Abstract
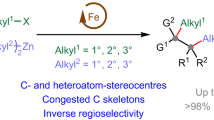
Metal complex catalysts typically comprise three components: ligands, central metals, and counterions. The counterion profoundly influences the activity and selectivity of numerous catalytic reactions, while such a phenomenon is rarely observed in carbozincation reactions. Herein, we discover that the catalytic efficiency of iron-catalyzed alkylzincation of alkynes is markedly enhanced when the weakly coordinating bulky counterion tetrakis(3,5-bis(trifluoromethyl)phenyl)borate ([BArF4]−) is incorporated into the reaction. This approach yields ionic iron catalysts with exceptional activity (up to 32,900 TON), regioselectivity (mostly >95:5), and stereoselectivity (mostly >95:5) in both alkylzincation reactions of terminal and internal alkynes. Notably, the methylzincation of non-activated terminal and internal alkynes is rendered feasible. This methodology also enables the efficient synthesis of a diverse array of biologically active molecules and their key intermediates. Mechanistic investigations suggest that a cationic Fe(II) species serves as the active catalytic species and the weakly coordinating bulky counterion stabilizes a cationic iron(II) alkyl complex without competing with the alkyne substrate.
Similar content being viewed by others
Introduction
Metal complex catalysts are widely employed in synthetic chemistry, typically comprising three components: ligands, central metals, and counterions. While research on these catalysts has primarily focused on the ligands and central metals, counterions have received comparatively little attention. Increasingly, studies have demonstrated that counterions significantly influence the activity and selectivity of numerous reactions, including rhodium-catalyzed asymmetric hydrogenation1, gold-catalyzed transformations of alkynes2,3, and coordination polymerization of ethene4. Introducing weakly coordinating anions with large steric hindrance and highly delocalized negative charge has been shown to markedly enhance the activity and selectivity of catalysts in these reactions5,6,7. The rational utilization of anions to enhance catalytic reaction activity or expand the types of catalytic reactions is of great significance.
Carbozincation reactions of alkynes have emerged as an effective method for synthesizing stereodefined polysubstituted alkenes8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30. However, significant limitations persist regarding both alkynes and organozinc reagents. For instance, the methylzincation of terminal alkynes has only been reported in one instance, achieving moderate yields for phenylacetylene (Fig. 1a), while the methylzincation of alkyl terminal alkynes and non-activated internal alkynes remains unsuccessful (Fig. 1b)14,21. Besides, the alkylzincation of non-activated internal alkynes has also been reported in just one case, facing issues such as high nickel catalyst loading (only up to 3.3 TON was obtained) and applicability limited to aryl-substituted internal alkynes and long-chain alkyl zinc reagents20,21. Additionally, the low catalytic efficiency of most previously reported carbozincation reactions hampers their practical application.
Our group has long been engaged in the study of iron group metal-catalyzed organic reactions13,14,31,32,33,34,35,36,37,38. In this work, we have identified that the weakly coordinating anion [BArF4]− significantly enhances the efficiency of the pyridyldiimine (PDI)-FeCl2-catalyzed alkylzincation of alkynes, thereby overcoming the limitations of previously known catalysts in this type of reaction. Specifically, we successfully achieved methylzincation of alkyl terminal alkynes and iron-catalyzed alkylzincation of internal alkynes using [BArF4]−-modified iron catalysts. This advancement provides an efficient method for the synthesis of polysubstituted alkenes and has been applied to the synthesis of various bioactive molecules and their key intermediates (Fig. 1c).
Results and discussion
Methylation reactions are extensively utilized in the synthesis of bioactive molecules39,40,41, however, the methylzincation of alkynes remains a significant challenge. In our previous work, we demonstrated that the methylzincation of Me2Zn and aryl terminal alkynes could be catalyzed by an Fe complex with a tridentate nitrogen ligand, although the reaction yield was limited to 53%14, and it failed to produce the target product for alkyl terminal alkynes (Table 1, entry 1). During optimization of the reaction conditions, we discovered that the addition of the weakly coordinating anion salt NaBArF4 effectively promoted the reaction of terminal alkynes with Me2Zn in weakly coordinating aromatic solvents using the PDI-FeCl2 complex C1 as a pre-catalyst (entries 2-4). With the appropriate pre-catalyst (C3), the reaction achieved a 75% yield for alkyl terminal alkyne, yielding a cis-, anti-Markovnikov methylation product of terminal alkyne (entry 4). The coordinating ability of the solvent significantly impacts the reaction: the coordinating solvent THF was proved incompatible (Table S6), and the yield increased to 92% using PhCF3, a solvent exhibiting weaker coordinating capacity yet enhanced solubility for ionic catalysts (entry 5). Addition of halide ions and most weakly coordinating anions failed to afford the target product (Table S2). Only the iron complex containing triflate (OTf−) exhibited marginal activity (13% yield). The weakly coordinating bulky anion [BArF4]− demonstrates a unique promotional effect in this catalytic reaction. Moreover, ligand and metal evaluation further demonstrated that iron combined with tridentate PDI-type ligands achieved optimal outcomes exclusively (Tables S3 and S5). Further studies revealed that most of the catalysts previously reported in the literature for the carbozincation of alkynes and alkenes were incapable of promoting this reaction (entries 6–14).
