Abstract
Iron-sulfur clusters fulfill numerous roles throughout biology. The reduced [2Fe-2S]+ cluster offers unique electronic and magnetic properties due to its mixed-valent nature and can serve as an essential model for understanding electron transfer, electron delocalization, and accessible spin states not only in mixed-valent dimers, but potentially larger iron sulfur clusters. Recently a series of mixed-valent diiron dichalcogenide complexes [L2Fe2Q2]− (Q = S (1), Se (2), Te (3), L = 2,6-diisopropylphenyl β-diketiminate ligand) were synthesized and characterized, where complex 1 showed a typical S = 1/2 spin state, while complexes 2 and 3 exhibited intermediate S = 3/2 spin states, potentially enabled by the minimization of vibronic coupling. Here we studied the vibrational dynamics of the Fe and Te centers in these complexes using 57Fe and 125Te nuclear resonance vibrational spectroscopy (NRVS), coupled with DFT calculations. The findings suggest that heavy character of larger chalcogen atoms results in decreased vibronic coupling. The observation of an intermediate spin state is shown to be unattainable for lighter Fe2Q2 cores. This highlights the crucial role of vibronic coupling in modulating the electronic structure of mixed-valence systems and should enhance understanding of the electronic structure in more complex biological Fe-S clusters.
Similar content being viewed by others
Introduction
The [2Fe-2S] motif represents the simplest form of Fe-S clusters and is a very common biological prosthetic group responsible for many important functions in living organisms, including electron transfer reactions. In electron transfer processes, these clusters switch between oxidized, diferric [Fe2S2]2+ and reduced, mixed-valent [Fe2S2]+, forms1,2. The majority of biological and biomimetic mixed-valent [2Fe-2S]+ clusters exhibit a Stot = 1/2 ground spin state, a result of the antiferromagnetic coupling of the high-spin Fe2+ (S = 2) and Fe3+ (S = 5/2) ions3,4,5,6,7. These [2Fe-2S] clusters can also be viewed as building blocks of larger Fe-S clusters, including [4Fe-4S] and other more complex biologically active sites such as the [7Fe9SMoC] cluster in many nitrogenases8,9,10. For higher nuclearity mixed-valent [4Fe-4S]+ clusters, various intermediate spin states (Stot = 3/2, and 5/2) and clear Fe2.5+-Fe2.5+ mixed-valent iron pairs are observed by Mössbauer spectroscopy and cannot be described by Heisenberg exchange coupling alone11. These more complex systems motivate the study and understanding of their electronic structure, particularly at the fundamental [2Fe-2S] unit level. In addition to the direct study of Fe-S clusters, it has been recently demonstrated that heavier chalcogenides, both Se and Te, can be introduced in biological and biomimetic Fe-S clusters as additional spectroscopic probes, offering additional site-specificity12,13,14,15,16. Replacement of S with heavier chalcogenides has been used to tune properties of various systems17,18,19 including proteins20,21,22, where they have an impact on electronic structure, thus it is important to evaluate the effect of chalcogen substitution on the electronic structure of biological and biomimetic clusters.
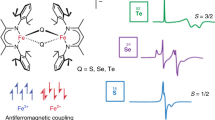
Previously, we reported on the synthesis and characterization of a series of mixed-valent diiron dichalcogenide clusters [Fe2Q2]+ (Q = S (1), Se (2), Te (3)), supported by bulky β-diketiminate ligands (Fig. 1a)23. The [Fe2S2]+ cluster exhibits a typical S = 1/2 ground spin state, while substitutions with selenide and telluride into the cluster significantly perturb the electronic structure and yield clear S = 3/2 spin state signatures that are well evidenced by electron paramagnetic resonance (EPR) spectroscopy. The EPR spectrum of the [Fe2Se2]+ cluster at 4 K evidenced a physical mixture of S = 1/2 and 3/2 spin states, while the [Fe2Te2]+ cluster demonstrated an isolated S = 3/2 intermediate spin state23. The formalism of Heisenberg exchange coupling, H = −2JS•S, is only able to rationalize the total high- (ferromagnetically coupled, J > 0) and low-spin (antiferromagnetically coupled J < 0) solutions, Stot, for mixed-valent dimers: Stot = 9/2 or Stot = 1/2, respectively. The introduction of double exchange coupling, B, to the single unpaired electron lifts the degeneracy of the Heisenberg spin states to yield the energy eigenvalues given by the following equation:
a Structures of previously synthesized mixed-valent diiron dichalcogenide clusters. b Energy levels determined by Eq. 1, as a function of B/|J| ratio, for antiferromagnetically coupled systems (J < 0). c Representation of PKS vibration for studied complexes. d, e Energy levels determined by Eq. 2 in the PKS coordinate for d S = 1/2 localized [Fe2S2]+ complex and e S = 9/2 delocalized [Fe2(OH)3(tmtacn)2]2+23,24.
Uniquely, at high ratios of B/|J| for antiferromagnetically coupled mixed-valent dimers, the total high-spin solution can be observed (B/|J|> 9, Fig. 1b).
Indeed, this formalism has been used to explain the S = 9/2 ground spin states of mixed-valent hydroxo-bridged iron dimers24,25,26,27,28 as well as in a ferredoxin [2Fe-2S]+ cluster29,30,31,32,33. The application of double exchange has not been limited to molecular iron dimers but also explains the high-spin ground states in other mixed-valent transition metal systems, including vanadium dimers34,35. In addition to Heisenberg and double exchange couplings, vibronic coupling can also have a significant influence on the cluster spin states by favoring electron localization and thus disfavoring the stabilization of intermediate spin states. First identified by Piepho, Krausz, and Schatz (PKS), the primary vibronic coupling mode, which favors electronic localization through desymmetrization of mixed-valent dimers, is a low-energy out-of-phase breathing mode (referred throughout here as the ‘PKS vibration’, Fig. 1c)36,37. The described Heisenberg double exchange model (Eq. 1) can be extended to include the influence of vibronic coupling by associating the electron-localizing PKS vibrational mode as a major vibronic contribution. The extended equation below (Eq. 2) is used to calculate the energy levels as a function of the vibronic coordinate (the PKS normal mode)24,38,39.
In this equation, (λ2/k–) is the vibronic coupling term (in cm−1). For this term, λ is calculated by λ = k–(ΔQ–) = k–(n1/2Δr), where Δr is the difference in metal-ligand bond lengths between oxidized and reduced monomeric subunits–an approximation for nuclear displacements in PKS normal mode, and n is the coordination number of each ion. Also in Eq. 2, x– is the dimensionless coordinate associated with normal mode in which ΔQ– is the vibrational normal coordinate, ν– is vibrational frequency, and k– = 4π2c2m(ν–)2) is a force constant for nuclear distortion along this coordinate24. Although double exchange coupling has been estimated to be stronger than Heisenberg coupling in most [Fe2S2]+ complexes24,40, the combination of localizing contributions of Heisenberg exchange and vibrational coupling leads to partially delocalized S = 1/2 ground states (Robin-Day class II, Fig. 1d). For antiferromagnetically coupled mixed-valent dimers that display complete electron delocalization (S = 9/2, Robin-Day class III)41, it has been shown that the large double exchange interaction prevails over the localizing effects of Heisenberg exchange and vibrational couplings (Fig. 1e)24,42,43. In our case, 57Fe Mössbauer experiments revealed two equally intense quadrupole doublets and isomer shift values for complexes 1 and 2, consistent with a partially delocalized electronic structure (class II)23. Complex 3, on the other hand, had only a single doublet, revealing a completely delocalized (class III) mixed-valent (Fe2.5+-Fe2.5+) electronic structure23. Previously, we proposed that the exchange of bridging chalcogens to heavier chalcogenide ions shifts the ‘out-of-phase’ PKS core vibration to lower energy. Therefore, we believe that a decreasing contribution of the PKS vibration plays a significant role in the ability to stabilize intermediate spin states for complexes 2 and 323.
While previous EPR experiments revealed intermediate spin states and Mössbauer experiments established the extent of electron delocalization, vibronic coupling, and its influence were not directly studied. To experimentally probe these low-energy vibrations, we employed nuclear resonance vibrational spectroscopy (NRVS). NRVS is a synchrotron-based vibrational spectroscopy technique enabling selective observation of vibrational modes originating only from Mössbauer active nuclei44,45,46. One of the most commonly employed nuclei for the NRVS experiments is 57Fe, which has proven to be particularly useful for analysis of complex, iron-containing biological systems, as it enables study of low-energy Fe normal modes without interference from complex environment contributions47,48,49,50,51. Besides 57Fe, other Mössbauer active nuclei can also be probed by the NRVS experiment. With brighter synchrotron sources capable of reaching the very high X-ray energies (>35 keV) needed for some Mössbauer transitions, and the development of high-resolution monochromators for these high energies52,53,54, the ability and success of more exotic NRVS is now realized16,55,56,57,58,59,60. Of particular interest here is 125Te NRVS, as it has recently been successfully used for the identification of low-energy vibrational modes in Fe-Te clusters16.
Replacement of the bridging S atoms with heavier Se atoms has previously been used to selectively probe local electronic structure in Fe-S proteins13,14. In a similar fashion, replacing bridging S atoms with heavier Te atoms unlocks the ability to perform both 57Fe and 125Te NRVS, which can offer us insight into the vibrations of Fe-Te clusters from two points of view, demonstrating the degree of iron or tellurium character in each mode. This site-selective advantage of NRVS spectroscopy is particularly beneficial to extract the Fe and Te core vibrations from the numerous vibrational modes involving mostly ligand, or the even more numerous protein background vibrations of Fe-Q metalloproteins. Previously, it was demonstrated that both 57Fe and 125Te NRVS can be used to understand the local structure of a 57Fe and 125Te in a labeled [4Fe-4Te]+ cluster16. The 125Te labeling offered a different perspective, enabling the extraction of weak breathing modes in the 125Te NRVS that were otherwise unobservable in the 57Fe NRVS.
Herein, we employed 57Fe NRVS to study the influence of chalcogen identity on vibrational dynamics of the series of mixed-valent diiron dichalcogenide clusters 1–3. The tellurium-bridged complex, 3, was also studied by 125Te NRVS to provide insight into predominantly tellurium-based vibrational modes. 125Te NRVS of the oxidized form of this complex was also collected to explore the applicability of this technique for studying electron transfer processes in these types of clusters. NRVS measurements were complemented by DFT calculations to provide insight into the nature of observed vibrational modes and help with their assignment.
