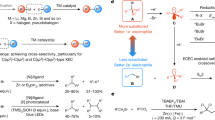
Abstract
Reformatsky reagents are commonly employed with activated electrophiles, such as aldehydes, ketones, or activated alkyl halides. However, their limited nucleophilicity remains a considerable challenge for direct reactions with unactivated alkyl halides, typically necessitating transition metal catalysis. Here, we present a transition-metal-catalyst-free approach that facilitates direct nucleophilic substitution between Reformatsky reagents and diverse unactivated alkyl halides, which enables formal reductive cross-electrophile coupling via a one-pot process. Mechanistic studies reveal the pivotal role of highly polar solvents and the formation of zincate enolate intermediates containing hindered alkyl groups, which streamlines the SN2 reaction with unactivated alkyl halides via open-frame transition states. The modular nature of the current protocol eliminates the need for strong bases and transition metal catalysts, allowing easy access to esters, amides, and ketones bearing all-carbon quaternary centers with a wide range of functional groups, thereby providing a simple and expedient synthetic avenue to build complex molecules.
Similar content being viewed by others
Introduction
Developing efficient methods to forge carbon–carbon bonds stands as a central research focus in organic chemistry1, given that these bonds underlie a vast range of organic compounds, from simple hydrocarbons to complex biomolecules such as pharmaceuticals, agrochemicals, and specialized materials2,3. Among these approaches, organometallic reagents demand particular attention, especially regarding the reactivity and selectivity of various types in C–C coupling reactions4,5,6. Early investigations demonstrated that Reformatsky reagents, known for their lower reactivity than organolithium or Grignard reagents but distinct selectivity among aldol-type reactions, found wide application in nucleophilic reactions with activated electrophiles, including aldehydes, ketones, acyl chlorides, epoxides, nitrones, aziridines, imines, or nitriles (Fig. 1a)7. However, for less reactive alkyl halides, the nucleophilicity of zinc-based Reformatsky reagents is often insufficient for direct SN2 reactions. To date, only highly reactive alkyl halides have been shown to undergo nucleophilic substitution with Reformatsky reagents, similar to other organozinc reagents8,9,10. Examples include iodomethane as well as allylic and benzylic bromides, which afford moderate yields, as reported by Orsini11,12,13, Bott14, and Watt15. In contrast, typical unactivated alkyl bromides (e.g., ethyl bromide) lack reactivity toward the Reformatsky reagents for nucleophilic substitution reactions. Instead, transition metal catalysts (Fe, Co, Ni, Pd, etc.) are usually used (Fig. 1b), facilitating transmetallation with organozinc reagents to form C(sp3)–C(sp3) bonds5. In this vein, Knochel’s group16,17,18 and Fu’s group19,20 have led key advancements in nickel catalysis, and other groups have likewise succeeded in achieving this coupling using various transition metal catalysts21,22,23,24,25,26.
a Reactions of the Reformatsky reagent. b Transition-metal-catalyzed cross-coupling of organozinc reagent with unactivated alkyl halides. c Zinc-mediated cross-coupling of α-bromo carbonyls with unactivated alkyl halides. d Representative orbital interactions between the Reformatsky reagent and diverse electrophiles. TM = transition metal, LP = lone pair electrons.
Crystallographic studies20,27,28,29 have shown that the Reformatsky reagents typically adopt a C-metallated dimer. During Aldol-type C–C bond-forming reactions, the oxygen lone pair on the carbonyl coordinates to the organozinc(II) center, which not only activates the electrophile but also facilitates the dissociation of the dimer from the thermodynamically stable C-metallated form to the more active O-metallated enolate, thereby promoting C–C bond formation via metalla-Claisen rearrangement30, with the πeno_zinc (HOMO) → π*C = O electron flow depicted in Fig. 1d. In contrast, unactivated alkyl halides, characterized by their low polarity and high-lying σ-antibonding orbitals (LUMO), exhibit minimal propensity for coordination with the organozinc(II) center. This effectively precludes a concerted coupling mechanism, represented by the Zimmerman-Traxler model pertinent to carbonyl compounds31. Additionally, the significant energy mismatch between the frontier orbitals of zinc enolate nucleophiles and unactivated alkyl halides creates a considerable reaction barrier32, rendering such nucleophilic reactions intrinsically challenging. In general, effective interaction requires sufficiently lowering of the alkyl halide’s LUMO energy to match the zinc-based nucleophile’s HOMO, as seen with allylic or benzylic halides.
To enable the direct cross-coupling between the Reformatsky reagent and unactivated alkyl halides under transition-metal-free conditions, the critical step is to increase the nucleophilicity of the Reformatsky reagent. To achieve this, we implemented three complementary strategies: introducing anionic ligands, installing alkyl substituents at the α-position of the carbonyl group, and utilizing highly polar solvents. The addition of anionic ligands leads to the accumulation of negative charge on the zinc metal, facilitating the dissociation of the C-metallated dimer enolate. Such a dissociation process could be further enhanced by the use of high-polarity solvents28,33 and the introduction of alkyl groups at the α-position of the carbonyl group. Additionally, the coordination of anionic ligand to the zinc cation could not only generate a more nucleophilic anionic zincate-based intermediate with a higher HOMO level8,9,10,34,35,36, but also prevent alkyl halide binding, favoring a low energy open-frame transition state over the classical six-membered Zimmerman–Traxler model. As such, the synergistic effects of these strategies collectively enable the potential SN2 reaction. In this work, we report a protocol for the direct alkylation of α-bromo-α-alkyl amides, esters, or ketones with unactivated alkyl halides under transition-metal-free conditions. A key aspect of this protocol involves the use of NaI as an anionic ligand: NaI not only exchanges with alkyl bromides to afford iodides with higher SN2 reactivity but more importantly coordinates with the organozinc(II) center to induce the formation of potential zincate enolate intermediates (Fig. 1c). These findings highlight the distinctive reactivity of Reformatsky reagents in the presence of anionic ligand and pave the way toward simplifying and expanding their roles in organic synthesis.
