Abstract
Small round cell sarcomas (SRCS) are highly aggressive tumors in soft tissues and bone of mostly children and young adults. Despite being different in many aspects, including genetics, possible cell-of-origin, and pathology, patients with any of these entities all receive the same therapeutic regimen. Although several pre-clinical models of Ewing sarcoma have been established, such as cell lines and patient-derived tumor xenografts, few models exist for other SRCS. Here, we describe a pediatric SRCS tumor organoid (tumoroid) biobank containing long-term tumoroid cultures with different translocations, including EWSR1::FLI1, EWSR1::ERG, CIC::DUX4, and BCOR-rearrangements. Using histology, whole genome sequencing and RNA sequencing, we demonstrate that these tumoroids retain histological characteristics, known marker gene expression and chromosomal rearangements of their matching patient tumors. In addition, we compare mutation clusters in the tumoroids across patient-matched longitudinal samples, which shows that cellular heterogeneity is maintained. Drug screening on the tumoroid models unveils entity-specific drug sensitivity to various cytotoxic compounds and targeted compounds, including MCL-1 inhibitors for CIC::DUX4 sarcomas. Taken together, this newly established SRCS patient-derived tumoroid biobank represents a promising source of material for future basic cancer research and drug screening.
Similar content being viewed by others
Introduction
Small round cell sarcomas (SRCS) such as Ewing sarcoma (ES), CIC::DUX4 sarcoma (CDS) and BCOR-rearranged sarcoma (BRS) are highly aggressive tumors that mainly affect children and young adults1. These tumors, which are composed of small round cells with a high nuclear-cytoplasmic ratio, are most often found in bone and soft tissues2. Genetically, these tumors are characterized by specific chromosomal translocations that result in the formation of a fusion gene. The hallmark translocation for ES generates a fusion of the Ewing Sarcoma Region 1 gene (EWSR1) with a member of the ETS family of transcription factors. The most common fusion is EWSR1::FLI1 (85%), followed by EWSR1::ERG (10%). The resulting fusion proteins contain the N terminal domain of EWSR1, an RNA binding protein, and the DNA binding domain of the ETS factor. The EWSR1::FLI1 oncoprotein disrupts normal gene expression in multiple ways. It can bind to canonical ETS sites, whereby the chimeric oncoprotein suppresses expression of target genes, and it can bind to GGAA repeats which in turn are converted into de novo enhancers3,4,5,6. Other SRCS lack the molecular hallmark of ES, EWSR1 fused with an ETS transcription factor. In CDS, the translocation results in a fusion between the CIC gene (19q13) and one of two DUX4 retro-genes (4q35 or 10q26)7,8,9. Due to the rarity of this tumor entity, not much is known about its biology, while its clinical behavior is still poorly defined. Similar to ES, BRS also arise from bone and soft tissue but are characterized by genetic alterations that involve the BCOR gene, most commonly fused with CCNB3. Typical for these BCOR fusion-driven tumors is the expression of genes like BCOR, TLE1 and SATB2 and their high mitotic activity10,11.
Despite being different in many aspects, including genetics, possible cell-of-origin, and pathology, patients with any of these entities receive the same therapeutic regimen. The ES treatment regimen is guided by standard protocols and includes systemic chemotherapy in addition to local therapy (surgery and/or radiotherapy)12. The prognosis for ES patients diagnosed with a primary tumor has improved in the last decades, while prognosis remains poor for patients that have refractory or relapsed disease13. CDS are treated with the same treatment regimen as ES but show little to no response to chemotherapy. Previous studies have shown that CDS patients often progress under treatment14,15. Taken together, this underlines the need to develop new entity-specific therapeutic options.
Although several pre-clinical models of ES have been established, such as cell lines and patient-derived tumor xenografts (PDX)16,17, few models exist for other SRCS. It’s possible to use cell lines in long-term and high-throughput screening analyses, but they have several disadvantages, including clonal outgrowth and very low establishment success percentages18. A recent study has described a screening platform for SRCS using short-term patient-derived in vitro cultures19. For some pediatric tumor entities, including Wilms tumors, rhabdomyosarcomas, atypical teratoid/rhabdoid tumors and small cell carcinoma of the ovary, hypercalcemic type, long-term patient-derived tumor organoid (tumoroid) models have been reported. These tumoroid models allow for sustained in vitro expansion and retain key properties of the original tumors, including cellular heterogeneity20,21,22,23,24,25. Several studies have shown that tumoroids are valuable tools to detect entity- and patient-specific drug sensitivities with higher fidelity than regular cell lines26,27,28,29,30,31,32,33,34. Patient-derived tumoroids can also be used to dissect the transforming mechanism in detail or to study the consequences of oncogenic mutations and investigate tumor cell heterogeneity20,21.
Here, we establish a pediatric tumoroid biobank of SRCSs containing different translocations, including EWSR1::FLI1, EWSR1::ERG, EWSR1::FEV, CIC::DUX4, and BCOR-rearrangements. We show that these long-term tumoroid cultures retain histological, genetic, and transcriptional features of their matching patient tumors, including cellular heterogeneity. Drug screening reveals subtype-specific responses, notably sensitivity to MCL-1 inhibition in CIC::DUX4 tumoroids. This biobank provides a robust platform for SRCS modeling and therapeutic discovery.
Results
A living biobank of small round cell sarcoma tumoroid models
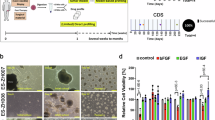
For the establishment of SRCS tumoroid models, we obtained fresh tumor tissue from patients that underwent a diagnostic fine-needle aspiration biopsy (FNAB) or surgical resection (Fig.1A; Supplemantary Data 1). Fresh PDX tumor specimens were obtained from the Innovative Therapies for Children with Cancer (ITCC-P4) consortium (Supplementary Data 1). Since the FNAB samples were taken for diagnostic purposes, these samples are treatment-naïve, while almost all resection material was collected following chemotherapy. As a starting point, we used a basic organoid culture medium (Advanced DMEM/F12 supplemented with penicillin/streptomycin, HEPES, Glutamax, N-2, B27 without vitamin A and n-acetyl-cysteine). Using a trial and error-based approach, we empirically tested growth factors which eventually led to the development of three types of Sarcoma Culture Medium (SCM), all including EGF, FGF2 and IGF1, but in different concentrations (Fig. 1A; Supplementary Data 2). We have thus been able to successfully culture and biobank a comprehensive collection of pediatric fusion-positive SRCS, including sarcomas with EWSR1::FLI1, EWSR1::ERG, EWSR1::FEV, BCOR::CCNB3, KMT2D::BCOR and CIC::DUX4 gene fusions (Fig. 1B). In 33 out of 37 (89%) samples, tumoroids were successfully established (Fig. 1B, Supplementary Data 1). The derivation efficiency was higher for treatment-naïve (10 out of 10, 100%) than for relapse and chemotherapy-treated patient samples (16 out of 20, 80%). Especially the ES chemotherapy-treated samples mainly consisted of necrotic tissue, which could explain the lower derivation efficiency. For PDX-derived tumoroids, we successfully established tumoroid lines in 7 out of 7 (100%) samples. Different media conditions were tested for all new samples, the media conditions that resulted in tumoroid growth per sample are shown in Supplementary Data 2. Depending on the amount of tumor tissue that was put in culture, a stable culture (defined as growth for at least 5 passages) was established within 5–9 weeks after receiving the tumor material. Upon establishment of a stable culture, cells were expanded for drug screen experiments, which were performed between passage 5 and 10. We collected DNA and RNA for further downstream analysis at the same timepoint. In general, tumoroid clusters appeared within 7–14 days after seeding on low attachment plates. The tumoroids could be split in a ratio between 1:2 and 1:6 every 8–14 days and could be passaged at least 20 times. Tumoroids were viably frozen down for long-term storage. We did not observe a clear difference in growth after a freeze-thaw cycle. Depending on the type of tumor, there was a modest difference in expansion rates between tumor entities. In particular, the tumoroids established from EWSR1::FLI1 tumors tended to expand somewhat faster (Fig. 1C; Supplementary Fig. 1A). We noticed a difference in tumoroid morphology between the different fusion-driven entities. Amongst the ES tumoroids, EWSR1::ERG and EWSR1::FEV tumoroids contained more compact cell structures, indicated by a darker appearance in the center of the tumoroid. They also displayed a less uniform shape compared to the EWSR1::FLI1 tumoroids (Fig. 1C). All ES tumoroids were quite loose in structure and easy to dissociate. The BCOR::CCNB3 cultures showed a mix of small to intermediate-sized tumoroids, which were exceptionally stiff and perfectly round, with some less dense pockets in the center of the tumoroid. H&E staining showed that the core of the BRS tumoroids contained fewer cells compared to other fusion-positive sarcomas (Supplementary Fig. 2B). In contrast, CDS tumoroids were more difficult to dissociate and typically appeared as grape-like clumps of cells (Fig. 1C).
A Flow diagram with an overview of the study design. Tumoroids were established from patient tumor and PDX material and subsequently characterized using whole genome sequencing, RNA sequencing and histology. Drug screens were performed on a subset of ES, CDS and BRS. Lastly, primary tumors and matching tumoroid lines were viably frozen and stored in liquid nitrogen. Created in BioRender. Ringnalda, F. (2025) https://BioRender.com/ovfd63d. B Donut chart (left) representing the different fusions and the number of patients per fusion that were included in the ES, CDS and BRS biobank. Bar chart (right) of the total number of established tumoroid lines from each fusion, subdivided in tumoroids derived from patient tumors and those derived from PDX material. C Representative brightfield microscopy images of EWSR1::FLI1, EWSR1::ERG, EWSR1::FEV, BCOR::CCNB3, KMT2D::BCOR and CIC::DUX4 tumoroids (n = 3, frames of individual area of interest). Scale bar: 200 µm, zoom-in 100 µm. ES Ewing sarcoma, CDS CIC::DUX4 sarcoma, BRS BCOR-rearranged sarcoma. Source data are provided as a Source Data file.
