Abstract
Organic radical materials have attracted significant attention for their unique photophysical properties and potential applications. Herein, we propose an auto triplet excitons supply system to generate radicals through the photoinduced electron transfer process for practical applications. By manipulating the n-π* transition and p-π conjugation, we achieve enhanced photoactivated phosphorescence coupled with rapid radical generation rates in the methoxy-substituted naphthylamine derivative (OCH3-DNaAPh) and fluorine-substituted naphthylamine derivative (F-DNaAPh), confirming the crucial role of the long-lived excitons supply in generating stable radicals upon UV irradiation. OCH3-DNaAPh is thus selected for implementing quantitative monitoring and an automated alarm system for UV radiation, featuring a two-stage risk warning model for excessive UV exposure. Furthermore, it acts as the photoinitiator for the polymerization of methyl methacrylate under UV irradiation, further enhancing phosphorescence emission above 473 K. The study describes a method to generate organic photogenerated radicals in the solid state, broadening their scope of practical applications.
Similar content being viewed by others
Introduction
Organic radicals have garnered much attention for their potential applications in flexible electronics1,2,3,4, quantum information science5,6,7, and molecular spintronics8,9,10. The efficient development of solid-state radical materials is crucial for real-world applications, though it continues to pose formidable challenges. In the past decades, great advancements in this field have been propelled by the development of methods for radical generation, which relied on redox reactions or homolysis triggered by thermal activation or photonic excitation11,12. Among these strategies, photosynthetic methods are particularly attractive in the solid state due to their precision, rapid, and controllable selectivity13,14,15, eliminating the need for multiple substances and high activation energy in traditional methods, and minimizing undesired side reactions and byproducts16,17,18,19,20. However, the low yields and limited air stability of radicals, coupled with the challenges of employing low-energy photonic excitation in the solid state, remain obstacles for modern manufacturing, especially in optoelectronics and photonic applications.
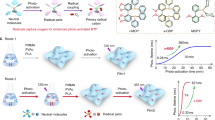
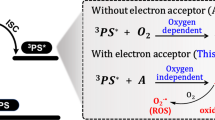
Actually, photoinduced electron transfer (PET) facilitated by UV or visible-light excitation has emerged as an effective strategy for simplifying radical generation in the solid state. By promoting the transition of ground state molecules to excited states, the PET process can occur to generate radicals (Fig. 1a). However, the fast decay rate of singlet excitons (Sn) still limited the photogenerated radical yield. According to chemical kinetics, given the same reactant concentration and reaction conditions, it must enhance the number of activated molecules and the stability of products to increase the reaction rate and yield. Triplet excitons (Tn), generated from singlet states via intersystem crossing (ISC) as the spin-forbidden process, typically exhibit much longer lifetimes than singlet species21,22,23,24, therefore offering potential to increase the number of activated molecules within the PET process, and therein enhancing photogenerated radical production. In 2021, our group had demonstrated that naphthylamine derivatives capable of generating radicals via the PET process also exhibited observable triplet excitons25. Although a straightforward design strategy based on the PET mechanism to facilitate the efficient generation of photogenerated radicals was not developed in that work, this discovery underscores the potential of incorporating an auto triplet exciton supply system, such as a photoactivated room-temperature phosphorescence (RTP) mechanism, to further enhance radical generation, thereby significantly advancing the practical applications of organic radicals (Fig. 1b)26,27.
a Schematic diagram of radicals generation in the solid state based on various mechanisms (ISC Intersystem crossing, PET Photoinduced electron transfer, M Moleculer, M·+/M·− Cationic/Anionic radical). b Schematic representation for the strategy of efficiently achieving radicals by building an auto triplet excitons supply system. c The design concept and chemical structures of guest molecules, and the performance of room temperature phosphorescence (τp) and photochromic behaviors (N·+) of doping guests in the poly(methyl methacrylate) (PMMA) matrix.
Theoretical studies suggest that the introduction of heteroatoms can enhance the generation of triplet excitons via n-π* transitions28,29, and stabilize radicals through spin delocalization to reduce the spin density at the radical center30,31,32,33,34, which aligns with the objectives of boosting the number of activated molecules and the stability of products as mentioned above. Consequently, in materials that generate radicals through the PET process, the introduction of heteroatoms may bridge the gap between triplet exciton production and photogenerated radical formation. Herein, as shown in Fig. 1c, we report the introduction of heteroatom substituents (O, F, etc.) into DNaAPh to yield a series of naphthylamine derivatives. Notably, after being doped into poly(methyl methacrylate) (PMMA), OCH3-DNaAPh@PMMA and F-DNaAPh@PMMA films with longer phosphorescence lifetime upon UV irradiation compared with other compounds demonstrated the fastest radical production rate with the strongest radical absorption, highlighting the significance of an auto triplet excitons supply system. By utilizing the ability to generate radicals quickly under UV irradiation, the issue of high-temperature quenching of phosphorescence was addressed, which is achieved through 3D printing technology based on photopolymerization. Leveraging the exceptional photochromic behavior of OCH3-DNaAPh@PMMA film with rapid response to UV light, the applications of quantitative monitoring and automated alarm for UV radiation based on organic materials were successfully implemented, and the UV irradiation dose alarm device exhibited effective warning efficacy. The auto triplet excitons supply system for the PET process developed in this work will offer valuable insights into materials design for photonics and optoelectronics, as well as for photopolymerization.
Results
Photoactivated RTP and photochromic behaviors
As shown in Supplementary Fig. 1, OCH3-DNaAPh, F-DNaAPh, Cl-DNaAPh, Br-DNaAPh, and CH3-DNaAPh were facilely synthesized. The molecular structure and purity of these compounds have been fully confirmed by 1H NMR, 13C NMR spectroscopy, high-resolution mass spectroscopy (HRMS), and high-performance liquid chromatography (HPLC) (Supplementary Figs. 2–18). Then, the thermal gravimetric analyses of these compounds were performed. As shown in Supplementary Fig. 19, their thermal decomposition temperatures were all around 300 °C, indicating high thermal stability. Meanwhile, the photophysical behaviors of these compounds in different solvents were also studied. As shown in Supplementary Figs. 20 and 21, although only a minor shift was observed in UV–Vis absorption spectra in organic solvents with various polarities, the emission peaks of the compounds exhibited a remarkable red-shift along with increasing solvent polarity. It should be ascribed to the intramolecular charge transfer effect, which could also narrow ΔEST and be advantageous for the ISC transition and the production of triplet excitons.
The newly synthesized dinaphthylamine derivatives were doped into the PMMA matrix at the concentration of 1 wt%, and their photo-responsive processes were carefully monitored and described as follows. As shown in Fig. 2a, OCH3-DNaAPh@PMMA film had a weak phosphorescence emission around 523 nm at the initial state with an RTP lifetime of 148.8 ms (Supplementary Fig. 22). After continuous irradiation with 365 nm UV irradiation for 60 s, a persistent phosphorescence was observed by the naked eyes after switching off the UV illumination. The phosphorescence intensity of OCH3-DNaAPh@PMMA film was gradually increased to three times of that of the original one, and the phosphorescence lifetime was also prolonged to 415.4 ms, showing a typical photoactivated RTP effect. Other films also displayed photoactivated RTP with different RTP duration times and intensities (Supplementary Figs. 23–26). When they were dispersed in the PMMA matrix at a low concentration of 1 wt%, the emission observed was similar to that in dilute solutions, as shown in Supplementary Fig. 27, indicating that the phosphorescence emission in the PMMA matrix should come from the triplet excited states of the small molecules. Furthermore, the photoactivated RTP effect of the films should also originate from the consumption of oxygen under UV irradiation, as reported in previous work (Supplementary Figs. 28–37)25. And the enhancement of the phosphorescence emission of the as-prepared films under vacuum conditions further validates the mechanism (Supplementary Fig. 38).
a The RTP spectra of OCH3-DNaAPh@PMMA film under continuous 365 nm UV irradiation for 60 s. b The RTP spectra of OCH3-DNaAPh@PMMA, F-DNaAPh@PMMA, Cl-DNaAPh@PMMA, Br-DNaAPh@PMMA, and CH3-DNaAPh@PMMA films after UV irradiation for 60 s. c The RTP lifetimes of OCH3-DNaAPh@PMMA, F-DNaAPh@PMMA, Cl-DNaAPh@PMMA, Br-DNaAPh@PMMA, and CH3-DNaAPh@PMMA films before and after UV irradiation for 60 s. d The photos of RTP behaviors for OCH3-DNaAPh@PMMA, F-DNaAPh@PMMA, Cl-DNaAPh@PMMA, Br-DNaAPh@PMMA, and CH3-DNaAPh@PMMA films before and after the 365 nm UV irradiation.
