Abstract
The environmental and health risks of silver nanoparticles (AgNPs) have driven the development of numerous engineering strategies to reduce the likelihood of exposure. Nonetheless, AgNP exposure is often inevitable, prompting a search for effective detoxification strategies at the organism level. Given the critical role of the gut microbiota in host health, we test its ability to mitigate the adverse effects of AgNPs by introducing various bacterial strains into the Caenorhabditis elegans gut and then comparing the nematode’s response with that of germ-free nematodes. Reproduction, the most sensitive toxicity endpoint tested herein, is significantly impaired by AgNPs but is rescued by colonization with Pseudomonas mendocina. Gene expression analyses reveal that this bacterium suppresses both the initiating and key events within the adverse outcome pathways triggered by AgNPs. Metabolomic profiling of gut bacteria and AgNP-exposed nematodes followed by verification with standard substances identifies two thiamine-derived metabolites, 4-methyl-5-thiazoleethanol and thiamine monophosphate, as pivotal in reducing the reproductive toxicity of AgNPs. Our study presents a promising approach to mitigate the adverse effects of nanoparticle exposure, through manipulation of the gut microbiota.
Similar content being viewed by others
Introduction
Silver nanoparticles (AgNPs) have received significant attention for their broad applications in biotechnology, electronics, environmental remediation, and the food industry1. The global production of AgNPs is projected to increase from 500 metric tons in 2017 to ~800 metric tons by 20252. The extensive production and utilization of AgNPs are expected to lead to significant environmental concentrations, with estimates of milligrams per liter in water and milligrams per kilogram in soil3,4. Concerns over the potential ecological and health risks of AgNPs have prompted extensive toxicity studies across various trophic levels5,6. The reported toxicological effects of AgNPs encompass oxidative stress, inflammation, DNA damage, protein denaturation, mitochondrial dysfunction, and cell apoptosis7,8. However, despite advances in engineering methods aimed at reducing the exposure risks from AgNPs (e.g., filtration, adsorption, coagulation and flocculation)4, effective organism-level detoxification strategies remain elusive. The pursuit of such strategies is imperative, as environmental AgNP exposure may well be inevitable.
The gut microbiota (GM) is critical for host health9,10, as it plays important roles in many host physiological processes, including digestion, metabolism, development, immunity, and aging11,12. AgNPs can alter GM composition, with effects that depend on the characteristics of the NPs as well as host factors13,14,15. For instance, the introduction of AgNPs with distinct morphologies into Sprague-Dawley rats was shown to impact different gut bacterial species16. Ma et al.17 reported that AgNPs disrupt GM diversity and structure in male, but not in female zebrafish. Such disruptions in GM have the potential to amplify AgNP toxicity. Our group’s prior research identified a significant correlation between 60% of the differentially expressed metabolites (DEMs) in the livers of AgNP-exposed mice and 23% of the differential microorganisms in the gut2. In a seminal study, AgNPs were shown to exhibit greater toxicity in germ-free zebrafish larvae than in their unsterilized counterparts18. The authors further found that microbiota-dependent TLR2 signaling reduces the toxicity of AgNPs19. These findings imply that modulation or colonization of the GM offers a viable strategy for mitigating the toxicity of AgNPs. Nevertheless, the specific mechanisms underlying the protective effects of the GM, including the actual microbial species involved and the associated molecular pathways, remain largely unexplored.
This study investigated the toxicity of AgNPs in the nematode Caenorhabditis elegans, comparing germ-free nematodes with those colonized by various gut-associated bacterial species: Pseudomonas mendocina MSPm1, Escherichia coli OP50, Comamonas sp. DA1877, and Comamonas piscis BIGb0172. These bacteria, previously reported in the literature20,21 and confirmed by our preliminary experiment (Supplementary Fig. 1), are highly prevalent gut microbes in wild-type nematodes. Their abundance also changed significantly in the presence of AgNPs, suggesting their potential effects on AgNP toxicity. We chose C. elegans as the model organism due to its bacterivorous nature, simple anatomy, suitability for germ-free culture, and the ability to manipulate its GM by inoculation with different bacterial strains22,23. The molecular mechanisms underlying AgNP toxicity and the potential effects of GM on AgNP toxicity were then examined using transcriptomics, quantitative real-time polymerase chain reaction (qPCR) of adverse outcome pathway (AOP) genes, and metabolomics. The bacterial metabolites were also analyzed to identify those responsible for the effects of GM on AgNP toxicity. Our results showed that, among the tested endpoints, the reproduction of C. elegans was most significantly affected by AgNP exposure. However, colonization with P. mendocina reduced the reproductive toxicity of AgNPs. Further investigation revealed that two vitamin-B1-derived metabolites in P. mendocina, 4-methyl-5-thiazoleethanol (MTE) and thiamine monophosphate (ThMP), play important roles in this protective effect. Collectively, our findings underscore the potential of GM colonization as a strategy to mitigate AgNP toxicity.
Results and discussion
Physicochemical properties of AgNPs
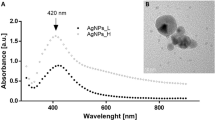
The AgNPs used in the present study were well dispersed in the experimental media (Fig. 1a, b). Their average diameter, determined from over 1000 randomly selected particles in transmission electron microscopy (TEM) images (Fig. 1a), was 21.5 ± 6.7 nm and their hydrodynamic diameter remained constant (88.5–96.1 nm) during a 96 h period (Fig. 1b). The zeta potential of the AgNPs was −27.3 mV, indicating that electrostatic repulsion between the particles contributed to their good dispersibility24. Steric repulsion arising from the polyvinylpyrrolidone coating, which has a molecular mass of 58 kDa, may have also played a role25. Additionally, the low-electron-density coating explained the discrepancy between the hydrodynamic diameter determined by dynamic light scattering (DLS) and the primary particle size observed via TEM26.
a Transmission electron microscopy and (b) dynamic light scattering results demonstrate the high dispersibility of AgNPs in deionized water. c Survival rate, head thrash, body length, and reproduction of germ-free (GF) C. elegans exposed to different concentrations (1.76, 5.3, 17.6, 53, 176, or 1144 μg/m2) of AgNPs versus the unexposed control treatment. d Reproduction in GF C. elegans and C. elegans colonized by active bacteria (P.m.—P. mendocina, E.c.—E. coli, Cmm.—Comamonas sp., C.p.—C. piscis) when exposed to different concentrations (1.76, 5.3, 17.6, 53, 176, or 1144 μg/m2) of AgNPs versus that in the respective unexposed control. e Top 20 Gene Ontology (GO) pathways enriched in differentially expressed genes of GF C. elegans exposed for 55 h to 17.6 μg/m2 of AgNPs as compared to the control treatment without any exposure of AgNPs. P values were corrected using the Bonferroni method for multiple testing following Fisher’s exact test. f Heat map of the expression of genes related to the MAPK and oocyte maturation pathways in GF nematodes exposed or not (Ctrl) to 17.6 μg/m2 of AgNPs and in those colonized by the four bacterial species and exposed to the same concentration of AgNPs. Lines in (c, d) are dose-response curves simulated by a four-parameter logistic model. To enable the logarithmic scale depiction of the data in the unexposed control treatment (c, d), a nominal AgNP concentration of 0.01 μg/m² was applied to this treatment, despite the absence of actual exposure. Data in (b–d) are the mean ± standard deviation (n = 3 independent experiments).
Bacterial colonization of the C. elegans gut
Dark-field microscopy revealed that all four bacterial species were rod-shaped (Supplementary Fig. 2a). Their average length and width were as follows: P. mendocina, 2.4 μm and 1.0 μm; E. coli, 2.1 μm and 0.9 μm; Comamonas sp., 2.6 μm and 0.9 μm; and C. piscis, 2.3 μm and 1.1 μm, respectively. Nutritional and caloric intake across the different toxicity assays described below were standardized using E. coli inactivated with 0.5% (w/v) paraformaldehyde as the dietary base for C. elegans27,28. Specifically, rather than administering 100% live C. piscis, Comamonas sp., P. mendocina, or E. coli to C. elegans during colonization, each species was mixed with inactive E. coli at a carbon mass ratio of 1:9, as described in previous studies29, prior to feeding the L1-stage nematodes. This approach ensured a consistent nutritional profile across different bacterial treatments. The growth and reproduction of C. elegans were thus comparable when fed on 100% inactive E. coli or a mixture of 90% inactive E. coli and 10% live C. piscis, Comamonas sp., P. mendocina, or E. coli (Supplementary Fig. 2b, c).
