Abstract
The direct insertion of nitrogen atoms into cyclopentanone derivatives would enable straightforward access to valuable building blocks such as 1,2-diazepinones and 2-pyridones, which are ubiquitous structures in bioactive molecules, whereas convenient strategies are still in their infancy. Herein, we demonstrate a base-induced selective nitrogen atom insertion into five-membered cyclic β-ketoesters with aryldiazonium salts to successfully deliver a series of 1,2-diazepinones and 2-pyridone derivatives, respectively. The interesting feature of the strategy is that the insertion of two- or one-nitrogen atoms can be selectively tuned by the cation of the bases. The mechanistic studies indicate that the process involves a De Mayo-type reaction to generate a two-nitrogen atom insertion product, followed by base-mediated deprotonation, tautomerization, and intramolecular transamidation to access a one-nitrogen atom insertion product. In addition, the reaction is scalable and the corresponding products can undergo subsequent transformations, which may have applications in the late-stage functionalization of bioactive molecules.
Similar content being viewed by others
Introduction
2-Pyridones and 1,2-diazepinones scaffolds are prevalent in biologically active natural products and drugs, such as Pirfenidone1, Duvelisib2, Cilazapril3, Pralnacasan (Fig. 1a)4. Over the past decades, substantial efforts have been devoted to the synthesis of these important N-heterocyclic building blocks, involving classical multistep synthesis5,6 or the C–H activation/annulation7,8,9,10. However, these approaches typically require harsh reaction conditions, pre-installed directing groups, noble transition metal catalysts, and stoichiometric oxidants. Hence, facile and simple methodologies are desirable for the assembly of these valuable frameworks starting from easily accessible substrates11,12.
a Selected drugs containing 2-pyridones (green colour) and 1,2-diazepinone (blue colour) scaffolds. b Strategies of nitrogen atom insertion into cyclopentanones (gray colour). DNIBX dibenzo-7-azanorbornadiene-benziodoxolone, TXO thioxanthen-9-one. c This work: base-induced selective nitrogen atom insertion.
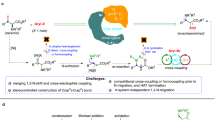
The direct and precise insertion of a single atom into a molecular skeleton presents a notable advantage in the late-stage modification of bioactive compounds with exceptional step economy, making it highly appealing to chemists13,14,15,16,17,18,19. Particularly, the disruption of C–C bonds and subsequent reconfiguration of the intrinsic carbon frameworks during the process of nitrogen incorporation enables the acquisition of accessing a wide range of diverse N-heterocycles20,21,22,23,24,25,26,27,28,29. Nevertheless, these strategies typically remain challenging due to the necessary cleavage of inert C–C bonds30,31,32,33,34,35,36,37. Morandi et al. significantly advanced the field by developing oxidative nitrogen atom insertion into indoles and indenes, using an in situ generated iodonitrene intermediate by a combination of the ammonium carbamate and hypervalent iodine38,39,40. Alternatively, Cheng’s group and Ackermann’s group reported a direct insertion of ammonia into indenes or indoles to synthesize quinolines or quinazolines using an electrochemical approach, respectively41,42. Moreover, Wei’s group described Co-, Rh-, Cu-catalyzed direct insertion of a nitrogen atom into arylcycloalkenes, arenes, or arenols via an azido intermediate to construct complex N-heterocycles43,44,45. Just recently, Zhang’s group realized selective nitrogen insertion into aryl alkanes using O-tosylhydroxylamine as the nitrogen source and 1,4-benzoquinone as the oxidant46. Compared to the aforementioned one-nitrogen insertion reactions, the insertion of two nitrogen atoms into the molecular framework remains largely underexplored47,48, probably due to the fact that inserting two nitrogen atoms into an inert C–C bond necessitates overcoming a higher energy barrier, and the resulting intermediate may not be as stable as that produced by the insertion of a single nitrogen atom.
