Abstract
Red, far red, or near-infrared photoswitchable drugs offer immense photo-pharmacological advantages due to the higher light penetration through the skin. Such photoactivation is achieved using processes such as two- and three-photon absorption, excited-state absorption, and triplet-triplet annihilation upconversion, which require higher photon fluences (W to kW cm−2) than the resilience constraints of skin (200 mW cm−2). Herein, a generalized approach of cis-to-trans photoisomerization of azobenzenes is demonstrated via triplet sensitization with NIR-I illumination (850 nm) of a new Zn-octa-substituted phthalocyanine photosensitizer, in aqueous medium at 2.62 mW cm−2. The approach is applied to control the heart rate of a frog tadpole via cis-to-trans photoisomerization of an azobenzene-functionalized muscarinic acetylcholine receptor M2 agonist in the phototherapeutic window (730 nm excitation: 42 mW cm−2). This advance highlights a powerful photo-pharmacological strategy for modulation of in vivo activity at 2-4 orders of magnitude lower photon fluences of NIR light compared to established methods.
Similar content being viewed by others
Introduction
Biological photoactivation upon far-red (FAR) and near infra-red (NIR) light excitation enables numerous in vivo applications such as bioimaging1, biosensing2, drug delivery3,4, optogenetic neural modulation5,6, cardiac defibrillation6,7, and photodynamic therapy8,9. This is due to the higher penetration of FAR/NIR light through living tissue in the phototherapeutic window (Fig. 1a and Supplementary Fig. 9)10. A very attractive phenomenon for photochemical control is a change in molecular shape. In this regard, the photoinduced geometrical interconversion of cis-trans isomers can enable control of biological activity when functionalized with a suitable drug molecule11. Photoisomerization offers several benefits compared to traditional photolysis techniques for in vivo photoactivation, as chemical by-products are not produced within the cell12. As a result, several photoswitches (azobenzene, thioindigo, hydrazone, diarylethene, spiropyran, donor-acceptor Stenhouse adducts13, and norbornadiene) have been tested for biological photoactivation employing linear14 or non-linear optical processes15,16,17. Azobenzenes have become widely recognized among the various photoswitches available. This is primarily due to their small size and molecular design flexibility, remarkable resistance to photobleaching and rapid photoisomerization, making them highly sought-after in the realm of biological activity. Consequently, these compounds have been extensively investigated in numerous research studies18,19,20,21,22. The trans-azobenzene is 10–12 kcal mol−1 more stable than the cis-azobenzene in the dark, hence the unsubstituted cis isomer eventually converts to the trans isomer via thermal relaxation in a few days. However, isomerization rates can be increased up to seconds via illumination at appropriate wavelengths. The trans-azobenzene (E) shows strong π-π* absorption at 320 nm and weak n-π* absorption at 440 nm. The cis-azobenzene (Z) shows n-π* absorption at 440 nm along with the π-π* absorptions at 280 and 250 nm23. Despite the desired photochemical stability of azobenzene, the low penetration depth of its absorption wavelengths is incompatible with in vivo applications. This issue has been addressed by red-shifting the photoswitching wavelengths with different strategies, including (i) chemical engineering of absorption wavelengths24,25 and (ii) indirect excitation with upconverted ultraviolet-blue photons via non-linear optical processes like two-and 3 photon absorption (2-3PA) or triplet-triplet annihilation (TTA)26,27,28,29,30,31. Both multiphoton absorption32,33 and excited state absorption/energy transfer upconversion (ESA/ETU)34,35 necessitate a high photon fluence (W cm−2 to kW cm−2) to achieve the simultaneous absorption of two or three coherent photons or excitation of a higher energy state (Supplementary Fig. 10a, b)36. On the contrary, TTA-UC involves a multi-component and multistep energy-transfer process that also demands high photon fluence for NIR-to-visible photon upconversion (Supplementary Fig. 10c)5,37. Hence, a feasible resolution lies in the utilization of an alternative photophysical mechanism that functions effectively under low photon fluences within the phototherapeutic window38. In the present study, triplet-sensitized photoisomerization (Fig. 1b, c) is explored for achieving biological photoactivation at low-energy excitation levels (λex = 730 or 850 nm).
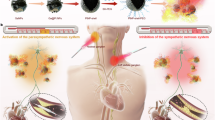
a Penetration depth of various wavelengths of solar energy in animal tissue. b Energy diagram of the low-energy excitation-based triplet-sensitized photoswitching. c Electron exchange via Dexter energy transfer due to the endothermic triplet energy gap between photosensitizer and photoisomer. d Triplet-sensitized photoswitching of azobenzene in aqueous solution after excitation-based 730 or 850 nm LED and modulation of the cardiac functions of the frog tadpole. S = photosensitizer, P = photoisomer, e = electron.
The triplet-sensitized photoisomerization enables the action spectrum of azobenzenes to be shifted into the therapeutic window (730–850 nm), with photon fluence ranging between 2-4 orders of magnitude lower compared to 2PA, 3PA, ESA, ETU, and TTA techniques32,33,34,35,36,37, as required for biological photoactivation (Fig. 1d). The strategy is extended to the in vivo photoisomerization of an azobenzene-modified muscarinic acetylcholine receptor M2 agonist15, which enabled regulation of the heart rate of a frog tadpole (Fig. 1d). While azobenzene-functionalized drugs have been increasingly utilized to selectively modulate cellular functions in vivo15,16,17,18,19,20,21,22,31, the present study showcases the pharmacological potential of triplet sensitization via low-intensity FAR or NIR light in a real-world biomedical application. Sensitized photoisomerization of various photoswitches (azobenzene, spiropyran, diarylethene, norbornadiene, and stilbene) via triplet manifolds has been known since the 1960s. However, most of these studies were carried out upon excitation with a pulsed laser in the UV-Vis region for mechanistic understanding (see Supplementary Table 1 refs. S15−S23). Studies on triplet-sensitized photoisomerization with FAR light have recently gained momentum, especially with azobenzenes39,40,41 and diazocines42. The lowest n-π* triplet energies of unsubstituted cis and trans azobenzene are reported at 1.26 eV and 1.53 eV, respectively43. Due to the energetic proximity of the triplets and the cis-trans photo-stationary state, and the preference of the triplet-state to relax to the trans form, it is possible to photoisomerize mainly the cis isomer to the trans isomer via triplet sensitization44, barring exceptional cases of disequilibration of azobenzene by sensitization under confinement39. Recently, the groups of Durandin40 and Moth-Poulsen41 reported proof-of-concept red/far-red triplet-sensitized cis-to-trans photoisomerization of azobenzene derivatives in de-aerated organic solvents and bioplastics, respectively40,41. However, further shifting the action spectrum into the NIR for deep tissue photoactivation45 is only one of a handful of design criteria that must be satisfied, together with the following: (1) the photoisomerization must operate in bio/biomimetic environments; (2) the photosensitizer and photoswitch must have suitable triplet energies for effective energy transfer; (3) the photosensitizer must be biocompatible; and (4) oxygen quenching of the chromophore triplet states must be limited. This study involves a comprehensive experimental investigation aimed at addressing the aforementioned criteria.
Results
Strategy of triplet sensitized photo-isomerization of azobenzenes
A systematic triplet-sensitized photoisomerization study has been carried out in biomimetic systems to investigate azobenzene photoswitches, and biocompatible FAR or NIR-absorbing triplet photosensitizers (Fig. 2) suitable for use in animal tissue.
Molecular structures of (a) azobenzenes (AZO-N, AZO-EH, AZO-MM and PAI) and (c) triplet photosensitizers (BC1, ZnPc1 and PdNc1). Normalized absorption spectra of (b) azobenzenes (Conc. = 40 µM) and (d) triplet photosensitizers (Conc. = 4 µM) dissolved in toluene. The absorption spectrum of PAI was recorded in phosphate buffer solution, pH 7.4 (Conc. = 10 µM).
First, given that animal skin is mainly comprised of collagen and lipids46, a biomimetic surrogate for animal skin has been constituted that is composed of collagen doped with a liquid surfactant forming a micellar membrane and containing photosensitizers and azobenzenes. Second, the skin biomimetic films were placed under an 8 mm thick dried Iberian ham layer to observe photoswitching with light that can penetrate animal tissue. Each azobenzene was examined for cis-to-trans photoswitching upon excitation with 730 or 850 nm LED light through triplet sensitization in the air at very low photon fluences. Third, further photoswitching experiments were carried out with the azobenzene-containing trans-Phthalimide-Azobenzene-Iperoxo (PAI) drug encapsulated in Pluronic F-127 (PF-127) micelles in combination with the triplet photosensitizer Zn phthalocyanine (ZnPc1) in a phosphate buffer solution at physiological pH to mimic the cellular environment. Fourth, the ZnPc1-PAI system, which exhibited cis-to-trans photoswitching upon 730 nm LED excitation in air, was utilized for an in vivo experiment involving frog tadpoles to regulate cardiac rate. Each of these is now described in depth.
