Abstract
Both SARS-CoV-2 mRNA and mucosal vaccines induce protective immunity against COVID-19 but showed different immune profiles. We conducted a longitudinal head-to-head analysis of the safety and immunogenicity of the aerosolized adenovirus-vectored and mRNA COVID-19 vaccines. 450 participants were enrolled and randomly assigned into three groups to be vaccinated with an aerosolized Ad5-vectored bivalent vaccine (wild-type and BA.5, Ad5-CoV5T), an intramuscular bivalent mRNA vaccine (mbO5), and an aerosolized wild-type Ad5-vectored vaccine (Ad5-nCoV). The primary outcomes were adverse reactions within 28 days and anti-XBB.1.5-specific neutralizing antibody titers at day 28 after vaccination. The secondary outcome assessed safety within 30 min, serious adverse event within 6 months, and the persistence of anti-XBB.1.5/BA.5-specific neutralizing antibodies during the 6 months. Both the vaccines were well tolerated, but participants vaccinated with mbO5 reported more adverse reactions (73.3% mbO5 vaccinees vs. 28.7% aerosol vaccinees). No serious adverse events were recorded. The Ad5-CoV5T vaccine induced a superior anti-XBB.1.5-specific neutralizing titer than Ad5-nCoV at day 28 (geometric mean titer ratio of 1.48, 95% CI 1.12–1.97), while the mbO5 vaccine induced the highest antibody titer. The neutralizing antibodies were declined at month 6 and were similar across the three groups. In the pre-specified exploratory analysis, the mbO5 and the aerosolized vaccines induced comparable antigen-specific memory B cells but the latter stimulated higher frequency of IgA isotype and higher expression of CXCR3. This trial met the main hypothesis; the findings may provide insights for the development of the next-generation COVID-19 vaccines. Clinical Trials.gov identifier: NCT05886790.
Similar content being viewed by others
Introduction
The COVID-19 vaccines help ensure protection against severe disease and death, but they may not adequately prevent infection or transmission of SARS-CoV-2 virus, especially as immunity declines and new variants emerge1,2. The updated COVID-19 booster vaccines were successively approved for use globally, including the bivalent Omicron BA.5/wild-type (WT) vaccines, and the monovalent XBB.1.5 vaccines, which improve the immune response and provided the better protection against infection, hospitalization and death3,4,5,6,7,8. US FDA authorized monovalent KP.2-adapted COVID-19 vaccines for 2024–2025 season in Aug, 20249.
A vaccine targeted directly to the respiratory tract that induces local mucosal immune responses have the potential to prevent virus infection, shedding and therefore transmission10. The aerosolized Ad5-nCoV, a human type 5 adenovirus which expresses the SARS-CoV-2 WT spike protein, was approved as booster dose in China and showed efficacy in preventing symptomatic SARS-CoV-2 infection during the Omicron BA.5 wave in China11,12. The aerosolized vaccine elicited the higher serum neutralizing antibodies and cellular response in the uninfected persons compared to the inactivated vaccines, the intramuscular adenovirus-vectored vaccine and the recombinant protein vaccine13,14,15. The aerosolized adenovirus-vectored COVID-19 vaccine induces both protective mucosal and systemic immunity and also reduce the likelihood of virus transmission in non-human primates16,17. The heterologous boosting of the COVID-19 mRNA vaccine CS-2034 (WT), developed by CanSino BIO, has a favorable safety profile and induces an effective immune response with protection against SARS-CoV-2 omicron infection18. However, there is a lack of in-depth understanding of the difference in human immune responses induced by the mucosal COVID-19 vaccines and intramuscular vaccines as boosters, including mucosal, cellular and humoral responses.
In this clinical trial, we evaluated the safety and immune profiles boosting with the aerosolized WT Ad5-nCoV, the aerosolized bivalent adenovirus-vectored vaccine encoding the WT/BA.5 SARS-CoV-2 spike proteins (Ad5-CoV5T) and an intramuscular bivalent mRNA vaccine (mbO5), also encoding the WT/BA.5 spike proteins, to gain insight into safety and the breadth, magnitude and durability of serum antibody responses, mucosal antibody responses, systemic memory B cells and T cell responses elicited by the aerosolized adenovirus-vectored vaccine and intramuscular mRNA vaccine. The primary hypothesis was the geometric mean titer (GMT) of anti-XBB.1.5 live-virus neutralizing antibody (LVN Ab) induced by Ad5-CoV5T is superior to that of Ad5-nCoV at day 28 (based on geometric mean titer ratio), the secondary hypothesis was the seroconversion rate of anti-XBB.1.5 LVN Ab induced by Ad5-CoV5T is superior to that of Ad5-nCoV at day 28 (based on the difference in the percentage of participants with a seroconversion). This trial met both hypotheses.
Results
Study design
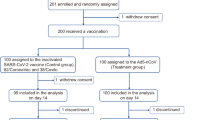
From 30 May to 6 June 2023, 487 individuals were screened for eligibility and 450 were randomly assigned, with 150 in each group (Fig.1). The participants received oral inhalation of an aerosolized bivalent Ad5-vectored COVID-19 vaccine (Ad5-CoV5T, including 5 × 109 viral particles (VPs) of Ad5-nCoV and 5 × 109 VPs of Ad5-nCoV-BA.5) or an aerosolized WT Ad5-vectored COVID-19 vaccine (Ad5-nCoV, 1 × 1010 VPs), or intramuscular injection of a bivalent mRNA COVID-19 vaccine (mbO5, comprised of 15 μg WT SARS-CoV-2 spike mRNA and 15 μg BA.5 spike mRNA, formulated with lipid nanoparticle). Any adverse reactions were monitored within 0–28 days in all subjects and serious adverse events were recorded up to 6 months. All participants were tested for LVN Ab against SARS-CoV-2 XBB.1.5 and BA.5.2 variants at day 0 and day 28, and the first 30 subjects in each group underwent immunogenicity testing up to 6 months, including serum LVN Ab, pseudovirus-neutralizing antibody (PsVN Ab) and anti-spike binding IgA and IgG antibody, nasal swab anti-spike binding IgA, IgG antibody and PsVN Ab, peripheral blood mononuclear cells (PBMCs) spike- or receptor-binding domain (RBD)- specific memory B cells and spike-specific cellular immune response.
Ad5-CoV5T = The participants who received one dose of bivalent Ad5-vectored COVID-19 vaccine (including 5 × 109 VPs of Ad5-nCoV and 5 × 109 VPs of Ad5-nCoV-BA.5) at day 0; mbO5 = The participants who received one dose of intramuscular bivalent mRNA COVID-19 vaccine (comprised of 15 μg WT SARS-CoV-2 spike mRNA and 15 μg BA.5 spike mRNA, formulated with lipid nanoparticle) at day 0; Ad5-nCoV = The participants who received one dose of aerosol Ad5-nCoV of 1 × 1010 VPs at day 0. a, 2 participants loss to the safety follow-up at 6 months; b, 1 participant loss to the sample collection follow-up at 6 months, and 1 participant loss to the safety follow-up at 6 months. c, 4 participants in Ad5-CoV5T group, 1 participant in mbO5 group, and 3 participants in Ad5-nCoV group were infected with SARS-CoV-2 during the 6-month follow-up period, and excluded from the long-term immunogenicity analysis.
Baseline demographics were similar across the study arms, including age, sex, BMI and COVID-19 history, both for the overall study population and the long-term immunogenicity subset (Supplementary Tables 1 and 2). All participants had been previously vaccinated with the COVID-19 vaccine. 71.3%, 73.3% and 78.0% of the participants in each group had been infected with SARS-CoV-2 within 6 months prior to enrollment in the national Omicron outbreak at the end of 2022 in China19, by self-reported positive SARS-CoV-2 PCR or antigenic test. All participants infected with SARS-CoV-2 during the 6-month follow-up period in the first 30 subjects in each group (4 in Ad5-CoV5T group, 1 in mbO5 group and 3 in Ad5-nCoV group), based on anti-nucleocapsid antibody, was excluded from the long-term immunogenicity analysis.
The intramuscular mRNA vaccine induced more adverse events than the aerosolized vaccines
We analyzed the incidence of adverse reactions within 28 days in all subjects as the primary safety endpoint. During this period, 196 (43.6%) of 450 participants reported at least one adverse reaction: 43 (28.7%) in the Ad5-CoV5T group, 110 (73.3%) in the mbO5 group, and 43 (28.7%) in the Ad5-nCoV group (Table 1). No significant different of the incidence of adverse reactions was observed between Ad5-CoV5T and Ad5-nCoV group, but there were more adverse reactions in the participants who received the intramuscular mRNA vaccine compared with those who received the aerosolized vaccines (p < 0.0001). Overall, the most common systematic adverse reactions were fever, headache, fatigue and dizziness, which reported in 26.0% to 40.0% participants in the mbO5 group, but only 6.0% to10.0% in the Ad5-CoV5T and Ad5-nCoV groups (p < 0.0001). The intramuscular mRNA vaccine induced more muscle pain (11.3% vs. 3.0%, p = 0.0004) and joint pian (14.0% vs. 1.7%, p < 0.0001), while the aerosolized vaccines induced more oropharyngeal pain (7.3% vs. 0.7%, p = 0.0051) and dry mouth (9.0% vs. 0%, p < 0.0001). Besides, the intramuscular mRNA vaccine induced significant adverse reactions at injection site, mainly including pain (59.3% vs. 0%, p < 0.0001), itch (25.3% vs. 0%, p < 0.0001), swelling (12.7% vs. 0%, p < 0.0001) and redness (10.7% vs. 0%, p < 0.0001) (Supplementary Table 3). The intramuscular mRNA vaccine also induced more grade 3 adverse reactions, with 22 (14.7% vs. 0.3%, p < 0.0001) participants reported at least one grade 3 adverse reactions, including fever (12.7% vs. 0%, p < 0.0001), headache (2.0%), fatigue (2.0%), muscle pain (0.7%), joint pain (0.7%), injection site pain and swelling (1.3%), while only one participant in the Ad5-nCoV group reported grade 3 oropharyngeal pain. No serious adverse events were reported during the 6 months follow-up. These data suggested that the aerosolized Ad5-vectored vaccine showed a better safety profile than that of the intramuscular mRNA vaccine.
