Abstract
SARS-CoV-2 has largely evolved to resist antibody pressure, with each successive viral variant becoming more and more resistant to serum antibodies in the population. This evolution renders all previously authorized anti-spike therapeutic monoclonal antibodies inactive, and it threatens the remaining pipelines against COVID-19. We report herein the isolation of a human monoclonal antibody with a broad but incomplete SARS-CoV-2 neutralization profile, but structural analyses and mutational scanning lead to the engineering of variants that result in greater antibody flexibility while binding to the viral spike. Three such optimized monoclonal antibodies neutralize all SARS-CoV-2 strains tested with much improved potency and breadth, including against subvariants XEC and LP.8.1. The findings of this study not only present antibody candidates for clinical development against COVID-19, but also introduce an engineering approach to improve antibody activity via increasing conformational flexibility.
Similar content being viewed by others
Introduction
Since the emergence of COVID-19 in late 2019, its causative agent, SARS-CoV-2, has undergone considerable evolution driven by immune pressure, with each successive viral variant becoming more resistant to serum antibodies elicited by prior infection and/or vaccination. By late 2020, mutations in the viral spike protein resulted in a discernible antigenic drift that yielded the Alpha variant, which evaded antibodies targeting the antigenic supersite on the N-terminal domain (NTD)1,2,3. Shortly thereafter, further antigenic drift led to the Beta and Gamma variants that evaded not only NTD-directed antibodies but also some antibodies directed to so-called class 1 and class 2 regions of the receptor-binding domain (RBD)1,4,5. These variants were, in turn, rapidly replaced by the Delta variant that dominated much of 2021, and it evaded antibodies directed to select class 2 and class 3 epitopes on the RBD6. Importantly, this initial wave of SARS-CoV-2 variants knocked out three therapeutic monoclonal antibodies (mAbs) authorized to treat COVID-19 infection—etesevimab, bamlanivimab, and casirvimab6,7,8,9,10.
The first major SARS-CoV-2 antigenic shift occurred with the emergence of the Omicron (BA.1) variant in late 2021 in southern Africa11,12, likely due to a saltatory event resulting in an accumulation of 34 spike mutations. This variant rapidly gained dominance in the population, as a consequence of its exceptional resistance to serum antibodies in the population13,14,15,16,17,18. Specifically, mutations in Omicron impaired the neutralizing activity of antibodies targeting all regions (classes 1–4) of the RBD, as well as two additional clinically authorized mAbs—imdevimab and tixagevimab9,13,19. Antigenic drift from BA.1 then ensued successively throughout 2022 and yielded subvariants BA.2, BA.5, and BQ.1.1, each being more antibody resistant than its predecessor. This evolutionary drift also knocked out the utility of three more clinically authorized mAbs— sotrovimab, ciligavimab, and bebtelovimab19,20,21,22,23.
The second major antigenic shift was detected in late 2022 when a recombination event between BA.2 and BA.2.75 resulted in the XBB subvariant, which promptly evolved into XBB.1.5, EG.5.1, HK.3, and HV.1 via further antigenic drift11,24,25,26. Again, each progeny subvariant was more antibody resistant than its predecessor, largely by evading more antibodies directed to the class 1 region of RBD. Sequentially, these subvariants dominated much of 2023 until the third major antigenic shift led to the emergence of BA.2.86, which presumably was due to another saltatory event, again in southern Africa, leading to an accumulation of >30 mutations in the BA.2 spike27. This new subvariant was again highly resistant to serum neutralizing antibodies, including those targeting the subdomain 1 (SD1) region of spike28,29,30. With one additional spike mutation, L455S, BA.2.86 became JN.1, and the JN.1 sublineage has been dominant worldwide from late 2023 until recently31,32.
In March 2024, a monoclonal antibody known as pemivibart (VYD222) was authorized for clinical use as pre-exposure prophylaxis for immunocompromised individuals who do not respond robustly to COVID-19 vaccines33. This antibody was specifically engineered to have broad activity against many known SARS-CoV-2 strains, including JN.1. However, as the JN.1 sublineage continued its antigenic drift, new forms such as XEC began to replace their predecessor in recent months. As of April 2025, the dominant SARS-CoV-2 strains are XEC and LP.8.134. Alarmingly, these subvariants are already ~18–28-fold more resistant to pemivibart in vitro than JN.135,36. This looming threat to the only authorized pre-exposure prophylaxis for millions of immunocompromised individuals who live in fear of COVID-19 is a stark reminder of the necessity to continue to develop more medical interventions to keep up with SARS-CoV-2 evolution. In addition, another mAb, AstraZeneca’s sipavibart (AZD3152), is also in clinical trials; however, this antibody has been shown to be affected by the F456L mutation37, which is present in multiple dominant JN.1 sublineages, such as XEC and LP.8.1.
We now describe the isolation and characterization of a human monoclonal antibody, 19-77, with a broad but incomplete SARS-CoV-2 neutralization profile. Structural analyses and mutational scanning led to the construction of engineered antibody variants, 19-77Δ, with a single amino acid substitution at residue R71 in the framework region 3 of the heavy chain. These modifications resulted in greater flexibility of the complementarity-determining regions (CDRs) of the heavy chain while binding to the viral spike, thereby enabling three 19-77Δ variants to neutralize all SARS-CoV-2 strains tested with much improved potency and breadth, including against the JN.1 sublineages. The findings of this study not only offer new antibody candidates for pre-exposure prophylaxis against COVID-19 for immunocompromised individuals, but also introduce a unique engineering approach to improve antibody activity.
Results
Isolation and characterization of mAb 19-77
To isolate new neutralizing mAbs against SARS-CoV-2, we studied Donor 19 who had been infected by the Omicron BA.5 subvariant despite having received three doses of the original monovalent vaccine (BNT162b2) and one dose of the WT/BA.5 bivalent vaccine (mRNA-1273.222) (Supplementary Fig. 1a). His serum, obtained ~7 months after breakthrough infection, strongly neutralized the D614G strain but less potently against XBB.1.5 and SARS-CoV (Supplementary Fig. 1b). Given the dominance of XBB.1.5 at the start of this study, XBB.1.5 and SARS-CoV spikes were used as probes to sort antigen-specific memory B cells from his peripheral blood mononuclear cells (PBMCs). The proportions of his antigen-specific B cells were 0.16% for XBB.1.5 spike, 0.067% for SARS-CoV, and 0.12% for both XBB.1.5 and SARS-CoV spikes; in contrast, PBMCs from two healthy donors showed negligible antigen-specific B cells (Supplementary Fig. 1c).
Donor 19’s memory B cells from quadrants 2 and 3 (Supplementary Fig. 1c) were then subjected to single-cell RNA sequencing using 10X Genomics to obtain paired heavy and light chain sequences of each B cell receptor, as previously described38. A total of 113 paired sequences were selected, and the corresponding antibodies were synthesized for in vitro characterization. A majority (75) of the mAbs bound the D614G spike at a concentration of 10 µg/ml, but only 29 neutralized either D614G or EG.5.1 in vitro (Supplementary Fig. 1d). Among the neutralizing mAbs, 19-77 was chosen for further studies, because it not only bound strongly to the D614G spike and RBD by immunoassays (Fig. 1a), but also showed broadly neutralizing activity against many SARS-CoV-2 strains (Fig. 1b). Specifically, in pseudovirus assays, 19-77 neutralized D614G, Alpha, Beta, Gamma, Delta, BA.1, BA.2, BA.5, BQ.1.1, XBB.1.5, EG.5.1, BA.2.86, and JN.1 with IC50 values < 0.05 µg/ml. However, its neutralizing activity was lower against HK.3 and JF.1, and not detectable against JD.1.1 or SARS-CoV.
a Binding of 19-77 to the indicated antigens of SARS-CoV-2 D614G tested by ELISA. b Neutralization IC50 values of 19-77 against pseudotyped SARS-CoV-2 variants and SARS-CoV, with the IC50 of each virus is denoted. Neutralization curves are shown as mean ± standard error of mean (SEM) from technical triplicates. c Complementarity-determining region 3 (CDR3) lengths and somatic hypermutations (SHM) of 19-77 heavy chain (HC) and light chain (LC), compared with other 3-53 and 3-66 antibodies. 19-77 is highlighted in red. d Cryo-EM reconstruction of 19-77 in complex with the SARS-CoV-2 D164G spike protein, at a resolution of 2.45 Å. e Key interactions between 19-77 heavy chain and the D614G RBD. Hydrogen bonds are shown as the orange dashed lines. f Key interactions between 19-77 light chain and the D614G RBD. Orange dashed lines indicate hydrogen bonds.