Azobenzenes and triplet photosensitizers
Four azobenzene compounds with distinct structural features (Fig. 2a) and absorption maxima (Fig. 2b) were chosen to understand the suitable molecular design for application in a biological setting. The parent azobenzene (AZO-N) was derivatized to increase the conformational flexibility for fast isomerization in restricted matrices such as films47. Derivatization also shifted the absorption energies. Compared to the native trans-azobenzene AZO-N (λabs = 320, 445 nm), AZO-EH and AZO-MM exhibit the π-π* absorption band red-shifted by 28 and 48 nm, respectively, whereas the n-π* band is blue-shifted by 9 nm (AZO-EH) or red-shifted by 15 nm (AZO-MM) (Fig. 2a, b). The synthesis procedure of the newly designed AZO-MM is provided in the experimental section of the Supplementary Information.
Three macrocycles were examined as photosensitizers (Fig. 2c), each of which absorbs strongly in the FAR or NIR region (Fig. 2d). Bacteriochlorin BC1 is a metal-free analog of Nature’s NIR absorber bacteriochlorophyll a48,49. BC1 shows a Qy band (S0 - S1, ε737nm = 8.6 × 104 M−1 cm−1) in the FAR region, the Qx band (S0-S2) in the blue-green region (499 nm), and nominal Bx (S0-S3) and By (S0 - S4) bands in the blue-violet (Soret) region at 370 and 354 nm, respectively50. A high quantum yield of intersystem crossing (ΦISC = 0.53)51 characteristic of bacteriochlorophylls makes such macrocycles potent triplet populators51. However, the short triplet lifetime makes it difficult to measure its phosphorescence at room temperature. Indeed, the phosphorescence spectrum of BC1 in a polystyrene matrix at 10 K could not be detected (Supplementary Fig. 11). Therefore, in this study, the triplet energy (T1 = 1.17 eV)51 has been considered as the approximate value for BC1.
For NIR sensitization in vivo, a new phthalocyanine photosensitizer, 1,4,8,11,15,18,22,25-octakis(2-ethylhexylsulfanyl) Zn(II) phthalocyanine (ZnPc1), which bears eight alkylthio substituents in non-peripheral positions, has been designed and synthesized to display a bathochromically shifted absorption maximum (see the experimental section of the Supporting Information). ZnPc1 exhibits a Q-band absorption maximum at 781 nm (ε781nm = 2.2 × 105 M−1 cm−1)52 with a tail extending to 900 nm (Fig. 2c, d). Attempts to measure its phosphorescence in a polystyrene matrix at 40 K did not yield a detectable spectrum (Supplementary Fig. 12). Owing to its structural similarity with a previously reported FAR-absorbing Zn phthalocyanine, the same triplet energy (T1 = 1.12 eV)41 has been employed in this study. Because the exact triplet energies of BC1 and ZnPc1 could not be determined, palladium naphthalocyanine PdNc1 (Fig. 2c, d), having Q-band absorption at 825 nm (ε825nm = 9.4 × 105 M−1 cm−1) and triplet state at 0.92 eV (Supplementary Fig. 13), has been used for proof-of-concept NIR sensitization studies in the skin biomimetic films.
Photoisomerization studies in the skin biomimetic film
Films were prepared by combining PdNc1 with the three azobenzene compounds dispersed in the interstitial liquid surfactant phase surrounded by collagen fibers (Fig. 3a)41. Hydrolyzed collagen, known as gelatin (G), has high solubility in water yet undergoes cross-linking upon dehydration to form a collagen-like triple-helix with a thick fibrous structure that acts as an oxygen barrier53. As a liquid surfactant, Triton-X-100 reduced (TXr) was utilized due to its globally accepted low toxicity (compared to Triton-X-100)54 and its ability to emulsify gelatin effectively41,55. The preparation of the films is described in the experimental section (see the Supplementary Information). The final concentrations of azobenzenes and PdNc1 in the films were 1.95 mmol kg−1 and 204 µmol kg−1, respectively, to keep the absorbance below 1.0. Upon dehydration, the expected triple-helix semi-crystalline structure of gelatin is retained in the G-TXr-AZO-PdNc1 films (P-XRD Supplementary Fig. 14a, b)41,56. Cross-section SEM images showed interstitial TXr-chromophores in the liquid dispersed between the fibrous structure of gelatin in the G-TXr-AZO-EH film (Supplementary Fig. 15)41. Compared to toluene, in the films the π-π* band underwent a 6 nm blue-shift (AZO-N) or a 2 nm red-shift (AZO-MM), which is indicative of aggregation (Supplementary Fig. 16a, b). By contrast, no shift was observed for AZO-EH in the G-TXr-AZO-EH film, consistent with molecular dispersion (Supplementary Fig. 16c).
a Schematic illustration of the biomimetic film. b Absorption profiles and illustrations of forward (trans-to-cis) and backward (cis-to-trans) photoisomerization of azobenzenes in the G-TXr-AZO-PdNc1 films via singlet absorption (λex = 340 or 365 nm, Io = 2.85 mW cm−2) and triplet sensitization (λex = 850 nm, Io = 107 mW cm−2), respectively.
The photoisomerization of the azobenzenes in the G-TXr-AZO-PdNc1 films in the air is shown in Fig. 3b. All films showed trans-to-cis photoisomerization upon LED excitation for 1 min at their normal absorption wavelengths (340 and 365 nm). In contrast, cis-to-trans photoisomerization occurred upon 850 nm excitation via triplet sensitization with PdNc1, thus shifting the action spectrum of the azobenzenes from 455 nm to 850 nm. The cis-to-trans photoisomerization of azobenzene at 850 nm surpasses the previous limits of 770 nm in solution40 and of 740 nm in the solid-state41. Interestingly, triplet sensitization occurred despite the low triplet energy of PdNc1 compared to that of cis-azobenzene compounds57,58,59,60. Hence, the electron exchange occurs via a Dexter energy-transfer mechanism (Fig. 1c)57,58. The involvement of triplet sensitization as the major driving force for cis-to-trans photoisomerization was confirmed by a control experiment in air-saturated AZO-EH-PdNc1 toluene solution, whereupon excitation at 850 nm elicited no photoisomerization. The kinetics of cis-to-trans photoisomerization were the fastest (2 min) in the G-TXr-AZO-EH-PdNc1 film. Therefore, AZO-EH was chosen for further studies with other photosensitizers in the film and in micellar solution.
The fast kinetics can be attributed to the higher conformational flexibility of AZO-EH in the condensed phase due to its liquid nature versus that of AZO-N and AZO-MM, which are solids at room temperature. The durability of photoisomerization in the G-TXr-AZO-EH-PdNc1 film was confirmed by measuring the photoisomerization for 13 consecutive cycles (Supplementary Fig. 17). The possible bio-incompatibility of PdNc1 due to the heavy metal prompted examination of BC1 and ZnPc1 for 730 nm and 850 nm sensitization (Fig. 2c, d) to cover the broad FAR and NIR absorption range in the phototherapeutic window. Both G-TXr-AZO-EH-BC1 and G-TXr-AZO-EH-ZnPc1 films showed efficient triplet-sensitized cis-to-trans photoisomerization in the films (10 s or 10 min) upon 730 nm (Io = 54.9 mW cm−2) or 850 nm (Io = 107 mW cm−2) LED excitations (Supplementary Fig. 18). The photoisomerization was faster upon excitation at 730 nm, consistent with a lesser energy difference between the triplet states of BC1 and AZO-EH versus those of ZnPc1 and AZO-EH. The slower observed photoisomerization process in ZnPc1 compared to PdNc1 can be attributed to the low extinction coefficient of ZnPc1 at 850 nm. The involvement of the triplet state in energy transfer was confirmed by experiments in AZO-EH-ZnPc1-toluene solution in the absence or presence of air, where photoisomerization was observed only in the de-aerated solution (Supplementary Fig. 19).