Next, we evaluated other types of alkynes and alkyl zinc reagents using the catalytic combination of PDI-FeCl2 and NaBArF4, and found that the weakly coordinating anion NaBArF4 played a crucial role in all reactions (Fig. 2). Similar to the case with alkyl terminal alkynes, aryl terminal alkynes and aryl methyl internal alkynes yielded trace amounts of methylzincation products in the absence of NaBArF4 (2aa-4bg). For the reaction of Et2Zn with internal alkynes, the addition of [BArF4]− significantly increased the yield. Comparable effects were observed for aryl primary alkyl internal alkynes, diaryl internal alkynes, and aryl secondary alkyl internal alkynes with large steric hindrance, resulting in yield enhancements ranging from 4.5- to 19-fold.
Reaction conditions: alkynes (0.5 mmol, 1.0 equiv), Me2Zn or Et2Zn (1 M in toluene, 0.55 mmol, 550 μL, 1.1 equiv), Cat. (0.01 mmol, 2 mol%), NaBArF4 (0.025 mmol, 0.05 mol%). The reactions were quenched with D2O (150 μL), rt (~30 °C), 30 min. aUsed C3 as pre-catalyst, PhCF3 (2 mL), −20 °C, 1 h, rt (~30 °C), 2 h. bUsed C2 as pre-catalyst, toluene (2 mL), rt (~30 °C), 3 h. cUsed C1 as pre-catalyst, PhCF3 (2 mL), rt (~30 °C), 3 h. Yields were determined by 1H NMR use 1,3,5-(OMe)3C6H3 as internal standard.
Subsequently, the methylzincation reaction of terminal alkynes was further investigated (Fig. 3). Methylzincation of various alkyl terminal alkynes was achieved using C3 as the pre-catalyst, PhCF3 as the solvent, and NaBArF4 as the additive. This approach provided moderate to high yields, ≥90:10 regio- and >95:5 stereoselectivities for long-chain alkyl terminal alkynes (2ae), benzyl acetylene (2af), and primary alkyl terminal alkynes with substituents such as alkoxyls (2aa-2ad), acetals (2al), halogens (2ag), aryl groups (2ah, 2ai), and heteroaryl groups (2aj, 2ak) on the side chains. In addition to primary alkyl terminal alkynes, this method was also applicable to the methylzincation of secondary alkyl terminal alkynes (2am, 2an) and tertiary alkyl (2ao) terminal alkynes (upper panel of Fig. 3).
Reaction conditions: alkynes (0.5 mmol, 1.0 equiv), Me2Zn (1 M in toluene, 0.55 mmol, 550 μL, 1.1 equiv), [Fe] cat. (0.01 mmol, 2 mol%), NaBArF4 (0.025 mmol, 5 mol%), solvent (2 mL). The reactions quenched with D2O (150 μL) or I2 (3.0 equiv), isolated yields were given. a Used C3 as pre-catalyst, PhCF3 as solvent, −20 °C, 1 h, rt (~30 °C), 2 h. b Used C3 as pre-catalyst, toluene as solvent, -78 °C to 0 °C, 3 h. c Quenched with PhC(O)Cl (3.0 equiv), CuCN (20 mol%), THF/PhCF3 = 1:1. d Used C2 as pre-catalyst, toluene as solvent, rt (~30 °C), 3 h. e Used C1 as pre-catalyst and PhCF3 as solvent rt (~30 °C), 3 h.