The intramuscular mRNA vaccine induced higher serum neutralizing antibody response and similar antigen-specific memory B cell response compared with the aerosolized vaccines
XBB.1.5 was the predominant Omicron variants in China during this clinical trial period. Firstly, we assessed the GMT of LVN Ab against XBB.1.5 at day 28 in all subjects as the primary immunogenicity endpoint, and the LVN Ab response against BA.5.2 in all subjects at day 28, the anti-XBB.1.5/ BA.5.2 LVN Ab responses at week 2, 12 and 24 for the first 30 subjects in each group as the secondary immunogenicity endpoints (Table 2, Fig. 2a, b). The LVN Ab GMTs against the XBB.1.5 variant was similar at the enrollment baseline among the three groups, with GMTs of 17.5 (95% confidence interval [CI] 14.3–21.6) in the Ad5-CoV5T group, 21.2 (95% CI 17.3–25.9) in the mbO5 group, and 18.6 (95% CI 15.3–22.6) in the Ad5-nCoV group. At day 28, the LVN Ab GMTs against XBB.1.5 increased to 56.9 (95% CI, 46.2–70.2) in the Ad5-CoV5T group, 275.2 (95% CI, 243.6–310.9) in the mbO5 group, and 38.4 (95% CI, 31.7–46.5) in the Ad5-nCoV group (Table 2). The Ad5-CoV5T vaccine induced a superior anti-XBB.1.5 LVN Ab GMT than that of Ad5-nCoV (p = 0.0032; GMT ratio 1.48, 95% CI, 1.12–1.97, met the superiority criterion). However, the mbO5 vaccine induced the highest LVN Ab response among the three groups (p < 0.0001). Seroconversions against XBB.1.5 variant at day 28 were 48.0% in the Ad5-CoV5T group, 86.7% in the mbO5 group, and 31.3% in the Ad5-nCoV group, with both Ad5-CoV5T and mbO5 groups were significantly higher than the Ad5-nCoV group (Ad5-CoV5T vs. Ad5-nCoV, p = 0.0032; mbO5 vs. Ad5-nCoV, p < 0.0001). The difference of the seroconversion rate between Ad5-CoV5T and Ad5-nCoV was 16.7% (95% CI, 5.8%–27.6%), which met the secondary superiority criterion. The tendency of LVN Ab GMTs against the BA.5.2 variant was similar with the XBB.1.5 variant, with GMTs of 182.1 (95% CI, 151.5–218.8) in the Ad5-CoV5T group, 785.2 (95% CI, 695.7–886.2) in the mbO5 group, and 122.2 (95% CI 103.4–144.4) in the Ad5-nCoV group at day 28 (Ad5-CoV5T vs. Ad5-nCoV, p = 0.0003; mbO5 vs. Ad5-nCoV, p < 0.0001). Seroconversion against BA.5.2 variant at day 28 was 52.0% in the Ad5-CoV5T group, 92.7% in the mbO5 group and 30.0% in the Ad5-nCoV group (Ad5-CoV5T vs. Ad5-nCoV, p = 0.0001; mbO5 vs. Ad5-nCoV, p < 0.0001). LVN Ab response against XBB.1.5 and BA.5.2 variant in the mbO5 mRNA vaccine group peaked at week 2 or week 4, while the LVN Ab responses in the aerosolized vaccine groups peaked at week 12 and remained stable from peak to week 24 (Fig. 2a, b). LVN GMTs in the mbO5 mRNA vaccine group decreased significantly from peak to week 24 (XBB.1.5, P < 0.0001; BA.5.2, P = 0.0001) and finally, the LVN GMTs against the variants were similar at week 24 among the three groups. We assessed the PsVN Ab response against SARS-CoV-2 variants as pre-specified exploratory endpoints, and a similar phenomenon of change was observed in the PsVN Ab response against BF.7, XBB.1.5, EG.5, JN.1 variants and WT strain (Supplementary Fig. 1). These results suggested the aerosolized vaccines elicited modest neutralizing antibody responses, but the mRNA vaccine produced more intense but faster-decaying neutralizing antibodies. Both the main hypothesis and the secondary hypothesis of this trial were met.
Participants were vaccinated with Ad5-CoV5T, mbO5 or Ad5-nCoV on day 0, serum samples were collected at week 0, 2, 4, 12, and 24 for antibody detection, PBMCs were collected at week 0, 4, and 24 for RBD or spike-specific memory B cell detection. Serum SARS-CoV-2 XBB.1.5 (a) and BA.5.2 (b) live virus neutralizing antibody titers over time. Dotted lines indicate the limit of detection for the assay, titers below the limit were assigned a value of 2. Bars show geometric mean values with the 95% confidence interval (CI). c Frequencies of SARS-CoV-2 RBD- or spike-specific (WT+ or BA.5+ or XBB.1.5+) memory B cells over time. Bars show geometric mean values with the 95% CI. d Multi-reactivity profiles of RBD-specific memory B cells. Frequencies (e) and percents (f) of WT+ BA.5+ XBB.1.5+, WT+ BA.5+ XBB.1.5-, WT+ BA.5- XBB.1.5+, WT- BA.5+ XBB.1.5+, WT+ BA.5- XBB.1.5-, WT- BA.5+ XBB.1.5- and WT- BA.5- XBB.1.5+ RBD-specific memory B cells over time. Plots show geometric mean values with the 95% CI in (e) and mean values with the 95% CI in (f). Statistics in (a, b, e, f) were calculated using unpaired nonparametric two-tailed Kruskal-Wallis test with Dunn’s correct for multiple comparisons. Statistics in (c) were calculated using paired nonparametric two-tailed Friedman test with Dunn’s correct for multiple comparisons. N = 26 for Ad5-CoV5T group, n = 29 for mbO5 group and n = 27 for Ad5-nCoV group. The neutralizing antibody detection was measured in duplicate, the antigen-specific memory B cell detection was measured in a single tube per sample due to the limited number of PBMCs. P-values marked in red in (e, f) indicate comparisons between Ad5-CoV5T group and mbO5 group, p-values marked in blue indicate comparisons between Ad5-nCoV group and mbO5 group.
The frequencies of SARS-CoV-2 RBD- and spike-specific memory B cells in PBMCs were measured for the first 30 subjects in each group as pre-specified exploratory endpoints by using a flow cytometric assay on the basic of binding to fluorescent antigens (Supplementary Fig. 2). The frequency of the RBD-specific memory B cells increased in all three groups at week 4, ranging from 0.09 to 0.12%, but significantly decreased at week 24 (Fig. 2c). Although the mbO5 mRNA vaccine elicited significantly higher peak neutralizing antibody responses, there was no difference in RBD-specific memory B cell response among the three groups, except for the frequency of BA. 5+ RBD-specific memory B cells at week 4 (mbO5 vs. Ad5-CoV5T, P = 0.041) (Fig. 2c, Supplementary Fig. 3a–c). The spike-specific memory B cells exhibited the similar trend to the RBD-specific memory B cells after the different vaccination, with a 2-fold increase in the percentage of total B cells compared to the RBD-specific memory B cells (Fig. 2c, Supplementary Fig. 3d–f).
The RBD-specific memory B cells exhibited broad-spectrum reactivity, with ~40% of the cells responding to the WT, BA.5 and XBB.1.5 RBD antigens simultaneously (Fig. 2d). The vaccination, whether it is mRNA vaccine or aerosolized vaccines, increased the proportion of WT+, BA.5+ and XBB.1.5+ memory B cells in the total B cells at week 4, but declined even below the baseline at week 24 (Fig. 2e). Although the Ad5-CoV5T vaccine contains the BA.5 antigen, no more BA.5+ memory B cells were observed compared to the Ad5-nCoV vaccine. In addition, compared to the aerosolized vaccine, mbO5 mRNA vaccine induced more BA.5+ XBB.1.5+, BA.5+ and XBB.1.5+ memory B cells, and increased the proportion of these cells in the RBD-specific memory B cells from week 4 to week 24 (Fig. 2e, f).