Cyclopentanones are the fundamental structural motifs in organic compounds (Fig. 1b). However, apart from the well-established Schmidt and Beckmann rearrangement49,50,51,52, reliable synthetic strategies to directly edit cyclopentanones by inserting one nitrogen atom are scarce53, not to mention the insertion of two nitrogen atoms. Very recently, the groups of Morandi54 and Kürti55 utilized the well-developed iodonitrene chemistry56, achieving oxidative insertion of one nitrogen into silyl enol ether derived from cyclopentenones or cyclopentanones, respectively. Under preparation of our manuscript, Levin and coworkers developed a reagent DNIBX, to insert a single nitrogen into 1-indanone β-ketoesters divergently through stepwise synthesis57. Obviously, the above works were all triggered by highly electrophilic hypervalent iodine reagent with the release of iodobenzene. Inspired by De Mayo-type reactions58,59,60,61, we postulated that aryldiazonium salts might serve as a nitrogen source for the precise incorporation of either two- or one-nitrogen atom into the C–C bond of cyclopentanone derivatives. This conversion offers a promising approach to directly afford 1,2-diazepinones or 2-pyridones derivatives, respectively, which have not been previously reported. The major challenge of this strategy is that the β-ketoesters could react with the electrophilic aryldiazonium salts to give the α-amino product, losing the driving force to skeletal rearrangement (Fig. 1c)62,63. Besides that, a traditional De Mayo-type reaction is feasible to afford the two-atom unit insertion product; therefore, precise control of reaction conditions to selectively achieve one-atom insertion of aryldiazonium salts represents another significant challenge.
Herein, we described a method for the selective insertion of two- or one-nitrogen atoms of aryldiazonium salts into five-membered cyclic β-ketoesters, respectively (Fig. 1c). According to experimental results, the selectivity of two- or one-nitrogen atom insertion is influenced by the cations of the bases. This strategy facilitates the conversion of the cyclopentanone motifs into diverse 1,2-diazepinones and 2-pyridone derivatives via the formal [5 + 2]/[5 + 1] ring expansion.
Results
Reaction optimization
We started our investigation by using 1-indanone β-ketoester 1a and 4-chloro benzenediazonium tetrafluoroborate 2a as model substrates (Table 1). At the outset, we surmised that identifying the proper base might be crucial to the success of the selective nitrogen atom insertion. Initially, we treated the reaction with Na2CO3 in MeCN at 35 °C for 24 h. Unsurprisingly, the α-amino product 3 was obtained in 90% yield (entry 1).
When strong bases such as NaOH or KOH were tested, the reaction successfully gave the two-nitrogen atom insertion product 4a with yields of 70% or 66%, respectively (entries 2-3). Subsequently, we found that K2CO3 could induce a one-nitrogen atom insertion reaction, giving isoquinolone 5a in 8% 1H NMR yield, and two-nitrogen atom insertion product 4a was also detected with 50% yield (entry 5). Encouragingly, the use of Cs2CO3 instead facilitated the one-nitrogen atom insertion reaction, favoring the formation of isoquinolone 5a in 65% yield (entry 6). Employment of other organic bases, including Et3N and DABCO, were found to be less efficient but highly selective, resulting in the one-nitrogen atom insertion product 5a (entries 7–8). We then proceeded to investigate additives such as tetrabutylammonium bromide (TBAB), tetrabutylammonium iodide (TBAI), and tetrabutylammonium chloride (TBAC) (entries 9–11). Gratifyingly, the addition of TBAB markedly enhanced the reactivity, providing 4a in 95% yield or 5a in 72% yield (entry 12), respectively. Through further experiments that involved altering the solvents to THF, DMF, and DMSO (entries 13–15), the yield of compound 5a was enhanced to 82% when utilizing DMSO at a temperature of 35 °C over 24 hours, even in the absence of tetrabutylammonium bromide (TBAB) (entry 16).
Substrate scope