Methylzincation of aryl and alkenyl terminal alkynes was achieved using the PDI-FeCl2 complex C2 as the pre-catalyst and toluene as the solvent (bottom panel of Fig. 3). The substituent electronic properties and steric hindrance on the aromatic ring of the aryl alkynes had unobvious impact on the reaction outcomes. This method also demonstrated good functional group tolerance: -Ph (o-Ph, m-Ph, p-Ph, 2ba-2bc), halogens (2bd-2bf), -CF3 (2bg), -SMe (2bh), -OTBS (2bi), -CO2Me (2bj), -C(O)NEt2 (2bk), and furyl (2bl) were all well tolerated, and all tested aryl terminal alkynes converted to the target products in high yields while maintaining ≥95:5 regio- and stereoselectivity. Besides, the 1,4-diethynylbenzene underwent dual methylzincation reactions, yielding the dizinc reagent (2bm). Additionally, 1,3-alkynyl substrates underwent methylzincation to afford conjugated dienyl zinc products, albeit with reduced stereoselectivity (2bn, 2bo). These findings suggest that the Fe catalyst containing the weakly coordinating anion [BArF4]⁻ can effectively prevent side reactions such as C-H bond zincation of the terminal alkyne, which is crucial for the success of these reactions.
Moreover, we found that the combination of PDI-FeCl2 and NaBArF4 efficiently catalyzed the alkylzincation of non-activated internal alkynes (Fig. 4). Less sterically hindered C1 exhibited better results for this larger steric hindrance of internal alkynes (Table S5). Using aryl methyl internal alkynes as model substrates, we evaluated the reaction’s tolerance to various functional groups (4aa-4ao). The reaction tolerated -F (4ab), -Cl (o-Cl, -m-Cl, -p-Cl, 4ac-4ae), -Br (4af), -CF3 (4ag), -CO2tBu (4ah), -SO2Me (4ai), -OMe (4aj), and -SMe (4ak), though the more coordinating -NMe2 substituent reduced stereoselectivity (4al). Furthermore, the reaction yielded good results (>85%) in terms of regioselectivity and stereoselectivity for internal alkynes containing other aromatic rings such as indole (4am), benzofuran (4an), ferrocene (4ao), thiophene (4ba), and furan (4bb).
Reaction conditions: alkynes (0.5 mmol, 1.0 equiv), alkylzinc reagents (0.55 mmol, 1.1 equiv), C1 (0.01 mmol, 2 mol%), NaBArF4 (0.025 mmol, 5 mol%), PhCF3 (2 mL), rt (~30 °C), 3 h, isolated yields were given. The reactions were quenched with D2O (150 μL) or I2 (3.0 equiv). a Used C3 (2 mol%) as pre-catalyst, rt (~30 °C), 12 h. b Used C3 as pre-catalyst, C3 (4 mol%), NaBArF4 (10 mol%), rt (~30 °C), 48 h.
Next, we evaluated the effect of various alkyl substituents on internal alkynes. For simple aryl alkyl internal alkynes, extending the alkyl groups did not affect yield or selectivity (4bc-4be). Increased steric hindrance from groups such as benzyl (4bf) and cyclopropyl (4bg) maintained regioselectivity and stereoselectivity. Internal alkynes with oxygen-containing alkyl groups yielded target products in >80% yield, high regioselectivity, and stereoselectivity (4bh-4bk). Notably, the regioselectivity of our addition was opposite to that reported for Ni-catalyzed alkylzincation21 of internal alkynes 4bj and 4bk, highlighting the complementary nature of our method.
We then explored the ethylzincation of dialkyl internal alkynes. For symmetric 4-octyne, the cis-addition product 4ca was obtained smoothly. Unsymmetrical dialkyl internal alkynes also underwent carbozincation, albeit only with 52:48 regioselectivity (4cb). Symmetric diaryl internal alkynes afforded trisubstituted alkenes in high yields and stereoselectivity (4da-4db).
Beyond Et2Zn, other alkyl zinc reagents, including Me2Zn (4ea and 4eb), n-butyl zinc reagent (4ec), long-chain alkyl zinc reagents prepared in situ, and even remotely functionalized alkyl zinc reagents (4ed-4eg), reacted smoothly, yielding the corresponding alkylzincation products with moderate to high yields and good regioselectivity and stereoselectivity.
