Abstract
Carbonic anhydrase (CA) mimics have received significant attention due to their promising applications in the enhanced hydration and sequestration of CO2. Herein, we report the assembly of sequence-defined peptoids into crystalline nanomaterials with controlled microenvironment of active sites as CA mimics for promoted hydration and sequestration of CO2. By incorporating specific ligands into self-assembling peptoids and coordinating these ligands with metal cations, we synthesize a variety of crystalline nanosheets and nanotubes as efficient CA mimics comparable to natural bovine CA. Molecular dynamics simulations reveal the critical roles of peptoid-Zn2+ binding energy and the active site local microenvironment on the catalytic performance of these CA mimics. CO2 precipitation results show that these CA mimics promote the hydration and sequestration of CO2 while retaining high thermal and chemical stabilities. This study offers essential guidance for the future design of high-performance CA-mimics suitable for applications in CO2 capture and sequestration.
Similar content being viewed by others
Introduction
CO2 is one of the most common greenhouse gases, and its fast rise in the atmosphere is believed to be the most important contributions to sever climate issues. In 2023, global CO2 emissions reached an all-time high with 35.8 Gt CO21. Consequently, it is urgent to develop new technologies that can effectively and efficiently reduce atmospheric CO2 concentrations. While marine organisms play a critical role in the global carbon cycle by using biomacromolecules to capture CO2 and convert it into stable carbonate minerals during biomineralization2,3,4, mimicking such CO2 sequestration process by developing biomimetic approaches suitable for industrial applications has been a long-standing challenge5,6,7.
While biomimetic approaches using carbonic anhydrases (CAs) have shown considerable promise in CO2 sequestration due to their high catalytic performance in converting CO2 to HCO3− for carbonate precipitation7,8,9, the use of natural CAs is restricted due to its intrinsic limitations, such as poor stability and high operational cost8,10,11. For that, various CA mimics have been developed for bio-inspired CO2 sequestration. Among them, peptide-based ones have received particular attention because self-assembly of peptides into various nanostructures enables the ability to mimic CA active sites via three-dimensional arrangement and investigate the correlations between assembled architectures and the catalytic activity12,13,14,15. Additionally, within the peptide sequence, the molecular-level arrangement of amino acids introduces tunability in catalytic efficiency and selectivity14,16. However, peptide assembly based CA mimics often face challenges in tuning the local environment of active sites without disrupting the assembled nanostructures due to their intrinsic structural complexity and low stability17,18. Compared to peptides, peptoids (or poly-N-substituted glycines) are a new class of sequence-defined synthetic polymers which have the identical backbone but the side chain of a peptoid is appended to the nitrogen rather than the α-carbon atoms19,20,21. Because of these differences, peptoids can be easily synthesized and offer precise controls over peptoid–peptoid and peptoid–substrate interactions solely through variations in side-chain chemistry, while still emulating the capacity of peptides/proteins for molecular recognition21,22,23. By designing a series of amphiphilic peptoids with controlled hydrophobic interaction and tuning their side chain chemistries, we and others have reported the self-assembly of peptoids into highly crystalline nanomaterials with various nanostructures16,20,21 including membrane-mimetic 2D nanosheets24,25 and 1D stiff and dynamic nanotubes26,27. We demonstrated that a wide range of functional groups, including various metal-ligand coordination active sites, can be incorporated and patterned within these nanomaterials to build biomimetic catalysts for lignin degradation28 and catalytic degradation of organophosphates29. The high stability and tunability of these peptoid crystalline nanomaterials make them unique advantages to be designed as CA mimics.
Inspired by the crystal structure of natural CA and our recent success in developing crystalline peptoid assemblies as enzyme mimics28,29, in this work, we design and synthesize a series of self-assembling peptoids with either six Nbrpe = N-([2-(4-bromophenyl)ethyl]glycine), Nclpe = N-([2-(4-chlorophenyl)ethyl]glycine), or Nbrpm = N-[(4-bromophenyl)methyl] glycine as the hydrophobic domains that are responsible for stabilizing the morphology of crystalline peptoid assemblies. 1,4,7,10-tetraazacyclododecane (cyclen), 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid (Do3a), [2-(4-pyridyl)ethyl]glycine (Npy), or [2-(4-imidazolyl)ethyl]glycine (Nhis) as the ligand in the hydrophilic domain for its coordination with metal cations to build active sites with tunable microenvironments (Fig. 1). We aim to use these assembled metal-containing crystalline nanomaterials containing well-aligned active sites as CA mimics for enhanced CO2 hydration and precipitation. Furthermore, we demonstrate the tuning of active site microenvironments for enhanced catalytic performance. The catalytic activities of these CA mimics are first investigated by the catalytic hydrolysis of 4-nitrophenyl acetate (4-NPA), a model reaction often used to evaluate the efficiency of CA mimics12,30. We demonstrate the control over catalytic activities of these CA mimics by tuning ligand and side chain chemistry, local microenvironment, and active sites arrangement. We find that these CA mimics are highly stable and effective in catalytic hydrolysis of 4-NPA. The tubular CA mimics assembled from peptoids containing three Nhis groups show the highest catalytic activity comparable to bovine carbonic anhydrases (BCAs), making them one of the most efficient CA mimics. We integrate experimental findings with computational studies through molecular dynamics (MD) simulations, revealing the critical roles of peptoid-Zn2+ binding energy and of the active site microenvironment on the catalytic performance of these peptoid-based CA mimics. The best CA mimic is further tested for its high efficacy in promoting CO2 hydration and precipitation as calcium carbonate materials. Finally, through in situ liquid-state NMR study, we show how peptoid-based CA mimics play critical roles in the reversible binding with CO2 to promote CO2 hydration, and in changing the CaCO3 formation pathways to accelerate the CO2 mineralization. As far as we know, this is the first example of CA mimics that are able to promote both CO2 hydration and conversion of HCO3− to CO32-. We expect that the self-assembly of peptoids into hierarchical crystalline nanomaterials will provide a fascinating strategy for the design and synthesis of robust and efficient enzyme mimetics, including high-performance CA-mimics suitable for applications in CO2 capture and sequestration.
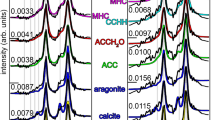
A A proposed model showing the assembly of peptoids into CA-mimicking nanosheet or nanotube structures including different ligands (L) and side chain groups (R) used to design Pep1-19. B AFM images of assembled nanosheet or nanotube structures. In all cases, height profile was used. C Representative XRD results of Pep4, Pep4-Zn2+ and Pep5-Zn2+ nanosheets (left) and of Pep12, Pep12-Zn2+ nanotubes. The values above each peak are the corresponding lattice spacing values.
Results and discussion
Design and synthesis of peptoid sequences
To mimic the active site of CAs and take advantage of high crystallinity and tunability of peptoid assemblies, we designed and synthesized a series of peptoids with either six Nbrpe, Nclpe, or Nbrpm groups as the hydrophobic domain and various ligands in the hydrophilic domain for their co-assembly with Zn2+ to form CA mimics (Fig. 1 and S1). The presence of six Nbrpe, Nclpe, and Nbrpm groups in the hydrophobic domain has been demonstrated to form highly crystalline nanosheets or nanotubes after self-assembly21,27,28,29,31,32. Ligands like cyclen, Do3a, Npy, or Nhis were introduced in the N-terminus to coordinate with Zn2+ for the formation of metal-ligand active sites on the surface of the peptoid assemblies. We further varied the number of ligands in each peptoid to tune the catalytic activity of peptoid-based CA mimics. These ligands were chosen because of their previous utilization in forming metal-ligand binding sites in enzyme mimic catalysts28,29,33,34. Because the local environment of the CA active site is important for CA catalytic activities8, besides varying specific ligands, we further modified the polar domain of peptoids with various side chains including N-(2-carboxyethyl)glycine (Nce), N-(2-methoxyethyl)glycine (Nme), N-(2-hydroxyethyl)glycine (Noe), and N-[2-(1H-indol-3-yl)ethyl]glycine (Ntrp) to tune the local microenvironment of the metal-ligand active site. We also added hydrophobic side chains like N-(i-methyl)ethylglycine (Nipr) and N-(phenylmethyl)glycine (Npm) close to the active site to create local hydrophobic environment. A list of all the peptoid sequences studied in this work with highlighting hydrophobic domain, hydrophobic and hydrophilic side chains, and ligands can be found in Fig. S1. All peptoids were synthesized by submonomer solid-phase synthesis method reported previously25,27,28,29. All synthesized peptoids were purified using reverse-phase high-performance liquid chromatography (HPLC) and characterized by ultra-performance liquid chromatography-mass spectrometry (UPLC-MS) to confirm the high purify. The detail synthesis and characterizations of these peptoids are shown in the Methods and in the supplementary Figs. 2–20.
