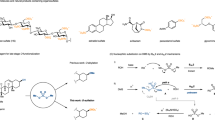
Abstract
O-sulfation is a widespread modification of both endogenous and exogenous biomolecules, where the primary objective is to identify effective sulfuryl donors. In nature, 3′-phosphoadenosine-5′-phosphosulfate (PAPS) and p-nitrophenyl sulfate (PNPS) are efficient sulfuryl donors. However, most chemical sulfuryl donors in O-sulfation, typically require harsh conditions and have not been demonstrated in complex molecules. Here we report a biomimetic O-sulfation method that is compatible with complex natural products and pharmaceutical scaffolds. Key to this approach is the use of tetrabutylammonium (nBu4N+) as a counterion for intrinsically anionic PNPS donor. The role of nBu4N+ goes far beyond simple charge balance; the coordination of nBu4N+ with sulfate in PNPS activates the sulfuryl donor by elongating the S–O bond and enhancing the leaving ability of nitrophenolate group. This unique activation model facilitates the transfer of sulfuryl group to diverse alcohols and phenols under simple and mild reaction conditions, thereby demonstrating its utility for site-selective O-sulfation with multiple hydroxyl groups.
Similar content being viewed by others
Introduction
O-sulfation of biomolecules is an essential process in all living organisms and is involved in blood clotting, pathogen infection, neurological health, and drug metabolism1,2,3,4. The installation of sulfate groups dramatically modulates polarity, solubility, and conformation, while also imparting electrostatic force and hydrogen bonding interaction5,6,7,8,9,10,11,12. Sulfotransferases (STs) catalyze this process by transfer of a sulfuryl group (alternatively referred to as sulfonate in O-sulfation) from a biologically active donor to a hydroxyl substrate, resulting in the formation of organosulfates (Fig. 1A). In eukaryotic systems, 3′-phosphoadenosine-5′-phosphosulfate (PAPS) is the universal sulfuryl donor, and PAPS-dependent O-sulfation greatly extends the structural complexity of biomolecules and also increases the diversity of their function13,14. In contrast, bacterial arylsulfate sulfotransferases (ASSTs) transfer SO3 group exclusively among phenolic compounds with p-nitrophenyl sulfate (PNPS) as the primary sulfuryl donor via a ping-pong bi-bi reaction mechanism15,16. Generally, biological O-sulfation is highly specific for its target molecules, which restricts its synthetic utility to access structurally diverse compounds17. The low availability of sulfotransferases and arylsulfate sulfotransferases, as well as their ambiguous structures and mechanisms for O-sulfation are additional impediments to understanding their biological function18,19. Therefore, a more effective approach to investigate the influence of O-sulfation is chemical synthesis.
While O-sulfation may seem like a simple one-step reaction, the sulfated-modification of complex natural products and drug molecules has remained a daunting challenge20,21,22. The inherent acid and temperature sensitivity of sulfate groups, coupled with their difficult handling after incorporation, precludes the possibility of other functional group transformations. This constraint essentially forces O-sulfation to be the final step in the synthetic scheme. Direct O-sulfation of structurally simple alcohols is known, and various sulfur trioxide-amine/amide complexes have been developed as sulfuryl donors (Fig. 1B)23,24,25,26,27. However, the harsh conditions associated with these sulfuryl donors, such as the need for stoichiometric excess and basic conditions, often lead to global sulfation on complex molecules containing multiple reactive sites, with few exceptions28. To circumvent the poor selectivity of these reagents, a strategy of de novo protected sulfate residues was developed. After building the target molecular structure, orthogonal removal of the protecting groups provides homogeneous organosulfates with pinpoint accuracy22,29,30,31,32,33,34,35,36,37. However, these protected groups complicate the synthetic route and reduce overall yields of the desired compound.
Inspired by the enzymatic O-sulfation that uses readily available PNPS as a sulfuryl donor, here we describe our strategy for manganese-catalyzed late-stage O-sulfation with tetrabutylammonium p-nitrophenyl sulfate (nBu4N-PNPS) (Fig. 1C)38,39. Key to this method is the use of tetrabutylammonium as a counterion for PNPS: the complexation of nBu4N+ with sulfate in PNPS generates an activated sulfuryl donor by elongating the sulfur-bridging oxygen (S–Ob) bond and enhancing the leaving ability of nitrophenolate group. This distinctive activation model enables efficient sulfuryl transfer to structurally complex alcohols and phenols under straightforward and mild reaction conditions, thereby achieving site-selective O-sulfation among various hydroxyl groups.
