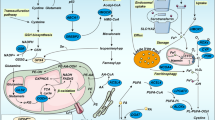
Abstract
The key to achieving synergistic ferroptosis immunotherapy is enhancing the iron content in tumor cells, improving specific immunity, and regulating the tumor microenvironment. In this study, a drug-free biohybrid system targeting ferritin is developed using M1 macrophage microvesicles and HKN15-modified Prussian blue nanoparticles for synergistic ferroptosis immunotherapy. HKN15-modified nanoparticles simultaneously enhance iron content by activating endogenous iron ions and replenishing exogenous iron ions, which disrupts iron homeostasis for inducing ferroptosis in tumor cells. Photothermally enhanced ferroptosis based on Prussian blue nanoparticles also stimulates dendritic cell maturation. Moreover, M1 vesicles and iron ions from Prussian blue nanoparticles promote macrophage polarization to improve specific immunity. The mutual promotion of ferroptosis and antitumor immunity effectively results in a synergistic therapeutic circuit for inhibiting tumor growth and preventing cancer recurrence and metastasis in 4T1 tumor-bearing female mice, thus offering a promising strategy for drug-free biohybrid system-mediated ferroptosis immunotherapy.
Similar content being viewed by others
Introduction
Owing to the unique biological behavior, clinicopathological characteristics, and poor prognosis of triple-negative breast cancer, targeted precision therapy and tumor prevention remain great challenges1,2. Tumor immunotherapy has emerged as an important method for treating breast cancer3,4. Enhancing the effect of immunotherapy on triple-negative breast cancer and comprehensively improving the specific immunity of the body are the keys to achieving efficient tumor immunotherapy. Ferroptosis is a special type of programmed cell death based on iron-dependent lipid peroxidation5,6,7. Notably, triple-negative breast cancer specifically expresses genes related to ferroptosis, promoting ferroptosis and making it an attractive therapeutic target8. Recently, the ferroptosis pathway of tumor cells was shown to be involved in T cell-mediated antitumor immune responses, suggesting that synergistic ferroptosis immunotherapy is a promising strategy for tumor immunotherapy9,10,11,12.
It is a key factor in improving iron accumulation in tumor cells to induce ferroptosis. There are two pathways whereby iron accumulation disrupts iron homeostasis in tumor cells: activation of endogenous iron ions and replenishment of exogenous iron ions. Ferritin is the main iron storage protein in cells during iron ions metabolism and is overexpressed in malignant tumors, including breast cancer6,13. An important factor of ferroptosis is the destruction of endogenous ferritin in tumor cells, as the increased intracellular free iron triggers endogenous ferroptosis. Thus, ferritin-rich tumor cells are an attractive endogenous resource for triggering ferroptosis14. HKN15 (HKNKGKKNGKHNGWK) exhibits strong ferritin homing ability, and HKN15-modified nanoparticles surround ferritin after being ingested by tumor cells, guaranteeing ferritin destruction to promote endogenous iron ion accumulation and trigger ferroptosis15. In contrast, exogenous iron ions are effective supplements for endogenous iron ions. Various iron-based nanomaterials can deliver exogenous iron to activate tumor ferroptosis16. Especially, Prussian blue nanoparticles (HMPB) are iron-organic frameworks with good biocompatibility and photothermal properties that stimulate ferroptosis and promote macrophage polarization after the release of iron ions through degradation17,18. Therefore, targeting intratumoral ferritin using HKN15-modified iron-based nanoparticles could simultaneously activate endogenous iron ions and replenish exogenous iron ions to enhance their accumulation in tumor cells through a combination of internal and external approaches, resulting in a more efficient ferroptosis effect.
Precise targeted delivery promotes the accumulation of iron ions in cells and regulates the tumor immune microenvironment, which is an important challenge for the synergistic treatment of ferroptosis and antitumor immunity. The inspiration from natural biological systems facilitated the rapid development of biological cell drug delivery systems19,20,21,22,23. Extracellular vesicles are circulating living cell-derived delivery systems that retain the biological function of live cell carriers and avoid safety issues, complicated preparation, and live cell carrier preservation. They have the advantages of low-immunogenicity, high-biocompatibility, high loading capacity, precise targeting, as well as easy chemical and genetic regulation24,25,26. Among them, M1 macrophage vesicles have intrinsic tumor-targeting ability owing to chemokine receptors on their surface and re-educate M2 macrophages into M1, phagocytize tumor cells, and activate innate immunity27,28,29. A biohybrid delivery system combining microvesicles and nanomaterials can integrate the advantages of biomimetic and nanodelivery systems to better achieve targeted tumor therapy. In previous studies, we developed a macrophage apoptotic body-based biohybrid delivery system for photothermal immunotherapy through targeted and precise delivery, which showed promising therapeutic effects on primary tumors, tumor metastasis, and the prevention of breast cancer30.
In this work, a drug-free biohybrid system targeting ferritin is constructed using M1 macrophage microvesicles (M1EV) and HKN15-modified Prussian blue nanoparticles (HMPB-H) for the photothermal-enhanced synergistic therapy of ferroptosis antitumor immunity (Fig. 1). The tumor-oriented ability of macrophage microvesicles is utilized for active targeted delivery to the tumor site. HMPB are homed around ferritin by the HKN15 targeting function, and increasing iron ions are achieved through two pathways: endogenous factors from photothermal-induced ferritin destruction and exogenous factors from nanoparticles degradation. The released Fe3+ is reduced to Fe2+ by high concentrations of reducing agents, such as glutathione (GSH), to catalyze the peroxidation of unsaturated lipids in tumor cells and liposomes, resulting in a large accumulation of lipid peroxide. The dual accumulation effects of iron ions amplify the oxidative stress in tumor cells, enhance tumor cell ferroptosis, and trigger an antitumor immune response. Photothermally enhanced ferroptosis based on HMPB also stimulates dendritic cell maturation. M1 vesicles and iron ions from HMPB promote macrophage polarization. The CD8+ T cells-secreted interferon γ stimulates liposome peroxidation and promotes ferroptosis, thus achieving synergistic therapeutic circuit via mutual promotion of ferroptosis and antitumor immunity.
A ferritin-targeted biohybrid system is constructed using M1 macrophage microvesicles (M1EV) and HKN15-modified Prussian blue nanoparticles (HMPB-H). The tumor-oriented ability of macrophage microvesicles is utilized for active targeted delivery to the tumor site. HKN15-modified nanoparticles simultaneously enhance iron content by activating endogenous iron ions and replenishing exogenous iron ions. The dual accumulation effects of iron ions amplify the oxidative stress in tumor cells, enhance tumor cell ferroptosis, and trigger an antitumor immune response. The mutual promotion of ferroptosis and antitumor immunity effectively results in a synergistic therapeutic circuit for inhibiting tumor growth and preventing cancer recurrence and metastasis in 4T1 tumor-bearing female mice.
