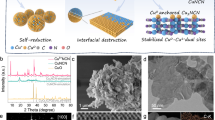
Abstract
The use of renewable electricity to drive the electrocatalytic coupling of CO with nitrogen-containing organics offers a promising strategy for producing high-value chemicals. In this work, we conduct a systematic investigation of the coordination effect between iodide and copper oxide to generate Cuδ+ active sites. These Cuδ+ sites enable the electrosynthesis of dimethylacetamide from CO and dimethylamine. Through precise regulation of the electrode surface microenvironment, a dimethylacetamide Faradaic efficiency of 45.6% is achieved at a partial current density of 182.4 mA·cm-2, with a production rate of 435.9 mmol·gcat.−1·h-1 and selectivity approaching 70%. Mechanistic studies reveal that specific adsorption of I- forms an iodide-enriched Cu0/Cu+ interface that synergistically promotes dimethylacetamide formation by enhancing adsorption of ketene intermediates (*CCO) and facilitating C–N bonds formation. This anion-coordination interfacial engineering strategy demonstrates broad applicability for synthesizing various acetamide derivatives from CO2/CO and amine, providing a foundational framework for electrocatalytic C-N coupling in acetamide synthesis.
Similar content being viewed by others
Introduction
The accelerating deployment of renewable energy infrastructure has driven significant cost reductions in green electricity, positioning electrochemical CO2/CO reduction reactions (ECRR) as a techno-economically viable pathway for carbon valorization1,2. Nitrogenous organic compounds, particularly those containing carbonyl-amine moieties, serve as mission-critical intermediates in pharmaceutical synthesis, specialty chemicals, and advanced polymer manufacturing3,4. This actuality motivates the development of electrocatalytic C-N coupling strategies that synergistically utilize CO2/CO and nitrogenous feedstocks5. Current research predominantly focuses on coupling ECRR with inorganic nitrogen sources (e.g., ammonia, nitrate, or nitrite) to produce single-carbon products such as urea, methylamine, and formamide6,7,8,9. Notably, acetamide derivatives (CH3−C(=O)−NR’R”) hold a wide array of applications and significant demand as versatile building blocks for organic reagents, agrochemicals, and dyestuffs, yet their industrial-scale production still relies on energy-intensive thermochemical routes employing acetic anhydride/acid under high-temperature, high-pressure acidic conditions10,11,12. The electrocatalytic acetylation of amines via ECRR presents a compelling green alternative, but faces intrinsic scientific barriers: the intricate multi-electron transfer processes and competing reaction pathways typically result in suboptimal performance and limited selectivity for acetamide derivatives, creating fundamental challenges in catalyst design and reaction microenvironment control (Fig. 1).
The electrocatalytic acetylation of amines via ECRR generally proceeds via a two-step mechanism: (1) electrochemical C–C coupling of CO to generate C2 intermediates, followed by (2) nucleophilic attack of amines at the activated carbonyl carbon, ultimately leading to C-N bond formation. While prior studies have established Cu+ stabilization strategies (via metal doping or surface modification) as effective promoters for C-C coupling, the subsequent C-N coupling—occurring predominantly at the electrode-electrolyte nanoconfined interface—demands precise interfacial microenvironment control13,14,15,16. For instance, the size of the hydrated cation is a key parameter affecting the interface, and the cationic solvation effect can not only regulate the local pH and CO2/CO concentration at the interface, but also promote the C-N coupling process17,18,19,20,21. In contrast, halide anions exhibit unique interfacial behaviors compared to cations: their strong chemisorption on copper catalysts induces dual functionality—simultaneously altering local proton activity (via Helmholtz layer restructuring) and reshaping catalyst morphology through surface reconstruction22,23,24,25. Nevertheless, the atomistic mechanisms underlying anions-mediated electronic interactions and their specific roles in accelerating C-N coupling kinetics remain insufficiently understood.
In this work, we develop a halide-mediated microenvironment regulation strategy through iodide-coordinated chemisorption on copper oxide substrates, establishing a dynamic Cu0/Cu+ interface that effectively suppresses Cu2O reduction while facilitating efficient electrocatalytic C-N coupling between CO and dimethylamine (DMA) for dimethylacetamide (DMAC) synthesis. The optimized electrolyte and catalyst deliver great performance, achieving a Faradaic efficiency (FE) of 45.6% for DMAC with a maximum production rate (PR) of 435.9 mmol·gcat.−1·h−1 at 400 mA·cm−2, accompanied by 70% selectivity. Through in situ electrochemical surface-enhanced Raman spectroscopy (SERS), we demonstrate that iodide species stabilize surface Cu+ species. Operando synchrotron radiation Fourier transform infrared spectroscopy (SR-FTIRS) further reveals that iodide coordination strengthens the adsorption energy of the carbon-containing intermediates on electrode surfaces, thereby promoting their C-N coupling with DMA to synthesize DMAC. The strong chemisorption of halide anions effectively blocks proton adsorption and inhibits the hydrogen evolution reaction (HER). Density functional theory (DFT) calculations corroborate that iodide-mediated electronic modulation promotes the coupling thermodynamics between CO and DMA. Remarkably, this strategy demonstrates generalizability for synthesizing diverse acetamide derivatives. This breakthrough establishes a paradigm for electrochemical synthesis of value-added amides using CO2/CO as sustainable carbon feedstocks, advancing practical solutions for carbon emission valorization.