Results and discussion
Reaction discovery and optimizations
Our initial studies focused on the zinc-mediated reductive cross-electrophile coupling (XEC) between 2-bromo-2-methyl-1-morpholin-4-yl-propan-1-one (1a) and (3-bromopropyl)benzene (8a) in DMF (0.4 M) using Zn (1.5 equiv.) as the reductant and LiBr (1.0 equiv.) as the additive. Under these conditions, the reaction proceeded smoothly and the desired cross-coupling product 9 was obtained after 2 h with a GC yield of 72%. The reaction was subsequently optimized by systematically evaluating the effects of different additives, solvents, and the equivalents of zinc powder used in the reaction (see Table S1 in the Supplementary Information for details). These optimizations revealed that employing 1.5 equivalents of NaI as the additive and N-methyl-2-pyrrolidone (NMP) as the solvent, along with 2.0 equivalents of zinc powder, significantly enhanced the reaction efficiency. Under these conditions, the reaction proceeded to full conversion of the starting materials within just 2 h at room temperature (25 °C), delivering the product 9 in an impressive 94% yield. Notably, this reaction system is distinct from both transition-metal-catalyzed and electricity-driven reductive XEC37,38,39,40,41,42,43,44,45,46. In this context, the metallic zinc is likely to react with the α-bromine of the carbonyl group to form the Reformatsky reagent that undergoes the subsequent desired transformation, rather than acting solely as a reducing agent, as is often the case in transition-metal-catalyzed XEC.
Mechanistic studies
A plausible explanation for the high conversion observed in the reaction could initially be the presence of trace amounts of transition metals, such as iron (Fe), nickel (Ni), or cobalt (Co), in the reagents, solvent, or additive. Such metal impurities, even in trace quantities, might theoretically act as unintended catalysts, promoting the XEC reaction47,48. To assess whether residual metals were indeed involved in the reaction (see Table S1 in the Supplementary Information), we performed control experiments comparing the performance of ultra-high-purity zinc pellets (99.999% purity) with that of unpurified zinc powder. Interestingly, the results were nearly identical (see Table S1, entries 2–3). Moreover, when zinc was replaced with manganese (Mn) as the reductant, the reaction was completely suppressed, suggesting that zinc itself plays an essential role in driving the XEC process. These observations strongly argue against the involvement of trace metal impurities as catalysts and highlight the critical nature of zinc in enabling the reaction.
Alternatively, the high reactivity observed in the catalyst-free reaction might be attributed to a direct electron transfer (ET) mechanism, potentially facilitated by zinc and NaI. Furthermore, metallic zinc is known to be a strong reducing agent, capable of directly transferring electrons to electrophilic substrates such as alkyl halides. In the absence of a catalytic system, zinc might reduce the brominated substrates via single-electron transfer (SET) to initiate the coupling reaction, while NaI might enhance the reactivity by stabilizing transient radical intermediates or participating in the halogen atom transfer (XAT) process. To probe whether a free radical was involved in this reaction, we embarked on various radical clock experiments (Fig. 2a). Specifically, 2-bromo-2-methyl-1-morpholinopropan-1-one (1a) was reacted with either (bromomethyl)cyclopropane (2) or 6-bromohex-1-ene (4) under standard conditions. In both cases, the cross-coupling products 3 and 5 were obtained in 77% and 96% yields, respectively. Importantly, there was no detection of by-products indicative of a radical pathway, such as the ring-opening product 3′ from 2 or the cyclization product 5′ from 4. These findings strongly argue against the involvement of a free radical in the carbon–carbon bond formation process. To gain more evidence against the ET pathway, we conducted a control experiment using optically pure secondary alkyl iodide 6. The formation of a radical intermediate would lead to racemization of the product. Experimentally, the cross-coupling of 1a with 6 only led to slight erosion of the stereochemistry of the product 7 (Fig. 2b). Additional experiments showed that the partial erosion of the product stereochemistry was attributed to partial racemization of 6 in the presence of iodide anions rather than any intrinsic ET mechanism (see Section S4.4). These findings strongly suggest that the carbon–carbon bond formation step of the reaction does not proceed via an ET process. Furthermore, they provide indirect evidence against the involvement of trace amounts of transition metals in the substrates as catalysts, since reported transition-metal-catalyzed XEC of alkyl halides often proceed via free radical intermediates, which were not observed in this case. Instead, these results indicated that an alternative, non-radical mechanism, such as a SN2 (bimolecular nucleophilic substitution) pathway, might be responsible for the high efficiency of the reaction.
It is well-known that the reaction between alkyl halides and zinc powder leads to the formation of organozinc reagents, which are versatile intermediates in organic synthesis. In particular, the reaction of α-bromo carbonyls with zinc generates the Reformatsky reagent, a nucleophilic zinc enolate, even though with low nucleophilicity, as mentioned in the introduction section. To probe whether this is the case, the Reformatsky reagent 1a-ZnBr (from 1a), the organozinc reagent 8a-ZnBr (from 8a), and 10-ZnBr (from 2-bromo-2-methylbutane 10) were generated independently and subjected to the optimized conditions. Under the optimized conditions, the reaction of 1a-ZnBr with 8a smoothly afforded the coupled product 9 in quantitative yield, while reactions of 8a-ZnBr with 1a and 10-ZnBr with 8a did not take place at all (Fig. 2c). In addition, it was found that mixing 1a with zinc in NMP in the presence of NaI generated 1a-ZnBr with a yield of 85% within 8 minutes. In contrast, under the same conditions, the formation of 8a-ZnBr from the reaction of 8a with zinc was not observed. Real-time monitoring revealed that the consumption of 1a and 8a occurred asynchronously, while a rapid equilibrium was established between 8a/NaI and (3-iodopropyl)benzene(8b)/NaBr, indicating that the initial stage of the reaction mainly involved the formation of the Reformatsky reagent, with NaI facilitated halogen exchange rather than the generation of the alkylzinc reagent (Fig. 3a, b). These results clearly demonstrated that zinc preferentially inserts into the C–Br bond of the activated alkyl bromide 1a, leading to the formation of the Reformatsky reagent 1a-ZnBr, rather than reacting with the unactivated alkyl bromide 8a. Additionally, these results also confirmed that the reactive intermediate in the reaction is the Reformatsky reagent 1a-ZnBr derived from 1a, and not the organozinc reagent 8a-ZnBr derived from 8a.
a Rapid consumption of 1a under standard conditions in the absence of its coupling partner. Blue line: remaining 1a. b Rapid bromine-iodine exchange of 8a under standard conditions in the absence of its coupling partner. Black line: remaining 8a; purple line: yield of 8b. c Modulation of reactivity by charge and side chain. Red line: yield of reaction between 8a and 1a-ZnBr·LiBr; blue line: yield of reaction between 8a and 1a-ZnBr; pink line: yield of reaction between 8a and 1b-ZnBr·LiBr; green line: yield of reaction between 8a and 1b-ZnBr; brown line: yield of reaction between 8a, 1b-ZnBr·LiBr, and NaI. Ph = phenyl, NMP = N-methyl-2-pyrrolidone.