Results
Characterization of biohybrid system
The drug-free biohybrid system comprised HMPB and M1EV. HMPB and HMPB-H nanoparticles exhibited irregular spherical structures (Fig. 2a and Supplementary Fig. 1a, b). As nanospheres comprise several small particles and have a larger specific surface area, they more easily degrade and release iron ions, thereby enhancing their biological efficacy in inducing ferroptosis. Following purification of M1EV using a well-established centrifugation protocol, the nanoparticles adhered to the surface of the cellular vesicles to form the HMPB-H@M1EV biohybrid system (Fig. 2b, c and Supplementary Fig. 1c). RAW264.7 cells (83.7%) were polarized to the M1 phenotype by treatment with lipopolysaccharide (LPS) (Fig. 2d). The DiD+RhB+ double positive signal represented that the all vesicles are efficiently loaded with nanoparticles without leakage (Supplementary Fig. 1e). According to the number ratio of feeding particles (Supplementary Fig. 1d) and the loading efficiency of nanoparticles on M1EV (97.2%) by ICP-MS, the number ratio of nanoparticle-to-vesicle was 4.97 after complexation. The size of HMPB-H increased to 241 nm compared to that of HMPB at 223 nm, whereas the negative potential (−6.5 mV) changed to a positive potential (7.6 mV) (Fig. 2e, f). HMPB and HMPB-H retained their stable particle size over 7 d in phosphate buffered saline (PBS; Supplementary Fig. 2). The hydrodynamic size and zeta potential of HMPB-H@M1EV are 652 nm and −37.0 mV, respectively, with slight changes compared to M1EV (569 nm, −38.3 mV). Elemental analysis (Fig. 2g) demonstrates that the three primary peaks at 721, 712.1, and 708 eV are derived from Fe 2p1/2 and Fe 2p3/2, respectively. Fe3+ is compatible with the peaks at 712.1 and 721 eV, whereas Fe2+ could be the cause of the other peak (708 eV) (Fig. 2h). Charge transfer jumps between Fe2+ and Fe3+ produced distinctive HMPB adsorption peaks at 720 nm and a red shift in the adsorption peak of HMPB-H was observed in the ultraviolet-vis spectra (Fig. 2i). In infrared spectroscopy of HMPB-H, the characteristic peaks at 2089 and 503 cm−1 are attributed to C ≡ N and Fe(II)-CN-Fe(III), corresponding to those of HMPB. The characteristic peaks at 1702 cm−1 are C = O generated by carboxyl group and amide bond, and the three between 900 and 700 cm−1 correspond to the bending vibration absorption peaks of the aromatic ring in HKN15, further confirming the successful preparation of HMPB-H (Supplementary Fig. 3). HMPB and HMPB-H could catalyze the overexpression of H2O2 in tumor microenvironment (TME) to the highly toxic ·OH via a POD-like enzyme-catalyzed reaction. Both HMPB and HMPB-H efficiently converted H2O2 to ·OH at pH 5.5, which is similar to the acidity of the tumor lysosome, whereas HMPB and HMPB-H decomposed H2O2 to non-toxic oxygen rather than to hydroxyl radicals at pH 7.4 (Fig. 2j–l). Thus, the catalytic activities of HMPB and HMPB-H continuously and selectively generates O2 and ·OH, and that the acid dependence of ·OH formation facilitated the prevention of damage to normal cells and tissues. In addition, the temperature of HMPB-H under irradiation reached >50 °C within 5 min and maintained an ideal photothermal stability with three irradiation cycles (Fig. 2m, n and Supplementary Fig. 4). The photothermal conversion efficiency of HMPB-H@M1EV is 29.46% (Supplementary Fig. 5). Elevated temperatures were positively correlated with the concentration of HMPB-H (Supplementary Fig. 6). The photothermal properties of HMPB-H guarantees the activation of endogenous iron ions and subsequent photothermal therapy (PTT). As the degradation of HMPB-H in the tumor environment is a prerequisite for the provision of exogenous iron ions, the biodegradation of HMPB-H was investigated based on the color change of the solution. HMPB-H degraded more rapidly under low pH conditions than it did under neutral conditions, changing from dark blue to nearly colorless after 48 h (Fig. 2o). Moreover, the in vitro degradation of HMPB-H and its ability to disrupt horse spleen ferritin (HSF) under irradiation were evaluated. The released iron ion from HMPB-H reached 16 μM, while the released iron ion from HMPB-H and HSF exceeded 40 μM with irradiation-triggered ferritin degradation (Fig. 2p). This confirmed the accumulation of endogenous iron ions and replenishment of exogenous iron ions, ensuring an enhanced ferroptosis therapeutic effect.
a–c TEM images of a HMPB-H, b M1EV, and c HMPB-H@M1EV. d Phenotypic transformation of macrophages analyzed using flow cytometry. e, f Hydrodynamic diameters and zeta potentials of HMPB, HMPB-H, M1EV, and HMPB-H@M1EV. g, h XPS spectrum of HMPB-H. i UV–vis adsorption spectrum of HMPB, HKN15, and HMPB-H. j, k UV–vis absorption spectra showing the degradation of MB in j pH 5.5 PBS and k pH 7.4 PBS under different conditions. l Oxygen production measured by a dissolved oxygen meter under different conditions. m Thermal images of PBS, HMPB, HMPB-H, and HMPB-H@M1EV under 808 nm laser irradiation for 5 min. n Temperature profile of HMPB-H solution for laser irradiation on/off cycles. o Change of HMPB-H in pH 5.5 and pH 7.4 solutions over 48 h. p Release profiles of iron ions from HMPB-H and HSF for 48 h. Data are presented as the mean ± SD (n = 3 independent experiments).
Cellular uptake and intracellular distribution of HMPB-HKN15
The drug-free biohybrid system for synergistic ferroptosis immunotherapy performs two main functions: inducing endogenous/exogenous ferroptosis and regulating the tumor immune microenvironment based on tumor-associated macrophage (TAM) reprogramming and photothermal stimulation effects. Effective uptake of HMPB by tumor cells is essential for inducing ferroptosis. Confocal laser scanning microscopy (CLSM) images (Supplementary Fig. 7) indicated that all tumor cells engulfed the nanoparticles after 12 h of co-incubation, and that they were mainly distributed in the cytoplasm. Notably, a stronger fluorescence signal was observed in the HMPB-HKN15 group than in the HMPB group. The average intracellular fluorescence intensity was significantly higher in HMPB-HKN15 -treated cells than in HMPB-treated cells (Supplementary Fig. 8); therefore, the increase in uptake efficiency may be due to the change from the negative to the positive charge of the nanoparticles after peptide modification. Generally, the nanoparticles are internalized by cells in an endocytic manner and are inevitably transported to endosomes/lysosomes. However, under laser irradiation, photothermally-triggered lysosomal escape facilitates the release of more nanoparticles into the cytoplasm (Supplementary Fig. 9), allowing them to target ferritin and subsequently supply endogenous iron ions.
Since HKN15 has a strong ferritin-tending ability31, HMPB-H were expected to aggregate around ferritin after uptake, being more conducive to the destruction of ferritin to release the stored iron through the photothermal effect of Prussian blue. The co-localization of ferritin with HMPB-H was observed using CLSM. A large overlap between the red fluorescence of HMPB-H and green fluorescence of ferritin was clearly observed (Fig. 3a). Moreover, the high coincidence of the two fluorescent signals through co-localization analysis further verified the remarkable aggregation of HMPB-H toward ferritin (Fig. 3b). Thus, the high cellular uptake and ferritin aggregation of HMPB-H nanoparticles have provided prerequisites for the disruption of ferritin to release iron ions.
a, b CLSM images and plot profile of HMPB-H and ferritin co-localization analysis in 4T1 cells. Scale bar: 20 µm. c The intracellular ROS levels were monitored using DCFH-DA. Scale bar: 50 µm. d Intracellular levels of GSH by CLSM. Scale bar: 50 µm. e GPX4 expression using western blotting. f LPO levels detected by Liperfluo probe. Scale bar: 50 µm. g, h LPO levels estimated by flow cytometric quantification. i, j Annexin V/PI staining by flow cytometric quantification. k Calcein/PI staining assay. Scale bar: 50 µm. l Intracellular oxygen levels by CLSM. Scale bar: 50 µM. m, n Cell viability and LPO levels in 4T1 cells treated with HMPB-H + L in the presence or absence of Fer-1. o, p Corresponding gray value analysis of GPX4 and SLC7A11 expression in 4T1 cells after different treatments. For (a, c–f, k, l), experiment was repeated three times independently with similar results. The data in (h, j, m–p) are presented as the mean ± SD (n = 3 independent experiments). Statistical differences were analyzed by one-way ANOVA with Tukey’s multiple comparisons test.
