Abstract
Previous evidence suggest bone and cartilage damage is the main pathogenesis of rheumatoid arthritis joint destruction. However, the role of meniscus usually has not been thoroughly explored. Here, we identify CD142+ synovial fibroblasts as a subset located at sublining layer in normal and osteoarthritis synovium, which is increased and distributed at lining layer in rheumatoid arthritis synovium. Injection of CD142+ fibroblasts into DBA/1 male mice’s knee destructs meniscus but has slight effect on cartilage. ABCC4 is highly expressed in CD142+ fibroblasts, whose blockage by MK571 attenuates CD142+ fibroblasts-induced meniscus destruction through cAMP/PKA signaling. Long-term follow-up of rheumatoid arthritis cohort indicates that enriched CD142+ fibroblasts at lining layer are a risk factor for severe knee joint destruction and eventually undergo total knee arthroplasty. Our results demonstrate CD142+ fibroblasts as an indicator to assess prognosis and a therapeutic target to inhibit meniscal destruction, thereby alleviating rheumatoid arthritis knee joint damage.
Similar content being viewed by others
Introduction
The global prevalence of rheumatoid arthritis (RA) is approximately 0.5–1%, affecting approximately 18 million people1. In China, the morbidity rate is 0.42%, with over 5 million diagnosed cases2. RA primarily involves small joints, such as the metacarpophalangeal and interphalangeal joints; however, some patients present with large joint involvement, including the knee joint, where severe cases may require total knee arthroplasty (TKA). The proportion of TKA for RA is 3–9% in the United States3,4 and approximately 6% in China5. The autoimmune response is the representative pathophysiology of RA6. Immune cells stimulate synovial fibroblasts (SF), which then undergo inflammatory and invasive phenotypes, damaging the cartilage and bone, and leading to joint dysfunction7. Recent single-cell RNA sequencing (scRNA-seq) studies revealed that FAPα+ THY1+ SF located at the synovial sublining layer (SL) resulted in severe and persistent inflammatory arthritis, whereas the FAPα+ THY1- SF located at the synovial lining layer selectively mediates bone and cartilage damage8. NOTCH3 signaling drives THY1+ sublining SF differentiation and exaggerates inflammation in RA9 cells.
Unlike other joints composed of bone, cartilage, and ligaments, the knee joint has a special structure, the meniscus10. The meniscus plays a vital role in knee-joint stability, shock absorption, and load distribution11. In osteoarthritis (OA), meniscus degeneration is an important pathogenesis for OA development12,13, and destabilization of the medial meniscus (DMM) is the most widely used model for traumatic OA in mice14. In RA, both direct observation during TKA surgery15 and magnetic resonance imaging (MRI) evaluation16 show severe meniscal damage. However, the role of the meniscus in knee damage in patients with RA remains unclear.
To determine the specific SF contributing to meniscal destruction in RA, we analyze the cellular interactions of the synovium, cartilage, and meniscus using scRNA-seq. We demonstrate that CD142+ SFs are elevated in both human and mouse RA, and their intra-articular injection invades and severely impairs the meniscus with only a minimal effect on cartilage destruction. Inhibiting CD142+ SFs invasion by MK571 attenuates meniscus damage. These results may offer insights into reducing RA knee damage by alleviating CD142+ SFs-induced meniscus destruction.
Results
Severe meniscus degradation in knee RA
We assessed meniscus degradation in human RA using radiology and histology. We compared the radiographs and MRI scans of knee joints of patients with OA (Kellgren–Lawrence grade 3–4) who required arthroplasty and those of patients with knee RA who also required arthroplasty. The radiograph at the weight-bearing position showed a significant reduction in both medial and lateral knee joint space in patients with RA compared with those having OA (Supplementary Fig. 1a, b, d). In addition to extensive synovial hyperplasia and cartilage destruction, the entire meniscus (lateral meniscus anterior horn, lateral meniscus body, lateral meniscus posterior horn, medial meniscus anterior horn, medial meniscus body, and medial meniscus posterior horn) was also drastically damaged compared with that in patients with OA (Supplementary Fig. 1a, c, e). In wrist meniscus, the MRI scans also showed severe destruction in RA (Supplementary Fig. 1f).
Meniscus degeneration in OA is accompanied by collagenous fiber disruption; Safranin O-positive areas appear in Safranin-O-Fast Green staining17. Compared with the OA meniscus, the RA meniscus showed a remarkable volume reduction and severe fiber structure destruction, and a large number of invaded areas emerged (Fig. 1a–c). Synovial hyperplasia is a common feature of RA, and various cell types, such as fibroblasts and immune cells in the synovium, play an important role in the destruction of the bone and cartilage of the knee joint7,18. Therefore, we assessed the relationship between histopathological synovitis score (Krenn Score) and meniscal degradation. The Krenn score evaluation included the width of the synovial lining layer, cell density of the stroma, and density of inflammatory infiltration19. The Krenn score of the RA synovium was significantly higher than that of the OA synovium, with similar results obtained when the lining layer (LL) scores were compared independently (Fig. 1d–f). Spearman’s correlation analysis indicated that the LL score was positively associated with meniscal degradation and invasion area (Fig. 1g, h). Collectively, these data demonstrate that severe meniscal destruction in RA is associated with synovitis and synovial invasion.
a Representative Safranin-O-Fast Green staining images of meniscus from OA patients (n = 10) and RA patients (n = 15). b Quantification of meniscus degeneration score, which consists of four components: femoral/tibial/inner border surface, cellularity, collagen organization/alignment, and fiber organization, matrix Safranin-O-Fast Green staining. c Quantification of synovial invasion area ratio to whole meniscus area. d Representative Hematoxylin and Eosin (HE) staining images of knee synovium from OA patients (n = 10) and RA patients (n = 15). e, f Quantification of total Krenn score (maximum point, 9) and lining cell layer component of Krenn score (maximum point, 3). g, h Spearman’s correlation between RA Krenn score (lining layer) and RA meniscus degeneration score and RA meniscus invasion area. Source data are provided in the Source Data file. Statistics: two-tailed Student’s t-test (b, c, e, f). Data are mean ± s.d.
CD142+ SF elevates in RA synovium
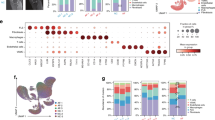
To investigate the mechanism of joint destruction by SF in RA, we performed scRNA-seq analysis of chondrocytes, meniscal cells, and synovial cells from patients with RA requiring TKA. Eight major cell types have been identified: meniscal cell, chondrocyte, SF, macrophage, smooth muscle cell, T cell, endothelial cell, and mast cell. SF was more closely distributed with meniscal cell than with chondrocyte in the uniform manifold approximation and projection (UMAP) (Fig. 2a, b, Supplementary Fig. 2a, b). Next, we re-analyzed SF separately and identified three distinct cell subpopulations: the PRG4+ cluster (expressing PRG4 and HBEGF) from the LL, the DKK3+ cluster (expressing DKK3 and CHI3L2) from the SL8, and the CD142+ cluster (expressing CD142 and CXCL12), an unreported cluster neighboring meniscal cell in UMAP (Fig. 2c, d). To validate whether CD142+ SF coincides with any previously reported SF cluster, we projected the scRNA-seq data from the AMP2 public dataset, including 9 SF clusters of 73 RA patients18, onto our fibroblast UMAP space. As a result, 7 SF clusters mapped with our data, and importantly the CXCL12+ SFRP1+ SF shared maximal similarity with CD142+ SF cluster (Fig. 2e). CellChat analysis showed that the interactions between CD142+ SF and meniscal cells were the most abundant, indicating a stronger relationship (Fig. 2f, Supplementary Fig. 2c).
a Single-cell RNA sequencing in UMAP projection shows 5368 cells of cartilage, 5256 cells of meniscus, and 4664 cells of synovium from one human RA knee sample. b Cells from three organs yield eight major clusters annotated according to the expression of known classical marker genes (Supplementary Fig. 2a, b). c Re-analysis of 3237 cells of synovial fibroblast (SF) revealed 3 SF subsets. d Expression of marker genes (y axes) for the three identified SF subsets (x axes). e Analysis of cell-to-cell interaction shows the heatmap of interaction numbers between SF subsets and meniscal cell or chondrocyte. The value in the colored block reveals the specific number of interactions between clusters (signaling ligand-receptor pairs listed in Supplementary Fig. 3c). f “Stromal cells” scRNA-seq from the AMP2 dataset, which contained nearly 60,000 single cells from 9 fibroblast clusters of 73 RA patients (excluding “Mu-0: Mural” cluster), projected and mapped on the fibroblast UMAP of this study. g Representative diagram of flow cytometry in the process of gating and sorting CD142+ SFs. h Percentage of CD142+ SFs to total CD31-CD45- SFs (normal synovium, n = 3; OA synovium, n = 6; RA synovium, n = 12; biological replicates). i Representative IHC images of CD142 in human knee synovial fluid deposit. j Quantification of CD142+ cells in synovial fluid deposit (n = 6; biological replicates). k–n Representative Safranin-O-Fast Green staining of mouse knee joint and IHC staining of CD142. k knee joint from wildtype mice and OA mice (DMM model) of C57BL/6 strain. l Quantification of CD142+ cell count by calculating the ratio of positive CD142 cells to total synovial cells (n = 6 mice, biological replicates). m knee joint from wildtype mice and CIA mice of DBA/1 strain. n Quantification of CD142+ cell count (n = 6 mice, biological replicates). Source data are provided in Source Data file. Statistics: one-way ANOVA with Tukey's multiple comparison test (h); two-tailed Student’s t-test (j, l, n). Data are mean ± s.d.