Prior investigations have established that Reformatsky reagents are moderate nucleophilic. Consequently, the high reactivity observed in the current protocol suggests the formation of a more nucleophilic species under the given conditions. To further explore this nucleophile, several experiments were conducted. First, the Reformatsky reagent 1a-ZnBr was prepared following established procedures, and confirmed as a C-bound enolate (Fig. 4a). The addition of 1.0 equivalent of LiBr to a solution of 1a-ZnBr in THF yielded an ON-chelated ate-type enolate 1a-ZnBr·LiBr. This species was fully characterized using 1H NMR spectroscopy, elemental analysis, and single-crystal XRD (Fig. 4b). When 1a-ZnBr·LiBr was reacted with 8a in NMP, full conversion was observed within 2.0 h, affording 9 in quantitative yield (red line, Fig. 3c). In contrast, the same reaction using 1a-ZnBr produced 9 in only 47% yield under identical conditions (blue line, Fig. 3c). Likewise, 1b-ZnBr and its ate-type derivative 1b-ZnBr·LiBr, which were confirmed to be in the C-bound form by their single-crystal XRD (Fig. 4c, d), were synthesized from secondary α-bromoamide 1b. Reaction of 1b-ZnBr·LiBr with 8a yielded the cross-coupled product in 22% after 2 h (pink line, Fig. 3c). Notably, the addition of 1.5 equivalents of NaI accelerated the reaction, affording the cross-coupled product in 58% yield (brown line, Fig. 3c). On the other hand, 1b-ZnBr failed to produce the desired product under the identical conditions (green line, Fig. 3c). These results clearly confirm that the addition of a halogenated salt can effectively transform Reformatsky reagents into their more reactive C-bound or O-bound ate-type enolates, thereby enabling direct alkylation with unactivated alkyl halides. Additionally, the reaction-accelerating effect of NaI could be explained by its capacity to convert the less reactive alkyl bromide into the more reactive alkyl iodide.
To gain deeper insights into the enhanced nucleophilicity of zincate, quantum chemical calculations were performed (Fig. 5). In alignment with previously reported crystal structures of Reformatsky reagents, the Reformatsky reagent derived from 2-bromo-N,N,2-trimethylpropanamide is observed to exist as a dimer, referred to as Dimert1. Upon the addition of 1.0 equivalent of NaBr, a more stable ionic dimer, designated as Dimert2, is formed. However, Dimert2 lacks nucleophilicity, necessitating dissociation to generate three distinct monomers—INTt1-O, INTt1-C, and INTt1-ON—for the subsequent SN2 reaction. DFT calculations revealed that the introduction of an α-alkyl group significantly increases steric repulsion with the anionic zincate moiety, inducing conformational distortion and thereby facilitating the dissociation process (see Section S5.4 in the Supplementary Information). Notably, among these monomers, INTt1-ON is most stable since the ON-chelation in INTt1-ON can lessen the 1,3-allylic strain. Despite O-metallated monomer INTt1-O is less stable than INTt1-C and INTt1-ON by 2.2 and 3.4 kcal/mol, respectively, INTt1-O demonstrates significantly higher nucleophilicity toward alkyl halides (see EHOMO in Fig. 5 and Section S5.6 in the Supplementary Information). The overall energy barrier for this process is calculated to be 24.0 kcal/mol, which is consistent with experimental findings. In contrast to the [1,3]-sigmatropic Aldol-type reaction, which requires the Bürgi–Dunitz angle of 105° ± 5°, the open-frame transition state TSt1-O-1 is favored due to its near-linear C–C–Br angle and the superior overlap between the HOMO of INTt1-O and the antibonding orbital of the C–Br bond of the alkyl halide (Fig. S18). As a comparison, the mechanistic pathway for the reaction of the Reformatsky reagent Dimert1 with an alkyl halide in the absence of any additive was also explored. Similarly, Dimert1 must dissociate to form monomers—INTt3-O, INTt3-C, and INTt3-ON. Similar to the case of the system in the presence of NaBr, the O-metallated monomer INTt3-O exhibits much higher reactivity than INTt3-C and INTt3-ON. The reaction of INTt3-O with an alkyl halide proceeds via a cyclic transition state (Note: neutral INTt3-O cannot undergo the open-frame transition state), designated as TSt2-O. However, the overall energy barrier for this pathway is 34.3 kcal/mol, rendering it unfeasible at room temperature. Consistent with the experimental results, these findings highlight the critical role of halogen anion on the reactivity of the monomer in determining the nucleophilicity and feasibility of zinc-mediated reactions, with the addition of NaBr significantly influencing the mechanistic pathways and energy barriers.
Solvated Gibbs free energies were performed at PBE0-D3(BJ)/ma-def2-SVP-SMD(DMF) for geometry optimizations and frequency analyses, PBE0-D3(BJ)/ma-def2-TZVP(-f) for single-point energies, and M05-2X/6-31 G*-SMD(DMF) for solvation energies. HOMO energies were calculated at PBE0-D3(BJ)/ma-def2-TZVP(-f)-SMD(DMF). Golden curve: pathway through a neutral TS; red curve: pathway through an ate TS; green curves: disfavored pathways. nPr = n-propyl, DMF = N,N-dimethylformamide, INT = intermediate, TS = transition state, P = product, HOMO = highest occupied molecular orbital.
