Abstract
Biomarker-enriched, chemotherapy-free treatments for patients with advanced gastric and gastroesophageal junction cancer have not been widely explored. In this multicenter, phase 2 trial (NCT04363801), we evaluated the efficacy and safety of second-line doublet immunotherapy, combining DKN-01, an immunomodulating antibody targeting Dickkopf-related protein 1 (DKK1), with the anti-programmed cell death-1 (PD1) antibody, tislelizumab in patients with advanced gastric/gastroesophageal junction cancer and elevated tumor DKK1 expression, a putative predictive biomarker for DKN-01. Here we report part B (second line cohort) of the larger DisTinGuish trial. The primary endpoint was safety and tolerability, with secondary endpoints including objective response rate (ORR), overall survival (OS), progression free survival (PFS), and disease control rate (DCR). The trial met the prespecified primary endpoint. In the safety population (n = 52), 21 (40.4%) patients reported at least 1 DKN-01-related adverse event, most of which were low-grade, with fatigue (15.4%) and nausea (9.6%) being most common. The ORR was 21.7% in the overall population (n = 46) and 31.8% in the programmed death-ligand 1 (PD-L1) ≥ 5% population. The median OS was 8.2 months, median PFS 1.4 months, and DCR rate 34.8% in the overall population. Although exploratory, the results of this trial compare favorably against second-line benchmarks of Keynote-061 and RAINBOW and support the safety and tolerability of DKN-01 combined with tislelizumab.
Similar content being viewed by others
Introduction
Gastric and gastroesophageal junction (GEJ) adenocarcinomas (gastroesophageal adenocarcinoma (GEA)) are the fifth most common malignancy, and the third leading cause of cancer mortality worldwide1. Despite recent improvements in outcome with combination chemo-immunotherapy in the first-line (1L)2,3,4, most patients with advanced/metastatic GEA develop treatment resistance and do not survive beyond 2 years. Second-line (2L) treatment for GEA primarily consists of taxane—[with or without the vascular endothelial growth factor receptor-2 antagonist, ramucirumab] or irinotecan-based chemotherapy. To date, there have not been any phase III trials that have shown improvement over standard paclitaxel plus ramucirumab5. The efficacy of 2L treatment is still limited and median overall survival (OS) remains in the 7–9 month range. Additionally, 2L treatment regimens based on cytotoxic chemotherapeutic agents decrease patient tolerance due to well-known toxicities, including bone marrow suppression and peripheral neurotoxicity. Notably, our current 2L standards do not rely on biomarker selection outside of the availability of trastuzumab deruxtecan for human epidermal growth factor receptor 2 (HER2)-positive tumors or an immune checkpoint inhibitor (ICI) for microsatellite instability (MSI)–high tumors, and biomarker-enriched 2L strategies remain a major unmet clinical need6,7.
Dickkopf-related protein 1 (DKK1) is a secreted protein that regulates Wnt and PI3K/AKT signaling pathways and has been implicated in promoting tumor growth and metastasis8. Increased DKK1 expression in gastric cancer is associated with shorter survival9. DKN-01 (Leap Therapeutics, Inc.) is a humanized IgG4 monoclonal antibody that neutralizes DKK1 and is in ongoing clinical development in multiple oncology indications (NCT04363801, NCT05480306, NCT05761951, NCT04166721). Preclinical models demonstrated that DKK1 contributes to an immunosuppressive tumor microenvironment10,11 and treatment with DKN-01 stimulates a pro-inflammatory anti-tumor response that largely depends on innate immunity11,12,13. DKN-01 can repolarize macrophage subsets toward an M1-like phenotype, increase CD8 T-cell recruitment, and inhibit myeloid-derived suppressor cell activity in the tumor microenvironment11,12,13. In a prior phase 1b trial, DKN-01 in combination with pembrolizumab, a programmed cell death-1 (PD1) inhibitor, showed encouraging safety and efficacy in patients with previously treated advanced GEA, particularly among tumors with elevated DKK1 expression14. Patients in the phase 1b trial with tumoral DKK1 expression corresponding to the upper tertile (H-score ≥ 35) demonstrated enriched antitumor activity.