Results and discussion
57Fe and 125Te NRVS results
57Fe NRVS was used to characterize complexes 1–3. Figure 2 shows the partial vibrational density of states (PVDOS) obtained in 57Fe NRVS experiments. The spectrum of complex 1 shows a range of transitions up to 400 cm−1 with the most dominant transition centered at 139 cm−1, overlapping with moderately intense transitions at 112 and 164 cm−1. At higher energies, a doublet at 214 cm−1 and a range of transitions between 280–400 cm−1 can be observed. Higher energy transitions (380, 399 cm−1) are expected to have significant Fe-S stretching character, due to the similarities with previously characterized Fe-S clusters that display transitions in the same region61,62,63,64,65,66,67. Considering a simple harmonic oscillator model for the Fe-Q vibrations (with the same force constant for all complexes), the transitions corresponding to the Fe-Q stretching vibrations are expected to shift toward lower energies with increased chalcogen mass (by a factor of 0.79 for Se and 0.72 for Te, compared to S)16. This indicates that the higher energy transitions in the spectrum of 1 (doublet between 380 and 400 cm−1) should shift to 300–315 cm−1 for 2 and 275–290 cm−1 for 3. Indeed, the 57Fe NRVS spectra of complexes 2 and 3 exhibit this expected trend as the highest energy transitions are centered ~303 cm−1 for 2 and 246/294 cm−1 for 3. The influence of Q’s mass is further observed in the differences between the 57Fe NRVS of 2 and 3 in the range of 220–310 cm−1. For 2, only a single broad peak is observed at 303 cm−1, whereas two distinct peaks at 246 and 294 cm−1 are observed for 3. Given the change in mass and the relative intensity differences observed for the ~300 cm−1 features in the 57Fe NRVS spectra of 2 and 3 (Fig. 2A), we assign the 246 cm−1 feature in 3 as having significant Fe-Te character. This feature is shifted significantly from the 294 cm−1 feature in the spectrum of 3. Conversely, the same Fe-Se feature for 2 is therefore overlapping significantly in the 303 cm−1 feature and is unresolved from the other Fe vibrations. The transition at 112 cm−1 in 1 also shifts significantly toward lower energies for 2 (99 cm−1) and 3 (78 cm−1) with lowering intensity of band, indicating that this vibrational mode also has significant chalcogen contribution (Fig. 2B). Unlike these features, transitions around 140 and 210 cm−1 are most intense features in all spectra and remain mostly unchanged with changing mass of chalcogen, which suggests that these transitions correspond to the normal modes with significant Fe and small chalcogen displacements.
Complex 3 was also examined by a 125Te NRVS measurement. Figure 2 compares the 57Fe and 125Te PVDOS for complex 3, and reveals a significantly different spectrum with the most intense 125Te features localized below 100 cm−1, corresponding to the largest displacements of the bridging Te atoms. The most intense features in the 57Fe spectrum (200–300 cm−1) have significantly lower relative intensity in the 125Te spectrum (compared to the most intense features), indicating smaller displacement of Te atoms in these modes, and suggesting that these modes are predominantly Fe-based, with minor Te character. The presence of a band around 250 cm−1 in both 57Fe and 125Te spectra suggests that this band indeed has significant Fe-Te character, as suggested from the initial analysis of 57Fe spectrum. The larger mass of Te compared to S leads to smaller displacements of the Te atoms in Fe-Q stretching vibrations, which would also result in lower intensity transitions in the 220–300 cm−1 region. Such observed transition localization is in agreement with a previously characterized 4Fe-4Te cluster16.
125Te NRVS was also used to characterize the oxidized [Fe2Te2]2+ (4) complex. Differences between the 125Te NRVS of 3 and 4 are related to the increased Fe oxidation state and help further refine the nature of observed normal modes in 125Te NRVS spectra. Both 3 and 4 have similarities in the region below 200 cm−1 (Fig. S4), indicating that oxidation state changes on the Fe centers do not have a significant influence on the predominantly Te-based vibrations (below 200 cm−1). However, major differences are observed above 200 cm−1, attributed to predominantly Fe-based vibrations, where the spectrum of 4 extends to approximately 310 cm−1, reflecting the increased Fe oxidation. Shifting of the spectral features to the higher energies is in accordance with observed changes when altering Fe oxidation states in Fe-S complexes68,69.
Experimentally, the collection of the 125Te NRVS is quite a bit more challenging. The determined 0 K Lamb-Mössbauer factor (fLM) for the 125Te nuclear forward scattering was 0.49, significantly less than the 57Fe fLM of 0.87. While NRVS measures the energy loss from recoilless absorption and emission (1-fLM), the signal-to-noise ratio of 125Te NRVS is notably lower than 57Fe, despite having a larger 1-fLM factor. This apparent paradox stems from the larger energy difference between the recoil energy and Debye energy of 125Te, which results in more multiphonon contributions compared to the predominantly single-phonon character of 57Fe experiments57. Although this multiphonon character significantly reduces data collection efficiency, distinct vibrations via 125Te NRVS remain resolvable, as demonstrated in our data and by others studying 125Te lattices57.
57Fe NRVS DFT results
Density functional theory (DFT) calculations utilizing the broken symmetry (BS) approach (S = 1/2) were used to obtain information about the nature of the observed NRVS transitions. Despite the inability of BS DFT to optimize the geometry of an intermediate spin state, like the S = 3/2 ground state of complexes 2 and 3, the S = 1/2 BS DFT optimized structures show good agreement with crystallographic data, indicating that the employed model can be further used (Figs. S5–S7, Table S1). Although there are ab initio methods that demonstrate the ability to calculate intermediate spin states70,71 they often rely on truncated models and BS DFT geometry-optimized structures.
The calculated 57Fe NRVS spectra for all complexes show good agreement with experimental data, with major features deviating less than 15 cm−1 from the experimental values (Figs. 3a and S8), further validating the usage of the BS DFT approach for studying these complexes. For completeness, we also calculated the S = 9/2 solutions for 1–3 and the corresponding NRVS spectra to more faithfully represent a fully electronically delocalized structure 3 (Figs. S9–S10). The S = 9/2 solution has much worse agreement with the experiment for 1 and does not reproduce the splitting of the high-energy features. For 2 and 3, however, the 57Fe NRVS spectra of the S = 1/2 and 9/2 solutions are more qualitatively like one another. As discussed later, though, the S = 1/2 solution has better agreement with the core’s geometric structure, and the 125Te NRVS also has better agreement with the experimental data (Fig. S10). This shows that the vibrational structure of 1 reflects a localized electronic structure; however, the distinction between localized vs. delocalized for 2 and 3 is much more challenging from the NRVS data alone, but the BS DFT calculated spectra have overall good agreement.
a Comparison of experimental (black line) and calculated (red line) 57Fe NRVS spectra of complexes 1–3. Individual transitions are depicted by red vertical lines. b 57Fe PVDOS along x (red line), y (blue line), and z (brown line) axes, for complexes 1–3. The x axis is defined along Fe-Fe bond, and z axis is perpendicular to the [Fe2Q2]+ core. c Representation of calculated PKS normal modes for complexes 1–3. For clarity, only the [Fe2Q2]+ cores and coordinating N atoms are shown.
The Fe mode composition factors were analyzed along the three molecular axes (x axis is defined along the Fe-Fe bond and z axis is perpendicular to the [Fe2Q2]+ plane). Figure 3b shows that for complex 1 high-energy transitions (in 250–400 cm−1 range) are predominantly localized in the xy plane, and thus are dominated by in-plane Fe vibrations influenced by the nature of the bridging Q atom and the Fe-Q bond length. Some features at lower energies also show significant contributions of in-plane vibrations, but features around 130 and 200 cm−1 are dominated by out-of-plane Fe vibrations (along z axis). High energy in-plane vibrations of complex 1 (250–400 cm−1) shift to lower energies for complexes 2 (200–350 cm−1) and 3 (160–330 cm−1). Additionally, for complex 1, the region below 250 cm−1 shows significant contributions of in-plane Fe vibrations, while for complexes 2 and 3 these contributions are mostly localized below 100 cm−1. Uniquely, the out-of-plane (z) vibrations remain mostly unchanged for all complexes (features around 130 and 200 cm−1), demonstrating that changes in bridging chalcogen are responsible for the shifting of in-plane Fe vibrational modes, while minimally impacting the out-of-plane Fe vibrational modes.
To gain more insight into the nature of observed vibrations as a function of pairs of atomic displacements, the DFT-based kinetic energy distribution (KED) spectra were calculated for all complexes (Fig. S11). The KED spectrum for complex 1 shows that the high-energy transitions above 350 cm−1 are the result of strong Fe-S vibrations with significant character along the S-S vector, and the region between 150 and 300 cm−1 is dominated by vibrations with significant Fe-Fe character. The KED spectra of complexes 2 and 3 show significant shifts and separation for the Fe-Q and Q-Q vibrations, in comparison with 1, potentially enabling a clearer observation of these vibrational modes in NRVS spectrum for 2 and 3. Unlike 1, the KED spectra of complexes 2 and 3 are dominated by Fe-Q vibration profile, and the similarity of this profile with the 57Fe NRVS spectra of 2 and 3 indicates that these normal modes are Fe-dominated and that these vibrations involve small Se (for 2) and Te (for 3) nuclei displacements. This suggests that vibrational couplings between Fe and Q for complexes 2 and 3 are much weaker than for complex 1. This is better illustrated by comparing calculated 57Fe and Q (32S, 80Se, 125Te) PVDOS spectra (Fig. S12), where 32S and 80Se are fictitious NRVS spectra as no Mössbauer active isotopes exist for these elements. Calculated PVDOS spectra of 57Fe and 32S for complex 1 have a similar profile, indicating strong vibrational coupling. Contrastingly, the spectra of 57Fe and Q for complexes 2 (80Se) and 3 (125Te) have significantly different profiles, indicating weaker coupling between Fe and Q in these complexes.