The aerosolized vaccines induced different spike-specific mucosal IgA and IgG response from the intramuscular mRNA vaccine
The participants’ nasal swabs for the first 30 subjects in each group were collected at the baseline, week 4 and 24, and the anti-spike binding IgA and IgG antibody responses against WT SARS-CoV-2 and their variants were assessed as pre-specified exploratory endpoints by MSD electro chemiluminescence multiplex assay and the values were normalized for the total IgA and IgG content of the nasal swabs. The nasal anti-spike IgA titers were consistent across three groups at the baseline and the geometric mean fold rise (GMFR) to the BA.5, XBB.1 and WT spike ranged from 1.7 to 3.0 at week 4 and remained from 1.0 to 1.8 at week 24 (Fig. 3a, Supplementary Fig. 4a). The nasal anti-spike IgA titers of the aerosolized Ad5-nCoV vaccine group were slightly higher than the other two groups at week 4 and week 24 (Supplementary Fig. 4a). However, the nasal anti-spike IgG response showed different profile with the IgA response after vaccination (Fig. 3b, Supplementary Fig. 4b). At week 4, the GMFR of the nasal IgG titers to the BA.5 and WT spike were 5.9 and 3.1 in the mbO5 vaccine group respectively, significantly higher than those in the aerosolized vaccine groups (BA.5, mbO5 vs. Ad5-COV5T, p = 0.0003, mbO5 vs. Ad5-nCoV, p = 0.0012; WT, mbO5 vs. Ad5-COV5T, p = 0.0075) (Fig. 3b). By week 24 post-vaccination, the anti-spike nasal IgA and IgG returned to the baseline levels, except for the IgA levels in the Ad5-nCoV group and IgG levels in the mbO5 group, which remained approximately twofold above baseline against most variants. Overall, the intramuscular mRNA vaccination produced the higher nasal anti-spike IgG response, and the aerosolized WT Ad5-nCoV, rather than the bivalent Ad5-COV5T, elicited a longer lasting nasal anti-spike IgA antibody response.
Participants were vaccinated with Ad5-CoV5T, mbO5 or Ad5-nCoV on day 0, nasal swabs were collected at week 0, 4, 24 and serum samples were collected at week 0, 2, 4, 12 and 24 for IgA and IgG concentrations detection, PBMCs were collected at week 0, 4 and 24 for antigen-specific memory B cell detection. Geometric mean fold rise of spike-specific IgA (a) and IgG (b) concentrations in nasal swab at 4- and 24-weeks post-vaccination. Geometric mean fold rise of spike-specific IgA (c) and IgG (d) concentrations in serum at 2-, 4-, 12- and 24-weeks post-vaccination. Plots show geometric mean values with the 95% CI; dotted lines indicate the fold rise equal to 1. e Correlation of nasal swab IgG concentrations with serum IgG concentrations at week 4. f Correlation of nasal swab IgA concentrations with serum IgA concentrations at week 4. g Frequencies of RBD+ IgG+, RBD+ IgA+ and RBD+ IgM+ memory B cells over time. h Frequencies of spike+ IgG+, spike+ IgA+ and spike+ IgM+ memory B cells over time. Plots show geometric mean values with the 95% CI. For (e, f), correlations were calculated using nonparametric two-tailed Spearman’s rank correlation. N = 26 for Ad5-CoV5T group, n = 29 for mbO5 group and n = 27 for Ad5-nCoV group. The IgA and IgG detection was measured in duplicate, the antigen-specific memory B cell detection was measured in a single tube per sample due to the limited number of PBMCs.
The anti-spike IgG and IgA antibody responses in serum were also assessed as exploratory endpoint that weren’t pre-specified. Anti-spike IgG and IgA antibody responses to the variants in the mbO5 vaccine group peaked at week 2, with GMFRs ranging from 5.9 to 13.6 for the different variants, whereas in the aerosolized vaccine groups, the anti-spike IgG antibody responses peaked at week 12 and the anti-spike IgA antibody responses peaked at week 4, with the GMFRs from 1.7 to 3.3 (Fig. 3c, d, Supplementary Fig. 4). There was a strong correlation between the serum anti-spike IgG and nasal anti-spike IgG antibody titers (BA.5, R2 = 0.7251, P < 0.0001; WT, R2 = 0.6700, P < 0.0001), but a weaker correlation existed between the serum anti-spike IgA and nasal anti-spike IgA titers (BA.5, R2 = 0.1018, P = 0,0035; WT, R2 = 0.1808, P < 0.0001) (Fig. 3e, f), indicating that the spike-specific IgG antibody in nasal may leak from the blood. The SARS-CoV-2 PsVN Ab response against WT, BA.5 and XBB.1.5 in nasal swabs were also assessed as exploratory endpoint that weren’t pre-specified, and the titers were normalized for the total IgA and IgG content. A slightly increase of PsVN Ab responses were detected at week 4, with the mbO5 mRNA vaccine induced a significantly higher BA.5 PsVN Ab titers than Ad5-COV5T (p = 0.047), which may be attributed to the high nasal IgG response induced by mbO5 (Supplementary Fig. 5a–c). There were good correlations between nasal PsVN Ab titers and nasal IgA concentrations against WT, BA.5 and XBB.1.5, with R2 of 0.4418–0.6384 in the Ad5-COV5T group, 0.3664–0.6781 in the mbO5 group, and 0.6074–0.6559 in the Ad5-nCoV group. While the correlations between nasal PsVN Ab titers and nasal IgG concentrations were lower, with R2 of 0.1673–0.3145 in the Ad5-COV5T group, 0.0675–0.288 in the mbO5 group, and 0.4367–0.4953 in the Ad5-nCoV group (Supplementary Fig. 5d–r). Those data indicated that the nasal IgA antibodies possess a neutralizing function.
At week 4 after vaccination, RBD-specific memory B cell isotype was dominant by IgG (85.0–89.3%), followed by IgA (7.2– 7.5%) and IgM (0–0.6%) among the three groups, with similar proportions at the baseline and week 24, and also the spike-specific memory B cell isotypes (Supplementary Fig. 6). The aerosolized vaccines stimulated the IgA+ RBD-specific memory B cell response at week 4, but the mbO5 mRNA vaccine didn’t, while both vaccines increased the frequency of IgG+ RBD-specific memory B cells at week 4 (Fig. 3g). The IgG+ and IgA+ spike-specific memory B cells also showed a similar trend of change (Fig. 3h). This indicated that inhalation of the aerosolized vaccine induced the IgA+ memory B cell response, but not the intramuscular vaccination.
The aerosolized vaccines stimulated distinct memory B cell phenotypes compared to the intramuscular mRNA vaccine
Memory B cells generated by mucosal immunity can express specific homing markers, for example, pulmonary viral infection elicits memory B cells in the lung that express CXCR320. CD71 is a surface marker to label proliferating peripheral blood B cells after immunization. We further analyzed the phenotype of RBD-specific memory B cells with the expression level of CXCR3, CXCR5 and CD71 as pre-specified exploratory endpoint. Compared to the total memory B cells, the expression levels of CXCR3 and CD71 increased in RBD-specific memory B cells at week 4. The mean fluorescence intensity (MFI) of CXCR3 and CD71 on RBD-specific memory B cells were significantly higher in the Ad5-CoV5T and Ad5-nCoV groups than that in the mbO5 group at week 4, and recovered to a similar level at week 24 among the three groups (Fig. 4a, b). All the vaccination decreased the expression level of CXCR5 on RBD-specific memory B cells at week 4 after vaccination, while the mRNA vaccine induced a lower CXCR5 expression on RBD-specific memory B cells compared to those of the aerosolized vaccines, and persisted until 24 weeks (Fig. 4c). The MFI of CXCR3, CD71 and CXCR5 on spike-specific memory B cells exhibited the similar trend to the RBD-specific memory B cells at week 4 (Fig. 4d). This suggested the aerosolized vaccine produced distinct phenotypes on the antigen-specific memory B cells compared to the intramuscular mRNA vaccine.
Participants were vaccinated with Ad5-CoV5T, mbO5 or Ad5-nCoV on day 0, PBMCs were collected at week 0, 4 and 24 for antigen-specific memory B cell detection. Mean fluorescence intensity (MFI) of CXCR3 (a), CD71 (b) and CXCR5 (c) on RBD-specific memory B cells or total memory B cells at week 0, 4, and 24. Bars show median values with the 95% CI. d MFI of CXCR3, CD71 and CXCR5 on spike-specific memory B cells or total memory B cells at week 4. Bars show median values with the 95% CI. Percents of activated (e), tissue-like (f), resting (g) and intermediate (h) RBD-specific memory B cell subsets at week 0, 4, and 24. Bars show median values with the 95% CI. Frequencies of activated (i), tissue-like (j), resting (k) and intermediate (l) RBD-specific memory B cell subsets at week 0, 4, and 24. Values less than 0.001 were assigned to 0.001. Lines connect the median values at different time points. Bars show median values with the 95% CI. For (a–h), statistics were calculated using unpaired nonparametric two-tailed Kruskal-Wallis test with Dunn’s correct for multiple comparisons. For (i–l), statistics between different time points within one group were calculated using paired nonparametric two-tailed Friedman test with Dunn’s correct for multiple comparisons, statistics between the different groups at week 4 were calculated using unpaired nonparametric two-tailed Kruskal-Wallis test with Dunn’s correct for multiple comparisons. N = 26 for Ad5-CoV5T group, n = 29 for mbO5 group and n = 27 for Ad5-nCoV group. The detection was measured in a single tube per sample due to the limited number of PBMCs. P values marked in red in (i, j) indicate comparisons between Ad5-CoV5T group and mbO5 group, p values marked in blue indicate comparisons between Ad5-nCoV group and mbO5 group. MBCs, total memory B cells.
Memory B cells can be subdivided into phenotypically and functionally distinct subsets in humans, including typical resting memory B cells (CD21+CD27+), activated memory B cells (CD21-CD27+), which contains a plasmablast-like subset, and exhausted tissue-like memory B cells (CD21-CD27-), which contains a CD11c+ subset features21. The proportion of CD21-CD27+ and CD21-CD27- RBD-specific memory B cells were significantly higher in the mbO5 group at week 4 after vaccination, while the proportion of the resting memory B cells was significantly higher in the Ad5-CoV5T and Ad5-nCoV groups, and no significant difference was observed in the proportion of intermediate memory B cells (CD21+CD27-) (Fig. 4e–h). The frequency of RBD-specific activated and exhausted tissue-like memory B cells in total B cells were significantly increased in mbO5 group at week 4, and declined to the baseline at week 24 (Fig. 4i, j). However, there were no increased in the frequency of RBD-specific activated and exhausted tissue-like memory B cells in the Ad5-CoV5T and Ad5-nCoV groups. The frequency of RBD-specific resting memory B cells was significantly increased in all three groups (Fig. 4k), but only a slight increase was observed in the intermediate memory B cells (Fig. 4l). A significantly higher response was observed in the frequency of RBD-specific activated and exhausted tissue-like memory B cells in mbO5 group at week 4, but no significant difference was observed in the frequency of RBD-specific resting and intermediate memory B cells among the three groups (Fig. 4i–l).
