Abstract
Molecular isomerization supports a variety of biological processes, and conformational regulation is a promising approach to achieve the desired physiological functions or inhibit adverse biological activities. Although extremely challenging, a controllable isomerism-modulated approach with features such as being molecule specific, non-invasive, and reversible is highly desirable for complex biosystems. Herein, based on the evidence from the molecular dynamic simulations of the controlled rotation around the σ bonds in retinal moiety and its generalizability to other systems, we present a strategy to achieve frequency-specific terahertz (THz) light-driven, controllable and reversible molecular isomerization. This strategy is attributed to the resonant energy transfer precisely from the THz irradiation to the rotational motion of the targeted molecular moieties by overcoming the energy barriers among the distinct isomers. This unique strategy is broadly applicable, as demonstrated in an extended study of rotation of an amino acid in aquaporin-4, and manifests significant implications for making precise molecular conformation manipulations and tuning controllable biochemical processes using state-of-the-art THz technologies.
Similar content being viewed by others
Introduction
Molecular conformation variations occur across a wide range of timescales and magnitudes and play crucial roles in numerous biochemical processes, such as protein folding, enzyme catalysis and cell signalling via neurotransmitter‒receptor interactions1,2,3,4,5. These structural variations are driven by isomerization around rotatable bonds, particularly the π bond, peptide bond, and σ bond and cause diverse biomolecular conformations6. For expamle, the cis-trans isomerization of certain amides, such as proline, plays a pivotal role in the rate-determining steps of protein folding7,8. Additionally, the rotation aroud disulfide σ bonds can significantly influence both the folding pathway and the final conformation of proteins9,10. In view of the importance of molecular-structure-biofunction relationship, isomer modulating is a promising strategy to modifying certain conformations and associated undesirable biological functionalities, thereby producing the desired physiological functionalities11.
Isomerization around stable π bonds involves a high energy barrier (90 ~ 170 kcal/mol)10,12 and typically needs visible light to induce photoisomerization, such as the transition of 11-cis to all-trans retinal in visual processes13,14,15. This ultrafast structural change and the thermally stable isomers can be probed by using time-resolved serial femtosecond crystallography (TR-SFX) at an X-ray free electron laser (XFEL)16,17,18. For peptide bonds with much lower energy barriers (∼20 kcal/mol), enzymes such as protein disulfide isomerase (PDI) and peptidyl-prolyl cis-trans isomerases (PPIs) are usually needed for bond rotations dedicated to protein folding19,20. Other candidates, such as heat21,22, free radicals23,24, and mid-infrared light25,26, can also be employed. Although peptide bonds are light-unresponsive, mid-infrared light can produce conformational changes by altering molecule‒environment interactions. For example, the 34.88 terahertz (THz) stimulus has been shown to resonate with the vibrations of Aβ fibrils, promoting the breakage of β-sheets and the formation of various coil and bend structures27.
For σ-bond isomerism between the s-cis and s-trans forms, the energy barrier is even lower (3 ~ 10 kcal/mol)28,29,30,31. This relatively low barrier facilitates the formation of various geometric isomers and is driven largely by molecular thermal motions; these motions increase the probability of this behaviour and the difficulty of finely tuning bond rotations with exceptional spatiotemporal precision. Notably, these isomers in some cases exhibit significant differences in their binding affinities, which enables specific s-cis or s-trans conformations to be advantageous in applications such as aggregation32,33, tumour imaging34,35, and anticancer36,37. While substantial progress has been made in regulating σ bond rotations through various methods, including environmental changes (e.g., temperature38,39 and substances40,41,42), strong laser pulses43,44, and molecular modifications34,36, these approaches often act globally or invasively and cause complications in the targeting of specific molecules in complex biological systems with fine-tuned interaction networks. As a consequence, achieving an effective isomerism-modulated approach that is molecule specific, non-invasive, and reversible remains extremely challenging for biosystems. Nevertheless, previous studies have shown that THz electric fields can drive the directed rotation of molecular surface motors45. Also, the concept of kinetic asymmetry further suggests that electromagnetic fields can promote directional conformational transformation of molecules by altering energy barriers or wells46,47. Notably, recent extensive work has demonstrated the superiority of THz radiation in selectively regulating the molecular motions (especially vibrations) and biological functions in a non-invasive and reversible way48,49,50,51,52,53,54,55,56. However, the potential of THz light to regulate molecular conformation in a controlled manner, along with the underlying mechanism, is still largely unknown.