Assembly and characterizations of peptoid-based CA mimics
To generate Zn2+-containing peptoid assemblies as CA mimics, the corresponding peptoids were incubated with Zn2+ (1:1 molar ratio) in water and acetonitrile (v/v = 50 : 50) at 4 °C for slow crystallization, similar to our previously reported methods28,29. Gel-like or suspension materials containing a large amount of crystalline nanosheets or nanotubes formed after a few days (See experimental section for details). Atomic force microscopy (AFM) results showed that peptoids with six Nbrpe side chains formed uniform 2D nanosheets with straight edges, with a thickness of ~4.0 nm. While those containing six Nbrpm groups formed nanotubes with a height of ~6.5 nm (Figs. 1B and S22, Table S2). Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images further confirmed the formation of sheet and tube nanostructures (Figs. S23-24), which are comparable to those we previously reported25,27. X-ray diffraction (XRD) results showed that these sheets and tubes are highly crystalline (Figs. 1C and S25). The XRD results of nanosheets with and without Zn2+ cations showed similar patterns to sheets assembled from other Nbrpe6 containing sequences29,32,35, confirming that metal-ligand coordination does not influence the formation of crystalline nanosheets and the ordered alignment of Nbrpe6 domains. Specifically, the peak at q = 1.4 Å−1 is due to the alignment of peptoid backbone chains along x-axis which leads to a spacing of 4.5 Å (Fig. 1). The 1.8 nm spacing corresponds to the distance between two packed peptoid backbones along y-axis with Nbrpe groups facing each other. The peak at q = 1.1 Å−1 with spacing of 6 Å corresponds to the Nbrpe side-chain length. Prominent XRD peaks with spacings of 4.0, 3.8, and 3.4 Å suggest the existence of extensive π-π interactions among Nbrpe groups36,37,38,39. The peak of 3.0 Å is corresponding to the distance between two adjacent side chains along the backbone chain direction (i.e., N···N distance) of a cis-conformation peptoid29,40,41. The XRD results of nanotubes with and without Zn2+ cations showed similar patterns to those of crystalline nanotubes assembled from Nbrpm6 containing sequences26,27,28,42. The first low q peak at d spacing of 3.1 nm corresponds to the wall thickness, and the peak of 1.67 nm spacing corresponds to the distance between two peptoid backbones. The prominent peak at 4.6 Å corresponds to the space of the alignment of peptoid chains along x-axis, and the peak at 5.7 Å refers to the ordered packing of Nbrpm side chains in the hydrophobic block. The presence of π-π interactions among the aromatic Nbrpm groups can be attributed to the peaks located at 4.3 and 3.8 Å36,37,38,39. In order to confirm the coordination between Zn²⁺ and the ligand-containing peptoids within the assembled metallopeptoid nanomaterials, we conducted the ultraviolet-visible (UV-vis) titration study which was previously used to characterize metal-peptoid complexes and reveal the binding stoichiometry of the peptoid-to-metal ratio43,44,45,46. Upon adding Zn(BF4)2 to the peptoid solution in acetonitrile, we noted an increase in the absorbance at 260 nm for Pep4, Pep6, Pep12, indicating the binding of peptoids with Zn2+ to form peptoid-Zn2+ complexes (Fig. S26). In contract, Pep1 without ligands showed no change in the absorbance, indicating no binding with Zn2+. A metal-to-peptoid ratio plot, constructed from these titration experiments, suggested 1 : 1 for Pep4, 1 : 2 for Pep6, and 3 : 4 for Pep12. Additionally, the binding of ligands with Zn2+ was further confirmed by the Fourier Transform Infrared (FTIR) analysis, where the appearance of characteristic peaks of metal-N bond coordination at the 400–550 cm−1 region was observed47 (Fig. S27). The amount of Zn2+ bound with peptoids within these assembled nanomaterials was determined using Eriochrome Black T as a complexometric indicator (See supporting information for details)29,48. The results demonstrated that 52 to 87% of the initially added Zn2+ was bound to the assembled nanomaterials prepared by co-assembling Zn2+ with Nhis-containing peptoids (Pep8, Pep11, and Pep12). In contrast, peptoids without ligands (Pep1 and Pep18) showed almost negligible binding to the initially added Zn2+. The coordination of Zn2+ ions with Nhis-containing peptoids within assembled crystalline nanomaterials was also confirmed by X-ray photoelectron spectroscopy (XPS) (Fig. S29). The presence of Zn2+ ions within assembled peptoid nanomaterials was further revealed by the SEM- energy dispersive X-ray analysis (EDX) (Fig. S30) and STEM/energy dispersive spectroscopy (STEM/EDS) (Fig. S31) results. The coordination of Zn2+ ions with ligand-containing peptoids was further confirmed by electrospray ionization-mass spectrometry (ESI-MS) results. As shown in Fig. S32, for Pep1 without ligand, the mass of [Zn(Pep)]2+ wasn’t observed (S32A), which is consistent with the result of our other characterizations showing the importance of having ligands for metal-peptoid coordination. In contrast, the metal-peptoid coordination was observed both ligand-containing peptoids: Pep4 and Pep12, which the mass of [Zn(Pep)]2+ species was clearly observed (Fig. S32B and C). In all cases, the assembled metallopeptoid nanomaterials were washed with water at least three times to confirm that there are no non-coordinated Zn2+ cations present.
CA mimic activity assessment
CA mimic activities of assembled peptoid nanosheets and nanotubes were determined by the colorimetric assay based on the hydrolysis of 4-NPA at room temperature to form 4-nitrophenol (4-NP), a model reaction frequently used to evaluate the CA mimic activity30. By tracing the UV-Vis absorbance of 4-NP at 400 nm, the hydrolysis rate can be determined (Fig. S33). In a standard condition, reaction was carried out in HEPES buffer at pH 7.4 and room temperature, with a peptoid catalyst concentration of 0.125 mM and a 4-NPA concentration of 0.25 mM (Fig. 2A).
A schematic representation of 4-NPA hydrolysis to 4-NP by peptoid nanosheets. B Time-dependent conversion of 4-NPA to 4-NP, triggered by different peptoid nanosheets. C 4-NPA hydrolysis activity of peptoid catalysts comprising of different ligands. Here, error bars represent standard deviation (SD), n = 3. D 4-NPA hydrolysis at different concentrations of Pep7-Zn2+ nanosheets.
Effect of ligand chemistry
Ligand-metal coordination chemistry plays a crucial role in the catalytic activity of biomimetic catalysts28,29, to mimic the coordination chemistry of CA active sites and investigate the influence of ligand chemistry. Zn2+-containing crystalline nanosheets assembled from Pep1 without ligand and from Pep2 to Pep7 containing four different ligands were used to evaluate the catalytic degradation of 4-NPA. As seen from Fig. 2A, the low conversion triggered by those without binding ligands showed the importance of metal-ligand coordination in the peptoid-triggered catalytic hydrolysis of 4-NPA. To further highlight the critical role of Nhis in the creation of active sites for efficient peptoid assembly catalysts, a new peptoid Pep17 with four non-ligand/binding methoxy groups was synthesized (Fig. S18) and used to co-assemble with Zn2+ to form crystalline nanosheets (Fig. S38). As seen from Fig. S28, the nanosheets co-assembled from Zn2+ and peptoids with a non-ligand group (Pep1 and Pep17) showed negligible binding to the initially added Zn2+. Such trivial coordination of Pep17 with Zn2+ was further confirmed by SEM-EDX analysis as no detectable Zn2+ was observed (Fig. S30H), and the very low 4-NPA hydrolysis activity (Table 1, Entry 28) by the nanosheets co-assembled from Pep17 with Zn2+ (Fig. S39). Among different ligands containing peptoids, Pep7- Zn2+ nanosheets containing Nhis ligands showed the highest activity (Fig. 2B). Reaction rate constants obtained from the catalytic hydrolysis of 4-NPA (Table 1) further confirmed the importance of ligand-Zn2+ coordination in the catalytic hydrolysis of 4-NPA, and crystalline nanosheets containing Nhis ligands are most effective. The catalytic activity of these peptoid nanosheets followed the order of Pep7-Zn2+ (k/kuncat = 17) > Pep4-Zn2+ (k/kuncat = 14) > Pep2-Zn2+(k/kuncat = 12.2) > Pep5-Zn2+(k/kuncat = 11.2) > Pep3-Zn2+ (k/kuncat = 6) ~ Pep6-Zn2+(k/kuncat = 6) (Fig. 2C). Very low activity was observed when no peptoid materials (k = 0.06 × 10−4 S−1, Table 1, Entry 1 and 2) or Pep1-Zn2+ nanosheets without ligands (k = 0.08 × 10−4 S−1, Table 1, Entry 3) were used. These results further confirmed the crucial roles of metal-ligand coordination and ligand chemistry in the peptoid-triggered catalytic hydrolysis of 4-NPA. As seen in Fig. 2D, the yield of 4-NP is proportional to the concentration of Pep7-Zn2+ nanosheets, showing the importance of peptoid-based catalysts in the catalytic hydrolysis of 4-NP. Among four different ligands, Nhis is the best ligand to assemble peptoid-Zn2+ nanosheets for efficient hydrolysis of 4-NPA.
Effect of the number of Nhis groups
Because Nhis-Zn2+ coordination is the most effective way to build active sites for CA mimics, we further optimized the performance of Nhis-Zn2+ containing peptoid assemblies by having varied number of Nhis groups. As shown in Fig. 1A, Pep8 with two Nhis, Pep11 with three Nhis, and Pep13 with four Nhis groups all formed well-defined nanosheets in the presence of Zn2+. When these sheets were used to evaluate the catalytic hydrolysis of 4-NPA, their activity showed an order of Pep8-Zn2+ (k/kuncat = 35.5) < Pep13-Zn2+ (k/kuncat of 50.2) < Pep11-Zn2+ (k/kuncat = 59.7) (Figs. 3A, B, S34A and Table 1), where Pep11-Zn2+ nanosheets from peptoid with three Nhis groups showed the highest activity, similar to CA active sites which typically contain three histidine residues around the Zn2+ center49. In contrast to Pep7-Zn2+ nanosheets (k/kuncat = 17), Pep11-Zn2+ sheets showed significantly higher activity which could be due to the increased Nhis-Zn2+ active sites within nanosheets. While the slightly lower activity of Pep13-Zn2+ than Pep11-Zn2+ nanosheets could be attributed to nonideal coordination between Nhis ligands and Zn2+ due to enhanced steric hindrance as well as reduced substrate bindings. These experimental findings were further supported by MD simulations (vide infra).
Effect of metal cations and counter anions of Zn2+ salts
The high catalytic activity of CA is attributed to the formation of tetrahedral Zn2+-ligand coordination for optimized activity50. Zn2+ acts as Lewis acid to produce hydroxyl anion from water, while at the same time, it directly takes part in the catalytic cycle via coordination geometry and electrostatic effects. CAs often have substantial changes in the catalytic activity when native Zn2+ is replaced with other transition metal ions50,51. To study the role of metal cations on peptoid-based catalysts, Pep8 was co-assembled with Co2+ (Co(BF4)2), or Cu2+ (Cu(BF4)2) salts. (Fig. S35A). As seen in Fig. S34B, Pep8-Zn2+ nanosheets exhibited the highest activity (k/kuncat = 35.5), followed by Pep8-Co2+ (k/kuncat = 20.1) and Pep8-Cu2+ (k/kuncat = 35.9) nanosheets. These results are similar to those found in the previously-reported theoretical study50, where Nhis coordinated with Zn2+ to form an tetrahedral structure during the catalytic mechanism, while Co2+ initially maintained a tetrahedral structure but later transformed to a less efficient octahedral structure. Finally, lower activity with Cu2+ could be due to the formation of suboptimal trigonal bipyramidal coordination geometry.
