Abstract
RAD51C is a tumor suppressor gene with over 285 variants of unknown significance (VUS) found in primary ovarian tumors. RAD51C is a paralog of the recombinase RAD51, and it forms complexes with other paralogs to regulate RAD51 activity. We screened 27 ovarian cancer-derived RAD51C VUS to identify those that affect the assembly of functional tetrameric RAD51B-C-D-XRCC2 (BCDX2) complex. With yeast 3-hybrid and biochemical analyses, we identify a mutation cluster of the RAD51C Walker B region affecting protein interactions with other RAD51 paralogs. By further analyzing these variants for homologous recombination (HR), replication fork regression, DNA binding and ATPase activity, and RAD51 filament formation, we identified separation-of-function alleles that uncouple RAD51C distinct enzymatic activities with HR and replication. Thus, our analysis of RAD51C identifies additional VUS with functional defects, which will aid in pathogenicity classification and inform future strategies to treat individuals harboring RAD51C loss-of-function alleles.
Similar content being viewed by others
Introduction
Homologous recombination (HR) is a high-fidelity mechanism of DNA double-strand break (DSB) repair with a strong connection to genome stability and cancer avoidance. HR defects have been identified in over 50% of high grade serous ovarian cancers (HGSOC) stemming from genetic mutations, copy number changes, and increased promoter methylation of related genes1. BRCA1 and BRCA2, the two most frequently mutated HR genes, are routinely screened for in breast and ovarian tumors to guide precision therapy2,3. Emerging studies show that mutations in several of the other HR genes, including RAD51C, RAD51D, PALB2, BARD1, BRIP1, may also create cancers susceptible to similar targeted therapies developed for BRCA1 and BRCA2 deficient tumors4,5. However, many of these genes, such as RAD51C and RAD51D, are less well characterized and thus point mutations identified are typically classified as variants of unknown significance (VUS)3,4,6. Uncertainty regarding possible pathogenicity of these VUS complicates cancer diagnosis and treatment.
The central step of HR is the formation of RAD51 filaments. These filaments comprise of the RAD51 recombinase wound around single-stranded DNA derived from the nucleolytic processing of DSB ends. These RAD51 nucleoprotein filaments perform a DNA homology search that leads to the engagement and invasion of a homologous chromosome to execute repair7,8. Many VUS occur in genes that regulate RAD51 activity such as BRCA2, PALB2, RAD51C, and RAD51D3,9. RAD51C and RAD51D belong to a family of related proteins called the RAD51 paralogs. The RAD51 paralogs aid in preservation of genome stability through regulation of RAD51 activity during HR, and in newly identified functions during DNA replication10. The canonical RAD51 paralogs consist of RAD51B, RAD51C, RAD51D, XRCC2 and XRCC3, which form two distinct complexes, namely, the BCDX2 complex (RAD51B, RAD51C, RAD51D, XRCC2) and the CX3 complex (RAD51C, XRCC3). These proteins share up to 30% sequence similarity with RAD51 with the majority of this similarity residing in the Walker A and Walker B ATPase motifs11. Along with ATP binding and hydrolysis, the region in and around the conserved Walker A motif of RAD51C was recently identified as crucial for both protein-protein interactions and HR12,13,14. A similar variant cluster in the Walker B region has yet to be identified.
RAD51C genetic alterations have been identified in 3% of familial ovarian cancers and ovarian cancer risk for RAD51C variant carriers is on par with BRCA2 variant carriers (Odds ratio RAD51C 8.3 vs BRCA2 9.94 [95% CI 5.43–12.48 and 8.09–12.25, respectively])3,15. Epidemiology studies have identified RAD51C variants in families with high incidences of breast and ovarian cancers that are otherwise wild-type for BRCA1 and BRCA216. Patient samples reveal that RAD51C variants are heterozygous within healthy patient tissue, and biallelic in the tumor itself, suggesting a loss-of-heterozygosity (LOH) event16. These tumors are also p53 deficient4,16. Several studies show that a subset of RAD51C missense variants are deleterious to RAD51C function as HR impairment and reduction in DNA damage-induced RAD51 foci are observed12,14,16,17,18,19,20,21,22. Furthermore, the same treatment strategies such as the use of PARP inhibitors, can be equally efficacious against BRCA1/BRCA2 and RAD51C-deficient tumors12,14,21,22.
Although RAD51C loss is closely associated with increased ovarian cancer risk, less than 50% of the reported ovarian cancer derived RAD51C VUS have been functionally screened for an HR defect (Supplementary Data 1). To close this knowledge gap, we recently created a framework to characterize RAD51C variants for altered protein function. Using a yeast-three-hybrid approach, we showed that RAD51C interaction with RAD51D is highly predictive of impaired HR and chemotherapeutic sensitivity12. In this study, we performed a comprehensive screen of 27 RAD51C ovarian cancer VUS for altered protein function and HR. Our results identify an ovarian cancer variant mutation cluster in and surrounding the Walker B motif. We show that variants in this region exhibit loss of protein function and identify separation-of-function alleles in the Walker B region that uncouple its enzymatic activities during HR and DNA replication. By performing biochemical analysis of reconstituted BCDX2 complex, we demonstrate that while both RAD51C HR and replication functions are needed for RAD51 filament formation, RAD51C HR function is needed for cisplatin and olaparib resistance whereas RAD51C replicative function require its ATPase activity. Our work thus provides evidence for an important role of the RAD51C Walker B region in RAD51C function and indicates that while variants that impact both RAD51C HR and replication function are found in ovarian cancer patients, those who harbor VUS that impact its HR function would most benefit from targeted therapies tailored against HR deficient tumors.
Results
Selection of 27 ovarian cancer VUS for functional analysis
We identified 50 RAD51C missense variants in ovarian cancer either reported in the literature, in cancer databases (COSMIC, TCGA, cBioPortal), or through our collaborations with clinicians (Supplementary Data 1, Supplementary Table 1). A subset of these 50 RAD51C ovarian cancer variants were functionally analyzed for DNA repair defects by our group and others (n = 23/50) and here we analyzed the remaining 27 variants (Summarized in Supplementary Data 1). The RAD51C ovarian cancer variants are primarily germline and a subset were also found in other cancer types (15 total), including breast (6 total). Furthermore, 24 of the cancer variants were also identified in population studies as single nucleotide polymorphisms (SNPs) through exome sequencing (gnomAD; https://gnomad.broadinstitute.org; Supplementary Data 1).
We clinically identified three ovarian cancer patients with RAD51C variants (F164L [c.492 T > G], L238R [c.713 T > G], V239G [c.716 T > A]) that had additional information about their treatment and disease progression. These patients were diagnosed with Stage III (or stage unknown) ovarian cancer and in each case, the RAD51C variants uncovered were germline, or likely germline. Two of these individuals (one with RAD51C-F164L and the other with RAD51C-V239G) showed pedigrees with familial breast and ovarian cancer (Supplementary Table 1, Supplementary Fig. 1). One of these patient families (RAD51C-F164L) also harbored a pathogenic BRCA1 variant, while the other two were BRCA wild-type. Each patient was treated with platinum-based chemotherapy, with the RAD51C-V239G ovarian cancer patient being in remission for 13+ years. In patients with RAD51C-L238R or F164L, the cancer recurred after platinum treatment and therefore continued to receive additional platinum-based therapy along with other targeted therapies including PARP inhibitors, VEGF inhibitors and/or CHK1 inhibitor. The patient with RAD51C-L238R ovarian cancer responded well to CHK1 inhibitor therapy but withdrew from the clinical trial during the COVID-19 pandemic and her status is currently unknown. The individual with RAD51C-F164L died from disease three years post diagnosis. We sought to analyze these patient derived variants as well as the other ovarian cancer variants that were publicly reported for functional changes (Fig. 1b; 27 total).