In this work, by modelling an 11-cis retinal protonated Schiff base (rpSb) initially linked to the dark-state opsin for a clear distinction of a limited number of ligand isomers, we exemplify frequency-specific THz light-driven controllable isomerization of retinal moiety without the Schiff base (referred to as rwSb) around either of two σ-bonds towards a desired s-cis or s-trans conformation (Fig. 1). This predicted feasibility is attributed to resonance between the intrinsic rotation of each rwSb conformation and the applied frequency-specific THz light; THz light promotes the rotational kinetic energy of the rwSb to overcome the energy barriers among distinct conformations. Furthermore, extensive simulations substantiate the generalizability of this phenomenon across different molecular systems. These findings not only identify the superior performance of THz light-driven molecular conformation regulation but also promote potential applications of THz technologies in pharmacology, biochemistry and supramolecular chemistry.
a An 11-cis retinal moiety (orange) is connected to Lys296 (red) in opsin (blue) via a protonated Schiff base (left image). Without external stimulation, the retinal moiety without the Schiff base (rwSb) exhibits four typical conformations with distinct occupancy probabilities: one dominant conformation (RET-A) and three secondary conformations (RET-B, RET-C, and RET-D). These conformation distribution probabilities are displayed in a pie chart. The double arrows imply possible interconversion of conformations. b−d Comparison of rwSb under different conformations along with surrounding residues. Orange, yellow, green, and purple sticks represent the RET-A, RET-B, RET-C, and RET-D, respectively.
Results
Figure 1a shows that in an equilibrated system, rwSb appears in four typical conformations, as identified by the moment of inertia in five parallel 2 µs simulations (Suppl. Fig. 1). The dominant conformation with the largest proportion (51.1 ± 3.9)% is named RET-A, whereas the three secondary conformations, with proportions of (26.9 ± 9.6)%, (16.4 ± 9.9)%, and (5.6 ± 2.0)%, are labelled RET-B, RET-C, and RET-D, respectively. These conformations originate from the isomerization around two rotatable σ-bonds, C8‒C9 and C12‒C13 (Fig. 1a and Suppl. Fig. 2). For example, rotation around the C8‒C9 bond induces a shift from the s-trans isomer (RET-A) to the s-cis isomer (RET-B), accompanied by changes in the dihedral angle between the C7, C8, C9, and C10 atoms. This type of isomerization is contradictory to the cis-trans photoisomerization, which occurs at stable π-bonds under visible or UV light exposure (Suppl. Fig. 3). Furthermore, by examining the snapshots of different conformations of the rwSb bound to protein, we find that there is sufficient space accommodating the spontaneous σ-bond isomerization within the protein (Fig. 1b-d). In a similar fashion, the π-bond isomerization has been experimentally observed, which needs little space at a 1-ps delay after photoactivation57.
Figure 2 shows that frequency-specific THz stimulation with sufficient strength (A) can promote the rwSb transition to the desired conformation. Specifically, a 4.7 THz stimulus induces a conformational isomerization from the dominant RET-A (in the s-trans state) to a secondary RET-B (s-cis) around the C8‒C9 bond (Fig. 1); these results are shown by the decreasing occupancy probability of RET-A and the increasing probability of RET-B with increasing A (Fig. 2a). A reverse transition from RET-B to RET-A can be accomplished using 10.6 THz irradiation (Fig. 2b). Similarly, 7.9 THz light shifts the rwSb conformation from the RET-A isomer to another secondary RET-C, whereas 11.1 THz light drives reverse isomerization (Fig. 2c, d). Additionallly, the time-dependent changes in the moments of inertia of rwSb during the THz simulations, along with the distributions of different conformations, indicate the conformational transition, as shown in Suppl. Fig. 4. To observe the transition from the thermally less stable RET-B or RET-C to the most stable RET-A more clearly, we prepare the samples to ensure the desired initial conformation in RET-B or RET-C (see Suppl. Note 3). Note that the percentages of the conformational transitions presented in Fig. 2 do not reach 100%. This occurs because the percentages reflect the statistical results of the conformational distributions over time, which include the events from the conformational transitions. A higher percentage indicates a greater probability of successful conversion. Moreover, for each case, the transition occurs when the stimulation strength surpasses a certain threshold value, and before this threshold, the rwSb conformation remains unchanged. More importantly, once the stimulation is removed, the changed rwSb returns to its initial conformation; these results show the reversibility of THz wave regulation (Suppl. Fig. 5). We have also performed additional MD simulations by using a different force field to confirm that the terahertz-driven controllable molecular isomerization remains valid, regardless of the force field adopted (Suppl. Fig. 6)58,59. With this new force field, the isomerization site shifts from the C8‒C9 and C12‒C13 bonds to the C6‒C7 bond. This shift is attributed to the lower energy barrier of the isomerization around C6‒C7, which falls within the reported range for σ-bond isomerization (<10 kcal/mol), whereas the barriers at C8‒C9 and C12‒C13 largely exceed this threshold (Suppl. Fig. 7).
a, b 4.7 THz stimulation induces a conformational shift from RET-A to RET-B (a), whereas 10.6 THz stimulation results in the reverse transition (b). c, d 7.9 THz stimulation promotes a conformational change from RET-A to RET-C (c), whereas the 11.1 THz stimulus results in the reverse transition (d). Here, 0.1 THz light is introduced into all cases as a contrast. Error bars represent the standard deviation (n = 5).