Substrate scope
Having the mechanism of the zinc-mediated cross-coupling reaction elucidated, our focus shifted towards exploring the generality of this protocol in synthesizing a diverse array of cross-coupling products featuring all-carbon quaternary centers. Firstly, we explored the cross-coupling of α-bromo-α,α-dialkyl amides with primary and secondary alkyl halides. As shown in Fig. 6, reaction of primary alkyl bromides with various functional groups, such as alkene (5), alkyne (13), silicon ether (14), Bpin (15), thioether (16), alkyl fluoride (22), alkyl chloride (23), trifluoromethyl group (24), aryl iodide (25), pyrrole (26–29, 33), furan (30), and thiophene (31), proceeded smoothly under the standard conditions. Notably, α-bromo-α,α-dialkyl lactam was also found to be a viable substrate (34). However, unactivated tertiary alkyl bromides failed to generate the desired product, probably due to excessive steric hindrance (38). Reaction of mono-alkyl substituted α-bromo-α-alkyl amides bearing a hindered alkyl group, such as tert-butyl or isopropyl group, occurred smoothly under the standard conditions to afford the products (39–41) in 47%, 53%, and 77% respectively. In contrast, α-bromo-α-ethyl amide bearing a less sterically hindered ethyl group reacted sluggishly at room temperature and required to increase the reaction temperature to 80 °C to afford the coupled product 42 in a low yield of 29%. Similarly, α-bromoamide without any alkyl substituents failed to give the desired product 43 even at 80 °C. Alkyl chlorides are much less reactive electrophiles than alkyl bromides. For these substrates, increasing the reaction temperature to 40 °C and elongating the reaction time to 48 h were required to enable full conversion. Under these conditions, alkyl chlorides containing alkene (5, 20), alkyl fluoride (12, 18, 21), alkyne (13), silicon ether (14, 19, 20), Bpin (15), thioether (16), thiophene (17), furan (18), cyclopropane (19), and acetal (21), were showed to be viable substrates to give the couple products in acceptable yields.
Conditions: aα-bromo-α-alkyl amide (0.40 mmol, 2.0 equiv.), alkyl chloride (0.20 mmol, 1.0 equiv.), Zn (3.0 equiv.), KI (3.0 equiv.), NMP (0.5 M), 40 °C, 48 h. bα-bromo-α-alkyl amide (0.30 mmol, 1.5 equiv.), alkyl bromide (0.20 mmol, 1.0 equiv.), Zn (2.0 equiv.), NaI (1.5 equiv.), NMP (0.4 M), 25 °C, 2 h. cα-bromo-α-alkyl amide (0.40 mmol, 2.0 equiv.), alkyl iodide (0.20 mmol, 1.0 equiv.), Zn (1.9 equiv.), NMP (0.5 M), 45 °C, 48 h. Et = ethyl, nPr = n-propyl, Ph = phenyl, Bpin = pinacolboryl, TBS = tributylsilyl.
Secondary alkyl bromides, due to their steric hinderance, are much less reactive in SN2 reactions. We found that activated secondary alkyl bromides, such as benzylic bromides and allylic bromides, could also couple with α-bromo-α,α-dialkyl amides to afford the corresponding coupled products (35–37) in good yields, whereas unactivated alkyl bromides reacted sluggishly even at elevated temperature. Notably, even though reactions of unactivated secondary alkyl iodides were initially sluggish under the standard conditions, optimization efforts led to full conversion after 48 h by increasing the amount of zinc powder to 1.9 equivalents and raising the reaction temperature to 45 °C. Under these optimized conditions, reactions of unactivated secondary alkyl iodides successfully afforded the desired products (44, 45) in 69% and 67% yields, respectively. In addition, cyclic alkyl iodides containing motifs such as cyclobutane (46), cyclopentane (47, 50), cyclohexane (48, 51–53), and cycloheptane (49), proved to be competent substrates, furnishing the desired products in good yields. Overall, the high reactivity of zincate derived from α-bromo-α-alkyl amide makes it quite valuable for the formation of new C(sp3)–C(sp3) bonds.
Encouraged by the success of zinc-mediated coupling of α-bromo-α-alkyl amides with unactivated alkyl halides, we turned to explore the coupling of α-bromo-α,α-dialkyl esters. As shown in Fig. 7, it was found that both primary and secondary alkylated α-bromo-α,α-dialkyl esters underwent successful cross-coupling with (3-bromopropyl)benzene (8a), generating the coupled products (54–61) in yields ranging from 73% to 95%. The scope of alkyl bromides was next studied in detail. Alkyl bromides bearing various functional groups such as alkene (56, 63, 64), aryl bromide (57, 69), aryl iodide (58), cyclopropane (62), acetal (65), thioether (66), secondary alkyl bromide (67), sulfonyl (68), Bpin (70) were compatible. Notably, secondary alkyl bromide and Bpin, which are usually incompatible under strongly alkaline conditions but are tolerant under the current reaction conditions. In addition, alkyl halides bearing pharmaceutically important structural motifs, such as furan (59), thiophene (60, 71), pyridine (61, 72), and pyrazole (73) would be successfully coupled. Not only primary alkyl bromides, but also activated secondary alkyl bromides such as benzylic or allylic bromide could also be employed under standard conditions to afford the coupled products (74–76) in good yields. With regard to the scope of α-bromo-α-alkyl esters, substrates containing more sterically hindered alkyl groups other than methyl, including n-propyl and ethyl, reacted smoothly to give the desired products (77, 78) with excellent yields of 94% and 93%, respectively. Likewise, reactions of α-bromo-α-cycloalkyl esters bearing cyclic alkyl rings such as cyclobutyl (79), cyclopentyl (80), cyclohexyl (81–84), tetrahydro-2H-pyran (82), gem-difluorocyclohexyl (84) also occurred in good to excellent yields. Moreover, reactions of α-bromo-α-alkyl lactones with one of the α-alkyl groups tethered to the ester moiety also proceeded smoothly to provide a variety of lactones with two different alkyl groups (86–99) in good to excellent yields. Notably, the coupling of monoalkyl-substituted α-bromo-α-isopropyl ester with a sterically hindered isopropyl group also proceeded smoothly to afford compound 85 in 39% yield.
Conditions: aα-bromo-α-alkyl ester (0.30 mmol, 1.5 equiv.), alkyl bromide (0.20 mmol, 1.0 equiv.), Zn (2.0 equiv.), NaI (1.5 equiv.), NMP (0.4 M), 25 °C, 2 h. bα-bromo-α-alkyl lactone (0.30 mmol, 1.5 equiv.), alkyl bromide (0.20 mmol, 1.0 equiv.), Zn (2.0 equiv.), Et4NI (2.0 equiv.), NMP/THF (4:1, 0.4 M), 25 °C, 12 h. Me = methyl, Et = ethyl, nPr = n-propyl, tBu = tert-butyl, nPent = n-pentyl, Ph = phenyl, Bn = benzyl, Bpin = pinacolboryl.