Over the 96 h period, C. elegans successfully accumulated substantial populations of the tested bacteria in their gut, with colonization levels rising over time (Supplementary Fig. 2d). However, the bacterial load per nematode was markedly higher in nematodes fed Comamonas sp. and E. coli than in those fed P. mendocina and C. piscis. Specifically, in C. elegans consuming a diet comprising 90% inactive E. coli and 10% active Comamonas sp., E. coli, P. mendocina, or C. piscis, the bacterial counts increased from 123.0, 133.4, 12.5, and 0.4 CFU/nematode after 36 h to 547.6, 433.7, 108.2, and 93.2 CFU/nematode after 96 h, respectively. Along with an increase in gut volume due to growth, the observed rise in bacterial colonization over time can also be explained by an age-related decline in the C. elegans pharyngeal crushing efficiency30. Colonization variability among different bacterial species has been attributed to their distinct capabilities to resist host pharyngeal breakdown31, adapt to gut environmental conditions32, and attract nematodes33. The colonization levels observed in the present study are at the lower range reported in the literature (1–106 cells per nematode)31,32,34, potentially due to the use of a bacterial diet mixture containing only a small proportion (10%) of active cells.
AgNP toxicity to C. elegans colonized by different bacteria
The exposure of germ-free C. elegans fed exclusively on non-viable E. coli to a gradient concentration (0–1144 μg/m2 agar plate) of AgNPs resulted in pronounced inhibitory effects across all four toxicity endpoints (Fig. 1c). Specifically, at the highest AgNP concentration (1144 μg/m2), survival rate, head thrash, body length, and reproduction were diminished by 38.3%, 89.0%, 77.1%, and 100.0%, respectively. The median effect concentrations (EC50) thus obtained were 2270 μg/m2 for survival, 135.5 μg/m2 for head thrash, 188.5 μg/m2 for body length, and 9.0 μg/m2 for reproduction. Although the EC50 value for survival exceeded the range of AgNP concentrations used in this study, these results still indicate that reproduction was the most sensitive parameter in response to AgNP exposure. This finding is consistent with the study by Starnes et al.35, in which C. elegans reproduction was shown to be more sensitive to both pristine and sulfidized AgNPs than mortality and growth. Integrating the molecular mechanisms of AgNP toxicity into an AOP framework pointed to oxidative stress as the molecular initiating event or an early key event that culminated in reproductive failure as the adverse outcome36,37. This hypothesis is further explored in subsequent sections.
Toxicity assays were then conducted on C. elegans colonized by P. mendocina, E. coli, Comamonas sp., or C. piscis, using the same method employed in the germ-free nematodes above. A comparison of the reproductive toxicity of AgNPs to germ-free nematodes versus nematodes colonized by the different bacteria (Fig. 1d) showed that P. mendocina significantly (p < 0.05) mitigated the inhibitory effects of AgNPs whereas mitigation by E. coli and Comamonas sp. was negligible and C. piscis exacerbated reproductive toxicity. The EC50 values obtained for the germ-free nematodes and those colonized by P. mendocina, E. coli, Comamonas sp., and C. piscis were 9.0, 52.7, 12.5, 9.2, and 3.7 μg/m2, respectively. Given that Ag accumulation is a critical factor in AgNP toxicity, the Ag content was assessed in nematodes colonized by different bacteria but no significant variation was detected (Supplementary Fig. 3a). Moreover, no significant amount of Ag ion was detected inside the bodies of C. elegans (Supplementary Fig. 3b). This finding indicates that the observed differences in AgNP-induced reproductive toxicity across various bacterial colonization treatments are not attributable to differential effects of the bacteria on Ag accumulation or speciation in C. elegans.
To further elucidate the molecular mechanisms underlying AgNP toxicity and the effects of bacterial colonization, we conducted RNA sequencing to examine the overall gene expression in C. elegans exposed to 17.6 μg/m2 of AgNPs across the five bacterial treatments described above. As shown in Supplementary Fig. 4, we identified a total of 2447 differentially expressed genes (DEGs) in C. elegans upon AgNP exposure, with 1436 genes upregulated and 1011 genes downregulated. Gene Ontology (GO) pathway enrichment analysis of these DEGs revealed that the top 20 pathways were predominantly associated with nematode reproduction and development (Fig. 1e). The reproductive toxicity of AgNPs in C. elegans thus appears to be caused by two main factors: disruption of reproduction and development, and regulation of signal transduction. Specifically, AgNPs significantly affected reproductive pathways, including abnormal meiosis regulation (upregulation of pmk-1 and fmo-2, which interferes with oocyte maturation), reproductive gland mechanical drive defects, impaired eggshell barrier function, and perturbation of developmental programs38. Meanwhile, AgNP exposure induced significant enrichment of the mitogen-activated protein kinase (MAPK) cascade and phosphatase activity, disrupting cellular signal homeostasis39. This led to reactive oxygen species (ROS) generation, upregulation of pmk-1, activation of hif-1, inhibition of glutathione S-transferase activity, and a decline in reproductive potential. Nevertheless, when the germ-free C. elegans was colonized with P. mendocina and exposed to AgNPs, their AgNP-induced gene expression change was most efficiently reversed (Fig. 1f), which is consistent with the toxicity results above.
The mitigating effects of P. mendocina on the reproductive toxicity of AgNPs were further confirmed by quantifying germline cell populations at various developmental stages in both germ-free C. elegans and C. elegans colonized by P. mendocina, under an exposure concentration of 17.6 μg/m2 of AgNPs. A control group of germ-free nematodes without AgNP exposure was also included. As depicted in Fig. 2a, b, AgNP exposure markedly diminished both the number of total germline cells and the number of cells in different developmental stages, including mitosis (mitotic zone), leptotene/zygotene (transition zone), pachytene, and diakinesis40. These reductions were partially mitigated in nematodes colonized by P. mendocina, as both the total germline cell count and the number of cells at each developmental stage were higher than in germ-free nematodes subjected to the same AgNP concentration.