Further calculations reveal that THz stimuli cause minimal changes in the root mean square displacement of the opsin protein (Suppl. Fig. 8), indicating its structural stability. As a result, the variation in the local electrostatic field generated by the protein on the rwSb is small (Suppl. Fig. 9); thus, this variation does not overshadow the direct resonance effect from the THz field on the rwSb, even though the local electrostatic field is 5−10 times stronger than the external THz field. Recall that spontaneous interconversion of conformations occurs without any stimulation; this fact initially explains the constitution of the four typical conformations instead of a single conformation. Hence, the secondary conformations RET-B and RET-C (Fig. 2b, d) tend to shift to other conformations, especially the dominant RET-A, which causes their occupancy possibilities to be <100% even without THz light (i.e., A = 0 V/nm). This further explains the occurrence of other unplanned conformations with trivial possibilities under specific THz stimuli (Suppl. Fig. 10). Furthermore, a nonspecific stimulus at 0.1 THz is introduced as a contrast, and the probabilities of rwSb conformations barely change. This finding indicates that the modulation of the conformational transition is frequency specific. Notably, variations in the ion concentration around the physiological solution value do not alter the overall trend of frequency-specific THz-induced isomerization (Suppl. Fig. 11). Moreover, the rwSb isomerization from the dominant conformation to either of the secondary conformations reduces the opsin-rwSb binding free energy (Suppl. Fig. 12); this result is reasonable since the dominant conformation accepts the strongest restriction from the opsin. Additionally, the energy changes result primarily from the contributions of neighbouring amino acids affected by rwSb isomerism (Suppl. Fig. 13). This indicates the potential regulatory or therapeutic effects on the linked receptor via THz light-modulated ligand isomerization since the altered interactions can be relayed by the protein scaffold.
Since the conformation switches mainly against two rotatable bonds (namely, C8‒C9 and C12‒C13 in Fig. 1), we investigate the rotation characteristics of the rwSb and determine the frequency specificity of THz stimulation accordingly. Similar to the infrared absorption, THz absorption also arises from the dynamic response of molecular dipole moments. However, THz fields specifically couple to torsional modes, rather than the bond stretching and bending modes excited via infrared light. Although rwSb is predominantly composed of hydrocarbons and is nonpolar, it is asymmetric and possesses a permanent dipole moment. Hence, torsion based on various rotational modes would induce fluctuations in its dipole moments, resulting in characteristic absorption features in the THz region. To identify specific molecular rotational modes and correlate them with THz absorption, we employ angular momentum as a straightforward variable representing torsional motion for spectral analysis. This approach enables the identification of THz frequencies that match the intrinsic frequency of the rotational modes. The rotational spectrum can be obtained by Fourier transforming the autocorrelation function of the angular momentum J(t) = ∑iri(t) × mivi(t), where mi, vi(t) and ri(t) are the mass, rotational velocity and displacement of the i-th atom with respect to the molecular centre of mass, respectively. Due to the polarization direction of the THz stimuli along the z direction (see Methods), the rwSb rotation is only considered in this direction. Figure 3 shows the rotational spectra of the dihedral atoms within 12 THz; beyond this range, the molecular vibrations dominate, and the rotational intensity is trivial. Apparently, despite being located at the same position, the dihedral atoms in different conformations possess largely different spectral peaks. For example, the peaks at 4.7 THz and 10.6 THz are unique for the dihedral atoms against the rotatable C8‒C9 bonds in RET-A and RET-B, respectively (Fig. 3a). Furthermore, the 7.9 THz and 11.1 THz peaks (Fig. 3b) exclusively result from the rotatable C12‒C13 bonds of RET-A and RET-C, respectively. This is reasonable as the THz rotational spectrum is specific to different single bonds in rpSb due to distinct torsion modes against each bond, and the torsion modes are influenced by factors such as bond/angle strength, steric hindrance of substitutes and surrounding environment. These differences lead to unique rotational frequencies for each bond, which can be selectively excited by a THz field that matches the resonant frequency of a particular bond. In this case, the THz field continuously exerts a time-varying torque on the local dipole of the atoms involved in the torsional motion around the bond, thereby selectively enhancing the kinetic energy of the associated atoms through resonance. More specifically, the 4.7 THz light is resonantly absorbed to drive the rotation of the left moiety of RET-A, which prompt RET-A to transform to RET-B. This trend also applies to other frequency-specific stimuli. In contrast, the intensity of the rotational spectrum at 0.1 THz is insignificant; thus, irradiation at this frequency barely drives the molecular rotation and causes a considerable conformation shift (Fig. 2). As a consequence, nonthermal THz light-driven conformational isomerization lies in the resonance between frequency-specific irradiation and rwSb rotation. In principle, the frequency at the resonant absorption peak can induce conformational transitions; for example, 2.1 THz stimulation can also drive the RET-A to RET-B transition (Suppl. Fig. 14). However, in practical applications, a frequency at which one conformation results in strong absorption needs to be selected, whereas the other conformations result in weak absorption.
a Rotational spectra of the dihedral atoms of φ1 (red) and φ2 (blue) at the same location but in different rwSb conformations (i.e., RET-A and RET-B). b Rotational spectra of the dihedral atoms of ψ1 (yellow) and ψ2 (violet) at the same location but in different rwSb conformations (i.e., RET-A and RET-C). Note that the dihedral atoms and their spectra are identically coloured.