By comparing the phosphorescence intensity of the films with the same content of luminogens after 365 nm UV activation (Fig. 2b), it was found that the introduction of heteroatoms from O to F, and then to Cl and Br, gradually enhanced the photoactivated RTP emission intensity. In particular, the heavy atom effect of Br made Br-DNaAPh@PMMA film exhibit nearly triple the phosphorescence emission intensity compared with others, while the CH3-DNaAPh@PMMA film exhibited weaker phosphorescence emission intensity. The phosphorescence lifetimes before and after photo-activation for these films can be found in Fig. 2c, and the corresponding RTP behaviors were shown in Fig. 2d. Among them, OCH3-DNaAPh@PMMA and F-DNaAPh@PMMA films gave the longest afterglow up to 8 s with a long photoactivated RTP lifetime, demonstrating the potential to build an auto triplet excitons supply system for the PET process to produce radicals.
As shown in Fig. 3a, in situ monitoring of the absorption spectra for OCH3-DNaAPh@PMMA film was carried out under continuous 365 nm UV irradiation for 60 s. With the irradiation time increasing, a new absorption band around 640 nm appeared at about 5 s, then rapidly increased and reached the top after UV irradiation for 60 s. The photochromic effect of OCH3-DNaAPh@PMMA film was more significant than DNaAPh@PMMA, indicating the enhancement of the photochromism performance based on radical formation (Supplementary Fig. 39c). It was inferred that the introduction of O heteroatom can form p-π conjugation, thus stabilizing OCH3-DNaAPh·+ radicals. After stopping UV irradiation, the absorption intensity at 640 nm gradually decreased for OCH3-DNaAPh@PMMA film, and then was undetectable after placing under an ambient environment for 20 min, demonstrating that the color change could be recovered (Fig. 3b). Then, in situ monitoring of the absorption spectra of F-DNaAPh@PMMA, Cl-DNaAPh@PMMA and Br-DNaAPh@PMMA films was also carried out and presented in Supplementary Figs. 40–42. These films all exhibited photochromic behavior along with the increased absorption intensity at 640 nm. Compared with OCH3-DNaAPh@PMMA film, the maximum absorption intensity at 640 nm, the radical generated rate, and the corresponding fading time of F-DNaAPh@PMMA, Cl-DNaAPh@PMMA, and Br-DNaAPh@PMMA films gradually decreased (Fig. 3c), indicating that the ability to produce radicals as well as the stability of the generated radicals have gradually deteriorated. This phenomenon could be attributed to the presence of heavy atoms, which facilitated the radiative transition process of triplet excitons, thus hindering the generation of radicals and leading to the gradual degradation of the photochromic effect from OCH3-DNaAPh@PMMA to Br-DNaAPh@PMMA film35.
a In situ absorption spectra for OCH3-DNaAPh@PMMA film under continuous 365 nm UV irradiation for 60 s. b Time-dependent UV–Vis spectra for the fading process of the OCH3-DNaAPh@PMMA film. c The absorption intensity curves at 640 nm of the coloring process for OCH3-DNaAPh@PMMA, F-DNaAPh@PMMA, Cl-DNaAPh@PMMA, Br-DNaAPh@PMMA, and CH3-DNaAPh@PMMA films. d The photochromic behavior and fading process of OCH3-DNaAPh@PMMA, F-DNaAPh@PMMA, Cl-DNaAPh@PMMA, Br-DNaAPh@PMMA and CH3-DNaAPh@PMMA films at room temperature. The photographs were taken at different times before and after turning off the 365 nm UV light under ambient conditions.
In addition, with poor photoactivated RTP property, CH3-DNaAPh@PMMA film exhibited only poor photochromic behavior due to the lack of an auto triplet exciton supply system (Supplementary Fig. 43). The absence of p-π conjugation to stabilize radicals also suppresses the photogenerated radical process. To investigate the influence of p-π conjugation on the generation of radicals, COOCH3-DNaAPh@PMMA film was fabricated. The introduction of the ester group promotes the n-π* transition in COOCH3-DNaAPh, thus leading to obvious photoactivated RTP behavior of COOCH3-DNaAPh@PMMA film (Supplementary Figs. 44 and 45). However, the ester group could not help to stabilize the nitrogen radical through p-π conjugation. As shown in Supplementary Fig. 46, only weak fluctuation of the absorption band around 640 nm after UV irradiation for 60 s was observed, indicating the poor ability of COOCH3-DNaAPh@PMMA film to produce radicals. At the same time, no obvious change could be observed in their appearance color before and after UV irradiation (Fig. 3d and Supplementary Fig. 46c). Based on these data, the important role of heteroatom and the corresponding p-π conjugation on the construction of the auto triplet excitons supply system for the generation of radicals and the resultant photochromic effect could be demonstrated.
Mechanism investigation
The electron spin resonance (ESR) analyses of the films before and after UV activation were carried out to certify the formation of radicals in the photochromic process (Fig. 4 and Supplementary Fig. 47). No obvious ESR signal was observed at the initial state of these films, while strong signals appeared in the range of 322–335 mT after 365 nm UV irradiation for 60 s, accompanied by the photochromic behavior. Further on, after heating at 80 °C for 10 min, the ESR signal of the films disappeared and the color of the films returned to the initial state. Based on these results, it could be well certified that the photochromic behavior of the films originated from the formation of radicals under UV irradiation. On the other hand, the ESR spectra of OCH3-DNaAPh@PMMA, F-DNaAPh@PMMA, Cl-DNaAPh@PMMA, and Br-DNaAPh@PMMA films after photo-activation were found to exhibit more complex fine structures compared with CH3-DNaAPh@PMMA film (Fig. 4b). Besides, to certify the involvement of the PMMA matrix in the formation process of radicals and photochromic behavior, the compounds were doped into the polystyrene (PS) matrix using the same preparation method. The ESR spectra of OCH3-DNaAPh@PS and F-DNaAPh@PS films exhibited a broad peak that was distinct from those found in the PMMA matrix (Fig. 4c and Supplementary Fig. 48d), but similar to that observed in the CH3-DNaAPh@PMMA film, while the ESR spectrum of CH3-DNaAPh@PS film was similar to that found in the PMMA matrix (Fig. 4d). According to the previous literature36, the PMMA matrix should have participated in the radical generation process for them. In the generation of nitrogen radical (N·+), the following reaction could happen:
Correspondingly, in Fig. 4a, the peaks of I and II should be ascribed to signals of nitrogen radical (N·+) based on luminogens, and the peaks of III to VI should be the signals of methyl radical (·CH3) from PMMA. As for the CH3-DNaAPh@PMMA film with weak RTP emission, no ESR signal from PMMA could be observed. Additionally, the absorption intensity around 640 nm was monitored to track the presence of nitrogen radical cations. When the color change of the films was recovered, the absorption intensity at 640 nm reduced to near zero, indicating the absence of nitrogen radical cations. Upon oxygen injection, the recovery process of the color changes was accelerated compared with that under an ambient environment (Supplementary Fig. 49), indicating that the disappearance of nitrogen radical cations is due to the quenching effect of oxygen.