a DAPI staining illustrates germline cell proliferation and differentiation (1–5 represent germline cells during mitosis, leptotene/zygotene, pachytene, diplotene, and diakinesis, respectively) in GF C. elegans, exposed or not (Ctrl) to 17.6 μg/m2 of AgNPs and in nematodes colonized by P. mendocina (P.m.) and exposed to the same concentration of AgNPs. b Number of germline cells at key developmental stages: mitosis (mitotic zone, Ctrl vs GF: p = 1.33 × 10−3), leptotene/zygotene (transition zone, Ctrl vs GF: p = 4.40 × 10−3), pachytene (Ctrl vs GF: p = 1.59 × 10−4, P.m. vs GF: p = 4.76 × 10−2), and diakinesis (Ctrl vs GF: p = 8.90 × 10−4, P.m. vs GF: p = 1.74 × 10−3), as well as their total number (Ctrl vs GF: p = 2.74 × 10−5, P.m. vs GF: p = 4.66 × 10−3). The number of diplotene cells was small and variable and was not included in this count. c Tetramethylrhodamine ethyl ester (TMRE) staining shows the mitochondrial membrane potential in GF C. elegans exposed or not (Ctrl) to 17.6 μg/m2 of AgNPs and in nematodes colonized by active bacteria (P.m.—P. mendocina, E.c.—E. coli, Cmm.—Comamonas sp., C.p.—C. piscis) and exposed to the same concentration of AgNPs. d Relative TMRE fluorescence intensity of the different treatments in (c). Ctrl vs GF: p = 5.36 × 10−6, P.m. vs GF: p = 2.75 × 10−6. e Gene expression in GF nematodes exposed or not (Ctrl) to 17.6 μg/m2 of AgNPs and in those colonized by the four bacterial species and exposed to the same concentration of AgNPs. The genes analyzed are pivotal to the adverse outcome pathways for AgNP reproductive toxicity. bli-3: Ctrl vs GF: p = 1.82 × 10−4, P.m. vs GF: p = 7.65 × 10−5, E.c. vs GF: p = 5.12 × 10−3, Cmm. vs GF: p = 8.92 × 10−3; pmk-1: Ctrl vs GF: p = 4.98 × 10−5, P.m. vs GF: p = 1.32 × 10−3, E.c. vs GF: p = 4.91 × 10−2, Cmm. vs GF: p = 8.94 × 10−4; ndx-4: Ctrl vs GF: p = 3.33 × 10−7, P.m. vs GF: p = 8.51 × 10−5, E.c. vs GF: p = 6.89 × 10−7, Cmm. vs GF: p = 1.55 × 10−4, C.p. vs GF: p = 5.34 × 10−7; nth-1: Ctrl vs GF: p = 4.05 × 10−6, P.m. vs GF: p = 3.24 × 10−5, E.c. vs GF: p = 2.82 × 10−2, Cmm. vs GF: p = 6.59 × 10−3, C.p. vs GF: p = 5.55 × 10−3; hif-1: P.m. vs GF: p = 2.54 × 10−3; fmo-2: Ctrl vs GF: p = 8.57 × 10−8, P.m. vs GF: p = 1.50 × 10−8, E.c. vs GF: p = 5.60 × 10−9, Cmm. vs GF: p = 6.19 × 10−8, C.p. vs GF: p = 5.94 × 10−6; ced-13: Cmm. vs GF: p = 3.73 × 10−3. One-way ANOVA was performed in (b–e) with two-sided comparisons, and post hoc comparisons were corrected using Dunnett’s method. *p < 0.05, **p < 0.01. Bar chart data represent the mean ± standard deviation (n = 3 independent experiments).
According to previous reports36,37 and supported by our transcriptomic data above, the generation of ROS, triggered by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activation, is identified as the molecular initiating event in the AOPs associated with AgNP-induced reproductive toxicity in C. elegans. ROS can then induce reproductive failure via three distinct pathways: 1) ROS generation leading to mitochondrial damage and germline cell apoptosis; 2) ROS-induced activation of the p38 MAPK ortholog PMK1 and of the transcription factor hypoxia-inducible factor (HIF-1), resulting in germline cell apoptosis; and 3) ROS-mediated PMK1/P38 MAPK activation, resulting in DNA damage and germline cell apoptosis. Given the central role of mitochondrial damage in the first pathway, the impact of AgNP exposure on the mitochondrial membrane potential of C. elegans colonized by the different bacterial species was investigated. As shown in Fig. 2c, the mitochondrial membranes of unexposed germ-free nematodes were highly polarized, evidenced by the intense red fluorescence. Exposure to AgNPs at a concentration of 17.6 μg/m2 diminished this fluorescence, signifying reduced membrane polarization and thus mitochondrial dysfunction41. In C. elegans colonized by active bacteria, particularly P. mendocina, fluorescence intensity was restored, suggesting the recovery of mitochondrial function. This effect became even more apparent by quantifying the relative red fluorescence intensity (Fig. 2d), which yielded comparable values for AgNP-exposed nematodes colonized by P. mendocina and unexposed germ-free nematodes.
We also assessed the expression profiles of genes central to the aforementioned AOP pathways, including bli-3 (which encodes a conserved NADPH oxidase responsible for ROS generation)42, the antioxidant gene sod-3, pmk-1 (encoding PMK-1), and hif-1 (encoding the transcription factor HIF-1) and its downstream target gene fmo-243,44,45. Genes implicated in apoptosis in response to DNA damage, such as ced-13, and those involved in DNA repair, such as ndx-4 and nth-1, were investigated as well46,47. As illustrated in Fig. 2e, the expression of most of these genes was significantly higher in germ-free nematodes exposed to 17.6 μg/m2 of AgNPs than in unexposed nematodes. These findings corroborate previous studies linking AgNP exposure to reproductive failure through the three pathways mentioned above36,37. Moreover, our results showed that, in nematodes colonized by active bacteria and exposed to AgNPs, the expression of genes upregulated in response to AgNPs was substantially reduced. Specifically, among the nematodes colonized by Comamonas sp., E. coli, P. mendocina, or C. piscis, those colonized by P. mendocina most effectively reversed the upregulation of the eight genes induced by AgNPs (Supplementary Table 1), as quantitatively assessed by the linear mixed effects model. The overall gene expression in this treatment was even comparable to that in the control treatment without AgNP exposure. These findings align with the mitigating effects of P. mendocina on AgNP-induced reproductive toxicity, suggesting that this bacterium is able to mitigate the adverse outcomes of AgNP exposure in C. elegans, by suppressing the molecular initiating event (ROS generation) and subsequent key events (PMK-1/MAPK activation, HIF-1 activation, apoptosis, DNA damage, and mitochondrial dysfunction). Nevertheless, future research should aim to quantitatively dissect the contributions of the three AOP pathways to the mitigating effects of P. mendocina.
Metabolites responsible for the mitigating effects of P. mendocina
To explore the chemical substances responsible for the protective effects of P. mendocina, a metabolomic analysis of C. elegans, both germ-free and colonized by the tested bacterial species, was conducted after exposure to 17.6 μg/m2 of AgNPs. In total, 178 metabolites were identified through database matching and iterative processing with MetDNA2. A comparative analysis between germ-free and bacterially colonized nematodes revealed a subset of DEMs with significantly different peak areas on mass spectrometry (MS) (Supplementary Data 1). The DEMs whose MS peak areas correlated significantly with at least one toxicity endpoint in nematodes treated with 17.6 μg/m2 of AgNPs are listed in Fig. 3a. According to the correlation analysis, reproduction was the endpoint most strongly associated with the majority of these DEMs. Subsequently, metabolic pathways were enriched from the DEMs that correlated significantly with reproduction across the four bacterial species. The number of enriched pathways from these DEMs was highest in P. mendocina (Fig. 3b), further substantiating the pronounced mitigating effect of this bacterium on AgNP-induced reproductive toxicity. Many of the enriched pathways, particularly those with a higher pathway impact, have been implicated in reproductive processes, either directly or indirectly. For instance, phenylalanine and its metabolite tyrosine play roles in alleviating oxidative stress and modulating reproduction48,49,50. Tryptophan, when metabolized to 3-hydroxyanthranilic acid, enhances resistance to oxidative stress and activates the Nrf2/SKN-1 antioxidant response in C. elegans51. Additionally, histidine has been shown to stimulate the transcriptional activity of HIF-152.
a Correlation between the four toxicity endpoints (survival rate, head thrash, body length, and reproduction) in nematodes colonized by different bacteria and exposed to AgNPs (17.6 μg/m2) and the MS peak areas of the DEMs in those nematodes versus those of GF nematodes exposed to the same concentration of AgNPs. Metabolites correlating significantly with at least one toxicity endpoint are shown; the endpoint most strongly associated with the majority of these DEMs was reproduction. b Enrichment of the metabolic pathways derived from the metabolites in (a) that correlated significantly with reproductive toxicity for each bacterial species (P.m.—P. mendocina, E.c.—E. coli, Cmm.—Comamonas sp., C.p.—C. piscis). The hypergeometric test was used in (b), which defaults to one-sided testing and employs the Bonferroni correction for multiple comparisons. *p < 0.05, **p < 0.01.
While P. mendocina was clearly capable of colonizing the gut of C. elegans, whether its mitigating effect was attributable to its metabolic activity or because it simply acted as a supplementary nutritional source was unclear27,53. This question was addressed by conducting a toxicity assay with C. elegans colonized by either metabolically active or inactive P. mendocina and then exposed for 96 h to 17.6 μg/m2 of AgNPs. Supplementary Fig. 5 shows that both active and inactive P. mendocina significantly mitigated the reproductive toxicity induced by AgNPs. However, more pronounced mitigating effects were obtained with active than inactive P. mendocina. This distinction suggests that the attenuation of AgNP toxicity is influenced not only by the nutritional contribution of P. mendocina but also by its metabolic activities associated with gut colonization.