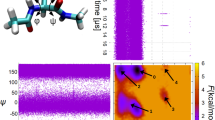
To explicitly interpret how specific THz light produces the conformational transition, we calculate the 2D free energy landscape in terms of dihedrals φ and ψ against the two key rotatable bonds (Fig. 4a). The four local energy minima represent the aforementioned four typical conformations, and the global minimum is denoted as the most stable RET-A. We further construct the 1D energy profile for each of the two dihedrals; this profile shows the energy barrier to overcome during the conformational change (Fig. 4b). These energy barriers also fall within the reported range for σ-bond isomerization. To validate these barriers, we perform quantum chemical calculations based on the XMS-CASPT2 method60,61. The calculation shows a maximum energy barrier of ~17.5 kJ/mol for the transition between s-cis and s-trans conformations (Suppl. Fig. 15), close to the value derived from our classical force fields. After THz stimulation, we monitor the changes in rwSb kinetic energy, which can be decomposed into rotational, vibrational, and translational kinetic energies (see Suppl. Fig. 16 and Suppl. Note 13 for details). Although kinetic energy can be redistributed among different components based on molecular relaxation at a short time scale, a thermostat is applied in the simulations to regulate the temperature and energy exchange, ensuring proper system relaxation and efficient energy redistribution. The thermostat uniformly rescales particle velocities across translational, rotational, and vibrational modes. However, Fig. 4c shows that the largest THz light-induced increase lies in the rotational kinetic energy (ER) of the rwSb compared with other energy components, that is, the observed enhancement is specific to the rotational component. This rules out the possibility that the THz-induced conformational transition is an artefact of indiscriminate energy redistribution into specific degrees of freedom, such as single-bond torsions. Additionally, as shown in Suppl. Fig. 17, ER increases on the sub-picosecond scale, matching the duration of THz stimulation, more rapidly than it dissipates via the thermostat. This finding indicates a short-term dynamic transfer of energy from THz radiation into the rwSb’s ER rather than a long-term accumulation effect. Furthermore, evolution of the dihedral angles and associated kinetic energies during THz-induced isomerization confirms that when the external THz field resonates with the torsional mode of a specific bond, the kinetic energy surges exclusively at the resonant site, accompanied by substantial changes of dihedrals therein (Suppl. Fig. 18). In other words, the THz irradiation can selectively promote single bond isomerization.
a, b 2D free energy surface (a) and 1D relative energy profiles (b) of the rwSb wrt. the two key dihedral angles φ and ψ are shown in the inset of Fig. 4b inset. c Rotational (ER), vibrational (EV), and translational (ET) kinetic energies of the rwSb with and without the application of a 10.6 THz stimulus. d Relationship between the mean rotational kinetic energy \(\bar{E}\)R and the strength of irradiation under the four key regulation frequencies.
As a further step, we analyse the relationship between the mean rotational kinetic energy (\(\bar{E}\)R) and the irradiation strength under different characteristic frequencies (Fig. 4d and Suppl. Fig. 19). Generally, stronger irradiation correlates to higher kinetic energy. For example, the baseline \(\bar{E}\)R (at A = 0 V/nm) is 96 ± 19 kJ/mol, and it reaches 116 ± 25 kJ/mol as the strength of the 7.9 THz irradiation increases to 0.8 V/nm. Recall that the conformational shift from RET-A to RET-C (corresponding to a dihedral change of ψ from 43° to −132°, as interpreted in Suppl. Fig. 20) results in an energy barrier of ~28.5 kJ/mol (Fig. 4b). As a result, due to the growth of ER by the 7.9 THz, 0.8 V/nm stimulus overcomes the energy barrier and enhances the conformational transition. This result agrees with the fact that the conformation starts to change at A = 0.8 V/nm, as shown in Fig. 2c. Before that, the kinetic promotion is insufficient to overcome the energy barrier. The cases for other THz stimuli follow the same trend. Hence, resonant absorption of frequency-specific THz light promotes the rotational kinetic energy of rwSb, improves its ability to overcome the free energy barrier at rotatable bonds, and ultimately prompts its conformational isomerization.