Results and Discussion
Electrochemical performance of obtained catalysts
Since copper oxides generally exhibit high multi-carbon product selectivity in ECRR systems, Cu2O nanocubes (NCs), synthesized via the liquid phase reduction method, were selected as model catalysts. The phase composition, size distribution, and crystal structure of the Cu2O NCs were characterized using X-ray diffraction (XRD), scanning electron microscopy (SEM), and transmission electron microscopy (TEM), as shown in Supplementary Fig. 1. A flow cell was employed to investigate product selectivity with the introduction of the organic amine DMA into the ECRR. In this cell, a Hg/HgO electrode was employed as the reference electrode, while a NiFeOx on Ni foam (NiFeOx/NF) electrode functioned as the anode. The cathode was fabricated by depositing synthesized Cu2O NCs onto a gas diffusion layer (GDL) through spray coating. The electrocatalytic performance of ECRR was subsequently evaluated through constant current electrolysis from 100 mA·cm-2 to 500 mA·cm-2 in 0.1 M K2SO4 and 1 M DMA electrolyte. Liquid and gas products were analyzed using hydrogen nuclear magnetic resonance (1H-NMR) spectroscopy and gas chromatography (GC), respectively (Supplementary Figs. 2 and 3). Comparative analysis of the 1H-NMR spectra obtained before and after electrolysis with standard samples confirmed the successful synthesis of the C-N coupling product DMAC (Supplementary Fig. 4). Furthermore, gas chromatography-mass spectrometry (GC-MS) was utilized for qualitatively detection and confirmation of DMAC presence (Supplementary Fig. 5). The FE of DMAC reached 13.2 ± 0.4% at 100 mA·cm−2 using the Cu2O NCs catalyst, with DMAC selectivity among carbon products reaching 36.4% (Supplementary Figs. 6 and 7, Supplementary Table 1). Both the FE and selectivity of DMAC exhibited a notable decline with increasing current density.
To enhance the generation of DMAC, regulating the reactivity of CO and DMA at the electrode-electrolyte interface is crucial. Based on this premise, we introduced halide anions into the electrolyte to investigate the specific adsorption mechanism at the electric double layer interface, aiming to promote the electrochemical C-N coupling reaction. Different halide ions, including Cl-, Br-, I-, were introduced into the electrolyte. As shown in Fig. 2a, the proton affinity of halide ions decreases, the current density of the ECRR process increases correspondingly, with iodide exhibiting the most significant enhancement (Fig. 2a). In contrast, the current densities decreased following the introduction of halide ions under the Ar gas atmosphere, which suggests that the incorporation of halide ions suppresses the HER process (Supplementary Fig. 8). The effects of different halide ions (Cl−, Br−, I−) on DMAC formation were systematically examined (Fig. 2b, Supplementary Figs. 9 and 10, Supplementary Tables 2–4). Comparative analysis of the FE and partial current density of DMAC revealed a remarkable enhancement in DMAC production upon the halide ions addition (Supplementary Figs. 11 and 12). Notably, the introduction of iodide ions resulted in an over tenfold increase in both the FE and partial current density of DMAC at 400 mA·cm−2. Specifically, the FE of DMAC increased from 4.5% to 45.6%, while the partial current density of DMAC increased from 18.0 mA·cm−² to 182.4 mA·cm−2, and DMAC selectivity approaching 70% (Fig. 2c and 2d). The maximum energy efficiency (EE) and PR of DMAC reached 25.1% and 435.9 mmol·gcat.−1·h−1, respectively, demonstrating that the introduction of iodide ions significantly enhances the electrochemical synthesis of DMAC from CO and DMA (Supplementary Fig. 13 and Fig. 2e).
a The linear sweep voltammetry (LSV) curves of Cu2O NCs catalyst during ECRR in 0.1 M K2SO4 and 1 M DMA containing various halide ions (KCl, KBr and KI) with the scan rate of 50 mV s-1. The potential value is non-iR corrected. b FE of products distribution on Cu2O electrodes in the electrolyte of 0.1 M K2SO4, 1 M DMA, and 20 mM KI at the current density from 100 mA·cm−2 to 500 mA·cm−2 under CO flow. The flow rate of CO gas is 20 mL·min−1. c The influence of the KI and DMA on the product FE. d Selectivity of products on Cu2O electrodes in the electrolyte of 0.1 M K2SO4, 1 M DMA, and 20 mM KI at the current density from 100 mA·cm−2 to 500 mA·cm-2 under CO flow. e PR of DMAC for Cu2O electrodes without or with KX (X = Cl, Br and I) from 100 mA·cm-2 to 500 mA·cm−2 in the electrolyte of 0.1 M K2SO4 and 1 M DMA under CO flow. f The impact of different FEs and current densities on the profit of DMAC electrosynthesis and the profits by using Cu2O NCs catalyst without or with KI. g Demonstration of 80-hour stability in the electrolyte of 0.1 M K2SO4, 1 M DMA, and 20 mM KI at the current density of 400 mA·cm−2 under CO flow. Error bars represent the standard deviation of three independent samples. Data are presented as mean values ± standard deviation. Source data for Fig. 2a–g are provided as a Source Data file.