a RAD51C ovarian cancer variants are highly conserved amongst vertebrate species. Multiple sequence alignment of human RAD51C with different species including monkey (M. fascicularis), mouse (M. musculus), zebrafish (D. rerio), and African clawed frog (X. tropicalis). The sequences were aligned using Clustal Omega and the conservation is indicated with an asterisk (*) for high conservation, a colon (:) for moderate conservation (conserved within amino acid groups of strongly similar properties), or a period (.) for some conservation (conserved within amino acid groups of weakly similar properties). Mutated amino acid residues are indicated in the gray shaded regions and/or are labeled above the residue with the amino acid change code. RAD51C ATPase domains are indicated with a purple box (Walker A motif) and a gold box (Walker B motif). The nuclear localization sequence (NLS) is shown with a light blue box. b Linear protein schematic of human RAD51C. The ovarian cancer variants analyzed in this study are labeled and shown to scale. c RAD51C ovarian cancer variants in the Walker A and Walker B regions exhibit reduced yeast-three-hybrid (Y3H) interaction with RAD51D. Y3H analysis of pGAD-RAD51C wild-type or the pGAD-RAD51C variant with pGBD-RAD51D was performed. pRS416-RAD51B was used to stabilize RAD51C. Yeast were transformed with the indicated plasmids and plated on selective medium (SC-LEU-TRP-URA-HIS). Growth indicates a Y3H interaction. Y3H interaction-deficient variants are indicated in gray. d Quantification of Y3H results was used to determine the relative growth of each RAD51C variant interaction with RAD51D, and mean growth was plotted with standard deviations. For variants E67K, K84N, T120A, M136I, G162A, M165L, V170G, and L182N, n = 4 biological replicates. For variants N71K, F164L, V166G, and G189R, n = 5. All other variants, n = 3. A RAD51C variant with a Y3H interaction with RAD51D between 50-100% of wild-type is indicated with green, whereas a Y3H interaction between 0–50% of wild-type RAD51C is indicated with red. Source data are provided as a Source Data file. e Western blot analysis of yeast cells transformed with the Y3H deficient RAD51C variants are expressed. RAD51C expression was analyzed using α-RAD51C antibodies and equal protein loading was assessed by Kar2 (α-Kar2). The experiment was performed in triplicate. Uncropped and unprocessed blots are provided as a Source Data file.
A subset of RAD51C ovarian cancer variants are conserved with other vertebrate species and RAD51
The RAD51C protein is highly conserved amongst vertebrate species, especially within the regions concerned with ATP binding and hydrolysis, including the Walker A and Walker B motifs. Of the 27 ovarian cancer VUS, nine of the residues are highly conserved (identical) in monkey, mouse, zebrafish, and frogs, six are moderately conserved and four somewhat conserved (Fig. 1a). These RAD51C vertebrate-conserved residues are concentrated in the ATPase core domain. RAD51C also shares sequence similarity to RAD51 itself. When comparing RAD51C to RAD51, five of the RAD51C residues are highly conserved in RAD51 and five are somewhat or moderately conserved (Supplementary Fig. 2a).
A subset of RAD51C ovarian cancer variants exhibit reduced protein interaction with RAD51D
RAD51C is an integral member of the BCDX2 complex and directly interacts with RAD51B and RAD51D23. The BCDX2 complex protein interactions can be readily assessed by yeast-2-hybrid and yeast-3-hybrid6,24. We previously found that RAD51C yeast-three-hybrid interaction with RAD51D is a significant predictor of HR proficiency (r = 0.85)12. Therefore, we analyzed RAD51C VUS for interaction with RAD51D by yeast-three-hybrid. Using this approach, we identified five RAD51C variants with 50% or more reduced interaction with RAD51D when compared to wild-type (WT) RAD51C (Fig. 1c, d; RAD51C-T120A [c.358 A > G], -G162A [c.485 G > C], -F164L, -V239G, -A279P [c.835 G > A]). To determine if RAD51C variant interaction loss is due to altered protein expression, we performed western blot analysis using protein extracted from the yeast-three-hybrid experiments. While all the RAD51C interaction deficient variants are expressed, a subset of these variants have reduced protein expression compared to wild-type RAD51C and this may explain the yeast-three-hybrid results (Fig. 1e; Supplementary Fig. 2b).
Testing RAD51C ovarian cancer variant expressing cells for HR
To examine the function of RAD51C ovarian cancer variants of interest, we complemented a CRISPR/Cas9 RAD51C knockout U2OS cell line (KO) with either wild-type RAD51C or the RAD51C variant of interest. We confirmed expression of the RAD51C variants in our polyclonal stable cell line by western blot (Fig. 2a). Note we do not detect expression of RAD51C-Q178E (c.532 C > G) (Fig. 2a).
a Polyclonal cell lines of the RAD51C variant of interest are largely stably expressed in mammalian RAD51C knockout U2OS cells. Note that M10R, L238R, H207R, and D318N have moderately reduced expression whereas Q178E is not expressed. Equal protein lysates of each cell line were analyzed for RAD51C (α-RAD51C) or tubulin (α-tubulin) or vinculin ((α-vinculin) expression by western blot. The experiment was performed in triplicate. Uncropped and unprocessed blots are provided as a Source Data file. b Homologous recombination is reduced in a subset of RAD51C ovarian cancer variants. A sister chromatid recombination assay was used to assess HR proficiency. In this assay, a nonfunctional copy of GFP containing an I-SceI restriction site and a GFP fragment that can be used as a repair template on the sister chromatid are stably integrated into the RAD51C knockout U2OS cell line. SCR can be assessed by monitoring the percentage of cells that become GFP+ when a plasmid expressing I-SceI enzyme is transfected. The RAD51C knockout cell line alone or stably expressing wild-type RAD51C or the indicated variant were assessed for the percentage of GFP+ cells, and mean ± standard deviations was plotted. For G162A, L238R, and A279P, n = 4 biological replicates. For M10R, E67K, and T120A, n = 5. For F164L, V166G, V170G, G189R, V239G, and D291V, n = 6. For the empty vector, n = 17. For all other variants, n = 3. All cell lines are normalized to wild-type RAD51C. A red box indicates a HR deficient variant (0–39%), yellow indicated a HR reduced variant (40–59%), whereas a green box indicates an HR proficient variant (60–100%). The color coding indicates variants with additional functional analysis and is used for the same variants throughout the remaining figures. Source data are provided as a Source Data file.
Next, we determined whether the RAD51C variants are impaired for HR activity. For this, we performed sister chromatid recombination assays on the 27 remaining ovarian cancer variants identified to date. In this assay, a nonfunctional copy of GFP has the unique I-SceI cut site inserted with an upstream GFP fragment. Upon transient expression of the I-SceI enzyme by transfecting the I-SceI endonuclease expressing plasmid, a DSB is created and GFP expression can be restored by use of the GFP fragment as a repair template. Using this assay, we find that five of the 27 RAD51C ovarian cancer variant cell lines were reduced in their HR capabilities when compared to the RAD51C complemented wild-type cell line (Fig. 2b; Q178E, Q181R [c.542 A > C], L238R, A279P, and D318N [c.952 G > A]). Note that while RAD51C-Q181R expressing cells are HR deficient, they are not reduced to the level of a RAD51C knockout cell line (Fig. 2b; indicated in yellow). Furthermore, RAD51C-Q178E is not expressed and therefore is unable to complement the HR deficiency of a RAD51C knockout cell.