In addition to metal cations, counter anions could also play a role in the catalytical activity of artificial enzymes52. To assess the role of counter anions of Zn2+ in the catalytic activity of assembled peptoid-Zn2+ catalysts, in addition to Pep8-Zn2+ nanosheets which Zn(BF4)2 was used, Pep8 was further co-assembled with Zn(NO3)2 or ZnCl2 to form nanosheets (Fig. S35A). As seen in Fig. S34B, The catalytic hydrolysis of 4-NPA results showed that Pep8-Zn2+ nanosheets from Zn(BF4)2 exhibited the highest activity with an order of Pep8-Zn(BF4)2 (k/kuncat = 35.5) > Pep8-Zn(NO3)2 (k/kuncat = 30.5) > Pep8-ZnCl2 (k/kuncat = 27.2), inversely correlating to the increase in their dissociation energy53,54. The highest activity of Pep8-Zn(BF4)2 could be due to the maximum number of Zn2+ coordinated with water molecules in the active sites as a result of the low dissociation energy and non-nucleophilic nature of BF4− anion. Interestingly, when Pep8 co-assembled with Zn(BF4)2 in a molar ratio of 1:2, instead of 1:1, a decrease in hydrolysis activity was observed (Table 1, Entry 15). As seen in Fig. S28, when Pep8 co-assembled with Zn2+ in a molar ratio of 1:2, 52% of initial Zn2+ were coordinated within crystalline nanomaterials. In contrast, the nanosheets co-assembled from Pep8 with Zn2+ in a molar ratio of 1:1 had 87% of the initially added Zn2+ coordinated with Pep8, suggesting the optimized molar ratio of Pep8 to co-assemble with Zn2+ is about 1:1. A further increase of the added Zn2+ ions results in a slight decrease in hydrolysis activity (Table 1, Entry 15) which could be due to the increased electrostatic repulsion as a result of excess Zn2+ ions, thus lowering the available Zn2+ active sites.
Role of Zn2+as a cofactor
Transition metals as cofactors are often credited for the unparalleled catalytic activity of natural enzymes14. However, cofactor-free histidine-rich enzyme mimics obtained from self-assembly have also been reported to have high catalytic activity55,56,57. The catalytic activity of Pep11-Zn2+ nanosheets for 4-NPA hydrolysis was compared to metal-free counterpart - Pep11 nanosheets. While Pep11 nanosheets performed significantly higher than the background activity with k/kuncat of 26.5, possibly due to the formation of imidazolyl-rich sites after self-assembly, leading to base mediated ester hydrolysis55. However, this catalytic activity is still more than two-fold lower than that of the Pep11-Zn2+ (Fig. 3B), underlining the importance of Zn2+ as a cofactor. To surmise, in our CA mimic peptoid catalysts, Zn2+ bound to three histidine molecules forming optimal tetrahedral geometry is more efficient in coordinating with a water molecule to form an -OH unit than imidazolyl groups.
A Conversion rate of 4-NPA hydrolyzed by different peptoid catalysts. B Comparison of 4-NPA hydrolysis activity using different metal free and metallopeptoid catalysts. C Effect of nanosheets crystallinity on 4-NPA hydrolysis activity: (I) Pep11- Zn2+ nanosheets, (II) Pep11 nanosheets + Zn2+, (III) amorphous Pep11-Zn2+ complex, (IV) histamine-Zn2+ complex. In (B, C), data are shown as the mean ± SD, n = 3. D AFM and SEM images of Pep11-Zn2+ nanosheets and Pep12-Zn2+ nanotubes after hydrolysis experiments.
Role of hydrophilic domain and hydrophobic pockets
The active site of CAs consists of Thr-199 hydroxyl group and Glu-106 carboxyl group, which form a hydrogen bonding network and stabilize Zn-bound hydroxide ions58. To study the effect of hydrophilic groups, Pep9-Zn2+ nanosheets with similar number of Nhis groups of Pep8-Zn2+ but with six polar Nce groups between hydrophobic domain and the active site was synthesized (Figs. S10 and S22). As seen in Fig. 3A, interestingly, Pep9-Zn2+ sheets showed a significant decrease in 4-NPA hydrolysis activity (k/kuncat = 7.8) in comparison to Pep8-Zn2+ sheets (k/kuncat = 35.5). We reasoned that the decreased activity of Pep9-Zn2+ nanosheets could be due to unwanted competition of six Nce groups with Nhis ligands for binding with Zn2+, leading to catalytically inactive sites14. Additionally, the presence of Nce residues could reduce the local hydrophobicity, thus disrupting the hydrophobic pocket14,59. In contrast, Pep8-Zn2+ nanosheets have six Nbrpe residues as the hydrophobic domain to create the hydrophobic pockets for stabilizing substrates and achieving enhanced catalytic activity60, while Nhis groups in the active sites form a hydrogen bonding network and participate in the stabilization of hydroxyl ions and the proton transfer mechanism57,61.
To further confirm the importance of local hydrophobic pockets in catalytic performance, we synthesized Pep14 with the hydrophilic domain containing five Nce and one Npm residues adjacent to the terminal Nhis groups (Fig. S15) and generated crystalline Pep14-Zn2+ nanosheets as confirmed by AFM, SEM and XRD results (Fig. S36A and B). We hypothesized that Npm groups adjacent to the metal active sites in the nanosheet could act as small hydrophobic pockets for effective substrate binding and substrate-catalyst complex stabilization29. As shown in Fig. S36C, Pep14-Zn2+ sheets exhibited a catalytic activity significantly higher than Pep9-Zn2+ sheets but similar to Pep8-Zn2+ sheets (Table 1, entry 10 and 25). These results further confirmed the importance of hydrophobic pockets of peptoid-Zn2+ nanosheets in their catalytic hydrolysis of 4-NPA. Further modification of sheet-forming peptoids by adding one Noe and one Nce residues between the hydrophobic domain and Nhis3 ligands led to Pep15 (Fig. S16) and crystalline Pep15-Zn2+ nanosheets characterized by AFM, SEM, and XRD (Fig. S37). 4-NPA hydrolysis results showed Pep15-Zn2+ nanosheets had a lower catalytic efficiency (k/kuncat = 27.5) (Table 1, Entry 26) than Pep11-Zn2+ nanosheets (k/kuncat = 54.0), which could be due to the negative impact of both Noe and Nce groups on the formation of ligand-Zn2+ active sites and their hydrophobic pockets. In contrast, Pep16 with two Nipr and one Ntrp residues (Fig. S17) between the hydrophobic domain and Nhis3 ligands led to nanosheets (Fig. S37A) with a catalytic activity (k/kuncat = 54.0; Table 1, Entry 27) comparable to Pep11-Zn2+ nanosheets. These findings conclude that Pep11-Zn2+ nanosheets with hydrophobic domains of six Nbrpe groups adjacent to the catalytic active site is crucial for substrate binding, while hydrophilic domains consisting of three Nhis groups form optimal coordination sites with Zn2+ and take part in proton transfer mechanism57,61 leading for highest catalytic activity. Additionally, we synthesized a new peptoid sequence Pep18 with two imidazole units similar to Pep8 but one Npm group sandwiched (Fig. S19). We hypothesized, similar to Pep14-Zn2+ nanosheets, we would observe an effect of hydrophobic pockets in the catalytic activity of assembled Pep18-Zn2+ nanomaterials. As expected, the co-assembly of Pep18 with Zn2+ led to the formation of crystalline nanosheets (Fig. S38). As shown in Fig. S39, Pep18-Zn2+ nanosheets showed a higher catalytic activity (k/kuncat = 40) than Pep8-Zn2+ nanosheets (k/kuncat = 35) for 4-NPA hydrolysis (Table 1, Entry 29). We hypothesize, similar to Pep14-Zn2+ nanosheets, Npm groups in the sheet surface play a crucial role in efficient binding to the substrate (4-NPA) by forming small hydrophobic pockets and through enhanced π-π interactions62,63.
Finally, to assess whether bromophenyl group within the skeleton plays any role in the catalytic activity, Pep19 was synthesized by replacing six Nbrpe groups of Pep8 with six Nclpe groups, while retaining the same number of Nhis ligands (Fig. S20). Co-assembly of Pep19 with Zn2+ resulted in the formation of nanosheets with similar height to that of Pep8-Zn2+ nanosheets (Fig. S38), despite the sheet formation process of Pep19 being relatively slower than Pep8, due to the enhanced hydrophobic interactions among Nbrpe groups than Nclpe groups35. Hydrolysis of 4-NPA using Pep19-Zn2+ nanosheets showed a similar catalytic activity (k/kuncat = 33.2) to Pep8-Zn2+ nanosheets(k/kuncat = 35.5)(Table 1 entry 10 and 30, Fig. S39), indicating the trivial role of bromo- or chloro-phenyl groups for catalytic effect.
Effect of active site arrangement and ordering
High efficiency and selectivity in natural enzymes can be attributed to folding into their highly ordered active conformation via self-assembly64. To underline the importance of highly ordered packing in our peptoid catalysts, 4-NPA hydrolysis activity was assessed by amorphous aggregates of Pep11-Zn2+ obtained by mixing amorphous Pep11 powder with Zn(BF4)2 salt in a 1:1 molar ratio. Pep11-Zn2 complex showed an approximate 4.5-fold decrease in catalytic activity compared to the Pep11-Zn2+ nanosheets (Fig. 3C, I and III, Table 1 Entry 19 and 20). We believed that the assembly of crystalline Pep11-Zn2+ nanosheets could lead to the formation of a high density of highly ordered active sites, which is crucial in increasing catalytic activity. To further demonstrate the importance of peptoid self-assembly process in obtaining highly ordered arrangement of active sites, metal-free Pep11 nanosheets were incubated with Zn(BF4)2 salt in 1:1 molar ratio in an aqueous solution for a week. This obtained complex of Pep11 sheets with Zn2+ exhibited a catalytic activity of k/kuncat of 27.7 (Fig. 3C) that is significantly lower than the assembled Pep11-Zn2+ nanosheets. Such decreased activity could be due to the low coordination efficiency of Nhis ligands as a result of their tight packing within Pep11 nanosheets29. These findings are consistent with our recent studies in utilizing highly crystalline nanosheets and nanotubes in developing biomimetic catalysts28,29. Finally, small molecule histamine-Zn2+ complex showed a catalytic activity which is nearly 6-fold lower than Pep11-Zn2+ nanosheets (Fig. 3C, IV, Table 1 Entry 29, and Fig. S21), further highlighting the importance of highly ordered active sites for efficient catalysis. Interestingly, both AFM and SEM results showed that Pep11-Zn2+ nanosheets remain the intact structure after hydrolysis experiments (Fig. S40), suggesting the high stability of these crystalline nanomaterials and the importance of maintaining these ordered Nhis-Zn2+ active sites for enhanced catalytic activity.