57Fe NRVS normal mode analysis
Individual key vibrations are best identified by analysis and visualization of individual normal modes and their relative intensity contributions to the NRVS spectrum. For complex 1, the highest energy transitions (374 and 390 cm−1) have a significant contribution of Fe-S bond stretching vibrations (Fig. S13). The features around 300 cm−1 (of complex 1) are dominated by in-plane Fe-S core vibrations (Fig. S13), as well as out-of-phase in-plane breathing mode (PKS vibration, Fig. 3c), with additional Fe-S stretching vibrations toward lower energies, around 260 cm−1. Transitions around 200 and 140 cm−1 are dominated by strong Fe out-of-plane vibrations (Fig. S14), as suggested by the calculated polarized NRVS spectrum. The spectral feature at 112 cm−1 in the experimental spectrum is also well reproduced with a shoulder at 102 cm−1 in the calculated spectrum, and the normal mode that contributes most to this transition is the [Fe2S2]+ core in-plane normal mode (Fig. S13).
The Fe-Q bond stretching vibrations shift from 370–390 cm−1 in 1 to 280–310 cm−1 in 2, and further to 250–270 cm−1 in 3, consistent with increasing chalcogen mass (Fig. S13). Similarly, the in-plane [Fe2Q2]+ core normal mode at 101 cm−1 in 1, shifts to 89 cm−1 in 2 and 84 cm−1 in 3 (Fig. S13). Other in-plane vibrations around 300 cm−1 in 1 also shift to around 200 cm−1 in 2 and 3 (Fig. S13) and overlap with the strong Fe out-of-plane vibrations around 200 cm−1 (Fig. S14). The out-of-plane Fe vibrations around 140 and 200 cm−1 remain constant among the series as these modes are not strongly influenced by changing the chalcogen mass (Fig. S14). The sharp feature at 246 cm−1 in the experimental spectrum of complex 3 is well reproduced by a Fe-Te stretching band at 253 cm−1 (Fig. S13), and the clearly resolved band at 294 cm−1 is well reproduced by a Fe-Fe stretching vibration band at 292 cm−1 (Fig. S13). Analogous Fe-Fe stretching modes in 1 (~220 cm⁻¹) and 2 (~260 cm⁻¹) are obscured by other features (Fig. S13). The reduction of the vibronic coupling between Fe and Q and resultant contraction of the spectra as the mass of Q increases leads to simpler, more resolved spectra with less contributions from Q. For complex 3, this enables the observation of vibrations that are otherwise obscured in complexes 1 and 2, highlighting the advantage of replacing S and Se with Te for characterization of normal modes in these complexes.
Identifying the out-of-phase breathing (PKS) mode, which leads to electronic localization in these complexes, is important for understanding the electronic structure of these mixed-valent [Fe2Q2]+ clusters. The agreement between calculated and experimental spectra supports the DFT model’s reliability in estimating the PKS vibration energy. DFT calculations predict a PKS vibration at 290 cm−1 for 1 (Fig. 3c). The unambiguous assignment of the experimental peak at 301 cm−1 to the PKS vibration is not possible due to the overlap of other equally intense in-plane Fe-S core vibrations (Fig. S15) calculated near the same energy and within the resolution of the experiment. Increasing chalcogen mass reduces the PKS mode energy to 158 cm−1 in 2 and 141 cm⁻¹ in 3, consistent with weaker vibronic coupling. Unfortunately, the resolution of the experimental 57Fe NRVS spectra prevent direct observation of the PKS vibration, motivating further investigations via 125Te NRVS for 3.
125Te NRVS DFT results
The calculated 125Te NRVS spectrum shows overall good agreement with the experimental data (Fig. 4a, S16). Weak transitions in the 57Fe NRVS spectrum, below 100 cm−1, are the most intense transitions in the 125Te NRVS spectrum, reflecting their significant Te character and providing an alternative view of the core vibrations compared to the 57Fe NRVS experiment. In contrast to the strongest transitions in 57Fe NRVS spectrum between 200 and 300 cm−1, transitions in this region are weaker and less resolved in the 125Te NRVS spectrum, indicating lower Te character in these transitions.
a Comparison of 125Te (top) and 57Fe (bottom) NRVS spectra of complex 3. Experimental spectra are depicted by black lines, calculated spectra by blue (125Te) and red (57Fe) lines, with selected individual transitions depicted by blue and red vertical lines. b Representations of selected calculated normal modes of complex 3. For clarity, only the [Fe2Te2]+ core and coordinating N atoms are shown. Magenta colored rectangles represent regions containing normal modes with significant Te displacements (a) and corresponding normal modes (b). Orange colored rectangles represent regions containing normal modes with significant Fe displacements (a) and corresponding normal modes (b).
Analysis of the 125Te mode composition factors along its molecular axes reveals that transitions below 50 cm−1 are dominated by out-of-plane Te vibrations (Fig. S17). Transitions in the range 50–100 cm−1 mostly consist of in-plane Te vibrations with significant out-of-plane character, while transitions above 100 cm−1 are dominated by in-plane Te vibrations. The KED spectrum of complex 3 (Fig. S11) shows that the transitions at 118 and 156 cm−1 have significant Te-Te character, and in general, unlike 57Fe NRVS, 125Te NRVS spectrum is better described by the Te-Te KED profile. The sharp band in experimental spectrum at 126 cm−1 is well reproduced by the band at 118 cm−1 (with the strongest individual transition at 119 cm−1) in the calculated spectrum (Fig. 4a). Visualization of this mode shows that this band can be attributed to the Te in-plane breathing mode (Fig. 4b). The experimental band at 160 cm−1 is also well reproduced with the calculated band at 156 cm−1 (with strongest individual transition at 157 cm−1) and this transition can be assigned to a Te-Te stretching vibration (Fig. 4b). Such an assignment agrees with KED analysis which indicated that both bands have significant Te-Te character.
At higher energies, the experimental spectrum shows weak broad transition centered at 252 cm−1 which was previously assigned from 57Fe NRVS to a Fe-Te stretching vibration (Fig. 4b). It can be noted that the same feature has greater relative intensity in 57Fe NRVS spectrum, due to significant involvement of Fe nuclei in this normal mode (Fig. 4a). Also, the intense Fe-Fe stretching mode at 294 cm−1 in 57Fe NRVS spectrum of 3 (Fig. 4a) can also be observed in the DFT calculated 125Te NRVS spectrum as a very weak transition. The PKS vibration of 3 overlaps with other strong, close-in-energy vibrations, in this case, the strong Te-Te stretching vibration at 156 cm−1, as seen in the slightly overlapping peaks of the x- (PKS) and y- (Te-Te stretch) polarized spectra (Fig. S17).
DFT calculations also reproduce the 125Te NRVS spectrum of 4 well (Fig. S18), and polarized analysis of the 125Te composition factors show the same trends as for complex 3 (Fig. S19). Overall, the same assignments exist for the 125Te NRVS of 4, as described for 3 above (see SI for more detail). While predominantly Te-based vibrations are largely unaffected by the Fe oxidation state change, vibrations with higher Fe contributions show small shifts (~10 cm−1) toward higher energies with increasing Fe oxidation state, which is in agreement with observed trends in experimental spectrum, as well as with expected changes upon Fe oxidation. This further supports that 125Te can provide information about electronic changes on neighboring Fe centers.
The direct comparison of 57Fe and 125Te NRVS spectra for complex 3, as well as intensities of selected normal modes in both spectra (Fig. 4a), highlights the advantages of each of these techniques and emphasizes the dual point-of-view of using both. The 57Fe NRVS best reveals the direct observation of Fe-Te and Fe-Fe stretching vibrational modes, while the 125Te NRVS spectrum enables identification of predominantly Te vibrations, whose intensities in the 57Fe spectrum are too low to be observed and/or overlap with various other intense out-of-plane Fe vibrations. Thus, the more selective characterization of the Te vibrations via 125Te NRVS provides a clear advantage for characterization of Te containing complexes, enabling direct observation of strong Te-Te vibrations that could not be identified in the 57Fe NRVS experiments.
Influence of vibronic coupling on the electronic structure
The vibronic couplings were obtained from the DFT calculated frequencies of PKS normal modes, described above. For complexes 1–3 an approximation for nuclear displacements in PKS normal mode (Δr) has been determined from comparison of their crystal structures with crystal structures of analogous oxidized complexes, where a smaller displacement is observed for the heavier bridges (Δr(1) = 0.100, Δr(2) = 0.067, Δr(3) = 0.057 Å)23,72. Complex 1 exhibits a significantly larger vibronic coupling (2300 cm−1) than complexes 2 (610 cm−1) and 3 (540 cm−1), consistent with both the shifts of the PKS vibration and decreased displacements of the heavier atoms. Calculated vibronic couplings and previously experimentally estimated values for J (= −55 (1), −50 (2), −200 (3) cm−1) and B ( = 110 (1), 165 (2), 750 (3) cm−1)23 can be used to construct ground and excited spin state potential energy surfaces in the PKS coordinate using Eq. 2 (Fig. 5).
Calculated energy levels show that complex 1 displays a double-well S = 1/2 ground state in agreement with the previous partially delocalized class II assignment of the Robin-Day system (Fig. 5a)23. Complex 2 can also be characterized as partially delocalized (class II, Fig. 5) with S = 1/2 and 3/2 states very close in energy, in agreement with experimentally observed mixture of these states23. Unlike these, complex 3 shows fully delocalized S = 3/2 ground state (class III, Fig. 5), which is also in line with experimentally observed intermediate S = 3/2 ground state23.
