Abstract
Heat nociception involves thermosensors like transient receptor potential channel V1 in dorsal root ganglion (DRG) neurons, but their loss only partially impairs heat sensing, suggesting other mechanisms. Autism frequently involves abnormal pain perception, but its mechanisms remain unclear. Here we show that dedicator of cytokinesis 4 (Dock4), an autism susceptibility gene, is decreased in DRG neurons across multiple pain models via histone H4K8 lactylation. DOCK4 deficiency in sensory neurons increases heat nociception in mice. Mechanistically, DOCK4 interacts with sodium channel Nav1.7 and mediates its trafficking from the membrane to the cytoplasm in DRG neurons. Acting an adaptor protein, DOCK4 binds the motor protein Dynein to form a Dynein/DOCK4/Nav1.7 complex, where Dynein provides the mechanical force for Nav1.7 trafficking. DOCK4 knockdown in sensory neurons also enhances heat nociception in male nonhuman primates. Thus, the Dynein/DOCK4/Nav1.7 complex represents a thermosensor-independent mechanism regulating heat nociception and provides insights into abnormal pain in autism.
Similar content being viewed by others
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by impairments in social communication and the presence of repetitive behaviors or restricted interests1,2,3. Additionally, individuals with ASD frequently exhibit aberrant reactivity to sensory stimuli, with a high prevalence estimated to be up to 85% in patients4,5. These sensory abnormalities vary considerably among individuals, manifesting as hypersensitivity or hyposensitivity to various stimuli, including auditory, olfactory, gustatory, tactile, and painful inputs4,5,6,7,8,9.
Dedicator of cytokinesis 4 (DOCK4), a member of the DOCK family, functions as a guanine nucleotide exchange factor, and its gene is located on chromosome band 7q31.1, a region shared by several candidate genes implicated in autism10,11. Clinical evidence and genetic studies identify an involvement of DOCK4 in autism susceptibility12,13. Experimental research demonstrates that knocking out DOCK4 induces autism-like behaviors in mice11. However, the role of autism susceptibility gene Dock4 in the regulation of sensory abnormalities remains largely unexplored.
Post-translational histone modifications are integral to the hierarchy of epigenetic regulatory mechanisms14. In recent years, various histone acylation marks derived from cellular metabolites have been identified, including propionylation, butyrylation, 2-hydroxyisobutyrylation, succinylation, malonylation, glutarylation, crotonylation, and β-hydroxybutyrylation15. More recently, lactate derived from glycolysis has been identified as a substrate for histone lactylation16. Notably, histone lactylation induced by lactate (e.g., at H4K12la) has been demonstrated to directly stimulate gene transcription16,17. It has been reported that histone lactylation functions to regulate macrophage M1/2 polarization16,18, somatic cell reprogramming19, neural development20, Alzheimer’s disease17, and tumorigenesis21.
Noxious stimulus (e.g., thermal, mechanical, and chemical stimuli) induce pain by activating cutaneous Aδ and C nociceptors, which are the peripheral terminals of small-diameter DRG neurons. The transduction of nociceptive signals involves specialized membrane proteins that are sensitive to specific stimuli. For instance, the transient receptor potential cation channel V1 (TRPV1), expressed in small DRG neurons, functions as a thermosensor ( >42 °C). However, mice with a knockout of the Trpv1 gene display only a partial reduction in nociceptive behaviors induced by intense heat stimuli ( >52 °C) and show no apparent change in response to noxious heat stimuli below 48 °C22,23. This suggests the presence of undiscovered mechanisms in heat nociception.
In the present study, we demonstrate that DOCK4 levels are significantly reduced in the DRG neurons of mice across four distinct pain models: nerve injury, inflammation, chemotherapy, and cancer. Further investigation revealed that histone H4K8 lactylation (H4K8la) mediates the downregulation of DOCK4 in sensory neurons. Loss of DOCK4 in sensory neurons selectively enhances heat nociception in both mice and nonhuman primates. Additionally, DOCK4 interacts with Nav1.7 and facilitates its trafficking from the membrane to the cytoplasm and the motor protein Dynein participates in DOCK4-modulated trafficking process of Nav1.7. Our data elucidate the mechanism by which DOCK4 regulates heat nociception, establishing that the Dynein/DOCK4/Nav1.7 complex is essential for heat nociception signal transmission. Furthermore, this study uncovers potential mechanisms underlying abnormal pain processing in autism, offering promising therapeutic strategies such as targeting DOCK4 for pain management in autistic patients.
Results
DOCK4 is decreased in DRGs under conditions of pathological pain
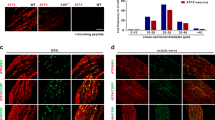
Various factors can lead to pathological pain, including nerve injury, chronic inflammation, chemotherapy drugs and cancer24. To identify common mechanisms and targets regulating different types of pathological pain, we established models for neuropathic pain induced by spinal nerve ligation (SNL), inflammatory pain induced by complete Freund’s adjuvant (CFA), chemotherapy pain induced by paclitaxel (PTX), and bone cancer pain induced by Lewis lung carcinoma (LLC) cells. We then collected dorsal root ganglion (DRG) tissues from mice for RNA-seq analysis (Fig. 1a and Fig. S1b). The results showed that three genes including Dock4, Afmid, and Rpl15-ps3 were simultaneously decreased in the DRGs of mice across four pain models (Fig. 1b). Notably, the level of downregulation of Dock4 was the highest (Fig. 1c). Dock4 is an autism susceptibility gene involved in ASD pathogenesis11,13. Given that individuals with ASD frequently experience sensory abnormalities, including abnormal pain perception4,5,25, we focused our investigation on Dock4 gene. Tissue expression profiling revealed widespread Dock4 mRNA expression in a series of tissues and organs (Fig. S1c), with particularly high expression in kidney, colon, brain, cerebellum, spinal cord and DRGs (Fig. S1c). Quantitative-PCR results confirmed significant downregulation of Dock4 mRNA expression in the DRG tissues of SNL, CFA, PTX, and LLC mice (Fig. 1d–g). Consistently, the protein levels of DOCK4 in the DRG tissues of neuropathic pain mice, inflammatory pain mice, chemotherapy pain mice, and bone cancer pain mice were also significantly decreased (Fig. 1h–k). These findings indicate decreased DOCK4 expression in DRGs under pathological pain conditions. Apart from the DRG, DOCK4 was also detected in the sciatic nerve (SN, peripheral axons of DRG) and the dorsal root (DR, central axons of DRG) (Fig. 1l), indicating that DOCK4, synthesized in the somata of DRG neurons, undergoes transportation to both their central and peripheral terminals. Immunostaining and in situ hybridization showed that DOCK4 protein and corresponding mRNA were expressed in most DRG neurons of mice (Fig. 1m). Size frequency analysis showed widespread DOCK4 distribution across small, medium, and large diameter DRG neurons (Fig. S1d). Double immunostaining confirmed DOCK4 expression in nonpeptidergic (IB4-positive) and peptidergic (CGRP-positive) small diameter nociceptors and also expressed in large diameter myelinated neurons (NF200-positive) (Fig. 1n). Colocalization analysis revealed that 88.33 ± 2.98% IB4-positive, 63.99 ± 5.86% CGRP-positive and 58.48 ± 4.36% NF200-positive DRG neurons expressed DOCK4 (Fig. S1e). Meanwhile, 44.97 ± 5.03%, 18.94 ± 2.17% and 25.82 ± 2.55% of DOCK4-positive DRG neurons presented IB4, CGRP and NF200, respectively (Fig. S1f). We further characterized the distribution pattern of DOCK4 in the peripheral and central terminals of the DRG. The results showed that DOCK4 co-localized with IB4, CGRP, and NF200-positive axons in the sciatic nerve (Fig. 1o). In spinal dorsal horn, DOCK4 was expressed in IB4- and CGRP-positive nerve terminals, and was scarcely expressed in NF200-positive nerve terminals (Fig. S1g). However, DOCK4 in the dorsal root colocalized with NF200-positive axons (Fig. S1h). Thus, these results demonstrate that DOCK4 is expressed in DRG sensory neurons, including their peripheral and central axons, with its expression significantly reduced in DRGs under conditions of pathological pain, including neuropathic, inflammatory, chemotherapy, and bone cancer.
a To identify common mechanisms and targets for different types of pathological pain, we established four pain models. We then collected L4-L6 DRG tissues from mice with pathological pain for RNA-seq analysis. (Created in BioRender. https://BioRender.com/xs51blc). b The expression of the three genes Dock4, Afmid, and Rpl15-ps3 was simultaneously decreased in the L4-L6 DRGs tissue of mice across the SNL, CFA, PTX, and LLC models. n = 3 samples per group. c Compared with naïve mice, the fold decrease of Dock4, Afmid, and Rpl15-ps3 in the L4-L6 DRGs of mice after SNL, CFA, PTX, and LLC treatment. d–g The mRNA expression of Dock4 in L4-L6 DRG tissues of SNL (Day 7), CFA (Day 3), PTX (Day 7) and LLC (Day 14) mice. n = 3 samples per group in d; n = 4 samples per group in e, f and g. t4 = 5.643, P = 0.0049 in d; t6 = 5.52, P = 0.0015 in e; t6 = 3.451, P = 0.0136 in f; t6 = 3.22, P = 0.0181 in (g). h–k The DOCK4 protein expression in L4-L6 DRG tissues of SNL (Day 7), CFA (Day 3), PTX (Day 7) and LLC (Day 14) mice. n = 3 samples per group. t4 = 4.58, P = 0.0102 in h; t4 = 3.169, P = 0.0339 in i; t4 = 6.461, P = 0.003 in j; t4 = 5.52, P = 0.0053 in k. l The expression abundance of the DOCK4 protein in sciatic nerve (SN), L4-L6 DRGs, and L4-L6 dorsal root (DR) tissues of Dock4WT (Dock4flox/flox) and AvCreERT2; Dock4CKO mice. m The immunostaining and in situ hybridization showed that DOCK4 protein and corresponding mRNA were expressed in most L4 DRG neurons of mice. Compared to Dock4WT mice, the lack of DOCK4 in the DRG of AvCreERT2; Dock4CKO mice following tamoxifen injection indicates the high specificity of both the DOCK4 antibody and probe. Scale bar, 100 μm. n, o Double immunostaining of DOCK4 with IB4, CGRP and NF200 in the L4 DRG and sciatic nerve of mice. Scale bar, left 100 μm, right 25 μm. d–k Two-tailed Independent Student’s t test. *P < 0.05, **P < 0.01. Data are presented as mean ± SEM. Complete sample size and sex is provided in the Supplementary Data. Source data are provided as a Source Data file.
Histone lactylation mediates the decrease of DOCK4
To further investigate the mechanism for the decrease of DOCK4 after pathological pain, we analyzed RNA-seq data and revealed that Ldha was increased in the DRG tissues of mice subjected to SNL, CFA, PTX, and LLC models of pathological pain (Fig. S2a). LDHA is a key enzyme in lactate production, and lactate serves as an important substrate for protein lactylation modification (Fig. S2b)16,26. Histone lactylation has been identified as a type of epigenetic modification that directly regulates gene transcription from chromatin16. To further determine the role of lactylation in regulating the expression of DOCK4, we first examined the changes in pan-lactylation (Pan Kla) levels in the DRG tissues following pathological pain. The data showed that in the DRG tissues of neuropathic pain, inflammatory pain, chemotherapeutic pain, and bone cancer pain mice, the Pan Kla was increased (Fig. 2a–d), concomitant with increased lactate levels (Fig. 2e). To further determine the effect of lactylation on DOCK4 expression, we injected sodium lactate (NaLA) intrathecally27 into mice (Fig. 2f) to increase the lactylation levels in the DRG tissues (Fig. S2c). This resulted in a significant reduction in DOCK4 expression in DRGs (Fig. 2g, h). Injection of sodium oxamate (an LDH inhibitor)27 (Fig. 2i), which decreased lactate levels (Fig. S2d) and Pan Kla (Fig. S2e), significantly increased the expression of DOCK4 in the DRG tissues (Fig. 2j, k). Sodium oxamate also significantly reversed the downregulation of DOCK4 in DRG tissues induced by SNL, CFA, PTX, and LLC (Fig. 2l–o). These results indicate that lactylation mediates the decrease in DOCK4 expression. We next examined the changes in histone lactylation levels in the DRG tissues after pathological pain. The data showed that nerve injury increased the levels of both H4K8la and H4K12la in DRG of mice, but did not alter the levels of H3K9la, H3K18la, H3K14la, H4K5la, or H4K16la (Fig. 2p). In CFA mice, only H4K8la levels increased in the DRGs, whereas the levels of H4K12la and H4K16la remained unchanged (Fig. 2q). Consistently, the levels of H4K8la were significantly elevated in DRG of mice after PTX and LLC treatment (Fig. 2r, s). Using chromatin immunoprecipitation (ChIP) combined with quantitative real-time PCR (qPCR), we found that H4K8la was significantly enriched in the promoter regions of the Dock4 gene in the DRGs following pathological pain (Fig. 2t–w). However, the inhibition of lactylation through sodium oxamate treatment greatly reduced the enrichment of H4K8la in the Dock4 promoter region (Fig. 2t–w). As anticipated, after administration of NaLA, we observed an increase in the levels of H4K8la in the Dock4 promoter (Fig. 2x). Collectively, these findings suggest that the downregulation of DOCK4 following pathological pain is regulated by histone lactylation (Fig. 2y).
a–d The pan-lactylation (Pan Kla) levels in the DRG tissues of SNL, CFA, PTX, and LLC mice. e Changes in lactate levels in the DRG tissues of mice subjected to SNL, CFA, PTX, and LLC. n = 6 mice in control, PTX and LLC groups; n = 8 mice in SNL group; n = 7 mice in CFA group. F(4, 28) = 7.84, P = 0.011 in SNL vs. control, P = 0.0002 in CFA vs. control, P = 0.0436 in PTX vs. control, P = 0.0007 in LLC vs. control. f The schematic diagram of intrathecal (i.t.) injection of sodium lactate (NaLA) (Created in BioRender. https://BioRender.com/xs51blc). g, h The effect of intrathecal injection of sodium lactate (NaLA, 50 μM) on the expression of DOCK4 in DRG tissues. n = 3 samples per group. t4 = 4.096, P = 0.0149 in (g); t4 = 2.894, P = 0.0444 in (h). i The schematic diagram of intraperitoneal (i.p.) injection of oxamate (Created in BioRender. https://BioRender.com/xs51blc). j, k The effect of intraperitoneal injection of oxamate (300 mg/kg) on the expression of DOCK4 in DRG tissues. n = 3 samples per group. t4 = 7.543, P = 0.0017 in (j); t4 = 2.837, P = 0.047 in (k). l–o The effect of oxamate on the expression of Dock4 mRNA in DRG tissues of SNL, CFA, PTX, and LLC mice. n = 3 samples per group in l, m and o; n = 4 samples per group in (n). F(2, 6) = 12.34, P = 0.0097 in sham vs. SNL, P = 0.0156 in SNL vs. SNL + oxamate in l; F(2, 6) = 11.14, P = 0.0224 in saline vs. CFA, P = 0.0113 in CFA vs. CFA + oxamate in m; F(2, 9) = 8.226, P = 0.039 in vehicle vs. PTX, P = 0.0093 in PTX vs. PTX + oxamate in n; F(2, 6) = 15.54, P = 0.0116 in IC vs. LLC, P = 0.0048 in LLC vs. LLC + oxamate in o. p The change of H3K9la, H3K18la, H3K14la, H4K5la, H4K8la, H4K12la, and H4K16la level in DRG of mice after SNL treatment. n = 3-4 samples per group. t4 = 6.515, P = 0.0029 in H4K8la; t4 = 4.34, P = 0.0122 in H4K12la. q The change of H4K8la, H4K12la, and H4K16la level in DRG of mice after CFA treatment. n = 4 samples per group. t6 = 2.691, P = 0.036. r The change of H4K8la level in DRG of mice after PTX treatment. n = 4 mice per group. t6 = 2.75, P = 0.0333. s The change of H4K8la level in DRG of mice after LLC treatment. n = 3 samples per group. t4 = 3.071, P = 0.0372. t–w ChIP-qPCR was used to assess the impact of oxamate on the relative occupancy of H4K8la with the Dock4 promoter in the DRGs of mice subjected to SNL, CFA, PTX, and LLC. n = 3 per group. F(2, 6) = 9.186, P = 0.0334 in sham vs. SNL, P = 0.0175 in SNL vs. SNL + oxamate in (t); F(2, 6) = 9.492, P = 0.0214 in saline vs. CFA, P = 0.0219 in CFA vs. CFA + oxamate in (u); F(2, 6) = 13.41, P = 0.0089 in vehicle vs. PTX, P = 0.0111 in PTX vs. PTX + oxamate in (v); F(2, 6) = 9.727, P = 0.0125 in IC vs. LLC, P = 0.048 in LLC vs. LLC + oxamate in (w). x ChIP-qPCR was used to assess the impact of sodium lactate (NaLA) on the relative occupancy of H4K8la with the Dock4 promoter in the DRGs. n = 3 per group. t4 = 4.164, P = 0.0141. y The schematic shows that pathological pain upregulates lactic acid in the DRG, leading to increased lactylation of histone H4K8, which inhibits Dock4 gene transcription (Created in BioRender. https://BioRender.com/xs51blc). g, h, j, k, p, q, r, s, x, Two-tailed Independent Student’s t test; e, l–o, t–w, One-way ANOVA followed by Tukey’s multiple comparisons test. *P < 0.05, **P < 0.01, ***P < 0.001, n.s. means not significant. Data are presented as mean ± SEM. Complete sample size and sex is provided in the Supplementary Data. Source data are provided as a Source Data file.