Genetic analysis revealed that the heavy chain of 19-77 utilized IGHV3-53 (Supplementary Fig. 1e), showing that this antibody belonged to the well-studied VH3-53/66 class of antibodies targeting the SARS-CoV-2 spike. Its light chain originated from IGKV3-11. Notably, the CDRH3 of 19-77 contained only 9 amino acids, one of the shortest among published VH3-53/66 class mAbs (Fig. 1c). Moreover, the level of somatic hypermutation of its heavy chain was the highest observed among VH3-53/66 mAbs, and its light chain was also quite hypermutated (Fig. 1c). These striking genetic features demonstrated the uniqueness of 19-77 and prompted further studies.
To understand the molecular basis for the neutralization breadth and potency of 19-77, we then visualized its Fab fragment in complex with the D614G spike (Fig. 1d, Supplementary Fig. 2, and Supplementary Fig. 3a, b) by single-particle cryo-electron microscopy (cryo-EM). The Fab was predominantly bound to the spike protein in a three-RBD-up conformation, like other VH3-53/66 class antibodies targeting the class 1 region39. The primary interaction between the 19-77 heavy chain and the RBD was mediated by CDRH1 and CDRH2, with hydrogen bonds to A475, N487, Y473, D420, and Y421 (Fig. 1e). The CDRH3 region formed two hydrogen bonds with the RBD: one between R97 and the backbone carbonyl of N487, and another between E102 and Y489. On the other hand, the 19-77 light chain made contact with the apical ridge of the RBD via its CDRL1 and CDRL3 (Fig. 1f).
Structural explanation for the loss of 19-77 activity against certain Omicron subvariants
The antibody footprint of 19-77 on the RBD largely overlapped with the human ACE2 receptor-binding site (Fig. 2a). Sequence conservation analysis indicated that most regions of its epitope are relatively conserved, except for the upper half of the left shoulder, which exhibits significant sequence variations at residues 455, 456, 475, 478, and 486. These specific residues have undergone frequent mutations in certain recent subvariants, such as EG.5.1, HK.3, JF.1, and JD.1.1. It is therefore not surprising that the neutralizing activity of 19-77 against HK.3 (XBB.1.9.2 carrying Q52H and L455F/F456L mutations) and JF.1 (XBB.1.16.6 carrying E180V, L455F/F456L, and K478R) decreased by 14- and 42-fold, respectively, compared with that against XBB.1.5 (Fig. 1b). The inactivity of 19-77 against JD.1.1 is likely due to its A475V mutation that causes a steric clash with CDRH1 (Fig. 2b). To further understand the resistance mechanism of L455F/F456L mutations to the neutralization by 19-77, we determined the structure of 19-77 Fab in complex with the RBD of EG.5.1 by X-ray crystallography, as well as the cryo-EM structures of the D614G RBD and the HK.3 RBD in complex with the Fabs of 19-77 and S309 (sotrovimab) (Supplementary Fig. 3), a non-overlapping mAb used to increase the molecular mass for visualization by cryo-EM. Compared with the D614G RBD (Fig. 2c), the change from phenylalanine (F) to leucine (L) at residue 456 in the EG.5.1 RBD reduced hydrophobic interactions with P100HC in the CDRH3 of 19-77 (Fig. 2d). Additionally, the L455F mutation in HK.3 introduced a minor van der Waals clash with P100HC (Fig. 2e).
a Top view of the RBD inner face in complex with the 19-77 antibody. 19-77 heavy chain and light chain are shown in marine and light blue, respectively. The residues in the RBD are colored by the sequence entropy in circulating SARS-CoV-2 variants. The blue and cyan boundaries show the footprints of 19-77 and human ACE2 (hACE2), respectively. b Structure modeling of how A475V on the RBD affects 19-77 neutralization. The clashes are shown as red plates. c–e Comparison of residues 455 and 456 on RBD and P100HC in D614G (c), EG.5.1 (d), and HK.3 (e) structures. The van der Waals clashes are shown as green plates.
Optimization of 19-77
Given the minor to moderate steric clashes between the RBDs of resistant viruses and 19-77, we explored the possibility of optimizing the antibody to accommodate the resistance mutations. Utilizing in silico modeling and the energy-calculation algorithm FoldX40, we performed saturation mutagenesis on each residue of the heavy chain, comparing the binding energy changes (ΔΔG) between each mutant and the original 19-77 in complex with the HK.3 RBD that contained the L455F/F456L mutations. Both beneficial and detrimental mutations were identified (Supplementary Fig. 4a). Interestingly, substitutions at residue R71 in framework region 3 of the heavy chain consistently predicted a moderately beneficial effect (~0.5 kcal/mol) on the binding of 19-77 to the HK.3 RBD. We then conducted alanine scanning on the antigen-contact residues of both heavy and light chains of 19-77, in addition to the residue R71, followed by testing each modified antibody for neutralization against JD.1.1, HK.3, EG.5, and EG.5-A475V (Fig. 3a, Supplementary Fig. 4b, and Supplementary Fig. 4c). Only the R71A mutation significantly enhanced the neutralization activity of 19-77 against the viruses tested, including the restoration of neutralizing activity against JD.1.1 and EG.5-A475V. We therefore introduced the other 18 possible amino acid substitutions to the R71 position of 19-77 and then evaluated their neutralization efficacy against the same panel of resistant viruses (Supplementary Fig. 4d). Except for R71K, all other substitutions resulted in not only improved neutralization activities against EG.5 and HK.3 relative to the original antibody, but also restoration of activity against JD.1.1 and EG.5-A475V. Overall, the extent of improvement for these mutant antibodies (19-77Δs) ranged from 3- to >100-fold, with aliphatic substitutions R71A, R71L, and R71V being the best (Fig. 3b and Supplementary Fig. 4d).
a Neutralization of 19-77 carrying individual mutations on both heavy and light chains, in comparison with the wild-type 19-77 antibody. b Neutralization of 19-77 carrying various amino acid substitutions at R71 on the heavy chain, compared with the wild-type 19-77 antibody. c Depiction of the interactions between R71 and the heavy chain CDRs H1 (cyan) and H2 (orange) in 19-77. Dashed lines indicate the presence of hydrogen bonds. d Structure modeling of K71 (blue) and A71 (green) with HCDRs of 19-77. Dashed lines indicate the presence of hydrogen bonds between 19-77∆K. e Comparison of the RMSF between the Fab regions of 19-77 and its mutant 19-77∆A. The Y-axis represents the fold change in RMSF per-residue in 19-77∆A relative to 19-77. The X-axis displays the positional numbering of residues within the antibody. RMSF, per-residue root-mean-square fluctuation.
Structural analysis showed that R71 of 19-77 forms hydrogen bonds with S30 of CDRH1 and P53 of CDRH2, likely contributing to antibody stability (Fig. 3c). Additional modeling indicated that mutating R71 could disrupt these hydrogen bonds, with the exception of R71K, which could also form similar interactions (Fig. 3d). We then hypothesized that R71 might restrict the motion of CDRH1 and CDRH2 of 19-77, and that mutating this arginine could lead to greater antibody flexibility. To test this hypothesis, we conducted molecular dynamics (MD) simulations for 19-77 with either arginine or alanine at position 71 of the heavy chain. The results showed that A71 increased the range of motion of the heavy–chain CDRs compared to R71 (Supplementary Movie 1). Moreover, the fluctuations in the movement of each residue for the A71 variant of 19-77 were noticeably greater than those in the original antibody, especially within the CDRs (Fig. 3e). These findings, in turn, suggested that the increased flexibility of the A71 variant could allow the antibody to better accommodate for certain mutations in the SARS-CoV-2 spike.
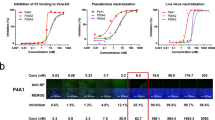
We next assessed the in vitro neutralizing activity of the R71A, R71L, and R71V versions of 19-77 (designated 19-77ΔA, 19-77ΔL, and 19-77ΔV, respectively) against a large pseudovirus panel comprising 36 SARS-CoV-2 variants and 5 SARS-CoV-2-like sarbecoviruses. Control antibodies included the original 19-77, along with the recently authorized pemivibart (VYD222)33 and a published broadly neutralizing mAb, P4J1541 (Fig. 4). The engineered 19-77 variants consistently showed better virus-neutralizing potency and breadth compared to the parental antibody. Importantly, against viruses that are widely circulating today, the improvements were substantial: 7.7-to-9.2-fold for JN.1, 6.2-to-7.8-fold for KP.2, 8.1-to-13.3-fold for KP.3, 16.2-to-21.6-fold for KP.3.1.1, and >50.0-to-147.1-fold for XEC, with the latter two being most relevant presently. The activity P4J15 was either severely impaired or knocked out by JN.1 and its progeny viruses. Pemivibart showed decreased potency against recent JN.1 sublineages, particularly KP.3.1.1 and XEC, and completely lost activity against MC.10.1. These results showed that the optimized 19-77 mAbs possess the requisite antiviral properties to be considered as candidates for clinical development, pending a full developability assessment. Reassuringly, the three mutations at R71 did not adversely impact antibody expression, aggregation propensity, or pharmacokinetics in mice (Supplementary Fig. 5).
