Abstract
Dielectric elastomer actuators (DEAs) exhibit large actuation strains, lightweight, and fast response, making them a promising candidate for soft robotics and soft grippers. Ionogels have been used as the electrodes in DEAs to offer thermostability and self-healability, however, typically the elastic modulus of the self-healing ionogel electrodes is of several tens of kPa (or higher), limiting the actuation strain performance and self-healing speed of the DEA. In this work, a poly(ionic liquid) (PIL) electrode with an ultralow elastic modulus of 3.4 kPa and rapid self-healing within 10 s in ambient and underwater conditions is achieved through ionic interaction regulation. The resultant DEAs realized an area strain of 63.2%, and maintained the strains after 10 s of self-healing at room temperature, outperforming other reported DEAs with self-healing electrodes. With the PIL electrode, a soft gripper composed of two bending DEAs is fabricated to gently handle soft and delicate objects in both air and underwater settings, retaining functionality even after damages due to self-healing of the PIL electrodes. The PIL electrode advances the development of electrically driven soft robotics for exploration in harsh environment or underwater settings.
Similar content being viewed by others
Introduction
Dielectric elastomer actuators (DEAs) are highly valued for their large actuation strains, light weight, flexibility, high energy density, high efficiency, and fast response, making them ideal for applications in soft robotics, soft grippers, haptics, and tunable lenses, etc1,2,3,4,5,6,7,8. DEAs consist of a dielectric elastomer (DE) layer sandwiched between two compliant electrodes9,10. When a high voltage is applied across the electrodes, the generated Maxwell pressure compresses DE in thickness direction and enables areal expansion, producing actuation. The actuation strains of DEAs depend on the high dielectric constant and low elastic modulus of DE materials, and can be enhanced by different strategies, including incorporating fillers11,12, chemical modifications13, adding plasticizers14, and thermal softening15,16.
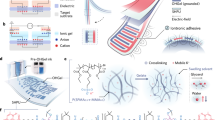
The role of compliant electrodes is equally critical in achieving high actuation strains. During actuation, the electrodes must deform in sync with the DE layer, requiring a much lower elastic modulus or an ultralow thickness of electrodes and an adhesive interface between electrodes and the DE to maximize the actuation strain17. Normally, rigid electrodes constrain the deformation of DEs, and non-healable electrodes can lead to a malfunction of DEAs when damaged (Fig. 1a). In contrast, having soft electrodes with minimal constraint on the deformation of DE enables conformable actuation of the resultant DEAs. The inclusion of fast self-healing electrodes further enhances the durability of DEAs, ensuring sustained performance even after damage (Fig. 1b). Therefore, ionic conductive gels have attracted attention as compliant electrodes due to their tunable modulus, high stretchability, adhesion, transparency, and self-healing capabilities18,19,20,21,22. Their ease of processing such as drop casting or directly attaching to the DE layer significantly simplify the fabrication process of DEAs, compared to spray coated or deposited rigid metal and carbon electrodes2,23,24,25.
a Schematic illustration of unconformable actuation by DEAs with rigid or non-healing electrodes, where rigid electrodes constrain the deformation of dielectric elastomer (DE) layers, and non-healing electrodes stop working with damage, resulting in low actuation strains. b Conformable actuation achieved by DEA with ultrasoft and fast self-healing electrode, which leads to a higher actuation strain and readily recoverable DEAs. c Schematic illustration of the chemical structure of PIL electrode, the size of the counterions relative to polycations influence the elastic modulus and self-healing speed of PILs.
DEAs with ionic conductive gel electrodes have demonstrated promising actuation performance and a wide range of applications, such as soft grippers with thermostable organohydrogel electrodes working at high or low temperatures26. Transparent gel electrodes paired with transparent DE layers allow for imperceptible actuation, useful for displays, tunable lenses, or camouflage27,28,29. The commonly used hydrogel electrodes such as polyacrylamide with LiCl undergo the inevitable evaporation of water inside the polymer network, causing thermostability issues30. Ionogels with nonvolatile ionic liquid are thermally stable and exhibit self-healing capability, which contributes to the durability of DEAs. Cao et al. developed a transparent and self-healing ionogel electrode composed of PVDF-HFP blended with EMIM OTF for DEAs, which showed an area strain of 22.6% at 5 kV, and maintained the strains after 24 h of self-healing18. Cheng et al. reported a water-stable PVDF-HFP-EMIM TFSI composite electrode for amphibious DEA based crawling robotics, where the electrode could self-heal in 5 min, highly shortening the recovering time for DEAs21. However, the Young’s moduli of these ionogel electrodes for DEAs are not low enough (several tens or hundreds of kPa), leaving much room for breakthroughs in soft electrodes with enhanced actuation strains of DEAs. Poly(ionic liquid) (PIL), a subcategory of ionogels, showcases excellent compatibility of ionic liquid with polymer network, and its elastic modulus can be adjusted to below 1 MPa and attained self-healing capability by blending with additional ionic liquid31, or increasing solvent amount in precursors32. Consequently, PIL has emerged as a promising candidate for DEA electrodes22.
