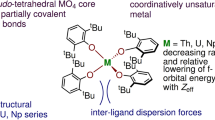
Abstract
The trivalent actinides are produced in the nuclear fuel cycle during power production and provide the largest long-term radiation dose in used nuclear fuel. It is ideal for these elements to be removed from used nuclear fuel for disposal and a necessity for fuel recycling. A key challenge to this is the similarity of chemical behavior of the trivalent actinides to the lanthanides that are also present as fission products in used fuel. Thus far, some of the most effective separations of actinides from lanthanides utilise chelating agents containing sulfur moieties such as dithiophosphinates that selectively bind to actinide ions because of a greater bond covalency relative to lanthanide ions. Typically, greater differences between actinide and lanthanide ions are observable the more ligands and chelators bonds have a covalent character. Here, a series of complexes of the trivalent actinides Np(III) through Cf(III) (excluding Bk(III)) with maleonitrile-1,2-dithiolate (mnt2–) are synthesized along with their lanthanide counterparts (La(III) – Nd(III), Sm(III) – Gd(III), Dy(III)), in order to characterize the nature of chemical bonds with these metal ions and a polarizable, non-innocent, sulfur-donor ligand. The metal-sulfur bonds in these complexes trend shorter than measured for lanthanides with equivalent ionic radii. However, particularly large deviations are observed in the neptunium and plutonium complexes in both structure and bonding, resulting in a nonlinear bond length trendline for the actinide series. Density Functional Theory (DFT) calculations with Quantum Theory of Atoms in Molecules (QTAIM) and Natural Bond Order (NBO) analyses indicate that for the neptunium and plutonium complexes, the presence of increased 5f-orbital participation, energy degeneracy of the metal and ligand orbitals, and the structure packing result in shortened M–S bonds. The stabilization of the energy of the 5f-orbitals and the decrease in f-contribution to bonding orbitals in the later actinides results in structural properties more similar to the lanthanide complexes.
Similar content being viewed by others
Introduction
It has been well established that although the trivalent actinides are similar to the lanthanide series in terms of chemistry and bonding, their structures and properties can diverge significantly depending on the compound complexing the ions1,2,3. Hard-donor ligands, such as oxygen containing ligands reinforce the similarities between actinides and lanthanides, while ligands with softer donor atoms like nitrogen or sulfur can result in shortened, more covalent bonds4,5. This is a property that is essential to the separation of actinides from lanthanides and is crucial for nuclear fuel recycling. Many ligands and chelating agents have been designed to exploit this effect and be used for separation purposes2,6. This is typically accomplished by including softer donor atoms in the ligands that preferentially bond to actinide ions, for example, nitrogen and sulfur, as opposed to oxygen. Ligands containing dithiophosphinic groups (such as Cyanex 301) have shown some of the highest separation factors (SF) between Am(III) and Eu(III) with SFs over 100,0002,7. It has been demonstrated in multiple cases that as the donor atoms become softer, such as with sulfur, significant differences between lanthanide and actinide bonding emerge, while for hard donors such as oxygen, little to no difference is observed3,4.
It has additionally been suggested that the actinide series itself may not always show monotonic changes in bonding based on the linear decrease in ionic radii such as in the lanthanide series. Computational studies by Yu et al. have shown that a break likely exists between plutonium and curium, where the Wiberg bond orders and natural f-orbital populations are much greater up to plutonium, after which it decreases8. It is self-evident that such a break would be expected as it is observed in properties such as redox potentials, preferred oxidation states, and even the structural properties of the metals and their temperature and pressure response, however it rarely manifests in any obvious form in coordination complexes9,10. Another break has been suggested to exist around californium, but a paucity of chemical studies of the late actinides makes this challenging to confirm experimentally, although it does find some support in DFT calculations, where increases in Wiberg bond orders and natural f-orbital participation values are observed11,12. In these computational studies, differences across the actinide series were observed to be greatest for soft-donor ligands such as Cl– and dimethyldithiophosphinate.
Since ligands containing soft, polarizable donor atoms are best suited for drawing out differences in the chemistry of the trivalent lanthanides and actinides, we have considered the potential for 1,2-dithiolate ligands to elicit such chemical behavior. 1,2-dithiolates have a rich history among transition metal chemistry, being one of the first instances of non-innocent behavior in which the oxidation state of the metal is not immediately obvious due to the ligand itself also being redox active13,14,15. For example, square planar nickel maleonitrile dithiolate (mnt2–) complexes first synthesized in the 1960s sparked debates over whether nickel was in the +2, +3, or even +4 oxidation state depending on the overall charge of the complex16. It is confirmed experimentally that nickel is formally +2 in all cases, with the electrons being removed from the ligand in the oxidized complexes. This is not, however, always the case, as in analogous complexes of copper where the metal has undergone formal oxidation17. The non-innocence of these ligands results from the delocalization of electron density around the S–C=C–S moiety, where two one-electron oxidation events can occur producing a radical form and a dithioketone form of the ligand that does not result in a drastic structural rearrangement of the ligand, but simply lengthening of the carbon–carbon bond and shortening of the carbon–sulfur bonds13. Complexes of 1,2-dithiolate ligands have displayed various properties of interest such as superconductivity, non-linear optical behavior, and three-dimensional aromaticity18,19,20. They were also found to be naturally occurring in molybdenum and tungsten containing enzymes spurring biochemical interest in their chemistry21,22.
Therefore, ligands containing a 1,2-dithiolate moiety make particularly good candidates for the synthesis of lanthanide and actinide complexes that may exhibit divergent structure and bonding due to the soft-donor sulfur atoms and their delocalized, polarizable electron density1,23. Despite this, examples of such complexes are surprisingly sparse. The earliest instances include work involving the synthesis of uranyl complexes with mnt24,25,26. After a couple decades, Tang et al. reported the synthesis of heteroleptic complexes of La(III), Nd(III), Sm(III), Gd(III), and Er(III) with 1,2-dithiole-2-thione-4,5-dithiolate (dmit2–) and phenanthroline27. Baux et al. also reports the synthesis of a gadolinium tetrathiooxalate complex28. Much of the work of f-element 1,2-dithiolate chemistry comes from Ephritikhine et al., in which they first report heteroleptic uranium complexes of 5,6-dihydro-1,2-dithiin-2,3-dithiolate (dddt2–) and cyclopentadienyl and cyclooctatetrenyl ligands, as well as other complexes with other various 1,2-dithiolate ligands via a reductive method starting with dithiocarbamates29,30,31. They then were able to synthesize various heteroleptic dddt complexes of cerium, neodymium, and uranium as well as homoleptic tris-dddt complexes that were notable for the unusually large folding of the M–(S2C2) metallacycle (greater than 80°)32,33. Later computational studies suggested a bonding interaction between uranium and the carbon double bond in the U(IV) tris-dddt complex34. Weis et al. report a simple synthesis of a homoleptic tetrakis-mnt complex of cerium by the reaction of the easily obtainable sodium salt of mnt with anhydrous CeI3 in tetrahydrofuran35. Most recently, there have been attempts to synthesize heteroleptic and homoleptic complexes of Np(IV) benzene dithiolate (BDT) complexes, the former producing crystalline product that was far too air-sensitive to allow for structural characterization by single-crystal X-ray diffraction36.