Impact of R71 mutations on other VH3-53/66 class of mAbs to the viral spike
Monoclonal 19-77 belonged to the VH3-53/66, multi-donor class of SARS-CoV-2-neutralizing mAbs that target the class 1 region of RBD39. We therefore explored whether the same set of R71 mutations could similarly benefit other members of this antibody class. Mutations R71A, R71L, and R71V were then introduced into five VH3-53/66 class mAbs, including BD56-130242, BD56-185442, Omi-343, 19-79, BD57-012942, and BD-51544, as well as two non-VH3-53/66 class mAbs, C68.5929, and Omi4243, serving as controls. The original mAbs and their modified antibody variants were then assayed for their neutralizing activity against a panel of pseudoviruses (Supplementary Fig. 6). All unmodified mAbs showed partial or complete loss of neutralizing activity against some later Omicron subvariants such as EG.5.1, HK.3, JD.1.1, and JN.1. In contrast, every modified VH3-53/66 mAb exhibited improved neutralization capability, albeit with varying magnitudes (3- to >100-fold), whereas no improvement was observed for non-VH3-53/66 mAbs (Fig. 5 and Supplementary Fig. 6). In fact, a number of modified mAbs regained the ability to neutralize subvariants that had been completely resistant to their unmodified counterparts. For example, BD57-0129 failed to neutralize JN.1 or HK.3, but its modified counterparts neutralized both viruses robustly. The extension of the benefit of R71 mutations to mAbs other than 19-77 suggests that the responsible mechanism is not likely to operate solely at the interface with the paratope. Instead, the broader benefit observed is consistent with the hypothesis of greater conformational flexibility of the antibody CDRs discussed previously. Additionally, antibodies using VH3-53 in the human B cell repertoire very rarely carry a mutation at R71 (Supplementary Fig. 7), highlighting the uniqueness of our engineered mAbs compared to those found after SARS-CoV-2 infections.
Molecular insights into enhanced virus
neutralization by 19-77ΔV
To further explore the mechanism of the enhancement of virus neutralization by mutating R71, we determined two additional cryo-EM structures: the Fabs of 19-77ΔV and S309 in complex with HK.3 or JD.1.1 RBD at 2.9 and 3.1 Å resolution, respectively (Fig. 6a and Supplementary Fig. 3). We then compared these structures by aligning their RBDs with that of the 19-77-D614G complex and measuring the alpha carbon (Cα) distances for each heavy chain residue in 19-77 against the D614G complex. The analysis showed that the Cα distances in 19-77ΔV, when bound to HK.3 and JD.1.1 RBDs, were significantly greater (0.99 and 0.91 Å, respectively) compared to the original 19-77 in complex with HK.3 RBD (0.76 Å) (Fig. 6b). Moreover, the motion of the CDRH1 and CDRH2 were observed directly across these structures, and the valine substitution at residue 71 resulted in the loss the hydrogen bonds that had existed between R71 and the residues S30 and P53 in the heavy chain (Fig. 6c). In addition, the CDRH3 in 19-77ΔV exhibited a shift of 1 Å compared to the original 19-77, resulting in an increased distance (from 3.3 to 3.6 Å) between residues P100 and F455 (Fig. 6d). This change mitigated the slight van der Waals clash between these two residues in HK.3 RBD and 19-77 complex (Fig. 2e). Lastly, the distance between V475 in the JD.1.1 RBD and N32 of the heavy chain of 19-77ΔV increased to 3.2 Å from the 2.0 Å found in the original 19-77 bound to D614G RBD (Fig. 6e), showing the mitigation of steric hindrance caused by the A475V mutation. Collectively, these findings support the notion that 19-77Δ mAbs are indeed more flexible and therefore more accommodating for the mutations found in emerging SARS-CoV-2 variants.
a Cryo-EM maps of 19-77 and 19-77∆V Fabs bound to SARS-CoV-2 RBD variants. b Distribution of Cα distance for the heavy chain residues of 19-77 in a compared with 19-77 in the D614G complex. The structures are aligned based on RBD. Comparisons were made by student’s t-tests. ***p < 0.001. ns not significant. c Comparison of CDRH1, CDRH2, and R71V in the alignments of 19-77 structures. The hydrogen bonds are shown as orange dashed lines. d Interaction details of P100HC in 19-77 and 19-77∆V with F455 and L456 in the HK.3 RBD. The orange and green dashed lines represent the distances between P100HC and F455 in 19-77 and 19-77∆V, respectively. e Interaction details of N32HC in 19-77 and 19-77∆V with A475V in the D614G and JD.1.1 RBDs. The orange and green dashed lines represent the distances between V475 and N32 in 19-77 and 19-77∆V, respectively.
In vitro selection of SARS
-CoV-2 resistant to 19-77ΔV
Although the SARS-CoV-2-neutralization breadth and potency of the optimized 19-77 variants were impressive (Fig. 4), our expectation was that the virus will still find a way to escape given its history of evading all COVID-19 mAbs authorized to date. We therefore undertook two sets of in vitro studies to define its mutational pathways to resist 19-77∆V. First, we performed serial passaging of the authentic JN.1 virus in increasing antibody concentrations (0.1 to 50 µg/mL) in Vero-ACE2-TMPRSS2 cells over 15 days (under 3 days per passage). After five passages, a resistant virus was sequenced and found to carry mutations F456S (TTT to TCT) and K554E (AAG to GAG, Fig. 7a). When these mutations were introduced into the JN.1 pseudovirus, the resultant pseudovirus showed a 33-fold greater resistance to 19-77∆V, and even more to 19-77. Additional studies showed that the antibody evasion was attributable solely to F456S, while K554E partially compensated for the loss in viral fitness (Supplementary Fig. 8a and Supplementary Fig. 8b). Notably, this combination of mutations is extremely rare (0.0002%) in the GISAID database.
a Properties of the authentic JN.1 escape variant selected under the pressure of 19-77∆V. b Properties of the replication-competent VSV-JN.1 escape variants selected under the pressure of 19-77∆V. c Structural modeling of how A475D, G476D, N487H, F456S, Y473S, and Y489H affect 19-77 neutralization. The clashes are shown as red plates, and the hydrogen bonds are shown as orange dashed lines.
Second, we also utilized a replication-competent VSV bearing the JN.1 spike to select for variants that could escape from 19-77∆V. This selection was performed in 66 replicates in 24-well plates, involving four rounds of selection over 8 days in Vero-E6-TMPRSS2-T2A-ACEs cells with increasing concentrations of 19-77∆V (0.4 to 50 µg/mL). At the end of this process, escape variants were detected in 11 wells, and sequencing of their spike genes revealed eight distinct single or double mutations (Fig. 7b). Notably, all these mutations resulted from single nucleotide changes but were rarely found in the GISAID database, with frequencies ranging from 0 to 0.01%, and each of the pseudoviruses constructed with these mutations exhibited impaired infectivity compared to the JN.1 pseudovirus, ranging from 1 to 33% (Fig. 7b and Supplementary Fig. 8c). Furthermore, these pseudoviruses demonstrated increased resistance to soluble hACE2 inhibition (Fig. 7b and Supplementary Fig. 8d), indicating a loss in receptor affinity, perhaps accounting in part for their reduced infectivity. Single mutations G485D (GGC to GAC) and Y489H (TAC to CAC), both residing in the RBD, decreased the neutralization activity of 19-77∆A/L/V considerably (9- to 121-fold), whereas the remaining mutations completely abolished the neutralization activity of the three optimized mAbs (Fig. 8b and Supplementary Fig. 8d).
In silico structural analysis showed that each of the escape viruses contained a mutation within the epitope of 19-77∆V that impaired antibody binding (Fig. 7c). A475D, G476D, and N487H caused steric hindrance to antibody binding, the F465S mutation and residue S455 in JN.1 significantly reduced hydrophobic interactions between the antibody and RBD. Furthermore, both Y473S and Y489H abolished hydrogen bonds with the antibody, contributing to the dramatic drop in neutralization observed for the escape viruses with these mutations.
Discussion
In this study, we present findings on the characterization of a human IGHV3-53-derived monoclonal antibody, 19-77, which exhibited neutralizing activity against most but not all SARS-CoV-2 variants (Figs. 1b, 4). Strikingly, 19-77 featured the shortest CDRH3 (nine amino acids) while containing the highest degree of somatic hypermutations among its class of mAbs (Fig. 1c). Structural analysis revealed that 19-77 recognized the spike RBD in the “up” position (Fig. 1d), and its epitope overlapped with the ACE2 footprint (Fig. 2a). Subsequent in silico energy calculations (Supplementary Fig. 4a) and empirical mutagenesis experiments (Figs. 3a, 4b) yielded modified antibodies 19-77∆A, 19-77∆L, and 19-77∆V, each of which neutralized all 36 SARS-CoV-2 variants tested, including KP.3.1.1, XEC and LP.8.1.1 (Fig. 4). These optimized antibodies joined a limited list of human mAbs that possess sufficiently potent neutralizing activity against all viral variants, such as SA5545, BD55-120546, and VIR-722947 that target an epitope overlapping with that of 19-77, as well as CYFN1006-148 that targets a RBD class 3 epitope. However, we showed that SARS-CoV-2 could easily escape from neutralization by 19-77∆V in vitro (Fig. 7). In fact, the virus found multiple solutions to evade our optimized antibody, albeit with a fitness cost. Such an outcome was, more or less, expected given how SARS-CoV-2 has evolved in the population to render inactive all clinical mAbs authorized prior to 2024. Indeed, pemivibart (VYD222) that was authorized only months ago for use as prophylaxis against COVID-19 in immune deficient persons is already seriously threatened by the emergence of KP.3.1.1 and XEC subvariants35,49.
