Abstract
Antibody-drug conjugates (ADCs) and tyrosine kinase inhibitors (TKIs) are widely used for HER2-positive metastatic breast cancer, but their efficacy in the neoadjuvant setting remains under investigation. The MUKDEN 06 trial (NCT05426486), a multicentre, randomised, phase 2b study, compared ARX788 (anti-HER2 ADC) plus pyrotinib (TKI) with the standard neoadjuvant regimen of docetaxel, carboplatin, trastuzumab, and pertuzumab (TCbHP) in female patients with early or locally advanced HER2-positive breast cancer. The primary endpoint was the pathological complete response (pCR, ypT0/is, ypN0) rate, analyzed in the intention-to-treat population. pCR was achieved in 70.6% (48/68) of patients receiving ARX788 plus pyrotinib, compared to 51.5% (35/68) in the TCbHP group, with a significant absolute difference of 19.1% (95% CI, 2.7–34.6; p = 0.023). No treatment-related deaths occurred. The most common grade 3–4 adverse events were diarrhea and hepatic dysfunction in the ARX788 plus pyrotinib group, and fatigue, nausea and anorexia in the TCbHP group. Interstitial lung disease (ILD)/pneumonitis and ocular events were observed with ARX788 plus pyrotinib, indicating a distinct safety profile. These findings offer clinical insights into the potential of dual HER2-targeted blockade with an ADC and TKI as an optional neoadjuvant strategy for patients with early or locally advanced HER2-positive breast cancer.
Similar content being viewed by others
Introduction
Approximately 20% of breast cancers overexpress human epidermal growth factor receptor 2 (HER2), a key driving factor for tumor growth, differentiation, and survival1,2. The advent of HER2-targeted agents has significantly improved the prognosis of this previously highly aggressive and high-risk breast cancer type3. Currently, the management of HER2-positive breast cancer has evolved with the FDA approval of three different categories of HER2-targeted agents: monoclonal antibodies (trastuzumab, pertuzumab), tyrosine kinase inhibitors (TKIs; lapatinib, neratinib, tucatinib), and antibody-drug conjugates (ADCs; T-DM1, T-DXd)4. Neoadjuvant regimens of dual HER2-targeted trastuzumab and pertuzumab in combination with cytotoxic agents are now the standard of care for early and locally advanced HER2-positive breast cancer. This therapeutic strategy has achieved a pathological complete response (pCR) rate of ~50%5,6, leaving nearly half of patients with residual disease at the time of surgery. Unfortunately, patients with residual disease usually have a dismal prognosis, even with adjuvant T-DM1 or intensified post-surgical anti-HER2 therapy7,8. Therefore, there is an urgent need to explore novel neoadjuvant strategies to improve prognosis in early and locally advanced HER2-positive breast cancer.
ADCs combine a monoclonal antibody targeting HER2 with a cytotoxic payload, allowing precise drug delivery to HER2-positive tumor cells4. T-DM1 and T-DXd have demonstrated clinical benefit in patients with HER2-positive advanced breast cancer and in those with residual invasive disease following neoadjuvant systemic therapy (T-DM1)8,9,10. However, the role of ADCs in the neoadjuvant setting remains incompletely understood. The KRISTINE trial found that T-DM1 plus pertuzumab resulted in an inferior pCR rate compared to trastuzumab plus pertuzumab and chemotherapy (44.4% vs 55.7%)11. The WSG-ADAPT study evaluated neoadjuvant T-DM1-based regimens in hormone receptor (HR)-positive, HER2-positive breast cancer and reported pCR rates of 41.5% for T-DM1 plus endocrine therapy, 41% for T-DM1 monotherapy, and 15.1% for trastuzumab plus endocrine therapy12. These findings suggest that while ADCs hold promise, further investigation is needed to assess their optimal use and potential combination strategies in the neoadjuvant setting.
Preclinical study indicates that TKIs may up-regulate HER2 expression, potentially enhancing the efficacy of ADCs when used in combination13. This synergistic effect has shown clinical benefit in advanced HER2-positive breast cancer14. In a phase 1b trial of patients with progressive HER2-positive metastatic breast cancer, the combination of T-DM1 with tucatinib achieved a median progression-free survival of 8.2 months14. The randomised HER2CLIMB-02 trial further reinforced these findings15, highlighting the potential of ADC-TKI combinations in earlier disease settings, including neoadjuvant therapy.