The aerosolized vaccines induced spike-specific CD4+ and CD8+ T cell responses similar to the intramuscular mRNA vaccine in peripheral blood
Spike-specific CD4+ and CD8+ T cell response was measured for the first 30 subjects in each group by a flow cytometry as exploratory outcome that weren’t pre-specified, including activation-induced marker (AIM) (CD4+CD40L+4-1BB+, CD8+4-1BB+), intracellular cytokine staining (ICS) assay and memory subsets (Supplementary Fig. 7). PBMCs were stimulated with an overlapping peptide pool covering the full-length amino acid sequence of spike. Antigen specific responses were quantified as the frequency of IFNγ+ or AIM+ T cells in stimulated samples with background subtraction from paired unstimulated controls.
SARS-CoV-2-specifc T cell response play an important role in protection against COVID-1922. Almost all individuals in our cohort showed positive spike-specific CD4+ and CD8+ T cell response before vaccination. A single inhalation of aerosolized Ad5-CoV5T or Ad5-nCoV or intramuscular injection of mbO5 mRNA vaccine induced a significant increase in frequency of IFNγ+ CD4+ T cells and peaked at week 2 post-vaccination (Fig. 5a). The response decreased significantly out to 24 weeks; however, all subjects showed a positive response at week 24. No significant differences were observed in IFNγ+ CD4+ T cell response among the three groups at any timepoint. A similar trajectory was observed in CD4+ AIM+ response, with a minor increase in Ad5-CoV5T and Ad5-nCoV group but a significant increase in mbO5 group at week 2, and significantly decreased out to 24 weeks in all groups (Fig. 5b). Ad5-CoV5T, Ad5-nCoV, and mbO5 vaccines induced multifunctional spike-specific CD4+ T cells, which peaked at 4 weeks post-vaccination and recovered to the baseline at 24 weeks (Fig. 5e). For CD8+ T cell response, both IFNγ+ and AIM+ responses peaked on week 2 to 4 post-vaccination among three groups, and decreased to the pre-vaccination level at week 24 (Fig. 5c, d). The spike-specific CD8+ T cells showed a less multifunctionality, with IL2+ CD8+ T cells dominating before vaccination and IFNγ+ CD8+ T cells dominating at week 4 (Fig. 5f). The spike-specific CD4+ and CD8+ T cell responses were comparable for the aerosolized vaccine and the mbO5 mRNA vaccine at all timepoints.
Participants were vaccinated with Ad5-CoV5T, mbO5 or Ad5-nCoV on day 0, PBMCs were collected at week 0, 2, 4 and 24 for spike-specific T cell detection. Frequencies of IFNγ+ CD4+ T cells (a), AIM+ CD4+ T cells (b), IFNγ+ CD8+ T cells (c) and AIM+ CD8+ T cells (d) over times in PBMCs. Data were background subtracted with the unstimulated control for each sample at each time point and values less than 0.001 were assigned to 0.001. Summary plots show median values with the 95% CI. Multifunctional profiles of spike-specific CD4+ T cells (e) and spike-specific CD8+ T cells (f). g Gating strategy of IFNγ+ CD4+ T helper subsets. The subsets were defined on the expression of chemokine receptor. h Frequencies of IFNγ+ CD4+ T helper subsets over time. Values less than 0.001 were assigned to 0.001. Lines connect the median values at different time points. Bars show median values with the 95% CI. Statistics were calculated using paired nonparametric two-tailed Friedman test with Dunn’s correct for multiple comparisons. N = 26 for Ad5-CoV5T group, n = 29 for mbO5 group and n = 27 for Ad5-nCoV group. The detection was measured in a peptide pool stimulated tube and an unstimulated control tube per sample.
Ad5-CoV5T, Ad5-nCoV and mbO5 vaccines also preferentially induced spike-specific CD4+ circulating T follicular helper cells (cTfh cells), T-helper 1 cells (Th1 cells) and Th17 cells, whereas Th2 and Th1/17 cells were detected at lower levels (Fig. 5g, h). Although no significant difference in CD4+ IFNγ+ cTfh and Th cell responses were observed among the three groups, the mbO5 mRNA vaccine induced a higher IFNγ+ cTfh cell response at week 2 and 4, which was consistent with the higher neutralizing antibody responses in mbO5 vaccinees and the spike-specific cTfh cells is crucial for supporting antibody responses following vaccination23. Consistent with the IFNγ+ cTfh cell response, both the aerosolized vaccines and the mRNA vaccine induced CD4+ TNF+ and IL2+ cTfh cell responses at week 2, and the mRNA vaccine induced a higher IL2+ cTfh cell response (Supplementary Fig. 8). The CD4+ and CD8+ T cells memory subsets were also detected. The frequency of IFNγ+ CD4+ and IFNγ+ CD8+ T cells memory subsets were comparable among the three groups. The IFNγ+ CD4+ T cells responses were mainly composed of predominantly effector memory (EM, CD45RA-CCR7-), while the IFNγ+ CD8+ T cells responses were composed of predominantly EM and effector memory cells re-expressing CD45RA (EMRA, CD45RA+CCR7-) (Supplementary Fig. 9).
Overall, the inhalation of the aerosolized vaccines produced systemic spike-specific CD4+ and CD8+ T cell responses similar to those of the intramuscular mbO5 mRNA vaccine. The spike-specific CD4+ T cells exhibited a polyfunctional profile, with a predominant EM subset, and the spike-specific IFNγ+ CD8+ T cells responses were dominated by EM and EMRA subsets. In addition, compared with the aerosolized vaccine, the mbO5 mRNA vaccine induced higher CD4+ IFNγ+ and IL2+ cTfh cell responses and rapid change during 6 months, consistent with the neutralizing antibody responses among three groups.
Discussion
The continued emergence of SARS-CoV-2 variants has necessitated the updating of COVID-19 vaccines, both in terms of antigens and modes of vaccination. It is hoped that novel vaccines will be developed that provide long-lasting protection against viral changes, stronger immunity, or better prevention of viral infection or transmission. A comprehensive understanding of the immunological profile of the existing vaccines will aid in the development of the next generation of COVID-19 vaccines. The aerosolized COVID-19 vaccine is a form of respiratory mucosal vaccination that can elicit secretory IgA and resident memory B and T cells in the respiratory mucosa, in addition to systemic adaptive immune response24. Our work provided the first comprehensive comparison of the potency and durability of mucosal and serum antibody responses, the characteristics of antigen-specific memory B cells and T cells response between the aerosolized adenovirus-vectored vaccine and the intramuscular mRNA vaccine.
Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection25. In this study, the bivalent Ad5-CoV5T vaccine elicited a superior anti-XBB.1.5 LVN Ab titer, as compared with Ad5-nCoV, and the magnitude of the difference in the seroconversion exceeded the recommended superiority criteria, at 28 days after vaccination. This result is consistent with the clinical findings of the bivalent omicron-containing mRNA-1273.214 booster vaccine26. The WT/BA.5 bivalent mbO5 mRNA vaccine by intramuscular injection induced a significant systemic neutralizing antibody response after a booster dose, which was significantly higher than those of the aerosolized adenovirus-vectored vaccines at week 4 after vaccination. However, the neutralizing antibody induced by the mRNA vaccine rapidly rose and declined, and reaching levels similar to those of the aerosolized vaccine at week 24. The LVN Ab responses induced by the aerosolized vaccines reached their peak at 12 weeks and kept at 80% of their peak at 24 weeks post-vaccination, while the response induced by the mRNA vaccine remained at about 40% of their peak at 24 weeks. The aerosolized vaccines stimulated a slower neutralizing antibody response, indicating that the intramuscular mRNA vaccine and the aerosolized vaccine have different trends in antigen presentation, antibody production and maturation. The antibody response is determined by the quantity of the newly generated plasma cells, and some extrinsic factors are critical for plasma cell maintenance, such as regulatory T cells, granulocytes monocytes and dendritic cells, et al in survival niche, which provide secreted factors and adhesion molecules promoting plasma longevity27. We further observed that the mbO5 mRNA vaccine induced a higher IFNγ+ and IL2+ cTfh cell response at week 2 and 4 after vaccination compared to the aerosolized vaccines, which contributed to the antibody production23.
Memory B cells play a critical role in protection against repeated infections, particularly from variant viruses21. The RBD-specific memory B cells induced by the mRNA vaccine and the aerosolized vaccines increased to the similar level at week 4, then declined to the level of baseline at 24 weeks, although the antibody response induced by the mRNA vaccine were obviously higher than those of the aerosolized vaccines. It seems that intramuscular mRNA vaccine has the advantage on stimulating more plasma cells, which secreted more specific antibody, rather than the humoral immune memory response including memory B cells and long-lived plasma cells. We observed that the aerosolized vaccines and mRNA vaccine induced different subsets of RBD-specific memory B cells, with the intramuscular mRNA vaccine stimulating a strong increase in RBD-specific activated and exhausted tissue-like memory B cells at week 4, which may be the reason for the rapid induction of antibody response by the mRNA vaccine. The expression level of homing marker on RBD-specific memory B cells is also different between the aerosolized vaccine and mRNA vaccine. The aerosolized vaccine induced a significant higher expression level of CXCR3, a marker known to facilitate homing to inflamed lung mucosae28,29, and demonstrated that SARS-CoV-2 specific memory B cells induced by the aerosolized vaccine may be better primed to home to the lung than those generated by the intramuscular mRNA vaccine. On the other hand, the mRNA vaccine induced a significant lower level of CXCR5, a homing marker for memory B cells migration to germinal centers30. The downregulation of CXCR5 on RBD-specific memory B cells may be associated with mature to plasmablasts rapidly to produce a higher antibody titer.