In this paper, we extend 1,2-dithiolate chemistry to the trivalent transuranium actinides as well as other lanthanides with the synthesis of tetrakis-mnt complexes using hydrated triiodides in the place of the anhydrous salts, simplifying the procedure. In the actinide series from neptunium to californium (excluding berkelium), these complexes exhibit a non-linear increase in the covalent character of the metal-sulfur bonds when moving toward the beginning of the series that manifests in significantly shorter M–S bond lengths from what would be expected.
Results and discussion
The reaction of hydrated f-element triiodides with Na2mnt undergoes a simple salt metathesis reaction resulting in the formation of a Na5(CH3CN)6[M(mnt)4] complex. Na2mnt is not entirely soluble in the amount of acetonitrile used, however upon reaction with the lanthanide or actinide triiodide, dissolution occurs as the complex is formed after a transitory cloudiness that may be due to the formation of a tris-dithiolate complex. The formation of the complex is not hindered by the coordinating water molecules of the starting triiodide salt due to the chelator effect and lability of water molecules. However, with the chloride salts that would contain coordinating chloride ions, the yield is drastically reduced. The hindrance of chloride ions to the formation of the complex was observed when using NpCl3(THF)4 for the synthesis of Np–β, in which the reaction did not go to completion and the crystalline yield was miniscule. The yield of crystalline product after vapor diffusion is greater than for the reaction reported by Weis et al. who performed the reaction in tetrahydrofuran, which was reported to be less than 10%, the remainder being an amorphous tan powder35. Carrying the reaction out in acetonitrile improves the yield of crystals to greater than 30% in the early lanthanides though some amorphous tan solid still forms. The yield decreases later in the series, approximately 25% for gadolinium and 16% for dysprosium. We predict that this solid is the tris-mnt complex as a greater amount of it forms when three equivalents or less of Na2mnt are used or in reactions with lanthanides past dysprosium that have smaller ionic radii and struggle to coordinate four mnt2– ligands.
Two structure types are observed across the lanthanide series, crystallizing in the C2/c (La–α – Eu–α) or P21/n (Gd–β, Dy–β) space groups, respectively (Fig. 1). In all structures, the metal ion is coordinated by four mnt2– ligands with a distorted D2d coordination geometry (Fig. 2). The ligands exhibit a folding as observed in previously reported 1,2-dithiolate complexes that ranges between 0° and 41° in each complex. All structures consist of a complex three-dimensional network with [M(mnt)4]5– units connected to one another by sodium or potassium ions coordinating between nitrogen and sulfur atoms on the mnt2– ligands, reminiscent of other reported lanthanide 1,2-dithiolate complexes32,33,35. Six acetonitrile molecules fill in voids within the network structure and coordinate around the sodium or potassium atoms in both structures, some of which are disordered over two or more positions. In the C2/c (α) structure, three of the mnt2– ligands of the complex engage in a π-stacking arrangement (Fig. 3) with neighboring complexes forming a corrugated sheet structure similar to the Na5(THF)10[Ce(mnt)4] structure reported by Weis et al., with the remaining ligand pointing perpendicular to the sheet35. The P21/n (β) structure does not have any kind of π-stacking arrangement between the mnt2– ligands and only have sodium or potassium ions connecting each ligand. The mnt2– ligands in all complexes are observed from the C=C and C–S bond lengths (1.37 Å and 1.73 Å, respectively) to only have 1,2-dithiolate character with no shortening or lengthening of bonds that would result from non-innocent behavior.
a C2/c (α) structure and (b) P21/n (β) structure as viewed along the b-axis. Acetonitrile molecules have been omitted for clarity. Teal and purple atoms correspond to Na/K, yellow, gray, and blue atoms correspond to S, C, and N, respectively, and the polyhedra represent the lanthanide and actinide ions.
As the ionic radius of the coordinated metal decreases a transition from the C2/c structure to the P21/n structure occurs at gadolinium. From the PXRD pattern gadolinium appears to crystallize in both forms (Supplementary Fig. S28). Interestingly, this trend is not exactly followed in the actinide series, where neptunium and plutonium crystallize with the P21/n structure, while americium crystallizes with the C2/c structure, and curium crystallizes in both the C2/c and P21/n structures, despite neptunium and plutonium having larger ionic radii than americium and curium37. Additionally, neptunium and plutonium serendipitously crystallized with potassium as the counterion rather than sodium. The potassium was likely introduced from reduction using KC8 for neptunium, while for plutonium it was likely carried over from the plutonium chloride stock solution that had been consolidated from past reactions. The P21/n structure then returns at californium in accordance with the decreasing radii of the actinide ions.
To test if the selective crystallization with potassium resulted in the P21/n structures, CeI3(H2O)n was reacted with four equivalents of Na2mnt and an excess of potassium iodide, which resulted in a structure incorporating both potassium and sodium (as evidenced by the differing alkali-nitrile bond distances) that was unique from both the C2/c and P21/n structures, crystallizing in the P21/c space group and having the formula Na2K3(CH3CN)8.5[Ce(mnt)4]. Whether or not the complex has sodium or potassium counterions has essentially no effect on the metal-ligand bond lengths; the average Ce–S bond length in the C2/c structure is 2.9494(15) Å, while 2.9505(14) Å in the P21/c structure.
