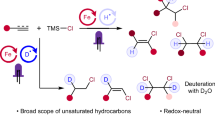
Abstract
Hydroazidation of alkenes provides a direct entry to alkyl azides, which are prevalent structural motifs in medicine development and chemical biology probes. While direct access to anti-Markovnikov hydroazidation products has seen recent progress, these protocols are restricted to highly oxidative hypervalent iodine reagents or superstoichiometric metal salts under photochemical conditions with moderate olefin generality. Thus, the development of a mild, catalytic, redox-neutral hydroazidation with high anti-Markovnikov regioselectivity compatible with diverse classes of alkene remains challenging. Here we report a photocatalytic anti-Markovnikov hydroazidation of alkenes enabled by cooperative ligand-to-metal charge transfer (LMCT) and hydrogen atom transfer (HAT). Critical to this protocol is the use of ligand to achieve efficient visible-light induced homolysis of iron azide species and the cooperation with thiol catalysts to promote this redox-neutral process and address previous challenging substrates. Additionally, the photocatalytic system enables a regioselective haloazidation via a tandem LMCT/ halogen atom transfer (XAT) process with judicious choice of halogenating reagents. Preliminary mechanistic studies support a radical nature of this cooperative system and suggest it to be a powerful manifold in olefin hydro- and di-functionalization.
Similar content being viewed by others
Introduction
Alkyl azides are prevalent structural motifs among pharmaceuticals, varied synthetic intermediates, and chemical biology probes1,2,3,4,5. This synthon can also serve as a compelling nitrene precursor and be leveraged for the construction of valuable carbon-nitrogen bonds of pivotal importance in natural product synthesis and medicinal chemistry6,7,8,9,10. Driven by these applications, hydroazidation of olefins presents one of the most direct strategies to access these useful building blocks from abundant feedstock hydrocarbons. Early approaches leveraged acid-mediated Markovnikov addition of explosive and gaseous HN3 to alkenes and proceeds through a carbocation pathway (Fig. 1)11,12. Toward avoiding this dangerous reagent, modern protocols developed by Carreira13,14 and Boger15 employing metal-hydride atom transfer (MHAT) provided a general and mild route to deliver the same Markovnikov substituted/branched alkyl azides products using alternative azide donors. However, access to linear, anti-Markovnikov alkyl azide product via these acid-mediated or MHAT mechanisms is unfavorable due to the intermediacy of less stable primary carbocations or alkyl radicals. Indeed, the anti-Markovnikov hydroazidation of alkenes usually requires multistep syntheses9 and/or stoichiometric use of boranes or electrophilic azide sources16,17 that resulted in low step and/or atom economy, and constrained generality, presenting challenges in direct anti-Markovnikov hydroazidation.
A Traditional Markovnikov hydroazidation via acid-mediated pathway or metal-hydride catalysis; multistep synthesis to access anti-Markovnikov selectivity. B Recent catalytic anti-Markovnikov hydroazidation reactions. C This work: Cooperative photocatalytic hydro- and haloazidation of alkenes. D Proposed mechanism of photocatalytic anti-Markovnikov hydro- and haloazidation enabled by cooperative ligand-to-metal charge transfer and hydrogen atom transfer or halogen atom transfer. OSET outer-sphere single electron transfer, RPC radical polar crossover.