Phenotypic characterization of small round cell sarcoma tumoroid models
Classification of bone and soft tissue sarcomas is based on pathological examination. To determine whether the SRCS tumoroids retained their phenotypic features in vitro, we first characterized the tumoroids models by histological analysis35. This showed that the different tumoroid models recapitulated their parental tumor tissue hallmarks (Fig. 2; Supplementary Fig. 2A). ES were characterized by strong CD99 staining and this was reflected in EWSR1::FLI1 and EWSR1::ERG tumoroids (Fig. 2B)36,37. EWSR1::FEV tumoroids showed reduced CD99 staining compared to their matching tumor tissue. A hallmark of CDS is the strong nuclear expression of Wilms tumor 1 (WT1) (Fig. 2C) and ETS variant transcription factor 4 (ETV4) (Supplementary Fig. 2A). Indeed, our CIC::DUX4 tumoroids strongly expressed these proteins. While these protein markers are adapted as helpful tools to distinguish CIC-rearranged sarcoma from ES and other histologic mimics, diagnosing fusion-positive sarcomas relies on the detection of the oncogenic fusion transcript, either by fluorescence in situ hybridization (FISH) or reverse-transcription PCR (RT-PCR)7,38. Before submitting tumoroids for whole genome sequencing (WGS), the presence of the pertinent oncogenic fusion transcript -found in the original tumors- was confirmed by RT-PCR in all established tumoroid lines, primers are listed in Supplementary Data 3. An overview of genetic breakpoints is shown in Supplementary Fig. 2C.
A Representative images of H&E staining of tumor tissue (top) and matching tumoroids (bottom) derived from the indicated fusion-positive tumor types (n = 3, individual tumoroids). Scale bars: 100 µm, zoom-in 50 µm. B Representative images of CD99 staining of tumor tissue (top) and matching tumoroids (bottom) (n = 3, individual tumoroids). Scale bars: 100 µm, zoom-in 50 µm. C Representative images of WT1 staining of tumor tissue (top) and matching tumoroids (bottom) (n = 3, individual tumoroids). Scale bars: 100 µm, zoom-in 50 µm.
Genetic characterization of small round cell sarcoma tumoroid models
To determine whether SRCS tumoroids genetically recapitulate the tumors from which they were derived, we performed WGS on 21 tumoroid samples and compared these, when available, to WGS of their matching tumor tissue. We created mutation matrices and used these to calculate Jaccard’s distance between the samples. This revealed that the tumoroids genetically resemble their tumor counterparts (Fig. 3B, Supplementary Fig. 3B, C). Some TT-TO paired samples were sequenced with different techniques, i.e., WXS versus WGS. These techniques cover different parts of the genome and share fewer variants, which explains why some pairs show relatively large Jaccard’s distances (e.g., ES-042 and ES-071). Also, several ES tumoroids showed CNAs on chromosomes 1, 8, 9 and 12 (Supplementary Fig. 3A, B), which is consistent with previous data39,40,41,42. Furthermore, mutations frequently found in ES were detected, such as STAG2 and TP53 (Fig. 3A) and these genetic characteristics were conserved in matching tumoroid models. In some samples (e.g., ES-027) additional mutations were detected in the tumoroid samples compared to their matching tumor sample. This could be an indication of de novo mutations or clonal selection. In a few cases, no recurrent somatic mutations other than the fusion gene could be detected. In CDS tumor and tumoroid samples, no additional oncogenic somatic mutations were found, except for an amplification of MYC in a relapse sample (CDS-045-TT)43. In the tumoroid corresponding to this relapse sample (CDS-045-TO), this MYC amplification was not detected. Moreover, we detected CDKN2A homozygous deletions in various ES samples and one BCOR-rearranged sample, which have been shown to be associated with poor prognosis44,45.
A Oncoplot depicting somatic mutations, copy number alterations (CNAs) and gene fusions identified in ES, CDS and BRS tumors and matching tumoroids. For samples in which the germline sample was not available (*), mutations in known tumor driver genes are indicated. For samples for which no sequencing data was available, the mutational information provided by the ITCC-P4 consortium is indicated (+). B Circos plots of ES-010 tumor and tumoroid. The circos plots show, from outside inwards: chromosomes, single-nucleotide variants (SNVs) (dark blue), copy number alterations (CNAs) as gained (red) or lost (light blue) regions and translocations (red line). C Circos plots of tumoroids derived from one EWSR1::FLI1 patient (top row; diagnostic [ES-016], first relapse [ES-055] and second relapse [ES-080] samples) and one CIC::DUX4 patient (bottom row; diagnostic [CDS-090], resection [CDS-032] and relapse [CDS-045] samples) at different timepoints during disease progression. The circos plots show, from outside inwards: chromosomes, single-nucleotide variants (SNVs) (dark blue), copy number alterations (CNAs) as gained (red) or lost (light blue) regions and translocations (red line). D River plot depicting differential clusters of mutations between time of diagnosis (ES-016), first relapse (ES-055) and second relapse (ES-080) tumor sample from one EWSR1::FLI1 patient. E The mean variant allele frequency of individual clusters of mutations in tumor and matching tumoroid samples in diagnostic (ES-016), first relapse (ES-055) and second relapse (ES-080) samples from one EWSR1::FLI1 patient. F Contribution of somatic mutational signatures per tumor and matching tumoroid model. SBS single base substitution, TT Tumor Tissue, TO TumorOid, ES Ewing sarcoma, CDS CIC::DUX4 sarcoma, BRS BCOR-rearranged sarcoma. Source data are provided as a Source Data file.
Genetic landscape during tumor evolution
Tumor progression is generally accompanied by an increase in mutational burden46. For tumoroids to be useful models to study disease progression, it is essential that they not only reflect the mutational landscape of primary tumors, but also of relapses and metastases. From 10 patients, we established tumoroid models at different time points during disease progression, namely primary tumor (diagnosis) and/or first and second relapse. We detected an increase in mutational load and CNAs, both in the relapsed tumors and in the tumoroids that were derived from them. However, this increase in mutational burden was different for ES, CDS and BRS tumors (Fig. 3A). As shown in Fig. 3C and Supplementary Fig. 3A, there was a striking increase in mutational burden and CNAs in tumoroids derived from patient M040AAB. The sample at diagnosis (ES-016) showed a few CNAs, gains on 12q, 14p and 15p and local losses on 1 and 4. The samples derived from first relapse and second relapse of the same patient showed a dramatic increase of CNAs (ES-055 and ES-080 respectively) (Fig. 3C). Especially ES-055 shows CNAs on nearly all major chromosomes (1, 2, 4, 5, 6, 7, 8, 9, 11, 12, 14, 15, 16, 17, 18, 19 and 21). In one CDS patient (M616AAA), we obtained tumor samples at diagnosis, resection, and relapse, but the increase in CNAs and mutational burden in samples from later timepoints was rather modest (Fig. 3C), in line with the more stable genome of CDS. We observed similar CNAs in tumors and tumoroids in both ES and CDS samples (Supplementary Fig. 3D). The genetic profiles of tumor progression were therefore reflected in our in vitro models.
Next, we more deeply analyzed tumor tissue and matching tumoroid lines from the 3 different time points (ES-016, ES-055 and ES-080) of one ES patient to better understand the mutational composition at different timepoints. We clustered all mutations found over all samples based on similarity of variant allele frequency and allele-specific copy numbers. This resulted in 16 different clusters of mutations (Fig. 3D, E). We noticed that not all clusters are present in all samples, indicating clonal evolution. In addition, we noticed that different groups of mutations could be either timepoint-specific or sample-specific. For instance, cluster 10 is present in all samples except for the first tumor sample (ES-016-TT). Possibly, in vitro, we selected for a clone already harboring this group of mutations, much like it seems to have happened in vivo. We also noticed clusters of mutations that only appeared in a single tumoroid sample, like cluster 12, 13, and 2. These mutations are only present in tumoroid samples and are likely a result of clonal selection or de novo mutations. In the second relapse sample (ES-080), an additional STAG2 mutation could be identified (Fig. 3A). We detected a TP53 mutation in the first relapse sample (ES-055, tumor and tumoroid), but not in the second relapse sample (ES-080, tumor and tumoroids) (Fig. 3A). This already indicates that there is variation in tumor development and clonal selection in vivo, as also indicated by the different clusters of mutations that are present in the tumor samples (Fig. 3D). Subsequently, we compared the mutational distribution of the tumoroids to their matching tumor samples. Although not identical, most clusters of mutations that were present in the tumors, were also present in the matching tumoroids, and vice versa (Fig. 3E; Supplementary Fig. 3E), with some exceptions that could be explained by either de novo mutations or clonal selection.