Additionally, the UV–Vis absorption spectra of OCH3-DNaAPh@PS, F-DNaAPh@PS, and CH3-DNaAPh@PS films did not show obvious change before and after UV irradiation, indicating the absence of the photochromic effect (Supplementary Fig. 48a–c). Based on these results, the involvement of the PMMA matrix in the formation of radicals and its promotion of photochromic behavior could be well-certified. Besides, the cyclic voltammetry (CV) curves of dinaphthylamine derivatives were measured (Fig. 4e). It was found that, except for CH3-DNaAPh, all other compounds exhibited good reversibility in their redox reactions, suggesting the instability of the oxidization product of CH3-DNaAPh. This instability likely prevented the participation of the PMMA matrix in the radical generation process in the CH3- DNaAPh@PMMA film.
To further verify the intrinsic mechanism of the photochromic effect, the configurations of the neutral molecules and the corresponding cationic radicals of the target compounds were optimized, and the internal reorganization energies were calculated. As shown in Fig. 5a, the internal reorganization energy reflects the energy change in the system due to geometric relaxation after gaining or losing electrons. The charged state represents the cationic radicals with a + 1 charge. According to the formula for calculating the reorganization energy: λ = λ(I) + λ(II), the value of the reorganization energy represents the energy required for the transformation between the radicals and neutral molecule. As shown in Fig. 5b, OCH3-DNaAPh had the highest reorganization energy, which meant the highest energy required for the radical to return to the neutral state. In other words, the radical was the most stable one, accompanied by the most significant photochromic behavior in the experiment. In addition, the frontier orbitals of the neutral molecules and radicals were also calculated (Supplementary Fig. 50). The HOMO orbitals for compounds OCH3-DNaAPh, F-DNaAPh, Cl-DNaAPh, and Br-DNaAPh were all delocalized to the heteroatoms of O/F/Cl/Br, indicating the existence of p-π conjugation. As for CH3-DNaAPh, no p-π conjugation between -CH3 and the phenyl ring could be observed.
a The diagram of reorganization energy. b The histogram of reorganization energies for OCH3-DNaAPh, F-DNaAPh, Cl-DNaAPh, Br-DNaAPh, and CH3-DNaAPh. c The spin populations on N atoms and heteroatoms for the radicals, reorganization energies of neutral molecules and radicals, and SOC constants of OCH3-DNaAPh, F-DNaAPh, Cl-DNaAPh, Br-DNaAPh, and CH3-DNaAPh. d The spin populations of OCH3-DNaAPh·+, F-DNaAPh·+, Cl-DNaAPh·+, Br-DNaAPh·+, and CH3-DNaAPh·+ radicals. e The energy level diagram and SOC constants of OCH3-DNaAPh, F-DNaAPh, Cl-DNaAPh, Br-DNaAPh, and CH3-DNaAPh.
Moreover, the spin population (ρ) distributions of radicals were also calculated (Supplementary Tables 1–6)37. The presence of heteroatoms in the molecule led to the formation of p-π conjugation, which facilitated the delocalization of charge. As the result, heteroatoms had relatively higher spin populations, which led to the lower spin populations on nitrogen atoms and stabilized the radicals. Based on the spin populations of OCH3-DNaAPh·+, F-DNaAPh·+, Cl-DNaAPh·+, and Br-DNaAPh·+ (Fig. 5d), it could be observed that the spin population on the O atom was the highest among all of the heteroatoms (O/F/Cl/Br), which made the corresponding radicals more stable and resulted in significant photochromic behavior. Besides, the radical lifetimes were calculated, which could refer to the period (t1/2) when the concentration of radicals fell to half. As shown in Supplementary Fig. 51, the lifetime of OCH3-DNaAPh·+ radical was found to be longer than those of F-DNaAPh·+, Cl-DNaAPh·+, Br-DNaAPh·+, and CH3-DNaAPh·+ radicals, verifying its high stability. In contrast, the C atom on the methyl group of CH3-DNaAPh, which could not form p-π conjugation, had a spin population close to zero (Fig. 5d). Therefore, the spin population on the N atom was relatively high for CH3-DNaAPh·+, leading to unstable radicals and inferior photochromic behavior.
On the other hand, although the spin population on the Br atom was higher than that of the F atom, Br-DNaAPh did not exhibit photochromic behavior more obvious than F-DNaAPh. The spin-orbit coupling (SOC) constants between singlet and triplet states were calculated. As shown in Fig. 5e, each compound had significant SOC constants, which also explained their significant photoactivated RTP effect. Notably, Br-DNaAPh had the largest SOC constant between T1 and the ground state due to the presence of the heaviest atom, resulting in the strongest phosphorescence emission intensity (Fig. 2b). Consequently, more triplet excitons were consumed in the radiative transition process rather than undergoing the PET process, resulting in the inferior generation of radicals in Br-DNaAPh@PMMA film.
Taking the above theoretical calculation results into account, the spin populations of the N atoms, the reorganization energy between the radical and neutral molecule, and the SOC constants were the main factors in constructing an auto triplet excitons supply system for radical generation in the PMMA matrix. As shown in Fig. 5c, in the pentagon factor analysis diagram, for compounds with a larger occupied area, there was a higher likelihood of establishing an automatic supply system for triplet excitons in the PET process. For example, the p-π conjugation on OCH3-DNaAPh gave the O atom a higher spin population, which lowered the spin population of the N atom. Along with a larger reorganization energy, enough number of ISC channels, and a smaller SOC constant between T1 and the ground state, the auto triplet excitons supply system was constructed, and stable radicals of OCH3-DNaAPh·+ were generated efficiently. For Br-DNaAPh, the smaller occupied area in the pentagon factor analysis diagram due to the largest SOC constant between T1 and the ground state made it tend to produce strong phosphorescence emission. CH3-DNaAPh exhibited the smallest area of the distribution in the diagram, leading to poor photochromic performance.
To further verify the mechanism of the construction of the auto triplet excitons supply system for radical generation, we tried to stabilize the nitrogen radicals through the introduction of steric hindrance protection adjacent to the nitrogen atom. Hence, o-CH3-DNaAPh was synthesized and doped into PMMA. As shown in Supplementary Figs. 52 and 53, o-CH3-DNaAPh@PMMA film exhibited photoactivated RTP with a lifetime of 638.7 ms. However, the photoactivated RTP intensity of o-CH3-DNaAPh@PMMA film was much weaker than that of Br-DNaAPh@PMMA film, but similar to that of OCH3-DNaAPh@PMMA and F-DNaAPh@PMMA films, and a small SOC constant between T1 and S0 could be found for o-CH3-DNaAPh (Supplementary Fig. 54). In addition, as shown in Supplementary Fig. 54c, the spin population of the nitrogen atom in the o-CH3-DNaAPh radical was 0.2576, which was even smaller than those of the nitrogen atoms in the OCH3-DNaAPh and F-DNaAPh radicals. As expected in Supplementary Fig. 55, remarkable photochromic behavior with a rapid response rate under UV irradiation was observed on o-CH3-DNaAPh@PMMA film. Comparing with OCH3-DNaAPh@PMMA and F-DNaAPh@PMMA films, o-CH3-DNaAPh@PMMA film also shows the fastest radical generation rate (Supplementary Fig. 56) and the slowest fading process (Supplementary Fig. 55b). These observations highlight the importance of factors such as the spin population on nitrogen atoms, SOC constants, and the introduction of steric hindrance adjacent to the nitrogen atom in constructing an efficient auto triplet exciton supply system for radical generation within the PMMA matrix. When the methyl group of o-CH3-DNaAPh was replaced by the chlorine atom with an electron-withdrawing effect, no photochromic behavior was observed on the o-Cl-DNaAPh@PMMA film due to the high spin population of the nitrogen atom in the o-Cl-DNaAPh radical (Supplementary Fig. 57), which reduces its stability.