To further reveal the intrinsic heterogeneity between SF clusters, we performed Gene Set Enrichment Analysis (GSEA). It revealed that CD142+ SF was enriched in gene ontology (GO) pathways of bone remodeling (ossification, osteoblast differentiation, bone remodeling), fibroblast invasiveness (integrin binding, epithelial to mesenchymal transition, positive regulation of cell migration, cell-matrix adhesion, tissue remodeling), and fibroblast proliferation (epithelial cell proliferation, fibroblast proliferation, response to fibroblast growth factor) (Supplementary Fig. 2d). The marker genes of CD142+ SF were involved in ossification (SFRP1, CDH11), positive regulation of cell migration (CD142), and fibroblast proliferation (SFRP1) (Supplementary Fig. 2e). Collectively, these data suggested that CD142+ SF has the potential to invade the meniscus.
We examined the number of CD142+ SF in normal, OA, and RA knee joints. Normal synovium was obtained from knees that underwent thigh amputation or hemipelvectomy, whereas OA and RA synovium were acquired from knee arthroplasty cases. Flow cytometry showed that the number of CD142+ SF in RA synovium was significantly elevated compared to that in normal and OA synovium (Fig. 2g, h). We also collected synovial fluid from patients with OA and RA during arthroplasty and obtained the deposits by centrifugation. The deposits were cut into histological sections, and immunohistochemical (IHC) staining revealed that the amount of CD142+ SF in RA synovial fluid deposits was also significantly higher than that in OA; however, the THY1+ and PRG4+ cells were not significantly changed (Fig. 2i, j, Supplementary Fig. 2f–h).
We evaluated the number of CD142+ SF in the mouse model. The surgical model of DMM in the C57BL/6 strain was used to study OA, whereas the model of collagen-induced arthritis (CIA) in the DBA/1 strain was used to induce autoimmune arthritis to study RA. CD142+ SF was slightly increased in OA mice and drastically increased in CIA mice (Fig. 2k–n). THY1+ and PRG4+ cells were slightly elevated in the CIA mice, but no significant changes were observed in the OA mice (Supplementary Fig. 2i–n).
Distribution of CD142+ SF converts from SL to LL in RA
Recently, scRNA-seq revealed that fibroblasts at the LL have a strong effect on cell adhesion and invasion, whereas fibroblasts at the SL exhibit severe proinflammatory function8. Therefore, we examined the spatial distribution of CD142+ SF in the synovium. We used multiplex immunohistochemistry (mIHC) staining to label different cell subtypes: PRG4 to label LL, THY1 to label SL, and CD146 to label blood vessels. Consistent with the cell flow cytometry results, CD142+ SF levels were markedly increased in patients with RA. Notably, the spatial distribution was also altered. In normal and OA synovium, CD142+ SFs were mainly located at the SL, whereas they were massively distributed at the LL in RA. Additionally, we observed that CD142+ SF was closer to the superficial layer than PRG4+ SF (Fig. 3a–c, Supplementary Fig. 3a, b).
a Multiplex immunohistochemistry (mIHC) staining of normal, OA, and RA knee synovium (normal, n = 3; OA, n = 5; RA, n = 5; biological replicates). The right column of images are spatial maps labeling the corresponding fluorescent cells in the middle column of sections. In spatial map, cells with no fluorescence but only stained with DAPI are defined as “Other” with gray color. We divided the synovium into lining layer (LL) and sublining layer (SL) based on the corresponding HE staining of the sections. Total cell number, lining layer cell number, and sublining layer cell number of CD142+, PRG4+, THY1+, CD146+ and Other were obtained based on the Akoya inForm (version 1.6). b Spatial distribution of CD142+ cells, which is calculated as the ratio of lining layer CD142+ number to total CD142+ number, or sublining layer CD142+ number to total CD142+ number (RA versus N, P = 0.001; RA versus OA, P = 0.0001). c Cell constituent in lining layer, which is calculated as the ratio of specific cell type number in lining layer to total lining layer cell number (CD142+ cell % in lining layer, RA versus N, P < 0.0001; RA versus OA, P < 0.0001). d Nearest spatial distance between CD142+ cells and CD146+ cells. Based on the spatial map acquired from Akoya inForm, we utilized R package PhenoptrReports (version 0.3.3) to process the data and yielded the nearest distances between CD142+ cells and CD146+ cells (μm). All distances were summed and taken average for one sample (normal, n = 3; OA, n = 5; RA, n = 5; biological replicates). e Co-expression of other SFs markers in CD142+ cells (normal, n = 3; OA, n = 5; RA, n = 5; biological replicates). Source data are provided in Source Data file. Statistics: one-way ANOVA with Tukey’s multiple comparison test (b, c, d, e). Data are mean ± s.d.
We calculated the distance from each CD142+ cell to the nearest CD146+ cell. No significant difference was observed between the normal and OA synovium, whereas the distance was significantly elongated in RA (Fig. 3d). To distinguish whether CD142+ SF is a distinct cell cluster or a population overlapping with previously identified subsets, we counted the number of CD142 single- or double-positive cells with PRG4 or THY1. In normal synovium, most CD142+ cells expressed THY1(52.2%) or PRG4 (28.9%), whereas in RA synovium, the majority of CD142+ cells (76.8%) did not overlap with THY1 or PRG4 (Fig. 3e, Supplementary Fig. 3c). Together, these results identified CD142+ fibroblasts as a distinct cluster significantly elevated in RA synovium, and the distribution also converted from the SL to the LL.
Next, we assessed the distribution of CD142+ SF in other joint synovia. Similar to the knee joint, the wrist joint also has the meniscus. Therefore, we obtained the human wrist RA synovial specimens during arthroscopic surgery, and the mIHC staining suggests CD142+ SFs are mainly expressed at the LL, which is similar to the the knee joint (Supplementary Fig. 4a). We also obtain RA synovial specimens from other joints during arthroplasty or arthroscopic surgery, and found that CD142+ SFs in the shoulder, elbow, and the first metacarpophalangeal joint are mainly distributed at the LL, while in the hip and ankle joints are mainly distributed at the SL (Supplementary Fig. 4b).