Toward overcoming these challenges in anti-Markovnikov hydroazidation, several distinct mechanistic approaches have been explored. Chiba, Gagosz, and coworkers leveraged the hypervalent iodine reagent azidobenziodoxlone (Zhdankin reagent) to achieve anti-Markovnikov hydroazidation via a radical addition/1,5 HAT/oxidative debenzylation cascade. While this reaction demonstrated the feasibility of anti-Markovnikov hydroazidation via a radical mechanism, this particular approach is only amenable to specific substrates designed for the full cascade process18. Yu and coworkers took a complementary noble-metal based photocatalysis approach to achieve hydroazidation of unsaturated aryl amides via outer-sphere single electron transfer (OSET) of azide, enabling hydroazidation though exhibiting moderate scope and requiring an expensive iridium photocatalyst19. By exploiting the reactive nature of hypervalent iodine reagents, which undergoes facile homolytic cleavage of I-N3 bonds and promote radical chain pathways in protic solvent, Xu20 and Liu21 independently reported elegant protocols to achieve direct anti-Markovnikov hydroazidation of alkenes. While synthetically enabling, the adoption of these highly energetic and oxidative reagents could still present barriers to oxidatively-labile functionalities. Additionally, this radical chain pathway is driven by the thermodynamically-favored hydrogen atom transfer (HAT) of in situ generated HN3 (BDE = 93.3 kcal/mol) to non-stabilized carbon-centered radical intermediates, making chain propagation less favorable with activated alkenes such as styrene due to endogenic HAT process to form a weaker bond (~ 86 kcal/mol for ethylbenzene)22. Therefore, a general anti-Markovnikov hydroazidation that can accommodate a diverse range of activated and unactivated alkenes and potentially reactive functional groups under mild conditions remains underdeveloped. Moreover, such system could also serve as a foundation for complementary functionalizations proceeding via open-shell azidyl radicals, such as haloazidation of alkenes, that would allow facile incorporation of two reactive handles. Previous protocols for haloazidation are largely confined to the in situ preparation of halogen azide in the presence of molecular halogen, affording poor regioselectivity and limited substrate scope23,24, and/or the requirement of directing group or specific substrate class for achieving high regioselectivity control25,26. We hypothesize that many of these challenges might be overcomed by an azidyl radical generation that is mild and amenable to broader functionalization. Taking these examples together, we recognized a mechanistically distinct azidyl radical generation method that avoids the usage of exogeneous oxidant and/or electrophilic/oxidative azidyl radical precursors might permit the efficient anti-Markovnikov hydroazidation and regioselective haloazidation of diverse alkenes, serving as a powerful and unified tool for accessing these useful azidated structural motifs.
Towards azidyl radical generation, previous methods have employed hypervalent iodine reagent BI–N3, which is able to undergo facile homolytic cleavage of the I–N bond due to its low bond dissociation energy (27.8 kcal/mol)27,28 or conventional photoredox approaches using noble-metal based photocatalysts, proceeding through a biomolecular, redox-potential matching, OSET oxidation of azide to azidyl radical29,30. A complementary approach is inner-sphere single electron transfer (ISET), with electron transfer occurring directly between a coordinated substrate and a metal. Light-driven ligand-to-metal charge transfer (LMCT) is one such ISET process that enables coordinated anionic, X-type ligands to be selectively oxidized to their radical form using metal catalysts of moderate ground state oxidation potential and short excited state lifetimes due to this intramolecular, excited state mechanism31,32. Gaining inspiration from Kochi’s pioneering work33 in LMCT of cupric chloride to generate chlorine radical for C-H chlorination and alkene dichlorination and rapid expansion of this LMCT reactivities to other earth-abundant metals in recent years34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56, our group57 and Shi and coworkers58 independently, developed the first stoichiometric photochemical diazidation of alkenes enabled by iron LMCT and radical ligand transfer as well as our subsequent work making this protocol photocatalytic59, where we postulated the iron-azide species formed in situ would undergo photolysis to release azidyl radical. Very recently, the azidyl radical generation from iron LMCT reactivity was elegantly adopted by Carreira and coworkers to achieve the non-catalytic iron-mediated photochemical hydroazidation of alkenes60. The protocol can accommodate a broad array of diversely substituted alkenes, with preliminary mechanistic studies showing water from iron hydrate as H-atom source. While demonstrating synthetic versatility, the superstoichiometric use of the iron salt still raises issues in sustainable synthesis, with a potential additional hazard through forming high concentration of metal-azide species in the system. Additionally, similar to previous hydroazidation protocols, activated alkenes such as styrene or α-heteroatom alkenes were not tolerated, possibly due to thermodynamically less favored C–H bond forming HAT (benzylic C-H, BDE ~ 86 kcal/mol; H-N3, BDE = 93 kcal/mol)61. We envisioned that this thermodynamic mismatch might be overcomed via HAT to the carbon-centered radical intermediate occurring from a redox-active hydrogen donor that can not only initiate facile HAT facilitated by BDE-driven thermodynamics and polarity-matching kinetics, but also enable reoxidation of lower valent iron formed after LMCT back to the high-valent photoactive state. We have obtained preliminary support for this design through development of iron LMCT/thiol HAT cooperative photocatalysis systems to achieve protodecarboxylation of carboxylic acids62 and hydrofluoroalkylation of alkenes63 with both reactions including these key elementary steps. From this, we hypothesized that this cooperative LMCT/HAT system could provide a redox-neutral, photocatalytic strategy to anti-Markovnikov hydroazidation of alkenes.
