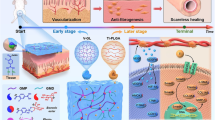
Abstract
In urethral damage/stricture prevention, open and harsh urethral microenvironments and isotropic compression and swelling properties of exogenous implants render urethral repair intractable. Here a dynamically urethra-adapted and obligations-oriented trilayer hydrogel was engineered to integrate scarless urethral repair. Therein, the diethylacrylamide-hydroxyethylacrylamide (HEAm) (D-H) hydrogel layer featuring high anti-fouling performance prevent adhesions of bacterial and blood cells, and its poor swelling avoids urethra occlusion. The upper swellable and verteporfin (VP)-loaded N,N’-methylenebisacrylamide-poly (N-isopropylacrylamide) (BP) layer encourages urethra regeneration through expediting cell migration and proliferation. The rigid and water-resistant Zein middle layer opposes urine voiding-arised BP shedding, urethral diastole/contraction, inward BP swelling-arised urethra occlusion and urine permeation. Importantly, systematic proteomic and genomic analysis reveals that such hydrogel scaffolds expedite epithelial & vascular regenerations, attenuate tight cell junction, oppose inflammation microenvironment and regulate extracellular matrix secretion and metabolism to realize integrated urethral repair. The microenvironment-adaptable design concepts provide reliable rationales to engineer urethral regeneration scaffolds.
Similar content being viewed by others
Introduction
Common urethral stricture often poses significant patient distress in male population, wherein various pathogeneses have been identified especially for elderly ones1, e.g., benign prostate hyperplasia-caused squeezing, urethral muscle flaccidity-arised collapse, etc. In particular, post-traumatic urethral hypertrophic scars induced by excessive collagen and fibrin depositions is prevalent across different ages and termed as post-traumatic urethral stricture (PTUS)2. In clinics, autologous graft replacement is a solution, which, however, suffers from limited donor source and potential complications (e.g., hair growth and stone formation)3,4. Urethra consist of fibroblasts, extracellular matrix and blood vessels-involved sparse layer and epithelial cells and basement membrane cells-involved mucosa layer5,6. After injures and trauma, they usually suffer from bacterial infection, recruited inflammation cells aggregation, excessive extracellular matrix secretion-induced scarring, resulting in PTUS7.
Although exogenous hydrogel scaffolds have been attempted to repair post-traumatic urethra and resist urethral stricture8,9,10, current hydrogel scaffolds that are composed of single composition alone or utmost with modification fail to achieve the expected results, because they are confronted with many issues. (1) Firstly, the isotropic property of single hydrogel scaffolds determines that these hydrogels fail to encourage outward swelling-expedited urethral regeneration and simultaneously inhibit inward swelling-arised urethra occlusion. (2) Importantly, the open and harsh urethral microenvironments and complex urethral structure compromised current hydrogels-based scaffolds-based repair in both laboratory and clinical scenarios. Typically, although hydrogels have shown many tunable properties to adapt various application domains after rational engineering11,12,13,14, the sufferings, e.g., various metabolite and inorganic salt invasions, moisture, bacteria adhesion, inflammation aggravation, urine permeation, mismatched urethral environment remain unresolved in current hydrogels-based urethral repair15,16. (3) Beyond them, these compressible hydrogels fail to avoid the repeated mechanical stimuli-arised wound tearing and fibrosis-originated scarring during voiding17, thereby necessitating frequent secondary surgeries18. Considering above intractable challenges, ideal hydrogels not only need to address above concerns, but also can emulate the structure of loose connective tissues, and exhibit specific expansion capabilities that effectively adapt to tissue deficits. Beyond that, they should have Janus sides with anti-fouling and tissue adhesiveness, respectively, and are able to eliminate voiding-induced mechanical stimuli and realize precise and controllable drug release. Engineering multilayer-structured hydrogels where each layer has different but oriented functions is a promising solution, which, however, is currently confined to injured skin repair especially associated with delayed wound healing in diabetes.
Here we construct dynamically urethra-adapted and obligation-oriented trilayer hydrogels with Janus sides. Therein, verteporfin (VP)-loaded poly (N-isopropylacrylamide) (PNIPAm), Zein and 50% diethylacrylamide (DEAm)−50% hydroxyethylacrylamide (HEAm) (D-H) served as the upper, middle and bottom layers, respectively, and each layer has their oriented but different obligations to address above concerns and accomplish integrated urethra repair (Fig. 1a). D-H hydrogels exhibit great anti-fouling performance19, determining that it can effectively prevent adhesions of biological bodies such as bacteria and blood cells. More significantly, its poor swelling together with anti-adhesion beneficially avoided urethra occlusion, and its hydrophobicity was designed to preliminarily hamper urine permeation or erosion to dampen inflammation. The urine temperature window (31–37 °C) dictates that upper PNIPAm hydrogel layers with a phase-transition temperature at 33 °C are highly desirable for controlled VP delivery20. Further, the crosslinking with N,N’-methylenebisacrylamide (Bis) (Bis-PNIPAm or BP) improves the mechanical properties (e.g., stiffness) without affecting their gelation properties. Therein, the non-swelling and anti-fouling D-H surface together with the swelling and hydrophilic BP surface constituted the Janus surface in such trilayer hydrogels (D-H/Zein/BP).
a Schematic illustration for the present study. Elements were created in BioRender. Ming, Y. (2025) b RNA sequencing results of clinical samples including normal tissues and scars in PTUS patients (n = 3); c Signaling pathway and action target of such trilayer hydrogels for scarless urethral repair, wherein VP release was used to counteract mechanical stimuli during urinary excretion. Elements were created in BioRender. Ming, Y. (2025). Source data is provided as a Source Data file.
Hydrogel swelling can provide adequate high stiffness and tensile stress21,22,23, which expedite cell migration, proliferation and differentiation23,24,25,26,27. Thereby, the outward swelling of isotropous BP hydrogels preferably favored urethra regeneration. However, the inward swelling-arised stress of BP hydrogels may endanger urethral occlusion through driving D-H movement towards urethral canal (Fig. 1a). Additionally, voiding-arised urethral diastole/contraction and urine metabolic stimuli undoubtedly delayed the wound healing of patients with urethral injuries, and induced fibrosis accumulation and scar formation17,28. To remove these concerns, Zein as middle layer that adhere to bottom D-H layer and upper BP layer was sutured to the defective tissues29. The Zein middle layer opposed urine voiding-arised BP hydrogels shedding, and its rigid and water-resistant properties could resist voiding-arised urethral diastole/contraction and inward BP hydrogels swelling (Fig. 1a). Moreover, its hydrophobicity further defends urine permeation against inflammation induced by metabolic stimuli in urine. Given this, it enabled the integrated trilayer hydrogels to favor cell migration/proliferation, attenuate fibrosis accumulation, prevent urine permeation, dampen scar formation and hamper urethra occlusion. Herein, the three components (Zein, D-H and BP hydrogels) have not been attempted for urethra repair, let alone their rational integration or synergy.
Voiding-arised mechanical stimulation activated kinase cascade reactions in Hippo and TGF-β signaling pathways, respectively30,31,32,33,34,35, where YAP in hippo pathway interacted with TGFβ downstream smad2/3 to promote fibrosis and scar formation36,37. Additionally, other targets such as immune cells, metabolites, excessive extracellular matrix secretion, etc., also correlated with tissue repair38,39,40,41. Our RNA sequencing based on clinical samples further verifies that both signaling pathways decide fibrosis and scarring through regulating extracellular matrix (ECM), wherein CLDN10, VEGFA, COL1A1 and MMP-1 are identified as targets (Fig. 1b). Although the delivered VP, one YAP inhibitor42,43, was only confined to skin repair against fibrosis, its underlying mechanisms of regulating Hippo and TGFβ signaling pathways can be expanded to scarless urethra repair. Its combination with above action principles in such trilayer hydrogels, e.g., counteracted mechanical tension changes during voiding, was encouraged to decrease fibrin accumulation and scar formation. Genomic and proteomic investigations unveiled that such dynamically urethra-adapted hydrogel systems with special obligation-oriented trilayer structure blockaded YAP signaling pathway and activated TFGβ signaling pathway. Further, they promoted epithelial & vascular regenerations (e.g., VEGFA, HIF-1α), attenuated tight cell junction (e.g., αSMA, CLDN-10, MYLK), opposed inflammation microenvironment (e.g., ARG1, IL-17), and regulated extracellular matrix secretion and metabolism (e.g., MMP1, COL-1, FGF23) to contribute to the integrated scarless repair (Fig. 1c). Ultimately, this hydrogel system not only realized scarless urethra integration repair, but also substituted normal urethra functions prior to repair on PTUS model. This research precisely adjusted and designed the properties of each hydrogel layer, which provided new ideas and treatment protocols for clinical urethral stenosis treatment. Beyond these urethral microenvironment and structure-matched reciprocal actions principles, great biosafety, simple processing procedures and cost also support strengthen the clinical translation.
Results
Urethra-adapted trilayer hydrogel construction
Trilayer hydrogels (i.e., BP/Zein/D-H), is consisted of upper BP, middle Zein and bottom D-H (Fig. 2a). D-H hydrogels were yielded through using Bis crosslinking agents to link DEAm with HEAm upon photo irradiation, while BP hydrogels originated from the crosslinking between PNIPAm with Bis in the presence of ammonium persulfate (APS) and N,N,N’,N’- Tetramethylethylenediamine accelerator (Fig. 2b). Fourier transform infrared (FTIR) spectroscopy and solid-state nuclear magnetic resonance (NMR) tests confirm the successful synthesis of D-H, BP and VP-loaded BPV hydrogels (Fig. 2c, d and Supplementary Figs. 1, 2). Comparing to HEAm and DEAm, C=C disappearance at 1620 cm−1, N-H retention, and the bathochromic C=O shift in the resulting D-H polymers demonstrates the successful D-H synthesis through covalent bonding (Fig. 2c). The 1H NMR spectra of DEAm and HEAm confirm their structural features with five and four chemical shifts, respectively (Supplementary Fig. 1). The 13C solid-state NMR analysis reveals distinct signals at 55–60 ppm and 40–45 ppm, verifying the successful copolymerization of HEAm (-OC2H4OH) and DEAm (-N(C2H5)2), wherein methylene bridges (-CH2-) from Bis-initiated crosslinking emerges at 15-25 ppm (Supplementary Fig. 2a, b). The complete disappearance of acrylamide monomers’ vinyl carbons (25-35 ppm) and new peaks at 35-45 ppm corresponding to saturated polymer backbones (CH2/CH groups) further confirm full D-H polymerization, and broad amide carbonyl resonances (50-65 ppm) and monomeric carbonyl signals attenuation are found. As for BP synthesis, the complete disappearance of C = C stretching peak at 1617 cm−1, the C=O stretching vibration shift to 1643 cm−1 with reduced intensity, and two emerging absorption bands at 1458 cm−1 and 1386 cm−1 corresponding to C-N stretching and CH3 symmetric deformation vibrations, respectively, are observed (Fig. 2d). These transformations associating with the disappearance of vinyl signatures and the emergence of polymer specific vibrations provide compelling evidences for the successful NIPAm polymerization into BP1.H NMR reveals complete NIPAm vinyl proton consumption (5.5–6.5 ppm disappearance), with aliphatic polymer backbone peaks (1.4–2.2 ppm) and isopropyl groups (-CH(CH3)2) at 1.0–1.2 ppm, further confirming BP synthesis (Supplementary Fig. 2c, d).