Finally, this THz-induced conformational isomerization is not limited to rwSb but has broader applicability to other biomolecules. To explore this further, we extended our study to the rotation of Arg216 in aquaporin-4, which plays a key role in switching the open and closed states of the water channel62. Similar to the isomerization around the C‒C σ-bond in rwSb, the frequency-specific THz stimulation induced the molecular isomerization of Arg216 around the C‒N single bond (see details in Suppl. Fig. 21 and Suppl. Note 18). Notably, as the strength of the THz stimulation increased, the isomerization effect became more pronounced.
Discussions
In conclusion, our simulations show that THz light can effectively switch the rwSb molecule among its conformations in a frequency-controlled manner even with low energy. These results are attributed to the resonant transfer of THz energy into the rotational kinetic energy of rwSb, enhancing its ability to overcome the free energy barriers and facilitate the conformational isomerization. In addition, the angular momenta-based spectra can be used to determine the molecular rotations and successfully pinpoint the effective THz frequencies. Although this study is primarily based on the rwSb model, in which the binding of rwSb to opsin lowers its rotational degrees of freedom and limits the number of isomers, such a model is ideal for demonstrating clear physical chemistry underlying our hypothesis, and the implication of the obtained findings extend to other molecular structures and bond types. The theoretical insights gained will help to understand the mechanisms underlying terahertz-driven controllable molecular isomerization. We hope this theoretical finding can promote future experiments for validation.
This work also elucidates a fundamental trend of the THz light-controlled reversible biomolecular isomerization between the s-cis and s-trans states. We note that many biological phenomena, e.g., enzyme catalysis and signal transduction, originate from local conformational changes, typically involving the repositioning of a few residues and small-scale torsional changes in the backbone, while the molecular conformation changes are driven by isomerization around various rotatable bonds, such as π bonds, peptide bonds, and σ bonds. These conformational changes contribute to vast diversity of biomolecular structures. Hence, the ability to regulate cis‒trans isomerism around various single bonds associated with numerous biochemical processes and diseases can be exploited in protein engineering and drug design.
Methods
Molecular dynamic simulations
The simulation system consist of a rhodopsin (PDB entry: 1U19) embedded within bilayer lipid membranes normal to the z direction and immersed in a 0.15 mol/L KCl solution (Suppl. Fig. 22). This KCl concentration represents the typical molarity of monovalent ions in physiological solutions. The 11-cis retinal moiety in rhodopsin is connected to Lys296 of opsin via a protonated Schiff base; thus, the compound is a nonstandard residue (Suppl. Fig. 23). This opsin-bound rwSb model can reduce the rotational degrees of freedom of the rwSb and limit its isomer number, making it an ideal system for demonstrating the clear physics behind terahertz-induced molecular isomerization. The charges and topology parameters of rpSb-Lys296 are exclusively generated via Multiwfn-3.863,64 and Sobtop-1.065 software, respectively, based on the electrostatic potential and Hessian matrix calculated via Gaussian66. The GAFF force fields (FFs) are chosen for rpSb integration with the Amber14sb FFs used to describe the opsin. This approach effectively accounts for the key interactions between rwSb and opsin while preserving the compatibility of the opsin protein’s force field.
During simulations, the all-atomic system is first effectively equilibrated at a temperature of 303.15 K and a pressure of 1 bar by using GROMACS-2020.767. For the cases with THz stimuli, the system runs for 100 ns in the NVT ensemble with atom trajectories recorded per ps. Under ambient conditions, the interaction between electromagnetic waves and charged particles is dominated by the electric component of the Lorentz force, which is much stronger than the magnetic component68. Therefore, the THz stimulation is modelled by an oscillating electric field E(t) = A · u·cos(2πft + θ), where A, f and θ denote the strength, frequency and initial phase of the stimulus, respectively69. u represents the polarization direction of the stimulus and is set to (0, 0, 1), i.e., perpendicular to the membrane plane. Consequently, the THz field interacts with molecules through the electrostatic forces on charged atoms. THz stimulation is applied to the entire system, considering its interactions with rwSb, the protein, the membrane, and solution. Notably, the system temperature is always maintained at ~303.15 K with the Nose‒Hoover thermostat during THz stimulation (Suppl. Fig. 24).
Metadynamics simulations
To compute the free energy landscape of the conformational interconversion of rwSb, the metadynamics simulations are conducted by using the PLUMED-2.9.070 patched to GROMACS, where two dihedrals φ and ψ around the rotational bonds (Fig. 1 and Suppl. Fig. 25) are used as the reaction coordinates, known as the collective variables (CVs), and are iteratively applied with biased potentials. Herein, the Gaussian potential hills with the initial height of 1.2 kcal/mol and width of 0.2 rad are deposited into both CVs every 500 timesteps. The temperature is 303.15 K and the bias factor is set 5. Each metadynamics simulation was performed for 200 ns. The utility sum_hills is used to sum the Gaussian kernels deposited during the simulation and get the 2D free energy landscape (Fig. 4a). Furthermore, by reweighting the biased simulation data, we have constructed the 1D PMF profiles wrt. each CV (Fig. 4b).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data generated in this study, including system setups, force field parameters, and GROMACS input files are available on Zenodo [https://doi.org/10.5281/zenodo.15337027]. Source data are provided as a Source Data file. Source data are provided in this paper. Source data are provided with this paper.