Applications of an auto triplet excitons supply system for radicals generation
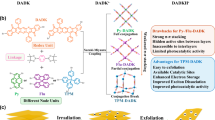
A photoinitiator plays a crucial role in initiating polymerization in light-driven processes, and its selection depends on the efficiency of the molecule to generate reactive species, such as radicals. Since OCH3-DNaAPh showed the ability to rapidly generate radicals under UV irradiation due to the existence of long-lived triplet excitons, we can dope OCH3-DNaAPh into MMA resin to initiate the photopolymerization and attempt to enhance the phosphorescence emission performance as the result. Radicals were also generated when OCH3-DNaAPh was dissolved in methyl methacrylate as demonstrated by ESR results (Supplementary Fig. 58). As depicted in Supplementary Fig. 59, OCH3-DNaAPh and MMA were exposed to UV light in the N2 atmosphere, and underwent photopolymerization, resulting in the solid product. In contrast, MMA without the luminogen did not undergo photopolymerization, and no solid product was obtained. Similarly, the addition of CH3-DNaAPh did not produce a solid product either. Consequently, the high rate of radical generation by OCH3-DNaAPh enabled it to act as a photoinitiator and participate in the photopolymerization process. As the result, the polymer derived from OCH3-DNaAPh and MMA exhibited enhanced high-temperature phosphorescence performance (Supplementary Figs. 59c and 60). Furthermore, a water cup in the shape of a “whale” was printed using light-curing 3D printing technology (Fig. 6a). In this process, OCH3-DNaAPh was dissolved in a commercial standard resin containing MMA monomers to ensure high dimensional precision and fabrication efficiency. As shown in Fig. 6b, c and Supplementary Fig. 61, the phosphorescence emission was detectable from 298 to 473 K, demonstrating the effective approach to achieving ultralong high-temperature phosphorescence. Triplet excitons were stabilized within the cured 3D printing material, characterized by highly entangled polymer chains that effectively isolated oxygen and restricted molecular motion, thereby resulting in visible phosphorescence emission even under daylight. It was worth noting that when the cup was placed in a high-temperature environment (Fig. 6d), bright phosphorescence could still be observed after the removal of UV irradiation. Therefore, a key aspect of applying this system in 3D printing technology is that OCH3-DNaAPh can participate in the in situ polymerization process of methyl methacrylate, resulting in the highly entangled polymer chains surrounding the OCH3-DNaAPh molecules to effectively isolate oxygen and restricted molecular motion, which is crucial for achieving strong phosphorescence emission, while no high-temperature phosphorescence effect was observed on the OCH3-DNaAPh@PMMA film due to the absence of the highly entangled polymer chains (Supplementary Fig. 62). Based on 3D light-curing printing technology with the auto triplet excitons supply system, we have successfully addressed the issue of the high-temperature (~473 K) quenching effect of afterglow materials, presenting significant implications for practical applications in extreme conditions in addition to anti-counterfeiting.
UV rays play a pivotal role in diverse fields, including biomedicine, industrial processes, and environmental protection38,39,40. Usually, UV rays are utilized in the biomedical field for sterilization, disinfection, and treatment of skin diseases41,42. Nevertheless, it is imperative to contemplate the potential ocular and dermal risks posed by excessive exposure to UV radiation. Therefore, the defense against the harm of UV radiation intensity through photochromic materials holds great importance in daily life. And the real-time monitoring of UV radiation and quantification of UV radiation intensity through simplified methods hold great importance in daily life. The materials derived from organic molecules are attractive due to their tunable optical and electronic properties, which can be modified through molecular engineering, as well as their inherent flexibility. On the basis of the significant flexibility and remarkable radical generation of OCH3-DNaAPh@PMMA film, we endeavored to convert the color signal from colorless to green into a monitored numerical indicator of UV radiation energy. As shown in Fig. 7a, the system for quantitative monitoring of UV radiation based on OCH3-DNaAPh@PMMA film was constructed, utilizing a photosensitive resistor with the photoelectric effect and a long-pass optical filter with a cutoff wavelength beyond 540 nm. As the duration of UV exposure increased, the color of OCH3-DNaAPh@PMMA film gradually changed to green, accompanied by the increased absorption band ranging from 500 to 750 nm. The filter could prevent interference from light with a wavelength less than 540 nm, enhancing the transparency changes and amplifying the color change signal induced by UV radiation, thus generating a significant resistance signal. In one word, the larger the resistance read, the greater the exposure of the film to UV radiation (Supplementary Fig. 63). As shown in Fig. 7b, the relationship between the photoresistor value and the corresponding UV radiation density could be quantitatively characterized by reading the photoresistor value after 10 s of UV radiation and performing data fitting. The fitting result showed that a linear regression equation could be obtained by taking the natural logarithm of both the photoresistor value (R) and the UV radiation density (E), which was ln R = 7.728 + 0.44 ln E. Therefore, the ultraviolet radiation density could be calculated by measuring the R-value, enabling quantitative monitoring of UV radiation density. By controlling the intensity of UV radiation, the resistance value of the photoresistor could also be adjusted, thereby achieving control of the circuit.
a Schematic diagram of the UV quantification monitoring system. b The relationship between the photoresistor value and UV radiation density. c The relationship between the resistance and UV dose. d Schematic and diagram of the UV automatic monitoring and alarm system, and the photos of the variations warning light changes and the recorded UV dose value as captured by the mobile application during UV exposure.
In addition to achieving quantitative monitoring of UV radiation density, the photo-responsive film, the photoresistor, the optical filter, the ESP32 and the red-light emitting diode (LED) could be connected according to the circuit shown in Supplementary Fig. 64 to form a UV automatic monitoring alarm system. The ESP32 was employed for voltage detection within the alarm system. The correlation between resistance and UV dose was examined and modeled through an equation, which was retrievable via the mobile application (Fig. 7d). As illustrated in Fig. 7e, the color of the film had minimal impact on the resistance value of the photoresistor under a lower UV dose. At this time, the red LED was not turned on. However, the color of the film gradually changed to deep green, and the resistance of the photoresistor also changed with the prolonged irradiation time. When the UV dose surpassed a threshold range of 100 mJ cm−², the red LED started flashing, alerting to potential skin damage from the UV dose. When UV radiation exceeded the dose of 200 mJ cm−², the red LED changed to a continuous light, exhibiting a two-stage risk warning model. The UV dose value could be viewed in the mobile application. Furthermore, the value of the UV dose was also recorded by the commercial photodetector, which aligned with the results provided by the application (Supplementary Fig. 65). When the UV lamp was turned off, the appearance color of the film gradually returned to the initial state, thus the warning light would stop shining. This simple and repeatable operation achieved the warning effect of exceeding UV radiation. In the industrial application of ultraviolet light, it can help people to avoid the harm caused by UV radiation. In addition, the films could also respond to UV radiation from sunlight. The quantitative characterization of the relationship between the intensity of sunlight radiation and the photochromic effect was realized, and the films could act as photochromic glass to effectively block the sunlight radiation (Supplementary Fig. 66).