DisTinGuish (NCT04363801) is a Phase 2, 3-part trial; the single-arm Part A [DKN-01 in combination with tislelizumab (an anti-PD1 antibody) plus chemotherapy] in 1L GEA patients is reported separately15; Part C is an ongoing and randomized trial (tislelizumab plus chemotherapy with or without DKN-01) in 1L GEA patients. Part B investigated two dosing cohorts of DKN-01 (300 mg and 600 mg) plus tislelizumab as 2L chemotherapy-free therapy in patients with DKK1-high expressing tumors. The overarching hypothesis for Part B was that DKN-01 plus tislelizumab would be safe and retain clinical activity in a DKK1-high selected population. The results of Part B are reported herein.
Results
Patient characteristics
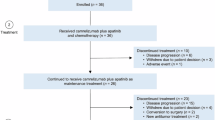
Between 27 October 2020 and 07 June 2022, a total of 291 patients underwent prescreening for assessment of DKK1 tumor expression at a central laboratory. Patients whose tumors expressed the previously defined cutoff for elevated DKK1 (H-score ≥ 35 based on prior trial data)14, were eligible for screening in Part B.
Of the 291 patients who were pre-screened, 228 failed pre-screening (66% DKK1 < 35; 18% insufficient tumor tissue available for testing; 10% not eligible; 6% did not consent) and 63 proceeded to screening. Of the 63 patients screened, 10 failed screening due to additional eligibility requirements (Supplementary Fig 1); 25 patients were enrolled in the 300 mg DKN-01 dose group (Part B1) and 28 patients in the 600 mg DKN-01 dose group (Part B2). One patient in Part B1 experienced clinical deterioration during screening and died due to cancer under trial. Enrolled and treated patients (n = 52) were predominantly Asian males (57.7%) and had a median age of 63 years (range: 29–76). Most patients (69.2%) had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 1 (Table 1). 34 patients (65.4%) had gastric adenocarcinoma and 18 patients (34.6%) had GEJ adenocarcinoma. The median time from diagnosis to trial enrollment was 9.6 months for patients with gastric and 8.3 months for those with GEJ adenocarcinoma. Based on central testing for microsatellite status and tumor mutation burden (TMB), 47 (94.0%) of 50 patients evaluated had microsatellite stable tumors. None had known MSI-high or mismatch repair deficiency (MMR) deficient tumors; MSI/MMR status was unknown for three patients. Of the 48 evaluated for TMB, 42 (87.5%) had tumors with TMB < 10 mutations per megabase, respectively. 36 (72%) of 50 patients evaluated had programmed death-ligand 1 (PD-L1) tumor area positivity (TAP)/visually-estimated combined positive score (vCPS) < 10%, and 25 (50%) of 50 patients had PD-L1 TAP/vCPS scores < 5%. Baseline tumor characteristics were similar between the 300 mg and 600 mg DKN-01 dose groups (Table 1).
Safety
In Part B, 21 (40.4%) patients reported at least 1 DKN-01-related treatment-emergent adverse event (TEAE), with the most common reported as fatigue (15.4%) and nausea (9.6%) (Table 2). Of these events, most were low-grade (Supplementary Table 1) and the higher DKN-01 dose of 600 mg was not associated with a higher proportion of DKN-01-related TEAEs. Overall, 27 (51.9%) patients reported at least 1 regimen-related TEAE (Supplementary Table 2), with the 2 most common events reported as fatigue and nausea. Six (11.5%) patients had a Grade ≥3 DKN-01-related TEAE, including single reports of increased alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase; decreased sodium and hemoglobin; vomiting; acute myocardial infarction, fatigue, and sepsis. Eight patients (15.4%) reported at least 1 of the following Grade ≥3 regimen-related TEAEs: decreased hemoglobin, lymphocyte count, and sodium; increased ALT, AST, and alkaline phosphatase; vomiting, acute myocardial infarction, immune-mediated hepatitis, sepsis, and rash (1 patient each, Supplementary Table 3). Serious adverse events (SAEs) occurring in more than one patient included abdominal pain (seven patients), vomiting, pyrexia, fatigue, hemoglobin decrease, and biliary obstruction (each, two patients). The higher DKN-01 dose of 600 mg was not associated with a higher frequency of SAEs (46.4% at 600 mg versus 54.2% at 300 mg, Supplementary Table 3). Three patients reported at least one of the following serious DKN-01-related TEAEs: vomiting, acute myocardial infarction, fatigue, and sepsis (one patient each). The higher DKN-01 dose of 600 mg was not associated with a higher proportion of DKN-01‑related SAEs. One patient receiving DKN-01 at 600 mg experienced a TEAE resulting in death following intestinal ischemia, with this event reported as unrelated to either DKN-01 or tislelizumab (Supplementary Table 3). DKN-01 infusion-related reactions were reported in two patients, both of whom received DKN-01 at 600 mg; neither event was severe or serious. No patients had treatment-emergent clinically meaningful electrocardiogram (ECG) abnormalities.
