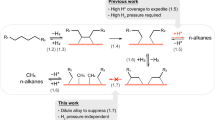
Abstract
Hydrogenolysis has emerged as a promising strategy for the chemical recycling of plastic waste, yet its reliance on high-pressure hydrogen poses significant challenges. Biomass- or CO2-derived formic acid (FA) is a renewable hydrogen carrier with the advantages of low toxicity and ease of storage and transport. Here, we use FA to replace high-pressure hydrogen to convert polyethylene (PE) into fuels and chemicals with only 4.1% gaseous products by a RuPt alloy catalyst. We demonstrate that the trace amounts of CO generated from the decomposition of FA do not poison the active sites, but rather induce the formation of Ruδ+, which facilitates the C–C bond cleavage during PE hydrogenolysis. This approach eliminates the need for high-pressure hydrogen and provides a more flexible and adaptable approach for decentralized plastic processing.
Similar content being viewed by others
Introduction
The escalating crisis of plastic pollution has become one of the most pressing environmental challenges due to the relentless accumulation of non-biodegradable plastics across terrestrial and marine ecosystems1,2,3. Polyethylene (PE), which accounts for approximately one-third of global plastic production, presents particularly persistent environmental hazards due to its high chemical stability and inherent hydrophobicity4,5,6. Current end-of-life management strategies for PE predominantly rely on landfilling and incineration, both of which carry substantial environmental burdens. Landfilling not only occupies valuable land resources but also poses long-term risks of soil pollution and groundwater contamination. Incineration releases CO2 and potentially toxic pollutants into the atmosphere, although it offers partial energy recovery. These critical limitations underscore the urgent need to develop advanced recycling technologies that can effectively transform plastic waste into valuable resources, thereby mitigating environmental impacts and advancing a sustainable circular economy7,8,9,10,11.
Catalytic hydrogenolysis has emerged as an attractive recycling method, as it breaks down the C–C bonds in PE at moderate temperatures (200–300 °C) and yields valuable hydrocarbons (40–90%) that can be repurposed as fuels or chemical feedstocks12,13,14,15. Among various catalytic systems, Ru-based and Pt-based catalysts have demonstrated excellent activity and selectivity in polyolefin hydrogenolysis16,17,18,19,20,21,22,23,24,25. However, the hydrogenolysis processes heavily rely on high-pressure H2 (2–6 MPa)23,26,27,28. Because H2 has a relatively low volumetric density and is highly flammable, the large-scale use of high-pressure hydrogen poses challenges related to infrastructure and requires rigorous safety protocols to prevent leakage and explosions29. Furthermore, if hydrogen is not produced on-site, its storage and transportation bring additional logistical hurdles30,31. These limitations significantly constrain the scalability and adaptability of hydrogenolysis as a versatile plastic recycling technology, particularly for decentralized or small-scale applications.
Liquid organic hydrogen carriers (LOHCs) is a class of organic compounds that remain liquid under ambient conditions while enabling reversible hydrogen storage and release through catalytic hydrogenation-dehydrogenation cycles32. A recent report indicates that, using methanol as a hydrogen donor, polystyrene can be depolymerized into alkylbenzenes over a Ru/SiO2 catalyst33. However, the considerable CO production accompanying methanol decomposition may accelerate catalyst deactivation over extended reaction periods34. Aqueous-phase reforming (APR) of methanol can effectively prevent this issue, and it has shown that almost no CO formation is detected during PE hydrogenolysis via in situ hydrogen generation through methanol APR under subcritical conditions35. Nevertheless, subcritical operation typically involves high temperature and pressure, which impose significant engineering and safety challenges. Therefore, it is necessary to explore and develop other LOHC-based hydrogenolysis systems that can enable sustainable plastic upcycling while addressing the technical and safety constraints of current approaches.
Formic acid (FA) is considered one of the most promising hydrogen storage materials36,37, due to its high volumetric capacity (53 g H2 L−1), minimal toxicity, and the ease of storage and transport. The growing interest in FA is further bolstered by recent advancements in its sustainable production from CO2 and diverse biomass sources, positioning it as a renewable hydrogen carrier38,39,40,41. FA has been extensively studied as an alternative hydrogen donor for biomass upgrading and lignin depolymerization42,43. While FA can generate hydrogen through the desirable dehydrogenation pathway (HCOOH → CO2 + H2), its parallel dehydration pathway (HCOOH → CO + H2O) presents a significant challenge by producing CO, a known catalyst poison that not only deactivates noble metal catalysts but also compromises hydrogen utilization efficiency. Therefore, to fully harness FA’s potential as an in situ hydrogen source for PE hydrogenolysis, it is essential to develop CO-tolerant catalysts with high activity and selectivity.
Herein, we report a CO-promoted PE hydrogenolysis using FA as a sustainable alternative to high-pressure H2 (Fig. 1). We demonstrate that, on a RuPt alloy catalyst, FA primarily undergoes dehydrogenation to supply H* required for hydrogenolysis. Meanwhile, the RuPt alloy catalyst exhibits excellent CO tolerance when the CO/H2 ratio is less than 20% (V/V). More importantly, we find that using FA enables faster C–C bond cleavage than when employing H2. Mechanistic studies indicate that trace amounts of CO formed during FA decomposition can adsorb onto the RuPt alloy surface, inducing the formation of Ruδ+ species that accelerate the C–C bond scission. We also demonstrate that this approach can convert real-life mixed polyolefin waste into liquid fuels and wax/lubricant base-oils with less than 4.2% gaseous products, validating the practical feasibility of this sustainable plastic upcycling strategy.
Results
Catalyst preparation and characterizations
The RuPt catalyst was prepared according to a previously reported method with slight modifications (see “Methods”). Inductively coupled plasma optical emission spectroscopy (ICP-OES) indicated that the molar ratio of Ru/Pt in the synthesized catalyst was approximately 1:3. Transmission electron microscopy (TEM) analysis revealed that the RuPt nanoparticles were uniformly dispersed, with an average diameter of approximately 2.6 ± 0.3 nm (Fig. 2a). The RuPt nanoparticles were well dispersed on a carbon support (Vulcan XC72) (Fig. 2b). For comparison, we also synthesized Ru/C and Pt/C catalysts (Supplementary Fig. 1). High-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) image of a representative RuPt nanoparticle revealed continuous lattice fringes (Fig. 2c), indicative of the single-crystalline nature of the particle. Energy-dispersive X-ray spectroscopy (EDS) elemental mapping demonstrated that Pt and Ru were evenly distributed within the nanoparticle (Fig. 2d). The X-ray diffraction (XRD) patterns of the RuPt nanoparticles displayed characteristic diffraction peaks of a face-centered cubic (fcc) structure (Supplementary Fig. 2), corresponding to the (111), (200), (220), and (311) crystal planes.
a TEM image of the synthesized RuPt nanoparticles (scale bar, 50 nm). The inset shows the diameter histogram. b TEM image of the supported catalysts (scale bar, 50 nm). c HAADF-STEM image of a representative RuPt nanoparticle (scale bar, 1 nm). d EDS mapping of a representative RuPt nanoparticle (scale bar, 1 nm). e, f Fourier transforms of EXAFS spectra of Pt L3-edge (e) and Ru K-edge (f). g, h XPS spectra of Ru 3p (g) and Pt 4f (h) for RuPt nanoparticles before and after reduction.