Testing RAD51C ovarian cancer variant expressing cells for cell growth
Recently, a saturation mutagenesis screen was used to examine the viability of RAD51C variants in a HAP1 cell line by bulk screening25. Therefore, we asked whether changes in cellular growth is indicative of HR proficiency in a representative subset of the variants (including both wild-type and HR deficient mutants). We analyzed the growth rate of each RAD51C variant cell line by monitoring cell number over time (Supplementary Fig. 3a). Using the growth rate, we also calculated the doubling time for each RAD51C variant tested (Supplementary Fig. 3b). Most of the RAD51C variant expressing cells grew at comparable rates to wild-type complemented RAD51C knockout U2OS cells. These findings are largely consistent with the results from the HAP1 cell line and our previous work demonstrating that RAD51C loss results in decreased cell proliferation25,26 (Supplementary Fig. 3c). However, Q181R, D318N (c.952 G > A), M10R (c.29 T > G), and V166G (c.497 T > G) from this study and D108Y (c.322 G > T), G125S (c.373 G > A), and V140G (c.419 T > G) from Prakash et al. do not correlate with the findings in HAP1 cells, which may be a functional limitation of the screening approach, a difference between cell lines, or a discrepancy between our findings (25; Supplementary Fig. 3c). Together, these results suggest that viability alone is not a surrogate for functional analysis. Note that comparison between our functional studies and the saturation mutagenesis analysis cannot be used as a basis for calibration in variant classification according to the American College of Medical Genetics guidelines.
Clustering of dysfunctional RAD51C ovarian cancer variants around the Walker B
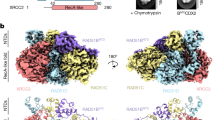
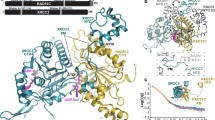
To analyze where the RAD51C HR deficient ovarian cancer variants are located on the RAD51C structure, we modeled the RAD51C variants on the recently solved cryo-EM, BCDX2-ssDNA complex structure (Fig. 3a, b; PDB ID:8GBJ)27,28. We previously identified the Walker A region as being crucial for RAD51C HR function12. Importantly, our current results affirm this finding of HR deficient ovarian cancer variants clustering in the Walker A region (Fig. 3b, c, d[Site II])12. In addition, we were surprised to find the newly characterized RAD51C variants (A279P, L238R, V239G) to be located in and around the Walker B motif (Fig. 3b, c, d[Site I]). The Walker B motif of RAD51C comprises a beta-strand that spans amino acids 238–242 and includes a highly conserved aspartate residue (D242) that plays a role in ATP binding and typically is involved in ATP hydrolysis. Importantly, two of the variants we identified reside within the motif itself (L238R, V239G) and an additional severely HR deficient variant, A279P, resides within a beta-strand that pairs with the Walker B motif. Therefore, the A279P mutation may disrupt strand pairing in the conserved beta-sheet of the RecA-like fold of RAD51C (Fig. 3c, d[Site II]). Notably, we identified additional HR deficient ovarian cancer variants in the literature in this Walker B region (L219S [c.656 T > C], L226P [c.677 T > G], R237P[c.710 G > C], and D242N [c.724 G > A]) (Fig. 3c, d[Site I])12,14. Collectively, the deleterious effects of these substitutions are likely due to perturbation of the structure/positioning of the Walker B motif, disruption of the conserved beta-sheet in the RAD51C fold, and/or substitution of residues known to be important for ATP binding and hydrolysis (e.g. D242).
a Linear schematic of RAD51C ovarian cancer variants with functional data for HR proficiency by SCR assay (circle), interaction with RAD51D by yeast-three-hybrid (Y3H, square), and sensitivity to olaparib and cisplatin (triangle). Note that the functional data from the starred (*) residues are from this study whereas the unstarred residues were previously reported in Prakash et al. and diagrammed here for direct comparison12. Variants sensitive to olaparib and cisplatin are indicated in red whereas insensitive variants are indicated in green. HR proficient variants are indicated in green (60–100%), intermediate variants are in yellow (40–59%), and deficient variants are in red (0–39%). The Walker A motif is indicated with a purple box, the Walker B motif with a gold box, the DNA binding loops in blue boxes and the NLS with a light blue box. b CryoEM structure of the BCDX2 complex with ssDNA (PDB ID:8GBJ). The sites highlighted in this study are labeled in black. c Domain organization and binding interfaces of RAD51C with RAD51D, DNA, RAD51B, and ATP binding pocket in the complex. In each case, the HR efficiency of the variant shown in (a) is indicated in red (deficient), yellow (intermediate), or green (proficient, with corresponding colored text. d Site I: The critical residues near the Walker B motif; Site II: RAD51C residues which are present near the ATP binding pocket and RAD51D interaction interface. Site III: RAD51C residues which are present near RAD51B. Site IV: N-terminal residues of RAD51C which are present on the RAD51D interaction interface. In each case, HR efficiency of the variant shown in (a) is indicated in red (deficient), yellow (intermediate), or green (proficient), with corresponding colored text.
Ovarian cancer RAD51C Walker B motif variant, V239G, possesses reduced ATPase activity
To assess the functional relevance of the RAD51C Walker B region, we biochemically analyzed RAD51C-L238R, V239G, and A279P variants for RAD51 paralog complex formation and potential defects of mutant protein complexes in DNA binding and ATP hydrolysis (Fig. 4). Notably unlike wild-type BCDX2, BCL238RDX2 complex displayed frequent dissociation into BC (RAD51B/RAD51C) and DX2 (RAD51D/XRCC2) subcomplexes during the affinity purification (Fig. 4a). However, we were able to purify BCDX2 complexes with the RAD51C- V239G, and A279P variants as stable complexes (Fig. 4b). Next, we compared ssDNA binding by purified mutant BCDX2 complexes with wild-type BCDX2 using DNA mobility shift assays in the presence of ATP and MgCl2 (Fig. 4c, d). BCDX2 complexes that harbor the Walker B motif variant V239G exhibit wild-type DNA binding activity, whereas the more distal RAD51C-A279P has a moderate DNA binding defect (Fig. 4c, d). ATPase activity of BCDX2 complex is stimulated by DNA29. Recently, we reported that the BC, but not DX2 subcomplex, can hydrolyze ATP, and BCDX2 complex harboring the RAD51C Walker A motif K131A substitution displays a strong defect in ATP hydrolysis27. Here, we first determined that ATP hydrolysis by the BCDX2 complex also requires a functional RAD51C Walker B motif as RAD51C-D242A substitution greatly impairs ATP hydrolysis by BCD242ADX2 both in the absence and presence of DNA (Fig. 4e, f). We further discovered that BCDX2 complex containing the Walker B motif variant V239G, has a 50% reduction in ATPase activity in the presence of DNA (Fig. 4e, f). In contrast, BCDX2 containing RAD51C-A279P, which is distal to the Walker B motif, maintains wild-type level of ATPase activity (Fig. 4e, f). Next, we assessed whether the RAD51C Walker B region variants would exhibit defects in CX3 complex formation. Interestingly, affinity purified CX3 complexes containing RAD51C-A279P and RAD51C-L238R emerges only in the void volume in size exclusion chromatography, suggesting that these mutant CX3 complexes form a soluble aggregate. In contrast, CV239GX3 fractionates between soluble aggregates as well as a monodispersed form, similar to wild-type CX3 complex. These results suggest that RAD51C-V239G can form more stable CX3 complex (Fig. 4g). Together, these results demonstrate that the Walker B region is critical for RAD51C biochemical function.