Efficacy
Overall, patients in the modified intent-to-treat (mITT) population (n = 46) in Part B had an overall response rate (ORR) of 21.7% (Table 3). A numerically higher ORR was observed in the 600 mg DKN-01 dose group versus the 300 mg DKN-01 dose group (6 responses, 25.0% versus 4 responses, 18.2%, respectively). All responses were partial responses(PRs) per Response Evaluation Criteria in Solid Tumors (RECIST, v1.1). No differences were noted in the depth or duration of response between the different dose levels (Fig. 1a, c). Consistent with the known relationship between PD-L1 expression and response, we observed an ORR of 31.8% in TAP/vCPS ≥ 5% and 13.6% in TAP/vCPS < 5%. (Table 3 and Fig. 1b, c, supplementary table 4). Among those who responded, the median duration of response (DoR) was 10.4 months (95% confidence interval [CI] 1.5—Not Reached). The overall disease control rate (DCR) was 34.8% and overall durable clinical benefit (DCB) rate was 26.1% (Table 3), with a higher DCR and DCB rate in patients with higher PD-L1 (≥5) compared with lower PD-L1 scores (<5) [45.5 vs 27.3% (DCR); 40.9 vs 13.6% (DCB rate)].
Waterfall plot for best percent change in sum of diameters per RECIST (v1.1) by Dose (A) and PD-L1 (B) in the overall modified intent-to-treat (mITT) population; C Spider plot for percent change in sum of diameters by PD-L1 in the overall mITT population. Source data is provided in the source Data file.
The median duration of DKN-01 treatment was 1.46 months and was similar in the 300 mg and 600 mg DKN-01 dose groups. The median duration on trial was 4.80 months and was longer in the 600 mg DKN-01 dose group (5.78 months) compared with the 300 mg DKN-01 dose group (2.61 months). At the time of data cutoff, 45 patients had discontinued treatment, most due to disease progression (n = 29). Seven patients were continuing treatment at the time of data cutoff, with three continuing treatment for more than 2 years (Supplementary Fig. 1). In terms of survival outcomes, the median progression-free survival (PFS) was 1.4 months (Fig. 2a), and was equivalent at both DKN-01 dose levels (Fig. 2b). The median OS was 8.2 months (Fig. 2d), with similar OS outcomes in the DKN-01 600 mg and 300 mg cohorts (7.7 months and 8.2 months, respectively, Fig. 2e). For both PFS and OS, there was a numerically longer survival in patients with PD-L1 scores ≥ 5% (Fig. 2c, f). Among 46 patients in the Part B mITT population, 43 had not previously received an ICI and were categorized as ICI-naïve. Among ICI-naïve patients with tumor PD-L1 TAP/vCPS scores > 10% (n = 12), the ORR was 50%, DCR was 67%, median PFS was 6.80 months, and median OS was not reached (Supplementary Table 4 and Supplementary Fig. 2). No correlation was observed between absolute DKK1 H-score values and best overall response (BOR) (Supplementary Table 5).
Kaplan-Meier curves for progression-free survival by Overall (A), Dose (B) and by PD-L1 (C) in the overall modified intent-to-treat population; Kaplan-Meier curves for overall survival by Overall (D), Dose (E) and by PD-L1 (F) in the overall population. Source data is provided in the source Data file.