The RuPt alloy structure was further elucidated by extended X-ray absorption fine structure (EXAFS) measurement. Figure 2e presents the Fourier transform of EXAFS at the Pt L3-edge, where the prominent peak at 2.46 Å—also observed in RuPt alloy—can be attributed to both Pt–Pt and Pt–Ru scattering paths, as corroborated by wavelet-transformed EXAFS (WT-EXAFS) (Supplementary Fig. 3). Fitting of the RuPt EXAFS data revealed a Pt–Ru coordination number (CN) of 2.0 (Supplementary Table 1), indicating the Ru is evenly distributed in Pt nanoparticles. In the k2-weighted Fourier transform of the EXAFS spectra at the Ru K-edge (Fig. 2f) and its complementary wavelet-transform EXAFS analysis (Supplementary Fig. 4), the feature at ~1.54 Å is attributed to Ru–O scattering, whereas the peak at ~2.45 Å arises from overlapping Ru–Pt and Ru–Ru scattering contributions. Quantitative EXAFS fitting (Supplementary Table 2) suggests an average coordination of 6.1 Pt atoms and 2.1 Ru atoms per Ru center, consistent with the formation of a Ru1Pt3 alloy. Furthermore, quasi in situ X-ray photoelectron spectroscopy (XPS) under reaction-relevant conditions confirmed that both Ru and Pt species remained in the metallic state after reduction (Fig. 2g, h).
FA as hydrogen donor for hydrogenolysis of PE
We began with a comprehensive evaluation of several different liquid hydrogen carriers (i.e., FA, methanol, ethanol, and isopropanol) for the PE hydrogenolysis (Fig. 3a). Specifically, 20 mg of RuPt alloy catalyst was mixed with 340 mg of PE (MW = 4000 g mol−1) in a 25 mL batch autoclave, supplemented with each hydrogen carrier (see “Methods”). Stoichiometric decomposition of the hydrogen carriers would yield hydrogen quantitatively corresponding to 0.5 bar of molecular H2 in the reaction system. In a control experiment where no hydrogen donor was added, the PE conversion was negligible, ruling out thermal pyrolysis at the reaction temperature. Remarkably, complete conversion of all solid was achieved when FA was used (Supplementary Table 3). The detailed carbon distribution of the product is shown in Supplementary Figs. 5 and 6. The main products were linear long-chain alkanes (C5–C40), with a gas product (C1–C4) selectivity of 4.1% (including 2.9% CH4). In contrast, methanol and ethanol exhibited limited efficacy, converting only 23.2% and 19.5% of PE, respectively (Supplementary Table 3). This poor performance correlated with substantial CO generation during their decomposition (Supplementary Fig. 7), implying catalyst poisoning by in situ generated CO. The decomposition of isopropanol generated less CO; however, the formation of acetone could occupy the active sites, hindering the progression of the reaction (Supplementary Fig. 7)44. FA’s exceptional performance stems from its clean decomposition profile, primarily generating H2 and CO2 with only trace CO (Supplementary Fig. 7). This distinctive characteristic makes FA stand out as the optimal hydrogen donor for the hydrogenolysis of PE among the tested candidates.
a PE hydrogenolysis with different hydrogen donors. Reaction conditions: 100 μL MeOH or 300 μL EtOH or 900 μL IPA or 150 μL FA, 300 °C, 340 mg PE, 20 mg catalyst, 12 h. b–d Time courses of PE hydrogenolysis on Ru/C (b), Pt/C (c), and RuPt/C (d). Reaction conditions: 150 μL FA, 300 °C, 340 mg PE, 20 mg catalyst. e Comparison of PE conversion and C–C bond cleavage on Pt/C, Ru/C, and RuPt/C. Reaction conditions: 0.5 bar H2, 300 °C, 340 mg PE, 20 mg catalyst, 8 h. f Comparison of the products generated from FA decomposition over blank, Pt/C, Ru/C, and RuPt/C. Reaction conditions: 150 μL FA, 300 °C, 20 mg catalyst, 1.5 h. g Proposed decomposition pathway of FA on RuPt/C catalyst.
We then compared the catalytic performance of different catalysts by tracking the evolution of the product distribution during the hydrogenolysis using FA. Over Ru/C, the residual solid gradually decreased as the reaction proceeded, though the reaction rate remained slow (Fig. 3b). The methane selectivity exceeded 20% throughout the reaction (Supplementary Fig. 8). Hydrogenolysis of PE over Pt/C produced only a small amount of methane, with long-chain alkanes (C10–C40) as the main products (Supplementary Fig. 9). Meanwhile, it proceeded at an even slower rate with only 16.1% conversion after 24 h (Fig. 3c). In comparison, PE hydrogenolysis on RuPt/C showed superior performance, the solid gradually decreased in the first 8 h of the reaction, and was completely converted by 12 h (Fig. 3d). The liquid and wax products initially centered around C28, and with an extended reaction time to 24 h, the product distribution shifted to a center around C18 (Supplementary Fig. 10). In addition, the RuPt/C catalyst exhibited good structural stability and reusability under the tested conditions, maintaining both activity and selectivity in the hydrogenolysis of polyethylene over multiple runs (Supplementary Fig. 11).