Results and discussion
Reaction validation and condition optimization
Early studies on sulfuryl transfer reaction support a concerted mechanism characterized by significant bond lengthening of the scissile S–Ob bond and dissociative sulfur trioxide-like transition state (Fig. 2A)40,41,42,43,44,45,46,47. The first fundamental rule of structure-reactivity correlations for sulfate monoesters, as proposed by Kirby and his colleagues, states that “the longer the bond, the faster it breaks”42. The slope of the structure-reactivity correlation for sulfate monoesters series (−4.7 × 10−3) corresponds to energetic cost of 435 kcal mol−1 Å−1, indicating the energetically expensive to stretch an S–Ob bond during the sulfuryl transfer reaction44. Varying stereoelectronic substituents on the aromatic ring of PNPS is a common approach to study structure-reactivity correlations45,46,47. Despite their potential, none of these substituents serves as an efficient and general sulfate donor for O-sulfation. In fact, PNPS is a negatively charged salt, typically with potassium as the native counterion (K-PNPS). The role of these counter-cations goes far beyond mere charge balance; it might significantly tailor the properties of intrinsically anionic PNPS through electrostatic interactions, covalent interactions, and solubility48,49,50,51,52,53,54,55. To the best of our knowledge, the use of counterions in structure-reactivity correlations of PNPS has not been exploited.
We synthesized nBu4N-PNPS via a scalable and practical procedure with nBu4N+ exchange. This compound is stable at room temperature for at least several months (Fig. 2B)56. A detailed analysis of its X-ray structure shows that the distances from nBu4N+ to the bridging oxygen (N–Ob) and terminal oxygen (N–Ot) are nearly equal, whereas K-PNPS exhibits a 1.88 Å difference between K–Ob and K–Ot; thus, the larger counterion nBu4N+ can coordinate both with Ob and Ot, while K+ can only coordinate with Ot47,57. This is further supported by the 7.37° smaller of Ob–S–Ot angle in nBu4N-PNPS than K-PNPS. The additional complexation between nBu4N+ and Ob reduces electron density on oxygen, attenuating its p-π conjugation with S=O bonds and, in turn, lowering the S–Ob bond energy. Consequently, the S–Ob bond length (1.658 Å) is longer than the expected 1.629 Å. Furthermore, the distortion of the bond angle of the C1–Ob away from planarity with the benzene ring is 2° for nBu4N+, in contrast to 5° for K+. This facilitates better conjugation of Ob in nBu4N-PNPS with the π system of p-nitrophenyl, which increases the C1–Ob bond energy (1.359 Å vs 1.398 Å) and improves the leaving ability of nitrophenolate. The kinetic isotope effect (KIE) for the bridging oxygen (Ob) in nBu4N-PNPS was measured in subsequent O-sulfation experiments (Fig. 2A), and an 18kOb value of 1.0177 confirms the S–Ob bond cleavage in the transition state43. The Na-PNPS structure reveals that Na⁺ counterion exhibits both coordinated and non-coordinated interactions with Ob. Notably, S–Ob bonds involved in Na⁺ coordination are longer (1.651 Å) than those not coordinated (1.640 Å) (Table S2).
With nBu4N-PNPS as an activated SO3 donor, its sulfuryl transfer potential was evaluated on testosterone 1a. O-sulfation with Mn(OTf)2 as a catalyst in MeCN at 25 °C afforded the desired sulfated testosterone 1 in 72% yield (Table 1, entry 1). Control experiments confirmed the essentiality of Mn(OTf)2 for O-sulfation reaction (Table 1, entry 2). The Lewis acid Mn(OTf)2 likely serves as an activator for the sulfate group in nBu4N-PNPS, and enhances the electrophilicity of the sulfur atom for the subsequent nucleophilic attack (Fig. S1).30 Other manganese salts and Fe(OTf)3 showed lower reactivity, while Ag(OTf)2 was ineffective (Table 1, entries 3-5). Although dichloromethane (DCM) gave a higher yield, MeCN was chosen as a less toxic solvent (Table 1, entry 6). The O-sulfation reaction proved relatively insensitive to air and temperature, proceeding efficiently under mild and simple conditions (Table 1, entries 7 and 8). The use of potassium, sodium, tetraethylammonium p-nitrophenyl sulfate provided the desired product in moderate yields; however, it is not as general and efficient as nBu4N-PNPS (Table 1, entries 9–11; Fig. S2). The corresponding potassium, sodium, and tetraethylammonium sulfate products were less stable during purification than their tetrabutylammonium (nBu4N⁺) counterparts53. Furthermore, their poor solubility in organic solvents required aqueous purification and ion-exchange chromatography, limiting their practical utility.