To demonstrate the potential application of the current method in synthesis, TON experiments were conducted (Fig. 5a). For the ethylzincation of phenyl methyl acetylene 3aa, a gram-scale reaction was performed with the 1/35,000 iron pre-catalyst and yielding the trisubstituted alkene product 4aa in 94% yield with well-maintained regioselectivity and stereoselectivity. This reaction achieved 32,900 TON, indicating that the active catalyst in this system possesses high stability and significant potential for large-scale applications.
a High TON and gram scale experiment. b Synthesis of tetrasubstituted alkenes by carbozincation of internal alkyne. Reaction conditions: (i) D2O (150 μL), rt (~30 °C), 30 min; (ii) NCS (N-chlorosuccinimide, 3.0 equiv), THF, rt (~30 °C), 12 h; (iii) CuCN (20 mol%), allyl bromide (5.0 equiv), THF, rt (~30 °C), 4 h; (iv) methyl propiolate (3.0 equiv), CuCN (3.0 equiv), LiCl (3.0 equiv), THF, −70 °C, 12 h; (v) Pd(PPh3)4 (2 mol%), 4-iodonitrobenzene (0.5 equiv), THF, rt (~30 °C), 12 h; (vi) benzoyl chloride (3.0 equiv), CuCN (20 mol%), LiCl (3.0 equiv), THF, 0 °C, 12 h; (vii) 4-tolyl isocyanate (0.5 equiv), THF, rt (~30 °C), 12 h.
The trisubstituted vinyl zinc reagents obtained from the alkylzincation of internal alkynes exhibit high reactivity and can be further utilized to synthesize a series of tetrasubstituted alkenes (Fig. 5b). Using 2-(prop-1-yn-1-yl)naphthalene 5 as the starting material, we synthesized a series of stereodefined tetrasubstituted alkenes through various one-pot transformations of the in situ generated trisubstituted vinyl zinc reagent 6 under standard conditions. For example, the alkenyl zinc product 6 could be directly reacted with D2O to produce deuterated trisubstituted alkene 6a in 91% yield, or with NCS to yield polysubstituted vinyl chloride 6b. 1,4-Diene 6c and conjugated dienyl ester 6d were obtained via CuCN-catalyzed allylation and Michael addition, respectively. Negishi coupling of 6 with p-nitroiodobenzene catalyzed by Pd(PPh3)4 produced tetrasubstituted alkene 6e in 87% yield and with well reserved configuration. Additionally, the alkenyl zinc reagent reacted with benzoyl chloride catalyzed by copper salts to form polysubstituted α,β-unsaturated ketone 6f in 96% yield, whose structure was confirmed by single-crystal X-ray diffraction. Finally, the alkenyl zinc reagent also reacted with isocyanate to generate the corresponding amide product 6 g in 77% yield.
Some aryl methyl-substituted Z-alkenes containing alkoxy groups are important bioactive molecules and intermediates for organic synthesis with numerous industrial applications42. The methylzincation method developed in this study allows for the convenient synthesis of these products from the corresponding aryl terminal alkynes. Furthermore, since the intermediate zinc reagent can undergo numerous transformations, this method can also be employed for the derivatization of the aforementioned bioactive molecules. For instance, the alkenylzinc reagents synthesized by our method can be quenched with D2O to yield a series of deuterated analogues (Fig. 6a), including d/1-trans-anethole (8a), d/1-trans-methyl isoeugenol (8b), d/1-polysphrin precursor (8c), and d/1-trans-isosafrole (8d), in high yields, and >95:5 stereo- and regioselectivities.
a Synthesis of industrially relevant E-Olefins. b Formal synthesis of naucleamides E. Reaction conditions: (i) alkyne 9 (1.0 mmol, 1.0 equiv), C3 (2 mol%), NaBArF4 (5 mol%), Me2Zn (1.1 equiv), toluene, -78 °C to 0 °C, 3 h; (ii) CuCN (3.0 equiv), LiCl (3.0 equiv), methyl propiolate (3.0 equiv), -70 °C, 12 h; (iii) Compound 10 (1.0 equiv), DIBAL-H (3.0 equiv), DCM, −78 °C to 0 °C, 3 h, then MnO2 (5.0 equiv), DCM, 25 °C, 12 h; c Fromal synthesis of (+)-cornexistin and mavacuran alkaloids. (iv) alkyne 12 (0.5 mmol, 1.0 equiv), C3 (2 mol%), NaBArF4 (5 mol%), Me2Zn (1.1 equiv), toluene, -78 °C to -40 °C, 2 h; (v) I2 (3.0 equiv), toluene/THF, rt (~30 °C), 3 h.