To further confirm that tumoroids maintained the genetic characteristics of their matching tumors, we extracted mutational signatures from WGS/WXS data of the tumoroids and matching tumors (Fig. 3F; Supplementary Fig. 3F). We compared these signatures to signatures reported in the Catalog of Somatic Mutations in Cancer (COSMIC) database47. A substantial number of signatures were found in our ES, CDS and BRS tumors, which were maintained in the tumoroids (Fig. 3F). ES samples harboring mutations in CDKN2A, STAG2 or TP53 showed a relatively high contribution of signatures SBS40 (etiology unknown), SBS6 (DNA mismatch repair), SBS12 (etiology unknown) and SBS3 (DNA-DSB repair by HR). ES samples that were treated with chemotherapy, mainly relapse samples, showed signatures that highly correlate to SBS18 (oxidative stress) and SBS25 (exposure to chemotherapy). Other signatures included SBS5 (cellular aging) and SBS12 (etiology unknown). The CDS tumors and tumoroids included signatures SBS6, SBS5 and SBS12, which stayed the same during disease progression and treatment. Overall, SRCS tumoroids displayed and maintained key features of the genomic landscape, such as cancer gene mutations, CNAs, and mutational signatures.
Gene expression profiles in small round cell sarcoma tumoroid models
To determine whether the tumoroids transcriptionally resembled the different SRCS entities, we performed paired-end RNA-sequencing on all samples. Firstly, STAR-Fusion confirmed the presence of the relevant fusion transcripts in all tumors and tumoroids48 (Supplementary Fig. 2C). A principal component analysis (PCA) based on the expression of the top 5000 most variable genes showed that all tumors and tumoroids within one tumor entity (ES versus BRS versus CDS) clustered closely together in the first principal component. The second principal component separated samples mostly based on sample type (tumor versus PDX versus tumoroid). PDXs and tumoroids clustered more closely together than tumors and tumoroids (Fig. 4A, Supplementary Fig. 4B). Known marker genes for the specific tumor entities were indeed highly expressed in the relevant tumors and PDXs, which was reflected in their corresponding tumoroids (Fig. 4B). Samples harboring EWSR1::FLI1, EWSR1::ERG and EWSR1::FEV fusions highly expressed CD99, a marker used to identify these tumors in a clinical setting. They also expressed other ES-specific markers such as NKX2.2 and PAX7. Analysis of BRS samples revealed that CCNB3 was highly expressed only in BCOR::CCNB3 samples, while NTRK3 was highly expressed only in KMT2D::BCOR samples. All BRS samples co-expressed TLE1 and SATB2, a combination that is used to diagnose BRS in the clinic. Interestingly, while CD99 is used to diagnose CDS in the clinic, little to no mRNA expression of CD99 could be observed in either tumor or tumoroid samples. Other markers such as ETV1/4, WT1 and DUX4 were highly expressed in both tumors and matching tumoroids11,35,36,37,49. Taken together, this confirmed the expression of most known marker genes in SRCS and showed conserved expression in their matching tumoroids.
A Principal component analysis (PCA) of top 5000 most variable genes, dots are colored based on tumor entity and shaped for tumoroid, tissue or PDX tissue. B Heatmap showing marker gene expression of the different tumor entities in tumor and tumoroid samples. C Heatmap showing the expression of the top 20 differentially expressed genes per tumor entity that have a CHIPseq binding peak of their characteristic (fusion-)protein in close proximity to the transcription start site. D Dot plot showing row-normalized mean expression values of genes that are targeted by our in-house drug library in the different tumor entities. Source data are provided as a Source Data file.
Identification of fusion protein-driven transcription
Next, we aimed to determine whether genes that are specifically expressed in each tumor entity and can possibly be attributed to the activity of the fusion oncoprotein, are also expressed in the matching tumoroids. In addition to tumoroid characterization, these genes might contain interesting entity-specific drug targets. To achieve this, gene expression levels of SRCS tumors and tumoroids were compared yielding a gene set specific to each tumor entity. Because the fusion gene can control gene expression in SRCS by binding to DNA elements in close proximity to target genes, CHIP-seq datasets were explored to identify which differentially expressed genes (DEGs) are likely targeted by the fusion protein50. Fusion protein-specific binding sites were extracted from datasets generated by Rivera et al.51 for the EWSR1::FLI1 fusion protein, by J. Taipale et al.50 for the EWSR1::ERG fusion protein, and by R.A. Okimoto et al.52 for the CIC::DUX4 fusion protein. For BCOR-rearranged fusion proteins, no fusion-specific dataset was available. Therefore, the locations of binding sites for the native BCOR protein were used as an approximation for binding sites of BCOR-rearranged fusion proteins (ENCODE, identifier: ENCFF186JKG53). Taken together, this allowed us to study genes that were differentially expressed when comparing SRCS with different fusions and showed a binding peak of the fusion gene in close proximity to the transcription start site (Fig. 4C). Most identified genes were known targets of the different tumors, such as NKX2.2 and ETS1 for EWSR1::FLI1 and CIC::DUX4, respectively. For example, MAPT was expressed in both EWSR1::FLI1 and EWSR1::ERG sarcomas, but not in the others54. This gene is a known target for Docetaxel and Paclitaxel, two anticancer drugs55. ETV5, which is downstream of the target of Trametinib7,54,56, could be identified as a DEG that was regulated specifically by the CIC::DUX4 fusion protein. Lastly, we aimed to identify tumor-specific DEGs that could be used as drug targets. Because of low sample numbers for EWSR1::FEV and EWSR1::ERG, we chose to group these. Doing so, we plotted the expression of a selection of known drug targets that are covered by our in-house drug library (Fig. 4D; Supplementary Data 4).
To determine which signaling pathways were active in the different entities, we performed a gene set enrichment analysis on the tumors and tumoroids. Notably, the comparison between different tumor entities demonstrated enrichment of a variety of pathways. This further supported the notion that the different translocations lead to activation or suppression of specific pathways. For example, in CDS, we verified that mitogen activated protein kinase (MAPK) phosphatase activity was increased, which has also been previously reported by Lin et al.52 (Supplementary Fig. 4A). In BRS samples, we identified increased SH3 domain binding (Supplementary Fig. 4A), which plays a role in tumor progression57.
In summary, the transcriptomic analyses confirmed that ES, CDS and BRS tumoroids retain key traits of their original tumors, and we identified pathways that might be targeted therapeutically in ES, CDS and BRS.
Drug screening of ES, CDS and BRS tumoroids reveals entity-specific sensitivities
To assess how our tumoroid models respond to drugs that are used in current treatment regimens, as well as to identify potential novel treatment options, we performed drug screens. In total, we screened twenty-one tumoroid lines derived from fourteen patients, including ten EWSR1::FLI1 lines, six CIC::DUX4 lines, two BCOR-rearranged lines, two EWSR1::ERG lines and one EWSR1::FEV line. Tumoroids were established from both primary and metastatic disease timepoints. Tumoroids were subjected to an in-house-developed drug library containing ~34 standard of care chemotherapeutics, including currently used drugs for ES, BRS and CDS, and 150 early-phase clinical trial compounds targeting various pathways (Supplementary Data 4). Cell viability was measured at T0 and T120 to determine growth rate (control-no drug) (Supplementary Fig. 1A, B). The drugs were added in six different concentrations, allowing us to generate dose-response curves (Fig. 5A; Supplementary Data 4−5).
A Schematic overview of the tumoroid drug screening workflow. Created in BioRender. Ringnalda, F. (2025) https://BioRender.com/mnd48e5. B Clustered heatmap of AUC values of each tumoroid model (x axis) and compound (y axis), showing AUC values after 120 h drug treatment in a dose-range of 0.1 nM to 10 µM. Low AUC (red) indicates high drug sensitivity while high AUC (blue) indicates low drug sensitivity. Highlighted clusters include (1) chemotherapy, proteases and histone deacetylase inhibitors, (2) mTOR inhibitors, (3) E3 ubiquitin-protein ligases and (4) MCL-1 inhibitors. C Clustered heatmap showing AUC values after chemotherapy treatment, compounds that are routinely used in ES and ELS patients marked in red. D Box plots showing the mean AUCs values of mTOR inhibitors in the indicated ES (n = 13, tumoroid lines), CDS (n = 6, tumoroid lines) and BRS (n = 2, tumoroid lines) tumoroids entities. The boxes represent the interquartile range, the line inside the box marks the median, and the whiskers extend to the lowest and highest values. Error bars represent SEM of individual indicated ES, CDS and BRS tumoroid enitities. P-values were calculated using a one-way Anova, Dunnet multiple comparisons test:*<0.05, **<0.005, ns not significant. Exact adjusted P-values: AZD8055, EWSR1::FLI1 vs. EWSR1::ERG/FEV = 0.0033, EWSR1::FLI1 vs. BCOR::CCNB3/KMT2D = 0.0157, EWSR1::FLI1 vs. CIC::DUX4 = 0.0035. Sirolimus, EWSR1::FLI1 vs. EWSR1::ERG/FEV = 0.0147, EWSR1::FLI1 vs. BCOR::CCNB3/KMT2D = ns, EWSR1::FLI1 vs. CIC::DUX4 = 0.0281. Temsirolimus, EWSR1::FLI1 vs. EWSR1::ERG/FEV = ns, EWSR1::FLI1 vs. BCOR::CCNB3/KMT2D = ns, EWSR1::FLI1 vs. CIC::DUX4 = ns. E Box plots showing the mean AUCs values of P53 pathway activators in the indicated ES, CDS and BRS tumoroids entities. The boxes represent the interquartile range, the line inside the box marks the median, and the whiskers extend to the lowest and highest values. Error bars represent SEM of individual indicated TP53 wildtype (p53WT) (n = 14) vs TP53 mutant (p53MUT) (n = 7) tumoroids lines. P-values were calculated using a two-tailed Student’s t test: ****<0.0001. Exact P-values: Idasanutlin ≤ 0.0001, Milademetan ≤ 0.0001, Siramadlin ≤ 0.0001. F Clustered heatmap of compounds targeting the apoptosis pathway, CDS tumoroids showing specific sensitivity towards MCL-1 inhibitors (in red). G Validation screen with MCL-1 inhibitors, dose-response curves of S63845, MIK665 and AZD5991 for indicated ES and CDS tumoroid cultures. Error bars represent SD, each consisting of triplicate measurements. SEM standard error of mean, SD standard deviation, ES Ewing sarcoma, CDS CIC::DUX4 sarcoma, BRS BCOR-rearranged sarcoma. Source data are provided as a Source Data file.