The average Ln–S bond distance decreases linearly from lanthanum (2.9729(12) Å) to dysprosium (2.829(2) Å) (Fig. 4). The average Ce–S bond distance in Ce–α is similar to what was reported for Na5(THF)10[Ce(mnt)4] (2.9494(15) Å and 2.957(2) Å, respectively)35. The average Nd–S bond distance in Nd–α is 0.06 Å longer than the homoleptic [Nd(dddt)3]3– complex reported by Roger et al. (2.9169(12) Å vs. 2.8564(19) Å), though this would be expected from the higher coordination number33. The average An–S bonds are overall shorter than those of the lanthanides when plotted against their six-coordinate ionic radii37. This is observed for other soft-donor systems and has been attributed to a combination of the greater amount of orbital overlap between the metal and ligand orbitals as well as a better energy match between the bonding orbitals of the ligand and actinide ion11. The average Am–S, Cm–S (α/β), and Cf–S bond distances (2.8944(9), 2.8843(18)/2.871(3) Å, and 2.8466(15) Å respectively), are similar to the An(S2CNEt2)3(phen) (An = Am, Cm, Cf) complexes reported by Cary et al. (2.874(2) Å, 2.862(2) Å, 2.835(6) Å respectively), though slightly longer, potentially due to the larger size and greater bite angle of the mnt2– ligand versus the dithiocarbamate ligand38. However, the average Cm–S bond length in the homoleptic tetrakis-pyrrolidine dithiocarbamate complex reported by Sperling et al. is 2.8841(14) Å, nearly identical to the average Cm–S bond length in Cm–α and slightly longer than in Cm–β, which suggests the size and geometry of the mnt2– ligand does not necessarily result in a large difference in bonding compared to 1,1-dithiolates39. A homoleptic tetrakis-diethyl-N,N-dithiocarbamate complex of neptunium is also reported by Brown et al. that has an average Np–S bond lengths of 2.87 Å, which is also similar, though shorter than what is measured for complex reported in this work (2.895(2) Å)40. They also report a synthesis of the plutonium analog of this compound but it was not crystallographically characterized.
M–S bond lengths in lanthanide (blue) and actinide (orange) mnt complexes (circles) and their average M–S bond lengths (stars) plotted against their reported six-coordinate ionic radii37. The bond lengths and average bond length for Cm–β are represented by triangles and pentagons, respectively. The line is a linear fit to the lanthanide series.
Unusually, the average Np–S bond is nearly the same as the average Am–S bond length, and the average Pu–S bond length is nearly the same as the average Cm–S bond length in Cm–α and shorter than the average Am–S bond length. This is unexpected, as with other coordination compounds with actinides, a linear decrease in average bond length with respect to ionic radius is observed4,41,42. Additionally, the range of the Np–S bond lengths only slightly overlaps with that of the Ce–S bond lengths despite having six-coordinate ionic radii within error; only the longest Np–S bond is longer than the shortest Ce–S bond. The observed bond lengths in the actinide complexes suggest that there are fundamental differences in bonding for the early actinides compared to the later actinides. This may also be occurring in the dithiocarbamate complexes mentioned above as the reported average Np–S bond lengths are very similar to the average Am–S bond lengths (2.87 Å vs. 2.874(2) Å). It is also observed that from americium to californium, the average An–S bond distances deviate further from the lanthanide trend line, which too occurs with the series of An(S2CNEt2)3(phen) complexes where the americium complex lies closest to the lanthanide trend line while californium shows the most deviation from it.
Additionally, the mnt2– ligands bind to the metals in an asymmetrical fashion such that there is a discrepancy between the two M–S bonds associated with each ligand (Fig. 5). The difference between the two bond lengths ranges between 0.001 and 0.060 Å for most lanthanides, typically averaging around 0.030 Å. Dy–β marks in increase in this bond length discrepancy due to the ionic radius becoming too small to coordinate four ligands due to steric hindrance of the sulfur atoms. The mnt2– in Np–β and Pu–β bond in a far more symmetrical fashion than observed for any of the lanthanides, 0.0125 Å and 0.0167 Å, respectively, while Am–α, Cm–α/β, and Cf–β, follow the lanthanide trend quite closely.
Spectroscopy
Overall, the absorption spectra of the Na5(CH3CN)6[Ln(mnt)4] complexes consist of a high energy ligand-based absorption band that extends to approximately 500 nm (20,000 cm–1) (Figs. S30–S37). The absorption transitions arising from the ligand are visible in the solution spectra obtained for the cerium, europium, and americium complexes (Supplementary Figs. S43 and S44). There are two transitions observed from the ligand around 26,250 cm–1 and 36,300 cm–1, the former resulting from a π → π* transition and the latter a σ → σ* transition of the ligand43.
When intense enough to be observed and not obscured by the ligand absorption, f → f transitions are also visible and are generally red-shifted from their positions when measured in hard-donor environments such as aqueous complexes due to an increased nephelauxetic effect from the mnt2– ligands44. In the spectrum of Ce–α what is likely a metal-ligand charge transfer (MLCT) absorption band is also observed overlapping with the π → π* transition but extending to longer wavelengths imparting a dark red color to the compound. Likewise, a low energy absorption band which is most likely ligand-metal charge transfer (LMCT) in Eu–α is observed around 700 nm which gives the compound a dark green color. These MLCT and LMCT absorption bands are the result of accessible higher oxidation and lower oxidation states, respectively. The mnt2– ligand is a particularly good electron donor and acceptor resulting in the sensitivity of the absorption spectra to the redox chemistry of the metal.
The spectrum of Np–β is dominated by an intense low energy absorption band that covers the entire visible range resulting in the compound being a dark black color, likely due to MLCT given the small III/IV oxidation potential (–0.15 V in acidic aqueous conditions) of neptunium (Fig. 6). Two f → f transitions of Np3+ are visible in the near-IR region of the spectrum corresponding to the 5I4 → 5I6 and 5I4 → 5I7 transitions and two others are seen as shoulders on the MLCT band. The MLCT band in Pu–β only extends slightly further than the ligand absorption band as observed in the trailing of the absorption band into lower energies, but the spectra are dominated by f → f transitions that are much more intense than in the lanthanide spectra. The f → f transitions in the actinide compounds are generally observed to be red–shifted to a greater degree than in the lanthanide compounds. This is particularly noticeable in the spectrum of Pu–β, where the transition around 550 nm is shifted on the scale of 1000 cm–1 from its position in hard-donor oxygen coordination environments such as in mellitate complexes, indicating a particularly strong nephelauxetic effect from coordination with the mnt2– ligands (Supplementary Fig. S45)45. The absorption spectra of both homoleptic tetrakis-diethyl-N,N-dithiocarbamate complexes of neptunium and plutonium are reported by Brown et al. and the f → f transitions appear to be similar in energy to what is observed in the mnt2– complexes, though the shapes of the transitions differ significantly40.