Herein, we report an ultrasoft and fast self-healing PIL electrode for enhancing the actuation strains and durability of DEAs. By utilizing counterions (TFSI−) with similar size of the side chain of polycations (poly(1-hexyl-3-vinylimidazolium), PC6+) to optimize ionic interaction as shown in Fig. 1c, PIL30-TFSI reaches a low elastic modulus of 3.4 kPa and short self-healing time of 10 s both in air and underwater, outperforming other polymers which self-heal in both environments at room temperature. The resultant DEAs with PIL30-TFSI electrodes demonstrate larger original and recovered area strains with shorter self-healing time than other reported self-healing electrodes, benefitting from the low product of Young’s modulus and thickness (0.116 Pa∙m). As PILs show excellent thermal stability, DEAs with PIL30-TFSI electrodes retained actuation strains at high (80 °C) or low temperatures (−20 °C), surpassing those with common hydrogel electrodes. A soft two-finger gripper was fabricated without the requirement of additional encapsulation, to grasp soft or delicate objects repeatedly in air and underwater, performing rapid self-healing in both media. Furthermore, this PIL electrode was proved effective in various DEA applications, including soft crawling robotics, artificial muscles, and tunable optical modulators, enhancing their recoverability and broadening their applicability.
Results
Anion-mediated ionic interaction in PILs
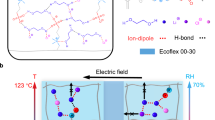
To realize the abovementioned ultrasoft and fast self-healing PIL electrodes for high-performance and durable DEAs, anions of varying sizes are selected to evaluate their ionic interaction with the polymer chains and the resultant mechanical properties. Previous studies have shown that larger anions can enhance the elastic modulus of imidazolium-based PILs when the size of the anion exceeds that of the cation’s side chain33. Thus, three anions (X = BF4−, OTF−, TFSI−) smaller than side-chain cations (C6+) were selected to investigate the relationship between ionic interactions and the softness and self-healing behavior of PILs (PIL30-X). The ratio of crosslinker and plasticizer of the PIL is optimized to obtain the lowest elastic modulus and highest stretchability, giving the composition of PIL30-X, which is elaborated in Supplementary Note 2 and Supplementary Fig. 3. The schematic illustration in Fig. 2a presents different chain mobility and anion mobility in PIL30-X controlled by anions-mediated ionic interaction. Three anions have the same charge but different size; the smaller anion such as BF4− has large surface electrostatic potential while the large anion (i.e., TFSI−) has lower surface electrostatic potential, as supported by the density functional theory (DFT) simulation. As plotted in Fig. 2b, the surface electrostatic potentials of three anions were calculated via DFT at the B3LYP/6-31 + G (d,p) level, from which the lowest surface charges of TFSI− was clearly observed. Since all PIL30-X variants share the same polycation (PC6+), the ionic interaction (electrostatic interaction) primarily depends on the surface electrostatic potential of anions and the distance between the anions and PC6+, as described by Coulomb’s law. Figure 2c displays the peaks associated with side chain correlation distance (qp) located at 13.6 nm−1, 13.9 nm−1, 14.9 nm−1 in PIL30-TFSI, PIL30-OTF, PIL30-BF4, respectively34,35. As the side chain correlation distance (dp) can be calculated from dp = 2π/qp, the dp in PIL30-TFSI is largest among PIL30-X, resulting from the largest volume and significant steric hindrance of the TFSI− anion36. Consequently, the anions in PIL30-BF4 and PIL30-OTF have stronger ionic interaction with PC6+ than that in PIL30-TFSI. This can be supported by DFT results in Supplementary Table 1, where ionic interaction energies are −82.6, −80.3, and −74.5 kcal mol−1 for PIL30-BF4, PIL30-OTF, and PIL30-TFSI, respectively. This stronger interaction in PIL30-BF4 causes a lower binding energy (401.7 eV) for the N 1 s peak related to nitrogen in the imidazolium ring compared to 402 eV for PIL30-TFSI (Fig. 2d). The relatively weak ionic interaction and steric hinderance lead to high polycation chain mobility and anion mobility in PIL30-TFSI (Fig. 2e, f). Therefore, PIL30-TFSI has the lowest Tg of −66.9 °C and highest ionic conductivity of 0.013 S/m among PIL30-X.
a Chemical structures of PIL30-X. Composition of PIL30-X is the same while three anions with increasing size are chosen for comparison, including BF4−, OTF−, TFSI−. b DFT simulation of the surface electrostatic potential of three anions, where it ranges from 0.1 a.u. (red) to −0.1 a.u. (blue). c Wide-angle X-ray scattering (WAXS) data of PIL30-X. d X-ray photoelectron spectroscopy (XPS) spectra of N 1 s peak for PIL30-X. e Glass transition temperature (Tg) of PIL30-X. f Ionic conductivity of PIL30-X.
Mechanical properties and self-healing performance of PIL30-X
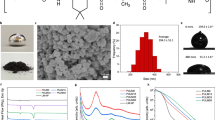
The chemical structure of PIL30-X is influenced by the anion-mediated ionic interaction, and thereby affecting the various mechanical properties and self-healing performance. Tensile stress-strain curves of PIL30-X in Fig. 3a show increasing stretchability and decreasing tensile strength from PIL30-BF4 (222% strain and 3549 kPa) to PIL30-TFSI (350% strain and 6.18 kPa). By calculating the slope of stress-strain curves at the first 5% strain, the Young’s moduli of PIL30-BF4, PIL30-OTF, and PIL30-TFSI are 26351.14 ± 8727.69, 15210.27 ± 5246.47, and 3.40 ± 1.00 kPa, respectively (Fig. 3b). The ultralow Young’s modulus of PIL30-TFSI indicates its easy deformation as it possesses the highest chain mobility and anion mobility. Remarkably, anion-mediated ionic interaction significantly influences the elastic modulus not only in PIL30-Xs but also in pristine PILs (PC6X) and PIL copolymers P(AAm-C6X) (refer to Supplementary Note 1 and Supplementary Figs. 1–2). Among the three tested anions, TFSI− consistently leads to the lowest Young’s modulus in the resultant PIL containing the polycation PC6+. This discovery offers valuable insights for tailoring the mechanical properties of PILs in various applications such as flexible electronics. Additionally, PIL30-TFSI exhibits stable cycling performance through 100 cycles at different strains and good elasticity as it showed the low hysteresis loss (14.1%) in cyclic tensile test (Supplementary Fig. 4). Furthermore, this material could withstand 50,000 cycles at 200% strain during the cyclic fatigue fracture test under a nominal strain rate of 4 s−1 (speed 480 mm/min) in Supplementary Fig. 5, which promises its long-term application in DEAs.
