Abstract
Diffuse interstitial fibrosis is associated with adverse outcomes in hypertensive heart disease and may be reversible. Sacubitril/valsartan could offer greater anti-fibrotic effects than valsartan alone. In the REVERSE-LVH phase 2 open-labelled trial (clinicaltrials.gov NCT: 03553810; funded by the National Medical Research Council of Singapore), 78 patients with essential hypertension and left ventricular hypertrophy (LVH) were randomized 1:1 to sacubitril/valsartan or valsartan for 52 weeks. Primary endpoint was a change in interstitial volume, assessed using cardiovascular magnetic resonance. Despite similar 24-hour systolic blood pressure at 52 weeks (125 ± 11 vs. 126 ± 11 mmHg; P = 0.762), sacubitril/valsartan resulted in a greater absolute reduction in interstitial volume compared to valsartan (−5.2 ± 5.4 vs. −2.5 ± 3.1 mL; P = 0.006). Secondary endpoints showed significant differences favoring sacubitril/valsartan in LV mass, left atrial volume, estimated LV filling pressure, and improved cardiac circulating biomarkers (N-terminal pro-B-type natriuretic peptide and high-sensitivity troponin T). Other markers of cardiac volumes, function and mechanics were similar between the two treatment arms. Here we show the potential myocardial benefits of sacubitril/valsartan beyond blood pressure control, though larger studies are needed to confirm their clinical relevance.
Similar content being viewed by others
Introduction
About 30% of the burden related to hypertension occurred in individuals with well-controlled blood pressures (BP)1. The updated definition of heart failure (HF) stages had identified more asymptomatic individuals in a pre-clinical stage2. This suggests that despite substantial benefits associated with BP lowering, there remains opportunities to improve risk stratification and develop therapeutic options beyond managing peripheral BP to slow/prevent the development of symptomatic HF.
In hypertensive heart disease (HHD), left ventricular hypertrophy (LVH) occurs in response to hemodynamic and neuro-hormonal stresses triggered by prolonged exposure to elevated BP3. Its pathophysiology is accompanied by an expansion in extracellular volume (ECV) due to accumulation of extracellular matrix components and collagen deposition throughout the myocardium – diffuse interstitial fibrosis. This type of fibrosis typically develops gradually and is initially reversible4. Myocardial fibrosis significantly contributes to cardiac dysfunction, arrhythmias, and reduced coronary perfusion, ultimately leading to HF and adverse cardiovascular outcomes4,5. Therefore in addition to peripheral BP, diffuse interstitial fibrosis is a potential marker for enhancing risk stratification and directing targeted therapies to prevent the progression of hypertensive LVH to advanced HF stages.
Angiotensin receptor–neprilysin inhibitor (ARNI) represents a novel drug class that inhibits the renin-angiotensin-aldosterone system (RAAS) and enhances natriuretic peptides. This dual agent combines a neprilysin inhibitor (sacubitril) and an angiotensin receptor blocker (ARB; valsartan). Sacubitril/valsartan has shown superior benefits over conventional ARB or angiotensin-converting enzyme inhibitors (ACEI) monotherapy in lowering BP and improving outcomes in those with HF and reduced LV ejection fraction (HFrEF)6,7. Pre-clinical studies of mouse models have demonstrated that sacubitril/valsartan is effective in reducing myocardial fibrosis and improving cardiac function by attenuating angiotensin II and transforming growth factor b1 fibrotic pathways8,9.
Using cardiovascular magnetic resonance (CMR) to quantify interstitial volume as a measure of diffuse interstitial fibrosis non-invasively10, the aim of the study was to compare sacubitril/valsartan versus valsartan in reducing diffuse interstitial fibrosis in patients with hypertensive LVH. The hypothesis was that 52 weeks of sacubitril/valsartan therapy would result in greater regression of diffuse interstitial fibrosis compared to valsartan, independent of BP control.
Results
Study participants
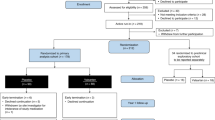
A total of 80 patients were recruited, and 78 individuals were successfully randomized to sacubitril/valsartan (n = 39) and valsartan (n = 39) (Fig. 1). The mean age at baseline was 58 ± 11 years, and 32 (41%) were males. The baseline 24 h mean SBP and DBP were 137 ± 14 and 81 ± 11 mmHg, respectively. The baseline clinical, renal function and CMR characteristics were well-balanced between the two treatment groups (Table 1). There was no change in body weight at the start and end of study.
