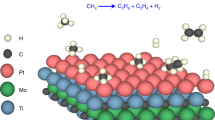
Abstract
A key challenge in heterogeneous catalysis is to design atomically dispersed catalysts with high surface density, while simultaneously preventing agglomeration and promoting electronic metal-support interaction. Transition metal dichalcogenides (TMDs), such as platinum diselenide (PtSe2), offer a promising solution due to their unique structural and electronic properties. This study proposes a catalyst design that utilizes atomically dispersed transition metal species within the topmost layer of TMD as catalytic reaction sites. The substantial presence of surface-exposed Pt species on PtSe2 and their role as catalytic reaction sites are elucidated using operando ambient-pressure X-ray photoelectron spectroscopy. Moreover, significantly high O2 coverage on PtSe2, achieved by mitigating the exclusive adsorption of carbon monoxide (CO), leads to enhanced CO oxidation performance. The characteristic d-band structure and resulting high O2 coverage of PtSe2 are further confirmed with density functional theory calculations. Overall, this study highlights the potential of densely distributed atomic transition metal on TMDs, which allows electronic metal-chalcogen interactions and diverse reaction mechanisms.
Similar content being viewed by others
Introduction
Designing a catalyst to intensify performance at each reaction site is a fundamental challenge in heterogeneous catalysis. This efforts has led to a reduction in the size of metal catalysts to increase surface free energy and expose a greater number of reaction sites on the surface, eventually resulting in the development of atomic catalysts1,2. Moreover, reducing the size to the atomic level enables metal catalysts to facilitate charge transfer through interaction with supporting materials3,4. This electronic metal-support interaction (EMSI) significantly alters the charge distribution of atomic catalysts and manipulates catalytic properties, such as binding energies of distinct adsorbates and activation energies, thereby enhancing catalytic performances5,6,7,8. However, designing atomically dispersed catalysts with high-density remains challenging, as metal atoms are prone to agglomeration and restructuring into nanoclusters during synthesis and reaction due to their high surface free energy9,10,11. As a result, excessive distance between metal atoms limits the ability to follow diverse reaction mechanisms and reduces overall catalytic activity12,13,14.
In this study, we propose a unique approach to address the issue of dense distribution by utilizing the metal element composing the transition metal dichalcogenide (TMD) as reaction sites in a solid-gas system. In heterogeneous catalysis, research on TMDs has been mainly focused on their capability as catalyst support, which effectively stabilizes atomic catalysts15,16,17,18. However, employing TMDs themselves as a catalyst allows us to utilize the densely distributed metal atoms. In the case of PtSe2, surface-exposed Pt atoms, formed due to Se vacancies, can interact with reactants and act as catalytic reaction sites19. Mono- or few-layer PtSe2 with Se-vacancy can be synthesized by selenizing Pt under a high vacuum or H2/Ar gas flow20,21. Since Se-vacancy has a low formation energy over a wide range of Se chemical potential, the formation of PtSe2 with substantial exposure of Pt atoms on the surface is likely22. In this configuration, Pt atoms form somewhat looser arrangements than those in Pt(111). Nevertheless, since Pt atoms are not too far apart, adsorbates can adsorb on various exposed Pt sites, such as atop, bridge, and hollow sites. Moreover, catalytic reactions can follow various catalytic mechanisms, such as the Langmuir-Hinshelwood (LH) mechanism, due to the interaction between adsorbates adsorbed on adjacent Pt sites.
The TMD catalyst also allows us to manipulate the electronic structure of the atomic catalyst through charge transfer between metal and chalcogen, thereby enhancing catalytic performance. Regarding the carbon monoxide (CO) oxidation reaction on Pt as a representative, there are difficulties due to the differences in binding energy between CO and O223,24. For optimal catalytic activity, CO and O2 should be adsorbed on the surface of platinum in adequate proportions. However, the binding energies of CO and O2 on the Pt(111) are −1.77 eV and −1.10 eV, respectively, making the adsorption of CO significantly stronger than O2. Consequently, the exclusive adsorption of CO inhibits the O2 adsorption and reduces catalytic activity while following the LH mechanism, known as the CO poisoning effect25,26. Nevertheless, by utilizing Pt atoms in PtSe2 as a catalyst, we can modulate the d-band center (εd) through Pt-Se charge transfer. According to d-band theory, the binding energy of adsorbate is closely related to the extent to which electrons are filled in the antibonding state of the metal-adsorbate bond. When the εd is lowered, the antibonding state also decreases, allowing more electrons to be filled and weakening the binding energy27. Therefore, the electronic metal-chalcogen interaction enables us to weaken the binding energy of CO significantly. The manipulation of the binding energy of CO and O2 induces resistance to the CO poisoning and highly increases the coverage of O2, thereby promoting rapid interaction between them.
Considering these factors, we showcase the potential of PtSe2 as a heterogeneous catalyst in a solid-gas system by utilizing atomically dispersed Pt as an active catalytic site under the CO oxidation reaction. Using operando ambient-pressure X-ray photoelectron spectroscopy (AP-XPS), we identified the sufficient exposure of Pt on the surface (i.e., Se-vacancy) and its function as catalytic reaction sites. Moreover, we elucidated the electronic metal-chalcogen interaction between Pt and Se and its role on CO oxidation through density functional theory (DFT) calculations. Subsequent experimental and theoretical comparisons with Pt(111) elucidate the superior catalytic performance of PtSe2 due to its significantly high O2 coverage and densely distributed Pt atoms on the surface.