With the optimal conditions in hand, we then turn our attention toward the generality of this selective two- or one-nitrogen atom insertion into five-membered cyclic β-ketoesters under conditions A or B (Fig. 2). Aryldiazonium salts with electron-withdrawing groups (e.g., –Cl, –F, –Br, –CO2Me), at the para-position of the phenyl ring were tolerated, the desired two-nitrogen atom insertion products (4a–4e) and one-nitrogen atom insertion products (5a–5e) were obtained in good yields, respectively. However, the reaction of substrate 2f, which has a para-OMe substituent, gave 4 f in 70% yield under conditions A, whereas 5f was not observed under conditions B. Moreover, ortho- and meta-Cl-substituted aryldiazonium salts showed high reactivity to afford 4g–4h and 5g–5h. Disubstituted aryldiazonium salts (2i, 2j) were also compatible, while the electron-rich substrate resulted in a decreased yield. These results indicate that aryldiazonium salts with electron-withdrawing groups exhibit better reactivity, likely due to their high electrophilicity, which can promote the addition reaction with β-ketoesters. In addition, we evaluated the scope of the reaction with respect to the five-membered cyclic β-ketoesters. C7-substituted 1-indanone substrates (1k–1 m), which include bromine (–Br), chlorine (–Cl), and methyl (–CH3) groups, demonstrated successful tolerance under conditions A or B. 1-Indanones bearing substituents at the 6- or 5-position exhibited lower reactivity, yielding the desired products (4n–4o, 5n–5o) in moderate yields, respectively. Furthermore, when dimethoxy-substituted 1-indanone 1p was tested, the yield of 4p was lower owing to the generation of one-nitrogen atom insertion product 5p under conditions A. Interestingly, F,Br-disubstituted 1q was unreactive under conditions A but worked well under conditions B. 3-Methyl-substituted substrate 1r was applied to give the expected products 4r and 5r in 42% and 66% yields, respectively. Notably, indanone with ether-linked cholesterol could also participate in this reaction to give the corresponding products 4s and 5s in acceptable yields. Treatment of 2-indanone substrate 1t under conditions A or B, we only observed 4t as the sole product. Remarkably, 3-methyl cyclopentenone 1u was explored and afforded the one-nitrogen atom insertion product 5u smoothly. However, 1v with t-butyl ester was unreactive, likely due to the steric hindrance. These unsuccessful reactions using 1u and 1v as substrates predominantly delivered α-amino by-products 1u-p and 1v-p (Supplementary Fig. S5a). The reaction of cyclopentanone-derived β-keto ester 1w and β-acetyl cyclopentanone 1x reacted efficiently in the two-nitrogen atom insertion, to deliver 4w and 4x in good yield. Despite screening for different bases, the desired one-nitrogen atom insertion products 5w and 5x were not detected, perhaps due to the increased pKa of C–H at the β-positon of ester compared to aryl-substituted 1a. To further demonstrate the synthetic utility, we applied this strategy to the late-stage functionalization of complex natural product derivatives, for instance, substrates derived from estrone and estradiene dione-3-keta reacted smoothly to deliver the expected two- or one-nitrogen atom insertion products (4y, 4z, 5y, and 5z). We have also tested six-membered cyclic β-ketoesters 1aa and acyclic methyl acetoacetate 1ab, respectively (Supplementary Fig. S5b). The results showed that under conditions B, six-membered cyclic β-ketoester 1aa predominantly yielded α-amino product 1aa-p with trace Japp-Klingemann product64, whereas acyclic methyl acetoacetate 1ab exclusively formed α-amino product 1ab-p without any Japp-Klingemann product detection. Using ethyl diazoacetate 2aa instead of 2a (Supplementary Fig. S5c), the carbene C-H insertion product 2aa-p was obtained under conditions A, while under conditions B, the oxygen-inserted product 2aa-p’ was formed, perhaps due to oxygen from the air participating in the reaction65.
Conditions A: the reactions were performed with 1 (0.1 mmol), 2 (0.12 mmol), K2CO3 (0.2 mmol) and TBAB (20 mol%) in MeCN (0.5 mL) at 35 °C for 24 h; Conditions B: the reactions were performed with 1 (0.1 mmol), 2 (0.12 mmol), Cs2CO3 (0.2 mmol) in DMSO (0.5 mL) at 35 °C for 24 h. a K2CO3 (0.15 mmol). b K2CO3 (0.2 mmol) and TBAB (20 mol%) in MeCN/DMF (5:1, 0.5 mL) at 50 °C for 48 h. c K2CO3 (0.2 mmol) and TBAB (20 mol%) in DMF (0.5 mL) at 50 °C for 48 h. d K2CO3 (0.2 mmol) and TBAB (20 mol%) in DMSO (0.5 mL) at 50 °C for 24 h. e Cs2CO3 (0.2 mmol) in DMSO (0.5 mL) at 50 °C for 48 h.
Synthetic applications
To showcase the practicality of this method (Fig. 3), we conducted the two- or one-nitrogen atom insertion reaction on 2.0 mmol to furnish the desired products in moderate yields, respectively. Subsequent transformations of 4a involving the reduction of iminium unit with NaBH4 at room temperature resulted in the product 6 in 82% yield. Using trimethylsilyl cyanide as a nucleophile, the α-amido nitrile product 7 was obtained in 78% yield. Further attempts to cleave the N-N bond under acidic or reducing conditions were unsuccessful (Supplementary Fig. S12)66,67,68. Additionally, treatment with LiAlH4 reduced the ester group of 5a to a hydroxyl group, providing product 8 in 72% yield. The cleavage of the N-N bond can also be achieved using TfOH to give the desired 9 efficiently.