a RAD51C-L238R cannot be stably purified in the BCDX2 complex. Ni-NTA and α-FLAG affinity purification of BCDX2 complexes harboring either wild-type or L238R variant of RAD51C. The lysate, flow through (FT), and elution was run on an SDS-PAGE gel and RAD51B-RAD51C (B/C) and RAD51D-XRCC2 (D/X2) subcomplexes are indicated. b RAD51C-V239G and A279P can be purified in the BCDX2 complex. Flow chart summarizing BCDX2 purification and Coomassie blue stained SDS-PAGE gel showing fractions of size exclusion chromatography for purification of BCDX2 complexes containing wild-type RAD51C or the indicated RAD51C Walker B variants. RAD51B-RAD51C (B/C) and RAD51D-XRCC2 (D/X2) subcomplexes are indicated. c BCA279PDX2 shows reduced DNA binding activity. ssDNA binding by the BCDX2 complexes containing wild-type RAD51C or the indicated RAD51C Walker B variants at increasing DNA concentrations (nM) assessed by electrophoretic mobility shift assays using an 80-nucleotide substrate. d BCV239GDX2 shows reduced ATPase activity. Quantification of ssDNA binding by the BCDX2 complexes from three independent experiments plotted as mean values ± s.d. e ATP hydrolysis with 1 μM BCDX2 complexes containing WT and indicated RAD51C Walker B variants with or without ssDNA (phix174) were assessed at the indicated time points (min). f Quantification of ATP hydrolysis measurements for three independent experiments plotted as mean values ± s.d. g RAD51C-V239G can be purified in the CX3 complex, but RAD51C-L238R and A279P cannot. CX3 complex formed with MBP tagged WT or the indicated RAD51C Walker B mutants and FLAG tagged XRCC3 were affinity purified from amylose resin followed by FLAG resin and loaded onto a Superdex200 increase 10/300 size exclusion column. Fractions were run on an SDS-PAGE gel and visualized by Coomassie staining, and void and monodispersed fractions are indicated. Gel images in Fig. 4a–c, g are representative of at least two independent experiments.
RAD51C Walker B ovarian cancer variant expressing cell lines, L238R and A279P, are sensitive to cisplatin and olaparib
HR deficient tumors are treated with platinum-based drugs like cisplatin, which introduce DNA crosslinks, as well as with PARP inhibitors such as olaparib, which causes PARP trapping and accumulation of ssDNA30,31,32. Therefore, we asked whether cell lines expressing RAD51C Walker B variants are sensitive to either cisplatin or olaparib. We treated cell lines stably expressing the RAD51C variants of interest (L238R, V239G, and A279P) with increasing concentrations of cisplatin or olaparib and performed clonogenic survival assays (Fig. 5). Even with a low drug concentration range, L238R and A279P expressing cells were killed upon introduction of these drugs. These severely HR deficient variant complemented cells showed exquisite sensitivity to both cisplatin and olaparib with low IC50 values comparable to the RAD51C knockout cell (Fig. 5, Supplementary Data 2; IC50 values L238R = 11.5 ± 3.52 nM, A279P = 48.5 ± 10.6 nM, KO = 32.9 ± 13.8 nM; olaparib IC50 values L238R = 2.28 ± 0.73 nM, A279P = 6.54 ± 1.40 nM, KO = 4.89 ± 3.64 nM). In contrast, RAD51C-V239G does not confer a significant sensitivity to either cisplatin or Olaparib (Fig. 5d). We also performed clonogenic survival assays on a subset of variants in the Walker A region that exhibit a reduction in their interaction with RAD51D and find that this reduction does not confer significant sensitivity to cisplatin or olaparib (Fig. 5). RAD51C-V166G maintains 67% of its RAD51D interaction. Although not significant, we find that T120A, F164L and V239G consistently trend towards olaparib sensitivity (Fig. 5c, d, Supplementary Data 2; IC50 values T120A = 191 ± 46.7 nM, F164L = 187 ± 111 nM, V239G = 112 ± 33.3 nM compared to WT = 696 ± 339 nM)). Note that these variants exhibit HR efficacy of 77%, 73% and 70% HR, respectively (Fig. 2b). Together these results suggest that HR proficiency is largely correlated with olaparib resistance.
RAD51C-L238R and V239G are sensitive to cisplatin and olaparib. Representative plate images of each RAD51C variant complemented cell line, RAD51C wild-type complemented cell line or parental RAD51C knockout U2OS cell line treated with increasing concentration of (a) cisplatin or (c) olaparib. Insensitive cell lines were treated with the higher dosage range (top; 0.125–2 µM) and sensitive cell lines were treated with the lower dosage range (bottom; 0.0156–0.25 µM). Colony area was quantified and normalized to the (b) untreated or (d) vehicle treated controls. Best fit dose-response curves are shown, with the mean of three to four trials plotted with standard deviation. Specific IC50 values can be found in Supplementary Data 2. In (b), trials were performed four times for T120A, V166G, and knockout cells, and three times for all other cell lines. In (d), trials were performed four times for G162A, F164L, and knockout cells, and three times for all other cell lines. Source data are provided as a Source Data file.
Analysis of replication fork dynamics reveals separation-of-function alleles in the Walker B region
RAD51C functions during HR to promote RAD51 activity as part of the BCDX2 and CX3 complexes and at the same time exhibits HR-independent functions during replication fork dynamics including fork regression33,34,35. Therefore, we asked whether RAD51C variants in the Walker B region would exhibit defects in replication fork regression. To address this, we performed DNA fiber analysis where a RAD51C knockout U2OS cell uncomplemented or complemented with RAD51C or the variant of interest was first labeled with CldU followed by IdU in the presence or absence of the Topoisomerase I inhibitor camptothecin (CPT; Fig. 6a, Supplementary Fig. 4). Indicative of replication fork regression and reversal defects and consistent with previous findings, loss of RAD51C results in a significant increase in IdU/CldU tract lengths upon CPT in comparison to WT-complemented RAD51C knockout cells, indicating impaired fork arrest and reversal (33; Fig. 6b). Importantly, cells expressing the HR-proficient RAD51C-V239G variant similarly have a significant decrease in replication fork regression and reversal whereas the two HR deficient variants, L238R and A279P, do not (Fig. 6b). These results suggest that RAD51C HR and replicative functions can be uncoupled and linked to distinct enzymatic activities (Summarized in Fig. 6c).
a Schematic of labeling of replication fork progression. The cells were first pulsed with CldU for 20 min followed by IdU with camptothecin (100 nM CPT) for 60 min subsequently medium with or without the S1 nuclease was added for 20 min. Replication fork speed in untreated cells was assessed in Supplementary Fig. 4. b RAD51C knockout cells and RAD51C-V239G expressing cells exhibit increased DNA tract lengths and increased ssDNA gaps. RAD51C CRISPR/Cas9 U2OS cells were complemented with the WT RAD51C or the indicated variant and DNA replication fork progression was determined. Each data point represents Idu/Cldu ratio for one fiber measurement. Approximately 200 fibers were analyzed from two experiments and bars represent median of each data set. Statistical significance was determined by Kruskal-Wallis with multiple Dunn’s comparison and is indicated (ns is not significant, ** p = 0.0071, *** p = 0.0034, **** p < 0.0001). From left to right, n = 204, 201, 203, 201, 203, 206, 202, 203, 203, or 204 fibers quantified between two biological replicates. c RAD51C variants uncouple the enzymatic, HR, and replicative functions. Summary of phenotypes of RAD51C variants V239G and L238R and A279P from Figs. 1–5. Green is indicative of wild-type RAD51C function, yellow is indicative of a partial function, and red is indicative of a reduced function. Note that the DNA binding and ATPase activities were analyzed for V239G and A279P only since L238R formed insoluble aggregates. Source data are provided as a Source Data file.