To understand the performance differences among Pt/C, Ru/C, and RuPt/C, we quantified the H2 consumption during the reaction to determine the C–C bond cleavage rates based on the 1:1 stoichiometric relationship between the two20. Notably, the rate of C–C bond cleavage in the reaction over Pt/C was much slower than that observed with Ru/C and RuPt/C (Fig. 3e). In other RuPt catalysts with varying ratios, an increase in the Ru content led to a significant rise in H2 consumption (Supplementary Fig. 12a). This result indicates that C–C cleavage predominantly occurs on Ru sites in the RuPt catalyst, consistent with the previous study45. We further performed in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) to monitor PE hydrogenolysis over Ru/C, Pt/C, and RuPt/C. As shown in Supplementary Fig. 13, we observed three characteristic bands at 2920 cm−1, 2850 cm−1, and 1462 cm−1 on all three catalysts, which can be assigned to the asymmetric CH2 stretching, symmetric CH2 stretching and CH bending of PE, respectively. Notably, Pt/C (Supplementary Fig. 13b) and RuPt/C (Supplementary Fig. 13c) exhibited an additional band at 1590–1650 cm−1 that remained detectable throughout the reaction. This signal can be attributed to the C=C stretching of dehydrogenated alkene intermediates accumulated on catalyst surface46. The accumulation of dehydrogenated species on Pt-containing catalysts reveals two key mechanistic insights: (1) the relatively slow C–C bond cleavage kinetics at Pt sites leads to intermediate buildup, and (2) Pt actively facilitates C–H bond activation in polyethylene chains, which is consistent with previous studies17.
Although Ru/C exhibited a faster C–C bond cleavage rate, the conversion of PE remained low (Fig. 3b). This may be related to the insufficient H2 generation from FA decomposition over the Ru/C catalyst (Fig. 3f). The time evolution of FA decomposition products over Ru/C, Pt/C, and RuPt/C catalysts revealed that FA decomposition over all three catalysts rapidly generated H2, with the H2 yield approaching its maximum within 30 min (Supplementary Fig. 14a). However, a key difference was observed on the Ru/C catalyst, where the H2 yield began to decline after 30 min. This trend correlates with the hydrogenation of CO to CH4 on Ru/C. The decreasing CO concentration after 30 min (Supplementary Fig. 14b), along with the continuous increase in CH4 content (Supplementary Fig. 14d), supports this interpretation, indicating that Ru/C has a higher CO conversion capability. The methanation of CO on Ru/C would lead to a substantial consumption of active H*, thereby inhibiting the hydrogenolysis of PE.
We further investigated the effect of reaction temperature on PE hydrogenolysis over RuPt/C (Supplementary Fig. 15). When the temperature was lowered from 300 to 270 °C, the methane selectivity increased from 2.8% to 5.5% while the yield of solid residues also rose (Supplementary Fig. 16). Meanwhile, the amount of CO in the reaction system decreased with decreasing temperature, suggesting that lower temperatures favor CO hydrogenation but impede PE hydrogenolysis. Control experiments in which CO and H2 were introduced without PE also showed that methane production from CO hydrogenation decreased as temperature increased (Supplementary Fig. 17). This trend aligns with recent observations in the depolymerization of polystyrene using methanol as the hydrogen donor33. Conversely, when the reaction temperature was raised from 300 to 320 °C, PE conversion increased from 88.3% to 100.0%, likely due to accelerated reaction kinetics. The center of the carbon number of the liquid and wax products shifted from C28 to C19 while the methane selectivity increased from 2.8% to 5.4%, presumably owing to secondary hydrogenolysis of initially formed products (Supplementary Fig. 18).
We also investigated the effect of FA dosage on PE hydrogenolysis. As shown in Supplementary Table 4, increasing the FA dosage from 50 to 500 μL led to proportional increase in the amount of H2, CO, and CO2 produced from FA decomposition. As the FA dosage increased from 0 to 200 μL, the conversion of PE increased, with complete PE conversion achieved at an FA dosage of 150 μL (Supplementary Fig. 19a). This is likely due to the increased generation of H2 from FA decomposition, which promoted the hydrogenolysis of PE. Notably, when the FA dosage exceeded 250 μL, the PE conversion began to decrease despite the greater availability of H2. To identify the reason of this conversion decrease, we performed a set of control experiments, where we individually added additional CO, CO2, or H2O in H2 at concentrations equivalent to those produced by 500 μL FA decomposition (Supplementary Fig. 19b). The results showed that only the CO-supplemented system exhibited a marked decline in PE conversion, whereas the addition of CO2 or H2O had negligible effect. This strongly indicates that the decline in activity is primarily due to catalyst poisoning by excessive CO, rather than other byproducts.
Promotional role of CO in PE hydrogenolysis over RuPt/C
To address the complex interplay between FA and PE, we performed a time-resolved study to track the products from FA decomposition and PE hydrogenolysis. It revealed that under standard reaction conditions at 300 °C (Fig. 4a), FA decomposed rapidly and completely within the first 30 min, generating stoichiometric amounts of H2, CO, and CO2. In contrast, the conversion of PE proceeded at a slower rate, with a measurable loss in solid weight observed only after 60 min of the reaction. This time disparity allows us to regard FA decomposition and PE hydrogenolysis as a semi-continuous process, in which FA first decomposes to generate gaseous reactive species that subsequently drive the hydrogenolysis of PE.
Subsequently, we conducted in situ DRIFTS experiments to investigate the key intermediates during the FA decomposition over the RuPt/C catalyst. As shown in Fig. 4b, at low temperatures, characteristic peaks corresponding to molecular FA were observed. The characteristic peaks which appeared at 1120, 1215, and 1750 cm−1 can be assigned to the stretching vibrations of C−OH, the coupling vibrations of C−O, O−H deformation vibrations, and the stretching vibrations of C=O in the HCOOH* species. Besides, typical peaks at 1362 cm−1 appeared on catalyst, which can be attributed to the O−C−O symmetric stretching vibration in HCOO* species. These bands gradually diminished and eventually disappeared completely as the temperature was ramped from ambient (0 min) to 300 °C (30 min). Concurrently, we observed the emergence of intense CO2 vibrational bands (~2350 cm−1), indicating complete decarboxylation of formate-type species, and CO-related bands in the 2100–2200 cm−1 region. Especially, we detected the adsorbed CO species (~2060 cm−1) on the RuPt/C surface. To better simulate the catalytic reaction in batch autoclave, we also conducted in situ DRIFT in a sealed system. Specifically, FA was continuously introduced into the DRIFTS reaction cell for a certain period at 300 °C, after which the system was closed (Fig. 4c). As the reaction proceeded, the peak at 1670 cm−1, corresponding to the HCOO* intermediate, gradually decreased and eventually disappeared. After FA was completely consumed, the resulting spectrum displayed strong gas-phase bands of CO2 and CO, with no other residual formate species observed on the surface. Notably, we detected adsorbed CO species on the catalyst surface, including linearly and bridge-bonded CO, with distinct peaks at ~2061 cm−1 and ~1770 cm−1, respectively. This suggests that CO and CO2 are the only thermodynamically stable species under the reaction conditions.