Effect of assembly morphology
The morphology of self-assembled nanostructures can play a crucial role in the catalytic performance of peptoid-based catalysts14,28,42. One of the significant advantages of peptoid assembly system is that the morphology of self-assembled peptoid nanostructures can be tuned and controlled based on the side chain chemistry of the hydrophobic domain21. Interestingly, self-assembled Pep10-Zn2+ and Pep12-Zn2+ nanostructures with nanotube morphologies showed slightly higher catalytic activities than Pep8-Zn2+ and Pep11-Zn2+ nanosheets (Fig. 3A, B, Table 1: Entry 17, 19, and 25). A similar finding was observed with peptoid/hemin enzymatic mimics for natural peroxidase-like activities28. We hypothesize this could be due to higher solvent accessible surface area containing active sites with nanotubular structures than nanosheet morphologies.
Effect of temperature and solution pH, and peptoid assembly stability
While CA enzymes show excellent CO2 hydration capability, they exhibit intrinsic disadvantages in thermal and chemical stability8,10,11. For example, CA enzymes have been reported to irreversibly denature and aggregate above 60 °C and in acidic conditions. In this aspect, peptoid-based biomimetic catalysts hold a significant advantage by exhibiting high stability21,23,28,29. AFM and SEM results showed both Pep11-Zn2+ nanosheets and Pep12-Zn2+ nanotubes retained after hydrolysis reactions (Fig. 3D). Interestingly, when 4-NPA hydrolysis was carried at elevated temperature (40 °C) and high pH (pH 8), Pep11-Zn2+ nanosheets and Pep12-Zn2+ nanotubes showed an increase in rate of 2.4 and 2.8-fold, respectively. Further hydrolysis reactions using Pep10-Zn2+ and Pep12-Zn2+ nanotubes at 60 °C and pH 7.4 showed a 4-fold increase in catalytic efficacy, while the uncatalyzed control reaction still maintained significantly low background activity (Table 1, Entry 33-35). When the stability of Pep11-Zn2+ nanosheets was assessed at 60 °C and 90 °C, acidic conditions, and organic solvent, in all cases the nanosheet structure was retained (Fig. S41). Similarly, Pep12-Zn2+ nanotubes also showed excellent stability in different conditions (Fig. S42). Additionally, when Pep12-Zn2+ nanotubes were recovered after heating in water at 60 °C for overnight, at 90 °C for 2 h, or after being stored at room temperature for 30 days, they showed catalytic activities comparable to freshly made nanotube samples (Fig. S43A). Finally, the recyclability of Pep11-Zn2+ nanosheets was evaluated for five consecutive 4-NPA hydrolysis cycles. After that, Pep11-Zn2+ sheets maintained nearly 85% of the catalytic activity (Fig. S43B). A slight decrease in catalytic activity could be due to the unavoidable mass loss during sheet recoveries. These results further confirmed that peptoid-based CA mimics are highly robust and show unique advantages in the catalytic hydrolysis of 4-NPA.
Enzyme-like activity
As Michaelis-Menten kinetics are typically used to for CA kinetics profile65, we further studied this kinetics with our best performing peptoid catalysts to compare their activity to CA. Our results showed that initial hydrolysis rate (V0) increased sharply at lower 4-NPA concentration before eventually subsided at higher concentration (Figs. S44–47), displaying enzyme-like behavior66. Parameters obtained from linear curve fitting into Lineweaver-Burke plots are given in Table 2. As seen in Table 2, the catalytic efficiency (kcat/Km) of Pep11-Zn2+ nanosheets measured at pH 7.4 and 25 °C is two-fold higher than metal-free Pep11 sheets. An increase of solution pH to 8 and temperature to 40 °C led to over a two-fold increase in maximum velocity (Vmax) and catalytic efficiency (kcat/Km). Interestingly, Pep12-Zn2+ nanotubes showed a moderate increase in catalytic efficiency (kcat/Km = 14) than Pep11-Zn2+ nanosheets (kcat/Km = 9). Interestingly, both Pep11-Zn2+ and Pep12-Zn2+ CA mimics showed very comparable Km values to the bovine carbonic anhydrase (BCA) (Table 2), suggesting efficient substrate binding. The high catalytic efficiency of Pep12-Zn2+ nanotubes was also observed for the slight decrease in the Michaelis-Menten constant (Km). Interestingly, at same solution pH and temperature, Pep12-Zn2+ nanotubes exhibited a very comparable high catalytic efficiency (kcat/Km) to Bovine CA (BCA) (Table 2, Entry 4 and 5), a natural enzyme often used as the point of reference for CA mimetics33. Additionally, catalytic performance of our peptoid assembly catalysts with different artificial CA mimics with or without crystalline motifs was also compared (Table S1). As seen in Table S1, the peptoid catalysts developed in this work outperformed various artificial CA mimics30,67,68,69,70,71,72 based on the derived Michaelis-Menten parameters, and hence they are highly qualified as efficient CA mimics.
Molecular dynamics (MD) simulation of peptoid-based CA mimics for catalytic performance
MD simulations have been integral in understanding the structure and assembly of peptoids, providing a powerful tool to investigate the structural basis of their function25,35,73,74,75,76,77,78. Leveraging this computational approach to gain more insight into the catalytic performance of peptoid-based CA mimics, we began by analyzing the structure of a natural enzyme, specifically the Human carbonic anhydrase II with a sulfonamide inhibitor (PDB-Entry: 3k34)79, as shown in Fig. 4A. This enzyme is used as model because the bound sulfonamide inhibitor exhibits conformational mimicry with 4-NPA, allowing us to draw meaningful parallels. Docking studies confirm that 4-NPA can bind into the enzyme’s active site with an orientation similar to the inhibitor’s (see simulation methods in the supporting information for details). The enzyme structure highlights the geometric arrangement of the active site, characterized by one Zn²⁺ ion coordinated with three histidine residues, accompanied by surrounding hydrophobic residues. These hydrophobic residues are essential for stabilizing the substrate within a well-defined pocket. Understanding this configuration provided critical insights into the factors influencing substrate recognition and catalytic efficiency. An active site is a region encompassing one Zn²⁺ ion coordinated by three histidine-type residues and the surrounding hydrophobic residues that facilitate substrate binding. Based on enzyme structure (Fig. 4A), we focused on two key features: 1) the active site, defined as the region encompassing one Zn²⁺ ion coordinated by three histidine-type residues, and 2) the hydrophobic binding regions, consisting of surrounding hydrophobic residues that facilitate substrate binding. This definition ensures that the active site represents the complete catalytic environment, where Zn²⁺ activation of the substrate is complemented by the hydrophobic residues’ role in stabilizing and orienting the substrate for efficient catalysis.
A Computational model built to understand the catalytic performance of peptoid-based CA mimics. Left: Detailed depiction of the active site within an enzyme (PDB-entry: 3k34), highlighting hydrophobic (green) and hydrophilic (blue) residues. Right: Model built to show the local environment of the Pep11-Zn²⁺ CA mimic based on MD simulations. Note that Zn²⁺ and hydroxide (OH⁻) ions are not present in the simulation but are included here solely for comparative visualizations. The inset illustrates the proposed catalytic hydrolysis of 4-NPA by the Pep11-Zn²⁺ CA mimic, detailing the sequence of steps involving substrate binding, catalysis, and product release. More detailed descriptions of ion modeling and simulation parameters are provided in the Supplementary Information (SI). B Comparison of the catalytic efficiency, predicted active site presence, and substrate binding affinity across different peptoids. The measured catalytic activity (gray bars) is compared to the simulation prediction (red line), which assumes that a catalytic site is formed when three histidine-like side chains coordinate with a Zn2+ ion and are within 5 Å of each other. The binding energy (blue circles) also indicates the substrate’s affinity for the potential active site. Here, experimental catalytic efficiencies were determined from three technical replicates (n = 3); individual replicate values are overlaid as dot plots, and error bars represent the standard deviation. Error bars for catalytic site prediction represent the standard deviation over all simulation frames from a 2 μs trajectory (n ≈ 200,000). Binding energy error bars reflect the standard deviation across eight equivalent binding sites within the simulation box (n = 8).
MD simulations focus on the effect of the number of histidines in the sequence and the inclusion of other hydrophilic residues. To gain more insights into the factors that determine the catalytic performance of peptoid-based CA mimics, we constructed models80 for CA mimics assembled from Pep7 – Pep9, Pep11, and Pep13 and performed MD simulations (see experimental methods and supporting information for details). We limit the simulations to the sheet-forming peptoids, as the atomistic structure is better studied than the tube-forming peptoids35,73. This allows us to model the hydrophobic core based on known structural features35,73,81,82. Simulations are performed, including only the sheet, water, and substrate. No ions are present.
The qualitative comparison of the enzyme and active site model of Pep11-Zn2+ assembly based on MD simulations is shown in Fig. 4. The hydrophobic residues (in green) form a hydrophobic pocket and play a vital role in substrate binding, thereby stabilizing the substrate within the active site. In contrast, the hydrophilic residues, shown in blue, interact with polar or charged parts of the substrate and other molecules, ensuring proper orientation and stabilization during the catalytic process. The spatial arrangement of these residues, with hydrophobic and hydrophilic regions distinct yet complementary, underscores the enzyme’s efficiency in substrate recognition and catalysis. Comparing our peptoid catalysts to CAs reveals a similarity in building a Zn2+-imidazolyl active site of three histidine residues and a hydroxide ion. Meanwhile, the peptoid side chains enable the creation of a hydrophobic pocket and tunable microenvironment. In both structures, the hydrophobic patch is a critical component of substrate binding. The peptoid nanosheet’s design replicates this feature, ensuring that the synthetic catalytic site can effectively interact with non-polar regions of substrates, akin to the natural enzyme. Furthermore, the high crystallinity of peptoid-based CA mimics enables the tuning of density and long-range order of active sites to achieve multivalence high performance.