Candidate bacterial endogenous metabolites involved in mitigating the AgNP-induced reproductive toxicity were identified in an HPLC-MS/MS analysis of the metabolomes of the four bacterial species, which, using MetDNA2, yielded 246 metabolites (Supplementary Data 1). A correlation analysis between the reproduction of C. elegans exposed to 17.6 μg/m2 of AgNPs and the MS peak area of each metabolite from different species of bacteria led to the identification of six metabolites with a significantly positive correlation with reproduction: MTE, ThMP, 4-pyridoxic acid, spermidine, thiamine, and cysteinylglycine (Fig. 4a). To establish a causal link between these bacterial metabolites and the change in C. elegans reproduction, toxicity assays were conducted with germ-free nematodes exposed to 17.6 μg/m2 of AgNPs and commercially available standard compounds of the six metabolites, tested at a concentration range (0–17.6 μg/m2) corresponding to their endogenous concentrations in bacteria. While all six metabolites correlated significantly with reproduction, only the supplementation of MTE and ThMP effectively mitigated the reproductive impairment caused by AgNP exposure (Fig. 4b).
a Spearman correlation analysis revealed significant associations between the reproduction of C. elegans colonized with different bacteria and exposed to 17.6 μg/m2 of AgNPs in toxicity tests and the MS peak areas of six metabolites (MTE—4-methyl-5-thiazoleethanol, ThMP—thiamine monophosphate, 4-PA—4-pyridoxic acid, spermidine, thiamine, and Cys-Gly—cysteinylglycine) from four bacterial species. b Reproduction of GF nematodes exposed to 17.6 μg/m2 of AgNPs in the presence of the six metabolites in (a) at concentrations of 0, 0.176, 1.76, and 17.6 μg/m2. MTE: 1.76 vs 0: p = 1.99 × 10−4; ThMP: 17.6 vs 0: p = 3.54 × 10−3; Cys-Gly: 17.6 vs 0: p = 9.85 × 10−4. c Gene expression in GF nematodes exposed to 17.6 μg/m2 of AgNPs and supplemented or not with 1.76 μg/m2 of MTE, 17.6 μg/m2 of ThMP, or 17.6 μg/m2 of thiamine. A control group (Ctrl), untreated with AgNPs, MTE, ThMP, or thiamine was also included. The genes analyzed are pivotal to the adverse outcome pathways mediating AgNP reproductive toxicity. bli-3: Ctrl vs GF: p = 2.03 × 10−2; pmk-1: Ctrl vs GF: p = 1.08 × 10−6, MTE vs GF: p = 1.39 × 10−4, ThMP vs GF: p = 3.89 × 10−2; ndx-4: Ctrl vs GF: p = 5.83 × 10−6; nth-1: Ctrl vs GF: p = 3.82 × 10−4, ThMP vs GF: p = 2.44 × 10−2; hif-1: ThMP vs GF: p = 1.91 × 10−10, Thiamine vs GF: p = 2.64 × 10−3; fmo-2: Ctrl vs GF: p = 2.0 × 10−5, ThMP vs GF: p = 3.59 × 10−3; Thiamine vs GF: p = 1.14 × 10−3; ced-13: Ctrl vs GF: p = 1.61 × 10−2, MTE vs GF: p = 2.47 × 10−2; sod-3: Thiamine vs GF: p = 5.05 × 10−3. d Metabolic pathway enrichment in GF nematodes exposed to 17.6 μg/m2 of AgNPs in the presence of 1.76 μg/m2 of MTE, 17.6 μg/m2 of ThMP, or 17.6 μg/m2 of thiamine. For comparison, the pathways enriched in AgNP-exposed nematodes colonized by P. mendocina (P.m.) are also presented. One-way ANOVA was performed in (b, c) with two-sided comparisons, and post hoc comparisons were corrected using Dunnett’s method. The hypergeometric test was used in (d), which defaults to one-sided testing and employs the Bonferroni correction for multiple comparisons. *p < 0.05, **p < 0.01. Data in (b, c) are presented as the mean ± standard deviation (n = 3 independent experiments).
Both MTE and ThMP are metabolically linked to thiamine (vitamin B1). Lau et al.54, showed that bacterial thiamine can be degraded to MTE, which, using phosphate from ATP, then forms 4-methyl-5-(2-phosphonooxyethyl)thiazole. The latter compound combines with 4-amino-5-hydroxymethyl-2-methylpyrimidine pyrophosphate to produce ThMP, which upon further phosphorylation yields thiamine pyrophosphate (ThDP)55, a cofactor essential for numerous cellular enzymes (e.g., pyruvate dehydrogenase, α-ketoglutarate dehydrogenase complex, transketolase, and branched chain α-ketoacid dehydrogenase complex)56. Thiamine can also be phosphorylated to ThDP, which can be dephosphorylated back to ThMP and eventually to thiamine56. Despite the metabolic interconversion among MTE, ThMP, ThDP, and thiamine, no direct metabolic functions have been reported for MTE, ThMP, and thiamine; ThDP is the only active form with previously known biological functions in various biochemical pathways (e.g., citric acid cycle, pentose phosphate pathway, and glycolysis)57. Nevertheless, neither thiamine nor ThDP exhibited significant effects on AgNP-induced reproductive toxicity (Fig. 4b and Supplementary Fig. 6). This observation suggests that MTE and ThMP have direct metabolic roles in mitigating AgNP toxicity.
The roles of MTE and ThMP within the AOPs of AgNP toxicity identified in this study were further investigated by examining the expression patterns of genes implicated in the above-described molecular initiating events and key events in the presence and absence of these two metabolites. As seen in Fig. 4c, the AgNP-induced upregulation of pmk-1 and hif-1 was substantially mitigated by the presence of MTE and ThMP. Additionally, MTE mitigated the AgNP-associated upregulation of bli-3 and ced-13, and ThMP reduced the AgNP-associated upregulation of ndx-4 and fmo-2. In contrast, thiamine had much less mitigating effects on the upregulated expression of these genes (Supplementary Table 2). These findings suggest that the mitigating effects of MTE and ThMP on AgNP-induced reproductive toxicity are exerted through both shared and distinct mechanisms. That all eight genes were significantly downregulated in the presence of P. mendocina suggests that mitigation by the bacterium reflected the combined action of MTE, ThMP, and potentially other, as-yet-unidentified bacterial metabolites or biomolecules.
The metabolic profiles of germ-free C. elegans exposed to 17.6 μg/m2 of AgNPs in the absence and presence of MTE or ThMP were also assessed. The addition of MTE and ThMP led to the identification of 35 and 39 DEMs, respectively. The metabolic pathways enriched by these metabolites are depicted in Fig. 4d. The pathways enriched by MTE and ThMP overlapped substantially with those of AgNP-exposed nematodes colonized by P. mendocina, although the number of enriched pathways was higher in the latter treatment. In contrast, much less pathways were enriched by thiamine and most of these pathways had a p-value close to 0.05, further confirming the negligible effects of thiamine on the reproductive toxicity of AgNPs. These findings verify our hypothesis that the mitigating effects of P. mendocina on AgNP-induced reproductive toxicity are only partially attributable to the production of MTE and ThMP; rather, additional unidentified metabolites or other biomolecules contributed by the bacterium also likely play an important role. Future research should also elucidate the detailed molecular mechanisms underlying the mitigating effects of MTE and ThMP on AgNP-induced reproductive toxicity, including the potential involvement of receptors such as TLR2 and AhR19,58.
In addition to germ-free nematodes, we cultured nematodes with forest soil extracts, allowing colonization of their gut by a natural bacterial community. Upon exposure of these wild-type nematodes to 17.6 μg/m² of AgNPs, reproductive toxicity was observed, which was significantly mitigated by further colonization of P. mendocina in their gut (Supplementary Fig. 7). Moreover, the reproductive capacity of C. elegans was partially restored with the supplementation of MTE and ThMP. These results indicate that the mitigating effects of P. mendocina and its metabolites (i.e., MTE and ThMP) apply not only to germ-free nematodes but also to wild-type nematodes colonized with natural microbial communities, highlighting their potential for broader applications in mitigating AgNP-induced reproductive toxicity.