To conduct deep tissue biomimetic experiments, an initial assessment of the viability of photoisomerization was done by positioning films of G-TXr-AZO-EH-BC1 and G-TXr-AZO-EH-ZnPc1 beneath an 8 mm thick layer of dried Iberian ham (Supplementary Fig. 20), followed by excitation at 730 or 850 nm (Fig. 4a).
a Schematic of the excitation with different LEDs of the biomimetic film placed below the 8 mm ham layer. The table shows the excitation intensities of different LEDs used in mW cm−2. b, c Absorption profile, and kinetics of cis-to-trans photoisomerization of the G-TXr-AZO-EH-BC1 film with ham-penetrated 730 nm LED excitation (IF = 0.51 mW cm−2). d, e Absorption profile, and kinetics of cis-to-trans photoisomerization of G-TXr-AZO-EH-ZnPc1 film with dried Iberian ham-penetrated 850 nm LED excitation (IF = 2.62 mW cm−2).
The real-time configuration can be observed in Supplementary Fig. 21. Excitation at 365 nm of both films was performed without the ham layer due to the absorption of various bio-absorbents in the ham layer at this wavelength (as shown in Supplementary Fig. 9). The table in Fig. 4a displays the recorded values of excitation power densities (Iex) of different LEDs without (Io) and with the ham layer (IF) utilized for the photoisomerization experiments. Indeed, only 0.0025% of 455 nm, 1% of 730 nm, and 2.44% of 850 nm light penetrated through the 8 mm ham layer. Prior to the photoisomerization experiments with IF, the intensity dependence of the cis-to-trans photoisomerization was examined for the G-TXr-AZO-EH-ZnPc1 film under 850 nm LED excitation (Io) for 10 min. A non-linear relationship between the excitation intensity and the NIR triplet-sensitized cis-to-trans isomerization of AZO-EH was observed (as depicted in Supplementary Fig. 22).
Subsequent cis-to-trans photoisomerization experiments were conducted in the films using LEDs at different IF (Fig. 4a–e). The penetration through the skin of different wavelengths is illustrated in Fig. 4b, where faster photoisomerization was observed upon a 15-min exposure with IF excitation of 730 nm versus the actual absorption wavelength of the azobenzene at 455 nm. The slow cis-to-trans isomerization (t1/2 = 16.4 min) was observed due to the low fluence of 730 nm irradiation (IF = 0.51 mW cm−2) as shown in Fig. 4c, consistent with the intensity dependence of isomerization (Supplementary Fig. 22) but perhaps altered in part by scattering of the incident light. A further shift in the action spectrum in the NIR-I region (850 nm) in the G-TXr-AZO-EH-ZnPc1 film was achieved, as indicated by cis-to-trans photoisomerization even at the low photon fluence of 2.62 mW cm−2 (Fig. 4d). The isomerization was slow (t1/2 = 24.3 min) due to the low fluence, low absorption coefficient of ZnPc1 at 850 nm, and the higher endothermic triplet energy gap between the cis-AZO-EH and ZnPc1 triplets. However, photoactivation via triplet sensitization is still possible at such low fluences after passing through the ham layer. The fluence for FAR/NIR triplet-sensitized photoactivation is between 2-4 orders of magnitude lower compared to 2-3PA, ESA, and TTA-based in vivo photoactivation applications32,33,34,35,36,37. Additionally, the maximum Io used here (54 or 107 mW cm−2) is well below the maximum tolerance limit of the human skin in these regions (200 mW cm−2)61. The past decade has witnessed remarkable advances in drugs containing azobenzene photoswitches for selective photoactivation in delivery, signaling, and channel activation15,16,17,18,19,20,21,22. The triplet-sensitized photoisomerization of azobenzenes within the phototherapeutic range and with fluences compatible with human skin constitutes an alternative strategy of remarkable potential for use in living organisms. To conduct real-time biological experiments, however, the photosensitizer-photoswitch pair must be delivered to the cell in an active state. Hence, to assess the viability of this method in a comparable setting, further experiments were conducted in aqueous micelles, as described in the next section.
Photoisomerization studies in PF-127 micelles
To explore conditions suitable for applications in biological systems, triplet-sensitized photoisomerization experiments were conducted in a phosphate buffer solution (25 mM, pH 7.4) with PF-127 micelles (2%) at room temperature. Concentrations of the photosensitizer (0.8 µM) and AZO-EH (24 µM) were comparable to those employed in photo-pharmacology. PF-127 (Fig. 5a and Supplementary Fig. 23) micelles are attractive due to their oxygen barrier capabilities5 and biocompatibility62. The experimental setup, featuring an 8 mm ham layer, is illustrated in Supplementary Fig. 24. In the micellar solution, AZO-EH exhibited typical trans-to-cis photoisomerization upon stimulation with a 365 nm LED, with a half-life of 0.37 min (Supplementary Fig. 25). When excited with an 850 nm LED with a ham layer, AZO-EH displayed cis-to-trans photoisomerization in the presence of air with a half-life of 1.28 min (Supplementary Fig. 26). The half-life of cis-to-trans isomerization, t1/2 cis-to-trans = 82 min Supplementary Fig. 27), is slow compared to the solution without the ham layer (t1/2 cis-to-trans = 1.28 min), which can be attributed to the excitation intensity-dependent nature of photoisomerization. Encouragingly, photoisomerization occurs even within an aqueous solution at physiological pH, suggesting potential applications of this technique to azobenzene-containing drugs.
a Illustration of chromophores in PF-127 micelles in phosphate buffer pH 7.4 (25 mM). b Illustration of trans↔cis photoisomerization of PAI upon 340 and 730 nm LED excitations. c, d Absorption spectra, and kinetics of cis-to-trans photoisomerization of PAI in PF-127-PAI-ZnPc1 micelles solution upon 730 nm LED excitation (Io = 46 mW cm−2). PAI = 50 µM, ZnPc1 = 3 µM and PF-127 = 2%. The spectra were recorded in a 0.1 cm pathlength quartz cuvette.
The long half-life of triplet-sensitized cis-to-trans isomerization of AZO-EH in the micellar environment prompted the examination of the contribution of temperature. The cis-to-trans isomerization studies of AZO-EH in PF-127 micelles were carried out at biologically relevant temperatures (30, 35 and 40 °C), where the half-life (t1/2 cis-to-trans) was found to be 1589, 888 and 488 min, respectively (Supplementary Fig. 28). These results demonstrate minimal thermal contribution to cis-to-trans photoisomerization of AZO-EH during triplet-sensitization at physiological temperature, (Supplementary Fig. 27). The effect of photosensitizer concentration on the kinetics of isomerization was also measured by titrating the same concentration of AZO-EH (30 µM) against varying ZnPc1 concentrations (0.5 µM to 10 µM) in PF-127 micelles in water (Supplementary Figs. 29 and 30). The results show that cis-to-trans isomerization could be achieved even at 0.5 µM concentration of ZnPc1. Moreover, the half-life (t1/2 cis-to-trans) decreased up to 5 µM concentration of ZnPc1, followed by stabilization thereafter (Supplementary Fig. 30).
The composition of the photostationary state (PSS) of AZO-EH in micellar solutions following triplet-sensitization was analyzed using 1H NMR spectroscopy. The measurements were first conducted in CDCl3 and PF-127-D2O solution without photosensitizer (ZnPc1), serving as a reference standard (Supplementary Figs. 31–42, 43–52). In the CDCl3 environment, irradiation at 365 nm for 2 min resulted in a maximum of 95% cis-AZO-EH in the PSS. Conversely, subsequent back-conversion through irradiation at 455 nm for one min reduced the cis-AZO-EH to 31% in the PSS (Supplementary Table 2). In the AZO-EH-PF-127-D2O solution, a 5 min exposure to 365 nm light achieved 99% cis-AZO-EH in the PSS, both in the absence of (Table S3) and the presence of ZnPc1 (Supplementary Figs. 53–57 and Table S4). Upon back-conversion with 455 (Supplementary Table 3) or 730 nm (Supplementary Figs. 58–62 and Supplementary Table 4) irradiation for 10 min, cis-AZO-EH decreased to 15%, and 18%, respectively, in the PSS. Therefore, the relative composition of cis and trans-AZO-EH in the PSS after back-conversion remains almost similar in micellar solution regardless of direct or indirect irradiation. The feasibility of triplet-sensitized cis-to-trans isomerization was also tested on the azobenzene-based drug molecule PAI15,33 in phosphate buffer solution of PF-127 micelles at pH 7.4 (Fig. 5a, b).