For a quantitative comparison among the sheet-forming peptoid sequences, the number of potential active sites was estimated from the simulation by identifying all viable triads of histidine residues that coordinate with a Zn²⁺ ion within five angstroms (See SI for detailed discussion). The substrate binding free energy was determined using metadynamics simulations (see experimental methods and SI for details). This technique allows us to explore the free energy landscape of the peptoid nanosheet systems by applying a bias that facilitates the exploration of rare conformational states, providing a detailed mapping of binding free energies across potential active sites. As shown in Fig. 4B, our experimental catalytic activities follow similar trends to the number of available catalytic sites. The average number of potential active sites increases with the number of histidine residues. To evaluate this relationship, Pearson’s correlation coefficient was calculated. The correlation analysis between the measured catalytic activity and the simulation predictions of the average number of catalytic sites for the peptoids reveals insightful trends and deviations. The initial analysis, which included all peptoids, yielded a moderate positive correlation coefficient of 0.66. This result suggests a general tendency for higher simulation predictions to correspond with higher measured catalytic activities but with notable variability (Fig. 4). Upon closer examination, Pep13 was identified as a significant outlier. When the data for Pep13 was excluded, the correlation coefficient dramatically increased to 0.99, indicating a robust positive correlation between the simulation predictions and measured activities for the remaining peptoids. Interestingly, for Pep9, a significant reduction compared to Pep8 is observed. The correlation analysis between the binding energy and the measured catalytic activity of the peptoids reveals a positive relationship. The Pearson’s correlation coefficient for these variables is 0.995, indicating an almost perfect linear correlation. This high correlation suggests that binding energy is a critical determinant of catalytic activity, and binding energies correlate with higher catalytic activities. This strong relationship underscores the importance of substrate binding affinity in the overall catalytic performance of the peptoids. It highlights that, in addition to the number of active sites, the ability to bind and stabilize the substrate near these sites is crucial for achieving high catalytic efficiency. Consequently, incorporating binding energy considerations into simulation models is essential for accurately predicting and optimizing peptoid catalytic activities.
Peptoid based CA mimics for enhanced hydration and sequestration of CO2
Although CAs and CA mimics hold great promise for CO2 sequestration, current CAs and CA mimics often suffer from reduced activity in CO2 hydration and precipitation at low pH (pH <9), leading to limited industrial applications83. Due to the unique advantages and high efficacy demonstrated in 4-NPA hydrolysis, our peptoid-based CA mimics are promising to overcome these challenges. Three most effective CA mimics were further tested for their capabilities of promoting the hydration and sequestration of CO2 sequestration (see details in the method section). During CO2 sequestration study, peptoid catalysts promoted the conversion of CO2 into HCO3-, and the deprotonation of HCO3− into CO32- to react with CaCl2 for the formation of CaCO3 precipitates.
As seen in Fig. S48, the weights of precipitated CaCO3 from the blank control, assembled Pep11, Pep11-Zn2+, and Pep12-Zn2+ CA-mimic samples were 1.2, 4.1, 6.9, and 7.3 mg, respectively. SEM with EDX characterizations confirmed the formation of CaCO3 precipitates (Fig. S49). The precipitated CaCO3 from Pep11-Zn2+ nanosheets and Pep12-Zn2+ nanotubes is nearly 7-fold higher than that of blank control, confirming that peptoid catalysts promote CO2 hydration and accelerate the CO2 sequestration. These findings imply that the active sites of peptoid catalysts directly involve in the coordination with CO2, leading to faster formation of carbonates in comparison to the blank sample. Additionally, metal containing peptoid catalysts showed higher efficiency than their metal-free counterparts, similar to the findings from the catalytic hydrolysis of 4-NPA. Finally, SEM results showed that Pep11-Zn2+ nanosheets and Pep12-Zn2+ nanotubes retained after their promoted CO2 sequestration experiments, further confirming their high stability and catalytic nature (Fig. S50). XRD characterization of all the precipitated CaCO3 showed characteristic peaks of calcite polymorph (Fig. S51).
13C and 1H NMR were utilized to understand the effect of peptoid CA mimics in CO2 hydration and HCO3− deprotonation. The best performing Pep12-Zn2+ nanotubes were used as our representative catalysts. Quantitative 13C NMR spectra (Fig. 5A) showed that, with saturated CO2 in 50 mM HEPES buffer at pH 8.0, 50 µM Pep12-Zn2+ tubes triggered the formation of nearly double [HCO3− & CO32−] compared to the control, reaching 77 ± 2 mM. The observed 13C chemical shift, reflecting a weighted average of CO32- and HCO3−, revealed that the fraction of CO32− increased from 0.22 ± 0.03% to 0.4 ± 0.2%. This corresponded to a four-fold increase in total [CO32−] in the presence of Pep12-Zn2+ nanotubes, suggesting that these CA mimics not only enhanced the hydration of CO2 to HCO3− but also accelerated the subsequent conversion of HCO3− to CO32−. This acceleration stems from the strong interaction between Pep12-Zn2+ nanotubes and HCO3−, as evidenced by a pronounced reduction in the 13C spin-spin relaxation time (T2). The 13C T2 of HCO3− and CO2 decreased from 3.5 s and 14.6 s in the control to 0.05 s and 7.4 s in the presence of 50 µM Pep12-Zn2+ nanotubes, respectively (Figs. 5B and S52). In the fast-exchanging limit, a shorter 13C T2 indicates slower chemical exchange between different sites or reduced molecular reorientation motions84,85. The notably two orders of magnitude decrease in 13C T2 of HCO3− suggests that bicarbonate ions that are interacting with Pep12-Zn2+ nanotubes experience highly restricted rotation motions and relatively slow exchange with bulk bicarbonate ions. This is consistent with the previous reports that bicarbonate can bind directly to the metal center of the enzyme or occupy the outer coordination shell of the metal, serving as substrate for the reverse dehydration process86,87. On the other hand, because the concentration of Pep12-Zn2+ nanotubes is three orders of magnitude smaller than the concentrations of CO2 or HCO3−, their presence does not affect the diffusion coefficients of CO2 and HCO3− (Table S3) as measured by 13C diffusion ordered spectroscopy (DOSY). Therefore, these crystalline CA mimics may provide a locally high-pH and stable environment that can attach to CO2 for its hydration and bind to HCO3− for its deprotonation, without impacting the overall mass transport of CO2 and HCO3− in solution.
A 13C single-pulse NMR spectra of the control and Pep12-Zn2+ tube solutions saturated with 13C -enriched CO2 at 1 atm. B Normalized 13C signal intensity as a function of spin echo delays during 13C T2 measurements showing that T2 of both CO2 and bicarbonate are reduced with Pep12-Zn2+ tubes. C Time-dependent 13C NMR spectra during CO2 sequestration with 0.1 M CaCl2 in the control solution and D in the presence of Pep12-Zn2+ tubes. E Evolution of concentrations of total bicarbonate (including fully solvated HCO3− and HCO3− in ion pairs and clusters) and dissolved CO2 during CO2 sequestration with and without Pep12-Zn2+ tubes. F A proposed model showing how crystalline Pep12-Zn2+ nanotubes function as CA mimics to promote CO2 hydration as well as the deprotonation of HCO3− into CO32 to achieve the accelerated mineralization of CO2 into stable calcite (CaCO3) solids.
Moreover, in situ 13C NMR was employed to monitor the effect of Pep12-Zn2+ nanotubes on the CO2 sequestration (Fig. 5C–E). After introducing CO2 in the presence of CaCl2, [HCO3−] in the control solution remained constant in the first 20 min, followed by a decrease in [HCO3−] and an increase in [CO2] at a molar ratio of 2:1, coupled with a broadening in the HCO3− signal followed by a subsequent sharpening. This behavior indicates that an incubation period is required for Ca2+ and HCO3− to form ion associates or clusters prior to the relatively slow decomposition reaction. Notably, the formation of Ca∙HCO3 dense liquid phase (DLP) was not observed in the control solution, as suggested by 1H spin-spin relaxation time of water (Figs. S53–S54) and time-dependent 13C NMR spectra at 5 °C (Fig. S55), primarily because the presence of 15 mM dissolved CO2 at pH 7.1 can highly affect the DLP formation88. In contrast, with 50 µM Pep12-Zn2+ CA mimic, [HCO3−] drops immediately and [CO2] rises sharply, again at a 2:1 ratio. After 20 min of reaction, the changes in the [HCO3−] and [CO2] indicate the generation of ~8 mM of CaCO3. While the control sample exhibited pseudo-zero-order kinetics after the incubation period with a reaction rate coefficient of 1.1 × 10−6 M/s (Fig. S56A), indicative of limited availability of CO32−; the presence of Pep12-Zn2+ nanotubes led to first-order kinetics with a reaction rate coefficient of 2.7 × 10−4 s−1 (Fig. S56B), suggesting the continuous and rapid conversion of HCO3− to CO32−. These results highlight that Pep12-Zn2+ nanotubes remain highly effective even under relatively acidic conditions, probably due to the crystalline nature and dense array of active sites that provide localized high-pH and stable microenvironment for CO2 hydration and sequestration (Fig. 5F).
In conclusion, by designing assembling peptoids with specific metal-ligand coordination, we developed peptoid assembly-based CA mimics for promoted hydration and sequestration of CO2. As peptoid chemistry offers high programmability, different peptoid sequences were carefully designed by varying side chain chemistry and terminal ligands, leading to control in their self-assembly behavior, surface chemistries, and local microenvironment. By tuning the local microenvironment, hydrophilicity, morphology, and ligand chemistries, peptoid catalysts with high efficiency in CO2 hydration were developed. Our studies further showed that these peptoid catalysts are highly stable and can be recycled multiple times while retaining catalytic activity at elevated temperatures. Experimental findings were further corroborated with MD simulation to facilitate our understanding of these peptoid catalysts and their catalytic process. Finally, peptoid catalysts exhibited excellent activities in CO2 hydration and precipitation even at pH 8 by hydration of CO2 to bicarbonate and fast conversion to carbonate. The catalytic CO2 sequestration by these peptoid-based CA mimics were thoroughly studied by NMR and MD simulations to unravel their catalytic activity in promoting CO2 hydration to form HCO3− as well as their promoted deprotonation of HCO3− to CO32−. In contrast to the control solution, Pep12-Zn2+ tube catalysts were able to trigger a complete bypass of the incubation period, thus accelerating the formation of stable calcite solids for durable storage of CO2. Overall, here we have developed a type of crystalline peptoid assemblies with tunable surface chemistries and microenvironment leading to promoted hydration and sequestration of CO2. This study paves a path in designing highly efficient CA mimics of high programmability and flexibility with elaborate understanding of catalytic CO2 hydration and sequestration processes. Further investigation of peptoid-based catalysts will be crucial in developing artificial enzymes rivaling natural CA enzyme’s activity while retaining excellent thermal and chemical stabilities in different practical applications. We believe that peptoid assembly-promoted CO2 hydration and mineral crystallization offer a wide range of applications in environment remediation, biomedical engineering, and in energy storage where a modulated crystallization is often required.
Methods
Materials
All materials were purchased from commercial suppliers and used as received unless otherwise specified. β-Alanine t-butyl ester hydrochloride was purchased from Chem-Impex International, Inc., which was deprotected by adding 1 M sodium hydroxide in dichloromethane (DCM) solution and stirred for one hour. β-Alanine t-butyl ester was then collected by extraction and evaporation of solvent.