a Tensile stress-strain curves of PIL30-X at a stretching speed of 100 mm min−1. The visible curve of PIL30-TFSI can be observed in Fig. 3f. b Comparison of Young’s modulus of PIL30-X. Error bars are the standard deviations of three independent samples. c Schematic illustration of different self-healing process in PIL30-X with small anions (BF4−, OTF−) or larger anion (TFSI−). d Optical microscopic images of PIL30-X autonomously self-healing from scratches in different time scale at room temperature. Scale bars are 500 μm. e Optical microscopic images of PIL30-TFSI autonomously self-healing from scars underwater at room temperature (left). DFT results depicting the ionic interaction between C6+ and TFSI− within PIL30-TFSI and interaction between water molecule and TFSI− (right). (f) Tensile stress-strain curves of pristine and self-healed PIL30-TFSI in the air and underwater. g In-situ FTIR spectra of PIL30-TFSI undergoing cut and healing in the air and h underwater. i Resistance change of PIL30-TFSI during cutting and self-healing in the air and j underwater. k Ashby plot comparing the elastic modulus and healing time of room temperature self-healing polymers both in the air and underwater. The solid symbols are for healing in air, and the open symbols are for healing under water. References are listed in Supplementary Table 2.
The schematic in Fig. 3c depicts the self-healing process of the PIL. With small anions like BF4− and OTF−, the limited chain mobility and high Young’s modulus of PIL30-BF4/OTF make it difficult for the separated segments to diffuse and merge at the damaged region. The anions with limited mobility makes the self-healing process relatively slow37,38. In contrast, PIL30-TFSI benefits from its softness, high chain mobility, and anion mobility, leading to easy diffusion and merge of the damaged region. As a result, PIL30-BF4 and PIL30-OTF took 2 days and 1 day to heal from the scratches, respectively, while PIL30-TFSI shows a perfect and almost invisible scar at the damage location after 10 s of healing, as observed in optical microscopic images in Fig. 3d.
PIL30-TFSI demonstrated similar fast self-healing (in 10 s) underwater as well, benefitting from the hydrophobicity of TFSI− with a contact angle of 107° (Fig. 3e and Supplementary Fig. 6)39. DFT simulations revealed that the interaction energy between C6+ and TFSI− was −74.54 kcal mol−1, significantly surpassed that between H2O and TFSI− (−25.88 kcal mol−1). This suggests that the damaged sections of the material are more likely to reconnect with each other than interacting with water, enabling fast underwater self-healing. After healing in the air or underwater for 10 s, the healed PIL30-TFSI kept 65% or 50% of tensile strength of the original sample, respectively (Fig. 3f). The reduction in tensile strength is attributed to the irreversible breakage of covalent bonds, while the recovery is driven by the reformation of reversible ionic interactions. When the sample went through multiple cycles of cutting and self-healing, the healing efficiency, which was calculated by the ratio of tensile strength of each cycle to that of the original sample in Supplementary Fig. 7b, stayed at around 26.5% (±6.6%) after 5 healing cycles. In-situ FTIR-ATR spectra of PIL30-TFSI in Fig. 3g recorded the peak at 1052 cm−1, which is attributed to the asymmetric stretching of S-N-S in TFSI−, it has been shifted to 1054 cm−1 when there was a cut in the middle of the sample in the air, reflecting the weakening of ionic interaction between PC6+ and TFSI− as they were separated40. This peak gradually shifted back to 1052 cm−1 when the damaged surfaces were brought into contact, which indicates the reformation of ionic interaction at the healed interface41,42. Similarly, when PIL30-TFSI was cut and healed underwater, the peak at 1053 cm−1 shifted to 1055 cm−1 due to damage and shifted back during healing (Fig. 3h). Subsequently, the resistance change of PIL30-TFSI was recorded during cut and healing both in the air and underwater (Fig. 3i, j). When the sample was cut in the middle, the electrical resistance drastically surged, and then it took ~7 s to return to the original value in the air and underwater. The fast recovery of its conductivity makes PIL30-TFSI a good electrode candidate for self-healing electronics. The ultrafast self-healing of PIL30-TFSI (in 10 s) makes it remarkable among the self-healing polymers both in the air and underwater at room temperature, which is correlated with its ultralow Young’s modulus (Fig. 3k and Supplementary Table 2). Apart from the rapid self-healing property, the adhesion and thermal stability of PIL30-TFSI are favorable for the construction of DEA (see Supplementary Fig. 8 and Supplementary Note 3). Compared with common hydrogel (PAAm as an example here), PIL30-TFSI demonstrated good adhesion at the interface with diverse materials such as wood, PTFE, glass, plastic, metal, and especially the different DE materials. This prevents electrode delamination from DE layers, enhancing device reliability. Among the temperature range from −20 to 80 °C, PIL30-TFSI exhibited stable weight retention as its decomposition temperature is around 400 °C based on TGA result (Supplementary Fig. 8e–g), which is advantageous for DEAs operating at high and low temperatures. Additionally, the ionic conductivity of PIL30-TFSI is 0.013 S/m at room temperature, outperforming other self-healing ionogel electrodes for DEAs (Supplementary Table 3). This high ionic conductivity of PIL30-TFSI electrode can help facilitate rapid charge distribution and minimize resistive losses for DEAs.