Code availability
The code used in this article can be found at https://doi.org/10.5281/zenodo.15333573.
References
T. Xie, T. Saleh, P. Rossi, C. G. Kalodimos, Conformational states dynamically populated by a kinase determine its function. Science 370, eabc2754 (2020).
Gong, G. Q. et al. A small-molecule PI3Kα activator for cardioprotection and neuroregeneration. Nature 618, 159–168 (2023).
Liang, S. & Blundell, T. L. Human DNA-dependent protein kinase activation mechanism. Nat. Struct. Mol. Biol. 30, 140–147 (2023).
García-Martínez, A., Zinovjev, K., Ruiz-Pernía, J. J. & Tuñón, I. Conformational changes and ATP hydrolysis in Zika helicase: the molecular basis of a biomolecular motor unveiled by multiscale simulations. J. Am. Chem. Soc. 145, 24809–24819 (2023).
Li, J. et al. Ligand binding initiates single-molecule integrin conformational activation. Cell 187, 2990–3005 (2024).
Dugave, C. & Demange, L. Cis−trans isomerization of organic molecules and biomolecules: implications and applications. Chem. Rev. 103, 2475–2532 (2003).
Wedemeyer, W. J., Welker, E. & Scheraga, H. A. Proline cis−trans isomerization and protein folding. Biochemistry 41, 14637–14644 (2002).
Englander, S. W. & Mayne, L. The nature of protein folding pathways. Proc. Natl. Acad. Sci. USA 111, 15873–15880 (2014).
Wedemeyer, W. J., Welker, E., Narayan, M. & Scheraga, H. A. Disulfide bonds and protein folding. Biochemistry 39, 4207–4216 (2000).
Fass, D. Disulfide bonding in protein biophysics. Annu. Rev. Biophys. 41, 63–79 (2012).
Lummis, S. C. R. et al. Cis–trans isomerization at a proline opens the pore of a neurotransmitter-gated ion channel. Nature 438, 248–252 (2005).
Nevesely, T., Wienhold, M., Molloy, J. J. & Gilmour, R. Advances in the E→Z isomerization of alkenes using small molecule photocatalysts. Chem. Rev. 122, 2650–2694 (2021).
Schoenlein, R. W., Peteanu, L. A., Mathies, R. A. & Shank, C. V. The first step in vision: femtosecond isomerization of rhodopsin. Science 254, 412–415 (1991).
Birge, R. R. & Hubbard, L. M. Molecular dynamics of cis-trans isomerization in rhodopsin. J. Am. Chem. Soc. 102, 2195–2205 (1980).
Polli, D. et al. Conical intersection dynamics of the primary photoisomerization event in vision. Nature 467, 440–443 (2010).
Nogly, P. et al. Retinal isomerization in bacteriorhodopsin captured by a femtosecond x-ray laser. Science 361, eaat0094 (2018).
Coquelle, N. et al. Chromophore twisting in the excited state of a photoswitchable fluorescent protein captured by time-resolved serial femtosecond crystallography. Nat. Chem. 10, 31–37 (2018).
Pande, K. et al. Femtosecond structural dynamics drives the trans/cis isomerization in photoactive yellow protein. Science 352, 725–729 (2016).
Okumura, M. et al. Dynamic assembly of protein disulfide isomerase in catalysis of oxidative folding. Nat. Chem. Biol. 15, 499–509 (2019).
Schmidpeter, P. A. M., Rheinberger, J. & Nimigean, C. M. Prolyl isomerization controls activation kinetics of a cyclic nucleotide-gated ion channel. Nat. Commun. 11, 6401 (2020).
Mallamace, F. et al. Energy landscape in protein folding and unfolding. Proc. Natl. Acad. Sci. USA. 113, 3159–3163 (2016).
Sivasankaran, R. P. et al. Polymer-mediated protein/peptide therapeutic stabilization: current progress and future directions. Prog. Polym. Sci. 156, 101867 (2024).
Schöneich, C., Pogocki, D., Hug, G. L. & Bobrowski, K. Free radical reactions of methionine in peptides: mechanisms relevant to β-amyloid oxidation and Alzheimer’s disease. J. Am. Chem. Soc. 125, 13700–13713 (2003).
Schwinn, J., Sprinz, H., Drossler, K., Leistner, S. & Brede, O. Thiyl radical-induced cis/trans-isomerization of methyl linoleate in methanol and of linoleic acid residues in liposomes. Int. J. Radiat. Biol. 74, 359–365 (1998).