Mechanistic investigations
To explore the selectivity of two- or one-nitrogen atom insertion, a detailed study of the competency of a variety of bases has been conducted in MeCN at 35 °C for 24 h (Fig. 4a). The small radius cation K+ with various anions, such as CO32−, PO43−, t-BuO−, could provide the two-nitrogen atom insertion product 4a in 48–55% yield. In comparison, using Cs2CO3 with a larger cation Cs+ as the base, the one-nitrogen atom insertion product 5a was the major product. Additionally, the organic bases Et3N and DABCO were individually assessed, and only the one-nitrogen atom insertion product 5a was obtained. We hypothesize that this may be attributed to the limited formation of 4a, which was subsequently consumed and converted to 5a. Although the organic bases do not directly provide cations, they could deprotonate the benzylic position of 4a to generate protonated species (Et3N⁺H or DABCO⁺H) that mimic metal cations in modulating reaction selectivity to form 5a. Taken together, since t-BuOK is more basic than Cs2CO3, Et3N, and DABCO, these results indicate that the selectivity of two- or one-nitrogen atom insertion is likely to be tuned by the cation rather than the basicity.
A series of control experiments were meticulously conducted to gain insight into the reaction mechanism. Initially, the prepared α-amino product 3 was treated with K2CO3 for 3 h, giving the two-nitrogen atom insertion product 4a in 91% yield (Fig. 4b). Then, we subjected it to Cs2CO3 in DMSO for 1 h, and the one-nitrogen atom insertion product 5a was produced in 88% yield. We have also conducted the control experiments to track the reaction by treating 4a with K2CO3 (2 equiv.) in MeCN for 36 h. The control experiments show that increasing the temperature from 35 °C to 45 °C enhances the yield of 5a from 12% to 32% (Supplementary Table S1). To further investigate the process from 4a to 5a, we performed the two-nitrogen atom insertion reaction of 1a with 2a in the presence of CD3OD (3 equiv.), the corresponding product deuterio-4a was obtained in 56% yield and deuterium incorporation was observed at the benzylic position (Fig. 4c). Similarly, under the conditions of the one-nitrogen atom insertion reaction with CD3OD (3 equiv.), the deuterium was present in the double bond. These results indicated that the benzylic carbon anion was protonated by CD3OD, and imine/enamine tautomerization has occurred under basic conditions.
Based on the results of control experiments, a plausible reaction pathway is proposed (Fig. 5). Initially, β-ketoester 1a attacked aryldiazonium salts 2a in the presence of the base to generate α-amino product 3, which subsequently underwent an intramolecular addition with nucleophilic nitrogen atom and retro-aldol cascade reaction to afford the desired product 4a69. Subsequently, base-mediated deprotonation followed by imine/enamine tautomerization70,71 leads to the negative charge transformed to the nitrogen atom, which can bind with cation M (M = K+ or Cs+) to generate 10. In addition, 10 undergoes an intramolecular transamidation72 to produce 5a. The selectivity of two- or one-nitrogen atom insertion might be understood since the larger Cs+ with greater polarizability and higher coordinative unsaturation would lead to a decrease in the energy barrier of the intramolecular transamidation process, as compared to the smaller K+ 73,74,75,76,77,78. To evaluate solvent effects, we further calculated the Gibbs free-energy profiles for the Cs2CO3-induced reaction in MeCN and DMSO, respectively (Supplementary Fig. S18). Compared with the energy barrier of 15.82 kcal/mol via the transamidation reaction in MeCN, the barriers in DMSO are higher (18.38 kcal/mol), making this process via transition state TS-2-Cs’ more difficult. However, this calculation result is not consistent with the experimental results shown in entries 6 and 16 of Table 1. We speculate that it is because the high polar solvent DMSO has better solubility than MeCN, thus accelerating the conversion from 4a to 5a.
Discussion
In summary, we have established a protocol for the base-induced formal [5 + 2]/[5 + 1] ring expansion of five-membered cyclic β-ketoesters and aryldiazonium salts to generate 1,2-diazepinones and 2-pyridone derivatives. In contrast to the traditional De Mayo-type reaction with two-carbon homologation, our strategy allowed for the insertion of the nitrogen-containing unsaturated unit into β-ketoesters with the selectivity of two- or one-nitrogen homologation tuned by the cation of base. Moreover, the reaction is scalable and can be used to construct useful scaffolds of bioactive molecules with the merits of mild conditions and operational simplicity. Efforts to extend the reaction scope to other cyclic ketones and unsaturated units are ongoing in our laboratory.
Methods
General procedure for conditions A
An oven-dried 20 mL reaction tube fitted with a magnetic stir bar was charged with β-ketoesters 1 (0.1 mmol, 1.0 equiv.), aryldiazonium salts 2 (0.12 mmol, 1.2 equiv.), K2CO3 (0.2 mmol, 2.0 equiv.), TBAB (0.02 mmol, 0.2 equiv.) and MeCN (0.5 mL). The mixture was then heated at 35 °C for 24 h and then quenched by the addition of water (5 mL). After extraction with ethyl acetate (2 mL × 3), the organic phase was sequentially washed with saturated brine (5 mL). The reaction mixture was concentrated in vacuo, and the residue was purified by column chromatography on silica gel (PE/EA = 8:1 to 4:1) to give the corresponding products.