Both the aerosolized vaccine and the intramuscular mRNA vaccine increased the anti-spike IgA and IgG antibody responses in the nasal swabs. The aerosolized vaccine had an advantage over the mRNA vaccine in producing the nasal IgA antibody and the nasal IgA titer did not correlate well with the serum neutralizing antibody and serum IgA titer, suggesting a compartmentalization of immune responses. In contrast, the mRNA vaccine produced higher levels of nasal IgG titer, which was closely related to the serum neutralizing antibodies and serum IgG titer, and maybe indicated the IgG’s spillover from the circulation to the mucosa. The IgA assay in this work was limited in that we were unable to differentiate between secretory IgA produced by plasma cells in the lamina propria of mucosal tissues, and monomeric IgA that is passively translocated from the circulation into the nasal mucosa. Compared with the IgG, IgA response in the nasopharyngeal samples, the anti-spike secretory IgA post-infection responses affected the viral RNA shedding dynamics and predicted the duration of infectious virus shedding31. Here, we also found out that the nasal IgA concentrations showed a good correlation with the nasal PsVN Ab titers, which confirmed the neutralizing function of the nasal IgA antibodies. Importantly, we found the aerosolized vaccine increased the antigen-specific IgA+ memory B cell response, but not the intramuscular mRNA vaccination. Other intramuscular mRNA vaccine also could not produce the antigen-specific IgA+ memory B cell response in SARS-CoV-2 recovered32. These observations fundamentally reflect established principles of mucosal immunity. The respiratory pathogens (e.g., influenza, SARS-CoV-2) and mucosal vaccines (e.g., intranasal live attenuated influenza vaccine) robustly induce IgA+ memory B cells that provide frontline defense at infection portals33,34,35,36. Nasal-associated lymphoid tissues (NALT)- and bronchial-derived IgA+ memory B cells exhibit tropism for distant mucosal sites, differentiating into plasmablasts that produce dimeric IgA for transcytosis as SIgA37,38, which mediates viral neutralization, blocks epithelial adhesion, and promotes pathogen clearance through immune exclusion39. The failure of intramuscular vaccines (including mRNA platforms) to induce durable IgA+ memory B cells stems from poor engagement of mucosal inductive sites16. Conversely, aerosol delivery mimics natural infection by directly activating NALT and lung-resident dendritic cells, initiating TGF-β/retinoic acid-dependent IgA class-switching40. Supporting this, intranasal adenoviral COVID-19 vaccines generate airway IgA+ B cells in humans41, while convalescents maintain spike-specific IgA+ memory B cells for 8 months42. Future studies should address the longevity and recall capacity of aerosol-induced IgA+ memory B cells.
In some clinical studies, the serum neutralizing antibody against SARS-CoV-2 and anti-spike binding antibody were considered to provide reliable correlate of protection against both infection and severe disease25,43,44. Other studies have reported a stronger protective effect of anti-spike mucosal IgA antibodies against infection compared to specific mucosal IgG antibodies31,45. In the SARS-CoV-2 controlled human infection trial, the baseline mucosal anti-spike antibody responses and the specific PBMCs IFNγ responses to a CD8+ T cells, not the serum neutralizing antibody, showed the significant difference in infected and uninfected volunteers46. Although the systemic spike-specific CD4+ and CD8+ T cell responses were comparable between the aerosolized vaccine and the intramuscular mRNA vaccine, respiratory mucosal vaccination, but not intramuscular vaccination, induces multifunctional respiratory mucosal tissue-resident memory T cell responses47. Tissue-resident CD8+ T cells in the respiratory tract have been shown to play important roles in prevention of respiratory virus transmission48. In a clinical trial conducted in Malaysia, the aerosolized Ad5-nCoV vaccine had an efficacy of 0.2 for the prevention of COVID-19 at day 120 post-vaccination compared with intramuscular injection of the BNT162b2 mRNA vaccine, although BNT162b2 induced significant higher neutralizing antibody49, suggesting the serum neutralizing antibody may not be a good indicator for prevention of COVID-19 infection.
There are some limitations in our studies. Due to difficulties in obtaining more samples related to the mucosa, we mainly targeted the systemic immune response and only detected the humoral immune response in the nasal swabs, and more detailed characterization of the immune responses to mucosal vaccination remains desirable. Second, no difference in the immunogenicity between the aerosolized vaccine and the mRNA vaccine was observed at 6 months after booster vaccination, the immune durability of the two kinds of vaccines for longer period is needed. Finally, this study was not designed to evaluate vaccine efficacy. Comparison of the efficacy of the aerosolized vaccine and the mRNA vaccine in preventing infection, disease and blocking transmission would need to undergo large and expensive phase III clinical trials, which are not currently available.
In conclusion, our study provides the comprehensive comparative evaluation of the aerosolized adenovirus-vectored vaccine and the intramuscular mRNA vaccine, which helps understand the characteristics of the mucosal and intramuscular vaccine. Advances in our understanding of mucosal immunity offer hope that development of safe, immunogenic and protective mucosal vaccination remains a prior choose both for the response to COVID-19 and more widely.
Methods
Study design and participants
This study was a randomized open-label trial to evaluate the safety and immunogenicity of the aerosolized bivalent (WT and BA.5) Ad5-vectored COVID-19 vaccine, the intramuscular bivalent mRNA vaccine and the aerosolized WT Ad5-vectored vaccine, in adults aged 18 years and older. Participants were recruited in the clinical trial center of Zhongnan Hospital (Wuhan, China). A total of 450 eligible adults were in general good health and HIV-negative. People with history of any allergies, psychiatric disorders, serious cardiovascular disease and other major chronic illnesses were excluded. Pregnant or breastfeeding women were also excluded. Prior COVID-19 vaccine inoculation or SARS-CoV-2 infection must be over 3 months before this trial. Evaluation of the history of COVID-19 was obtained through the inquiry of the medical history by researchers. A COVID-19 antigen rapid test based on immune colloidal gold technique was conducted during the screening to exclude the subjects who are in the infection period of COVID-19. Sex was recorded at the time of enrollment for each participant based on self-report. Gender was not included in the data collection or analysis. Participants will receive compensation to acknowledge their time, travel, and participation in study procedures, with ¥1000 payment for a safety and sample collection follow-up and ¥500 payment for a safety follow-up. All participants provided written informed consent and completed an assessment of understanding before screening. The protocol of this study (Supplementary information), the informed consent, and other information related to participants were approved by the medical ethics committee of Zhongnan Hospital. This trial followed the Declaration of Helsinki, Good Clinical Practice (GCP) requirements, and other regulations issued by authorities. The study was registered with ClinicalTrial.gov, NCT05886790.
Vaccines
The Ad5-CoV5T vaccine contains recombinant replication deficient human adenovirus type 5 expressing the spike protein of the SARS-CoV-2 prototype strain or BA.5 strain, each with 2.5 × 1010 VPs in 0.5 mL per bottle. The Ad5-nCoV vaccine contains recombinant replication deficient human adenovirus type 5 expressing the spike protein of the SARS-CoV-2 prototype strain, with 5 × 1010 VPs in 0.5 mL per bottle. The mbO5 vaccine contains mRNA encoding the spike protein of the SARS-CoV-2 prototype strain with four Beta mutations or encoding the spike protein of BA.5 strain, formulated with lipid nanoparticle, and each with 15 μg mRNA in 0.3 mL per bottle. The lipid nanoparticle capsule composed of ionizable cationic lipid ALC-0315, 1,2-distearoyl-sn-glycero-3-phosphocholine, cholesterol, DMG PEG2000, and was formulated in a fixed ratio of mRNA and lipid. All the vaccines were produced by Cansinotech under Good Manufacturing Practice of Medical Products. Ad5-nCoV has been approved for use in China, Ad5-CoV5T and mbO5 have been approved for clinical trials.
Randomization and masking
All eligible participants were randomized using SAS software (version 9.4) to one of three groups in a 1:1:1 ratio by block randomization with block size of six. Randomization was completed by research assistants who were not involved in data collection and statistical analyses. The participants were obtained a unique study number which matched to the labeled vaccine. Participants could not be masked to group assignment because of the different operation between aerosol inhalation and intramuscular injection, and no vaccine blinding was performed in this trial. The laboratory staff were masked to the treatment and they identified samples by serial numbers.