To further strengthen the synthetic potential of this methodology and inspired by the broad scope and functional group tolerance across a range of substrate classes of intermolecular cross-coupling, we next sought to extend intermolecular cross-coupling to intramolecular cross-coupling. Spirolactone is a common structural motif in a large number of natural products with diverse biological activities. Yet, due to the unfavorable entropy and enthalpy effects, spirolactones, particularly those with 8- and 9-membered rings, are relatively challenging to synthesize49. To our delight, after additional optimization of reaction parameters, we found a straightforward method to access these lactones that differs from existing synthetic methods. As shown in Fig. 8, a diverse range of spirolactones with [4 + 6], [6 + 6], [4 + 8], [5 + 8], [6 + 8], and [6 + 9] skeletons (101–106) were synthesized smoothly in good yields, including those of with 8- and 9-membered rings (103–106).
Regioselective control of α-alkylation in unsymmetrical ketones is a longstanding research topic in organic chemistry. Achieving selective alkylation at the more hindered α-position, as opposed to the less hindered α-position, presents a significant challenge50,51,52. This difficulty mainly stems from the fact that the traditional nucleophilic alkylation process requires the use of a strong alkaline base to deprotonate the ketone, and strong alkalinity itself favors the formation of less substituted enolates, making it difficult to generate the more substituted enolates required for the selective alkylation at the more hindered α-position. Notably, under the standard conditions, α-bromo-α-alkyl ketones also reacted with alkyl halides smoothly to afford the couped products in good yields. As shown in Fig. 9, a wide variety of alkyl halides with functional groups readily participated in the reaction, affording products with an all-carbon quaternary center in high yields. As such, functional groups including alkene (108), alkyne (109), secondary alkyl bromide (110), ester (111), acetal (112, 119), silicon ether (113), Bpin (114), pyrrole (115), imide (116), oxazolone (117), thiophene (118), alkyl fluoride (120), alkyl chloride (121). In addition, activated secondary alkyl bromides, such as benzylic or allylic bromide, could also couple with α-bromo-α-alkyl ketones to afford the corresponding products (123, 124) in 72% and 63% yields, respectively.
Applications and extensions
The robustness and synthetic utility of our protocol are further demonstrated by a 100-gram-scale experiment with comparable efficiency and subsequent derivatization. As shown in Fig. 10, the 100-gram-scale synthesis of compound 54 and the gram-scale synthesis of compound 83 were achieved under standard conditions without any apparent loss in yields, emphasizing the potential and practicality of this synthetic strategy for accessing various building blocks with all-carbon quaternary centers. Following this, specific downstream transformations of ester-containing compounds 54 and 83 were explored. Hydrolysis of ester 54 quantitatively gave carboxylic acid 125, which underwent fluorination to produce acyl fluoride 126 in 78% yield. Decarboxylative chlorination of acid 125 under silver catalysis generated tertiary alkyl chloride 127 in 64% yield. Additionally, the amide derivative of loratadine 128 was synthesized in 96% yield through a three-step sequence of hydrolysis, acid chloride formation, and subsequent amide formation. Michael addition product 129 was obtained in 82% yield via a three-step sequence involving hydrolysis, redox-active ester formation, and nickel-catalyzed coupling. Likewise, stereoselective formation of (Z)-monofluoroalkene 130 was achieved in 88% yield via a similar three-step sequence involving hydrolysis, redox-active ester formation, and zinc-mediated coupling.
Me = methyl, Et = ethyl, nBu = n-butyl, Ph = phenyl, Tf = triflyl, DCC = N,N′-dicyclohexylcarbodiimide, DIPEA = diisopropylethylamine, DMAc = N,N-dimethylacetamide, DMAP = 4-dimethylaminopyridine, DMP = Dess–Martin periodinane, NHPI = N-hydroxyphthalimide, NMP = N-methyl-2-pyrrolidone, Phen = 1,10-phenanthroline, TFAA = trifluoroacetic anhydride.
In another series of transformations, the ester group in compound 83 was efficiently converted into an aldehyde 131 and an alkene 132 functionality in 83% and 54% yields, respectively. The cyclic product 133 was synthesized from compound 83 through hydrolysis followed by Friedel–Crafts acylation in 90% yield. Similarly, bicyclo[1.1.1]pentane-containing compound 134 was prepared in 96% yield via a two-step sequence of hydrogenation and condensation. Furthermore, the α-bromo-α,α-dialkyl amide derived from the drug molecule paroxetine was readily converted into the cross-coupling product 138 in 89% yield. Additionally, α-bromo-α,α-dimethyl esters derived from natural alcohols such as l-menthol, l-carveol, and cholesterol exhibited robust reactivity under standard conditions, affording the corresponding products (135–137) in 96%, 94%, and 86% yields, respectively.
After a century of developing strategies for Reformatsky reaction, a simple and straightforward protocol to enhance the nucleophilicity of Reformatsky reagent has been identified, which enables the XEC between α-bromo-α-alkyl esters, amides, or ketones with unactivated alkyl halides, both intermolecularly or intramolecularly, thereby providing fresh ideas for the construction of C(sp3)–C(sp3) bonds bearing quaternary carbon centers. Considering that the carbonyl groups, including ester, amide, and ketone, rank among the most prevalent functional groups in natural products and synthetic compounds, easily convertible into other valuable functional groups, the current protocol has great potential as a powerful method for the formation of C(sp3)–C(sp3) bonds. We anticipate that this strategy will soon spur more efforts to develop cross-electrophile coupling with other challenging substrates under zinc-mediated, transition-metal-catalyst-free conditions. Finally, it is worth noting that transition-metal-catalyzed XEC reactions offer a more streamlined route for constructing chiral centers compared to the methodology described herein. This is primarily because our current method relies on the use of enantiopure alkyl halide substrates, whereas transition-metal-catalyzed XEC typically necessitates only catalytic quantities of chiral ligands. Consequently, a central objective of our future work is to achieve asymmetric construction of racemic substrates with the aid of chiral ligands under transition-metal-free conditions.
Methods
Zinc-mediated intermolecular cross-coupling reactions of α-bromo-α-alkyl amides/esters with alkyl bromides
In an argon-filled glove box, an oven-dried 4 mL screw-cap vial, pre-loaded with a magnetic stir bar, was charged with Zn (26.2 mg, 0.400 mmol, 2.00 equiv.), NaI (45.0 mg, 0.300 mmol, 1.50 equiv.), and anhydrous NMP (0.5 mL, 0.4 M). Subsequently, α-bromo-α-alkyl amide/ester (0.300 mmol, 1.50 equiv.) was added, followed by the addition of alkyl bromide (0.200 mmol, 1.00 equiv.). The vial was sealed with a Teflon-lined screw cap and removed from the glove box. The mixture was stirred at 25 °C for 2 h at 800 rpm. The reaction was quenched by the addition of 3.0 mL of H2O and extracted 3 times with 5.0 mL of EtOAc. The combined organic extracts were washed with 3.0 mL of saturated NaCl solution and dried over anhydrous MgSO4. After filtration, the solution was concentrated under vacuum to afford the crude product. For quantification via GC analysis, 20 μL of n-dodecane was added as an internal standard. Finally, the product was purified via flash column chromatography (petroleum ether/EtOAc).