The Am–α spectrum exhibits the intense Group E and H (7F0 → 5L6 and 7F0 → 7F6) transitions observed in most americium spectra46. The most intense f → f transitions of Cm3+ are obscured by the ligand absorption, however the weaker Group A and B transitions are both observed, the latter as a shoulder on the ligand absorption band47. The photoluminescence of curium, which is typically very intense, appears to be nearly entirely quenched by the mnt2– ligands when excited with either 365, 420, or 536 nm light. With long collection times, very weak photoluminescence is observed (Supplementary Fig. S46), however, it is uncertain whether this is from the compound itself or a degradation product containing curium. Quenching of curium’s photoluminescence has also been observed in a (Cpʹ3Cm)2(μ–4,4ʹ–bpy) compound which was attributed to resonance with the C–H bonds of the 4,4ʹ–bpy ligand48. It is probable that the mnt2– ligands behave similarly and allow for efficient non-radiative relaxation pathways. Similar to Np–β and Pu–β, the f → f transitions in Am–α, Cm–α/β and Cf–β are similar in energies to those reported in the An(S2CNEt2)3(phen) complexes by Cary et al. and similar in peak shape38.
In Cf–β, a high-energy absorption band unusually extends to lower energies than the ligand absorption resulting in the compound being a reddish orange. Californium does not have an easily accessible +4 oxidation state and although the +2 oxidation state is achievable, samarium does not exhibit the same effect despite having a similar redox potential. However, a higher degree of covalency in the Cf–S bonds may result in a lowering in energy of the LMCT band due to easier donation of electron density to the ligand. The f → f transitions of californium are also observed to be quite strong despite them typically being very weak in other complexes and the splitting corresponds closely to what is observed in californium halides at liquid helium temperatures, indicating strong ligand-field splitting resolving the various energy levels49.
Detailed plots of the absorption spectra of each compound are in the supplementary information with assignments of the f → f transitions based off of work by Carnall for the actinides and Binnemans for the lanthanides44,46,47,49,50,51.
The vibrational Raman spectra of lanthanide complexes (Figs. S35–S43) were collected and all contain two strong vibrational modes around 1450 cm–1 and 2250 cm–1 that correspond to the symmetric stretching of the C=C bonds and the symmetric and antisymmetric stretching of the nitrile bonds respectively as determined from a simple DFT calculation on the mnt2– ligand. Weaker modes are observed around 500 cm–1 and 1100 cm–1 corresponding to the symmetric stretching of the C–S bonds and rocking of the C=C bond, respectively. Weaker Raman peaks in the spectra correspond to the lattice acetonitrile molecules.
Bonding
To better understand the nature of the actinide-mnt interaction, topological analyses and localized orbitals were calculated using DFT molecular densities (Computational Details). While attempts to use CASSCF densities were made, it became evident that impractical active spaces were needed to properly describe the actinide-mnt interaction. The topology of the molecular electron density was examined using the Quantum Theory of Atoms in Molecules (QTAIM) approach and the orbital analysis was performed using Natural Localized Molecular Orbitals (NLMOs).
Several QTAIM metrics were used to examine the nature of bonding at the bond critical points (BCPs) of the M–S bonds in the complexes including the electron density (ρ), the energy density (H), and the delocalization index (δ)52. For organic molecules, a formally covalent bond will have values of ρ of greater than 1.35 e/Å3, while values less than this are more typically assigned to closed-shell interactions. Generally speaking, larger ρ values are attributed to an increase in covalent contributions. Likewise, a decrease in the total energy density H, which is the sum of the potential and kinetic energy densities, is suggestive of increasing electron sharing. These metrics are usually contrasted with the delocalization index δ that quantifies the amount to which electron pairs are exchanged at the BCP. The values of ρ for all complexes are similar to other homoleptic sulfur-containing actinide complexes with averages between 0.27 and 0.29 e ∙ Å–3. When plotted against their ionic radii, the average ρ at the BCPs is similar across the series and exhibits a dependency on the structure type where the β-structure has greater values of ρ than the α-structure (Fig. 7 and Supplementary Fig. S56) resulting from the shorter M–S bond lengths in the β-structure. Nevertheless, there is a slight decrease in ρ across the series (0.283 e ∙ Å–3 for Np–β, 0.273 e ∙ Å–3 for Am–α). However, Cf–β contains the highest value of ρ in one of its bonds and a range that nearly encompasses the whole series, which is a result of the asymmetrical bonding of each mnt2– ligand. A similar trend is observed for the average H values, which also appears correlated with the structure type where the β-structures have shorter average M–S bond lengths and thus more negative energy densities (Fig. 7 and Supplementary Fig. S56). Again, as for ρ, the values of H become less negative across the series (–113 kJ∙mol–1 ∙ Å–3 for Np–β and –98.6 kJ∙mol–1 ∙ Å–3 for Cm–α). The values of δ, however, do not show a correlation with the structure type, which is particularly evident from both curium structures having nearly the same values. The value of δ is nearly identical for both Np–β and Pu–β (0.387 and 0.386, respectively) and then decreases to its lowest at Cm–α (0.347) before increasing slightly for Cf–β (0.356). When plotted against average M–S bond length, it becomes evident that the bonding for neptunium and plutonium is differing from the rest of the series and that there is increased electron sharing occurring in Np–β and Pu–β (Supplementary Fig. S56). Additionally, the values of H are lower and the delocalization indices are higher than in similar dithiocarbamate and dithiophosphate complexes. Overall, the QTAIM metrics suggest a greater covalent component for the earlier members of the actinide series, which may be resulting from a combination of packing structure as well as a more fundamental difference in the bonding of neptunium and plutonium versus the trans-plutonium actinides.