The specific loss of DOCK4 in sensory neurons enhances heat nociception
To investigate the involvement of DOCK4 in sensory modulation, we injected DOCK4-siRNA via the sciatic nerve to suppress the expression of DOCK4 in DRGs (Fig. 3a). Immunofluorescence analysis revealed a reduction in DOCK4 expression in the DRGs of mice treated with DOCK4-siRNA (Fig. S3a, b). The behavioral data demonstrated that mice injected with DOCK4-siRNA exhibited normal touch sensitivity in the cotton (Fig. S3c) and foot tape (Fig. S3d) tests. Mice treated with DOCK4-siRNA maintained normal withdrawal thresholds when subjected to von Frey filament-induced punctate mechanical stimulation (Fig. S3e) and brush-induced dynamic mechanical stimulation (Fig. S3f). DOCK4 knockdown in mice DRGs did not result in a significant alteration in the sensitivity to intense mechanical stimulation in the pinprick test (Fig. S3g), but significantly reduced the thermal withdrawal threshold induced by the Hargreaves test in both male and female mice, thereby enhancing heat nociception (Fig. 3b, S3h). There were no differences in responses to the evaporative cooling test between mice injected with nontargeting (NT)-siRNA and those injected with DOCK4-siRNA (Fig. S3i). This suggests that DOCK4 is involved in the regulation of thermal nociception. To confirm these results, we conducted a short-term conditioned place aversion (CPA) assay28 (Fig. 3c). During the experiment, we measured the time the mice spent in the dark chamber pre- and post-stimulus. The difference between these two-time intervals (pre- minus post-defined as an aversion score) was then used to assess the extent of CPA. We observed that the average withdrawal latency for the NT-siRNA injected mice was approximately 10 seconds (Fig. 3b), whereas it was approximately 6 seconds for DOCK4-siRNA injected mice in Hargreaves thermal radiation test (Fig. 3b). Based on these findings, we conducted the CPA experiments utilizing a cutoff value of 8 seconds for the thermal radiation stimulation. The data showed that mice repeatedly stimulated with a thermal radiation source (cutoff 8 seconds) in a dark chamber developed CPA after DOCK4-siRNA injection, unlike controls (Fig. 3d). The punctate (von Frey 1.0 g) and dynamic (brush) mechanical stimuli did not induce CPA in either control or DOCK4-siRNA injected mice (Fig. S3j, k). Further, we performed intrathecal injection of an adeno-associated virus (AAV) delivery vector called AAV-hSyn-Cre-GFP, which contained the Cre gene controlled by the neuronal promoter hSyn (Fig. 3e). The injections were administered in Dock4flox/flox mice, resulting in a conditional knockout of DOCK4 expression in the DRG neurons. Indeed, the presence of GFP in the DRG neurons of the control AAV (AAV-GFP)- and experimental AAV (AAV-Cre-GFP)-injected mice provides confirmation of transgene expression specifically in the DRG neurons (Fig. S4a). The infection rates in the DRG were comparable between the AAV-GFP and the AAV-Cre-GFP groups, regardless of whether the mice are male or female (Fig. S4a). Additionally, significant reductions in the expression of DOCK4 had been observed in both the mRNA (Fig. S4b) and protein (Fig. S4c) levels in DRG tissues. Based on the behavioral data, it was observed that the injection of AAV-Cre into mice did not induce any significant changes in their tactile sensitivity towards cotton (Fig. S4d), back tape (Fig. S4e), and foot tape (Fig. S4f) stimulation. Similarly, AAV-Cre administration did not have an impact on the mice’s mechanical pain responses to punctate (Fig. S4g), brush (Fig. S4h), pinprick (Fig. S4i), or tail clip (Fig. S4j) stimuli. Significantly, it was found that the injection of AAV-Cre led to a notable reduction in the thermal nociceptive threshold of mice in response to Hargreaves (Fig. 3f), tail-flick (Fig. 3g), and hot plate tests (Fig. 3h), indicating increased sensitivity to thermal pain stimuli. No significant differences were observed in the responses to the evaporative cooling test between the mice injected with control AAV and those injected with AAV-Cre (Fig. S4k). These results suggest that DOCK4 specifically influences thermal pain modulation without affecting other sensory modalities like touch, mechanical pain, or cooling sensitivity. Furthermore, we investigated the role of DOCK4 in spontaneous pain. We assessed the spontaneous pain behavior of mice by measuring the number and duration of licking or flinching within a one-hour period. The results showed that AAV-Cre injection did not affect the spontaneous pain behavior of mice, regardless of the number or duration of licking or flinching (Fig. S4l, m). These findings indicate that DOCK4 may not play a significant role in modulating spontaneous pain.
a Diagram illustrating the injection of non-targeting (NT)-siRNA or DOCK4-siRNA into the sciatic nerve of mice, followed by behavioral and immunostaining tests conducted 2 days post-injection. b The behaviors were tested in NT-siRNA or DOCK4-siRNA injected mice by Hargreaves experiment. n = 16 mice/group. t30 = 4.411, P = 0.0001. c Short-term conditional place aversion (CPA) assays pairing in dark chamber with heat (cutoff 8 s), punctate (1.0 g) and dynamic stimuli were performed in NT-siRNA and DOCK4-siRNA injected mice. d The short-term CPA scores induced by Hargreaves stimuli was assessed in NT-siRNA and DOCK4-siRNA injected mice. n = 7 mice/group. t12 = 2.213, P = 0.047. e Diagram illustrating the intrathecal injection of AAV-hSyn-GFP or AAV-hSyn-Cre-GFP into Dock4flox/flox mice, followed by behavioral and molecular biology tests conducted 21 days post-injection. f–h The behaviors were tested in AAV-GFP and AAV-Cre injected Dock4flox/flox mice by Hargreaves, tail-flick, and hot plate experiments. n = 6 mice/group. t10 = 5.439, P = 0.0003 in (f). t10 = 3.303, P = 0.008 in 46 °C; t10 = 3.053, P = 0.0122 in 48 °C; t10 = 6.82, P = 0.000046 in 50°C in (g). t10 = 4.76, P = 0.0008 in 50 °C; t10 = 3.952, P = 0.0027 in 52 °C; t10 = 3.379, P = 0.007 in 55°C in (h). i The CPA scores induced by Hargreaves training was assessed in AAV-GFP and AAV-Cre injected Dock4flox/flox mice. n = 6 mice/group. t10 = 8.817, P = 0.000005. j Diagram depicting the intraperitoneal injection (i.p.) of tamoxifen (TAM) into Dock4WT (Dock4flox/flox) and AvCreERT2; Dock4CKO mice, followed by subsequent behavioral and molecular biology tests. k–r The behaviors were tested in Dock4WT and AvCreERT2; Dock4CKO mice by Hargreaves, tail-flick, and hot plate experiments with or without TAM injection. n = 8 mice in Dock4WT group, n = 10 mice in AvCreERT2; Dock4CKO group. t18 = 8.834, P = 0.00000006 in (k); t18 = 7.735, P = 0.0000008 in (l); t18 = 4.432, P = 0.0003 in (m); t18 = 4.089, P = 0.0007 in (n); t18 = 3.913, P = 0.001 in (o); t18 = 4.486, P = 0.0003 in (p); t18 = 5.998, P = 0.000011 in (q); t18 = 4.628, P = 0.0002 in (r). s The CPA scores induced by Hargreaves training was assessed in with or without TAM injected Dock4WT and AvCreERT2; Dock4CKO mice. n = 8 mice in Dock4WT group, n = 10 mice in AvCreERT2; Dock4CKO group. t18 = 3.745, P = 0.0015. t-v The behaviors were tested in Dock4WT and Trpv1-Cre; Dock4CKO mice by Hargreaves, tail-flick, and hot plate experiments. n = 13 mice in Dock4WT group, n = 12 mice in Trpv1-Cre; Dock4CKO group. t23 = 3.088, P = 0.0052 in t. t23 = 2.377, P = 0.0261 in 46 °C; t23 = 7.244, P = 0.0000002 in 48 °C; t23 = 4.257, P = 0.0003 in 50 °C; t23 = 2.875, P = 0.0086 in 52 °C in u. t23 = 4.481, P = 0.0002 in 50 °C; t23 = 2.171, P = 0.0405 in 52 °C; t23 = 2.287, P = 0.0318 in 55 °C in v. b, d, f–i, k–v, Two-tailed Independent Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001, n.s. means not significant. Data are presented as mean ± SEM. Complete sample size and sex is provided in the Supplementary Data. Source data are provided as a Source Data file.
To evaluate the emotional and cognitive aspects of thermal pain, we conducted a long-term CPA assay29,30 (Fig. S4n). During the experiment, we measured the time the mice spent in the dark chamber A on the first day of preconditioning (pre) and the eighth day of postconditioning (post). The difference between these two-time intervals (pre-post) was then used to assess the extent of CPA. This assay allowed us to gauge the aversive response associated with thermal pain and provided insights into the emotional and cognitive processing of pain in the mice. The results showed that Hargreaves (cutoff 8 seconds) conditioning had no effect on the time spent by control mice in the dark chamber, but significantly reduced the time spent by AAV-Cre mice in the dark chamber (Fig. S4o). Compared to control mice, AAV-Cre mice exhibited significantly higher aversion scores (Fig. 3i). The punctate (von Frey 1.0 g) and dynamic conditioning stimuli had no impact on the time spent in the dark chamber by both control and AAV-Cre mice (Fig. S4p, r). And, these stimuli did not alter the aversion scores in either of the two groups (Fig. S4q, s). These findings suggest that DOCK4 is specifically involved in the processing of affective and/or cognitive aspects of thermal pain, but not mechanical pain.
To determine the specific role of DOCK4 expressed by DRG neurons in pain regulation, we generated a conditional, tamoxifen (TAM)-inducible DRG neurons DOCK4 knockout mouse line (AvCreERT2; Dock4CKO) by crossing Dock4flox/flox mice with AvCreERT2 mice (Fig. 3j). The AvCreERT2 mouse line enables TAM-induced activation of the Cre recombinase, driven by the Advillin promoter specifically in DRG neurons, to facilitate gene knockout in adult mice31,32. After TAM injections, the protein and mRNA expression levels of DOCK4 in DRGs of AvCreERT2; Dock4CKO mice were significantly reduced (Fig. 1m), which also suggests that the antibody and probe used have good specificity. Behavioral data showed that TAM injection did not alter tactile or nociceptive behaviors in Dock4WT (Dock4flox/flox) mice (Fig. 3k–s, S5a–k), indicating TAM itself did not affect the mice’s pain perception. Furthermore, AvCreERT2; Dock4CKO mice exhibited normal touch (Fig. S5a–c) and mechanical pain (Fig. S5d–g) sensitivity after TAM treatment. Importantly, TAM-induced conditionally knock-out of DOCK4 in DRG neurons significantly decreased thermal nociceptive threshold in mice (Fig. 3k–r), eliciting thermal hyperalgesia. No significant differences were observed in the responses to the evaporative cooling test in AvCreERT2; Dock4CKO mice injected with TAM (Fig. S5h). Conditional knock out of DOCK4 in DRG neurons also did not change the spontaneous pain behavior of mice (Fig. S5i, j). Moreover, in the long-term CPA test, Hargreaves (cutoff 8 seconds) conditioning had no effect on the time spent by Dock4WT mice in the dark chamber, but significantly reduced the time spent by AvCreERT2; Dock4CKO mice in the dark chamber after TAM treatment (Fig. S5k). TAM administration resulted in a significant increase in aversion scores in AvCreERT2; Dock4CKO mice as measured by Hargreaves conditioning CPA test (Fig. 3s). Thus, specific loss of DOCK4 in sensory neurons of adult mice enhances heat nociception.
Research indicates that TRPV1-positive small-sized DRG neurons play a crucial role in the perception of thermal nociceptive stimuli22. Further, we crossed Trpv1-Cre mice with Dock4flox/flox mice (Trpv1-Cre; Dock4CKO) to conditionally knock out DOCK4 in nociceptive neurons and observed its effects on pain. Behavioral data revealed that compared with Dock4WT mice, Trpv1-Cre; Dock4CKO mice showed comparable mechanical sensitivity responses to cotton (Fig. S5l), punctate of vonFrey (Fig. S5m), dynamic of brush (Fig. S5n) and pinprick (Fig. S5o) stimulation. Consistently, compared to Dock4WT mice, Trpv1-Cre; Dock4CKO mice exhibited heightened sensitivity to heat pain in the thermal radiation (Fig. 3t), tail-flick (Fig. 3u), and hot plate (Fig. 3v) tests. No significant differences were observed in the responses to the evaporative cooling test between Dock4WT and Trpv1-Cre; Dock4CKO mice (Fig. S5p). Thus, specific deletion of DOCK4 in TRPV1-positive nociceptive sensory neurons also increases thermal pain sensitivity. Considering the broad expression of DOCK4 in DRG neurons, we further assessed the impact of DOCK4 in low-threshold mechanoreceptors on touch sensitivity and motor function in mice. Aβ fibers are primarily involved in mediating low-threshold mechanical sensation, and NF200 is commonly used as a marker for Aβ fibers33,34. To further investigate the role of DOCK4 in low-threshold mechanoreceptors, we crossed Nefh (gene of NF200)-Cre mice with Dock4flox/flox mice to achieve the conditional knockout of DOCK4 in NF200-positive low-threshold mechanosensory neurons (Nefh-Cre; Dock4CKO). Behavioral analyses revealed that, compared with Dock4WT mice, Nefh-Cre; Dock4CKO mice showed no differences in tactile sensitivity (Fig. S5q–s) or motor coordination (Fig. S5t, u). This suggests that DOCK4 in low-threshold mechanoreceptors does not regulate tactile sensitivity or motor function.
Overexpression of DOCK4 decreases heat nociception and alleviates pathological heat hyperalgesia
To further confirm the role of DOCK4 in pain modulation, we intrathecally injected CAS9-Easy-SAM lentivirus vector to overexpress DOCK4 in the DRGs. CAS9-Easy-SAM is a lentiviral vector system that incorporates the clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) technology along with the synergistic activation mediator (SAM) system35. The SAM system enables efficient gene activation by targeting specific genomic loci and recruiting transcriptional activation domains35. This approach allows for precise and targeted gene activation, making it a valuable tool for studying the function of genes in various biological processes and disease models36. We designed three specific sgRNAs to target the Dock4 gene and employed the SV40-MS2-P65-HSF1 activation factor with GFP labeling (Fig. 4a). After screening in HEK293T cells, sgRNA3 showed the best overexpression of Dock4 mRNA (Fig. S6a). We then injected CAS9-Easy-SAM lentivirus (LV) carrying sgRNA3 intrathecally (LV-sgRNA3-GFP). The results demonstrated significant green fluorescence expression in DRG neurons, indicating successful transgene expression (Fig. S6b). The infection rates in the DRGs of male and female mice were comparable between the control LV (LV-GFP) and the LV-sgRNA3-GFP groups (Fig. S6b). Moreover, both DOCK4 mRNA and protein levels significantly increased in DRGs, confirming the effectiveness of CAS9-Easy-SAM lentivirus injection (Fig. S6c, d). However, it is important to note that the injection of CAS9-Easy-SAM lentivirus carrying sgRNA3 did not affect the expression of pain-regulating genes, such as sodium channels and TRP channels (Fig. S6e). Behavioral data showed that overexpression of DOCK4 did not alter touch sensitivity in mice (Fig. S6f-h). Mice overexpressing DOCK4 exhibited comparable mechanical pain sensitivity to control mice, regardless of whether the mechanical stimulation was mild (Fig. S6i, j) or intense (Fig. S6k, l). Notably, overexpression of DOCK4 significantly increased thermal nociceptive threshold in mice, as observed in tests involving thermal radiation (Fig. 4b), tail-flick (Fig. 4c), and hot plate (Fig. 4d). In contrast, mice overexpressing DOCK4 displayed normal cold sensitivity in the evaporative cooling test (Fig. S6m). Then, we investigated the impact of DOCK4 overexpression on the long-term CPA assay with thermal pain stimulation. Considering that the average withdrawal latency was approximately 12 seconds in control mice, and approximately 16 seconds in DOCK4-overexpressed mice in the Hargreaves test (Fig. 4b), we conducted the long-term CPA conditioning experiment using a cutoff value of 14 seconds for Hargreaves thermal radiation stimulation. The CPA data showed that Hargreaves (cutoff 14 seconds) conditioning decreased the time spent by control mice in the dark chamber, but had no effect on the time spent by DOCK4-overexpressed mice in the dark chamber (Fig. 4e, left). This suggests that the induction of CPA was significant in control mice but not in DOCK4-overexpressed mice (Fig. 4e, left). Moreover, the overexpression of DOCK4 substantially decreased the scores of CPA compared to the control mice (Fig. 4e, right). To explore the role of DOCK4 in pathological pain, we conducted further investigations. The behavioral data demonstrated that the overexpression of DOCK4 significantly relieved thermal hyperalgesia (Fig. 4f, g), but not mechanical allodynia (Fig. S6n) induced by CFA. Consistently, the overexpression of DOCK4 lessened heat hyperalgesia (Fig. 4h, i), but not mechanical allodynia (Fig. S6o) induced by SNL. Considering that overexpressing DOCK4 increased the baseline threshold for thermal pain in mice, we standardized the baseline values to determine whether the alleviating effect of DOCK4 overexpression on thermal hyperalgesia was due to changes in the baseline threshold. We found that after standardizing the baseline values, overexpressing DOCK4 still significantly alleviated thermal hyperalgesia induced by CFA (Fig. 4j) and SNL (Fig. 4k). This indicates that the relieving effect of DOCK4 on thermal hyperalgesia is not solely dependent on changes in the baseline threshold. DOCK4 overexpression also significantly alleviated heat hyperalgesia (Fig. 4l-q), but did not affect mechanical allodynia (Fig. S6p, q) induced by PTX and LLC. Thus, these data suggest that overexpression of DOCK4 decreases heat nociception and alleviates pathological heat hyperalgesia in mice.
a Diagram illustrating the intrathecal injection of CAS9-Easy-SAM lentivirus (LV) vector into mice, followed by behavioral and molecular biology tests conducted 14 days post-injection (Created in BioRender. https://BioRender.com/xs51blc). b–d The impact of DOCK4 overexpression on behavioral responses to Hargreaves, tail-flick, and hot plate experiments. n = 10 mice in control, n = 8 mice in DOCK4-over in b and d; n = 9 mice in control, n = 12 mice in DOCK4-over in c. t16 = 3.662, P = 0.0021 in (b). t19 = 2.819, P = 0.011 in 46 °C; t19 = 2.406, P = 0.0256 in 48 °C; t19 = 5.578, P = 0.000022 in 50 °C; t19 = 3.683, P = 0.0016 in 52°C in (c). t16 = 2.612, P = 0.0189 in 50 °C; t16 = 3.913, P = 0.0012 in 52 °C; t16 = 2.49, P = 0.0242 in 55°C in (d). e Left: the absolute time spent in the dark A chamber before (pre) versus after (post) Hargreaves pairing in control and DOCK4-overexpressed mice. Right: The CPA scores induced by Hargreaves training was assessed in control and DOCK4-overexpressed mice. n = 6 mice per group. t10 = 4.026, P = 0.0024 in left; t10 = 2.434, P = 0.0352 in right. f-i The impact of DOCK4 overexpression on the pathological thermal hyperalgesia induced by CFA and SNL (Created in BioRender. https://BioRender.com/xs51blc). n = 6 mice/group. F(1, 40) = 63.57, P = 0.049 in 0 d, P = 0.0103 in 1 d, P = 0.000019 in 3 d, P = 0.000082 in 5 d in g; F(1, 50) = 170.8, P = 0.0015 in 0 d, P = 0.000007 in 4 d, P = 0.000003 in 7 d, P = 0.00000007 in 10 d, P = 0.000000007 in 14 d in i. j, k The impact of DOCK4 overexpression on the thermal hyperalgesia induced by CFA and SNL (normalized to 0 day). n = 6 mice/group. F(1, 40) = 22.99, P = 0.0016 in 3 d, P = 0.0051 in 5 d in j; F(1, 50) = 39.9, P = 0.0219 in 4 d, P = 0.028 in 7 d, P = 0.0015 in 10 d; P = 0.0003 in 14 d in k. l-q The impact of DOCK4 overexpression on the pathological thermal hyperalgesia induced by PTX and LLC (Created in BioRender. https://BioRender.com/xs51blc). n = 8 mice in control, n = 9 mice in DOCK4-over in m and n; n = 9 mice in control, n = 10 mice in DOCK4-over in p and q. F(1, 60) = 183.5, P = 0.000041 in 0 d, P = 0.000000002 in 5 d, P = 0.0000000004 in 7 d, P = 0.00000001 in 14 d in m; F(1, 60) = 27.76, P = 0.0029 in 5 d, P = 0.0005 in 7 d, P = 0.0231 in 14 d in n; F(1, 68) = 94.76, P = 0.0048 in 0 d, P = 0.0001 in 7 d, P = 0.00001 in 10 d, P = 0.00000005 in 14 d in p; F(1, 68) = 18.08, P = 0.0325 in 10 d, P = 0.0005 in 14 d in q. r-t Schematic diagram for the in vivo calcium imaging of sensory neurons and the representative images of GCaMP6 fluorescence as a measure of intracellular calcium following stimulation to the hindpaw (Created in BioRender. https://BioRender.com/xs51blc). Scale bar, 100 μm. u In comparison to Dock4WT mice, small, medium, and large-sized DRG neurons in AvCreERT2; Dock4CKO mice exhibited no significant changes in their responses to brush stimulation ( < 500 μm2 Dock4WT, n = 33 cells; AvCreERT2; Dock4CKO, n = 35 cells; 500-1000 μm2 Dock4WT, n = 28 cells; AvCreERT2; Dock4CKO, n = 25 cells; >1000 μm2 Dock4WT, n = 11 cells; AvCreERT2; Dock4CKO, n = 12 cells). v Compared to Dock4WT mice, all sized DRG neurons in AvCreERT2; Dock4CKO mice showed no significant changes in response to pinch stimulation ( < 500 μm2 Dock4WT, n = 105 cells; AvCreERT2; Dock4CKO, n = 116 cells; 500-1,000 μm2 Dock4WT, n = 52 cells; AvCreERT2; Dock4CKO, n = 61 cells; >1,000 μm2 Dock4WT, n = 13 cells; AvCreERT2; Dock4CKO, n = 15 cells). w In comparison to Dock4WT, small and medium-sized DRG neurons in AvCreERT2; Dock4CKO mice showed significantly increased responses to heat stimulation ( < 500 μm2 Dock4WT, n = 61 cells; AvCreERT2; Dock4CKO, n = 132 cells; 500-1,000 μm2 Dock4WT, n = 9 cells; AvCreERT2; Dock4CKO, n = 25 cells; >1,000 μm2 Dock4WT, n = 3 cells; AvCreERT2; Dock4CKO, n = 5 cells). t191 = 3.535, P = 0.0005 in <500 μm2; t32 = 3.415, P = 0.0018 in 500-1,000 μm2. x Compared to Dock4WT mice, all sized DRG neurons in AvCreERT2; Dock4CKO mice showed no significant alters in response to capsaicin stimulation ( < 500 μm2 Dock4WT, n = 43 cells; AvCreERT2; Dock4CKO, n = 51 cells; 500-1,000 μm2 Dock4WT, n = 2 cells; AvCreERT2; Dock4CKO, n = 5 cells; >1,000 μm2 AvCreERT2; Dock4CKO, n = 2 cells). b-e, u-x, Two-tailed Independent Student’s t test; g, i–k, m, n, p, q, Two-way ANOVA followed by Bonferroni’s multiple comparisons test. *P < 0.05, **P < 0.01, ***P < 0.001, n.s. means not significant. Data are presented as mean ± SEM. Complete sample size and sex is provided in the Supplementary Data. Source data are provided as a Source Data file.