A total of 78 participants were randomized in the trial. One patient in the sacubitril/valsartan group withdrew at week 4 due to concerns of medication side effects. One patient in the valsartan group withdrew at week 26 due to difficulties with trial commitment. This participant had final visit investigations (including cardiovascular magnetic resonance) performed at time of withdrawal, according to study protocol. Primary and secondary endpoints were analyzed as intention-to-treat.
The median dose prescribed in the sacubitril/valsartan group was 200 [100, 200] mg twice a day and 160 [160, 320] mg once a day in the valsartan group, respectively. A total of 50 participants required additional anti-hypertensive medications (sacubitril/valsartan group, n = 26 and valsartan group, n = 24; P = 0.571; Supplementary Table 2). The overall treatment adherence rate achieved in the trial was 97% (sacubitril/valsartan group, 97%; valsartan group, 96%).
Primary trial endpoint
The baseline interstitial volume in the sacubitril/valsartan group was 29.2 ± 12.8 mL compared to 28.0 ± 11.3 mL in the valsartan group. After 52 weeks, sacubitril/valsartan resulted in greater regression in diffuse interstitial fibrosis compared to valsartan alone (−5.2 ± 5.4 versus −2.5 ± 3.1 mL, P = 0.006), with an absolute between-group difference of −2.8 (95% CI: −4.8 to −0.8) mL. Sacubitril/valsartan resulted in an 18% reduction in interstitial volume from baseline, compared to an 8.9% reduction with valsartan. The reduction in diffuse interstitial fibrosis remained greater in the sacubitril/valsartan group compared to valsartan alone after accounting for baseline interstitial volume, prior ACEI or ARB use, and baseline 24 h SBP (Fig. 2).
The primary endpoint in 78 participants. The effect estimates were presented in mean and 95% confidence intervals, with corresponding P values from one-way ANCOVA testing before and after adjustment for baseline covariates. ACEI angiotensin-converting enzyme inhibitor, ARB angiotensin receptor blocker.
Exploratory subgroup analysis suggested treatment heterogeneity in favor of sacubitril/valsartan in individuals with 24 h median SBP > 135 mmHg, with an absolute between-group difference of −5.2 (95% CI: −8.0 to −2.5) mL compared to −0.5 (95% CI: −3.2 to 2.2) mL in those with 24 h SBP ≤135 mmHg (P = 0.016 for interaction). There was no significant treatment heterogeneity in the other subgroups stratified by age, sex, LV ejection fraction, baseline interstitial volume, prior ACEI or ARB use and body mass index (Supplementary Table 3).
Secondary trial endpoints
Compared to valsartan alone, sacubitril/valsartan resulted in greater reduction in LV mass (−21.9 ± 25.4 versus −11.0 ± 9.0 g, P = 0.014) and myocyte volume (−16.0 ± 19.5 versus −8.0 ± 7.1 mL, P = 0.019). Sacubitril/valsartan also led to a greater decrease in LA volume (−18.3 ± 16.6 versus −8.6 ± 13.6 mL, P = 0.006) and LV filling pressures (−1.8 ± 1.5 versus −0.9 ± 1.1 mmHg, P = 0.003) compared to valsartan, suggestive of improved diastolic function.
There were no significant differences in changes in cardiac volumes, systolic function, and other measures of myocardial mechanics between the treatment groups. Although sacubitril/valsartan was associated with a statistically significant improvement in global longitudinal strain (GLS) compared to valsartan (−0.9 ± 2.3 versus 0.2 ± 1.9, P = 0.027), the effect size was small and the isolated improvement in GLS was not associated with improvement in the other strain components (Table 2). Similar findings were observed when relevant CMR metrics were indexed to body surface area (Supplementary Table 4). Compared to valsartan, sacubitril/valsartan was associated with a significant reduction from baseline to 52 weeks in NT-proBNP (ETR = 0.72; 95% CI: 0.54–0.91; P = 0.006) and hsTnT (ETR = 0.83; 95% CI: 0.69–0.99; P = 0.005).
Treatment effects on blood pressure control
Participants in both groups achieved similarly lowered 24 h mean BP at all timepoints assessed in the trial (Fig. 3). At the end of 52 weeks, the 24 h mean SBP was 125 ± 11 mmHg with sacubitril/valsartan versus 126 ± 11 mmHg with valsartan (P = 0.762 for comparison). This corresponded to a decrease of 13.3 ± 13.1 and 10.6 ± 13.4 mmHg from baseline in the sacubitril/valsartan and valsartan groups, respectively. Treatment difference between the two groups was not significant (−2.7 [95% CI: −8.7 to 3.3] mmHg, P = 0.379).