ARX788 is an ADC consisting of a trastuzumab-based antibody conjugated to two tubulin inhibitors (AS269) via a site-specific para-acetyl phenylalanine (pAF) linker, forming a stable oxime bond and yielding a drug-to-antibody ratio (DAR) of two16. Clinical studies have demonstrated the efficacy and safety of ARX788 in HER2-positive metastatic breast cancer16,17. Pyrotinib is an irreversible TKI targeting HER1, HER2, and HER4, approved in China for the treatment of HER2-positive early and advanced breast cancer18,19.
In this work, we conducted the multicentre, randomised phase 2b MUKDEN 06 trial to evaluate the efficacy and safety of neoadjuvant ARX788 plus pyrotinib versus the standard TCbHP regimen (docetaxel, carboplatin, trastuzumab and pertuzumab) in patients with early or locally advanced HER2-positive breast cancer. ARX788 plus pyrotinib significantly improved the pCR rate over TCbHP, highlighting the potential of dual HER2-targeted blockade with an ADC and TKI as a neoadjuvant strategy for early or locally advanced HER2-positive breast cancer.
Results
Patient characteristics
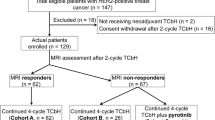
Between May 23, 2022 and October 7, 2023, a total of 152 patients from seven centres in China were screened, and 136 eligible patients were randomised to receive ARX788 plus pyrotinib (n = 68) or TCbHP (n = 68) for efficacy and safety analysis (Fig. 1). Baseline demographic and clinical characteristics were well balanced between the two groups (Table 1). Among patients receiving ARX788 plus pyrotinib, 31 (45.6%) had stage III disease, 48 (70.6%) had lymph node metastasis (N1 or higher), and 38 (55.9%) had HR-positive disease. In the TCbHP group, 30 (44.1%) patients had stage III disease, 45 (66.2%) had lymph node metastasis, and 34 (50.0%) had HR-positive disease.
Efficacy
The pCR rate was significantly higher in the ARX788 plus pyrotinib group compared to the TCbHP group (70.6% [48/68] vs. 51.5% [35/68]; absolute difference: 19.1%, 95% CI: 2.7 to 34.6; odds ratio [OR]: 2.3; p = 0.023; Table 2). Sensitivity analysis confirmed the robustness of the primary pCR findings (adjusted difference: 20.0%; adjusted OR: 2.5; adjusted p = 0.019; Table 2). The breast pCR (bpCR) rate was 73.5% (50/68) in the ARX788 plus pyrotinib group, compared to 55.9% (38/68) in the TCbHP group, with an absolute difference of 17.6% (95% CI: 1.5–32.9; p = 0.031). The proportion of patients achieving residual cancer burden (RCB)−0 or I was similar between the two groups: 75.0% (51/68) in the ARX788 plus pyrotinib group vs. 70.6% (48/68) in the TCbHP group (absolute difference: 4.4%; 95% CI: –10.6 to 19.3; p = 0.56). The objective response rate (ORR) was 94.1% (64/68) in the ARX788 plus pyrotinib group and 91.2% (62/68) in the TCbHP group (absolute difference: 2.9%; 95% CI: –6.6 to 12.9; p = 0.51). OS and EFS remain undetermined due to ongoing follow-up and insufficient follow-up time.
Subgroup analysis of pCR by treatment across baseline characteristics is presented in Fig. 2. The results were generally consistent with the main analysis across all subgroups, except for the N0 subgroup (55.0% vs. 56.5%), where no significant difference was observed. Notably, patients with node-positive disease (77.1% vs. 48.9%) and those with stage IIIA-IIIC disease (74.2% vs. 46.7%) appeared to derive greater benefit from ARX788 plus pyrotinib.
Data are presented as pCR rates (%) and 95% confidence intervals (CI). Black squares represent the differences in pCR rates between the ARX788 plus pyrotinib group and the TCbHP group across stratified subgroups. TCbHP, docetaxel plus carboplatin, trastuzumab and pertuzumab; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; FISH, fluorescence in situ hybridization; CI, confidence intervals. Source data are provided as Supplementary Table 1.