Loss of DOCK4 enhances the activity of sensory neurons in response to heat stimulation
Further, we utilized in vivo intracellular calcium imaging to assess the activity of DRG neurons, aiming to determine whether DOCK4 influences neuronal activity induced by heat stimulation. Dock4WT and AvCreERT2; Dock4CKO mice were intrathecally injected with an AAV delivery vector encoding the calcium indicator GCaMP6 (rAAV-CAG-GCaMp6s) (Fig. 4r). Following this, we observed the activity of L5 DRG neurons using Multiphoton Laser Confocal Microscope (Fig. 4s). The change of fluorescence signal after the application of stimulations in the hindpaw indicates the activity of DRG neurons. Following stimulations of brush, pinch, heat and capsaicin into the hindpaw, we observed robust activation of sensory neurons in L5 DRG (Fig. 4t). Furthermore, we isolated the neuronal responses by cell profile area and found that brush and pinch stimuli induced comparable intracellular calcium levels in DRG neurons of both Dock4WT and AvCreERT2; Dock4CKO mice, which include small- ( <500 μm2), medium- (500-1000 μm2), and large- ( > 1000 μm2) diameter neurons (Fig. 4u, v). Importantly, in comparison with Dock4WT mice, the small- ( <500 μm2) and medium- (500-1,000 μm2), but not larger- ( > 1000 μm2) diameter DRG neurons exhibited a significant hyper-responsiveness to heat stimulation applied to the hindpaw in AvCreERT2; Dock4CKO mice (Fig. 4w). However, the number of heat-activated neurons with a diameter > 1000 μm2 was very small (Fig. 4w). This indicates that the loss of DOCK4 increases the activity of sensory neurons in response to heat stimulation, but not to mechanical stimulation. The TRPV1 channel is an important ion channel expressed in DRG neurons that detects heat nociceptive stimuli37. Furthermore, we investigated whether DOCK4 regulates TRPV1 signaling in DRG neurons. The data showed that deletion of DOCK4 in sensory neurons did not affect the intracellular calcium responses in DRG neurons of different diameters following capsaicin (TRPV1 agonists)37 application to the hindpaw of mice (Fig. 4x). This suggests that DOCK4 may not be involved in regulating the TRPV1 signaling pathway in sensory neurons.
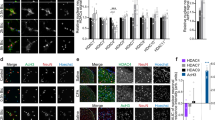
DOCK4 interacts with Nav1.7 and mediates trafficking of Nav1.7 from the membrane to the cytoplasm in sensory neurons
To investigate the underlying mechanism by which DOCK4 controls pain sensation, we employed the co-immunoprecipitation (co-IP) method to examine the interactions between DOCK4 and key channels involved in pain signaling, including TRP channels (such as TRPV1, TRPA1, and TRPM3)38 and Nav channels (such as Nav1.6, Nav1.7, Nav1.8, and Nav1.9)39 in the DRG. In co-IP experiments utilizing the DOCK4 antibody for pull-down, a potential interaction between DOCK4 and Nav1.7 was observed, with no detectable binding to TRPV1, TRPA1, TRPM3, Nav1.6, Nav1.8, and Nav1.9 in mouse DRG protein extracts (Fig. 5a–d). Similarly, using Nav1.7 antibody for co-IP pull-down experiments, we also observed a potential interaction between Nav1.7 and DOCK4 in mice DRGs (Fig. 5e). Immunofluorescence double staining revealed a co-localization of DOCK4 and Nav1.7 in DRGs (Fig. 5f). Co-localization analysis showed that 64.0 ± 2.92% of DOCK4-positive neurons expressed Nav1.7, while 78.18 ± 1.22% of Nav1.7-positive neurons expressed DOCK4 (Fig. S7a). To further confirm the specificity of the Nav1.7 antibody, we intrathecally injected AAV-hSyn-Cre-GFP into Nav1.7flox/flox mice to induce a conditional knockout of Nav1.7 in DRG neurons (Fig. S7b). The antibody specificity was validated by the lack of staining in the DRG after co-incubation with the blocking peptide (Fig. S7b). A previous study showed that Nav1.7 is not expressed in the cerebellum40. Staining was then performed on the cerebellum, and no positive Nav1.7 staining was observed (Fig. S7c), consistent with previous research findings41,42, confirming the antibody’s specificity for Nav1.7. Then, we employed structured illumination microscopy (SIM) to conduct high-resolution imaging, revealing that 26.15 ± 3.30% of DOCK4 exhibited colocalization with Nav1.7, while 23.07 ± 1.26% of Nav1.7 exhibited colocalization with DOCK4 in DRG neurons (Fig. 5g and Fig. S7d). To further confirm the potential DOCK4/Nav1.7 interaction, we conducted a proximity ligation assay (PLA) on dissociated DRG neurons30,43. The PLA analysis revealed positive fluorescence signals on the cell bodies of cultured DRG neurons from Dock4WT mice (Fig. 5h and Fig. S7e), indicating the presence of the DOCK4/Nav1.7 interaction. In contrast, no staining was observed in cultured DRG neurons from AvCreERT2; Dock4CKO mice DRG neurons (Fig. S7e). These results suggest a close spatial relationship between DOCK4 and Nav1.7, hinting at a potential interaction between these two molecules in DRG neurons. It is known that the expression and membrane trafficking of Nav1.7 in DRG neurons are crucial for the transmission of pain signals. We then examined the role of DOCK4 in regulating the membrane, cytoplasm, and total expression of Nav1.7 in DRGs of mice. The data revealed that compared to Dock4WT mice, the membrane expression of Nav1.7 was increased in the DRGs of AvCreERT2; Dock4CKO mice (Fig. 5i). Additionally, in AvCreERT2; Dock4CKO mice, there was a decrease in the cytoplasmic expression of Nav1.7 in DRGs (Fig. 5j), while the total expression of Nav1.7 remained unchanged (Fig. 5k). Compared to Dock4WT mice, the trafficking ratio of Nav1.7 was significantly increased in DRGs of AvCreERT2; Dock4CKO mice (Fig. S7f). We assessed the subcellular distribution of Nav1.7 in cultured DRG neurons derived from control AAV- and AAV-Cre-injected Dock4flox/flox mice. Notably, we observed a distinct membrane staining of Nav1.7 in DRG neurons from AAV-Cre mice when compared with control mice (Fig. 5l and Fig. S7g). The intensity of Nav1.7 immunoreactivity across the cell body was measured by the profile plots, and cells with a membrane-to-cytoplasm ratio greater than 1.5 were defined as Nav1.7 membrane positive30,44. (Fig. 5l). The proportion of Nav1.7 membrane-positive neurons was significantly increased in DRGs of AAV-Cre mice compared to controls (Fig. S7h). In contrast, we observed a significant decrease in Nav1.7 membrane expression (Fig. 5m) and a concurrent increase in Nav1.7 cytoplasmic expression (Fig. 5n), while the overall expression of Nav1.7 remained unaffected (Fig. 5o) in DRGs following DOCK4 overexpression. Overexpression of DOCK4 resulted in a decreased membrane trafficking ratio of Nav1.7 in DRGs (Fig. S7i). Thus, these suggest that DOCK4 interacts with Nav1.7 and promotes the trafficking of Nav1.7 from the membrane to the cytoplasm in DRG neurons. Further, we investigated the impact of DOCK4 on the Nav1.7 currents in DRG neurons. Nav1.7 currents were recorded in primary cultured DRG neurons by isolating them through the subtraction of ProTxII (a selective Nav1.7 channel blocker)-resistant Na+ currents from the total Na+ currents45. The current-voltage (I-V) curves demonstrated that compared with Dock4WT mice, the Nav1.7 current density in DRG neurons was significantly increased in AvCreERT2; Dock4CKO mice (Fig. 5p). Conditional knockout of DOCK4 had no impact on the activation, inactivation or recovery of Nav1.7 channels in DRG neurons (Fig. 5q, r). Further, we assessed the excitability of DRG neurons using current-clamp, and the data revealed that the number of action potentials elicited by the same stimulus was significantly enhanced in the AvCreERT2; Dock4CKO mice compared to the Dock4WT mice, and this increase could be reversed by the Nav1.7 blocker ProTxII (Fig. 5s, t). Additionally, we examined whether DOCK4 regulates Nav1.8 currents in DRG neurons. The results showed that knockout of DOCK4 in DRG neurons had no effect on Nav1.8 currents (Fig. S7j). Therefore, these data suggest that the loss of DOCK4 may increase the excitability of DRG neurons and the Nav1.7 current density by regulating the trafficking of Nav1.7 without altering its channel properties.
a–d DRG lysates were immunoprecipitated with DOCK4 antibody and immunoblotted with Nav1.6, TRPV1, Nav1.7, TRPA1, Nav1.8, Nav1.9, TRPM3 and DOCK4 antibody as indicated. This experiment was repeated three times. e DRG lysates were immunoprecipitated with Nav1.7 antibody and immunoblotted with DOCK4 and Nav1.7 antibody as indicated. This experiment was repeated three times. f Double immunostaining of DOCK4 and Nav1.7 in DRGs. Scale bar, 100 μm. g High-resolution images showing the colocalization of DOCK4 and Nav1.7 in DRG neurons. Scale bar, 10 μm. h Proximity ligation assay (PLA) showed positive signals of DOCK4/Nav1.7 interaction in cultured DRG neurons (5 images from two repeats). Scale bar, 20 μm. i–k Expression of Nav1.7 in the membrane, cytoplasmic, and total lysate from DRG neurons of Dock4WT and AvCreERT2; Dock4CKO mice. n = 3 samples per group. t4 = 4.187, P = 0.0138 in i; t4 = 16.44, P = 0.00008 in (j). l Representative images depicting Nav1.7 membrane and cytoplasmic staining in DRG neurons from AAV-GFP and AAV-Cre injected Dock4flox/flox mice are presented. Profile plots were utilized to delineate membrane staining. Subsequently, a ratio of membrane to cytoplasm was computed, with cells exhibiting a ratio exceeding 1.5 times defined as Nav1.7 membrane positive. Scale bar, 10 μm. m–o Expression of Nav1.7 in the membrane, cytoplasmic, and total lysate from DRG neurons of control and DOCK4 overexpressed mice. n = 3 samples per group. t4 = 6.05, P = 0.0038 in m; t4 = 4.389, P = 0.0118 in (n). p The I-V curves showed the peak Nav1.7 currents recorded under different potentials in primary cultured DRG neurons from Dock4WT and AvCreERT2; Dock4CKO mice. n = 6 neurons per group. F(1, 370) = 92.99, P = 8.87×10⁻²⁰. q, r Impacts of DOCK4 deletion on the activation, inactivation, and recovery of Nav1.7 channels in DRG neurons. n = 6-8 neurons per group. s, t The number of action potentials in DRG neurons was increased in the AvCreERT2; Dock4CKO mice compared to the Dock4WT mice, and this increase could be reversed by the Nav1.7 blocker ProTxII. n = 8 neurons per group. F(2, 21) = 12.76, P = 0.0005 in AvCreERT2; Dock4CKO vs. Dock4WT, P = 0.0012 in AvCreERT2; Dock4CKO vs. AvCreERT2; Dock4CKO + ProTxII. i–k, m–o, Two-tailed Independent Student’s t test; t, One-way ANOVA followed by Tukey’s multiple comparisons test; p–r, Two-way ANOVA followed by Bonferroni’s multiple comparisons test. *P < 0.05, **P < 0.01, ***P < 0.001, n.s. means not significant. Data are presented as mean ± SEM. Complete sample size and sex is provided in the Supplementary Data. Source data are provided as a Source Data file.
To further investigate whether DOCK4 regulates the function of the TRPV1, TRPM3, and TRPA1 channels, a trio of TRP channels mediating heat sensing38, we examined the impact of knocking out DOCK4 in DRG on spontaneous pain induced by capsaicin (a TRPV1 agonist)37, CIM0216 (a TRPM3 agonist)46, and isothiocyanate (AITC, a TRPA1 agonist)47. The behavioral data revealed that intraplantar injection of capsaicin (1 nmol/paw), CIM0216 (2.5 nmol/paw), or AITC (10 nmol/paw) elicited rapid spontaneous pain (measured by the number or duration of licking or flinching behaviors within 2 minutes) in Dock4WT mice (Fig. S8a–c). However, the deletion of DOCK4 in DRG neurons did not alter the spontaneous pain induced by capsaicin, CIM0216, or AITC (Fig. S8a–c). Further, we examined the effect of DOCK4 knockout on calcium signaling induced by activation of TRPM3, TRPA1, and TRPV1 in cultured DRG neurons. The results showed that knocking out DOCK4 in DRG neurons had no effect on calcium signaling triggered by TRPM3, TRPA1, or TRPV1 agonists (Fig. S8d, e). These suggest that DOCK4 may not play a role in regulating the function of TRPV1, TRPM3, or TRPA1 channels.
Disrupting DOCK4/Nav1.7 interaction enhances thermal pain sensitivity and increases Nav1.7 membrane expression
Although DOCK4 binds to Nav1.7, the specific interaction region remains unclear. Subsequently, we aimed to investigate the precise binding region between DOCK4 and Nav1.7. It has been proven that the C-terminal region of Nav1.7 channels plays a crucial role in channel modulation and protein-protein interactions48,49. Further, we determined whether DOCK4 interacted with the C-terminal region of Nav1.7 (Nav1.7CT). A GST pull-down assay showed a direct interaction between the purified DOCK4 and Nav1.7CT, and the 1825–1899 aa (Nav1.7CT-F2) in Nav1.7CT was the major binding site for DOCK4 (Fig. 6a, b). On the other hand, we divided DOCK4 into the N-terminal (DN, 1–655 aa), middle segment (DM, 656–1310 aa), and C-terminal (DC, 1311–1966 aa). We found that the specific region of DOCK4 mediating its interaction with Nav1.7CT was the C-terminal (DC, 1311–1966 aa) (Fig. 6c, d). To confirm the contribution of the DOCK4/Nav1.7 interaction to thermal sensation regulation, we constructed rAAV-hSyn-Nav1.7CT-F2-GFP for overexpressing the 1825–1899 aa fragment of Nav1.7CT-F2 (Fig. 6e) and it was aimed to block the endogenous DOCK4/Nav1.7 interaction as a competitive peptide. The GFP expression in the DRG neurons of the AAV-injected mice serves as a confirming indicator of Nav1.7-CT-F2 expression (Fig. 6f). The expression of the Nav1.7-CT-F2 fragment significantly inhibited the endogenous DOCK4/Nav1.7 interaction in the DRGs (Fig. 6g, h). Behavioral data showed that the thermal withdrawal latency of the AAV-Nav1.7-CT-F2-treated mice had a significant reduction in Hargreaves, tail-flick, and hot plate tests, compared to the control group (Fig. 6i–k). In addition, AAV-Nav1.7-CT-F2 injected mice showed significant positional aversion to the conditional chamber after a pairing with an 8-second cutting time Hargreaves stimulation in long-term CPA test, compared to control mice (Fig. 6l). The expression of the Nav1.7-CT-F2 fragment did not alter the mechanical pain sensitivity induced by pinprick (Fig. 6m). Thus, these data suggest that the disruption of DOCK4/Nav1.7 interaction enhances thermal pain sensation. Moreover, we explored the distribution of Nav1.7 immunoreactivity across the cell body. The profile plots showed significantly more Nav1.7 positive membrane expression on dissociated DRG neurons of AAV-Nav1.7-CT-F2 treated mice than that of control mice (Fig. 6n and Fig. S8f, g). Additionally, we recorded the Nav1.7 currents in cultured DRG neurons. The results showed that the Nav1.7 currents increased in the DRG neurons of AAV-Nav1.7-CT-F2 injected mice, compared to control mice (Fig. 6o). Together, disrupting the DOCK4/Nav1.7 interaction inhibits DOCK4-mediated trafficking of Nav1.7 from the membrane to the cytoplasm, leading to an increase in Nav1.7 membrane localization and Nav1.7 currents, which may intensify thermal nociception.
a Schematic diagram of the full-length C-terminus of Nav1.7 (Nav1.7CT) and various truncated fragment variants. b A GST pull-down assay with two purified proteins, GST-flag-Nav1.7CT and DOCK4, shows the direct interaction between DOCK4 and Nav1.7. The major interaction site is F2 (1825-1899 aa) of Nav1.7CT. This experiment was repeated three times. c Schematic diagram of the DOCK4 full-length and various truncated fragment variants. d A GST pull-down assay reveals the interaction between DOCK4 and Nav1.7, with the interaction site identified as the DC (1311-1966 aa) region of DOCK4. This experiment was repeated three times. e Nav1.7CT-F2 fragment (1825-1899 aa) AAV overexpression vector construction schematic. f The expression of GFP in the DRG neurons of AAV-injected mice indicates successful Nav1.7CT-F2 overexpression. Scale bar, 100 μm. g, h The effect of Nav1.7CT-F2 overexpression on the interaction between DOCK4 and Nav1.7. n = 3 repeats per group. t4 = 5.18, P = 0.0066. i–k The impact of Nav1.7CT-F2 overexpression on behavioral responses to Hargreaves, tail-flick, and hot plate tests. n = 6 mice per group. t10 = 3.296, P = 0.0081 in (i). t10 = 3.155, P = 0.0103 in 48 °C; t10 = 4.859, P = 0.0007 in 50 °C; t10 = 4.03, P = 0.0024 in 52°C in (j). t10 = 4.551, P = 0.0011 in 50 °C; t10 = 2.611, P = 0.026 in 52 °C; t10 = 4.679, P = 0.0009 in 55°C in k. l The CPA scores induced by Hargreaves training was assessed in control and Nav1.7CT-F2 overexpressed mice. n = 6 mice per group. t10 = 5.667, P = 0.0002. m The impact of Nav1.7CT-F2 overexpression on behavioral responses to intense mechanical pinprick tests. n = 6 mice per group. n The influence of Nav1.7CT-F2 overexpression on the distribution of Nav1.7 in the membrane and cytoplasm of cultured DRG neurons. Profile plots were utilized to delineate membrane staining. Scale bar, 10 μm. o The impact of Nav1.7CT-F2 overexpression on the peak Nav1.7 currents in cultured DRG neurons. n = 7 neurons in control group, n = 6 neurons in Nav1.7CT-F2 group. F(1, 407) = 74.63, P = 1.31 × 10⁻¹⁶. p The diagram illustrates the experimental procedure. The hindpaws of the mice were immersed in a 43 °C water bath for 30 seconds, after which the mice were placed at RT for 5, 10, 20, or 40 minutes. Behavioral and biochemical experiments were then conducted (Created in BioRender. https://BioRender.com/xs51blc). q The impact of heat stimulation on the behaviors of mice response to the Hargreaves. n = 8 mice in RT group, n = 10 mice in 43 °C group. F(1, 80) = 10.88, P = 0.0072 in 5 min, P = 0.0004 in 10 min. r The impact of heat stimulation on the interaction between DOCK4 and Nav1.7 in DRG lysates of mice. s The impact of heat stimulation on the membrane expression of Nav1.7 in DRGs of mice. n = 3 samples per group. F(2, 6) = 23.14, P = 0.0021 in RT vs. 10 min, P = 0.0032 in 10 min vs. 40 min. t, u The impact of heat stimulation on the total expression of Nav1.7 and DOCK4 in DRGs of mice. n = 3 samples per group. v The diagram outlines the experimental procedure. Isolated DRG neurons were immersed in a 43 °C water bath for 30 seconds and then placed at RT for either 10 or 40 minutes. Subsequently, the neurons were lysed to assess the membrane abundance of Nav1.7 (Created in BioRender. https://BioRender.com/xs51blc). w Nav1.7 membrane expression in isolated DRG neurons was evaluated at different time points after direct heat stimulation. n = 4 repeats. F(2, 9) = 25.61, P = 0.0002 in RT vs. 10 min, P = 0.0021 in 10 min vs. 40 min. x, y The effect of DOCK4 deleted in DRG neurons on the behaviors of mice response to the Hargreaves after heat stimulation. n = 8 mice in AAV-GFP group, n = 11 mice in AAV-Cre group, n = 9 mice in Dock4WT group, n = 10 mice in AvCreERT2; Dock4CKO group. F(4, 68) = 19.05, P = 0.0115 in 5 min, P = 0.00000002 in 10 min, P = 0.0007 in 20 min in x; F(4, 68) = 13.79, P = 0.0002 in 5 min, P = 0.00000005 in 10 min, P = 0.0085 in 20 min in y. h–m, t, u, Two-tailed Independent Student’s t test; s, w, One-way ANOVA followed by Tukey’s multiple comparisons test; o, q, x, y, Two-way ANOVA followed by Bonferroni’s multiple comparisons test. *P < 0.05, **P < 0.01, ***P < 0.001, n.s. means not significant. Data are presented as mean ± SEM. Complete sample size and sex is provided in the Supplementary Data. Source data are provided as a Source Data file.