a Overview and structure of the D-H/Zein/BP hydrogel scaffold. Elements were created in BioRender. Ming, Y. (2025). b Chemical synthesis reaction formula of D-H and BP. c FTIR spectra of DEAm, HEAm, and D-H hydrogel; and d FTIR spectra of NIPAm, BIS, BP and BPV. e Scanning electron microscopy (SEM) images of D-H hydrogel, Zein and Bis-PNIPAm (BP) hydrogels; and f Cross-sectional SEM of D-H/Zein/BP trilayer hydrogels in a hydrated state via Cryo-SEM, more than three experiments were conducted, and the results were repeatable. g Temperature-correlated rheological assay of upper Bis-PNIPAm (BP) hydrogels layer. D-H hydrogel; h Detection of the change in heat flow under varying temperatures in BP hydrogel using Differential Scanning Calorimetry (DSC). i–k Time-correlated rheological assay of upper Bis-PNIPAm (i) and bottom D-H (k); and j Stress-strain curves during compression testing of D-H and BP hydrogels. l Volume expansion curves for D-H, Zein and BP; and m expansion rate after 24 h (n = 3 independent samples). n Values in Depth-Sensing Indentation (DSI) testing (n = 6 independent samples), Er: Reduced Young’s Modulus, H: Nanoscale Hardness of Indentation, Pmax: Maximum Load; o Water contact angle testing for hydrated D-H hydrogels. p Time-dependent degradation rates of D-H, BP and Zein hydrogels in urine environment under static conditions (n = 3 independent samples); and q Residual mass of of D-H, BP, Zein and D-H/Zein/BP hydrogels after 60 days in urine environment (n = 3 independent samples). Data are expressed as mean ± standard error of the mean (SEM). Source data is provided as a Source Data file.
The synthesized BP exhibits the typical diffraction pattern of PNIPAm (Supplementary Fig. 3). D-H hydrogels experience gelation upon UV photoirradiation (Supplementary Fig. 4 and Supplementary Movie 1). BP hydrogels can respond to temperature and gelate into solid phase (Supplementary Fig. 4). Gelated BP encompasses hydrophobic long-chain alkyls, and Zein itself is hydrophobic in nature, which means that hydrophobic interaction between BP and Zein can be expected. Smooth D-H surface disfavors the adhesions of bacteria or blood cells, while the abundant pores in BP allow VP loading (Fig. 2e). The middle layer, Zein, is a natural protein, and instantly gelate into rigid solid phase once touching water (Fig. 2e and Supplementary Fig. 4)44, resulting in the trilayer structure of D-H/Zein/BP (Fig. 2f). Notably, a transition layer between D-H and Zein denotes the reciprocally physical entanglement between D-H framework and Zein framework.
Each component obligation-determined physiochemical tests
The phase-change temperature of BP hydrogels resides at 32.9 °C in both rheological test and differential scanning calorimetry (DSC) analysis (Fig. 2g, h), and it has poor rigidity and mechanical strength (Fig. 2i, j). Comparing to BP hydrogels, D-H hydrogels are equipped with larger mechanical properties to resist stress since no structure collapse is obtained even under large strain and stress (Fig. 2j, k). Additionally, D-H hydrogels have much lower swelling rates in both water and urine (Fig. 2l, m and Supplementary Fig. 5a). These properties are highly preferable for further avoiding urethral occlusion induced by self-expansion or self-deformation of D-H hydrogels. Owing to the high swelling property, BP can absorb water to induce volume expansion with approximately 2.0 times and 1.26 times increases in volume and height (Fig. 2l, m and Supplementary Figs. 5a, 6), respectively. The swelling property not only enables drug release and tissue defects filling, but also favorably produces mechanical stress to promote cell proliferation and wound healing after absorbing tissue fluid.
Although D-H hydrogels show a low swelling rate and rigidity, the upper BP hydrogel layer may give birth to internal stress to push D-H layer and pose D-H shedding as BP hydrogels are equipped with the high swelling property. To remove this risk, the middle Zein layer featuring inherent high tissue adhesion was inserted to bind with D-H hydrogels (Fig. 2a). Intriguingly, solid metastructure without evident porosity in Zein (Fig. 2e) explains why Zein is imparted with high mechanical strength and poor elasticity or compressibility (Fig. 2n and Supplementary Fig. 7). Zein can withstand the pressure from gel expansion with a maximum load (P max) of 996.41 ± 0.08 μN, a reduced Young’s modulus (Er) of 4.57 ± 0.75 GPa, and a nanoindentation hardness (H) of 0.23 ± 0.05 GPa (Fig. 2n). The large and stable mechanical rigidity and low swelling rate encourage Zein to perform like a baffle to unload BP swelling-induced tensile stress (Supplementary Fig. 6), enabling unidirectional BP swelling opposite to Zein, which further resist hydrogels’ swelling or detachment against urethral occlusion. Additionally, the impermeability against water makes them become ideal barriers to hamper urine permeation without shedding. The hydrophobicity property imparts D-H hydrogels with anti-fouling ability, which is expected to resist urethral stricture via inhibiting the adhesions and accumulations of blood cells and bacteria (Fig. 2o). Besides physical entanglement (Fig. 2f), the hydrophobic interaction will also contribute to the high affinity of D-H with Zein since both hydrogels are hydrophobic. The chemical crosslinking in both D-H and BP hydrogels endows them with high stability, resulting in no obvious degradation under static conditions (Fig. 2p). Inspiringly, in real scenarios-simulated conditions featuring squeezing/vibration and dynamical urine erosion, the three components can not only retain the structure to substitute normal urethra functions prior to complete repair in dynamical urine, but also have desirable clearance rates to ensure biosafety (Fig. 2q and Supplementary Fig. 5b).
Interlamellar affinity and mechanical properties in trilayer D-H/Zein/BP scaffolds
High interlamellar adhesion affinity in both interfaces including D-H/Zein and Zein/BP can guarantee the stability of such trilayer D-H/Zein/BP hydrogels scaffold for practical applicability. To investigate the macroscopical interface interaction, molecular dynamic simulation was firstly carried out. Results show that the binding barriers of both Zein—D-H and Zein—BP resemble D-H—D-H and BP—BP, respectively (Fig. 3a), suggesting that the adhesion affinities of both interfaces between D-H and Zein and between Zein and BP are high and approach to intermolecular affinities of D-H and BP, respectively. To uncover the detailed types of interfacial interactions, surface potential, NMR and FTIR analysis were harnessed. Besides the reciprocally physical entanglement and hydrophobic interaction mentioned above, electrostatic interaction exists in D-H/Zein since positively-charged D-H and negatively-charged Zein are found (Fig. 3b). NMR analysis further validates the presence of hydrogen bonding between D-H (-NH) and Zein (-COO⁻/-CONH) since Zein carbonyl signals are broadened and downfield-shifted (26-39 ppm) and D-H acrylamide carbonyls are shifted upfield (31–45 ppm) (Supplementary Fig. 2a, e). Simultaneously, the restricted mobility of BP’s methylene (35–45 ppm) and isopropyl (20–25 ppm) groups, along with the altered Zein α-carbon intensity (55–60 ppm), further supports hydrogen-bond-mediated entanglement between Zein and BP (Supplementary Fig. 2c, f). Especially, the D-H/Zein/BP trilayer hydrogels exhibit spectral overlapping with binary systems (D-H/Zein and Zein/BP) but with enhanced peak intensities, underpinning the successful assembly of three layers with enhanced structural cohesion (Supplementary Fig. 2g).
a Molecular dynamic simulation of various intermolecular interactions in the dominant component of Zein, BP and D-H for calculating the binding barrier using Gaussian 16. b Surface potential of various samples at pH=6 and 7, respectively (n = 3 independent samples). c FTIR spectra of D-H/Zein, Zein/BP and D-H/Zein/BP (a.u. represents arbitrary units). d Rheological properties of D-H/Zein/BPV hydrogels after incubation in urine for 7 days. e–g Tensile stress-strain curves of Zein (d), D-H and BP (f), and Zein/BP, D-H/Zein/BP and D-H/Zein/BPV (g). Er, H and Pmax values in DSI tests (h, j, l) and the load-depth curves obtained from multiple nanoindentation tests (i, k, m) on Zein/BP, D-H/Zein/BP and D-H/Zein/BPV (n = 5 independent samples). Data are expressed as mean ± SEM. Source data is provided as a Source Data file.
Beyond Zeta potential and NMR analysis, hydrogen bonds are observed in the FTIR spectra of Zein, D-H and D-H/Zein (Figs. 2c, d and 3c), and the vibrational peak intensities at 1480 and 1150 cm−1 in D-H/Zein are significantly attenuated compared to D-H due to the hydrogen bonding-mediated intermolecular interactions between the -NH/-OH groups in D-H and Zein (Fig. 3c), respectively. This result indicates that hydrogen bonding interaction also contributes to the high adhesion between D-H and Zein. As for the interaction between Zein and BP, hydrogen bonding interactions between -NH or -OH groups in BP and Zein are also observed in Zein/BP, and simultaneously the hydrophobic interaction brings about the decline in characteristic peak intensities of Zein/BP at 1220, 1480, and 1400 cm−1 compared to BP (Fig. 3c). Additionally, the hydrogen bonding peak of BP at 3550 cm−1 is replaced by new intermolecular interactions upon binding with Zein (Fig. 3c). As the three layers are assembled into D-H/Zein/BP hydrogels, enhanced hydrogen bonding and intermolecular interactions are observed, as evidenced by the attenuated carbonyl peak intensity at 1630 cm−1 compared to Zein/BP or D-H/Zein.
The strong noncovalent interactions improve the adhesion of Zein with BP and D-H, thus enabling the assembled trilayer D-H/Zein/BP hydrogels to withstand the high rotary shear force without delamination (Fig. 3d and Supplementary Fig. 8). Under tensile stress-strain tests, Zein retains the high rigidity and mechanical strength, which results in neglectable tensile strain even under above 20 MPa (Fig. 3e). In contrast, D-H hydrogels show poor tensile resistance (0.003 MPa, Fig. 3f), but receive high tensile strain (27%). Consequently, the assembly of Zein with D-H optimizes the mechanical properties of trilayer D-H/Zein/BP hydrogels, where they resist 0.08 MPa tensile stress and simultaneously retain above 10% tensile strain (Fig. 3g). More significantly, BP and D-H hydrogels fail to detach from the assembled trilayer D-H/Zein/BP hydrogels under 0.08 MPa tensile stress, further demonstrating the high adhesion affinities between Zein and BP and between Zein and D-H. Digital Image Correlation (DIC) test further shows that the physical entanglement, hydrophobic interaction, electrostatic interaction and hydrogen bonding cooperatively bring about the high adhesion between D-H and Zein (Supplementary Fig. 9 and Supplementary Movie 2) where large shear stress fails to bring about D-H delamination or detachment from D-H/Zein/BP hydrogels. Additionally. the high adhesion between Zein and BP not only hampers BP delamination or detachment from D-H/Zein/BP and retains complete trilayer structure without curling, but also even inhibits width and length side expansions of BP with only vertically unidirectional expansion opposite to the adherent Zein (Supplementary Fig. S). Eventually, the assembled trilayer D-H/Zein/BP hydrogels show desirable mechanical properties, e.g., reduced Er representing rigidity decline, and enhanced Pmax representing stress resistance increase, wherein D-H exerts the dominant roles (Figs. 2j, k and 3h–m).