Results
Synthesis and characterization of high-quality PtSe2
To synthesize PtSe2 multilayer, Pt film with a thickness of 0.75 nm was deposited on a SiO2/Si substrate using an e-beam evaporator. Subsequently, Pt film was selenized via a chemical vapor deposition (CVD) method. The synthesized PtSe2 showed a 1T-hexagonal structure, which can be differentiated into Se-Pt-Se sublayers, as shown in Fig. 1a28. The structure of PtSe2 was characterized by cross-sectional high-angle annular dark-field scanning transmission electron microscopy (HADDF-STEM) and bright-field high-resolution transmission electron microscopy (HRTEM). The STEM image in Fig. 1b shows the atomically dispersed nature of Pt with appropriate distance for catalytic reaction requiring multiple adsorption sites while supported by Se atoms in the upper and lower sublayers. The corresponding energy-dispersive X-ray spectroscopy (EDS) was performed to show the elemental distribution of synthesized PtSe2 (Supplementary Fig. 1)29. The HRTEM image in Fig. 1c shows that the multilayer PtSe2 is well resolved to parallel the underlying SiO2 substrate. The van der Waals (vdW) gap was observed via the corresponding fast Fourier transform (FFT) pattern with a spacing of ~0.55 nm, consistent with the previously reported interlayer spacing of PtSe2 along the (001) direction20,30.
a Geometric scheme of PtSe2 unit cell with top-view and side-view of PtSe2 layer. b Cross-sectional HAADF-STEM image of PtSe2 deposited on the SiO2/Si film. c Cross-sectional HRTEM image and corresponding FFT pattern of PtSe2. d Pt L3 edge XAS spectra and e corresponding FT-EXAFS spectra of PtSe2, with Pt and PtO2 as references.
The bonding characteristics and electronic structure of PtSe2 were investigated using Pt L3 edge X-ray absorption spectroscopy (XAS), in comparison with the spectra of metallic Pt and PtO2. The XAS data were normalized using the signal from a standard Pt foil. In the X-ray absorption near-edge structure (XANES) spectrum, the whiteline intensity of PtSe2 was measured to be 1.66, which lies between that of metallic Pt (1.29) and PtO2 (2.02) (Fig. 1d). The L3 edge peak position of PtSe2 (11567.5 eV) also appeared at a slightly higher photon energy than that of metallic Pt (11567.1 eV), but lower than that of PtO2 (11568.1 eV). The increase in whiteline intensity is known to indicate a higher number of unoccupied 5d states, whereas the peak shift reflects an increased energy separation between the 2p3/2 core level and the 5d orbitals31,32. Together, these observations indicate that the Pt atoms in PtSe2 are moderately oxidized, which enables fine-tuning of the binding energies of gas molecules adsorbed on Pt sites33.
To further investigate the local coordination environment of Pt in PtSe2, extended X-ray absorption fine structure (EXAFS) analysis was performed (Fig. 1e). The EXAFS spectra were obtained by k3-weighted Fourier transform (FT) over a k-range of 3.0 to 12.0 Å−1. The PtSe2 spectra exhibited a single prominent peak at 2.16 Å, corresponding to the Pt-Se bond. In addition, comparison with the spectra from metallic Pt revealed that peaks corresponding to Pt-Pt bonds (2.35 and 2.93 Å) were not observed in the PtSe2 spectra. These results indicate that the catalytic performance of PtSe2 does not originate from undesired bonding or phases such as Pt nanoclusters, but rather from surface-exposed Pt atoms at the Se vacancies in the topmost Se sublayer19,34.
CO oxidation properties and structural stability
The catalytic performance of the PtSe2 was evaluated in an ultrahigh vacuum (UHV) batch reactor under the CO oxidation reaction35. As a reference sample, Pt film with a thickness of 20 nm was also prepared using an e-beam evaporator. We performed the CO oxidation reaction as a model reaction in the batch reactor from 180 °C to 220 °C under 40 Torr of CO, 100 Torr of O2, and 620 Torr of He to match the total pressure to the atmospheric pressure. Catalytic activities of PtSe2 and Pt film were evaluated with turnover frequency (TOF) as a function of temperature (Fig. 2a). As a result, PtSe2 showed higher catalytic activity compared to Pt over the entire temperature range, particularly 3.26 times higher from 92.4 ± 0.4 to 301.1 ± 19.3 at 220 °C. The average TOF values were replotted on the logarithmic-Arrhenius scale, as shown in Fig. 2b. The activation energies (Ea), derived from the slopes of the Arrhenius plots, were 19.3 and 17.8 kcal/mol for PtSe2 and Pt, respectively. These Ea values show only marginal differences compared to the variation in TOF, suggesting that Ea value is not the primary factor influencing the differences in catalytic performance. The similar Ea values also suggest that the reaction mechanism on PtSe2 might be similar to that on Pt. It is well known that CO oxidation on Pt follows the LH mechanism, indicating that CO oxidation on PtSe2 likely involves two or more adjacent Pt sites23. To further verify the stable catalytic performance of PtSe2, we conducted repeated tests that yielded consistent TOF values (51.7 ± 0.3 at 180 °C) and Ea values (19.3 ± 1.1 kcal/mol) based on Arrhenius plots (Supplementary Figs. 2a, b and 3). In the separate long-term test, the TOF remained stable over 10 hours, further confirming the durability of PtSe2 (Supplementary Fig. 2c, d).