CD142+ SFs invade and destruct meniscus
To examine the role of CD142+ SF in meniscal destruction, we performed histological staining of the synovial invasion area in human RA and CIA mouse menisci. IHC staining of the invaded area showed strong CD142 expression in both human RA menisci and CIA mouse menisci, whereas only a small number of cells expressed PRG4 and THY1(Fig. 4a–c). In the CIA mouse wrist joint, the wrist meniscus was also invaded by CD142+ SFs (Supplementary Fig. 5a). We established a knee joint injection mouse model for SF invasion. CD142+ and CD142- SFs were sorted by flow cytometry and cultured, which were then labeled with fluorescence before each injection. The knee joint of DBA/1 mice was injected CD142+ or CD142- SFs at 8, 9, 10, and 11-week of age, and these mice were sacrificed at 12- or 16-week of age for histological assessment (Fig. 4d).
a CD142 expression markedly elevates in synovial invasion area in RA meniscal matrix. Safranin-O-Fast Green staining shows the invasion area in meniscal matrix, with the corresponding image in the same section of IHC staining of CD142, PRG4, THY1. b Quantification of human meniscus (n = 6, biological replicates) invaded area cell count, calculated by the ratio of specific cell to total invaded area cell. c Quantification of CIA mice meniscus (n = 6 mice, biological replicates) invaded area cell count. d Diagram of establishing mice invasive SFs arthritis model. DBA/1 male mice were applied knee intra-articular injection of CD142+ and CD142- SFs at age of 8, 9, 10, and 11 weeks once a week. Knee samples were obtained at age of 12- and 16-week. e Representative fluorescent image of knee joint of invasive SFs arthritis model at 16-week of age provides direct evidence for CD142+ SFs invading meniscus (n = 3 mice, biological replicates). Before intra-articular injection, SFs are labeled with autofluorescence by CellTrace CFSE dye, which shows green color excited at 488 nm channel. DAPI labels nuclei. f Representative Safranin-O-Fast Green staining image of knee joint of invasive SFs arthritis model (n = 6 mice for both 12-week and 16-week, biological replicates). All sections displayed are sagittal view of knee lateral compartment. g Quantification of meniscus degeneration score of CD142- and CD142+ injection group. h Quantification of synovial invasion area in meniscus of CD142- and CD142+ injection group. i Quantification of Krenn score of CD142- and CD142+ injection group. j Quantification of cartilage OARSI score of CD142- and CD142+ injection group. k Co-culture of SFs-meniscal cells and quantification of expression of COL1A1 mRNA in meniscal cells (n = 6, biological replicates). Source data are provided in Source Data file. Statistics: one-way ANOVA with Tukey’s multiple comparison test (b, c, k); two-tailed Student’s t-test (g, h, i, j). Data are mean ± s.d.
Frozen sections from 16-week-old mice showed significant invasion of CD142+ SF, particularly in the contact area of the meniscus and synovium, whereas the invasion of CD142- SF was attenuated. Cartilage invasion was weaker than that of the meniscus, with only one or two layers of cells invading the cartilage surface (Fig. 4e). Paraffin sections from both groups showed severe synovitis; however, meniscal synovial invasion and destruction were more severe in the CD142+ group. The meniscus volume was smaller, and the meniscal white zone (without blood supply) was completely disrupted and replaced by synovial tissue (Fig. 4f–i). Notably, cartilage damage was mild in both groups. At 12-week, both groups showed minimal cartilage damage with no significant difference in OARSI scores, while OARSI scores in the CD142+ group were higher than those in the CD142- group at 16-week (Fig. 4j). This suggests secondary cartilage damage resulting from meniscus degradation rather than direct synovial inflammation or cartilage invasion.
ABCC4 blockade attenuates CD142+ SF invasion
We examined the invasive and migratory abilities of CD142+ SFs. Compared with CD142- SF, CD142+ SF exhibited higher invasion and migration abilities (Supplementary Fig. 5b, c). The mRNA expression of cell migration- and invasion-related genes (SNAI1, SNAI2, and TWIST1) and matrix metalloproteinase genes (MMP3, MMP9, and MMP13) were also significantly upregulated, whereas the migration suppressor gene CDH1 (encoding E-cadherin)20 was significantly downregulated (Supplementary Fig. 5d, e). Co-culture of human RA meniscal cells with CD142+ or CD142- SFs showed that COL1A1 mRNA expression in human meniscal cells co-cultured with CD142+ SFs was significantly decreased (Fig. 4k).
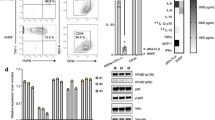
We then identified potential targets of CD142+ SF invasion using Bulk RNA-seq to compare gene expression between CD142+ and CD142- SF. In the five genes with the highest upregulated expression, we found three genes, including RELN (3.87-fold increase)21, P2RY2 (3.44-fold increase)22, and ABCC4 (3.19-fold increase)23 associated with cell migration and invasion (Fig. 5a–b, Supplementary Data 1). We next used siRNAs to inhibit the respective gene and indicated that ABCC4 knockdown can significantly attenuate CD142+ SFs migration and invasion. ABCC4, also known as multidrug resistance protein 4, a member of the C subfamily of ABC transporters, is reported to be associated with cell migration and adhesion24. Gene Ontology (GO) of Cellular Component (CC) analysis revealed enrichment of cell migration and adhesion-related components in CD142+ SF (Fig. 5c). IHC staining confirmed increased ABCC4 expression in the synovial LL and synovial fluid deposits of patients with RA (Fig. 5d–g).
a Bulk RNA sequencing of CD142- SFs and CD142+ SFs (n = 3, biological replicates) reveals 25 up-regulated genes and 89 down-regulated genes in CD142+ SFs, displayed on volcano plot with red color for up-regulated and blue color for down-regulated. b Heatmap of all up- and down- regulated genes for CD142- SFs (three columns on the left) and CD142+ SFs (three columns on the right), with top 10 genes labeled. c GO analysis in Cellular Component (CC) category for CD142+ SFs. d Representative images of IHC staining of ABCC4 in human RA and OA synovium. e Quantification of synovium ABCC4+ cell count (ratio to total synovial cells in the section, n = 6, biological replicates). f Representative images of IHC staining of ABCC4 in human RA and OA synovial fluid deposit. g Quantification of synovial fluid deposit ABCC4+ cell count (ratio to total deposit cells in the section, n = 6, biological replicates). h MK571 slightly influences on CD142- SFs in transwell assays. MK571: 5 μM. i Quantification of CD142- SFs transwell cell count (n = 3, biological replicates). j MK571 drastically represses the aggressiveness of CD142+ SFs in transwell assays. MK571: 5 μM. k Quantification of CD142+ SFs transwell cell count (n = 3, biological replicates). l MK571 suppresses the expression of related classical cell migration genes (mRNA) for CD142+ SFs by qPCR (n = 3, biological replicates). m MK571 suppresses the expression of extracellular matrix degradation enzymes genes (mRNA) for CD142+ SFs by qPCR (n = 3, biological replicates). n Co-culture of CD142+ SFs-meniscal cells in MK571 and quantification of expression of COL1A1 mRNA in meniscal cells (n = 6, biological replicates). Source data are provided in Source Data file. Statistics: one-way ANOVA with Tukey’s multiple comparison test (n); two-tailed Student’s t-test (e, g, i, k, l, m). Data are mean ± s.d.
We further assessed whether pharmacological blockade of ABCC4 using MK571, a selective ABCC4 blocker25, could inhibit CD142+ SF invasion. While MK571 slightly inhibited migration and invasion of CD142- SF(Fig. 5h, i), CD142+ SF treated with MK571 resulted in prominently reduced migration and invasion(Fig. 5j, k), accompanied by decreased mRNA expression of SNAI1, SNAI2, TWIST1, MMP3, MMP9, and MMP13, and increased CDH1 expression (Fig. 5l, m). Additionally, MK571 alleviated the effect of COL1A1 mRNA inhibition in human RA meniscal cells co-cultured with CD142+ SFs (Fig. 5n).
MK571 attenuates meniscus degradation driven by CD142+ SF invasion
We investigated whether MK571 could inhibit CD142+ SF invasion and attenuate meniscal degradation in vivo. The knee joints of DBA/1 mice were injected with CD142+ SF separately or in combination with MK571 at 8, 9, 10, and 11-week of age, and sacrificed at 12- or 16-week of age for histological assessment, as previously described (Fig. 6a). Frozen sections from 16-week mice showed that MK571 treatment protected the meniscus from CD142+ SF invasion (Fig. 6b). Safranin-O-Fast Green staining of paraffin sections revealed that MK571 treatment significantly reduced the meniscal invasion area, lowered the Krenn score at both 12- and 16-week, and decreased the OARSI score at 16 weeks (Fig. 6c–g). Collectively, these results demonstrate that MK571 attenuates meniscal damage by inhibiting CD142+ SF invasion, thereby protecting against secondary damage to the articular cartilage.
a Diagram of establishing mice invasive SFs arthritis model. DBA/1 male mice were applied knee intra-articular injection of CD142+ SFs and MK571 (5 μM) + CD142+ SFs at age of 8, 9, 10, and 11 weeks once a week. Knee samples were obtained at age of 12- and 16-week. b Representative fluorescent image of knee joint of invasive SFs arthritis model at 16-week of age proves the blockage effect of MK571 toward CD142+ SFs invading meniscus (n = 3 mice, biological replicates). c Representative Safranin-O-Fast Green staining image of knee joint of invasive SFs arthritis model (n = 6 mice for both 12-week and 16-week, biological replicates). All sections displayed are sagittal view of knee lateral compartment. d Quantification of meniscus degeneration score. e Quantification of synovial invasion area in meniscus. f Quantification of Krenn score. g Quantification of cartilage OARSI score. Source data are provided in Source Data file. Statistics: two-tailed Student’s t-test (d, e, f, g). Data are mean ± s.d.