From the NLMOs perspective, the expected bonding interactions are observed where the primary orbitals responsible for the metal-ligand bonds are predominately composed of a hybrid p/s-orbital representing the sulfur lone pair electrons. Bonding mainly occurs by donation from the p-orbital oriented parallel to the bond while the p-orbitals oriented perpendicular to the plane of the ligand provides a minor contribution and the remaining p-orbital a negligible contribution (Fig. 8). The most significant bonding NLMOs have a metal contribution ranging between 7% to 13%. The Wiberg bond indices (WBIs) are greatest in the neptunium complex, averaging 0.555 for all M–S bonds, a value that indicates an increased covalent contribution. The WBI decreases to its lowest at curium at 0.462 (0.466 for the β-structure,) but increases slightly at californium to 0.474 (Fig. 9b). Figure 9a shows the average percent metal contribution to the eight bonding NLMOs for each metal. The highest metal contributions to bonding are seen for neptunium, averaging 11.1% and ranging between 8.4% and 13.4%. The metal contribution decreases with increasing atomic number up to curium which has the lowest average metal contribution of 9.25% (ranging between 6.80% and 11.8%) in the α-structure and 10.1% (ranging between 6.73% and 12.9%) in the β-structure. The metal contribution slightly increases in Cm–β due to the shorter average Cm–S bond length. The metal contribution then increases again for californium (10.1%). Figure 9c captures the breakdown of this metal contribution. The metal orbitals involved in bonding are the 6 d, 7 s, and 5 f, with a negligible 6p contribution. The 6 d and 7 s contribution remains relatively constant across the series with a slight decrease around americium and curium. The 5 f contribution decreases from neptunium (1.87% average) relatively linearly to curium (0.39% and 0.41% for the α and β-structures, respectively) and subsequently increases again with californium (0.60% averaged). These results reflect the behavior observed in the computational examination of Cyanex 301 and other ligand systems of the trivalent actinides by Yu et al. where the 5f-orbital participation significantly decreases toward the middle of the actinide series but slightly increases again further in the series8.
In addition, the 5f-orbitals are energetically degenerate with the sulfur p-orbitals from neptunium to americium, while for curium and californium they are deeper in energy and degenerate with the delocalized π-orbitals of the mnt2– ligand (Fig. 10). This indicates that there could also be an energy degeneracy driven enhancement to the covalency of the M–S bonds in Np–β and Pu–β53.
These results suggest that the participation of the 5f-orbitals early in the actinide series leads to significant M–S bond shortening and bond stabilization in Np–β and Pu–β. The reasoning would be that as electron repulsion is overcome by the bonding interaction the electrons subsequently become spatially delocalized and electron density can then accumulate between the bonds (as observed in the QTAIM metrics). Because of the attractive interaction between the atomic nuclei and this electron density, the bond is shortened from the sum of the ionic radii of the two atoms. Additionally, this produces a stronger bonding interaction and more stable bond as observed in the values of H. In the case of Np–β and Pu–β, the shortened M–S bond lengths also likely cause the adoption of the β-structure, which further enforces shorter bonds due to the crystal packing.
As the 5f-orbital becomes more localized, the electron density between the bonds is reduced and bonding becomes more ionic in nature (as suggested by the WBIs) and thereby more dependent on the ionic radii of the metal, which causes the increase in average bond length between plutonium and americium. The small increase in electron density and metal contribution in the Cf–S bonds of Cf–β may be a result of increased polarization of the sulfur orbitals by the harder, smaller californium cation, consequently resulting in greater participation of the valence orbitals.
The increased strength of the metal-ligand bonds in Np–β and Pu–β due to participation of the 5f-orbitals is also observed in the discrepancy of M–S bond lengths in each mnt2– ligand (vide supra). For both neptunium and plutonium, the mnt2– ligands bond more symmetrically to the metal cations compared to the lanthanide series, while americium, curium, and californium follow the lanthanide series quite closely. For californium, this discrepancy in bond length is highest (averaging 0.036 Ǻ) due to its smaller ionic radius and the steric bulk of coordinating four mnt2– ligands and also results in a wide range in the character of its bonds. For example, the bonds with the highest metal contributions and electron densities are similar to the highest in Np–β and Pu–β, while the lowest are similar to those observed in Am–α and Cm–α.
Summary
In the vast majority of series of compounds of lanthanides, the structure and bonding are driven primarily by the ionic radii of the lanthanide cations, with the 4f-orbitals having only a minimal effect. The trivalent actinides often exhibit small deviations in structure in comparison to their lanthanide counterparts in the same series of compounds, due to increased overlap of their valence orbitals with ligand orbitals. These differences in structure are highly ligand dependent with larger differences observed for ligands that are more polarizable, but overall ionic radius is still a major driving force behind the structure actinide compounds adopt. Complexes of trivalent actinides with maleonitrile-1,2-dithiolate appear to engage the 5f-orbitals to such a degree that their role in bonding has substantial effects in the resulting structures. Neptunium and plutonium have metal-ligand bond lengths and structure types more akin to smaller actinide ions later in the series. Through QTAIM and NBO calculations, this can be attributed to a combination of the increased covalent contribution of the 5f-orbitals, energy degeneracy of the bonding orbitals, and the packing structure. Additionally, the participation of the 5f-orbitals does not continuously decrease along the actinide series, but californium potentially marks a reversal of the trend and exhibits a covalent character to its bonding that is comparable to or greater than observed in curium. In all, these compounds highlight the importance of the f-orbitals in actinide bonding and that when paired with charged ligands with diffuse, polarizable electron density, their chemistry cannot be completely described by simple electrostatic interactions.
Methods
Caution! Neptunium-237 (t1/2 = 2,144,000 yr), Plutonium-239 (t1/2 = 24,110 yr), Americium-243 (t1/2 = 7364 yr), Curium-248 (t1/2 = 348,000 yr), and Californium-249 (t1/2 = 351 yr) are all α-emitters with concomitant γ-emissions, particularly for Cf-249 (γ = 388.15 keV), that pose a serious external and internal radiologic hazard. All reactions were performed in a class–II nuclear hazard facility in fume hoods and gloveboxes with HEPA filters. Lead bricks and vests were used to shield researchers and exposure to radioactive isotopes were kept as low as possible.
Materials
Acetonitrile, diethyl ether, tetrahydrofuran (THF), and dimethoxyethane (DME) were purchased from Sigma Aldrich (reagent grade ≥ 99.8%, ≥ 99.0%, ≥ 99.9%, ≥ 99.5%, respectively) and were dried by distillation over calcium hydride (acetonitrile) or sodium metal (diethyl ether, THF, and DME) for 24 h. Sodium maleonitriledithiolate hydrate (>95.0%) was purchased from Sigma Aldrich and was dried by dissolving in methanol and allowing to sit over molecular sieves for several days in an argon-filled inert glove box then filtering and evaporating down the solution. Ammonium hydroxide (30.33%), hydroiodic acid (no stabilizer, 57 wt% in H2O) were purchased from Sigma Aldrich and used as is. KC8 was prepared by the combination of potassium metal and eight molar equivalents of graphite with heating in a glovebox.