Safety
All patients in both treatment groups experienced at least one treatment-emergent adverse event (TEAE). A summary of TEAEs is presented in Table 3. The most common TEAEs in the ARX788 plus pyrotinib group were diarrhea (67 [99%]), hypokalemia (56 [82%]), elevated alanine aminotransferase (ALT) (54 [79%]) and elevated aspartate aminotransferase (AST) (49 [72%]). Grade 3 TEAEs were reported in 38 (56%) patients, with diarrhea being the most frequent (31 [46%]). No grade 4 or 5 TEAEs were observed. Due to AEs, dose reductions of ARX788 and pyrotinib were required in 31 (46%) and 26 (38%) patients, respectively.
Interstitial lung disease (ILD)/pneumonitis and ocular toxicities were the most common ARX788-related adverse events. ILD/pneumonitis occurred in 29 (43%) patients, with 26 (38%) experiencing grade 1 or 2 and three patients developing grade 3 ILD/pneumonitis. Among these, 27 (93%) patients recovered following steroid treatment, while two (7%) self-recovered without specific intervention. Early diagnosis through routine monitoring and prompt steroid administration minimized the risk of severe complications. The median time to onset of ILD/pneumonitis was five cycles (range: 2–6). Surgery was delayed in five patients due to ILD/pneumonitis (median delay: 16 days, range: 11–19), whereas 24 patients proceeded with surgery without delay. Notably, the pCR rate among patients with ILD/pneumonitis was 86.2% (25/29). Ocular AEs were reported in 51 (75%) patients, with the most common being blurred vision (32 [47%]), dry eye syndrome (29 [43%]), and corneal epitheliopathy (24 [35%]). Two patients developed grade 3 corneal epitheliopathy. All ocular AEs, including the grade 3 cases, were reversible with supportive care, such as ocular lubricants, topical antibiotics, anti-inflammatory agents, and other corneal recovery interventions.
The most common TEAEs in the TCbHP group were fatigue (62 [91%]), nausea (58 [85%]), anorexia (58 [85%]), diarrhea (51 [75%]), anemia (50 [73%]), vomiting (42 [62%]), decreased white blood cell (42 [62%]), and decreased neutrophil count (40 [59%]). Grade 3 or 4 TEAEs were reported in 39 (57%) patients, with the most common being decreased neutrophil count (28 [41%]) and decreased white blood cell count (16 [24%]). Due to AEs, 20 (29%) patients required dose reductions, primarily due to hematologic toxicities, hepatotoxicity, and fatigue.
Treatment compliance
All 136 patients received neoadjuvant therapy, underwent surgery, and had a pathological evaluation. Two patients in the ARX788 plus pyrotinib group switched to an alternative neoadjuvant regimen due to stable disease, while three patients in the TCbHP group switched regimens (two due to stable disease and one due to progressive disease). In the ARX788 plus pyrotinib group, 61 patients completed at least four treatment cycles: 18 completed six cycles, 15 completed five cycles, and 28 completed four cycles. Reasons for not completing all six cycles included stable disease (two [2.9%]), patient decision (nine [13.2%]), and adverse events (AEs; 39 [57.4%]). In the TCbHP group, ten (14.7%) patients discontinued neoadjuvant therapy, with one (1.5%) due to progressive disease, two (2.9%) due to stable disease, and seven (10.3%) due to AEs. The dose reduction rate for ARX788 was 45.6% (31/68), and the discontinuation rate was 73.5% (50/68). For pyrotinib, the dose reduction and discontinuation rates were 38.2% (26/68) and 73.5% (50/68), respectively. The average relative dose intensity (RDI) for ARX788 was 96.89% (range, 66%-100.00%), and for pyrotinib, it was 89.35% (range, 39%-100.00%) in the ARX788 plus pyrotinib group. In the TCbHP group, the average RDI were 98.25% (77%-100.00%) for both docetaxel and carboplatin, and 100% (range, 100%-100%) for trastuzumab and pertuzumab.
Discussion
The MUKDEN 06 trial evaluated the efficacy and safety of a neoadjuvant dual HER2 blockade strategy combining an ADC with a small-molecule TKI compared to the standard trastuzumab-pertuzumab-chemotherapy (TCbHP) regimen in HER2-positive breast cancer. The results demonstrated that ARX788 plus pyrotinib significantly improved the pCR rate compared to TCbHP (70.6% vs 51.5%). Of note, while the ARX788 plus pyrotinib regimen did not cause any grade 4 adverse events, diarrhea and ILD/pneumonia require careful consideration. These findings indicate the potential of combining an anti-HER2 ADC with a TKI as a neoadjuvant strategy for HER2-positive breast cancer, warranting further clinical investigation.