Discussion
Despite the improvement in frontline outcomes with biomarker selected approaches, 2L therapies have poor outcomes and fewer advances. Development of 2L and later therapies that achieve a balance of efficacy and safety remains a major unmet clinical need in gastroesophageal cancers. For 2L treatment, cytotoxic agents remain the standard backbone; with taxanes (paclitaxel or docetaxel) or irinotecan monotherapy showing ORRs of 0–16%, median PFS rates of 1.7–3.6 months, and median OS rates of 4.0–9.5 months in phase 3 trials16,17,18,19,20,21. In 2014, paclitaxel plus ramucirumab became the most recommended 2L treatment in areas with ramucirumab approval. In the phase 3 RAINBOW trial the ORR was 28%, the median PFS was 4.4 months, and the median OS was 9.6 months for paclitaxel plus ramucirumab versus an ORR of 16%, a median PFS of 2.9 months, and a median OS of 7.4 months for paclitaxel monotherapy5. Efforts to introduce anti-PD1 agents into 2L GEA failed to improve outcomes, most notably in the phase 3 Keynote-061 trial comparing pembrolizumab against paclitaxel in unselected patients with 2L gastric/GEJ cancer22. In this trial, the ORR to single-agent pembrolizumab was 11% with a median PFS of 1.5 months and OS of 6.7 months in the overall population. Even among PD-L1+ (CPS ≥ 1) the ORR with pembrolizumab in Keynote-061 was 16%, suggesting a large portion of gastric/GEJ cancer harbor intrinsic resistance to anti-PD1 therapy. In fact, trastuzumab-deruxtecan in HER2+ disease and anti-PD1 in MSI-high patients represent the only biomarker selected 2L strategies. Our prior work suggested DKK1 may serve as a predictive biomarker for identifying patients who may benefit from the chemotherapy-free combination of DKN-01 and anti-PD114. Mechanistically, there are multiple potential mediators of PD1 resistance and elevated tumor expression of DKK1 is associated with a poor prognosis and resistance to chemotherapy23,24. DKK1 has direct pro-tumor effects and contributes to an immunosuppressive tumor microenvironment, which can be modulated by DKN-01 in preclinical models9,12. Based on the body of preclinical work and retrospective biomarker analysis from our prior trial in unselected patients, we hypothesized that the combination of DKN-01 with tislelizumab may expand the portion of patients benefiting from an anti-PD114.
Part B of the DisTinGuish trial evaluated 2 different dose levels of DKN-01 (300 mg and 600 mg) in the 2L advanced/metastatic GEA patient population. The 600 mg dose was chosen based on modeling from prior studies showing a dose-dependent decrease in free DKK1 maximum observed serum concentrations, supporting the hypothesis that higher doses of DKN-01 may lead to greater DKK1 neutralization. No new safety signals were identified at either dose level in this current trial. The overall safety profile was comparable at the lower and higher dose levels. Consistent with previous studies, gastrointestinal adverse events (AEs) were common at both dose levels, however the majority were low-grade. The higher DKN-01 dose was not associated with a higher proportion of TEAEs, Grade ≥ 3 TEAEs, TEAEs leading to DKN-01 discontinuation, or SAEs. In terms of anti-tumor activity, a dose-response relationship was not observed although the ORR was numerically higher in the 600 mg DKN-01 dose group (25.0%; 6/24) versus the 300 mg dose group (18.2%; 4/22), but the median PFS (1.4 months versus 1.4 months) and the median OS (7.7 months versus 8.2 months) were similar between the DKN-01 600 mg and 300 mg cohorts. This observation should be interpreted with caution, given the smaller sample size and notably the trial was not designed to directly compare the two dose levels.
We noted an ORR of 21.7% in the overall population and 31.8% in the TAP/vCPS ≥ 5%, with a DCR rate of 34.8% in the overall population. In an exploratory landmark analysis, we observed a 1-year OS rate of 48% with three patients on trial for over 2 years. These numbers compare favorably against 2L benchmarks of Keynote-061 and RAINBOW and may hint at an activity of the chemotherapy-free DKN-01 and tislelizumab combination in a biomarker enriched population (DKK1-high). As anticipated, patients with higher tumor PD-L1 scores performed better in Part B of DisTinGuish, with improvement in all efficacy outcome measures. Additionally, the OS in the population of patients with tumor PD-L1 ≥ 10 also exceeded that observed in this same population in Keynote-061 (median OS not reached versus 10.4 months). However, considering that the number of enrolled patients was small (n = 52), efficacy data in our trial need to be considered exploratory and would require validation in larger trials. A key limitation and area of future focus is a deeper exploration of additional biomarkers that may ultimately refine patient selection. Based on prior work, tumor intrinsic CTNNB1 gain-of-function mutations or other drivers of Wnt-pathway activity are relatively rare in gastroesophageal cancers. We hypothesize that exploring the TME heterogeneity via RNA-seq may help define features associated with benefit from the combination of DKN-01 and tislelizumab, but unfortunately, this data is not available within our study cohort. We acknowledge that while selected dual DKK1-high and PD-L1 high tumors may select patients more likely to benefit, additional biomarker work will be important. Although our trial met its safety endpoints, the low overall PFS (median 1.4 months) of DKN-01 plus tislelizumab underscores the need to improve patient selection. Additionally, during the course of our trial, multiple anti-PD1 agents were approved in the frontline setting and most of our patients were PD1 naïve, so we cannot comment on the activity of DKN-01 with tislelizumab after prior frontline PD1-containing combination therapy. Taken together, the safety and clinical activity demonstrated here, coupled with the evolving shift of anti-PD1 therapy to frontline, supported the co-development of DKN-01 with tislelizumab and fluoropyrimidine/oxaliplatin chemotherapy in a frontline cohort (Part A15) and the ongoing randomized frontline trial (Part C).