ABCC4 promotes CD142+ SF invasion by down-regulating cAMP/PKA signaling
We next investigate how ABCC4 regulates CD142+ SF invasion. The cytokines including IL-1β, TNF-α, IL-17α, TGF-β, and INF-γ were used to stimulate CD142- SFs, and the flow cytometry revealed that IL-1β and TNF-α significantly promoted the transformation of CD142- SFs to CD142+ SFs (Fig. 7a, b), and qRT-PCR suggested that IL-1β and TNF-α significantly upregulated the marker genes of CD142+ SFs, including CD142, CXCL12, SFRP1, and the expression of CDH11, CD34, and ABCC4, while INF-γ played the opposite effect (Fig. 7c). These results suggest that IL-1β and TNF-α may contribute to the produce of CD142+ SFs in the progression of RA.
a Representative images of flow cytometry on cytokines stimulating CD142- SFs after 96 h, assessing percentage of CD142+ SFs (PE). The concentration of each cytokine is 10 ng/ml. b Quantification of percentage of CD142+ SFs for each group (n = 3, biological replicates). c Quantification of expression of on CD142+ SF marker genes and ABCC4 by qPCR (n = 3, biological replicates). d,e Intracellular cAMP concentration of each group by ELISA. f H89 restores aggressiveness of CD142+ SFs in transwell assays under the background of MK571. H89: 15 μM. MK571: 5 μM. g Quantification of CD142- SFs transwell cell count (n = 3, biological replicates). h H89 restores the expression of related classical cell migration genes for CD142+ SFs by qPCR (n = 3, biological replicates). i H89 restores the expression of extracellular matrix degradation enzymes genes for CD142+ SFs by qPCR (n = 3, biological replicates). Source data are provided in Source Data file. Statistics: one-way ANOVA with Tukey’s multiple comparison test (b, d, e); two-tailed Student’s t-test (c, g, h, i). Data are mean ± s.d.
ABCC4 is responsible for cAMP efflux26,27. To further explore how ABCC4 regulates the invasion of CD142+ SFs, we measured the intracellular cAMP concentration and found that intracellular cAMP concentration in CD142+ SFs was remarkably lower than CD142- SFs, and the blockage of ABCC4 by MK571 elevated intracellular cAMP in CD142+ SFs (Fig. 7d). IL-1β and TNF-α stimulation also promoted cAMP efflux in stimulated CD142- SFs (Fig. 7e). As a second messenger involved in vital signaling pathways, cAMP usually activates downstream PKA (cAMP-dependent protein kinase A) to modulate cell migration and invasion28. We next validated whether ABCC4 regulated CD142+ SFs in cAMP/PKA manner; therefore, we used H89 to inhibit cAMP-dependent PKA. CD142+ SFs were separately stimulated by MK571 or MK571 plus H89. In the background of MK571, H89 does not change intracellular cAMP concentration (Fig. 7d) but largely restores the invasion of CD142+ SFs (Fig. 7f, g), and elevates the expression of SNAI1, SNAI2, TWIST1, and MMPs (Fig. 7h, i). Together, these results indicate ABCC4 promotes the invasion of CD142+ SFs by down-regulating cAMP/PKA signaling (Fig. 8g).
a X-ray for RA patients who haven’t progressed to knee deformity (non-TKA) and have received total knee arthroplasty with knee deformity (TKA). b Quantification of Knee Society score (KSS score) for non-TKA (n = 10) and TKA (n = 7) patients at the end-point of follow-up. We chose Objective Knee Indicators for assessment, containing four categories (each category scoring 25 points): alignment, instability, joint motion and symptoms. c mIHC staining of knee synovia from non-TKA patients (n = 10, biological replicates) and TKA patients (n = 7, biological replicates). All knee synovia were acquired with knee parker-pearson fine needle biopsy at the time of earliest onset of knee symptoms. The right column of images are spatial maps labeling the corresponding fluorescent cells in the middle column of sections. d Spatial distribution of CD142+ cells for non-TKA synovia and TKA synovia, which is calculated as the ratio of lining layer CD142+ number to total CD142+ number, or sublining layer CD142+ number to total CD142+ number (non-TKA versus TKA, P < 0.0001). e Cell constituent in lining layer for non-TKA synovia and TKA synovia, which is calculated as the ratio of specific cell type number to total lining layer cell number (CD142+ cell % in lining layer, non-TKA versus TKA, P = 0.001). f Nearest spatial distance between CD142+ cells and CD146+ cells for non-TKA synovia (n = 10, biological replicates) and TKA synovia (n = 7, biological replicates). g Schematic diagram of the mechanism of CD142+ SFs driving meniscus destruction in RA and ABCC4 regulates CD142+ SF invasion by down-regulating cAMP/PKA signaling. Source data are provided in Source Data file. Statistics: two-tailed Student’s t-test (b, d, e, f). Data are mean ± s.d.
CD142+ SF distributed at LL is a risk factor for knee dysfunction in RA
To investigate the role of CD142+ SF in the progression of knee dysfunction in RA, we followed up with patients with RA having long-term knee symptoms. Seventeen patients with RA having knee symptoms were included in this study, and all patients underwent synovial biopsy at an early stage of knee pain or swelling. The follow-up duration was from 2013 to 2024, and the patients were categorized into two groups based on whether they eventually underwent TKA. We defined synovial biopsy as the starting point for this study, with the time of TKA surgery as the endpoint of follow-up for the TKA group (seven knees, mean follow-up time 82.4 months, 64–108 months), and May 2024 as the endpoint for the non-TKA group (10 knees, mean follow-up time 92.3 months, 60–120 months). Detailed demographic data are presented in Supplementary Table 1.
The New Knee Society Score System (KSS Score)29 was used to evaluate knee function. The TKA group showed significantly lower KSS scores than the non-TKA group, including worse knee alignment, stability, and range of motion (Fig. 8a, b). We analyzed the histological sections from synovial biopsies using mIHC staining. Although the number of CD142+ SFs at the SL and the distance of each CD142+ SF from the nearest CD146+ cells did not differ, the CD142+ SFs at the LL were significantly higher in the TKA group than in the non-TKA group (Fig. 8c–f), suggesting that increased CD142+ SFs at the synovial lining layer are a risk factor for severe knee deformity in patients with RA who require TKA.
Discussion
Recently, many studies have investigated the phenotype of SF in RA and clarified their spatial distribution and corresponding functions30,31. Synovial B cells, T cells, and macrophages promote SF proliferation, invasion, inflammatory mediator production, and matrix metalloproteinase secretion, eventually leading to joint destruction6,32. However, most studies have focused on bone and cartilage damage and ignored a specific anatomical part of the knee joint, the meniscus. In our study, we identified CD142+ SF as a SF subcluster, which is located at the SL in normal and OA synovium but is located at the LL in the RA synovium, and is activated by inflammatory stimuli in RA synovium to be aggressive. Intra-articular injection of CD142+ SF can quickly and drastically lead to meniscus damage, but has a slight effect on cartilage, while CD142- SF only leads to slight meniscus destruction. ABCC4, which is highly expressed in CD142+ SF, could be targeted to block CD142+ SF-induced meniscal degradation. Using the synovia from synovial biopsy in our long-term follow-up RA cohort, we found that CD142+ SFs were highly expressed at the LL of patients who eventually underwent TKA due to severe joint destruction.
Croft et al. reported two distinct SF subsets, FAPα+ THY1+ SF located at the sublining for persistent inflammation, and FAPα+ THY1− SF located at the LL for bone and cartilage damage8. However, our CD142+ SF did not belong to the preceding two cell populations but are classified as FAPα- SF (Fig. 2d) and were located variably. These differentiated results may be due to the different synovial sources, as our synovium was obtained from TKA, whereas other studies used synovial biopsies of different joints. Zhang et al. recently used scRNA-seq and surface protein histology of synovial tissues from 82 donors to classify them into six groups18. According to Zhang’s criteria, the synovium used for scRNA-seq in our study was fibroblast-abundant, the type with the poorest response to different treatments. Furthermore, in a separate fibroblast cluster analysis conducted by Zhang et al., CD142+ SF coincided with CXCL12+ SFRP1+ SF. These data indicate that CD142+ SFs are mainly present in RA synovial tissue with poor prognosis, which is also consistent with the synovial biopsy results from our long-term follow-up RA cohort.