Neoadjuvant therapy plays a critical role in early or locally advanced HER2-positive breast cancer by improving surgical outcomes and enabling early assessment of treatment response to guide postoperative therapy. Achieving pCR serves as a strong predictor of favorable long-term outcomes20. Previous clinical trials have demonstrated that dual HER2-targeted therapy with trastuzumab, pertuzumab and chemotherapy achieves a pCR rate of ~50%20. However, intertumoral heterogeneity-including differences in tumor genomics and the tumor microenvironment, significantly impacts pCR rates in HER2-positive breast cancer21,22. De novo or acquired resistance remains a major challenge, underscoring the need for novel strategies to overcome therapeutic resistance.
Although HER2-targeted ADCs have shown remarkable efficacy in metastatic HER2-positive breast cancer, their role in early or locally-advanced disease still remains under investigation. Several trials have explored HER2 ADC-based neoadjuvant regimens, but results have been suboptimal. The KRISTINE trial reported a lower pCR rate with neoadjuvant T-DM1 plus pertuzumab compared to TCbHP (44.4% vs. 55.7%)11. Similarly, the WSG-ADAPT study found that neoadjuvant T-DM1 plus endocrine therapy yielded pCR rates of 41.5% in HR-positive, HER2-positive breast cancer, comparable to T-DM1 monotherapy (41%)12. These findings underscore the need for more effective synergistic partners for ADCs to enhance therapeutic efficacy in the neoadjuvant setting.
Preclinical study suggests that ADC-TKI combinations may upregulate HER2 expression and exert synergistic therapeutic effects, providing a strong rationale for ADC plus TKI therapeutic strategies in HER2-positive breast cancer. In the MUKDEN 06 study, neoadjuvant therapy with ARX788 plus pyrotinib achieved a significantly higher pCR rate (70.6%) than TCbHP (51.5%, p = 0.023). Moreover, this regimen demonstrated superior efficacy compared to T-DM1 plus pertuzumab (70.6% vs. 44.4%), suggesting that TKIs may be more potent synergistic partners for ADCs. These findings in the neoadjuvant, treatment-naïve population align with preclinical data supporting the potential of ADC-TKI combinations. Furthermore, in the MUKDEN 06 study, ARX788 plus pyrotinib led to more rapid disease control and greater tumor shrinkage than TCbHP, particularly in the stage III subgroup. Given the inherently aggressive nature of HER2-positive breast cancer, achieving rapid tumor shrinkage and downstaging through neoadjuvant therapy is of critical importance for complete surgical resection and improved treatment outcomes. Thus, ARX788 plus pyrotinib may offer a promising neoadjuvant optional strategy for patients requiring a rapid therapeutic response. Additionally, the pCR rate observed with TCbHP group in the MUKDEN 06 study (51.5%) was similar compared to that observed with the same regimen (TCbHP, 18 weeks) in the KRISTINE study (55.7%)11. In contrast, in the BERENICE23 and TRAIN-224 trials, which extended neoadjuvant therapy duration (20–24 weeks in BERENICE, 27 weeks in TRAIN-2), achieved pCR rates of 60.7% and 68%, respectively, suggesting that prolonged treatment may enhance response rates. Furthermore, the identification of predictive biomarkers for ARX788 plus pyrotinib is currently underway to facilitate patient selection and optimize therapeutic efficacy.
In terms of safety, ARX788 plus pyrotinib was primarily associated with diarrhea, hypokalemia, and liver dysfunction, whereas TCbHP was more frequently associated with fatigue, gastrointestinal and hematological toxicities. The incidence of grade 3 TEAEs was comparable between the two groups (56% vs 57%). In the ARX788 plus pyrotinib group, diarrhea was the most common grade 3 TEAE, whereas neutropenia, leukopenia and fatigue predominated in the TCbHP group. Notably, grade 3 or 4 neutropenia occurred in 41% of TCbHP-treated patients but only 1% of those receiving ARX788 plus pyrotinib. Similarly, grade 3 fatigue was frequent with TCbHP (22%), compared to ARX788 plus pyrotinib (13%). These distinct toxicity profiles suggest that the two regimens may be better suited for different patient populations, highlighting the need for individualized treatment strategies.