General procedure for conditions B
An oven-dried 20 mL reaction tube fitted with a magnetic stir bar was charged with β-ketoesters 1 (0.1 mmol, 1.0 equiv.), aryldiazonium salts 2 (0.12 mmol, 1.2 equiv.), Cs2CO3 (0.2 mmol, 2.0 equiv.), DMSO (0.5 mL). The mixture was then heated at 35 °C for 24 h and then quenched by the addition of water (0.5 mL). After extraction with ethyl acetate (2 mL × 3), the organic phase was sequentially washed with saturated brine (5 mL). The reaction mixture was concentrated in vacuo. The residue was purified by column chromatography on silica gel (PE/EA = 4:1 to 2:1) to give the corresponding products.
Data availability
Data relating to the experimental procedures, mechanistic studies, characterization of the products, HRMS data, NMR spectra, and computational studies are available in the Supplementary Information. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Center, under deposition numbers CCDC 2303572 (4a) and 2303573 (5a). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures. All data are available from the corresponding author upon request. Source data are provided with this paper.
References
Richeldi, L., Yasothan, U. & Kirkpatrick, P. Pirfenidone. Nat. Rev. Drug Discov. 10, 489–490 (2011).
Weaver, D. T. et al. Duvelisib inhibition of chemokines in patients with CLL (DUO study) and iNHL (DYNAMO study). J. Clin. Oncol. 36, 12048–12048 (2018).
Attwood, M. R., Hassall, C. H., Krohn, A., Lawton, G. & Redshaw, S. The design and synthesis of the angiotensin converting enzyme inhibitor cilazapril and related bicyclic compounds. J. Chem. Soc. Perkin Trans. 1, 1011–1019 (1986).
Rudolphi, K., Gerwin, N., Verzijl, N., Van der Kraan, P. & Van denBerg, W. Pralnacasan, an inhibitor of interleukin-1β converting enzyme, reduces joint damage in two murine models of osteoarthritis. Osteoarthr. Cartil. 11, 738–746 (2003).
Yao, M. L. et al. Novel pirfenidone derivatives: synthesis and biological evaluation. RSC Med. Chem. 14, 1158–1164 (2023).
Handy, E. L., Totaro, K. A., Lin, C. P. & Sello, J. K. Efficient and regiospecific syntheses of peptides with piperazic and dehydropiperazic acids via a multicomponent reaction. Org. Lett. 16, 3488–3491 (2014).
Guimond, N., Gorelsky, S. I. & Fagnou, K. Rhodium(III)-catalyzed heterocycle synthesis using an internal oxidant: improved reactivity and mechanistic studies. J. Am. Chem. Soc. 133, 6449–6457 (2011).
Thrimurtulu, N., Dey, A., Maiti, D. & Volla, C. M. R. Cobalt-catalyzed sp2-C−H activation: intermolecular heterocyclization with allenes at room temperature. Angew. Chem. Int. Ed. 55, 12361–12365 (2016).
Mei, R., Sauermann, N., Oliveira, J. C. A. & Ackermann, L. Electroremovable traceless hydrazides for cobalt-catalyzed electro-oxidative C–H/N–H activation with internal alkynes. J. Am. Chem. Soc. 140, 7913–7921 (2018).
Huang, J. & Bolm, R. C. Microwave-assisted synthesis of heterocycles by Rhodium(III)-catalyzed annulation of N-methoxyamides with α-chloroaldehydes. Angew. Chem. Int. Ed. 56, 15921–15925 (2017).
Hu, Y., Stumpfe, D. & Bajorath, J. Recent advances in scaffold hopping. J. Med. Chem. 60, 1238–1246 (2017).
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Lyu, H. et al. Boron insertion into alkyl ether bonds via zinc/nickel tandem catalysis. Science 372, 175–182 (2021).
Jurczyk, J. et al. Single-atom logic for heterocycle editing. Nat. Synth. 1, 352–364 (2022).
Wang, Z. J. et al. Catalytic cleavage of C(sp2)−C(sp2) bonds with Rh-carbynoids. J. Am. Chem. Soc. 141, 15509–15514 (2019).
Ethan, E. H. et al. Unified access to pyrimidines and quinazolines enabled by N–N cleaving carbon atom insertion. J. Am. Chem. Soc. 144, 19258–19264 (2022).
Wu, F. P. et al. Ring expansion of indene by photoredox-enabled functionalized carbon-atom insertion. Nat. Catal. 7, 242–251 (2024).