General procedure
M-α/β (Na 5 (CH 3 CN) 6 [M(mnt) 4 ]) (M = La–Nd, Sm–Gd, Dy; Am, Cm, Cf)
M(H2O)nI3 was synthesized by dissolving M2(CO3)3 with a minimal amount of hydroiodic acid. Once dissolved, the solution was dried under a stream of N2. The solid was then washed with diethyl ether until the residual iodine was completely removed. The clean solid was dried under N2 and then brought into a glovebox. 10 mg of M(H2O)nI3 was dissolved in 1–2 mL of acetonitrile and four molar equivalents of Na2mnt was added. The solution initially becomes cloudy when stirred but then clears. Some excess Na2mnt typically remains undissolved. The solution is then filtered and set up for vapor diffusion overnight with diethyl ether. Crystals of Na5(CH3CN)6[M(mnt)4] form with the vapor diffusion along with a small amount of amorphous powder which may be the tris–mnt complex. The crystals were isolated by removing the supernatant and washing with diethyl ether, suspending the amorphous powder into the ether. The yield of crystalline product is typically around 30%, but decreases past gadolinium. Two different structure types occur for the crystallized products, one having the C2/c space group and the other having the P21/n space group, referred to as the α and β structure types, respectively.
For the actinides (An3+ = Am3+, Cm3+, Cf3+), the An(H2O)nI3 starting material was synthesized by dissolving An(OH)3 in hydroiodic acid, that was obtained by precipitation of the hydroxide from an actinide stock solution in hydrochloric acid with ammonium hydroxide and washing with water three times. For the reaction with curium, both structure types were obtained.
Np–β (K5(CH3CN)6[Np(mnt)4])
Due to the instability of Np3+ in aqueous solutions when exposed to oxygen, NpCl3(THF)4 was produced by the reduction of NpCl4(dme)2 with KC8 in THF in a glovebox following a peviously published procedure54. The resulting solution was filtered and the THF was removed by vacuum. Acetonitrile was used to attempt to dissolve the residue, but the NpCl3(THF)4 appeared to be poorly soluble. Four molar equivalents of Na2mnt were added and the reaction was stirred for a few hours. Due to poor solubility of both reagents, the solution never cleared. The solution was filtered and set up for vapor diffusion with diethyl ether and produced a very small yield of bluish-black crystals of Np–β along with a much larger yield of residual ligand. The product also selectively crystallized with potassium likely due to the introduction of potassium ions by KC8.
Pu–β (K5(CH3CN)6[Pu(mnt)4])
The reaction for Pu–β was set up in the same way as for Am, Cm, and Cf. No special procedure was used to produce Pu(III) or prevent it from oxidizing as plutonium autoreduces to the +3 oxidation state when the hydroxide is dissolved in HI and is stable for the time frame of the experiment. However, vapor diffusion of diethyl ether did not initially produce crystals but resulted in an oil. The reaction was attempted a second time using less material as concentrated solutions had a tendency to produce oils. This attempt, however, also did not produce crystals. The solution and diethyl ether were then consolidated into a single vial and the vial was left to stand for two days, after which crystals of Pu–β serendipitously formed. The structure also selectively crystallized with potassium ions that are most likely carried over from the plutonium stock solution.
Crystallography
Single-crystals of each compound were isolated under Parabar immersion oil and placed on 75 μm MiTeGen loop. Single-crystal X-ray crystallography was performed on a Bruker D8 Quest diffractometer at 100 K under a stream of N2. The unit cell and collection strategy determination, integration, scaling, and space group determination were performed using APEX4 software. Structure solution and refinement was done using ShelXT and ShelXL on the Olex2 v1.5 GUI55,56,57. The powder X-ray diffraction patterns were collected on a Bruker D2 Phaser between 2θ angles of 5° and 50°.
UV-Vis-NIR absorption and photoluminescence spectroscopy
Single crystals were isolated on a microscope slide under Parabar immersion oil. The solid-state absorption spectra of the crystals were collected using a CRAIC Technologies 20/20 PV microspectrophotometer along with the photoluminescence spectrum of Cm–α/β. Solution spectra were collected of the complex dissolved in acetonitrile on an Agilent Technologies Cary Series 6000i UV-Vis-NIR spectrometer.
Raman spectroscopy
Dried crystals of the lanthanide compounds were sandwiched between two glass slides and the Raman spectra were collected using a Bruker Senterra II Raman spectrometer using a 785 nm laser.
Computational methods
All calculations were performed using the Amsterdam Modeling Suite molecular ADF program version 2024.10558,59. The DFT wavefunctions were calculated for each (K/Na)5[An(mnt)4] complex using the hybrid generalized gradient approximation (GGA) Perdew-Burke-Ernzerhof (PBE0) functional with the triple-zeta potential (TZP) basis set. Scalar relativistic effects were incorporated using the zeroth-order relativistic approximation (ZORA). Calculations were performed on geometries optimized from the coordinates obtained from the single-crystal X-ray structures. The PBE/TZP level of theory was used for the geometry optimizations keeping the Na/K atoms within 10 angstroms frozen to simulate the constraints imposed by the crystal packing. A topological analysis of the resulting wavefunctions was performed via Bader’s Quantum Theory of Atoms in Molecules (QTAIM) in the ADF program60. Natural Bond Order (NBO) analyses were also performed and the natural localized molecular orbitals (NLMOs) were calculated with the NBO software in the ADF program.
Data availability
Source data are provided with this manuscript. The UV-vis-NIR, Raman, PXRD, and computational coordinates data generated in this study have been deposited in the figshare database under accession code https://doi.org/10.6084/m9.figshare.29420633. Supplementary crystallographic, spectral, and computational data generated in this study are provided in the Supplementary Information file.
The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Center (CCDC), under deposition numbers 2423135–2423148. These data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif. All data are available from the corresponding author upon request. Source data are provided with this paper.
References
Gaunt, A. J. et al. Experimental and theoretical comparison of actinide and lanthanide bonding in M[N(EPR2)2]3 complexes (M = U, Pu, La, Ce; E = S, Se, Te; R = Ph, iPr, H). Inorg. Chem. 47, 29–41 (2008).
Bessen, N. P., Jackson, J. A., Jensen, M. P. & Shafer, J. C. Sulfur donating extractants for the separation of trivalent actinides and lanthanides. Coord. Chem. Rev. 421, 213446 (2020).
