P., Fegley, B. & Seward, T.) 393–460. https://doi.org/10.1515/rmg.2018.84.11 (De Gruyter, 2018).
Creech, J. B. & Moynier, F. Tin and zinc stable isotope characterisation of chondrites and implications for early Solar System evolution. Chem. Geol. 511, 81–90 (2019).
Sossi, P. A., Nebel, O., O’Neill, H. S.tC. & Moynier, F. Zinc isotope composition of the Earth and its behaviour during planetary accretion. Chem. Geol. 477, 73–84 (2018).
Acknowledgements
K.F.Q. acknowledges financial support from the National Key R&D Program of China (grant 2023YFF0804200). F.M. acknowledges funding from the ERC under the European Community’s H2020 framework program/ERC grant agreement No. 101001282 (METAL) and an International Emerging Action grant from the CNRS. Z.Y.L. acknowledges the financial support of the China Scholarship Council (202206400044). R.C. acknowledges financial support from Labex UnivEarthS and ICUB. J.D. acknowledges support from the NASA Solar System Workings Program (80NSSC22K0098). The calculations were performed thanks to the eDARI stl2816 grants. This study is also financially supported by the Frontiers Science Center for Deep-Time Digital Earth (grant 2652023001). We thank Samia Hidalgo for her assistance with the ICP-QMS analyses. We thank NASA and the National Institute for Polar Research, Japan, for the meteorites. Parts of this work were supported by IPGP multidisciplinary program PARI, by Region Île-de-France SESAME Grants no. 12015908, EX047016, and the IdEx Université de Paris grant, ANR−18-IDEX-0001 and the DIM ACAV+.
Author information
Authors and Affiliations
Contributions
Z.Y.L. and F.M. designed the project. Z.Y.L., L.F., T.H.L. and D.R. conducted the experiments and chemical analyses. T.F.J.B. and R.C. conducted the first-principles calculations. M.P., F.J., K.F.Q., J.D. and J.M.D.D. contributed to the interpretation and discussion of the results. All authors participated in writing, reviewing, and editing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Philippe Claeys and the other, anonymous, reviewer for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Long, ZY., Moynier, F., Bögels, T.F.J. et al. Impact-induced sublimation drives volatile depletion in carbonaceous meteorites. Nat Commun 16, 6146 (2025). https://doi.org/10.1038/s41467-025-61115-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-61115-3