Subsequently, we systematically investigated the influence of iodide concentration on the reaction performance (Supplementary Fig. 14). The FE of ECRR products demonstrated a significant increase with increasing KI concentration, reaching an optimal value at 20 mM. At this concentration, the flow cell achieved maximum FE (45.6%) for DMAC production while showing noticeable suppression of HER process. However, when the KI concentration exceeded this optimal value, a gradual decrease in DMAC FE was observed. This phenomenon can be attributed to the excessive adsorption of iodide species on the catalyst surface, which likely occupies active sites and consequently reduces the efficiency of the ECRR process. The influence of DMA concentrations on product selectivity was investigated in Supplementary Fig. 15. To ensure the consistency of the pH of the electrolyte, the evaluation of ECRR performance was conducted in the electrolyte both with and without iodide in the electrolyte of 0.1 M K2SO4 and 0.1 M KOH (Supplementary Fig. 16, Supplementary Tables 5 and 6). The primary ECRR products were identified as n-propanol, ethylene, acetate, and ethanol. Comparative analysis revealed that iodide addition significantly enhanced the FE of C2+ products. However, upon DMA introduction, a substantial decrease in the FE of C2+ products was observed, while the FE of DMAC products showed a remarkable increase. This phenomenon can be explained by the relatively lower energy barrier of electrochemical C-N coupling reactions compared to ECRR processes. Notably, when the DMA concentration exceeded 2 M, a decrease in product FE was observed. This reduction is attributed to the increased amine concentration in the electrolyte, which impedes mass transfer and consequently leads to deteriorated electrochemical performance.
To evaluate the economic feasibility of renewable electricity-driven DMAC electrosynthesis, we conducted a technical and economic analysis (TEA) of the process (Fig. 2f). The analysis incorporated various cost factors, including separation processes, electrolyzer components, catalyst materials, membrane, maintenance requirements, balance of plant equipment, input chemicals, and electricity consumption26,27. Sensitivity analysis revealed that the plant-gate levelized cost primarily depends on the cost of electricity pricing, input materials, and electrochemical performance parameters, particularly the FE of DMAC, current density and full-cell potential. At a renewable electricity price of 2 cents/kWh−1, the reaction system incorporating KI maintained profitability within the current density range of 200–500 mA·cm−2 (pentagram dots), benefiting from the high FE at elevated current densities. Furthermore, durability testing performed under constant current density of 400 mA·cm−2 exhibited stable operation for over 80 hours, while maintaining a DMAC FE above 30% throughout the testing period, thereby demonstrating the system’s capacity for efficient and sustained DMAC productions (Fig. 2g).
Physical and chemical characterization of the prepared Cu electrocatalysts
To elucidate the enhanced performance in DMAC synthesis, we propose that several factors may contribute to this improvement: (1) an increase in the active surface area of the catalyst; (2) the specific adsorption between iodide and the catalyst, which preserves the active sites of Cuδ+; and (3) enhanced CO adsorption capacity. To validate these hypotheses, we first measured the electrochemical active surface area (ECSA) of the catalyst with and without iodide addition. As demonstrated in Supplementary Fig. 17, the ECSA of KI-modified Cu2O NCs (0.129 mF·cm-2) remains virtually identical to that of pristine Cu2O NCs (0.131 mF·cm−2), directly excluding the possibility that iodide-induced active surface area enhancement. In addition, the SEM images of the electrodes after the electrolysis exhibited the introduction of iodine ions resulted in minimal changes to the electrode structure (Supplementary Fig. 18). The nanocube morphology was preserved, suggesting that the catalyst’s active surface area remained unchanged during the reconstruction process.
Subsequently, the electronic structural evolution of the Cu2O NCs catalyst was examined using Auger electron spectroscopy (AES) (Fig. 3a). In the iodide-free electrolyte, the catalyst predominantly existed in the Cu0 state at 568.2 eV after the electrolysis28. In contrast, when iodide was present, the catalyst maintained a mixed valence state of Cu0/Cu+. Furthermore, distinct signals of halide anions on the electrode after electrolysis were observed, indicating the adsorption interaction between Cu2O and halide ions during the electrochemical process (Supplementary Fig. 19).
a XPS spectra of Cu2O catalysts before and after the electrolysis with or without 20 mM KI at −0.6 VRHE in 0.1 M K2SO4 and 1 M DMA. In situ surface enhanced Raman spectra of Cu2O during electrolysis (b) without or c with 20 mM KI at −0.6 VRHE in 0.1 M K2SO4 and 1 M DMA. d, e) TEM images and elemental mappings of the catalyst after the coupling reaction. f The differential capacitance curve of Cu2O in K2SO4 solution with or without KI additive. The potential value is non-iR corrected. Source data for Fig. 3a-c and f are provided as a Source Data file.