We calculated the area under the curve (AUC) values of the individual drugs in all tumoroid models (Fig. 5B). Members of four classes of drugs appeared to have tumor entity-specific effects: chemotherapeutics, mTOR inhibitors, p53 activators and MCL-1 inhibitors (Fig. 5B). We first focused on chemotherapy sensitivity. Currently, patients with ES, CDS and BRS are treated with the same chemotherapy regimen, including either vincristine, ifosfamide, doxorubicin and etoposide (VIDE) (Ewing 2008 protocol) or vincristine, doxorubicin and cyclofosfamide, followed by ifosfamide and etoposide (VDC/IE)58,59. The drugs used in these regimens and included in our drug library are marked in red in Fig. 5C. Although the response varied between the different tumoroid lines, the CDS tumoroids overall responded less to the chemotherapeutics when compared to the other groups (Fig. 5C). Previous retrospective studies have shown that CDS sarcomas are less chemo-sensitive than ES14,60,61,62, which was reflected in our tumoroids, indicating that tumoroid drug response can be predictive of in vivo response and highlighting the need for fusion gene-specific treatment options.
We noticed that mTOR inhibitors (AZD8055, sirolimus and temsirolimus) were particularly effective in EWSR1::FLI1 tumoroids compared to the other groups (Fig. 5D), in line with previous literature that showed that mTOR inhibition leads to reduced EWSR1::FLI1 protein expression63,64. As the response to mTOR inhibition was independent of the TP53 mutational status in ES tumoroids (Supplementary Fig. 5A), this response seems to be EWSR1::FLI1-specific. As expected, compounds that activate the p53 signaling pathway (idasanutlin, milademetan and siremadlin) were highly effective against TP53 wildtype tumoroids compared to TP53 mutant lines (Fig. 5E). We also tested chemotherapeutics that are currently used in ES patients. All EWSR1::FLI1 tumoroids responded to currently used chemotherapy agents. We observed that EWSR1::FLI1 tumoroid lines derived from chemotherapy-treated tumors responded slightly less to chemotherapeutics than those derived from treatment-naïve samples. This difference was not significant, possibly due to the small sample size (Supplementary Fig. 5B). Looking at the MCL-1 inhibitors, we noticed that specifically CDS tumoroids were highly sensitive to S63845, MIK665, AZD5991 and -to a lesser extent- to Tapotoclax (Fig. 5F, G; Supplementary Fig. 5D, E). Looking back at the transcriptome analysis, we noticed that CDS indeed expressed higher levels of MCL1, the target of these drugs, compared to the other groups (Fig. 4D). In contrast to the anti-apoptotic MCL1, NOXA is a pro-apoptotic protein that binds and neutralizes MCL1, and we found that NOXA mRNA expression is lower in CDS compared to ES (Supplementary Fig. 6A). In a Western blot analysis, we saw a similar trend in MCL1 and NOXA expression in two CDS compared to two ES tumoroid samples (Supplementary Fig. 6B). We also confirmed that CDS tumoroids have high MAPK phosphatase (MKPs) expression (Supplementary Fig. 4A), which may contribute to the MAPK/MEK inhibitor resistance that we observed in CDS tumoroids compared to ES tumoroids (Supplementary Fig. 5C)52,56.
To confirm the specific vulnerability of CDS tumoroids to MCL-1 inhibitors, we performed validation screens on a selection of six ES (five EWSR1::FLI1 and one EWSR1::ERG line(s)) and six CIC::DUX4 tumoroid lines. The validations were performed with more drug concentrations (12) to accurately determine half-maximal inhibitory concentration (IC50) values for the different tumoroid lines. CDS tumoroids were markedly more sensitive to MCL-1 inhibitors compared to the ES tumoroids (Fig. 5G; Supplementary Fig. 5D, E). MCL-1 inhibitors S63845 and MIK665 showed very similar dose-response curves in all tumoroid models. Since compound MIK665 is a derivative of S63845, this confirmed the robustness of our tumoroid platform. The IC50 values for S63845 and MIK665 in the CDS tumoroids ranged from 0.9 nM to 39 nM, while IC50 values for the ES tumoroids could only be calculated from two lines, being 3235 nM and 4349 nM (Fig. 5G). For the other ES lines, the full inhibitory plateau was not reached. The MCL-1 inhibitors AZD5991 and Tapotoclax gave a less strong response in all CIC::DUX4 models, with IC50 values ranging from 20 to 110 nM and 160 to 550 nM, respectively (Fig. 5G; Supplementary Fig. 5D, E). These results implied that MCL-1 plays an important role in the survival of CIC::DUX4 cells and that MCL-1 inhibition showed promise as a possible targeted therapy. To determine whether the MCL-1 inhibition reduces cell viability in CDS tumoroids by inducing apoptosis, we performed Annexin V and PI imaging experiments upon treatment with the MCL-1 inhibitor MIK665. This confirmed that MCL-1 inhibition induced apoptosis already after 1 h in CDS tumoroids but not in ES tumoroids (Supplementary Fig. 7).
Taken together, we show that ES, CDS and BRS tumoroids can be used for drug testing, which enabled us to find tumor entity-specific drug vulnerabilities. In particular, MCL-1 inhibitors stood out as potentially promising targeted drugs for CDS treatment.
Discussion
ES, CDS and BRS are highly aggressive tumors that mainly affect children and young adults. In the last decades, the survival rate has improved for patients that are diagnosed with a primary tumor, but remains poor for patients that have refractory or relapsed disease13. Despite being different in many aspects, including genetics and pathology, patients with any of these entities receive the same therapeutic regimen. In this study, we show that tumoroids of different ES, CDS and BRS entities reflect the genetic and transcriptomic profiles of their matching primary tumors and exhibit different responses to drug screens. We identify several classes of drugs with entity-specific responses, including MCL-1 inhibitors which specifically target CDS tumoroids.
2D cell lines derived from SRCS have contributed tremendously to our understanding of the tumor biology. However, in most cases these were derived from late-stage cancers and thereby lack characteristics of early disease timepoints. Moreover, the currently available cell lines do not reflect the heterogeneous nature of these tumor entities, while the corresponding patient histories are typically not recorded65,66,67,68. Patient-derived tumoroids have many advantages compared to 2D cell lines and are already applied in pre-clinical settings for multiple adult and pediatric cancers20,21,24,34,69,70,71,72. PDX models are currently the gold standard preclinical models, but these are time-consuming, take rates are generally low and they are very costly to produce and maintain. Tumoroid models can be established in a matter of weeks with high take rates, and because we can efficiently expand them, they are suitable for large-scale high-drug screening platforms69,70,71,72. Several studies have shown that tumoroids of different cancer entities, including rectal cancer, colorectal cancer, lung cancer, head and neck cancer, pancreatic cancer, and ovarian cancer, can predict patient responses to radiation therapy, chemotherapy and targeted therapy26,27,28,29,30,31.
This study describes the establishment of a broad panel of ES, CDS and BRS patient-derived tumoroids which can be passaged and maintained long-term, including those with rare fusions such as EWSR1::FEV, EWSR1::ERG and CIC::DUX4. Importantly, we demonstrate that the tumoroids retain tumor heterogeneity in culture. By establishing tumoroids from different disease timepoints, including primary and relapsed tumors, we show that tumor evolution was reflected in the tumoroids. These tumoroids are therefore of value as representative pre-clinical models. A living tumoroid biobank can be used in future studies, not only for translational studies using drug screens as we did in this study, but also for more fundamental studies, e.g., by performing gene editing.
Several studies have revealed that the genetic landscape of ES, CDS and BRS is heterogeneous39,40,45,73. In ES, chromosomal aberrations are common, while recurrent somatic mutations other than the fusion gene are sparse but most commonly present in TP53, STAG2 and CDKN2A. Mutations in genes such as TP53 and STAG2 often co-occur in a subset of patients with refractory ES40,44,74. In our dataset, we noticed that several EWSR1::FLI1 sarcomas also harbored a loss of CDKN2A/B, either homo- or heterozygous. In CDS, loss of chromosome 1p and gain of chromosome 8 (including MYC amplification) has been reported previously; we detected this also in one CDS in our dataset. Additional recurrent driver mutations are scarce in CIC::DUX4 sarcomas43,75. Similar to ES, BRS frequently show a loss of CDKN2A/B, which we also detected in our KMT2D::BCOR samples, both tumoroid and tumor44.