Peptoid synthesis and self-assembly
Synthesis of all the peptoid sequences was carried out on rink amide resins by following manual or auto synthesis protocols reported in our previous works, unless mentioned otherwise27,28. Synthesis protocols of each peptoid sequence can be found in the supplementary materials. Synthesized peptoids were cleaved from resins by treating with 95%(v/v) trifluoroacetic acid (TFA) in H2O in a cartridge for 1 h with agitation. Afterwards, the solution was collected by filtration, and TFA was removed by evaporating at reduced pressure at 40 °C. Peptoid sequences containing N-(2-hydroxyethyl)glycine (Noe) side chains were further treated with excess acetic acid/water mixture to remove the protecting group. After TFA evaporation, the obtained crude samples were fully dissolved in acetonitrile/water and purified using reverse-phase high performance liquid chromatography (HPLC). The purification procedure utilized a Waters 1525 system equipped with an XBridgeTM Prep C18 OBDTM column (10 µm, 19 mm × 100 mm). Purified peptoids were analyzed using Waters ACQUITY reverse phase UPLC (a gradient of acetonitrile ranging from 5 - 95 % at 0.4 mL/min over 7.0 min at 40 °C with an ACQUITYBEH C18, 1.7 μm, 2.1 mm × 50 mm column) that was connected with a Waters SQD2 mass spectrometry system. The final purified peptoid product was lyophilized at least twice from its acetonitrile/water solution.
Peptoid self-assembly was conducted by dissolving 1 µmol of corresponding peptoid into 200 µl acetonitrile/water mixture (1:1 by volume) to make a 5.0 mM clear solution. For co-assembly, metal salt solution was added into this and left for slow evaporation at 4 °C. The pH of the peptoid solution was adjusted by adding 0.5 M NaOH dropwise, where needed. Gel-like materials containing crystalline nanomaterials were obtained in a week.
Synthesis of histamine-Zn2+ and Cyclen-Zn2+ complexes
Histamine-Zn2+ complex was synthesized by mixing histamine (1 eq, 0.36 mmol) and zinc tetrafluoroborate (1 eq, 0.36 mmol) in 3.0 ml of acetonitrile. The reaction mixture was left at 40 °C shaking for overnight. Cyclen-Zn2+ complex was synthesized following similar protocol. Formation of the complexes were studied by 1H-NMR and confirmed with previous literature reports89,90. 1H-NMR spectra of these complexes are given in Fig. S21.
General protocol for evaluating catalytic activity of peptoid catalysts using 4-NPA hydrolysis
The hydrolysis reactions were performed in a Tecan Safire 2 microplate reader. For each reaction, a total volume of 300 µl containing 37.5 µl of peptoid catalyst solution (1 mM catalyst solution in DI water), 255 µl of HEPES buffer (50 mM, pH = 7.4), and 7.5 µl of 4-nitrophenyl acetate (4-NPA) (10 mM solution in acetonitrile). The final concentration of catalyst and 4-NPA was 0.125 mM and 0.25 mM, respectively. After the addition of the substrate, mixture solution was transferred to the reader immediately to study absorbance at 400 nm for the formation of 4-nitrophenol (4-NP).
General protocol for recycling peptoid-based catalysts
Peptoid catalysts were recycled by centrifugation after each catalytic hydrolysis cycle. After hydrolysis, the reaction mixture solution was transferred into a 1.5 mL Eppendorf tube and centrifuged for 10 min at 12,000 rpm. Then the supernatant was removed by pipette, and collected peptoid catalysts were resuspended in water for second round of centrifugation and removal of supernatant.
CO2 hydration and sequestration
CO2 hydration and sequestration experiments were assessed by studying the conversion of CO2 to CaCO3. These reactions were studied in a solution containing 1 mL HEPES buffer (50 mM, pH 8) of 400 mM CaCl2 and 0.025 µmol of peptoid catalyst (5.0 µl from 5 mM peptoid catalyst solution). Into that solution, CO2 gas was bubbled for 2 h. After 2 h, CaCO3 was collected by centrifugation and then dried. For the blank control experiment, no peptoid catalyst was used.
Molecular dynamics (MD) simulations
MD simulations were performed using Gromacs 2020.291. Initial structures were built using tleap with the STEPs force field for peptoids as previously described80,92,93. Nhis side chain and hydrogen capped Nhis side chains were created and added to STEPs. 4-NPA substrate was generated using mol2 and gaff2 parameters were generated using amber tools. Initial structures were aligned based on the previously reported hydrophobic core crystal packing model using VMD and duplicated to be an eight-by-four infinite sheet35. All sheets were solvated using TIP3P water. Steepest descent energy minimization was performed before the simulation. Gromacs restraints were applied to the hydrophobic core and simulated for 1 microsecond at 600 K and 1 bar NPT using a Berendsen barostat and thermostat94. All the starting boxes were size 3.6, 7.2, 6.0 nm (x, y, z) except for Pep9, which was 3.6, 7.2, 8.0 nm (x, y, z). These microsecond runs were analyzed using a custom C code to determine the number of potential catalytic binding sites. Final peptoid structures were de-solvated and a single 4-NPA substrate was added. Then, the combined structure was solvated and minimized using the steepest descent energy minimization. These systems were subjected to parallel biased metadynamics (PBMetad) for 1 µs NPT simulation at 323.15 K and 1 bar NPT (Berendsen barostat and thermostat) with the bias on the y and z components of the center of mass of the substrate95,96. Free energy values for the binding sites of the substrate relative to the surface were calculated from the reweighted trajectory using a Python script. Energies were normalized to the minimum value calculated for the substrates’ center of mass located in the bulk water, defined as a 3 Å band in the center of the water box for each simulation. A more detailed description of the generation of the initial structures, the analysis of potential catalytic sites, and the free-energy calculations is provided in the Supplementary Information (SI). We also include all input files, codes, and scripts necessary to reproduce the simulations in an accompanying zip file.
Nuclear magnetic resonance (NMR)
NMR studies were performed using a Bruker 500 MHz spectrometer. CO2 hydration and sequestration NMR experiments were performed on a Bruker Avance Neo spectrometer operating at a field strength of 14.1 T (600 MHz 1H and 150 MHz 13 C) and equipped with a 5 mm TCI H&F/C/N-D cryogenically cooled probe with Z-axis gradient. 13C single-pulse NMR spectra were collected using pulse sequence “zg” with a single scan at 90° pulse angle and a relaxation delay of 5 min for quantitative measurements. 13C T2 and DOSY experiments were measured using pulse sequence “cpmg” with a spin echo delay of 5 ms and “ledbpgp2s” with a diffusion time of 1 s and a gradient duration of 2 ms.
Ultra-violet visible (UV-vis) spectroscopy
UV-vis spectroscopy measurements for the hydrolysis of 4-NPA and quantitative metal-peptoid binding capacity by using Eriochrome Black T (EBT) were carried out in a Tecan Safire 2 microplate reader. All UV–vis spectra for evaluating Zn2+-peptoid binding were recorded using an Agilent Cary 60 UV–vis spectrophotometer, operating with a 1.0 nm bandwidth and a scan speed of 400 nm/min. Measurements were conducted using a quartz cuvette with a 1.0 cm optical path length.
Atomic force microscopy (AFM) spectroscopy
Ex situ AFM imaging was carried out with a Bruker Nanoscope 8 instrument in ScanAsyst mode using silicon tips at ambient conditions. Sample was prepared by adding 1.0 µl of self-assembled gel-like materials into 400 µl of DI water and then deposited onto a freshly cleaved mica substrate. After 5 min of incubation, water was removed from the mica surface by blotting with Whatman filter paper. Surface was further dried by blowing N2 gas.
Scanning electron microscopy (SEM)
SEM imaging was carried out with a Apreo Low Vac 2S microscope. Sample was prepared by adding 1.0 µl of self-assembled gel-like materials into 400 µl of DI water and then deposited onto a silicon wafer. After 5 min of incubation, water was removed from the mica surface by blotting with Whatman filter paper. Surface was further dried by blowing N2 gas.
Fourier transform infrared (FTIR) spectroscopy
FTIR study on peptoid powder was carried out in a Bruker ALPHA II Compact FT-IR Spectrometer including ATR probe.
Transmission electron microscopy (TEM)
TEM imaging was performed on an FEI Tecnai F20 operating at an accelerating voltage of 200 keV for bright field (BF) and annular dark-field (ADF) imaging. To prepare TEM samples, 1.0 µl of self-assembled gel-like materials was diluted in 200 μL of deionized water and drop-cast onto carbon-coated copper grids, and excess liquids were removed with filter paper after 5 min. For negative staining, 5.0 μl of phosphotungstic acid (wt 2%, pH = 7) was dropped onto the TEM grid, and excess liquids were removed with filter paper after 1 min.
Scanning TEM(STEM) imaging
STEM images were collected on an FEI Titan 80-300 High-resolution ETEM operated at an accelerating voltage of 300 kV using a high-angle annular dark field (HAADF) detector in STEM mode. EDS spectra were collected on a Bruker XFlash 6 T | 60 detector and analyzed using the Bruker Esprit software.
X-ray photoelectron spectroscopy (XPS) analysis
XPS analysis was used to analyze the surface composition and chemical state of elements in the Pep8 nanosheets, Pep8-Zn2+ nanosheets and Zn2+ samples. The measurements were performed with a Kratos Axis Ultra DLD spectrometer, which featured a focused Al Kα monochromatic X-ray source (λ = 1486.6 eV). Operating at 150 W, the spectrometer incorporated a high-resolution spherical mirror analyzer. Photoelectrons were collected at the analyzer’s entrance slit, positioned perpendicular to the sample surface. The analysis area measured 700 × 300 μm, and the analyzer chamber pressure was maintained at 5 × 10⁻⁹ Torr. Survey spectra were recorded at a pass energy of 160 eV with a step size of 0.5 eV, while high-resolution spectra were acquired at a pass energy of 40 eV with a step size of 0.1 eV. Data processing and spectral analysis were conducted using the CasaXPS software.
X-ray diffraction (XRD) analysis
Powder XRD data of peptoid assemblies were collected using a multiple-wavelength anomalous diffraction and monochromatic macromolecular crystallography beamline, 8.3.1 at the Advanced Light Source located at Lawrence Berkeley National Laboratory. Data characterization and processing were carried out following protocols from our earlier reports.