Similarly, the 24 h mean DBP at the end of study was 75 ± 9 mmHg with sacubitril/valsartan versus 75 ± 12 mmHg with valsartan (P = 0.943 for comparison), corresponding to a decrease of 7.1 ± 7.1 and 5.6 ± 9.6 mmHg from baseline in the two respective treatment groups. The treatment difference in 24 h DBP reduction between the two groups was also not significant (−1.5 [95% CI: −5.4 to 2.3] mmHg, P = 0.426).
Adverse events
Treatment with sacubitril/valsartan and valsartan were very well-tolerated in this trial. One patient developed nocturnal cough with sacubitril/valsartan three months after randomization. Symptoms improved upon dose reduction, and the participant continued with the trial. Renal function remained stable throughout the trial (Table 3). There were no major adverse cardiovascular events such as hospitalizations for HF, myocardial infarction, strokes and deaths reported during the trial.
Discussion
The main finding of REVERSE-LVH was that sacubitril/valsartan resulted in a reduction in diffuse interstitial fibrosis in hypertensive patients with LVH following 52 weeks of treatment compared to valsartan alone. This was accompanied by a favorable change in LV mass, circulating cardiac biomarkers and diastolic function (LA size and estimated LV filling pressure). There was no significant difference in systolic function improvement between the two treatment groups.
The use of CMR in REVERSE-LVH has allowed reliable quantification of fibrosis without invasive endomyocardial biopsies, a procedure that was required in previous studies on anti-fibrotic therapies11,12,13,14. Novel CMR mapping techniques have dramatically advanced tissue characterization non-invasively, allowing us to investigate novel anti-fibrotic therapies in diverse populations.
ECV fraction represents the ratio of interstitium to the total myocardial volume. Although it is widely used to measure diffuse interstitial fibrosis, its utility may be limited where treatments affect both myocyte and interstitial compartments. In such cases, therapies that reduce both compartments may lead to an unchanged or even increased ECV fraction. For instance, in REVERSE-LVH, despite reductions in interstitial and myocyte volumes, both treatment groups showed a slight increase in ECV fraction, likely due to a greater reduction in myocyte volume relative to interstitial volume. Instead, deeper mechanistic insights can be accomplished by separately assessing the interstitial and myocyte compartments, particularly for novel treatments or interventions with potential effects on both compartments.
Myocardial fibrosis is a hallmark of HF, and not all BP medications have anti-fibrotic properties. Among conventional anti-hypertensive therapies, ACEIs or ARBs that target the RAAS have shown the most promise in regressing biopsy-confirmed myocardial fibrosis in HHD, independent of BP lowering effects. Similarly, mineralocorticoid receptor antagonists (MRAs) like spironolactone and eplerenone reduce serum markers of collagen turnover and improve diastolic function on echocardiography15,16,17.
Sacubitril/valsartan, a first-in-class ARNI used primarily for HFrEF, is also an effective anti-hypertensive medication18. While sacubitril/valsartan is approved for hypertension in some countries, its effectiveness in regressing myocardial fibrosis in patients with hypertension has not been established. Through the REVERSE-LVH trial using CMR to assess interstitial volume, sacubitril/valsartan demonstrated a two-fold greater reduction in diffuse interstitial fibrosis compared to valsartan alone. Both treatment groups achieved similar BP throughout the trial, an important feature to isolate the anti-fibrotic effects of sacubitril/valsartan from any potential influence of BP control. It is also noteworthy that the other classes of anti-hypertensive medications prescribed were similar between the two treatment groups.
Sacubitril/valsartan also led to greater LV mass regression compared to valsartan alone, findings that align with results from a previous trial comparing sacubitril/valsartan and olmesartan19. Overall, these results reinforced the beneficial impact of sacubitril/valsartan on reverse remodeling in patients with HHD.
PARAGON-HF (Prospective Comparison of ARNI with ARB Global Outcomes in HF with Preserved Ejection Fraction) reported that sacubitril/valsartan did not result in significantly lower rates of HF hospitalizations and cardiovascular deaths in patients with HF and preserved ejection fraction (HFpEF) compared to valsartan20. Considering these results, it is important to discuss the relevance of REVERSE-LVH after PARAGON-HF and possible implications for future HFpEF trials.