Neidig, M. L., Clark, D. L. & Martin, R. L. Covalency in f-element complexes. Coord. Chem. Rev. 257, 394–406 (2013).
Beck, N. B. et al. Two neptunium(III) mellitate coordination polymers: completing the series Np–Cf of trans-uranic An(III) mellitates. Inorg. Chem. 61, 17730–17737 (2022).
Guillaumont, D. Actinide(III) and lanthanide(III) complexes with nitrogen ligands: counterions and ligand substituent effects on the metal–ligand bond. J. Mol. Struct. THEOCHEM 771, 105–110 (2006).
Bhattacharyya, A. & Mohapatra, P. K. Separation of trivalent actinides and lanthanides using various ‘N’, ‘S’ and mixed ‘N,O’ donor ligands: a review. Radiochim. Acta 107, 931–949 (2019).
Klaehn, J. R. et al. Synthesis of symmetric dithiophosphinic acids for “minor actinide” extraction. Inorg. Chim. Acta 361, 2522–2532 (2008).
Yu, X., Sergentu, D. C., Feng, R. & Autschbach, J. Covalency of trivalent actinide ions with different donor ligands: do density functional and multiconfigurational wavefunction calculations corroborate the observed “breaks”?. Inorg. Chem. 60, 17744–17757 (2021).
Benedict, U., Holzapfel, W. B. Chapter 113 high-pressure studies — structural aspects. In Handbook on the Physics and Chemistry of Rare Earths 17, 245–300 (Elsevier, 1993).
Morss, L. R. Chapter 122 Comparative thermochemical and oxidation-reduction properties of lanthanides and actinides. In Handbook on the Physics and Chemistry of Rare Earths 18. 239–291 (Elsevier, 1994).
Kelley, M. P. et al. On the origin of covalent bonding in heavy actinides. J. Am. Chem. Soc. 139, 9901–9908 (2017).
Cary, S. K. et al. Emergence of californium as the second transitional element in the actinide series. Nat. Commun. 6, 6827 (2015).
Eisenberg, R. & Gray, H. B. Noninnocence in metal complexes: a dithiolene dawn. Inorg. Chem. 50, 9741–9751 (2011).
Schrauzer, G. N. & Mayweg, V. Reaction of diphenylacetylene with nickel sulfides. J. Am. Chem. Soc. 84, 3221–3221 (1962).
Gray, H. B., Williams, R., Bernal, I. & Billig, E. A spin-free square planar cobaltous complex. J. Am. Chem. Soc. 84, 3596–3597 (1962).
Stiefel, E. I., Waters, J. H., Billig, E. & Gray, H. B. The myth of nickel(III) and nickel(IV) in planar complexes. J. Am. Chem. Soc. 87, 3016–3017 (1965).
Chuang, Y. C., Sheu, C. F., Lee, G. H., Chen, Y. S. & Wang, Y. Charge density studies of 3d metal (Ni/Cu) complexes with a non-innocent ligand. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 73, 634–642 (2017).
Kwik, W. L. & Stiefel, E. I. Single-crystal electron paramagnetic resonance study of a trigonal vanadium tris(dithiolate) complex. Inorg. Chem. 12, 2337–2342 (1973).
Cummings, S. D., Cheng, L. T. & Eisenberg, R. Metalloorganic compounds for nonlinear optics: molecular hyperpolarizabilities of m(diimine)(dithiolate) complexes (M = Pt, Pd, Ni). Chem. Mater. 9, 440–450 (1997).
Cassoux, P. Molecular (super)conductors derived from bis-dithiolate metal complexes. Coord. Chem. Rev. 185, 213–232 (1999).
Joshi, H. K., Cooney, J. A., Inscore, F. E. & Enemark, J. H. Investigation of metal–dithiolate fold angle effects: implications for molybdenum and tungsten enzymes. Proc. Natl. Acad. Sci. USA 100, 3719–3724 (2003).
Westcott, B. L., Gruhn, N. E. & Enemark, J. H. Evaluation of molybdenum−sulfur interactions in molybdoenzyme model complexes by gas-phase photoelectron spectroscopy. The “electronic buffer” effect. J. Am. Chem. Soc. 120, 3382–3386 (1998).
Eisenberg, R. Trigonal prismatic coordination in tris(dithiolene) complexes: guilty or just non-innocent?. Coord. Chem. Rev. 255, 825–836 (2011).
Zimmer, L. & Lieser, K. H. Isomere uranyl-chelate mit dithiolat-liganden. Inorg. Nucl. Chem. Lett. 7, 563–567 (1971).
Zimmer, L. & Lieser, K. H. Uranyl-bis(dithiolat)-komplexe mit pyridin-n-oxid, triphenylphosphinoxid und triphenylarsinoxid als liganden. Inorg. Nucl. Chem. Lett. 7, 1163–1168 (1971).
Dietzsch, W. & Hoyer, E. Dithiolenchelate des urans mit Cis-1,2-dicyanoathylen-1,2-dithiolat. Inorg. Nucl. Chem. Lett. 5, 635–638 (1969).
Tang, Y., Gan, X., Tan, M. & Zheng, X. Synthesis and characterization of mixed-ligand 1,3-dithiole-2-thione-4,5-dithiolate and 1,10- phenanthroline complexes of lanthanide chloride and their iodinated materials. Polyhedron 17, 429–432 (1998).
Baux, C. et al. New sulfur rich lanthanide based materials: synthesis and magnetic properties. J. Alloy. Compd. 344, 114–119 (2002).
Arliguie, T., Fourmigué, M. & Ephritikhine, M. The first dithiolene complexes of an f-element, including the cyclooctatetraene derivative [Na(18-Crown-6)(THF)][U(η8-C8H8)(C4H4S4)2], a unique example of a uranium(V) compound with metal−sulfur bonds. Organometallics 19, 109–111 (2000).
Arliguie, T., Thuéry, P., Fourmigué, M. & Ephritikhine, M. Reduction of dithiocarbonates as a novel route to dithiolene compounds of uranium. crystal structure of the first bimetallic dithiolene complex of an f-element. Organometallics 22, 3000–3003 (2003).
Arliguie, T., Thuéry, P., Fourmigué, M. & Ephritikhine, M. Monocyclooctatetraenyl(dithiolene)uranium compounds: monocyclooctatetraenyl(dithiolene)uranium compounds. Eur. J. Inorg. Chem. 2004, 4502–4509 (2004).