Zinc-mediated intermolecular cross-coupling reactions of α-bromo-α-alkyl amides with primary alkyl chlorides
In an argon-filled glove box, an oven-dried 4 mL screw-cap vial, pre-loaded with a magnetic stir bar, was charged with Zn (39.2 mg, 0.600 mmol, 3.00 equiv.), KI (99.6 mg, 0.600 mmol, 3.00 equiv.), and anhydrous NMP (0.4 mL, 0.5 M). Subsequently, α-bromo-α-alkyl amide (0.400 mmol, 2.00 equiv.) was added, followed by the addition of primary alkyl chloride (0.200 mmol, 1.00 equiv.). The vial was sealed with a Teflon-lined screw cap and removed from the glove box. The mixture was stirred at 40 °C for 48 h at 800 rpm. The reaction was quenched by the addition of 3.0 mL of H2O and extracted 3 times with 5.0 mL of EtOAc. The combined organic extracts were then washed with 3.0 mL of saturated NaCl solution and dried over anhydrous MgSO4. After filtration, the solution was concentrated under vacuum to afford the crude product. For quantification via GC analysis, 20 μL of n-dodecane was added as an internal standard. Finally, the product was purified via flash column chromatography (petroleum ether/EtOAc).
Zinc-mediated intermolecular cross-coupling reactions of α-bromo-α-alkyl amides with secondary alkyl iodides
In an argon-filled glove box, an oven-dried 4 mL screw-cap vial, pre-loaded with a magnetic stir bar, was charged with Zn (24.8 mg, 0.380 mmol, 1.90 equiv.) and anhydrous NMP (0.4 mL, 0.5 M). Subsequently, α-bromo-α-alkyl amide (0.400 mmol, 2.00 equiv.) was added, followed by the addition of secondary alkyl iodide (0.200 mmol, 1.00 equiv.). The vial was sealed with a Teflon-lined screw cap and removed from the glove box. The mixture was stirred at 45 °C for 48 h at 800 rpm. The reaction was quenched by the addition of 3.0 mL of H2O and extracted 3 times with 5.0 mL of EtOAc. The combined organic extracts were then washed with 3.0 mL of saturated NaCl solution and dried over anhydrous MgSO4. After filtration, the solution was concentrated under vacuum to afford the crude product. For quantification via GC analysis, 20 μL of n-dodecane was added as an internal standard. Finally, the product was purified by flash column chromatography (petroleum ether/EtOAc).
Zinc-mediated intermolecular cross-coupling reactions of α-bromo-α-alkyl lactones with alkyl bromides
In an argon-filled glove box, an oven-dried 4 mL screw-cap vial, pre-loaded with a magnetic stir bar, was charged with Zn (26.2 mg, 0.400 mmol, 2.00 equiv.), Et4NI (103 mg, 0.400 mmol, 2.00 equiv.), and anhydrous NMP/THF (4:1, 0.4 mL + 0.1 mL). Subsequently, α-bromo-α-alkyl lactones (0.300 mmol, 1.50 equiv.) were added, followed by the addition of alkyl bromides (0.200 mmol, 1.00 equiv.). The vial was sealed with a Teflon-lined screw cap and removed from the glove box. The mixture was stirred at 25 °C for 12 h at 800 rpm. The reaction was quenched by the addition of 3.0 mL of H2O and extracted 3 times with 5.0 mL of EtOAc. The combined organic extracts were then washed with 3.0 mL of saturated NaCl solution and dried over anhydrous MgSO4. After filtration, the solution was concentrated under vacuum to afford the crude product. For quantification via GC analysis, 20 μL of n-dodecane was added as an internal standard. Finally, the product was purified by flash column chromatography (petroleum ether/EtOAc).
Zinc-mediated intramolecular cross-coupling reactions to form lactones
In an argon-filled glove box, an oven-dried 4 mL screw-cap vial, pre-loaded with a magnetic stir bar, was charged with Zn (26.2 mg, 0.400 mmol, 2.00 equiv.), Et4NI (77.1 mg, 0.300 mmol, 1.50 equiv.), and anhydrous NMP (0.5 mL, 0.4 M). Subsequently, substrate (0.200 mmol, 1.00 equiv.) was added. The vial was sealed with a Teflon-lined screw cap and removed from the glove box. The mixture was stirred at 25 °C for 2 h at 800 rpm.
For medium-sized rings: In an argon-filled glove box, an oven-dried 4 mL screw-cap vial, pre-loaded with a magnetic stir bar, was charged with Zn (26.2 mg, 0.400 mmol, 2.00 equiv.), NaI (45.0 mg, 0.300 mmol, 1.50 equiv.), and anhydrous NMP (2.0 mL, 0.10 M). Subsequently, substrate (0.200 mmol, 1.00 equiv.) was added. The vial was sealed with a Teflon-lined screw cap and removed from the glove box. The mixture was stirred at 60 °C for 2 h at 800 rpm.
The reaction was quenched by the addition of 3.0 mL of H2O and extracted 3 times with 5 mL of EtOAc. The combined organic extracts were then washed with 3.0 mL of saturated NaCl solution and dried over anhydrous MgSO4. After filtration, the solution was concentrated under vacuum to afford the crude product. For quantification via GC analysis, 20 μL of n-dodecane was added as an internal standard. Finally, the product was purified by flash column chromatography (petroleum ether/EtOAc).