PAI is an agonist of muscarinic acetylcholine receptor subtype 2 (M2 mAChR)15,33. The M2 mAChRs belong to the class A G-protein coupled receptors (GPCRs) and are primarily found in the heart. Stimulation of the M2 mAChRs by the parasympathetic nervous system leads to a decrease in heart rate and an increase in atrioventricular conduction time. Previous studies have shown that PAI can act as a dualsteric drug, allowing for the photo-control of M2 mAChR activity using one-photon (460 nm LED)15, two-photon (840 nm pulsed laser)15 and three-photon (1560 nm pulsed laser)33 absorption techniques, albeit at high photon fluences. Here, PAI isomerization was controlled through photoactivation using triplet sensitization upon excitation with a 730 nm continuous wave LED at a significantly reduced excitation power density (46 mW cm−2) compared to that of 2PA (Fig. 5c, d). The successful result in an aqueous, cellular-like environment within the phototherapeutic window prompted in vivo experiments to explore the application of triplet-sensitized photoswitching in photo-pharmacology.
In vivo Photomodulation of Cardiac function via TSP
The development of red-light photoswitchable drugs must satisfy challenging criteria of aqueous solubility, reversible switching behavior, and absence of immunogenicity13. Activation of photoswitchable drugs has been demonstrated by two-photon excitation at NIR wavelengths (800–900 nm)30,63,64,65 and three-photon excitation at 1560 nm (mid-IR)11, but the requirement for large laser and optical equipment confines the methods to research laboratories. Upconversion in optogenetics5 and photo-pharmacology66 has been achieved with continuous wave (CW) light sources (LED or lasers) at 980 nm, but requires injecting potentially toxic, heavy metal-laden nanoparticles. Optogenetic defibrillation, which utilizes red light to regulate arrhythmias in the heart of transgenic mice, is another emerging technique; however, it necessitates genetic mutation of the receptive cardiac tissue with light-sensitive channelrhodopsin-2 (ChR2)6. Optical defibrillation through the reversible photoswitching of azobenzene-labeled azobupivacaine 2 (AB2) represents another promising approach for the modulation of cardiac electrophysiology by reversibly inhibiting voltage-gated Na+ and K+ channels. Nonetheless, this technique relies on UV-blue light for the reversible photoswitching67. Developing synthetic biocompatible photosensitizers that allow photoactivation of drugs with CW IR light by simple triplet sensitization could have a wide impact in photo-pharmacology and phototherapies. Accordingly, the feasibility of controlling cardiac activity in vivo with 730 nm illumination via triplet-sensitized photoisomerization of the azobenzene-containing PAI was examined. PAI has been demonstrated to serve as a photoswitchable agonist capable of activating muscarinic M2 receptors at picomolar potency with subtype selectivity15. The experiments were conducted on albino Xenopus laevis tadpoles, refs. S7−S11, which are translucent and allow non-invasive real-time visualization and quantification of cardiac activity and efficient photoswitching of drugs by CW light sources such as LEDs to dynamically control cardiac function (Fig. 6a–d) 6,15.
a Apparatus to optically record cardiac activity in frog tadpoles, including a binocular microscope equipped with a camera and LEDs with 365, 455, and 730 nm wavelengths. b Freely swimming tadpoles at stages 46–48. c Image representing the application of 0.23 mM pancuronium dibromide solution to immobilize the tadpole (stage 47) for long-term imaging of cardiac activity under dim light at 32 frames per second (fps) video recording. A region of interest (ROI) is placed at the edge of the heart to record the beating. d Image representing the setup for applying compounds to the medium and illuminating at wavelengths of interest (365, 455 and 730 nm LED in the photo). e Time course of the mean ROI intensity of heartbeat under the conditions indicated in the labels: control in the absence of PAI and light, inactive 365 nm-preilluminated (Io = 10.9 mW cm−2) 30 µM PAI together with 10 µM ZnPc1 photosensitizer in the dark, and effect of illuminating the mix with 730 nm light (Io = 42 mW cm−2) in consecutive 15 min periods. f Time course of the amplitude of cardiac contraction during controls, compound application in the dark, and three consecutive 10 min periods of 730 nm illumination (shaded in red). The black line represents inactive 365 nm-preilluminated 30 µM PAI with 10 µM ZnPc1, and 2% PF127 in 0.1X MBS diluted buffer, the gray line shows the control experiment in the absence of the triplet photosensitizer ZnPc1, and the dotted line represents the control corresponding to the triplet sensitizer alone (10 µM ZnPc1 and 2% PF127 in 0.1X MBS). The corresponding frequency plots are shown in the Supplementary Information. g Quantification of the experiments in panels (e, f). White bars represent the mean amplitude of contraction measured at intervals of 15 s during each experimental phase (baseline, application, 730 nm 10 min, 20 min, and 30 min) following treatment with the sensitizer (ZnPc1) alone (n = 3 animals, biological replicates). Gray bars represent measurements with PAI alone (n = 2 animals, biological replicates), and black bars represent the combination of ZnPc1 and PAI (n = 3 animals, biological replicates). Each data point shown represents independent biological replicates derived from separate animals. Due to ethical considerations regarding animal use, the number of biological replicates was kept minimal. Error bars indicate the standard error of the mean (SEM). Statistical significance (*) indicates p < 0.05 when comparing the combination treatment (PAI + sensitizer) to sensitizer alone, multiple unpaired t test. “nd” indicates no significant difference.
The drug PAI was dissolved in an aqueous micellar solution along with the triplet photosensitizer ZnPc1 for testing (Fig. 1d). The micelles act as carriers to deliver both PAI and ZnPc1 to the M2 mAChR membrane receptor. Before the introduction of this formulation into a tadpole water, followed by 730 nm excitation, photoswitching experiments with UV-blue light were performed to demonstrate feasibility at normal absorption wavelengths. PAI reversibly photoisomerizes between the trans form (dark-relaxed and under blue 455 nm LED illumination) and cis form (under 365 nm UV illumination), displaying higher and lower pharmacological activity, respectively (Supplementary Fig. 63a–e).
Application of 30 μM trans PAI to water decreases the tadpole heartbeat, leading to cardiac arrest (Supplementary Fig. 63b), which can be reversed by UV illumination with a 365 nm LED (Supplementary Fig. 63c), as reported15. This effect is reversible due to PAI isomerization from cis-to-trans (Supplementary Fig. 63d). The results are quantified by the averaged amplitude of cardiac contraction (Supplementary Fig. 63e) and by the heartbeat frequency (Supplementary Fig. 64). After control experiments, here, an in vivo triplet-sensitized photoswitching assay of PAI combined with ZnPc1 was implemented (Fig. 6e). First, a 3 min waiting period was recorded to test the stabilization and health status of the tadpoles. Then, the tadpole medium was replaced with an inactive, pre-irradiated 30 μM cis-PAI solution combined with or without 10 μM ZnPc1 encapsulated by 2% PF127 in 0.1X MBS. This solution produced only minor changes in heartbeat (Fig. 6e). Following this, 730 nm LED illumination was applied in consecutive 10 min intervals to allow triplet sensitization of PAI for cis-to-trans isomerization while minimizing sample warming. Figure 6f shows that in the initial absence of PAI, the tadpole cardiac activity (amplitude of cardiac contraction and cardiac frequency, see Supplementary Fig. 65) remained stable and only changed slightly upon application of 30 μM cis-PAI. In contrast, cardiac activity was reduced during consecutive 10 min 730 nm LED illumination periods, while no significant effects were observed with illumination in the absence of ZnPc1 or PAI (Fig. 6f). These controls rule out possibilities like PAI being activated thermally or cardiac activity being reduced by ZnPc1-photoemitted species. The decrease in amplitude of cardiac contraction is quantified in Fig. 6g and is comparable to that observed with active trans-PAI, although changes are significantly slower, probably due to limited penetration of micelles in tissue and/or photoconversion in the experimental conditions. Future studies will be focused on testing different molecular weight Pluronics for enhanced tissue penetration and proximity of photoswitchable drugs to photosensitizers to accelerate overall triplet sensitization, in addition to designing covalently linked photosensitizer-photoswitch pairs. Overall, the results to date indicate that PAI can be converted from the inactive cis form to the active trans form in vivo by means of ZnPc1-mediated triplet sensitization under 730 nm illumination. Since the light sources and reagents are widely available, the triplet photosensitization method opens an alternative avenue to activate photoswitchable drugs using tissue-penetrating, non-invasive illumination.
Discussion
The field of photo-pharmacology aims to exploit the rich array of photochemical phenomena for non-invasive applications in the biomedical arena. Here, triplet-sensitized photoswitching has been examined as a means of utilizing light in the phototherapeutic window to exercise structural control of the azobenzene motif. A comprehensive experimental investigation conducted across various matrices such as a skin mimic and micelles has demonstrated that (1) the action spectrum of azobenzenes can be effectively shifted towards the NIR-I region (850 nm) by 400 nm, and (2) this red-shift could be achieved at an excitation power of only 2.62 mW cm−2, after passing through animal tissue. The strategy is a viable alternative to 2PA, ESA, and TTA-UC for achieving biological photoactivation at very low levels of photon fluence within the phototherapeutic window. As one example, the heart rate of frog tadpoles is modulated through triplet-sensitized cis-to-trans photoisomerization of a dualsteric drug, Phthalimide-Azo-Iperoxo (PAI), upon excitation of a photosensitizer, ZnPc1, with 730 nm LED light at an excitation power of 42 mW cm−2. This excitation power level is substantially below the established tolerance limit of skin (200 mW cm−2).