Therefore, to decouple the effects of CO2 and CO, we conducted controlled atmosphere experiments with varying CO/H2 and CO2/H2 ratios (based on the actual gas-phase product distributions from FA decomposition) while maintaining constant hydrogen levels. Surprisingly, the introduction of a small amount of CO promoted PE hydrogenolysis compared with when pure H2 was used. When the CO/H2 ratio was 0.05, the PE conversion increased from 52.1% to 79.9% (Fig. 5a). This promotional effect diminished at higher CO/H2 ratios (>0.3), likely because more active sites on the catalyst became occupied by CO, leading to reduced catalytic performance. Different to CO, the introduction of CO2 did not show a significant impact on PE conversion (Fig. 5b), though when the CO2/H2 ratio was increased from 0 to 3, the PE conversion moderately increased from 52.1% to 64.7%. CO2 temperature-programmed desorption (CO2-TPD) results indicated facile CO2 desorption from the RuPt catalyst below the reaction temperature (Supplementary Fig. 20), while additional experiments indicated that the mild promotional effect of CO2 stemmed from its hydrogenation to CO (Supplementary Fig. 21), consistent with the results observed under the H2/CO atmosphere. When CO is formed during FA decomposition, H2O is generated simultaneously. Control experiments further indicated that the addition of trace amounts of H2O exerted minimal influence on the hydrogenolysis of PE (Supplementary Fig. 22).
a, b Effects of CO (a) and CO2 (b) on PE hydrogenolysis over RuPt/C catalyst. Reaction conditions: 0.5 bar H2, 300 °C, 340 mg PE, 20 mg catalyst, 8 h. c The time-dependence of DRIFTS spectra for CO adsorption (left) and desorption (right) over RuPt/C. d Comparison of DRIFTS spectra of Ru/C and Pt/C after CO adsorption. e XPS spectra of Ru 3p for the RuPt/C after hydrogenolysis using H2 and FA as the hydrogen donors, respectively. f XPS spectra of Ru 3p for the RuPt/C after hydrogenolysis in H2/CO and H2/CO2 atmospheres, respectively. Reaction conditions: 300 °C, 340 mg C36, 20 mg catalyst, 8 h. The error bars represent the standard deviations of three independent measurements.
To further understand how the addition of CO promotes the hydrogenolysis reaction, we employed in situ DRIFTS to identify the forms of CO present (Fig. 5c). The double peaks observed at approximately 2175 cm−1 and 2115 cm−1 were attributed to gaseous CO47. The bands at 2035–2051 cm−1 can be assigned to the linearly bonded CO on Pt/Ru ensembles. The peak at 2035–2051 cm−1 remained even after 30 min of Ar purging, indicating the presence of chemisorbed CO on RuPt under the reaction temperature. The weak peak near 1827 cm−1 was associated with bridge-bonded CO adsorption on the RuPt alloy (Supplementary Fig. 23). Even after introducing H2, CO remained strongly adsorbed on the catalyst surface (Supplementary Fig. 24). For CO adsorption on Ru/C and Pt/C, similar features of CO adsorption bands were also observed (Fig. 5d). For Pt/C, the band at 2080 cm−1 was assigned to linearly adsorbed CO on Pt sites47, while the shoulder peak at approximately 2024 cm−1 corresponded to CO adsorbed on low-coordination Pt sites. A weak peak near 1830 cm−1 was attributed to bridge-bonded CO adsorption on Pt. For Ru/C, the band around 2024 cm−1 was assigned to linearly adsorbed CO on Ru sites. The linear adsorption peak of CO on Ru sites appeared at a lower frequency compared to Pt, indicating a stronger binding interaction of CO-Ru than CO-Pt48. The linear CO adsorption band for RuPt appeared at 2078 cm−1, which is very close to the linear CO band on Pt/C. No significant red- or blue-shift was observed. This indicates that, under the DRIFTS conditions employed, the CO adsorption environment on RuPt is electronically similar to that of Pt alone. A likely reason is that the high Pt content in the RuPt alloy (Pt:Ru = 3:1) results in a surface that is predominantly Pt-rich.
We then conducted XPS analysis on the catalyst after reactions using H2 and FA as hydrogen donors, respectively. Compared with molecular H2, the catalyst reacted with FA showed a higher proportion of Ruδ+ species (Fig. 5e). The XPS spectra indicated that the Ruδ+ proportion was 17.9% for the catalyst after the reaction in H2, whereas it increased to 38.6% after the reaction in FA. Similarly, after the reaction in an H2/CO atmosphere, the Ruδ+ proportion was 35.5%, while it was only 20.3% after the reaction in an H2/CO2 atmosphere (Fig. 5f). Moreover, across all tested conditions, no significant change was observed in the Pt 4f binding energy or peak shape (Supplementary Fig. 25). It has been revealed that the presence of Ruδ+ can promote C−C bond cleavage during hydrogenolysis49. Therefore, the excellent activity of the RuPt alloy catalyst in the PE hydrogenolysis using FA can arise from the small amount of CO generated during FA decomposition, which adsorbs on the catalyst surface and induces the formation of Ruδ+ species.
Density functional theory (DFT) calculations provide further mechanistic insights into CO adsorption behavior on the RuPt alloy. Computational results show that when CO adsorbs at the Ru-Pt bridge site and the Pt-Pt-Ru hollow site, it preferentially migrates to the Ru top site. This result demonstrates stronger CO binding affinity on Ru compared to Pt sites, which aligns perfectly with prior CO-DRIFTS results (Fig. 5d). To quantitatively understand the electronic effects of CO adsorption, we performed detailed DFT analysis of three representative configurations: CO adsorption at the Ru top site (Fig. 6a), Pt-Pt bridge site (Fig. 6b), and Pt top site (Fig. 6c). Bader charge analysis, where blue and yellow represent charge loss and accumulation respectively, revealed that when CO adsorbs at the Ru top site, Ru transfers 0.5|e| to CO due to π-back donation (Fig. 6d). Interestingly, even when CO adsorbs at Pt sites, neighboring Ru atoms experience electron loss (0.42|e| at Pt-Pt bridge site, Fig. 6e; 0.3|e| at Pt top site, Fig. 6f), demonstrating long-range electronic effects in the alloy. These computational findings quantitatively corroborate our XPS measurements of Ruδ+ formation (Fig. 5e, f). The collective computational and experimental evidence conclusively demonstrates that CO adsorption serves as an effective electronic modulator of the RuPt alloy surface. Through both direct (Ru site adsorption) and indirect (neighboring Pt site adsorption) mechanisms, CO induces electron depletion from Ru atoms, generating catalytically active Ruδ+ species that are crucial for enhancing hydrogenolysis activity.