HFpEF represents a syndrome characterized by phenotypic heterogeneity, underpinned by complex underlying mechanisms, and contributed by various coexisting comorbidities. This heterogeneity is exemplified by the observation that despite similar E/e’ (echocardiographic marker of LV filling pressure), the prevalence of LVH ranges between 14 and 50% in recent HFpEF trials (21% in PARAGON-HF)21. This observation hints at the presence of additional factors contributing to elevated LV filling pressure (such as chronic lung disease, atrial fibrillation and/or chronic kidney disease). Consequently, it is plausible that not all these patients had derived the anticipated benefits from the HF medications being investigated.
To enhance the effectiveness of future HFpEF trials, one key consideration lies in the strategic inclusion of patients with common phenotypes driving the underlying pathophysiology. This approach will enable more precise selection of pharmaceutical therapies tailored at specific mechanistic phenotypes22,23. For instance, pirfenidone, a small-molecule anti-fibrotic agent without hemodynamic effects, reduced myocardial fibrosis in patients with HFpEF24. This reduction in interstitial space without significant changes in myocyte compartment explains for the overall decrease in ECV fraction. In contrast, sacubitril/valsartan reduces both myocardial fibrosis and hypertrophy because of its effects on hemodynamics, natriuretic and RAAS system.
The effectiveness of hypertension treatment is often assessed by the BP targets achieved, with the fundamental goal to minimize end-organ complications such as HF. However, cardiac remodeling in the hypertensive heart is complex and the peripheral BP may not adequately reflect the abnormalities in the myocardium. A moderate correlation was demonstrated between interstitial volume and 24 h SBP (r = 0.36, P < 0.001), worse with office SBP (r = 0.21; P < 0.01)25. This poses a challenge in defining the optimal BP targets in hypertensive patients with LVH, particularly if BP values are adequately controlled.
Because the apparent amelioration of diffuse interstitial fibrosis is associated with improved outcomes, the interstitium is an attractive target for drug repurposing and development26,27. Sacubitril/valsartan resulted in a greater reduction in diffuse interstitial fibrosis compared to valsartan alone after 52 weeks of treatment. The observed association with an improvement in diastolic function and circulating biomarkers was encouraging.
The findings from REVERSE-LVH strengthened the case for considering sacubitril/valsartan in selected individuals with hypertensive LVH, especially those who failed to achieve adequate reduction in diffuse interstitial fibrosis with ACEIs or ARBs. Individuals with 24 h SBP greater than 135 mmHg (median SBP in this study) may have further reduction in diffuse interstitial fibrosis with sacubitril/valsartan but this finding from the post-hoc subgroup analysis was exploratory and should be investigated in future trials.
It was by chance that a higher proportion of female participants (59%) were recruited. Despite the challenges posed by COVID-19 between 2020 and 2021, the trial had very low dropout rates (5%) and exceptionally high treatment adherence rates, surpassing the 80% threshold widely used to define “good adherence”. Both treatment arms achieved similar BP reduction at the end of the trial. These observations highlighted the meticulous conduct of the trial.
The study had some limitations. Two participants (one in each treatment group) were given MRAs, which may confound regression of diffuse interstitial fibrosis. This was inevitable because their BPs were not optimally controlled despite being prescribed the maximum tolerated doses of trial medications and other classes of anti-hypertensives. Although REVERSE-LVH was adequately powered as a phase 2 trial, the effect size observed is small and warrants further investigation in larger studies powered for clinical outcomes. Prior exposure to ACEIs and ARBs in the participants may have introduced confounding, although no significant treatment interactions were observed.
In conclusion, compared to valsartan alone, sacubitril/valsartan appears to exert beneficial myocardial effects beyond blood pressure reduction in patients with hypertensive LVH. However, larger and longer-term studies are necessary to determine the clinical significance of the observed effect sizes.
Methods
Trial design
The Role of ARNI in the Ventricular Remodeling in Hypertensive LVH (REVERSE-LVH) was a prospective, randomized, open-label, blinded endpoint (PROBE) phase 2 trial (ClinicalTrials.gov identifier: NCT03553810). The original design and methods of the trial has been published28 (Supplementary note 1). All study procedures were conducted at the National Heart Centre Singapore (NHCS). This was an investigator-initiated trial. All trial-related expenses, including investigations and medications were funded by the National Medical Research Council. Novartis did not participate in the design of the trial, analysis and interpretation of the data.
REVERSE-LVH was reviewed and approved by the SingHealth Centralized Institutional Review Board (CIRB ref: 2018/2182) and the Health Sciences Authority. All participants provided written informed consent.