Although the tumoroids closely resembled their matching tumors in many aspects, we also observed some differences. For instance, some somatic variants that were present in the tumor were not detected in the matching tumoroids, e.g., a MYC gene amplification in sample CDS-045. Vice versa, some tumoroids carried additional somatic variants compared to the matching tumor, e.g., in sample ES-041 (TP53 mutant) and BRS-057. We reason that there are several explanations for this. First, the tumor tissue pieces used for diagnostic sequencing and for tumoroid derivation are different and there could be regional differences. In another study, we have analyzed clonal selection and tumor heterogeneity in three primary, previously untreated colon cancers76. We found genetic differences even in tumor cells which are closely related within the same tumor. We assume the same could hold true for ES, CDS and BRS tumors. Second, discrepancies can result from clonal outgrowth of mutations in cells that were already present, but in too low numbers to be detected. Third, additional de novo mutations might have occurred while culturing. For some samples, we see relatively large Jaccard’s distances between TT-TO pairs, which is related to the different sequencing methods that were used (WXS versus WGS). Overall, we see more similarities than differences, meaning that these tumoroids can provide useful information, e.g., about tumor-specific drug sensitivities.
In the REGOBONE study, regorafenib has been shown to modestly delay progression-free survival in ES patients with metastatic disease77. However, in our ES tumoroids, we did not observe any response to regorafenib. In vivo, regorafenib is heavily metabolized by the liver. Therefore, not all active metabolites such as the M-2 and M-5 metabolites are present in our in vitro model, potentially explaining the lack of response to regorafenib in our ES tumoroids.
Our data show that MCL-1 inhibitors, particularly S63845 and MIK665, induce strong and effective cell killing at low nM dose range. In line with this, the expression of MCL1 was increased in CDS samples, a feature that can be linked to drug resistance, in particular to chemotherapeutic agents78. Several MCL inhibitors are currently being evaluated in phase I clinical trials for acute myeloid leukemia (AML) and relapsed lymphoma as a single compound treatment or in combination with BLC2 inhibitors (clinicaltrials.gov: NCT02992483, NCT02979366, NCT03672695). One of the first trials with AMG-397 was put on hold because of possible concerns about on-target/off-tumor toxicity that caused cardiac side effects79. Because MCL-1 is implicated in normal cardiac myocyte functioning, side effects like these are probably directly related to the on-target activity of these compounds which may limit the therapeutic window of MCL-1 inhibitors80. Nonetheless, since the MCL-1 inhibitors already affected CDS tumoroids at low dose, these could have potential for future treatment of CDS patients, for whom effective treatment options currently do not exist. In future studies, it would be interesting to perform combination screens to identify drugs with synergistic effects, which could lower required dosing and thereby reduce MCL-1-related toxicity. For example, a recent study in zebrafish xenografts showed that dual targeting of MCL-1 and BCL-XL effectively targeted ES cells81.
Here, we report that tumoroid models can be generated with high efficiency from ES, CDS and BRS, while preserving the molecular and phenotypic heterogeneity of their matching tumors. Furthermore, the tumoroid models confirm distinct entity-specific vulnerabilities towards chemotherapy and show specific sensitivities for targeted drugs, including ones that have not been described previously. In particular, MCL-1 inhibition appears to be a potentially useful therapeutic strategy for CDS. Future studies could focus on combination screens to identify drugs with synergistic effects to further decrease the required doses. This newly established ES, CDS and BRS patient-derived tumoroid biobank generates a unique source of material for future research.
Methods
Ethics statement tumor tissue
Tumor samples were obtained from the Netherlands Cancer Institute (Amsterdam, The Netherlands) and as part of the biobank initiative of the Princess Máxima Center for pediatric oncology, Utrecht, The Netherlands, and are listed in Supplementary Data 1. The biobank initiative was approved by the medical ethical committee NedMec, and the biobank and data access committee of the Máxima approved the use of tumor tissues for this project. For tumoroids derived from patient-derived xenografts (PDX) (Supplementary Data 1), approval to collect patient samples for development of PDX was granted by the Innovative Therapies for Children with Cancer Paediatric Preclinical Proof-of-concept Platform (ITCC-P4) in Europe. All patients and/or their legal guardians signed informed consent for biobank usage of their tumor samples.
Patient material processing
Tumor material obtained via diagnostic FNAB or surgical resection was collected in Advanced Dulbecco’s Modified Eagle’s Medium with Nutrient Mixture Ham’s F-12 (Adv-DF) (#12634, Invitrogen), supplemented with 1% pen/strep (#15140-122, Invitrogen), 1% Glutamax (#35050-038, Invitrogen) and 1% Hepes (#15630-056, Invitrogen) (hereafter referred to as Adv-DF+++). Using scalpels, tumor tissues were minced into 1-3mm3 pieces, and was subdivided into three parts, one part was used for tumoroid establishment, one for downstream histopathological analysis and one part for cryopreservation. The part used for tumoroid establishment, was mechanically dissociated by repetitive pipetting with a P1000 pipet. After washing with ice-cold Adv-DF+++ medium, the cells were taken into culture (see: generation long-term tumoroid cultures).
Patient-derived xenografts
The PDX samples were shipped on ice by courier. On arrival, tumor tissues were washed in Adv-DF+++ and cut into 1–3 mm3 pieces using scalpels. If tumor material contained fatty tissue, a pair of scissors and a tweezer were used to remove this prior to further handling. At this point, the tumor tissue was subdivided into four parts, one part was used for tumoroid establishment, one for downstream histopathological analysis, one for DNA/RNA isolation and one part for cryopreservation. When the sample contained too many red blood cells, red blood cell lysis buffer (#11814389001, Roche) was used according to the manufacturer’s guidelines. The part used for tumoroid establishment, was mechanically dissociated by repetitive pipetting with a P1000 pipet. After washing with ice-cold Adv-DF+++ medium, the cells were taken into culture (see: generation long-term tumoroid cultures).
Generation long-term tumoroid cultures
Cells were seeded in one of the three freshly prepared Sarcoma Culture Medium (SCM) (Supplementary Data 3). The culture medium contained the following components; Adv-DF+++ with 1x B27 supplement without Vitamin A (#12587-010, Gibco), 1× N2 (#17502-048, Gibco), 0.2% BME (#3533-010-02, Bio-Techne), 1.25 mM N-Acetylcystein (#A9165, Sigma Aldrich). For the different SCM media the following growth factors were added: SCM1: human epidermal growth factor (hEGF; 50 ng/mL, Peprotech), human fibroblast growth factor (hFGF2; 50 ng/mL, Peprotech) and human insulin-like growth factor-I (hIGF1; 100 ng/mL, Peprotech), SCM2; hEGF; 50 ng/mL, hFGF2; 10 ng/mL and hIGF1; 100 ng/mL and SCM3; hEGF; 15 ng/mL, hFGF2; 15 ng/mL and hIGF1; 100 ng/mL. Every 3–4 days, 50% of the medium was removed and fresh medium was added. Cultures were passaged every 1–3 weeks in a 1:2 to 1:6 ratio. For passaging, tumoroids were disrupted by mechanical dissociation by using a P1000 pipet. Following the addition of 5-10 mL ice-cold Adv-DF+++, cells were centrifuged at 300 × G for 5 min at 4 °C and subsequently reseeded in fresh STS medium. All tumoroid models were established in suspension plates (#665102, Greiner) and grown at 37 °C with 5% CO2. Tumoroids were regularly (every 6–8 weeks) checked for mycoplasma contamination using the MycoAlert Mycoplasma Detection Kit (#LT07-318, Lonza). Tumoroids were frozen down for long-term storage in CellBanker 2 (#11914, Amsbio).
Fusion detection by RT-qPCR
For the detection of fusion transcripts, snap-frozen cell pellets were collected after passaging the tumoroid cultures for at least five times. Total RNA was isolated from tumoroid lines using an Rneasy Mini Kit (#74104, Qiagen) according to the manufacturer’s instructions, including the recommended DNAse I treatment (#79254, Qiagen). A total of 500 ng RNA was used for cDNA synthesis, using an oligo-dT primed Superscript III Reverse Transcriptase (#18080044, Invitrogen) base reaction according to the manufacturer’s protocol. RT-PCR for the different fusion transcripts was performed with the obtained cDNA using iQ SYBR Green (Bio-Rad, #1708882). PCR reactions were run on 1.5% agarose gel. PCR products were cut out and purified with an QIAquick Gel Extraction Kit (#28704, Qiagen). Purified PCR products were sequenced with Sanger sequencing to confirm the identity and determine the exact fusion transcripts. Primers used in this study are listed in Supplementary Data 3.
Histology and immunohistochemistry
Tumor tissues and tumoroids were fixed in 4% paraformaldehyde, dehydrated, and subsequently embedded in paraffin. Subsequently, 4 µm thick sections were cut and used for H&E and immunohistochemical staining according to standard protocols. The following primary antibodies were used: CD99 (#915604, Biolegend, 1:150), WT1 (#05-753, Sigma-Aldrich, 1:200), BCOR (#Z2334MP, Zeta Corporation, 1:100) and ETV4 (#LS-C800519_200, LS Bio, 1:200). Images were acquired with a Leica DMi8 microscope.