DEA actuation performance
The enhanced actuation performance of DEAs equipped with PIL30-TFSI electrodes was validated through three distinct actuation modes. For DEAs working in buckling mode, DEA with the softest electrode, i.e., PIL30-TFSI electrode showed the largest displacement of 2.95 ± 0.26 mm among those using PIL30-X electrodes (see Supplementary Note 4 and Supplementary Fig. 9). This PIL30-TFSI electrode was demonstrated in DEAs with diverse DE materials without deteriorating the breakdown strength of DEAs using VHB and PDMS, and long-term stability at high or low temperatures (Supplementary Fig. 9d–i). Figure 4a displays the schematic of DEAs operating in area strain mode, which was fabricated using prestrained elastomer VHB 4905 (biaxially 100% by 100%). The resultant DEAs with different PIL30-X electrodes were compared in Fig. 4b, where DEAs with PIL30-TFSI electrodes exhibited an area strain of 63.2% at 32 V/μm, higher than 32.9% for PIL30-OTF electrodes and 11.9% for PIL30-BF4 electrodes at the same electric field. This verifies that the soft electrodes contribute to high actuation strains. As PIL30-TFSI is able to self-heal rapidly, the area strain of DEAs with PIL30-TFSI electrodes after cutting and subsequent healing was measured. Almost 96% of the original area strain is retained when measured at 32 V/μm (Fig. 4c). When comparing with other reported DEAs with self-healing electrodes, the PIL30-TFSI electrode achieved high pristine and self-healed area strain due to its low elastic modulus, low thickness, and excellent self-healing capability (Fig. 4d and Supplementary Table 4)18,19,21,43,44. By calculating the product of Young’s modulus (Y) and thickness (d) for both the electrode and DE layer, Y × d is 0.116 Pa∙m for PIL30-TFSI, significantly lower than 31.25 Pa∙m for VHB 4905 biaxially prestrained 100% by 100%. This leads to the minimization of constrain imposed by the electrodes on deformation of DE layer, thereby maximizing actuation strains.
a Schematic illustration of DEAs working in area strain mode. The DE layer is prestrained and then mounted on a frame. b Area strain of DEAs with PIL30-X electrodes under different electric field. c Area strain of DEAs with pristine and self-healed PIL30-TFSI electrodes under different electric field. d Ashby chart summarizing area strains and healing time of DEAs with self-healing electrodes. The solid symbols are for DEAs with pristine electrodes, and the open symbols are for self-healed electrodes. References are listed in Supplementary Table 4. e Schematic illustration of unimorph bending DEAs. The DE layer is uniaxially prestrained and attached to a passive layer. f X-axis displacement of bending DEAs with original and self-healed PIL30-TFSI electrodes. Inset is the overlapping photographs of x-axis movement of DEA at 0 and 19.2 V/μm. g Blocking forces of bending DEA with original and self-healed PIL30-TFSI electrodes. h X-axis displacement of bending DEAs as a function of time. i Cyclic actuation under 11 V/μm and 1 Hz (square wave) for bending DEA with PIL30-TFSI electrodes at different humidity.
DEAs working in bending mode, known as bending DEAs, consist of a single DE layer sandwiched between two electrodes and an additional passive layer. The passive layer, characterized by a high elastic modulus and minimal stretchability compared to the DE, induces a bending motion towards it when the DE undergoes area expansion at applied voltage. With a uniaxially 20% prestrained VHB, the bending DEA exhibits an initial curvature when unactuated, which decreases as the voltage increases (Fig. 4e). The displacement of the tip of bending DEA in x axis was recorded by a laser displacement sensor and the equilibrium state reached within 2 s (Fig. 4h). The equilibrated displacements at different voltages were plotted in Fig. 4f, where the bending DEA reached 1.74 mm at 19.2 V/μm and retained 91% of the original x-axis displacement after self-healing from cut of PIL30-TFSI electrode, which presented the good recoverability of DEAs. Additionally, the blocking force of bending DEA with original PIL30-TFSI electrodes achieved 1.27 mN at 19.2 V/μm and 1.13 mN after self-healing in 10 s, respectively, proving its functionality and recoverability for a soft gripper (Fig. 4g). When operating at different humidity especially the 100% RH in Fig. 4i, the bending DEA could stably actuate in 1000 cycles, which benefits from the fatigue resistance and hydrophobicity of PIL30-TFSI.
Soft Robotics by DEA with PIL30-TFSI electrodes
With the successful verification of high actuation strains and fast self-healing in the electrodes of DEAs, a soft gripper composed of two bending DEAs was developed to gently and repeatedly grip objects both in the air and underwater. As shown in Fig. 5a, PET membrane was chosen as the passive layer for the bending DEAs, while the exposed electrode remains grounded. The two-finger DEA gripper opens when voltage is applied, but can grasp and hold objects without the need for continuous voltage, thus working with low and short-term energy consumption. As shown in Fig. 4h, the displacement of bending DEAs with PIL30-TFSI electrodes returned to 0 mm within 1 s when voltage was switched off, enabling the soft gripper to rapidly close its fingers and grasp objects. This gripper is capable of grasping versatile and soft objects such as a soft cotton ball (Fig. 5b). Besides, it can gently and promptly pick up a delicate microcube, lifting it steadily and releasing it within 1 s (Supplementary Movie 1). Even if the exposed PIL30-TFSI electrode sustains damage, such as a cut by a scalpel, the gripper can recover from the damage and maintain its functionality (Fig. 5c). Moreover, the bending DEA can overcome water resistance, ensuring its functionality in aquatic environments. Owing to the underwater self-healing capability of the PIL30-TFSI electrode, the gripper can effectively grasp a metal column or a leaf, and healed from cuts, demonstrating its potential as a durable underwater soft gripper (Fig. 5d, Supplementary Fig. 10 and Supplementary Movie 2). The soft gripper, equipped with low elastic modulus (3.4 kPa) electrodes, offers a gentle platform for capturing delicate marine animals and enables rapid capture due to the short response time of DEAs45. With tailored designs of DEA geometries and utilization of this ultrasoft and fast self-healing PIL30-TFSI electrode, high-performing soft robotics could be fabricated with versatile underwater motion.