Tissue factor (TF) encoded by CD142, is normally expressed in endothelial and mural cells, engaging in the blood clotting process, but recent studies have found that CD142 overexpression is associated with increased tumor aggressiveness and poor prognosis in various cancers33. Therefore, the overexpression of CD142 is thought to be one marker of invasive cancer cells34,35,36. This resembles our study, because we found that CD142+ SFs in normal and OA synovium remain at a low amount, while in RA synovium, they remarkably increase and acquire invasiveness. CD142+ fibroblast is found to be implicated in inflammatory bowel disease37 and carotid atherosclerosis38, but has not been reported in RA. Evidence from this study indicated that CD142 could be regarded as a marker of an invasive fibroblast subcluster in RA. ABCC4 is involved in regulating the invasiveness of CD142+ SF, while the function of CD142 itself in promoting invasion needs further study.
Previous studies have evaluated meniscal damage in RA using morphology15, histology39, and radiology16, which showed that both the cartilage and meniscus were severely damaged; however, it is unclear which was damaged first. This may be because we could not obtain the data at the early stage of knee RA, and radiological evaluation requires expensive MRI, whereas histological evaluation of the cartilage and meniscus was derived from arthroplasty for end-stage knee RA. Our study found that CD142+ SF intra-articular injection first invaded and damaged the meniscus, whereas the effect on the cartilage was slight, suggesting that knee joint dysfunction in RA might originate from meniscal destruction, and cartilage erosion might partly result from the weakened protective effect after meniscal damage. In our clinical study, we noted that patients with RA who had mild knee damage supported this hypothesis. For example, during knee arthroplasty in a 62-year-old woman with RA, we found that the lateral meniscus was completely defective, and only a small part of the medial meniscus remained. However, the articular cartilage was mainly worn in the weight-bearing area, and the cartilage is still present in the non-weight-bearing areas (Supplementary Fig. 6). The meniscus body is connected to the synovium, and the anatomical distance is shorter than the distance between the cartilage and synovium, which may explain why synovial tissue is more likely to invade the meniscus.
In conclusion, our study identified a RA fibroblast subset that proliferates and converts distribution from the SL to the LL in RA. The meniscus destruction driven by the invasion of CD142+ SF may be the primary cause of knee damage in RA. Additionally, CD142+ SF is infiltrated in multiple RA joint synovia and also invades the wrist meniscus. IL-1β and TNF-α could transform CD142- SFs into CD142+ SFs, fueling the progression of RA. High CD142+ SF expression at the LL of patients with early RA is a risk factor for severe knee destruction, ultimately requiring TKA (Fig. 8g). CD142+ SF could be used as an indicator for assessing prognosis and as a therapeutic target for the destruction of the RA knee joint.
Methods
Human subjects research
This study does not involve sex-based analysis, and sex was not considered in the study design, since RA involves both. Therefore, for human participants, both male and female patients were included. All patients included in this study signed a written informed consent form for the use of abandoned specimens (meniscus, cartilage, synovium, and synovial fluid) and publication of any potentially identifiable images or data, as approved by the Ethics Committee of Sun Yat-sen Memorial Hospital (2009-06, 2020-126). We included patients with RA who met the 1987 American Rheumatism Association criteria40 and the 2010 ACR/EULAR criteria41.
For patients with RA who underwent systemic treatment without remission of severe knee pain or knee deformity (knee varus or knee valgus), we performed knee arthroplasty and obtained meniscus, cartilage, synovium, and synovial fluid samples. For patients with OA who met Kellgren-Lawrence III or IV grading42 and had severe knee pain that could not be relieved by NSAIDs, we performed knee arthroplasty and obtained knee tissue samples. For the control knee synovium samples, we obtained normal knees by thigh amputation or hemipelvectomy. For RA shoulder and hip joint synovia, we obtained them by arthroplasty surgery. For RA elbow, wrist, and ankle joint synovia, we obtained them by arthroscopic surgery. For RA first metatarsophalangeal joint synovium, we obtained it by Parker-Pearson fine needle biopsy. Radiological data from patients with OA and RA were used for radiological analysis, and samples from normal, OA, and RA knees were used for histological analysis, single-cell RNA sequencing, cell cytometry, cell culture, in vitro and in vivo experiments, and transcriptomic RNA sequencing.
The cohort was established in 2013 for long-term follow-up in RA cohort studies. All patients with RA underwent knee Parker-Pearson fine needle biopsy at the onset of knee symptoms (knee pain and swelling) to obtain knee synovium tissue samples. After the biopsy, all patients underwent systematic antirheumatoid treatment. By the end of May 2024, 16 female and 1 male patients completed the follow-up period (detailed in Supplementary Table 1). We categorized the patients into non-TKA and TKA groups based on whether they underwent TKA. For the TKA group, the follow-up endpoint was set at the time of surgery; for the non-TKA group, the follow-up endpoint was set at May 2024. Knee joint function was assessed using the KSS score29 for both groups.
Radiological analysis of human knee data
An X-ray in the anteroposterior view in the standing posture was used to measure the medial joint space and the lateral joint space by selecting the minimum segment between the medial and lateral compartments43. All measurements were based on a real-world plotting scale. MRI with a T2-weighted sequence was used for knee and wrist menisci measurement. In the knee coronal sequence, the image plan in which the medial tibial spine volume was maximal was selected for measuring the thickness and width of the meniscal body segment, whereas in the sagittal sequence, the image plan on the midpoint of the medial/lateral compartment was selected for measuring the thickness and width of the medial/lateral meniscal anterior and posterior horn44.
Histological morphology analysis and scoring of human synovium and meniscus
For histological sections, fresh meniscus, cartilage, and synovial tissues obtained from knee arthroplasty surgery (for RA and OA subjects) were fixed in 4% paraformaldehyde and embedded in paraffin. Human knee synovial fluid was centrifuged at 770 g for 5 min, and the deposit was fixed in 4% paraformaldehyde and embedded in paraffin. All paraffin-embedded specimens were dissected into 5 μm sections and stored at room temperature. Sections of the human synovium and meniscus were stained with haematoxylin and eosin (HE), and Safranin-O-Fast Green. The lining (cell) layer of the synovium was identified on HE staining as the membrane facing the joint cavity, characterised by ellipsoidal cells arranged in layers45. Synovial sections were scored using the total Krenn synovitis score (ranging from 0–9 points) and the enlargement of the synovial lining cell layer component of the Krenn synovitis score (ranging from 0–3 points)19.
Meniscal sections were scored using the Pauli degeneration score17 (ranging from 0–18 points). Synovium invasion area on the meniscus was defined as the area containing limited clustered or widely infiltrated cells, usually accompanied by new angiogenesis. The area was calculated as the ratio of the invasion area to the total meniscal area using ImageJ software (version 1.54).
Immunohistochemistry and mIHC
For immunohistochemistry (IHC), antigen retrieval was performed at pH 9.0 using Tris-EDTA for 20 min by microwave heating. Sections were incubated with primary antibodies of CD142 (Bioss, 1:200), PRG4 (Abcam, 1:200), THY1 (Proteintech, 1:200), and ABCC4 (Proteintech, 1:200) at 4 °C overnight, followed by incubation with anti-rabbit horseradish peroxidase (HRP) secondary antibody (Servicebio, 1:200) at room temperature for 50 min. HRP staining was performed using DAB Peroxidase HRP Substrate (Servicebio).
For mIHC, antigen retrieval was performed at pH 9.0 using Tris-EDTA for 20 min by microwave heating. The main steps of mIHC are as follows: sections were incubated with primary antibody at 4 °C overnight, and then with anti-rabbit HRP secondary antibody at room temperature for 50 min, followed by tyramide signal amplification (TSA) staining at room temperature for 10 min. Antigen retrieval was performed again for the next primary antibody incubation. Primary antibodies against PRG4 (Abcam, 1:1000), CD146 (Proteintech, 1:1000), CD142 (Cell Signaling Technology, 1:600), and THY1 (Abcam, 1:600) were incubated. IF440-TSA (anti-CD146, Servicebio, 1:500), iF488-TSA (anti-CD142, Servicebio, 1:500), iF555-TSA (anti-PRG4, Servicebio, 1:500) and iF647-TSA (anti-THY1, Servicebio, 1:500) were used for TSA staining. DAPI staining (Servicebio) was performed last to label nuclei.