Heat stimulation inhibits DOCK4/Nav1.7 interaction and maintains Nav1.7 level in cell membrane
It has been demonstrated that heat stimulation (43 °C) to DRG neurons can alter the molecular interactions and membrane expression of ion channels46,48. We further examined the effects of heat stimulation on the interaction between DOCK4 and Nav1.7, as well as on the membrane expression of Nav1.7. The hindpaws of mice were immersed in a 43 °C water bath for 30 seconds, after which the mice were kept at room temperature (RT) for 5, 10, 20, or 40 minutes. Behavioral and biochemical experiments were then conducted (Fig. 6p). The results showed that the thermal withdrawal threshold was significantly decreased in mice at 5 and 10 minutes after exposure to 43 °C heat stimulation, but not at 20 or 40 minutes (Fig. 6q). The interaction between DOCK4 and Nav1.7 in DRG tissues was decreased at 10 minutes after heat stimulation (Fig. 6r). The membrane abundance of Nav1.7 in DRG tissues increased at 10 minutes following heat stimulation but returned to normal levels by 40 minutes (Fig. 6s), which is consistent with the behavioral results. Heat stimulation to the paws did not affect the total expression levels of Nav1.7 or DOCK4 in DRG tissues (Fig. 6t, u). We further investigated the effects of direct heat stimulation on the membrane expression of Nav1.7 in isolated DRG neurons (Fig. 6v). The results indicated that membrane Nav1.7 levels increased at 10 minutes but were not elevated at 40 minutes following direct heat stimulation of isolated DRG neurons (Fig. 6w). Furthermore, we examined the role of DOCK4 in behavioral changes induced by heat stimulation and found that knocking out DOCK4 in DRG neurons significantly inhibited the behavioral changes caused by heat stimulation (Fig. 6x, y). Thus, these results suggest that the heat-inhibited DOCK4/Nav1.7 interaction may stabilize Nav1.7 channels on the cell membrane.
Dynein participates in DOCK4-modulated trafficking of Nav1.7
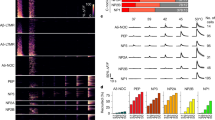
Considering the binding between DOCK4 and Nav1.7, as well as the fact that DOCK4 overexpression reduces the membrane expression of Nav1.7 while increasing its cytoplasmic expression, these findings suggest that DOCK4 may facilitate the transport of Nav1.7 from the cell membrane to the cytoplasm. Motor proteins are a class of cellular cytoskeletal movement proteins that travel along microtubules within the cell50. These motor proteins can convert the chemical energy stored in ATP into mechanical energy, providing the driving force for the transport and precise localization of various cellular cargoes50. Studies indicate that, so far, Dynein is the sole motor protein in neurons known to regulate the retrograde transport of neuronal cargoes, such as ion channels and receptors50,51. Dynein is a large molecule composed of multiple subunits, where the heavy chain primarily binds to microtubules and provides the driving force, while the intermediate chain (IC) mainly associates with the adaptors or cargos in neurons50,52. We then investigated the interaction between DOCK4 and Dynein-IC (hereafter simply Dynein) to determine whether the retrograde motor protein Dynein is involved in the DOCK4-mediated trafficking of Nav1.7 from the membrane to the cytoplasm. The results of co-IP pull-down exhibited that DOCK4 potentially interacted with Dynein in DRGs of mouse (Fig. 7a, b), and the DOCK4 N-terminal (DN, 1–655 aa) was the major binding site for Dynein (Fig. 7c). Double immunofluorescence staining unveiled a co-localization of DOCK4 and Dynein in DRG neurons (Fig. 7d). High-resolution imaging using SIM further validated the co-localization of DOCK4 and Dynein in DRG neurons (Fig. 7e). To corroborate the potential interaction between DOCK4 and Dynein, we performed a proximity ligation assay (PLA) on dissociated DRG neurons. The PLA analysis demonstrated positive fluorescence signals on the cell bodies of cultured DRG neurons from WT mice (Fig. 7f), providing evidence for the existence of the DOCK4/Dynein interaction. Next, we explored whether Dynein participates in DOCK4-mediated trafficking of Nav1.7 from the membrane to the cytoplasm in DRG neurons. The co-IP and PLA experiments unveiled a potential interaction between Dynein and Nav1.7 in the DRGs of Dock4WT mice (Fig. 7g, h). Interestingly, the interaction between Dynein and Nav1.7 was inhibited in the DRGs of AvCreERT2; Dock4CKO mice (Fig. 7g, h), suggesting an adaptor role of DOCK4 in mediating the association between Nav1.7 and Dynein. Considering the potential interactions among Dynein, DOCK4, and Nav1.7, we conducted triple immunostaining and observed that Dynein, DOCK4, and Nav1.7 were colocalized in DRG neurons and sciatic nerves of mice (Fig. 7i and Fig. S9a). In addition, we conducted high-resolution imaging data through SIM, which revealed that 12.24 ± 1.29% of DOCK4 was colocalized with Dynein and Nav1.7, and 10.21 ± 1.40% of Dynein was co-localized with DOCK4 and Nav1.7, and 12.47 ± 1.26% Nav1.7 was co-localized with DOCK4 and Dynein in DRG neurons (Fig. 7j and Fig. S9b). We next intrathecally injected AAV-Dynein-shRNA (rAAV-hSyn-EGFP-5’miR-30a-shRNA (dync1i1)-3’-miR30a-WPREs) to knockdown the expression of Dynein in DRG neurons (Fig. S9c), and found that knockdown of Dynein significantly increased the membrane expression of Nav1.7 in DRG neurons (Fig. 7k). Moreover, the DRG neurons of mice injected with AAV for overexpressing Dynein (rAAV-hSyn-dync1i1-2A-EGFP-WPRE) (Fig. S9d) had a decreased level of membrane Nav1.7 (Fig. 7l). The behavior data showed that knockdown and overexpression of Dynein significantly decreased and increased thermal withdrawal thresholds in the Hargreaves and tail-flick tests, respectively (Fig. 7m–p). Furthermore, Dynein-shRNA reversed the reduction of Nav1.7 expression in the membrane induced by DOCK4 overexpression (Fig. 7q). Consistently, Dynein-shRNA also reversed the elevation of thermal withdrawal threshold induced by DOCK4 overexpression (Fig. 7r). Thus, these results suggest that the motor protein Dynein plays an important role in DOCK4-mediated trafficking of Nav1.7 in DRG neurons.
a DRG lysates were immunoprecipitated with DOCK4 antibody and immunoblotted with Dynein and DOCK4 antibody as indicated. This experiment was repeated three times. b DRG lysates were immunoprecipitated with Dynein antibody and immunoblotted with DOCK4 and Dynein antibody as indicated. This experiment was repeated three times. c The Co-IP assay reveals the interaction between DOCK4 and Dynein, with the interaction site identified as the DN (1-655 aa) region of DOCK4. This experiment was repeated three times. d The colocalization of DOCK4 and Dynein in DRG neurons of mice. The box represents magnification. Scale bar, 100 μm. e High-resolution images showing the colocalization of DOCK4 and Dynein in DRG neurons of mice. Scale bar, 10 μm. f Proximity ligation assay (PLA) showed positive signals of DOCK4/Dynein interaction in cultured DRG neurons (6 images from two repeats). Scale bar, 50 μm. g The interaction level between Dynein and Nav1.7 was examined by co-IP in Dock4WT and AvCreERT2; Dock4CKO mouse DRG lysates. DRG lysates were immunoprecipitated with Dynein antibody and immunoblotted with Nav1.7 and Dynein antibody as indicated. This experiment was repeated three times. t4 = 17.34, P = 0.000065. h Proximity ligation assay (PLA) shows positive signals of Dynein/Nav1.7 interaction in cultured DRG neurons of Dock4WT mice, and signals was loss in cultured DRG neurons of AvCreERT2; Dock4CKO mice (7 images from two repeats). Scale bar, 50 μm. i Immunofluorescence triple staining demonstrates the co-localization of Dynein, DOCK4, and Nav1.7 in DRG neurons of mice. Scale bar, 100 μm. j High-resolution images show the colocalization of Dynein, DOCK4, and Nav1.7 in DRG neurons of mice. Scale bar, 2 μm. k Changes in membrane expression of Nav1.7 following Dynein knockdown in the DRGs. n = 3 samples per group. t4 = 7.381, P = 0.0018. l Changes in membrane expression of Nav1.7 following Dynein overexpression in the DRGs. n = 3 samples per group. t4 = 6.923, P = 0.0023. m–p The effects of Dynein knockdown and overexpression on mouse Hargreaves and tail-flick behaviors. n = 6 mice per group in m and o, n = 5 mice per group in (n and p). t10 = 2.83, P = 0.0179 in m; t8 = 3.079, P = 0.0151 in n; t10 = 3.054, P = 0.0122 in o; t8 = 4.236, P = 0.0029 in (p). q Dynein knockdown reversed the effect of DOCK4 overexpression on membrane expression of Nav1.7 in the DRGs. n = 3 samples per group. F(2, 6) = 18.28, P = 0.0034 in control vs. DOCK4-over, P = 0.0071 in DOCK4-over vs. DOCK4-over + Dynein-shRNA. r Dynein knockdown reversed the effect of DOCK4 overexpression on mouse Hargreaves behavior. n = 6 mice per group. F(2, 15) = 6.149, P = 0.0203 in control vs. DOCK4-over, P = 0.0226 in DOCK4-over vs. DOCK4-over + Dynein-shRNA. g, k–p, Two-tailed Independent Student’s t test; q, r, One-way ANOVA followed by Tukey’s multiple comparisons test. *P < 0.05, **P < 0.01, ***P < 0.001. Data are presented as mean ± SEM. Complete sample size and sex is provided in the Supplementary Data. Source data are provided as a Source Data file.
Interfering with DOCK4 enhances heat nociception in nonhuman primates
Because nonhuman primates closely resemble humans phylogenetically53, we conducted triple immunostaining in monkey and observed that Dynein, DOCK4 and Nav1.7 were co-localized in monkey DRG neurons (Fig. S10a). Co-localization of Dynein/DOCK4/Nav1.7 was further observed in axons in monkey sciatic nerves (Fig. S10b), suggesting the axonal transport of these three proteins. High-resolution imaging data through SIM also revealed that Dynein, DOCK4, and Nav1.7 proteins were spatially close to each other in monkey DRG neurons (Fig. 8a). Further, we investigated whether intrathecal (i.t.) injection of cholesterol-modified DOCK4-siRNA could enhance thermal pain sensitivity in monkeys. The knockdown effect of DOCK4-siRNA was verified in monkey Vero cells (Fig. S10c). The behavioral data showed that DOCK4-siRNA significantly increased thermal pain sensitivity of monkeys (Fig. 8b-d and Movie S1). The increased thermal pain sensitivity began at day 2 and returned to normal at day 10 after injection of DOCK4-siRNA (Fig. 8b-d), indicating that siRNA was metabolically cleared at day 10 in vivo. The sensitivity of monkeys to tactile stimulation remained unaltered in both the cotton (Fig. S10d) and foot tape (Fig. S10e) tests following the administration of DOCK4-siRNA. Monkeys injected with DOCK4-siRNA exhibited a normal withdrawal threshold in response to von Frey filament-evoked punctate mechanical stimulation (Fig. S10f) and brush-evoked dynamic mechanical stimulation (Fig. S10g). To determine the role of DOCK4 in regulating Nav1.7 of monkeys, we established an effective method for knocking down DOCK4 expression in dissociated monkey DRG neurons using siGLO-labeled siRNA54. In Fig. 8e, it is demonstrated that siGLO-labeled siRNA can be taken up by all monkey DRG neurons in primary cultures. The treatment with selective DOCK4-siRNA (100 nM) resulted in a partial reduction in DOCK4 expression in monkey DRG neurons (Fig. 8e, f). Further, we explored the distribution of Nav1.7 immunoreactivity across the siGLO-labeled neuron cell body. Profile plots demonstrated a significant increase in Nav1.7 positive membrane expression on dissociated monkey DRG neurons after DOCK4-siRNA treatment (Fig. 8g and Fig. S10h, i), suggesting that the trafficking of Nav1.7 from the membrane to the cytoplasm in these neurons may be inhibited. Patch clamp recordings in siGLO-labeled monkey DRG neurons revealed a significant increase in Nav1.7 currents following treatment with DOCK4-siRNA (Fig. 8h). These suggest that the knockdown of DOCK4 inhibits the trafficking of Nav1.7 from the membrane to the cytoplasm, resulting in increased Nav1.7 membrane expression and enhanced Nav1.7 currents in monkey DRG neurons. These changes may contribute to heat nociception in monkeys.
a High-resolution images show the colocalization of Dynein, DOCK4, and Nav1.7 in DRG neurons of a monkey. Scale bar, 10 μm. b–d Heat sensitivity changes in monkeys after intrathecal injection of DOCK4-siRNA (20 nmol) in tail-flick and hot plate tests. n = 4 monkeys per group. F(1, 24) = 21.87, P = 0.0000001 in (b); F(1, 24) = 2.807, P = 0.0068 in (c); F(1, 24) = 1.607, P = 0.0134 in (d). e Immunocytochemistry showing knockdown of DOCK4 expression by DOCK4-siRNA treatment (50 nM, 48 h) in cultured monkey DRG neurons. Scale bar, 100 μm. f Intensity of DOCK4-immunoreactivity in monkey DRG neurons after DOCK4-siRNA and nontargeting (NT)-siRNA treatment. n = 33 neurons in NT-siRNA, n = 32 neurons in DOCK4-siRNA. t63 = 6.622, P = 0.000000009. g The influence of DOCK4 knockdown on the distribution of Nav1.7 in the membrane and cytoplasm of cultured monkey DRG neurons. Profile plots were utilized to delineate membrane staining. Scale bar, 25 μm. h The impact of DOCK4 knockdown on the peak Nav1.7 currents in cultured monkey DRG neurons. n = 8 neurons in NT-siRNA, n = 9 neurons in DOCK4-siRNA. F(1, 555) = 116.4, P = 9.19×10⁻²⁵. i The expression of DOCK4 protein in human DRG sections. Scale bar, left: 500 μm, right: 200 μm. j The size frequency distribution of DOCK4-positive (DOCK4+) and total neurons was examined in human DRG sections. A total of 2706 neurons from three human DRGs were analyzed. k The co-localization of Dynein, DOCK4, and Nav1.7 in human DRG neurons. Scale bar, 100 μm. l High-resolution images show the colocalization of Dynein, DOCK4, and Nav1.7 in DRG neurons of human. Scale bar, 1 μm. m Proximity ligation assay (PLA) shows positive signals of DOCK4/Nav1.7 interaction in cultured human DRG neurons (5 images from two repeats). Scale bar, 100 μm. n Proximity ligation assay (PLA) shows positive signals of DOCK4/Dynein interaction in cultured human DRG neurons (6 images from two repeats). Scale bar, 100 μm. o Proximity ligation assay (PLA) shows positive signals of Dynein/Nav1.7 interaction in cultured human DRG neurons with NT-siRNA treatment, and signals was loss in cultured human DRG neurons with DOCK4-siRNA treatment (6 images from two repeats). Scale bar, 100 μm. p The influence of DOCK4 knockdown on the distribution of Nav1.7 in the membrane and cytoplasm of cultured human DRG neurons. Profile plots were utilized to delineate membrane staining. Scale bar, 25 μm. q The impact of DOCK4 knockdown on the peak Nav1.7 currents in cultured human DRG neurons. n = 9 neurons in NT-siRNA, n = 8 neurons in DOCK4-siRNA. F(1, 620) = 69.65, P = 4.61 × 10⁻¹⁶. r The model shows that DOCK4 acts as an adaptor, helping assemble the Nav1.7/DOCK4/Dynein complex to traffic Nav1.7 from the membrane to the cytoplasm in DRG neurons, affecting thermal pain perception. Loss of DOCK4 disrupts this process, increasing Nav1.7 membrane expression and causing heat hyperalgesia. f, Two-tailed Independent Student’s t test; b–d, h, q, Two-way ANOVA followed by Bonferroni’s multiple comparisons test. *P < 0.05, **P < 0.01, ***P < 0.001, n.s. means not significant. Data are presented as mean ± SEM. Complete sample size and sex is provided in the Supplementary Data. Source data are provided as a Source Data file.