The methylzincation reaction of alkyl terminal alkynes can be utilized to synthesize important intermediates of complex natural products. For example, a key intermediate (11) in the total synthesis of the natural product naucleamides E was readily prepared starting with silyl-protected propargyl alcohol 9 through iron-catalyzed methylzincation coupled with a copper-mediated conjugate addition with methyl propiolate in a one-pot (10), following with two consecutive redox reactions (Fig. 6b). This method offers advantages in yield and steps compared to the literature method43 and avoids the use of precious palladium catalysts. Besides, we used commercially available silyl-protected propargyl alcohol 12 for the methylzincation reaction, and the resulting zinc reagent was quenched by iodine in one-pot to give alkenyl iodide compounds 13, which is a key intermediate in the total synthesis of (+)-cornexistin44 and mavacuran alkaloids45 (Fig. 6c). Compared to the reported method, our protocol has advantages in steps with an improved overall yield.
To understand the reaction mechanism, we attempted to prepare the active catalysts (Fig. 7a). When the catalyst precursor C1 was reacted with NaBArF4 in PhF and recrystallized in ether, it was found that Cl⁻ on Fe was not exchanged by [BArF4]−. Single-crystal X-ray diffraction indicated that only a complex of the catalyst and NaBArF4 was obtained. When this complex was further mixed with an excess of Et2Zn, the crystallinity of the catalyst deteriorates significantly and the color turns purple-black to dark yellow. Although we did not directly obtain single crystals of the pure cationic complex C1b, we successfully obtained corresponding single crystals when coordinated with THF C1c, and the structure indicated that the iron was divalent. Notably, although our reaction could not proceed in the coordinating solvent THF, the complex with THF could still catalyze the reaction smoothly (Fig. 7b).
The cationic PDI-Fe(II) complex obtained above is structurally similar to Fe(II) complexes reported by Chirik et al., formed via the reaction of dialkyl Fe(II) complexes with B(C6F5)3 or by one-electron oxidation of alkyl Fe(I) complexes, which were successfully applied in ethylene polymerization reactions without the need for an external co-catalyst46,47. Accordingly, we hypothesized that ionic Fe(II)-alkyl species was the real active catalytic species. To validate this postulate, we synthesized the PDI-Fe(I)-Me complex C4a from pre-catalyst C4 following Chirik’s method48, which was subsequently mixed with single-electron oxidation FcBArF4 to afford the [BArF4]−-modified PDI-Fe(II)-Me complex C4b (Fig. 7c)47. Remarkably, C4b demonstrated efficient catalytic activity in the reaction between internal alkyne 3ea and Me2Zn (Fig. 7d). Furthermore, stoichiometric insertion of C4b and alkyne 3ea proceeded smoothly, delivering the methylated product in 76% yield (Fig. 7e). Therefore, we propose that the alkylzincation reactions of alkynes described in this paper also follows a catalytic cycle with cationic Fe(II)-Me species as the active catalysts. Wherein, [BArF4]⁻ acts as a weakly coordinating anion to stabilize the highly reactive cationic iron catalyst and promote its coordination to the alkynes.
We thus propose a possible catalytic cycle: with the assistance of the zinc reagent and NaBArF4, the catalyst precursor transforms into the cationic PDI-Fe(II)-Cl species int1, which may react with NaBArF4 and Me2Zn to generate the active cationic PDI-Fe(II)-Me species int2. Coordination of int2 with the alkyne yields intermediate int3, which undergoes migratory insertion transition state TS1 to form the Fe-alkenyl intermediate int4. The int4 undergoes transmetalation with Me2Zn through σ-bond metathesis to afford the alkenyl zinc product and be coordinated with alkyne to regenerate the int3 (Fig. 8a).
DFT calculations were then performed to verify the rationality of the mechanism in both high-spin (S = 2, 1) and low-spin (S = 0) states at the (dispersion-corrected) unrestricted UωB97XD/6-31G*/TZVP level of theory with SMD-UωB97XD/def2-TZVPP single-point energy calculations (see Table S10 for details). The calculations indicate that the migratory insertion (int3 to TS1) is irreversible and can proceed smoothly at room temperature. Notably, the activation energy of this step can be lowered by a spin crossover from the quintet to the triplet (Fig. 8b)49.