In summary, triplet-sensitized photoisomerization in biological environments presents an alternative approach to trigger photoswitchable drugs through non-invasive, tissue-penetrating illumination. As such, the demonstrated approach represents an important advance in the domain of photopharmacology. The co-localization of the photosensitizer and of the photoswitch is crucial for triplet-sensitization. We assume that the colocalization was accomplished here due to the presence of the orthosteric site of M2 mAChR within the hydrophobic core of the membrane, which serves as an ideal environment for housing hydrophobic chromophores. However, while it is crucial to understand the mechanism by which chromophores encapsulated in PF-127 micelles are internalized, this would deserve a separate study about the colocalization of the dyes upon their delivery using micelles. Another key issue is the trans-to-cis photoisomerization with the UV light and singlet oxygen generation during the interaction of photosensitizer with the molecular oxygen in vivo. Future molecular designs are envisaged that extend beyond the present proof-of-principle experiments wherein the photosensitizer and photo-drug are linked together along with the singlet oxygen quencher, and the electronic structure of azobenzene is tailored to shift the action spectrum of trans-to-cis isomerization towards the red region. For example, recent work by Thorn-Seshold and co-workers (submitted in chemrxiv) demonstrated in vitro photocontrol of the SNAP-mGluR2 expression in granule cells of the dentate gyrus brain slices due to cis-to-trans photoisomerization of a bioactive azobenzene-photosensitizer dyad upon irradiation with 660 or 740 nm light68. Taken together, the foundation is solid for the next step: exploring such systems for efficient and broad-spectrum photocontrol of pharmacology in the phototherapeutic window.
Methods
The biological research carried out in this work complies with all relevant ethical regulations set by the ethical commission of the University of Barcelona.
Preparation procedure of G-TXr-AZO-N-PdNc1, G-TXr-AZO-MM-PdNc1, and G-TXr-AZO-EH-PdNc1 biomimetic films
Films were prepared using the following procedure. A solution composed of 12 µL of AZO-N, or AZO-MM or AZO-EH (40 mM in toluene) and 70 µL of PdNc1 (716 µM in toluene) was concentrated under a N2 atmosphere in a glass vial. The residual mixture was treated with 0.026 g of TXr and stirred at 60 °C for complete solubilization of chromophores. The resulting solution was treated with 1 mL of Millipore grade water followed by stirring. The aqueous solution of chromophores and TXr was treated with 0.22 g of gelatin type A (G), followed by stirring at 70 °C for 10 min. The hot solution was allowed to rest at room temperature for 2 min, followed by drop-casting of 260 μl of the solution on a 3 × 1 cm glass plate and air drying for 24 h. The resulting air-dried films contained the following constituents: TXr = 10.5%, gelatin = 89.4%, PdNc1 = 203 µmol kg−1and AZO compounds (AZO-N, AZO-MM and AZO-EH = 1.95 mmol kg−1. The faint yellow semi-transparent film obtained after air drying for 24 h was used for the photoisomerization measurements upon excitation with 340, 365 and 850 nm LEDs.
Preparation of the G-TXr-AZO-EH-BC1 biomimetic film
A solution composed of 12 µL of AZO-EH (40 mM) and 100 µL of BC1 (500 µM) in toluene was concentrated under N2 atmosphere in a glass vial. To the residual mixture added 0.026 g of TXr and stirred at 60 °C until the chromophores were fully dissolved. To the resulting solution added added 1 mL of Millipore grade water, followed by stirring. Into the aqueous solution of chromophores added 0.22 g of gelatin type A (G), followed by stirring at 70 °C for 10 min. The hot solution was allowed to rest at room temperature for 2 min, followed by drop-casting of 260 μl of the solution on a 3 × 1 cm glass plate and air drying for 24 h. The air-dried films contain TXr = 10.5%, gelatin = 89.4%, BC1 = 203 µmol.kg-1and AZO-EH = 1.95 mmol kg−1.
Preparation of the G-TXr-AZO-EH-ZnPc1 biomimetic film
A solution composed of 12 µL of AZO-EH (40 mM) and 157 µL of ZnPc1 (317.5 µM) in toluene was concentrated under a N2 atmosphere in a glass vial. The residual mixture was treated with 0.026 g of TXr and stirred at 60 °C until the chromophores were fully dissolved. The resulting solution was treated with 1 mL of Millipore grade water followed by stirring. The aqueous solution of chromophores was treated with 0.22 g of gelatin type A (G), followed by stirring at 70 °C for 10 min. The hot solution was allowed to rest at room temperature for 2 min, followed by drop-casting of the 260 μl of the solution on a 3 × 1 cm glass plate and air drying for 24 h. The resulting air-dried films contained TXr = 10.5%, gelatin = 89.4%, BC1 = 203 µmol kg−1 and AZO-EH = 1.95 mmol kg−1.
Preparation of PF-127-AZO-EH-ZnPc1 micelles solutions
A solution composed of 12 µL of AZO-EH (40 mM), 50 µL of ZnPc1 (317.5 µM) and 0.02 g of PF-127 in toluene was concentrated under a N2 atmosphere in a glass vial. The residual mixture was treated with 1 mL of Millipore grade H2O, followed by stirring in an ice bath to dissolve PF-127. The resulting solution was brought to room temperature. The resulting micellar solution contained the following constituents: AZO-EH = 0.48 mmol L−1, ZnPc1 = 15.85 µmol L−1 and PF-127 = 2%. This stock solution was diluted 20x with 2% PF-127 in water. Photoswitching measurements were carried out in a 0.1 cm pathlength quartz cuvette with the following concentrations, AZO-EH = 24 µmol L−1, ZnPc1 = 0.8 µmol L−1 and PF-127 = 2%. Temperature dependent experiments were carried out in 1 cm pathlength quartz cuvette. For this 185 µL of AZO-EH-PF-127 aqueous solution (480 µM) is added to 2.315 mL of PF-127 (2%) aqueous micellar solution to keep the absorbance below 1.0. Final AZO-EH concentration = 35.5 µM. The samples for UV-Vis titrations of AZO-EH (30 µM) against different concentrations of ZnPc1 (0.5, 2.5, 5, and 10 µM) were prepared by mixing 62.5 µL of AZO-EH (480 µM in 2% PF-127 in water) with 10, 50, 100, and 200 µL of ZnPc1 (50 µM in 2% PF-127 in water), and 927.5, 887.5, 837.5 and 737.5 µL of 2% PF-127 in water respectively.
Preparation of AZO-EH CDCl3 solution and 1H NMR characterization to measure composition of AZO-EH photostationary state in CDCl3 as reference
A 5 mM solution of AZO-EH was prepared by dissolving 1.55 mg of AZO-EH in CDCl3. The solution was irradiated with 365 nm LED, for 15, 30, 60, 120, 180 and 240 s. 1H NMR spectra were recorded (Supplementary Figs. 31–36). The relevant peaks of the AZO-EH between 8.5-6 ppm, 4.5-3.5 ppm, and 2-0.5 ppm undergo shifts and a decrease/increase in intensity. The conversion could also be observed by a change in the color of the solution from yellow to orange (Supplementary Fig. 31). The peaks were identified to originate from the AZO-EH by comparison to the solution without the AZO-EH present. The sample solution was irradiated in a second irradiation experiment with 455 nm for 15, 30, 60, 150, 300 s for back-conversion. 1H NMR spectra were recorded, and the changes were seen to be reversible (Supplementary Figs. 37–42) The color change is also reversible (Supplementary Fig. 37). The composition of the photostationary state was determined for both irradiation experiments by integrating the changes in relevant peak areas and is provided in Supplementary Table 2.