Subsequently, we examined whether the level of iron within the tumor cells could be elevated by the degradation of exogenous HMPB and destruction of endogenous ferritin. FerroOrange was used to detect intracellular ferrous ion levels. The degradation of HMPB-H and HMPB resulted in a limited iron content (Supplementary Fig. 10). The strongest FerroOrange signal intensity was observed in the HMPB-H + L group, whereas the intracellular iron level in the HMPB + L group was only moderately elevated owing to the absence of ferritin targeting. Therefore, the decomposition of HMPB in an acidic environment releases exogenous iron for the production of toxic hydroxyl radicals, coupled with the targeting and photothermal destruction of ferritin in a neutral cytoplasmic environment, thus collectively constituting a synergistic process. Accordingly, accumulated levels of intracellular iron from exogenous HMPB degradation and endogenous ferritin induce stronger ferroptosis in tumor cells.
We further investigated the uptake of HMPB-H@M1EV using flow cytometry and ferritin co-localization using laser confocal imaging to verify its ferritin targeting ability. No significant difference was observed in the uptake efficiencies of HMPB-H@M1EV and HMPB-H (Supplementary Fig. 11). The co-localization of ferritin with HMPB-H@M1EV affirmed its ability to target ferritin (Supplementary Fig. 12). Macrophage vesicles did not affect their targeting owing to the surface loading of the nanoparticles. The cellular uptake mechanism of HMPB-H@M1EV and HMPB-H was investigated by treating the cells with various endocytic pathway inhibitors using flow cytometry (Supplementary Fig. 13). The results demonstrated that both HMPB-H and HMPB-H@M1EV were taken up by tumor cells in an energy-dependent manner, and clathrin-mediated endocytosis was mainly involved. In addition, the internalization partly involved caveolin-mediated endocytosis and macropinocytosis.
In vitro HMPB-HKN15-induced ferroptosis
Typical markers were used to demonstrate HMPB-HKN15-induced ferroptosis. The intensity of intracellular green fluorescence moderately increased in the HMPB and HMPB-H groups relative to that in the PBS group, whereas the enhanced fluorescence intensity suggested that laser irradiation remarkably elevated reactive oxygen species (ROS) content (Fig. 3c). HMPB-H + L-treated cells produced the highest levels of intracellular ROS. However, there was no significant accumulation of intracellular iron in HMPB + L-treated cells owing to the lack of ferritin targeting, and cellular ROS levels remained elevated to some extent. This suggests that temperature may be another important factor affecting ROS generation efficiency, thus strengthening ferroptosis32.
As an intracellular antioxidant, GSH converts oxidized GSH via GPX4, thereby protecting cells from ferroptosis by inhibiting cytotoxic LPO. Therefore, GSH depletion can indirectly inhibit GPX4, enhance the ferroptotic Fenton reaction, and cause LPO accumulation. As expected, GSH levels and GPX4 activity were remarkably decreased in the HMPB + L and HMPB-H + L groups relative to that in the HMPB or HMPB-H groups. Due to the limited exogenous iron from Prussian blue, the level of GSH did not significantly decrease in the HMPB and HMPB-H groups, whereas GSH consumption in the HMPB + L treatment was significantly enhanced. In HMPB-H + L-treated cells, the iron release triggered by intracellular ferritin degradation caused the greatest rate of GSH depletion (Fig. 3d). Accordingly, GPX4 expression showed the same trend, and its activity was inhibited in the HMPB-H + L group, suggesting that photothermally enhanced ferroptosis further damaged the cellular antioxidant defense (Fig. 3e).
LPO accumulation ultimately disrupts cellular redox homeostasis and causes ferroptosis. Relative to the decreased GSH levels and GPX4 activity, the strongest green fluorescence signals from the LPO probe were observed in HMPB-H + L-treated cells (Fig. 3f ). HMPB-H + L-induced exogenous and endogenous ferroptosis led to LPO accumulation in 84.5% of 4T1 cells (Fig. 3g, h). The killing effect of ferroptosis-induced lipid peroxidation was further verified by apoptosis detection and live cell staining analysis. The overall Annexin V+cells was approximately 60% in the HMPB-H + L group, and the proportion of dead cells was increased (Fig. 3I, j). Using PTT resulted in a nearly tenfold (HMPB + L vs HMPB) and sixfold increase (HMPB-H + L vs HMPB-H) in the overall Annexin V+cells rate, demonstrating the role of PTT in accelerating LPO and enhancing ferroptosis. Moreover, HMPB-H + L treatment led to an obvious increase in Annexin V+ PI+ cells rate (24.4%) compared to the HMPB + L treatment (9.54%), indicating that endogenous ferroptosis or synergistic effects caused an eventual increase in the total Annexin V+cells rate. The calcein/propidium iodide staining assay performed on the 4T1 cells after different treatments demonstrated similar results (Fig. 3k).
Hydrogen peroxide levels are higher in tumors compared to that in normal tissues, and Prussian blue could efficiently catalyze H2O2 into O2 with a positive correlation to pH value18. Here, the decrease in [Ru(dpp)3]Cl2 fluorescence intensity reflects an elevation in intracellular O2 content. Laser irradiation effectively promoted intracellular O2 production via Prussian blue catalysis (Fig. 3l), implying that this ferroptosis-inducing system may simultaneously alleviate the symptoms of tumor hypoxia, which is conducive to synergistic tumor treatment.
Furthermore, we used the ferroptosis inhibitor, Ferrostatin-1 (Fer-1) to confirm ferroptosis in the biohybrid system. The cytotoxicity of HMPB-H + L was alleviated in the presence of Fer-1, and cell viability was restored to 80% of baseline levels (Fig. 3m). In contrast, the fluorescence signal of LPO decreased from 79.6% in the HMPB-H + L group to 36.7% in the HMPB-H + L+Fer-1 group, indicating that ferroptosis-induced lipid peroxidation accumulation was dramatically blocked by Fer-1 (Fig. 3n and Supplementary Fig. 14). Furthermore, the western blotting analysis of GPX4 was consistent with the LPO levels, affirming that HMPB-H + L effectively induced ferroptosis in tumor cells.
The SLC7A11-GSH-GPX4 signaling axis is a crucial antioxidative defense system. A reduction in the expression of the downstream regulator, GPX4, was observed along this signaling axis. Similarly, SLC7A11 expression was downregulated in the HMPB-H + L-treated groups, decreasing GSH synthesis and lipid peroxidation. However, the addition of Fer-1 promoted the expression of SLC7A11, indicating a restoration of the antioxidant capacity of the tumor cells, further confirming the important role of SLC7A11 in ferroptosis (Fig. 3o, p and Supplementary Fig. 15).
The above results not only illustrate the successful induction of exogenous and endogenous ferroptosis in tumor cells but also reveal the multiple functions of Prussian blue in the whole system. It serves as a source of exogenous iron, an initiator for the photothermal destruction of ferritin, and a facilitator of tumor hypoxia alleviation. The photothermal effect further enhances ROS production, accelerates lipid peroxidation, and amplifies ferroptosis. This positive feedback ferroptosis-triggering system maximizes tumor suppression through synergistic roles.
Effects of ferroptosis on macrophages and 4T1 tumor cells
Another important component of this biohybrid system for synergistic therapy was the macrophage external vesicle (M1EV). Synergistic ferroptosis immunotherapy is achieved via M1EV-induced TAM reprogramming and a PTT-triggered immune response. Therefore, it is necessary to avoid ferroptosis-induced damage to immune cells. Relevant studies have shown that triple-negative breast cancers were particularly susceptible to ferroptosis inducers6,13. Moreover, the gene expression level of ferritin was upregulated in triple-negative breast cancer tissues compared to that in normal tissues. These biological characteristics are not only favorable for inducing ferroptosis in tumor cells, but also for avoiding potential damage to normal cells from HMPB-HKN15-induced endogenous ferroptosis. Herein, the ferritin level in tumor cells was confirmed to be significantly higher than that in macrophages (M1 and M2 phenotypes) by western blot analysis (Fig. 4a and Supplementary Fig. 16). Ferroptosis-induced LPO aggregation in macrophages was lower than that in 4T1 tumor cells (Fig. 4b and Supplementary Fig. 17); thus, macrophages maintained their viability without significant adverse effects of ferroptosis. In addition, consistent with previous studies, M1 macrophages expressed higher levels of iNOS (Fig. 4c and Supplementary Fig. 18), resulting in higher levels of nitric oxide free radicals than M2 macrophages33, inhibiting LPO to a certain extent and resisting ferroptosis-induced injuries34 (Fig. 4d). Therefore, it could be inferred that endogenous/exogenous ferroptosis induced by ferroptosis synergistic immunotherapy has less of an effect on M1 macrophages than on 4T1 tumor cells, ensuring the feasibility of the combined macrophage modulation strategy.