In conclusion, using iron complex catalysts containing large steric hindrance and weakly coordinating anion [BArF4]−, we efficiently achieved two types of alkylzincation of terminal and internal alkynes, which are not well addressed in the literature. These reactions exhibit broad substrate scope, high regioselectivity (mostly >95:5), and stereoselectivity (mostly >95:5), and provide 32900 TON in catalytic carbometallation reactions, offering an effective method for synthesizing polysubstituted alkenes. This method enabled the synthesis of various biologically active molecules and their key intermediates with enhanced synthetic efficiency. Mechanistic studies revealed that the weakly coordinating bulky anion stabilizes Fe(II)-alkyl complex, which serves as the active species to promote the desired transformations.
Methods
General procedure of methylzincation of alkyl alkynes
In an argon-filled glovebox, C3 (6.5 mg, 0.01 mmol, 0.02 equiv), NaBArF4 (22.1 mg, 0.025 mmol, 0.05 equiv), PhCF3 (2.0 mL) and alkyl terminal alkyne (0.5 mmol) were added sequentially to a 10 mL capped tube. After complete dissolution, Me2Zn (550 μL, 0.55 mmol, 1.0 M in toluene, 1.1 equiv) was added, and the reaction was stirred for 1 h at −20 °C, then the reaction mixture was reacted for another 2 h at 30 °C. Then the reaction was quenched by electrophilic reagent. [When E = D, D2O (150 μL) was added and reacted for 30 min; When E = I, I2 (381 mg, 1.5 equiv) was added and reacted for 3 h at 30 °C, excessive I2 was reacted with Na2S2O3; When E = benzoyl, the PhCF3 was removed in vacuo, THF (2 mL), CuCN (134 mg, 0.1 mmol), LiCl (63.6 mg, 1.5 mmol) in sequence, benzoyl chloride (211 mg, 1.5 mmol, 3.0 equiv) were added while stirring at 0 °C.] After quenching, the solvent was removed in vacuum, and the product was purified through silica gel flash chromatography.
Data availability
The authors declare that all other data supporting the findings of this study are available within the article and Supplementary information files, and also are available from the corresponding author upon request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 2306007 (6f), CCDC 2349497 (C1a) and 2349498 (C1c). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. The Cartesian coordinates for the optimized structures in this study have been save as Source Data and provided with this manuscript. Source data are provided with this paper.
References
Verendel, J. J., Pàmies, O., Diéguez, M. & Andersson, P. G. Asymmetric hydrogenation of olefins using chiral Crabtree-type catalysts: scope and limitations. Chem. Rev. 114, 2130–2169 (2014).
Jia, M. & Bandini, M. Counterion effects in homogeneous gold catalysis. ACS Catal. 5, 1638–1652 (2015).
Lu, Z., Li, T., Mudshinge, S. R., Xu, B. & Hammond, G. B. Optimization of catalysts and conditions in gold(I) catalysis-counterion and additive effects. Chem. Rev. 121, 8452–8477 (2021).
Chen, E. Y.-X. Cocatalysts for metal-catalyzed olefin polymerization: activators, activation processes, and structure-activity relationships. Chem. Rev. 100, 1391–1434 (2000).
Strauss, S. H. The search for larger and more weakly coordinating anions. Chem. Rev. 93, 927–942 (1993).
Krossing, I. & Raabe, I. Noncoordinating anions−fact or fiction? a survey of likely candidates. Angew. Chem. Int. Ed. 43, 2066–2090 (2004).
Riddlestone, I. M., Kraft, A., Schaefer, J. & Krossing, I. Taming the cationic beast: novel developments in the synthesis and application of weakly coordinating anions. Angew. Chem. Int. Ed. 57, 13982–14024 (2018).
Normant, J. F. & Alexakis, A. Carbometallation (C-metallation) of alkynes: stereospecific synthesis of alkenyl derivatives. Synthesis 1981, 841–870 (1981).
Cavender, H., Sklute, G. & Marek, I. Carbozincation reactions of carbon-carbon multiple bonds. Org. React. 87, 507–763 (2015).
Murakami, K. & Yorimitsu, H. Recent advances in transition-metal-catalyzed intermolecular carbomagnesiation and carbozincation. Beilstein J. Org. Chem. 9, 278–302 (2013).
Lorthiois, E. & Meyer, C. Carbozincation of alkenes and alkynes. Patai’s Chemistry of Functional Groups, (Wiley: New York, 2009).
Cheung, C. W., Zhurkin, F. E. & Hu, X.-L. Z-Selective olefin synthesis via iron-catalyzed reductive coupling of alkyl halides with terminal arylalkynes. J. Am. Chem. Soc. 137, 4932–4935 (2015).