Lastly, we sought to determine if the increased tract lengths observed upon loss of RAD51C or upon expression of RAD51C-V239G were due to increased replication fork gaps. To address this, we repeated the IdU and CldU labeling upon CPT in the presence of the S1 nuclease, which digests ssDNA. We find that indeed, both loss of RAD51C and expression of RAD51C-V239G results in ssDNA gaps, thus explaining the increased tract lengths observed (Fig. 6b). In contrast, the HR deficient variants, L238R and A279P, do not accumulate ssDNA gaps (Fig. 6b).
Both RAD51C HR and replicative functions are needed to efficiently form RAD51 filaments by EM
While RAD51C is known to promote RAD51 filament formation27, whether RAD51C HR or replicative functions contribute to its mediator activity has not been addressed to date. Using separation-of-function alleles in RAD51C that uncouple its replicative and HR activities, we sought to determine if either of these functions are required for RAD51 filament formation by negative stain EM. As expected, recombinant WT BCDX2 complex promotes assembly of interconnected RAD51 nucleoprotein filaments in EM analysis by increasing the local concentration of RAD51 and ssDNA (Fig. 7; No vs WT BCDX2). In contrast to WT BCDX2, we find that BCDX2 complexes with either the replication deficient RAD51C variant (V239G) or the HR deficient RAD51C variant (A279P) have significantly lower RAD51 filaments per frame and fewer junctions (Fig. 7a, b, d; WT vs. mutant BCDX2). However, both mutant BCDX2 complexes retain partial ability to promote RAD51 filament assembly from no BCDX2 levels and RAD51 filament length is unchanged in either mutant complex in comparison to wild-type BCDX2 (Fig. 7a–c). Together these findings suggest that both RAD51C HR and replicative functions are necessary to efficiently form RAD51 filaments and uniquely contribute to RAD51 filament assembly.
Box plots depict (a) number of RAD51 nucleoprotein filaments per frame, (b) number of junctions formed by filaments per frame and (c) length of filaments in nanometers measured by negative stain electron microscopy in absence or presence of BCDX2 and indicated BCDX2 variants. The P values for the significance of differences in median values were calculated by the two-sided Mann–Whitney–Wilcoxon test. For box plots, each box depicts the interquartile range (IQR) containing 50% of the data, intersected by the median, and whiskers extend up to 1.5 IQR value. d Representative micrographs depicting RAD51nucleoprotein filaments seen by negative stain electron microscopy in absence or presence of BCDX2, and BCDX2 with RAD51C mutants V239G and A279P. Data represent at least 30 electron micrographs for each sample captured in two independent experiments.
Discussion
RAD51C is a tumor suppressor and is included on high-risk breast and ovarian cancer screening panels11,12,36,37,38. Fifty RAD51C missense variants have been identified in patients with ovarian cancer and 27 of these variants have little to no functional analysis. Therefore, how each individual variant contributes to cancer development has yet to be determined. Furthermore, how RAD51C distinct functions in HR-dependent DSB repair and HR-independent role during replicative damage contribute to cancer and response to therapy is largely unknown. Here we identified a mutation cluster in the Walker B region of RAD51C. We find that ovarian cancer variants in this region can differentially impact its role in HR and replication-associated repair. By identifying separation-of-function alleles, we find that RAD51C ATPase activity is critical for its role in replication fork regression and reversal. Lastly, we find that RAD51C HR and replication-associated functions both contribute to RAD51 filament formation by EM. Together our results suggest that RAD51C HR and replication-associated functions may uniquely contribute to ovarian cancer development and response to HR-deficient targeted therapy.
The classification of RAD51C variants is critical for risk management, early surveillance, and effective cancer treatment38,39. In this study, we identified five HR deficient RAD51C variants including one hypomorphic or intermediate RAD51C variant. When we consider our results with our previously published data, the emergence of several patterns in variant clustering are identified12. First, our results support previous findings of a dysfunctional variant cluster surrounding the Walker A motif. The addition of our new variant analysis establishes the boundaries of this region as being between approximately amino acids 121 and 162. Secondly, through this comprehensive functional analysis, we identified a second critical region for RAD51C in the Walker B motif that is important for its cellular and biochemical functions. Together, our findings uncover new RAD51C HR deficient variants while helping to pinpoint and define new regions and activities of RAD51C that may be important for tumor suppression.
We find the RAD51C Walker B region to be functionally critical for its DNA repair function and response to therapy. Interestingly, our previous analysis of ovarian cancer RAD51C variants did not identify functionally deficient variants in the Walker B motif12. With the newly published BCDX2 complex structure, we can infer this to be due to the differences in the local structure between these two regions. While, the Walker B motif resides within aβ-pleated sheet and is therefore prone to allosteric effects from more distant variants (e.g. A279P), the Walker A motif resides within a solvent exposed flexible loop that is less susceptible to such allosteric effects from substitutions at positions outside of the motif (Fig. 3b, c, d)27,28. In this study, we identified two functionally deficient variants in the Walker B (L238R and V239G) as well as a proximal Walker B disrupting variant (A279P). Although A279 does not appear to be located by the Walker B motif based on the primary sequence, the structure reveals that A279 is immediately proximal to the Walker B motif and its mutation likely disrupts the beta pleated sheet structure of this motif (Fig. 3a, d; [Site II]). This finding is further supported by previous analysis which identified two deleterious Walker B region variants R237P and D242N that disrupt HR14. Additionally, HR deficient variants that alter hydrophobic interactions with V239 in the Walker B motif (L219S and L226P) were also found to be HR deficient12. Upon further analysis, we find that the V239G variant within the Walker B has a direct impact on ATPase activity and that this variant links this biochemical activity to its replicative function (Fig. 4e, f; Fig. 6). Surprisingly, the Walker B variant, V239G shows a reduction in ATPase activity and replication fork regression but still retains HR efficiency (70%). This is in stark contrast to the more distal Walker B disrupting variant, A279P, which exhibits near wild-type ATPase activity and replication fork progression, but a reduced HR efficacy of 3% (Figs. 2b, 6). Additionally, Walker B variant L238R shown deficient protein-protein interactions both through yeast-three hybrid analysis and upon BCL238RDX2 and CL238RX3 purification. These results suggest that the RAD51C Walker B motif not only links its ATPase activity to replication fork regression but is also responsible for a host of functions including protein-protein interactions and DNA binding, which ultimately influence HR efficacy and replicative repair. Recently the CX3 structure was solved from the metazoan Alvinella pompejana35. When analyzing the conserved residues in RAD51C with humans, similar to the BCDX2 complex, the HR deficient variants are clustering between the CX3 interphase and around the ATP binding pocket (Supplementary Fig. 6;35). Future studies are needed to determine how these variants impact CX3 function.
Determining the impact of the amino acid change and intermediate HR phenotypes is critical for understanding cancer risk and patient outcomes. This study identified five HR deficient RAD51C variants (T120A, G162A, F164L, V166G and V239G) with intermediate HR efficiency (70–88% of wild-type RAD51C). These variants are in the Walker A (G162A, F164L, V166G) and Walker B region (T120A, V239G). In addition to variant location and conservation, the amino acid change itself can also be critical for predicting HR function. For example, RAD51C-G162A and G162E have very different HR outcomes (Supplementary 3; 78% and 14%, respectively)12. Since both glycine and alanine are nonpolar amino acids, an alanine substitution (as in G162A) is likely better tolerated than glutamic acid (as in G162E), which is negatively charged. Therefore, it is possible that a more significant change in charge can create a severe impairment in HR function at amino acid residues where intermediate HR variants currently exist. How a moderate reduction in HR affects ovarian cancer risk is not well understood, but a recent study examining hypomorphic variants in BRCA2 has determined that these variants produce a moderate impact on breast cancer risk40. Additionally, our clonogenic survival studies identified an insignificant sensitivity to olaparib in the RAD51C-V239G cell line. In the clinic, an ovarian cancer patient harboring this Walker B variant responded well to platinum-based chemotherapy and is in complete remission with no sign of disease (13+ years; Supplementary Table 1). Additional studies are needed to understand how hypomorphic HR variants and replication deficient variants impact disease development and response to treatment.