The combination of ARX788 and pyrotinib exhibited a unique safety profile requiring careful monitoring and proactive management. The incidence of grade 3 diarrhea was 46%; however, no severe diarrhea-related complications were observed. Symptoms were effectively managed through dose adjustments and loperamide, following clinical guidelines25. These findings suggest a potential additive toxicity effect of ARX788 and pyrotinib, underscoring the necessity for further investigation into dose reduction strategies. Similarly, ILD/pneumonia associated with ARX788 also warrants close attention. To mitigate risks, we implemented strict eligibility criteria, patient education, routine monitoring, and standardized management protocols. Most cases (38%) were grade 1-2, while only a small proportion (4%) were grade 3, consistent with prior data onARX788 (2.9%)16,17. Although five patients experienced a median surgery delay of 16 days due to ILD/pneumonitis, they achieved a pCR rate of 86.2%. Notably, treatment delays of less than three months have not been shown to negatively impact survival outcomes26,27. We are currently conducting research to identify biomarkers predictive of ADC-induced ILD/pneumonitis. Ocular adverse events, another known toxicity of ARX788, were mostly mild (grade 1-2), with only two patients developing grade 3 corneal epitheliopathy. These toxicities were manageable with sodium hyaluronate eye drops and supportive care. The underlying mechanism may be related to the intracellular accumulation of monomethyl auristatin F28. Consequently, these adverse events impacted treatment completion in the experimental group. Notably, despite the lower-than-expected treatment completion rates, pCR rates remained unaffected: 61.1% for six cycles, 86.7% for five cycles, and 82.1% for four cycles. This suggests that a five- or four-cycle regimen may still achieve a favorable pCR rate, warranting further investigation. These findings provide valuable insights for ongoing and future research, as the combination of ADCs (e.g., T-DM1, T-DXd, SHR-A1811) and TKIs (e.g., tucatinib, pyrotinib) is currently being investigated in multiple clinical trials (NCT04457596, NCT06245824, NCT05635487).
We acknowledge several limitations in our study. First, the relatively small sample size and the inclusion of only Asian patients may limit generalizability. Second, long-term survival outcomes remain to be determined with ongoing follow-up. Additionally, the higher incidence of ILD/pneumonitis and diarrhea with ARX788 plus pyrotinib underscores the need for optimized management strategies. Biomarker analyses are in progress to identify patient subgroups most likely to benefit from this regimen and to develop predictive markers for ADC-induced ILD/pneumonitis.
In conclusion, neoadjuvant therapy with ARX788 plus pyrotinib significantly improved pCR rate compared to the standard TCbHP regimen in early or locally advanced HER2-positive breast cancer. While this combination demonstrated a distinct safety profile requiring further refinement, it represents a therapeutic strategy for patients who may not respond optimally to conventional neoadjuvant regimens. Ongoing research into biomarker-driven patient selection and toxicity management will be essential to optimizing the clinical utility of ADC-TKI combinations in HER2-positive breast cancer.
Methods
The study was designed and conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice (GCP) Guidelines. Ethics approvals were obtained from independent ethics committees of Shengjing Hospital of China Medical University, Anshan Cancer Hospital, Liaohe Oilfield General Hospital, Yan’an People’s Hospital, Liaoning Cancer Hospital and Institute, Cancer Hospital of Harbin Medical University, and the First Affiliated Hospital of Jinzhou Medical University. All patients provided written informed consent. This trial is registered with ClinicalTrials.gov (NCT05426486). The first patient was enrolled on May 23, 2022, which was the same day we received notification of ethics committee approval for patient enrollment. However, the upload of the ethics approval document to the clinical trial registry was slightly delayed due to time limitations.
Trial design and patients
The MUKDEN 06 trial is a multicentre, open-label, randomised phase 2b study conducted across seven centres in China. Eligible participants were women aged 18–75 years with pathologically confirmed HER2-positive breast cancer considered amenable to neoadjuvant therapy. Additional inclusion criteria included: stage II-III HER2-positive breast cancer; Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; at least one measurable lesion per the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1; and adequate bone marrow and organ function. HER2-positive status was defined as immunohistochemistry (IHC) 3 + , or 2+ with positive fluorescence in situ hybridization (FISH) (amplification rate ≥2.0). There were no restrictions on HR status. HR-positive tumors were identified by IHC positivity for estrogen receptor (ER) in ≥1% of tumor cells and/or for progesterone receptor (PR) in ≥1% of tumor cells.