Discussion
In summary, a series of dinaphthylamine derivatives were synthesized. Through doping them into the PMMA matrix, an auto triplet excitons supply system was constructed and efficient photoinduced radicals generation was observed. Our investigation revealed that the SOC constants, spin populations, and reorganization energy were the three primary factors essential for constructing the system. Compounds with large SOC constants between S1 and Tn states but small SOC constants between T1 and S0 states are available to generate increased triplet excitons in the PET process in the PMMA matrix. Along with low spin populations on the nitrogen atoms in radicals and large reorganization energy, an auto triplet excitons supply system can be established to efficiently generate radicals upon light irradiation.
By using photopolymerization 3D printing technology with the compound exhibiting a fast radical generation rate, the phosphorescence emission performance was enhanced as the result, and the high-temperature (~473 K) quenching effect of phosphorescence was overcome. Based on the improved performance of the materials, the quantitative monitoring and automated alarm for UV radiation were successfully implemented. A two-stage risk warning model for excessive UV exposure was constructed. In the first stage, a blinking light was triggered when the UV dose exceeded 100 mJ cm−2. And a continuous light was observed in the second state when the UV dose exceeded 200 mJ cm−2. Overall, an effective design strategy to develop intelligence with superior optical properties is presented, which will be beneficial for potential real-world applications43,44,45,46,47,48,49,50.
Methods
Reagents
Unless otherwise noted, all reagents used in the experiments were purchased from Jiangtian Chemical Co., Ltd. (Tianjin, China). The poly(methyl methacrylate) (PMMA) was purchased from Macklin Biochemical Technology Co., Ltd (Shanghai, China). The Polystyrene (PS), potassium tert-butoxide (purity: 95%), and sodium tert-butoxide (purity: 98%) was purchased from Aladdin Scientific. Di(naphthalene-2-yl)amine (purity: 98%), 1-bromo-4-methoxybenzene (purity: 98%), 1-bromo-4-fluorobenzene (purity: 98%), 1-bromo-4-chlorobenzene (purity: 98%), 1,4-dibromobenzene (purity: 98%), 1-bromo-4-methylbenzene(purity: 98%), 1-bromo-2-methylbenzene (purity: 98%), 1-bromo-2-chlorobenzene (purity: 98%), methyl 4-bromobenzoate and tri-butylphosphine solution were purchased from Heowns and used as received without further purification. Palladium acetate, tris(dibenzylideneacetone) dipalladium and copper(I) iodide were purchased from Energy Chemical.
Measurements
1H NMR spectra and 13C NMR spectra were recorded on a 400-MHz Bruker AVANCE III spectrometer using dimethyl sulfoxide (DMSO-d6) as solvent. The chemical shift references were as follows: (1H) DMSO-d6, 2.50 ppm, (13C) DMSO-d6, 39.50 ppm. High-resolution mass spectra (HRMS) were measured on a UHPLC/Q-TOF mass spectrometer. HPLC spectra were recorded on an Agilent 1100 HPLC chromatograph using acetonitrile as solvent. Photoluminescence spectra and time-resolved PL-decay curves at room temperature were produced by a Hitachi F-4700 fluorescence spectrophotometer. Time-resolved PL-decay curves of fluorescence were determined by an FLS1000 spectrometer. Time-dependent UV–Vis spectra were collected by a QE65 Pro (Ocean Optics). ESR spectra were recorded on a JEOL JES-FA200 spectrometer. The power of the UV light for the photopolymerization was 300 W. The GPC spectrum was measured by Waters 2690 D and 2410. Photopolymerization 3D printing was utilizing the Shape 1 HD 3D printer. Optical photographs of the samples were obtained using iPhone 13. The power density of the UV light used in the photophysical characterizations was 100 mW cm−2, while that in the photopolymerization process was 50 mW cm−2.
Applications
For the 3D printing process, 4.4 mL of standard resin from Rayshape Intelligence Technology Co., Ltd. was used, and the “Standard V1” program in the ShapeWare software for 3D printing was applied directly. The printing process lasted 225 min, during which 1255 layers were printed, each with a thickness of 0.050 mm.
A CdS photoresistor and an NPN bipolar junction transistor were used to implement quantitative monitoring and an automated alarm system for UV radiation. When the film is colorless, the incident light intensity on the photoresistor is high, resulting in low resistance and allowing a substantial current to flow through it. Conversely, when the color of the film gradually changes to deep green, the incident light intensity decreases, leading to a significant increase in resistance, which effectively reduces the current flow. This behavior enables the photoresistor to function as a light-sensitive switch within circuits. When combined with a transistor as an electronic switch, the current supplied to the LED can be regulated. In the presence of sufficient ambient light when the film remains colorless, the photoresistor exhibits low resistance, which results in an insufficient voltage drop across the transistor’s base-emitter junction to activate it. As the result, the transistor remains in its cut-off state, preventing current from reaching the LEnsequently, the LED is illuminated and starts flashing.
Theoretical calculation
All density functional theory (DFT) calculations were performed using the Gaussian 09 program (revision D.01). The B3LYP functional and 631 G(d) basis set were used to optimize the geometries of the ground states (S0 and D0). Based on the optimized ground state geometry, the excited state energy levels were calculated by the TD-DFT method, and the spin-orbit coupling constants between S0/S1 and the excited triplet states were calculated by the PySOC program. The spin population was calculated by the Multiwfn program and visualized using the VMD program. The neutral and radical structures were both further optimized using the b31yp/6-31 g* method to obtain the molecular structures of the compounds, and the HOMO/LUMO energy levels as well as reorganization energy were calculated.
General procedure for synthesis of compounds
N-(4-methoxyphenyl)-N-(naphthalen-2-yl)naphthalen-2-amine
Synthesis of OCH3-DNaAPh: A mixture of di(naphthalene-2-yl)amine (1.35 g, 5.000 mmol), 1-bromo-4-methoxybenzene (0.75 mL, 6.000 mmol), palladium acetate (0.06 g, 0.250 mmol), potassium tert-butoxide (0.84 g, 7.500 mmol) and tri-butylphosphine solution (0.30 mL, 0.130 mmol) were dissolved in toluene (40 mL) in a Schlenk tube, then refluxed at 110 °C for 12 h under N2 atmosphere. The solution was concentrated under reduced pressure. The crude product was extracted with dichloromethane and water, and dried over anhydrous MgSO4. After removing the solvent, the residue was purified by silica gel column chromatography to afford OCH3-DNaAPh as a light green solid (1.50 g, 79.8%). 1H NMR (400 MHz, DMSO-d6) δ (ppm) 7.83–7.80 (d, J = 12 Hz, 4H), 7.77–7.65 (d, J = 4.8 Hz, 2H), 7.41–7.33 (m, 6H), 7.25–7.23 (dd, J1 = 8 Hz, J2 = 2.3 Hz, 2H), 7.13–7.11 (dt, J1 = 8 Hz, J2 = 2.8 Hz, 2H), 7.98–7.96 (dt, J1 = 8 Hz, J2 = 2.8 Hz, 2H), 7.77 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ (ppm) 156.69, 145.85, 140.38, 134.58, 129.87, 129.50, 127.94, 127.82, 127.23, 126.91, 124.85, 123.86, 118.79, 115.66, 55.73. HRMS (ESI) m/z calcd for C27H22NO+ (M + H)+ 376.1696, found 376.1687.