a Schematic of the working principle and structure of a two-finger gripper. The gripper opens when voltage is on and close for grasping when voltage is off. b Photographs showing the gripper opens at 11 V/μm, and close at 0 V/μm to grasp and lift a cotton ball (weight of 50 mg). c Photographs showing the gripper opens at 11 V/μm, and close at 0 V/μm to grasp and lift a microcube (1.5 mm3, weight of 4 mg), which can maintain the ability to grasp after cutting and healing of the PIL30-TFSI electrode. d Photographs showing the gripper works underwater to grasp a metal column (weight of 64 mg) and keeps working after underwater cutting and healing of PIL30-TFSI electrode (it opens at 12 V/μm and closes at 0 V/μm). Scale bars are 1 cm for photos.
Beyond soft grippers with recoverability, the application of PIL30-TFSI electrodes was further explored in soft robotics, including crawling robots, artificial muscles, and tunable optical modulators (Supplementary Fig. 11 and 12). The ultralow Young’s modulus and rapid self-healing capability of PIL30-TFSI enabled these DEA-based systems to achieve excellent actuation performance and quick recovery from damage. Notably, the high transparency (~94%) of self-healing PIL30-TFSI supports its use as a tunable optical modulator, addressing the demand for soft, transparent electrodes or electrolytes in optical applications and soft wearable electronics.
Discussion
We presented an ultrasoft and fast self-healing PIL30-TFSI electrode for high-performing and durable dielectric elastomer actuators (DEAs). By optimizing anion-mediated ionic interaction among three different anions (BF4−, OTF−, TFSI−), the weak ionic interaction between TFSI− and PC6+ led to high mobility of both the polycation chains and the anions. As a result, PIL30-TFSI achieved an exceptionally low elastic modulus of 3.4 kPa and rapid self-healing within 10 s in both air and underwater. These characteristics surpass those of other polymers that self-heal at room temperature in both environments. As such, DEAs with PIL30-TFSI electrodes demonstrated higher original and recovered actuation strains with shorter healing time compared to other self-healing electrodes. The stable mechanical properties of PIL30-TFSI through cyclic tensile test and fatigue test at a cyclic load contributed to the consistent cycling actuation of the resultant DEAs. Additionally, the PIL30-TFSI electrodes enable DEAs to perform reliably at high or low temperatures (80 and −20 °C) and in submerged environments. A two-finger soft gripper composed of bending DEAs successfully grasped soft or delicate objects such as cotton, microcube, and leaf, maintaining its functionality after the damage of electrode both in air and underwater owning to its rapid self-healing property. Furthermore, this PIL30-TFSI electrode was demonstrated in soft crawling robotics, artificial muscles, and tunable optical modulators for recoverability from damage, proving its wide applicability. These findings highlight the potential of PIL30-TFSI electrodes for electric field-driven soft actuators, especially in applications like soft robotics and marine exploration.
Methods
Preparation of PIL monomers
Firstly, 1-hexyl-3-vinylimidazolium bromide (C6Br) was synthesized by the reaction of 1-vinylimidazole and 1-bromohexane. 1-bromohexane (76.0 g, 0.460 mol) was added dropwise into 1-vinylimidazole (40.0 g, 0.425 mol) over a period of 2 h and stirred at 35 °C for 3 days. The resulting viscous liquid was washed with ethyl acetate three times and then diethyl ether three times in a separation funnel. The liquid product was dried in vacuo for completely removing solvent. Secondly, monomers with different anions X− such as BF4−, OTF−, TFSI−, were obtained by ion exchange of C6Br with NaBF4, LiOTF, LiTFSI, respectively. The molar ratio of each salt to C6Br was 1.2. For example, C6Br (20 g, 0.077 mol), NaBF4 (10.1 g, 0.092 mol), and 40 g DI water were mixed together and stirred at 35 °C for 2 days. The product was washed with DI water three times and then dried in vacuo to get pure C6BF4.
Preparation of PC6X
The pristine PILs with different anions (PC6X) were prepared by free radical polymerization of monomers. The molar ratio of photoinitiator diphenyl(2,4,6 trimethylbenzoyl)phosphine oxide (TPO) to monomer is 0.004. A small amount of acetonitrile (its molar ratio to monomer is 0.32) was added for dissolving TPO and removed after dissolution. The obtained mixture was polymerized under ultraviolet (UV) light (395 nm) for 1 min to obtain the PILs with different anions.
Preparation of copolymers
The copolymer P(AAm-C6X) was synthesized from the comonomers AAm and C6X in a 1:1 molar ratio. Relative to the weight of AAm, 4 wt.% of photoinitiator TPO and 30 wt.% of solvent ethanol were added. The mixture experienced 5 min of photopolymerization under UV light and subsequential 0.5 h of heating at 80 °C to obtain the copolymer.