Procedures
From 30 May to 6 June 2023, 487 individuals were screened with 450 participants were enrolled. Eligible adults were randomly assigned to one of the following groups: Ad5-CoV5T group (inhalation of 5×109 VPs of Ad5-nCoV and 5 × 109 VPs of Ad5-nCoV-BA.5), mbO5 group (intramuscular injection of 15 μg of WT SARS-CoV-2 spike mRNA and 15 μg of BA.5 spike mRNA, formulated with lipid nanoparticle) and Ad5-nCoV group (inhalation of 1 × 1010 VPs of Ad5-nCoV). For aerosolized vaccination, the inhaling aerosol was generated from the vaccine liquid using a nebulizer (Aerogen), and inhaled by mouth. Briefly, a total of 1 × 1010 VPs of Ad5-nCoV or 5 × 109 VPs of Ad5-nCoV and 5 × 109 VPs of Ad5-nCoV-BA.5 in a volume of 0.1 mL were aerosolized for 25 s and filled into a closed cup with a suction nozzle on lid. After taking a deep breath, the subjects inhaled the vaccine slowly until there was no fog in the cup and held their breath for 5 s. To avoid exposure, the immunization was administered in three completely independent areas for each of the three groups. All participants were monitored for 30 min after vaccination for immediate adverse reactions. Adverse events were self-reported and were verified by investigators every day during the first 14 days via a diary card issued at day 0. From day 15 to 28, adverse events were recorded by the participants on contact cards, and retrospectively verified by the investigators on day 28. At the 3rd and 6th months after vaccination, all subjects except the first 90 subjects were followed up by telephone for safety. Serum samples were collected at day 0 and day 28 in all participants for LVN Ab detection. For the first 30 participants in each group, serum samples were also collected at week 2, 12 and 24 and nasal swabs were collected at week 0, 4, and 24 for antibody detection, and PBMCs were isolated for memory B cells or cellular immune response detection at week 0, 2, 4 and 24.
Outcomes
The primary endpoint for safety was the incidence of adverse reactions within 28 days after vaccination in all subjects, the primary immunogenicity outcome was anti-XBB.1.5-specific neutralizing antibody on day 28 after vaccination in all subjects. The secondary safety endpoints were the incidence of adverse reactions within 30 min after vaccination and the incidence of serious adverse event and adverse event of special interest within 6 months in all subjects. The secondary endpoints for immunogenicity were anti-BA.5-specific neutralizing antibody response on day 28 after vaccination in all subject, and anti-XBB.1.5- and anti-BA.5-specific neutralizing antibody response in the first 90 participants on day 14, month 3 and month 6 after vaccination. The exploratory endpoints for immunogenicity were SARS-CoV-2 specific IgA response in nasal swab, SARS-CoV-2 spike protein-specific cellular response and memory B cell response in PBMCs, and PsV Ab response in serum, in the first 90 participants.
Isolation of serum and PBMCs
Venous blood was collected into pro-coagulation tubes for serum separation and EDTA tubes for PBMCs isolation. The pro-coagulation tubes were centrifuged at 2000 g for 10 minutes, serum was transferred into Eppendorf tubes and stored at -80 °C for antibody analysis. PBMCs were isolated by density-gradient sedimentation. Whole blood in EDTA tubes was diluted 1:1 with phosphate buffer saline (PBS) and layered onto human lymphocyte separation medium (DAKEWE) in the 50 mL tubes. After centrifuged at 800 g for 25 minutes, the PBMCs were collected into new tubes, washed twice with R1 [RPMI1640 + 1% fetal bovine serum (FBS) + 100 U penicillin/streptomycin] and re-suspended in R10 (RPMI1640 + 10% FBS + 100 U penicillin/streptomycin). The cells were counted on cellometer K2 fluorescent cell counter (Nexcelom) and cryopreserved in 90% FBS 10% dimethyl sulfoxide (DMSO) with 5-10 × 106 cells per tube. The PBMCs were stored in liquid nitrogen until used in the T cell and B cell assays.
Collection of nasal swabs
Before collection, the subjects were asked to blow their nose to remove secretions from the nose cavity. When collecting, the subjects kept their head slightly tilted upwards, and the nurse held the tail of the swab and inserted it into one nostril. The swab was slowly penetrated 1 to 1.5 centimeters backwards along the bottom of the lower nasal passage, rotated at least 4 times against the nasal cavity, and held for at least 15 s. Repeated the same procedure using the same swab in the other nasal cavity, and placed the head of the swab into an Eppendorf tube. The swabs were soaked in PBS for at least 1 hat 4 °C, vortexed, and centrifuged at 5000 g for 10 min, the supernatant was transferred into Eppendorf tubes and stored at −80 °C for antibody analysis. To ensure the homogeneity of the operation, we conducted a training before the trial started, and tried to arrange the same nurse to complete this operation as much as possible.
Detection of SARS-CoV-2 PsVN Ab
SARS-CoV-2 pseudovirus bearing the full-length spike protein of SARS-CoV-2 WT and BA.5, BF.7, XBB.1.5, EG.5, JN.1 variants were produced in a Env-defective, luciferase-expressing HIV-1 backbone by con-transfecting the HEK293T cells (ATCC, CRL-1573). 100 µL of heat-inactivated serially diluted serum samples or nasal swab samples were mixed with 50 µL of 1 × 103 50% tissue culture infective dose (TCID50) pseudovirus in 96-well microplate and incubated at 37 °C in 5% CO2 for 1 h. Then, 50 µL of ACE2-293T cells at a density of 4 × 105 cells per mL was seeded into the plates, and cultured in a 37 °C, 5% CO2 humidified incubator for 2 days. Wells without sample or with ACE2-293T cells only were also included as positive and negative controls. Cells were lysed 48 h later and luciferase activity was measured on Glomax® Navigator microplate luminometer (Promega). EC50 neutralization titers were calculated for each sample using Reed-Muench method. The initial dilution of serum (1: 30) was set as the lower limit of confidence of the assay, titers below the lower limit were assigned a value of 15. For nasal swab samples, the initial dilution was 1: 2, the PsVN Ab titers were normalized for the total IgA and IgG content, after that, the titers below the lower limit were assigned a value of 0.01.
Detection of SARS-CoV-2 LVN Ab
The SARS-CoV-2 LVN Ab detection was based on the virus-induced cytopathic effects (CPE) assay. Briefly, 50 µL of heat-inactivated serially diluted serum samples were mixed with the same volume of 100 TCID50 live SARS-CoV-2 virus (SARS-CoV-2/E6/FJH/2022/ZJ104 (Omicron/BA.5.2), or SARS-CoV-2/E6/YF/2023/ZJ78 (Omicron/XBB.1.5)) in 96-well plate, and incubated at 37 °C in 5% CO2 for 2 h. Then, 100 µL of Vero E6 cells (ATCC, CRL-1586) at a density of 1 × 105 cells per mL was seeded into the plates, and cultured in a 35 °C, 5% CO2 humidified incubator for 3 days. Wells without serum sample or with Vero E6 cells only were also included as positive and negative controls. The plates were observed under microscope (Olympus) for the presence of CPE on day 3, and LVN Ab titers were calculated as the reciprocal of serum dilution resulting in an 100% reduction of the CPE. The initial dilution of serum (1: 4) was set as the lower limit of confidence of the assay, and the final dilution (1: 2048) was set as the upper limit. Titers below the lower limit were assigned a value of 2, and above the upper limit were assigned a value of 2048. Seroconversion was defined as a ≥ 4-fold increase in LVN Ab titer from the baseline, or a baseline LVN Ab titer below the lower limit of detection accompanied by a titer above the lower limit at the specific time point.
Quantitative detection of human total IgA and IgG in nasal swab
The concentration of human total IgA and IgG antibody in nasal swab was quantified by human IgA and IgG enzyme-linked immunosorbent assay (ELISA) kits (Thermo Fisher) according to the manufacturer’s instructions. Briefly, prediluted samples and standard (7 concentration gradients from 100 ng/mL to 1.6 ng/mL) were added into the pre-coated microwell strips with 100 μL per well, and then 50 μL of diluted HRP-conjugated antibody was added to all wells. Samples and standard were in duplicated and two blank wells were contained in each plate. The plates were covered with adhesive film and incubated at room temperature (RT) for 1 h on a microplate shaker. After 4 times of washing, 100 μL of TMB substrate solution was added to all wells, and incubated for 30 min in the dark at RT for color development. The enzyme reaction was stopped by adding 100 μL of stop solution into each well, and the absorbance of each microwell was read at 450 nm on a spectrophotometer (Tecan). The concentration of IgA and IgG in nasal swab was calculated by the 5-parameter standard curve.
Detection of SARS-CoV-2 spike-specific IgA and IgG antibodies
SARS-CoV-2 spike-specific IgA and IgG antibodies in nasal swab and serum were quantified by V-PLEX SARS-CoV-2 Panel 32 (IgA) Kit and V-PLEX SARS-CoV-2 Panel 27 (IgG) Kit (Meso Scale Discovery) according to the manufacturer’s instructions. Briefly, the 10 spots precoated plates were blocked with Blocker A solution by shaking at ~700 rpm at RT for at least 30 min. The plates were washed 3 times with 1 × MSD Wash buffer, the calibrators, diluted samples and controls were added to the plate with 50 μL per well, and incubated at RT with shaking (~700 rpm) for 2 h. After washing, 50 μL/well of 1 × detection antibody solution was added to each well, and incubated at RT with shaking for 1 h. Wash the plate 3 times again, and add 150 μL/well of the MSD GOLD read buffer to each well. The plates were read on the MSD instrument immediately after adding the read buffer. Antibody unit concentrations in diluted samples and controls were determined from their electrochemiluminescence signals by backfitting to the calibration curve. Spike-specific antibody concentrations in serum were recorded as AU/mL, the antibody concentrations in nasal swab were divided by the total IgA and IgG concentrations, and recorded as AU/μg. V-PLEX SARS-CoV-2 Panel 32 (IgA) Kit was specific to SARS-CoV-2 WT spike and BA.1, BA.2.75, BA.2.75.2, BA.4.6, BA.5, BF.7, BQ.1, BQ.1.1, XBB.1 spike. V-PLEX SARS-CoV-2 Panel 27 (IgG) Kit was specific to SARS-CoV-2 WT spike and B.1.351, AY.4, BA.2, BA.2.12.1, BA.2 + L452M, BA.2 + L452R, BA.3, BA.4, BA.5 spike.