In vitro wound healing test and mechanism exploration
The bottom D-H hydrogels directly face urine microenvironment, and easily encounter bacteria and cell adhesions that may cause infection and poor repair and occlude urethral passage. Fortunately, D-H hydrogels are hydrophobic, and its anti-fouling property enables D-H/Zein/BP scaffold to resist bacteria and cell adhesions. As expected, D-H hydrogels receive the lowest E. coli coverage area (2.65 ± 0.08%) (Fig. 4a, b), indicating the ability of D-H hydrogels to prevent bacteria adhesion. Direct microscopic observation also reveals the considerably-decreased bacteria density (Fig. 4c), further suggesting the strongest anti-fouling property of D-H hydrogels. Similar anti-adherence phenomenon is observed on other bacteria, e.g., Staphylococcus aureus (S. aureus) and Proteus where the significant decline in colony proportion is obtained (Supplementary Fig. 10a, c), suggesting the generality of D-H-mediated anti-bacteria adherence. Live cell staining images further verify the anti-adherence property of D-H layers against E. coli, S. aureus and Proteus (Supplementary Fig. 10b). Intriguingly, the bottom D-H hydrogels also oppose blood cell adhesion (Supplementary Fig. 11), which unites with the anti-bacteria adherence to avoid urethral occlusion. As for tissue adherence, the upper BP hydrogels in such D-H/Zein/BPV system directly touch wound tissues, showing high adhesions on various surfaces (Fig. 4d), thereby favoring practical clinical applicability.
a Evaluation of anti-fouling effects for DEAm, HEAm, and D-H hydrogels against E. coli, and b assessment of colony area after 48 h of cultivation (n = 3 independent samples). c Biological scanning electron microscope images of E. coli on DEAm, HEAm, and D-H hydrogels. d Adhesion photos of BP hydrogel on surfaces of different objects. e Release of dye from BP hydrogel at different temperatures after loading; and f Time-correlated VP release curve from BPV hydrogel in urine (n = 3 independent samples). Analysis of migration photos (g) and rates (h) in Control (Zein) group, D-H/Zein/BP and D-H/Zein/BPV groups by comparing the area occupied by proliferating and migrating ADSCs (n = 3 independent samples). Processed with image J software. Elements were created in BioRender. Ming, Y. (2025). i Three-dimensional reconstructed images of live and dead ADSCs stained after 7 days of co-culture with cells on Control (Zein), D-H/Zein/BP and D-H/Zein/BPV scaffolds, with sodium alginate scaffold used as a control. j Construction of a model using canine ADSCs sheet to study the impact of tension on cell signaling. k Schematic on tissue softening via activating TGFβ signaling pathway. l Comparison of qPCR results for the expression of TGF-β2, SMAD2, and YAP1 in canine ADSCs after 48 h of treatment in normal group, normal group with VP, stretched group, and stretched group with VP (n = 3 independent samples); and m Western blot results depicting the protein expressions in canine ADSCs including active-YAP1, SMAD2/3, COL I, FGF23, and VEGFA. n qPCR results depicting the expression levels of TGF-β2, SMAD2/3,VEGFA, FGF23, YAP1, CLDN-10 and α-SMA in human fibroblasts after 72 h incubation (n = 3 independent samples). o Western blot results depicting the protein expression of TGF-β2, SMAD2/3,VEGFA, FGF23, YAP1, CLDN-10 and α-SMA in human fibroblasts after 72 h incubation. Data are shown as mean ± SEM. Unpaired two-sided Student’s t test was used to analyze the significance, and *P < 0.05, **P < 0.01, ***P < 0.001. Source data is provided as a Source Data file.
The phase transition temperature of BP is beyond 32.9 °C (Fig. 2g, h), while the temperature in the rabbit urethra resides between 31.5–32.9 °C (Supplementary Fig. 12). Only when urine passes through urethral canal, the temperature rises to beyond 37 °C (Supplementary Fig. 12), but the temperature only sustains for less than 30 s since urine voiding process consume less than 30 s. Therefore, the temperature in vivo at most time is below 32.9 °C for sustaining the BP swelling state, and we explored BP swelling-induced migration, proliferation and fibrosis inhibition at below 32.9 °C. Since both states below and beyond phase-change temperature contributes to VP release via diffusion and phase change-arised burst, respectively, we investigated drug release profiles at 25 °C and 37 °C to mimic the real in vivo scenarios despite urine voiding-arised temperature rise is short-lived. It is found that the phase change at 37 °C can be leveraged to expedite the release of loaded VP or other representative dyes from urine and water (Fig. 4e, f, Supplementary Figs. 13–15 and Supplementary Movie 3). In the course of VP-blockaded YAP pathway against fibrosis, excessive VP also affect cell proliferation and oppose urethra repair43. To ascertain the optimal VP concentration, VP with varied concentrations was used to treat fibroblasts. Below 500 ng/ml, cell morphology and vitality resemble normal cells (Supplementary Fig. 16a), but long-term incubation (7 days) inhibits their proliferation (Supplementary Fig. 16b). Comprehensive consideration finds that 200 ng/ml is identified as the optimal concentration, under which high safety is accessible. Subsequently, time-dependent scratch experiment was proceeded to investigate the influences of D-H/Zein/BPV trilayer hydrogels on mesenchymal stem cells (MSCs). The upper BP layer swelling significantly promotes the migration of adipose-derived stem cells (ADSCs) (Fig. 4g, h), which is advantageous to wound repair. Additionally, 3D live-dead cell imaging also indicates BP layer in D-H/Zein/BP hydrogel system favors the proliferation of ADSCs (Fig. 4i). Herein, the loaded VP can limit uncontrollable ADSCs expansion under the optimal safe concentration (200 ng/mL) (Fig. 4i and Supplementary Fig. 17), which is expected to suppress swelling stress-arised excessive proliferation for fibrosis and scar formation.
Although BP swelling after absorbing tissue fluid can produce internal stress to expedite cell expansion and migration, the swelling-induced tension increases the risk of fibrosis. Additionally, the enhanced tension in stainless steel hoop-imposed ADSCs sheet is found to activate Yap and TGF-β signaling pathways towards fibrosis progression (Fig. 4j–l). VP introduction attenuates this risk via blockading the fibrosis-encouraged communication between SMAD2/3 and YAP/TAZ, as indicated by RNA decline in SMAD2 and YAP1. Especially, the nuclear distribution of YAP is decreased owing to YAP phosphorylation (pYAP) and YAP migration to cytoplasm (Supplementary Fig. 18). Notably, the upregulations of YAP1 and SMAD2/3 expression upon exposure to VP in the normal group may be probably attributed to the mild cytotoxicity of DMSO solvent, VP dose-decided double-edged ‘sword’ (Supplementary Fig. 16) and inactivation state-arised poor sensitivity in normal tissues, which might activate YAP expression not through YAP-TEAD binding. However, this hypothesis has certain limitations and requires more in-depth and comprehensive research in future study. Inspiringly, the wound healing motive is retained, as indicated in the upregulations of FGF23, COL and free SMAD2/3 proteins (Fig. 4m and Supplementary Fig. 19). Additionally, ELISA quantitation analysis validates the concentrations of VEGFA and FGF23 are gradually increased in both D-H/Zein/BP and D-H/Zein/BPV (Supplementary Fig. 20). Inspired by it, the ability of D-H/Zein/BPV scaffolds to promote early repair via mechanical stress-activated TGFβ (free SMAD2/3) and VEGFA upregulations is retained. Simultaneously, the fibrosis and scar risks are reduced by the sustained VP release since cell adhesion-associated CLDN10 and cell stiffness-associated αSMA in human fibroblasts are downregulated to avert tight cell junction (Fig. 4n, o and Supplementary Fig. 21). Additionally, macrophage polarization test reveals that D-H/Zein/BPV hydrogels treatment indeed encourages M0 macrophages’ polarization into anti-inflammatory M2 ones, which future preferably hampers fibrosis and scar formation (Supplementary Fig. 22a). Moreover, the pro-angiogenic marker, VEGF, is increased after hydrogels treatment, undoubtedly benefiting scarless repair (Supplementary Fig. 22b).
In vivo urethral reconstruction and scarless wound healing
Rabbit urethral reconstruction was performed on PTUS model since nearly all patients developed varying degrees of hypertrophic scars, wherein urethral mucosa was removed to establish PTUS model 1 month earlier before urethral reconstruction surgery of adult New Zealand rabbit (Fig. 5a). Herein, the middle Zein layer was saturated with tissues to ensure the stability (Supplementary Fig. 23), and the rigid Zein failed to induce adjacent tissue damages. Even though inflammation and operation trauma emerge at the early stage (Day 0), inflammation rapidly recedes and tissue repair proceeds well after 14 days without detachment (Supplementary Fig. 24). In a long-term monitoring (28 days), there is still no obvious chronic inflammation (Supplementary Fig. 24). This anti-inflammation property is be probably attributed to VP-mediated inflammation inhibition, bacterial adhesion inhibition, urine permeation occlusion and the great biocompatibility and appropriate repair-matched degradation rate of D-H/Zein-BP hydrogels (Supplementary Fig. 25). Additionally, it also suggests that the degraded products have no toxic reactions in surrounding tissues. Especially, the transient temperature rise beyond the phase-change temperature determines that the temperature-induced hydrophobicity and volumetric shrinkage of BP layers are short-lived, which has neglectable influences on repair efficacy and treatment biosafety.
a Schematic representation of animal experiments validating the efficacy and mechanism of action of the D-H/Zein/BPV scaffold in urethral repair. Elements were created in BioRender. Ming, Y. (2025). b Schematic representation of the D-H/Zein/BPV scaffold replacing the urethra. Elements were created in BioRender. Ming, Y. (2025). c Conceptual diagram depicting the urinary response after substituting the urethra with the D-H/Zein/BPV scaffold. d Surgical photographs illustrating the replacement of the urethra with the urethral scaffold. e, f Urethral contrast images and urethral patency assessment in the control group (injury only), D-H/Zein/BP scaffold group, D-H/Zein/BPV scaffold group, D-H scaffold group and BP scaffold group after 8 weeks post-surgery. g Macroscopic views of the urethral repair sites in animals from above each group at 4 and 8 weeks. h Maximum urine flow rate (Qmax) in animals from each group at 8 weeks. Histological images (i, k) and quantitative scar thickness (j, l) of neo-tissues stained with hematoxylin-eosin (upper, i, j) and Masson’s trichrome (bottom, k, l) at 4 and 8 weeks, depicting the injury sites in animals from above each group. Sirius red staining of neo-tissues in G1, G2 and G3 groups at 4 (m) and 8 (n) weeks, respectively. Immunofluorescence staining for AE1/AE3 and CD31 was performed to assess epithelialization and vascularization in each group at 4 (o) and 8 (p) weeks. Semi-quantitative analysis of epithelial cell layers and vascular count at 4 (q) and 8 (r) weeks. Data are shown as mean ± SEM (n = 3). Unpaired two-sided Student’s t test was used to analyze the significance, and *P < 0.05, **P < 0.01, ***P < 0.001. G1–G5 represent Control (injury only), D-H/Zein/BP, D-H/Zein/BPV, D-H and BP, respectively. Source data is provided as a Source Data file.