Furthermore, the crystal structure and surface morphology of the catalyst after-electrolysis were comprehensively characterized using XRD and TEM. As shown in Supplementary Fig. 20, XRD pattern revealed that the catalyst underwent complete reduction to metallic Cu in the absence of iodide, while in the iodide-containing electrolyte, the catalyst maintained a mixed phase of Cu and Cu2O. As shown as Fig. 3d, the catalyst preserved its nanocube morphology throughout the electrolysis process, which agrees with the SEM images. However, HR-TEM images identified two distinct types of lattice fringes of the catalyst in the iodide-containing electrolyte, with measured spacings of 0.208 nm and 0.245 nm, which were assigned to the Cu (111) and Cu2O (111) planes, respectively. In contrast, only the Cu (111) plane was detected in the control experiment without iodide (Supplementary Fig. 21). Furthermore, energy-dispersive spectroscopy (EDS) mapping verified the uniform distribution of iodide on the catalyst surface, aligning with the XPS results (Fig. 3e).
To monitor the structural evolution of the catalyst during the electrochemical coupling process, in situ Raman spectra were collected at five-minutes intervals before and after iodide introduction (Fig. 3b, c and Supplementary Fig. 22). At the open-circuit potential, characteristic peaks at 151 cm−1, 217 cm−1 and 636 cm−1, corresponding to Cu2O, along with a peak at 288 cm−1 associated with the Cu-CO bond formed from CO adsorption on the electrode surface were observed in the Raman spectra29,30. In the iodide-free electrolyte, the Cu2O characteristic peaks exhibited rapid attenuation and eventually vanished during the electrochemical coupling process, indicating complete reduction to metallic Cu. Concurrently, the intensity of Cu-CO peak decreased significantly, suggesting the reduced CO adsorption affinity on Cu0 sites. Conversely, the introduction of iodide maintained the Cu2O characteristic peaks, demonstrating effective suppression of Cu2O reduction. Moreover, the persistent Cu-CO signal confirmed enhanced CO adsorption on Cu+ sites, providing a plausible explanation for the improved ECRR performance.
To further validate the enhanced CO adsorption capacity on the iodide-rich Cu0-Cu+ catalyst, the DFT calculations were performed to determine the CO adsorption energy (Supplementary Figs. 23 and 24, and Supplementary Data 1). The results demonstrated that the CO adsorption energy on the iodide-rich Cu0-Cu+ catalyst surface was more negative (−0.95 eV) compared to that on the Cu surface (−0.69 eV). These findings confirmed that the introduction of iodide substantially enhances the CO adsorption on the electrode surface.
To investigate the iodide adsorption behavior on the catalyst, the differential capacitance curves of the catalyst in electrolytes with and without iodide were tested using a three-electrode system. Notably, the surface capacitance showed a substantial increase following iodide addition. This phenomenon can be attributed to iodide adsorption on the Cu2O surface, where iodide ions displace water molecules in the electric double layer (EDL), establishing short-range interactions with the electrode surface to form the inner Helmholtz layer. This structural modification reduces the effective thickness of the EDL, thereby increasing the differential capacitance (Fig. 3f). The compression of the EDL thickness substantially enhances the interfacial electric field strength, which facilitates the charge transfer kinetics and promotes CO transport to the electrode surface by reducing the diffusion layer thickness. These combined effects ultimately lower the activation energy barrier for CO adsorption31.
The investigation of coupling reaction pathways
To elucidate the reaction pathway of the electrochemical coupling between CO and DMA, operando SR-FTIR spectroscopy was employed (Supplementary Fig. 25). Figure 4a and b present the time-dependent infrared spectra obtained with and without iodide addition during electrolysis. The appearance of C-OH stretching vibrations in the spectral range of 1000–1200 cm−1 signified the initial formation of *COCOH intermediates, demonstrating that iodide significantly facilitates the C-C coupling process32,33. Subsequently, the spectra display intensified absorption bands corresponding to C=O bonds at approximately 1613 cm-1. Additionally, the emergence of a characteristic *CH3 peak at 1314 cm−1 provides further evidence for acetyl group formation34,35,36 A prominent peak at 1401 cm−1, attributed to C–N bond formation, exhibited enhanced intensity in the presence of iodide, highlighting its crucial role in promoting the C–N coupling reaction, which are essential precursors for DMAC synthesis37,38. Based on these observations, we conclude that iodide plays a dual role: not only does it promote C-C coupling on the catalyst surface to generate C2 intermediates, but also facilitates the coupling of carbon-containing intermediates with DMA, thereby enabling crucial C-N bond formation.
a, b Operando SR-FTIR spectra in the range of 950–2000 cm−1 for adsorbed intermediates under various time (a) without I- and (b) with I- in 0.1 M K2SO4 and 1 M DMA. c Schematic diagram of the coupling reaction path on the obtained catalyst. Source data for Fig. 4a, b are provided as a Source Data file.
The proposed reaction pathway was exhibited at Fig. 4c. In the initial stage, adsorbed CO undergoes electrochemical reduction, initiating C-C coupling to form the *COCOH intermediate. Subsequently, the intermediate undergoes electrochemical hydrogenation, generating *COHCOH. Subsequent dehydration of this species yields the ketene intermediate *CCO. The unique electronic configuration of *CCO induces charge polarization in the C=O bond, with the carbon atom acquiring a partial positive charge. This charge redistribution thermodynamically favors C-N coupling with nucleophilic nitrogen sources over further electrochemical hydrogenation. Concurrently, C-N bond formation activates the N-H bond, enabling proton transfer to generate the intermediate *CHCON(CH3)2. Finally, the target product CH3CON(CH3)2 (DMAC) is synthesized via a two-step electrochemical hydrogenation sequence.