Preparation of PIL-Y
The precursors of PIL-Y (where Y denotes the weight percentage of additional IL to monomer) were prepared by mixing monomer C6TFSI, 2 wt.% of the crosslinker poly(ethylene glycol) diacrylate (PEGDA 700, Mn 700), 0.5 wt.% of photoinitiator TPO, and plasticizer BMIM TFSI, whose weight percentage to monomer varies from 0 to 40 wt.%. The solutions were sonicated for 10 min by a digital ultrasonic cleaner (DIGITAL PRO). PIL-Ys were obtained by drop casting the precursors and polymerizing under UV light (395 nm) for 1 min.
Preparation of PIL30-X
With the characterizations of PIL-Y, the softest while solid PIL was confirmed as PIL-30. To achieve the same composition of PIL30-X except the anions, the precursors of PIL30-X were prepared at the same molar ratio of photoinitiator, crosslinker, and plasticizer BMIM X (BMIM BF4 for PIL30-BF4, BMIM OTF for PIL30-OTF, BMIM TFSI for PIL30-TFSI, respectively). Specifically, in the precursor of PIL30-BF4, 1 g of C6BF4, 0.012 g of PEGDA 700, 0.28 g of BMIM BF4, and 2.9 mg of TPO were mixed and sonicated to a well-dispersed solution.
Fabrication of DEAs
Three different types of DEAs were fabricated, including buckling mode, area strain mode, and bending mode. (1) For DEAs working in buckling mode, a piece of 3 M VHB 4905 tape (thickness: 500 μm) was directly mounted on a PMMA ring (inner diameter: 30 mm, outer diameter: 50 mm) without prestrain. 5 μL of the PIL30-X precursors was drop cast and UV cured within an overlapping central circle with a diameter of 10 mm on the top and bottom of VHB 4905 tape subsequently. The PIL30-X electrodes with a thickness of ~34 μm were extended by a copper-nickel tape and connected to a Trek 610E high voltage power source. The voltage applied to the DEAs increased by 1 kV to enable a perpendicular displacement of VHB tape. The displacements at the central point of DEAs were recorded by a laser displacement sensor (Epsilon optoNCDT 2300). (2) The planar DEAs working in area strain mode were fabricated through prestraining the VHB 4905 tape biaxially 100% by 100% on the same PMMA ring and UV curing 5 μL of PIL30-X electrodes in the abovementioned same steps. The area expansion of the overlapping electrodes was captured by a video camera and then analyzed using the software Image J. The applied voltage increased by 1 kV until the breakdown voltage was reached. Area strains (ΔA) were calculated using the equation: ΔA = (Af −A0)/A0 × 100%, where Af represents the area at different voltages and A0 is the initial area of the overlapping region. (3) Bending DEAs were fabricated with 20% prestrained VHB 4905, PIL30-TFSI electrodes, and a piece of PET membrane with a thickness of 100 μm serving as the passive layer. When attached to the non-stretchable passive layer, the prestrained VHB 4905 induced an initial curvature in the DEAs. With the top of bending DEA clamped, the bottom moves as a free end. Upon voltage application, the DEAs bent towards the passive layer. The bending motion along the x-axis was recorded by a laser displacement sensor (Epsilon optoNCDT 2300). The blocking force was obtained by contacting the free end to a load cell (LSB200, Futek). When preparing the DEAs with PIL electrodes undergoing cutting and self-healing, a force sensor was placed under the DEAs (Supplementary Fig. 13). The cutting force was controlled to be around 0.5 N, which was sufficient to cut through the 500 μm-thick PIL30-TFSI sample without damaging the DE layer.
Fabrication of soft grippers
The soft gripper consists of two bending DEAs. These bending DEAs utilize VHB 4905 tape with a 20% prestrain and employ 100 μm-thick PET membrane as the passive layer, which enables the bending motion while protecting the DEAs from premature breakdown in underwater environments. In this configuration, the electrode exposed to air or water is grounded, while the electrode covered by the passive layer is connected to a high voltage. The passive layers of the DEAs face outward, causing the DEAs to bend outward and open the gripper when voltage is applied. Conversely, when the voltage is turned off, the DEAs return to their original position, closing the gripper and allowing it to grasp objects. To prevent adhesion to objects, the free end of the bending DEAs, which acts as the fingertip of the gripper, is covered with dextran powders. One end of each bending DEA is fixed, allowing the free end to move freely as the gripper operates.
Fabrication of soft crawling robotics
The soft crawling robot consisted of a single-layer VHB body, PIL30-TFSI electrodes, and PET feet. A VHB 4910 tape (thickness: 1 mm) was uniaxially prestrained by 200% and mounted on a rigid PMMA frame. Two 200 μm-thick PET films were cut into outer frames of 34 × 50 mm2 with a 20 × 30 mm2 central hole using a Cricut Maker 3. One PET frame was directly adhered to the top of the prestrained VHB tape, while the other was cut in the middle of the hole and then attached to the bottom of the VHB layer. After securing the wires, 20 μL of PIL precursor was drop-cast onto both sides of the VHB 4910 within the hole area of PET and UV cured to ensure a strong connection with the wires. Finally, the excess VHB extending beyond the PET frame was trimmed, and the DEA-based crawling robotic was released from the PMMA frame. The displacement along the x-axis of this soft crawling robotic was recorded by a laser displacement sensor (Epsilon optoNCDT 2300), for which we applied square-wave 8 kV with frequency varying from 1 to 100 Hz.