During the urethral reconstruction, the scar was firstly removed, and then the Zein/BPV scaffold was sutured to the defective tissues. Afterwards, D-H precursor solution was dropped on Zein/BPV scaffolds, and then received ultraviolet light irradiation to solidify D-H hydrogels and eventually obtain D-H/Zein/BPV (Fig. 5b–d). The temperature in the urethra and bladder of rabbits (Supplementary Fig. 12) is below the phase-change temperature (beyond 32.9 °C) of BP, ensuring the long-term retention of BP at the swelling state. Urethrogram observation shows D-H alone (G4) or BP alone (G5) only reaches a patency rate approaching to Control (G1), which is lower than trilayer D-H/Zein/BP scaffolds (G2), suggesting the excellence of such trilayer-structured scaffolds (Fig. 5e, f). The thickness of scaffolds is appropriate for in vivo application, and even though BP swelling exists, no obstruction is found (Fig. 5e and Supplementary Movie 4 and Supplementary Fig. S24). Inspiringly, the almost complete patency rate (97.66 ± 2.31%) in D-H/Zein/BPV group (G3) after 8 weeks post-surgery, representing the complete urethral reconstruction. The general view of repaired urethra also evidences the successful urethral reconstruction in D-H/Zein/BPV group especially after 3 weeks (Fig. 5g). Beyond that, the maximum urinary flow rate (Qmax) further validates the repair ability of D-H/Zein/BPV since it receives the highest Qmax (4.71 ± 0.22 ml/s) (Fig. 5h). More significantly, the patency rate, reconstructed urethral intactness and Qmax in such dynamically urethra-adapted trilayer D-H/Zein/BPV hydrogels resemble those in the normal undamaged urethra, directly validating that the oriented but different obligations of each layer collaboratively promoted urethra repair.
To assess scar status, histological examination was carried out. The dense collagen thickness representing scar thickness was firstly evaluated as the repair indicator. Minimum scar thickness (4.67 ± 2.08 μm) is found in the D-H/Zein/BPV group (G3) compared to Control (211.33 ± 33.50 μm, G1) and D-H/Zein/BP groups (135.67 ± 18.01 μm, G2) at 4 weeks (Fig. 5i, j). Even though the time is prolonged to 8 weeks, D-H/Zein/BPV group still receives the minimum scar thickness (6.5 ± 2.12 μm) in comparison to Control (567.5 ± 188.07 μm) and D-H/Zein/BP (211.5 ± 34.64 μm) groups (Fig. 5k, l). Notably, the trilayer structure in D-H/Zein/BP performs better in inhibiting inflammatory cells recruitment and favoring complete epithelial regeneration without damaging normal tissues compared to D-H (G4) and BP (G5). Consequently, they harvest much thinner scar thickness, more blood vessel formation and more complete epithelial coverage at both 4 weeks and 8 weeks (Fig. 5i–l). Additionally, no deformation and no detachment or delamination in vivo are observed, and no degraded products bring about toxic reactions to surrounding tissues. In terms of Type I collagen deposition, the highest accumulation emerges in the Control group, while the lowest deposition is observed in the D-H/Zein/BPV group (Fig. 5m, n), implying that D-H/Zein/BPV disfavors scar formation in the course of repair.
Epithelialization and vascularization as another crucial indicators for evaluating wound healing outcomes were tacked. Both D-H/Zein/BP and D-H/Zein/BPV harvest complete epithelial layer growth and abundant blood vessels (Fig. 5o–r). D-H/Zein/BPV induces the significant increase from ~3–5 layers at 4 weeks to 7–8 weeks at 8 weeks in epithelial layer (Fig. 5q). By contrast, the number in both Control and D-H/Zein/BP groups is still lower than that in D-H/Zein/BPV, e.g., 1–2 layers at 4 weeks, and 3–5 layers at 8 weeks. Identical results are found in vascularization detection, wherein D-H/Zein/BPV treatment generates the most discernible vessels (24 ± 3 and 38 ± 5) at 4 weeks and 8 weeks within 400 μm2, respectively (Fig. 5r). These indicators reveal that the integrated scarless urethra repair unlocked by such trilayer D-H/Zein/BPV hydrogels is realized through promoting vascular and epithelial regenerations and blockading fibrosis-correlated YAP and TFG-β signaling pathways. Considering that persistent inflammatory stimulation promotes fibroblast activation and excessive collagen deposition to ultimately lead to fibrotic complications, the neglectable or low-level chronic inflammation enabled by the implanted urethral scaffold within 28 days (Supplementary Fig. S24) is also responsible for the scar formation inhibition.
Mechanism explorations associated with scarless repair
Generally, urethra has higher sensitivity and more rapid immune response to grafts compared to subcutaneous tissues, where rapid immune activation under sustained mechanical stresses brings about chronic inflammation to promotes epithelial hyperplasia and stricture formation, while subcutaneous tissue elicits a delayed inflammatory response after graft implantation. This explains why urethral pair is challenging and no satisfactory solution has been proposed yet. Additionally, it also highlights the significance of our dynamically urethral microenvironment-adapted and obligation-oriented trilayer hydrogels in dampening immune activation and attenuating inflammation to inhibit epithelial hyperplasia and stricture formation, and further underscores the rationale for conducting independent in-depth mechanistic investigations into the urethral repair. In order to investigate the underlying mechanisms of scarless urethra repair, RNA sequencing was firstly implemented. Principal Component Analysis (PCA) reveals significant differences in RNA levels among D-H/Zein/BPV, D-H/Zein/BP and control groups at day 7 (Fig. 6a), preliminarily validating that D-H/Zein/BP and loaded VP could interfere with signaling pathways during the repair process. However, at 4 weeks post-operation, the differences are not remarkably evident (Supplementary Figs. 26, 27), inconsistent with aforementioned histological changes. This phenomenon suggests that the regulated genes associated with scar formation in the late stages of urethral repair have undergone early modifications. Until 4th week, signaling pathway activation becomes less apparent, yet the secretion of extracellular matrix continues. To figure out the decisive factors in scarless urethral repair, we conducted a comparative analysis between D-H/Zein/BPV and Control since they represent the thinnest and thickest scar layers, respectively. The volcano plot displays substantial differences with 3204 differentially expressed genes (p < 0.05) and 2667 significantly different genes (Fig. 6b). The top 20 significantly-altered genes are enriched in Focal adhesion, hedgehog, ECM-receptor interaction, TGF-β and Hippo signaling pathways (Fig. 6c, d).
a Principal Component Analysis (PCA) of RNA sequencing results at 7 days and 4 weeks for the normal group and other experimental groups. b Volcano plot depicting differentially expressed genes between the D-H/Zein/BPV group and the control group at 4 weeks. c, d Top 20 downregulated signaling pathways in the KEGG enrichment analysis for the D-H/Zein/BPV group and the control group at 4 weeks. e Gene expression levels related to wound healing, M2 type macrophages, YAP-associated, and scar formation at 4 weeks. f Heatmap of gene expression related to scar formation and scarless healing in the D-H/Zein/BPV group and the control group at 4 weeks. g–j Gene Set Enrichment Analysis (GSEA) and corresponding heatmaps for the Hippo signaling pathway and TGF-β signaling pathway in the D-H/Zein/BPV group and the control group at 4 weeks. Data are shown as mean ± SEM (n = 3). Source data is provided as a Source Data file.
Subsequently, we conducted a comparative analysis of mRNA counts associated with wound repair, anti-inflammatory M2 macrophages, YAP and scar formation (Fig. 6e). On Day 7 post-surgery, gene transcription into mRNA is enough to translate related proteins, and the related protein expressions are hysteretic and delayed to launch wound healing. Thereby, it is not difficult to understand why those post-transcriptional mRNAs including MYLK, YAP, COL1a1 in the YAP signaling pathway and Col1a1/3a1, WNT2, SMAD4 and CTNNB1 mattering scar formation are downregulated in the D-H/Zein/BPV group. Undoubtedly, higher mRNA levels associated with wound repair (e.g., VEGFA, MMP1) and anti-inflammation activation (AGR1, CD163, JAK3 in M2 macrophages) are a matter of course. Further categorization of differentially-expressed genes reveals a higher expression of genes associated with scarless healing in the D-H/Zein/BPV group (Fig. 6f). Additionally, Hippo and TGF-β signaling pathways that are crucial for wound healing and fibrosis were inspected, and considerably-downregulated genes in the D-H/Zein/BPV group are obtained (Fig. 6g–j and Supplementary Fig. 28).
Early competition between regeneration motive and scaring
Immediately afterwards, qPCR was further proceeded to analyze several pivotal differential genes mattering wound healing and scar formation in the early stage (Fig. 7a). With differing from late-stage RNA sequencing findings, the upregulations of TGF-β2, COL1, FGF23 MMP-1 and VEGFA are observed in the D-H/Zein/BPV group. Notably, although late RNA sequencing analysis shows no apparent differences in the downstream genes (SMAD2 and SMAD3), a significant increase in qPCR results is obtained, meaning that SMAD2/3-YAP/TAZ-TEAD1-4 complexation is inhibited. Thanks to the timely upregulations of transcriptional mRNA, the early expressions of TGF-β2, SMAD2/3 and COL-1 proteins in the D-H/Zein/BPV group are activated compared to Control (Fig. 7b and Supplementary Fig. 29) for expediting tissue regeneration, but terminated at late stage before excessive ECM secretion for scarring (Fig. 7e). The direct fibrosis- and scar-related a-YAP1 expression in D-H/Zein/BPV is completely inhibited by the loaded VP and the compromised mechanical tension at both early and late stages, resembling the cell tight junction-related proteins including CLDN-10 and a-SMA. These results further uncover that scar formation inhibition is earlier initiated and remains sustained in the course of regeneration repair, resulting in thin scar thickness. By contrast, the control group suffers from poor integrated repair, and the hard scar tissues are dominant in those limited regenerated tissues owning to the persistent activations of TGFβ and YAP. All above results uncover that D-H/Zein/BPV indeed blockaded TGFβ- and YAP-associated fibrosis signaling pathway, inactivated inflammation pathway, and activated vascular & epithelial regeneration and proliferation & migration signaling pathways, all of which contributed to the integrated scarless urethral repair with soft tissues (Fig. 7c). Specifically, the oriented obligations of each later in such urethra-adapted D-H/Zein/BPV hydrogels expedited epithelial and vascular regenerations to favor integrated repair at early stage. But at late stage, the loaded VP compromised excessive extracellular matrix secretion-induced fibrosis, tissue stiffening and scar formation especially stimulated by BP swelling-originated mechanical stress or tension-induced overgrowth.
a mRNA expression levels of TGF-β2, SMAD2, SMAD3, YAP1, MMP1, and VEGFA in the control group, D-H/Zein/BPV group, and normal group at 4 days post-surgery of rabbit tissue. b Western blot results depicting the expression of key proteins in the TGF-β signaling pathway and proteins related to scarless wound healing in the control group, D-H/Zein/BPV group, and normal group at 4 days post-surgery of rabbit tissue. More than three experiments of different samples were conducted, and the results were repeatable. c Schematic on tight and loose cell junctions at the normal and scar of urethral epithelial layer. Elements were created in BioRender. Ming, Y. (2025). Immunofluorescence staining (d) and semi-quantitative analysis (e) of HIF-1α, MYLK, and YAP1 protein expression at neotissues at 4 weeks in the control group, D-H/Zein/BP group, and D-H/Zein/BPV group. Top 20 upregulated and downregulated signaling pathways in the KEGG enrichment analysis at 4 weeks after comparing control group with D-H/Zein/BP (f) and D-H/Zein/BPV (g) group. Data are shown as mean ± SEM (n = 3). Unpaired two-sided Student’s t test was used to analyze the significance, and ‘ns’ no significance, *P < 0.05, **P < 0.01, ***P < 0.001. Source data is provided as a Source Data file.