Whole-genome and whole-exome sequencing
Genomic DNA was extracted between passage 5–10 using QIAGEN blood and tissue DNA kit (QIAGEN, Hilden, Germany). DNA library preparation was done using the KAPA HyperPlus kit (Roche, Basel, Switzerland) according to the manufacturer’s instructions. Tumors (60–90× coverage), whole blood (30x coverage) and tumoroids (30× coverage) were sequenced on a NovaSeq 6000 (Illumina). Reads were mapped against the human reference genome (GRCh38) with the Burrows-Wheeler Aligner (v0.7.5a) mapping software with settings ‘bwa mem -c 100 -M’. Duplicate reads were marked with Sambamba (v0.6.8). Variants were annotated using the Genome Analysis Toolkit (GATK) (v3.8-1-0). A detailed description of the complete data analysis pipeline is available at: https://github.com/UMCUGenetics/IAP and https://github.com/Hubrecht-Clevers/NF-IAP_exome for WGS and WXS, respectively. Postprocessing of variants was done using the SMuRF pipeline with default settings. A detailed overview of the postprocessing pipeline can be found at https://github.com/ToolsVanBox/SMuRF. Briefly, variants on all autosomal and sex chromosomes were used in this analysis. Variants were filtered on mapping quality >40, base coverage of at least 10, a blacklist was used to filter out known false positives (available on request). Variants were also filtered to a matched normal sample when available. Furthermore, variants with a mean allele frequency of above 0.01 in the general population were filtered out unless these variants had a COSMIC identifier. For samples that did not have a matched normal, only known gene altering variants with a COSMIC identifier in a known oncogene (COSMIC cancer consensus genes) were taken into account. VCF-files from postprocessing were converted to MAF-files using a personalized script available at https://github.com/Hubrecht-Clevers/Convert_maf. Variants were further analyzed in Rstudio with R (v4.4.0), maftools (v2.19), ggplot2 (v3.5.1), data.table (v1.15.4), RColorBrewer (v1.1-3), NMF (v0.27), viridis (v0.6.5), gggenes (v0.5.1), Seurat (v5.0.3), ggrepel (v0.9.5) and readxl (v1.4.3). The full script is available at https://github.com/Hubrecht-Clevers/small_cell_sarcomas. Briefly, variants were divided into coding silent mutations such as synonymous variants, intron variants and in-frame deletions or insertions and damaging mutations such as nonsynonymous variants, frameshifts and gene fusions. Visualization of mutations was further processed in R using maftools (v2.19.0). The detailed script is available at https://github.com/Hubrecht-Clevers/small_cell_sarcomas.
Mutational signature analysis
All single nucleotide variants, both non-synonymous and synonymous were loaden into R. A trinucleotide matrix was constructed using the UCSC h38 genome. Mutational signatures were extracted using the extractSignatures function from Maftools. Signatures were compared with known SBS signatures form the COSMIC database. Cosine distance was computed and the best match for each signature was annotated to the extracted signatures. Ggplot2 was used for further visualization of the mutational signatures. The full script is available at https://github.com/Hubrecht-Clevers/small_cell_sarcomas.
Clonal evolution on longitudinal tumor samples
A full description of the pipeline and variables is available at https://github.com/Hubrecht-Clevers/small_cell_sarcomas. Briefly, allele specific copy number profiles were constructed using CNV-facets(v0.5.14) using standard settings82. Doing so, for all variants (synonymous and non-synonymous) the minor and major copy number was computed. Next, subclones were identified using pyclone-vi83. Pyclone fit function was run with the following settings “-c 40 -d beta-binomial -r 100”, followed by the write-results-file function. Visualization of subclones over the different samples was done in R using the ggalluvial package (v0.12.5).
Bulk RNA sequencing
Total RNA was extracted from tumoroid lines using a Rneasy Mini Kit (#74104, Qiagen) following manufacturer’s instructions, including a recommended DNAse I treatment (#79254, Qiagen). In case of tumor tissue, total RNA was extracted using an AllPrep DNA/RNA/miRNA universal Kit (#80224, Qiagen) following manufacturer’s instructions. RNA sequencing of tumor tissues and tumoroids, unless specified, was performed as previously described84. In short, cDNA libraries were generated using the KAPA RNA HyperPrep Kit with RiboErase (#08098140702, Roche) and sequenced on a NovaSeq 6000 (2 × 150 bp) (Illumina). Reads were mapped to the human26,27,28,29,30,31 (GRCh38 and gencode version 32) by STAR(v2.7.2 d). Fusions were detected by STARfusion (v1.8.0) using standard settings. Raw read counts were obtained via featureCounts function of the subread package (v1.6.6). Fusion transcripts were compared between tumor and tumoroid to confirm matching tumor/tumoroid pairs. Readcounts were further analyzed in R using DESEQ2 (v1.44.0) for differential expression analysis. Pathway analyses were preformed using the Enrichr packages (v3.2). The full script used in this analysis and to generate figures is available at https://github.com/Hubrecht-Clevers/small_cell_sarcomas.
Medium-throughput drug screens
Tumoroids were harvested, washed in ice-cold Adv-DF+++, filtered using a 70 μm nylon cell strainer (#542070, Greiner Bio-One) and resuspended in SCM. Subsequently, ~800 tumoroid clusters (one cluster consisting of ~6 cells) were plated in a volume of 40 µl per well in black 384-well plates (#3764, Corning) using a Multi-drop Combi Reagent Dispenser (#5840340, Thermo Scientific). Two days after plating, compounds were added (six different concentrations) using an acoustic liquid handler Echo550. Positive control samples were treated with staurosporine (final concentration 10 μM) and negative control samples were treated with DMSO (final concentration of 0.25%). All medium-throughput drug screens were carried out in duplicate. The drug screens were performed using a library containing 186 drugs. Drugs are listed in Supplementary Data 4. Readouts were performed at T = 0 (no drugs—control plate) and T = 120 (120 h after drugs added—readout) using CellTiter-Glo 3D reagent (#G9683, Promega) according to the manufacturer’s instructions on the Spectramax i3x luminescence microplate reader. The results were normalized to the DMSO vehicle (100% viability) (Supplementary Data 5). Data were analyzed with software GraphPad Prism v10.02. Forced group clustering was visualized using the R package ComplexHeatmap v2.2.0 with default values.
Validation drug screen
Validations drug screens were performed in the same workflow as mentioned above, with the exeption that compounds were added using the Tecan D300e Digital Dispenser. The following drugs were used: Bortezomib (#HY-10227), AZD5991 (#HY-101533), Tapotoclax (#HY-101565), S63845 (#HY-10074), MIK665 (#HY-112218), and A − 1210477 (#HY-12468) were all purchased from MedChemExpress. For the validation screens, twelve concentrations (0.03 nM, 0.1 nM, 0.3 nM, 1 nM, 3 nM, 10 nM, 30 nM, 100 nM, 300 nM, 1 µM, 3 µM and 10 µM) were used. Drugs were added in triplicate for each compound per experiment. Validation experiments were performed at least twice. Bortezomib was taken along on every validation and was used as internal positive control. The results were normalized to the DMSO vehicle (100% viability). Data were analyzed with software GraphPad Prism v10.02.
Apoptosis assay
ES and CDS tumoroids were seeded in 96-well plates 2 days prior to treatment. The seeded tumoroids were incubated with single agent MIK665 (50 nM) for 1, 3 and 6 h at 37 °C. Individual wells with tumoroids were washed with PBS, and Annexin V binding buffer (#422201, Biolegend), followed by staining with Annexin V-FITC (#940906, Biolegend) for 15 min in the dark at room temperature. Then, propidium iodide (#421301, Biolegend) was added 5 min prior imaging. Images were acquired with a Leica STELLARIS8 FALCON microscope.
Western blotting assay
ES and CDS tumoroids were collected and washed 3× with PBS. Whole-cell lysates were prepared using RIPA lysis buffer (50 mM Tris, 150 mM NaCl, 0.1% sodium dodecyl sulfate, 0.5% sodium deoxycholate, 0.1% Triton X-100) to which protease/phosphatase inhibitor cocktails (#11873580001, Roche) were freshly added in a 1:100 ratio. Protein concentrations were determined using a BCA protein assay kit (#23227, Pierce™, Thermo Scientific™) per manufacturer’s instructions. Lysate samples containing 20 μg of protein were prepared with Laemmli loading buffer and separated on 12% SDS-polyacrylamide gels and transferred to 0.45 µM nitrocellulose membranes using wet transfer. Membranes were probed overnight at 4 °C with the following primary antibodies: MCL-1 (#CST#39224, Cell Signaling), NOXA (#MA5-32679, Invitrogen™) and Vinculin (CST#13901, Cell Signaling). Membranes were subsequently incubated with rabbit (#G21234, Invitrogen™) horseradish peroxidase-conjugated secondary antibody and protein bands were visualized using ECL Western Blotting Substrate (#32209, Pierce™, Thermo Scientific™). Images were captured using Bio-Rad ChemiDoc, and densitometric analysis was carried out using ImageJ (V2.0.0-rc-69/1.52i).
Original western blots are available in the Source data file
Statistics analysis
AUC was calculated using Prism software version 10.0.2 with a 4PL regression curve. Values (AUC) were plotted on a matrix to evaluate sample to sample differences. Differences in AUC values of specific drugs were evaluated using a one-way ANOVA (more than 2 groups) or a students’ T-test (when only 2 groups) in Prism. Differential gene expression was calculated using the DEseq2 package using standard settings. nbinomLRT likelyhood test was used to identify DEGs and pvalues were adjusted with a false discovery rate by methods of Benjamini and Hochberg. Euclidian sample distance matrices were calculated based on RNA expression values. Samples were then clusterd using hiarchical clustering. For WGS, mutation matrices were generated in a binary format to allow a distance matrix calculaton in Jaccard distance. Samples were then again clustered using hiarchical clustering (R package stats v4.4.0).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The transcriptomic and genomic data are deposited in the EGA study database under accession number EGAS00001007941, RNA-sequencing data is available under accession number EGAD00001015419, WGS data is available under accession number EGAD00001015418 and WXS data is available under accession number EGAD00001015417. Datasets mentioned above are available under restricted access due to patient privacy and regulatory requirements. Access to the sequencing data is managed by the Biobank and Data Access Committee (BDAC) of the Princess Máxima Center. All researchers can request access by submitting a project proposal to the BDAC (biobank@prinsesmaximacentrum.nl). Requests are typically reviewed within ~2 weeks. The duration of access will be determined by the BDAC. The ChIP-seq publicly available dataset used in this study were accessed from ENCODE experiments ENCFF186JKG [https://www.encodeproject.org/files/ENCFF186JKG/] for BCOR. Requests to use the tumoroid lines that were generated in this paper will be evaluated on a case-by-case basis by the BDAC of the Princess Máxima Center. When approved, specific material transfer agreements will be drawn up. To contact the BDAC, email biobank@prinsesmaximacentrum.nl. The remaining data are available within the Article, Supplementary Information or Source Data file. Source data are provided with this paper.