N-(4-fluorophenyl)-N-(naphthalen-2-yl)naphthalen-2-amine
Synthesis of F-DNaAPh: A mixture of di(naphthalene-2-yl)amine (1.35 g, 5.000 mmol), 1-bromo-4-fluorobenzene (0.66 mL, 6.000 mmol), palladium acetate (0.06 g, 0.250 mmol), potassium tert-butoxide (0.84 g, 7.500 mmol) and tri-butylphosphine solution (0.30 mL, 0.130 mmol) were dissolved in toluene (40 mL) in a Schlenk tube, then refluxed at 110 °C for 12 h under N2 atmosphere. The solution was concentrated under reduced pressure. The crude product was extracted with dichloromethane and water, and dried over anhydrous MgSO4. After removing the solvent, the residue was purified by silica gel column chromatography to afford F-DNaAPh as a white solid (1.54 g, 84.6%). 1H NMR (400 MHz, DMSO-d6) δ (ppm) 7.86–7.82 (t, J = 8 Hz, 4H), 7.70–7.68 (d, J = 8 Hz, 2H), 7.43–7.36 (m, 6H), 7.26–7.24 (dd, J1 = 8 Hz, J2 = 2.3 Hz, 2H), 7.22–7.14 (m, 4H). 13C NMR (100 MHz, DMSO-d6) δ (ppm) 160.24, 157.84, 145.51, 144.03, 144.01, 134.56, 130.17, 129.74, 127.98, 127.37, 127.27, 127.19, 126.98, 125.18, 124.20, 119.88, 117.11, 116.88. HRMS (ESI) m/z calcd for C26H19FN+ (M + H)+ 364.1497, found 364.1459.
N-(4-chlorophenyl)-N-(naphthalen-2-yl)naphthalen-2-amine
Synthesis of Cl-DNaAPh: A mixture of di(naphthalene-2-yl)amine (1.35 g, 5.000 mmol), 1-bromo-4-chlorobenzene (1.15 g, 6.000 mmol), palladium acetate (0.06 g, 0.250 mmol), potassium tert-butoxide (0.84 g, 7.500 mmol) and tri-butylphosphine solution (0.30 mL, 0.130 mmol) were dissolved in toluene (40 mL) in a Schlenk tube, then refluxed at 110 °C for 12 h under N2 atmosphere. The solution was concentrated under reduced pressure. The crude product was extracted with dichloromethane and water, and dried over anhydrous MgSO4. After removing the solvent, the residue was purified by silica gel column chromatography to afford Cl-DNaAPh as a white solid (1.60 g, 84.2%). 1H NMR (400 MHz, DMSO-d6) δ (ppm) 7.87–7.83 (m, 4H), 7.72–7.70 (m, 2H), 7.49 (d, J = 2.1 Hz, 2H), 7.43–7.38 (m, 4H), 7.36–7.34 (dt, J1 = 8 Hz, J2 = 2.6 Hz, 2H), 7.28–7.25 (dd, J1 = 12 Hz, J2 = 2.3 Hz, 2H), 7.09–7.07 (dt, J1 = 8 Hz, J2 = 2.6 Hz, 2H). 13C NMR (100 MHz, DMSO-d6) δ (ppm) 146.69, 145.02, 134.54, 130.46, 129.99, 129.89, 128.00, 127.48, 127.19, 127.01, 125.65, 125.45, 124.63, 120.97. HRMS (ESI) m/z calcd for C26H19ClN+ (M + H)+ 380.1206, found 380.1210.
N-(4-bromophenyl)-N-(naphthalen-2-yl)naphthalen-2-amine
Synthesis of Br-DNaAPh: A mixture of di(naphthalene-2-yl)amine (1.35 g, 5.000 mmol), 1,4-dibromobenzene (0.77 mL, 6.000 mmol), palladium acetate (0.06 g, 0.250 mmol), potassium tert-butoxide (0.84 g, 7.500 mmol) and tri-butylphosphine solution (0.30 mL, 0.130 mmol) were dissolved in toluene (40 mL) in a Schlenk tube, then refluxed at 110 °C for 12 h under N2 atmosphere. The solution was concentrated under reduced pressure. The crude product was extracted with dichloromethane and water, and dried over anhydrous MgSO4. After removing the solvent, the residue was purified by silica gel column chromatography to afford Br-DNaAPh as a white solid (1.70 g, 80.5%). 1H NMR (400 MHz, DMSO-d6) δ (ppm) 7.90–7.85 (m, 4H), 7.76–7.73 (m, 2H), 7.51 (d, J = 2.2 Hz, 2H), 7.50–7.48 (m, 2H), 7.46-7.39 (m, 4H), 7.30–7.28 (dd, J1 = 8 Hz, J2 = 2.3 Hz, 2H), 7.05–7.03 (dt, J1 = 8 Hz, J2 = 2.6 Hz, 2H). 13C NMR (100 MHz, DMSO-d6) δ (ppm) 147.12, 144.94, 134.55, 132.87, 130.50, 129.91, 128.01, 127.50, 127.02, 125.88, 125.49, 124.70, 121.12, 115.10. HRMS (ESI) m/z calcd for C26H19BrN+ (M + H)+ 424.0701, found 424.0705.
N-(naphthalen-2-yl)-N-(p-tolyl)naphthalen-2-amine
Synthesis of CH3-DNaAPh: A mixture of di(naphthalene-2-yl)amine (1.35 g, 5.000 mmol), 1-bromo-4-methylbenzene (0.74 mL, 6.000 mmol), palladium acetate (0.06 g, 0.250 mmol), potassium tert-butoxide (0.84 g, 7.500 mmol) and tri-butylphosphine solution (0.30 mL, 0.130 mmol) were dissolved in toluene (40 mL) in a Schlenk tube, then refluxed at 110 °C for 12 h under N2 atmosphere. The solution was concentrated under reduced pressure. The crude product was extracted with dichloromethane and water, and dried over anhydrous MgSO4. After removing the solvent, the residue was purified by silica gel column chromatography to afford CH3-DNaAPh as white solid (1.02 g, 56.7%). 1H NMR (400 MHz, DMSO-d6) δ (ppm) 7.92–7.89 (m, 2H), 7.71–7.67 (t, J = 8 Hz, 2H), 7.61 (d, J = 12 Hz, 1H), 7.60 (d, J = 8 Hz, 1H), 7.41–7.29 (m, 6H), 7.23–7.17 (m, 6H), 7.37 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ (ppm) 143.80, 137.82, 137.01, 134.88, 133.97, 133.94, 130.93, 130.53, 130.03, 129.81, 129.16, 128.83, 128.47, 128.43, 127.92, 126.80, 126.67, 126.48, 125.34, 124.54, 123.00, 122.96, 120.03, 108.83, 21.40. HRMS (ESI) m/z calcd for C27H22N+ (M + H)+ 360.1747, found 360.1738.
Methyl 4-(di(naphthalen-2-yl)methyl)benzoate
Synthesis of COOCH3-DNaAPh: A mixture of di(naphthalene-2-yl)amine (1.35 g, 5.000 mmol), methyl 4-bromobenzoate (1.29 g, 6.000 mmol), palladium acetate (0.06 g, 0.250 mmol), potassium tert-butoxide (0.84 g, 7.500 mmol) and tri-butylphosphine solution (0.30 mL, 0.130 mmol) were dissolved in toluene (40 mL) in a Schlenk tube, then refluxed at 110 °C for 12 h under N2 atmosphere. The solution was concentrated under reduced pressure. The crude product was extracted with dichloromethane and water, and dried over anhydrous MgSO4. After removing the solvent, the residue was purified by silica gel column chromatography to afford COOCH3-DNaAPh as white solid (1.69 g, 86.7%). 1H NMR (400 MHz, DMSO-d6) δ (ppm) 7.94–7.92 (d, J = 8 Hz, 2H), 7.90–7.88 (t, J = 4 Hz, 2H), 7.85–7.83 (d, J = 8 Hz, 2H), 7.79–7.76 (t, J = 6 Hz, 2H), 7.63 (d, J = 2 Hz, 2H), 7.47–7.43 (m, 4H), 7.35–7.32 (dd, J1 = 12 Hz, J2 = 2.2 Hz, 2H), 7.06–7.04 (d, J = 8 Hz, 2H). 13C NMR (100 MHz, DMSO-d6) δ (ppm) 167.46, 151.67, 144.39, 134.52, 131.48, 130.98, 130.13, 128.07, 127.72, 127.09, 125.93, 125.45, 123.96, 122.89, 120.93. HRMS (ESI) m/z calcd for C27H20NO2+ (M + H) + 390.1494, found 390.1499.