To exclude the interference that protein expression was later than mRNA transcription in the course of wound repair, immunofluorescence detection was enforced to verify some key molecules after 4 weeks post-surgery, e.g., HIF-1α and MYLK that regulate vascularization-correlated VEGFA45, tight junction-decided a-SMA46,47, and tension-sensitive factor YAP. The considerably-elevated expression of HIF-1α provides a convincing explanation for vascular regeneration in the D-H/Zein/BPV group. The low MYLK and YAP expressions imply the increased softness and the reduced scar formation in the D-H/Zein/BPV-mediated repair process (Fig. 7d, e), which favors the progressive elasticity restoration of neo-tissues. Intriguingly, no significant differences in transcriptional mRNA levels of neo-tissues are observed among all groups at 4 weeks (Fig. 7f, g). This point highlights that the timely gene transcription termination treated with D-H/Zein/BPV is of great importance against scarring even though the accumulated extracellular matrix remains to support urethra repair.
Arg1, a marker of M2 macrophage polarization, is highly expressed in in the D-H/Zein/BPV group at 4 weeks (Supplementary Fig. 30a, b), but is recovered to normal level (Supplementary Fig. 30c, d). This process records the inflammation evolution from anti-inflammation activation to eventual inflammation termination during urethral repair. Interleukin-17 (IL-17) that regulates HIF signaling pathway is activated, which is partially responsible for epithelial regeneration, angiogenesis and scarless healing (Supplementary Fig. 31)48,49. RNA sequencing analysis also indicates that VP may potentially facilitate scarless healing through mediating immune modulation and ribosome metabolism (Supplementary Figs. 32, 33).
Signaling pathway analysis
Collectively, aforementioned oriented obligations of each layer, e.g., anti-fouling, non-swelling and anti-bacteria adhesion in the bottom D-H layer, high rigidity against BP swelling stress and high binding affinity with D-H in the middle Zein layer, as well as high swelling-favored pro-proliferation and expansion in upper BP layer have been verified. These distinctive characteristics collaborated to oppose urethral stricture, which united with the loaded VP to attenuate fibrosis and scar formation, and expedite the integrated scarless repair. The detailed mechanism and signaling pathway of such trilayer D-H/Zein/BPV hydrogels are generalized as below (Fig. 1c). Gene transcription associated with scar formation and wound repair (e.g., COL1a1/1a3, TGFβ2, WNT, CTNNB1, YAP1, etc.) was timely inhibited on Day 7. Under this condition, although the delayed protein expression accumulation was higher for promoting wound repair, their levels were still inferior or equal to those in normal tissues, which avoided overproliferation-arised fibrosis and scar formation especially strengthened by BP swelling-originated tension. During this process, proteomic and genomic analysis validated that anti-inflammation (ARG1, IL-17), angiogenesis (HIF-1α, VEGFA), extracellular matrix (MMP1, FGF23) were activated to expedite epithelial & vascular regenerations and wound healing via regulating urethral immune microenvironment and ribosome metabolism. However, their degrees remained not to surpass those in Normal group, thus guaranteeing few scar formations. Moreover, the downregulations of cell tight junction activation (MYLK, CLDN-10, aSMA) explained tissue softening and collagen secretion decrease in repaired tissues, and answered why such urethra-adapted and obligations-oriented trilayer D-H/Zein/BPV hydrogels successfully integrated scarless urethral repair.
In summary, we identified urethral damage microenvironment and scarring-associated signaling pathways and key targets from clinical samples. Based on them, a dynamically urethra-adapted and obligations-oriented trilayer hydrogel consisting of BP, Zein and D-H was rationally designed and constructed to integrate scarless urethral repair. The high swelling in the upper BP layer enabled more MSCs’ migration and proliferation, benefiting urethra repair. The anti-fouling and anti-bacteria adhesion properties in D-H layer dampened bacterial infection and inflammation accumulation, which was also advantageous to urethra repair. The non-swelling, high mechanical strength and rigidity and high binding affinity of Zein with D-H inhibited urine voiding-arised BP hydrogels shedding and urethral diastole/contraction, resisted potential urethral occlusion induced by inward BP swelling, and occluded urine permeation, preferably dampening fibrosis and scar formation. More significantly, the loaded VP blockaded fibrosis-correlated YAP signaling pathway, which combined with their oriented obligations of each layer to synergistically accelerate vascular and epithelial regenerations, reduce collagen accumulations and ECM secretions, hamper tight cell junction and inhibit fibrosis. Contributed by them, innate immune microenvironment (e.g., inflammation microenvironment) and ribosome metabolism in damaged urethra were remodeled to eventually realize the integrated urethra repair without evident scar. The dynamically urethra-adapted and obligations-oriented trilayer hydrogels demonstrated promising outcomes in urethral injury repair research, providing meaningful insights into the mechanisms of scarless urethral repair in specific environments.
Methods
Ethical statement
For human urethral samples, this study was part of a clinical research project approved by the Institutional Review Board (IRB) of Shanghai Jiao Tong University Affiliated SixthPeople’s Hospital with the clinical trial number of 2020-141. This study is part of the clinical research project and reports partial research findings. The main results of this clinical study are not yet published. Participants were aged 33–51 years old and were diagnosed with urethral stricture and received urethral repair and reconstruction surgery at Shanghai Sixth People’s Hospital. Inclusion requirements: patients with a clear diagnosis of urethral scar, willing to undergo surgery, and who sign the informed consent for this study. The scar tissue was the tissue that needed to be resected during the surgery, and we obtained it in accordance with the clinical trial protocol. The samples were collected prior to the intervention. The normal tissues were derived from patients undergoing urethral repair and reconstruction surgery, specifically the urethral tissues that needed to be trimmed according to standard surgical procedures during the operation. Limitations of sample selection: Since normal urethral tissue can only be obtained from patients with urethral stricture, the problem of stricture genes being ignored in the comparison may occur. The removed scar tissue and normal tissues trimmed for suturing purposes are collected and processed as research samples at this stage. The collection of all tissues was conducted in strict accordance with the clinical trial protocol, ensuring that the patients’ interests were not compromised. All patients were recruited in accordance with the clinical research protocol and signed the informed consent form in person.The study has been registered on the Chinese Clinical Trial Registry website (https://www.chictr.org.cn/) with registration number ChiCTR2000035416. The research adhered to the principles of the Declaration of Helsinki, and written informed consent was obtained from all human participants. Human urethral fibroblast samples were used only for RNA-sequencing and qPCR and Westernblot detection of related gene expression.
Animal experiments involving New Zealand rabbits and Beagle dogs were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Shanghai Jiao Tong University Affiliated SixthPeople’s Hospital, and all procedures were approved by the committee under the ethical review number No:DWLL2024-0477.
Characterizations
The morphological characteristics were detected by scan electronic microscope (JEOL JSM-5610LV, Japan), XRD (X-ray diffraction) data were acquired on Bruker AXSD8-Focus diffractometer using Cu Kαradiation, 40 kV, 30 mA, test angle: 5–120°. Characteristic FTIR peaks were detected on Nicolet 670, USA,Laser confocal microscopy (LASAFSP5, China) and fluorescent microscope (Olympus IX70-S1F2, USA) were used for observing cell behaviors. Bruker Hysitron TI980 was used for the Depth-Sensing Indentation (DSI) testing. The contact angles were measured on a goniometer (JY-82B Kruss DSA,China). The rheological properties of the hydrogels were characterized on a stress-controlled rheometer (HAAKE RheoStress 6000, Thermo Scientific, USA). UV-vis absorbance spectra of verteporfin was measured using a UV spectrophotometer (UV-3600, Shimadzu, Japan). The surface potential of the hydrogel was obtained by solid zeta potential measurement (Anton Paar surpass3, Austria). The surface hardness of the hydrogel was tested by Depth-Sensing Indentation Technique (Bruker Hysitron TI 950, Germany). The morphology of the hydrogel in the hydrated state was tested by cryo-scanning electron microscopy (FEI Quanta 450). The hydrogel structure and composition were characterized by solid-state NMR (Bruker Avance Neo 400WB, Germany).
Three layers scaffold construction
The surface layer is composed of a D-H hydrogel synthesized with a 1:1 ratio of DEAm and HEAm under the influence of a photoinitiator and Bis. The middle layer is obtained by molding Zein in the aqueous phase and subsequently drying it. The bottom layer is formed by dissolving 1.5% wt/wt PNIPAm in double-distilled water containing 500 ng/ml Verteporfin. The gelation process occurs overnight under the action of Bis, Ammonium persulfate (APS), and the accelerator N,N,N’,N’- Tetramethylethylenediamine.
In brief, a precursor solution of D-H hydrogel was obtained by dissolving 10%wt DEAm, 10%wt HEAm, 1%wt Bis, and 1%wt LAP in 2 ml of deionized water. Upon exposure to UV light at a wavelength of 305 nm, the solution transformed into D-H hydrogel. The synthesis of Zein film involved dissolving 2 g of Zein powder in an 80% ethanol solution at room temperature for 6 h, followed by molding with a 0.3 mm depth of mold and drying to obtain Zein film. The precursor for BP hydrogel was prepared by dissolving 0.18 mmol APS, 0.33 mmol Bis and 15 mmol PNIPAm monomer in 20 ml deionized water, stirring at 60 °C, and cooling to room temperature. Subsequently, 40 μl of accelerator was added for Bis-PNIPAm hydrogel formation. The D-H/Zein/BPV scaffold synthesis involved preparing Zein film, uniformly mixing 500 μg/ml VP into the BP hydrogel precursor, pouring the mixture into the Zein film mold. The resulting bilayer scaffold was used for urethral repair, and the top layer of D-H precursor solution was dropped onto the surface before cross-linking and obtain the D-H/Zein/BPV scaffold.
To remove monomers, initiators, and accelerators, the as-prepared hydrogels were placed in a dialysis bag with a molecular weight cutoff of 5 kDa and immersed in PBS buffer. Dialysis was carried out under stirring at 4 °C, with the dialysis solution being replaced every 6 h for a total duration of 48 h. Cell viability experiments confirmed the absence of cytotoxicity (Supplementary Fig. S20). Drug loading method: After synthesizing and purifying the blank hydrogel, it was heated to 40 °C to ensure the removal of internal moisture. The hydrogel was then immersed in a 200 ng/mL VP solution and allowed to swell and adsorb the drug at 4 °C.