Wang, X. & Zhang, Y. Potential terahertz therapeutic strategy for the prevention or mitigation of Alzheimer’s disease pathology. Light Sci. Appl. 12, 254 (2023).
López-Lorente, ÁI. & Mizaikoff, B. Mid-infrared spectroscopy for protein analysis: potential and challenges. Anal. Bioanal. Chem. 408, 2875–2889 (2016).
Peng, W. et al. High-frequency terahertz waves disrupt Alzheimer’s β-amyloid fibril formation. eLight 3, 18 (2023).
Clayden, J., Greeves, N., Warren, S. Organic Chemistry, 2nd edn, Vol. 520 (Oxford University Press, 2012).
Ebrahimi, A., Habibi, S. M., Sanati, A. & Mohammadi, M. A comparison of C-C rotational barrier in [2] staffane,[2] tetrahedrane and ethane. Chem. Phys. Lett. 466, 32–36 (2008).
Park, S., Shin, J., Yoon, H. & Lim, M. Rotational isomerization of carbon-carbon single bonds in ethyl radical derivatives in a room-temperature solution. J. Phys. Chem. Lett. 13, 11551–11557 (2022).
Zheng, J., Kwak, K., Xie, J. & Fayer, M. D. Ultrafast carbon-carbon single-bond rotational isomerization in room-temperature solution. Science 313, 1951–1955 (2006).
Qiu, R. et al. Cis-trans isomerization of peptoid residues in the collagen triple-helix. Nat. Comm. 14, 7571 (2023).
Hu, H. Y. et al. Probing the dynamic environment-associated conformational conversion from secondary to supersecondary structures in oligo (phenanthroline dicarboxamide) s. J. Org. Chem. 74, 4949–4957 (2009).
Zhang, X., Gao, J. & Tang, Y. Bioorthogonally activatable cyanine dye with torsion-induced disaggregation for in vivo tumor imaging. Nat. Comm. 13, 3513 (2022).
Chen, H. J. et al. S-cis diene conformation: a new bathochromic shift strategy for near-infrared fluorescence switchable dye and the imaging applications. J. Am. Chem. Soc. 140, 5224–5234 (2018).
Yang, J., Yan, W. & Yu, Y. The compound millepachine and its derivatives inhibit tubulin polymerization by irreversibly binding to the colchicine-binding site in β-tubulin. J. Bio. Chem. 293, 9461–9472 (2018).
Immormino, R. M., Kang, Y., Chiosis, G. & Gewirth, D. T. Structural and quantum chemical studies of 8-aryl-sulfanyl adenine class Hsp90 inhibitors. J. Med. Chem. 49, 4953–4960 (2006).
Wei, J. et al. Conformation-dependent dynamic organic phosphorescence through thermal energy driven molecular rotations. Nat. Commun. 14, 627 (2023).
Chen, K. et al. Twofold rigidity activates ultralong organic high-temperature phosphorescence. Nat. Commun. 15, 1269 (2024).
Zhang, C. et al. Twist-diameter coupling drives DNA twist changes with salt and temperature. Sci. Adv. 8, eabn1384 (2022).
Rablen, P. R., Miller, D. A., Bullock, V. R., Hutchinson, P. H. & Gorman, J. A. Solvent effects on the barrier to C−N bond rotation in N, N-dimethylaminoacrylonitrile. J. Am. Chem. Soc. 121, 218–226 (1999).
Liu, K. et al. Modulation of antimicrobial peptide conformation and aggregation by terminal lipidation and surfactants. Langmuir 36, 1737–1744 (2020).
Madsen, C. B. et al. Manipulating the torsion of molecules by strong laser pulses. Phys. Rev. Lett. 102, 073007 (2009).
Dalton, J. et al. Experimental and computational analysis of para-hydroxy methylcinnamate following photoexcitation. Molecules 26, 7621 (2021).
Neumann, J., Gottschalk, K. E. & Astumian, R. D. Driving and controlling molecular surface rotors with a terahertz electric field. ACS Nano 6, 5242–5248 (2012).
Astumian, R. D. Nonequilibrium steady states, ratchets, and kinetic asymmetry. Matter 6, 2533–2536 (2023).
Astumian, R. D. Kinetic asymmetry and directionality of nonequilibrium molecular systems. Angew. Chem. Int. Ed. 136, e202306569 (2024).
Li, Y., Chang, C., Zhu, Z., Sun, L. & Fan, C. Terahertz wave enhances permeability of the voltage-gated calcium channel. J. Am. Chem. Soc. 143, 4311–4318 (2021).
Wu, K. et al. Terahertz wave accelerates DNA unwinding: a molecular dynamics simulation study. J. Phys. Chem. Lett. 11, 7002–7008 (2020).