a TOF plots and b Arrhenius plots at elevated temperatures from 180 °C to 220 °C. Values (unit in kcal/mol) on the Arrhenius plot indicate the activation energy (Ea) of the CO oxidation reaction with each sample. Error bars represent standard deviations derived from the mean values of the 1st and 2nd experiments. c Kinetic study of the CO oxidation reaction as a function of the CO partial pressure with 100 Torr of O2 at 210 °C. d XRD characterization of PtSe2 before and after the CO oxidation reaction. e Raman profile and f area ratio of Eg (~179 cm−1) and A1g (~207 cm−1) peaks before and after the CO oxidation reaction, calculated based on the Raman mapping data covering the 5 × 5 μm area represented in Supplementary Fig. 5.
Moreover, TOF of PtSe2 and Pt film were evaluated while changing the CO partial pressure, with the O2 partial pressure fixed at 100 Torr (Fig. 2c). The TOF of PtSe2 showed a slight decrease with increasing CO partial pressure, whereas the TOF of Pt decreased rapidly. This trend arises as the adsorption of O2 becomes more difficult with the increasing partial pressure of CO, thereby reducing the likelihood of CO reacting with the adsorbed oxygen24. Therefore, the gradual decrease trend of PtSe2 verifies its high resistance to CO poisoning and implies that the binding energy of O2 is similar to that of CO. Since the binding energy of adsorbate is closely related to the charge transfer through a metal-chalcogen bond, it can be inferred that the binding energies of CO and O2 on PtSe2 were affected by the charge transfer through the Pt-Se bond5. Investigations on the charge transfer and surface coverage of reactant molecules will be explained further with DFT calculations.
To analyze possible structural variations and their impact on catalytic activity, structural characterizations of PtSe2 were conducted. The crystal structure of PtSe2 was explored by X-ray diffraction (XRD) at the macroscale. As shown in Fig. 2d, a distinctive (001) diffraction peak of the layered PtSe2 (JCPDS PDF#: 01-088-2266) was observed, manifesting that PtSe2 was grown along the c-direction without any other structures36. The detectable structural variations were rarely observed in either XRD or cross-sectional HRTEM, indicating that vdW layered structure still exists without significant deformation after the reaction (Supplementary Fig. 4a). Accordingly, EDS analysis exhibits that the ratio of Se to Pt is similar before and after the reaction with a change of less than 0.2 % (Supplementary Fig. 4b). As shown in Fig. 2e, two dominant phonon modes were detected by Raman spectroscopy: Eg (~179 cm−1) and A1g (~207 cm−1) corresponding to the in-plane and out-of-plane vibration motions of top and bottom Se atoms inside a layer, respectively. Meanwhile, the intensity ratio between Eg and A1g is around 4.1:1 (Fig. 2f, Supplementary Fig. 5). The peak positions and the intensity ratio of A1g/Eg, similar to previous reports, indicating that vdW layer structure of PtSe2 was horizontally established with high-quality20,37,38. Also, neither Raman spectroscopy nor the distribution map for the intensity ratio of A1g/Eg reveals any meaningful variations.
Surface-exposed Pt and its role in CO oxidation
To identify the stability of atomically spread Pt species and their role in the CO oxidation reaction, we conducted an AP-XPS study in the synchrotron facility39. Considering the reaction conditions in Fig. 2, XPS was performed under four different environments: i) UHV at room temperature (RT), ii) near ambient pressure (NAP) at RT, iii) NAP at 200 °C, and iv) NAP at 250 °C. The NAP conditions were established with 0.3 mbar of CO and 0.7 mbar of O2, following the pressure ratio of the reactions conducted in the UHV batch reactor.
Figure 3a displays the XPS core spectra of Pt 4f obtained under the four different environments. The photon energy is set to 760 eV. Considering the asymmetric degree and the instrumental signal broadening error, the spectral envelope of Pt 4f can be attributed to the superposition of two different species, PtSe2 and PtSex, where the spin-orbit splitting values and the weight ratio between spin-orbit peaks are set to 3.33 eV and 4:3, respectively. Li et al. investigated defects of PtSe2 at the atomic scale and frequently found the presence of Se-vacancy on both the top and bottom Se sublayers in the PtSe2 layer22. Since the formation energy of Se-vacancy is calculated to be between 1.55 and 1.85 eV within the Se chemical potential range of −0.35 to 0 eV, the authors addressed the frequent occurrence of Se-vacancy due to its low formation energy over a wide range of Se chemical potential. Therefore, in our XPS result, PtSex 4f7/2 spectrum at 71.88 ± 0.03 eV is attributable to surface-exposed Pt species (i.e., Se-vacancy), while PtSe2 4f7/2 spectrum at 73.08 ± 0.03 eV corresponds to stoichiometric PtSe240. The spectral envelope of Se 3d spectra in Supplementary Fig. 6 can be similarly attributed to the superposition of PtSe2 at 54.28 ± 0.04 eV and PtSex at 54.71 ± 0.04 eV, with spin-orbit couple between 3d5/2 and 3d3/2. The spin-orbit splitting values and the weight ratio between spin-orbit peaks are set to 0.83 eV and 3:2, respectively. Detailed peak positions and area of Pt 4f and Se 3d spectra are organized in Supplementary Table 1.