a Ferritin expression in 4T1 and macrophages are analyzed Western blotting. b Flow cytometric analysis of LPO levels in HMPB-H and laser treated 4T1 macrophages. c Flow cytometric analysis of iNOS expression in 4T1 macrophages. d Calcein/PI staining assay in HMPB-H and laser treated-4T1 macrophages. Scale bar: 50 µm. e, f Quantification of CD206+ and CD86+ cells in M2 macrophages treated with PBS, M0EV, M1EV, and HMPB-H@M1EV. g, h ELISA assay of TNF-α and IL-6 in M2 macrophages post-treatment with PBS, M0EV, M1EV, and HMPB-H@M1EV. ND stands for not detected. i Representative CLSM images of M2-like macrophages phagocytosing 4T1 cells. Macrophages and 4T1 cells were labeled with RhB (red) and CFSE (green), respectively. Scale bar: 50 µm. j 4T1 phagocytosis by macrophages. k Schematic diagram of the in vitro co-culture process of immature DCs and treated 4T1 cells. Created with MedPeer (www.medpeer.cn). l, m CD80+ CD86+ DCs by flow cytometric analysis. For (a–d, i), experiment was repeated three times independently with similar results. The data in (e–h, m) are presented as the mean ± SD (n = 3 independent experiments). Statistical differences were analyzed by one-way ANOVA with Tukey’s multiple comparisons test.
Re-education of macrophages and dendritic cells (DC) maturation in vitro
Macrophage re-education was assessed by detecting the expression of CD206 (an M2 macrophage marker) and CD86 (an M1 macrophage marker). After treating interleukin (IL)-4-induced M2-type macrophages with different preparations, the proportion of CD206+ macrophages significantly decreased in the M1EV and HMPB-H@M1EV groups compared to that in the M0EV group (Fig. 4e and Supplementary Fig. 19). In contrast, CD86 expression was greatly elevated by approximately tenfold in the M1EV and HMPB-H@M1EV groups relative to the M0EV group (Fig. 4f and Supplementary Fig. 20), indicating that M1EV has a potent macrophage polarization effect. Increased TNF-ɑ and IL-6 expression levels also indicated better macrophages activity (Fig. 4g, h). CLSM and flow cytometry further demonstrated that macrophage phagocytosis of tumor cells increased from 2.74% (M0EV) to 31.0% (M1EV) and 37.3% (HMPB-H@M1EV) after M1EV treatment (Fig. 4I, j and Supplementary Fig. 21).
Macrophage polarization was attributed to the accumulation of M1 and HMPB. M1 macrophage vesicles retain a function similar to that of their mother cells and can continuously re-educate M2 macrophages into M1 macrophages, phagocytize tumor cells, and activate inherent immunity. The release of iron ions after HMPB degradation also promotes macrophage polarization. Cancer cells can compete with macrophages for iron35, causing a higher iron content. We also demonstrated a difference in ferritin levels between the two cell types with the iron restriction inducing or maintaining the immunosuppressive polarization state of M2 macrophages in the TME36,37. Related reports indicate that supplementing iron with iron-based nanoparticles replaces M2 macrophages with proinflammatory macrophages through the interferon-regulatory factor 5 pathway38. Prussian blue induces M2-TAM to the M1 phenotype17. Herein, HMPB-H@M1EV had an improved ability to polarize M2 macrophages to a certain extent compared to M1EV, indicating that Prussian blue-supplemented iron ions combined with M1 vesicles co-polarized macrophages, thereby increasing their ability to engulf tumor cells. These results further confirm the importance and feasibility of a therapeutic strategy based on tumor cell ferroptosis combined with macrophage regulation.
DCs maturation triggers adaptive immunity. Photothermally enhanced ferroptosis based on HMPB stimulates DCs maturation for antitumor immune responses. The expression of costimulatory molecules was evaluated by co-incubating DCs with pretreated 4T1 cells (Fig. 4k), and found that HMPB-H pretreated tumor cells showed increased CD80 and CD86 expression. Furthermore, the stimulatory effect of laser-irradiated tumor cells on DC was stronger than that of non-laser-irradiated cells, and the proportion of CD80+CD86+ DCs that interacted with HMPB-H-treated tumor cells increased from 34% to 46.8% under irradiation, whereas the proportion of mature DCs that interacted with HMPB-H@M1EV-treated tumor cells increased from 45.6% to 60.2% (Fig. 4l, m). The increase in TNF-ɑ expression level also reflected the enhanced activity of DC (Supplementary Fig. 22).
Tumor targeting and photothermal properties
Targeted delivery is a primary strategy for achieving effective treatment. Compared to other traditional synthetic carriers, M1EVs have multiple advantages that make them promising vehicles for drug delivery. The natural membrane structure of M1EV allows them to remain stable in systemic circulation, and chemokine receptors on the surface confer intrinsic tumor-targeting ability. Therefore, M1EVs are more susceptible to accumulation at tumor site owing to their biological characteristics, including circulatory stability, tumor-targeting ability, and permeability or retention effects. They maintain the biological function of living cell carriers and avoid the safety concerns of living cell carriers. First, flow cytometry was conducted to evaluate the uptake of HMPB-H@M1EV by 4T1 cells and normal cells (3T3 and HUVEC), revealing a significantly higher uptake in 4T1 cells (Supplementary Fig. 23). Next, tumor accumulation of HMPB-H@M1EV was evaluated using rhodamine as an indicator in 4T1 tumor mice using in vivo fluorescence imaging. The ex vivo fluorescence imaging indicated that the intratumoral fluorescence signal of the HMPB-H@M1EV group was clearly observed after the 24 h injection (Fig. 5a, c), and the mean fluorescence intensity was significantly higher than that in the HMPB-H group (Fig. 5b, d). From the tumor tissue section images, HMPB-H nanoparticles were mainly dispersed in a small area close to the edge of the blood vessels, whereas HMPB-H@M1EV penetrated deeper tissue sites along the blood vessels with a uniform fluorescence signal (Fig. 5e, f). Thus, M1EVs facilitated the accumulation of more nanoparticles at the tumor site and promoted their diffusion into deeper tumors for better therapeutic effects.
a, b Ex vivo fluorescence imaging and quantification 24 h after HMPB-H/RhB and HMPB-H/RhB@M1EV injections. c, d Fluorescent imaging and quantification of isolated tumors. e, f Fluorescence images of tumor tissue displaying fluorescence distribution around (e) tumor margins and (f) blood vessels. Scale bar: 200 μM. g, h In vivo photothermal imaging and time-temperature profiles under 808 nm irradiation 24 h intravenous post-injection. For (a, e, f), experiment was repeated three times independently with similar results. The data in (b, d, h) are presented as the mean ± SD (n = 3 mice). Statistical differences were analyzed by two-tailed Student t-test.
PTT is an important link between ferroptosis and synergistic immunotherapy in this treatment system as it triggers endogenous ferroptosis, amplifies ferroptosis, and is a prerequisite for inducing the immunogenic death of tumor cells to initiate immunity. Figure 5g confirms that HMPB has excellent photothermal conversion ability. The local temperatures at the tumor site in the HMPB-H and HMPB-H@M1EV groups showed an obvious increase. The temperature rose to approximate 44 °C in 2 min in both groups (Fig. 5h), potentially triggering ferroptosis and immune responses simultaneously via the photothermal effect. Furthermore, we confirmed that tumor cells exhibit higher sensitivity to temperature than representative immune cells macrophages (Supplementary Fig. 24), thus maybe ensuring the desired photothermal effect while minimizing the damage to normal tissues.