Huang, Q. et al. Iron-catalyzed vinylzincation of terminal alkynes. J. Am. Chem. Soc. 144, 515–526 (2022).
Huang, Q., Wang, W.-N. & Zhu, S.-F. Iron-catalyzed alkylzincation of terminal alkynes. ACS Catal. 12, 2581–2588 (2022).
Wang, W.-N., Huang, Q., Jin, Y., Zhou, Q.-L. & Zhu, S.-F. Iron-catalyzed alkenylzincation of internal alkynes. Chin. J. Chem. 41, 3547–3552 (2023).
Nishikawa, T., Yorimitsu, H. & Oshima, K. Cobalt-catalyzed regio- and stereoselective allylzincation of 1-phenyl-1-alkynes regio- and stereoselective allylzincation of 1-phenyl-1-alkynes. Synlett 9, 1573–1574 (2004).
Murakami, K., Yorimitsu, H. & Oshima, K. Cobalt-catalyzed benzylzincation of alkynes. Chem. Eur. J. 16, 7688–7691 (2010).
Murakami, K., Yorimitsu, H. & Oshima, K. Cobalt-catalyzed arylzincation of alkynes. Org. Lett. 11, 2373–2375 (2009).
Wu, J.-L. & Yoshikai, N. Cobalt-catalyzed alkenylzincation of unfunctionalized alkynes. Angew. Chem. Int. Ed. 55, 336–340 (2016).
Stüdemann, T. M. & Knochel, P. New nickel-catalyzed carbozincation of alkynes: a short synthesis of (Z)-tamoxifen. Angew. Chem. Int. Ed. Engl. 36, 93–95 (1997).
Stüdemann, T., Ibrahim-Ouali, M. & Knochel, P. A nickel-catalyzed carbozincation of aryl-substituted alkynes. Tetrahedron 54, 1299–1316 (1998).
Gourdet, B. & Lam, H. W. Stereoselective synthesis of multisubstituted enamides via rhodium-catalyzed carbozincation of ynamides. J. Am. Chem. Soc. 131, 3802–3803 (2009).
Gourdet, B., Rudkin, M. E., Watts, C. A. & Lam, H. W. Preparation of multisubstituted enamides via rhodium-catalyzed carbozincation and hydrozincation of ynamides. J. Org. Chem. 74, 7849–7858 (2009).
Xie, M., Wang, J., Gu, X., Sun, Y. & Wang, S. Highly regio- and stereoselective synthesis of tetrasubstituted olefins by the three-component tandem reaction of allylzinc bromide, acetylenic sulfone, and halohydrocarbon. Org. Lett. 8, 431–434 (2006).
Sklute, G., Bolm, C. & Marek, I. Regio- and stereoselective copper-catalyzed carbozincation reactions of alkynyl sulfoximines and sulfones. Org. Lett. 9, 1259–1261 (2007).
Kadikova, R. N., Ramazanov, I. R., Gabdullin, A. M., Mozgovoi, O. S. & Dzhemilev, U. M. Carbozincation of substituted 2-alkynylamines, 1-alkynylphosphines, 1-alkynylphosphine sulfides with Et2Zn in the presence of catalytic system of Ti(O-iPr)4 and EtMgBr. Catalyst, 9, https://doi.org/10.3390/catal9121022 (2019).
Nakamura, M., Hirai, A. & Nakamura, E. Iron-catalyzed olefin carbometalation. J. Am. Chem. Soc. 122, 978–979 (2000).
Krämer, K., Leong, P. & Lautens, M. Enantioselective palladium-catalyzed carbozincation of cyclopropenes. Org. Lett. 13, 819–821 (2011).
Müller, D. S. & Marek, I. Asymmetric copper-catalyzed carbozincation of cyclopropenes en route to the formation of diastereo- and enantiomerically enriched polysubstituted cyclopropanes. J. Am. Chem. Soc. 137, 15414–15417 (2015).
Flynn, A. B. & Ogilvie, W. W. Stereocontrolled synthesis of tetrasubstituted olefins. Chem. Rev. 107, 4698–4745 (2007).
Hu, M.-Y. et al. Ligands with 1,10-phenanthroline scaffold for highly regioselective iron-catalyzed alkene hydrosilylation. Nat. Commun. 9, 221–231 (2018).
Hu, M.-Y. et al. Iron-catalyzed regiodivergent alkyne hydrosilylation. J. Am. Chem. Soc. 142, 16894–16902 (2020).