Trial participants and recruitment
Participants were recruited between June 2019 and June 2023. These participants were screened and recruited from the REMODEL cohort, an ongoing non-interventional prospective study to examine the role of CMR in patients with hypertension (ClinicalTrials.gov Identifier: NCT02670031). Eligible participants were adults at least 21 years old with essential hypertension and LVH, diagnosed using Asian specific thresholds on CMR29.
Essential hypertension was defined as systolic BP (SBP) ≥140 mmHg and/or diastolic BP (DBP) ≥90 mmHg at time of diagnosis, on at least one medication for BP control. Individuals with known secondary causes of hypertension, inherited cardiomyopathies, atrial fibrillation, and history of cardiovascular complications (such as myocardial infarction, HF and stroke) were excluded. Other exclusion criteria included intolerance to ARBs, contraindications for CMR, stage IV or V chronic renal disease, presence of disease with limited life expectancy (<3 years), pregnancy and/or breast-feeding.
Randomization, treatment and blinding
Study participants were randomized to either sacubitril/valsartan or valsartan in a 1:1 fashion. Randomization was performed using sealed and sequentially numbered envelopes, prepared by the Singapore Clinical Research Institute. Crossover of treatment allocation was not allowed, and the treatment duration was 52 weeks. Treatment allocation was not concealed from patients. Treatment assignment and trial endpoints were concealed from the core-lab staff analyzing the CMR images.
Sacubitril/valsartan was initiated at 50–100 mg twice a day, uptitrated to maximum 200 mg twice a day; valsartan was initiated at 80–160 mg once a day, uptitrated to maximum 320 mg once a day. Medications in both treatment groups were uptitrated as tolerated to achieve a target SBP of <140 mmHg according to contemporary guidelines at the time of study conception30,31. Additional non-RAAS inhibiting anti-hypertensive agents were prescribed where necessary. Participants who were on ACEI or ARB at the time of enrollment underwent a washout period of two weeks before commencing trial medication to eliminate residual RAAS-intervening effects, during which alternative medicines or uptitration of existing concomitant anti-hypertensive agents were prescribed for BP control.
Study medications were dispensed by NHCS pharmacists after each study visit. Treatment adherence was assessed by pill counting by the pharmacists.
Study procedures
Baseline demographic and clinical information were obtained from participants upon randomization. Participants were seen at the clinic with 24 h ambulatory BP at weeks 4, 8, 26 and 52. Renal function tests were performed during these visits as part of safety monitoring. Phone calls were made to the participants at weeks 12 and 38. Additional un-scheduled visits were arranged if deemed necessary by the study team (Supplementary Table 1). If withdrawal of participation occurred after 12 weeks of treatment allocation, final visit procedures (including CMR to assess endpoints) would be performed at the time of withdrawal. At each clinic visit, adverse events related to study treatment were assessed by the study team. If present, they were recorded and reported to the local ethics board and the Health Sciences Authority within the required timeline.
Cardiac volumes, function, myocardial mass, and interstitial volumes were assessed at baseline and after 52 weeks of treatment using standardized imaging CMR protocols on the Siemens Aera 1.5 T scanner (Siemens Healthineers, Erlangen, Germany). Balanced steady-state free precession cine images were acquired in the long-axis 2-, 3- and 4-chamber views; and short axis cines extending from the mitral valve annulus to the apex (acquired voxel size of 1.6 × 1.3 × 8.0 mm3 and 30 phases per cardiac cycle). T1 mapping was performed with the Modified Look-Locker Inversion-recovery sequence, where native T1 maps were acquired using a heartbeat scheme of 5(3)3, and post-contrast T1 maps were acquired 15 min after administration of 0.1 mmol/kg gadobutrol (Gadovist; Bayer Pharma AG, Germany) using a heartbeat scheme of 4(1)3(1)2. Details of the imaging protocol were published in the trial protocol28.
All CMR images were de-identified and analyzed by trained personnel at the National Heart Research Institute Singapore CMR core-lab using CVI42 software (Circle Cardiovascular Imaging, Calgary, Canada) according to the published analysis protocols25,29.
Primary trial endpoint
The primary endpoint was the difference in absolute change in interstitial volume from baseline to post-treatment between the two treatment groups. Interstitial volume, reflecting diffuse interstitial fibrosis in the absence of infiltrative diseases, was derived as the product of ECV fraction × myocardial volume. This approach of quantifying interstitial volume was previously validated against histological fibrosis10 and demonstrated prognostic value in patients with HHD32.
ECV fraction was measured using the T1 mapping module in CVI42. Hematocrit for calculating ECV fraction was taken on the day of CMR. Myocardial volume (mL) was calculated as myocardial mass (g) divided by the specific density of the myocardium (1.05 g/mL).