Harvesting and culture of canine adipose-derived stem cells (ADSCs) and human urethral fibroblasts
All animal experiments have been approved by all experiments were purchased from the Experimental Animal Center of Shanghai Sixth People’s Hospital and approved by the animal welfare ethics committee of Shanghai Sixth People’s Hospital with the approval number (2020-0267). Human urethral fibroblasts were derived from excess urethral tissue removed during surgery and approved by ethics committee of Shanghai Sixth People’s Hospital. Briefly, fresh tissues were washed three times in phosphate-buffered saline (PBS) and then cut into small pieces. They were digested with 0.1% collagenase I (Sigma) under continuous oscillation at 37 °C for 1 h, and then neutralized with basal DMEM containing 10% FBS. The cell suspension was filtered through a 50 μl-specialized mesh filter and then centrifuged. The primary ADSCs were collected and maintained in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin. Culturing of cell sheet consistent with our previous research2. When culturing with trilayer scaffold, temperature will be set at 30 °C.
Swelling ratio (SR) detection
The samples (D-H, Zein, BP hydorgel) were immersed in PBS at room temperature for 0.5 h, 1 h, 2 h, 6 h, 12 h and 24 h, respectively. After PBS was removed, the hydrogels were gently scrubbed using absorbent paper to remove the residual solvent and weighed at each time points. The SR of hydrogels was defined as:
Wherein, Ws is designated as the weight of hydrogels at different time points; and Wd is designated as the weight of original hydrogels.
In vitro degradation test
In vitro mass change rate of each layer (D-H, Zein and BP hydrogel respectively) with fixed weight (W0) was assessed in 2 ml of simulated body fluid (SBF, cz0400, leading biotechnology, pH = 7.4) and acidic urine. The process was performed at 30 °C. The mass change was calculated according to Eq. (2), as follows:
Note, Wt is the mass of the residual dried hydrogels after incubating in urine for 10, 40, 60, 100, 130, 160, 190 and 220 s, while W0 is the initial mass of hydrogels. Two conditions were set, (1) mimicking the real scenarios with pulsed vibrating and squeezing operations (once per 12 h); (2) static placement.
Three layers scaffold construction
The surface layer is composed of a D-H hydrogel synthesized with a 1:1 ratio of DEAm and HEAm under the influence of a photoinitiator and Bis. The middle layer is obtained by molding Zein in the aqueous phase and subsequently drying it. The bottom layer is formed by dissolving 1.5% wt/wt PNIPAm in double-distilled water containing 500 ng/ml Verteporfin. The gelation process occurs overnight under the action of Bis, Ammonium persulfate (APS), and the accelerator N,N,N’,N’- Tetramethylethylenediamine.
In brief, a precursor solution of D-H hydrogel was obtained by dissolving 10%wt DEAm, 10%wt HEAm, 1%wt Bis, and 1%wt LAP in 2 ml of deionized water. Upon exposure to UV light at a wavelength of 305 nm, the solution transformed into D-H hydrogel. The synthesis of Zein film involved dissolving 2 g of Zein powder in an 80% ethanol solution at room temperature for 6 h, followed by molding with a 0.3 mm depth of mold and drying to obtain Zein film. The precursor for BP hydrogel was prepared by dissolving 0.18 mmol APS, 0.33 mmol Bis and 15 mmol PNIPAm monomer in 20 ml deionized water, stirring at 60 °C, and cooling to room temperature. Subsequently, 40 μl of accelerator was added for Bis-PNIPAm hydrogel formation. The D-H/Zein/BPV scaffold synthesis involved preparing Zein film, uniformly mixing 500 μg/ml VP into the BP hydrogel precursor, pouring the mixture into the Zein film mold. The resulting bilayer scaffold was used for urethral repair, and the top layer of D-H precursor solution was dropped onto the surface before cross-linking and obtain the D-H/Zein/BPV scaffold.
To remove monomers, initiators, and accelerators, the as-prepared hydrogels were placed in a dialysis bag with a molecular weight cutoff of 5 kDa and immersed in PBS buffer. Dialysis was carried out under stirring at 4 °C, with the dialysis solution being replaced every 6 h for a total duration of 48 h. Cell viability experiments confirmed the absence of cytotoxicity (Supplementary Fig. S20). Drug loading method: After synthesizing and purifying the blank hydrogel, it was heated to 40 °C to ensure the removal of internal moisture. The hydrogel was then immersed in a 200 ng/mL VP solution and allowed to swell and adsorb the drug at 4 °C.
Molecular dynamical simulation
The Density Functional Theory calculations were performed using the Gaussian 16 software that was provided by Prof. Liang Wu from Shanghai Jiaotong University with an authorization by Gaussian, Inc. The B3LYP functional was adopted for all calculations in combination with the D3BJ dispersion correction. For geometry optimization and frequency calculations, 6–31 G(d,p) basis set was used for all atmos. The single point calculations under the level of M062x-D3/6-311 G(d, p).
Mechanical properties
The compressive properties and tensile strain of the hydrogels were measured by a universal material testing machine (INSTRON-5542, China) at 1 mm/min. There composite hydrogel scaffolds, i.e., D-H hydrogel (diameter of 1 cm, height of 0.5 cm), BP (diameter of 1 cm, height of 0.5 cm) were tested at room temperature, respectively.
Rheology analysis
The rheological properties of different hydrogels were characterized on a stress-controlled rheometer (HAAKE RheoStress 6000, Thermo Scientific, USA) with a diameter of 10 mm plate geometry and a thermostatic bath at a temperature of ~25.0 °C. To achieve an intimate contact between hydrogels and the geometry surfaces, each sample was pre-sheared for 1 min prior to test. To examine the rheological behaviors of the hydrogel under oscillatory shear, a stress-sweep test was performed at an oscillatory frequency of 1 Hz and shear of 1 N, and both the storage (G’) and loss (G”) moduli as a function of the oscillatory stress were acquired. The rheological response of BP to temperature variations is investigated by configuring the machine temperature within the range of 25–40 °C, with a temperature change rate of 1 °C/min. This controlled experimentation allows for the acquisition of the storage modulus (G’) and the loss modulus (G”) of the BP hydrogel.
Differential scanning calorimetry (DSC) for characterization of BP hydrogel
TA, NETZSCH was used for DSC testing of BP hydrogel.The experimental procedure are as follow: Set the machine temperature from 25 to 45 °C for the analysis of BP hydrogels. Implement a controlled temperature change rate of 1 °C/min to mimic realistic thermal conditions. After the test temperature rises from 25 °C to 45 °C, it then drops back to 25 °C, we recorded and analyzed the thermal behavior of the BP hydrogel during the temperature ramp.
Anti-fouling experiment
The anti-fouling capacity of the hydrogels against E. coli, Proteus, and Staphylococcus aureus was assessed via the agar diffusion assay. Briefly, various hydrogels (DEAm, HEAm, and D-H) were formed into spheres with a diameter of 0.5 cm. First, sterilized hydrogel specimens were placed in 24-well culture plates, and 1 ml of E. coli, Proteus, or Staphylococcus aureus suspension (1 × 10⁶ CFU/ml) was added to submerge the specimens. The plates were then incubated in a biochemical incubator at 37 °C for a total of 24 h. After incubation, the specimens were removed and rinsed with sterilized water to eliminate non-adherent bacteria from their surfaces. The treated specimens were transferred to centrifuge tubes containing 5 ml of sterile PBS, sonicated for 30 s, and then serially diluted to 1/1000 via gradient dilution. The total colony count was determined using the plate assay. Bacterial adhesion on the surface of untreated hydrogels was visualized using scanning electron microscopy (SEM). Sample preparation for SEM was conducted as follows: specimens co-cultured with bacterial suspensions were retrieved, rinsed three times in PBS, and then fixed with 2.5% glutaraldehyde solution for 2 h of immersion. Gradient dehydration of the fixed, surface-adherent bacteria was performed by sequential immersion in 3%, 50%, 70%, 90%, and 100% ethanol solutions, followed by isoamyl acetate, with each step lasting 10 min. Finally, the specimens were dried using a CO₂ critical point dryer. Prior to confocal microscopic imaging, bacteria were stained with DMAO fluorescent dye and subsequently observed via laser confocal microscopy.
Biocompatibility
Verteporfin first dissolved in a solution containing 10% dimethyl sulfoxide, 10% Tween, and 80% double-distilled water, achieving a final VP concentration of 1 mg/ml. Cells were cultured in 6-well plates using VP solutions at concentrations of 50 μg/ml, 5 μg/ml, 500 ng/ml, 50 ng/ml, 5 ng/ml, and 0.5 ng/ml for 24 h. Cell shape and viability was observed under an optical microscope. VP with varied concentrations at 10 ng/ml, 50 ng/ml, 100 ng/ml, 200 ng/ml, 500 ng/ml, 1000 ng/ml and 2000 ng/ml were cultured with ADSCs for evaluation of cell viability.
The viabilities of ADSCs in BP and BPV hydorgels were evaluated by live/dead cell staining kit in vitro at the endpoint (7 days). Sodium alginate was set as control. The cells-contained scaffolds were washed by PBS three times, and then treated with propidium iodide (PI, 4 μM) and calcein-AM (2 μM) in medium minus FBS at room temperature and incubated in the darkness for 15 min. Then, it was rinsed with PBS 3 times and photographed on confocal laser scanning microscope (CLSM) at the excitation/emission wavelengths of 494 nm/528 nm. The percentage of living and dead cells was calculated after counting by image J software. All groups were reconstructed as three-dimensional photograph.
Cell Counting Kit-8 (CCK-8) was further used to assess the biosafety of hydrogels. Briefly, ADSCs were co-cultured with D-H/Zein/BP and D-H/Zein/BPV scaffolds and incubated for 24 h, followed by further incubations for 3 days, 7 days, respectively. At each time point, scaffolds were removed and cells were incubated with 100 µL of fresh cell culture medium containing 10 µL of CCK-8 at 37 °C for 4 h. The absorbance of the supernatant at 450 nm was analyzed by using a microplate reader, and viability was recorded in relation to control group without any treatment.
We followed sterile surgical procedures to embed D-H/Zein/BPV hydrogel subcutaneously in the abdominal area of New Zealand rabbits. Ultrasound imaging was then immediately performed to capture the ultrasound images of the hydrogel and to mark its location. Antibiotic injections were administered intramuscularly to the experimental animals. Fourteen days later, ultrasound imaging was performed at the designated location, followed by tissue sampling at the embedded site.
As well, to observe the safety, urethra damages were implanted with D-H/Zein/BPV hydrogels, and at day 0, day 14 and day 28, HE, IL-6 and Masson’s trichrome immunohistochemical staining were used to observe the local conditions between the hydrogel scaffolds and the surrounding tissues.
Tensile stress-promoted fibrosis tests
The edge of the ADSCs sheet was gently lifted, and a stainless steel ring (1.5-mm thick, 1.5-cm diameter) was positioned at the center of the culture dish. The cell sheet was then carefully overlaid onto the stainless steel ring, with its peripheral edges repositioned to their original orientation and securely anchored, producing 3.8 × 10−3 μN per cell. Following 48 h of incubation, RNA and protein were extracted from the cell sheet for subsequent quantitative polymerase chain reaction (qPCR) analysis and Western blotting detection.