Substrate scope and synthetic application
The mild conditions and simple reaction setup make it applicable to a variety of structurally diverse medicinal agents and natural products. As demonstrated in Fig. 3, this protocol was found to be general and robust, with remarkable functional group tolerances. Several natural steroid hormones, including diosgenin (2), cholesterol (3), epiandrosterone (4), and estrone (5), could be readily sulfated with high efficiency. Phenolic motifs in sesamol (6), mecarbinate (7), and monobenzone (8) were viable in this O-sulfation, albeit with moderate yields. These lower yields relative to alkyl sulfates resulted from their inherent instability and mass losses during purification. For other substrates, moderate or poor yields arose from poor reaction conversion; no significant by-products were detected. Terpenoids perilla alcohol (9), mevastatin (10), β-citronellol (11), and oleanic acid (12) underwent sulfation smoothly. We further established that O-sulfation is efficient for a diverse range of quinones and nucleosides, such as aloe emodin (13), idebenone (14), stavudine (15), and azidothymidine (16). Other structurally complex substrates were sulfated directly with this method, including the genital warts agent podophyllotoxin (17), insect repellent picaridin (18), selective estrogen receptor modulator ospemifene (19), proxyphylline (20), ezetimibe (21), antibiotic florfenicol (22), and muscle relaxant methocarbamol (23). Additionally, tertiary alcohol 24 and disaccharide (25) were also amenable to these reaction conditions. Protic groups such as carboxylic acid (12), phenols (13, 21), primary (22) and secondary amides (23) are tolerated; however, basic amines are incompatible due to their deactivation to Lewis acid Mn(OTf)2 catalyst.
a Reactions were conducted as in Table 1. b DCM, 25 °C. c DCM, 50 °C. d NMR yields. e MeCN, 50 °C. f MeCN, nBu4N-PNPS (2.0 equiv).
nBu4N-PNPS demonstrates a mild profile and high chemoselectivity compared to other sulfuryl donor reagents, such as sulfur trioxide-amine/amide complex and sulfurochloridic acid. For example, mevastatin (10) was successfully sulfated with nBu4N-PNPS under nearly neutral conditions to avoid decomposition. In contrast, the use of sulfur trioxide-pyridine complex or sulfurochloridic acid was less productive and produced elimination by-products (Fig. S3). They also led to the formation of both monosulfated and disulfated analogues upon reaction with pleuromulin (26) due to their inability to differentiate between primary and secondary alcohols. O-sulfation with nBu4N-PNPS can discriminate among different hydroxyl groups and afford a sulfated analogue with synthetically useful selectivity; for instance, a 55% yield of the sulfated forskolin analogue 27 was obtained, despite the presence of three hydroxyl groups in forskolin. We identified several trends to predict the O-sulfation site among multiple hydroxyl groups: (1) alcohols are selectively sulfated in the presence of phenols; (2) primary alcohols are preferentially sulfated over secondary and tertiary alcohols; (3) β, β′-dibranched secondary alcohols typically react much more slowly; (4) tertiary alcohols are unreactive unless they are less sterically-hindered. These guidelines have the potential to accurately predict the reactivity and selectivity of O-sulfation for the evaluated substrates.
Hydroxyl-containing amino acids, including tyrosine (28), threonine (29), and proline (30) could be efficiently sulfated (Fig. 4). This method was then extended to oligopeptides with protected serine and threonine residues. We found that common side chains in canonical amino acids are compatible, such as methionine (31, 34), tryptophan (32), valine (33) and phenylalanine (33, 34), demonstrating broad functional group tolerance. Tripeptide ritonavir (35) could be modified to access its sulfate-conjugates. Such post-translational modification of peptide sequences is a significant challenge via traditional O-sulfonation method.
a Reactions were performed as in Table 1. b DCM, 25 °C. c Acetone, 50 °C, NMR yields.
In conclusion, we have developed an efficient and general method for the late-stage O-sulfation with tetrabutylammonium p-nitrophenyl sulfate (nBu4N-PNPS). The often-overlooked interplay of the instrinsically anionic PNPS with counterions is essential to modulate the reactivity, solubility, and function of the parent compound PNPS. Therefore, the bioinspired sulfuryl donor with the activation by tetrabutylammonium enables this O-sulfation reaction to proceed under simple and mild conditions, demonstrating a broader substrate scope and greater functional group tolerance than any reported sulfation protocols. We anticipate that this method will be a valuable addition to the late-stage sulfated conjugation with significant implications for biological functions and drug discovery.