To investigate the electronic interactions at the atomic level, we calculated the differential charge density distribution for *CCO intermediate adsorption on both Cu (111) and Cu/Cu2O-I− surface using DFT calculations (Fig. 5a, b). Comparative analysis revealed that the Cu/Cu2O-I− surface exhibits pronounced interlaced charge transfer, indicating a stronger electron interaction between the *CCO intermediate and the catalyst compared to the Cu (111) surface. Through comparative Bader charge analysis, we observed distinct electron distribution patterns: C1 site receives more electrons on the Cu/Cu2O-I- surface (0.37 e) compared to the pure Cu surface (0.33e), suggesting enhanced stabilization of *CCO intermediates through stronger adsorption. Conversely, C2 site exhibits greater electron losses (−0.72 e on the Cu/Cu2O-I− compared to −0.68 e on Cu), rendering it more susceptible to nucleophilic attack and thereby facilitating the C-N coupling process. Furthermore, the adsorbed iodide species receives a significant charge transfer of 0.30 e, which likely contributes to the observed suppression of Cu2O reduction. Additionally, transition state calculations for the electrochemical C-N coupling of *CCO intermediates with DMA demonstrate that the energy barrier for C-N coupling is substantially lower on the Cu/Cu2O-I− surface (0.50 eV) than on the Cu (111) surface (1.87 eV) (Fig. 5c, Supplementary Figs. 26 and 27).
Charge density difference of key intermediates *CCO on (a) Cu (111) and (b) Cu/Cu2O-I−. c Relative energy profiles of the C-N coupling to form *CCONHMe2 intermediate. d Free energy diagrams of reaction pathway for the synthesis of DMAC on Cu and Cu/Cu2O-I− surface. Cu, I, C, O, and H atoms were shown as orange, purple, gray, red and pink sphere, respectively. Source data for Fig. 5c, d are provided as a Source Data file.
Furthermore, DFT calculations were performed to determine the energy barriers along the entire reaction pathway on Cu and Cu/Cu2O-I− surface (Supplementary Figs. 28 and 29). As shown in Fig. 5d, CO molecules initially adsorbed on the catalyst surface. Subsequently, two *CO couple to form *COCOH intermediates through an electrochemical C-C coupling process, which is a critical step for the generation of C2 products and also the rate-determining step of this reaction. The presence of iodide significantly lowers the energy barrier for this coupling from 0.67 eV to 0.35 eV, consistent with infrared spectroscopy results. The ketene intermediate *CCO is generated through electrochemical dehydration of *COHCOH, followed by nucleophilic attack by HNMe2 to produce *CCONHMe2 intermediates. After C-N coupling, *CCONHMe2 undergoes proton migration to form *CHCONMe2. Finally, DMAC is produced through a two-step electrochemical hydrogenation and subsequent desorption. These findings demonstrate that iodide incorporation not only promotes the initial C-C coupling of CO but also reduces the reaction barriers for coupling *CCO and HNMe2.
To substantiate the versatility of this approach, the nitrogen source was expanded to other substrates, such as ammonia, ethylamine, ethanolamine, benzylamine, and morpholine. The products were determined by GC-MS, and subsequently quantified by 1H-NMR (Supplementary Figs. 30–39). The FE of acetylation products was summarized in Supplementary Table 7. An analysis of the C-N coupling products revealed that the nucleophilicity of the nitrogen source significantly influenced the FE, thereby confirming the hypothesis of a competitive reaction between the nucleophilic coupling of *CCO intermediates and electrochemical hydrogenation. Furthermore, CO₂ molecules were employed instead of CO for electrolysis, resulting in the generation of DMAC with a FE of up to 15.8% (Supplementary Fig. 40). Additionally, the presence of another coupling product, dimethylformamide, with an FE of up to 15.4% was detected. Moreover, the FE content of DMAC also increased after the introduction of iodide, indicating the universality of the coordination strategy of iodide. In conclusion, the general synthesis route of electrochemical acetylation by ECRR can be broadened to a wider array of C-N coupling reactions, demonstrating the potential for diverse applications in the synthesis of nitrogen-containing compounds.
In summary, the synthesis of DMAC was successfully achieved by the electrochemical coupling of CO and DMA on the Cu2O catalyst through the coordination effect between halide ions and metal oxides. SERS and differential capacitance tests confirmed the specific adsorption of iodide on the catalyst, which inhibits the reduction of Cu2O and stabilizes the active Cu+ sites, thereby enhancing CO adsorption and promoting the ECRR process. SR-FTIR analysis revealed an enhanced adsorption of the key ketene intermediates (*CCO) and the formation of C-N bonds, demonstrating a two-step pathway involving C-C and C-N coupling in the electrosynthesis of DMAC. DFT calculations further proved that iodide facilitates the initial C-C coupling of *CO and lowers the coupling reaction barriers of *CCO and HNMe2. The addition of iodide to the electrolyte fine-tuned the electrode surface microenvironment, achieving a DMAC FE of 45.6% at a partial current density of 182.4 mA·cm−2, and a PR of 435.9 mmol·gcat.−1·h−1. The flow cell maintained a stable DMAC electrosynthesis for over 80 hours at 400 mA·cm−2. The TEA suggests that the ECRR-based DMAC synthesis possesses cost advantage and economic viability. Finally, the synthesis of acetylated value-added chemicals was achieved from various nitrogen sources and CO2, confirming the universality of this strategy. This research paves the way for the efficient electrosynthesis of N-containing compounds from CO gas.