Methods
Study design and conduct
DisTinGuish is a phase 2a, open label, global trial of DKN-01 in combination with tislelizumab with or without chemotherapy as 1L or 2L therapy in locally advanced unresectable or metastatic GEA. The first patient was enrolled in the trial on 18 September 2020 and data cutoff for Part B was 31 January 2023. The trial is being conducted in the United States, South Korea, and Germany. Part B was designed to evaluate the safety, tolerability, and efficacy of the combination therapy of DKN-01 and tislelizumab as 2L treatment in GEA patients whose tumors expressed high levels of DKK1. The trial protocol and all amendments were approved by the local institutional review boards. A complete list of involved institutional review boards is included in the supplementary information as a supplementary note. The trial was performed in accordance with the protocol, its amendments, and good clinical practice guidelines. All patients provided written informed consent as per the Declaration of Helsinki. This trial was registered with clinicaltrials.gov (NCT04363801). The full protocol is provided as a supplementary note within the supplementary information file.
Following institutional standard premedication, patients in Part B received intravenous (IV) DKN-01 (300 mg [Part B1] or 600 mg [Part B2]) on Days 1 and 15 and IV tislelizumab (200 mg) on Day 1 of each 21-day cycle. The order of IV administration was DKN-01 followed by tislelizumab. Dose modification, including dose reduction, delay, and omission, was conducted as specified in the protocol (See Supplementary Note). Trial treatments were maintained until disease progression, unacceptable toxicity, withdrawal of consent by the patient, or at the discretion of treating physicians. Patients were allowed to discontinue tislelizumab treatment and permitted to continue in the trial with DKN-01 monotherapy if they were receiving clinical benefit from treatment.
Patients
Part B enrolled adult patients aged ≥18 years with histologically or cytologically confirmed GEA who had received one prior line of systemic treatment with a platinum and fluoropyrimidine-based regimen for unresectable or metastatic disease. Patients may have received prior neoadjuvant or adjuvant therapy; if progression occurred within 6 months from the last dose of neoadjuvant or adjuvant treatment, the regimen was considered as 1L therapy for advanced disease. Prior trastuzumab treatment for patients with HER2-positive GEA and/or prior therapy with an ICI (anti-PD1 or anti-PD-L1) in any treatment setting was allowed but not required. Documentation of high DKK1 tumor RNA expression by central testing was required. To determine the H-score, a fresh tumor biopsy sample (preferred) or archived specimen was sent to a Sponsor-designated, College of American Pathologists (CAP)/Clinical Laboratory Improvement Amendments (CLIA)-certified central laboratory for evaluation of DKK1 messenger ribonucleic acid (mRNA) in the tumor cells. An H-score was calculated by determining the percentage of low (1–3 dots/cell), medium (4–9 dots/cell), and high (10+ dots/cell) DKK1 staining tumor cells and using the following formula: H-score = (%High)\(\,*\) 3 + (%Medium)\(\,*\) 2 + (%Low)\(\,*\) 1. The theoretical maximal H-score was 300.
Other key eligibility criteria included ECOG PS 0-1, histologically confirmed gastric or Siewert I-III GEJ adenocarcinoma and at least one measurable lesion as defined by RECIST, v1.1. Patients were required to have adequate hepatic function defined as total bilirubin ≤2.0 times upper limit of normal (ULN), and AST and ALT ≤3 times ULN (if liver metastases were present, ≤5 times ULN was allowed); serum creatinine ≤1.5 times ULN or estimated GFR ≥ 30 mL/min; and hematologic parameters including an absolute neutrophil count of ≥1.5 × 10^9/L, platelet count ≥75 × 10^9/L, and hemoglobin ≥9 g/dL. Key exclusion criteria were major surgery within 4 weeks of first dose of trial drug, partial or complete bowel obstruction, squamous or undifferentiated histology, active autoimmune disease, any condition requiring >10 mg daily prednisone or equivalent or with unstable brain metastases. Due to hypothetical bone remodeling effects of DKN-01 based on known DKK1 expression in normal bone, patients with a history of osteonecrosis of the hip or osteoblastic bony metastases were excluded.