a Pt 4f spectra under UHV-RT, NAP-RT, NAP-200 °C, NAP-250 °C using a photon energy of 760 eV. b The stoichiometric ratio of PtSe2 and PtSex spectrum under UHV-RT, NAP-RT, NAP-200 °C, NAP-250 °C using a photon energy of 760 eV. c Pt 4f spectra acquired using a photon energy of 240, 650, and 760 eV under NAP-200 °C. d The stoichiometric ratio of PtSe2 and PtSex spectrum in Pt 4f spectra acquired using a photon energy of 240, 650, and 760 eV under NAP-200 °C. e Reaction scheme of CO oxidation reaction on PtSe2 layer.
The stoichiometric ratio of the PtSex spectrum was 16.1 % at UHV-RT, 17.0% at NAP-RT, and gradually increased up to 22.1 % at NAP-250 °C (Fig. 3b). Se 3d spectra at the same environments also show a similar increasing trend. Several studies have reported that once mono Se-vacancy is sufficiently formed in PtSe2, the migration of Se-vacancy and formation of multi vacancies can be preferably formed due to their high diffusivity41,42,43. Therefore, a slight increase of PtSex spectrum during the reaction indicates that the adsorption of CO and O2 on the surface-exposed Pt, along with the subsequent catalytic reactions, stabilizes the Pt and induces migration of Se-vacancy. In addition, structural analysis and stability tests on the catalytic ability confirm that Se-vacancy migration does not lead to any structural deformation or catalytic activity degradations (Fig. 2d–f, Supplementary Figs. 2 and 3).
Then, the thickness dependency of Se-vacancy was investigated by manipulating the penetration depth of the photon beam. After fixing the beam spot at a specific point, we sequentially acquired core spectra of PtSe2 using three different photon energies (hν = 240, 650, and 760 eV) under NAP-200 °C (Fig. 3c). The relative area intensity between the PtSex and PtSe2 spectra exhibits a clear increase with decreasing photon energy, varying from 21.2 % at 760 eV to 28.8 % at 240 eV (Fig. 3d). The distinct variation indicates that Pt species corresponding to PtSex are predominantly present at the surface rather than in the sub-surface44. Specifically, by considering the electron attenuation length together with the variation in the area ratio between the PtSex and PtSe2 spectra, the Pt-to-Se ratio in the topmost layer was estimated to be 1:1.75 (± 0.05). Similar photon energy dependencies were observed for Pt 4f and Se 3d under all four different reaction conditions, corroborating that more Se-vacancy is mainly present at the surface (Supplementary Figs. 6 and 7). Therefore, it is inferred that a substantial fraction of Pt species is dispersed on the surface, serving as catalytically active sites (Fig. 3e).
Effect of Pt-Se charge transfer on the reaction mechanism
We performed DFT calculations to provide further insight into the improved activity of CO oxidation on PtSe2, referring to the AP-XPS analysis. Such AP-XPS results indicate that the PtSe2 layer has Se- and Pt-terminated adsorption sites. Therefore, we constructed two catalyst models: a Se-terminated PtSe2 referring to the pristine PtSe2 monolayer and a Pt-terminated PtSe2 by exfoliating the topmost Se layer of the pristine PtSe2 monolayer (Supplementary Fig. 8). The calculated binding energies (Ebind) of CO (−0.10 eV) and O2 (−0.11 eV) on Se-terminated PtSe2 indicate that Se sites cannot effectively bind the reactants (Supplementary Fig. 9). On the other hand, the Ebind of CO (−1.77 eV and −1.10 eV) and O2 (−0.85 eV and −1.06 eV) on Pt(111) and Pt-terminated PtSe2 indicate that Pt sites initiate CO oxidation (Fig. 4a). Since Se sites do not participate in CO oxidation, we used the Pt-terminated PtSe2 model to study the catalytic activity of the Pt sites of PtSe2.
a The difference between CO and O2 binding energy, ΔEbind. Surface coverage diagrams of CO and O2 on b Pt-terminated PtSe2 and c Pt(111) under p(CO): p(O2) = 0.05: 0.013 (atm). Projected DOS (pDOS) diagrams and the location of ɛd, COHP, and integrated COHP (ICOHP) of d Pt-terminated PtSe2 and e Pt(111), where Ef is the Fermi energy.
Even a slight difference of around 0.1 eV between the Ebind of CO and O2 may make a single adsorbate exclusively cover the catalyst surface45. In this manner, the large ΔEbind (ΔEbind = Ebind (CO) − Ebind (O2)) of Pt(111) (−0.92 eV) makes the Pt(111) poisoned by CO (Fig. 4c). On the other hand, the marginal ΔEbind of −0.04 eV calculated in Pt-terminated PtSe2 indicates that competitive adsorption between CO and O2 is available. The Langmuir adsorption isotherm in Fig. 4b shows that the O2 coverage of the Pt-terminated PtSe2 is about 20 % under the experimental CO oxidation condition. Our DFT calculation results suggest that the coverage issue accompanies the experimental findings in Fig. 2c, which elucidates the ability of PtSe2 to exhibit high TOF and resistance to the CO poisoning effect due to the sufficient adsorption of oxygen.