Detection of SARS-CoV-2-specific memory B cells
The detection of SARS-CoV-2-specific memory B cells (MBCs) was performed using B cell probes as previously described32. Full-length SARS-CoV-2 spike and RBD of WT and BA.5, XBB.1.5 variants were purchased from Sino Biological or Vazyme Biotech. The proteins were biotinylated using an EZ-Link Micro NHS-PEG4 Biotinylation Kit (Thermo Fisher) according to the manufacturer’s instructions. Zebra Spin Desalting Columns 7 K MWCO (Thermo Fisher) were used to remove the excess biotin from the antigens. After quantified with Pierce BCA assay (Thermo Fisher), the proteins were multimerized with fluorescently labeled streptavidin (SA) individually for 1 h at 4 °C in dark and neutralized with excess free D-biotin (avidity biosciences) before mixed to minimize cross-reactivity. The molar ratio of the protein and SA-fluorophore was about 4:1, with mass ratio was 10:1 for spike (200 ng spike with 20 ng SA-fluorophore) and 2:1 for RBD (25 ng RBD with 12.5 ng SA-fluorophore). For cell staining, about 5 × 106 PBMCs per sample were recovered and placed in U-bottom 96 well plates. Cells were first stained with Live/Dead Fixable Near-IR dye (Thermo Fisher) and Fc receptor block (Biolegend, 1:200) for 15 min at 4 °C. After washing, cells were then stained with 50 μL antigen probe mixture containing spikes (WT-PE, WT-AF647, WT-AF700, BA.5-PE, BA.5-AF647, BA.5-PE/Cy5, XBB.1.5-PE, XBB.1.5-AF647 and XBB.1.5-BV421, 200 ng per probe) or RBDs (WT-PE, WT-AF647, WT-AF700, BA.5-PE, BA.5-AF647, BA.5-PE/Cy5, XBB.1.5-PE, XBB.1.5-AF647 and XBB.1.5-BV421, 25 ng per probe) for 1 h at 4 °C. Cells were washed again and stained with APC/Cy7 anti-CD3, APC/Cy7 anti-CD56, BUV396 anti-CD19, BV605 anti-IgD, BUV805 anti-CD27, PerCP/Cy5.5 anti-CD38, BUV496 anti-CD20, FITC anti-IgA, BV480 anti-IgG, BV786 anti-IgM, PE/Cy7 anti-CD71, BUV737 anti-CD21, BV650 anti-CXCR3, and BV711 anti-CXCR5 for 30 min at 4 °C. The SA-fluorophores and fluorescently labeled antibodies were purchased from Biolegend or BD Biosciences, except for FITC anti-IgA (Miltenyi Biotec). Cells were finally washed twice, resuspended in flow cytometry staining buffer (FACS) buffer, and acquired on BD FACSymphonyTM A3 cell analyzer (BD Biosciences) and analyzed by FACSDiva software (BD Biosciences). The frequency of SARS-CoV-2-specific MBCs was expressed as a percentage of total B cells.
Detection of SARS-CoV-2-specific T cells
PBMCs were recovered, resuspended in R10, counted on cellometer K2 fluorescent cell counter and adjusted the density to 1 × 107 cells/mL. Cells were plated in duplicate in U-bottom 96 well plate with 2 × 106 cells per well and rested overnight at 37 °C, 5% CO2 in a humidified incubator. After at least 12 hours, anti-human CD40 antibody were added to the cultures. 15 min later, co-stimulation (anti-human CD28 and CD49d, Biolegend) and a spike peptide pool with a final concentration of 1 μg/mL per peptide were added, and the cells were stimulated for 24 h. The peptides were 15 amino acids length, overlapped by 11 amino acids, and covering the full-length amino acid sequence of SARS-CoV-2 XBB.1.5 spike. Unstimulated controls for each sample were set and treated with co-stimulation alone. About 18 h later, BUV395 anti-CXCR3, BV605 anti-CXCR5, BUV805 anti-CCR4, BUV496 anti-CCR6 and BB515 anti-CCR7 were added to the culture for a 6-h stain at 37 °C, and brefeldin A and monensin (GolgiPlus and GolgiStop, BD Biosciences) were also added to block the secretion process of cytokines. Cells were washed with FACS buffer and stained with Live/Dead Fixable Near-IR dye (Thermo Fisher) and Fc receptor block (Biolegend) for 15 min in the dark at RT. After one wash with FACS buffer, a cocktail of surface antibodies in brilliant stain buffer (BD Biosciences) containing APC/Cy7 anti-CD14, APC/Cy7 anti-CD16, APC/Cy7 anti-CD19, PerCP/Cy5.5 anti-CD3, AF700 anti-CD4, BV480 anti-CD8, PE/Cy7 anti-CD45RA, BUV737 anti-CD69, PE/Cy5 anti-OX40 and BV650 anti-PD-1 was added and incubated in the dark at RT for 30 min. Cells were washed once in FACS buffer, fixed and permeabilized for 30 min at RT in Cytofix/Cytoperm buffer (BD Biosciences), washed once in 1 × Perm/Wash buffer (BD Biosciences), and stained with intracellular cytokines and markers for 30 min at RT. The intracellular antibodies were in 1 × Perm/Wash buffer and brilliant stain buffer containing PE anti-IFNγ, AF647 anti-TNFα, BV421 anti-IL2, BV711 anti-IL4, BV711 anti-IL13, BV785 anti-IL17A, BB630 anti-IL21, PE/CF594 anti-Granzyme B, BUV563 anti-CD40L, BUV661 anti-4-1BB, and BB790 anti-MIP1β. All the fluorescently labeled surface and intracellular antibodies were purchased from Biolegend or BD Biosciences. Cells were washed successively with Perm/Wash buffer and FACS buffer and resuspended in FACS buffer. Data were acquired on BD FACSymphonyTM A3 cell analyzer and analyzed by Flowjo (BD Biosciences). SARS-CoV-2 spike-specific responses were expressed as a percentage of CD4+ or CD8+ T cells, and calculated by subtraction of the unstimulated controls from the peptide-stimulated samples. Values less than 0.001 were assigned a value of 0.001.
Statistical analysis
The sample size for this trial was based on the superiority test of the GMT of neutralizing antibodies against Omicron XBB.1.5 variant in the population aged 18 and above. The coefficient of variation of GMT was derived as 1.37 based on the article (doi.org/10.1038/s41591-022-02162-x) in that the GMT of XBB.1.5 neutralizing antibody was 103 at 28 days after the BA.5 bivalent vaccination. Using a one-sided α = 0.025 and a total certainty of 1-β = 0.90, the predicted ratio of test group to control group of the GMT of neutralizing antibodies against XBB.1.5 was assumed to be 3.0 on day 28, and the superiority boundary value was 1. The ratio of test group to control group was 1:1, and the PASS 16.0 software (Superiority by a Margin Tests for the Ratio of Two Means) calculated a sample size of 137 cases for each group. Considering a 10% shedding, each group would be approximately 150 subjects, and 450 participants were included in this trial.
The main hypothesis of the study was that the GMT of anti-XBB.1.5 LVN Ab induced by Ad5-CoV5T was superior to that of Ad5-nCoV at day 28 after vaccination, superiority was established when the lower bound of the two-side 95% CI for the ratio of the GMT of anti-XBB.1.5 LVN Ab induced by Ad5-CoV5T and Ad5-nCoV was great than 1. The secondary hypothesis was that the seroconversion rate of anti-XBB.1.5 LVN Ab induced by Ad5-CoV5T was superior to that of Ad5-nCoV at day 28, superiority was established when the lower bound of the two-side 95% CI for the difference of the seroconversion rate between Ad5-CoV5T and Ad5-nCoV was greater than 0. The tests were based on a two-sided alpha level of 0.05.
χ² test and Fisher’s exact test were used to analyze the safety and data, which were performed with SAS (version 9.4). The immunogenicity data were analyzed on GraphPad Prism v.10.1.2. LVN Ab titers compared to the Ad5-nCoV group on day 28 were calculated using two-tailed unpaired t test, statistics on seroconversion data were calculated using χ² test. For the long-term immunogenicity data, statistics between different time points within one group were calculated using paired nonparametric two-tailed Friedman test with Dunn’s correct for multiple comparisons, statistics between the different groups at a specific time point were calculated using unpaired nonparametric two-tailed Kruskal-Wallis test with Dunn’s correct for multiple comparisons. Correlations were assessed by nonparametric two-tailed Spearman’s rank-correlation tests. No specific analyzes were performed stratified by sex due to the small sample size and expected results.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Source data are provided with this paper.
References
Barouch, D. H. Covid-19 vaccines - Immunity, Variants, Boosters. N. Engl. J. Med. 387, 1011–1020 (2022).
Wu, N. et al. Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022. Lancet Respir. Med. 11, 439–452 (2023).
Chalkias, S. et al. Original SARS-CoV-2 monovalent and Omicron BA.4/BA.5 bivalent COVID-19 mRNA vaccines: phase 2/3 trial interim results. Nat. Med. 29, 2325–2333 (2023).
Hansen, C. H. et al. Short-term effectiveness of the XBB.1.5 updated COVID-19 vaccine against hospitalisation in Denmark: a national cohort study. Lancet Infect. Dis. 24, e73–e74 (2024).
Huiberts, A. J. et al. Effectiveness of Omicron XBB.1.5 vaccine against infection with SARS-CoV-2 Omicron XBB and JN.1 variants, prospective cohort study, the Netherlands, October 2023 to January 2024. Euro Surveill 29, 2400109 (2024).