Methods
A typical procedure for O-sulfation reaction
To a 4 mL borosilicate vial equipped with a stir bar was added substrate (0.1 mmol, 1.0 equiv), Mn(OTf)2 (3.6 mg, 0.01 mmol, 10 mol%), tetrabutylammonium 4-nitrophenyl sulfate (nBu4N-PNPS, 69.1 mg, 0.15 mmol, 1.5 equiv), and solvent (0.5 mL, c = 0.2 M). The reaction mixture was stirred at 25 °C or 50 °C for 4 h, and then diluted with CH2Cl2 (2 mL). The resulting mixture was concentrated by rotary evaporation. The residue was purified by flash chromatography on silica gel eluting with CH2Cl2/MeOH (100:1 \(\longrightarrow\) 50:1\(\longrightarrow\) 30:1, v/v) to afford tetrabutylammonium sulfate salt.
Data availability
All data is available in the main text or the supplementary materials. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2414336 (nBu4N-PNPS) and 2465453 (Na-PNPS). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. All data are available from the corresponding author upon request.
References
Mulder, G. J. Sulfation of drugs and related compounds (CRC Press, 1981).
Hemmerich, S. in Handbook of Neurochemistry and Molecular Neurobiology: Neural Protein Metabolism and Function (eds Lajtha, A & Banik, N) 283−302 (Springer, 2007).
Huang, C. et al. Structures of the CCR5 N terminus and of a tyrosine-sulfated antibody with HIV-1 gp120 and CD4. Science 317, 1930–1934 (2007).
D’Agostino, G. D., Chaudhari, S. N. & Devlin, A. S. Host-microbiome orchestration of the sulfated metabolome. Nat. Chem. Biol. 20, 410–421 (2024).
Schug, K. A. & Lindner, W. Noncovalent binding between guanidinium and anionic groups: Focus on biological- and synthetic-based arginine/guanidinium interactions with phosph[on]ate and sulf[on]ate residues. Chem. Rev. 105, 67–113 (2005).
Yang, J. et al. Structure-guided discovery of bile acid derivatives for treating liver diseases without causing itch. Cell 187, 7164–7182 (2024).
Xu, Z. et al. Integrated chemoenzymatic synthesis of a comprehensive sulfated ganglioside glycan library to decipher functional sulfoglycomics and sialoglycomics. Nat. Chem. 16, 881–892 (2024).
Zhang, B. B. et al. Discovery of selective cyclic D-sulfopeptide ligands of the chemokine CCL22 via mirror-image mRNA display with genetic reprogramming. J. Am. Chem. Soc. 146, 34253–34259 (2024).
Wang, L. et al. Efficient platform for synthesizing comprehensive heparan sulfate oligosaccharide libraries for decoding glycosaminoglycan–protein interactions. Nat. Chem. 15, 1108–1117 (2023).
Gao, T. et al. Chemoenzymatic synthesis of O-mannose glycans containing sulfated or nonsulfated HNK-1 epitope. J. Am. Chem. Soc. 2019, 19351–119359 (2019).
Chopra, P. et al. The 3-O-sulfation of heparan sulfate modulates protein binding and lyase degradation. Proc. Natl. Acad. Sci. USA 118, e2012935118 (2021).
Wang, Z. et al. Increased 3-O-sulfated heparin sulfate in Alzheimer’s disease brain is associated with genetic risk gene HS3ST1. Sci. Adv. 9, eadf6232 (2023).
Chapman, E., Best, M. D., Hanson, S. R. & Wong, C.-H. Sulfotransferases: Structure, mechanism, biological activity, inhibition, and synthetic utility. Angew. Chem. Int. Ed. 43, 3526–3548 (2004).
Tang, X. et al. A two-step sulfation in antibiotic biosynthesis requires a type III polyketide synthase. Nat. Chem. Biol. 9, 610–615 (2013).
Malojčić, G. et al. A structural and biochemical basis for PAPS-independent sulfuryl transfer by aryl sulfotransferase from uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 105, 19217–19222 (2008).
Stressler, T., Seitl, I., Kuhn, A. & Fischer, L. Detection, production, and application of microbial arylsulfatases. Appl. Microbiol. Biotechnol. 100, 9053–5067 (2006).
van Kasteren, S. I. et al. Expanding the diversity of chemical protein modification allows post-translational mimicry. Nature 446, 1105–1109 (2007).