Preparation of PF-127-AZO-EH-D2O micelles solution for 1H NMR characterization to measure the composition of the photostationary state
A 5 mM solution of AZO-EH in D2O was prepared by mixing 1.55 mg of AZO-EH with 0.02 g via heating at 70 °C for 5 min, followed by the addition of 1000 µL of D2O and stirring in the ice bath for 10 min for complete solubilization of all components. As a reference experiment, the AZO-EH (5 mM)-PF127 (2%)-D2O solution was irradiated with 365 and 455 nm, for 15, 30, 60, 120, 180 and 240 s, followed by recording of their 1H NMR spectra after each irradiation. First, the sample was irradiated with 365 nm LED, and 1H NMR spectra were recorded (Supplementary Figs. 43–47). The relevant peaks of the AZO-EH between 6-8 ppm, and 0.75-0.4 ppm undergo shifts and a decrease/increase in intensity. The conversion could also be observed by a color change of the solution from yellow to orange (Supplementary Fig. 43). The peaks were identified to originate from the AZO-EH by comparison to the solution without the AZO-EH present. The sample solution was irradiated in a second irradiation experiment with 455 nm for 15, 30, 60, 150, 300 s for back-conversion. 1H NMR spectra were recorded, and the changes were seen to be reversible (Supplementary Figs. 48–52). The color change is also reversible (Supplementary Fig. 48). The composition of the photostationary state was determined for both irradiation experiments by integrating the changes in relevant peak areas and is provided in Supplementary Table 3.
Preparation of PF-127-AZO-EH-ZnPc1-D2O micellar solution for 1H NMR characterization to measure the composition of the photostationary state
A 5 mM solution of AZO-EH doped with 50 µM of ZnPc1 in D2O was prepared by mixing 1.55 mg of AZO-EH with 0.02 g of PF-127, via heating at 70 °C for 5 min, followed by the addition of 784 µL of D2O, and 216 µL of ZnPc1-PF-127 (2%)-D2O solution (231 µM). The solution was stirred in an ice bath for 10 min for complete solubilization of all components. First, the NMR sample was irradiated with 365 nm LED, for a total of 5 min. 1H NMR spectra were recorded (Supplementary Figs. 53–57). The relevant peaks of the AZO-EH between 6-8 ppm, and 0.75-0.4 ppm undergo shifts and a decrease/increase in intensity. The conversion could also be observed by a change in the color of the solution from yellow to orange (Supplementary Fig. 53). The peaks were identified to originate from the AZO-EH by comparison to the solution without the AZO-EH present. When irradiated in a second irradiation experiment with 730 nm for in total of 10 min, while a 1H NMR spectrum was recorded after various time steps, and the changes were seen to be reversible (Supplementary Figs. 58–62). The color change is also reversible (Supplementary Fig. 58).
Preparation of PF-127-PAI-ZnPc1 micelles solution
2.5 µL of ZnPc1 (317.5 µM in toluene) and 0.005 g of PF-127 dissolved in toluene were evaporated under N2 atmosphere in a glass vial. The residual mixture was treated with 250 µL of PAI solution in phosphate buffer pH. 7.4, followed by stirring in an ice bath to dissolve PF-127. The resulting solution was brought to room temperature for the photoswitching measurements in a 0.1 cm pathlength quartz cuvette. The final measurement concentrations were as follows: AZO-EH = 50 µmol L−1, ZnPc1 = 3 µmol L−1 and PF-127 = 2%.
Animal housing
Albino Xenopus laevis (Nasco Ltd) ref. S7 embryos were obtained by natural mating and sustained until 3-4 days post-fertilization (dpf) in 0.1X Marc’s modified Ringer’s (MMR) solution within a dark incubator set at 24 °C. Subsequently, the tadpoles were relocated to tanks containing Xenopus water, prepared by dissolving 8 g of Instant Ocean salt (Instant Ocean) in 20 L of distilled water. Tadpoles were maintained at a density of 20–30 animals per litre, at a constant temperature of 24 °C, and were fed spirulina daily. All procedures adhered to the ethical standards set by the ethical commission of the University of Barcelona. Xenopus tropicalis tadpoles, corresponding to developmental stages 44–48 according to Nieuwkoop and Faber ref. S8 were immobilized in 0.23 mM Pancuronium dibromide solution (PCD) (Merck, Cat no. P1918) for 10 min due to its higher cardiac tolerance and non-UV dependent effects ref. S10 before being transferred to a custom chamber containing 150 μL of 0.1x Modified Barth’s Saline (MBS) prepared by 10x stock containing 800 mM NaCl, 10 mM KCl, 10 mM MgSO4·7H2O, 50 mM HEPES free acid, 25 mM NaHCO3; adjust the pH to 7.8 with NaOH and diluted in milli-Q water ref. S11.
Characterization techniques
Scanning electron microscopy
The cross-section scanning electron microscopy (SEM) images of the G-TXr-AZO-EH film were obtained using JEOL JSM-7800F Prime FEG SEM. The acceleration voltage is set to 10 kV, and a secondary electron detector is used. Before SEM observation, the sample is dried under vacuum for two days and coated with gold using the Edwards S150B gold sputter. The cross-section is obtained by breaking the film in half with hands.
X-ray diffraction measurements
X-ray diffractograms of the G-TXr-AZO-EH film is measured using Bruker the D8 DISCOVER is an X-ray diffraction instrument equipped with four motorized axes stage, having as typical applications: XR Reflectometry, Rocking measurements, RSM measurements and structural phase identification.
UV-Vis spectroscopy measurements
UV-Vis measurements of the chromophores solution and solid sample is done using a UV-Vis-NIR spectrophotometer Jasco V-770 with operational range of 190–3300 nm and FLS980 (Edinburgh Instruments). Samples were excited with at 365, 340, 455, 730, and 850 nm LED Thorlabs integrated with collimator and 340 nm UV-lamp, and 850 nm laser. LED emission power is measured with a standard Photodiode Power Sensor, Thor-Labs S120VC. Solution state spectra were recorded in a quartz cuvette of 1 cm to 0.1 cm path length.
Fluorescence measurements
The steady-state FL emission spectra were measured using a back-thinned CCD spectrometer PMA-12 (Hamamatsu). Phosphorescence was measured with a time-gated iCCD camera, New iStar DH340T (Andor), after exciting the samples with the emission of a tunable-wavelength optical amplifier (Ekspla) pumped by a nanosecond Nd3+ :YAG laser (wavelength – 850 nm, pulse duration – 5 ns, repetition rate – 1 kHz).
1H NMR measurements
The 1H NMR spectra were recorded using a Bruker NMR Ascend 400 of 400 MHz equipped with two probes for a liquid and solid samples.
Cardiac activity measurements
Video recordings of tadpole hearts were conducted using a microscope equipped with an OptixCam Summit Series OCS-D3K2-5 camera that can capture more than 30 frames per second to resolve the ~ 1 Hz tadpole heartbeats. The recordings were acquired using ToupLite software, which enhances visual contrast to improve cardiac imaging, and the video streams were converted to the AVI format12 recordings were briefly paused during compound addition and changes in illumination conditions. Video information and data analysis were extracted and executed with custom scripts based on ImageJ13 for AVI file analysis and subsequently converted to csv format for further analysis ref. S12.
The illumination protocol established for control and treatment video recordings followed the procedure described below. LED irradiance values were measured by a Thorlabs PM101/PD TH optical power meter equipped with a Thorlabs S120VC sensor with a diameter of 9.5 mm. A three-minute dim light period (0.26 mW cm−2) is allocated solely for imaging to establish baseline activity, followed by a 10 min incubation of 30 μM PAI (with or without 10 μM ZnPc1, encapsulated in 2 % PF127 in 0.1X MBS) pre-irradiated under UV (365 nm 11 mW cm−2) for 5 min to allow for enough trans-to-cis isomerization. Subsequently, 10 min 730 nm illumination (42 mW cm−2) with a Thorlabs M730L5 LED is applied for three cycles to achieve trans-to-cis isomerization, with 2-min intervals of waiting period under dim light between each cycle.
Tadpoles (n = 6) were initially paralyzed with PCD as described earlier, placed in MBS, and video recorded under the established illumination protocol in the absence of PAI and ZnPc1 combination to assess the effect of light alone on cardiac activity and to verify control conditions. Subsequently, each tadpole is exposed to a 30 μM PAI with 10 μM ZnPc1 (n = 3) and without ZnPc1 (n = 3) in MBS with 2% PF127 micelles and is subjected to the same illumination protocol to observe the light-dependent effects of the drug alone. One additional animal is tested to evaluate the effect of 10 μM ZnPc1 alone in MBS with 2% PF127 without illumination cycles.
Quantification of the experiments in panels (f, g) in Fig. 6 main manuscript is performed by calculating the amplitude and frequency of cardiac contraction as a function of time and illumination in the presence and in the absence of ZnPc1 (black and white bars, respectively). The results were obtained in 5 different animals (n = 2 control tadpoles without ZnPc1, n = 3 tadpoles treated with ZnPc1) and analyzed statistically using unpaired t tests with data from experiments with inactive PAI + ZnPc1 versus inactive PAI without ZnPc1 as the main factors, along with baseline measurements, compound application, and consecutive 730 nm illumination cycles as their interactions.