N-(naphthalen-2-yl)-N-(o-tolyl)naphthalen-2-amine
Synthesis of o-CH3-DNaAPh: A mixture of di(naphthalene-2-yl)amine (1.35 g, 5.000 mmol), 1-bromo-2-methylbenzene (0.72 mL, 6.000 mmol), tris(dibenzylideneacetone) dipalladium (0.23 g, 0.250 mmol), potassium tert-butoxide (0.84 g, 7.500 mmol) and tri-butylphosphine solution (0.30 mL, 0.125 mmol) were dissolved in toluene (25 mL) in a Schlenk tube, then refluxed at 85 °C for 5 h under N2 atmosphere. The solution was concentrated under reduced pressure. The crude product was extracted with dichloromethane and water, and dried over anhydrous MgSO4. After removing the solvent, the residue was purified by silica gel column chromatography to afford o-CH3-DNaAPh as a white solid (1.20 g, 66.7%). 1H NMR (400 MHz, DMSO-d6) δ (ppm) 7.83–7.78 (m, 4H), 7.66–7.64 (d, J = 8 Hz, 2H), 7.41–7.32 (m, 5H), 7.30–7.25 (td, J1 = 20 Hz, J2 = 1.6 Hz, 2H), 7.24 (d, J = 1.6 Hz, 2H), 7.22–7.20 (dd, J1 = 8 Hz, J2 = 2.3 Hz, 2H), 7.17–7.15 (dd, J1 = 8 Hz, J2 = 1.6 Hz, 1H), 2.02 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ (ppm) 145.35, 145.19, 136.07, 134.53, 132.42, 129.71, 129.69, 129.52, 128.28, 127.95, 127.19, 126.95, 124.73, 122.98, 117.43, 18.69. HRMS (ESI) m/z calcd for C27H22N+ (M + H)+ 360.1752, found 360.1708.
N-(2-chlorophenyl)-N-(naphthalen-2-yl)naphthalen-2-amine
Synthesis of o-Cl-DNaAPh: A mixture of di(naphthalene-2-yl)amine (1.35 g, 5.000 mmol), 1-bromo-2-chlorobenzene (1.15 g, 6.000 mmol), cuprous iodide (0.05 mg, 0.250 mmol) and sodium tert-butoxide (0.72 mg, 7.500 mmol) were dissolved in 1,4-dioxane (40 mL) in a Schlenk tube, then refluxed at 60 °C for 12 h under N2 atmosphere. The solution was concentrated under reduced pressure. The crude product was extracted with dichloromethane and water, and dried over anhydrous MgSO4. After removing the solvent, the residue was purified by silica gel column chromatography to afford o-Cl-DNaAPh as a white solid (0.8 g, 42.1%). 1H NMR (400 MHz, DMSO-d6) δ (ppm) 7.83–7.78 (m, 4H), 7.66–7.64 (d, J = 8 Hz, 2H), 7.41–7.32 (m, 5H), 7.30–7.25 (td, J1 = 20 Hz, J2 = 1.6 Hz, 2H), 7.24 (d, J = 1.6 Hz, 2H), 7.22–7.20 (dd, J1 = 8 Hz, J2 = 2.3 Hz, 2H), 7.17–7.15 (dd, J1 = 8 Hz, J2 = 1.6 Hz, 1H), 2.02 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ (ppm) 145.35, 145.19, 136.07, 134.53, 132.42, 129.71, 129.69, 129.52, 128.28, 127.95, 127.19, 126.95, 124.73, 122.98, 117.43, 18.69. HRMS (ESI) m/z calcd for C27H22N+ (M + H)+ 380.1206, found 380.1190.
General procedure for synthesis of hybrids
Poly(methyl methacrylate) (PMMA) solution (100 mg/mL): Stirring the solution of PMMA (10 g) in tetrahydrofuran (100 mL) in a 250 mL flask at room temperature for three days until completely dissolved.
Polystyrene (PS) solution (100 mg/mL): Stirring the solution of PS (10 g) in tetrahydrofuran (100 mL) in a 250 mL flask at room temperature for three days until completely dissolved.
OCH3-DNaAPh@PMMA film (1 wt%): The mixture of OCH3-DNaAPh (1 mg) and PMMA solution (1 mL) were added to the vessel, then ultrasonic concussion, evaporated, and dried naturally to get the highly transparent film.
The preparation methods of F-DNaAPh@PMMA, Cl-DNaAPh@PMMA, Br-DNaAPh@PMMA, CH3-DNaAPh@PMMA, COOCH3-DNaAPh@PMMA, and o-CH3-DNaAPh@PMMA films were similar to OCH3-DNaAPh@PMMA film.
Data availability
All data are available from the corresponding author upon request. The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files. Source data are available. The source data generated in this study have been deposited in the Figshare database under accession code https://doi.org/10.6084/m9.figshare.28912340. Coordinate files for DFT experiments are available in Supplementary Data 1. Source data are provided with this paper.
References
Domínguez, I. F., Distler, A. & Lüe, L. Stability of organic solar cells: the influence of nanostructured carbon materials. Adv. Energy Mater. 7, 1601320 (2017).
Gu, S. et al. Modulation of radical intermediates in rechargeable organic batteries. Adv. Mater. 36, 2306491 (2024).
Abdurahman, A. et al. Understanding the luminescent nature of organic radicals for efficient doublet emitters and pure-red light-emitting diodes. Nat. Mater. 19, 1224–1229 (2020).
Ai, X. et al. Efficient radical-based light-emitting diodes with doublet emission. Nature 563, 536–540 (2018).
Olshansky, J. H. et al. Using photoexcited core/shell quantum dots to spin polarize appended radical qubits. J. Am. Chem. Soc. 142, 13590–13597 (2020).
Rugg, B. K. et al. Photodriven quantum teleportation of an electron spin state in a covalent donor–acceptor–radical system. Nat. Chem. 11, 981–986 (2019).
Schaller, R. D. & Wasielewski, M. R. Photogenerated spin-correlated radical pairs: from photosynthetic energy transduction to quantum information science. J. Am. Chem. Soc. 143, 15508–15529 (2021).
Herrmann, C., Solomon, G. C. & Ratner, M. A. Organic radicals as spin filters. J. Am. Chem. Soc. 132, 3682–3684 (2010).
Quintes, T., Mayländer, M. & Richert, S. Properties and applications of photoexcited chromophore–radical systems. Nat. Rev. Chem. 7, 75–90 (2023).
Ratera, I. & Veciana, J. Playing with organic radicals as building blocks for functional molecular materials. Chem. Soc. Rev. 41, 303–349 (2012).
Hatakeyama-Sato, K. & Oyaizu, K. Redox: organic robust radicals and their polymers for energy conversion/storage devices. Chem. Rev. 123, 11336–11391 (2023).
Chen, Z. X., Li, Y. & Huang, F. Persistent and stable organic radicals: design, synthesis, and applications. Chem 7, 288–332 (2021).
Chang, L., An, Q., Duan, L., Feng, K. & Zuo, Z. Alkoxy radicals see the light: new paradigms of photochemical synthesis. Chem. Rev. 122, 2429–2486 (2022).