Piecing all evidence together, we proposed a plausible mechanism for the PE hydrogenolysis on RuPt surface using FA as the hydrogen donor. In conventional H2-driven hydrogenolysis, molecular hydrogen dissociates to form surface H*, and PE undergoes adsorption and partial dehydrogenation to yield dehydrogenated intermediates. Subsequently, these dehydrogenated intermediates undergo C–C bond cleavage at the Ru sites, forming two shorter alkyl fragments. The alkyl fragments could either combine with H* and subsequently desorb or experience further dehydrogenation and consecutive C–C bond scission. A high H* coverage on the catalyst surface (i.e., high H2 pressure) is often required to prevent the undesirable consecutive C–C bond scission45.
The FA-mediated process introduces distinct mechanistic features. FA decomposition generates H2, CO, and CO2 simultaneously, with H* participating in intermediate hydrogenation. While Ru/C catalysts suffer from H* depletion due to rapid CO/CO2 hydrogenation to methane, and Pt/C exhibits intrinsically slow C–C cleavage rates, the RuPt alloy demonstrates unique synergistic effects (Fig. 5g). CO adsorption induces Ruδ+ formation through two complementary pathways: (1) direct adsorption on Ru sites, where d-orbital electrons back-donate to CO’s π* orbitals; (2) adsorption on adjacent Pt sites, inducing electron redistribution through Ru-Pt metal bonding. In both scenarios, the resulting Ruδ+ species facilitate C–C cleavage by withdrawing electron density from polyolefin carbon atoms, effectively lowering the activation barrier49.
FA-mediated hydrogenolysis of real-life plastic waste
To demonstrate the potential of this approach for practical applications, we employed FA as the hydrogen donor for the hydrogenolysis of real-life plastic waste (Fig. 7), including LDPE bag, HDPE bottle, PP pipette tips, PS foams, and mixtures of several polyolefin-based plastics (59% PE, 29% PP, and 12% PS). Nearly complete degradation was achieved for all tested plastic categories (Fig. 7a). Specifically, the yield of liquid-phase products was more than 79.3% for LDPE, HDPE, PP, PS, and mixed plastics. The liquid-phase product distribution varied among the different materials. For example, the hydrogenolysis products of polyethylene-based plastics were mainly waxes/lubricant base-oils, whereas polypropylene conversion favored diesel-range hydrocarbons (Fig. 7b). Polystyrene hydrogenolysis produced valuable monocyclic aromatic hydrocarbons, with ethylbenzene (47.4%) as the major products, accompanied by toluene (4.1%) and cumene (9.1%) (Fig. 7f). For hydrogenolysis of mixed polyolefin plastics, the methane selectivity was only 3.0%, with the yield of C5–C40 yields no less than 95.0% (Supplementary Fig. 26).
a The product distributions of LDPE bag, HDPE bottle, PP pipette tips, PS foams, and mixed plastics (59% PE, 29% PP, and 12% PS). Reaction conditions: 300 °C, 340 mg substrate, 40 mg catalyst, 150 μL FA, 24 h. b Selectivity of the products based on carbon number range: gasoline, C5–C12; jet fuel, C8–C16; diesel, C9–C22; and waxes/lubricant base-oils, C20–C40. c–f Detailed carbon distributions of the hydrogenolysis products from LDPE bag (c), HDPE bottle (d), PP pipette tips (e), and PS foams (f).
Discussion
In summary, we have developed a sustainable strategy for PE hydrogenolysis utilizing renewable FA as an efficient hydrogen donor. We discover that the trace amounts of CO generated from FA decomposition plays a crucial role by adsorbing on the RuPt surface and inducing the formation of highly active Ruδ+ species, which facilitate C–C bond cleavage. This FA-mediated approach enables complete conversion of real-life polyolefin feedstocks into valuable products with minimal gas formation (4.2% selectivity). By eliminating the requirement for high-pressure hydrogen infrastructure, our method presents a transformative solution for decentralized plastic upcycling, offering the potential for modular, scalable waste treatment facilities.
Methods
Synthesis of RuPt alloy nanoparticles
The synthesis of RuPt alloy nanoparticles was based on previously reported protocols50. In a typical synthesis, 0.3 mmol of Pt(acac)2 and 0.1 mmol of Ru(acac)3 were dissolved in 10 mL of oleylamine (OAm) in a three-neck round-bottom flask. The solution was stirred at 50 °C for 30 min until fully dissolved and then gradually heated up to 120 °C at a rate of 5 °C min−1 under a nitrogen atmosphere. Once the temperature reached 120 °C, 0.3 mmol of borane tert-butylamine complex (TBAB) was added, and the reaction was maintained at this temperature for an additional 30 min. Subsequently, the temperature was increased to 300 °C at the same ramp rate and held for 1 h. After cooling down to room temperature, the nanoparticles were isolated by centrifugation. To purify the product, approximately 20 mL of acetone was added into the reaction mixture, which was then centrifuged at 6791 × g for 1 min. The supernatant was discarded, and the nanoparticles were redispersed in a mixture of 10 mL toluene and 20 mL acetone, followed by another centrifugation step. This washing process was repeated twice. Finally, the purified nanoparticles were redispersed in 10 mL of toluene and stored for subsequent use.
Synthesis of Pt nanoparticles
The Pt/C catalyst was synthesized according to a previously reported method19. In a typical synthesis, 0.1 mmol of K2PtCl4, 505 mg of C14TAB, and 222 mg of PVP (Mw = 40,000) were added into 20 mL of ethylene glycol in a two-neck round-bottom flask. The mixture was purged with nitrogen, then heated at 140 °C for 2 h. After cooling down to room temperature, the reaction mixture was transferred to a centrifuge tube, and acetone was added to precipitate the Pt nanoparticles, which was then centrifuged at 6791 × g for 1 min. Afterward, the precipitate was further washed with a mixed solution of ethanol and n-hexane and then centrifuged at 10612 × g for 1 min. This process was repeated for three times. The supernatant was discarded, and the nanoparticles were dispersed in 10 mL of ethanol for storage.