To examine the influence of vibronic coupling on the electronic structure of these complexes, cases with varying vibronic coupling contribution (with other parameters constant) are considered (Figs. S22–S24). For complex 1, lowering of vibronic coupling to the level observed for complex 3 results in lowering energies of excited states, but ground state remains partially delocalized S = 1/2 (Fig. 5a). Further lowering of vibronic coupling would lead to the completely delocalized ground state (class III, Fig. S22), which would still remain S = 1/2. While this indicates that vibronic coupling has a direct impact on the degree of electron delocalization, ground spin state should remain same for complex 1. For complex 2, increased vibronic coupling leads to a well-isolated ground spin state of S = 1/2, while decreased vibronic coupling may result in a delocalized S = 1/2 ground spin state with almost isoenergetic S = 3/2 excited state (Fig. S23). On the other hand, increasing vibronic coupling for complex 3 to the levels corresponding to the complex 1 result in a partially delocalized mixture of the S = 1/2 and 3/2 states (similar to the scenario for complex 2, Fig. S24), while a further increase results in larger separation of spin states and well isolated partially delocalized S = 1/2 ground state (class II, Fig. 5c). This analysis further confirms that vibrational coupling can have significant influence not only on the electron localization, but consideration of its contribution is crucial for the stabilization of intermediate spin states. For the studied complexes, it is shown that decreased vibronic coupling in complexes 2 and 3 leads to the stabilization of intermediate spin states. The analysis of Eq. 2 furthermore supports that the larger mass of Se and Te, and their decreased vibronic displacements in comparison to S is a major factor in the successful stabilization of these rare intermediate spin states. This demonstrates that minimization of vibronic coupling results in the stabilization of excited states, and more accessible excited states should improve reactivity of the Fe-S clusters70.
Understanding the vibronic coupling in mixed-valent transition metal cores is essential to our ability to design complexes and materials with tailored properties, including specified electronic spin states. In summary, we demonstrated here the successful application of 57Fe NRVS to probe the vibrational dynamics of Fe centers in a series of diiron dichalcogenide complexes and identify normal modes with the help of DFT calculations. Additionally, it was shown that 125Te NRVS can be employed as a complementary method to the 57Fe NRVS, providing further information about low-energy Te-based normal modes. Such an approach enables studying these complexes from two different vantage points. Substitution with heavier chalcogens leads to contraction of 57Fe NRVS spectra due to the smaller coupling between Fe center and bridging chalcogen. This results in spectra that have less influence from the chalcogen, leading to isolation of normal modes that could not be observed in complexes with lighter chalcogens. While the PKS vibration, responsible for electron localization in mixed-valent complexes, could not be directly identified in the experimental spectra, pairing with DFT calculations helps identify their signatures in the NRVS spectra and shows that the energy of this vibration decreases with heavier chalcogenides, consistent with smaller vibronic coupling between Fe and heavier chalcogens. These findings support that a decrease of the vibronic coupling in complexes with heavier chalcogens leads to the observed stabilization of intermediate spin states.
Although now two decades old, the application of NRVS spectroscopy to (bio)inorganic molecules is still developing, and extending the accessible nuclei beyond 57Fe to 125Te offers unique opportunities for the characterization of iron chalcogenide clusters. While Te, unlike S and Se, is not widely present in biological systems, Te labeling of proteins has been successfully accomplished in the past and opens up the possibility of using Te as a probe to study biological systems20,21,73,74,75,76. In our attempts to understand vibronic contributions to the electronic structure of the mixed-valent diiron dichalcogenide cluster, 125Te offered the best extraction of important low-energy core vibrations. The systematic decrease in the contribution of the PKS to the electronic localization in the [Fe2Q2]+ cluster, with increased chalcogenide mass, reveals that Se and Te substitutions in Fe-S clusters may not be strictly innocent. In this system, where the Fe-Fe distance does not change significantly, the dampening of the vibronics has allowed for double-exchanged intermediate spin states.
In other biological systems, such as nitrogenase which catalyzes reduction of N2 to ammonia and contains FeMo cofactor ([7Fe9SMoC]) which consists of [Fe4S3] and [MoFe3S3] subunits bridged by three μ2 sulfides (belt sulfides) and one μ6 carbide9,10, Se substitution of the belt sulfides under turnover conditions has been exploited as an atom and site-specific spectroscopic marker to understand the local electronic structure12, particularly the redox level, of the neighboring iron ions, via X-ray absorption spectroscopy (XAS)13. While Se substitution appears innocent enough via XAS, more recent EPR spectroscopy of these Se-turnover samples exhibits redistributions of the rhombicity of the S = 3/2 zero-field splitting tensor, indicating a clear but perhaps not fully understood change in the electronic structure15. Our diiron dichalcogenide dimers further support that Se and further Te substitution can have significant electronic influences that are sometimes the result of a change in the delicate balances of Heisenberg, double exchange, and vibronic couplings.
Methods
General considerations
Unless indicated otherwise, all manipulations were performed using oven-dried glassware in an M-Braun nitrogen-atmosphere glovebox or on a Schlenk line using standard Schlenk techniques. Molecular sieves were activated by heating at 200 °C for 48 h under high vacuum. THF, toluene, diethyl ether, hexane, and pentane were purchased anhydrous from Sigma, further dried over sodium/benzophenone ketyl, vacuum-transferred before use, and stored over 4 Å molecular sieves. KC8, FeBr2, C10H8, PCy3 (Cy = cyclohexyl), PMe3 (1.0 M in PhMe), 2,6-diisopropylaniline, acetylacetone, (Me3Si)2S, MeSO3H, S8, Se0, Te0, naphthalene, concentrated HCl (37%), and n-BuLi (2.5 M in hexanes) were purchased from Sigma and used as received. 57Fe metal (≥96% 57Fe) was purchased from Campro Scientific GmbH (Berlin), and 125Te metal (88% 125Te) was purchased from US Services (Summit, NJ, USA) and used as received. 1H, 31P, and 125Te NMR spectra were recorded on a Bruker Avance III HD 500 NMR spectrometer.
Synthesis of SePMe3
The synthesis was adapted from the literature77. A solution of Me3P in PhMe (1.0 M, 14.0 ml, 14.0 mmol, 1.1 eq) was added to solid Se (0.9915 g, 12.6 mmol, 1.0 eq) in a 20 ml vial with rapid stirring. A moderate exothermic reaction took place, resulting in complete consumption of the Se solids. As the reaction cooled back to room temperature, white microcrystalline solids precipitated from the solution. After stirring for 12 hours at room temperature, the solids were collected on a glass frit and washed with pentane (12 ml) and dried under vacuum to afford 1.1921 g (ca. 61%) of the desired product. 1H and 31P data are consistent with reported values77. 1H NMR (CDCl3), δ (ppm): 1.92 (d, 13 Hz, 9H, P(CH3)3). 31P NMR (CDCl3), δ (ppm): 8.76 (s, SePMe3, ca. 93% abundance (non-77Se)), 8.78 (d, 677 Hz, 77SePMe3, ca. 7% abundance 77Se).
Synthesis of TePCy3
The synthesis was adapted from the literature78. Solid Te0 (0.3201 g, 2.51 mmol, 1.4 eq) was added to a solution of PCy3 (0.5084 g, 1.81 mmol, 1.0 eq) in PhMe (10 ml) and the reaction was left to stir at room temperature for four days. The resulting suspension was filtered through a pad of celite to remove unreacted Te0, and the resulting yellow filtrate was concentrated under vacuum. The residue was triturated with pentane (5 ml), collected on a glass frit, and washed with Et2O (10 ml). The resultant pale-yellow solid was dried under vacuum to afford 0.5262 g (ca. 71%) of the desired product. 1H and 31P data are consistent with reported values78. 1H NMR (C6D6), δ (ppm): 1.99–1.91 (m, 6H), 1.80–1.70 (m, 3H), 1.65–1.57 (m, 6H), 1.49–1.43 (m, 3H), 1.43–1.32 (m, 6H), 1.10–0.94 (m, 9H). 31P NMR (C6D6), δ (ppm): 27.73 (s, TePCy3, ca. 94% abundance (non-125Te)), 27.75 (d, 1J(125Te) = 1710 Hz, 125TePCy3, ca. 6% abundance 125Te).
Synthesis of OAsPh3
The synthesis was adapted from the literature79. Under air, H2O2 (50%, 0.55 ml, 9.7 mmol, 1.3 eq) was added dropwise to an ice-water bath cooled solution of AsPh3 (2.3 g, 7.5 mmol, 1.0 eq) in acetone (20 ml). Upon complete addition, a significant white precipitate formed. The mixture was removed from the ice bath, and upon warming, the precipitate resolubilized. After stirring for 1 hour at room temperature, the volatiles were removed under vacuum to afford a white microcrystalline residue. The residue was triturated with PhMe (5 ml), collected on a glass frit, and washed with additional PhMe (2 × 5 ml). The white microcrystalline product was then dried under vacuum to a weight of 2.3 g (95%). 1H NMR data are consistent with reported values79. 1H NMR (CDCl3), δ (ppm): 7.65–7.55 (m, 6H, o-ArH), 7.45–7.36 (m, 9H, m,p-ArH).
Synthesis of SAsPh3
The synthesis was adapted from the literature80. In the glovebox, (Me3Si)2S (1.50 ml, 7.11 mmol, 1.1 eq) was added dropwise to a stirring suspension of OAsPh3 (2.0143 g, 6.25 mmol, 1.0 eq) in MeCN (12 ml). With each drop, a blue-green flash was observed, which quickly faded to yellow over one or two seconds. Upon complete addition, the OAsPh3 had completely dissolved, and the resulting yellow solution was warm to the touch. After about 30–60 seconds, a large quantity of off-white microcrystals had precipitated from the solution. The reaction was left to stir overnight at room temperature. The precipitates were then collected on a glass frit and washed with Et2O (20 ml). The resultant off-white microcrystalline solid was then dried under vacuum to afford 1.7423 g (82%) yield of the desired product. 1H NMR data is consistent with reported values81. 1H NMR (CDCl3), δ (ppm): 7.76–7.73 (m, 6H, o-ArH), 7.56–7.51 (m, 3H, p-ArH), 7.51–7.46 (m, 6H, m-ArH).