It is well known that the transition metal surface with the higher εd generally more strongly binds an adsorbate, keeping the antibonding states empty45. The partial density of states (DOS) diagrams of Pt(111) and Pt-terminated PtSe2 show that the εd decreases from −2.11 eV to −2.52 eV upon introducing Se, suggesting that the Pt-CO interaction in PtSe2 is relatively weak (Fig. 4d, e). Besides, the crystal orbital Hamilton population (COHP) analysis results also present the relatively prominent antibonding states near the Fermi level of Pt-terminated PtSe2, originating from downshifting of the 2\(\pi\)* and 5\(\sigma\)* orbitals of gas-phase CO upon adsorption (Supplementary Fig. 10). The increased integrated COHP value in Pt-terminated PtSe2 (−2.183) compared with Pt(111) (−2.432) indicates that the antibonding becomes dominant. Our DFT calculation results concurrently suggest that the modulated electronic structure of PtSe2 adjusts the adsorption trend of PtSe2 to be beneficial for facile CO oxidation.
The DFT-calculated energetics of the overall CO oxidation reaction via the LH mechanism on Pt-terminated PtSe2 and Pt(111) are presented in Fig. 5. The morphologies of the initial reaction stage, S0, are determined based on the equilibrium CO coverage (Supplementary Note 1). The morphologies on S1 to S3 show the formation of the O-C-O type intermediate by the interaction between CO and oxygen adsorbed on adjacent Pt sites, elucidating that densely distributed Pt sites on PtSe2 enable CO oxidation through the LH mechanism. The reactions on Pt-terminated PtSe2 and Pt(111) require 0.70 eV and 0.66 eV of activation energy barrier, respectively. These values reproduce the experimental apparent activation energy barriers presented in Fig. 2b. Subsequent CO2 production from the O-C-O intermediate is exothermic in both cases (ΔE = −0.19 eV in Pt-terminated PtSe2 and −0.83 eV in Pt(111), respectively). After the first oxidation cycle, the residual oxygen atom readily oxidizes the second CO molecule46,47.
Discussion
This study establishes atomically dispersed Pt on the surface of PtSe2 as a high-performance heterogeneous catalyst for CO oxidation, providing the first experimental and theoretical evidence of its activity in gas-phase reactions. The PtSe2 catalyst exhibited superior catalytic performance compared to a Pt film, which is attributed to the unique structural and electronic properties of the atomically dispersed Pt on PtSe2.
Operando AP-XPS analysis identified surface-exposed Pt atoms of PtSe2 and clarified their role as active sites. In addition, DFT calculations demonstrated that charge transfer within the Pt-Se bond modulates the d-band structure of Pt within PtSe2, resulting in comparable binding energies of CO and O2. Integrated analysis with CO partial pressure-dependent experiments confirmed the balanced adsorption behavior of CO and O2 on PtSe2. This behavior facilitates the adsorption of O2 and leads to significantly higher oxygen coverage on PtSe2 than on the Pt film, ultimately accelerating CO oxidation and enhancing catalytic performance. We also demonstrated that the dense distribution of Pt atoms on the PtSe2 surface significantly contributes to the observed catalytic enhancement. Arrhenius analysis and DFT calculations revealed that high-density reaction sites promote the CO oxidation reaction via the LH mechanism.
Overall, these findings reveal that the outstanding catalytic performance of PtSe2 stems from the synergistic interplay between electronic metal-chalcogen interactions and the high-density of atomically dispersed Pt sites, which collectively facilitate efficient O2 adsorption and promote favorable reaction pathways. Importantly, these characteristics are not exclusive to PtSe2 and can be generalized to other TMDs with varied metal-chalcogen pairings. Furthermore, the ability to tune adsorbate binding energies suggests broader applicability of this approach to a range of gas-phase reactions beyond CO oxidation.
Methods
Fabrication of PtSe2 multilayer and Pt film
PtSe2 multilayer was synthesized using a horizontal quartz tube furnace (Lindberg/Blue M Mini-Mite) through CVD. The Pt seed with a thickness of 0.75 nm was deposited on SiO2/Si wafers (~300 nm; SiO2 thickness). The deposition was conducted using an e-beam evaporator (Thermionics VE-100) with a deposition rate of 0.05 Å/s. After deposition, Pt seed on SiO2/Si substrate was placed in the central zone of the CVD furnace, while an alumina boat containing Se powder (99.99 %, Millipore Sigma) was positioned at the upstream side of the furnace at around 200 °C. For optimal growth conditions, the quartz tube was evacuated around 10 mTorr and purged with Ar gas to remove any residual molecules in it. After the evacuation, the furnace was heated to 400 °C for 50 minutes and then maintained for an additional 50 minutes. Throughout the growth process, the flow rate of Ar gas was maintained at around 200 standard cubic centimeters per minute at 100 mTorr37.