It has been a daunting challenge, as well as a frustrating endeavor, to develop therapeutic or prophylactic mAbs to keep up with the rapid pace of SARS-CoV-2 evolution. Yet the reality is that millions of individuals worldwide are sufficiently immunocompromised that they cannot benefit from the protection conferred by COVID-19 vaccines. Such persons need effective prophylaxis with passively administered virus-neutralizing mAbs, as was conferred previously by the combination of tixagevimab and cilgavimab known as Evusheld50 and presently by pemivibart33. Going forward, we believe one of our optimized 19-77∆ mAbs would qualify as a compelling candidate for clinical development given its superior potency and breadth against all SARS-CoV-2 variants known to date (Fig. 4), as well as its lack of discernible developability challenges to date (Supplementary Fig. 5). The potential clinical utility of an optimized 19-77∆ could be further improved if used in combination with a non-competing, broadly neutralizing mAb like CYFN1006-148. Additionally, the use of antibody cocktails may help reduce the likelihood of generating escape variants during antiviral mAb therapy51,52.
Herein, we also present a unique strategy for optimizing the activity of a monoclonal antibody by increasing its conformational flexibility. Traditionally, antibody engineering had been focused primarily on increasing the binding affinity using a number of approaches, including chain shuffling53,54, site-directed mutagenesis55, phage and other display technologies56,57,58,59,60, and structure-based or artificial-intelligence-guided methods61,62,63. Such methodologies, typically, had been designed to increase antibody affinity by modifying the CDR residues at the antibody-antigen interface. Other antibody approaches to optimize antibody activity included the use of multivalency with the creation of “multabodies”64, or the strategic addition of a glycan to bulk up a steric hindrance effect65. The framework regions of a mAb were seldom touched, because they are crucial for maintaining the proper orientation and conformation of the CDRs66,67. In our study on 19-77, however, in silico energy calculations indicated that a single amino acid substitution at residue 71 in framework region 3 of the heavy chain could improve antibody binding to the viral spike (Supplementary Fig. 4a), which was proven correct experimentally by replacing the arginine with any other natural amino acid except for lysine (Figs. 3a, b, 4). The improved activity of optimized 19-77∆ mAbs was attributable to the loss of hydrogen bonding by R71 (Figs. 3c, 6c), leading to greater flexibility of the CDRs as shown by molecular dynamics (Fig. 3e) and structural analyses (Fig. 6). This enhanced conformation flexibility, in turn, allowed each optimized antibody to be more tolerant of mutations within or near its epitope, ultimately resulting in the striking SARS-CoV-2 neutralization potency and breadth observed (Fig. 4). Remarkably, this optimization strategy was also successfully applied to 6 other IGHV3-53/3-66-derived human mAbs that target the same epitope cluster as 19-77 (Fig. 5). But its applicability did not extend to other SARS-CoV-2-neutralizing mAbs that utilize other VH germline genes, suggesting a specific mutation at framework residue 71 is not likely to confer a generalizable benefit to other mAbs. Nevertheless, the general concept of modifying antibody conformational flexibility should be further explored in other settings by other means. Increasing conformational rigidity could lead to improved antibody-antigen affinity, as has been reported for an IGHV4-59-encoded anti-lysozyme mAb68. On the other hand, increasing conformational flexibility, as shown in this study, could allow the antibody to better tolerate sequence variations in the target antigen. Such an approach may be useful for monoclonal antibodies directed to polymorphic antigens or surface proteins of rapidly evolving viruses such as HIV-1, coronaviruses, influenza viruses, and hepatitis C virus. It may be another important conceptual tool in our antibody engineering armamentarium.
Methods
Human participant
Blood sample from Donor 19, a 41-year-old Asian male, was collected at Columbia University Irving Medical Center. Donor 19 was confirmed for a BA.5 infection by PCR sequencing (single-nucleotide polymorphisms) and provided written informed consent. Sample collections were performed under protocols reviewed and approved by the Institutional Review Board of Columbia University. Clinical information of Donor 19 is provided in Supplementary Fig. 1a.
Cell lines
Vero-E6 (CRL-1586) and HEK293T (CRL-3216) cells were purchased from the American Type Culture Collection (ATCC). Expi293 cells (A14527) were purchased from Thermo Fisher Scientific. Vero-E6-TMPRSS2-T2A-ACE2 (NR-54970) were obtained from BEI Resources. 293T-ACE2 were kindly provided by Dr. Jesse D. Bloom. The morphology of each cell line was confirmed visually before use. All cell lines tested mycoplasma negative. Vero-E6 and Vero-E6-TMPRSS2-T2A-ACE2 cell lines are from African green monkey kidneys. HEK293T, 293T-ACE2, and Expi293 cells are of female origin.
Plasmid construction
SARS-CoV-2 spike-expressing plasmids for D614G, Alpha, Beta, Gamma, Delta and Omicron were previously generated13,20,21,27. Expressing constructs for the spike proteins of SARS-CoV-2-like sarbecoviruses were either previously generated69 or newly generated by synthesizing (GenScript) spike genes and then cloned into the pCMV3 vector. To generate the expression constructs for soluble spike trimer (S2P) proteins, the ectodomains (1-1208aa, numbering based on WA1) of the spikes were PCR amplified and cloned into the paH vector and then introduced K986P and V987P substitutions, as well as a “GSAS” substitution of the furin cleavage site (682-685aa in WA1) into the spikes70. SARS-CoV S2P was fused with an AVI tag at the C terminus and D614G and XBB.1.5 S2P spikes were tagged with a 6×His tail also at the C terminus. To make the SARS-CoV-2 RBD-expressing construct, the RBD region (319–537aa) of each variant was fused with a 6×His tag and then cloned into the p3BNC vector. All constructs were confirmed by Sanger sequencing.
Antibody-expressing constructs were generated as previously described38. The variable regions of heavy and light chains for each antibody were synthesized (GenScript) and then cloned into the gWiz vector. To make spike/antibody plasmid constructs carrying individual mutations, the Q5 Site-Directed Mutagenesis Kit (NEB) was utilized following the manufacturer’s instructions.
Protein purification
To make human ACE2-Fc (hACE2) protein, pcDNA3-sACE2-WT(732)-IgG171 (Addgene plasmid #154104, gift of Erik Procko) plasmid was transfected into Expi293 cells using 1 mg/mL polyethyleneimine (PEI) at a ratio of 1:3, and the supernatants were collected after 5 days. hACE2 was purified from the cell supernatant by using rProtein A Sepharose (GE). For antibody purification, both heavy and light chains of each antibody were transfected at a ratio of 1:1 into Expi293 cells using PEI. And the expressed antibody in the cell supernatant was purified using the same method as for hACE2 purification. For the spike trimer proteins or RBD proteins, paH-spike or p3BNC-RBD, respectively, was transfected into Expi293 cells using PEI at a ratio of 1:3, and the supernatants were collected five days later. The His-tagged and the AVI-tagged proteins were purified using Excel resin (Cytiva) and Agarose-bound Galanthus nivalis lectin (VectorLabs, AL-1243-5) according to the manufacturers’ instructions. The molecular weight and purity were checked by running the proteins on SDS-PAGE.
After purification of 19-77 and 19-77\(\triangle\)A/L/V, 100 µg of each antibody was prepared and run through a Superdex 200 Increase 10/300 GL column to generate their size exclusion chromatography (SEC) profiles. AVI-tagged SARS-CoV S2P protein were biotinylated using the BirA biotin-protein ligase standard reaction kit (Avidity LLC; BirA500) following the manufacturer’s instructions.