Synthesis of LH
The synthesis was adapted from the literature82. 2,6-diisopropylaniline (23.5 ml, 0.125 mmol, 2.0 eq), acetylacetone (6.4 ml, 0.0623 mmol, 1.0 eq), and methanesulfonic acid (8.1 ml, 0.125 mmol, 2.0 eq) were combined with toluene (200 ml) in a 500 ml round-bottom flask charged with a stir bar and fitted with a Dean-Stark condenser. The reaction was then heated to reflux (135 °C, oil bath) for 12 hours. After cooling to room temperature, the volatiles were removed under vacuum via rotary evaporation until a viscous oil remained. A saturated solution of Na2CO3 (250 ml) was added to the residue, followed by CHCl3 (150 ml). The resulting mixture was stirred for 5 min and then transferred to a 1000 ml separatory funnel. After mixing well, the layers were separated, and the aqueous phase was washed with additional CHCl3 (2 ×100 ml). The combined organics were dried over Na2SO4, filtered, and then concentrated to a viscous oil via rotary evaporation. MeOH (150 ml) was added to the oil, resulting in the formation of a white crystalline solid. The mixture was heated to 60 °C for 50 minutes, then filtered hot on a glass frit. The solid was washed with additional MeOH (200 ml total) and then dried under vacuum to afford 15.4 g (ca. 59%) of the desired product. 1H NMR data is consistent with reported values82. 1H NMR (CDCl3), δ (ppm): 12.15 (s, broad, 1H, OH), 7.16–7.13 (m, 6H, m,o-ArH), 4.89 (s, 1H, (CNAr)2CH), 3.14 (sept, 6.9 Hz, 1H, CH(CH3)2), 1.73 (s, 6H, H3C(CNAr)2CH), 1.23 (d, 6.9 Hz, 6H, CH(CH3)2), 1.14 (d, 6.9 Hz, 6H, CH(CH3)2).
Synthesis of 57FeCl2
The synthesis was adapted from the literature83. 57Fe metal (0.5021 g, 8.81 mmol) was added to a 50 ml round-bottom flask charged with a stir bar and fitted with an N2 inlet and reflux condenser. Under N2, concentrated HCl (37%, degassed, 10 ml) was added. The resulting mixture was heated to 100 °C overnight until H2 evolution had ceased and visual inspection confirmed all of the solid 57Fe had been consumed. The solution was then cooled to room temperature and the volatiles removed under high vacuum (0.001 mbar). To remove the residual coordinated water, the residue was heated to 200 °C under high vacuum (0.001 mbar) for 2 hours, affording the desired anhydrous 57FeCl2 as a light beige solid (1.0426 g, ca. 93%).
Synthesis of LFe(PhMe)
The synthesis was adapted from the literature84. n-Butyllithium (2.0 ml, 5.0 mmol) was added dropwise to a solution of LH (2.0155 g, 4.81 mmol) in THF (10 ml). Upon complete addition, the pale-yellow solution was stirred for 30 min. Then FeBr2 (1.0422 g, 4.83 mmol) was added and the reaction was stirred for 1 hour. All volatiles were then removed under vacuum and the residue was reconstituted with PhMe (10 ml). KC8 (0.8344 g, 6.18 mmol) was added, and the mixture was left stirring for 72 hours at room temperature. The volatiles were then removed under vacuum, and the residue was extracted with hexane (3 × 10 ml). The hexane extracts were filtered through a glass microfiber pad and the filtrates concentrated under vacuum. The residue was again extracted with hexane (10 ml), filtered, and the filtrate reduced to half-volume under vacuum. The concentrated solution was then placed in the freezer (−35 °C) and stored overnight to afford dark-red crystals. The supernatant was pipetted off, again concentrated to half-volume and a second crop of crystals were grown overnight. Combined yield 1.3176 g (ca. 48%) of dark-red crystals.
Synthesis of 125TePCy3
In a Schlenk tube charged with a stir bar and fitted with a Teflon screw-in pin, 125Te metal (0.1926 g, 1.54 mmol) and excess PCy3 (1.4847 g, 5.29 mmol) were combined in PhMe (20 ml). The Schlenk tube was sealed, removed from the glovebox, and heated to 100 °C in an oil bath with stirring for 16 hours. At this time, visual inspection revealed all the Te metal had been consumed. After cooling to room temperature, the Schlenk tube was brought back into the glovebox, the reaction was filtered through a glass microfiber pad, and volatiles were removed under vacuum from the filtrate. The resulting residue was triturated with Et2O (20 ml), and the off-white solid collected on a glass frit. The solid was further washed with Et2O to ensure excess PCy3 was removed (ca. 60 ml total). The resulting solid was then collected and dried under vacuum to afford 0.4922 g (ca. 78%) of a pale-yellow powder. 31P NMR analysis revealed the major product to be 125TePCy3 (ca. 80% by 31P NMR), with a small amount of OPCy3 (ca. 5% by 31P NMR, δ = 46.8 ppm) and an unidentified phosphorus-containing impurity (ca. 15% by 31P NMR, δ = 33.6 ppm). 125Te NMR revealed 125TePCy3 as the only tellurium-containing product (δ = -938.0 ppm). The material was used without further purification. 31P NMR (C6D6), δ (ppm): 27.47 (d, 1J(125Te) = 1720 Hz). 125Te NMR (C6D6), δ (ppm): −937.98 (d, 1J(31P) = 1710 Hz).
Synthesis of L2Fe2 125Te2
The synthesis was adapted from the natural abundance isotopologue previously reported23. Filtered solutions of LFe(PhMe) (0.5461 g, 0.965 mmol, 1.0 eq) and 125TePCy3 (0.4550 g, 1.12 mmol, 1.2 eq) in PhMe (4 ml each) were combined in a 20 ml scintillation vial, which was then capped and inverted several times to thoroughly mix the solutions. Upon standing overnight, the mixture had produced dark brown precipitates, which were collected on a glass frit, washed with additional PhMe until the filtrates ran clean, and then dried under vacuum to afford 0.4518 g (ca. 39%) of the desired product as a maroon-black powder.
Synthesis of [K(THF)5][L2Fe2 125Te2]
The synthesis was adapted from the natural abundance isotopologue previously reported23. A solution of KC10H8 was generated by stirring KC8 (0.0737 g, 0.545 mmol, 1.7 eq) with naphthalene (0.0507 g, 0.396 mmol, 1.2 eq) in THF (3 ml). After 30 min, the dark-green KC10H8 solution was added dropwise to a stirring suspension of L2Fe2125Te2 (0.3866 g, 0.323 mmol, 1.0 eq) in THF (5 ml). Upon complete addition, the L2Fe2125Te2 had solubilized to give a dark-red solution. The reaction mixture was stirred for 1 h, and then all volatiles were removed under vacuum. The resulting dark residue was triturated with Et2O (5 ml) and filtered through a glass microfibre filter pad. The resulting black-red solid was washed with additional Et2O until the filtrate ran clear (ca. 5 ml). The black-red solid was then resolubilized in THF (ca. 5 ml), filtered through the glass microfibre pad, further concentrated to half-volume under vacuum, and stored at –35 °C to afford 0.1564 g (ca. 30%) of the desired product as black-red crystals.
Synthesis of L57Fe(PhMe)
The synthesis was adapted from the natural abundance isotopologue reported above. n-Butyllithium (2.0 ml, 5.0 mmol) was added dropwise to a solution of LH (2.0224 g, 4.83 mmol) in THF (10 ml). Upon complete addition, the pale-yellow solution was stirred for 30 min. Then, 57FeCl2 (0.6063 g, 4.74 mmol) was added, and the reaction was stirred overnight at room temperature. All volatiles were then removed under vacuum, and the residue was reconstituted with PhMe (10 ml). KC8 (0.8583 g, 6.35 mmol) was added, and the mixture was left stirring for 72 hours at room temperature. The volatiles were then removed under vacuum, and the residue was extracted with hexane (3 ×10 ml). The hexane extracts were filtered through a glass microfiber pad, and the filtrates concentrated under vacuum. The residue was again extracted with hexane (10 ml), filtered, and the filtrate reduced to half-volume under vacuum. The concentrated solution was then placed in the freezer (−35 °C) and stored overnight to afford dark-red crystals. The supernatant was pipetted off, again concentrated to half-volume, and a second crop of crystals was grown overnight. Combined yield 1.5621 g (ca. 58%) of dark-red crystals.
Synthesis of L2 57Fe2S2
The synthesis was adapted from the literature23. Filtered solutions of L57Fe(PhMe) (0.5082 g, 0.897 mmol, 1.0 eq) and SAsPh3 (0.2999 g, 0.886 mmol, 1.0 eq) in PhMe (4 ml each) were combined in a 20 ml scintillation vial, which was then capped and inverted several times to thoroughly mix the solutions. Upon standing overnight, the mixture had produced dark-red-brown precipitates, which were collected on a glass frit, washed with additional PhMe until the filtrates ran clean, and then dried under vacuum to afford 0.3365 g (ca. 75%) of the desired product as a burgundy powder.
Synthesis of [K(THF)6][L2 57Fe2S2]•2THF
The synthesis was adapted from the literature23. A solution of KC10H8 was generated by stirring KC8 (0.0553 g, 0.409 mmol, 1.7 eq) with naphthalene (0.0395 g, 0.308 mmol, 1.2 eq) in THF (3 ml). After 30 min, the dark-green KC10H8 solution was added dropwise to a stirring suspension of L257Fe2S2 (0.2502 g, 0.247 mmol, 1.0 eq) in THF (5 ml). Upon complete addition, the L257Fe2S2 had solubilized to give a dark-red solution. The reaction mixture was stirred for 1 h, and then all volatiles were removed under vacuum. The resulting dark residue was triturated with Et2O (5 ml) and filtered through a glass microfibre filter pad. The resulting dark-red solid was washed with additional Et2O until the filtrate ran clear (ca. 5 ml). The red solid was then resolubilized in THF (ca. 5 ml), filtered through the glass microfibre pad, further concentrated to half-volume under vacuum and stored at –35 °C to afford 0.1886 g (ca. 47%) of the desired product as dark-red crystals.
Synthesis of L2 57Fe2Se2
The synthesis was adapted from the literature23. Filtered solutions of L57Fe(PhMe) (0.5222 g, 0.921 mmol, 1.0 eq) and SePMe3 (0.1439 g, 0.928 mmol, 1.0 eq) in PhMe (4 ml each) were combined in a 20 ml scintillation vial, which was then capped and inverted several times to thoroughly mix the solutions. Upon standing overnight the mixture had produced dark-green precipitates, which were collected on a glass frit, washed with additional PhMe until the filtrates ran clean, and then dried under vacuum to afford 0.4165 g (ca. 41%) of the desired product as a dark-green powder.