Trial assessments
The primary endpoint of safety was evaluated by assessing the incidence of TEAEs, grade ≥ 3 TEAEs, treatment-related TEAEs, treatment-emergent SAEs, treatment-related SAEs, and TEAEs leading to trial drug discontinuation. Additional safety evaluations included the incidence of treatment-emergent immune-related AEs, changes from baseline in clinical laboratory parameters (serum chemistry and hematology), changes from baseline in vital signs and ECG parameters, and a shift from baseline in ECOG PS. AEs were graded and documented according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE v5.0 guidelines). Radiological assessment of tumor-response status was performed every 6 weeks (±7 days) after cycle 1 day 1 (C1D1) for the first 24 weeks, then every 9 weeks (±7 days) after 24 weeks based on RECIST v1.1. Tumor response was assessed by the investigator. Immune-related Response Criteria was assessed in patients continuing treatment beyond progressive disease (PD). The following were secondary endpoints of Part B: ORR, defined as the proportion of patients with BOR of complete response (CR) plus PR, as assessed by the Investigator; DoR, defined as the time from initial response (CR or PR) until radiographically documented PD or death due to any cause; duration of complete response, defined as the time from initial CR until radiographically documented PD or death due to any cause; PFS, defined as the time from first trial drug dose (i.e., C1D1) to first radiographically documented PD or death due to any cause; OS, defined as the time from C1D1 to death due to any cause; duration of clinical benefit (DoCB), defined as the time from C1D1 to the time of PD or death due to any cause in patients who had a BOR of CR, PR, or SD of ≥6 weeks; DCB, defined as DoCB ≥180 days and DCR, defined as the proportion of patients with CR + PR + SD (≥6 weeks).
Statistical analyzes
The sample size for Part B is not based on formal statistical calculations, as this was a pilot study designed primarily to seek information on the safety, efficacy, and pharmacokinetics/pharmacodynamics of DKN-01 in combination with tislelizumab. Data collected from previous clinical studies indicate that DKN-01 is well tolerated, therefore, 20 patients for each of the two dose levels, or 40 patients in total, were determined to be sufficient to assess the safety and tolerability of DKN-01 in combination with tislelizumab. The primary population for the safety analysis (intent-to-treat, ITT population) was defined as patients who provided informed consent and received at least 1 dose of DKN-01. The main efficacy population was the mITT population, defined as patients who received more than 1 dose of DKN-01. BOR was determined in accordance with RECIST v1.1. Statistical analyzes were conducted using SAS Version 9.4. Kaplan-Meier curves were generated using R 4.4.0 with the ggsurvplot package.
Continuous and categorical variables were summarized descriptively. Selected statistics for categorical variables included 2-sided 95% CIs for the percent based on the exact Clopper–Pearson methodology for binomial proportions. Time-to-event variables were derived using Kaplan–Meier methods. For Kaplan–Meier analyzes, CIs were calculated using log-log transformation.
Biomarkers
Patients were required to submit either a fresh or archival biopsy specimen during prescreening. Tumor DKK1 RNA expression was centrally assessed using an analytically validated chromogenic in-situ hybridization assay using RNAscope technology and an H-score (0–300) was determined for the tumor compartment (Flagship Biosciences, Broomfield, CO; Advanced Cell Diagnostics, Newark, CA)25. An DKK1 H-score ≥ 35 was considered high. PD-L1 immunohistochemistry (IHC) was centrally performed using the SP263 antibody and a TAP/vCPS score (as a %) was reported (Roche Tissue Diagnostics, Tucson, AZ)25. Circulating tumor DNA was assessed for TMB and microsatellite status (Foundation Medicine Inc.) (Supplementary table 6).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting the presented results are provided in the manuscript, supplementary files and source data file. Additional de-identified patient data is not available. Questions regarding any of the presented data can be directed to S.J.K., sklempner@mgb.org. The full protocol is provided as a supplementary note within the supplementary information file. In accordance with International Council for Harmonization Good Clinical Practice guidelines, the trial monitor had direct access to the Investigator’s source documentation to verify the data recorded in the electronic case report forms for consistency. Representatives of regulatory authorities or the sponsor may have conducted inspections or audits at any time during or after completion of this clinical trial. Results of this trial were disclosed on www.clinicaltrials.gov and other public regulatory websites per regulatory requirement. Source data are provided with this paper.