Zinc-mediated intermolecular cross-coupling reactions of α-bromo-α-alkyl ketones with alkyl bromides
In an argon-filled glove box, an oven-dried 4 mL screw-cap vial, pre-loaded with a magnetic stir bar, was charged with Zn (26.2 mg, 0.400 mmol, 2.00 equiv.), NaI (60.0 mg, 0.400 mmol, 2.00 equiv.), and anhydrous NMP (0.5 mL, 0.4 M). Subsequently, α-bromo-α-alkyl ketones (0.300 mmol, 1.50 equiv.) were added, followed by the addition of alkyl bromides (0.200 mmol, 1.00 equiv.). The vial was sealed with a Teflon-lined screw cap and removed from the glove box. The mixture was stirred at 25 °C for 4 h at 800 rpm. The reaction was quenched by the addition of 3.0 mL of H2O and extracted 3 times with 5.0 mL of EtOAc. The combined organic extracts were then washed with 3.0 mL of saturated NaCl solution and dried over anhydrous MgSO4. After filtration, the solution was concentrated under vacuum to afford the crude product. For quantification via GC analysis, 20 μL of n-dodecane was added as an internal standard. Finally, the product was purified by flash column chromatography (petroleum ether/EtOAc).
Data availability
The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Center (CCDC), under deposition numbers CCDC 2416944 (1a-ZnBr), 2416945 (1a-ZnBr·LiBr), 2416946 (1b-ZnBr), and 2416947 (1b-ZnBr·LiBr). These data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif. The coordinates of the optimized structures generated in this study are provided in the Source Data file. The experimental, spectroscopic, crystallographic, and other computational data generated and analyzed in this study are provided in the Supplementary Information file. All data are available from the corresponding author upon request. Source data are provided with this paper.
References
Wender, P. A. & Miller, B. L. Synthesis at the molecular frontier. Nature 460, 197–201 (2009).
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Geist, E., Kirschning, A. & Schmidt, T. sp3–sp3 Coupling reactions in the synthesis of natural products and biologically active molecules. Nat. Prod. Rep. 31, 441–448 (2014).
Kambe, N., Iwasakia, T. & Terao, J. Pd-catalyzed cross-coupling reactions of alkyl halides. Chem. Soc. Rev. 40, 4937–4947 (2011).
Choi, J. & Fu, G. C. Transition metal–catalyzed alkyl-alkyl bond formation: Another dimension in cross-coupling chemistry. Science 356, eaaf7230 (2017).
Kranthikumar, R. Recent advances in C(sp3)–C(sp3) cross-coupling chemistry: A dominant performance of nickel catalysts. Organometallics 41, 667–679 (2022).
Reformatsky, S. Neue Synthese zweiatomiger einbasischer Säuren aus den Ketonen. Ber. Dtsch. Chem. Ges. 20, 1210–1211 (1887).
Procter, R. J. et al. A zinc catalyzed C(sp3)–C(sp2) Suzuki–Miyaura cross-coupling reaction mediated by aryl-zincates. Chem. Eur. J. 23, 15889–15893 (2017).
Barroso, S. et al. Cine substitution with arylzinc reagents: scope and mechanistic studies. J. Org. Chem. 81, 2804–2816 (2016).
Ellwart, M., Makarov, I. S., Achrainer, F., Zipse, H. & Knochel, P. Regioselective transition-metal-free allyl–allyl cross-couplings. Angew. Chem. Int. Ed. 55, 10502–10506 (2016).
Orsini, F. & Pelizzoni, F. A Reformatsky reagent. C–C bonds formation by substitution reactions. Synth. Commun. 13, 523–530 (1983).
Orsini, F. & Pelizzoni, F. Organometallic compounds of zinc as selective nucleophilic reagents in organic synthesis. Synth. Commun. 14, 805–816 (1984).
Orsini, F., Pelizzoni, F. & Ricca, G. C-metallated reformatsky intermediates. Structure and reactivity. Tetrahedron 40, 2781–2787 (1984).
Bott, K. Neue anwendungsmöglichkeiten des reformatzky-reagenz zur syntheses substituierter essigsäureethylester. Tetrahedron Lett. 35, 555–556 (1994).
Spencer, T. A., Britton, R. W. & Watt, D. S. Chemistry of enolates from zinc reduction of.alpha.-bromo ketones. New method of substitution.alpha. to carbonyl groups. J. Am. Chem. Soc. 89, 5727–5729 (1967).
Devasagayaraj, A., Stüdemann, T. & Knochel, P. A new nickel-catalyzed cross-coupling reaction between sp3 carbon centers. Angew. Chem. Int. Ed. 34, 2723–2725 (1996).
Giovannini, R., Stüdemann, T., Dussin, G. & Knochel, P. An efficient nickel-catalyzed cross-coupling between sp3 carbon centers. Angew. Chem. Int. Ed. 37, 2387–2390 (1998).
Piber, M., Jensen, A. E., Rottländer, M. & Knochel, P. New efficient nickel- and palladium-catalyzed cross-coupling reactions mediated by tetrabutylammonium iodide. Org. Lett. 1, 1323–1326 (1999).
Zhou, J. & Fu, G. C. Cross-couplings of unactivated secondary alkyl halides: room-temperature nickel-catalyzed Negishi reactions of alkyl bromides and iodides. J. Am. Chem. Soc. 125, 14726–14727 (2003).
Tong, X., Schneck, F. & Fu, G. C. Catalytic enantioselective α-alkylation of amides by unactivated alkyl electrophiles. J. Am. Chem. Soc. 144, 14856–14863 (2022).
Zhou, J. & Fu, G. C. Palladium-catalyzed Negishi cross-coupling reactions of unactivated alkyl iodides, bromides, chlorides, and tosylates. J. Am. Chem. Soc. 125, 12527–12530 (2003).
Terao, J., Todo, H., Watanabe, H., Ikumi, A. & Kambe, N. Nickel-catalyzed cross-coupling reaction of alkyl halides with organozinc and Grignard reagents with 1,3,8,10-tetraenes as additives. Angew. Chem. Int. Ed. 43, 6180–6182 (2004).
Phapale, V. B., Buñuel, E., García-Iglesias, M. & Cárdenas, D. J. Ni-catalyzed cascade formation of C(sp3)–C(sp3) bonds by cyclization and cross-coupling reactions of iodoalkanes with alkyl zinc halides. Angew. Chem. Int. Ed. 46, 8790–8795 (2007).
Mitamura, Y. et al. Silver-catalyzed benzylation and allylation of tertiary alkyl bromides with organozinc reagents. Chem. Asian J. 5, 1487–1493 (2010).
Palao, E. et al. Formation of quaternary carbons through cobalt-catalyzed C(sp3)–C(sp3) Negishi cross-coupling. Chem. Commun. 56, 8210–8213 (2020).
Zhang, Q. et al. Iron-catalyzed C(sp3)–C(sp3) coupling to construct quaternary carbon centers. J. Am. Chem. Soc. 146, 5051–5055 (2024).