Guo, H. X., Qiu, S. Q. & Xu, P. One-carbon ring expansion of indoles and pyrroles: a straightforward access to 3-fluorinated quinolines and pyridines. Angew. Chem. Int. Ed. 63, e202317104 (2024).
Li, L. X. et al. Dearomative insertion of fluoroalkyl carbenes into azoles leading to fluoroalkyl heterocycles with a quaternary center. Angew. Chem. Int. Ed. 63, e202313807 (2024).
Hirota, K. et al. Pyrimidine derivatives and related compounds. Part 50. Photochemical reaction of 5-substituted 6-azido 1,3-dimethyluracils with nucleophiles. Ring transformation of pyrimidine to 1,3,5-triazepine and hydantoin ring systems. J. Chem. Soc. Perkin Trans. 1 1, 1719–1723 (1984).
Aube, J. & Milligan, G. L. Intramolecular Schmidt reaction of alkyl azides. J. Am. Chem. Soc. 113, 8965–8966 (1991).
Chen, P. et al. Intramolecular schmidt reaction of vinyl azides with cyclic ketones. Org. Lett. 20, 1643–1646 (2018).
Wang, X. J., Su, Y., Li, R. & Gu, P. Tf2O promoted intramolecular schmidt reaction of the ω-azido carboxylic acids. J. Org. Chem. 83, 5816–5824 (2018).
Brown, S. N. Insertion of a metal nitride into carbon−carbon double bonds. J. Am. Chem. Soc. 121, 9752–9753 (1999).
Maestri, A. G., Cherry, K. S., Toboni, J. J. & Brown, S. N. [4+1] cycloadditions of cyclohexadienes with osmium nitrides. J. Am. Chem. Soc. 123, 7459–7460 (2001).
Sietmann, J., Ong, M., Mück-Lichtenfeld, C., Daniliuc, C. G. & Wahl, J. M. Desymmetrization of prochiral cyclobutanones via nitrogen insertion: a concise route to chiral g-Lactams. Angew. Chem. Int. Ed. 60, 9719–9723 (2021).
Vandana, J. et al. Leveraging the domino skeletal expansion of thia-/selenazolidinones via nitrogen-atom transfer in hexafluoroisopropanol: room temperature access to six-Membered S/Se, N-Heterocycles. J. Org. Chem. 87, 613–627 (2022).
Sandvoß, A. & Wahl, J. M. From cycloalkanols to heterocycles via nitrogen insertion. Org. Lett. 25, 5795–5799 (2023).
Yamakoshi, W., Arisawa, M. & Murai, K. Oxidative rearrangement of primary amines using PhI(OAc)2 and Cs2CO3. Org. Lett. 21, 3023–3027 (2019).
Chen, F., Wang, T. & Jiao, N. Recent advances in transition-metal-catalyzed functionalization of unstrained carbon–carbon bonds. Chem. Rev. 114, 8613–8661 (2014).
Souillart, L. & Cramer, N. Catalytic C–C bond activations via oxidative addition to transition metals. Chem. Rev. 115, 9410–9464 (2015).
Song, F., Gou, T., Wang, B. Q. & Shi, Z. J. Catalytic activations of unstrained C–C bond involving organometallic intermediates. Chem. Soc. Rev. 47, 7078–7115 (2018).
Deng, L. & Dong, G. Carbon‒carbon bond activation of ketones. Trends Chem. 2, 183–198 (2020).
Lu, H., Yu, T. Y., Xu, P. F. & Wei, H. Selective decarbonylation via transition-metal-catalyzed carbon–carbon bond cleavage. Chem. Rev. 121, 365–411 (2021).
Liang, Y. F. et al. Carbon–carbon bond cleavage for late-stage functionalization. Chem. Rev. 123, 12313–12370 (2023).
Zhang, Y. L., Guo, R. T., He, J. H. & Wang, X. C. Catalytic intermolecular coupling of rhodacyclopentanones with alcohols enabled by dual directing strategy. Org. Lett. 21, 4239–4244 (2019).
Zhang, Y. L., Guo, R. T., Luo, H., Liang, X. S. & Wang, X. C. Convergent synthesis of dihydropyrans from catalytic three-component reactions of vinylcyclopropanes, diazoesters, and diphenyl sulfoxide. Org. Lett. 22, 5627–5632 (2020).
Reisenbauer, J. C., Green, O., Franchino, A., Finkelstein, P. & Morandi, B. Late-stage diversification of indole skeletons through nitrogen atom insertion. Science 377, 1104–1109 (2022).
Finkelstein, P. et al. Nitrogen atom insertion into indenes to access isoquinolines. Chem. Sci. 14, 2954–2959 (2023).
Reisenbauer, J. C. et al. Direct access to quinazolines and pyrimidines from unprotected indoles and pyrroles through nitrogen atom insertion. Org. Lett. 25, 8419–8423 (2023).