Pt film with a thickness of 20 nm was deposited on the SiO2/Si wafers (500 nm; SiO2 thickness) using an e-beam evaporator (A-tech system). The deposition rate was 0.3 Å/s until the thickness reached 5 nm, then 0.7 Å/s until the thickness reached 20 nm, under 2.0 × 10−6 Torr. After deposition, Pt film was cooled down for 20 min under UHV conditions and for 1 hr under 760 Torr of N2.
Catalytic performance measurements
Catalytic performance was measured in a batch reactor (1 L). The reactor was evacuated to UHV conditions (~10−8 Torr) using rotary and turbomolecular pumps. After that, CO, O2, and He gases were sequentially injected into the reactor. To detect the reactants and products, a gas chromatograph (GC, Donam, DS 6200) equipped with a thermal conductivity detector was used. A stainless-steel molecular sieve 5 A (with a 0.5 nm nominal opening) was employed to separate the mixed gases. The mixed gases in the reactor were circulated through the reaction line at a rate of 2 L min−1 using a Metal Bellows recirculation pump (Senior Metal Bellows, MB-41) to synchronize the partial pressures within the reactor and GC. The reaction temperature was controlled using a ceramic boron nitride heater. A temperature controller provided feedback on the current applied to the heater, maintaining temperature fluctuations below 0.5 °C.
TOF (sec−1) was calculated in terms of CO2 produced per Pt active surface site per reaction time (sec), as shown in the following equation26,48:
where PCO is the partial pressure of CO (Torr), V is the volume of the batch chamber (L), R is the ideal gas constant (Torr⋅L/mol⋅K), T is the reaction temperature (K), and NA is the Avogadro’s number (mol−1).
All the reaction data were obtained under low conversion conditions with a CO conversion rate below ~20%, assuming that the initial reaction rate was within a kinetically controllable regime. The number of active sites in the PtSe2 was estimated based on the STEM images from the reference article37.
Structural characterizations
XRD (RIGAKU, SmartLab) was performed to analyze the structural characteristics. Analysis was done on 2θ scan (grazing incidence of diffraction) mode with a scan speed of 5˚/min and a step size of 0.01˚. A structural image was obtained using TEM instrument (FEI, Titan cubed G2 60-300) capable of spherical aberration (Cs)-corrected HADDF-STEM and HRTEM imaging at 300 kV. The EDS mapping and count ratio between Pt and Se was analyzed using Super-X detector equipped on the same TEM instrument. A thin cross-section of PtSe2 was prepared using a focused ion beam (FIB, Thermo Fisher Scientific, Helios G5 and FEI, Helios NanoLab 450 F1) with ~70 nm thickness for cross-sectional TEM analysis. Sample preparation with FIB was done after the carbon coating on the PtSe2 and Pt film for protection. Raman spectroscopy (Horiba Jobin Yvon, ARAMIS) was performed to confirm the uniform growth and stability of PtSe2. Phonon modes of samples were calibrated in reference to Si mode (~520 cm−1).
Synchrotron-based analysis
Surface chemical states of PtSe2 were analyzed via AP-XPS at the Pohang Accelerator laboratory (KBSI-PAL beamline, Pohang, South Korea)39,49. PtSe2 was loaded under UHV (~10−12 bar) and then filled to near ambient pressure (10−3 bar). XPS data was calibrated with the C 1 s peak at 284.8 eV. Chemical bonding environment and oxidation state of PtSe2 were analyzed via XAS Pt L3 edge spectra at the PAL 10 C beamline. PtSe2 and Pt was loaded as film deposited on SiO2/Si wafer, and PtO2 was loaded as fine power (Platinum (IV) oxide, Sigma-Aldrich) using transparent tape (Scotch). XAS data was analyzed using Demeter software.
Computational details for density functional theory calculations
We constructed the three DFT models: Pt(111) 8×8×3, Se-terminated PtSe2 6×6×3, and Pt-terminated PtSe2 6×6×2 slabs. The Pt-terminated PtSe2 model was constructed by exfoliating the topmost Se layer from the Se-terminated PtSe2 6×6×3 slab. This model was selected to emphasize the chemical factors that bring the experimentally observed catalytic functionality of PtSe2. The Pt- and Se-terminated PtSe2 models were designed to represent the surface morphology of the experimentally synthesized PtSe2. All three models were used to study the CO oxidation activity comparatively. A 15 Å of vacuum space was added along the z-axis to avoid interference between the periodic supercells. All spin-polarized DFT calculations were conducted using the Vienna Ab-initio Simulation Package (VASP) code with the Perdew-Burke-Ernzerhof (PBE) exchange-correlation functional50,51. The interaction between the ionic core and valence electrons was described using the projector-augmented wave method52. Valance electron wave functions were expanded in a plane-wave basis set with an energy cutoff of 400 eV. The Brillouin zone was sampled at the Γ-point for all calculations. All electronic structure data was acquired using a 4×4×1 k-points sampling. Convergence criteria were set to 10−4 eV for the electronic structure and 0.05 eV/Å for the atomic geometry. A Gaussian smearing function with a finite temperature width of 0.05 eV was employed to enhance the convergence of states near the Fermi level. The location and energy of transition states were calculated using the climbing-image nudged-elastic-band (CI-NEB) method53. In addition, details about Langmuir adsorption models are described in Supplementary Note 2. Refer to Supplementary Note 3 for calculation quality test results.