Change history
06 February 2026
A Correction to this paper has been published: https://doi.org/10.1038/s41467-026-69394-0
References
Siegel, R. L. et al. Cancer statistics, 2023. CA Cancer J. Clin. 73, 17–48 (2023).
Janjigian Y. Y. et al. First-Line Nivolumab Plus Chemotherapy for Advanced Gastric, Gastroesophageal Junction, and Esophageal Adenocarcinoma: 3-Year Follow-Up of the Phase III CheckMate 649 Trial. J. Clin. Oncol. https://doi.org/10.1200/JCO.23.01601 (2024).
Rha, S. Y. et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 24, 1181–1195 (2023).
Qiu, M. Z. et al. Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first line treatment for advanced gastric or gastro-oesophageal junction adenocarcinoma: RATIONALE-305 randomised, double blind, phase 3 trial. BMJ 385, e078876 (2024).
Wilke, H. et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 15, 1224–1235 (2014).
Shitara, K. et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N. Engl. J. Med. 382, 2419–2430 (2020).
Van Cutsem, E. et al. Trastuzumab deruxtecan in patients in the USA and Europe with HER2-positive advanced gastric or gastroesophageal junction cancer with disease progression on or after a trastuzumab-containing regimen (DESTINY-Gastric02): primary and updated analyses from a single-arm, phase 2 study. Lancet Oncol. 24, 744–756 (2023).
Kagey, M. H. & He, X. Rationale for targeting the Wnt signalling modulator Dickkopf1 for oncology. BJP 174, 4637–4650 (2017).
Shi, T. et al. DKK1 promotes tumor immune evasion and impedes anti-PD-1 treatment by inducing immunosuppressive macrophages in gastric cancer. Cancer Immunol. Res. 10, 1506–1524 (2022).
Malladi, S. et al. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell 165, 45–60 (2016).
D’Amico, L. et al. Dickkopf-related protein 1 (Dkk1) regulates the accumulation and function of myeloid derived suppressor cells in cancer. J. Exp. Med. 213, 827–840 (2016).
Haas, M. et al. mDKN-01, a novel anti-DKK1 mAb, enhances innate immune responses in the tumor microenvironment. Mol. Cancer Res. 19, 717–725 (2021).
Wise, D. R. et al. Dickkopf-1 can lead to immune evasion in metastatic castration-resistant prostate cancer. JCO Precis. Oncol. 29, PO.20.00097 (2020).
Klempner, S. J. et al. Safety, efficacy, and biomarker results from a phase Ib study of the anti-DKK1 antibody DKN-01 in combination with pembrolizumab in advanced esophagogastric cancers. Mol. Cancer Ther. 20, 2240–2249 (2021).
Klempner S. J. et al. DKN-01 in combination with tislelizumab and chemotherapy as first-line therapy in advanced gastric or gastroesophageal junction adenocarcinoma: DisTinGuish. J. Clin. Oncol. 43, 339–349 (2024).
Thuss-Patience, P. C. et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer—a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur. J. Cancer 47, 2306–2314 (2011).
Ford, H. E. et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 15, 78–86 (2014).
Kang, J. H. et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J. Clin. Oncol. 30, 1513–1518 (2012).
Hironaka, S. et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J. Clin. Oncol. 31, 4438–4444 (2013).
Lee, K. W. et al. A phase III study to compare the efficacy and safety of paclitaxel versus irinotecan in patients with metastatic or recurrent gastric cancer who failed in first-line therapy (KCSG ST10-01). Oncologist 24, 18–e24 (2019).
Shah M. A. et al. Randomized, double-blind, placebo-controlled phase III study of paclitaxel ± napabucasin in pretreated advanced gastric or gastroesophageal junction adenocarcinoma. Clin. Cancer Res. 28, 3686–3694 (2022).
Shitara, K. et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 392, 123–133 (2018).
Kagey, M. H. & He, X. Rationale for targeting the Wnt signalling modulator dickkopf-1 for oncology. Br. J. Pharm. 174, 4637–4650 (2017).
Zhao, Y., Wang, C. & Goel, A. Andrographis overcomes 5-fluorouracil-associated chemoresistance through inhibition of DKK1 in colorectal cancer. Carcinogenesis 42, 814–825 (2021).
Caldwell, C. et al. Validation of a DKK1 RNAscope chromogenic in situ hybridization assay for gastric and gastroesophageal junction adenocarcinoma tumors. Sci. Rep. 11, 9920 (2021).