The key exclusion criteria were: any prior systemic anti-tumor therapy; bilateral, inflammatory, or occult breast cancer; other malignancies within the past 5 years, except for malignant skin melanoma and cured carcinoma in situ of the cervix; severe dysfunction of vital organs (heart, liver, or kidney); history or clinical evidence of ILD or radiation pneumonitis requiring steroid therapy; current eye disease requiring intervention (e.g., keratitis, corneal disease, retinal disease, or active eye infections); inability or unwillingness to discontinue contact lens use during the study; pregnancy or lactation. The trial protocol is available in Supplement 1.
The randomisation sequence was generated by a randomisation specialist. Investigators registered patients and assigned them according to the randomisation sequence, using a 1:1 allocation ratio. Patients were randomised to receive either ARX788 plus pyrotinib, or TCbHP (docetaxel, carboplatin, trastuzumab, and pertuzumab). As an open-label trial, both investigators and patients were aware of the treatment assignment.
Procedures
Patients in the ARX788 plus pyrotinib group received intravenous ARX788 (1.5 mg/kg, day 1) combined with oral pyrotinib (320 mg once daily). Patients in the TCbHP group received intravenous docetaxel (75 mg/m2, day 1), carboplatin (area under the concentration-time curve [AUC] 6 mg/mL × min, day 1), trastuzumab (8 mg/kg loading dose, followed by 6 mg/kg maintenance dose, day 1) and pertuzumab (840 mg loading dose, followed by 420 mg maintenance dose, day 1). Both treatment regimens were administered every three weeks for six cycles as neoadjuvant therapy. To manage potential pyrotinib-related diarrhea, patients in the ARX788 plus pyrotinib group received oral loperamide (up to 16 mg/day). Additionally, baseline eye examinations were conducted, and patients were advised to use sodium hyaluronate eye drops for the prevention of ocular adverse effects. Management guidelines for ARX788-related pulmonary and ocular toxicities were detailed in the study protocol (Supplement 1). Dose reductions were permitted to manage toxicities. If needed, the ARX788 dose was reduced stepwise from 1.5 mg/kg to 1.3 mg/kg to 1.1 mg/kg, while the pyrotinib dose was gradually reduced from 320 mg to 240 mg to 160 mg to manage toxicities. Trastuzumab dose reduction was permitted only if body weight decreased by more than 10%. The minimum allowable doses for docetaxel and carboplatin were 60 mg/m² and AUC 4, respectively. Treatment interruptions and discontinuations were permitted based on clinical judgment. Detailed dose adjustment guidelines are available in the protocol (Supplement 1).
Breast cancer surgery was performed 2-6 weeks after the final neoadjuvant therapy dose. According to National Comprehensive Cancer Network (NCCN) guidelines, both groups received postoperative HER2 targeted therapy, radiotherapy (if indicated), and endocrine therapy for HR-positive patients. In the TCbHP group, patients with non-pCR were advised to receive adjuvant T-DM1, or intensified adjuvant TKI (neratinib) following trastuzumab, while in the ARX788 plus pyrotinib group, patients were recommended to receive trastuzumab plus pertuzumab and chemotherapy.
Breast ultrasound was conducted at baseline, after each cycle of neoadjuvant therapy, and before surgery. Magnetic resonance imaging (MRI) was performed at baseline, after two and four cycles, and prior to surgery. Clinical responses were assessed using MRI, independently evaluated by two senior radiologists at each centre per RECIST 1.1. Pathological responses and RCB were evaluated from surgical tumor samples by two senior pathologists at each centre, following the Expert Panel Consensus on Pathological Diagnosis of Breast Cancer with Neoadjuvant Therapy, version 202029. RCB classification was based on five indexes: area of primary tumor bed, overall cancer cellularity, percentage of cancer that was in situ disease, number of positive lymph nodes, and diameter of the largest metastatic lesion30. RCB was categorized as RCB-0 (no residual disease), RCB-I (minimal residual disease), RCB-II (moderate residual disease), and RCB-III (extensive residual disease)30. Adverse events (AEs) in all patients were monitored until four weeks after the final neoadjuvant therapy dose and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 5.0.