Code availability
All code used to generate figures can be accessed on https://github.com/Hubrecht-Clevers/small_cell_sarcomas.
References
Dehner, C. A., Lazar, A. J. & Chrisinger, J. S. A. Updates on WHO classification for small round cell tumors: Ewing sarcoma vs. everything else. Hum. Pathol. 147, 101–113 (2024).
Ross, K. A., Smyth, N. A., Murawski, C. D. & Kennedy, J. G. The biology of Ewing sarcoma. ISRN Oncol. 2013, 759725 (2013).
Aurias, A., Rimbaut, C., Buffe, D., Zucker, J. M. & Mazabraud, A. Translocation involving chromosome 22 in Ewing’s sarcoma. A cytogenetic study of four fresh tumors. Cancer Genet. Cytogenet. 12, 21–25 (1984).
Delattre, O. et al. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature 359, 162–165 (1992).
Lessnick, S. L., Braun, B. S., Denny, C. T. & May, W. A. Multiple domains mediate transformation by the Ewing’s sarcoma EWS/FLI-1 fusion gene. Oncogene 10, 423–431 (1995).
May, W. A. et al. Ewing sarcoma 11;22 translocation produces a chimeric transcription factor that requires the DNA-binding domain encoded by FLI1 for transformation. Proc. Natl. Acad. Sci. USA 90, 5752–5756 (1993).
Kawamura-Saito, M. et al. Fusion between CIC and DUX4 up-regulates PEA3 family genes in Ewing-like sarcomas with t(4;19)(q35;q13) translocation. Hum. Mol. Genet. 15, 2125–2137 (2006).
Chebib, I. & Jo, V. Y. Round cell sarcoma with CIC-DUX4 gene fusion: discussion of the distinctive cytomorphologic, immunohistochemical, and molecular features in the differential diagnosis of round cell tumors. Cancer Cytopathol. 124, 350–361 (2016).
Okimoto, R. A. et al. CIC-DUX4 oncoprotein drives sarcoma metastasis and tumorigenesis via distinct regulatory programs. J. Clin. Invest 129, 3401–3406 (2019).
Peters, T. L. et al. BCOR-CCNB3 fusions are frequent in undifferentiated sarcomas of male children. Mod. Pathol. 28, 575–586 (2015).
Kao, Y. C. et al. BCOR-CCNB3 fusion positive sarcomas: a clinicopathologic and molecular analysis of 36 cases with comparison to morphologic spectrum and clinical behavior of other round cell sarcomas. Am. J. Surg. Pathol. 42, 604–615 (2018).
Dirksen, U. et al. High-dose chemotherapy compared with standard chemotherapy and lung radiation in ewing sarcoma with pulmonary metastases: results of the European Ewing Tumour Working Initiative of National Groups, 99 Trial and EWING 2008. J. Clin. Oncol. 37, 3192–3202 (2019).
Kovar, H. et al. The first European interdisciplinary ewing sarcoma research summit. Front. Oncol. 2, 54 (2012).
Brahmi, M. et al. Patterns of care and outcome of CIC-rearranged sarcoma patients: a nationwide study of the French sarcoma group. Cancer Med. 12, 7801–7807 (2023).
Kyriazoglou, A. & Bagos, P. Meta-analysis of BCOR rearranged sarcomas: challenging the therapeutic approach. Acta Oncol. 60, 721–726 (2021).
Nanni, P. et al. Bone sarcoma patient-derived xenografts are faithful and stable preclinical models for molecular and therapeutic investigations. Sci. Rep. 9, 12174 (2019).
Orth, M. F. et al. Systematic multi-omics cell line profiling uncovers principles of Ewing sarcoma fusion oncogene-mediated gene regulation. Cell Rep. 41, 111761 (2022).
Sato, T. et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141, 1762–1772 (2011).
Al Shihabi, A. et al. The landscape of drug sensitivity and resistance in sarcoma. Cell Stem Cell 31, 1524–1542.e1524 (2024).
Calandrini, C. et al. An organoid biobank for childhood kidney cancers that captures disease and tissue heterogeneity. Nat. Commun. 11, 1310 (2020).
Meister, M. T. et al. Mesenchymal tumor organoid models recapitulate rhabdomyosarcoma subtypes. EMBO Mol. Med. 14, e16001 (2022).
Kluiver, T. A. et al. Divergent WNT signaling and drug sensitivity profiles within hepatoblastoma tumors and organoids. Nat. Commun. 15, 8576 (2024).
Kim, S. Y. et al. Organoid drug profiling identifies methotrexate as a therapy for SCCOHT, a rare pediatric cancer. Sci. Adv. 11, eadq1724 (2025).
Paassen, I. et al. Atypical teratoid/rhabdoid tumoroids reveal subgroup-specific drug vulnerabilities. Oncogene 42, 1661–1671 (2023).
Kim, S.-Y., van de Wetering, M., Clevers, H. & Sanders, K. The future of tumor organoids in precision therapy. Trends Cancer. https://doi.org/10.1016/j.trecan.2025.03.005 (2025).
Driehuis, E. et al. Pancreatic cancer organoids recapitulate disease and allow personalized drug screening. Proc. Natl. Acad. Sci. USA 116, 26580–26590 (2019).
Vlachogiannis, G. et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 359, 920–926 (2018).
Driehuis, E. et al. Oral mucosal organoids as a potential platform for personalized cancer therapy. Cancer Discov. 9, 852–871 (2019).
Ganesh, K. et al. A rectal cancer organoid platform to study individual responses to chemoradiation. Nat. Med. 25, 1607–1614 (2019).
Ooft, S. N. et al. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci. Transl. Med. 11, eaay2574 (2019).
Tan, T. et al. Unified framework for patient-derived, tumor-organoid-based predictive testing of standard-of-care therapies in metastatic colorectal cancer. Cell Rep. Med. 4, 101335 (2023).
Fujii, M. et al. A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell 18, 827–838 (2016).
Liu, L., Yu, L., Li, Z., Li, W. & Huang, W. Patient-derived organoid (PDO) platforms to facilitate clinical decision making. J. Transl. Med. 19, 40 (2021).
Hirt, C. K. et al. Drug screening and genome editing in human pancreatic cancer organoids identifies drug-gene interactions and candidates for off-label treatment. Cell Genom. 2, 100095 (2022).
Yoshida, A. Ewing and Ewing-like sarcomas: a morphological guide through genetically-defined entities. Pathol. Int 73, 12–26 (2023).
Watson, S. et al. Transcriptomic definition of molecular subgroups of small round cell sarcomas. J. Pathol. 245, 29–40 (2018).
Tsuda, Y. et al. Ewing sarcoma with FEV gene rearrangements is a rare subset with predilection for extraskeletal locations and aggressive behavior. Genes Chromosomes Cancer 59, 286–294 (2020).
Hung, Y. P., Fletcher, C. D. & Hornick, J. L. Evaluation of ETV4 and WT1 expression in CIC-rearranged sarcomas and histologic mimics. Mod. Pathol. 29, 1324–1334 (2016).
Crompton, B. D. et al. The genomic landscape of pediatric Ewing sarcoma. Cancer Discov. 4, 1326–1341 (2014).
Brohl, A. S. et al. The genomic landscape of the Ewing Sarcoma family of tumors reveals recurrent STAG2 mutation. PLoS Genet. 10, e1004475 (2014).
Grünewald, T. G. et al. Chimeric EWSR1-FLI1 regulates the Ewing sarcoma susceptibility gene EGR2 via a GGAA microsatellite. Nat. Genet. 47, 1073–1078 (2015).
Riskjell, A. I., Makinen, V. N., Sandfeld-Paulsen, B. & Aggerholm-Pedersen, N. Targeted treatment of soft-tissue sarcoma. J. Personal. Med. 13, 730 (2023).
Smith, S. C. et al. CIC-DUX sarcomas demonstrate frequent MYC amplification and ETS-family transcription factor expression. Mod. Pathol. 28, 57–68 (2015).
Bui, N. Q. et al. A clinico-genomic analysis of soft tissue sarcoma patients reveals CDKN2A deletion as a biomarker for poor prognosis. Clin. Sarcoma Res. 9, 12 (2019).
Lin, D. I. et al. Genomic profiling of BCOR-rearranged uterine sarcomas reveals novel gene fusion partners, frequent CDK4 amplification and CDKN2A loss. Gynecol. Oncol. 157, 357–366 (2020).
Liu, K. X. et al. Risk stratification by somatic mutation burden in Ewing sarcoma. Cancer 125, 1357–1364 (2019).
Sondka, Z. et al. COSMIC: a curated database of somatic variants and clinical data for cancer. Nucleic Acids Res. 52, D1210–D1217 (2024).
Haas, B. J. et al. Accuracy assessment of fusion transcript detection via read-mapping and de novo fusion transcript assembly-based methods. Genome Biol. 20, 213 (2019).