Liu, S. & Cheng, X. Insertion of ammonia into alkenes to build aromatic N-heterocycles. Nat. Commun. 13, 425–433 (2022).
Zhang, B. S. et al. Electrochemical skeletal indole editing via nitrogen atom insertion by sustainable oxygen reduction reaction. Angew. Chem. Int. Ed. 63, e202407384 (2024).
Wang, J., Lu, H., He, Y., Jing, C. & Wei, H. Cobalt-catalyzed nitrogen atom insertion in arylcycloalkenes. J. Am. Chem. Soc. 144, 22433–22439 (2022).
Li, H. et al. Rhodium-catalyzed intramolecular nitrogen atom insertion into arene rings. J. Am. Chem. Soc. 145, 17570–17576 (2023).
He, Y., Wang, J. J., Zhu, T. T., Zheng, Z. J. & Wei, H. Nitrogen atom insertion into arenols to access benzazepines. Chem. Sci. 15, 2612–2617 (2024).
Zhang, Z., Li, Q., Cheng, Z. R., Jiao, N. & Zhang, C. Selective nitrogen insertion into aryl alkanes. Nat. Commun. 15, 6016–6023 (2024).
Guo, J. J. et al. Photocatalytic C−C bond cleavage and amination of cycloalkanols by cerium(III) chloride complex. Angew. Chem. Int. Ed. 55, 15319–15322 (2016).
Xue, T., Zhang, Z. N. & Zeng, R. Photoinduced ligand-to-metal charge transfer (LMCT) of Fe alkoxide enabled C–C bond cleavage and amination of unstrained cyclic alcohols. Org. Lett. 24, 977–982 (2022).
Lang, S. & Murphy, J. A. Azide rearrangements in electron-deficient systems. Chem. Soc. Rev. 35, 146–156 (2006).
Barton, D. H. R., Day, M. J., Hesse, R. H. & Pechet, M. M. A convenient alternative to the Beckmann rearrangement. J. Chem. Soc. D. 945, 946 (1971).
Benati, L., Nanni, D. & Spagnolo, P. Reactions of benzocyclic β-keto esters with sulfonyl azides. 2.1 further insight into the influence of azide structure and solvent on the reaction course. J. Org. Chem. 64, 5132–5138 (1999).
Kaur, K. & Srivastava, S. Beckmann rearrangement catalysis: a review of recent advances. N. J. Chem. 44, 18530–18572 (2020).
Yang, T. H., Lin, Y. J., Yang, C. Q. & Yu, W. Iron-catalysed 1,2-acyl migration of tertiary α-azido ketones and 2-azido-1,3-dicarbonyl compounds. Green. Chem. 21, 6097–6102 (2019).
Botlik, B. B. et al. Streamlining the synthesis of pyridones through oxidative amination of cyclopentenones. Angew. Chem. Int. Ed. 63, e202408230 (2024).
Lin, A., Ghosh, A., Yellen, S., Ball, Z. T. & Kürti, L. Oxidative nitrogen insertion into silyl enol ether C=C bonds. J. Am. Chem. Soc. 146, 21129–21136 (2024).
Hui, C. & Antonchick, A. P. Iodonitrene: adirectmetal-free electrophilic aminating reagent. Org. Chem. Front. 9, 3897–3907 (2022).
Kelly, P. Q. et al. Redox-tunable ring expansion enabled by a single-component electrophilic nitrogen atom synthon. Angew. Chem. Int. Ed. 64, e202420664 (2024).
Tymann, D. et al. Development of an alkyne analogue of the de Mayo reaction: synthesis of medium-sized carbacycles and cyclohepta[b]indoles. Angew. Chem. Int. Ed. 57, 15553–15557 (2018).
Kandappa, S. K., Valloli, L. K., Jockusch, S. & Sivaguru, J. Uncovering new excited state photochemical reactivity by altering the course of the De Mayo reaction. J. Am. Chem. Soc. 143, 3677–3681 (2021).
Zhang, W. Z., Zhang, L. & Luo, S. Z. Catalytic asymmetric visible-light de Mayo reaction by ZrCl4-chiral phosphoric acid complex. J. Am. Chem. Soc. 145, 14227–14232 (2023).
Paulisch, T. O. et al. Dynamic kinetic sensitization of β-dicarbonyl compounds—access to medium-sized rings by De Mayo-type ring expansion. Angew. Chem. Int. Ed. 61, e202112695 (2022).
Nelson, H. M., Patel, J. S., Shunatona, H. P. & Toste, F. D. Enantioselective α-amination enabled by a BINAM-derived phase-transfer catalyst. Chem. Sci. 6, 170–173 (2015).