ELISA
50 ng per well of an antigen such as S2P spike, NTD (ACROBiosystems, S1D-C52H6), RBD (ACROBiosystems, SPD-C52H1), RBD-SD1 (Exonbio, 19Cov-S130), S1 (ACROBiosystems, S1N-C52H3), or S2 (ACROBiosystems, S2N-C52H2) was coated onto ELISA plates at 4 °C overnight. The ELISA plates were then blocked with 300 μL of blocking buffer consisting of phosphate-buffered saline (PBS) with 1% bovine serum albumin and 20% bovine calf serum (Sigma-Aldrich) at 37 °C for 2 h. Afterward, 100 μL of fivefold serially diluted antibodies was added and then incubated at 37 °C for 1 h. Next, 100 μL of 10,000-fold diluted Peroxidase AffiniPure goat anti-human IgG Fcγ fragment-specific antibody (Jackson ImmunoResearch, catalog no. 109-035-170, RRID: AB_2810887) was added into each well and incubated for another 1 h at 37 °C. The plates were washed between each step with PBST (0.5% Tween-20 in PBS). Last, 3,3′,5,5′-tetramethylbenzidine (TMB) substrate (Sigma-Aldrich) was added and incubated before the reaction was stopped using 1 M sulfuric acid. Absorbance was measured at 450 nm.
Antibody pharmacokinetics in mice
About 100 µg (1 mg/mL) of each antibody was intraperitoneally (ip) injected into Balb/c mice (three mice for each antibody). Mouse blood was collected on days 2, 4, 7, and 10 post-IP injection and antibody concentrations in serum were measured by ELISA. Briefly, 100 ng goat anti-human IgG Fc antibody (Cat: 109-005-008, Jackson ImmunoResearch) was coated per well in 96-well plates overnight and the ELISA plates were then blocked with 300 μL of blocking buffer consisting of PBS with 1% bovine serum albumin and 20% bovine calf serum (Sigma-Aldrich) at 37 °C for 2 h. Afterward, 100 μL of threefold serially diluted serum or twofold serially diluted purified antibody was added and then incubated at 37 °C for 1 h. Next, 100 μL of 10,000-fold diluted Peroxidase AffiniPure goat anti-human IgG H + L HRP (Cat: 109-035-088, Jackson ImmunoResearch) was added into each well and incubated for another 1 hour at 37 °C. The plates were washed between each step with PBST (0.5% Tween-20 in PBS). Last, TMB substrate (Sigma-Aldrich) was added and incubated before the reaction was stopped using 1 M sulfuric acid. Absorbance was measured at 450 nm.
Pseudovirus production and infectivity
SARS-CoV-2 pseudoviruses were generated as previously described38. In brief, HEK293T cells were transfected with a spike-expressing construct using 1 mg/mL PEI and then infected with VSV-G pseudotyped ΔG-luciferase (G*ΔG-luciferase, Kerafast) 1 day post-transfection. Two hours after infection, cells were washed three times with PBS, changed to fresh medium, and then cultured for one more day before the cell supernatants were harvested. Pseudoviruses in the cell supernatants were clarified by centrifugation, aliquoted, and stored at −80 °C.
To evaluate the infectivity of the pseudotyped escape variants of 19-77 and 19-77∆V, fresh pseudoviruses without freezing and thawing were serially titrated from 50 µL with a dilution factor of 3 and then inoculated into Vero-E6 cells. After overnight culture, Vero-E6 cells were harvested and quantified for luciferase activity using the Luciferase Assay System (Promega).
Pseudovirus neutralization assay
To normalize the viral input between assays before conducting the neutralization assays, pseudoviruses of SARS-CoV and SARS-CoV-2 variants and SARS-CoV-2-like sarbecoviruses were titrated on Vero-E6 cells and 293T-ACE2 cells. Heat-inactivated serum from Donor 19 was serially diluted starting from 1:25 with a dilution factor of four. Monoclonal antibodies were fivefold serially diluted starting from 20 µg/mL in 96-well plates in triplicate. Then, 50 µL of diluted pseudovirus was added and incubated with 50 µL serial dilutions of serum or mAb for 1 h at 37 °C. During the coculture, target cells were trypsinized, resuspended with fresh medium, and then added into the virus-sample mixture at a density of 4–10 × 104 cells/well. The plates were incubated at 37 °C for ~12 h before luciferase activity was quantified using the Luciferase Assay System (Promega) using SoftMax Pro v.7.0.2 (Molecular Devices). Neutralization ID50 values for sera and IC50 values for antibodies were calculated by fitting a nonlinear five-parameter dose-response curve to the data in GraphPad Prism v.10.
Selection of escape mutations
SARS-CoV-2 isolate Omicron JN.1 (BEI NR-59693) was mixed with serial fivefold dilutions of 19-77∆V antibody at an MOI of 0.2 and incubated for 1 h. Following incubation, the mix was overlaid on a 24-well plate bearing a monolayer of Vero-ACE2-TMPRSS2 cells (BEI NR-54970) to a final volume of 1 mL. Plates were incubated at 37 °C/ 5% CO2 for 70 h till cytopathic effect (CPE) was complete (100%) in virus control wells bearing no antibody. At this time, all wells with antibody dilutions were scored to determine the 50% inhibition titer (EC50) and supernatant collected from this well was used for the subsequent round of selection. Passaging of the progeny over new Vero-ACE2-TMPRSS2 cells continued till each of the virus variant was able to form CPE in the presence of 50 µg/mL of the antibody. The resulting supernatant was then collected, and RNA was extracted using QiaAMP Viral RNA kit (Qiagen 57704). cDNA was obtained using the Superscript IV enzyme (Thermo Scientific 18090010). Spike gene from the cDNA was amplified using limiting dilution nested PCR and sequenced using Sanger sequencing (Genewiz). Multiple clones from limiting dilution nested PCR were sequenced to confirm the dominant mutants in the pool of the resulting progeny viruses, and a percentage of their prevalence was calculated from the total number sequenced. At least eight clones were sequenced from each of the passages reported in Fig. 7.
To generate recombinant replication-competent VSV-ΔG bearing the JN.1 spike protein (VSV-ΔG-JN.1), the pVSVΔG-SARS-CoV-2-S_nLucP plasmid was purchased from Kerafast (Cat# EGA292) and its encoding SARS-CoV-2-S gene was replaced with the JN.1 spike gene to create pVSVΔG-JN.1_nLucP. The pVSVΔG-JN.1_nLucP plasmid and a set of helper plasmids, including VSV-N, VSV-P, VSV-L, and VSV-G (Kerafast, Cat# EH1012), were then transfected into 293T cells at a ratio of 5:3:5:1:8 using 1 mg/mL PEI-MAX. Before transfection, 293T cells were rinsed with serum-free DMEM, incubated with Vaccinia vTF7-3 (Imanis Life Sciences, Cat# REA006) at an MOI of 5 for 45 min, and then replaced with fresh medium. Two days post-transfection, the supernatant was harvested and filtered through a 0.22 µm filter to remove cell debris and Vaccinia vTF7-3. The rVSV-ΔG-JN.1 generated from 293T cells was then serially diluted with a dilution factor of 5 and inoculated into Vero-E6-TMPRSS2-T2A-ACE2 cells in 24-well plates for 1 h. The virus was then washed away, and the cells were cultured at 37 °C/5% CO2 for 16–24 h. Vero-E6-TMPRSS2-T2A-ACE2 cells were monitored, and the virus was harvested from wells in which only one plaque was observed, then filtered and stored at −80 °C. The rVSV-ΔG-JN.1 generated from Vero-E6-TMPRSS2-T2A-ACE2 cells was titrated on Vero-E6-TMPRSS2-T2A-ACE2 cells before use.
To select escape viruses, rVSVΔG-JN.1 was incubated with 0.4 µg/mL of 19-77∆V for 1 h before being added to Vero-E6-TMPRSS2-T2A-ACE2 cells in 24-well plates at an MOI of 0.01. A total of 66 replicates were set up. Two days after coculture, cell supernatants from wells with CPE were harvested, and 100 µL of each supernatant was incubated with 4 µg/mL of 19-77∆V for 1 hour before another round of infection in pre-seeded Vero-E6-TMPRSS2-T2A-ACE2 cells in 24-well plates. Two days later, supernatants containing escape viruses were further harvested and selected the same way using 20 µg/mL, and then 50 µg/mL of 19-77∆V in a stepwise manner. mRNAs of the escape viruses in the supernatants were then extracted using the viral RNA/DNA purification kit (MACHEREY-NAGEL, Cat# 740643) and reverse-transcribed to cDNA using the SuperScript VILO cDNA Synthesis Kit (Invitrogen, Cat# 11754). The RBD genes of the escape viruses were then amplified using primers 5’-GGGCATCTACCAGACCAGCAACTTCA-3’ and 5’-GAACACATTGCTGCCTGTGCTGT-3’ and sequenced.