We proposed the reaction beginning with the photo-induced cleavage of a pre-associated N3-FeIII species to generate azidyl radical I and lower valent iron species. The azidyl radical can then add to an alkene, forming a carbon-centered radical intermediate. Redox-active thiol could subsequently function as hydrogen atom donor to this intermediate via HAT to furnish anti-Markovnikov hydroazidation product II with concurrent generation of thiyl radical (step B). Finally, thiyl radical can further oxidize lower valent iron back to its photoactive state, followed by protonation by H2O co-solvent to form the active thiol catalyst, closing both catalytic cycles (step A). Furthermore, by replacing H2O with cheap D2O, the deuteroazidation isotopologue is expected to form, providing a unified route to prepare D-labelled azidation products. The intermediacy of carbon-centered radicals can also be utilized in conjunction with somophiles such as NCS or NBS via halogen atom transfer (XAT) to achieve a regioselective haloazidation, providing a versatile protocol to access varied haloazidated products.
Herein, we report a general, photocatalytic anti-Markovnikov hydroazidation of alkenes enabled by cooperative LMCT and HAT. Following our original discovery of azidyl radical generation via LMCT of iron-azide species, we combine this chemistry with HAT of redox-active thiol to permit anti-Markovnikov hydroazidation process to proceed with no additional redox reagents or superstoichiometric mediator. Critical to success of this reaction is presence of a supporting ligand for iron, where the efficiency is improved compared with the “ligand-free” condition and milder, lower energy visible light (427 nm) irradiation can be used instead of high-energy near UV irradiation. Zuo and coworkers reported a pioneering study of iron terpyridine catalysis utilizing LMCT to achieve highly efficient aerobic carbonylation of methane, showcasing the unique photochemical properties of ligated iron complexes in LMCT processes64. Notably, this cooperative protocol not only accommodates previously unreactive substrates classes but also allows D-labelled isotopologue preparation, dramatically expanding the applications of this reaction. Additionally, common halogenation reagents can be used in place of thiol to perform efficient XAT to sequester alkyl radical intermediates and achieve regioselective chloro- and bromo-azidation of alkenes. Together, these cooperative strategies enable facile preparation of diverse alkyl azides under mild and sustainable iron photocatalysis, introducing a powerful new tool for azidofunctionalization of olefins.