Lin, D. Y. et al. Effectiveness of bivalent boosters against severe Omicron infection. N. Engl. J. Med. 388, 764–766 (2023).
Wang, Q. et al. Antibody response to Omicron BA.4-BA.5 bivalent booster. N. Engl. J. Med. 388, 567–569 (2023).
Wang, Q. et al. XBB.1.5 monovalent mRNA vaccine booster elicits robust neutralizing antibodies against XBB subvariants and JN.1. Cell Host Microbe 32, 315–321.e3 (2024).
FDA news release. August 22, 2024., <https://www.fda.gov/news-events/press-announcements/fda-approves-and-authorizes-updated-mrna-covid-19-vaccines-better-protect-against-currently>
Knisely, J. M. et al. Mucosal vaccines for SARS-CoV-2: scientific gaps and opportunities-workshop report. NPJ Vaccines 8, 53 (2023).
Li, J. X. et al. Safety, immunogenicity and protection of heterologous boost with an aerosolised Ad5-nCoV after two-dose inactivated COVID-19 vaccines in adults: a multicentre, open-label phase 3 trial. Lancet Infect. Dis. 23, 1143–1152 (2023).
Wang, F. Z. et al. An observational prospective cohort study of vaccine effectiveness against severe acute respiratory syndrome coronavirus 2 infection of an aerosolized, inhaled adenovirus type 5-vectored coronavirus disease 2019 vaccine given as a second bBooster dose in Guangzhou city, China. J. Infect. Dis. 229, 117–121 (2024).
Li, J. X. et al. Safety and immunogenicity of heterologous boost immunisation with an orally administered aerosolised Ad5-nCoV after two-dose priming with an inactivated SARS-CoV-2 vaccine in Chinese adults: a randomised, open-label, single-centre trial. Lancet Respir. Med. 10, 739–748 (2022).
Tang, R. et al. Safety and immunogenicity of aerosolised Ad5-nCoV, intramuscular Ad5-nCoV, or inactivated COVID-19 vaccine CoronaVac given as the second booster following three doses of CoronaVac: a multicentre, open-label, phase 4, randomised trial. Lancet Respir. Med. 11, 613–623 (2023).
Zhang, Z. et al. Boosting with an aerosolized Ad5-nCoV elicited robust immune responses in inactivated COVID-19 vaccines recipients. Front. Immunol. 14, 1239179 (2023).
Gagne, M. et al. Mucosal adenovirus vaccine boosting elicits IgA and durably prevents XBB.1.16 infection in nonhuman primates. Nat. Immunol. 25, 1913–1927 (2024).
McMahan, K. et al. Mucosal boosting enhances vaccine protection against SARS-CoV-2 in macaques. Nature 626, 385–391 (2024).
Wu, J. D. et al. Safety, immunogenicity, and efficacy of the mRNA vaccine CS-2034 as a heterologous booster versus homologous booster with BBIBP-CorV in adults aged >/=18 years: a randomised, double-blind, phase 2b trial. Lancet Infect. Dis. 23, 1020–1030 (2023).
Zheng, L., Liu, S. & Lu, F. Impact of National Omicron Outbreak at the end of 2022 on the future outlook of COVID-19 in China. Emerg. Microbes Infect. 12, 2191738 (2023).
Allie, S. R. et al. The establishment of resident memory B cells in the lung requires local antigen encounter. Nat. Immunol. 20, 97–108 (2019).
Inoue, T. & Kurosaki, T. Memory B cells. Nat. Rev. Immunol. 24, 5–17 (2024).
Tan, A. T. et al. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep. 34, 108728 (2021).
Mudd, P. A. et al. SARS-CoV-2 mRNA vaccination elicits a robust and persistent T follicular helper cell response in humans. Cell 185, 603–613.e15 (2022).
Lavelle, E. C. & Ward, R. W. Mucosal vaccines - fortifying the frontiers. Nat. Rev. Immunol. 22, 236–250 (2022).
Khoury, D. S. et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 27, 1205–1211 (2021).
Chalkias, S. et al. A bivalent Omicron-containing booster vaccine against Covid-19. N. Engl. J. Med. 387, 1279–1291 (2022).
Khamyath, M., Melhem, H., Balabanian, K. & Espeli, M. New insights into the mechanisms regulating plasma cell survival and longevity. Curr. Opin. Immunol. 88, 102442 (2024).
MacLean, A. J. et al. Secondary influenza challenge triggers resident memory B cell migration and rapid relocation to boost antibody secretion at infected sites. Immunity 55, 718–733.e8 (2022).
Onodera, T. et al. Memory B cells in the lung participate in protective humoral immune responses to pulmonary influenza virus reinfection. Proc. Natl. Acad. Sci. USA 109, 2485–2490 (2012).
Havenar-Daughton, C. et al. CXCL13 is a plasma biomarker of germinal center activity. Proc. Natl. Acad. Sci. USA 113, 2702–2707 (2016).
Miyamoto, S. et al. Infectious virus shedding duration reflects secretory IgA antibody response latency after SARS-CoV-2 infection. Proc. Natl. Acad. Sci. USA 120, e2314808120 (2023).
Goel, R. R. et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 374, abm0829 (2021).
Liew, F. Y. et al. Cross-protection in mice infected with influenza A virus by the respiratory route is correlated with local IgA antibody rather than serum antibody or cytotoxic T cell reactivity. Eur. J. Immunol. 14, 350–356 (1984).
Quinti, I. et al. IgA antibodies and IgA deficiency in SARS-CoV-2 infection. Front. Cell. Infect. Microbiol. 11, 655896 (2021).
Tomoda, T. et al. Prevention of influenza by the intranasal administration of cold-recombinant, live-attenuated influenza virus vaccine: importance of interferon-gamma production and local IgA response. Vaccine 13, 185–190 (1995).
Liu, J. et al. Turbinate-homing IgA-secreting cells originate in the nasal lymphoid tissues. Nature 632, 637–646 (2024).
Brandtzaeg, P. Secretory IgA: designed for anti-microbial defense. Front. Immunol. 4, 222 (2013).
Oh, J. E. et al. Intranasal priming induces local lung-resident B cell populations that secrete protective mucosal antiviral IgA. Sci. Immunol. 6, eabj5129 (2021).
Li, Y. et al. The effects of secretory IgA in the mucosal immune system. Biomed. Res. Int. 2020, 2032057 (2020).
Cerutti, A. The regulation of IgA class switching. Nat. Rev. Immunol. 8, 421–434 (2008).
Sun, B. et al. An intranasally administered adenovirus-vectored SARS-CoV-2 vaccine induces robust mucosal secretory IgA. JCI Insight 9, e180784 (2024).
Dan, J. M. et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 371, eabf4063 (2021).
Feng, S. et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 27, 2032–2040 (2021).
Perez-Saez, J. et al. Long term anti-SARS-CoV-2 antibody kinetics and correlate of protection against Omicron BA.1/BA.2 infection. Nat. Commun. 14, 3032 (2023).
Havervall, S. et al. Anti-Spike Mucosal IgA Protection against SARS-CoV-2 Omicron Infection. N. Engl. J. Med. 387, 1333–1336 (2022).
Jackson, S. et al. Safety, tolerability, viral kinetics, and immune correlates of protection in healthy, seropositive UK adults inoculated with SARS-CoV-2: a single-centre, open-label, phase 1 controlled human infection study. Lancet Microbe 5, 655–668 (2024).
Afkhami, S. et al. Respiratory mucosal delivery of next-generation COVID-19 vaccine provides robust protection against both ancestral and variant strains of SARS-CoV-2. Cell 185, 896–915.e19 (2022).
Uddback, I. et al. Prevention of respiratory virus transmission by resident memory CD8(+) T cells. Nature 626, 392–400 (2024).
Chew, C. K. et al. Safety, efficacy and immunogenicity of aerosolized Ad5-nCoV COVID-19 vaccine in a non-inferiority randomized controlled trial. NPJ Vaccines 9, 209 (2024).
Acknowledgements
The authors would like to thank all the participants who volunteered for this study. We also thank Di Hu, Hui Yuan (Zhongnan Hospital of Wuhan University) for their contributions to this work. This study was partly supported by the Special Project for Significant New Drug Research and Development in the National Major Science & Technology Major Project (2020ZX09201007, J.H.). The sponsors of the study participated in study design, but had no role in data collection, data analysis and manuscript writing.
Author information
Authors and Affiliations
Contributions
L.H., X.W., Y.Z., S.W., and B.W. designed the study. S.W., Z. Zhang, B.W., J.Z., and Z. Zhao isolated the serum and PMBCs, J. Li, Y.Z., H.M., and H.Y. performed the PsVN Ab detection. B.W., S.W., X.S., and S.H. performed the LVN Ab and IgA, IgG antibodies detection. S.W., N.H., J. Long, H.H., M.Z., and J.D. perform the memory B cells and T cells detection. J.H., J.W., and L.L. led and participated in the clinical site work. S.W., B.W., and Z.C. analyzed the data, S.W., L.H., and B.W. drafted the initial version of the manuscript. All authors reviewed the manuscript and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Bo Zhang, who co-reviewed with Zhe Chen; Kohtaro Fujihashi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, S., Huang, J., Wang, B. et al. Safety and Immunogenicity of aerosolized adenovirus-vectored COVID-19 vaccine and intramuscular mRNA vaccine bivalent boosters: a randomized open-label clinical trial. Nat Commun 16, 7281 (2025). https://doi.org/10.1038/s41467-025-62698-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-62698-7