Yu, Y., Hoffhines, A. J., Moore, K. L. & Leary, J. A. Determination of the sites of tyrosine O-sulfation in peptides and proteins. Nat. Methods 4, 583–588 (2006).
Hartoga, A. F. & Wever, R. Substrate engineering and its synthetic utility in the sulfation of primary aliphatic alcohol groups by a bacterial arylsulfotransferase. Adv. Synth. Catal. 357, 2629–2632 (2015).
Al-Horani, R. A. & Desai, U. R. Chemical sulfation of small molecules advances and challenges. Tetrahedron 66, 2907–2918 (2010).
Alshehri, J. & Jones, A. Chemical approaches to the sulfation of small molecules: current progress and future directions. Essays Biochem. 68, 449–466 (2024).
Stone, M. J. & Payne, R. J. Homogeneous sulfopeptides and sulfoproteins: Synthetic approaches and applications to characterize the effects of tyrosine sulfation on biochemical function. Acc. Chem. Res. 48, 2251–2261 (2015).
Czapek, E. Zur Kenntnis der aromatischen esterschwefelsäuren. Monatsh. Chem. 35, 635–642 (1914).
Hoiberg, C. P. & Mumma, R. O. Preparation of sulfate esters. Reactions of various alcohols, phenols, amines, mercaptans, and oximes with sulfuric acid and dicyclohexylcarbodiimide. J. Am. Chem. Soc. 91, 4273–4278 (1969).
Burg, A. B. The behavior of trimethylamine, trimethylammino-sulfur trioxide and trimethylamine oxide toward sulfur dioxide. J. Am. Chem. Soc. 65, 1629–1635 (1943).
Wolfrom, M. L. & Shen Han, T. M. The sulfonation of chitosan1,2. J. Am. Chem. Soc. 81, 1764–1766 (1959).
Gill, D. M., Male, L. & Jones, A. M. Sulfation made simple: a strategy for synthesising sulfated molecules. Chem. Commun. 55, 4319–4322 (2019).
Benedetti, A. M., Gill, D. M., Tsang, C. W. & Jones, A. M. Chemical methods for N- and O-sulfation of small molecules, amino acids and peptides. ChemBioChem 21, 938–942 (2020).
Simpson, L. S. & Widlanski, T. S. A comprehensive approach to the synthesis of sulfate esters. J. Am. Chem. Soc. 128, 1605–1610 (2006).
Yue, S. et al. Dimethyl sulfate and diisopropyl sulfate as practical and versatile reagents in O-sulfation. Nat. Commun. 15, 1861 (2024).
Chen, W. et al. Synthesis of sulfotyrosine-containing peptides by incorporating fluorosulfated tyrosine using an Fmoc-based solid-phase strategy. Angew. Chem. Int. Ed. 55, 1835–1838 (2016).
Liu, C. et al. A general approach to O-sulfation by a sulfur(VI) fluoride exchange reaction. Angew. Chem. Int. Ed. 59, 18435–18441 (2020).
Liu, X. Y. et al. Site-selective solid-phase synthesis of a CCR5 sulfopeptide library to interrogate HIV binding and entry. ACS Chem. Biol. 9, 2074–2081 (2014).
Taleski, D., Butler, S. J., Stone, M. J. & Payne, R. J. Divergent and site-selective solid-phase synthesis of sulfopeptides. Chem. - Asian J. 6, 1316–1320 (2011).
Taylor, S. D. & Desoky, A. Rapid and efficient chemoselective and multiple sulfations of phenols using sulfuryl imidazolium salts. Tetrahedron Lett. 52, 3353–3357 (2011).
Ali, A. M. & Taylor, S. D. Efficient solid-phase synthesis of sulfotyrosine peptides using a sulfate protecting-group strategy. Angew. Chem. Int. Ed. 48, 2024–2026 (2009).
Bunschoten, A. et al. A general sequence independent solid phase method for the site specific synthesis of multiple sulfated-tyrosine containing peptides. Chem. Commun. 21, 2999–3001 (2009).
Beaudry, C. M., Malerich, J. P. & Trauner, D. Biosynthetic and biomimetic electrocyclizations. Chem. Rev. 105, 4757–4778 (2005).
Bao, R., Zhang, H. & Tang, Y. Biomimetic synthesis of natural products: a journey to learn, to mimic, and to be better. Acc. Chem. Res. 54, 3720–3733 (2021).