Secondary trial endpoints
Secondary endpoints were absolute change in CMR markers of cardiac remodeling that included LV morphology, function (including multi-directional LV strain) and mechanics. LV concentricity was defined by the ratio between LV mass and end-diastolic volume (EDV). Myocyte volume was derived as the product of (1-ECV fraction) and myocardial volume. Maximal left atrial volume was estimated based on the biplane area-length method using the 2- and 4-chamber views, at end atrial diastole29.
Several CMR measures of myocardial mechanics were assessed. The Remodeling Index (RI) was used as a marker of LV wall stress25,33. The RI was calculated as \(\frac{\root 3 \of {{EDV}}}{t}\), where t is the maximal wall thickness (cm) across the 16 myocardial segments. The ventricular-arterial coupling ratio (VCR) was used to describe the relationship between LV and arterial system. It was calculated as the ratio of LV end-systolic volume to stroke volume34. LV filling pressures were estimated using a CMR model consisting of left atrial (LA) volume and LV mass, which had been validated with invasively measured pulmonary wedge pressure and demonstrated to have prognostic implications35.
Blood samples were collected on the first and final study visit; and stored at −80 °C. Biochemical analyses were conducted in a single freeze-thaw cycle across two assay runs at a College of American Pathologists-accredited laboratory (Changi General Hospital, Singapore). Serum NT-proBNP (N-terminal pro-B-type natriuretic peptide; proBNP II STAT, Roche Diagnostics, Germany) and hsTnT (high-sensitivity cardiac troponin T; Trop T hs STAT; Roche Diagnostics, Germany) were measured using electrochemiluminescence immunoassay on the Cobas E801 analyzer (Roche Diagnostics Asia Pacific, Singapore). The lab-reported lower detection limits for NT-proBNP and hsTnT were 11 pg/mL and 3 pg/mL, respectively. All biochemical concentrations below detection thresholds were assigned a value of half the detection limit.
Sample size estimation
An estimated sample size of 70 (35 per treatment group) would provide effective power of 80% at a 5% significance level (two-sided) to detect superiority of sacubitril/valsartan over valsartan for the primary endpoint, assuming an absolute minimum difference of 3.5 mL/m2 in mean interstitial volume change between the two groups, a standard deviation (SD) of 5.8 mL/m2 (data subsequently published34) and a moderate correlation of 0.60 between interstitial volume at baseline and week 52. This effect size was based on an estimate of the magnitude of myocardial fibrosis regression that could be expected to translate into improved cardiac function and clinical outcomes8. The study plan was to recruit 80 participants, allowing a withdrawal rate of up to 15%.
Statistical analysis
The endpoint comparison was based on intention-to-treat analysis, i.e., all participants were analyzed as part of the group to which they had been randomized. Continuous variables were presented as mean ± SD and categorical variables were expressed as count (percentage). Skewed data were presented as medians and inter-quartile range. Comparisons between treatment groups were performed using independent samples t-tests for continuous variables and χ2 test for dichotomous variables.
For primary endpoint, analysis-of-covariance (ANCOVA) was performed to adjust for baseline covariates. Treatment group at randomization was included as fixed effect. Baseline interstitial volume, 24 h SBP and prior ACEI/ARB use were included as covariates. Results were reported as adjusted mean difference with 95% confidence intervals (CI). Post-hoc subgroup analyses of the primary endpoint were performed using two-way analysis of variance (ANOVA) to test heterogeneity of treatment effects in the following subgroups: age, sex, body mass index, whether washout was performed, LV ejection fraction, interstitial volume and 24 h SBP. Continuous covariates were categorized by median values. The hypothesis testing on secondary endpoints was considered exploratory. The analyses were presented with effect estimates and unadjusted 95% CI. Adjustment for multiple comparisons was not performed.
The change in NT-proBNP and hsTnT were reported as the ratio of geometric mean values at baseline and 52 weeks. For treatment comparisons, the effective treatment ratio (ETR) was calculated as the ratio of geometric mean changes in biomarkers between the two groups. An ETR closer to 0 indicates greater effectiveness of sacubitril/valsartan in reducing biomarker level compared to valsartan.