Methods
Plasmids, yeast strains and cell lines
A complete list of plasmids and primers used in this study are found in Supplementary Data 1. RAD51C variants were created in the pGAD-RAD51C or pCMV-2B-RAD51C vector using site-directed mutagenesis as in Kondrashova et al. or made by Gene Universal. Yeast-3-hybrid experiments were performed with S. cerevisiae strain PJ69-4α. The RAD51C CRISPR/Cas9 U2OS knockout cell line containing a sister chromatid recombination assay (SCR#18) was generously provided by Mauro Modesti26. These cells were grown in DMEM with 10% fetal bovine serum. U2OS cell lines were cultured in DMEM with 10% FBS and 1% penstrep with or without plasmocin prophylactic (InvivoGen). Cells were grown at 37 °C with 5% CO2. Polyclonal stable U2OS cell lines containing pCMV-2B-RAD51C or the pCMV-2B-RAD51C variant of interest plasmids were selected by culturing the parental RAD51C knockout U2OS cell line with the indicated plasmid harboring G418 resistant marker in G418-containing medium for two weeks.
Yeast-3-hybrid assays
The indicated pGAD-C1, pGAD-RAD51C wild-type or variant plasmids (1 µl of miniprepped plasmid, roughly 100–500 ng/µl) were transformed into competent yeast, PJ69-4α, with RAD51C binding partners, pGBD-C1-RAD51D and pRS-ADH-416-RAD51B. Transformants were plated on synthetic complete media lacking leucine, tryptophan, and uracil (SC-Leu-Trp-Ura) and incubated for 48–72 h at 30 °C. Three colonies of each variant were inoculated and grown in 5 mL YPD overnight (30° C). Overnight cultures were diluted to 0.2 OD600. The cultures were incubated for 3–4 h at 30 °C to 0.5 OD600. The 5 µl of 0.5 OD units of culture were plated on SC-Leu-Trp-Ura medium and synthetic complete medium lacking leucine, tryptophan, uracil and histidine (SC-His-Leu-Trp). The plates were incubated for 72 h at 30° C. Plates were imaged and edited for brightness and contrast using Adobe photoshop and yeast spots were quantified using ImageJ software. The relative growth of each RAD51C variant was measured by subtracting the growth of the RAD51C variant with the empty pGBD plasmid. This value was normalized to wild-type RAD51C which was set to 100% as the maximum value.
Yeast western blot analysis
Three colonies of yeast expressing the indicated plasmids were inoculated for growth overnight at 30 °C in 3 mL of YPD. The 0.75 OD600 of cells were pelleted and washed in 1 mL of water. The cells were lysed by TCA precipitation and the protein pellet was resuspended in 50 µl of loading buffer (HU) as described (Knop, 1999) except that the HU buffer was supplemented with DTT. Fifteen µL of protein was run on a 4–20% gradient SDS-PAGE gel at 120 V for 75 min. The protein was transferred to a PVDF membrane at 100 V for 2 h and the membrane was blocked for 1 h using Oddessy Licor TBS blocking buffer. RAD51C and Kar2 loading control was visualized on a Licor CLX scanner using a RAD51C antibody (Abcam Cat# ab55728, RRID:AB_945135, 1:500), Kar2 antibody (sc-33630, 1:2000) and secondary antibodies (Licor IRDye 680RD goat anti-rabbit 926-68071 and Licor IRDye 800CW goat anti-rabbit 926-32211).
Mammalian western blot analysis
One million cells of each RAD51C knock out or variant complemented cell line were seeded in a 60 mm plate and collected 24 h later. Cells were lysed in RIPA buffer supplemented with PMSF, 1X PhosStop and 1X protease inhibitor cocktails (ThermoFischer) and 0.2 µL Benzonase (Sigma). Cells were incubated on ice for 30 min. Lysates were pelleted and 20–40 µL of supernatants were run on a 10% SDS-PAGE gel at 120 V for 75 min. The protein was transferred to a PVDF membrane at 100 V for 2 h and the membrane was blocked for 1 h using Oddessy Licor TBS blocking buffer. RAD51C and αTubulin loading control was visualized on a Licor CLX scanner using a RAD51C antibody (Abcam Cat# ab55728, RRID:AB_945135, 1:500), αTubulin antibody (Cell Signaling Antibody#2144S, 1:1000) and secondary antibodies (Licor IRDye 680RD goat anti-rabbit 926-68071 and Licor IRDye 800CW goat anti-rabbit 926-32211).
Sister chromatid recombination assays
RAD51C knockout U2OS cells alone or stably expressing wild-type RAD51C or the RAD51C variant of interest were transfected with 4 µg of plasmid expressing I-SceI (pCBSceI) using 5 µL Lipofectamine2000. Three days post-transfection, the cells were collected and immediately analyzed or fixed in 2% PFA for analysis. The percentage of HR performed was determined by measuring GFP expression of 20,0000 events by flow cytometry (CytoFlex 6 L;). Percentage of GFP was normalized to wild-type RAD51C and RAD51C knockout cell line was ran as a negative control.
Growth rate and doubling times
Three hundred thousand RAD51C knockout U2OS cells alone or stably expressing wild-type RAD51C or the RAD51C variant of interest were seeded in a 12 well plate. The cells were collected from individual wells and counted every day for four days. The number of cells collected each day was plotted as the growth rate. Doubling times were calculated for each 24-h period using the following equation:
For each cell line, the fastest doubling time, indicative of log phase for that cell line, was graphed.
Clonogenic survival assays
Six hundred RAD51C knockout U2OS cells alone or stably expressing wild-type RAD51C or the RAD51C variant of interest were seeded on a 60 mm plate in triplicate. The next day, cells were treated with cisplatin (Sensitive cell lines; 0, 0.015625, 0.03125, 0.0625, 0.125 and 0.25 µM. Insensitive cell lines; 0.125, 0.25, 0.5, 1, and 2 µM) or olaparib (Sensitive cell lines; 0, 0.015625, 0.03125, 0.0625, 0.125 and 0.25 µM. Insensitive cell lines; 0.125, 0.25, 0.5, 1, and 2 µM) containing media. The cells were incubated in cisplatin-containing media for two days (one cell cycle), after which the cells were grown in fresh media for two weeks. Olaparib containing media was continuously replaced every three days for a two-week treatment. Olaparib containing media was supplemented with equal volumes of DMSO (vehicle). At the end of either the cisplatin or olaparib treatment, the cells were washed with PBS, fixed with 100% methanol for 20 min and stained with Crystal Violet for two hours. Plates were imaged using a BioRad imager, and the colony area was quantified in ImageJ. Colony area data was normalized to the no-treatment control for each cell line. Colony survival was graphed vs cisplatin or olaparib concentration. The data were fit to the following IC50 model:
where top is 100% survival and bottom is the final % survival in Graphpad Prism. Data are reported as IC50 values with standard error of the mean.