To understand the influences of hydrogels-arised internal stress on fibrosis tests, 1 × 10⁶ urethral fibroblasts were seeded in 10-cm diameter cell culture dishes. After 48 h of culture, we replaced the medium and co-culture the cells with prepared D-H/Zein/BP or D-H/Zein/BPV hydrogels (with thickness matching the height of culture dish) for 72 h, during which rigid cap was covered on the dishes to resist hydrogels swelling and induce internal stress. Subsequently, harvest the cells and extract both RNA and protein for qPCR and Western blot experiments. ADSC cell slides were prepared and co-cultured with hydrogels, and the nuclear and extranuclear distribution of YAP and pYAP were observed by immunofluorescence staining.
Macrophage differentiation and secretion assay
M0 macrophages were co-cultured with D-H/Zein/BPV hydrogel for 24 h, with non-co-cultured M0 macrophages serving as the control group. Flow cytometry was performed to detect CD206 and CD68 expression using specific antibodies (abcam: CD206, Cat# ab270634; CD68, Cat# ab283654). Secreted cytokines were analyzed using ELISA kits (VEGFA: Elabscience, Cat# E-EL-R2603; FGF23: Elabscience, Cat# E-EL-R3031) following the manufacturer’s protocols. Cell culture supernatants were collected. 96-well plates were coated with 100 μL/well of capture antibody (2 μg/mL in PBS, pH 7.4) overnight at 4 °C. After washing three times with PBS containing 0.05% Tween-20 (PBST), wells were blocked with 300 μL/well of 1% bovine serum albumin (BSA) in PBS for 2 h at room temperature (RT).Standards (recombinant IL-6, 0–500 pg/mL) and thawed supernatants (diluted 1:5 in assay diluent) were added (100 μL/well) in triplicate and incubated for 2 h at RT. Plates were washed, followed by addition of biotinylated detection antibody (100 μL/well, 200 ng/mL in PBST) for 1 h at RT. After washing, streptavidin-HRP conjugate (1:200 dilution, 100 μL/well) was applied for 30 min in the dark. Absorbance was measured at 450 nm using a microplate reader.
In vivo biosafety evaluation
The abdominal epidermis of rabbits was surgically incised, and hydrogels were implanted subcutaneously in the abdominal region, adjacent to the rectus abdominis muscle. Post-suturing, the hydrogel implantation site was marked with a surgical marker. Hydrogel thickness was monitored longitudinally using ultrasound imaging. At predetermined time points, hydrogels were explanted, cryosectioned, and subjected to subsequent histological and biocompatibility analyses.
Red blood cell adhesion assay
Whole rabbit blood was collected into heparin-anticoagulated tubes and allowed to settle for 1 h. The lower layer containing red blood cells (RBCs) was isolated, and the RBC concentration was adjusted to 107 cells/mL using phosphate-buffered saline (PBS). Hydrogel samples were placed in a 24-well plate, and 10 μL of the RBC suspension was pipetted onto the surface of each hydrogel. After incubation for 30 min at 37 °C, non-adherent cells were removed by gentle washing with PBS under ultrasonication (three times, 5 min each). The adhered RBCs were fixed with 4% paraformaldehyde for 15 min and rinsed with PBS. Digital images of the adhered RBCs were acquired using an optical microscope (Nikon Eclipse Ti2) equipped with a CCD camera (DS-Ri2).
ELISA detection
To measure the concentration of VEGFA and FGF23 secreted by ADSCs, an enzyme-linked immunosorbent assay (ELISA) was performed. Briefly, a pre-prepared 96-well ELISA plate was coated with 100 µL of antibody solution (a 1:1000 dilution of the primary antibody specific to the target protein) in each well. The plate was incubated at 4 °C overnight. After incubation, the liquid in the wells was discarded, and 200 µL of blocking solution (5% bovine serum albumin, BSA) was added to each well. The plate was incubated at room temperature for 1 h. Following blocking, samples were added to each well (1:100) with 100 µL per well. The plate was incubated at room temperature for 2 h. After each step, the ELISA plate was washed 3 times with PBS containing 0.05% Tween-20. 100 µL of horseradish peroxidase (HRP)-labeled secondary antibody corresponding to the target protein (1:5000 dilution) was added to each well. The plate was incubated at room temperature for 1 h. 100 µL of TMB substrate solution was added to each well, and the reaction was allowed to proceed in the dark at room temperature for 10–30 min and terminated the reaction. The absorbance (OD value) of each well was measured at 450 nm using a microplate reader. The concentration of the target protein in the samples was determined by comparing the absorbance to a standard curve.
Drug release experiment
HPLC detection using 0.02 mg/ml, 0.05 mg/ml, 0.1 mg/ml, 0.2 mg/ml, 0.3 mg/ml verteprofin to obtain a standard curve, then we put 1 cm3 BPV hydrogel at 100 ml deionized water in different temperature, collect 100 µL solution after 1 min, 5 min, 10 min, 30 min, 1 h, 12 h and 24 h. Calculate concentration using standard curve. To demonstrate drug release more vividly, synthetic pigments were used instead of VP in the Supplementary Movie.
Cell migration experiment in vitro
ADSCs were cultured on Zein, D-H/Zein/BP and D-H/Zein/BPV scaffold respectively for 48 h, then 0.5 cm diameter cylinder of scaffold and cells were move into a new plate, culturing with 5% CO2 at 30 °C. Subsequently, digital photos of the wound area were captured by optical microscope (Olympus, Japan) at 0 h, 12 h, 24 h and 48 h, respectively. The cell-covered area was measured and analyzed by Image J.
In vivo construction of urethral defect model and urethral reconstruction surgery on rabbits
40 male rabbits (6–8 months old/2.5–4-kg weight) were divided into 6 groups: control group, D-H/Zein/BP group and D-H/Zein/BPV group, observing time point of 4 weeks and 8 weeks for each group. The ventral side wound healing is easily interfered after multi-layer suture, but the dorsal side is close to the cavernous body without redundant tissue damage, which makes it appropriate for observation and evaluation, so the dorsal side damage model was adopted in this study. After double layer scaffold (with or without VP) was sutured on the defect site, D-H hydrogel precursor is dropped on the surface and cross-linked by UV light irradiation.
Histological, immunohistochemistry and immunofluorescence analysis
Rabbit urethral specimens and cell migration sheets were fixed with 4% neutral buffered formalin for 48 h. After dehydration using graded ethanol, these tissues were embedded in paraffin and cut into thin slices with 3 mm thickness, and stained with hematoxylin & eosin (H&E) and Masson staining. The expressions of AE1/AE3, CD31, HIF-1α, Arg1, MYLK and YAP1 were analyzed by immunofluorescence staining, and IL-6 was analyzed by immunohistochemistry staining. The pictures were captured on a phase contrast microscope.
Urethrography analysis
Rabbits in the treatment groups underwent urethrographic assessment prior to euthanasia at the 8-week mark of the experiment. An 8F catheter was inserted into the urethra, and X-ray images were acquired following the injection of iodine-based contrast media into the urethral lumen. By comparing images across all groups, the severity of urethral stricture was assessed using the ratio of stricture width to total urethral width.
Maximum flow rate (Qmax)
After 8 weeks of surgery, the rabbits were anesthetized, 200 ml normal saline was injected into the bladder. Then wake up the rabbit, wait for the rabbit to urinate, and detect the maximum urine flow rate of the rabbit through the urine flow rate detector.
Flow cytometry
The following experimental procedures were performed: 100 μL of cells per tube (~1 × 10⁶cells) were placed into flow cytometry tubes. According to the antibody instruction manual, a fluorescently labeled antibody (5 μL of CD86-FITC) was added to stain the cell surface antibodies. After thorough mixing, the tubes were incubated at room temperature in the dark for 30 min. Following incubation, 2 mL of PBS was added to resuspend the cells, which were then centrifuged at 300 × g for 5 min. The supernatant was discarded, and the cell pellet was resuspended in 200 μL of PBS. Subsequently, 200 μL of Fixation Buffer was added, and the mixture was incubated at room temperature in the dark for 60 min. Next, 1 mL of 1× Permeabilization Working Solution was added, and the cells were centrifuged at 600 × g for 5 min. The supernatant was discarded, and the cell pellet was resuspended in 100 μL of 1× Permeabilization Working Solution. According to the antibody instruction manual, the corresponding intracellular staining antibody (5 μL of CD206-PE) was added. After mixing, the tubes were incubated at room temperature in the dark for 30 min. Subsequently, 2 mL of PBS was added, and the cells were centrifuged at 600 × g for 5 min. The supernatant was discarded, and the cells were resuspended in an appropriate volume of PBS for flow cytometry analysis. Data were analyzed using FlowJo software. Sysmex Partec CyFlow Cube8, Sysmex Partec
The scatter plot was plotted with forward scatter (FSC) as the horizontal axis and side scatter (SSC) as the vertical axis. The voltages of FSC and SSC were adjusted, and the target cell population was gated by a polygon to exclude non-target cells such as debris and cell clusters. Another scatter plot was constructed with the CD68-FITC fluorescence channel as the horizontal axis and the CD206-PE fluorescence channel as the vertical axis. Cross-quadrant gating was set based on the blank control and single positive tubes (with confirmed expression of the target antibody) to distinguish between negative and positive cells. After multiple adjustments, the optimal gating strategy was applied to the sample groups to obtain the best analytical results. According to the observation and analysis of the sample group results, information such as the fluorescence proportion and fluorescence intensity of the indicator in the cells was obtained.
RNA sequencing
We collected the new and peripheral tissues of urethral wounds in control, D-H/Zein/BP group, and D-H/Zein/BPV group after 7 days post-surgery. Total RNA was extracted with TRIzol reagent (Beyotime, China). The primers sequences are summarized in Supplementary Table S1. Pathway enrichment was performed with KEGG pathway analysis (OE Biotech, China).
Quantitative real time polymerase chain reaction (qRT-PCR) analysis
Gene expression levels of cells extracted from new tissues in the 7th day after different treatments were analyzed by qRT-PCR. PrimeScript™ RT Reagent Kit (Takara Bio Inc., China) was used for reverse transcription (RT) of total RNA to obtain cDNA. qRT-PCR was performed using 2 × Color SYBR Green Master Mix (EZBioscience, USA) on a detection system. The cycling conditions for PCR were 95 °C for 3 min, followed by 40 cycles consisting of 95 °C for 3 s and then 60 °C for 30 s. The forward and reverse primer sequences of canine and rabbit for GAPDH, TGF-β2, SMAD2, SMAD3, MMP-1, VEGFA, YAP1, are listed in Supplementary Tables S1–S3. The expression levels of mRNA were quantified with β-actin as an internal control and the data were determined based on the cycle threshold (Ct) method as R = 2−ΔΔCT. All primers are listed in Supplementary Table S1. -A stands for antisense primer, -S stands for sense primer.
Western blotting analysis
Cells extracted from new tissue on the 7th day post-different treatment were harvested, and Benzosulfonyl fluoride, a protease and phosphatase inhibitor, was added and dissolved in RIPA buffer. The cell lysates were cultured on ice for 3 h and then centrifuged to collect the supernatant. A total of 60 μg of protein loaded in each lane and 10% SDS-PAGE gel was used for electrophoresis. The target proteins on the SDS-PAGE gel were transferred to polyvinylidene fluoride membranes (PVDF; 0.45μm), then blockaded with 5% blocking buffer at 37 °C for 1 h. PVDF membranes were treated with primary antibodies against β-actin, TGF-β2, SMAD2/3, MMP-1, Col I, active-YAP1, VEGFA, FGF23, CLDN-10, α-SMA at 4 °C for overnight, respectively. Subsequently, the membranes were rinsed with TBST three times, and secondary antibody was incubated for 1 h. An imaging system was used to scan the membranes, and gray values were measured to present the expressions of differential proteins.