Doll, S. et al. Proteomic characterization of undifferentiated small round cell sarcomas with EWSR1 and CIC::DUX4 translocations reveals diverging tumor biology and distinct diagnostic markers. Mod. Pathol. 37, 100511 (2024).
Wei, G. H. et al. Genome-wide analysis of ETS-family DNA-binding in vitro and in vivo. EMBO J. 29, 2147–2160 (2010).
Riggi, N. et al. EWS-FLI1 utilizes divergent chromatin remodeling mechanisms to directly activate or repress enhancer elements in Ewing sarcoma. Cancer Cell 26, 668–681 (2014).
Lin, Y. K., Wu, W., Ponce, R. K., Kim, J. W. & Okimoto, R. A. Negative MAPK-ERK regulation sustains CIC-DUX4 oncoprotein expression in undifferentiated sarcoma. Proc. Natl. Acad. Sci. USA 117, 20776–20784 (2020).
Luo, Y. et al. New developments on the Encyclopedia of DNA Elements (ENCODE) data portal. Nucleic Acids Res. 48, D882–d889 (2020).
Hu-Lieskovan, S. et al. EWS-FLI1 fusion protein up-regulates critical genes in neural crest development and is responsible for the observed phenotype of Ewing’s family of tumors. Cancer Res. 65, 4633–4644 (2005).
McGrogan, B. T., Gilmartin, B., Carney, D. N. & McCann, A. Taxanes, microtubules and chemoresistant breast cancer. Biochim. Biophys. Acta 1785, 96–132 (2008).
Wang, B. et al. ATXN1L, CIC, and ETS transcription factors modulate sensitivity to MAPK pathway inhibition. Cell Rep. 18, 1543–1557 (2017).
Hua, X.-Y., Bie, X.-X., Cheng, X. & Zhang, S.-G. High expression of CIN85 promotes proliferation and invasion of human esophageal squamous cell carcinoma. Mol. Med. Rep. 23, 12 (2021).
Gaspar, N. et al. Ewing sarcoma: current management and future approaches through collaboration. J. Clin. Oncol. 33, 3036–3046 (2015).
Whelan, J. et al. Interval compressed vincristine, doxorubicin, cyclophosphamide alternating with ifosfamide, etoposide in patients with advanced Ewing’s and other small round cell sarcomas. Clin. Sarcoma Res 2, 12 (2012).
Connolly, E. A. et al. Systemic treatments and outcomes in CIC-rearranged Sarcoma: a national multi-centre clinicopathological series and literature review. Cancer Med. 11, 1805–1816 (2022).
Antonescu, C. R. et al. Sarcomas With CIC-rearrangements are a distinct pathologic entity with aggressive outcome: a clinicopathologic and molecular study of 115 cases. Am. J. Surg. Pathol. 41, 941–949 (2017).
Murphy, J. et al. Clinical outcomes of patients with CIC-rearranged sarcoma: a single institution retrospective analysis. J. Cancer Res. Clin. Oncol. 150, 112 (2024).
Prieur, A., Tirode, F., Cohen, P. & Delattre, O. EWS/FLI-1 silencing and gene profiling of ewing cells reveal downstream oncogenic pathways and a crucial role for repression of insulin-like growth factor binding protein 3. Mol. Cell. Biol. 24, 7275–7283 (2004).
Mateo-Lozano, S., Tirado, O. M. & Notario, V. Rapamycin induces the fusion-type independent downregulation of the EWS/FLI-1 proteins and inhibits Ewing’s sarcoma cell proliferation. Oncogene 22, 9282–9287 (2003).
Oyama, R. et al. Generation of novel patient-derived CIC-DUX4 sarcoma xenografts and cell lines. Sci. Rep. 7, 4712 (2017).
Nakai, S. et al. Establishment of a novel human CIC-DUX(4) sarcoma cell line, Kitra-SRS, with autocrine IGF-1R activation and metastatic potential to the lungs. Sci. Rep. 9, 15812 (2019).
Fevre, R. et al. Combinatorial drug screening on 3D Ewing sarcoma spheroids using droplet-based microfluidics. iScience 26, 106651 (2023).
Nitsch, A. et al. Effective combination of cold physical plasma and chemotherapy against Ewing sarcoma cells in vitro. Sci. Rep. 14, 6505 (2024).
Guillen, K. P. et al. A human breast cancer-derived xenograft and organoid platform for drug discovery and precision oncology. Nat. Cancer 3, 232–250 (2022).
Karkampouna, S. et al. Patient-derived xenografts and organoids model therapy response in prostate cancer. Nat. Commun. 12, 1117 (2021).
Gao, J. et al. Promising preclinical patient-derived organoid (PDO) and xenograft (PDX) models in upper gastrointestinal cancers: progress and challenges. BMC Cancer 23, 1205 (2023).
Kim, J., Koo, B.-K. & Knoblich, J. A. Human organoids: model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 21, 571–584 (2020).
Ohali, A. et al. High frequency of genomic instability in Ewing family of tumors. Cancer Genet. Cytogenet. 150, 50–56 (2004).
Casey, D. L. et al. TP53 mutations increase radioresistance in rhabdomyosarcoma and Ewing sarcoma. Br. J. Cancer 125, 576–581 (2021).
Lazo de la Vega, L. et al. Targeted next-generation sequencing of CIC-DUX4 soft tissue sarcomas demonstrates low mutational burden and recurrent chromosome 1p loss. Hum. Pathol. 58, 161–170 (2016).
Roerink, S. F. et al. Intra-tumour diversification in colorectal cancer at the single-cell level. Nature 556, 457–462 (2018).
Duffaud, F. et al. Regorafenib in patients with advanced Ewing sarcoma: results of a non-comparative, randomised, double-blind, placebo-controlled, multicentre Phase II study. Br. J. Cancer 129, 1940–1948 (2023).
Lee, K. M. et al. MYC and MCL1 cooperatively promote chemotherapy-resistant breast cancer stem cells via regulation of mitochondrial oxidative phosphorylation. Cell Metab. 26, 633–647.e637 (2017).
Yuda, J. et al. Selective MCL-1 inhibitor ABBV-467 is efficacious in tumor models but is associated with cardiac troponin increases in patients. Commun. Med. 3, 154 (2023).
Wang, X. et al. Deletion of MCL-1 causes lethal cardiac failure and mitochondrial dysfunction. Genes Dev. 27, 1351–1364 (2013).
Grissenberger, S. et al. High-content drug screening in zebrafish xenografts reveals high efficacy of dual MCL-1/BCL-XL inhibition against Ewing sarcoma. Cancer Lett. 554, 216028 (2023).
Shen, R. & Seshan, V. E. FACETS: allele-specific copy number and clonal heterogeneity analysis tool for high-throughput DNA sequencing. Nucleic Acids Res. 44, e131 (2016).
Gillis, S. & Roth, A. PyClone-VI: scalable inference of clonal population structures using whole genome data. BMC Bioinform. 21, 571 (2020).
Hehir-Kwa, J. Y. et al. Improved gene fusion detection in childhood cancer diagnostics using RNA sequencing. JCO Precis Oncol. 6, e2000504 (2022).
Acknowledgements
We thank all children and their families for participating in our research as well as the clinical teams involved in approaching patients for consent and collecting tissue. The ITCC-P4 consortium has received funding from the Innovative Medicines Initiative 2 Joint Undertaking, which has been supported by the European Union’s Horizon 2020 research and innovation program and the European federation of Pharmaceutical Industries and Associations. Research reported in this publication was supported by the Children Cancer Free Foundation (KiKa) (M.v.d.W. and H.C.), by Oncode Institute (H.C.) and by Oncode Accelerator, a Dutch National Growth Fund Project under grant number NGFOP2201 (K.S. and M.v.d.W.).
Author information
Authors and Affiliations
Contributions
Conceptualization: F.R., M.v.d.W., H.C.; Designed experiments: F.R., M.v.d.W.; Performed experiments and data analyses: F.R.; Performed Western blot experiments: S.Y.K; Data curation: F.R., L.V., V.A.A., H.K., M.S., J.J.M., R.v.B.; Performed histopathological evaluations: U.F., L.H.J.; Formal Analysis: F.R., G.v.S., L.V.; Performed bioinformatic analyses: G.v.S.; Project administration: F.R., M.v.d.W., K.S., H.C.; Resources: K.L., J.B., L.H., M.v.d.S., W.v.H., M.v.N., G.S., O.D., K.Sc., B.G., J.H.M.M. and J.J.M.; Provided clinical context and guidance: K.L., M.v.d.S., and J.H.M.M.; Developed pipelines for data analyses: R.v.B.; Supervision: M.v.d.W., K.S., H.C.; Writing—original draft: F.R.; Writing—review & editing: F.R., G.v.S., M.v.d.W., K.S., H.C.; Funding acquisition: M.v.d.W., K.S., H.C.
Corresponding authors
Ethics declarations
Competing interests
H.C. is an inventor on patents held by the Royal Netherlands Academy of Arts and Sciences that cover organoid technology. He is now head of Pharma Research and Early Development (pRED) at Roche, Basel, Switzerland. H.C.’s full disclosure is given at https://uu.nl/staff/JCClevers/. All other authors declare that they have no competing interests.
Peer review
Peer review information
Nature Communications thanks Joseph Ludwig, Alice Soragni, and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ringnalda, F.C.A.S., van Son, G.J.F., Verweij, L.H.G. et al. Small round cell sarcoma tumoroid biobank reveals CIC::DUX4 sarcoma vulnerability to MCL-1 inhibition. Nat Commun 16, 7689 (2025). https://doi.org/10.1038/s41467-025-62673-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-62673-2