Quantitative and spatial analysis of mIHC
Human synovial sections stained with mIHC were scanned using Akoya whole-slide multispectral imaging in fluorophore Spectral DAPI (excitation: 368; emission: 461), Opal Polaris 480 (excitation: 450; emission: 500), Opal 520 (excitation: 494; emission: 525), Opal 570 (excitation: 550; emission: 570), and Opal 690 (excitation: 6676; emission: 694). Three fields (923 μm × 692 μm) in each section were chosen for analysis, and their data were summed as one sample. Akoya InForm (version 1.6) loaded the chosen fields to output phenoptic data, followed by tissue segmentation, cell segmentation, and phenotyping. For tissue segmentation, the lining and sublining were segmented using haematoxylin and corresponding HE staining. For cell segmentation, the minimum nuclear size was set at 10, the cytoplasm thickness was set at 5, and the membrane search distance was set at 10. For cell phenotyping, 20 cells for each fluorophore were manually chosen for classifier training, and the classifier was used for field phenotyping to label each segmented cell. The R package PhenoptrReports (version 0.3.3) processed the phenoptics data and calculated all phenotyping cells in the LL and SL of each section and the nearest distance between each pair of phenotyping cells. The results from three fields were summed and averaged for each sample.
Human meniscus, cartilage, and synovium tissue processing for cell suspensions and cell culturing
Fresh meniscus, cartilage, and synovial tissues were obtained from knee arthroplasty (for RA and OA subjects) and thigh amputation or hemipelvectomy (for control subjects). For flow cytometry, cell culturing and RNA sequencing, fresh synovium tissues were dissected in 2 mm pieces and then digested in collagenase I solution (2 mg/mL, Gibco; +10% FBS, Gibco; +1% penicillin-streptomycin, Gibco) for 2 h in 37 °C, after which solution was filtered by 70 nm cell strainer (BIOFIL) and centrifuged at 430 g for 5 minutes to obtain cells and made into single cell suspensions. Meniscal and cartilage tissues were dissected into 2 mm pieces and then digested in collagenase P solution (2 mg/mL for meniscus and 0.25 mg/mL for cartilage, Roche; +10% FBS, Gibco; +1% penicillin-streptomycin, Gibco) for 6–8 h at 37 °C for cell suspensions.
For human primary meniscal cells and SF, we cultured them in Dulbecco’s modified Eagle’s medium/Hams F12 (DMEM/F-12, Gibco) with 10% FBS and 1% penicillin-streptomycin in an incubator at 37 °C and 5% CO2.
Flow cytometry and synovial fibroblast collection
For the flow cytometric analysis of human synovial cells, red blood cell lysis buffer (Solarbio) was added to the cell suspensions for 10 min at room temperature, and the suspensions were centrifuged, washed, and resuspended. After removing red blood cells, synovial cells were stained with antibodies against CD31 (BV421, BioLegend), CD45 (APC, BioLegend), CD142 (PE, BioLegend), and 1% BSA in PBS for 20 min at room temperature. 7-Aminoactinomycin D (7-AAD, BioLegend) was added to cell suspensions, and cells were passed through a 100 μm filter. Data were acquired using BD FACSverse and analysed using FlowJo (version 10.8). Based on the above protocol, we sorted CD31-/CD45- living fibroblasts from primary human synovial cells and collected CD142+ and CD142- fibroblasts using BDJAzz for further in vivo and in vitro experiments.
Generation and analysis of single-cell RNA sequencing data
Fresh meniscus, cartilage, and synovial tissues obtained from the same RA knee by TKA were made into cell suspensions, in which meniscal cells, chondrocytes, and synovial cells were captured with 10x Genomics based on the Illumina NovaSeq 6000 platform provided by NovelBio Bio-Pharm Technology Co., Ltd. Fastp was applied with a default parameter for filtering the adaptor sequence and removing low-quality reads46. Umi tools were used for single-cell transcriptome analysis to identify the cell barcode whitelist, extract the cell barcode UMIs, and calculate the cell expression counts based on the filtered clean FASTQ data47. Downstream analysis was performed using the Seurat R package (version 4.0.2) as follows: cells with >20% mitochondrial reads, <200 genes, or <200 UMI were excluded from the analysis. We merged cells from the meniscus, cartilage, and synovium, and the per-cell counts were normalized and scaled. The fastMNN function (k = 5, d = 50, approximate = TRUE) in the R package scran (version 1.12.1) was used to apply the mutual nearest-neighbor method to correct for batch effects among samples. The first ten principal components were retained for the UMAP projection. GraphCluster and K-means were used for cell clustering (resolution set at 0.8 for the full analysis), and the Wilcoxon rank-sum test was used for marker gene analysis (min.pct = 0.1, logfc. threshold = 0.25). We identified the meniscal cells as COL1A1+/CEMIP+, chondrocytes as ACAN+/COL2A1+, SF as PRG4+/COL1A1+, macrophages as CD14+/LYZ+, smooth muscle cells as MYL9+/NOTCH3+, T cells as CD3D+/CD8A+, endothelial cells as MCAM+(CD146+)/PECAM1+, and mast cells as CD79A+/TPSB2+. SF were re-analysed to identify subclusters in the fastMNN-based UMAP space with a resolution set at 1.8, and marker genes were calculated (min.pct = 0.1, logfc. threshold = 0.25). For public scRNA-seq data projection, public scRNA-seq data were downloaded according to the reference guidance. R package scmap (version 1.24.0) was used. Fibroblast subsets of public data were set as referenced clusters by indexCluster, and mapped with the current fibroblast data of this study by scmapCluster, projecting to the same UMAP space. GO and Gene Set Enrichment Analysis (GSEA) was completed by R package clusterProfiler (version 4.10.0) and visualized by R package GseaVis (version 0.1.0).
Cell-to-cell interaction analysis
After identifying the three subclusters of SF, we performed a cell-to-cell interaction analysis using the R package CellChat (version 1.6.1). We created a CellChat object with clusters of meniscal cells and chondrocytes and three subclusters of SF. CellChat database for humans (CellChatDB.human) was loaded, and the “Secreted Signaling” category was chosen for analysis. Overexpressed genes and interactions were identified and projected onto the protein-protein interactions (PPI) of the CellChat object. We then computed the communication probability based on the algorithm’s truncated mean (cutoff = 20%) and filtered out the cell-to-cell communication if there were <10 cells in certain cell groups, which were aggregated into the final cell-to-cell communication network. Three subclusters of SF were set as communication senders, while meniscal cells and chondrocytes were set as communication receivers, and the interaction numbers were output for a heatmap and bubble plot.
Transwell assays for SF
CD142+ and CD142- fibroblasts were used for in vitro experiments. For transwell assays, we used 8.0 μm PET membrane in a 24-well format (Corning) for migration assays and 8.0 μm Matrigel-coated PET membrane in a 24-well format (Corning) for invasion assays. Note that MK571 (Selleck) and H89 (MedChemExpress) were dissolved in DMSO, and when added to the experimental system of DMEM/F-12, the concentration of MK571 was 5 μM, and H89 was 15 μM, and DMSO was lower than 0.1%. We added 1 × 105 fibroblasts and 200 μL DMEM/F-12 (0% FBS, 1% penicillin-streptomycin, and 5 μM MK571 or 15 μM H89 for the specified group) in the upper chamber, and 600 μL DMEM/F-12 (10% FBS, 1% penicillin-streptomycin) in the nether chamber. Twenty-four well plates were placed in a 37 °C incubator for 24 h for migration assay and 48 h for invasion assay.
Cytokines stimulation on CD142- SFs
CD142- SFs were sorted by flow cytometry and planted on 6-well plates with 1 × 105 per well. Each identical group of CD142- SFs was seperately stimulated with IL-1β (novoprotein), TNF-α (novoprotein), IL-17α (novoprotein), TGF-β (novoprotein), and INF-γ (novoprotein), which are dissolved in distilled water and with a working concentration of 10 ng/mL. CD142- SFs were placed in a 37 °C incubator for 96 h, and then collected for flow cytometry, ELISA, and qPCR.
Co-culture of SFs-meniscal cells
Primary human RA meniscal cells were used in this study. We used 0.4 μm PET membrane in a 6-well format (Corning). We added 1 × 105 CD142+ or CD142- fibroblasts, 1 ml DMEM/F-12 (10% FBS, 1% penicillin-streptomycin, and 5 μM MK571 for the certain group) in the upper chamber, 1 × 105 meniscal cells, and 2 mL DMEM/F-12 (10% FBS, 1% penicillin-streptomycin) in the lower chamber. Six well plates were placed in a 37 °C incubator for 72 h; then, meniscal cells were washed with PBS and extracted to total RNA (details written below) for further analysis.