Protein expression and purification
BCDX2 and CX3 purification was carried out as previously described12. Briefly BCDX2 (RAD51B-His and XRCC2-Flag) and CX3 (MBP-RAD51C and XRCC3-FLAG) were expressed in Hi5 insect cells using baculoviruses and all the purification steps were carried out at 0–4 °C. Cell pellets were resuspended in T buffer (25 mM Tris-HCl, pH 7.5, 10% glycerol, 0.5 mM EDTA, 1 mM DTT, 0.05% IGEPAL CA-630 (Sigma), 1 mM PMSF and protease inhibitors) containing 300 mM KCl (T300), 2 mM ATP, and 2 mM MgCl2 and lysed by sonication. BCDX2 complex was affinity purified from Ni-NTA resin (Qiagen) and for further purification protein was fractionated on HiTrap Q HP column with 150-600 mM KCl gradient and concentrated pooled fractions were subject to size exclusion chromatography in a Superose6 Increase 10/300 GL column in T300 buffer with 2 mM each of ATP and MgCl2. CX3 complex was first affinity purified from amylose resin (NEB) and then anti-FLAG resin (Sigma) and eluted protein complex was concentrated and subject to size exclusion chromatography in a Superdex200 Increase 10/300 GL column in T300 buffer with 2 mM each of ATP and MgCl2.
DNA binding and ATPase assays
For DNA binding, 1 nM of 5’ Cy5-80-nt ssDNA (Cy5-iSp9-TTATGTTCATTTTTTATATCCTTTACTTTATTTTCTCTGTTTATTCATTTACTTATTTTGTATTATCCTTATCTTATTTA) was incubated with the indicated concentration of purified BCDX2 complex in 10 μl reaction buffer (50 mM Tris-HCl, pH 7.5, 155 mM KCl, 1 mM DTT, 1 mM ATP, 1 mM MgCl2 and 100 ng/μl BSA) for 10 min at 37 °C. Nucleoprotein complexes were resolved on 5% polyacrylamide gels in Tris-borate buffer (45 mM each). Gels were visualized using the ChemiDoc imaging system (Bio-Rad) and proportion of bound versus unbound DNA was quantified using the ImageJ software.
BCDX2 complexes or its sub-complexes (1 μM) were incubated with or without viral ssDNA (20 μM nucleotides, phiX174 virion) in 10 μl of reaction buffer (20 mM HEPES, pH 7.5, 1 mM DTT, 1 mM MgCl2 and 30 mM KCl) containing 0.05 mM ATP with 0.25 μCi [γ−32P] ATP at 37 °C. At indicated time points aliquots of 2 μl were withdrawn and mixed with 2 μl of 0.5 M EDTA to stop the reaction. The ATP hydrolysis was determined by thin layer chromatography on PEI cellulose F sheets (Millipore, 105579) in 0.375 M potassium phosphate (pH 3.5), followed by phosphorimaging analysis with Amersham Typhoon phosphorimager (Cytiva) and the ImageQuant software (Cytiva).
DNA fiber assays
DNA fiber assays were performed as previously described (DNA Fiber Analysis: Mind the Gap!, Quinet et al). In short, 105 cells were plated in each well of a 12-well plate. 24 h later, cells were washed with PBS and media was replaced with fresh media mixed with 20 µM Cldu. After 20 min of incubation with Cldu, media was replaced with fresh media containing 200 µM IdU and 100 nM camptothecin (CPT). Cells were incubated for 1 h with IdU/CPT. Media was then removed and cells were washed with PBS and permeabilized with CSK100 buffer (100 mM NaCl, 10 mM MOPS, pH 7, 3 mM MgCl2, 300 mM sucrose, 0.5% Triton X-100) at room temperature for 7 min, followed by S1 nuclease digestion in S1 buffer (30 mM sodium acetate, pH 4.6, 10 mM zinc acetate, 5% glycerol, and 50 mM NaCl) at 37 °C for 20 min. Cells were then collected by scraping, pelleted by centrifugation, and resuspended in 100-200 µl PBS. 2 µl of cell suspension was dropped onto positively charged slides, lysed with lysis buffer for 6 min (0.5% SDS, 200 mM Tris-HCl, pH 7.4, and 50 mM EDTA) and spread by tilting slides at a 45° angle. Slides were then fixed in 3:1 methanol:acetic acid for 10 min, then washed with PBS, and denatured in 2.5 M HCl for 1 h. Slides were then neutralized in 400 mM Tris-HCl (pH 7.4), washed with PBS, and blocked with 5% BSA/10% goat serum. Slides were then treated with primary antibody (1:150-Rat anti-Cldu, 1:40-Mouse anti-IdU) for 1 h. After washing, slides were treated with secondary antibody (goat anti-rat 647, goat anti-mouse 488, 1:100 for both). Slides were then washed with PBS and mounted with ProLong Gold antifade reagent. Slides were then imaged with a Nikon Eclipse Ti2 microscope. Fiber lengths were then measured using DNA Stranding software41.
Electron microscopy
For presynaptic filament assembly, RAD51 (1 μM) was incubated with 150-nt oligonucleotide (3 μM nucleotides) with or without 25 nM BCDX2 variants in reaction buffer (25 mM HEPES pH 7.5, 25 mM KCl, 1 mM MgCl2, and 1 mM ATP) at 37 °C for 30 min. A droplet of 4 μl of a reaction mixture was blotted onto a glow-discharged carbon-coated grid (EMS CF300 Cu) for 1 min. After blotting with filter paper, the grid was immersed in a 20 μl droplet of the staining solution (2% uranyl acetate). The stain was removed from the grid by blotting with filter paper and the grid was washed twice with water for 1 min. Following excess stain removal, grids were dried in air for about 3 min. Negatively stained samples of hRAD51 presynaptic filaments were examined by transmission electron microscopy (TEM). Images were processed in Fiji ImageJ image analysis package (v2.90/1.53t) and filaments were quantified using ImageJ plugin Ridge detection.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information. Source data are provided with this paper.
References
Konstantinopoulos, P. A., Ceccaldi, R., Shapiro, G. I. & D’Andrea, A. D. Homologous Recombination Deficiency: Exploiting the Fundamental Vulnerability of Ovarian Cancer. Cancer Discov. 5, 1137–1154 (2015).
Maxwell, K. N. et al. BRCA locus-specific loss of heterozygosity in germline BRCA1 and BRCA2 carriers. Nat. Commun. 8, 319 (2017).
Pennington, K. P. et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin. Cancer Res. 20, 764–775 (2014).
Kondrashova, O. et al. Secondary Somatic Mutations Restoring RAD51C and RAD51D Associated with Acquired Resistance to the PARP Inhibitor Rucaparib in High-Grade Ovarian Carcinoma. Cancer Discov. 7, 984–998 (2017).
Ollier, M. et al. DNA repair genes implicated in triple negative familial non-BRCA1/2 breast cancer predisposition. Am. J. Cancer Res. 5, 2113–2126 (2015).
Baldock, R. A. et al. RAD51D splice variants and cancer-associated mutations reveal XRCC2 interaction to be critical for homologous recombination. DNA Repair (Amst.) 76, 99–107 (2019).
Jasin, M. & Rothstein, R. Repair of strand breaks by homologous recombination. Cold Spring Harb. Perspect. Biol. 5, a012740 (2013).
Godin, S. K., Sullivan, M. R. & Bernstein, K. A. Novel insights into RAD51 activity and regulation during homologous recombination and DNA replication. Biochem. Cell Biol. 94, 407–418 (2016).
Tung, N. et al. Frequency of Germline Mutations in 25 Cancer Susceptibility Genes in a Sequential Series of Patients With Breast Cancer. J. Clin. Oncol. 34, 1460–1468 (2016).
Rein, H. L., Bernstein, K. A. & Baldock, R. A. RAD51 paralog function in replicative DNA damage and tolerance. Curr. Opin. Genet Dev. 71, 86–91 (2021).
Sullivan, M. R. & Bernstein, K. A. RAD-ical New Insights into RAD51 Regulation. Genes (Basel) 9 (2018). https://doi.org/10.3390/genes9120629
Prakash, R. et al. Homologous recombination-deficient mutation cluster in tumor suppressor RAD51C identified by comprehensive analysis of cancer variants. Proc. Natl. Acad. Sci. USA 119, e2202727119 (2022).