Methods
Materials and synthesis
NaOH (99.0%), CuCl2·H2O (99.5%), KOH (99.0%), KCl (99.5%), KBr (99.5%), KI (99.5%) and L- ascorbic acid (99.9%) were purchased from Sinopharm Chemical Reagent Co., Ltd. Dimethylamine (40% in H2O), ammonia (25% in H2O), ethylamine (70% in H2O), benzylamine (99.0%), ethanolamine (99.0%), morpholine (99.0%) were purchased from Aladdin. All reagents were obtained from commerce without further purification. Deionized water was used in all electrolyte solution with 18.2 MΩ·cm−1. CO (99.999%) and Ar (99.999%) come from Changsha ChangDa Special Gas Company.
Preparation of Cu2O electrocatalysts
First, 0.483 g CuCl2·H2O was dissolved in 100 mL deionized water and then heated to 55 °C in an oil bath. At the same time, 0.8 g of NaOH was dissolved in 10 mL of deionized water, and then slowly dropped into the above solution and stirred for 30 min. Further, 1.057 g L-ascorbic acid was dissolved in 10 mL deionized water and added into the above solution, and aged at 55 °C for 3 h. Finally, the obtained samples were centrifugally cleaned with water and ethanol and vacuum dried at 60 °C.
Electrocatalysts characterization
The X-ray diffraction (XRD, Bruker D8 Advance X-ray diffractometer) was operated at 40 kV and 40 mA, and Cu tube with 1.5418 Å. Scanning electron microscopy (SEM, Regulus 8100) and transmission electron microscopy (TEM, Themis Z-3.2) were performed at 15 kV and 200 kV. The X-ray photoelectron spectroscopy (XPS, Thermo Scientific ESCALAB Xi + , America) was used by a 1486.6 eV X-ray source and equipped with Al kα, and the work voltage was 12.5 kV, filament current was 16 mA.
Electrochemical measurements
The electrolytic testing process is carried out in a flow cell using a standard three-electrode system at room temperature (298 K). Among them, the cathode catalyst is composed of synthetic Cu2O (4 mg·cm−2, 1 cm2) supported on the gas diffusion electrode, and the anode is supported on the foam nickel substrate with NiFe oxide (NiFeOx/NF) as the catalyst. The reference electrode is selected Hg/HgO filled with 1 M KOH. An anion exchange membrane was selected for the electrolyte separator (Alkymer, W-25). The anion exchange membrane was pre-treated by soaking it in 1 M KOH solution for 12 hours and stored in pure water. The cathode electrolyte was used 0.1 M K2SO4 and 1 M dimethylamine (pH = 12.8 ± 0.2, which determined by pH meter), in which a certain amount of halide ions was added as accelerators. All potentials were recorded against the reversible hydrogen electrode (RHE, ERHE = 0.0591 × pH + 0.098 V + EHg/HgO) without special explanation. The anode uses 1 M KOH as the electrolyte. The anode and cathode electrolytes are circulated through the peristaltic pump into the reactor for reaction. The linear sweep voltammetry (LSV) test was performed at a rate of 50 mV s-1, and the potential was not iR corrected. The differential capacitance curve was calculated using the equation: C = -(2πfZim)−1. Where C is differential capacitance, f denotes the angular frequency, and Zim corresponds to the imaginary part of impedance. A specific frequency of 100 Hz was selected for this calculation. Electrochemical surface area (ECSA) was calculated from scan-rate dependence of CV with scan rates of 20–100 mV·s−1.
Product analysis
The liquid products were analyzed quantitatively using nuclear magnetic resonance spectroscopy (1H-NMR, AVANCE NEO-400). The procedure involved mixing 500 uL of electrolytic product stock solution with 100 uL D2O and 100 uL internal standard solution containing 250 ppm phenol in the nuclear magnetic tube. The water peak was suppressed during testing, with a total of 32 test cycles. Additionally, the product underwent qualitative analysis via gas chromatography-mass spectrometry (GCMS, SHIMADZU 2030QP). Gas products were analyzed using a flow cell and gas chromatography (GC). In real-time, tail gas was injected into GC to determine the content of H2, C2H4, and other gas products.
The Faradaic efficiency for the liquid products was calculated using the following equation:
where n is the number of electrons transferred from CO to DMAC (n = 4), F is Faraday’s constant, x is the mol of DMAC formed, Qtot is the electric quantity.
The Faradaic efficiency for the gas products was calculated using the following equation:
where n is the number of electrons transferred, F is Faraday’s constant, x is the mole fraction of product, V is the total molar flow rate of gas and jtot is the total current.
The energy efficiency for the formation of DMAC is defined as follows:
The selectivity for the products was calculated using the following equation:
where x is the mole fraction of target product, xtot is the total mole fraction of ECRR product11.