Intracellular cAMP concentration assessment by ELISA
Cells cultured in a 6-well plate were washed with PBS and then added with 500 μL lysis buffer (EZ Bioscience). 50 μL of lysis buffer for each group was fetched for assessment. Human Cyclic Adenosine Monophosphate (cAMP) ELISA kit (JONLNBIO) was used according to product guidance. OD values were obtained at 450 nm, and concentration were converted based on the product’s standard curve.
Mouse OA and RA models establishment
This study does not involve sex-based analysis, and sex was not considered in the study design. Therefore, in order to control for the variable, we used only male mice in this study. The animal study design was reviewed and approved by the Ethics Committee of Sun Yat-sen Memorial Hospital (2020-B0270). All animal experiments in this study abide by the ARRIVE guidelines. Mice were housed under standard laboratory conditions (22 ± 2 °C, 50 ± 10% humidity, 12:12 light-dark cycle) with ad libitum access to a conventional rodent chow (containing ~20% protein, ~5% fat, and ~5% fiber) and autoclaved water. Bedding and cages were changed weekly. The euthanasia method of cervical dislocation in mice was applied. We created OA models by surgically destabilizing the medial meniscus (DMM) in C57BL/6 male mice at 8 weeks of age14. After 8 weeks (16 weeks of age), DMM mice were sacrificed to obtain knee samples. We created an RA model with CIA in DBA/1 male mice48. Mice were then immunized with 200 µg chick type II collagen (CII, Chondrex) emulsified 1:1 in complete Freund’s adjuvant (CFA, Chondrex) at 8 weeks of age, and were boosted 3 weeks later with 200 µg CII emulsified 1:1 in Freund’s incomplete adjuvant (IFA, Chondrex). At 16 weeks of age, CIA mice were sacrificed to obtain knee samples.
Mouse invasive SF arthritis model establishment
Human RA CD142+ and CD142- SF were used to create an invasive synovial fibroblast arthritis model. Living CD142+ and CD142- fibroblasts were first labeled with autofluorescence using CellTrace CFSE dye (Thermo Fisher Scientific), which diffused into cells and bound covalently to intracellular amines, resulting in fluorescent staining (excitation: 488 nm; Ex/Em: 592/571 nm). Resuspended fibroblasts in PBS were then added to the CFSE dye at a working concentration of 5 μM and incubated for 20 min at room temperature, protected from light. Five times the original staining volume of the culture medium was added to the fibroblasts, incubated for 5 min, centrifuged, and resuspended in DMEM/F-12. For intra-articular injection, each knee joint was injected with 4 μl cell suspensions (5 × 104 fibroblasts in DMEM/F-12, without FBS and penicillin-streptomycin), while in the MK571 group, each knee joint was injected 2 μl cell suspensions (5 × 104 fibroblasts in DMEM/F-12, without FBS and penicillin-streptomycin) plus 2 μL of MK571 (10 μM).
DBA/1 male mice were anaesthetised using isoflurane as mentioned above. We made a 1 cm vertical incision above the ligamentum patellae to fully expose it, then used a microinjector at a range of 0–10 μL to fetch cell suspensions. The needle tip was parallel to the platform and pointed at the midpoint of the medial or lateral side of the ligamentum patellae. The knee joint was then penetrated, and cell suspensions were injected until visible swelling was observed on the joint without spillage. The tip of the needle was withdrawn, the penetration point was pressed for 2 min using a cotton ball, and the skin was sutured. Intra-articular injection was administered once a week at 8, 9, 10, and 11 weeks of age. Mice were sacrificed at the age of 12 weeks for paraffin-embedded slices and at 16 weeks for both frozen OCT and paraffin-embedded slices. OCT-embedded slices were stained with DAPI and directly observed under a fluorescence microscope to detect the invasive fibroblasts. OCT and paraffin slices were prepared according to the protocol described above.
Histological analysis of mouse joint slices
The knee and wrist joints of mice were decalcified for 3 weeks with 10% EDTA solution (pH 7.2, Solarbio) and embedded in paraffin. Consecutive slices were made (paraffin in 5 μm thick, OCT in 10 μm thick) in the knee lateral compartment at sagittal view and at wrist coronal view. We used the degeneration score and invasion area to assess the destruction of the mouse meniscus, as discussed above. The Krenn score was calculated to assess synovitis in the mouse knees. To evaluate cartilage damage, the OARSI score was calculated, with a maximum of six scores49. The scores of three slices (one slice after an interval of nine slices) were summed and averaged as one joint, whereas both the left and right joints were summed and averaged as an independent sample.
Quantitative reverse transcription PCR
RNA was isolated from the cell suspensions using an RNA isolation kit (EZ Bioscience) according to the manufacturer’s instructions. cDNA synthesis was performed for all samples (500 ng of RNA was transcribed) using the RT Premix cDNA synthesis kit (Accuracy Biology). Reverse transcription quantitative real-time PCR (qRT-PCR) was performed using the SYBR GreenPro Taq qPCR kit (Accuracy Biology) on a real-time PCR detection system (Roche LightCycler480 II). The gene expression levels were quantified using the 2−ΔΔCt method. Each primer (forward, F; reverse, R) was designed based on the sequences available in the NCBI database (Supplementary Table 2).
Bulk RNA sequencing data
Human RA primary CD142+ and CD142- fibroblasts with three independent samples sorted by flow cytometry from the RA knee synovium obtained by TKA surgery were used to extract RNA. Bulk RNA-seq was performed by NovelBio Bio-Pharm Technology Co., Ltd. Fastp was applied with the default parameters to filter the adaptor sequence and remove low-quality reads. The clean reads were aligned to 9606 (Taxonomy ID) genomes (version: GRCh38) using Hisat2. HTseq was used to calculate the gene counts. Reads/fragments per kilobase million reads (RPKM/FPKM) were used to standardize expression data. We applied the DEseq2 algorithm to filter the differentially expressed genes and then filtered the fold change and FDR under the following criteria: i) log2FC > 0.585 or < -0.585; ii) FDR < 0.05. Volcano plots and heat maps were drawn using R based on the analysis of differentially expressed genes, and the color was determined using the filtering criteria. GO analysis was performed to elucidate the biological implications of unique genes in the significant or representative profiles of the differentially expressed genes in the experiment50. We downloaded from the National Center for Biotechnology Information (NCBI; http://www.ncbi.nlm.nih.gov/), UniProt (http://www.uniprot.org/), and GO (http://www.geneontology.org/) databases. Fisher’s exact test was used to identify significant GO categories, and FDR was used to correct p-values.
Statistical analysis
Statistical analyses were performed as described in each section using GraphPad Prism 9 software. Data are presented as mean ± s.d. from at least three independent experiments. Spearman’s correlation analysis was used to test the correlations between ordinal variables. Differences were considered significant at P < 0.05. Multiple testing corrections were applied where appropriate. All measurements of sample size (n) were taken from distinct biological replicates.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Sequencing data from the AMPII dataset are available on Synapse (10.7303/syn52297840)18. The raw and processed RNA sequencing data of this study are provided and accessible through GEO Series accession number GSE299518. Source data are provided with this paper.
References
Smolen, J. S., Aletaha, D. & McInnes, I. B. Rheumatoid arthritis. Lancet 388, 2023–2038 (2016).
Tian, X. et al. 2018 Chinese guidelines for the diagnosis and treatment of rheumatoid arthritis. Rheumatol. Immunol. Res 2, 1–14 (2021).
Agarwal, A. R. et al. Trend of using cementless total knee arthroplasty: a nationwide analysis from 2015 to 2021. Arthroplasty 6, 24 (2024).
Harb, M. A. et al. Have the annual trends of total knee arthroplasty in rheumatoid arthritis patients changed?. J. Knee Surg. 31, 841–845 (2018).
Long, H. et al. Length of stay and inpatient charges of total knee arthroplasty in China: analysis of a national database. Chin. Med J. (Engl.) 136, 2050–2057 (2023).
Nishide, M., Shimagami, H. & Kumanogoh, A. Single-cell analysis in rheumatic and allergic diseases: insights for clinical practice. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-024-01043-3 (2024).