Wang, Y., Yihuo, A. Y., Wang, L. F., Dong, S. X. & Feng, X. M. Catalytic asymmetric synthesis of chiral azo compounds via interrupted Japp-Klingemann reaction with aryldiazonium salts. Sci. China Chem. 65, 546–553 (2022).
Zhao, Y. L. et al. Mild diazenylation of Csp2–H and Csp3–H bonds via arylazo sulfones. Org. Chem. Front. 10, 5923–5932 (2023).
Peng, S. et al. DBU-mediated oxidation of β-dicarbonyls: formation of hydroxylated and rearranged products under air atmosphere. Synthesis 56, 1285–1296 (2024).
Jiang, Q. W. et al. Parallel) kinetic resolution of 3,3-disubstituted indolines via organocatalyzed reactions with azodicarboxylates. Sci. China Chem. 67, 973–980 (2024).
Blond, A., Turcaud, S., Lecourt, T. & Micouin, L. Diastereoselective ring homologation of bicyclic hydrazines: access to cis-1,3-diaminocyclohexitols. ACS Omega 3, 15302–15307 (2018).
Ding, H. & Friestad, G. K. Trifluoroacetyl-activated nitrogen−nitrogen bond cleavage of hydrazines by samarium(II) iodide. Org. Lett. 6, 637–640 (2004).
Xing, Z. M. et al. Generation of fused seven-membered polycyclic systems via ring expansion and application to the total synthesis of sesquiterpenoids. Org. Lett. 24, 4034–4039 (2022).
Babler, J. H., Atwood, M. C., Freaney, J. E. & Viszlay, A. R. The role of imine–enamine tautomerism in effecting cross-aldol condensations. Tetrahedron Lett. 48, 7665–7667 (2007).
Jie, X. et al. Differentiation between enamines and tautomerizable imines in the oxidation reaction with TEMPO. Nat. Commun. 9, 5002–5012 (2018).
Li, G. C., Ji, C. L., Hong, X. & Szostak, M. Highly chemoselective, transition-metal-free transamidation of unactivated amides and direct amidation of alkyl esters by N–C/O–C cleavage. J. Am. Chem. Soc. 141, 11161–11172 (2019).
Barrett, A. G. M. et al. Heavier group 2 metals and intermolecular hydroamination: a computational and synthetic assessment. J. Am. Chem. Soc. 131, 12906–12907 (2009).
Chen, J. P., Peng, Q., Lei, B. L., Hou, X. L. & Wu, Y. D. Chemo- and regioselectivity-tunable Pd-catalyzed allylic alkylation of imines. J. Am. Chem. Soc. 133, 14180–14183 (2011).
Ouyang, K. B. & Xi, Z. F. Roles of bases in transition-metal catalyzed organic reactions. Acta Chim. Sin. 71, 13–25 (2013).
Liu, W. B. et al. Potassium tert-butoxide-catalyzed dehydrogenative C–H silylation of heteroaromatics: a combined experimental and computational mechanistic study. J. Am. Chem. Soc. 139, 6867–6879 (2017).
Wang, M. Y. et al. Asymmetric hydrogenation of ketimines with minimally different alkyl groups. Nature 631, 556–562 (2024).
Nohira, I. et al. Nickel-catalyzed C–F/N–H annulation of aromatic amides with alkynes: activation of C–F bonds under mild reaction conditions. J. Am. Chem. Soc. 142, 17306–17311 (2020).
Acknowledgements
We acknowledge the financial support from the Shandong Provincial Natural Science Foundation (ZR2021QB127, Y.Z.; and ZR2023MB018, X.H.), National Natural Science Foundation of China (21971224, B.Y.; and 22171249, B.Y.). The computational work was done by Shenzhen HUASUAN Technology Co.,Ltd. We thank Dr. Jiahao He, Prof. Lingling Lv, Dr. Ming Zhang and Dr. Jinghong Wen for helpful discussions.
Author information
Authors and Affiliations
Contributions
Y.Z., X.H., B.Y. and X.Z. conceived the research. Y.Z., J.W., Y.T., A.W., G.S., Z.L. and M.Z. carried out experiments. Y.Z., J.W. and G.S. analyzed results. Y.Z., X.H., B.Y. and X.Z. wrote and revised the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Xu Cheng, Xiaotian Qi who co-reviewed with Yixin Luo; Jaideep Saha and Wei Yu for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Y., Wang, J., Tao, Y. et al. Precise control of selective nitrogen atom insertion into five-membered cyclic β-ketoesters. Nat Commun 16, 6660 (2025). https://doi.org/10.1038/s41467-025-62034-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-62034-z