In summary, the present study provides compelling evidence that components of the GM, and specifically P. mendocina, significantly mitigate the reproductive toxicity of AgNPs in the model organism C. elegans, by suppressing both the molecular initiating event (ROS generation) and subsequent key events (PMK-1/MAPK activation, HIF-1 activation, apoptosis, DNA damage, and mitochondrial dysfunction) in the AOPs of AgNPs. Further analysis of the bacterial metabolites revealed two thiamine-derived metabolites MTE and ThMP, with no previously known biological effects, as crucial to this protective mechanism. These findings have profound implications for environmental health management, as they suggest that modulation of the GM is a viable strategy to reduce the adverse effects of nanoparticle exposure. Moreover, the identification of specific metabolites may contribute to the development of targeted therapies aimed at counteracting nanoparticle toxicity. Given the varying modes of action of different nanoparticles in distinct organisms, additional research is required to identify a broader range of GM in various hosts that are able to mitigate the potential hazards associated with nanoparticle exposure.
Methods
Physicochemical characterization of the AgNPs
The AgNPs employed in this study were purchased from XFNANO, Inc. (Nanjing, China) and surface-coated with polyvinylpyrrolidone to enhance their aqueous dispersibility. Prior to each toxicity assay, the AgNPs were washed a minimum of six times using a regenerated cellulose filter membrane with a 3 kDa molecular mass cut-off (Millipore, Merck KGaA, Darmstadt, Germany) to eliminate any soluble impurities present in the suspension. The concentration of AgNPs was determined by inductively coupled plasma mass spectrometry (ICP-MS, NexION 300, PerkinElmer, MA, USA) following digestion of the particles with concentrated HNO3 and H2O259. The morphology of the AgNPs was visualized by TEM (JEM-200CX, JEOL, Tokyo, Japan). Their hydrodynamic diameter and zeta potential were determined by DLS (ZetaPALS, Brookhaven Instruments, NY, USA).
Nematode and bacterial culture
C. elegans Bristol N2 was obtained from the Caenorhabditis Genetics Center (CGC, Minneapolis, MN, USA) and maintained at 20 °C in Nematode Growth Media (NGM) agar plates seeded with a lawn of E. coli OP50. Germ-free eggs were obtained using a 10 mL lysis solution containing 0.5 M of NaOH and 1% (v/v) of NaClO. The eggs were washed three times with sterile M9 medium and then incubated overnight on 6-cm-diameter unseeded NGM agar plates at 20 °C. The nematodes thus hatched were at the L1 stage.
The bacterial strains P. mendocina MSPm1, E. coli OP50, Comamonas sp. DA1877, and C. piscis BIGb0172 were purchased from the CGC and cultivated in Luria broth (LB) medium. Specifically, E. coli and Comamonas sp. were incubated in a shaking incubator at 37 °C set at 120 rpm; P. mendocina and C. piscis were incubated at 20 °C under the same shaking conditions. Bacterial growth was monitored by measuring the absorbance at 600 nm using a UV-visible spectrophotometer (Shimadzu UV-1800, Kyoto, Japan). The bacteria were examined morphologically using a dark-field microscope (Cytoviva, Inc., Auburn, AL, USA). Their biomass was quantified based on the bacterial carbon content, measured using a total organic carbon analyzer (Elemental Instruments Vario TOC, Frankfurt, Germany).
Bacterial colonization
E. coli OP50 inactivated by 0.5% (w/v) paraformaldehyde was used as the dietary base for C. elegans. Preliminary experiments indicated that neither the growth nor the reproduction of C. elegans was affected by the inactive E. coli, based on a comparison with their live counterpart. Active forms of the four species of bacteria (P. mendocina, E. coli, Comamonas sp., and C. piscis) were mixed with inactive E. coli at a carbon mass ratio of 1:9. Suspensions of the four types of bacterial mixture were then seeded onto the NGM agar plates in triplicate, followed by the transfer of 20 L1-stage C. elegans individuals to each plate. After 36, 48, 72, and 96 h, C. elegans from each replicate was collected, washed three times with sterile M9, and homogenized using a homogenizer (Bioprep-24, Hangzhou Allsheng Instrument Co., Ltd., China) at 2430 rpm. The homogenization was conducted in four cycles, each lasting 15 s, with a 15 s interval between each cycle, in M9 on ice. The resulting homogenates were serially diluted in M9 and spread onto a series of LB agar plates. After a 48-h incubation, the number of bacterial colonies on each plate was enumerated, and the average bacterial load per C. elegans individual was calculated.
AgNP toxicity tests
Toxicity tests were performed using germ-free C. elegans (fed exclusively with inactive E. coli) and C. elegans colonized by P. mendocina, E. coli, Comamonas sp., and C. piscis. Each test comprised seven exposure concentrations, with one of the five bacterial diets (either 100% inactive E. coli or a 9:1 mix of inactive E. coli with the active form of the respective bacterial species) combined with AgNPs at concentrations of 0, 0.1, 0.3, 1, 3, 10, or 65 mg/L. The bacterial diets mixed with the varying AgNP concentrations were then applied to peptone-free NGM agar plates in six replicates, resulting in final AgNP concentrations on the plates of 0, 1.76, 5.3, 17.6, 53, 176, or 1144 μg/m2. Approximately 20 L1-stage nematodes were then introduced onto each plate. After a 96 h exposure, the survival and progeny production of C. elegans from three out of the six replicates were assessed. Head thrash and body length in these replicates were measured using the methodologies outlined in Yang et al60. To generate dose-response curves, a four-parameter logistic model was fitted to the toxicity data. For the purpose of plotting the concentration data on a logarithmic scale, a nominal AgNP concentration of 0.01 μg/m² was assigned to the control treatment without any exposure of AgNPs. The nematodes in the other three replicates were collected, depurated on clean NGM agar plates without AgNPs, rinsed three times with deionized water, and then digested in concentrated HNO3 and H2O259. The Ag concentration in the digest was measured by ICP-MS, based on which the accumulation of AgNPs in C. elegans was calculated.
To elucidate the potential impact of the bacterial metabolites MTE, ThMP, 4-pyridoxic acid, spermidine, thiamine, cysteinylglycine, and ThDP on C. elegans reproduction, approximately 20 germ-free L1-stage nematodes were exposed in triplicate to 17.6 μg/m2 of AgNPs in the presence of these metabolites at concentrations of 0, 0.176, 1.76, or 17.6 μg/m2 for 96 h. Selecting this concentration of AgNPs (17.6 μg/m2) ensures that any mitigating effects of the metabolites on AgNP-induced reproductive toxicity would be clearly observable. Subsequently, the number of progenies was counted for each replicate. Whether the mitigating effects of P. mendocina on AgNP-induced reproductive toxicity were solely dependent on the metabolic activity of this bacterium was assessed by subjecting approximately 20 L1-stage nematodes in triplicate to 17.6 μg/m2 of AgNPs in the presence of inactive E. coli (90%) supplemented with either active or inactive P. mendocina (10%). After a 96-h exposure, the number of offspring from each replicate was recorded.
To assess the capacity of P. mendocina and its metabolites to mitigate the reproductive toxicity of AgNPs under real-world conditions, wild-type C. elegans with its gut colonized by natural microbiota was prepared. Briefly, forest soil rich in natural organic matter was collected near our university. The soil was mechanically sieved through a 2 mm mesh, and 10 g of the pre-treated soil was placed in a 250 mL Erlenmeyer flask with 90 mL of sterile phosphate-buffered saline. The mixture was shaken at 300 rpm for 5 min and then vacuum filtered through a 220 μm nylon filter membrane. The resulting filtrate, termed the forest soil extract, was spread onto NGM agar plates and C. elegans cultured on these plates was designated as wild-type nematodes. The wild-type C. elegans was then exposed to 0 or 17.6 μg/m2 of AgNPs for 96 h, and their gut microbiota was analyzed using 16S rRNA sequencing2. Additionally, the wild-type C. elegans was exposed to 17.6 μg/m2 of AgNPs in the presence of either 100% inactive E. coli, a mixture of 90% inactive E. coli and 10% active P. mendocina, or 100% inactive E. coli supplemented with 1.76 μg/m2 of MTE, 17.6 μg/m2 of ThMP, 17.6 μg/m2 of thiamine, or 17.6 μg/m2 of ThDP for 96 h. A control treatment with 100% inactive E. coli but without any exposure of AgNPs was also included. The number of progeny was then counted for each replicate.