Data availability
The data that support the finding of this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Yang, X.-F. et al. Single-atom catalysts: a new frontier in heterogeneous catalysis. Acc. Chem. Res. 46, 1740–1748 (2013).
Wang, A., Li, J. & Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2, 65–81 (2018).
Zhu, Y. et al. Strain engineering of a defect-free, single-layer MoS2 substrate for highly efficient single-atom catalysis of CO oxidation. ACS Appl. Mater. Interfaces 11, 32887–32894 (2019).
Li, H. et al. Atomic structure and dynamics of single platinum atom interactions with monolayer MoS2. ACS Nano 11, 3392–3403 (2017).
Shi, Y. et al. Electronic metal-support interaction modulates single-atom platinum catalysis for hydrogen evolution reaction. Nat. Commun. 12, 3021 (2021).
Lykhach, Y. et al. Counting electrons on supported nanoparticles. Nat. Mater. 15, 284–288 (2016).
Liu, L. & Corma, A. Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles. Chem. Rev. 118, 4981–5079 (2018).
Lee, S. W. et al. Surface chemistry of hot electron and metal-oxide interfaces. Surf. Sci. Rep. 76, 100532 (2021).
Liu, J. Catalysis by supported single metal atoms. ACS Catal. 7, 34–59 (2017).
Yuan, W., Ma, Y., Wu, H. & Cheng, L. Single-atom catalysts for CO oxidation, CO2 reduction, and O2 electrochemistry. J. Energy Chem. 65, 254–279 (2022).
Kumar, P. et al. High-density cobalt single-atom catalysts for enhanced oxygen evolution reaction. J. Am. Chem. Soc. 145, 8052–8063 (2023).
Liu, X. et al. Superior catalytic performance of atomically dispersed palladium on graphene in CO oxidation. ACS Catal. 10, 3084–3093 (2020).
Krishnan, R., Wu, S.-Y. & Chen, H.-T. Single Pt atom supported on penta-graphene as an efficient catalyst for CO oxidation. Phys. Chem. Chem. Phys. 21, 12201–12208 (2019).
Li, Y., Zhou, Z., Yu, G., Chen, W. & Chen, Z. CO catalytic oxidation on iron-embedded graphene: computational quest for low-cost nanocatalysts. J. Phys. Chem. C. 114, 6250–6254 (2010).
Luo, Y. et al. Unsaturated single atoms on monolayer transition metal dichalcogenides for ultrafast hydrogen evolution. ACS Nano 14, 767–776 (2020).
Xuan, N. et al. Single-atom electroplating on two dimensional materials. Chem. Mater. 31, 429–435 (2019).
Shi, Y. et al. Site-specific electrodeposition enables self-terminating growth of atomically dispersed metal catalysts. Nat. Commun. 11, 4558 (2020).
Li, X., Rong, H., Zhang, J., Wang, D. & Li, Y. Modulating the local coordination environment of single-atom catalysts for enhanced catalytic performance. Nano Res. 13, 1842–1855 (2020).
He, Y. et al. Amorphizing noble metal chalcogenide catalysts at the single-layer limit towards hydrogen production. Nat. Catal. 5, 212–221 (2022).
Kim, J. H. et al. Revealing Pt-seed-induced structural effects to tribological/electrical/thermoelectric modulations in two-dimensional PtSe2 using scanning probe microscopy. Nano Energy 91, 106693 (2022).
Gong, Y. et al. Two-dimensional platinum diselenide: synthesis, emerging applications, and future challenges. Nano Micro Lett. 12, 174 (2020).
Li, J. et al. Edge and point-defect induced electronic and magnetic properties in monolayer PtSe2. Adv. Funct. Mater. 32, 2110428 (2022).
Allian, A. D. et al. Chemisorption of CO and mechanism of CO oxidation on supported platinum nanoclusters. J. Am. Chem. Soc. 133, 4498–4517 (2011).
Bunluesin, T., Cordatos, H. & Gorte, R. J. Study of CO oxidation kinetics on Rh/Ceria. J. Catal. 157, 222–226 (1995).
Langmuir, I. The mechanism of the catalytic action of platinum in the reactions 2CO + O2 = 2CO2 and 2H2+ O2 = 2H2O. Trans. Faraday Soc. 17, 621–654 (1922).
Han, G., Song, H. C., Kim, S. H. & Park, J. Y. Role of the support oxidation state on the catalytic activity of two-dimensional Pt/TiOx catalysts. J. Phys. Chem. C. 127, 4096–4103 (2023).
Nørskov, J. K., Abild-Pedersen, F., Studt, F. & Bligaard, T. Density functional theory in surface chemistry and catalysis. PNAS 108, 937–943 (2011).
Yu, X. et al. Atomically thin noble metal dichalcogenide: a broadband mid-infrared semiconductor. Nat. Commun. 9, 1545 (2018).
Ma, H. et al. Controlled synthesis of ultrathin PtSe2 nanosheets with thickness-tunable electrical and magnetoelectrical properties. Adv. Sci. 9, 2103507 (2022).
Zhang, L. et al. Precise layer-dependent electronic structure of MBE-grown PtSe2. Adv. Electron. Mater. 7, 2100559 (2021).