Study endpoints
The primary endpoint was the rate of pCR, defined as the disappearance of invasive tumors in the breast and axillary lymph nodes (ypT0/is, ypN0). Patients who did not undergo surgery or changed neoadjuvant regimens were considered non-pCR.
Secondary endpoints included bpCR (ypT0/is), RCB, ORR, overall survival (OS), event-free survival (EFS), and safety. ORR was defined as the proportion of patients achieving complete or partial response per RECIST 1.1.
Statistical analysis
The study was designed to provide at least 80% power to detect an increase in the pCR rate from 56% (TCbHP, based on the KRISTINE study)11 to 78% (ARX788 plus pyrotinib, according to historical data), assuming a two-sided α level of 5%. Thus, 68 patients per treatment arm were planned.
Efficacy analyses, including pCR rate, bpCR rate, RCB, ORR and treatment-emergent adverse events (TEAEs), were conducted in the intention-to-treat (ITT) population (all randomly assigned patients). Response rates were summarized as n (%) with the corresponding exact two-sided 95% confidence interval (CI) estimated by the Clopper-Pearson method. Risk difference (RD) and odds ratio (OR) for pCR analyzed using the Cochran-Mantel-Haenszel (CMH) test. 95% CI for RD and OR was estimated using the Miettinen-Nurminen (score) method. A sensitivity analysis of the CMH test was conducted to assess the impact of baseline confounders (T stage, N stage, and HR status) on the comparison between treatment groups. All statistical tests were two-sided, with α = 0.05 considered statistically significant. All statistical analyses were performed using SAS, version 9.4.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The raw clinical data are protected due to patient privacy laws. De-identified study data are available for research purposes for a period of 10 years to the corresponding author, Caigang Liu, subject to approval from the Institutional Ethics Committees. Once approved, the de-identified data will be transferred to the requesting investigator via a secure file transfer protocol and will remain accessible for approximately two weeks after transfer. The Source Data and the study protocol are provided in the Supplementary Information. Source data are provided with this paper.
References
Giaquinto, A. N. et al. Breast cancer statistics, 2022. CA Cancer J. Clin. 72, 524–541 (2022).
Ross, J. et al. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. oncologist 14, 320–368 (2009).
Choong, G., Cullen, G. & O’Sullivan, C. Evolving standards of care and new challenges in the management of HER2-positive breast cancer. CA: a cancer J. clinicians 70, 355–374 (2020).
Swain, S., Shastry, M. & Hamilton, E. Targeting HER2-positive breast cancer: advances and future directions. Nat. Rev. Drug Discov. 22, 101–126 (2023).
Gianni, L. et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 13, 25–32 (2012).
Schneeweiss, A. et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann. Oncol. 24, 2278–2284 (2013).
Martin, M. et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 18, 1688–1700 (2017).
von Minckwitz, G. et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 380, 617–628 (2019).
Dieras, V. et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 18, 732–742 (2017).
Hurvitz, S. et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet (Lond., Engl.) 401, 105–117 (2023).
Hurvitz, S. A. et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 19, 115–126 (2018).
Harbeck, N. et al. De-escalation strategies in human epidermal growth factor receptor 2 (HER2)–positive early breast cancer (BC): final analysis of the west german study group adjuvant dynamic marker-adjusted personalized therapy trial optimizing risk assessment and therapy response prediction in early BC HER2- and hormone receptor–positive phase ii randomized trial—efficacy, safety, and predictive markers for 12 weeks of neoadjuvant trastuzumab emtansine with or without endocrine therapy (ET) versus trastuzumab plus ET. J. Clin. Oncol. 35, 3046–3054 (2017).
Scaltriti, M. et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene 28, 803–814 (2009).
Borges, V. et al. Tucatinib combined with ado-trastuzumab emtansine in advanced ERBB2/HER2-positive metastatic breast cancer: a phase 1b clinical trial. JAMA Oncol. 4, 1214–1220 (2018).
Hurvitz, S. et al. Abstract OT-28-01: HER2CLIMB-02: a randomized, double-blind, phase 3 study of tucatinib or placebo with T-DM1 for unresectable locally-advanced or metastatic HER2+ breast cancer. Cancer Res. 81, OT-28-01–OT-28-01 (2021).
Zhang, J. et al. Phase I trial of a novel anti-HER2 antibody-drug conjugate, ARX788, for the treatment of HER2-positive metastatic breast cancer. Clin. Cancer Res., 29, OF1-OF10 (2022).