Benkovic, S. J. & Benkovic, P. A. Studies on sulfate esters. I. Nucleophilic reactions of amines with p-nitrophenyl sulfate. J. Am. Chem. Soc. 123, 5504–5511 (1966).
Bourne, N., Hopkins, A. & Williams, A. Single transition state for sulfuryl group (-SO3−) transfer between pyridine nucleophiles. J. Am. Chem. Soc. 107, 4327–4331 (1985).
Edwards, M. R., Jones, P. G. & Kirby, A. J. Anatomy of an SN1 reaction. Crystal structure-reactivity correlations for 1-arylethanol derivatives. J. Am. Chem. Soc. 108, 7067–7073 (1986).
Hoff, R. H., Larsen, P. & Hengge, A. C. Isotope effects and medium effects on sulfuryl transfer reactions. J. Am. Chem. Soc. 123, 9338–9344 (2001).
Fendler, E. J. & Fendler, J. H. Hydrolysis of nitrophenyl and dinitrophenyl sulfate esters1. J. Org. Chem. 33, 3852–3859 (1968).
Hopkins, A., Day, R. A. & Williams, A. Sulfate group transfer between nitrogen and oxygen: evidence consistent with an open “exploded” transition state. J. Am. Chem. Soc. 105, 6062–6070 (1983).
Williams, A. Bonding in phosphoryl (-PO32−) and sulfuryl (-SO3−) group transfer between nitrogen nucleophiles as determined from rate constants for identity reactions. J. Am. Chem. Soc. 107, 6335–6339 (1985).
Mandal, S., Bera, T., Dubey, G., Saha, J. & Laha, J. K. Ground state structures of sulfate monoesters and sulfamates reveal similar reaction coordinates for sulfuryl and sulfamyl transfer. Chem. Commun. 314, 316 (2006).
Misra, A., Kozma, K., Streb, C. & Nyman, M. Beyond charge balance: counter-cations in polyoxo metalate chemistry. Angew. Chem. Int. Ed. 59, 596–612 (2020).
Golomb, M. J., Tolborg, K., Calbo, J. & Walsh, A. Role of counterions in the structural stabilisation of redox-active metal-organic frameworks. Chem. Eur. J. 29, e202203843 (2023).
Zhao, Z. et al. Stereospecific C–O sulfation via persulfate-induced 1,4-metallate migration. Nat. Synth. 3, 1529–1537 (2024).
Xia, Z. et al. Benzylic C–H radical sulfation by persulfates. Angew. Chem. Int. Ed. 64, e202413847 (2025).
Mihai, M. T., Williams, B. D. & Phipps, R. J. Para-selective C−H borylation of common arene building block enabled by ion-pairing with a bulky countercation. J. Am. Chem. Soc. 141, 15477–15482 (2019).
Sheng, W., Peng, H., Gao, B., Song, C. & Li, J. Electrochemical benzylic C–H sulfation beyond the O-sulfonation limitation. Angew. Chem. Int. Ed. 64, e202507048 (2025).
Xia, Z., Deng, T., Song, C. & Li, J. Decarboxylative sulfation by persulfates. Chem. Sci. 16, 11568–11573 (2025).
Hamilton, G. L., Kang, E. J., Mba, M. & Toste, F. D. A powerful chiral counterion strategy for asymmetric transition metal catalysis. Science 317, 496–499 (2007).
Zhou, J. & Jones, A. M. Rearrangement of arylsulfamates and sulfates to para-sulfonyl anilines and phenols. Molecules 29, 1449 (2024).
Brandão, T. A. S. et al. Bond length-reactivity correlations for sulfate monoesters. The crystal structure of potassium 4-nitrophenyl sulfate, C6H4KNO6S. J. Mol. Struct. 734, 205–209 (2005).
Acknowledgements
This work was supported by the Natural Science Foundation of Hunan Province (No. 2025JJ40012, C.S.). We thank Analytical Instrumentation Center of Hunan University for mass spectrometry analysis.
Author information
Authors and Affiliations
Contributions
J.L. conceived the idea, directed the project, and wrote the manuscript. Y.Z. and L.H. performed the experiments. Y.Z., L.H., and C.S. analyzed the data and participated in the preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Umesh Desai, who co-reviewed with Bharath Villuri, Alan Jones, and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zheng, Y., Huang, L., Song, C. et al. Late-stage O-sulfation with a bioinspired sulfuryl donor. Nat Commun 16, 6920 (2025). https://doi.org/10.1038/s41467-025-62093-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-62093-2