DOCK4 knockdown increases Nav1.7 currents in human DRG neurons
To challenge the translational relevance of our findings, we further tested if DOCK4 would regulate Nav1.7 in humans. Immunostaining and in situ hybridization showed that DOCK4 protein and mRNA were expressed in most human DRG neurons (Fig. 8i and Fig. S11a). Size frequency analysis revealed a broad expression of DOCK4 protein in human DRG neurons (Fig. 8j). We also observed the co-localization of DOCK4 with NF200 and CGRP in human DRG (Fig. S11b) and sciatic nerve (Fig. S11c). Immunostaining showed the co-localization of Dynein, DOCK4 and Nav1.7 in the DRG neurons of human (Fig. 8k) and also colocalized within the axons of human sciatic nerve (Fig. S11d). High-resolution imaging data obtained from SIM further disclosed the colocalization of Dynein, DOCK4, and Nav1.7 proteins in human DRG neurons (Fig. 8l and Fig. S12a). To further confirm the potential interactions among Dynein, DOCK4, and Nav1.7 in human DRG neurons, we conducted a proximity ligation assay (PLA) in dissociated human DRG neurons. The PLA analysis unveiled potential interactions between DOCK4 and Nav1.7 (Fig. 8m), as well as DOCK4 and Dynein (Fig. 8n) in human DRG neurons. Similarly, PLA experiments also demonstrated potential interactions between Dynein and Nav1.7 in human DRG neurons (Fig. 8o and Fig. S12b). Importantly, in cultured human DRG neurons, treatment with DOCK4-siRNA significantly inhibited the interaction between Dynein and Nav1.7 (Fig. 8o and Fig. S12b). This further substantiates the role of DOCK4 as an adapter in mediating the association between Dynein and Nav1.7 in human DRG neurons. Moreover, we investigated the distribution of Nav1.7 immunoreactivity in cultured human DRG neurons after siGLO-labeled DOCK4-siRNA treatment. The profile plots revealed a notably increased membrane Nav1.7 expression in dissociated human DRG neurons following treatment with DOCK4-siRNA (Fig. 8p and Fig. S12c, d). Patch clamp recordings on human DRG neurons demonstrated that DOCK4-siRNA increased Nav1.7 currents in human sensory neurons (Fig. 8q). This suggests that disrupting DOCK4 leads to increased Nav1.7 surface expression and enhanced Nav1.7 currents in human DRG neurons. Collectively, these findings reveal that DOCK4 potentially functions as an adaptor, aiding the assembly of the Nav1.7/DOCK4/Dynein complex, thereby facilitating the trafficking of Nav1.7 from the membrane to cytoplasm in DRG neurons, ultimately influencing the thermal pain perception (Fig. 8r). The loss of DOCK4 hinders this process, leading to an increase in the membrane expression of Nav1.7 and causing heat hyperalgesia (Fig. 8r).
Discussion
In the present study, we uncovered that DOCK4 selectively regulates thermal nociception in both mice and nonhuman primates. Furthermore, our findings reveal that DOCK4 may act as an adaptor, contributing to the assembly of the Nav1.7/DOCK4/Dynein complex, which regulates the trafficking of Nav1.7 from the membrane to the cytoplasm in DRG neurons, and impacts thermal pain perception. DOCK4, a member of the DOCK family, acts as a guanine nucleotide exchange factor, and its gene is located at chromosome band 7q31.110. This location maps to the locus shared by several candidate genes implicated in autism11. Studies have reported that the single-nucleotide variations or chromosome mutations of Dock4 may be one of the suspicious gene phenotypes leading to ASD12,55. ASD is also characterized by a broad spectrum of sensory abnormalities, encompassing issues related to touch, smell, taste, and pain4,5,6,7,8,9. The occurrence of pain abnormalities in individuals with ASD has drawn significant attention from researchers. However, the mechanisms underlying pain abnormalities in patients with ASD remain largely unclear. Our study provides insights into the role of DOCK4 in pain regulation. Initially, we observed that DOCK4 is widely expressed in the peripheral sensory nervous system, encompassing DRG neurons and the sciatic nerve in mice, nonhuman primates (NHPs), and humans. Secondly, inducing DOCK4 deficiency in mice through DOCK4 siRNA or utilizing DOCK4 conditional knockout results in thermal hypersensitivity. Importantly, decreasing DOCK4 expression in nonhuman primates leads to heightened heat sensitivity. Thirdly, the overexpression of DOCK4 reduces thermal sensitivity and notably diminishes heat hyperalgesia caused by inflammation, nerve injury, chemotherapy, and cancer. Previous study indicated that knocking out DOCK4 in the central nervous system can lead to autism-like behaviors in mice11. Our data show that knocking out DOCK4 in the peripheral nervous system results in significant heat hyperalgesia. Research shows that by delivering AAVs loaded with specific genes into the body, it is feasible to induce the overexpression of relevant proteins in targeted tissues, thereby facilitating the treatment of various diseases56. Therefore, the approaches for pain management in patients with autism are twofold: the first approach is to search for agonists of DOCK4 or drugs that increase DOCK4 expression. The second approach is to endogenously increase the expression level of DOCK4 in central and peripheral nervous systems by AAV. These two approaches could provide potential strategies for treating autism and its associated pain abnormalities. However, further experiments and clinical research are required to validate their safety and feasibility. Studies have shown that DOCK4 is an important GTP exchange factor that activates the small GTPase Rac111,57. Rac1 plays a crucial role in growth cone development58, but it remains unclear whether DOCK4 is involved in regulating the development and structural properties of DRG neurons, and further research is needed to explore this. Our results show that DOCK4 mainly co-localizes with IB4- and CGRP-positive small-diameter neurons, as well as NF200-positive large-diameter neurons in the DRG. However, in the spinal dorsal horn, DOCK4 mainly co-localizes with IB4- and CGRP-positive nerve endings, while it almost does not co-localize with NF200-positive nerve endings. Interestingly, in the dorsal root tissue, DOCK4 is expressed in NF200-positive axons. This suggests that DOCK4 is transported to the NF200-positive dorsal root axons, but its expression is excluded from the NF200-positive nerve terminals in the spinal dorsal horn. It is currently unclear what causes this expression pattern of DOCK4, and the relationship between this expression pattern and DOCK4’s specific regulation of heat pain is also unknown.
Individuals with autism exhibit differences in pain sensitivity; some have a decreased sensitivity to pain stimuli, while others have an increased sensitivity59,60,61,62. A recent clinical study has also indicated that individuals with autism have heightened sensitivity to thermal pain stimuli63. Dock4 is an autism susceptibility gene, and its deletion can lead to autism-like behaviors in mice11,12,13. Our results show that knocking out DOCK4 in sensory neurons significantly enhances the sensitivity of mice to thermal pain stimuli. These findings may help explain the increased pain sensitivity observed in individuals with autism. In addition to pain sensitivity, studies have also shown that individuals with autism have abnormal tactile sensitivity4,64. However, we found that knocking out DOCK4 in sensory neurons did not affect mice’s tactile sensitivity. This may be due to the fact that different genetic mutations leading to autism result in different types of sensory abnormalities, which requires further research.
Our data indicate that the levels of DOCK4 are significantly decreased in the DRG neurons of mice across all four pathological pain models: nerve injury, inflammation, chemotherapy, and cancer. Additionally, we found that the levels of lactic acid, pan-lactylation (Pan Kla), and histone H4K8 lactylation (H4K8la) in the DRG tissues were significantly increased in these pathological pain model mice. Further investigation revealed that histone lactylation (H4K8la) mediated the downregulation of DOCK4 in sensory neurons. Lactylation is a recently discovered form of protein modification that is involved in the regulation of the occurrence and progression of various diseases, such as Alzheimer’s disease17, and tumorigenesis21. However, research on lactylation is still in its early stages, and there is currently no report on its role in pain or autism. Further studies are needed.
Voltage-gated sodium channel Nav1.7 is one of the critical determinants of nociceptor excitability, which is encoded by Scn9a and widely expressed in the sensory neurons and autonomic neurons39,65. In humans, mutations that enhance the function of Nav1.7 led to heightened sensitivity to heat and severe heat hyperalgesia. Conversely, mutations that impair Nav1.7 function result in a total insensitivity to painful heat stimuli66,67,68. Our data show that DOCK4 interacts with Nav1.7 and barely interacts with other Nav channels, such as Nav1.6, Nav1.8, and Nav1.9 in DRG neurons. Overexpressing DOCK4 in sensory neurons reduces Nav1.7’s membrane expression and increases its cytoplasmic presence, without changing Nav1.7’s total expression levels. This indicates that DOCK4 interacts with Nav1.7 and mediates trafficking of Nav1.7 from the membrane to cytoplasmic, leading to changes in the expression of Nav1.7 on the neuronal cell membrane. This could be a potential mechanism by which DOCK4 modulates thermal pain perception. Our immunostaining results indicate that knockout of DOCK4 increases the membrane expression of Nav1.7 in DRG neurons. We acknowledge that since the antibody recognizes an antigen in the cytoplasmic region of the Nav1.7 channel, the immunostaining experiment cannot distinguish whether the Nav1.7 channel is inserted into the cell membrane or located near the membrane. Studies have shown that gain-of-function mutations in Nav1.7 cause not only thermal hyperalgesia but also mechanical pain66,67, while our data indicate that knockout of DOCK4 has no effect on mechanical pain. A possible explanation is that DOCK4 may compensate by regulating other molecules to counteract mechanical pain, which requires further investigation. Studies have shown that Nav1.7 co-trafficking with other Nav (Nav1.6, Nav1.8, and Nav1.9) and voltage-gated potassium (Kv) channels, as well as other membrane proteins, within the same vesicles69,70,71. Although DOCK4 does not interact with other Nav channels (Nav1.6, Nav1.8, and Nav1.9), we cannot exclude the possibility that DOCK4 may influence the trafficking of other Nav and Kv channels. Studies have shown that heat stimulation (43 °C) of DRG neurons can alter the molecular interactions in DRG neurons46,48. Our results showed that exposing one paw of mice to a 43 °C water bath inhibits the interaction between DOCK4 and Nav1.7, and increases the membrane expression of Nav1.7 in DRGs. Direct ex vivo heat stimulation of DRG neurons also significantly increases the membrane expression of Nav1.7. However, it must be pointed out that our study does not fully represent the direct heat stimulation of DRG in vivo situation. Research indicates that the TRPV1, TRPA1, and TRPM3 channels play a significant role in thermal pain perception38. Although triple knockout of the TRPV1, TRPA1, and TRPM3 channels eliminates most heat nociceptive behaviors, it does not completely abolish heat nociceptive responses, as some of the triple knockout mice are still capable of sensing heat pain38. This suggests that other molecules, apart from TRPV1, TRPA1, and TRPM3, are involved in the regulation of heat pain. Our data show that the knockout of DOCK4 enhances heat pain sensitivity in mice, while the knockout of DOCK4 does not affect spontaneous pain or neuronal activity induced by activation of TRPV1, TRPA1, or TRPM3 channels, indicating that the regulation of heat pain sensitivity by DOCK4 may not depend on these channels. However, it must be noted that to confirm this, interference with DOCK4 in the triple knockout TRPV1, TRPA1, and TRPM3 mice would be necessary to observe the effects on heat pain behaviors. Therefore, we cannot completely rule out the possibility that DOCK4 regulates heat pain through TRPV1, TRPA1, or TRPM3 channels. The expression of most proteins in DRG neurons is gradient, with varying expression levels even within the same size of neurons28,48,54. This is a common phenomenon. The expression of DOCK4 in the DRG is also gradient, and even within the same size of neurons, and the relationship between this expression pattern of DOCK4 and heat nociception behavioral outcomes is currently unclear. Our results indicate that DOCK4 is broadly expressed in DRG neurons and a portion of DOCK4-positive neurons does not express Nav1.7. The role of DOCK4 in neurons that do not express Nav1.7 is unknown and may be involved in regulating other sensory modalities, such as itch or proprioception, requiring further research.
TRPV1 channel, expressed in DRG neurons, functions as a thermosensor. However, mice with a knockout of the Trpv1 gene only displayed a partial reduction in nociceptive behaviors induced by intense heat stimuli ( > 52 °C) and did not show an apparent reduction in response to noxious heat stimuli below 48 °C22,23. This suggests the presence of undiscovered mechanisms in heat nociception. Our data indicate that the temperature range regulated by DOCK4, which spans from 46 °C to 55 °C, is broader than that of TRPV1. Moreover, the knockout of DOCK4 in sensory neurons does not affect the spontaneous pain or neuronal activity induced by the TRPV1 agonist capsaicin, suggesting that the regulation of heat nociception by DOCK4 may not be mediated through the TRPV1 channel. Although our results indicate that the modulation of heat pain by DOCK4 is not mediated through the TRPV1 channel, this does not mean that DOCK4 can explain the remaining heat nociception in TRPV1 knockout mice. Interfering with DOCK4 in TRPV1 knockout mice to observe its effect on heat pain behavior could be an important approach to addressing this issue.
Dynein, a retrograde motor protein, facilitates the transport of various cargos, including ion channels and receptors, in a retrograde manner along microtubules. It assembles together with adaptors and cargos to form a complex, essential for efficient transportation50,51. Researches indicate that Dynein is widely expressed in the nervous system and participates in regulating various neuronal functions associated with retrograde transport72,73. For instance, in cortical neurons, Dynein mediates the retrograde transport of lysosomes and autophagosomes73. Activation of Dynein promotes the retrograde transport of tropomyosin receptor kinase (TrkB)-brain-derived neurotrophic factor (BDNF) endosomes in hippocampal neurons74. In HEK293 cell lines stably expressing Kv1.5 channels, enhancement of Dynein function promotes the retrograde trafficking of Kv1.5 channels, thereby inhibiting Kv1.5 membrane expression75. Our data showed that DOCK4 acts as an adaptor protein, interacting with Nav1.7 and Dynein, mediating the formation of a complex of the three in sensory neurons. DOCK4, Dynein and Nav1.7 are co-expressed in DRG neurons of mice, monkeys, and humans. Knockdown of Dynein evoked heat nociception and increased Nav1.7 membrane accumulation. Consistently, Dynein knockdown reversed the reduction in Nav1.7 membrane expression and the decrease in heat nociception induced by DOCK4 overexpression. This suggests that Dynein is involved in the DOCK4-regulated trafficking of Nav1.7. Dynein transports Nav1.7 from the cell membrane to the cytoplasm. However, it is currently unclear whether the Nav1.7 transported to the cytoplasm undergoes degradation or is transported to other regions, such as axon terminals. Further research is needed to elucidate this. The motor protein Dynein converts the chemical energy stored in ATP into mechanical kinetic energy, driving movement along microtubules and Nav1.7 trafficking. However, the underlying mechanism by which Dynein transforms chemical energy into mechanical energy remains unknown and warrants further investigation. Given that Dynein plays a crucial role in the retrograde transport of various neuronal cargos72,73, knockdown of Dynein is likely to have multiple effects on DRG neurons. In addition to heat pain, it remains unclear whether the impairment of Dynein function will affect other sensory modalities, such as proprioception and itch sensation, and this requires further investigation. During the experiments, we conducted multiple behavioral tests on the same group of mice, with an interval of at least 2 hours between each behavioral test. Based on our experience and research from peers29,30,46, a 2-hour testing interval does not have an impact on behavioral results. However, we are still unable to rule out potential influences between the behavioral tests.
Collectively, our data reveal the underlying mechanism by which the ASD-related gene Dock4 regulates heat nociception. Specifically, we demonstrate that the Dynein/DOCK4/Nav1.7 complex is essential for transmitting heat nociceptive signals. Additionally, we identify potential mechanisms underlying abnormal pain nociception in autism, offering promising strategies for pain management through targeting DOCK4 in patients with autism.
Methods
Animals/mice
C57BL/6 male and female mice were obtained from the Institute of Experimental Animals of Sun Yat-sen University. Dock4flox/flox, AvCreERT2, Nefh-Cre and Trpv1-Cre mice were purchased from Cyagen Biosciences Inc. Nav1.7flox/flox mice were purchased from GemPharmatech Co. Adult mice (8–10 weeks) were used for behavioral and biochemical studies. Young mice (5–7 weeks) were used for electrophysiological studies in DRG neurons. All animals were housed in individually ventilated cages (no more than 5 mice per cage) in a temperature-controlled (24 ± 1) °C] and humidity-controlled (50 – 60%) room under a 12-h/12-h light/dark cycle. The mice were provided with free access to water and standard laboratory food. All animal experimental procedures were approved by the Research Ethics Committee of Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences (Approval No.: KY-D-2020-094-03) and were carried out in accordance with the guidelines of the National Institutes of Health (NIH) on animal care and ethics. Animals were randomly assigned to their respective experimental groups, with both males and females included in each group in a sex-matched manner. The data from both sexes were pooled and utilized relatively equally throughout this study, as no significant sex differences were observed. For WT mice, those with abnormal baseline pain threshold values were excluded, with the exclusion criterion being a von Frey threshold of less than 0.4 g. The total number of animals used, as well as the number and sex of animals in each experimental group, are listed in Supplementary Data. For euthanizing animals, mice were first anesthetized the mice with 10% urethane (0.02 ml/g, i.p.), followed by euthanasia through carbon dioxide asphyxiation. For different experiments, the mice tissue collection methods varied. For western blot and PCR experiments, mice were first anesthetized with 10% urethane (0.02 ml/g, i.p.), then perfused through the ascending aorta with ice-cold PBS, followed by rapid dissection of the lumbar DRG, lumbar spinal dorsal cord, sciatic nerve, and dorsal root nerve on ice. For immunohistochemistry experiments, we first anesthetized the mice with 10% urethane (0.02 ml/g, i.p.), then perfused through the ascending aorta with ice-cold PBS, followed by 4% paraformaldehyde until the limbs became rigid, and then dissected the lumbar DRG, lumbar spinal dorsal cord, sciatic nerve, and dorsal root nerve. For primary DRG neurons culture and electrophysiology experiments, after anesthetizing the mice with 10% urethane (0.02 ml/g, i.p.), mouse spinal column was directly opened and the DRG tissues were rapidly collected on ice.
Mice pain models
All animals underwent baseline testing before the establishment of the pain models. To produce inflammatory pain, complete Freund’s adjuvant (CFA, 20 μL) was injected into the plantar surface of the hindpaw, then the behavioral tests were performed on days 1, 3 and 5 after CFA treatment. To produce spinal nerve ligation (SNL) neuropathic pain, the mice were anaesthetized with isoflurane delivered in oxygen (4% for induction and 1.5–2% for maintenance), and the left L5 spinal nerve was isolated adjacent to the vertebral column, and tightly ligated with a 4–0 silk suture as previously described46. The L5 spinal nerves of sham-operated mice were identically exposed but not ligated. Postoperatively, no analgesics were used for pain relief. The behavioral tests were performed on days 4, 7, 10, and 14 after SNL treatment. Chemotherapy-induced neuropathy was induced through intraperitoneal injection of paclitaxel (PTX) at a dosage of 4 mg/kg (0.2 mg/ml) on days 1, 3, 5, 7, and 1476. The behavioral tests were performed on day 5, 7 and 14 after the first PTX treatment. Paclitaxel was sourced from Sichuan Huiyu Pharmaceutical Co., Ltd., located in Neijiang City, Sichuan Province, China. The bone cancer pain model was established using Lewis lung carcinoma (LLC, ATCC, catalogue no.: CRL-1642) cells. LLC cells were digested with 0.25% trypsin, centrifuged, and resuspended in PBS. 10 μL of the tumor cell suspension (2 × 106 cells) was injected into the left femoral bone marrow cavity, followed by sealing of the injection site with bone wax77. The behavioral tests were performed on days 7, 1,0 and 14 after LLC treatment.
Behavioral tests in mice
The mice were acclimatized to the behavioral testing apparatus for at least 2 days prior to behavioral testing. All behavioral tests were conducted in a blinded manner. Mice with different genotypes or treatments underwent multiple behavioral tests over a 5-day period in the following sequence: von Frey, dynamic, and tail clip on day 1; back tape and foot tape on day 2; cotton, Hargraves, and cooling on day 3; hot plate and pinprick on day 4; tail-flick on day 5 (Fig. S1a). The interval between different tests was at least 2 hours. Following behavioral tests, different cohorts of mice were used to perform pain models, biochemical, or electrophysiology experiments (Fig. S1a).
Von Frey mechanical test in mice
The mice were positioned in plastic chambers with a mesh floor. Von Frey filaments, progressively increasing in force (0.008g–4 g), were applied to the hindpaws of the mice. Each filament was administered 5 times during the test. The minimum filament force that elicited paw withdrawal more than 3 times throughout the test was designated as the mechanical threshold.