Synthesis of Ru/C catalyst
Ru/C was synthesized following a modified version of a previously reported procedure51. In a typical synthesis, 0.05 mmol of RuCl3·3H2O, 27.8 mg of PVP, and 100 mg of Vulcan XC72 were mixed with 12.5 mL of ethylene glycol in a two-neck round-bottom flask. The mixture was heated up to 200 °C in an oil bath and maintained at 200 °C for 3 h. After cooling to room temperature, the reaction mixture was transferred to a centrifuge tube and centrifuged at 10612 × g for 5 min. The supernatant was discarded, and the solid residue was washed 3 times with water and ethanol. The washed solid powder was then dried overnight at 65 °C in an oven and subsequently calcined in air at 200 °C in a muffle furnace to remove remaining organic ligands.
Catalyst loading on support
In a typical synthesis, Vulcan XC72 carbon were first dispersed in toluene through sonication for 5 min. Next, a stock nanoparticle solution was added dropwise to the carbon dispersion under sonication, and the mixture was sonicated for 1 h. The resulting catalysts had the following metal loadings: 5.0 wt% Pt for Pt/C and 5.0 wt% Pt for RuPt/C. The suspension was then transferred to a centrifuge tube and centrifuged at 6791 × g for 3 min. After discarding the supernatant, the solid residue was washed twice with toluene. The washed powder was dried overnight at 65 °C and subsequently calcined in air at 200 °C in a muffle furnace for 3 h to remove remaining organic ligands. The final carbon-supported catalysts were then stored for further use, and all catalysts in this work were pre-reduced under H2 at 300 °C for 2 h prior to reaction.
Catalytic hydrogenolysis of PE using FA
PE hydrogenolysis was carried out in a 25 mL batch autoclave. A mixture of 340 mg PE, 20 mg catalyst, and 150 μL FA was loaded into a glass-lined autoclave equipped with a glass magnetic stirrer. A 4 mm diameter glass thermowell was custom-fabricated and fused to the bottom of the glass liner. A 1.5 mm K-type thermocouple was bent and inserted so that its tip rested at the base of the thermowell, allowing accurate measurement of the reactant temperature. The autoclave was then sealed, evacuated, and purged 3 times with Ar. After purging, the autoclave was pressurized with 0.5 MPa of Ar at room temperature. Specially, high-pressure of Ar was used to facilitate gas collection. Once the reaction proceeded for the specified duration, the autoclave was allowed to cool down to ambient temperature. The released gas was collected using a gas bag and analyzed by gas chromatography (GC). The liquid products were dissolved in CHCl3 and analyzed by GC. The solid residue was quantified by measuring the mass difference of the glass liner before and after the reaction. Under the same conditions as described above, catalytic tests were carried out using other liquid hydrogen donors, with the addition of 100 μL methanol (or 300 μL ethanol, or 900 μL isopropanol). Stoichiometric decomposition of the hydrogen donors would yield H2 quantitatively corresponding to 0.05 MPa of molecular H2 in the reaction system.
When using H2 as the hydrogen source, the reactor was purged three times with Ar followed by three purges with H2. Subsequently, the high-pressure reactor was charged with 0.05 MPa H2 and 0.5 MPa Ar. Otherwise, catalytic hydrogenolysis was performed under the same conditions as described above.
Product analysis
Gas and liquid products were analyzed using an Agilent Technologies 8890 GC system equipped with both a thermal conductivity detector (TCD) and a flame ionization detector (FID). For gas analysis, a certain volume of N2 was purged into the gas bag as an internal standard. The equation was shown below:
Vg: The volume of gas products.
\({V}_{{N}_{2}}\): The volume of N2.
\({\Phi }_{{N}_{2}}\): The volume fraction of N2 measured by GC.
The GC system was configured with two separate channels: the first channel utilized two Hayesep Q (G3591-81020) columns in series with a MolSieve 5 A column (G3591-81022) to separate hydrogen, nitrogen, methane, ethane, propane, and butane, which were subsequently quantified by the TCD. The second channel employed a DB-1 capillary column (123–1032, 0.32 mm, 30 m, 0.25 µm) to separate pentane, hexane, and heptane, which were measured using the FID. We used Aspen Plus to calculate the mass of gas products. The interaction parameters in Redlich-Kwong-Soave equation were automatically provided in the software. The volume of the gas products was calculated through N2 internal standard. The temperature was set at 25 °C.
Liquid products were dissolved in 50 mL of chloroform, with mesitylene added as an internal standard. These samples were analyzed using the DB-1 capillary column (123–1032, 0.32 mm, 30 m, 0.25 µm) to separate alkanes ranging from C7H16 to C40H88, which were detected and quantified by the FID. Calibration was performed using retention times of C7–C40 saturated alkane standards (Supelco, Lot 49452-U) at a concentration of 1000 μg mL−1. Additionally, the FID response factors for each carbon number were calibrated, and the positions of isomers for each carbon length were determined relative to the linear-chain alkanes.
Material characterizations
TEM images were taken on a Hitachi HT-7800 microscope operated at 80 kV. HAADF-STEM and EDS analyses were conducted on aberration-corrected microscopes, specifically the FEI-Titan Cubed Themis G2 300 and FEI-Themis Z, both operated at 300 kV. Powder XRD patterns were obtained with a Rigaku MiniFlex 600 utilizing Cu-Kα radiation. Inductively coupled plasma optical emission spectroscopy (ICP-OES) measurements were performed using a Thermo Scientific iCAP 6300 instrument.
X-ray absorption spectroscopy
X-ray absorption spectroscopy (XAS) measurements were performed at the BL11B and BL14W1 beamlines of the Shanghai Synchrotron Radiation Facility and the 1W1B beamline at the Beijing Synchrotron Radiation Facility. Calibration for the Pt L3-edge was conducted using a Pt foil standard. All data analyses were executed with Artemis and Athena software from the Demeter suite, employing bulk references via the FEFF software. For EXAFS fitting, the amplitude reduction factor (S02) was determined by fitting the first-shell scattering of the reference metal foils and was subsequently fixed for the sample analyses.
Quasi in situ X-ray photoelectron spectroscopy
Quasi in situ XPS experiments were carried out using an Axis Supra system (Kratos Analytical Ltd., UK) equipped with an Al Kα X-ray source (1486.6 eV) operating at 200 W for survey scans and 300 W for core-level spectra. The binding energies were calibrated against the C 1 s peak at 284.8 eV. The XPS system features a reaction chamber connected through an ultra-high vacuum chamber.