Dekker, J., Boersma, J. & Van der Kerk, G. J. M. The structure of the Reformatsky reagent. J. Chem. Soc., Chem. Commun. 553, 555 (1983).
Dekker, J., Budzelaar, P. H. M., Boersma, J., Van der Kerk, G. J. M. & Spek, A. J. The nature of the Reformatsky reagent. Crystal structure of (BrZnCH2COO-t-Bu·THF)2. Organometallics 3, 1403–1407 (1984).
Hlavinka, M. L. & Hagadorn, J. R. The first structural characterization of α-zincated (Reformatsky) amides and phosphine oxides. Organometallics 24, 4116–4118 (2005).
Maiz, J. et al. Transition structures for the Reformatsky reaction. A theoretical (MNDO-PM3) study. Tetrahedron Lett. 34, 6111–6117 (1993).
Zimmerman, H. E. & Traxler, M. D. J. Am. Chem. Soc. 79, 1920–1923 (1957).
Mori, S., Hirai, A., Nakamura, M. & Nakamura, E. Correlation of reactivities of organocuprate(I) and zincate(II) with d-orbital energies of ate complexes. Tetrahedron 56, 2805–2809 (2000).
Hunter, H. N. et al. Identification of a higher-order organozincate intermediate involved in Negishi cross-coupling reactions by mass spectrometry and NMR spectroscopy. Chem. Eur. J. 17, 7845–7851 (2011).
Koszinowski, K. & Bohrer, P. Formation of organozincate anions in LiCl-mediatedz insertion reactions. Organometallics 28, 771–779 (2009).
Koszinowski, K. & Bohrer, P. Aggregation and reactivity of organozincate anions probed by electrospray mass spectrometry. Organometallics 28, 100–110 (2009).
Achonduh, G. T. et al. On the role of additives in alkyl–alkyl Negishi cross-couplings. Chem. Commun. 46, 4109–4111 (2010).
Yu, X., Yang, T., Wang, S., Xu, H. & Gong, H. Nickel-catalyzed reductive cross-coupling of unactivated alkyl halides. Org. Lett. 13, 2138–2141 (2011).
Johnston, C. P., Smith, R. T., Allmendinger, S. & MacMillan, D. W. C. Metallaphotoredox-catalysed sp3–sp3 cross-coupling of carboxylic acids with alkyl halides. Nature 536, 322–325 (2016).
Smith, R. T. et al. Metallaphotoredox-catalyzed cross-electrophile Csp3–Csp3 coupling of aliphatic bromides. J. Am. Chem. Soc. 140, 17433–17438 (2018).
Chen, R. et al. Alcohol-alcohol cross-coupling enabled by SH2 radical sorting. Science 383, 1350–1357 (2024).
Zhang, B. et al. Ni-electrocatalytic Csp3–Csp3 doubly decarboxylative coupling. Nature 606, 313–318 (2022).
Zhang, B. et al. Complex molecule synthesis by electrocatalytic decarboxylative cross-coupling. Nature 623, 745–751 (2023).
Hioki, Y. et al. Overcoming the limitations of Kolbe coupling with waveform-controlled electrosynthesis. Science 380, 81–87 (2023).
Fu, H. et al. An asymmetric sp3–sp3 cross-electrophile coupling using ‘ene’-reductases. Nature 610, 302–307 (2022).
Nedelec, J. Y., Mouloud, H. A. H., Folest, J. C. & Perichon, J. Electrochemical cross-coupling of alkyl halides in the presence of a sacrificial anode. J. Org. Chem. 53, 4720–4724 (1988).
Zhang, W. et al. Electrochemically driven cross-electrophile coupling of alkyl halides. Nature 604, 292–297 (2022).
Buchwald, S. L. & Bolm, C. On the role of metal contaminants in catalyses with FeCl3. Angew. Chem. Int. Ed. 48, 5586–5587 (2009).
Rowsell, B. J. S. et al. The iron-catalysed Suzuki coupling of aryl chlorides. Nat. Catal. 7, 1186–1198 (2024).
Shiina, I. Total synthesis of natural 8- and 9-membered lactones: recent advancements in medium-sized ring formation. Chem. Rev. 107, 239–273 (2007).
Hosomi, A., Araki, Y. & Sakurai, H. Remarkably high regioselective deprotonation and alkylation of unsymmetrical imines at the more substituted.alpha.-carbon atom. J. Am. Chem. Soc. 104, 2081–2083 (1982).
Saito, S., Ito, M. & Yamamoto, H. Highly regioselective alkylation at the more-hindered α-site of unsymmetrical ketones by the combined use of aluminum tris(2,6-diphenylphenoxide) and lithium diisopropylamide. J. Am. Chem. Soc. 119, 611–612 (1997).
Li, M.-M. et al. Ketone α-alkylation at the more-hindered site. Nat. Commun. 14, 3326–3333 (2023).
Acknowledgments
We gratefully acknowledge the financial support from the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB0590000, Q. Shen). We acknowledge the Beijing Beilong Super Cloud Computing Co., Ltd (URL: http://www.blsc.cn/) for providing HPC resources that have contributed to the research results reported within this paper. We also thank Mr. Wenju Wan, Mr. Yuxuan Jin, Mr. Shengjie Huang, and Miss Shunli Chen for conducting some experiments, and Dr. Yongrui Luo for discussions on the mechanism.
Author information
Authors and Affiliations
Contributions
L. Meng and Q. Shen conceived the idea and designed the research. L. Meng performed optimization studies. L. Meng, T. Zhang, Y. Xu, and Y. Li performed substrate scopes. B. Wu performed crystallographic studies. L. Meng performed UV and ECD experiments. Z. Li simulated UV and ECD spectra. Z. Li calculated reaction coordinate diagrams, proposed the mechanism, and investigated the structure–activity relationship. Z. Li designed products 39–42 and 85. L. Meng and Z. Li designed mechanistic studies. L. Meng performed mechanistic studies. L. Meng, Z. Li, B. Wu, and Q. Shen wrote and revised the manuscript. Z. Li completed the author checklist.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interest.
Peer review
Peer review information
Nature Communications thanks Chinmoy Kumar Hazra, Wei Li, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Meng, L., Li, Z., Zhang, T. et al. Revitalizing reformatsky reagent for catalyst-free direct alkylation with unactivated alkyl halides. Nat Commun 16, 7627 (2025). https://doi.org/10.1038/s41467-025-62833-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-62833-4