Dynamic mechanical test in mice
The dynamic test was assessed following previously published methods29. Mice were acclimated in von Frey chambers. The hindpaw was gently stroked with a 5/0 brush from heel to toe. Responses were scored as follows: 0 = no response; 1 = very short, fast movement/lifting of the paw; 2 = sustained lifting of the paw for more than 2 seconds towards the body or strong lateral lifting above the body level; and 3 = flinching, licking, or flicking of the affected paw. The average score of 3–5 trials per mouse was reported as the dynamic score.
Tail clip mechanical test in mice
A small alligator clip (force, 700 × g) was applied 1 cm from the base of the tail. The latency to attack/bite the clip was measured. Once an attack occurred, the clip was removed, and each mouse was tested once and then returned to its cage. To avoid injury to the mice, the cutoff time for the tail clip test is set to 15 s.
Back tape touch test in mice
The mice were conditioned to a round plexiglass container. A 1-inch piece of laboratory tape was gently applied to the bottom center of the mouse’s back. The mice were observed for 5 minutes, and the total number of responses to the tape was recorded. Any instance of biting or grabbing the tape, or displaying an evident ‘wet dog shake’ movement to remove the tape from the back, was considered a response.
Foot tape touch test in mice
Mouse were placed in a cylindrical container with a diameter of 15 cm. A circular piece of tape (0.2 cm in diameter) was affixed to the sole of its foot, and mice were allowed to move freely, and the time was recorded from affixing to the time when the mouse finds the tape piece, up to 5 minutes. Mice responses, such as licking, flapping, or staring at the sole of the foot were considered the end of the timing. Each mouse was tested individually.
Cotton touch test in mice
A cotton swab from a Q-tip was manually pulled, expanding to three times its original size. The mouse’s withdrawal response, characterized by paw retraction upon stimulation underneath the paw with the swab, was considered a positive reaction. Five sweeps were conducted with a minimum interval of 10 seconds between each. The number of withdrawals out of 3–5 trials was calculated and reported as a percentage for each mouse.
Hargreaves thermal test in mice
Thermal hypersensitivity was assessed utilizing a plantar test following the methodology outlined by Hargreaves. In brief, a radiant heat source positioned beneath a glass floor was directed towards the fat pad on the plantar surface of the hindpaw. The time taken for the hindpaw withdrawal response was measured. The hindpaw of each mouse was submerged in a 43 °C water bath for 30 seconds, after which the mice were immediately placed in a plastic chamber. Behavioral experiments were conducted 5, 10, 20, or 40 minutes later. The mice were tested individually. To avoid injury to the mice, the cutoff time for the Hargreaves test was set to 25 s.
Evaporative cooling test in mice
The mice were acclimated to a plastic chamber with a mesh floor. Subsequently, a drop (10–20 μL) of acetone was administered to the hindpaws of the mice. The duration of withdrawal, shaking, lifting, flinching or licking behaviors within a 1-minute timeframe was recorded.
Hot plate thermal test in mice
The mice were placed into a clear Plexiglas cylinder on top of a metal surface maintained at 50 °C, 52 °C, or 55 °C. The latency to shaking, lifting, flinching, or licking the paw was measured. The cutoff times for 50 °C, 52 °C, and 55 °C were set at 70 s, 50 s, and 30 s, respectively.
Pinprick mechanical test in mice
The mice were habituated to the von Frey chamber for 30 minutes and 27 g needle was applied to the hairless skin of the hindpaw. Paw withdrawal, shaking, lifting, flinching or licking was rated as a response and the percentage of the response to the total test was recorded. Each mouse was tested 10 times with a minimum interval of 1 minute between each test.
Tail-flick thermal test in mice
The mice were gently restrained inside a cloth/cardboard pocket with their tails outside the pocket. The distal half of the tail was immersed in a 46 °C, 48 °C, 50 °C, or 52 °C water bath, and the latency to vigorous withdrawal of the tail from the water was measured. The cutoff times for 46 °C, 48 °C, 50 °C, and 52 °C were set at 40 s, 20 s, 15 s, and 10 s, respectively.
Spontaneous pain test in mice
Long-term spontaneous pain was assessed in mice without plantar injection by quantifying the number and duration of instances in which the mouse licked and flinched its hindpaw within a one-hour period. Acute spontaneous pain was assessed in mice with plantar injection of capsaicin (1 nmol/20 μL/paw, Tocris Bioscience, catalogue no.: 0462), CIM0216 (2.5 nmol/20 μL/paw, Tocris Bioscience, catalogue no.: 5521), and AITC (10 nmol/20 μL/paw, Sigma, catalogue no.: W203408) by quantifying the number and duration of licking or flinching behaviors within 2 minutes.
Rotarod test in mice
The mice were tested on a rotarod with a velocity that increased from 4 rpm to 40 rpm within 5 minutes. The mice were pre-trained for 2 days for adaptation. Then, the amount of time that each mouse spent on the rotarod before it fell off was recorded.
The open field test in mice
The mice were put in an open field (45 ×45 cm) and their locomotor activities within 30 minutes were recorded and analyzed. The total moving distance and the moving velocity were measured for the evaluation of the locomotor activity.
Short-term CPA assay in mice
Mice were habituated for 3 days (30 minutes each) to a small custom-designed two chamber CPA apparatus. The CPA apparatus comprises two chambers, namely a dark chamber (A) and a light chamber (B). A partition, featuring a black side (facing A) and a white side (facing B), along with a rectangular aperture at the bottom center, was introduced between the two chambers. On the final day of adaptation, baseline preferences were video-recorded for 10 minutes and the total duration in the black chamber was recorded as pre-test time. Based on the baseline measures, mice were isolated to their preferred chamber. The experiment was performed by repeatedly stimulating the hindpaw using the radiant heat source (cutoff 8 seconds) once every 40 seconds for 10 minutes, which was generally innocuous and did not induce withdrawal responses or CPA in naive mice. After stimulation, mice were placed into their previous cage for 20 minutes, after which they were sent back to the CPA chamber and enjoyed equal access to each chamber. Post-conditional-stimulus behaviors of the mice were recorded via videotaping for 10 minutes, and the duration spent in the black chamber was recorded as post-test time. Similarly, to further assess punctate and dynamic mechanical sensation, the hindpaw was repeatedly stimulated using a 1.0 g filament (once every 10 seconds) and a 5/0 brush (once every 10 seconds) for a duration of 10 minutes. CPA score was defined as the value of the pre-test time minus the post-test time28.
Long-term CPA assay in mice
The long-term CPA test was conducted following the procedures outlined in a prior study29. The CPA apparatus comprises two chambers, namely a dark chamber (A) and a light chamber (B). A partition, featuring a black side (facing A) and a white side (facing B), along with a rectangular aperture at the bottom center, was introduced between the two chambers. The duration of time that each mouse spent in dark chamber (A) was documented. On Day 1, each mouse was introduced to the bright compartment (B) and given a 30-minute period to explore freely between chambers A and B (pre-test). Under the device’s design, the majority of naïve mice initially exhibited a preference for the dark chamber. The mice underwent a 6-day training regimen. On Days 2, 4, and 6, the hole in the central wall was obstructed, and the mouse was positioned in the light chamber (B) without any stimulation for 30 minutes. Conversely, on Days 3, 5, and 7, the hole in the central wall was obstructed, and the mouse was placed in the dark chamber (A). Subsequently, hindpaw stimulation was administered using Hargreaves (cutoff 8 seconds or 14 seconds), punctate (1.0 g), and dynamic (paintbrush from heel to toe) methods continuously for a duration of 30 minutes. The interval between punctate and dynamic stimuli was approximately 10 s, while the interval for Hargreaves stimuli was approximately 40 seconds. On Day 8, the aperture in the central wall was unblocked. Initially, the mice were situated in a well-lit compartment, granting them unrestricted access to explore the entire apparatus for 30 minutes—a test aimed at assessing the mice’s compartmental preference post-training (post-test). The aversion score was computed as the disparity between the time spent in the dark chamber during the pre-test and post-test phases, represented as follows: aversion score (s) = (pre-test time in the dark chamber) – (post-test time in the dark chamber).
Drug injection in mice
For sciatic nerve injection, mice were anesthetized with isoflurane delivered in oxygen (4% for induction and 1.5–2% for maintenance), and a mixture of 2 μg DOCK4-siRNA (Dharmacon, catalog: L-043254-01-0005) and 1.5 μg transfection reagent (chimeric rabies virus glycoprotein fragment, RVG-9R, AnaSpec, catalog: AS-62565) in 6 μL D5W (5% deoxygenated water) was injected into the sciatic nerve. Take care to prevent mixture entering the epineurium of the sciatic nerve. For intrathecal injection, mice were anesthetized with isoflurane delivered in oxygen (4% for induction and 1.5-2% for maintenance), and spinal cord puncture was performed with 30-G needle at the level of the intervertebral space between L4 and L5 to transfer the reagent (10 μL) to cerebrospinal fluid. For adeno-associated virus (AAV) and lentivirus (LV) intrathecal injection, AAV vectors for Cre expression (serotype 2/9, rAAV-hSyn-Cre-EGFP), Dynein overexpression (serotype 2/9, rAAV-hSyn-dync1i1-2A-EGFP), Dynein shRNA (serotype 2/9, rAAV-hSyn-EGFP-5’miR-30a-shRNA (dync1i1)-3’-miR30a, GCTTGCCGTTCCACACAATGA) or Nav1.7-CT-F2 overexpression (serotype 2/9, rAAV-hSyn-Nav1.7-CT-F2-2A-EGFP) were purchased from BrainVTA Biotechnology and were injected intrathecal 21 days before the experiments. The CAS9-Easy-SAM lentivirus vector for DOCK4 overexpression (U6-sgRNA-SV40-MS2-P65-HSF1-CMV-EGFP, TATGCTGTGCCGTCACATTT, 10 μL) was purchased from GENECHEM and was injected intrathecal 14 days prior to the experiments. Sodium lactate (NaLA, Sigma, catalogue no.: 71718-10 G) was dissolved in saline solution and the concentration of sodium lactate for intrathecal injection is 50 μM, with 10 μL injected per mouse. Sodium oxamate was purchased from MedChemExpress (catalogue no.: HY-W013032A), and was dissolved in saline solution, with an intraperitoneal injection concentration of 300 mg/kg.
Animals/monkeys
Four adults male cynomolgus monkeys (Macaca fascicularis), aged between 7 and 10 years old, were sourced from Guangzhou Xiangguan Biotechnology Co. Ltd. (Guangzhou, China). They were housed in a nonhuman primate (NHP) experimental facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. The animals were individually housed in temperature- and humidity-controlled rooms maintained on a 12-hour light/12-hour dark cycle. Prior to the behavioral assay, all monkeys underwent a period of acclimatization. All animal care and experimental procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals standards established by the NIH. Additionally, these procedures were approved by the Research Ethics Committee of Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences (Approval No.: KY-D-2020-094-03) and the Laboratory Animal Ethics Committee of the Institute of Zoology, Guangdong Academy of Sciences (Approval No.: G2Z20210712).
Intrathecal siRNA in the monkeys
According to previous method30, the cynomolgus monkeys were anesthetized by intramuscular injection of zoletil (3–5 mg/kg). The hair on the lower back was removed in the cynomolgus monkeys. Next, the monkeys were placed on the animal operating table and maintained lateral decubitus position. Then the operative area was disinfected twice with Anerdian and laid the surgical hole towel. The surgical assistant fixed the cynomolgus monkey’s body position so that it could keep a maximal spine flexion. The anterior superior iliac spine was used to locate the L5 spinous process. A 2.5 ml syringe was hold in the right hand and the needle was injected into the L4 intervertebral space. In the process of injection, the needle was slowly pushed until there was a sense of breakthrough, then the needle bolt was retracted to see the clear cerebrospinal fluid, and the injection tube was discarded. We first validated DOCK4-siRNA with monkey-derived Vero cells (ATCC, catalogue no.: CRL-1586) and then methylated and cholesterol-modified siRNA for in vivo intrathecal injection. Another 2.5 ml syringe was used to draw 400 μL DOCK4-siRNA (CACAAACATTCCCGAGAAA for Macaca fascicularis purchased from Ribobio) mixture (20 nmol siRNA mixed in 400 μL saline) and connected the original injection needle to inject the mixture into the subarachnoid space. Principle of asepsis should be followed in the whole process.
Behavioral tests in monkeys
In order to explore the translational potential of our research, we conducted experiments involving monkeys due to their close phylogenetic relationship with humans. The behavioral tests in monkeys were carried out as previously outlined28,30,43. The monkeys were acclimated to the restraint chair for 1 hour per day for a week before the behavioral tests.
Tail-withdrawal thermal test in monkeys
The hair of the lower third of the tail was shaved and the monkey was adapted to monkey cage for 2 days. The monkey was set to primate restraint chair with its tail outside the chair. The lower third of the tail was immersed in a water bath at 46 °C and 50 °C in turn (10 minutes apart, 3 times for each temperature) and skin of the tail was dried every time. Tail withdrawal latency was recorded after each immersion.
Hot plate thermal test in monkeys
A customized digital temperature-controlled silicone rubber heating plate was used. The heating plate was a heating double-sided silicone rubber whose size is 1 cm × 5 cm. The temperature of digital display temperature controller was set to rise gradually from 25 °C with the upper limit of 60 °C. During the test, monkeys were allowed to hold one end of the silicone rubber heating plate with their hindpaw. And the digital display temperature value was recorded when the monkeys released the heating plate and tightened their paws. Each monkey was tested 3 times, and each interval was 10 minutes.
Von Frey mechanical test in monkeys
Monkeys were adapted to a monkey restraint chair for 1 hour with their feet kept free. Von Frey filaments with progressively increasing force were applied to the hindpaw of monkeys. During the test, each filament was applied to the monkey’s hindpaws 5 times, with a minimum interval of 10 minutes. The minimum filament force that caused paw withdrawal more than 3 times during the test was defined as the mechanical threshold.
Dynamic mechanical test in monkeys
Monkeys were adapted to a monkey restraint chair for 1 hour. The outside of the rear paw was gently stroked from heel to toe with a 5/0 brush. The criterion for the response score was as follows: 0 = no response; 1 = very short, fast movement/lifting of the paw; 2 = sustained lifting of the paw lasting for more than 2 seconds or strong lifting of the paw; and 3 = flinching, licking, or flicking of the affected paw. Each monkey underwent 3 tests, with a minimum interval of 10 minutes between each test. The average score from the 3 tests was defined as the dynamic score.
Cotton touch test in monkeys
First, cotton was pulled at the top of a medical cotton swab to 3 times its original size. The center of the monkey’s feet was gently touched from the bottom up with cotton, and the reaction of their feet was observed. A paw withdrawal motion in response to a stroke of the swab underneath the monkey paw was scored. Five sweeps were performed with at least 10 seconds between each. The number of withdrawals out of five trials was counted and reported as the percentage withdrawal for each monkey.
Foot tape touch test in monkeys
Before the experiment, the monkeys were trained to adapt to the process of grasping with their feet. In the experiment, a baffle was used to block the monkey’s view, and while grasping the monkey’s feet, transparent tape was randomly attached to the sole of one of the monkey’s feet. The transparent tape used in the experiment was cut to a size of 3 cm × 3 cm. The time was recorded from releasing the monkey’s foot to their discovery of the tape, with an upper limit of 5 minutes. Longer latencies indicated decreased tatile sensitivity.
Immunohistochemistry
After the behavioral tests, the mice were anesthetized with 10% urethane (0.02 ml/g, ip), the cynomolgus monkeys were anesthetized by intramuscular injection of zoletil (3-5 mg/kg). Then the ascending aorta was perfused with ice-cold PBS, followed by 4% paraformaldehyde until the limbs became rigid, and the lumbar DRG, lumbar spinal dorsal cord, sciatic nerve, and dorsal root nerve were dissected. The nerve tissues were immediately postfixed in 4% paraformaldehyde for 1 hour. Human thoracic segment DRGs and sciatic nerves were collected from patients at Sun Yat-sen University Cancer Center (five males and three females, age from 12 to 78 years old). Informed written consent was obtained from all participants or their next of kin prior to the research, and the study was approved by the Research Ethics Committee of Sun Yat-sen University Cancer Center (Approval No.: SL-G2021-099-02). Each donor was undergoing surgery for disease treatment where the DRGs and sciatic nerves were discarded as the standard of care. The DRGs and sciatic nerves were immediately postfixed in 4% paraformaldehyde for 24 hours. The nerve tissues from mice, monkeys, and humans were dehydrated in 30% sucrose for 3 days until the tissues settled at the bottom. The tissues were then embedded in OCT frozen section embedding agent (Sakura, catalogue no. 4583) and sectioned using a cryostat (Leica). After washing the frozen sections with PBS, the sections were blocked at room temperature for 1 hour using the blocking solution containing Triton X-100 (Beyotime, catalogue no. P0102). The sections were incubated with primary antibodies against DOCK4 (Rabbit, 1:200, Abcam, catalogue no.: ab85723; Mouse, 1:200, Santa Cruz, catalogue no.: sc-100718), Nav1.7 (Rabbit, 1:100, Alomone, catalogue no.: ASC-008), cytoplasmic Dynein intermediate chain (Rabbit, 1:200, Bioss, catalogue no.: 10470 R), IB4 (1:50, sigma, catalogue no.: L2895), NF200 (mouse, 1:200, sigma, catalogue no.: N0142) and CGRP (mouse, 1:200, Abcam, catalogue no.: ab81887) at 4 °C overnight. The tissue sections were rinsed 3 times with PBS. Then the tissue sections were incubated with secondary antibodies (1:400) for 1 hour at room temperature (RT), and then photographed under a fluorescence microscope (EVOS FL, Thermo; LSM880, Carl Zeiss). Three-dimensional super-resolution images were captured using a three-dimensional structured illumination microscope equipped with the N-SIM System by Nikon, Japan. To confirm the specificity of the Nav1.7 antibody, blocking experiments were conducted in DRG sections using a mixture of anti-Nav1.7 antibody and Nav1.7 blocking peptide (10 times the molar concentration of the antibody, Alomone labs, catalogue no.: BLP-SC008) based on an immunizing peptide blocking protocol (https://www.abcam.com/protocols/blocking-with-immunizing-peptide-protocol-peptide-competition). During the microscope imaging process, we controlled the laser intensity and exposure time to remain consistent across the same batch of experiments. In the data analysis process, we ensured that the analysis parameters were kept consistent. We used ImageJ software to quantify the positive neuronal fluorescence signals in IHC images. The specific steps were as follows: 1. Open the image. 2. Set the threshold: Go to Image-Adjust-Threshold. Adjust the sliders until the fluorescence signal is separated from the background. 3. Quantify the fluorescence signal: Go to Analyze-Set Measurements and select the parameters you needed (e.g., Area, Mean Gray Value, Integrated Density). Then, select Analyze-Measure to view the measurement results. As in previous methods78, the threshold for positive neurons was set to 2.25 times the average intensity in the control images, which were not incubated with the primary antibody. Similarly, we used ImageJ to measure the diameter of neurons. The specific procedure was as follows: 1. Open the image. 2. Set the scale: First, click the straight-line selection tool in the ImageJ toolbar, then create a line selection of a known length in the image (e.g., 20 μm). After that, click the Analyze menu and select the Set Scale command. In the dialog box that appears, set the Known distance to the known length (e.g., 20), set the Unit of length to the unit of measurement (e.g., μm), and click OK. 3. Set the measurement options: Open the Set Measurements option from the Analyze menu, and in the dialog box that appears, select the option to measure. Then, select Analyze-Measure to view the measurement results.