Li, W.-T., Hu, M.-Y., Xiong, J.-W., Zhang, X.-Y. & Zhu, S.-F. Iron-catalyzed hydroalumination of internal alkynes. Chem. Sci. 13, 7873–7879 (2022).
Zhang, Y.-D. et al. Highly regioselective cobalt-catalyzed hydroboration of internal alkynes. Angew. Chem. Int. Ed. 61, e202208473 (2022).
Sun, W. et al. Iron-catalyzed stereoconvergent 1,4-hydrosilylation of conjugated dienes. Angew. Chem. Int. Ed. 62, e202315473 (2023).
He, P. et al. Spin effect on redox acceleration and regioselectivity in Fe-catalyzed alkyne hydrosilylation. Natl Sci. Rev. 11, nwad324 (2024).
Zhang, Q. et al. Iron-catalyzed C(sp3)-C(sp3) coupling to construct quaternary carbon centers. J. Am. Chem. Soc. 146, 5051–5055 (2024).
Li, L.-J. et al. Recent advances in Mn, Fe, Co, Ni-catalyzed organic reactions. CCS Chem. 6, 537–584 (2024).
Mao, E. & MacMillan, D. W. C. Late-stage C(sp3)-H methylation of drug molecules. J. Am. Chem. Soc. 145, 2787–2793 (2023).
Schönherr, H. & Cernak, T. Profound methyl effects in drug discovery and a call for new C−H methylation reactions. Angew. Chem. Int. Ed. 52, 12256–12267 (2013).
Barreiro, E. J., Küummerle, A. E. & Fraga, C. A. M. The methylation effect in medicinal chemistry. Chem. Rev. 111, 5215–5246 (2011).
Kapat, A., Sperger, T., Guven, S. & Schoenebeck, F. E-Olefins through intramolecular radical relocation. Science 363, 391–396 (2019).
Li, L., Aibibula, P., Jia, Q. & Jia, Y. Total syntheses of naucleamides A–C and E, geissoschizine, geissoschizol, (E)-isositsirikine, and 16-epi-(E)-isositsirikine. Org. Lett. 19, 2642–2645 (2017).
Steinborn, C., Wildermuth, R. E., Barber, D. M. & Magauer, T. Total synthesis of (+)-cornexistin. Angew. Chem. Int. Ed. 59, 17282–17285 (2020).
Mauger, A. et al. Collective total synthesis of mavacuran alkaloids through intermolecular 1,4-addition of an organolithium reagent. Angew. Chem. Int. Ed. 62, e202302461 (2023).
Bouwkamp, M. W., Lobkovsky, E. & Chirik, P. J. Bis(imino)pyridine iron(II) alkyl cations for olefin polymerization. J. Am. Chem. Soc. 127, 9660–9661 (2005).
Tondreau, A. M. et al. Synthesis and electronic structure of cationic, neutral, and anionic bis(imino)pyridine iron alkyl complexes: evaluation of redox activity in single-component ethylene polymerization catalysts. J. Am. Chem. Soc. 132, 15046–15059 (2010).
Bouwkamp, M. W. et al. Square planar bis(imino)pyridine iron halide and alkyl complexes. Chem. Commun. 3406–3408 (2005).
He, P. & Zhu, S.-F. Spin crossover and its application in organometallic catalysis: concepts and recent progress. Chem. Eur. J. 30, e202403437 (2024).
Acknowledgements
We thank the National Key R&D Program of China (2021YFA1500200, S.-F.Z.), National Natural Science Foundation of China (22301230, Q.Z.; 22221002, S.-F.Z.; 92256301, S.-F.Z.), Fundamental Research Funds for the Central Universities (S.-F.Z.), and New Cornerstone Science Foundation through the XPLORER PRIZE (S.-F.Z.) for financial support.
Author information
Authors and Affiliations
Contributions
S.-F. Z. and L.-J. L. conceived the project. S.-F. Z. supervised the experiments. L.-J. L. Q. Z., P. H. and M.-Y. H. performed the experiments and DFT calculation. S.-F. Z., L.-J. L. and Q. Z. prepared this manuscript for publication. All authors commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The Authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Samuel Dagorne, Gunnar Werncke, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, LJ., Zhang, Q., He, P. et al. Iron-catalyzed alkyne alkylzincation affected by counterions. Nat Commun 16, 7161 (2025). https://doi.org/10.1038/s41467-025-62460-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-62460-z