Data analysis and statistics
The videos capturing cardiac movement were processed to generate sinusoidal signals representing the grayscale intensity distribution within a selected region of interest (ROI) Fig. 6c (manuscript). This allowed recording tadpole cardiac movements and quantification of contraction amplitude and frequency over time (Fig. 6d, e and Supplementary Figs. 63 and 64). The amplitude of contraction data is extracted from the signal peaks occurring in 15 s intervals, after filtering out excessive noise to identify actual beating events. This involved identifying maximum peaks with high standard deviation within adjacent peaks, the range of which is determined empirically by examining the baseline dataset for each experiment. In addition, cardiac frequency is calculated based on the number of real beating events observed within every 15 s. Both the time course of cardiac parameters (amplitude of contraction and cardiac frequency) is plotted as a function of time (Fig. 6f, g and Supplementary Fig. 65).
Statistical analyses were conducted using an unpaired t test, incorporating data from experiments involving inactive PAI + ZnPc1 and inactive PAI without ZnPc1 as main factors, along with baseline measurements, compound applications, and 730 nm illumination cycles as their interactions ref. S13.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data that support the findings of this study are presented in the manuscript and Supplementary Information, or are available from the corresponding author upon request. Source data are also provided in this paper.
References
Liu, Q., Feng, W., Yang, T., Yi, T. & Li, F. Upconversion luminescence imaging of cells and small animals. Nat. Protoc. 8, 2033–2044 (2013).
Jewell, M. P., Greer, M. D., Dailey, A. L. & Cash, K. J. Triplet-triplet annihilation upconversion based nanosensors for fluorescence detection of potassium. ACS Sens. 5, 474–480 (2020).
Wang, Y. & Kohane, D. S. External triggering and triggered targeting strategies for drug delivery. Nat. Rev. Mater. 2, 17020 (2017).
Yang, D. et al. Current advances in lanthanide ion (Ln3+)-based upconversion nanomaterials for drug delivery. Chem. Soc. Rev. 44, 1416–1448 (2015).
Chen, S. et al. Near-infrared deep brain stimulation via upconversion nanoparticle–mediated optogenetics. Science 359, 679–684 (2018).
Bruegmann, t et al. Optogenetic defibrillation terminates ventricular arrhythmia in mouse hearts and human simulations. J. Clin. Invest. 126, 3894–3904 (2016).
Crocini, C. et al. Optogenetics design of mechanistically based stimulation patterns for cardiac defibrillation. Sci. Rep. 35628, 1–7 (2016).
Swamy, P. C. A. et al. Near Infrared (NIR) absorbing dyes as promising photosensitizer for photo dynamic therapy. Coord. Chem. Rev. 411, 213233 (2020).
Lo, P.-C. et al. The unique features and promises of phthalocyanines as advanced photosensitisers for photodynamic therapy of cancer. Chem. Soc. Rev. 49, 1041–1056 (2020).
Dąbrowski, J. M. et al. Engineering of relevant photodynamic processes through structural modifications of metallotetrapyrrolic photosensitizers. Coord. Chem. Rev. 325, 67–101 (2016).
Vickerman, B. M., Zywot, E. M., Tarrant, T. K. & Lawrence, D. S. Taking phototherapeutics from concept to clinical launch. Nat. Rev. Chem. 5, 816–834 (2021).
Klán, P. et al. Photoremovable protecting groups in chemistry and biology: reaction mechanisms and efficacy. Chem. Rev. 113, 119–191 (2013).
Castagna, R., Maleeva, G., Pirovano, D., Matera, C. & Gorostiza, P. Donor–acceptor stenhouse adduct displaying reversible photoswitching in water and neuronal activity. J. Am. Chem. Soc. 144, 15595–15602 (2022).
Samanta, S. et al. Photoswitching azo compounds in vivo with red light. J. Am. Chem. Soc. 135, 9777–9784 (2013).
Riefolo, F. et al. Optical control of cardiac function with a photoswitchable muscarinic agonist. J. Am. Chem. Soc. 141, 7628–7636 (2019).
Garrido-Charles, A. et al. Fast photoswitchable molecular prosthetics control neuronal activity in the cochlea. J. Am. Chem. Soc. 144, 9229–9239 (2022).
Boyer, J.-C., Carling, C.-J., Gates, B. D. & Branda, N. R. Two-way photoswitching using one type of near-infrared light, upconverting nanoparticles, and changing only the light intensity. J. Am. Chem. Soc. 132, 15766–15772 (2010).
Beharry, A. A. & Woolley, G. A. Azobenzene photoswitches for biomolecules. Chem. Soc. Rev. 40, 4422–4437 (2011).
Vepřek, N. A. et al. Optical control of G-Actin with a photoswitchable latrunculin. J. Am. Chem. Soc. 146, 8895–8903 (2024).
Dolai, A. et al. Photoisomerization and light-controlled antibacterial activity of fluoroquinolone-azoisoxazole hybrids. ChemBioChem25, e202300851 (2024).
Xiao, D. et al. Azobenzene-based linker strategy for selective activation of antibody–drug conjugates. Angew. Chem. Int. Ed. 63, e202310318 (2024).
Volarić, J. et al. Visible light control over the cytolytic activity of a toxic pore-forming protein. ACS Chem. Biol. 19, 451–461 (2024).
Griffiths, J. Photochemistry of azobenzene and its derivatives. Chem. Soc. Rev. 1, 481–493 (1972).
Dong, M. et al. Near Infrared photoswitching of azobenzenes under physiological conditions. J. Am. Chem. Soc. 139, 13483–13486 (2017).
Dong, M., Babalhavaeji, A., Samanta, S., Beharry, A. A. & Woolley, G. A. Red-shifting azobenzene photoswitches for in vivo use. Acc. Chem. Res. 48, 2662–2670 (2015).
Mandl, G. A., Rojas-Guterrez, P. A. & Capobianco, J. A. A NIR-responsive azobenzene-based supramolecular hydrogel using upconverting nanoparticles. Chem. Commun. 54, 5847–5850 (2018).
Wu, W. et al. NIR-light-induced deformation of cross-linked liquid-crystal polymers using upconversion nanophosphors. J. Am. Chem. Soc. 133, 15810–15813 (2011).
Jiang, Z., Xu, M., Li, F. & Yu, Y. Red-light-controllable liquid-crystal soft actuators via low-power excited upconversion based on triplet–triplet annihilation. J. Am. Chem. Soc. 135, 16446–16453 (2013).
Cabré, G. et al. Rationally designed azobenzene photoswitches for efficient two-photon neuronal excitation. Nat. Commun. 10, 907 (2019).
Izquierdo-Serra, M. et al. Two-photon neuronal and astrocytic stimulation with azobenzene-based photoswitches. J. Am. Chem. Soc. 136, 8693–8701 (2014).
Jerca, F. A., Jerca, V. V. & Hoogenboom, R. Advances and opportunities in the exciting world of azobenzenes. Nat. Rev. Chem. 6, 51–69 (2022).
Ye, C., Zhou, L., Wang, X. & Liang, Z. Photon upconversion: from two-photon absorption (TPA) to triplet–triplet annihilation (TTA). Phys. Chem. Chem. Phys. 18, 10818–10835 (2016).
Sortino, R. et al. Three-Photon Infrared Stimulation of Endogenous Neuroreceptors in vivo. Angew. Chem. Int. Ed. Engl. 62, e202311181 (2023).
Zhou, B., Shi, B., Jin, D. & Liu, X. Controlling upconversion nanocrystals for emerging applications. Nat. Nanotechnol. 10, 924–936 (2015).
Yang, Y. et al. Switching the NIR upconversion of nanoparticles for the orthogonal activation of photoacoustic imaging and phototherapy. Nat. Commun. 13, 3149 (2022).
Sanders, S. N. et al. Triplet fusion upconversion nanocapsules for volumetric 3D printing. Nature 604, 474–478 (2022).
Bharmoria, P., Bildirir, H. & Moth-Poulsen, K. Triplet-triplet annihilation based near infrared to visible molecular photon upconversion. Chem. Soc. Rev. 49, 6529–6554 (2020).
Alachouzos, G., Schulte, A. M., Mondal, A., Szymanski, W. & Feringa, B. L. Computational design, synthesis, and photochemistry of Cy7-PPG, an efficient NIR-activated photolabile protecting group for therapeutic applications. Angew. Chem. Int. Ed. Engl. 61, e202201308 (2022).