Extraction of plasma membrane proteins
For the preparation of membrane proteins, DRG tissues and cultured DRG neurons were homogenized on ice using a plasma membrane protein extraction kit (Invent Biotechnologies, catalogue no.: SM-005). The efficacy of the membrane protein extraction has been previously demonstrated in our work45. The detailed protocol was as follows: DRG tissues were lysed with buffer A. The filter cartridge was capped and centrifuged at 16,000 × g for 30 s. The filter was discarded, and the pellet was resuspended and centrifuged at 700 × g for 1 minute (the pellet contained the intact nuclei). The supernatant was carefully transferred to a new tube and centrifuged for 30 minutes at 16,000 × g. The supernatant (the cytosolic fraction) was removed, and the pellet (the total membrane protein fraction including organelles and plasma membranes) was saved. The total membrane protein fraction was resuspended in buffer B and centrifuged at 7800 × g for 5 minutes. The pellet contained the organelle membrane proteins (in our study, cytoplasmic protein comprised the cytosolic fraction and organelle membrane fraction). The supernatant was carefully transferred to a fresh microcentrifuge tube, and mixed a few times by inverting and centrifuged at 16,000 × g for 30 minutes. The supernatant was discarded, the pellet (isolated plasma membrane proteins) was saved, and the BCA method was used to determine the protein concentration. Protein samples of different fractions were denatured and prepared for immunoblotting.
Western blotting
Mice were anesthetized with 10% urethane (0.02 ml/g, ip), followed by perfusion through the ascending aorta with ice-cold PBS. The lumbar DRG, lumbar spinal dorsal cord, sciatic nerve, and dorsal root nerve were then rapidly dissected on ice. Lumbar spinal dorsal cord, dorsal roots, DRGs, sciatic nerve tissues, cultured DRG neurons, and cultured HEK293 cells were lysed and homogenized in cold RIPA buffer supplemented with protease (Roche, catalogue no.: 5892970001) and phosphatase (Roche, catalogue no.: 4906845001) inhibitors. Centrifuge at 12,000–14,000 rpm for 20 minutes at 4 °C to remove cellular debris. Collect the supernatant and measure the protein concentration using BCA kit (Thermo Fisher, catalogue no.: 23227). Prepare an SDS-PAGE gel appropriate for the size of the target protein. Mix equal amounts of protein with SDS-PAGE sample buffer and heat at 95 °C for 5 minutes to denature the proteins. Load the protein samples into the gel wells and run the gel at a constant voltage until the dye front reaches the bottom. After electrophoresis, transfer the proteins onto a PVDF membrane. The membranes were then immersed in blocking buffer (5% BSA in TBS-T) for 1 hour at room temperature and then incubated with primary antibodies targeting DOCK4 (Rabbit, 1:2500, Abcam, catalogue no.: ab85723; Mouse, 1:200, Santa Cruz, catalogue no.: sc-100718), Nav1.6 (Rabbit, 1:200, Alomone, catalogue no.: ASC-009), Nav1.7 (Rabbit, 1:200, Alomone, catalogue no.: ASC-008), Nav1.8 (Rabbit, 1:200, Alomone, catalogue no.: ASC-016), Nav1.9 (Rabbit, 1:200, Alomone, catalogue no.: ASC-017), TRPM3 (Rabbit, 1:1000, Bioss, catalogue no.: bs-9046R), TRPV1 (Rabbit, 1:200, Alomone, catalogue no.: ACC-030), TRPA1 (Rabbit, 1:1000, ABclonal, catalogue no.: A12544), L-Lactyllysine (Rabbit, 1:1000, PTM BIO, catalogue no.: PTM-1401RM), LactyI-histone antibody sample kit (Rabbit, 1:1000, PTM BIO, catalogue no.: PTM-7093), TfR (Mouse, 1:1000, Thermo Fisher Scientific, catalogue no.: 13-6800), β-tubulin (Mouse, 1:2000, Argio, catalogue no.: ARG62347), β-actin (Mouse, 1:2000, Affinity, T0022), cytoplasmic Dynein intermediate chain (Rabbit, 1:1000, Bioss, catalogue no.: 10470 R), flag (Rabbit, 1:1000, Cell Signaling Technology, catalogue no.: 14793) and myc (Mouse, 1:1000, Santa Cruz, catalogue no.: sc-40) overnight at 4 °C with gentle agitation. Wash the membrane 3 times for 5 minutes each with TBS-T (Tris-buffered saline with 0.1% Tween-20) to remove unbound primary antibody. Incubate the membrane with an appropriate HRP-conjugated secondary antibody (e.g., anti-rabbit or anti-mouse) diluted in blocking solution for 1 hour at room temperature with gentle agitation. Wash the membrane 3 times for 5 minutes each with TBS-T to remove excess secondary antibody. Develop the membrane using ECL hypersensitive luminescent solution (Millipore, catalogue no.: WBKLS0100) and visualize the signal with the Tanon chemiluminescent imager. Unprocessed scans of the blots are provided in the Source Data file.
Metabolic assays
Mice were anesthetized with 10% urethane (0.02 ml/g, ip), then perfused through the ascending aorta with ice-cold PBS, followed by rapid dissection of the DRG tissue on ice. To measure lactate levels in DRG, the DRG was homogenized with lysis buffer on ice, and the supernatant was obtained by centrifugation at 12,000 g for 10 minutes at 4 °C. The supernatants were collected, and lactate levels were measured using an l-Lactate Assay Kit (Elabscience, catalogue no.: E-BC-K044-M) following the manufacturer’s instructions.
Preparation of the GST-Fused proteins
GST-fused proteins were expressed in Escherichia coli BL21. The bacteria were grown in 2 × YTA media, and protein expression was induced by adding 1 mM Isopropyl β-D-thiogalactopyranoside. Subsequently, the bacteria were centrifuged, resuspended, and subjected to ultrasonic treatment to release the proteins. The proteins were then purified using Glutathione-Sepharose beads (GE Healthcare), concentrated, and quantified before further use.
Coimmunoprecipitation and GST Pull-Down
The cultured cells or dissected tissues were lysed in ice-cold RIPA buffer. The lysates were centrifuged, and 5% of each supernatant was taken for the input sample. The remaining supernatant was incubated overnight with 5 μg DOCK4 (Santa Cruz, catalogue no.: sc-100718), Nav1.7 (Alomone, catalogue no.: ASC-008), cytoplasmic Dynein intermediate chain (Abcam, catalogue no.: ab23905) or myc (Santa Cruz, catalogue no.: sc-40) antibody at 4 °C, and then incubated with protein A/G beads (GE Healthcare) at 4 °C for 4 h. Denaturation of the immunoprecipitation sample and preparation of western blotting for immunoprecipitation was carried out with antibodies against Nav1.6 (Rabbit, 1:200, Alomone, catalogue no.: ASC-009), Nav1.7 (Rabbit, 1:200, Alomone, catalogue no.: ASC-008), Nav1.8 (Rabbit, 1:200, Alomone, catalogue no.: ASC-016), Nav1.9 (Rabbit, 1:200, Alomone, catalogue no.: ASC-017), TRPM3 (Rabbit, 1:1000, Bioss, catalogue no.: bs-9046R), TRPV1 (Rabbit, 1:200, Alomone, catalogue no.: ACC-030), TRPA1 (Rabbit, 1:1000, ABclonal, catalogue no.: A12544), DOCK4 (Rabbit, 1:2500, Abcam, catalogue no.: ab85723; Mouse, 1:200, Santa Cruz, catalogue no.: sc-100718), cytoplasmic Dynein intermediate chain (Rabbit, 1:1000, Bioss, catalogue no.: 10470 R), myc (Mouse, 1:1000, Santa Cruz, catalogue no.: sc-40) and flag (Rabbit, 1:1000, Cell Signaling Technology, catalogue no.:14793). Washed and detected the immunocomplexes. The GST-fused protein (5 μg) was incubated with the cell lysate at 4 °C overnight. Then, the GST-fused protein was precipitated with 10 μL of Glutathione-Sepharose beads at 4 °C for 2 hours. The precipitant was washed, denatured and prepared for immunoblotting. To investigate the direct interaction between DOCK4 and Nav1.7CT, we obtained purified DOCK4 by cleaving GST from GST-DOCK4 using thrombin (Sigma-Aldrich). The GST-DOCK4 slurry (100 μL, ~1 mg/mL) underwent digestion with 0.02 units of thrombin at room temperature for 12 h. The beads were then removed, GST and undigested GST-DOCK4 proteins via centrifugation, leaving the purified DOCK4 protein in the supernatant. Subsequently, we incubated 10 μL of the supernatant (diluted in 400 μL of RIPA buffer) with 5 μg of GST-flag-Nav1.7CT or its fragments to detect their direct interaction.
Proximity ligation assay (PLA)
PLA was performed using Duolink reagents (Sigma‒Aldrich, DUO92101) on cultured DRG neurons from mice, monkey, and human43. The DRG neurons were cultured 2 days, the cells were fixed with 4% paraformaldehyde at RT for 15 minutes and permeabilized with 0.1% Triton X-100 for 30 minutes. PLA was performed according to the Duolink PLA Protocol of Sigma‒Aldrich to examine the interactions between proteins. Briefly, add primary antibody solution with primary antibody against a pairwise combination of DOCK4 (Mouse, 1:200, Santa Cruz, catalogue no.: sc-100718; Rabbit, 1:200, Abcam, catalogue no.: ab85723), Nav1.7 (Rabbit, 1:100, Alomone, catalogue no.: ASC-008) and cytoplasmic Dynein intermediate chain (Mouse, 1:100, Abcam, catalogue no.: ab23905) to each coverslip. Negative control cells are incubated without primary antibodies. Wash twice and incubate the two PLA probes mixture (Duolink II anti-mouse MINUS and Duolink II anti-Rabbit PLUS) with each coverslip. Wash with Buffer and add the ligase solution to each coverslip. Apply the mixture of polymerase and Amplification stock and incubate for 100 minutes. Let coverslips dry, add Dapi Fluoromount-G and seal coverslip edges. Observe samples with a fluorescence microscope (LSM880, Carl Zeiss) the next day. During the microscope imaging process, we controlled the laser intensity and exposure time to remain consistent across the same batch of experiments. In the data analysis process, we ensured that the analysis parameters were kept consistent.
Quantification of immunostained membrane Nav1.7
DRG neurons from mice, monkeys, and human were cultured for 1–2 days. Following fixation, DRG coverslips were treated with primary antibodies against Nav1.7 (Rabbit, 1:100, Alomone, catalogue no.: ASC-008) and the appropriate secondary antibodies. Coverslips were then imaged using a confocal microscope. Using ImageJ software, profile plots of each cell were made spanning the cell diameter for Nav1.7 immunoreactivity, and a background reading was also taken. An average of the signal intensity was then taken for the portion of the plot relating to the membrane and that relating to the cytoplasm (avoiding the nucleus). Only signal intensities greater than background were used. The ratio of membrane to cytoplasm was calculated, and those cells with a ratio greater than 1.5 times were defined as Nav1.7 membrane positive positive30,44.
Quantitative real-time polymerase chain reaction (q-PCR)
A series of tissues were rapidly isolated in RNase-free conditions, which included lung, heart, muscle, intestine, skin, kidney, colon, brain, cerebellum, spinal cord, and DRGs of mice. Total RNA was extracted using TRIzol reagent (Invitrogen, USA). Reverse transcription was carried out using M-MLV reverse transcriptase (Promega, USA) following the manufacturer’s protocol. Primer sequences of genes for PCR are list in Table S1. Quantitative real-time PCR was conducted using SYBR Green qPCR SuperMix (Invitrogen, USA) and an ABI PRISM 7500 Sequence Detection System. The reactions were set up according to the manufacturer’s protocol. The PCR conditions were incubation at 95 °C for 3 minutes followed by 40 cycles of thermal cycling (10 seconds at 95 °C, 20 seconds at 58 °C, and 10 seconds at 72 °C). The relative expression ratio of mRNA was quantified using the 2-ΔΔCT method.
DRG extraction and RNA sequencing
Mice were anesthetized with the 10% urethane (0.02 ml/g, ip), then perfused through the ascending aorta with ice-cold PBS, followed by rapid dissection of the DRG tissues on ice. Total RNA was then isolated from the DRG using the TaKaRa MiniBEST Universal RNA Extraction Kit (Takara, Kyoto, Japan), with quantity and quality verified by an Agilent 2100 Bioanalyzer (Agilent, CA, USA). As in the previous study79, library preparation was performed using the Optimal Dual-mode mRNA Library Prep Kit, which involved mRNA enrichment, fragmentation, cDNA synthesis, end repair, A-tailing, adapter ligation, and PCR amplification. The prepared libraries were circularized and then amplified via RCA to form DNA nanoballs (DNBs), each carrying over 300 copies. These DNBs were subsequently loaded onto a patterned nanoarray for the generation of PE 150 base reads on a G400 platform. For data analysis, the raw sequencing data underwent stringent filtering with SOAPnuke. This process involved: (1) eliminating reads containing sequencing adapters; (2) removing reads where the ratio of low-quality bases ( ≤ 15) exceeded 20%; (3) discarding reads with an unknown base (‘N’ base) ratio greater than 5%. The resulting high-quality clean reads were then saved in FASTQ format.
Chromatin immunoprecipitation (ChIP) assay
ChIP assays were performed using anti-IgG or anti-H4K8la antibodies, and the process was performed with a SimpleChIP Enzymatic Chromatin IP Kit (Cell Signaling, catalogue no.: 9003) according to the manufacturer’s protocols. The specific DNA fragments were subjected to q-PCR.
Tamoxifen (TAM) injections
The TAM injection test was conducted according to a previously described protocol31. 15 mg of TAM (Sigma) were dissolved in 1 ml of 100% corn oil and prepared freshly each day before use. The mice received intraperitoneal injections (i.p.) of 150 mg/kg TAM for 5 consecutive days. Each mouse was weighed prior to injection to standardize for weight differences. Behavioral tests were performed between 7 and 21 days after tamoxifen injection. Prior to TAM injections, AvCreERT2; Dock4CKO mice were healthy and viable, showing no differences compared to Dock4WT littermate controls in various somatosensory assays. However, TAM injections led to a visible difference in behavior of AvCreERT2; Dock4CKO but not Dock4WT mice that started ~7 days after the last injection.
Cell culture and transfection
The mouse, monkey and human DRGs were isolated from their surrounding connective tissue sheath and placed in ice-cold DMEM/F (Gibco, catalogue no.: C11330500BT). The DRG was trimmed to leave only the cell bodies, and excess moisture is removed using filter paper. The tissue was then minced into a slurry using scissors. Subsequently, the tissue was incubated in pre-prepared digestion solution (5 ml DMEM/F; 3 mg collagenase, Sigma-Aldrich, catalogue no.: C9891; 2 mg trypsin, Sigma-Aldrich, catalogue no.: T9201) for 15–25 minutes until no visible tissue clumps remain. Digestion was terminated by adding 5 ml of pre-prepared complete medium (10% fetal bovine serum, Ozfan, catalogue no.: FBSKM0501; 1% Penicillin-Streptomycin, Gibco, catalogue no.: 15140-122). The mixture is then centrifuged at 1000 rpm for 5 minutes, and the supernatant is discarded. The pellet is resuspended in 5 ml of complete medium and centrifuged again for 5 minutes. After discarding the supernatant, the pellet is resuspended in an appropriate volume of complete medium and evenly seeded onto glass coverslips pre-coated with poly-L-lysine (Sigma, catalogue no.: P7890). The coverslips are then incubated in a humidified incubator at 37 °C with 5% CO2. After approximately 4 to 48 h of plating, the cells were utilized for electrophysiological recordings, immunofluorescence staining and proximity ligation assay (PLA). The same protocol was applied for primary DRG culture from mice, monkeys and humans, with digestion times adjusted according to the specific needs of the tissue. For siRNA transfection, 12 hours after plating, 50 nM siRNA for DOCK4 (CACAAACATTCCCGAGAAA for Macaca fascicularis purchased from Ribobio; for Human, catalogue no.: L-017968-01-0005 purchased from Dharmacon) was mixed with siGLO control (Dharmacon) and Transfection Reagent in Opti-MEM. After 48 hours, cells were used for electrophysiology or immunocytochemistry. HEK293T cells (ATCC, catalogue no.: CRL-3216) were cultured in DMEM with 10% fetal bovine serum. Full-length GST-flag-Nav1.7-CT, GST-flag-Nav1.7-CT (aa 1749–1824, 1825–1899, 1900–1977), full-length myc-DOCK4, myc-DOCK4 (aa 1–655, 656–1310, 1311–1966) and full-length Flag-DYNC1I1 plasmids were obtained from Synbio Technologies and all plasmid vectors are based on the pcDNA3.4 vector. The flag and myc fusion proteins were co-expressed in HEK293T cells. The cells were transfected with 1–2 μg plasmid per 35-mm dish or 2–3 μg plasmid per 60-mm dish using Lipofectamine 3000 reagent (Invitrogen). The cells were used for subsequent experiments 24–48 hours after transfection.
Electrophysiological recordings
Whole-cell patch clamp recordings of sodium currents and action potentials were performed at room temperature using an EPC-10 amplifier and the PULSE program (HEKA Electronics, Lambrecht, Germany), as previously described42. Data were recorded with glass pipettes (35 MΩ resistance) fabricated from borosilicate glass capillaries using a Sutter P-97 puller (Sutter Instruments, Novato, CA). Data were low-pass filtered at 2 kHz and sampled at 10 kHz. Voltage errors were minimized by using 80% series resistance compensation, and leak subtraction was performed. Neurons with leak currents >500 pA or series resistance >10 MΩ were excluded. For voltage-clamp recordings, the extracellular solution contained (in mM): 30 NaCl, 20 TEA-Cl, 90 choline chloride, 3 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose, and 0.1 CdCl2 (adjusted to pH 7.4 with Tris base). The pipette solution contained (in mM): 135 CsF, 10 NaCl, 10 HEPES, 5 EGTA, and 2 Na2ATP (adjusted to pH 7.2 with CsOH). Nav1.7 current was obtained by subtracting the ProTxII-resistant Na+ current from the total current, as outlined in the published protocol42,45. We used 300 nM TTX to block TTX-sensitive currents for Nav1.8 recording. To study the I-V curve, Na+ current was recorded at depolarized voltages (-100 mV to +80 mV, 5 mV steps). Current density was calculated by normalizing maximal peak currents with cell capacitance. For building activation curves, the neuron was clamped at a holding potential of -90 mV, and a pre-pulse voltage of -120 mV for 200 ms was applied, followed by a stepped depolarized test voltage pulse from -80 to +100 mV for 50 ms. For inactivation curves, the cell was clamped at a holding potential of -90 mV, and a stepped pre-pulse from -120 to +40 mV with 5 mV increments for 1000 ms was applied, followed by a 50 ms pulse of voltage at 0 mV. Time constants for recovery from inactivation of sodium channels were measured with a double-pulse protocol. A first pulse (P1) for 250 ms to -10 mV caused inactivation, and Na+ current evoked by the test pulse (P2) to -10 mV after variable intervals was compared with the sodium current at P1 of the same episode. For current-clamp recordings, the extracellular solution contained (in mM): 140 NaCl, 3.5 KCl, 1 MgCl2•6H2O, 2 CaCl2•2H2O, 10 D-glucose, 10 HEPES, 1.25 NaH2PO4•2H2O (adjusted to pH 7.4 with NaOH). The pipette solution contained (in mM): 5 NaCl, 140 K-Gluconate, 0.1 CaCl2•2H2O, 1 MgCl2•6H2O, 10 HEPES, 1 EGTA, 2 Mg-ATP (adjusted to pH 7.2 with KOH). The osmolality of all solutions was adjusted to 310 mOsm. The action potential (AP) of DRG neurons, evoked by a depolarizing current stimulation (1 sec, 200 pA current intensity), was recorded.