Xichun, H. et al. ACE-Breast-02: a randomized phase III trial of ARX788 versus lapatinib plus capecitabine for HER2-positive advanced breast cancer. Signal Transduct Target Ther 17, 56 (2025).
Xu, B. et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 22, 351–360 (2021).
Wu, J. et al. Neoadjuvant pyrotinib, trastuzumab, and docetaxel for HER2-positive breast cancer (PHEDRA): a double-blind, randomized phase 3 trial. BMC Med. 20, 498 (2022).
von Minckwitz, G. et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol.: J. Am. Soc. Clin. Oncol. 30, 1796–1804 (2012).
Debora, F. et al. RNA Sequencing to Predict Response to Neoadjuvant Anti-HER2 Therapy: A Secondary Analysis of the NeoALTTO Randomized Clinical Trial. JAMA Oncol 3, 227–234 (2017).
Carey L. A. et al. Molecular heterogeneity and response to neoadjuvant human epidermal growth factor receptor 2 targeting in CALGB 40601, a randomized phase III trial of paclitaxel plus trastuzumab with or without lapatinib. J. Clin. Oncol. 34, 542–549 (2015).
Swain, S. M. et al. Pertuzumab, trastuzumab, and standard anthracycline- and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): a phase II, open-label, multicenter, multinational cardiac safety study. Ann. Oncol. 29, 646–653 (2018).
van Ramshorst, M. S. et al. Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 19, 1630–1640 (2018).
Rugo, H. et al. The characterization, management, and future considerations for ErbB-family TKI-associated diarrhea. Breast Cancer Res. Treat. 175, 5–15 (2019).
Sainsbury, R., Johnston, C. & Haward, B. Effect on survival of delays in referral of patients with breast-cancer symptoms: a retrospective analysis. Lancet (Lond., Engl.) 353, 1132–1135 (1999).
Richards, M., Westcombe, A., Love, S., Littlejohns, P. & Ramirez, A. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet (Lond., Engl.) 353, 1119–1126 (1999).
Sharma, A. et al. Reversible HER2 antibody-drug conjugate-induced ocular toxicity. Can. J. Ophthalmol. J. canadien d.’ophtalmologie 57, 118–126 (2022).
MoBCEPoC. Expert panel consensus on pathological diagnosis of breast cancer with neoadjuvant therapy, the 2020 version. Zhonghua Bing Li Xue Za Zhi 49, 296–304 (2020).
Symmans, W. et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J. Clin. Oncol.: J. Am. Soc. Clin. Oncol. 25, 4414–4422 (2007).
Acknowledgements
NovoCodex Biopharmaceuticals provided the study drug ARX788 free of charge and Jiangsu Hengrui Pharmaceuticals provided the study drug pyrotinib with a discount for patients enrolled in the study. The drug provider was not involved in the study design, data collection, and analysis as well as the paper writing. We thank the patients, their families, other investigators, and investigational site members involved in this study. We gratefully acknowledge Dr. Neeha Zaidi, MD. from Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University for her insightful critique on the manuscript.
Author information
Authors and Affiliations
Contributions
C.L. conceived, designed and supervised the study. N.N., J.X., G.C., F.Q., Q.X., X.Z., X.G., Yi.Z., H.X., H.Z., G.H., K.L., P.L., X.C., Y.L., S.W., D.Z., T.L., F.X., Y.X., Y.H., M.T. and C.L. recruited patients. N.N., Ch.L., Y.Z., M.L., G.J. and X.J. were responsible for collection and assembly of data. C.L. and N.N. verified the data in the study. All authors contributed to the analysis and interpretation of data. N.N. drafted the manuscript. C.L., N.N., Z.P., and T.Y. contributed to the critical revision of the manuscript for important intellectual content. All authors approved the final version of manuscript for submission. All authors had full access to the raw data and the corresponding author had full responsibility for the decision to submit it for publication.
Corresponding authors
Ethics declarations
Competing interests
ZP is employee of Hengrui Pharmaceuticals. The other authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Niu, N., Xue, J., Chen, G. et al. Neoadjuvant ARX788 plus pyrotinib versus trastuzumab, pertuzumab, docetaxel and carboplatin for HER2-positive breast cancer: a randomised phase 2b trial. Nat Commun 16, 6036 (2025). https://doi.org/10.1038/s41467-025-61213-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-61213-2