Transcriptomic analysis
Approximately 2000 nematodes in triplicate were exposed to 17.6 μg/m2 of AgNPs in the presence of five different bacterial diets mentioned above. A control treatment, unexposed to AgNPs and fed exclusively on inactive E. coli, was also included. To reduce offspring-related interference, the exposure duration was shortened from 96 h (full adulthood) to 55 h (young adulthood), thus prior to the onset of the reproductive phase. Otherwise, the procedure was the same as that of the aforementioned toxicity tests. Total RNA was extracted from each replicate and purified using a DNA-free™ DNA removal kit (Thermo Fisher Scientific, USA). Eighteen cDNA libraries were constructed from the mRNA isolated from the six treatments and sequenced on the Illumina HiSeq 4000 platform. Gene expression levels were quantified using the transcripts per kilobase million method. DEGs were identified using a threshold of >2- or <0.5-fold change and a false discovery rate adjusted p-value of <0.05. GO analysis was then performed to elucidate the mechanisms underlying AgNP toxicity in C. elegans.
Fluorescence microscopy
The concentration of Ag ions released from AgNPs accumulated within the nematodes was measured using a method based on aggregation-induced emission61. For this purpose, approximately 20 nematodes per replicate were exposed to 1144 μg/m2 of AgNPs in the presence of either 100% inactive E. coli or a mixture of 90% inactive E. coli and 10% active P. mendocina, E. coli, Comamonas sp., or C. piscis. A control treatment using germ-free nematodes without AgNP exposure was also included. After 96 h of exposure, the nematodes were collected and depurated using the same procedure as that employed for the determination of total AgNP accumulation in the toxicity tests above. Subsequently, the nematodes were incubated in M9 containing 5 μM of the fluorogenic Ag ion probe tetrazole-functionalized tetraphenylethylene derivative 1 (TEZ-YPE-1). After 30 min, the nematodes were thoroughly washed with fresh M9 and imaged using fluorescence microscopy (Nikon Eclipse Ti2, Tokyo, Japan) with an excitation wavelength of 405 nm and an emission wavelength range of 450–550 nm. To convert the fluorescence signal into Ag mass, approximately 40 germ-free nematodes per replicate were exposed to 0, 0.1, 1, and 10 mg/L of AgNO3 for 96 h. The concentration of Ag ions in the nematodes was then determined using both ICP-MS and TEZ-YPE-1. The limit of quantification for this method was ~0.005 ng/cm of Ag ion in C. elegans.
The impact of AgNPs on gonadal development and thus on reproduction was assessed by quantifying the number of germline cells in C. elegans. Approximately 20 nematodes in triplicate were exposed to 17.6 μg/m2 of AgNPs in the presence of either 100% inactive E. coli or a mixture of 90% inactive E. coli and 10% active P. mendocina for 55 h. A control treatment consisting of C. elegans unexposed to AgNPs and fed exclusively on inactive E. coli was also included. At the end of the incubation, nematodes from each treatment were washed three times with 90% v/v ethanol and stained for 30 min with 20 μL of 4’,6-diamidino-2-phenylindole (Beyotime, Shanghai, China). Excess dye was removed and the stained nematodes were imaged using an inverted confocal laser scanning fluorescence microscope (Zeiss LSM 880, Oberkochen, Germany). The number of germline cells at various developmental stages was determined from these images.
Mitochondrial membrane potential in C. elegans was determined using the tetramethylrhodamine ethyl ester (TMRE) staining method62. For this purpose, approximately 20 nematodes in triplicate were subjected for 55 h to 17.6 μg/m2 of AgNPs and each of the five bacterial diets. A control group maintained on inactive E. coli and not exposed to AgNPs was also included. Thereafter, 15 nematodes from each treatment (five per replicate) were placed in K buffer (2.36 g/L of KCl and 3 g/L of NaCl, pH 6.0) supplemented with 30 μM TMRE, incubated for 2 h at 20 °C, and then fixed with 4% (w/v) formaldehyde. Images were obtained using fluorescence microscopy (Zeiss Axio Imager, Oberkochen, Germany), with an excitation wavelength of 550 nm and an emission wavelength of 575 nm.
qPCR assay
Approximately 2000 nematodes in triplicate were exposed for 55 h to 17.6 μg/m2 of AgNPs in the presence of five different bacterial diets. A control group unexposed to AgNPs and fed exclusively on inactive E. coli was also included. Total RNA was then isolated from the nematodes in each replicate using Trizol reagent (Invitrogen, Thermo Fisher Scientific, MA, USA). Complementary DNA was synthesized from 1 μg of total RNA, devoid of genomic DNA, using the PrimeScript qPCR kit (Takara Bio, Inc., Tokyo, Japan). qPCR was conducted for selected genes (bli-3, ced-13, fmo-2, hif-1, ndx-4, nth-1, pmk-1, and sod-3) using SYBR Premix and a CFX96TM real-time detection system, with cdc-42 serving as the endogenous control. Relative gene expression levels were determined using the 2-ΔΔCT method36. Primers were designed using the online primer BLAST tool (www.ncbi.nlm.nih.gov); the sequences are listed in Supplementary Table 3. To elucidate the effects of MTE, ThMP, and thiamine on gene expression, an additional ~2000 nematodes in triplicate were exposed for 55 h to 17.6 μg/m2 of AgNPs and completely inactive E. coli with or without the addition of 1.76 μg/m2 of MTE, 17.6 μg/m2 of ThMP, or 17.6 μg/m2 of thiamine. The subsequent procedures followed those used in the qPCR experiment described above.
To quantitatively assess the impact of different bacteria and metabolites on gene expression, we fitted a linear mixed effects model in R (4.2.2) to log2-transformed expression values, using log2(value) ~ group × gene + (1 | replicate), with a random intercept for biological replicates. We employed Kenward–Roger degrees of freedom and conducted pairwise contrasts using P. mendocina and thiamine as controls. Dunnett-type adjustments were applied to control the family-wise error rate, with 5 comparisons for P. mendocina and 4 comparisons for thiamine.
Metabolomic analysis
Approximately 2000 nematodes for each of six replicates were exposed to 17.6 μg/m² of AgNPs and fed either one of the five bacterial diets or an entirely inactive E. coli diet supplemented with 1.76 μg/m² of MTE, 17.6 μg/m² of ThMP, or 17.6 μg/m² of thiamine. After 55 h, the resultant nematodes from each replicate were harvested, rinsed at least three times with cold M9, flash-frozen in liquid nitrogen to halt metabolic activity, and freeze-dried. Post-weighing, the freeze-dried nematodes were combined with 12.5 μL of an internal standard (Supplementary Table 4) and ice-cold methanol (80% v/v, 400 μL), then sonicated in an ice bath for 2 min. Thereafter, 1.0 mL of methyl tertiary-butyl ether was added and the mixture was agitated at 120 rpm for 1 h. Phase separation was achieved by the addition of 250 μL of water, followed by a 10-min incubation at 4 °C. The sample was then centrifuged (14,000 g) for 15 min at 4 °C and 300 µL of the interphase was transferred to an HPLC vial, evaporated under a stream of nitrogen to near dryness, and reconstituted with 150 µL of methanol. In parallel, metabolites within the four bacterial species were quantified. For this purpose, approximately 109 cells of P. mendocina, E. coli, Comamonas sp., and C. piscis were pelleted by centrifugation at 8800 g for 8 min, washed extensively with sterile deionized water, subjected to seven cycles of freezing and thawing in liquid nitrogen, and freeze-dried. Metabolite extraction was performed using the same protocol employed in C. elegans. Full-process blank samples were included to eliminate potential contamination from the medium or the sample processing steps.