Gemen, J. et al. Disequilibrating azobenzenes by visible-light sensitization under confinement. Science 381, 1357–1363 (2023).
Isokuortti, J. et al. Expanding excitation wavelengths for azobenzene photoswitching into the near-infrared range via endothermic triplet energy transfer. Chem. Sci. 12, 7504–7509 (2021).
Bharmoria, P. et al. Far-red triplet sensitized Z-to-E photoswitching of azobenzene in bioplastics. Chem. Sci. 13, 11904–11911 (2022).
Isokuortti, J. et al. Triplet sensitization enables bidirectional isomerization of diazocine with 130 nm redshift in excitation wavelengths. Chem. Sci. 14, 9161–9166 (2023).
Monti, S., Gardini, E., Bortolus, P. & Amouyal, E. The triplet state of azobenzene. Chem. Phys. Lett. 77, 115–119 (1981).
Bandara, H. M. D. & Burdette, S. C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 41, 1809–1825 (2012).
Pitruzzello, G. Seeing into deep tissue. Nat. Photon. 17, 376–377 (2023).
Tobin, D. J. Biochemistry of human skin-our brain on the outside. Chem. Soc. Rev. 35, 52–67 (2006).
Masutani, K., Morikawa, M. -a & Kimizuka, N. A liquid azobenzene derivative as a solvent-free solar thermal fuel. Chem. Commun. 50, 15803–15806 (2014).
Pucelik, B., Sułek, A. & Dąbrowski, J. M. Bacteriochlorins and their metal complexes as NIR-absorbing photosensitizers: properties, mechanisms, and applications. Coord. Chem. Rev. 416, 213340 (2020).
Tran, V.-P. et al. Bacteriochlorin Syntheses – Status, Problems, and Exploration. J. Porphyr. Phthalocyanines 27, 1502–1551 (2020).
Vairaprakash, P. et al. Extending the short and long wavelength limits of bacteriochlorin near-infrared absorption via dioxo- and bisimide-functionalization. J. Phys. Chem. B 119, 4382–4395 (2015).
Takiff, L. & Boxer, S. G. Phosphorescence spectra of bacteriochlorophylls. J. Am. Chem. Soc. 110, 4425–4426 (1988).
Topal, S. Z. et al. Modulation of the electronic and spectroscopic properties of Zn(II) phthalocyanines by their substitution pattern. Dalton Trans. 43, 6897–6908 (2014).
Tobolskv, A. V. Cross-linking of gelatine by dehydration. Nature 215, 509–510 (1967).
Farcet, J.-B., Kindermann, J., Karbiener, M. & Kreil, T. R. Development of a Triton X-100 replacement for effective virus inactivation in biotechnology processes. Eng. Rep. 1, e12078 (2019).
Bharmoria, P. et al. Recyclable optical bioplastics platform for solid state red light harvesting via triplet–triplet annihilation photon upconversion. J. Mater. Chem. A 10, 21279–21290 (2022).
Holmes, D. F., Lu, Y., Starborg, T. & Kadler, K. E. Collagen fibril assembly and function. Curr. Top. Dev. Biol. 130, 107–142 (2018).
Isokuortti, J. et al. Endothermic and exothermic energy transfer made equally efficient for triplet−triplet annihilation upconversion. J. Phys. Chem. Lett. 11, 318–324 (2020).
Goulet-Hanssens, A. et al. Electrocatalytic Z → E isomerization of azobenzenes. J. Am. Chem. Soc. 139, 335–341 (2017).
Sandros, K. & Bäckström, H. L. J. Transfer of Triplet-state energies in the fluid solutions. Acta Chem. Scand. 16, 958–968 (1962).
Sandros, K. Transfer of triplet-state energies in the fluid solutions. III reversible energy transfer. Acta Chem. Scand. 16, 2355–2374 (1964).
Smith, M. H. Safe delivery of optical power from space. Opt. Express 8, 537–546 (2001).
Akash, M. S. H. & Rehman, K. Recent progress in biomedical applications of Pluronic (PF127): Pharmaceutical perspectives. J. Control Release 209, 120–138 (2015).
Carroll, E. C. et al. Two-photon brightness of azobenzene photoswitches designed for glutamate receptor optogenetics. PNAS 112, E776–E785 (2015).
Pittolo, S. et al. Reversible silencing of endogenous receptors in intact brain tissue using 2-photon pharmacology. PNAS 116, 13680–13689 (2019).
Kellner, S. & Berlin, S. Two-photon excitation of azobenzene photoswitches for synthetic optogenetics. Appl. Sci. 10, 805 (2020).
Zhao, J. & Ellis-Davies, G. C. Intracellular photoswitchable neuropharmacology driven by luminescence from upconverting nanoparticles. Chem. Commun. 56, 9445–9448 (2020).
Fehrentz, T. et al. Optical control of cardiac electrophysiology by thephotochromic ligand azobupivacaine 2. Br. J. Pharm. 182, 1125–1142 (2025).
Baumgartner, B. et al. A general method for near-infrared photoswitching in biology, demonstrated by the >700 nm photocontrol of GPCR activity in brain slices. Preprint at https://doi.org/10.26434/chemrxiv-2024-vm4n3 (2024).
Acknowledgements
This work is developed within the scope of the La-Caixa junior research leadership-post doctoral project PHOLCEB (ID: 100010434, fellowship code: LCF/BQ/P122/11910023). P.B. and M.M. acknowledge La-Caixa foundation, the State Investigation Agency, through the Severo Ochoa Program for Centers of Excellence in R&D (CEX2023-001263-S) and project PID2021-123873NB-I00 for financial support. K.M.P. acknowledges funding from the European Research Council (No. 101002131), the Swedish Energy Agency, the Göran Gustafsson Foundation, the Swedish Research Council, Swedish Research Council Formas, the European Research Council (ERC) under grant agreement CoG, PHOTHERM –101002131, the Catalan Institute of Advanced Studies (ICREA) and the European Union’s Horizon 2020 Framework Program under grant agreement no. 951801. L.N. acknowledges the Erasmus + Traineeship Program. M.D., J.L., and K.K. acknowledge the “Universities‘ Excellence Initiative” program by the Ministry of Education, Science and Sports of the Republic of Lithuania under the agreement with the Research Council of Lithuania (project No. S-A-UEI-23-6). P.G. acknowledges EU Horizon 2020 Framework Program for Research and Innovation, including the European Innovation Council Pathfinder Open (Phototheraport, 101130883), Human Brain Project (WaveScalES, SGA3, 945539), and Information and Communication Technologies (Deeper, ICT-36-2020- 101016787). It is also supported by the Government of Catalonia (CERCA Program; AGAUR 2021-SGR-01410) and by the Spanish Ministry of Science and Innovation (DEEP RED, grant PID2019-111493RB-I00; EPILLUM, grant AEI/10.13039/501100011033; Research Network in Biomedicine eBrains-Spain, RED2022-134823-E). J.S.L. acknowledges support from the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences and Biosciences Division, under Award Number DE-FG0205ER15661. Galyna Malieieva, Núria Camarero (Institute for Bioengineering of Catalonia), and Javier Palacios Sánchez (Parc Científic de Barcelona) are acknowledged for help with Xenopus ethical procedures, mating and manipulation, which were authorized by the Barcelona Biomedical Research Park (PRBB) Animal Research Ethics Committee and the Catalan Government (code #9891), and by Parc Científic de Barcelona (CEEA-PCB 21-036). Nuria Aliaga-Alcalde and Daniel Herrera Miranda are acknowledged for performing electrocatalytic measurements.
Author information
Authors and Affiliations
Contributions
P.B., L.N., K.M.P., and P.G. conceptualized the idea of this work. L.N., P.B., and M.M. carried out the photoswitching studies in different materials. M.D., J.L., E.R., and K.K. carried out photoluminescence emission and quenching studies. P.G. group (E.O., P.B., V.C.M., F.R., C.M.) synthesized PAI and carried out in vivo studies in frog tadpoles. H.H. synthesized AZO-MM and carried out photoswitching studies using NMR spectroscopy, M.-a-M. and N.K. synthesized AZO-EH, K.H. and F.D. synthesized ZnPc1, and M.T. and J.S.L. synthesized BC1. P.B., K.M.P., E.O., and P.G. contributed to writing the first draft of the manuscript. M.T., J.S.L. and F.D. edited the first draft of the manuscript. All authors contributed to revise the final draft of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Naimovičius, L., Miroshnichenko, M., Opar, E. et al. Noninvasive cardiac modulation via triplet-sensitized photoswitching in the phototherapeutic window. Nat Commun 16, 6377 (2025). https://doi.org/10.1038/s41467-025-61301-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-61301-3