In vitro calcium imaging
Ca2+ imaging was conducted in cultured DRGs from Dock4WT and AvCreERT2; Dock4CKO mice after loading cultured DRGs with 3 μL of Fluo-4 diluent (50 μg Fluo-4AM dissolved in 45.6 μL DMSO) for 30 minutes in DMEM/F-12 medium at room temperature (avoid light). Then discard the DMEM/F-12 medium, add the Ca2+ imaging buffer for Ca2+ imaging. The imaging buffer includes 136 mM NaCl, 1.0 mM MgCl2, 2.0 mM CaCl2, 5.4 mM KCl, 10 mM D-Glucose, 0.33 mM NaH2PO4, 10 mM HEPES and pH = 7.4. The fluorescence reflecting the Ca2+ signal was imaged in a Laser Scanning Confocal Microscope (Leica SP5-FCS) and calcium flux was analysed from the mean fluorescence measured with LAS AF system. DRG plates were recorded for more than 12 minutes, which was divided in 1 minute of initial reading (0-s mark, baseline values, F0), following by stimulation with CIM0216 (TRPM3 agonist, 1 μM, Tocris Bioscience, catalogue no.: 5521), AITC (TRPA1 agonist, 200 μM, Sigma, catalogue no.: W203408), capsaicin (TRPV1 agonist, 100 nM, Tocris Bioscience, catalogue no.: 0462), and KCL (40 mM). Ca2+ signal amplitudes were presented as ΔF/F0 = (Ft-F0)/F0 as ratio of fluorescence difference (Ft-F0) to basal value (F0). The average fluorescence intensity of the baseline period was set as F0 and the fluorescence intensity of the record time (t) was remarked as Ft.
In vivo calcium imaging of DRG
The mice received an intrathecal injection of 10 μL of rAAV-CAG-GCaMp6s (BrainVTA, serotype 9, 2.0 × 1012 vg/ml) and were allowed to recover for three weeks before undergoing calcium imaging, in accordance with the protocols established in previous studies44,80,81. Before imaging, an initial dose of 0.3 ml of urethane (12.5% w/v) was administered intraperitoneally. After approximately 15 minutes, additional doses were titrated to achieve the desired level of anesthesia until surgical depth was reached. A constant temperature heating pad was used to maintain the core temperature at approximately 37 °C, with temperature monitored via a rectal probe. The skin of the mice was prepared, and an incision was made between the L3 and L5 spinal segments. Connective tissue and muscle surrounding the vertebrae were carefully resected, followed by a small transverse laminectomy around the L5 DRG, ensuring that the dura mater and perineurium remained intact. The mice were positioned prone on a customized microscope table, and their spines were stabilized using specially designed clamps to minimize movement during imaging. Imaging was conducted using the Multiphoton Laser Confocal Microscope (FVMPE-RS, Olympus) at a frame rate of 10 seconds per frame, with a resolution of 800 × 800 pixels for the DRG. An Argon ion laser with a 488 nm excitation line was utilized, and the GCaMP signal was collected in the wavelength range of 500-550 nm. During the imaging of the DRG response, brush strokes were applied to the ipsilateral paw at approximately 1–2 Hz, moving from medial to lateral. Additionally, pinch stimuli were applied using an arterial clamp at the pads and toes of the ipsilateral hindpaw. Thermal stimulation at 50 °C was applied to the hindpaw using a digital temperature-controlled silicone rubber heating plate. A 10% capsaicin cream was applied to both the hairy and plantar surfaces of the skin, and responses were assessed for 5 minutes following the completion of the application. The raw data underwent background subtraction and normalization by first subtracting the baseline. The difference was then divided by the baseline to calculate ΔF/F. Regions of interest (ROIs) surrounding the neuronal cell bodies were selected using the freehand selection tool in Fiji/ImageJ Version 1.48 v. ROIs for assessing GCaMP fluorescence were selected stringently, minimizing overlap to reduce interference from adjacent somata. In contrast, ROIs for calculating neuronal cell body size were chosen with less stringency, permitting overlapping regions where cells are in close proximity. This approach allowed for a more accurate estimation of cell diameter. Activation of a cell was defined as an increase in fluorescence intensity (ΔF) ≥ 30% of baseline. The responses of these cells were then compared between Dock4WT and AvCreERT2; Dock4CKO mice.
Data analysis
The gray values of the western blot bands were quantified by Tanon Gis software. The gray value of the images and colocalization were quantified by ImageJ software. All western blotting, immunostaining, calcium imaging, electrophysiological, and behavioral data are expressed as the mean ± SEM and were analyzed with GraphPad Prism 9.5.1. The western blotting, immunostaining, and calcium imaging data were analyzed by Two-tailed Independent Student’s t test and One-way ANOVA followed by Tukey’s multiple comparisons test. The behavioral data were analyzed by Two-tailed Independent Student’s t test, One-way ANOVA followed by Tukey’s multiple comparisons test and Two-way ANOVA followed by Bonferroni’s multiple comparisons test. The electrophysiological data were analyzed by One-way ANOVA followed by Tukey’s multiple comparisons test and Two-way ANOVA followed by Bonferroni’s multiple comparisons test. The threshold for statistical significance was P < 0.05. *P < 0.05, **P < 0.01, ***P < 0.001, n.s. means not significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The Seq data generated in this study have been deposited in NCBI GEO under accession code GSE283287. There are no restrictions on data availability in the manuscript. The data generated in this study are provided in the Source Data file. Source data are provided with this paper.
References
Sharma, S. R., Gonda, X. & Tarazi, F. I. Autism spectrum disorder: Classification, diagnosis and therapy. Pharm. Ther. 190, 91–104 (2018).
Lord, C., Cook, E. H., Leventhal, B. L. & Amaral, D. G. Autism spectrum disorders. Neuron 28, 355–363 (2000).
Buch, A. M. et al. Molecular and network-level mechanisms explaining individual differences in autism spectrum disorder. Nat. Neurosci. 26, 650–663 (2023).
Robertson, C. E. & Baron-Cohen, S. Sensory perception in autism. Nat. Rev. Neurosci. 18, 671–684 (2017).
Whitney, D. G. & Shapiro, D. N. National prevalence of pain among children and adolescents with autism spectrum disorders. JAMA Pediatr. 173, 1203–1205 (2019).
Bennetto, L., Kuschner, E. S. & Hyman, S. L. Olfaction and taste processing in autism. Biol. Psychiatry 62, 1015–1021 (2007).
Orefice, L. L. et al. Targeting peripheral somatosensory neurons to improve tactile-related phenotypes in ASD Models. Cell 178, 867–886.e824 (2019).
Chen, Q. et al. Dysfunction of cortical GABAergic neurons leads to sensory hyper-reactivity in a Shank3 mouse model of ASD. Nat. Neurosci. 23, 520–532 (2020).
Wang, C. et al. Impaired cerebellar plasticity hypersensitizes sensory reflexes in SCN2A-associated ASD. Neuron 112, 1444–1455.e1445 (2024).
Pagnamenta, A. T. et al. Characterization of a family with rare deletions in CNTNAP5 and DOCK4 suggests novel risk loci for autism and dyslexia. Biol. psychiatry 68, 320–328 (2010).
Guo, D. et al. Autism-like social deficit generated by Dock4 deficiency is rescued by restoration of Rac1 activity and NMDA receptor function. Mol. psychiatry 26, 1505–1519 (2019).
Maestrini, E. et al. High-density SNP association study and copy number variation analysis of the AUTS1 and AUTS5 loci implicate the IMMP2L-DOCK4 gene region in autism susceptibility. Mol. Psychiatry 15, 954–968 (2010).
Yang, Y. et al. KnockoffTrio: A knockoff framework for the identification of putative causal variants in genome-wide association studies with trio design. Am. J. Hum. Genet. 109, 1761–1776 (2022).
Handy, D. E., Castro, R. & Loscalzo, J. Epigenetic modifications: basic mechanisms and role in cardiovascular disease. Circulation 123, 2145–2156 (2011).
Sabari, B. R., Zhang, D., Allis, C. D. & Zhao, Y. Metabolic regulation of gene expression through histone acylations. Nat. Rev. Mol. cell Biol. 18, 90–101 (2017).
Zhang, D. et al. Metabolic regulation of gene expression by histone lactylation. Nature 574, 575–580 (2019).
Pan, R. Y. et al. Positive feedback regulation of microglial glucose metabolism by histone H4 lysine 12 lactylation in Alzheimer’s disease. Cell Metab. 34, 634–648.e636 (2022).
Irizarry-Caro, R. A. et al. TLR signaling adapter BCAP regulates inflammatory to reparatory macrophage transition by promoting histone lactylation. Proc. Natl Acad. Sci. USA 117, 30628–30638 (2020).
Li, L. et al. Glis1 facilitates induction of pluripotency via an epigenome-metabolome-epigenome signalling cascade. Nat. Metab. 2, 882–892 (2020).
Dai, S. K. et al. Dynamic profiling and functional interpretation of histone lysine crotonylation and lactylation during neural development. Development 149, https://doi.org/10.1242/dev.200049 (2022).
Yu, J. et al. Histone lactylation drives oncogenesis by facilitating m(6)A reader protein YTHDF2 expression in ocular melanoma. Genome Biol. 22, 85 (2021).
Caterina, M. J. et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Sci. (N. Y., N. Y.) 288, 306–313 (2000).
Davis, J. B. et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 405, 183–187 (2000).
Scholz, J. et al. The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain 160, 53–59 (2019).
Allely, C. S. Pain sensitivity and observer perception of pain in individuals with autistic spectrum disorder. The Scientific World Journal 2013, 916178 (2013).
Valvona, C. J., Fillmore, H. L., Nunn, P. B. & Pilkington, G. J. The Regulation and Function of Lactate Dehydrogenase A: Therapeutic Potential in Brain Tumor. Brain Pathol. (Zur., Switz.) 26, 3–17 (2016).
Chen, Y. et al. Metabolic regulation of homologous recombination repair by MRE11 lactylation. Cell 187, 294–311.e221 (2024).
Donnelly, C. R. et al. STING controls nociception via type I interferon signalling in sensory neurons. Nature 591, 275–280 (2021).
Cheng, L. et al. Identification of spinal circuits involved in touch-evoked dynamic mechanical pain. Nat. Neurosci. 20, 804–814 (2017).
Xie, M. X. et al. Endophilin A2 controls touch and mechanical allodynia via kinesin-mediated Piezo2 trafficking. Mil. Med. Res. 11, 17 (2024).
Ranade, S. S. et al. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 516, 121–125 (2014).
Zurborg, S. et al. Generation and characterization of an Advillin-Cre driver mouse line. Mol. pain. 7, 66 (2011).
Lawson, S. N. & Waddell, P. J. Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J. Physiol. 435, 41–63 (1991).
Abraira, V. E. & Ginty, D. D. The sensory neurons of touch. Neuron 79, 618–639 (2013).
Konermann, S. et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature 517, 583–588 (2015).
Narváez-Pérez, L. F. et al. CRISPR/sgRNA-directed synergistic activation mediator (SAM) as a therapeutic tool for Parkinson´s disease. Gene Ther. https://doi.org/10.1038/s41434-023-00414-0 (2023).
Caterina, M. J. et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824 (1997).
Vandewauw, I. et al. A TRP channel trio mediates acute noxious heat sensing. Nature 555, 662–666 (2018).
Dib-Hajj, S. D., Cummins, T. R., Black, J. A. & Waxman, S. G. Sodium channels in normal and pathological pain. Annu. Rev. Neurosci. 33, 325–347 (2010).
Schaller, K. L. & Caldwell, J. H. Expression and distribution of voltage-gated sodium channels in the cerebellum. Cerebellum 2, 2–9 (2003).
Li, Y. et al. DRG Voltage-Gated Sodium Channel 1.7 Is Upregulated in Paclitaxel-Induced Neuropathy in Rats and in Humans with Neuropathic Pain. J. Neurosci. 38, 1124–1136 (2018).
Zhang, X. L. et al. Foxo1 selectively regulates static mechanical pain by interacting with Nav1.7. Pain 162, 490–502 (2021).
Wang, Z. et al. Anti-PD-1 treatment impairs opioid antinociception in rodents and nonhuman primates. Sci. Transl. Med. 12, https://doi.org/10.1126/scitranslmed.aaw6471 (2020).
Dawes, J. M. et al. Immune or genetic-mediated disruption of CASPR2 causes pain hypersensitivity due to enhanced primary afferent excitability. Neuron 97, 806–822.e810 (2018).
Xie, M. X. et al. Nuclear factor-kappaB Gates Nav1.7 Channels in DRG Neurons via Protein-Protein Interaction. iScience 19, 623–633 (2019).
Xie, M. X. et al. ATF4 selectively regulates heat nociception and contributes to kinesin-mediated TRPM3 trafficking. Nat. Commun. 12, 1401 (2021).
Jordt, S. E. et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427, 260–265 (2004).
Yang, L. et al. FGF13 Selectively Regulates Heat Nociception by Interacting with Nav1.7. Neuron 93, 806–821.e809 (2017).
Lampert, A. et al. A pore-blocking hydrophobic motif at the cytoplasmic aperture of the closed-state Nav1.7 channel is disrupted by the erythromelalgia-associated F1449V mutation. J. Biol. Chem. 283, 24118–24127 (2008).
Reck-Peterson, S. L., Redwine, W. B., Vale, R. D. & Carter, A. P. The cytoplasmic dynein transport machinery and its many cargoes. Nat. Rev. Mol. cell Biol. 19, 382–398 (2018).
Cason, S. E. & Holzbaur, E. L. F. Selective motor activation in organelle transport along axons. Nat. Rev. Mol. Cell Biol. 23, 699–714 (2022).
Pfister, K. K. Distinct functional roles of cytoplasmic dynein defined by the intermediate chain isoforms. Exp. cell Res. 334, 54–60 (2015).
Ding, H. et al. A bifunctional nociceptin and mu opioid receptor agonist is analgesic without opioid side effects in nonhuman primates. Sci. Transl. Med. 10, https://doi.org/10.1126/scitranslmed.aar3483 (2018).
Han, Q. et al. SHANK3 deficiency impairs heat hyperalgesia and TRPV1 signaling in primary sensory neurons. Neuron 92, 1279–1293 (2016).
Iossifov, I. et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature 515, 216–221 (2014).
Ling, Q., Herstine, J. A., Bradbury, A. & Gray, S. J. AAV-based in vivo gene therapy for neurological disorders. Nat. Rev. Drug Discov. 22, 789–806 (2023).
Yajnik, V. et al. DOCK4, a GTPase activator, is disrupted during tumorigenesis. Cell 112, 673–684 (2003).
Kozma, R., Sarner, S., Ahmed, S. & Lim, L. Rho family GTPases and neuronal growth cone remodelling: relationship between increased complexity induced by Cdc42Hs, Rac1, and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol. Cell Biol. 17, 1201–1211 (1997).
Furniss, F. & Biswas, A. B. Recent research on aetiology, development and phenomenology of self-injurious behaviour in people with intellectual disabilities: a systematic review and implications for treatment. J. Intellect. Disabil. Res. 56, 453–475 (2012).
Mandell, D. S., Novak, M. M. & Zubritsky, C. D. Factors associated with age of diagnosis among children with autism spectrum disorders. Pediatrics 116, 1480–1486 (2005).
Nader, R., Oberlander, T. F., Chambers, C. T. & Craig, K. D. Expression of pain in children with autism. Clin. J. pain. 20, 88–97 (2004).
Rattaz, C. et al. How do children with autism spectrum disorders express pain? A comparison with developmentally delayed and typically developing children. Pain 154, 2007–2013 (2013).
Failla, M. D., Gerdes, M. B., Williams, Z. J., Moore, D. J. & Cascio, C. J. Increased pain sensitivity and pain-related anxiety in individuals with autism. Pain. Rep. 5, e861 (2020).
Mikkelsen, M., Wodka, E. L., Mostofsky, S. H. & Puts, N. A. J. Autism spectrum disorder in the scope of tactile processing. Developmental Cogn. Neurosci. 29, 140–150 (2018).
Bennett, D. L. & Woods, C. G. Painful and painless channelopathies. Lancet Neurol. 13, 587–599 (2014).
Dib-Hajj, S. D. et al. Gain-of-function mutation in Nav1.7 in familial erythromelalgia induces bursting of sensory neurons. Brain: a J. Neurol. 128, 1847–1854 (2005).
Fertleman, C. R. et al. SCN9A mutations in paroxysmal extreme pain disorder: allelic variants underlie distinct channel defects and phenotypes. Neuron 52, 767–774 (2006).
Cox, J. J. et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature 444, 894–898 (2006).
Higerd-Rusli, G. P. et al. Depolarizing Na(V) and hyperpolarizing K(V) channels are co-trafficked in sensory neurons. J. Neurosci. 42, 4794–4811 (2022).
Higerd-Rusli, G. P. et al. Inflammation differentially controls transport of depolarizing Nav versus hyperpolarizing Kv channels to drive rat nociceptor activity. Proc. Natl Acad. Sci. USA 120, e2215417120 (2023).
Higerd-Rusli, G. P. et al. The fates of internalized Na(V)1.7 channels in sensory neurons: Retrograde cotransport with other ion channels, axon-specific recycling, and degradation. J. Biol. Chem. 299, 102816 (2023).
Guedes-Dias, P. & Holzbaur, E. L. F. Axonal transport: Driving synaptic function. Science (New York, N.Y.) 366, https://doi.org/10.1126/science.aaw9997 (2019).
Canty, J. T. & Yildiz, A. Activation and regulation of cytoplasmic dynein. Trends biochemical Sci. 45, 440–453 (2020).
Olenick, M. A., Dominguez, R. & Holzbaur, E. L. F. Dynein activator Hook1 is required for trafficking of BDNF-signaling endosomes in neurons. J. cell Biol. 218, 220–233 (2019).
Choi, W. S. et al. Kv1.5 surface expression is modulated by retrograde trafficking of newly endocytosed channels by the dynein motor. Circulation Res. 97, 363–371 (2005).
Mao, Q. et al. DNMT3a-triggered downregulation of K2p 1.1 gene in primary sensory neurons contributes to paclitaxel-induced neuropathic pain. Int J. Cancer 145, 2122–2134 (2019).
Wang, K. et al. STING suppresses bone cancer pain via immune and neuronal modulation. Nat. Commun. 12, 4558 (2021).
Mason, M. R. et al. Comparison of AAV serotypes for gene delivery to dorsal root ganglion neurons. Mol. Ther. 18, 715–724 (2010).
Wang, M. et al. A dual-action Rhein-peptide hydrogel synergistically inhibits inflammation and matrix degradation for therapeutic mitigation of abdominal aortic aneurysm. Chem. Eng. J. 498, 155723 (2024).
Meixiong, J. et al. Activation of mast-cell-expressed mas-related g-protein-coupled receptors drives non-histaminergic Itch. Immunity 50, 1163–1171.e1165 (2019).
Xie, M. X. et al. ATF4 inhibits TRPV4 function and controls itch perception in rodents and nonhuman primates. Pain 165, 1840–1859 (2024).
Acknowledgements
This study was supported by National Natural Science Foundation of China (82271241 to X.L.Z., 82171230 to M.X.X.). Guangdong Basic and Applied Basic Research Foundation (2024A1515013015 to X.L.Z., 2024A1515013199 to M.X.X.). Science and Technology Projects in Guangzhou (2023A04J1758 to M.X.X.). Excellent Young Talents Project of Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences (KY012021188 to X.L.Z.). Young Talents Program of Sun Yat-sen University Cancer Center (M.X.X.).
Author information
Authors and Affiliations
Contributions
M.X.X., R.C.L., Y.B.X., S.Y.Z., and H.T. performed the experiments and prepared the Figs. X.Y.C., J.K.L., Z.S.Z., S.H.W., X.F.L., Y.H,. and Y.X. analyzed and interpreted the data. X.L.Z. designed the project and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xie, MX., Lai, RC., Xiao, YB. et al. Histone lactylation regulates DOCK4 to control heat nociception and supports Dynein-mediated Nav1.7 trafficking. Nat Commun 16, 7165 (2025). https://doi.org/10.1038/s41467-025-62343-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-62343-3
This article is cited by
-
Beyond inflammation: a comprehensive microglial regulation model in chronic pain
Molecular Biology Reports (2025)