The extracted metabolites were analyzed by ultra-performance liquid chromatography coupled to electrospray ionization quadrupole time-of-flight mass spectrometry (UPLC-QTOF, X500R, SCIEX, USA), following the procedure used in a previous study by our group2. MS-DIAL version 4.70 was used to extract ion peaks, with a mass tolerance of 0.002 Da for MS and 0.01 Da for MS/MS. Sample peak alignment was conducted with a retention time tolerance of 0.4 min and a mass tolerance of 0.015 Da. Peaks consistently detected in >80% of the samples were considered as candidate metabolites. Those metabolites were identified using the MS-DIAL databases and through iterative processing with MetDNA2. DEMs were identified as those present in C. elegans colonized by different bacteria and with MS peak areas significantly different from those in germ-free nematodes under the same AgNP exposure concentration. Potential correlations between the four toxicity endpoints (survival rate, head thrash, body length, reproduction) and the MS peak areas of the DEMs in C. elegans colonized by the same species of bacterium and exposed to the same concentration of AgNPs (17.6 μg/m2) were assessed in a Spearman rank correlation analysis. A similar analysis was conducted to explore the correlations between the reproduction of C. elegans colonized by different bacteria and exposed to 17.6 μg/m2 of AgNPs in the toxicity tests above and the MS peak areas of the endogenous metabolites associated with each bacterium. Subsequently, metabolic pathways were enriched from the DEMs that correlated significantly (p < 0.01) with reproduction across different bacterial species, using MetaboAnalyst (6.0). The Pathway Impact value, which represents the overall importance and influence of a metabolic pathway in a biological process, was calculated by multiplying the Pathway Topology Score (reflecting the importance of metabolites within the pathway based on their positions and connections in the pathway network) and the Differential Metabolites Score (indicating the extent of changes in metabolite levels within the pathway). Pathway enrichment analysis was also conducted on the DEMs resulting from treatment with 1.76 μg/m2 of MTE, 17.6 μg/m2 of ThMP, or 17.6 μg/m2 of thiamine and based on comparisons with germ-free C. elegans without these metabolites.
Statistical analysis
Significant differences (p < 0.05) were determined using one- or two-way analysis of variance (ANOVA) with post-hoc multiple comparisons (Tukey or Tamhane) or Student’s t-test (IBM SPSS Statistics 22.0, NY, USA). The normality (Kolmogorov-Smirnov and Shapiro-Wilk tests) and homogeneity of variance (Levene’s test) of the data were examined during the ANOVAs. Spearman rank correlation analysis was performed using SPSS Statistics 22.0 and the results were plotted using R (4.2.2).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data supporting the findings of this study are available within this manuscript and its Supplementary Information files. All other relevant data are available from the corresponding authors upon reasonable request. Source data for Figs. 1–4 and Supplementary Figs. 1–7 are available in the associated source data file. RNA sequencing data generated in this study have been deposited in the NCBI Sequence Read Archive under BioProject ID: PRJNA1274181. Source data are provided with this paper.
References
Tortella, G. R. et al. Silver nanoparticles: Toxicity in model organisms as an overview of its hazard for human health and the environment. J. Hazard. Mater. 390, 121974 (2020).
Wang, X. L. et al. Changes in gut microbiota structure: A potential pathway for silver nanoparticles to affect the host metabolism. ACS Nano 16, 19002–19012 (2022).
Zhu, D. et al. Exposure of a soil collembolan to Ag nanoparticles and AgNO(3) disturbs its associated microbiota and lowers the incidence of antibiotic resistance genes in the gut. Environ. Sci. Technol. 52, 12748–12756 (2018).
Islam, M. A., Jacob, M. V. & Antunes, E. A critical review on silver nanoparticles: From synthesis and applications to its mitigation through low-cost adsorption by biochar. J. Environ. Manag. 281, 111918 (2021).
Fabrega, J., Luoma, S. N., Tyler, C. R., Galloway, T. S. & Lead, J. R. Silver nanoparticles: Behaviour and effects in the aquatic environment. Environ. Int. 37, 517–531 (2011).
Ahamed, M., Alsalhi, M. S. & Siddiqui, M. K. Silver nanoparticle applications and human health. Clin. Chim. Acta 411, 1841–1848 (2010).
Xu, L. et al. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 10, 8996–9031 (2020).
McShan, D., Ray, P. C. & Yu, H. Molecular toxicity mechanism of nanosilver. J. Food Drug Anal. 22, 116–127 (2014).
Lee, W. J. & Hase, K. Gut microbiota-generated metabolites in animal health and disease. Nat. Chem. Biol. 10, 416–424 (2014).
Jin, Y., Wu, S., Zeng, Z. & Fu, Z. Effects of environmental pollutants on gut microbiota. Environ. Pollut. 222, 1–9 (2017).
Yang, W. et al. The inducible response of the nematode Caenorhabditis elegans to members of its natural microbiota across development and adult life. Front. Microbiol. 10, 1793 (2019).
McCarville, J. L., Chen, G. Y., Cuevas, V. D., Troha, K. & Ayres, J. S. Microbiota metabolites in health and disease. Annu. Rev. Immunol. 38, 147–170 (2020).
Ma, Y. et al. Effect of nanomaterials on gut microbiota. Toxics 11, 384 (2023).
Utembe, W., Tlotleng, N. & Kamng’ona, A. W. A systematic review on the effects of nanomaterials on gut microbiota. Curr. Res. Microb. Sci. 3, 100118 (2022).
Li, J., Tang, M. & Xue, Y. Review of the effects of silver nanoparticle exposure on gut bacteria. J. Appl. Toxicol. 39, 27–37 (2018).
Javurek, A. B. et al. Gut dysbiosis and neurobehavioral alterations in rats exposed to silver nanoparticles. Sci. Rep. 7, 2822 (2017).
Ma, Y. et al. Sex dependent effects of silver nanoparticles on the zebrafish gut microbiota. Environ. Sci. Nano 5, 740–751 (2018).
Brinkmann, B. W., Koch, B. E. V., Spaink, H. P., Peijnenburg, W. J. G. M. & Vijver, M. G. Colonizing microbiota protect zebrafish larvae against silver nanoparticle toxicity. Nanotoxicology 14, 725–739 (2020).
Brinkmann, B. W., Koch, B. E. V., Peijnenburg, W. J. G. M. & Vijver, M. G. Microbiota-dependent TLR2 signaling reduces silver nanoparticle toxicity to zebrafish larvae. Ecotoxicol. Environ. Saf. 237, 113522 (2022).
Zhang, F. et al. Natural genetic variation drives microbiome selection in the Caenorhabditis elegans gut. Curr. Biol. 31, 2603–2618 (2021).
Kim, D. H. & Flavell, S. W. Host-microbe interactions and the behavior of Caenorhabditis elegans. J. Neurogenet. 34, 500–509 (2020).
Dirksen, P. et al. CeMbio – The Caenorhabditis elegans microbiome resource. G3 Genes Genom. Genet. 10, 3025–3039 (2020).
Berg, M. et al. Assembly of the Caenorhabditis elegans gut microbiota from diverse soil microbial environments. ISME J. 10, 1998–2009 (2016).
Onugwu, A. L. et al. Nanotechnology based drug delivery systems for the treatment of anterior segment eye diseases. J. Control. Release 354, 465–488 (2023).
Heurtault, B., Saulnier, P., Pech, B., Proust, J. E. & Benoit, J. P. Physico-chemical stability of colloidal lipid particles. Biomaterials 24, 4283–4300 (2003).
Badawy, A. M. E. et al. Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environ. Sci. Technol. 44, 1260–1266 (2010).
Clark, L. C. & Hodgkin, J. Commensals, probiotics and pathogens in the Caenorhabditis elegans model. Cell Microbiol. 16, 27–38 (2014).
Slowinski, S. et al. Interactions with a complex microbiota mediate a trade-off between the host development rate and heat stress resistance. Microorganisms 8, 1781 (2020).