Fabrication of soft artificial muscles
A pure-shear DEA was fabricated using VHB 4905 tape with overlapping PIL30-TFSI electrodes (22 × 20 mm2). A load of 110.6 g was lifted by the pure-shear DEA, voltage by a step of 1 kV was applied until its breakdown. The displacement was analyzed by Image J, and the specific energy was calculated through \(E=\frac{{m}_{L}gh}{{m}_{a}}\), where mL, ma, h denote mass of load, mass of active area of DE, height change of the load, respectively.
Fabrication of optical modulators
The optical modulator consisted of the area-strain-mode DEA with a rectangular electrode area (10 × 10 mm2), and additional layers of silver nanowires on the top of PIL30-TFSI electrodes. The silver nanowires (L50, XFNANO) were diluted in isopropyl alcohol into 1 mg/mL and sprayed at the PIL30-TFSI layer at a distance of 20 cm until it formed a network.
Computational method
The theoretical calculations of surface electrostatic potential and ionic interaction energy were performed via the Gaussian 16 suite of programs. The structures of cation C6+, three anions, and H2O were optimized at the B3LYP/6-31 + G (d,p) level of theory. The surface electrostatic potential of three anions is ploted using Visual Molecular Dynamics (VMD) program. The interaction energies (ΔE) of the optimized complexes, including monomers C6X and TFSI−-H2O, were evaluated to compare the ionic interaction strength for three monomers and the affinity of TFSI− to water. These energies were computed using the B3LYP/6-31 + G (d,p) level of theory. The interaction energy ΔE is defined as the difference between the total energy of the complex and the sum of the individual energies of its monomer components, according to the equation ΔE = E(AB) − E(A) − E(B).
Data availability
The data that supports the findings of this work can be found in the Main Text and the Supplementary Information. Coordinate files are provided as Supplementary Data 1. Source data generated in this study have been deposited in figshare database under https://doi.org/10.6084/m9.figshare.29323175. All data are available from the corresponding author upon request.
References
Feng, W. et al. A large-strain and ultrahigh energy density dielectric elastomer for fast moving soft robot. Nat. Commun. 15, 4222 (2024).
Duduta, M., Hajiesmaili, E., Zhao, H., Wood, R. J. & Clarke, D. R. Realizing the potential of dielectric elastomer artificial muscles. Proc. Natl. Acad. Sci. USA 116, 2476–2481 (2019).
Shi, Y. et al. A processable, high-performance dielectric elastomer and multilayering process. Science 377, 228–232 (2022).
Qiu, Y., Zhang, E., Plamthottam, R. & Pei, Q. Dielectric elastomer artificial muscle: materials innovations and device explorations. Acc. Chem. Res. 52, 316–325 (2019).
Lee, D.-Y. et al. A wearable textile-embedded dielectric elastomer actuator haptic display. Soft Robot 9, 1186–1197 (2022).
Ji, X. et al. Untethered feel-through haptics using 18-μm thick dielectric elastomer actuators. Adv. Funct. Mater. 31, 2006639 (2021).
Nam, S. et al. A robust soft lens for tunable camera application using dielectric elastomer actuators. Soft Robot 5, 777–782 (2018).
Pu, J. et al. A unimorph nanocomposite dielectric elastomer for large out-of-plane actuation. Sci. Adv. 8, eabm6200 (2022).
Guo, Y., Liu, L., Liu, Y. & Leng, J. Review of dielectric elastomer actuators and their applications in soft robots. Adv. Intell. Syst. 3, 2000282 (2021).
Li, M., Pal, A., Aghakhani, A., Pena-Francesch, A. & Sitti, M. Soft actuators for real-world applications. Nat. Rev. Mater. 7, 235–249 (2022).
Pan, C. et al. A liquid-metal-elastomer nanocomposite for stretchable dielectric materials. Adv. Mater. 31, e1900663 (2019).
Ankit et al. High- k, ultrastretchable self-enclosed ionic liquid-elastomer composites for soft robotics and flexible electronics. ACS Appl. Mater. Interfaces 12, 37561–37570 (2020).
Vatankhah-Varnoosfaderani, M. et al. Bottlebrush elastomers: a new platform for freestanding electroactuation. Adv. Mater. 29, 1604209 (2017).
Ni, Y. et al. Plasticizer-induced enhanced electromechanical performance of natural rubber dielectric elastomer composites. Compos. Sci. Technol. 195, 108202 (2020).
Sangian, D. Improving the performance of dielectric-elastomer actuators at elevated operating temperatures by means of thermal softening. Smart Mater. Struct. 29, 025013 (2020).
Tan, M. W. M., Bark, H., Thangavel, G., Gong, X. & Lee, P. S. Photothermal modulated dielectric elastomer actuator for resilient soft robots. Nat. Commun. 13, 6769 (2022).
Hajiesmaili, E. & Clarke, D. R. Dielectric elastomer actuators. J. Appl. Phys. 129, 151102 (2021).
Cao, Y. et al. A transparent, self-healing, highly stretchable ionic conductor. Adv. Mater. 29, 1605099 (2017).
Zhang, Y. et al. Highly transparent, underwater self-healing, and ionic conductive elastomer based on multivalent ion–dipole interactions. Chem. Mater. 32, 6310–6317 (2020).
Chen, B. et al. Highly stretchable and transparent ionogels as nonvolatile conductors for dielectric elastomer transducers. ACS Appl. Mater. Interfaces 6, 7840–7845 (2014).
Cheng, Z. et al. A highly robust amphibious soft robot with imperceptibility based on a water-stable and self-healing ionic conductor. Adv. Mater. 35, 2301005 (2023).