Zhang, C. et al. Driving DNA origami assembly with a terahertz wave. Nano Lett. 22, 468–475 (2021).
Zhu, Z., Chen, C., Chang, C. & Song, B. Terahertz-light induced structural transition and superpermeation of confined monolayer water. ACS Photonics 8, 781–786 (2020).
Lou, J. et al. Calibration-free, high-precision, and robust terahertz ultrafast metasurfaces for monitoring gastric cancers. Proc. Natl. Acad. Sci. USA 119, e2209218119 (2022).
Sun, T. & Zhu, Z. Light resonantly enhances the permeability of functionalized membranes. J. Membr. Sci. 662, 121026 (2022).
Liu, X. et al. Nonthermal and reversible control of neuronal signaling and behavior by midinfrared stimulation. Proc. Natl. Acad. Sci. USA 118, e2015685118 (2021).
Zhang, Q. L. et al. Ultrahigh-flux water nanopumps generated by asymmetric terahertz absorption. Phys. Rev. Lett. 132, 184003 (2024).
Li, Y. et al. Physicochemical insights on terahertz wave diminished side effects of drugs from slow dissociation. ACS Nano 16, 8419–8426 (2022).
Gruhl, T. et al. Ultrafast structural changes direct the first molecular events of vision. Nature 615, 939–944 (2023).
Prima, D. D., Reinholdt, P. & Kongsted, J. Color tuning in bovine rhodopsin through polarizable embedding. J. Phys. Chem. B 128, 2864–2873 (2024).
Church, J. R., Olsen, J. M. H. & Schapiro, I. The impact of retinal configuration on the protein–chromophore interactions in bistable jumping spider rhodopsin-1. Molecules 27, 71 (2022).
Sen, S. & Schapiro, I. A comprehensive benchmark of the XMS-CASPT2 method for the photochemistry of a retinal chromophore model. Mol. Phys. 116, 2571–2582 (2018).
Shiozaki, T., Győrffy, W., Celani, P. & Werner, H. J. Communication: Extended multi-state complete active space second-order perturbation theory: energy and nuclear gradients. J. Chem. Phys. 135, 081106 (2011).
Wang, Y., Schulten, K. & Tajkhorshid, E. What makes an aquaporin a glycerol channel? A comparative study of AqpZ and GlpF. Structure 13, 1107–1118 (2005).
Lu, T. & Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Lu, T. A comprehensive electron wavefunction analysis toolbox for chemists, Multiwfn. J. Chem. Phys. 161, 082503 (2024).
Lu, T. Sobtop, Version 1.0 (dev3.1). http://sobereva.com/soft/Sobtop (2023).
Frisch, A. et al. Gaussian 09W Reference. (Gaussian, Inc., 2009).
Abraham, M. J. et al. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1, 19–25 (2015).
Zhu, Z., Chang, C., Shu, Y. & Song, B. Transition to a superpermeation phase of confined water induced by a terahertz electromagnetic wave. J. Phys. Chem. Lett. 11, 256–262 (2020).
Zhu, Z. et al. Tunable surface wettability via terahertz electrowave controlled vicinal subnanoscale water layer. Nano Lett. 24, 3243–3248 (2024).
Bonomi, M. et al. PLUMED: A portable plugin for free-energy calculations with molecular dynamics. Comput. Phys. Commun. 180, 1961–1972 (2009).
Acknowledgements
We would like to thank Dr. Yindong Huang for his helpful discussions. This work is supported by the National Natural Science Foundation of China Projects (T2241002 and 12225511 to C.C., 12204547 to Y.L., and 12374214 to Z.Z.), and the Shanghai Rising-Star Program (23QA1404200 to Z.Z.). X.C.Z. acknowledges support from the Hong Kong Global STEM Professorship Scheme and a GRF grant (11204123) from the Research Grants Council of Hong Kong. We would also like to thank the computing resources and technical support provided by Shanghai Yonovate Technology Co. Ltd.
Author information
Authors and Affiliations
Contributions
Z.Z., C.C., Y.L., J.S.F., and X.C.Z. conceived the concepts. Z.Z., S.G., H.Q., and Y. L. performed the simulations. Z. X. and C. W. provided valuable ideas. Z.Z., S.G., C.C., Y.L., J.S.F., and X.C.Z. conducted the data analysis. C.C., Y.L., J.S.F., and X.C.Z. polished the language and supervised this work. Z.Z., C.C., Y.L., J.S.F., and X.C.Z. wrote this paper. All authors discussed the results and commented on the manuscript at all stages.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Raymond Astumian, Ruibin Liang and the other anonymous reviewer for their contribution to the peer review of this work. [A peer review file is available].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhu, Z., Gu, S., Chang, C. et al. Computational study of terahertz-driven controllable molecular isomerization. Nat Commun 16, 7081 (2025). https://doi.org/10.1038/s41467-025-62521-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-62521-3