Synthesis of [K(THF)6][L2 57Fe2Se2]•2THF
The synthesis was adapted from the literature23. A solution of KC10H8 was generated by stirring KC8 (0.0618 g, 0.457 mmol, 1.7 eq) with naphthalene (0.0465 g, 0.363 mmol, 1.35 eq) in THF (3 ml). After 30 min, the dark-green KC10H8 solution was added dropwise to a stirring suspension of L257Fe2Se2 (0.2971 g, 0.268 mmol, 1.0 eq) in THF (5 ml). Upon complete addition, the L257Fe2Se2 had solubilized to give a dark brown solution. The reaction mixture was stirred for 1 h, and then all volatiles were removed under vacuum. The resulting dark residue was triturated with Et2O (5 ml) and filtered through a glass microfibre filter pad. The resulting black solid was washed with additional Et2O until the filtrate ran clear (ca. 5 ml). The black solid was then resolubilized in THF (ca. 5 ml), filtered through the glass microfibre pad, further concentrated to half-volume under vacuum, and stored at –35 °C to afford 0.1977 g (ca. 43%) of the desired product as black crystals.
Synthesis of L2 57Fe2Te2
The synthesis was adapted from the natural abundance isotopologue previously reported23. Filtered solutions of L57Fe(PhMe) (0.5245 g, 0.925 mmol, 1.0 eq) and TePCy3 (0.3847 g, 0.943 mmol, 1.0 eq) in PhMe (4 ml each) were combined in a 20 ml scintillation vial, which was then capped and inverted several times to thoroughly mix the solutions. Upon standing overnight, the mixture had produced dark brown precipitates, which were collected on a glass frit, washed with additional PhMe until the filtrates ran clean, and then dried under vacuum to afford 0.3891 g (ca. 70%) of the desired product as a black-brown powder.
Synthesis of [K(THF)5][L2 57Fe2Te2]
The synthesis was adapted from the natural abundance isotopologue previously reported23. A solution of KC10H8 was generated by stirring KC8 (0.0477 g, 0.353 mmol, 1.5 eq) with naphthalene (0.0384 g, 0.299 mmol, 1.25 eq) in THF (3 ml). After 30 min, the dark-green KC10H8 solution was added dropwise to a stirring suspension of L257Fe2Te2 (0.2882 g, 0.239 mmol, 1.0 eq) in THF (5 ml). Upon complete addition, the L257Fe2Te2 had solubilized to give a dark-red solution. The reaction mixture was stirred for 1 h, and then all volatiles were removed under vacuum. The resulting dark residue was triturated with Et2O (5 ml) and filtered through a glass microfibre filter pad. The resulting black-red solid was washed with additional Et2O until the filtrate ran clear (ca. 5 ml). The black-red solid was then resolubilized in THF (ca. 5 ml), filtered through the glass microfibre pad, further concentrated to half-volume under vacuum and stored at –35 °C to afford 0.2021 g (ca. 53%) of the desired product as black-red crystals.
NRVS sample preparation
Samples for 57Fe and 125Te NRVS studies were prepared by grinding between 100 and 200 mg of material in an M-Braun nitrogen-atmosphere glovebox and placed into custom sample cells sealed with Kapton tape. Samples were then flash frozen in liquid nitrogen and stored in liquid nitrogen until measurements. The frozen powder samples were transported to the beamlines in a dry-shipper cooled to liquid nitrogen temperatures.
57Fe NRVS
57Fe NRVS data were collected at the synchrotron radiation facility SPring-8 at BL19LXU, operating in the C-mode bunch pattern with a 145.5 ns interval between x-ray pulses, as previously described65. The samples were placed in a helium flow cold finger cryostat maintained nominally at 10 K.
The x-ray beam after the undulator was monochromated at a high heat load Si(111) monochromator to ~1 eV bandwidth, and then further by the Ge(331) x2 Si(975) high-resolution monochromator (HRM) to ~0.8 meV linewidth centered at the nuclear resonance energy (~14.4 keV)85. A 2×2 avalanche photodiode (APD) array detector was used to detect the delayed 57Fe nuclear fluorescence and Fe K fluorescence (from internal conversion) following the nuclear resonance excitation.
125Te NRVS
125Te NRVS measurements were conducted at the Dynamics beamline P01 at PETRA III (DESY, Hamburg)86 with a ring current of 100 mA in the 40 bunch mode, with a 192 ns separation between bunches. The X-ray beam was monochromatized to the 125Te nuclear transition energy (35.5 keV) using a sapphire backscattering monochromator54 with an energy resolution of 0.9 meV. The sample was placed in a closed-cycle cryostat and cooled to 22 K, with the temperature estimated via detailed balance analysis using Bose-Einstein statistics. This low temperature was selected to minimize multiphonon contributions, which are significant for 125Te at room temperature. The nuclear resonance signal, contributing to the NRVS spectra, was separated in time from electronic X-ray fluorescence and scattering, and measured using a stack of two Si APD detectors (10 mm×10 mm), positioned close to the sample and detecting mainly the 27.4 keV internally converted nuclear fluorescence following the nuclear resonance excitation.
DFT calculations
All calculations including geometry optimizations and analytical frequencies calculations were performed using ORCA version 5.0487,88,89. Experimentally obtained crystal structures of all studied complexes were used as starting points for geometry optimizations. Structures of all complexes were optimized without counter ion. Calculations were performed utilizing bp86 functional90,91, along with atom-pairwise dispersion correction with the Becke-Johnson damping scheme (D3BJ)92,93. For the inclusion of relativistic effects zeroth-order regular approximation (ZORA)94,95 was used along with relativistically contracted def2 Ahlrichs basis set96,97. Used basis sets included triple ζ ZORA-def2-tzvp basis set for Fe, N, S, Se atoms, double ζ ZORA-def2-svp basis set for C, H atoms and old-ZORA-TZVP basis set for Te atoms. The resolution of identity approximation for Coulomb integrals (RI)98 was used to speed up calculations. To account for solvation effects, the conductor-like polarizable continuum model (CPCM)99 with THF solvent was used. Calculation of antiferromagnetic ground states of studied complexes was achieved starting from ferromagnetic solution and using spin flip feature to produce broken symmetry (BS) solution.
DFT calculated NRVS spectra were represented as PVDOS, which is defined as100,101:
where \({e}_{j\alpha }^{2}\) represents mode composition factor of atom j in normal mode α, while \(L(\bar{\nu }-{\bar{\nu }}_{\alpha })\) represents a line shape function. Normal mode composition factors represent a fraction of kinetic energy associated with displacement, \({r}_{j\alpha }\), of nuclei j with mass of mj, in normal mode α100,102,103:
Calculated mode composition factors were broadened by convolution with a Lorentzian function, with 15 cm−1 full width at half maximum (fwhm), to account for the resolution of NRVS experiments. The DFT calculated NRVS spectra were obtained by summing all broadened mode composition factors across all normal modes.
Normal mode composition factors were additionally analyzed as their projections along axes, where axes were defined such that x axis was along Fe-Fe bond, [Fe2Q2]+/2+ core was in xy plane and z axis was perpendicular to [Fe2Q2]+/2+ core. In that way normal modes involving displacements of specific nuclei along x and y axes can be attributed to in-plane vibrational modes of those nuclei, while normal modes involving displacements of specific nuclei along z axis are attributed to out-of-plane vibrations. Spectra displaying normal mode composition factors along different axes are obtained by broadening of calculated normal modes along axes by convolution with a Lorentzian function, with 15 cm−1 fwhm, and summing all broadened mode composition factors across all normal modes.
For KED analysis, in terms of relative displacements of nuclear pairs, displacements of both nuclei were projected on the bond between nuclei of interest (4Fe-Q, 4Fe-N, 1 Fe-Fe, and 1 Te-Te bond), and mode composition factors were calculated by summing contributions of both nuclei. To focus on the normal modes with significant displacements along the bond between nuclei of interest, only modes in which the angle between the bond and nuclear displacements is less than 30° are considered. For creating KED spectra, normal mode composition factors were broadened by convolution with a Lorentzian function, with 15 cm−1 FWHM and summed over all normal modes.
Chemcraft software104 was used for visualization of individual normal modes as well as for comparison of experimental and optimized structures of studied complexes.
Data availability
The data that support the conclusions of this study are either presented in the paper or in its Supplementary Information. Source data are provided with this paper, and all data are available from the corresponding author upon request. Source data are provided with this paper.
References
Beinert, H., Holm, R. H. & Munck, E. Iron-sulfur clusters: nature’s modular, multipurpose structures. Science 277, 653–659 (1997).
Solomon, E. I., Xie, X. J. & Dey, A. Mixed valent sites in biological electron transfer. Chem. Soc. Rev. 37, 623–638 (2008).
Albers, A. et al. The complete characterization of a reduced biomimetic [2Fe-2S] cluster. Angew. Chem. Int. Edit. 50, 9191–9194 (2011).
Saouma, C. T., Kaminsky, W. & Mayer, J. M. Protonation and concerted proton-electron transfer reactivity of a bis-benzimidazolate ligated [2Fe-2S] model for rieske clusters. J. Am. Chem. Soc. 134, 7293–7296 (2012).
Albers, A., Bayer, T., Demeshko, S., Dechert, S. & Meyer, F. A Functional model for the Rieske center: full characterization of a biomimetic N-Ligated [2Fe-2S] cluster in different protonation states. Chem. Eur. J. 19, 10101–10106 (2013).
Albers, A. et al. Fast proton-coupled electron transfer observed for a high-fidelity structural and functional [2Fe-2S] Rieske model. J. Am. Chem. Soc. 136, 3946–3954 (2014).
Reesbeck, M. E. et al. Oxidized and reduced [2Fe-2S] clusters from an iron(I) synthon. J. Biol. Inorg. Chem. 20, 875–883 (2015).
Van Stappen, C. et al. The spectroscopy of nitrogenases. Chem. Rev. 120, 5005–5081 (2020).
Spatzal, T. et al. Evidence for interstitial carbon in nitrogenase FeMo cofactor. Science 334, 940–940 (2011).
Lancaster, K. M. et al. X-ray emission spectroscopy evidences a central carbon in the nitrogenase iron-molybdenum cofactor. Science 334, 974–977 (2011).
Carney, M. J., Papaefthymiou, G. C., Spartalian, K., Frankel, R. B. & Holm, R. H. Ground spin state variability in [Fe4S4(SR)4]3− - synthetic analogs of the reduced clusters in ferredoxins and other iron sulfur proteins - cases of extreme sensitivity of electronic state and structure to extrinsic factors. J. Am. Chem. Soc. 110, 6084–6095 (1988).