Dai, J. et al. Hydrogen spillover in complex oxide multifunctional sites improves acidic hydrogen evolution electrocatalysis. Nat. Commun. 13, 1189 (2022).
Fang, S. et al. Uncovering near-free platinum single-atom dynamics during electrochemical hydrogen evolution reaction. Nat. Commun. 11, 1029 (2020).
Shimizu, K. -i et al. Quantitative determination of average rhodium oxidation state by a simple XANES analysis. Appl. Catal. B: Environ. 111-112, 509–514 (2012).
Zhu, Y. et al. Facilitating alkaline hydrogen evolution reaction on the hetero-interfaced Ru/RuO2 through Pt single atoms doping. Nat. Commun. 15, 1447 (2024).
Song, H. C. et al. Engineering nanoscale interfaces of metal/oxide nanowires to control catalytic activity. ACS Nano 14, 8335–8342 (2020).
Zeng, L. et al. Ultrafast and sensitive photodetector based on a PtSe2/silicon nanowire array heterojunction with a multiband spectral response from 200 to 1550 nm. NPG Asia Mater. 10, 352–362 (2018).
Han, S. S. et al. Horizontal-to-vertical transition of 2d layer orientation in low-temperature chemical vapor deposition-grown PtSe2 and its influences on electrical properties and device applications. ACS Appl. Mater. Interfaces 11, 13598–13607 (2019).
Gulo, D. P., Yeh, H., Chang, W.-H. & Liu, H.-L. Temperature-dependent optical and vibrational properties of PtSe2 thin films. Sci. Rep. 10, 19003 (2020).
Kim, G. et al. AP-XPS beamline, a platform for operando science at Pohang accelerator laboratory. J. Synchrotron Radiat. 27, 507–514 (2020).
Sojková, M. et al. High carrier mobility epitaxially aligned PtSe2 films grown by one-zone selenization. Appl. Surf. Sci. 538, 147936 (2021).
Gao, J. et al. Structure, stability, and kinetics of vacancy defects in monolayer PtSe2: a first-principles study. ACS Omega 2, 8640–8648 (2017).
Kumar, P., Meng, A. C., Jo, K., Stach, E. A. & Jariwala, D. Interfacial reaction and diffusion at the one-dimensional interface of two-dimensional PtSe2. Nano Lett. 22, 4733–4740 (2022).
Chen, J. et al. Atomic-level dynamics of point vacancies and the induced stretched defects in 2D monolayer PtSe2. Nano Lett. 22, 3289–3297 (2022).
Powell, C. J. Practical guide for inelastic mean free paths, effective attenuation lengths, mean escape depths, and information depths in X-ray photoelectron spectroscopy. J. Vac. Sci. Tech. 38, 023209 (2020).
Hoffmann, R. Solids and Surfaces: a Chemist’s View of Bonding in Extended Structures. (VCH Publishers, 1988).
Kim, H. Y., Lee, H. M. & Henkelman, G. CO oxidation mechanism on CeO2-supported Au nanoparticles. J. Am. Chem. Soc. 134, 1560–1570 (2012).
Kim, H. Y. & Liu, P. Complex catalytic behaviors of CuTiOx mixed-oxide during CO oxidation. J. Phys. Chem. C. 119, 22985–22991 (2015).
Song, H. C. et al. Synergistic interactions between water and the metal/oxide interface in CO oxidation on Pt/CeO2 model catalysts. Catal. Today 411-412, 113825 (2023).
Kim, T.-S. et al. Catalytic boosting on AuCu bimetallic nanoparticles by oxygen-induced atomic restructuring. Appl. Catal. B: Environ. 331, 122704 (2023).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Acknowledgements
J.Y.P. and H.Y.K. acknowledge support from the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2022R1A2C3004242), (RS-2023-NR077216), (RS-2021-NR060128). Y. Jung acknowledges financial support from the US National Science Foundation (CAREER: 2142310). J.H.K. acknowledges the support from Inha University. S. S. Han acknowledges financial support from the Preeminent Postdoctoral Program (P3) at UCF. S.W.L. acknowledges the support from the Inha University Research Grant. Synchrotron-based AP-XPS research at the KBSI-PAL 8A2 AP-XPS beamline and XAS research at the 10C beamline were supported in part by Pohang Accelerator Laboratory/POSTECH and Korea Basic Science Institute under the R&D program (Project No. A426220) supervised by the Ministry of Science and ICT.
Author information
Authors and Affiliations
Contributions
G.H., H.C., and J.H.K. contributed equally. G.H., J.H.K., and J.Y.P. conceptualized the research. S.S.H. performed the synthesis under the guidance of Y.J. G.H., D.K., H.P., K.K., J.K., and M.G.K. performed the characterizations and experimental analysis. G.H. performed the catalytic performance measurements. H.C. and H.Y.K. performed the theoretical analysis. G.H. and H.C. wrote the manuscript with feedback from all authors. J.H.K., S.W.L., and J.Y.P. provided major supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Yu Jing and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Han, G., Choi, H., Kim, J.H. et al. Enhanced catalytic activity on atomically dispersed PtSe2 two-dimensional layers. Nat Commun 16, 6139 (2025). https://doi.org/10.1038/s41467-025-61320-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-61320-0