Staveness, D., Bosque, I. & Stephenson, C. R. J. Free radical chemistry enabled by visible light-induced electron transfer. Acc. Chem. Res. 49, 2295–2306 (2016).
Luo, M., Xiao, Q. & Li, J. Electro-/photocatalytic alkene-derived radical cation chemistry: recent advances in synthetic applications. Chem. Soc. Rev. 51, 7206–7237 (2022).
Ghosh, I., Ghosh, T., Bardagi, J. I. & König, B. Reduction of aryl halides by consecutive visible light-induced electron transfer processes. Science 346, 725–729 (2014).
Jin, J. et al. Photo-controllable luminescence from radicals leading to ratiometric emission switching via dynamic intermolecular coupling. Angew. Chem. Int. Ed. 62, e202214281 (2023).
Lee, M., Song, I., Hong, M., Koo, J. Y. & Choi, H. C. Single-component-based white light photoluminescence emission via selective photooxidation in an organic–polymer hybrid system. Adv. Funct. Mater. 28, 1703509 (2018).
Li, Y. et al. Photoinduced radical emission in a coassembly system. Angew. Chem. Int. Ed. 60, 23842–23848 (2021).
Yang, Y. et al. Photo-response with radical afterglow by regulation of spin populations and hole-electron distributions. Angew. Chem. Int. Ed. 62, e202218994 (2023).
Zhao, W., He, Z. & Tang, B. Z. Room-temperature phosphorescence from organic aggregates. Nat. Rev. Mater. 5, 869–885 (2020).
Wang, J. et al. Management of triplet excitons transition: fine regulation of Förster and Dexter energy transfer simultaneously. Light Sci. Appl. 13, 35 (2024).
Xiao, F. et al. Guest-host doped strategy for constructing ultralong-lifetime near-infrared organic phosphorescence materials for bioimaging. Nat. Commun. 13, 186 (2022).
Wang, Y. et al. High performance of simple organic phosphorescence host–guest materials and their application in time-resolved bioimaging. Adv. Mater. 33, 2007811 (2021).
Yang, Y. et al. Tunable photoresponsive behaviors based on triphenylamine derivatives: the pivotal role of π-conjugated structure and corresponding application. Adv. Mater. 33, 2104002 (2021).
Jockusch, S. & Yagci, Y. The active role of excited states of phenothiazines in photoinduced metal free atom transfer radical polymerization: singlet or triplet excited states?. Polym. Chem. 7, 6039–6043 (2016).
Rahal, M. et al. Investigation of pyrene vs Anthracene-based oxime esters: role of the excited states on their polymerization initiating abilities. Eur. Polym. J. 177, 111452 (2022).
Wang, Z. et al. Recent advances in organic room-temperature phosphorescence of heteroatom (B/S/P)-containing chromophores. CCS Chem. 5, 292–309 (2023).
Zhang, Y. et al. Cross-linked polyphosphazene nanospheres boosting long-lived organic room-temperature phosphorescence. J. Am. Chem. Soc. 144, 6107–6117 (2022).
Wang, Z., Zhou, J., Zhang, Y., Zhu, W. & Li, Y. Accessing highly efficient photothermal conversion with stable open-shell aromatic nitric acid radicals. Angew. Chem. Int. Ed. 61, e202113653 (2022).
Zhang, Z. et al. Near-infrared emission beyond 900 nm from stable radicals in nonconjugated poly(diphenylmethane). Angew. Chem. Int. Ed. 63, e202403827 (2024).
Kato, K. & Osuka, A. Platforms for stable carbon-centered radicals. Angew. Chem. Int. Ed. 58, 8978–8986 (2019).
Jiao, Y. et al. A supramolecularly activated radical cation for accelerated catalytic oxidation. Angew. Chem. Int. Ed. 55, 8933–8937 (2016).
Shimizu, D., Furukawa, K. & Osuka, A. Stable subporphyrin meso-aminyl radicals without resonance stabilization by a neighboring heteroatom. Angew. Chem. Int. Ed. 56, 7435–7439 (2017).
Sindt, A. J. et al. Guest inclusion modulates concentration and persistence of photogenerated radicals in assembled triphenylamine macrocycles. J. Am. Chem. Soc. 142, 502 (2020).
Tanaka, M., Yoshida, H. & Ichikawa, T. Thermal and photo-induced reactions of polymer radicals in γ-irradiated poly(alkyl methacrylate). Polym. J. 22, 835 (1990).
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Kang, C. H. et al. High-speed colour-converting photodetector with all-inorganic CsPbBr3 perovskite nanocrystals for ultraviolet light communication. Light Sci. Appl. 8, 94 (2019).
Lampel, A. et al. Polymeric peptide pigments with sequence-encoded properties. Science 356, 1064–1068 (2017).
Li, J., Duan, H. & Pu, K. Nanotransducers for near-infrared photoregulation in biomedicine. Adv. Mater. 31, 1901607 (2019).
Zuo, J. et al. Near infrared light sensitive ultraviolet−bluenanophotoswitch for imaging-guided “off−on” therapy. ACS Nano 12, 3217–3225 (2018).
Guo, K., Wu, Z., Chen, C. & Fang, J. UV/chlorine process: an efficient advanced oxidation process with multiple radicals and functions in water treatment. Acc. Chem. Res. 55, 286–297 (2022).
Wang, J., Yang, Y., Li, K., Zhang, L. & Li, Z. Purely organic fluorescence afterglow: visible-light-excitation, inherent mechanism, tunable color, and practical applications with very low cost. Angew. Chem. Int. Ed. 62, e202304020 (2023).
Peng, F. et al. Color-tunable, excitation-dependent, and water stimulus-responsive room-temperature phosphorescence cellulose for versatile applications. Adv. Mater. 35, 2304032 (2023).
Zheng, Z. et al. Digital photoprogramming of liquid-crystal superstructures featuring intrinsic chiral photoswitches. Nat. Photon. 16, 226–234 (2022).
Zhang, Y. et al. Ultraviolet irradiation-responsive dynamic ultralong organic phosphorescence in polymeric systems. Nat. Commun. 12, 2297 (2021).
Zheng, J. et al. Photochromism from wavelength-selective colloidal phase segregation. Nature 617, 499–506 (2023).
Crespi, S., Simeth, N. A. & König, B. Heteroaryl azo dyes as molecular photoswitches. Nat. Rev. Chem. 3, 133–146 (2019).
Li, D. et al. Completely aqueous processable stimulus responsive organic room temperature phosphorescence materials with tunable afterglow color. Nat. Commun. 13, 347 (2022).
Chen, X. et al. Engineering stable radicals using photochromic triggers. Nat. Commun. 11, 945 (2020).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 22235006, received by Z.L.; 22405200, received by Y.Y.) and the starting Grants of Tianjin University and the Open Project (received by Z.L.). The Open Fund of the State Key Laboratory of Luminescent Materials and Devices (South China University of Technology, No. 2024-skllmd-04, received by Z.L.).
Author information
Authors and Affiliations
Contributions
M.F. and Z.L. conceived the experiments. Y.Y. was primarily responsible for the experiments, then further purified all compounds, carried out the photophysical properties survey, analyzed the optical data and conducted corresponding applications. J.W. conducted the theoretical calculations. J.Y. provided support for the mechanism investigation of photochromism and analyzed the optical data. L.T. and X.C. provided support on the application of the films. M.F. and Z.L. provided support and suggestions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Yinyin Bao, Yuan Li, and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, Y., Wang, J., Yang, J. et al. Constructing an auto triplet excitons supply system for photogenerated radicals in the solid state. Nat Commun 16, 8356 (2025). https://doi.org/10.1038/s41467-025-61353-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-61353-5