In situ surface-enhanced Raman spectrum (SERS) tests
The catalyst Cu2O underwent electrochemical changes during the reaction, which were analyzed using Raman spectroscopy. To enhance the signal strength before the test, a layer of Au@SiO2 was applied onto the catalyst surface as a surface enhancer. Throughout the test, constant potential electrolysis was conducted at −0.5 VRHE, with data collection occurring every five minutes. Subsequently, a Raman spectrum was obtained for detailed analysis, revealing characteristic peaks of Cu2O at 151 cm−1, 217 cm−1 and 636 cm−1, Cu-CO at 288 cm−1, respectively.
Operando synchrotron radiation Fourier transform infrared spectroscopy (SR-FTIR) measurements
The Operando SR-FTIR test was conducted at the infrared beamline BL01B of the National Synchrotron Radiation Laboratory (NSRL) using a homemade roof reflection infrared device and the FT-IR spectrometer (Bruker, 66 V/s) with ZnSe crystals as the infrared transmission window. Vertical incidence reflection mode of infrared light was applied in the test with a range of 1000–2000 cm−1. Infrared spectrum was recorded every 2 minutes under constant potential, averaging 512 scans per spectrum with a resolution of 2 cm−1. The in-situ electrochemical device continuously introduced CO gas to maintain its concentration in the reaction environment. The Ag/AgCl electrode served as the reference electrode, while a platinum wire was used as the counter electrode.
Theoretical calculation
All calculations were carried out using density functional theory (DFT) as implemented in the Vienna Ab initio Simulation Package (VASP). Electron-ion interactions were described using the projector-augmented-wave (PAW) potentials. The Perdew-Burke-Ernzerhof (PBE) exchange-correlation functional within the generalized gradient approximation (GGA) was employed in this study. A k-points mesh of 3 × 3 × 1 was used for geometry optimization with the Gamma-centered method, and a plane wave energy cutoff of 400 eV was applied in all computations. The electronic energy and forces were converged to within 1.0 × 10−5 eV and 0.03 eV/Å, respectively. Transition state intermediates were identified using the Climbing-image nudged elastic band (CI-NEB) method. In the slab model, a vacuum layer thickness of 15 15 Å was adopted, which is sufficiently large to minimize spurious interactions.
Data availability
All data that support the findings of this study are available in the paper and its Supplementary Information. Source data for Figs. 1–5 are provided as a Source Data file. Source data are provided in this paper. Source data are provided with this paper.
References
Jin, J. et al. Constrained C2 adsorbate orientation enables CO-to-acetate electroreduction. Nature 617, 724–729 (2023).
Jiang, M. et al. Review on strategies for improving the added value and expanding the scope of CO2 electroreduction products. Chem. Soc. Rev. 53, 5149–5189 (2024).
Yang, X., Zhang, Y., Sun, P. & Peng, C. A review on renewable energy: conversion and utilization of biomass. Smart Mol. 2, e20240019 (2024).
Lu, Y. et al. Anodic electrosynthesis of amide from alcohol and ammonia. CCS Chem. 6, 125–136 (2023).
Lee, G. et al. Electrochemical upgrade of CO2 from amine capture solution. Nat. Energy 6, 46–53 (2021).
Hu, Q. et al. Pulsed co-electrolysis of carbon dioxide and nitrate for sustainable urea synthesis. Nat. Sustain. 7, 442–451 (2024).
Guo, C. et al. Electrochemical upgrading of formic acid to formamide via coupling nitrite co-reduction. J. Am. Chem. Soc. 144, 16006–16011 (2022).
Wu, Y., Jiang, Z., Lin, Z., Liang, Y. & Wang, H. Direct electrosynthesis of methylamine from carbon dioxide and nitrate. Nat. Sustain. 4, 725–730 (2021).
Li, J. & Kornienko, N. Electrochemically driven C–N bond formation from CO2 and ammonia at the triple-phase boundary. Chem. Sci. 13, 3957–3964 (2022).
Xiao, Y. et al. Screening efficient C–N coupling catalysts for electrosynthesis of acetamide and output ammonia through a cascade strategy of electrochemical CO2 and N2 reduction using Cu-based nitrogen–carbon nanosheets. ACS Appl. Mater. Inter. 16, 12486–12499 (2024).
Jouny, M. et al. Formation of carbon–nitrogen bonds in carbon monoxide electrolysis. Nat. Chem. 11, 846–851 (2019).
Patil, S. B. et al. Porifera-like nickel nanodendrite for the efficient electrosynthesis of C–N compounds from carbon dioxide and nitrate anions. J. Mater. Chem. A. 11, 11495–11506 (2023).
Li, H. et al. CO electrolysis to multicarbon products over grain boundary-rich Cu nanoparticles in membrane electrode assembly electrolyzers. Nat. Commun. 15, 4603 (2024).
Yang, Y. et al. Operando studies reveal active Cu nanograins for CO2 electroreduction. Nature 614, 262–269 (2023).
Wang, Y., Han, P., Lv, X., Zhang, L. & Zheng, G. Defect and interface engineering for aqueous electrocatalytic CO2 reduction. Joule 2, 2551–2582 (2018).
Yang, R. et al. How local electric field regulates C–C coupling at a single nanocavity in electrocatalytic CO2 reduction. Nat. Commun. 15, 7140 (2024).