XRD data of CaCO3 minerals were collected in a Rigaku SmartLab XE diffractometer. Specimens for powder diffraction were loaded into thin-walled glass capillaries (500 μm diameter, 10 μm wall thickness, Charles Supper Co., MA) and positioned on the θ-2θ axis of a Rigaku SmartLab XE diffractometer equipped with a Cu rotating anode (λ = 1.5418 Å) operated at 45 kV, 200 mA. A parallel incident beam from a parabolic x-ray mirror passed through a 0.4 mm slit onto the specimen, and the diffracted intensities were recorded with a HyPix 3000 detector operating in 1D mode scanning a 2θ range from 10 to 100 °.
Data availability
All data are available within the Article and Supplementary Files, or available from the corresponding authors upon request.
Code availability
Custom code is available as Supplementary File or from the corresponding author upon request.
References
Liu, Z., Deng, Z., Davis, S. J. & Ciais, P. Global carbon emissions in 2023. Nat. Rev. Earth Environ. 5, 253–254 (2024).
Meldrum, F. C. Calcium carbonate in biomineralisation and biomimetic chemistry. Int. Mater. Rev. 48, 187–224 (2003).
Porter, S. M. Seawater chemistry and early carbonate biomineralization. Science 316, 1302–1302 (2007).
Stanley, S. M. Effects of global seawater chemistry on biomineralization: past, present, and future. Chem. Rev. 108, 4483–4498 (2008).
Yu, X. et al. Trends in research and development for CO2 capture and sequestration. ACS Omega 8, 11643–11664 (2023).
Sommerdijk, N. & de With, G. Biomimetic CaCO3 mineralization using designer molecules and interfaces. Chem. Rev. 108, 4499–4550 (2008).
Verma, M., Bhaduri, G. A., Phani Kumar, V. S. & Deshpande, P. A. Biomimetic catalysis of CO2 hydration: a materials perspective. Ind. Eng. Chem. Res. 60, 4777–4793 (2021).
Sillu, D. & Achal, V. Carbon dioxide sequestration with carbonic anhydrase nanobiocatalysts: a review. Environ. Chem. Lett. https://doi.org/10.1007/s10311-024-01755-x (2024).
Bierbaumer, S. et al. Enzymatic conversion of CO2: from natural to artificial utilization. Chem. Rev. 123, 5702–5754 (2023).
Steger, F. et al. Comparison of carbonic anhydrases for CO2 sequestration. Int. J. Mol. Sci. 23 https://doi.org/10.3390/ijms23020957 (2022).
Talekar, S., Jo, B. H., Dordick, J. S. & Kim, J. Carbonic anhydrase for CO2 capture, conversion and utilization. Curr. Opin. Biotechnol. 74, 230–240 (2022).
Al-Garawi, Z. S. et al. The amyloid architecture provides a scaffold for enzyme-like catalysts. Nanoscale 9, 10773–10783 (2017).
Navarro, S. et al. Amyloid fibrils formed by short prion-inspired peptides are metalloenzymes. ACS Nano 17, 16968–16979 (2023).
Zozulia, O., Dolan, M. A. & Korendovych, I. V. Catalytic peptide assemblies. Chem. Soc. Rev. 47, 3621–3639 (2018).
Liu, S., Du, P., Sun, H., Yu, H.-Y. & Wang, Z.-G. Bioinspired supramolecular catalysts from designed self-assembly of DNA or peptides. ACS Catal. 10, 14937–14958 (2020).
Shao, L. et al. Hierarchical materials from high information content macromolecular building blocks: construction, dynamic interventions, and prediction. Chem. Rev. 122, 17397–17478 (2022).
Mandal, D., Nasrolahi Shirazi, A. & Parang, K. Self-assembly of peptides to nanostructures. Org. Biomol. Chem. 12, 3544–3561 (2014).
Brooks, S. C. et al. Single amino acid modifications for controlling the helicity of peptide-based chiral gold nanoparticle superstructures. J. Am. Chem. Soc. 145, 6546–6553 (2023).
Zuckermann, R. N., Kerr, J. M., Kent, S. B. H. & Moos, W. H. Efficient method for the preparation of peptoids oligo(N-substituted glycines) by submonomer solid-phase synthesis. J. Am. Chem. Soc. 114, 10646–10647 (1992).
Sun, J. & Zuckermann, R. N. Peptoid polymers: a highly designable bioinspired material. ACS Nano 7, 4715–4732 (2013).
Li, Z., Cai, B., Yang, W. & Chen, C.-L. Hierarchical nanomaterials assembled from peptoids and other sequence-defined synthetic polymers. Chem. Rev. 121, 14031–14087 (2021).
Yang, W., Yin, Q. & Chen, C.-L. Designing sequence-defined peptoids for biomimetic control over inorganic crystallization. Chem. Mater. 33, 3047–3065 (2021).
Cai, B., Li, Z. & Chen, C.-L. Programming amphiphilic peptoid oligomers for hierarchical assembly and inorganic crystallization. Acc. Chem. Res. 54, 81–91 (2021).
Jiao, F. et al. Self-repair and patterning of 2D membrane-like peptoid materials. Adv. Funct. Mater. 26, 8960-8967 (2016).
Jin, H. et al. Highly stable and self-repairing membrane-mimetic 2D nanomaterials assembled from lipid-like peptoids. Nat. Commun. 7, 12252 (2016).
Luo, Y. et al. Bioinspired peptoid nanotubes for targeted tumor cell imaging and chemo-photodynamic therapy. Small 15, 1902485 (2019).
Jin, H. et al. Designable and dynamic single-walled stiff nanotubes assembled from sequence-defined peptoids. Nat. Commun. 9, 270 (2018).
Jian, T. et al. Highly stable and tunable peptoid/hemin enzymatic mimetics with natural peroxidase-like activities. Nat. Commun. 13, 3025 (2022).
Trinh, T. K. H. et al. Designed metal-containing peptoid membranes as enzyme mimetics for catalytic organophosphate degradation. ACS Appl. Mater. Interfaces 15, 51191–51203 (2023).
Liang, S., Wu, X.-L., Zong, M.-H. & Lou, W.-Y. Zn-triazole coordination polymers: Bioinspired carbonic anhydrase mimics for hydration and sequestration of CO2. Chem. Eng. J. 398, 125530 (2020).
Ma, J. et al. Controlling mineralization with protein-functionalized peptoid nanotubes. Adv. Mater. 35, 2207543 (2023).
Ma, J. et al. Nanoparticle-mediated assembly of peptoid nanosheets functionalized with solid-binding proteins: designing heterostructures for hierarchy. Nano Lett. 21, 1636–1642 (2021).
Arifuzzaman, M. D. & Zhao, Y. Artificial zinc enzymes with fine-tuned active sites for highly selective hydrolysis of activated esters. ACS Catal. 8, 8154–8161 (2018).
Koziol, L. et al. Toward a small molecule, biomimetic carbonic anhydrase model: theoretical and experimental investigations of a panel of zinc(II) Aza-macrocyclic catalysts. Inorg. Chem. 51, 6803–6812 (2012).
Hammons, J. A. et al. Early-stage aggregation and crystalline interactions of peptoid nanomembranes. J. Phys. Chem. Lett. 12, 6126–6133 (2021).
Chen, C. L. & Beatty, A. M. Guest inclusion and structural dynamics in 2-D hydrogen-bonded metal-organic frameworks. J. Am. Chem. Soc. 130, 17222–17223 (2008).
Chen, C. L., Goforth, A. M., Smith, M. D., Su, C. Y. & zur Loye, H. C. [Co-2(ppca)(2)(H2O)(V4O12)(0.5)]: A framework material exhibiting reversible shrinkage and expansion through a single-crystal-to-single-crystal transformation involving a change in the cobalt coordination environment. Angew. Chem., Int. Ed. 44, 6673–6677 (2005).
Chen, C. L. et al. Multidimensional frameworks assembled from silver(I) coordination polymers containing flexible bis(thioquinolyl) ligands: role of the intra- and intermolecular aromatic stacking interactions. Inorg. Chem. 42, 3738–3750 (2003).
Chen, C. L. et al. A non-interpenetrating 2D coordination polymer from a (CH2)(8) spacer-based highly flexible linear ligand and AgCF3CO2. New. J. Chem. 27, 790–792 (2003).
Song, Y. et al. Highly bright and photostable two-dimensional nanomaterials assembled from sequence-defined peptoids. ACS Mater. Lett. 3, 420–427 (2021).
Greer, D. R. et al. Universal relationship between molecular structure and crystal structure in peptoid polymers and prevalence of the cis backbone conformation. J. Am. Chem. Soc. 140, 827–833 (2018).
Song, Y. et al. Assembly of highly efficient aqueous light-harvesting system from sequence-defined peptoids for cytosolic microRNA detection. Nano Res 17, 788–796 (2024).
Behar, A. E. & Maayan, G. A cocktail of Cu2+- and Zn2+-peptoid-based chelators can stop ROS formation for Alzheimer’s disease therapy. Chem. Sci. 15, 18855–18864 (2024).
Ghosh, P. & Maayan, G. A rationally designed peptoid for the selective chelation of Zn2+ over Cu2+. Chem. Sci. 11, 10127–10134 (2020).
Ricano, A. et al. Combinatorial design of multimeric chelating peptoids for selective metal coordination. Chem. Sci. 10, 6834–6843 (2019).
Knight, A. S., Zhou, E. Y., Pelton, J. G. & Francis, M. B. Selective chromium(VI) ligands identified using combinatorial peptoid libraries. J. Am. Chem. Soc. 135, 17488–17493 (2013).
Köse, D. A. & Necefoğlu, H. Synthesis and characterization of bis(nicotinamide) m-hydroxybenzoate complexes of Co(II), Ni(II), Cu(II) and Zn(II). J. Therm. Anal. Calorim. 93, 509–514 (2008).
Yilmaz, M. D. & Oktem, H. A. Eriochrome Black T–Eu3+ complex as a ratiometric colorimetric and fluorescent probe for the detection of dipicolinic acid, a biomarker of bacterial spores. Anal. Chem. 90, 4221–4225 (2018).
Wright, A. M. et al. A structural mimic of carbonic anhydrase in a metal-organic framework. Chem 4, 2894–2901 (2018).
Kim, J. K. et al. Elucidating the role of metal ions in carbonic anhydrase catalysis. Nat. Commun. 11, 4557 (2020).
Xu, Y., Feng, L., Jeffrey, P. D., Shi, Y. & Morel, F. M. M. Structure and metal exchange in the cadmium carbonic anhydrase of marine diatoms. Nature 452, 56–61 (2008).