Spatzal, T., Perez, K. A., Howard, J. B. & Rees, D. C. Catalysis-dependent selenium incorporation and migration in the nitrogenase active site iron-molybdenum cofactor. eLife 4, e11620 (2015).
Henthorn, J. T. et al. Localized electronic structure of nitrogenase FeMoco revealed by selenium K-edge high resolution X-ray absorption spectroscopy. J. Am. Chem. Soc. 141, 13676–13688 (2019).
Henthorn, J. T. & DeBeer, S. Selenium valence-to-core X-ray emission spectroscopy and Kβ HERFD X-ray absorption spectroscopy as complementary probes of chemical and electronic structure. Inorg. Chem. 61, 2760–2767 (2022).
Heidinger, L. et al. Analysis of early intermediate states of the nitrogenase reaction by regularization of EPR spectra. Nat. Commun. 15, 4041 (2024).
Wittkamp, F. et al. Insights from 125Te and 57Fe nuclear resonance vibrational spectroscopy: a [4Fe–4Te] cluster from two points of view. Chem. Sci. 10, 7535–7541 (2019).
Heine, T. Transition metal chalcogenides: ultrathin inorganic materials with tunable electronic properties. Acc. Chem. Res. 48, 65–72 (2015).
Hoover, G. C. & Seferos, D. S. Photoactivity and optical applications of organic materials containing selenium and tellurium. Chem. Sci. 10, 9182–9188 (2019).
He, X. M. & Baumgartner, T. Conjugated main-group polymers for optoelectronics. RSC Adv. 3, 11334–11350 (2013).
Mao, S. Z. et al. Semisynthetic tellurosubtilisin with glutathione peroxidase activity. J. Am. Chem. Soc. 127, 11588–11589 (2005).
Liu, X. M. et al. Incorporation of tellurocysteine into glutathione transferase generates high glutathione peroxidase efficiency. Angew. Chem. Int. Ed. 48, 2020–2023 (2009).
Kertess, L. et al. Chalcogenide substitution in the [2Fe] cluster of [FeFe]-hydrogenases conserves high enzymatic activity. Dalton Trans. 46, 16947–16958 (2017).
Henthorn, J. T., Cutsail, G. E., Weyhermüller, T. & DeBeer, S. Stabilization of intermediate spin states in mixed-valent diiron dichalcogenide complexes. Nat. Chem. 14, 328–333 (2022).
Gamelin, D. R., Bominaar, E. L., Kirk, M. L., Wieghardt, K. & Solomon, E. I. Excited-state contributions to ground-state properties of mixed-valence dimers: spectral and electronic-structural studies of [Fe2(OH)3(tmtacn)2]2+ related to the [Fe2S2]+ active sites of plant-type ferredoxins. J. Am. Chem. Soc. 118, 8085–8097 (1996).
Saal, C. et al. Magnetic investigations on a valence-delocalized dinuclear Fe(II)-Fe(III) complex. Ber. Bunsenges. Phys. Chem. 100, 2086–2090 (1996).
Ding, X. Q. et al. Exchange interactions, charge delocalization, and spin relaxation in a mixed-valence diiron complex studied by Mossbauer-spectroscopy. J. Chem. Phys. 99, 6421–6428 (1993).
Ding, X. Q. et al. Mossbauer and electron-paramagnetic resonance study of the double-exchange and heisenberg-exchange interactions in a novel binuclear Fe(II/III) delocalized-valence compound. J. Chem. Phys. 92, 178–186 (1990).
Drueke, S. et al. The novel mixed-valence, exchange-coupled, class III dimer [L2Fe2(µ-OH)3]2+(L =N,N’,N”-trimethyl-1,4,7-triazacyclononane). J. Chem. Soc. Chem. Commun. 59–62 (1989).
Crouse, B. R., Meyer, J. & Johnson, M. K. Spectroscopic evidence for a reduced Fe2S2 cluster with a S = 9/2 ground state in mutant forms of Clostridium pasteurianum 2Fe ferredoxin. J. Am. Chem. Soc. 117, 9612–9613 (1995).
Achim, C., Golinelli, M. P., Bominaar, E. L., Meyer, J. & Munck, E. Mössbauer study of Cys56Ser mutant 2Fe ferredoxin from Clostridium pasteurianum: evidence for double exchange in an [Fe2S2]+ cluster. J. Am. Chem. Soc. 118, 8168–8169 (1996).
Achim, C., Bominaar, E. L., Meyer, J., Peterson, J. & Münck, E. Observation and interpretation of temperature-dependent valence delocalization in the [2Fe-2S] cluster of a ferredoxin from Clostridium pasteurianum. J. Am. Chem. Soc. 121, 3704–3714 (1999).
Subramanian, S. et al. Spectroscopic and redox studies of valence-delocalized [Fe2S2]+ centers in thioredoxin-like ferredoxins. J. Am. Chem. Soc. 137, 4567–4580 (2015).
Johnson, M. K., Duin, E. C., Crouse, B. R., Golinelli, M. P. & Meyer, J. In Spectroscopic methods in bioinorganic chemistry Vol. 692 ACS Symposium Series Ch. 16, 286–301 (American Chemical Society, 1998).
Shores, M. P. & Long, J. R. Tetracyanide-bridged divanadium complexes: redox switching between strong antiferromagnetic and strong ferromagnetic coupling. J. Am. Chem. Soc. 124, 3512–3513 (2002).
Bechlars, B. et al. High-spin ground states via electron delocalization in mixed-valence imidazolate-bridged divanadium complexes. Nat. Chem. 2, 362–368 (2010).
Piepho, S. B. Vibronic coupling model for the calculation of mixed-valence line-shapes - a new look at the creutz taube ion. J. Am. Chem. Soc. 112, 4197–4206 (1990).
Piepho, S. B., Krausz, E. R. & Schatz, P. N. Vibronic coupling model for calculation of mixed-valence absorption profiles. J. Am. Chem. Soc. 100, 2996–3005 (1978).
Girerd, J. J. Electron-transfer between magnetic ions in mixed-valence binuclear systems. J. Chem. Phys. 79, 1766–1775 (1983).
Blondin, G. & Girerd, J. J. Interplay of electron exchange and electron-transfer in metal polynuclear complexes in proteins or chemical-models. Chem. Rev. 90, 1359–1376 (1990).
Mouesca, J. M. & Lamotte, B. Iron-sulfur clusters and their electronic and magnetic properties. Coord. Chem. Rev. 178, 1573–1614 (1998).
Robin, M. B. & Day, P. In Advances in Inorganic Chemistry and Radiochemistry Vol. 10 (eds H. J. Emeléus & A. G. Sharpe) 247–422 (Academic Press, 1968).
Noodleman, L., Peng, C. Y., Case, D. A. & Mouesca, J. M. Orbital interactions, electron delocalization and spin coupling in iron-sulfur clusters. Coord. Chem. Rev. 144, 199–244 (1995).
Gaudette, A. I. et al. Electron hopping through double-exchange coupling in a mixed-valence diiminobenzoquinone-bridged Fe2 complex. J. Am. Chem. Soc. 137, 12617–12626 (2015).
Scheidt, W. R., Durbin, S. M. & Sage, J. T. Nuclear resonance vibrational spectroscopy - NRVS. J. Inorg. Biochem. 99, 60–71 (2005).
Scheidt, W. R., Li, J. F. & Sage, J. T. What can be learned from nuclear resonance vibrational spectroscopy: vibrational dynamics and hemes. Chem. Rev. 117, 12532–12563 (2017).
Cramer, S. P. X-ray spectroscopy with synchrotron radiation: fundamentals and applications. (Springer International Publishing, 2020).
Cramer, S. P., Xiao, Y. M., Wang, H. X., Guo, Y. S. & Smith, M. C. Nuclear resonance vibrational spectroscopy (NRVS) of Fe-S model compounds, Fe-S proteins, and nitrogenase. Hyperfine Interact. 170, 47–54 (2006).
Guo, Y. S. et al. Characterization of the Fe site in iron-sulfur cluster-free hydrogenase (Hmd) and of a model compound via nuclear resonance vibrational spectroscopy (NRVS). Inorg. Chem. 47, 3969–3977 (2008).
Wang, H., Alp, E. E., Yoda, Y. & Cramer, S. P. In Metalloproteins: Methods and Protocols (eds. J. C. Fontecilla-Camps & Y. Nicolet) 125–137 (Humana Press, 2014).
Ogata, H. et al. Hydride bridge in [NiFe]-hydrogenase observed by nuclear resonance vibrational spectroscopy. Nat. Commun. 6, 7890 (2015).
Gee, L. B., Wang, H. X. & Cramer, S. P. In: Fe-S Cluster Enzymes Part B Vol. 599 (ed S. David Sheila) 409–425 (Academic Press, 2018).
Shvyd’ko, Y. V. & Gerdau, E. Backscattering mirrors for X-rays and Mossbauer radiation. Hyperfine Interact. 123, 741–776 (1999).
Sergueev, I. et al. Milli-electronvolt monochromatization of hard X-rays with a sapphire backscattering monochromator. J. Synchrotron Radiat. 18, 802–810 (2011).
Alexeev, P. et al. The sapphire backscattering monochromator at the Dynamics beamline P01 of PETRA III. Hyperfine Interact. 59, 237 (2016).
Wille, H. C. et al. Nuclear resonant forward scattering of synchrotron radiation from Sb at 37.13 keV. Europhys. Lett. 74, 170–176 (2006).
Sergueev, I. et al. Nuclear forward scattering for high energy mossbauer transitions. Phys. Rev. Lett. 99, 097601 (2007).
Wille, H. C. et al. Nuclear forward and inelastic spectroscopy on 125Te and Sb2125Te3. Epl 91, 62001 (2010).
Bessas, D. et al. Lattice dynamics in Bi2Te3 and Sb2Te3: Te and Sb density of phonon states. Phys. Rev. B 86, 224301 (2012).
Klobes, B., Desmedt, A., Sergueev, I., Schmalzl, K. & Hermann, R. P. 129Xe nuclear resonance scattering on solid Xe and 129Xe clathrate hydrate. Epl 103, 2192–2201 (2013).