Gu, J. et al. Modulating electric field distribution by alkali cations for CO2 electroreduction in strongly acidic medium. Nat. Catal. 5, 268–276 (2022).
Weng, S., Toh, W. L. & Surendranath, Y. Weakly coordinating organic cations are intrinsically capable of supporting CO2 reduction catalysis. J. Am. Chem. Soc. 145, 16787–16795 (2023).
Fan, M. et al. Cationic-group-functionalized electrocatalysts enable stable acidic CO2 electrolysis. Nat. Catal. 6, 763–772 (2023).
Le, J.-B., Chen, A., Kuang, Y. & Cheng, J. Molecular understanding of cation effects on double layers and their significance to CO-CO dimerization. Nat. Sci. Rev. 10, nwad105 (2023).
Wang, D. et al. Modulating microenvironments to enhance CO2 electroreduction performance. eScience 3, 100119 (2023).
Peng, C. et al. Surface co-modification of halide anions and potassium cations promotes high-rate CO2-to-ethanol electrosynthesis. Adv. Mater. 34, 2204476 (2022).
Xia, Z. et al. Enhancing the electrocatalytic hydrogenation of furfural via anion-induced molecular activation and adsorption. J. Am. Chem. Soc. 146, 24570–24579 (2024).
Varela, A. S., Ju, W., Reier, T. & Strasser, P. Tuning the catalytic activity and selectivity of Cu for CO2 electroreduction in the presence of halides. ACS Catal. 6, 2136–2144 (2016).
Nguyen, D. L. T. et al. Effect of halides on nanoporous Zn-based catalysts for highly efficient electroreduction of CO2 to CO. Catal. Commun. 114, 109–113 (2018).
Lanigan, R. M., Starkov, P. & Sheppard, T. D. Direct synthesis of amides from carboxylic acids and amines using B(OCH2CF3)3. J. Org. Chem. 78, 4512–4523 (2013).
Krause, T., Baader, S., Erb, B. & Gooßen, L. J. Atom-economic catalytic amide synthesis from amines and carboxylic acids activated in situ with acetylenes. Nat. Commun. 7, 11732 (2016).
Li, H. et al. High-rate CO2 electroreduction to C2+ products over a copper-copper iodide catalyst. Angew. Chem. Int. Ed. 60, 14329–14333 (2021).
Teh, W. J. et al. Selective electroreduction of acetylene to 1,3-butadiene on iodide-induced Cuδ+–Cu0 sites. Nat. Catal. 7, 1382–1393 (2024).
Jiang, Y., Li, H., Chen, C., Zheng, Y. & Qiao, S.-Z. Dynamic Cu0/Cu+ interface promotes acidic CO2 electroreduction. ACS Catal. 14, 8310–8316 (2024).
Schott, C. M. et al. How to assess and predict electrical double layer properties. implications for electrocatalysis. Chem. Rev. 124, 12391–12462 (2024).
Ding, J. et al. Molecular tuning boosts asymmetric C-C coupling for CO conversion to acetate. Nat. Commun. 15, 3641 (2024).
Sun, W. et al. V-doped Cu2Se hierarchical nanotubes enabling flow-cell CO2 electroreduction to ethanol with high efficiency and selectivity. Adv. Mater. 34, 2207691 (2022).
Pan, L. et al. Single-atom or dual-atom in TiO2 nanosheet: which is the better choice for electrocatalytic urea synthesis?. Angew. Chem. Int. Ed. 62, e202216835 (2023).
Li, X., Wang, S., Li, L., Sun, Y. & Xie, Y. Progress and perspective for in situ studies of CO2 reduction. J. Am. Chem. Soc. 142, 9567–9581 (2020).
Li, J. et al. Intercepting elusive intermediates in Cu-mediated CO electrochemical reduction with alkyl species. J. Am. Chem. Soc. 144, 20495–20506 (2022).
Lv, C. et al. A defect engineered electrocatalyst that promotes high-efficiency urea synthesis under ambient conditions.ACS Nano.16, 8213–8222 (2022).
Shi, Y. et al. Localized geometry determined selectivity of iodide-derived copper for electrochemical CO2 reduction. Adv. Energy Mater. 13, 2203896 (2023).
Acknowledgements
This work was supported by the National Key Research and Development Program of China (No. 2023YFA1507400), the National Natural Science Foundation of China (Nos. 22425021, U24A20498), the Guangdong Basic and Applied Basic Research Foundation (2024A1515012702), and the Hunan Provincial Innovation Foundation for Postgraduate (CX20240377). Thanks to the Analytical Instrumentation Center of Hunan University for NMR and Raman.
Author information
Authors and Affiliations
Contributions
S. W. and Y. Z. lead the present work. Y. F. carried out the experiments, collected and analyzed the data. Y. Y. carried out the DFT calculations. Q. A. and Q. L. assisted with Operando SR-FTIR measurements and data analysis. Z. X., Y. Lu., Y. P., R. W., Z. Z. and Y. Li. helped with data analysis. Y. F. and Y. Z. wrote and revised the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fan, Y., Yan, Y., An, Q. et al. Electrochemical coupling of carbon monoxide and amine on iodide coordination stabilized Cuδ+ site. Nat Commun 16, 6917 (2025). https://doi.org/10.1038/s41467-025-62291-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-62291-y