Antigen-specific memory B cell sorting and single-cell B cell receptor sequencing
Peripheral blood mononuclear cells from Donor 19, and two healthy donors were stained with the LIVE/DEAD Fixable Yellow Dead Cell Stain Kit (Invitrogen) at ambient temperature for 20 min, followed by washing with RPMI 1640 complete medium [RPMI 1640 + 10% fetal bovine serum (FBS) + penicillin/streptomycin (P/S) (100 U/mL)] and incubation with 10 μg/mL XBB.1.5 S2P protein and biotinylated SARS-CoV S2P at 4 °C for 45 min. Afterwards, the cells were washed again and incubated with a cocktail of flow cytometry and Hashtag antibodies, consisting of CD3 PerCP-Cy5.5, CD19 APC/Cyanine 7, CD27 APC, IgM FITC, anti-His PE/DazzleTM 594, Streptavidin BV421, and human Hashtag 3 at 4 °C for 1 h. Stained cells were then washed, resuspended in RPMI 1640 complete medium, and sorted for SARS-CoV and/or XBB.1.5 S2P trimer-specific memory B cells (CD3 − CD19 + CD27+IgM−antigen+ live single lymphocytes) by flow cytometry. The sorted cells were mixed with spike-in CD3+ cells and loaded into a 10X Chromium chip of the 5′ Single Cell Immune Profiling Assay (10X Genomics) at the Columbia University Single-Cell Analysis Core. Library preparation and quality control were performed according to the manufacturer’s protocol and sequenced on a NextSeq 500 sequencer (Illumina).
Identification of spike-specific antibody transcripts
Antibody transcripts specific to the XBB.1.5 S2P spike and SARS-CoV spike trimers were identified following previously established methods38. The assembly of full-length antibody transcripts was performed utilizing the Cell Ranger V(D)J analysis software (version 3.1.0, 10X Genomics), employing default settings with the GRCh38 V(D)J germline sequence version 2.0.0 as the reference genome. To differentiate between cells captured in the antigen-specific sorting process and those added as spike-ins, the count module of Cell Ranger was used to quantify the presence of all hashtag oligonucleotides in each cell based on Next Generation Sequencing (NGS) raw data. Identification of high-confidence antigen-specific cells was achieved using the following criteria: (1) A minimum of 100 copies of the antigen-specific hashtag was required for a cell to be classified as antigen-specific, (2) Given that hashtags might detach from their original cells and attach to others within the sample, a cell was considered truly antigen-specific only if the copy number of its specific hashtag was at least 1.5 times higher than that of any non-specific hashtag present, (3) Cells deemed to be of low quality were excluded based on the cell quality assessment algorithm used by Cell Ranger, (4) Only cells expressing both productive heavy and light chain antibody gene pairs were retained, (5) In instances where a cell exhibited more than two transcripts for heavy and/or light chains, transcripts supported by fewer than three unique molecular identifiers (UMIs) were discarded, and 6) Cells sharing identical heavy and light chain sequences, potentially indicative of mRNA contamination, were consolidated into a single cell entry.
Antibody transcript annotation
Transcripts specific to the antigen were analyzed and annotated with SONAR version 2.0, following previously established procedures. Assignment of V(D)J gene segments to each transcript was conducted via BLASTn, employing specialized parameters against a germline gene repository sourced from the International ImMunoGeneTics (IMGT) information system database. The identification of the complementarity-determining region 3 (CDR3) utilized BLAST alignments of the V and J segments, focusing on the conserved second cysteine within the V segment and the WGXG (for heavy chains) or FGXG (for light chains) motifs in the J segment, with “X” indicating any amino acid. Isotype determination for heavy chain transcripts was achieved by analyzing Constant domain 1 (CH1) sequences against a human CH1 gene database from IMGT, using BLASTn with standard parameters. The CH1 allele presenting the lowest E-value was selected for precise isotype classification, adhering to a BLAST E-value cutoff of 10e-6. Transcripts with incomplete V(D)J segments, frameshifts, or extraneous sequences beyond the V(D)J region were discarded. The filtered transcripts were then aligned to their corresponding germline V gene using CLUSTALO, and levels of somatic hypermutation were quantified through the Sievers method. In instances where cells possessed multiple high-quality heavy or light chains, potentially indicative of doublets, combinations of all H and L chains were generated.
Crystallization and data processing
19-77 Fab was produced by digestion of IgG with immobilized Endoproteinase Lys-C (Sigma-Aldrich) equilibrated with 25 mM Tris pH 8.5 and 1 mM EDTA for 3 h. The resulting Fab was further purified from the cleaved Fc domain by cation exchange chromatography. Fab purity was analyzed by SDS-PAGE and buffer-exchanged into 20 mM Tris, 150 mM, pH 7.4 prior to cryo-EM/Crystallization experiments.
19-77/SARS-CoV-2-RBD and 19-77/EG5.1-CoV-2-RBD complexes were prepared by mixing each of the protein components at an equimolar concentration and incubating overnight at 4 °C. Protein complexes were then isolated by gel filtration on a Superdex 200 column (Cytiva, GE Healthcare). Fractions containing complexes were pooled and concentrated to 12.0 mg/mL in SEC buffer. Screening for initial crystallization conditions was carried out in 96-well sitting drop plates using the vapor-diffusion method with a mosquito crystallization robot (TTP LabTech) using various commercially available crystallization screens: MSCG-1 (Anthracene), Proplex and LMB (Molecular dimensions). Diffraction quality crystals were obtained after 7 days in the following conditions for 19-77/SARS-CoV-2-RBD: 0.1 M NaCl, 0.1 M Tris pH 7.5, 12% w/v PEG 4000, and the following conditions for 19-77/ EG5.1-CoV-2-RBD: 75% MPD and 0.1 M HEPES pH 7.5.
Prior to data collection, crystals were cryoprotected with 40% ethylene glycol supplemented in mother liquor and flash frozen in liquid nitrogen. X-ray diffraction data extending to 2.8 Å (19-77/SARS-CoV-2-RBD) and 3.2 Å (19-77/ EG5.1-CoV-2-RBD) resolution were collected at 100 K on beam line 17-ID-1 (AMX) at Brookhaven National Laboratory. Diffraction data were processed with XDS72 and scaled using AIMLESS73 from the CCP4 software suite (Collaborative Computational Project Number 4, 1994)74. Molecular replacement was performed with PHASER75, using a previously reported RBD structure (PDB 7L5B) and for 19-77 Fab, heavy chain (PDB 7XIK), light chain (3FIK) used as search models. Manual rebuilding of the structure using COOT76 was alternated with refinement using Phenix refine77. The Molprobity server was used for structure validation78 and PyMOL (version 2.1, Schrödinger, LLC) for structure visualization. A summary of the X-ray data collection and refinement statistics are shown in Supplementary Fig. 3c.
Cryo-EM sample preparation
Fab fragments of antibodies were produced by digestion of IgG with immobilized Endoproteinase Lys-C (Sigma-Aldrich) equilibrated with 25 mM Tris, pH 8.5, and 1 mM EDTA for 3 h. The resulting Fabs were purified by ion-exchange chromatography on a mono-Q column.
For the structure of 19-77 bound to D614G spike, the complex was made by mixing purified SARS-CoV-2 S2P D614G spike protein with Fab in a 1:3 molar ratio (spike potomer:Fab) in PBS, pH 7.4, such that the final concentration of spike was 1 mg/mL. This mixture was incubated on ice for 1 h.
For the structures of the ternary complex of 19-77, RBDs, and S309, complexes were made by mixing purified SARS-CoV-2 RBD with Fabs in a 1:1.2 molar ratio in PBS, pH 7.4, and further purified using size exclusion chromatography (SEC) using a Superdex 200 Increase column. The resulting complex peak was then concentrated to 4 mg/mL and held on ice until vitrification.
Before freezing, 0.005% (w/v) n-dodecyl β-D-maltoside (DDM) was added to deter preferred orientation and aggregation during vitrification. Cryo-EM grids were prepared by applying 3 µL of sample to a freshly glow-discharged carbon-coated copper grid (CF 1.2/1.3 300 mesh); the sample was then vitrified in liquid ethane using a Vitrobot Mark IV with a wait time of 30 s, a blot time of 3 s, and a blot force of 0.
Cryo-EM data collection and analysis
Cryo-EM data for single particle analysis were collected at the Columbia Cryo-EM Facility on a Titan Krios electron microscope operating at 300 kV, equipped with a Gatan K3-BioQuantum detector and energy filter, using the Leginon7 software package. Exposures were taken at a magnification of 105,000x (pixel size of 0.83 Å), using a total electron flux of 58 e-/Å2 fractionated over 50 frames with an exposure time of 2.5 s. A random defocus range of −0.8 to −2.0 µm was used.
Data processing was performed using cryoSPARC v3.3.1.8. Raw movies were aligned and dose-weighted using patch motion correction, and the micrograph contrast transfer function (CTF) parameters were estimated using patch CTF estimation. Micrographs were picked using a blob or template picker, and an initial particle set was selected using 2D classification. Further heterogeneous refinement was used to 3D-classify particles and remove debris. The resulting curated particle sets were corrected for local motion and refined using homogenous refinement. Local refinement was performed for the spike dataset using a mask enveloping the RBD+Fab variable region. The default cryoSPARC auto-sharpened maps were then used to build the models. Cryo-EM data collection and consensus refinements are summarized in Supplementary Fig. 3.