Ferroptosis synergistic immunotherapy efficacy in vivo
HMPB-HKN15-based exo- and endogenous ferroptosis synergistic immunotherapy and related mechanisms are discussed in detail in vitro. Subsequently, whether this combination therapy system produced effective antitumor effects and regulated the immune microenvironment in vivo was further investigated (Fig. 6a). Compared to the PBS group, M1EV, HMPB-H, and HMPB-H@M1EV exhibited moderately inhibitory effects on tumor growth, whereas the addition of laser irradiation significantly enhanced tumor suppression. Specifically, HMPB-H@M1EV + L treatment almost completely controlled tumor growth (Fig. 6b, c), and the ex vivo tumor tissue data (Fig. 6d, e) showed a similar trend post-treatment. As expected, after M1EV-assisted targeted delivery of HMPB-HKN15 to tumor tissues, the release of endogenous iron triggered by the photothermal effect further amplified ferroptosis, which combined with antitumor immunity exerted the strongest tumor-killing effect. In addition, body weight did not change significantly during treatment (Fig. 6f ).
a Treatment schedule. b Individual tumor volume growth curves. c Tumor growth curves for each treatment group. d Resected tumors at treatment endpoint. The red dotted line outlines the tumor-free area. e Weight of resected tumors. f Changes in body weight of mice. (n = 6 mice). g, h Immunofluorescence staining of ferritin and GPX4 in 4T1 tumor tissue. Scale bar: 50 µm. i, j Detection of GSH and LPO in 4T1 tumor tissue. Scale bar: 50 µm. For (g–j), experiment was repeated three times independently with similar results. The data in (c, e) are presented as the mean ± SD (n = 6 mice). Statistical differences were analyzed by one-way ANOVA with two-tailed Tukey’s multiple comparisons test.
The synergistic antitumor mechanism was revealed by measuring representative factors in ferroptosis, including ferritin, GPX4, GSH, and LPO, in the immune microenvironment. The HMPB-H@M1EV + L group exhibited the greatest reduction in ferritin levels (Fig. 6g). Owing to the lack of targeted delivery of M1EVs in the HMPB-H + L group, and the intratumoral accumulation of HMPB-H was lower than that of HMPB-H@M1EV + L, leading to decreased photothermal degradation of ferritin. Moreover, compared to the other treatment groups, the HMPB-H@M1EV + L group showed the largest reduction in GPX4 expression (Fig. 6h) and GSH consumption (Fig. 6i), and the highest LPO generation (Fig. 6j). Thus, HMPB-H@M1EV destroyed the antioxidant capacity of tumor cells through the synergistic effect of exogenous and endogenous ferroptosis under laser irradiation, and PTT might further enhance ferroptosis.
Tumor microenvironment analysis and peripheral immune response
In our proposed therapy, M1EVs directly regulated immunity using macrophage polarization. Therefore, various immune cells were first analyzed using flow cytometry (Supplementary Fig. 25) and immunofluorescence staining of tissue sections (Fig. 7a, b). Phenotypic analysis of intratumoral macrophages showed that the proportion of CD86+ M1 macrophages was significantly increased in the M1EV-loaded nanoparticle treatment (HMPB-H@M1EV and HMPB-H@M1EV + L) groups compared to that in the simple nanoparticle (HMPB-H and HMPB-H + L) groups (Fig. 7c and Supplementary Fig. 26). Correspondingly, the proportion of CD206+ M2 macrophages decreased drastically in the M1EVs participation groups, indicating a switch in the intratumoral macrophage phenotype (Fig. 7d and Supplementary Fig. 27). In addition to macrophages, other key immune cells were detected to analyze the regulation of the intratumoral immune microenvironment. Overall, the intratumoral microenvironment was becoming immune-promoting rather than immune-suppressive, which was manifested by an increase in the proportion of CD8+ (10.9%); (Fig. 7e and Supplementary Fig. 28), CD4+ (25.9%), T cells (Fig. 7f and Supplementary Fig. 29), and mature DCs (35.9%) (Fig. 7g and Supplementary Fig. 30) in the HMPB-H@M1EV + L groups. High interstitial pressure and hypoxia within tumors severely hinder T-cell infiltration, and DCs are mostly found in an immunosuppressive state. Therefore, the increase in T cell infiltration and DCs activity effectively improved the tumor immunosuppressive microenvironment, facilitating an effective cooperation with ferroptosis to exert antitumor effects, preventing tumor metastasis or recurrence by establishing the body’s immunological memory. In addition, MDSC, another immunosuppressive cell type in the tumor, were significantly reduced post-treatment (Fig. 7h and Supplementary Fig. 31), and the level of the corresponding inhibitory cytokine IL-10 was reduced (Fig. 7i). In contrast, the level of the immune-promoting cytokines (TNF-ɑ, IFN-γ, and IL-2) increased in HMPB-H@M1EV + L groups relative to other treatment groups (Fig. 7j–l). Notably, IFN-γ downregulates the expression of SLC7A11, hinders GSH synthesis, and causes lipid peroxidation, thereby augmenting ferroptosis in tumor cells39. Them, the various immunostimulatory molecules released from ferroptotic tumor cells promote DC maturation and further enhance CD8+ T cells infiltration6,40. Moreover, the immunosuppressive M2 type macrophages are more susceptible to ferroptosis than the M1-type macrophages; thus, ferroptosis in these immunosuppressive cells further regulates the tumor immunosuppressive microenvironment33. Therefore, this combination treatment system effectively inhibits tumor growth through the synergistic effects of exo- and endogenous ferroptosis and immune microenvironment regulation.
a, b Immunofluorescence analysis of CD8 and CD206 in 4T1 tumor tissue at treatment endpoint. Scale bar: 50 μm. c, d M1-like (CD86+) and M2-like (CD206+) macrophages (CD45+ F4/80+) in tumors. e, f CD8+ and CD4+ T cells (CD45+ CD3+) in tumors. g CD80+ CD86+ DCs (CD45+ CD11c+) in tumors. h MDSCs (CD45+ CD11b+ Gr-1+) in tumors. i–l IL-10, IL-2, IFN-γ, and TNF-α in TME. m, n CD8+ IFN-γ+ and CD4+ IFN-γ+ T cells (CD3+) in spleen. o–q Cytokine secretion (TNF-α, IL-2, and IFN-γ) by splenocytes. ND stands for not detected. r Splenocytes specifically killing 4T1 cells. E: T stands for effector: target cells. For (a, b), experiment was repeated three times independently with similar results. The data in (c–r) are presented as the mean ± SD (n = 4 mice). Statistical differences were analyzed by one-way ANOVA with Tukey’s multiple comparisons test.
The analysis of immune cells in the spleen can directly reflect the strength of immunity during tumor treatment. Cytotoxic T and Th1 cells or total T cells were remarkably increased in the HMPB-H@M1EV + L group after tumor antigen re-stimulation compared with that in the other groups (Fig. 7m, n and Supplementary Figs. 32 and 33). This also suggests that M1EVs and photothermal-triggered endogenous ferroptosis must be co-involved in the therapeutic process to trigger specific immunity. The levels of cytokines in the splenocyte supernatants of HMPB-H@M1EV + L were significantly increased (Fig. 7o–q). Furthermore, in vitro lysis experiments detecting LDH release, further confirmed the enhanced, specific lysis of tumor cells (Fig. 7r). Therefore, the strong immune response generated by peripheral immune organs may prevent tumors by eliciting a specific immune memory post-treatment with HMPB-H@M1EV + L.