Roger, M., Arliguie, T., Thuéry, P., Fourmigué, M. & Ephritikhine, M. Homoleptic tris(dithiolene) and tetrakis(dithiolene) complexes of uranium(IV). Inorg. Chem. 44, 594–600 (2005).
Roger, M., Arliguie, T., Thuéry, P., Fourmigué, M. & Ephritikhine, M. Tris(dithiolene) complexes of neodymium and cerium: mononuclear species, chains, and honeycomb networks. Inorg. Chem. 44, 584–593 (2005).
Meskaldji, S. et al. Density functional theory investigations of the homoleptic tris(dithiolene) complexes [M(dddt)3]−q (q = 3, 2; M = Nd3+ and U3+/4+) related to lanthanide(III)/actinide(III) differentiation. Inorg. Chem. 49, 3192–3200 (2010).
Weis, E. M., Barnes, C. L. & Duval, P. B. Na5(THF)10Ce(Mnt)4]∞: a honeycomb network polymer that yields homoleptic cerium(III) tetrakis(dithiolene) complexes in donor solvents. Inorg. Chem. 45, 10126–10130 (2006).
Hall, G. et al. Probing F-Orbital Covalency through the Fold Angles of Transuranium Dithiolene Complexes PNNL-33295 (Pacific Northwest National Laboratory, 2022).
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 32, 751–767 (1976).
Cary, S. K. et al. A series of dithiocarbamates for americium, curium, and californium. Dalton Trans. 47, 14452–14461 (2018).
Joseph M. et al. Compression of curium pyrrolidine-dithiocarbamate enhances covalency. Nature 583, 396–399 (2020).
Brown, D., Holah, D. G. & Rickard, C. E. F. N,N-diethyldithiocarbamate complexes of trivalent lanthanide and actinide elements and the crystal structure of tetraethylammonium neptunium(III) tetrakis-(N,N-diethyldithiocarbamate). J. Chem. Soc. A. 0, 786–790 (1970).
Pennenvan, R. A., Ryan, R. R. & Rosenzweig, A. Structural systematics in actinide fluoride complexes. In Structure and Bonding 13: Rare Earths. 1–52 (Springer, 1973).
Apostolidis, C. et al. Tris-{hydridotris(1-pyrazolyl)Borato}actinide complexes: synthesis, spectroscopy, crystal structure, bonding properties and magnetic behaviour. Chem. Eur. J. 26, 11293–11306 (2020).
Shupack, S. I., Billig, E., Clark, R. J. H., Williams, R. & Gray, H. B. The electronic structures of square-planar metal complexes. V. Spectral properties of the maleonitriledithiolate complexes of nickel, palladium, and platinum. J. Am. Chem. Soc. 86, 4594–4602 (1964).
Binnemans, K. & Görller-Walrand, C. On the color of the trivalent lanthanide ions. Chem. Phys. Lett. 235, 163–174 (1995).
Sperling, J. M. et al. Structural and spectroscopic investigation of two plutonium mellitates. Inorg. Chem. 59, 3085–3090 (2020).
Pappalardo, R. G., Carnall, W. T. & Fields, P. R. Low-temperature optical absorption of americium halides. J. Chem. Phys. 51, 1182–1200 (1969).
Carnall, W. T. & Rajnak, K. Electronic energy level and intensity correlations in the spectra of the trivalent actinide aquo ions. II. Cm3+. J. Chem. Phys. 63, 3510–3514 (1975).
Long, B. N. et al. Altering the spectroscopy, electronic structure, and bonding of organometallic curium(III) upon coordination of 4,4′−bipyridine. Nat. Commun. 14, 3774 (2023).
Carnall, W. T., Fried, S. & Wagner, F. Absorption spectrum of CfCl3. J. Chem. Phys. 58, 1938–1949 (1973).
Carnall, W. T. et al. Energy level analysis of Np3+:LaCl3 and Np3+:LaBr3. J. Chem. Phys. 72, 5089–5102 (1980).
Carnall, W. T., Fields, P. R. & Pappalardo, R. G. Absorption spectrum of PuCl3. J. Chem. Phys. 53, 2922–2938 (1970).
Huang, Q. R., Kingham, J. R. & Kaltsoyannis, N. The strength of actinide–element bonds from the quantum theory of atoms-in-molecules. Dalton Trans. 44, 2554–2566 (2015).
Su, J. et al. Energy-degeneracy-driven covalency in actinide bonding. J. Am. Chem. Soc. 140, 17977–17984 (2018).
Whitefoot, M. A., Perales, D., Zeller, M. & Bart, S. C. Synthesis of non-aqueous neptunium(III) halide solvates from NpO2. Chem. Eur. J. 27, 18054–18057 (2021).
Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. C. Struct. Chem. 71, 3–8 (2015).
Sheldrick, G. M. SHELXT – Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 71, 3–8 (2015).
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Cryst. 42, 339–341 (2009).
te Velde, G. et al. Chemistry with ADF. J. Comp. Chem. 22, 931–967 (2001).
ADF 2024.1, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands, URL: http://www.scm.com, accessed May, 2024.
Bader, R. F. W. Atoms in molecules: a quantum theory. in International Series of Monographs on Chemistry (Oxford University Press, 1994).
Acknowledgements
All experiments were supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, Heavy Elements Chemistry Program under award number DE-SC0023693. We thank Oak Ridge National Laboratory for supplying all the radioisotopes used in the study.
Author information
Authors and Affiliations
Contributions
N.B.B. carried out the bulk of the development and completion of the experiments and characterization and the writing of the manuscript. C.C.B. and M.C.M. performed the computational study and provided much feedback regarding theory. J.M.S. provided feedback throughout the project and aided in the purifying of radioisotopes used in the experiments as well as running experiments with radioisotopes. Z.B., J.P.B., D.G.M., Z.K.H., B.N.L., B.M.R., and T.N.P. aided in the purifying of radioisotopes used in the experiments as well as running experiments with radioisotopes. K.N.M. aided in the collection of PXRD spectra of the complexes. R.W.M. aided in preparing lanthanide samples for further characterization. T.E.A. was the principal investigator for the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Chao Xu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Beck, N.B., Celis-Barros, C., Martelles, M.C. et al. Non–linear bonding trends in maleonitrile-1,2–dithiolate complexes of the transuranium actinides. Nat Commun 16, 7759 (2025). https://doi.org/10.1038/s41467-025-63129-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-63129-3