Stepanenko, I. et al. 187Os nuclear resonance scattering to explore hyperfine interactions and lattice dynamics for biological applications. Sci. Adv. 11, eads3406 (2025).
Han, S., Czernuszewicz, R. S. & Spiro, T. G. Vibrational-spectra and normal mode analysis for [2Fe-2S] protein analogs using S-34, Fe-54, and H-2 substitution - coupling of Fe-S stretching and S-C-C bending modes. J. Am. Chem. Soc. 111, 3496–3504 (1989).
Smith, M. C. et al. Normal-Mode Analysis of FeCl4− and Fe2S2Cl42− via Vibrational Mössbauer, Resonance Raman, and FT-IR Spectroscopies. Inorg. Chem. 44, 5562–5570 (2005).
Xiao, Y. M. et al. Dynamics of rhodobacter capsulatus [2Fe-2S] ferredoxin VI and aquifex aeolicus ferredoxin 5 via nuclear resonance vibrational spectroscopy (NRVS) and resonance raman spectroscopy. Biochemistry 47, 6612–6627 (2008).
Serrano, P. N. et al. Nitrosylation of nitric-oxide-sensing regulatory proteins containing [4Fe-4S] clusters gives rise to multiple iron-nitrosyl complexes. Angew. Chem. Int. Edit. 55, 14575–14579 (2016).
Gee, L. B. et al. NRVS and DFT of MitoNEET: understanding the special vibrational structure of a [2Fe-2S] cluster with (Cys)3(His)1 ligation. Biochemistry 60, 2419–2424 (2021).
Mitra, D. et al. Characterization of [4Fe-4S] cluster vibrations and structure in nitrogenase fe protein at three oxidation levels via combined NRVS, EXAFS, and DFT analyses. J. Am. Chem. Soc. 135, 2530–2543 (2013).
Xiao, Y. M. et al. Dynamics of an [Fe4S4(SPh)4]2− cluster explored via IR, Raman, and nuclear resonance vibrational spectroscopy (NRVS)-analysis using 36S substitution, DFT calculations, and empirical force fields. Dalton Trans. 2006, 2192–2201 (2006).
Mitra, D. et al. Dynamics of the [4Fe-4S] Cluster in Pyrococcus furiosus D14C ferredoxin via nuclear resonance vibrational and resonance raman spectroscopies, force field simulations, and density functional theory calculations. Biochemistry 50, 5220–5235 (2011).
Lauterbach, L. et al. Characterization of the [3Fe–4S]0/1+ cluster from the D14C variant of Pyrococcus furiosus ferredoxin via combined NRVS and DFT analyses. Dalton Trans. 45, 7215–7219 (2016).
Sharma, S., Sivalingam, K., Neese, F. & Chan, G. K. L. Low-energy spectrum of iron-sulfur clusters directly from many-particle quantum mechanics. Nat. Chem. 6, 927–933 (2014).
Atanasov, M., Spiller, N. & Neese, F. Magnetic exchange and valence delocalization in a mixed valence [Fe2+Fe3+Te2]+ complex: insights from theory and interpretations of magnetic and spectroscopic data. Phys. Chem. Chem. Phys. 24, 20760–20775 (2022).
Castillo, R. G. et al. Probing physical oxidation state by resonant X-ray emission spectroscopy: applications to iron model complexes and nitrogenase. Angew. Chem. Int. Ed. 60, 10112–10121 (2021).
Moroder, L. Isosteric replacement of sulfur with other chalcogens in peptides and proteins. J. Pept. Sci. 11, 187–214 (2005).
Budisa, N. et al. Bioincorporation of telluromethionine into proteins: a promising new approach for X-ray structure analysis of proteins. J. Mol. Biol. 270, 616–623 (1997).
Bassan, J. et al. TePhe, a tellurium-containing phenylalanine mimic, allows monitoring of protein synthesis in vivo with mass cytometry. Proc. Natl Acad. Sci. USA 116, 8155–8160 (2019).
Vurgun, N. & Nitz, M. Validation of l-tellurienylalanine as a phenylalanine isostere. ChemBioChem 21, 1136–1139 (2020).
García-Rodríguez, R. & Liu, H. T. Mechanistic study of the synthesis of CdSe nanocrystals: release of selenium. J. Am. Chem. Soc. 134, 1400–1403 (2012).
McDonough, J. E. et al. Thermodynamic, kinetic, and computational study of heavier chalcogen (S, Se, and Te) terminal multiple bonds to molybdenum, carbon, and phosphorus. Inorg. Chem. 47, 2133–2141 (2008).
Grudzien, K., Basak, T., Barbasiewicz, M., Wojciechowski, T. M. & Fedorynski, M. Synthesis, properties and application of electronically-tuned tetraarylarsonium salts as phase transfer catalysts (PTC) for the synthesis of gem-difluorocyclopropanes. J. Fluor. Chem. 197, 106–110 (2017).
Wang, J. J., Kryatova, O. P., Rybak-Akimova, E. V. & Holm, R. H. Comparative kinetics and mechanism of oxygen and sulfur atom transfer reactions mediated by bis(dithiolene) complexes of molybdenum and tungsten. Inorg. Chem. 43, 8092–8101 (2004).
Transue, W. J. et al. Anthracene as a launchpad for a phosphinidene sulfide and for generation of a phosphorus-sulfur material having the composition P2S, a vulcanized red phosphorus that is yellow. J. Am. Chem. Soc. 141, 431–440 (2019).
Feldman, J. et al. Electrophilic metal precursors and a beta-diimine ligand for nickel(II)- and palladium(II)-catalyzed ethylene polymerizations. O. rganometallics 16, 1514–1516 (1997).
Berto, T. C. et al. Structural and electronic characterization of non-heme Fe(II)–nitrosyls as biomimetic models of the FeB center of bacterial nitric oxide reductase. J. Am. Chem. Soc. 133, 16714–16717 (2011).
Spitzer, F. et al. Influence of the nacnac ligand in Iron(I)-mediated P4 transformations. Angew. Chem. Int. Ed. 55, 4340–4344 (2016).
Yoda, Y., Okada, K., Wang, H. X., Cramer, S. P. & Seto, M. High-resolution monochromator for iron nuclear resonance vibrational spectroscopy of biological samples. Jpn. J. Appl. Phys. 55, 122401 (2016).
Wille, H. C., Franz, H., Röhlsberger, R., Caliebe, W. A. & Dill, F. U. Nuclear resonant scattering at PETRA III: brillant opportunities for nano - and extreme condition science. J. Phys.: Conf. Ser. 217 (2010).
Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2, 73–78 (2012).
Neese, F., Wennmohs, F., Becker, U. & Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 152, 224108 (2020).
Neese, F. Software update: the ORCA program system-version 5.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 12, e1606 (2022).
Perdew, J. P. Density-functional approximation for the correlation-energy of the inhomogeneous electron-gas. Phys. Rev. B 33, 8822–8824 (1986).
Becke, A. D. Density-functional exchange-energy approximation with correct asymptotic-behavior. Phys. Rev. A 38, 3098–3100 (1988).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Vanlenthe, E., Baerends, E. J. & Snijders, J. G. Relativistic regular 2-component Hamiltonians. J. Chem. Phys. 99, 4597–4610 (1993).
van Wüllen, C. Molecular density functional calculations in the regular relativistic approximation: method, application to coinage metal diatomics, hydrides, fluorides and chlorides, and comparison with first-order relativistic calculations. J. Chem. Phys. 109, 392–399 (1998).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Pantazis, D. A., Chen, X. Y., Landis, C. R. & Neese, F. All-electron scalar relativistic basis sets for third-row transition metal atoms. J. Chem. Theory Comput. 4, 908–919 (2008).
Weigend, F. A fully direct RI-HF algorithm: Implementation, optimised auxiliary basis sets, demonstration of accuracy and efficiency. Phys. Chem. Chem. Phys. 4, 4285–4291 (2002).
Cossi, M., Rega, N., Scalmani, G. & Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 24, 669–681 (2003).
Leu, B. M. et al. Quantitative vibrational dynamics of iron in nitrosyl porphyrins. J. Am. Chem. Soc. 126, 4211–4227 (2004).
Sage, J. T. et al. Nuclear resonance vibrational spectroscopy of a protein active-site mimic. J. Phys. Condens. Matter 13, 7707–7722 (2001).
Chumakov, A. I., Rüffer, R., Leupold, O. & Sergueev, I. Insight to dynamics of molecules with nuclear inelastic scattering. Struct. Chem. 14, 109–119 (2003).
Gee, L. B., Wang, H. & Cramer, S. P. In Bioorganometallic Chemistry (eds W. Wolfgang & A. Ulf-Peter) 353–394 (De Gruyter, 2020).
Zhurko, G. A. Chemcraft - graphical program for visualization of quantum chemistry computations. Ivanovo, Russia (2005).
Acknowledgements
The authors thank the Max Planck Society (GEC) and the National Institutes of Health (GM65440 to SPC). We acknowledge DESY (Hamburg, Germany), a member of the Helmholtz Association HGF, for the provision of experimental facilities. 125Te NRVS synchrotron radiation experiments were carried out at DESY at beamline P01. Beamtime was allocated for proposal I-20220762. 57Fe NRVS synchrotron radiation experiments were performed at BL19LXU in SPring-8 with the approval of RIKEN (Proposal No. 20230009). The authors thank the Max Planck Computing and Data Facility for allocation of high-performance computing time (on Raven).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
A.R. contributed to data analysis and the writing of the original draft. J.T.H. contributed to project conceptualization, sample synthesis, data acquisition, and manuscript editing. H.W., D.P., I.S., N.N., and Y.Y. each contributed to data collection and manuscript editing. S.P.C. contributed to conceptualization, manuscript editing, acquisition of resources, and funding acquisition. G.E.C. supervised and contributed to conceptualization, data analysis, writing of the original draft, acquisition of resources, and funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Dimitrios Pantazis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Radović, A., Henthorn, J.T., Wang, H. et al. 125Te and 57Fe nuclear resonance vibrational spectroscopic characterization of intermediate spin state mixed-valent dimers. Nat Commun 16, 6843 (2025). https://doi.org/10.1038/s41467-025-62118-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-62118-w