Statistics and reproducibility
All data are expressed as means ± standard error of mean (SEM). Unpaired two-sided Student’s t test was used for comparisons, and *P < 0.05 was considered significant. All statistical analyses were performed using SPSS 25.0 software (SPSS Inc., USA). All data are presented as the means ± SDs. All error bars represent SD. Differences between the values were evaluated using one-side unpaired t-test with P < 0.05 considered statistically significant. Source data are provided as a Source Data file.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The authors declare that all data supporting of results in this study are available within the paper and its Supplementary Information, or from the corresponding authors upon request. The data generated in this study are provided in the Source Data file. The transcriptome sequencing data used in this study are available in the Gene Expression Omnibus database under accession number GSE301213. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE301213. RNA-Seq data are provided with this paper. Source data are provided with this paper.
References
Lumen, N. et al. European association of urology guidelines on urethral stricture disease (Part 1): Management of male urethral stricture disease. Eur. Urol. 80, 190–200 (2021).
Martin, P. Wound healing - Aiming for perfect skin regeneration. Science 276, 75–81 (1997).
Xu, Y. M. et al. Intermediate-term outcomes and complications of long segment urethroplasty with lingual uucosa grafts. J. Urol. 198, 401–406 (2017).
Xu, Y. M. et al. Substitution urethroplasty of complex and long-segment urethral strictures: a rationale for procedure selection. Eur. Urol. 51, 1093–1099 (2007).
Dalghi, M. G., Montalbetti, N., Carattino, M. D. & Apodaca, G. The urothelium: life in a liquid environment. Physiol. Rev. 100, 1621–1705 (2020).
Kulkarni, S. B., Barbagli, G., Kulkarni, J. S., Romano, G. & Lazzeri, M. Posterior urethral stricture after pelvic fracture urethral distraction defects in developing and developed countries, and choice of surgical technique. J. Urol. 183, 1049–1054 (2010).
Tritschler, S., Roosen, A., Füllhase, C., Stief, C. G. & Rübben, H. Urethral stricture: etiology, investigation and treatments. Dtsch. Arztebl. Int. 110, 220–226 (2013).
Yang, B. Y. et al. Porous Se@SiO nanosphere-coated catheter accelerates prostatic urethra wound healing by modulating macrophage polarization through reactive oxygen species-NF-κB pathway inhibition. Acta Biomater. 88, 392–405 (2019).
Fang, W. Z. et al. Hydrogels for 3D bioprinting in tissue engineering and regenerative medicine: current progress and challenges. Int. J. Bioprinting 9, 207–238 (2023).
Fang, W. Z. et al. Review on additives in hydrogels for 3D bioprinting of regenerative medicine: from mechanism to methodology. Pharmaceutics 15, 1700 (2023).
Wang, D. et al. Extracellular matrix viscosity reprogramming by in situ Au bioreactor-boosted microwavegenetics disables tumor escape in CAR-T immunotherapy. ACS Nano 17, 5503–5516 (2023).
Ge, J. C. et al. Endogenous Zinc-ion-triggered in situ gelation enables Zn capture to reprogram benign hyperplastic prostate microenvironment and shrink prostate. Adv. Mater. 36, 2307796 (2024).
Zhou, H. et al. Wrecking neutrophil extracellular traps and antagonizing cancer-associated neurotransmitters by interpenetrating network hydrogels prevent postsurgical cancer relapse and metastases. Bioact. Mater. 39, 14–24 (2024).
Zhang, X. N. et al. Tumor microenvironment-triggered intratumoral in-situ biosynthesis of inorganic nanomaterials for precise tumor diagnostics. Coord. Chem. Rev. 484, 215115 (2023).
Gu, X. et al. Preparation of a photocured biocompatible hydrogel for urethral tissue engineering. ACS Appl. Polym. Mater. 3, 3519–3527 (2021).
Cao, N. et al. Prevascularized bladder acellular matrix hydrogel/silk fibroin composite scaffolds promote the regeneration of urethra in a rabbit model. Biomed. Mater. 14, 015002 (2019).
Li, W., Yang, Z., Yang, C. & Guo, W. Novel hollow ultrasound-triggered ZnFe2O4-Bi2MoO6 S-scheme heterojunction for efficient ferroptosis-based tumor therapy. J. Colloid Interface Sci. 683, 132–146 (2024).
Kargi, E. et al. Fascia lata grafts for closure of secondary urethral fistulas. Urology 62, 928–931 (2003).
Chan, D. et al. Combinatorial polyacrylamide hydrogels for preventing biofouling on implantable biosensors. Adv. Mater. 34, 2109764 (2022).
Haq, M. A., Su, Y. L. & Wang, D. J. Mechanical properties of PNIPAM based hydrogels: a review. Mat. Sci. Eng. C. Mater. 70, 842–855 (2017).
Zhang, K. et al. Extravascular gelation shrinkage-derived internal stress enables tumor starvation therapy with suppressed metastasis and recurrence. Nat. Commun. 10, 5380 (2019).
Zhang, Y., Fang, C., Zhang, W. & Zhang, K. Emerging pyroptosis-engineered nanobiotechnologies regulate cancers and inflammatory diseases: a double-edged sword. Matter 5, 3740–3774 (2022).
Cao, H., Duan, L., Zhang, Y., Cao, J. & Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Sig. Transduct. Target. Ther. 6, 426 (2021).
Zheng, L. et al. Intensified stiffness and photodynamic provocation in a collagen-based composite hydrogel drive chondrogenesis. Adv. Sci. 6, 1900099 (2019).
Zhou, L. et al. Hybrid gelatin/oxidized chondroitin sulfate hydrogels incorporating bioactive glass nanoparticles with enhanced mechanical properties, mineralization, and osteogenic differentiation. Bioact. Mater. 6, 890–904 (2021).
Hadjipanayi, E., Mudera, V. & Brown, R. A. Close dependence of fibroblast proliferation on collagen scaffold matrix stiffness. J. Tissue Eng. Regen. Med. 3, 77–84 (2009).
Baker, B. M. et al. Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat. Mater. 14, 1262–1268 (2015).
Wang, L. et al. Urethral microenvironment adapted sodium alginate/gelatin/reduced graphene oxide biomimetic patch improves scarless urethral regeneration. Adv. Sci. 11, 2302574 (2024).
Zhang, Y. et al. Zein-based films and their usage for controlled delivery: origin, classes and current landscape. J. Control Rel. 206, 206–219 (2015).
Yang, M. et al. Urine-microenvironment-initiated composite hydrogel patch reconfiguration propels scarless memory repair and reinvigoration of the urethra. Adv. Mater. 34, 2109522 (2022).
Yu, F. X. et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 150, 780–791 (2012).
Ibar, C. & Irvine, K. D. Integration of Hippo-YAP signaling with metabolism. Dev. Cell 54, 256–267 (2020).
Csibi, A. & Blenis, J. Hippo-YAP and mTOR pathways collaborate to regulate organ size. Nat. Cell Biol. 14, 1244–1245 (2012).
Hua, Y. J. et al. Four-dimensional hydrogel dressing adaptable to the urethral microenvironment for scarless urethral reconstruction. Nat. Commun. 14, 7632 (2023).
Meng, X. M., Nikolic-Paterson, D. J. & Lan, H. Y. TGF-β: the master regulator of fibrosis. Nat. Rev. Nephrol. 12, 325–338 (2016).
Oh, S. H., Swiderska-Syn, M., Jewell, M. L., Premont, R. T. & Diehl, A. M. Liver regeneration requires Yap1-TGFβ-dependent epithelial-mesenchymal transition in hepatocytes. J. Hepatol. 69, 359–367 (2018).
Kollmannsberger, P., Bidan, C. M., Dunlop, J. W. C., Fratzl, P. & Vogel, V. Tensile forces drive a reversible fibroblast-to-myofibroblast transition during tissue growth in engineered clefts. Sci. Adv. 4, eaao4881 (2018).
Lokwani, R. et al. Pro-regenerative biomaterials recruit immunoregulatory dendritic cells after traumatic injury. Nat. Mater. 23, 147–157 (2024).
Lu, Y. Z. et al. CGRP sensory neurons promote tissue healing via neutrophils and macrophages. Nature 628, 604–611 (2024).
Mehrotra, P. et al. Oxylipins and metabolites from pyroptotic cells act as promoters of tissue repair. Nature 631, 207–215 (2024).
Petsakou, A. et al. Cholinergic neurons trigger epithelial Ca2+ currents to heal the gut. Nature 623, 122–131 (2023).
Chen, K. et al. Hyaluronic acid-modified and verteporfin-loaded polylactic acid nanogels promote scarless wound healing by accelerating wound re-epithelialization and controlling scar formation. J. Nanobiotechnol. 21, 241 (2023).
Mascharak, S. et al. Preventing Engrailed-1 activation in fibroblasts yields wound regeneration without scarring. Science 372, eaba2374 (2021).
Zubair, M. et al. Trends in protein derived materials for wound care applications. Biomater. Sci. 13, 130–160 (2024).
Rey, S. & Semenza, G. L. Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc Res. 86, 236–242 (2010).
Horowitz, A., Chanez-Paredes, S. D., Haest, X. & Turner, J. R. Paracellular permeability and tight junction regulation in gut health and disease. Nat. Rev. Gastro Hepat. 20, 417–432 (2023).
Otani, T. & Furuse, M. Tight junction structure and function revisited. Trends Cell Biol. 30, 805–817 (2020).
Zhou, Z. P. et al. Snail-inspired AFG/GelMA hydrogel accelerates diabetic wound healing via inflammatory cytokines suppression and macrophage polarization. Biomaterials 299, 122141 (2023).
Konieczny, P. et al. Interleukin-17 governs hypoxic adaptation of injured epithelium. Science 377, eabg9302 (2022).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grants Number: 82022033, awarded to K.Z.; 82170694, awarded to Q.F.; 82370684, awarded to Q.F.), Sichuan Science and Technology Program (2024NSFJQ0048, awarded to K.Z.), the Key Interdisciplinary Program of Shanghai Jiao Tong University (YG2022ZD020, awarded to Q.F.). We are appreciated for the help of Shiyanjia Lab (www.shiyanjia.com) for the schematic drawing.
Author information
Authors and Affiliations
Contributions
Yang, M.C. Zuo, R.X. Yang and K.L. Zhang contributed equally to this work. K. Zhang conceived this project, K. Zhang, Q. Fu and M. Yang designed the experimental contents and plans. M. Yang, R.X. Yang, M.C. Zuo, K.L. Zhang, R.N. Jia, B.X. Yin, Y. Wang, M. Liu, W.Z. Fang, H.J. Guo, Y.W. Jin performed the experiments. K. Zhang and M. Yang analyzed the data and wrote the original manuscript, and K. Zhang meticulously scrutinized and revised manuscript. K. Zhang and Q. Fu supported the project. K. Zhang supervised the project and all authors commented on this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Yunsheng Chen, and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, M., Zuo, M., Yang, R. et al. Dynamically urethra-adapted and obligations-oriented trilayer hydrogels integrate scarless urethral repair. Nat Commun 16, 7333 (2025). https://doi.org/10.1038/s41467-025-62851-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-62851-2