Results
Reaction design and optimization
We began exploring the possibility of cooperative anti-Markovnikov hydroazidation by using pent-4-en-1-yl benzoate as our model substrate, combined with trimethylsilyl azide (TMSN3) as the anionic azide source, catalytic loading of earth-abundant iron salt, bi- or tridentate ligand, redox-active thiol, 19:1 HCCl3/water as solvent, and irradiation with 427 nm LEDs at room temperature. Excitingly, the inexpensive Fe(NO3)3·9H2O (10 mol%) and tridendate terpyridine ligand (terpy, 10 mol%) in cooperation with 4-F-thiophenol (10 mol%) could facilitate the production of highly anti-Markovnikov product 1 in 76% isolated yield, supporting our hypothesized cooperative catalysis scheme (Table 1, entry 1). To differentiate our system from previous protocols that are based on radical chain propagation of HN3 or stoichiometric use of iron hydrate and provide support that ligand is indispensable in achieving high efficiency, we tested 30 mol% of iron(III) nitrate nonahydrate in the absence of both ligands and thiol co-catalyst under 390 nm and observed drastically decreased yields, supporting the reaction is not likely to operate via the same radical chain mechanism seen in previous reports (Table 1, entry 2). Furthermore, the ligated iron complex exhibited a notable red-shift for effective illumination wavelength, with 390 nm irradiation showing significantly reduced reactivity compared to 427 nm (Table 1, entry 3, for more details, see supporting information). We next observed that unligated iron exhibits almost absent reactivity at 427 nm (Table 1, entry 4), further emphasizing the enabling effect of ligand. Toward further exploring the influence of ligand on the system, we tested a variety of polypyridyl ligands. While bidentate and tridentate pyridine-based ligands showed drastically increased reactivities compared with entries without ligands, the tridentate 2,2′:6′,2″-terpyridine was found to be the optimal ligand of those tested (Table 1, entry 5 and supporting information). Next, we found the identity of thiol to have a strong influence on reaction efficiency, finding electron-rich aryl thiol showed unsatisfying efficiency and electron-deficient aryl thiol or disulfide could provide improved yields, with 4-F-thiophenol performing most efficiently (Table 1, entries 6 and supporting information). Interestingly, non-protic solvents such as acetonitrile and acetone, which are effective for diazidation in our previous work, gave trace hydroazidation product (Table 1, entries 7), leading us to continue using the initial mixed CHCl3/H2O solvent system. Higher intensity blue light (50%) and extended reaction time allowed the product yield to be improved to 82% (Table 1, entry 8). Lastly, control experiments indicated no conversion in the absence of iron or light, supporting azidyl radical generation from light-induced homolysis of iron-azide species (Table 1, entries 9–10).
Scope of anti-Markovnikov hydroazidation of alkenes
With the optimized conditions in hand, we next investigated the functional group compatibility of our photocatalytic hydroazidation (Fig. 2). First, simple, aliphatic alkenes (2, 3) and the tosylate protecting group (6) were tolerated, providing corresponding primary alkyl azides in moderate to high yields. Substituted arenes (4, 5) with electronically distinct functionalities were compatible with this hydroazidation system. 2,2,2-Trichloroethoxycarbonyl (Troc) (7) and N-methylated sulfonamides (8) were also tolerated, giving good yields of hydroazidation products, further demonstrating compatibility of this reaction with synthetically and medicinally relevant functionality. Next, a broad range of heterocycles such as thiophene (9), pyrrole (10), N-phthalimide (11), tetrahydropyran (12), and benzofuran (13) also underwent efficient hydroazidation under our photocatalytic conditions, overcoming limitation in protocols exploiting highly oxidizing conditions. We next sought to test substrates bearing strained ring structures and acid-, nucleophilic substitution-, and redox-labile functionalities. After observing high yields of terminal azides featuring strained ring such as tetramethylated cyclopropane (14) and oxetane (15), we were excited to see smooth transformation of an alkene substituted with an alkyl bromide handle which remains intact without any azide displacement, providing dual (pseudo)halogenated (Br- and N3-) alkanes in 72% yield (16). Importantly, various redox-labile functionalities including ester (17), sulfide (18), and alcohol (19) all afforded corresponding primary azides in moderate to good yields, showcasing a powerful solution to accessing azidated molecules with these reactive functional groups, which suggests another advantage of our mild photocatalytic system in comparison with protocols using highly oxidizing hypervalent iodine reagents. We next shifted our investigation to different alkene substitutions, finding 1,1-disubstituted alkenes (21-24) behave well, without forming detected Markovnikov products (> 20:1 r.r.). Similarly, 1,2-disubstituted alkenes (25, 26) and disubstituted cyclized alkene (27) both demonstrated encouraging reactivities and provided expected near equal quantities of regioisomers. Trisubstituted alkenes (29, 30) showed excellent compatibility to our system, giving moderate to high yields of corresponding alkyl azides in exclusive anti-Markovnikov regioselectivities. More sterically hindered tetrasubstituted alkene (31) also gave good yield of corresponding product in a mixture of both regioisomers. Finally, R-carvone, a substrate containing two chemically distinct olefinic functionalities (32), was tested to investigate the chemoselectivity of this system and expectedly provided reaction at the non-conjugated site in exclusive anti-Markovnikov regioselectivity.