Model building and refinement
Initial molecular models for Fabs were generated using Alphafold Multimer79 using paired heavy and light sequences. An RBD from PDB 7KNI, an RBD-up spike structure, was used as a starting model. For the initial 19-77 structure, the 14-7 structure (PDB 8F89), RBD (PDB 8IOV), and S309 Fab (PDB 7XSW) were used. The initial models were rigid body docked into the density map using UCSF Chimera’s “fit to map” tool and combined. The Fab CDR loops were manually fitted to the density map using Coot real-space refinement. The models were fit to density using the ISOLDE package in ChimeraX80. Ramachandran outliers were corrected using ISOLDE’s “flip peptide bond” feature. Real-space refinement in Phenix81 was performed to remove geometry outliers. The remaining manual adjustments were performed in Coot. Models were validated using MolProbity16 in Phenix and the PDB validation server and deposited to the PDB with accession codes: 19-77 + SARS-CoV-2 D614G RBD (PDB 9CFE), 19-77 + HK.3 RBD (PDB 9CFF), 19-77∆V + HK.3 RBD (PDB 9CFG), and 19-77∆V + JD.1.1 RBD (PDB 9CFH). A summary of data collection, processing, and model refinement statistics is shown in Supplementary Fig. 3.
In silico antibody engineering
The energy changes for the binding of both the mutant and original 19-77 to the HK.3 RBD were calculated using FoldX software40. Initially, the 19-77 and HK.3 complex was repaired and optimized using the “Optimize” function. Subsequently, every position in the heavy and light chains was subjected to saturation mutagenesis to all other amino acids using the “BuildModel” function. This process generated both an unmutated model and a mutant model for each mutation. The binding energies were estimated with the “AnalyseComplex” function, and the changes in Gibbs free energy (ΔΔG) were calculated based on the energy difference between each mutant and its corresponding original antibody-RBD complex.
Molecular dynamics analysis
Antibody 19-77, along with other VH3-53/66 class antibodies, was meticulously aligned through the RBD within each complex to ensure structural consistency. The R71A mutant antibodies were precisely engineered using the “Mutagenesis” function in PyMOL version 2.5.4, provided by Schrödinger, LLC. Subsequently, the Fab regions of both the original and mutant antibodies underwent molecular dynamics simulations employing GROMACS on the WebGro server. The preprocessing was executed using the GROMOS96 54a7 force field and incorporated an SPC water model within a cubic simulation box, supplemented with 0.15 M NaCl to mimic physiological ionic strength. The initial energy minimization step was set to 5000 iterations to stabilize the system. This was followed by equilibration and simulation phases under NVT/NPT conditions, conducted at a physiological temperature of 310 K for 10 ns, with all other parameters set to default. To ensure the reliability of the simulation, the root mean square deviation (RMSD) of each system was manually monitored to verify that each simulation reached equilibrium. Additionally, the per-residue root-mean-square fluctuation (RMSF) analysis was conducted to assess the flexibility of residues within each Fab region.
Structure comparison analysis
The paratope and epitope residues for 19-77 was identified using PISA with the default parameters. The gene-specific substitution profiles (GSSP) for 19-77 germline genes were obtained from the cAb-Rep database (https://cab-rep.c2b2.columbia.edu/). The 19-77 and its mutant complexes were first superimposed by using the “align” function in PyMOL 2.5.4. The distance between the Cα from identical residues within the 19-77 heavy chains were then determined using the rms_cur function in PyMOL by in-house Python script.
Data analysis
IC50 values were determined by fitting the data to five-parameter dose-response curves in GraphPad Prism v.10.3. GraphPad Prism v.10.3 was also used for data visualization. PISA was used for identifying antibody-spike interface residuals. PyMOL V.2.5.4 was used to perform mutagenesis and to generate structural plots.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All experimental data were provided in this manuscript. SARS-CoV-2 spike sequences were sourced from the Global Initiative on Sharing All Influenza Data (GISAID) database (https://www.gisaid.org/). The sequences for 19-77 and 19-79 are deposited in NCBI under accession: PV010127 to PV010130 [https://www.ncbi.nlm.nih.gov/nuccore/PV010127, https://www.ncbi.nlm.nih.gov/nuccore/PV010128, https://www.ncbi.nlm.nih.gov/nuccore/PV010129, https://www.ncbi.nlm.nih.gov/nuccore/PV010130]. Structures are available in the Protein Data Bank (PDB) under the following IDs: 9CFE, 9CFF, 9CFG, 9CFH, and 9CAV. All data supporting the results of this study are available in the article, supplementary, and source data files or from the corresponding author upon request. Source data are provided with this paper.
References
Wang, P. et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 593, 130–135 (2021).
Cerutti, G. et al. Potent SARS-CoV-2 neutralizing antibodies directed against spike N-terminal domain target a single supersite. Cell Host Microbe 29, 819–833.e817 (2021).
McCallum, M. et al. N-terminal domain antigenic mapping reveals a site of vulnerability for SARS-CoV-2. Cell 184, 2332–2347 e2316 (2021).
Wang, P. et al. Increased resistance of SARS-CoV-2 variant P.1 to antibody neutralization. Cell Host Microbe 29, 747–751.e744 (2021).
Barnes, C. O. et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature 588, 682–687 (2020).
Planas, D. et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 596, 276–280 (2021).
Shi, R. et al. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature 584, 120–124 (2020).
Jones, B. E. et al. The neutralizing antibody, LY-CoV555, protects against SARS-CoV-2 infection in nonhuman primates. Sci. Transl. Med. 13, eabf1906 (2021).
Hansen, J. et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science 369, 1010–1014 (2020).
Park, Y. J. et al. Antibody-mediated broad sarbecovirus neutralization through ACE2 molecular mimicry. Science 375, 449–454 (2022).
Shu, Y. & McCauley, J. GISAID: Global initiative on sharing all influenza data - from vision to reality. Euro Surveill. 22, 30494 (2017).
Scott, L. et al. Track Omicron’s spread with molecular data. Science 374, 1454–1455 (2021).
Liu, L. et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature 602, 676–681 (2022).
Carreno, J. M. et al. Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature 602, 682–688 (2022).
Cameroni, E. et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature 602, 664–670 (2022).
Planas, D. et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 602, 671–675 (2022).
Cao, Y. et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 602, 657–663 (2022).
Cele, S. et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 602, 654–656 (2022).
Zost, S. J. et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature 584, 443–449 (2020).
Wang, Q. et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature 608, 603–608 (2022).
Wang, Q. et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell 186, 279–286.e278 (2023).
Pinto, D. et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature 583, 290–295 (2020).
Westendorf, K. et al. LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants. Cell Rep. 39, 110812 (2022).
Wang, Q. et al. Antibody neutralisation of emerging SARS-CoV-2 subvariants: EG.5.1 and XBC.1.6. Lancet Infect. Dis. 23, e397–e398 (2023).
Yue, C. et al. ACE2 binding and antibody evasion in enhanced transmissibility of XBB.1.5. Lancet Infect. Dis. 23, 278–280 (2023).
Jian, F. et al. Convergent evolution of SARS-CoV-2 XBB lineages on receptor-binding domain 455-456 synergistically enhances antibody evasion and ACE2 binding. PLoS Pathog. 19, e1011868 (2023).
Wang, Q. et al. Antigenicity and receptor affinity of SARS-CoV-2 BA.2.86 spike. Nature 624, 639–644 (2023).
Hong, Q. et al. Molecular basis of receptor binding and antibody neutralization of Omicron. Nature 604, 546–552 (2022).
Guenthoer, J. et al. Identification of broad, potent antibodies to functionally constrained regions of SARS-CoV-2 spike following a breakthrough infection. Proc. Natl Acad. Sci. USA 120, e2220948120 (2023).
Liu, L. et al. Antibodies targeting a quaternary site on SARS-CoV-2 spike glycoprotein prevent viral receptor engagement by conformational locking. Immunity 56, 2442–2455.e2448 (2023).
Looi, M. K. Covid-19: WHO adds JN.1 as new variant of interest. BMJ 383, 2975 (2023).
Wang, Q. et al. XBB.1.5 monovalent mRNA vaccine booster elicits robust neutralizing antibodies against XBB subvariants and JN.1. Cell Host Microbe 32, 315–321.e313 (2024).
U.S. Food and Drug Administration. FDA Roundup: March 22, 2024, https://www.fda.gov/news-events/press-announcements/fda-roundup-march-22-2024 (2024).
Wang, Q. et al. Antibody evasiveness of SARS-CoV-2 subvariants KP.3.1.1 and XEC. Cell Rep. 44, 115543 (2024).
Wang, Q., Guo, Y., Ho, J. & Ho, D. D. Activity of research-grade pemivibart against recent SARS-CoV-2 JN.1 sublineages. N. Engl. J. Med. 391, 1863–1864 (2024).
Wang, Q. et al. Antibody evasiveness of SARS-CoV-2 subvariants KP.3.1.1 and XEC. Cell Rep. 44, 115543 (2025).