Kim, M.-C. & Lee, S.-Y. Comparative study on the catalytic hydration of carbon dioxide by catalysts that mimic carbonic anhydrase prepared with zinc salts. ChemCatChem 7, 698–704 (2015).
Keum, C., Kim, M.-C. & Lee, S.-Y. Effects of transition metal ions on the catalytic activity of carbonic anhydrase mimics. J. Mol. Catal. A Chem. 408, 69–74 (2015).
Cresswell, A. J., Davies, S. G., Roberts, P. M. & Thomson, J. E. Beyond the Balz–Schiemann reaction: the utility of tetrafluoroborates and boron trifluoride as nucleophilic fluoride sources. Chem. Rev. 115, 566–611 (2015).
Zhang, X., Wang, Y., Dai, Y. & Xia, F. Tuning the enzyme-like activity of peptide–nanoparticle conjugates with amino acid sequences. Nanoscale 15, 8148–8152 (2023).
Li, Z., Joshi, S. Y., Wang, Y., Deshmukh, S. A. & Matson, J. B. Supramolecular peptide nanostructures regulate catalytic efficiency and selectivity. Angew. Chem., Int. Ed. 62, e202303755 (2023).
Liu, Q. et al. Cofactor-free oxidase-mimetic nanomaterials from self-assembled histidine-rich peptides. Nat. Mater. 20, 395–402 (2021).
Christianson, D. W. & Fierke, C. A. Carbonic anhydrase: evolution of the zinc binding site by nature and by design. Acc. Chem. Res. 29, 331–339 (1996).
Sun, S., Zhang, Z., Xiang, Y., Cao, M. & Yu, D. Amino acid-mediated synthesis of the ZIF-8 nanozyme that reproduces both the zinc-coordinated active center and hydrophobic pocket of natural carbonic anhydrase. Langmuir 38, 1621–1630 (2022).
Supuran, C. laudiuT. Structure and function of carbonic anhydrases. Biochem. J. 473, 2023–2032 (2016).
Fisher, Z. et al. Structural and Kinetic Characterization of Active-Site Histidine as a Proton Shuttle in Catalysis by Human Carbonic Anhydrase II. Biochemistry 44, 1097–1105 (2005).
Ge, Q.-C. et al. Role of trinuclear Zn (II) complexes in promoting the hydrolysis of 4-nitrophenyl acetate. Can. J. Chem. 82, 409–417 (2004).
Cai, Y.-P. et al. Self-assembly of silver(I) polymers with single strand double-helical structures containing the ligand O,O′-bis(8-quinolyl)-1,8-dioxaoctane. J. Chem. Soc., Dalton Trans. 2429-2434 (2001).
Raynal, M., Ballester, P., Vidal-Ferran, A. & van Leeuwen, P. W. N. M. Supramolecular catalysis. Part 2: artificial enzyme mimics. Chem. Soc. Rev. 43, 1734–1787 (2014).
Zhang, Z. et al. Zinc-based deep eutectic solvent–an efficient carbonic anhydrase mimic for CO2 hydration and conversion. Sep. Purif. Technol. 276, 119446 (2021).
Johnson, K. A. & Goody, R. S. The original michaelis constant: translation of the 1913 Michaelis–Menten Paper. Biochemistry 50, 8264–8269 (2011).
Zastrow, M. L., Peacock, A. F. A., Stuckey, J. A. & Pecoraro, V. L. Hydrolytic catalysis and structural stabilization in a designed metalloprotein. Nat. Chem. 4, 118–123 (2012).
Zhang, Q. et al. Artificial hydrolase based on carbon nanotubes conjugated with peptides. Nanoscale 8, 16851–16856 (2016).
Zhang, C. et al. Self-assembled peptide nanofibers designed as biological enzymes for catalyzing ester hydrolysis. ACS Nano 8, 11715–11723 (2014).
Ma, N. et al. A remote optically controlled hydrolase model based on supramolecular assembly and disassembly of its enzyme-like active site. Nanoscale 11, 3521–3526 (2019).
Burton, A. J., Thomson, A. R., Dawson, W. M., Brady, R. L. & Woolfson, D. N. Installing hydrolytic activity into a completely de novo protein framework. Nat. Chem. 8, 837–844 (2016).
Mikolajczak, D. J. & Koksch, B. Peptide–gold nanoparticle conjugates as artificial carbonic anhydrase mimics. Catalysts 9 (2019).
Xuan, S. T. et al. Atomic-level engineering and imaging of polypeptoid crystal lattices. PNAS 116, 22491–22499 (2019).
Zhao, M. et al. Hierarchical self-assembly pathways of peptoid helices and sheets. Biomacromolecules 23, 992–1008 (2022).
Swanson, H. W. A., Lau, K. H. A. & Tuttle, T. Minimal peptoid dynamics inform self-assembly propensity. J. Phys. Chem. B 127, 10601–10614 (2023).
Swanson, H. W. A., van Teijlingen, A., Lau, K. H. A. & Tuttle, T. Martinoid: the peptoid martini force field. Phys. Chem. Chem. Phys. 26, 4939–4953 (2024).
Mannige, R. V. et al. Peptoid nanosheets exhibit a new secondary-structure motif. Nature 526, 415–420 (2015).
Ma, X. et al. Tuning crystallization pathways through sequence engineering of biomimetic polymers. Nat. Mater. 16, 767–775 (2017).
Behnke, C. A. et al. Atomic resolution studies of carbonic anhydrase II. Acta Crystallogr. D. Biol. Crystallogr 66, 616–627 (2010).
Harris, B. S., Bejagam, K. K. & Baer, M. D. Development of a systematic and extensible force field for peptoids (STEPs). J. Phys. Chem. B 127, 6573–6584 (2023).
Seidler, M. et al. Atomic-scale imaging of condensed counterions. Macromolecules 57, 10016–10022 (2024).
Jiang, X. et al. Atomic-scale corrugations in crystalline polypeptoid nanosheets revealed by three-dimensional cryogenic electron microscopy. ACS Macro Lett. 12, 632–638 (2023).
Huang, Y. et al. A ZInc Coordination Complex Mimicking Carbonic Anhydrase for CO2 hydrolysis and sequestration. Inorg. Chem. 58, 9916–9921 (2019).
Mann, B. E. Dynamic 13C NMR spectroscopy. Prog. Nucl. Magn. Reson. Spectrosc. 11, 95–114 (1977).
Ilkaeva, M. et al. Assessing CO2 capture in porous sorbents via solid-state NMR-assisted adsorption techniques. J. Am. Chem. Soc. 145, 8764–8769 (2023).
Yeagle, P. L., Lochmüller, C. H. & Henkens, R. W. 13-C nuclear magnetic resonance studies on the mechanism of action of carbonic anhydrase. PNAS 72, 454–458 (1975).
Steiner, H., Jonsson, B.-H. & Lindskog, S. The catalytic mechanism of carbonic anhydrase. Eur. J. Biochem. 59, 253–259 (1975).
Jin, B. et al. Formation, chemical evolution and solidification of the dense liquid phase of calcium (bi)carbonate. Nat. Mater. 24, 125–132 (2025).
López-Martínez, L. M., Santacruz-Ortega, H., Navarro, R. E., Caro-León, F. J. & Ochoa-Terán, A. Studies by 1H NMR and UV-Vis spectroscopy of the molecular recognition of histamine by copper and zinc complexes of polyazamacrocyclic ligands. J. Mol. Struct. 1204, 127545 (2020).
Vargová, Z. et al. Ternary complexes of zinc(II), cyclen and pyridinecarboxylic acids. Eur. J. Inorg. Chem. 2007, 3974–3987 (2007).
Abraham, M. J. et al. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1-2, 19–25 (2015).
Yadav Schmid, S. et al. Influence of peptoid sequence on the mechanisms and kinetics of 2D assembly. ACS Nano 18, 3497–3508 (2024).
Zhang, S. et al. Hierarchical assembly of peptoids on MoS2. Mater. Today Phys. 101406 https://doi.org/10.1016/j.mtphys.2024.101406 (2024).
Berendsen, H. J. C., Postma, J. P. M., van Gunsteren, W. F., DiNola, A. & Haak, J. R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 81, 3684–3690 (1984).
Pfaendtner, J. & Bonomi, M. Efficient sampling of high-dimensional free-energy landscapes with parallel bias metadynamics. J. Chem. Theory Comput. 11, 5062–5067 (2015).
Bonomi, M. et al. PLUMED: a portable plugin for free-energy calculations with molecular dynamics. Comput. Phys. Commun. 180, 1961–1972 (2009).
Acknowledgements
This work was supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences, Materials Sciences and Engineering Division, Biomolecular Materials program, FWP80124. BSH and MDB performed and analyzed molecular dynamics simulations supported under FWP77876. This research used resources of the National Energy Research Scientific Computing Center (NERSC), a U.S. Department of Energy Office of Science User Facility located at Lawrence Berkeley National Laboratory (LBNL), operated under Contract No. DE-AC02-05CH11231 using NERSC award BES-ERCAP0023738. XRD work was conducted at the Advanced Light Source (ALS) of LBNL, which was supported by the Office of Science (No. DE-AC02-05CH11231). The authors would like to thank Dr. Emily G. Succuzzo for her assistance in CO2 hydration experiments. PNNL is multi-program national laboratory operated for DOE by Battelle under Contracts No. DE-AC05−76RL01830.
Author information
Authors and Affiliations
Contributions
C.-L.C. conceived and directed the project. P.C. conducted all the peptoid synthesis, assembly/coassembly, and catalytic efficiency study. P.C. conducted all the characterizations unless mentioned otherwise. B.S.H., M.D.B., Y.W.E., and C.J.M. conducted the molecular dynamics simulations. Y.C. conducted the NMR studies. C.-L.C. did XRD experiments of peptoid assemblies. P.C. and C.-L.C. analyzed XRD data. R.Z. and A.B.B. conducted the TEM characterizations. M.E.B. collected the XRD data of CaCO3. T.K.H.T. conducted the UV-vis titrations of peptoid-metal bindings. V.S. conducted the XPS experiments. X.Z. conducted the high-resolution mass spectrometry analysis. P.C., Y.C., B.S.H., M.D.B., and C.-L.C. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chakma, P., Chen, Y., Harris, B.S. et al. Assembled peptoid crystalline nanomaterials as carbonic anhydrase mimics for promoted hydration and sequestration of CO2. Nat Commun 16, 7348 (2025). https://doi.org/10.1038/s41467-025-62366-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-62366-w
This article is cited by
-
Self-assembled cellulosic superstructures with unanticipated high quantum yields
Nature Communications (2025)