Statistical significance was set at two-sided P value < 0.05. All analyses were performed with SPSS, version 24 (SPSS; IBM, INC, Armonk, NY) and R, version 4.3.3 (R foundation for statistical computing, Vienna, Austria). Graphs were created using GraphPad Prism 8.1.2 (GraphPad Software, Inc, San Diego, CA). Statistical analyses were performed by team members who were not involved in image analysis and participants’ follow up.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
De-identified data for this study is available for non-commercial academic purposes. Request for access can be made with the corresponding author and should include a clear research plan with statistical considerations. Requests will be reviewed by the Data Access Committee at the National Heart Centre Singapore within 6 weeks. The data required for the approved, specified purposes will be provided after completion of a data sharing agreement that include instructions on publications, reporting and usage policy. Source data are provided with paper. Source data are provided with this paper.
References
Forouzanfar, M. H. et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 19902015. JAMA. 317, 165–182 (2017).
Mohebi, R. et al. Effect of 2022 ACC/AHA/HFSA criteria on stages of heart failure in a pooled community cohort. J. Am. Coll. Cardiol. 81, 2231–2242 (2023).
Messerli, F. H., Rimoldi, S. F. & Bangalore, S. The transition from hypertension to heart failure: contemporary update. JACC Heart Fail. 5, 543–551 (2017).
González, A. et al. Myocardial Interstitial fibrosis in hypertensive heart disease: from mechanisms to clinical management. Hypertens 81, 218–228 (2024).
González, A., Schelbert, E. B., Díez, J. & Butler, J. Myocardial interstitial fibrosis in heart failure. J. Am. Coll. Cardiol. 71, 1696–1706 (2018).
Ruilope, L. M. et al. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet 375, 1255–1266 (2010).
McMurray, J. J. V. et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 371, 993–1004 (2014).
Suematsu, Y. et al. LCZ696, an angiotensin receptor-neprilysin inhibitor, improves cardiac function with the attenuation of fibrosis in heart failure with reduced ejection fraction in streptozotocin-induced diabetic mice. Eur. J. Heart Fail. 18, 386–393 (2016).
Burke, R. M., Lighthouse, J. K., Mickelsen, D. M. & Small, E. M. Sacubitril/Valsartan decreases cardiac fibrosis in left ventricle pressure overload by restoring pkg signaling in cardiac fibroblasts. Circ. Heart Fail. 12, e005565 (2019).
Chin, C. W. L. et al. Myocardial fibrosis and cardiac decompensation in aortic stenosis. JACC Cardiovasc. Imaging 10, 1320–1333 (2017).
Brilla, C. G., Funck, R. C. & Rupp, H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation 102, 1388–1393 (2000).
Díez, J. et al. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation 105, 2512–2517 (2002).
López, B. et al. Effects of loop diuretics on myocardial fibrosis and collagen type I turnover in chronic heart failure. J. Am. Coll. Cardiol. 43, 2028–2035 (2004).
Izawa, H. et al. Mineralocorticoid receptor antagonism ameliorates left ventricular diastolic dysfunction and myocardial fibrosis in mildly symptomatic patients with idiopathic dilated cardiomyopathy: a pilot study. Circulation 112, 2940–2945 (2005).
Mak, G. J. et al. Natural history of markers of collagen turnover in patients with early diastolic dysfunction and impact of eplerenone. J. Am. Coll. Cardiol. 54, 1674–1682 (2009).
Kosmala, W. et al. A randomized study of the beneficial effects of aldosterone antagonism on LV function, structure, and fibrosis markers in metabolic syndrome. JACC Cardiovasc. Imaging 4, 1239–1249 (2011).
Kosmala, W., Przewlocka-Kosmala, M., Szczepanik-Osadnik, H., Mysiak, A. & Marwick, T. H. Fibrosis and cardiac function in obesity: a randomised controlled trial of aldosterone blockade. Heart 99, 320–326 (2013).
Bavishi, C., Messerli, F. H., Kadosh, B., Ruilope, L. M. & Kario, K. Role of neprilysin inhibitor combinations in hypertension: insights from hypertension and heart failure trials. Eur. Heart J. 36, 1967–1973 (2015).
Schmieder, R. E. et al. The effect of sacubitril/valsartan compared to olmesartan on cardiovascular remodelling in subjects with essential hypertension: the results of a randomized, double-blind, active-controlled study. Eur. Heart J. 38, 3308–3317 (2017).
Solomon, S. D. et al. Angiotensin–neprilysin inhibition in heart failure with preserved ejection fraction. N. Engl. J. Med. 381, 1609–1620 (2019).
Shah, A. M. et al. Echocardiographic features of patients with heart failure and preserved left ventricular ejection fraction. J. Am. Coll. Cardiol. 74, 2858–2873 (2019).