HMPB-HKN15@M1EV to prevent tumor recurrence and metastasis
In vivo imaging results showed that PBS-, M1EV-, and HMPB-H-treated mice had severe abdominal or lung metastases 28 d post-treatment, whereas mice treated with HMPB-H@M1EV had milder tumor metastasis (Fig. 8a, b). Meanwhile, only two mice had recurrence at the surgical site without obvious metastases in the HMPB-H@M1EV + L group. Moreover, in situ tumor recurrence may be caused by residual microtumors post-surgery. Ex vivo lung tissue staining further confirmed tumor lung metastasis status (Fig. 8c, d and Supplementary Fig. 34). No evident metastatic nodules were observed in the HMPB-H@M1EV + L group. Additionally, the HMPB-H@M1EV + L-treated mice generated the highest percentage of CD44+CD62L+ central memory T cells (Fig. 8e, f).
a Treatment schedule for metastasis. b In vivo bioluminescence images in different groups of mice on days 20 and 28. c Digital image of lungs stained with Bouin’s fluid. d lung tissue staining. Scale bar: 100 μm. e, f Tcm (CD3+ CD8+ CD44+ CD62L+) in the spleen on day 28. Data are presented as the mean ± SD (n = 3 mice). Statistical differences were analyzed by ordinary one-way ANOVA with Tukey’s multiple comparisons test.
Biosafety of biohybrid system
The impact of ferroptosis on untargeted tissues was also a factor to consider. We first verify the susceptibility of 4T1 cells and normal cells to ferroptosis with a widely recognized ferroptosis inducer erasin. As shown in Supplementary Fig. 35, erasin had a weaker killing effect on normal cells compared to 4T1 cells, which suggested that the normal cells were relatively resistive to ferroptosis. Moreover, we examined HMPB-H@M1EV-induced cytotoxicity (Supplementary Fig. 36) and LPO accumulation (Supplementary Fig. 37) in normal cells. 3T3 and HUVEC cells had no significant change in viability and LPO level, indicating that HMPB-H@M1EV-induced ferroptosis had minimal impact on normal cells.
Next, the pathological examination of major organs and complete blood count analysis did not reveal any significant tissue or systemic toxicity postinjection 24 h (Supplementary Table 1 and Supplementary Fig. 38). A long-term safety assessment was performed over 30 d, including changes in body weight, complete blood count analysis, pathological examination of major organs, and serum biochemical analysis (Supplementary Table 2 and Supplementary Figs. 39–41). No histopathological or toxicological changes were observed. Furthermore, the pharmacokinetic behavior of HMPB-H@M1EV was determined with the percentage of injected dose per gram (%ID/g) by the quantifying the labelled Gd content. The structure of Gd-doped Prussian blue was verified by XRD and absorption spectrum. The formed Fe-C ≡ N-Gd affirmed the successful doping of Gd into Prussian blue (Supplementary Fig. 42a, b). Owing to metabolic clearance, the Gd content in the blood decreased gradually with time-dependence. With the rapid removal of nanoparticles from the blood, the nanoparticle biodistribution coefficients has dropped below 1%ID/g after 4 h (Supplementary Fig. 42c).
Discussion
Since ferritin is overexpressed in breast cancer, ferritin-rich breast cancer cells can act as endogenous iron pools. Disrupting iron homeostasis to release iron ions is an important strategy to trigger endogenous ferroptosis41,42. For instance, NCOA4 can increase intercellular iron levels to trigger spontaneous ferroptosis in tumor cells43. Photodynamic-mediated ferritin destruction is an effective endogenous iron ion release strategy15. In this study, HKN15 peptide-modified Prussian blue nanomaterials triggered both endogenous and exogenous ferroptosis. Prussian blue has been clinically proven to be a highly, biosafe antidote that was approved by the FDA44. HMPB are iron-organic framework-structured materials that have excellent dispersion, low toxicity, high stability, easy degradation, and biocompatibility. Importantly, they release Fe ions to stimulate ferroptosis after degradation17. Targeting intratumoral ferritin by HMPB-H can simultaneously enhance iron content by activating endogenous iron ions and replenishing exogenous iron ions for more efficient ferroptosis treatment. In this study, we confirmed the cumulative effects of endogenous and exogenous ferroptosis induced by HMPB-H.
For photothermal combination therapy based on a drug delivery system, three key components—laser, drug, and delivery vector—are typically required. Our therapeutic system simplified this approach by using drug-free nanoparticles instead of conventional drugs. Drug-free active biomaterials have unique applications in tumor treatment. The physicochemical and biological properties of active biomaterials triggers various endogenous biological effects to avoid the toxic side effects caused by drugs. Various drug-free active nanomaterials have made significant progress in tumor therapy, including mixed-charge nanoparticles, upconversion nanoparticles, poly(salicylic acid) nanocarriers, and cationic anticancer polypeptide nanoparticles45,46,47,48. Therefore, the development of biomaterials with highly effective antitumor activities to prevent tumor recurrence and metastasis is an important challenge. In addition, appropriately-sized nanomaterials allow for better transport and distribution within the organism, thereby improving drug efficacy and reducing side effects49. The properties of nanoparticles influence their biological processes, enhanced permeation and retention (EPR) effect, and therapeutic outcomes50. Extracellular vesicles as a next-generation drug delivery platform are applied in ongoing clinical trials51. Extracellular vesicle-loaded nanomaterials can effectively improve the efficiency of tumor-targeted delivery and enhance the therapeutic effects. In this study, the M1EV and HMPB-H biohybrid delivery system integrated the advantages of biomimetic and nanodelivery systems. Functional HMPB serve as inducers of ferroptosis, whereas macrophage microvesicles have a dual tumor-targeted delivery function and deliver nanoparticles accurately owing to their biological characteristics. Moreover, they can improve the immunosuppressive microenvironment by regulating the macrophage phenotype and effectively promoting tumor-targeted immunotherapy. The biohybrid delivery system exerted a highly effective synergistic therapeutic effect on ferroptosis and antitumor immunity, effectively inhibited tumor growth, and prevented tumor metastasis.
In summary, a drug-free biohybrid system targeting ferritin was successfully developed considering the high expression of ferritin in triple-negative breast cancer. This system utilizes M1EV and HMPB-H for photothermally enhanced ferroptosis immunosynergistic cancer therapy. HMPB-H enables the activation of endogenous iron ions and replenishment of exogenous iron ions, leading to its enhanced accumulation in tumor cells. The dual accumulation of iron ions amplifies oxidative stress, enhances ferroptosis, and triggers an antitumor immune response. Furthermore, interferon γ stimulates liposome peroxidation and promotes ferroptosis, thus achieving synergistic treatment through the mutual promotion of ferroptosis and antitumor immunity, effectively preventing tumor recurrence and metastasis. This biohybrid system avoids the use of traditional drugs and reduces toxic side effects in the body, providing a promising strategy for biohybrid system-mediated ferroptosis tumor immunotherapy.
Methods
Animals and ethical statement
All animal experiments in this study were carried out in compliance with all relevant ethical regulations and approved by the Experimental Animal Ethics Committee of the Institute of Radiological Medicine, Chinese Academy of Medical Sciences (IRM-DWLL-2023028). Six-week-old female BALB/c mice (16–18 g) were purchased from Beijing Huafukang Biotechnology Co., Ltd. (Beijing, China). All animals were raised together under standard conditions (23–26 °C, 40–60% humidity, 12 h light–dark cycle, 5 mice/cage), allowing free access to food and water. When the tumor burden exceeds 10% of the animal’s own body weight, the body weight decreases by 20% of the normal body weight, or the ulcers at tumor occurs, the experimental animal should be euthanized. The experimental animals in this study did not exceed the maximum tumor burden. Since breast cancer mainly affects female in clinical practice, female mice are chosen.
In vitro degradation and ferritin destruction of HMPB-H
To evaluate the in vitro degradation of HMPB-H and its ability to disrupt HSF under irradiation, iron release was measured using a total iron ion colorimetric assay kit. HSF (1 mg/mL) was incubated with HMPB-H solution (200 μg/mL) and irradiated with an 808 nm laser (1 W/cm2) for 5 min. Excess HMPB-H and HSF were removed by ultrafiltration and the iron content was determined according to the manufacturer’s protocol.
In vitro O2 and ROS generation
For O2 generation, 4T1 cells (5 × 104 per well at 24-well plates) were incubated for 8 h in a simulated hypoxic atmosphere in an anaerobic oxygen bag (5% anaerobic, MGC). Subsequently, after 2 h treatment with [Ru(dpp)3]Cl2 (10 μM), the cells were incubated with HMPB or HMPB-H (HMPB: 80 μg/mL) for another 12 h incubation and then irradiated with an 808 nm laser (1 W/cm2) for 5 min. All images were captured using CLSM.