Reaction conditions: alkene (0.1 mmol, 1.0 equiv.), TMSN3 (4.0 equiv.), Fe(NO3)3·9H2O (10 mol%), terpyridine (10 mol%), 4-F-thiophenol (10 mol%), and HCCl3/H2O (19:1, 0.1 M), 24 h, RT, 427 nm Kessil LED (25%). a With 50% 427 nm light, 60 h. b With 25% light, 60 h. c NMR yield is determined due to volatile nature. d With 50% light, 48 h. e With 50% 427 nm light, 72 h. f With 25% 427 nm light, 96 h. g With Fe(OAc)2, isolated yield indicates anti-Markovnikov product yield.
To further illustrate the importance of this anti-Markovnikov hydroazidation and showcase these alkyl azide handle could be useful for chemical biology probe development, we next endeavored to explore a wide array of alkenes derived from commercially available pharmaceutical ingredients (APIs) and natural products to evaluate viability of our method in late-stage functionalization65, providing a direct and versatile route to diverse alkyl azides featuring medicinally-relevant motifs. Olefins derived from the NSAID Ibuprofen (33), Oxaprozin (39), lipid-lowering Clofibric acid (34), Benzafibrate (37), sulfonamide-containing Probenecid (35), and natural product Flavone-derived alkene (36) provided hydroazidated products smoothly in moderate to good yields (43%–76%). Notably, Proline-derived alkene (38) featuring low-BDE sp3 α-C-H bonds adjacent to a heteroatom that are the liability for other hydroazidation methods, performs well in our reaction, further demonstrating its chemoselectivity. Alkenes derived from natural product L-Menthol (40), ketal-protected monosaccharide (41), and the complex steroid 18β-Glycyrrhetinic acid (42) behaved well and afforded hydroazidated products in useful yields. Taken together, the high catalytic reactivity, regioselectivity, and broad tolerance of our hydroazidation to structurally diverse alkenes recommend our method as a powerful route to efficiently install azide handles in alkenes featuring biologically active fragments that could help accelerate medicinal chemistry campaigns.
To further differentiate our cooperative LMCT/HAT system from the established radical chain pathways and stoichiometric metal-mediated methods and address challenging substrates20,21,60, we then investigated problematic olefins in previous protocols. A common incompatible substrate class is styrene-type activated alkenes and we reason this could be correlated to thermodynamically less favored HAT process between H-N3 and benzylic radicals (benzylic C-H, BDE = 86 kcal/mol; H-N3, BDE = 93 kcal/mol) when proceeding via a radical chain pathway. We envisioned that leveraging an aryl thiol HAT cocatalyst (PhS-H, BDE = 84 kcal/mol)66 should provide more thermodynamic incentive for C–H bond formation and permit anti-Markovnikov hydroazidation of activated alkenes. Excitingly, we found this to be the case, with diversely substituted styrene-type alkenes affording anti-Markovnikov products (44–46) in moderate to good yields. Additionally, this cooperative system can also be applied to activated alkenes adjacent to halogen (47) and heteroatom (48), indicating a general solution for working with these classes of substrates. Furthermore, alkenes substituted with sensitive functionalities such as carboxylic acid (43) or carbazole (49) that are categorized as incompatible examples in previous work were also tolerated under this photocatalytic condition60, providing corresponding products in moderate to good yields (46–62%). The enhanced tolerance of our protocol, in conjunction with other synthetic advantages in this photocatalytic strategy, demonstrates it to be a general and mild pathway to access anti-Markovnikov hydroazidation.