For catalyst characterization, the catalyst was pressed into a tablet and placed in a glass holder. The holder was evacuated and transferred to the reaction chamber, where the catalyst was pretreated at 300 °C for 2 h under a hydrogen flow of 10 mL min−1. After cooling to room temperature under H2 flow, the reaction chamber was evacuated, and the glass holder was moved to the XPS chamber for analysis. The catalyst after reaction was not pretreated with H2.
In situ diffuse reflectance infrared Fourier transform spectroscopy
In situ DRIFTS was conducted using a Thermo Nicolet iS50 Fourier transform infrared spectrometer equipped with a diffuse reflection accessory (Harrick Inc.). To prepare the samples, 20 mg of catalyst was first combined with 200 mg of KBr. The prepared sample was loaded into a high-temperature in situ DRIFTS reactor (Harrick Inc.) and pretreated at 300 °C under a hydrogen flow of 5 mL min−1 for 2 h. After pretreatment, the temperature was maintained at 300 °C to initiate the DRIFTS measurements. In the CO DRIFTS experiment, the sample was first purged by CO for 30 min and the DRIFTS signals were collected continuously, and then the CO was switched to Ar to sweep away the gaseous CO and weakly adsorbed CO. During the whole process, the DRIFTS signals were collected with a resolution of 4 cm−1. In the DRIFTS study of PE hydrogenolysis, 20 mg of the catalysts was first mixed with 200 mg of KBr. Separately, 100 mg of PE was dissolved in 10 mL of toluene at 120 °C. Subsequently, 5 mL of PE solution was added into the catalyst mixture and stirred until dry. The sample was further dried out in oven at 65 °C overnight. Prior to measurement, the sample was loaded in a high temperature in situ DRIFTS reactor and then heated up to 300 °C under a H2 flow to initiate the reaction.
Data availability
All data generated in this study are provided in the Supplementary Information.
References
MacLeod, M., Arp, H. P. H., Tekman, M. B. & Jahnke, A. The global threat from plastic pollution. Science 373, 61–65 (2021).
Garcia, J. M. & Robertson, M. L. The future of plastics recycling. Science 358, 870–872 (2017).
Jambeck, J. R. et al. Plastic waste inputs from land into the ocean. Science 347, 768–771 (2015).
Zhang, F. et al. Polyethylene upcycling to long-chain alkylaromatics by tandem hydrogenolysis/aromatization. Science 370, 437–441 (2020).
Jia, X., Qin, C., Friedberger, T., Guan, Z. & Huang, Z. Efficient and selective degradation of polyethylenes into liquid fuels and waxes under mild conditions. Sci. Adv. 2, e1501591 (2016).
Conk, R. J. et al. Catalytic deconstruction of waste polyethylene with ethylene to form propylene. Science 377, 1561–1566 (2022).
Korley, L. T. J., Epps, T. H., Helms, B. A. & Ryan, A. J. Toward polymer upcycling—adding value and tackling circularity. Science 373, 66–69 (2021).
Gancedo, J. et al. Investigation into the shape selectivity of zeolites for conversion of polyolefin plastic pyrolysis oil model compound. Appl. Catal. A: Gen. 669, 119484 (2024).
Zhang, W. et al. Low-temperature upcycling of polyolefins into liquid alkanes via tandem cracking-alkylation. Science 379, 807–811 (2023).
Li, H. et al. Aliphatic amines from waste polyolefins by tandem pyrolysis, hydroformylation, and reductive amination. Green. Chem. 26, 8718–8727 (2024).
Gan, L. et al. Beyond conventional degradation: catalytic solutions for polyolefin upcycling. CCS Chem. 6, 313–333 (2024).
Hancock, J. N. & Rorrer, J. E. Hydrogen-free catalytic depolymerization of waste polyolefins at mild temperatures. Appl. Catal. B: Environ. 338, 123071 (2023).
Wang, C. et al. Polyethylene hydrogenolysis at mild conditions over ruthenium on tungstated zirconia. JACS Au 1, 1422–1434 (2021).
Chen, L. et al. Disordered, sub-nanometer Ru structures on CeO2 are highly efficient and selective catalysts in polymer upcycling by hydrogenolysis. ACS Catal. 12, 4618–4627 (2022).
Chen, S. et al. Ultrasmall amorphous zirconia nanoparticles catalyse polyolefin hydrogenolysis. Nat. Catal. 6, 161–173 (2023).
Chauhan, M. et al. Isoreticular metal–organic frameworks confined mononuclear Ru-hydrides enable highly efficient shape-selective hydrogenolysis of polyolefins. JACS Au 3, 3473–3484 (2023).
Sun, C. et al. Pt enhanced C–H bond activation for efficient and low-methane-selectivity hydrogenolysis of polyethylene over alloyed RuPt/ZrO2. Appl. Catal. B: Environ. Energy 353, 124046 (2024).
Chu, M. et al. Site-selective polyolefin hydrogenolysis on atomic Ru for methanation suppression and liquid fuel production. Research 6, 0032 (2023).
Wu, X. et al. Size-controlled nanoparticles embedded in a mesoporous architecture leading to efficient and selective hydrogenolysis of polyolefins. J. Am. Chem. Soc. 144, 5323–5334 (2022).
Tennakoon, A. et al. Two mesoporous domains are better than one for catalytic deconstruction of polyolefins. J. Am. Chem. Soc. 145, 17936–17944 (2023).
Celik, G. et al. Upcycling single-use polyethylene into high-quality liquid products. ACS Cent. Sci. 5, 1795–1803 (2019).
Liu, S., Kots, P. A., Vance, B. C., Danielson, A. & Vlachos, D. G. Plastic waste to fuels by hydrocracking at mild conditions. Sci. Adv. 7, eabf8283 (2021).
Rorrer, J. E., Beckham, G. T. & Román-Leshkov, Y. Conversion of polyolefin waste to liquid alkanes with ru-based catalysts under mild conditions. JACS Au 1, 8–12 (2021).
Wang, C. et al. A general strategy and a consolidated mechanism for low-methane hydrogenolysis of polyethylene over ruthenium. Appl. Catal. B: Environ. 319, 121899 (2022).
Zhou, Q. et al. Mechanistic understanding of efficient polyethylene hydrocracking over two-dimensional platinum-anchored tungsten trioxide. Angew. Chem. Int. Ed. 62, e202305644 (2023).
Nakaji, Y. et al. Low-temperature catalytic upgrading of waste polyolefinic plastics into liquid fuels and waxes. Appl. Catal. B: Environ. 285, 119805 (2021).