Lo, W. S., Han, Z., Witte, H., Roseler, W. & Sommer, R. J. Synergistic interaction of gut microbiota enhances the growth of nematode through neuroendocrine signaling. Curr. Biol. 32, 2037–2050 (2022).
Maynard, C. & Weinkove, D. Bacteria increase host micronutrient availability: Mechanisms revealed by studies in C. elegans. Genes Nutr. 15, 4 (2020).
Gomez, F. et al. Delayed accumulation of intestinal coliform bacteria enhances life span and stress resistance in Caenorhabditis elegans fed respiratory deficient E. coli. BMC Microbiol. 12, 300 (2012).
Ambat, A. et al. A Caenorhabditis elegans based system for high-throughput functional phenotyping of human gut microbiota. bioRxiv https://doi.org/10.1101/2024.02.27.582212 (2024).
Chai, V. Z. et al. Chemical basis of microbiome preference in the nematode C. elegans. Sci. Rep. 14, 1350 (2024).
Portal-Celhay, C., Bradley, E. R. & Blaser, M. J. Control of intestinal bacterial proliferation in regulation of lifespan in Caenorhabditis elegans. BMC Microbiol. 12, 49 (2012).
Starnes, D. L. et al. Impact of sulfidation on the bioavailability and toxicity of silver nanoparticles to Caenorhabditis elegans. Environ. Pollut. 196, 239–246 (2015).
Jeong, J. et al. Developing adverse outcome pathways on silver nanoparticle-induced reproductive toxicity via oxidative stress in the nematode Caenorhabditis elegans using a Bayesian network model. Nanotoxicology 12, 1182–1197 (2018).
Lim, D. et al. Oxidative stress-related PMK-1 P38 MAPK activation as a mechanism for toxicity of silver nanoparticles to reproduction in the nematode Caenorhabditis elegans. Environ. Toxicol. Chem. 31, 585–592 (2012).
Xu, Q. H. et al. Silver nanoparticles impair zebrafish skeletal and cardiac myofibrillogenesis and sarcomere formation. Aquat. Toxicol. 200, 102–113 (2018).
Liu, Z., Wang, B., He, R., Zhao, Y. & Miao, L. Calcium signaling and the MAPK cascade are required for sperm activation in Caenorhabditis elegans. Biochim. Biophys. Acta 1843, 299–308 (2014).
Shin, N., Cuenca, L., Karthikraj, R., Kannan, K. & Colaiacovo, M. P. Assessing effects of germline exposure to environmental toxicants by high-throughput screening in C. elegans. PLoS Genet. 15, e1007975 (2019).
Du, H. et al. Reproductive toxicity of endosulfan: Implication from germ cell apoptosis modulated by mitochondrial dysfunction and genotoxic response genes in Caenorhabditis elegans. Toxicol. Sci. 145, 118–127 (2015).
Ewald, C. Y. Redox signaling of NADPH oxidases regulates oxidative stress responses, immunity and aging. Antioxidants 7, 130 (2018).
Inoue, H. et al. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev. 19, 2278–2283 (2005).
Chatterjee, N., Eom, H. J. & Choi, J. Effects of silver nanoparticles on oxidative DNA damage-repair as a function of p38 MAPK status: A comparative approach using human Jurkat T cells and the nematode Caenorhabditis elegans. Environ. Mol. Mutagen. 55, 122–133 (2014).
Eom, H. J., Ahn, J. M., Kim, Y. & Choi, J. Hypoxia inducible factor-1 (HIF-1)–flavin containing monooxygenase-2 (FMO-2) signaling acts in silver nanoparticles and silver ion toxicity in the nematode, Caenorhabditis elegans. Toxicol. Appl. Pharmacol. 270, 106–113 (2013).
Jeong, J. et al. Toxicity assessment of nano-sized MAX phases: Considerations for safe-by-design approaches. Environ. Sci. Nano 11, 186–199 (2024).
Fausett, S. et al. Germ cell apoptosis is critical to maintain Caenorhabditis elegans offspring viability in stressful environments. PLoS One 16, e0260573 (2021).
Simonet, P. et al. Disruption of phenylalanine hydroxylase reduces adult lifespan and fecundity, and impairs embryonic development in parthenogenetic pea aphids. Sci. Rep. 6, 34321 (2016).
Calvo, A. C., Pey, A. L., Ying, M., Loer, C. M. & Martinez, A. Anabolic function of phenylalanine hydroxylase in Caenorhabditis elegans. FASEB J. 22, 3046–3058 (2008).
Leitao-Goncalves, R. et al. Commensal bacteria and essential amino acids control food choice behavior and reproduction. PLoS Biol. 15, e2000862 (2017).
Dang, H. et al. On the benefits of the tryptophan metabolite 3-hydroxyanthranilic acid in Caenorhabditis elegans and mouse aging. Nat. Commun. 14, 8338 (2023).
Edwards, C. et al. Mechanisms of amino acid-mediated lifespan extension in Caenorhabditis elegans. BMC Genet. 16, 8 (2015).
Cabreiro, F. & Gems, D. Worms need microbes too: microbiota, health and aging in Caenorhabditis elegans. EMBO Mol. Med. 5, 1300–1310 (2013).
Lau, S. K. et al. Metabolomic profiling of Burkholderia pseudomallei using UHPLC-ESI-Q-TOF-MS reveals specific biomarkers including 4-methyl-5-thiazoleethanol and unique thiamine degradation pathway. Cell Biosci. 5, 26 (2015).
Chatterjee, A. et al. Reconstitution of ThiC in thiamine pyrimidine biosynthesis expands the radical SAM superfamily. Nat. Chem. Biol. 4, 758–765 (2008).
Mrowicka, M., Mrowicki, J., Dragan, G. & Majsterek, I. The importance of thiamine (vitamin B1) in humans. Biosci. Rep. 43, BSR20230374 (2023).
Stuetz, W. et al. Thiamine diphosphate in whole blood, thiamine and thiamine monophosphate in breast-milk in a refugee population. PLoS One 7, e36280 (2012).
Peng, G. et al. Graphene oxide elicits microbiome-dependent type 2 immune responses via the aryl hydrocarbon receptor. Nat. Nanotechnol. 18, 42–48 (2023).
López-Serrano Oliver, A. et al. Quantification of silver nanoparticles taken up by single cells using inductively coupled plasma mass spectrometry in the single cell measurement mode. J. Anal. At. Spectrom. 33, 1256–1263 (2018).
Yang, Y. et al. Effect of ionic strength on bioaccumulation and toxicity of silver nanoparticles in Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 165, 291–298 (2018).
Yan, N., He, X., Tang, B. Z. & Wang, W. X. Differentiating silver nanoparticles and ions in medaka larvae by coupling two aggregation-induced emission fluorophores. Environ. Sci. Technol. 53, 5895–5905 (2019).
Ahn, J. M., Eom, H. J., Yang, X., Meyer, J. N. & Choi, J. Comparative toxicity of silver nanoparticles on oxidative stress and DNA damage in the nematode, Caenorhabditis elegans. Chemosphere 108, 343–352 (2014).
Acknowledgements
This work was supported by the National Key R&D Program of China 2022YFA1205603 (A.J.M.), the National Natural Science Foundation of China 21822605 (A.J.M.), 22176093 (A.J.M.) and 21677068 (A.J.M.), the Fundamental Research Funds for the Central Universities 021114380234 (A.J.M.) and the Key Program for Science and Technology of CNTC 110202202030 (J.W).
Author information
Authors and Affiliations
Contributions
A.J.M. conceived the idea. J.X.G., X.L.W., C.X.L., and X.Y.L. performed the experiments. A.J.M., J.X.G., X.L.W. and C.X.L., J.W., Q.G.T. and L.Y. analyzed the data and drew the figures. A.J.M. wrote the manuscript with contributions from all co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Bregje (W) Brinkmann and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gong, JX., Wang, XL., Lin, CX. et al. Gut microbiota mitigate the reproductive toxicity of silver nanoparticles through thiamine-derived metabolites. Nat Commun 16, 7294 (2025). https://doi.org/10.1038/s41467-025-62595-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-62595-z