4T1 cells (5 × 104 per well) were incubated with PBS, HMPB, or HMPB-H (HMPB: 80 μg/mL) in 24-well plates for 12 h. After which, the cells were irradiated with an 808 nm laser (1 W/cm2) for 5 min and then incubated for 30 min with DCFH-DA (20 μM). Finally, DCF fluorescence intensity was immediately observed using CLSM.
GSH and GPX4 detection
For intracellular GSH depletion, 4T1 cells (5 × 104 per well at 24-well plates) were treated with HMPB or HMPB-H (HMPB: 80 μg/mL) for 12 h, and then were irradiated with an 808 nm laser (1 W/cm2) for 5 min. Finally, cells were stained with ThiolTrackerTM Violet (Glutathione Detection Reagent, 20 μM) for 30 min and imaged using CLSM. For GPX4 expression levels, the 4T1 cells (2 × 106) were collected and total protein was extracted for western blotting after various treatments.
Lipid peroxidation detection and cytotoxicity evaluation
Liperfluo is specifically oxidized by lipid peroxides and emits fluorescence. 4T1 cells (1 × 105 per well at 12-well plates) were exposed to PBS, HMPB NPs, or HMPB-H NPs (HMPB: 80 μg/mL) for 12 h. Subsequently, the cells were irradiated with an 808 nm laser (1 W/cm2) for 5 min, and then incubated for 30 min with Liperfluo. After Hoechst staining, cells were subjected to CLSM and FCM analyses. In addition, cytotoxicity was directly observed using calcein-AM/PI staining. Apoptotic cells were quantified using a FITC-Annexin V/PI probe and analyzed using FCM.
Effect of specific inhibitor Fer-1 on ferroptosis induced by HMPB-H
The 4T1 cells were exposed to HMPB-H or HMPB-H+Fer-1 (HMPB: 80 μg/mL; Fer-1: 2 μM) for 12 h. Cell viability (1 × 104 per well at 96-well plates) and LPO levels in 4T1 cells (1 × 105 per well at 12-well plates) were measured after an 808 nm laser irradiation (1 W/cm2) for 5 min. The expression of SLC7A11 and GPX4 in 4T1 cells (2 × 106) after different treatments (with or without Fer-1) was analyzed using western blotting.
In vitro ferritin and iNOS expression assays
To detect ferritin expression in different cells, RAW264.7 cells (5 × 105 per well at 6-well plates) were first stimulated with IL-4 (40 ng/mL) and LPS (100 ng/mL) for 36 h to obtain M2-type and M1-type macrophages, respectively. Ferritin expression in 4T1 cells, M1-type macrophages, and M2-type macrophages was determined by western blotting. To detect cellular iNOS expression, 4T1 cells, M1-type macrophages, and M2-type macrophages were harvested for staining.
In vitro macrophage phagocytosis assay
After successful induction of M2-type macrophages, PBS, M0EV, M1EV, and HMPB-H@M1EV were added, and the cells were harvested after 24 h of incubation and stained with RhB. RAW264.7 cells (3 × 105) and CFSE-stained 4T1 cells (1 × 105) were co-incubated for 2 h. Macrophage phagocytosis of tumor cells was then observed using CLSM and quantified using FCM.
In vitro DCs stimulation
4T1 cells (1 × 105) were incubated for 6 h after treatment with PBS, M1EV, HMPB-H, HMPB-H@M1EV, HMPB-H+ laser, and HMPB-H@M1EV+ laser. Cells were exposed to an 808 nm laser (1 W/cm2) for 5 min. Then, the 4T1 cells and supernatant were transferred and co-cultured with pre-prepared immature DCs for 24 h. Finally, non-adherent cells were collected and stained with anti-CD11c, anti-CD80, and anti-CD86 antibodies to detect the maturation level of DCs using FCM. In addition, the level of cytokine TNF-α was analyzed using enzyme linked immunosorbent assay (ELISA).
In vivo antitumor effect
Female Mice with 4T1 tumors were randomly divided into six groups (each n = 6 mice): G1, PBS; G2, M1EV; G3, HMPB-H; G4, HMPB-H@M1EV; G5, HMPB-H+laser; and G6, HMPB-H@M1EV+ laser. On days 0 and 7, the various formulations (HMPB, 10 mg/kg; M1EV, 10 mg/kg) were administered intravenously to each group of mice. On days 1 and 8, an 808 nm laser irradiation was performed at the tumor sites (0.75 W/cm2 at 5 min), and the tumor volume was measured continuously every 2 d. To investigate ferroptosis in vivo, ferritin and GPX4 protein expression was detected by immunohistochemistry using anti-ferritin and anti-GPX4 antibodies.
In vivo GSH and LPO production assays
In addition, at 24 h post-administration, the tumor site was irradiated with or without an 808 nm laser (0.75 W/cm2 at 5 min). ThiolTrackerTM Violet (20 μM, 50 μL) was injected intratumorally and maintained for 30 min. Subsequently, the tumors were excised for freeze-section analysis, and GSH fluorescence images of tumor sections were captured using CLSM. Similarly, Liperfluo probe (15 μM, 50 μL) was injected intratumorally after an 808 nm laser irradiation (0.75 W/cm2 at 5 min). After 30 min, the tumor tissues were frozen and sectioned, and fluorescence images were obtained using CLSM.
Anti-metastasis study
An antitumor metastasis model was established by inoculating mice with 1 × 106 4T1-Luc cells (n = 5 mice). After the mice were subjected to various therapeutic treatments, their tumors were surgically removed, and their bioluminescence was recorded using an in vivo imaging system (IVIS) spectrum (Caliper Life Sciences, USA) on days 20 and 28. Finally, the lungs were harvested for Bouin’s along with hematoxylin and eosin staining, and the metastatic nodules were counted.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting the findings of this study are available within the article and the Supplementary Information. All data underlying this study are available from the corresponding author upon request. Source data are provided with this paper.
References
Bianchini, G., De Angelis, C., Licata, L. & Gianni, L. Treatment landscape of triple-negative breast cancer - expanded options, evolving needs. Nat. Rev. Clin. Oncol. 19, 91–113 (2022).
Carey, L., Winer, E., Viale, G., Cameron, D. & Gianni, L. Triple-negative breast cancer: disease entity or title of convenience?. Nat. Rev. Clin. Oncol. 7, 683–692 (2010).
Chen, D. S. & Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 541, 321–330 (2017).
Sharma, P. & Allison, J. P. The future of immune checkpoint therapy. Science 348, 56–61 (2015).
Stockwell, B. R. Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell 185, 2401–2421 (2022).
Lei, G., Zhuang, L. & Gan, B. Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer 22, 381–396 (2022).
Sun, L. L., Linghu, D. L. & Hung, M. C. Ferroptosis: a promising target for cancer immunotherapy. Am. J. Cancer Res. 11, 5856–5863 (2021).
Verma, N. et al. Synthetic lethal combination targeting BET uncovered intrinsic susceptibility of TNBC to ferroptosis. Sci. Adv. 6, eaba8968 (2020).
Wang, W. et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 569, 270–274 (2019).
Zhao, L. et al. Ferroptosis in cancer and cancer immunotherapy. Cancer Commun.42, 88–116 (2022).
Liu, J. et al. Ultrathin clay nanoparticles-mediated mutual reinforcement of ferroptosis and cancer immunotherapy. Adv. Mater. 36, e2309562 (2023).
Du, Y. et al. A ‘Closed-Loop’ therapeutic strategy based on mutually reinforced ferroptosis and immunotherapy. Adv. Funct. Mater. 13, 32 (2022).
Yang, F. et al. Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy. Cell Metab. 35, 84–100 e108 (2023).
Salnikow, K. Role of iron in cancer. Semin. Cancer Biol. 76, 189–194 (2021).