Braybrooke, J. P., Spink, K. G., Thacker, J. & Hickson, I. D. The RAD51 family member, RAD51L3, is a DNA-stimulated ATPase that forms a complex with XRCC2. J. Biol. Chem. 275, 29100–29106 (2000).
Hu, C. et al. Functional and clinical characterization of variants of uncertain significance identifies a hotspot for inactivating missense variants in RAD51C. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-22-2319 (2023).
LaDuca, H. et al. A clinical guide to hereditary cancer panel testing: evaluation of gene-specific cancer associations and sensitivity of genetic testing criteria in a cohort of 165,000 high-risk patients. Genet. Med. 22, 407–415 (2020).
Meindl, A. et al. Germline mutations in breast and ovarian cancer pedigrees establish RAD51C as a human cancer susceptibility gene. Nat. Genet. 42, 410–414 (2010).
Clague, J. et al. RAD51C germline mutations in breast and ovarian cancer cases from high-risk families. PLoS One 6, e25632 (2011).
Thompson, E. R. et al. Exome sequencing identifies rare deleterious mutations in DNA repair genes FANCC and BLM as potential breast cancer susceptibility alleles. PLoS Genet. 8, e1002894 (2012).
Osorio, A. et al. Predominance of pathogenic missense variants in the RAD51C gene occurring in breast and ovarian cancer families. Hum. Mol. Genet 21, 2889–2898 (2012).
Blanco, A. et al. RAD51C germline mutations found in Spanish site-specific breast cancer and breast-ovarian cancer families. Breast Cancer Res. Treat. 147, 133–143 (2014).
Sullivan, M. R. et al. Long-term survival of an ovarian cancer patient harboring a RAD51C missense mutation. Cold Spring Harb Mol Case Stud 7 (2021). https://doi.org/10.1101/mcs.a006083
Somyajit, K., Subramanya, S. & Nagaraju, G. Distinct roles of FANCO/RAD51C protein in DNA damage signaling and repair: implications for Fanconi anemia and breast cancer susceptibility. J. Biol. Chem. 287, 3366–3380 (2012).
Masson, J. Y. et al. Identification and purification of two distinct complexes containing the five RAD51 paralogs. Genes Dev. 15, 3296–3307 (2001).
Schild, D., Lio, Y. C., Collins, D. W., Tsomondo, T. & Chen, D. J. Evidence for simultaneous protein interactions between human Rad51 paralogs. J. Biol. Chem. 275, 16443–16449 (2000).
Olvera-Leon, R. et al. High-resolution functional mapping of RAD51C by saturation genome editing. Cell 187, 5719–5734 e5719 (2024).
Garcin, E. B. et al. Differential Requirements for the RAD51 Paralogs in Genome Repair and Maintenance in Human Cells. PLoS Genet 15, e1008355 (2019).
Rawal, Y. et al. Structural insights into BCDX2 complex function in homologous recombination. Nature 619, 640–649 (2023).
Greenhough, L. A. et al. Structure and function of the RAD51B-RAD51C-RAD51D-XRCC2 tumour suppressor. Nature https://doi.org/10.1038/s41586-023-06179-1 (2023).
Sigurdsson, S. et al. Mediator function of the human Rad51B-Rad51C complex in Rad51/RPA-catalyzed DNA strand exchange. Genes Dev. 15, 3308–3318 (2001).
Lheureux, S., Braunstein, M. & Oza, A. M. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J. Clin. 69, 280–304 (2019).
Dasari, S. & Tchounwou, P. B. Cisplatin in cancer therapy: molecular mechanisms of action. Eur. J. Pharm. 740, 364–378 (2014).
Cong, K. et al. Replication gaps are a key determinant of PARP inhibitor synthetic lethality with BRCA deficiency. Mol. Cell 81, 3227 (2021).
Berti, M. et al. Sequential role of RAD51 paralog complexes in replication fork remodeling and restart. Nat. Commun. 11, 3531 (2020).
Somyajit, K., Saxena, S., Babu, S., Mishra, A. & Nagaraju, G. Mammalian RAD51 paralogs protect nascent DNA at stalled forks and mediate replication restart. Nucleic Acids Res 43, 9835–9855 (2015).
Longo, M. A. et al. RAD51C-XRCC3 structure and cancer patient mutations define DNA replication roles. Nat. Commun. 14, 4445 (2023).
Kuznetsov, S. G., Haines, D. C., Martin, B. K. & Sharan, S. K. Loss of Rad51c leads to embryonic lethality and modulation of Trp53-dependent tumorigenesis in mice. Cancer Res. 69, 863–872 (2009).
Pelttari, L. M. et al. RAD51C is a susceptibility gene for ovarian cancer. Hum. Mol. Genet. 20, 3278–3288 (2011).
Sopik, V., Akbari, M. R. & Narod, S. A. Genetic testing for RAD51C mutations: in the clinic and community. Clin. Genet. 88, 303–312 (2015).
Liu, J., Konstantinopoulos, P. A. & Matulonis, U. A. Genomic testing and precision medicine–What does this mean for gynecologic oncology?. Gynecol. Oncol. 140, 3–5 (2016).
Shimelis, H. et al. BRCA2 Hypomorphic Missense Variants Confer Moderate Risks of Breast Cancer. Cancer Res. 77, 2789–2799 (2017).
Li, L. et al. Automatic DNA replication tract measurement to assess replication and repair dynamics at the single-molecule level. Bioinformatics 38, 4395–4402 (2022).
Acknowledgements
This work was supported by the National Institutes of Health grants [R01 ES031796 (K.A.B.), R01 ES030335 (K.A.B.), F31 CA264889 (H.L.R.), R01 ES030396 (P.L.O.), GM115568 (S.K.O), GM128731 (S.K.O), R35 CA241801 (P.S.), R35 GM118026 (E.C.G.), R01 CA236606 (E.C.G.), P01 CA275717 (E.C.G. & P.S.), R01 CA293655 (E.C.G. & S.K.O.), and R01 GM127569 (J.L.Y.)], the American Cancer Society (DBG-24-1243614-01-DMC) to K.A.B., the Penn Center for Genome Integrity, the Basser Center for BRCA and The Intramural Research Program of the Center for CCR of NCI, NIH (ZIA BC011525, to J.-M.L.). A.P. is supported by NIEHS (5T32ES019851-10). S.K.O. is the recipient of a CPRIT Rising Star Award (RR200030). S.R.H. is supported by the NIEHS (K99/R00-ES033738), F31 ES027321 (M.R.S.).
Author information
Authors and Affiliations
Contributions
H.L.R., Y.R., A.L.P., G.G., P.P., R.R. and K.D. all conducted experiments. H.L.R., G.G. and M.S. contributed to manuscript writing. M.A. conducted data analysis and helped with figure creation. S.R.H. helped with data analysis. M.R.R., J.M.L., S.M.D. and E.M.S. supplied patient information. P.L.O., J.L.Y. and E.C.G. provided aid in conceptual experimental design. S.K.O., P.S. and K.A.B. designed experiments, and aided in interpreting results and writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
: Nature Communications thanks Matthew Wakefield and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rein, H.L., Rawal, Y., Palovcak-Lightbourn, A.L. et al. Comprehensive RAD51C ovarian cancer variant analysis uncouples homologous recombination and replicative functions. Nat Commun 16, 6539 (2025). https://doi.org/10.1038/s41467-025-61283-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-61283-2
This article is cited by
-
The role of homologous recombination in human retrovirus-associated diseases
Virus Genes (2026)