Palazzuoli, A. et al. Current gaps in HFpEF trials: time to reconsider patients’ selection and to target phenotypes. Prog. Cardiovasc. Dis. 67, 89–97 (2021).
Sweeney, M., Corden, B. & Cook, S. A. Targeting cardiac fibrosis in heart failure with preserved ejection fraction: mirage or miracle?. EMBO Mol. Med. 12, e10865 (2020).
Lewis, G. A. et al. Pirfenidone in heart failure with preserved ejection fraction: a randomized phase 2 trial. Nat. Med. 27, 1477–1482 (2021).
Goh, V. J. et al. Novel Index of maladaptive myocardial remodeling in hypertension. Circ. Cardiovasc. Imaging 10, e006840 (2017).
Schelbert, E. B., Fonarow, G. C., Bonow, R. O., Butler, J. & Gheorghiade, M. Therapeutic targets in heart failure: refocusing on the myocardial interstitium. J. Am. Coll. Cardiol. 63, 2188–2198 (2014).
Schelbert, E. B., Butler, J. & Diez, J. Why clinicians should care about the cardiac interstitium. JACC Cardiovasc. Imaging 12, 2305–2318 (2019).
Lee, V. et al. Sacubitril/valsartan versus valsartan in regressing myocardial fibrosis in hypertension: a prospective, randomized, open-label, blinded endpoint clinical trial protocol. Front. Cardiovasc. Med. 10, 1248468 (2023).
Le, T. T. et al. Cardiovascular magnetic resonance reference ranges for the heart and aorta in Chinese at 3T. J. Cardiovasc. Magn. Reson. 18, 21 (2016).
Weber, M. A. et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J. Clin. Hypertens. 16, 14–26 (2014).
Ministry of Health, Singapore. MOH clinical practice guidelines on hypertension 1/2017. Ministry of Health, Singapore; https://www.moh.gov.sg/docs/librariesprovider4/guidelines/cpg_hypertension-booklet---nov-2017.pdf (2017).
Iyer, N. R. et al. Markers of focal and diffuse nonischemic myocardial fibrosis are associated with adverse cardiac remodeling and prognosis in patients with hypertension: the REMODEL study. Hypertens 79, 1804–1813 (2022).
Le, T. T. et al. The remodelling index risk stratifies patients with hypertensive left ventricular hypertrophy. Eur. Heart J. Cardiovasc. Imaging 22, 670–679 (2021).
Holm, H., Magnusson, M., Jujić, A., Bozec, E. & Girerd, N. How to calculate ventricular–arterial coupling?. Eur. J. Heart Fail. 24, 600–602 (2022).
Garg, P. et al. Cardiac magnetic resonance identifies raised left ventricular filling pressure: prognostic implications. Eur. Heart J. 43, 2511–2522 (2022).
Cai, J. et al. Fractal analysis of left ventricular trabeculations is associated with impaired myocardial deformation in healthy Chinese. J. Cardiovasc. Magn. Reson. 19, 102 (2016).
Acknowledgements
The authors wish to thank all the study participants, the radiographers at the Department of Cardiovascular Magnetic Resonance, the pharmacists at the Department of Pharmacy and the research coordinators at the Clinical Trial Research Office, National Heart Centre Singapore for their assistance in the study. REVERSE-LVH is funded by the National Medical Research Council of Singapore (MOH-CTGIIT17may-0001).
Author information
Authors and Affiliations
Contributions
C.W.L.C., T.T.L., S.A.C., A.M.R. and C.H.L. contributed to the conception and design of the study. Q.Z. designed the statistical analysis plan. V.L. and D.F.T. led the data collection and management. V.L., M.D., and Q.Z. performed the data analysis. J.A.B. and T.T.L. led the CMR image analysis. T.A. analyzed the circulating biomarkers. J.B., J.D., R.F. and C.S.P.L. provided important inputs to the overall trial conduct. V.L. and M.D. wrote the first draft of the manuscript. C.W.L.C. secured funding for the trial. All authors made critical revisions for important intellectual content, contributed to the article, and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
C.S.P.L. has received consulting fees from Novartis. J.B. is a consultant to Novartis. The other authors do not have any relevant conflicts of interest to disclose.
Peer review
Peer review information
Nature Communications thanks Sheldon Litwin, and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lee, V., Dalakoti, M., Zheng, Q. et al. Effects of sacubitril/valsartan on hypertensive heart disease: the REVERSE-LVH randomized phase 2 trial. Nat Commun 16, 6981 (2025). https://doi.org/10.1038/s41467-025-62203-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-62203-0