Zare, M. et al. Effect of reaction media on hydrogenolysis of polyethylene plastic waste: polymer-surface interactions in small alkane/polymer blends. Appl. Catal. B: Environ. Energy 351, 123969 (2024).
Vance, B. C., Najmi, S. & Vlachos, D. G. Polystyrene hydrogenolysis to high-quality lubricants using Ni/SiO2. ACS Catal. 14, 5389–5402 (2024).
Sun, Y.-Q. Explosions of hydrogen storages and the safety considerations in hydrogen-powered railway applications—A review. Hydrogen 5, 901–918 (2024).
Faye, O., Szpunar, J. & Eduok, U. A critical review on the current technologies for the generation, storage, and transportation of hydrogen. Int. J. Hydrog. Energy 47, 13771–13802 (2022).
Abdalla, A. M. et al. Hydrogen production, storage, transportation and key challenges with applications: a review. Energy Convers. Manag. 165, 602–627 (2018).
Rao, P. C. & Yoon, M. Potential liquid-organic hydrogen carrier (LOHC) systems: a review on recent progress. Energies 13, 6040 (2020).
Zeng, L. et al. Recycling valuable alkylbenzenes from polystyrene through methanol-assisted depolymerization. Angew. Chem. Int. Ed. 63, e202404952 (2024).
Abdel-Mageed, A. M., Widmann, D., Olesen, S. E., Chorkendorff, I. & Behm, R. J. Selective CO methanation on highly active Ru/TiO2 catalysts: identifying the physical origin of the observed activation/deactivation and loss in selectivity. ACS Catal. 8, 5399–5414 (2018).
Lee, J., Kwon, T., Kang, K. H., Won, W. & Ro, I. Tandem catalysis for plastic depolymerization: in situ hydrogen generation via methanol APR for sustainable PE hydrogenolysis. Angew. Chem. Int. Ed. 64, e202420748 (2025).
Mellmann, D., Sponholz, P., Junge, H. & Beller, M. Formic acid as a hydrogen storage material – development of homogeneous catalysts for selective hydrogen release. Chem. Soc. Rev. 45, 3954–3988 (2016).
Zhu, Q.-L. & Xu, Q. Liquid organic and inorganic chemical hydrides for high-capacity hydrogen storage. Energy Environ. Sci. 8, 478–512 (2015).
Valentini, F. et al. Formic acid, a biomass-derived source of energy and hydrogen for biomass upgrading. Energy Environ. Sci. 12, 2646–2664 (2019).
Sun, R. et al. Heterogeneous catalysts for CO2 hydrogenation to formic acid/formate: from nanoscale to single atom. Energy Environ. Sci. 14, 1247–1285 (2021).
Ra, E. C. et al. Recycling carbon dioxide through catalytic hydrogenation: recent key developments and perspectives. ACS Catal. 10, 11318–11345 (2020).
Benson, E. E., Kubiak, C. P., Sathrum, A. J. & Smieja, J. M. Electrocatalytic and homogeneous approaches to conversion of CO2 to liquid fuels. Chem. Soc. Rev. 38, 89–99 (2009).
Løhre, C., Barth, T. & Kleinert, M. The effect of solvent and input material pretreatment on product yield and composition of bio-oils from lignin solvolysis. J. Anal. Appl. Pyrolysis 119, 208–216 (2016).
Oregui Bengoechea, M., Hertzberg, A., Miletić, N., Arias, P. L. & Barth, T. Simultaneous catalytic de-polymerization and hydrodeoxygenation of lignin in water/formic acid media with Rh/Al2O3, Ru/Al2O3 and Pd/Al2O3 as bifunctional catalysts. J. Anal. Appl. Pyrolysis 113, 713–722 (2015).
Liu, Y. et al. Poisoning of acetone to Pt and Au electrodes for electrooxidation of 2-propanol in alkaline medium. Electrochim. Acta 76, 174–178 (2012).
Hu, Q. et al. Polyethylene hydrogenolysis by dilute RuPt alloy to achieve H2-pressure-independent low methane selectivity. Nat. Commun. 15, 10573 (2024).
Choudhury, N., Kim, A., Kim, M. & Kim, B.-S. Mechanochemical degradation of Poly(vinyl chloride) into nontoxic water-soluble products via sequential dechlorination, heterolytic oxirane ring-opening, and hydrolysis. Adv. Mater. 35, 2304113 (2023).
Pereira-Hernández, X. I. et al. Tuning Pt-CeO2 interactions by high-temperature vapor-phase synthesis for improved reducibility of lattice oxygen. Nat. Commun. 10, 1358 (2019).
Mojet, B. L., Ebbesen, S. D. & Lefferts, L. Light at the interface: the potential of attenuated total reflection infrared spectroscopy for understanding heterogeneous catalysis in water. Chem. Soc. Rev. 39, 4643–4655 (2010).
Hu, P. et al. Stable interfacial ruthenium species for highly efficient polyolefin upcycling. J. Am. Chem. Soc. 146, 7076–7087 (2024).
Li, J. et al. Anisotropic strain tuning of L10 ternary nanoparticles for oxygen reduction. J. Am. Chem. Soc. 142, 19209–19216 (2020).
Kusada, K. et al. Discovery of face-centered-cubic ruthenium nanoparticles: facile size-controlled synthesis using the chemical reduction method. J. Am. Chem. Soc. 135, 5493–5496 (2013).
Acknowledgements
This work was supported by Beijing Natural Science Foundation (Z240029) and the National Natural Science Foundation of China (22375113 and 22422602). We acknowledge the BL11B, BL14W1 stations in Shanghai Synchrotron Radiation Facility (SSRF) for the collection of XAFS data.
Author information
Authors and Affiliations
Contributions
Z.N. conceptualized this work. Z.N. and J.Z.(Zhang) guided this work. Z.N. and Y.W. designed the experiments. Y.W. and Q.H. performed the catalyst preparation and hydrogenolysis. Y.C. and S.Q. performed the DFT calculations. Z.N., Y.W., Q.H., and S.Q. wrote the paper. J.Z.(Zhao), J.M., and J.Z.(Zhang) revised the manuscript. All the authors participated in the data analysis and commented on the manuscript. These authors contributed equally: Y.W. and Q.H.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Y., Hu, Q., Qian, S. et al. CO-promoted polyethylene hydrogenolysis with renewable formic acid as hydrogen donor. Nat Commun 16, 7821 (2025). https://doi.org/10.1038/s41467-025-63189-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-63189-5