Komatsu, N. & Takayanagi, H. Mechanisms of joint destruction in rheumatoid arthritis - immune cell-fibroblast-bone interactions. Nat. Rev. Rheumatol. 18, 415–429 (2022).
Croft, A. P. et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature 570, 246–251 (2019).
Wei, K. et al. Notch signalling drives synovial fibroblast identity and arthritis pathology. Nature 582, 259–264 (2020).
Kwon, H. et al. Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat. Rev. Rheumatol. 15, 550–570 (2019).
Makris, E. A., Hadidi, P. & Athanasiou, K. A. The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials 32, 7411–7431 (2011).
Sun, H. et al. Silencing of NOTCH3 signaling in meniscus smooth muscle cells inhibits fibrosis and exacerbates degeneration in a HEYL-dependent manner. Adv. Sci. (Weinh.) 10, e2207020 (2023).
Sun, H. et al. Single-cell RNA-seq analysis identifies meniscus progenitors and reveals the progression of meniscus degeneration. Ann. Rheum. Dis. 79, 408–417 (2020).
Glasson, S. S., Blanchet, T. J. & Morris, E. A. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthr. Cartil. 15, 1061–1069 (2007).
Kimura, C. & Vainio, K. The pattern of meniscus damage in the rheumatoid arthritis. Arch. Orthop. Unfallchir 83, 145–151 (1975).
Meng, X. H., Wang, Z., Zhang, X. N., Xu, J. & Hu, Y. C. Rheumatoid arthritis of knee joints: mri-pathological correlation. Orthop. Surg. 10, 247–254 (2018).
Pauli, C. et al. Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthr. Cartil. 19, 1132–1141 (2011).
Zhang, F. et al. Deconstruction of rheumatoid arthritis synovium defines inflammatory subtypes. Nature 623, 616–624 (2023).
Krenn, V. et al. Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology 49, 358–364 (2006).
Nobre, A. R. et al. ZFP281 drives a mesenchymal-like dormancy program in early disseminated breast cancer cells that prevents metastatic outgrowth in the lung. Nat. Cancer 3, 1165–1180 (2022).
Riva, M. et al. De novo monoallelic Reelin missense variants cause dominant neuronal migration disorders via a dominant-negative mechanism. J. Clin. Invest. 134, https://doi.org/10.1172/jci153097 (2024).
Tomas Bort, E. et al. Purinergic GPCR-integrin interactions drive pancreatic cancer cell invasion. Elife 12, https://doi.org/10.7554/eLife.86971 (2023).
van de Ven, R. et al. A role for multidrug resistance protein 4 (MRP4; ABCC4) in human dendritic cell migration. Blood 112, 2353–2359 (2008).
Yerushalmi, G. M. et al. ABCC4 is a PGE2 efflux transporter in the ovarian follicle: A mediator of ovulation and a potential non-hormonal contraceptive target. Faseb j. 37, e22858 (2023).
Hara, Y. et al. Inhibition of MRP4 prevents and reverses pulmonary hypertension in mice. J. Clin. Investig. 121, 2888–2897 (2011).
Cheepala, S. B. et al. The ABCC4 membrane transporter modulates platelet aggregation. Blood 126, 2307–2319 (2015).
Rodríguez González, A. et al. MRP4/ABCC4 expression is regulated by histamine in acute myeloid leukemia cells, determining cAMP efflux. Febs j. 288, 229–243 (2021).
Stoufflet, J. et al. Primary cilium-dependent cAMP/PKA signaling at the centrosome regulates neuronal migration. Sci Adv 6, https://doi.org/10.1126/sciadv.aba3992 (2020).
Scuderi, G. R. et al. The new knee society knee scoring system. Clin. Orthop. Relat. Res 470, 3–19 (2012).
Zhang, F. et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 20, 928–942 (2019).
Wu, X. et al. Single-cell sequencing of immune cells from anticitrullinated peptide antibody positive and negative rheumatoid arthritis. Nat. Commun. 12, 4977 (2021).
McGrath, S. et al. Correlation of professional antigen-presenting tbet(+)CD11c(+) B cells with bone destruction in untreated rheumatoid arthritis. Arthritis Rheumatol, https://doi.org/10.1002/art.42857 (2024).
Ahmadi, S. E. et al. Tissue factor (coagulation factor III): a potential double-edge molecule to be targeted and re-targeted toward cancer. Biomark. Res. 11, 60 (2023).
Leppert, U. & Eisenreich, A. The role of tissue factor isoforms in cancer biology. Int. J. cancer 137, 497–503 (2015).
Ruf, W. Tissue factor and cancer. Thrombosis Res. 130, S84–S87 (2012).
Goldin-Lang, P. et al. Tissue factor expression pattern in human non-small cell lung cancer tissues indicate increased blood thrombogenicity and tumor metastasis. Oncol. Rep. 20, 123–128 (2008).
Kinchen, J. et al. Structural remodeling of the human colonic mesenchyme in inflammatory bowel disease. Cell 175, 372–386.e317 (2018).
Bashore, A. C. et al. High-dimensional single-cell multimodal landscape of human carotid atherosclerosis. Arteriosclerosis, thrombosis, Vasc. Biol. 44, 930–945 (2024).
Fuhrmann, I. K., Steinhagen, J., Rüther, W. & Schumacher, U. Comparative immunohistochemical evaluation of the zonal distribution of extracellular matrix and inflammation markers in human meniscus in osteoarthritis and rheumatoid arthritis. Acta Histochem 117, 243–254 (2015).
Arnett, F. C. et al. The american rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis rheumatism 31, 315–324 (1988).
Radner, H., Neogi, T., Smolen, J. S. & Aletaha, D. Performance of the 2010 ACR/EULAR classification criteria for rheumatoid arthritis: a systematic literature review. Ann. Rheum. Dis. 73, 114–123 (2014).
Kellgren, J. H. & Lawrence, J. S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 16, 494–502 (1957).
Vignon, E. et al. Measurement of radiographic joint space width in the tibiofemoral compartment of the osteoarthritic knee: comparison of standing anteroposterior and Lyon schuss views. Arthritis rheumatism 48, 378–384 (2003).
Hunter, D. J. et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis rheumatism 54, 795–801 (2006).
Saaibi, D. L. & Schumacher, H. R. Jr. Percutaneous needle biopsy and synovial histology. Baillieres Clin. Rheumatol. 10, 535–554 (1996).
Chen, S., Zhou, Y., Chen, Y. & Gu, J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890 (2018).
Smith, T., Heger, A. & Sudbery, I. UMI-tools: modeling sequencing errors in Unique Molecular Identifiers to improve quantification accuracy. Genome Res 27, 491–499 (2017).
Brand, D. D., Latham, K. A. & Rosloniec, E. F. Collagen-induced arthritis. Nat. Protoc. 2, 1269–1275 (2007).
Glasson, S. S., Chambers, M. G., Van Den Berg, W. B. & Little, C. B. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr. Cartil. 18, S17–S23 (2010).
Ashburner, M. et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet 25, 25–29 (2000).
Acknowledgements
This study was funded by the National Key R&D Program of China (No. 2021YFA1102600, Hao Sun and Yulong Wei), National Natural Science Foundation of China (No. 82272443, Ruofan Ma; No. 82372435, Hao Sun), and Guangdong Basic and Applied Basic Research Foundation (No. 2023A1515010501, Ruofan Ma).
Author information
Authors and Affiliations
Contributions
H.Sun and X.Lin conceptualized the project, performed experiments, analyzed data, and co-wrote the manuscript. Y.Zhang and Z.Huang collected and analyzed radiological data. F.Liu and L.Yao established mice models. H.Sun and X.Lin performed IHC and mIHC staining and analyzed data. X.Lin, J.Huang, and M.Zhang analyzed scRNA-seq data and RNA-seq data. J.Ma and L.Dai completed the long-term RA cohort follow-up and collected knee synovium samples. H.Sun, X.lin, F.Liu and J.Huang generated paraffin and frozen sections. H.Sun, X.Lin, and M.Chen completed statistical data analysis. R.Ma, L.Dai, Y.Wei, and J.Xu conceived the project, supervised the work, and co-wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Christopher Buckley, Kevin Wei, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sun, H., Lin, X., Liu, F. et al. CD142-positive synovial fibroblasts drive meniscus destruction in rheumatoid arthritis. Nat Commun 16, 6942 (2025). https://doi.org/10.1038/s41467-025-61842-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-61842-7
This article is cited by
-
CD142+ synovial fibroblasts attack the meniscus in knee RA
Nature Reviews Rheumatology (2025)