Acknowledgments
The authors would like to thank all the patients and their caregivers, and the trial staff at all the trial sites. The sponsor acknowledges Michelle Currie for assistance in the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
C.A.S. conceived and designed the trial. K.W.L., D.M., B.Y.S., I.H.K., D.Y.O., H.U., S.J.S., M.S., K.A., M.A.T., J.A.A., and S.J.K. enrolled patients and collected data for the trial. The sponsors, K.W.L. and S.J.K. analyzed and interpreted the data. C.A.S, R.A., K.W.L., and S.J.K. wrote the initial draft and all authors approved the final version of the manuscript for submission. All authors approved the final version of the manuscript for submission. The sponsor acknowledges Rachel Altura and Michelle Currie for assistance in the preparation of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
K.W.L. reports research funding to his institution for conducting clinical trials from Leap Therapeutics in relation to this manuscript. He also reported research funding to his institution for conducting clinical trials from MSD, AstraZeneca, Ono Pharmaceutical, Roche, Merck KGaA, BeiGene, Astellas Pharma, Amgen, Daiichi Sankyo, ALX Oncology, GlaxoSmithKline, Macrogenics, Taiho Pharmaceutical, Seagen, Y-BIOLOGICS, Bolt Biotherapeutics, Trishula Therapeutics, InventisBio, MedPacto, Ildong Pharmaceutical, Genome & Company, Arcus Biosciences, Elevar Therapeutics, Jazz Pharmaceuticals, TRIO Oncology, Exelixis, IgM Biosciences, Panolos Bioscience, Metafines, Wellmarker Bio, Medicenna, and Erasca; consulting fees from Daiichi Sankyo, MSD, Astellas Pharma, AbbVie, and Metafines; honoraria for lectures or presentations from Sanofi/Aventis, Astellas Pharma, Bayer, Daiichi Sankyo, and Merck KGaA. D.M. has received research funding from Amgen, Merck, Oncolytics and Rafael; scientific advisory board for Actuate, Qurient, and an advisory/speaker bureau for Amgen, BMS, Eisai, and Exelixis; has received funding paid to their institution from Acepodia, Actuate Therapeutics, ADC Therapeutics, Amgen, AVEO, Bayer, Blueprint Medicines, BMS, BioNTech, Dialectic Therapeutics, Epizyme, Fujifilm, ImmuneSensor, Immune-Onc Therapeutics, Leap Therapeutics, Lycera Corp, Merck, Millennium, MiNA Alpha, NGM Biopharmaceuticals, Novartis, Oncolytics, Orano Med, Puma, Qurient, Rafael, Repare Therapeutics, Triumvira Immunologics, Vigeo Therapeutics, Warewolf Therapeutics. H.J.C. has received honoraria from Eisai, Roche, ONO, MSD, Bristol Myers Squibb, BeiGene, Sanofi, Servier, AstraZeneca, Aptamer Science, and Boryung; served on advisory boards for Eisai, Roche, ONO, MSD, Bristol Myers Squibb, BeiGene, Servier, AstraZeneca, Boryung, IMBDx, and Aptamer Science; and received research grants from Roche, BeiGene, IMBDx, Dong-A ST, and Boryung Pharmaceuticals. M.H.K., M.S., C.J., R.A. and C.A.S. are employees of Leap Therapeutics and report stock ownership in Leap Therapeutics. S.J.K. has served a consultant/advisory role for Bristol Myers Squibb, Merck, Roche, Astellas, Daiichi-Sankyo, Pieris, Natera, Novartis, AstraZeneca, Mersana, Sanofi-Aventis, I-Mab, Taiho Oncology, Eisai, Gilead, BeiGene, and Elevation Oncology, Boehringer-Ingelheim. S.J.K. reports research support (institutional) from Arcus Biosciences, I-Mab, AstraZeneca, Parabilis, Debbie’s Dream Foundation, Degregorio Foundation, StandUp2Cancer, AACR, NIH/NCI, Gastric Cancer Foundation, and the Korean Ministry of Health. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Yingjie Qiu and the other anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lee, KW., Mahalingam, D., Shim, B.Y. et al. DKN-01 and tislelizumab as second-line therapy in DKK1-high gastroesophageal adenocarcinoma: DisTinGuish trial part B. Nat Commun 16, 6393 (2025). https://doi.org/10.1038/s41467-025-61420-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-61420-x
This article is cited by
-
Mechanisms and therapeutic strategies for immunotherapy resistance in gastric cancer
Cancer Cell International (2025)