Cai, Y. et al. AZD3152 neutralizes SARS-CoV-2 historical and contemporary variants and is protective in hamsters and well tolerated in adults. Sci. Transl. Med. 16, eado2817 (2024).
Liu, L. et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature 584, 450–456 (2020).
Yuan, M. et al. Structural basis of a shared antibody response to SARS-CoV-2. Science 369, 1119–1123 (2020).
Schymkowitz, J. et al. The FoldX web server: an online force field. Nucleic Acids Res. 33, W382–W388 (2005).
Fenwick, C. et al. Broadly potent anti-SARS-CoV-2 antibody shares 93% of epitope with ACE2 and provides full protection in monkeys. J. Infect. 87, 524–537 (2023).
Cao, Y. et al. Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution. Nature 614, 521–529 (2022).
Nutalai, R. et al. Potent cross-reactive antibodies following Omicron breakthrough in vaccinees. Cell 185, 2116–2131.e2118 (2022).
Cao, Y. et al. Potent neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell sequencing of convalescent patients’ B cells. Cell 182, 73–84.e16 (2020).
Cao, Y. et al. Rational identification of potent and broad sarbecovirus-neutralizing antibody cocktails from SARS convalescents. Cell Rep. 41, 111845 (2022).
Jian, F. et al. Viral evolution prediction identifies broadly neutralizing antibodies to existing and prospective SARS-CoV-2 variants. Nat. Microbiol. https://doi.org/10.1038/s41564-025-02030-7 (2025).
Rosen, L. E. et al. A potent pan-sarbecovirus neutralizing antibody resilient to epitope diversification. Cell 187, 7196-–7213.e26 (2024).
Yu, L. et al. Potent and broadly neutralizing antibodies against sarbecoviruses elicited by single ancestral SARS-CoV-2 infection. Commun. Biol. 8, 378 (2025).
Planas, D. et al. Escape of SARS-CoV-2 variants KP.1.1, LB.1, and KP3.3 from approved monoclonal antibodies. Pathog. Immun. 10, 1–11 (2024).
Tixagevimab and cilgavimab (Evusheld) for pre-exposure prophylaxis of COVID-19. JAMA 327, 384–385 (2022).
Casadevall, A. & Focosi, D. Lessons from the use of monoclonal antibodies to SARS-CoV-2 spike protein during the COVID-19 pandemic. Annu. Rev. Med. 76, 1–12 (2025).
Casadevall, A. & Focosi, D. Anti-spike monoclonal antibody monotherapies and immune escape risk minimization strategies. Clin. Infect. Dis. 80, 484–485 (2025).
Marks, J. D. et al. By-passing immunization: building high affinity human antibodies by chain shuffling. Biotechnology 10, 779–783 (1992).
Yoshinaga, K. et al. Ig L-chain shuffling for affinity maturation of phage library-derived human anti-human MCP-1 antibody blocking its chemotactic activity. J. Biochem. 143, 593–601 (2008).
Kim, H. Y., Stojadinovic, A. & Izadjoo, M. J. Affinity maturation of monoclonal antibodies by multi-site-directed mutagenesis. Methods Mol. Biol. 1131, 407–420 (2014).
Banach, B. B. et al. Antibody-directed evolution reveals a mechanism for enhanced neutralization at the HIV-1 fusion peptide site. Nat. Commun. 14, 7593 (2023).
Pan, Y. et al. Screening of potent neutralizing antibodies against SARS-CoV-2 using convalescent patients-derived phage-display libraries. Cell Discov. 7, 57 (2021).
Hess, G. T. et al. Directed evolution using dCas9-targeted somatic hypermutation in mammalian cells. Nat. Methods 13, 1036–1042 (2016).
Mason, D. M. et al. High-throughput antibody engineering in mammalian cells by CRISPR/Cas9-mediated homology-directed mutagenesis. Nucleic Acids Res. 46, 7436–7449 (2018).
Wellner, A. et al. Rapid generation of potent antibodies by autonomous hypermutation in yeast. Nat. Chem. Biol. 17, 1057–1064 (2021).
Desautels, T. A. et al. Computationally restoring the potency of a clinical antibody against Omicron. Nature 629, 878–885 (2024).
Shan, S. et al. Deep learning guided optimization of human antibody against SARS-CoV-2 variants with broad neutralization. Proc. Natl Acad. Sci. USA 119, e2122954119 (2022).
Li, L. et al. Machine learning optimization of candidate antibody yields highly diverse sub-nanomolar affinity antibody libraries. Nat. Commun. 14, 3454 (2023).
Burn Aschner, C. et al. A multi-specific, multi-affinity antibody platform neutralizes sarbecoviruses and confers protection against SARS-CoV-2 in vivo. Sci. Transl. Med. 15, eadf4549 (2023).
Song, R., Oren, D. A., Franco, D., Seaman, M. S. & Ho, D. D. Strategic addition of an N-linked glycan to a monoclonal antibody improves its HIV-1-neutralizing activity. Nat. Biotechnol. 31, 1047–1052 (2013).
Ovchinnikov, V., Louveau, J. E., Barton, J. P., Karplus, M. & Chakraborty, A. K. Role of framework mutations and antibody flexibility in the evolution of broadly neutralizing antibodies. Elife 7, e33038 (2018).
Tramontano, A., Chothia, C. & Lesk, A. M. Framework residue 71 is a major determinant of the position and conformation of the second hypervariable region in the VH domains of immunoglobulins. J. Mol. Biol. 215, 175–182 (1990).
Yanaka, S., Moriwaki, Y., Tsumoto, K. & Sugase, K. Elucidation of potential sites for antibody engineering by fluctuation editing. Sci. Rep. 7, 9597 (2017).
Liu, L. et al. An antibody class with a common CDRH3 motif broadly neutralizes sarbecoviruses. Sci. Transl. Med. 14, eabn6859 (2022).
Hsieh, C. L. et al. Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science 369, 1501–1505 (2020).
Chan, K. K. et al. Engineering human ACE2 to optimize binding to the spike protein of SARS coronavirus 2. Science 369, 1261–1265 (2020).
Kabsch, W. Integration, scaling, space-group assignment and post-refinement. Acta Crystallogr. D. Biol. Crystallogr. 66, 133–144 (2010).
Evans, P. R. & Murshudov, G. N. How good are my data and what is the resolution?. Acta Crystallogr. D. Biol. Crystallogr. 69, 1204–1214 (2013).
Winn, M. D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D. Biol. Crystallogr. 67, 235–242 (2011).
McCoy, A. J. Solving structures of protein complexes by molecular replacement with Phaser. Acta Crystallogr. D. Biol. Crystallogr. 63, 32–41 (2007).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D. Biol. Crystallogr. 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. Biol. Crystallogr. 66, 213–221 (2010).
Davis, I. W., Murray, L. W., Richardson, J. S. & Richardson, D. C. MOLPROBITY: structure validation and all-atom contact analysis for nucleic acids and their complexes. Nucleic Acids Res. 32, W615–W619 (2004).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Meng, E. C. et al. UCSF ChimeraX: Tools for structure building and analysis. Protein Sci. 32, e4792 (2023).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D. Struct. Biol. 75, 861–877 (2019).
Acknowledgements
This study was supported by funding from the Gates Foundation (INV019355) and donations from Andrew and Peggy Cherng.
Author information
Authors and Affiliations
Contributions
D.D.H. and L.L. conceived this project. J.Y., J.H., and C.C.T. constructed antibody expression plasmids. R.G.C. conducted cryo-EM and E.R.R. conducted x-ray. J.Y. studied antibody PK in mice. M.S.N. and Y.H. performed escape virus selection on live virus. Y.G. did bioinformatic and structural analyses. Q.W., I.A.M., M.W., H.H., C.C.T. and L.L. conducted pseudovirus neutralization assays. Q.W. and L.L. conducted all other experiments including antigen-specific B cell sorting, protein expression plasmid construction, ELISA, protein/antibody purification, rVSV-JN.1 escape variants selection, etc. L.S., L.L., and D.D.H. directed and supervised the project. Q.W., Y.G., R.G.C., L.L., L.S., and D.D.H. analyzed the results and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
Q.W., J.Y., R.G.C., Y.G., L.S., L.L., and D.D.H. are inventors on the patent application (WH Ref: 0019240.01338US1) or the provisional patent application (63/619,716) filed by Columbia University for a number of SARS-CoV-2-neutralizing antibodies described in this manuscript. Both sets of applications are under review. D.D.H. is a co-founder of TaiMed Biologics and RenBio, a consultant to WuXi Biologics and Brii Biosciences, and board director for Vicarious Surgical. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Emanuele Andreano, Mario Borgnia and Daniele Focosi for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Q., Guo, Y., Casner, R.G. et al. Optimizing a human monoclonal antibody for better neutralization of SARS-CoV-2. Nat Commun 16, 6195 (2025). https://doi.org/10.1038/s41467-025-61472-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-61472-z