Ming, X. et al. Highly transparent, stretchable, and conducting ionoelastomers based on poly(ionic liquid)s. ACS Appl. Mater. Interfaces 13, 31102–31110 (2021).
Peng, Z., Shi, Y., Chen, N., Li, Y. & Pei, Q. Stable and high-strain dielectric elastomer actuators based on a carbon nanotube-polymer bilayer electrode. Adv. Funct. Mater. 31, 2008321 (2021).
Hubertus, J., Fasolt, B., Linnebach, P., Seelecke, S. & Schultes, G. Electromechanical evaluation of sub-micron NiCr-carbon thin films as highly conductive and compliant electrodes for dielectric elastomers. Sens. Actuators Phys. 315, 112243 (2020).
Rosset, S. & Shea, H. R. Flexible and stretchable electrodes for dielectric elastomer actuators. Appl. Phys. A 110, 281–307 (2013).
Gao, D. et al. A supramolecular gel-elastomer system for soft iontronic adhesives. Nat. Commun. 14, 1990 (2023).
Keplinger, C. et al. Stretchable, transparent, ionic conductors. Science 341, 984–987 (2013).
Zhang, H., Dai, M. & Zhang, Z. The analysis of transparent dielectric elastomer actuators for lens. Optik 178, 841–845 (2019).
Li, T. et al. Fast-moving soft electronic fish. Sci. Adv. 3, e1602045 (2017).
Chen, B. et al. Stretchable and transparent hydrogels as soft conductors for dielectric elastomer actuators. J. Polym. Sci. Part B Polym. Phys. 52, 1055–1060 (2014).
Xiang, S., Zheng, F., Chen, S. & Lu, Q. Self-healable, recyclable, and ultrastrong adhesive ionogel for multifunctional strain sensor. ACS Appl. Mater. Interfaces 13, 20653–20661 (2021).
Wang, H. et al. A highly stretchable, self-healable, transparent and solid-state poly(ionic liquid) filler for high-performance dielectric elastomer actuators. J. Mater. Chem. A 11, 14159–14168 (2023).
Matsumoto, A. et al. Introducing large counteranions enhances the elastic modulus of imidazolium-based polymerized ionic liquids. Macromolecules 51, 4129–4142 (2018).
Iacob, C. et al. Polymerized ionic liquids: correlation of ionic conductivity with nanoscale morphology and counterion volume. ACS Macro Lett. 6, 941–946 (2017).
la Cruz, D. S. et al. Correlating backbone-to-backbone distance to ionic conductivity in amorphous polymerized ionic liquids. J. Polym. Sci. Part B Polym. Phys. 50, 338–346 (2012).
Cui, J. et al. Novel imidazolium-based poly(ionic liquid)s with different counterions for self-healing. J. Mater. Chem. A 5, 25220–25229 (2017).
Yang, Y. & Urban, M. W. Self-healing polymeric materials. Chem. Soc. Rev. 42, 7446–7467 (2013).
Utrera-Barrios, S., Verdejo, R., López-Manchado, M. A. & Hernández Santana, M. Evolution of self-healing elastomers, from extrinsic to combined intrinsic mechanisms: a review. Mater. Horiz. 7, 2882–2902 (2020).
Huynh, T.-P., Khatib, M. & Haick, H. Self-healable materials for underwater applications. Adv. Mater. Technol. 4, 1900081 (2019).
Kong, Z. et al. Ultrafast underwater self-healing piezo-ionic elastomer via dynamic hydrophobic-hydrolytic domains. Nat. Commun. 15, 2129 (2024).
Moschovi, A. M., Ntais, S., Dracopoulos, V. & Nikolakis, V. Vibrational spectroscopic study of the protic ionic liquid 1-H-3-methylimidazolium bis(trifluoromethanesulfonyl)imide. Vib. Spectrosc. 63, 350–359 (2012).
Liew, C.-W., Ramesh, S. & Durairaj, R. Impact of low viscosity ionic liquid on PMMA-PVC-LiTFSI polymer electrolytes based on AC -impedance, dielectric behavior, and HATR-FTIR characteristics. J. Mater. Res. 27, 2996–3004 (2012).
Liu, Y. et al. Self-powered and self-healable extraocular-muscle-like actuator based on dielectric elastomer actuator and triboelectric nanogenerator. Adv. Mater. n/a, 2309893 (2023).
Lee, J. et al. Water-processable, stretchable, self-healable, thermally stable, and transparent ionic conductors for actuators and sensors. Adv. Mater. 32, 1906679 (2020).
Sinatra, N. R. et al. Ultragentle manipulation of delicate structures using a soft robotic gripper. Sci. Robot. 4, eaax5425 (2019).
Acknowledgements
H.W. acknowledges the scholarship awarded by the Nanyang Technological University, Singapore. This project is supported by the Ministry of Education, Singapore, under AcRF Tier 2 grant no. MOE-T2EP50122-0002 and AcRF Tier 1 grant no. RG74/24.
Author information
Authors and Affiliations
Contributions
H.W. and P.S.L. conceived the idea and designed the research; H.W. synthesized the materials. H.W., A.G., W.W., X.Hu, and X.Huang characterized the materials and analyzed the results. H.W. designed the actuators and measured their actuation performance. H.W., Q.L., and X.W. carried out device photography and videography. H.W. and P.S.L. prepared the manuscript with input from all authors. P.S.L. supervised the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications David Clarke and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, H., Gupta, A., Lu, Q. et al. Ultrasoft and fast self-healing poly(ionic liquid) electrode for dielectric elastomer actuators. Nat Commun 16, 7405 (2025). https://doi.org/10.1038/s41467-025-62796-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-62796-6