Zhu, L. et al. Ferritin-Hijacking nanoparticles spatiotemporally directing endogenous ferroptosis for synergistic anticancer therapy. Adv. Mater. 34, e2207174 (2022).
Wang, Y., Sun, T. & Jiang, C. Nanodrug delivery systems for ferroptosis-based cancer therapy. J. Control. Release. 344, 289–301 (2022).
Hou, L., Gong, X., Yang, J., Zhang, H., Yang, W. & Chen, X. Hybrid-Membrane-Decorated prussian blue for effective cancer immunotherapy via tumor-associated macrophages polarization and hypoxia relief. Adv. Mater. 34, e2200389 (2022).
Zhou, L. et al. Metal-polyphenol-network coated prussian blue nanoparticles for synergistic ferroptosis and apoptosis via triggered GPX4 inhibition and concurrent in situ bleomycin toxification. Small 17, e2103919 (2021).
Yang, L., Yang, Y., Chen, Y., Xu, Y. & Peng, J. Cell-based drug delivery systems and their in vivo fate. Adv. Drug Deliv. Rev. 187, 114394 (2022).
Yu, X. et al. Neutrophil camouflaged stealth nanovehicle for photothermal-induced tumor immunotherapy by triggering pyroptosis. Adv. Sci. 10, e2207456 (2023).
Zhang, Y. et al. A platelet intelligent vehicle with navigation for cancer photothermal-chemotherapy. ACS Nano 16, 6359–6371 (2022).
Sheng, S. et al. A twindrive precise delivery system of platelet-neutrophil hybrid membrane regulates macrophage combined with CD47 blocking for postoperative immunotherapy. ACS Nano 18, 4981–4992 (2024).
Xing, G. et al. Macrophages-based biohybrid microrobots for breast cancer photothermal immunotherapy by inducing pyroptosis. Small 20, e2305526 (2023).
Zhou, M. et al. Apoptotic bodies for advanced drug delivery and therapy. J. Control. Release. 351, 394–406 (2022).
Zheng, L. et al. In vivo monocyte/macrophage-hitchhiked intratumoral accumulation of nanomedicines for enhanced tumor therapy. J. Am. Chem. Soc. 142, 382–391 (2020).
Zhao, D. et al. Apoptotic body-mediated intercellular delivery for enhanced drug penetration and whole tumor destruction. Sci. Adv. 7, eabg0880 (2021).
Wang, X. et al. Exploration and functionalization of M1-macrophage extracellular vesicles for effective accumulation in glioblastoma and strong synergistic therapeutic effects. Signal Transduct. Target Ther. 7, 74 (2022).
Tang, L. et al. Extracellular vesicles-derived hybrid nanoplatforms for amplified CD47 blockade-based cancer immunotherapy. Adv. Mater. 35, e2303835 (2023).
Sun, Z. et al. Engineered extracellular vesicles expressing Siglec-10 camouflaged AIE photosensitizer to reprogram macrophages to active M1 phenotype and present tumor-associated antigens for photodynamic immunotherapy. Small 20, e2307147 (2023).
Sheng, S. et al. An apoptotic body-based vehicle with navigation for photothermal-immunotherapy by precise delivery and tumor microenvironment regulation. Adv. Funct. Mater. 33, 2212118 (2023).
Coffman, L. G., Parsonage, D., D’Agostino, R. Jr., Torti, F. M. & Torti, S. V. Regulatory effects of ferritin on angiogenesis. Proc. Natl. Acad. Sci. USA 106, 570–575 (2009).
Lv, R., Yang, P., Chen, G., Gai, S., Xu, J. & Prasad, P. N. Dopamine-mediated photothermal theranostics combined with up-conversion platform under near infrared light. Sci. Rep. 7, 13562 (2017).
Kapralov, A. A. et al. Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat. Chem. Biol. 16, 278–290 (2020).
Xu, S., Min, J. & Wang, F. Ferroptosis: an emerging player in immune cells. Sci. Bull.66, 2257–2260 (2021).
Chi, Y. et al. Cancer cells deploy lipocalin-2 to collect limiting iron in leptomeningeal metastasis. Science 369, 276–282 (2020).
Sun, J. L. et al. Tumor cell-imposed iron restriction drives immunosuppressive polarization of tumor-associated macrophages. J. Transl. Med 19, 347 (2021).
Zhang, Y. Y., Han, Y., Li, W. N., Xu, R. H. & Ju, H. Q. Tumor iron homeostasis and immune regulation. Trends Pharm. Sci. 45, 145–156 (2024).
Zanganeh, S. et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 11, 986–994 (2016).
Song, R. et al. Acidity-activatable dynamic nanoparticles boosting ferroptotic cell death for immunotherapy of cancer. Adv. Mater. 33, e2101155 (2021).
Yu, B., Choi, B., Li, W. & Kim, D. H. Magnetic field boosted ferroptosis-like cell death and responsive MRI using hybrid vesicles for cancer immunotherapy. Nat. Commun. 11, 3637 (2020).
Du, J. et al. Disrupting intracellular iron homeostasis by engineered metal-organic framework for nanocatalytic tumor therapy in synergy with autophagy amplification-promoted ferroptosis. Adv. Funct. Mater. 33, 2215244 (2023).
Song, T. et al. Enhanced ferroptosis therapy with a “nano-destructor” by disrupting intracellular redox and iron homeostasis. Nano Today 51, 101896 (2023).
Mancias, J. D., Wang, X., Gygi, S. P., Harper, J. W. & Kimmelman, A. C. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 509, 105–109 (2014).
Qin, Z., Li, Y. & Gu, N. Progress in applications of prussian blue nanoparticles in biomedicine. Adv. Healthc. Mater. 7, e1800347 (2018).
Borkowska, M. et al. Targeted crystallization of mixed-charge nanoparticles in lysosomes induces selective death of cancer cells. Nat. Nanotechnol. 15, 331–341 (2020).
Shen, W. et al. Antineoplastic drug-free anticancer strategy enabled by host-defense-peptides-mimicking synthetic polypeptides. Adv. Mater. 32, e2001108 (2020).
Wang, J. et al. Spatiotemporally light controlled “drug-free” macromolecules via upconversion-nanoparticle for precise tumor therapy. Nano Today 42, 101360 (2022).
You, X., Wang, L., Wang, L. & Wu, J. Rebirth of aspirin synthesis by-product: prickly poly(salicylic acid) nanoparticles as self-anticancer drug carrier. Adv. Funct. Mater. 31, 2100805 (2021).
Sousa de Almeida, M., Susnik, E., Drasler, B., Taladriz-Blanco, P., Petri-Fink, A. & Rothen-Rutishauser, B. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem. Soc. Rev. 50, 5397–5434 (2021).
Shi, J., Kantoff, P. W., Wooster, R. & Farokhzad, O. C. Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer 17, 20–37 (2017).
Herrmann, I. K., Wood, M. J. A. & Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 16, 748–759 (2021).
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2023YFD1800105, F.L.), National Natural Science Foundation of China (32371391, F.L. and 82272154, L.M.), the Natural Science Foundation of Tianjin, China (23JCYBJC00270, X.D., 23JCYBJC00430, Y.Z.), the Fundamental Research Funds for the Central Universities (2019PT320028, F.L.) and the CAMS Innovation Fund for Medical Sciences (2021-I2M-1-058, F.L.).
Author information
Authors and Affiliations
Contributions
L.M., X.D., and F.L. conceived and designed the study. L.M., X.D., and F.L.supervised the project. S.S., Y.Z., L.J., and W.S. performed the experiments. S.S., Y.Z., D.Z., L.M., X.D., and F.L. analyzed the data and participated in the discussion. S.S., L.M., X.D., and F.L. contributed to the writing of this paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sheng, S., Zhang, Y., Jin, L. et al. A ferritin-targeted biohybrid triggering ferroptosis immunotherapy via activating endogenous iron and replenishing exogenous iron simultaneously. Nat Commun 16, 6045 (2025). https://doi.org/10.1038/s41467-025-61419-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-61419-4
This article is cited by
-
Iron and metabolic rewiring in cancer
Oncogenesis (2026)