In our proposed mechanism, thiol transfers a hydrogen atom originating as a proton from H2O via HAT to the carbon-centered radical, resulting in C–H bond formation. Accordingly, this origin suggests that simply replacing H2O with D2O could result in deuterium atom transfer67,68 to access D-labelled alkyl azides. Implementing this design, we carried out preliminary testing on alkenes of different substitution patterns (terminal, 1,1-, tri-substituted) to see if this deuteroazidation can behave well over these representative examples. Excitingly, deuteroazidation isotopologues could be prepared using a similar catalytic system, with adjustment of iron salt and increased light intensity, allowing product formation in moderate yields with good deutero-incorporation rate (80–89% D). These initial results suggest the feasibility of this approach and offer a synthetically useful solution to deuterated alkyl azides.
Scope of regioselective chloro- and bromo-azidation of alkenes
Encouraged by the broad scope of our photocatalytic hydroazidation, we sought to investigate if this iron-azide LMCT process can cooperate with other reaction schemes, such as using electrophilic halogen sources to permit XAT and achieve photocatalytic regioselective haloazidation, which would provide an efficient route to diverse alkyl azides bearing halide reaction handles. In our hypothesis, an ideal electrophilic halogen source could not only deliver halogen functionality efficiently and regioselectively via homolytic XAT, but also serve as single electron oxidant that could reoxidize lower valent iron to its higher valent photoactive state for the next LMCT catalysis cycle. Thus, this tandem LMCT/XAT could provide a competitive strategy of redox-neutral, regioselective haloazidation. Notably, while pioneering studies by Finn24, Kappe25, Feng26, Burns27, and coworkers have provided intriguing approaches to this transformation, the reliance on stoichiometric exogenous oxidant or molecular halogen often results in low regioselectivity control due to unselective ring opening of cationic halonium intermediates. Further, the requirement of directing group or specific substrate class for efficient reaction have rendered these protocols less general towards the development of unified strategies that accommodate broad scope of alkenes.
Tackling these challenges, we were delighted to find electrophilic N-chloro- and N-bromo-succinimides (NCS, NBS) can be efficiently leveraged in photocatalytic, regioselective chloro- and bromoazidation (Fig. 3). A broad range of unactivated alkenes (53–70; 78–90) and activated alkenes (71–74; 91–94) were converted to the corresponding haloazidated products in moderate to good yields. Reminiscent to our hydroazidation scope, simple aliphatic alkenes (53, 78) and protecting groups including benzoate (54, 55, 79), tosylate (80), and 2,2,2-trichloroethoxycarbonyl (Troc) (82) afforded corresponding chloroazidation and bromoazidation products efficiently. Unactivated alkenes containing heterocycles that are commonly used in structure-activity relationship studies were also compatible with our chloroazidation and bromoazidation protocols. Sensitive functionalities including sulfonamides (56) and benzamides (57) with acidic N–H protons, along with N-methylated sulfonamides (81) and strained rings (61, 62, 87), underwent smooth chloro- and bromoazidation without compromising structural integrity and labile functionalities that are prone to nucleophilic substitution (63, 86) and oxidation or reduction (64–67, 85) were individually examined to explore the generality of our protocols, furnishing difunctionalized products in moderate to good yields. The encouraging compatibility with these sensitive and redox-labile functionalities further showcases the synthetic advantage of our system over previous protocols that were dependent on strong exogenous oxidants and molecular halogen, where the decomposition of these functionalities would be expected. Substitution patterns were also investigated, demonstrating tolerance of 1,1-disubstituted alkenes (68, 69, 88, 89) and sterically hindered tri-substituted alkenes (70, 90). Importantly, these difunctionalizations indicate generality to activated alkenes including styrene-type (71, 91) and alkenes adjacent to heteroatom functionality (72–74; 92–94), with these entries all delivering the chloro- and bromo- azidation products smoothly. Similar to the hydroazidation protocol, these haloazidation conditions can also be employed on olefins containing API fragments (75, 76, 95), agrochemical moieties (96), and a natural product (77), which allows the simultaneous incorporation of two (pseudo)halides reaction handles into bioactive compounds for chemical biology studies. The versatility and broad compatibility of these protocols towards various functional groups and low-toxicity of the earth-abundant iron catalyst used in these haloazidation systems further demonstrate cooperative LMCT/XAT as a powerful approach to regioselective heterodifunctionalization of olefins.
Reaction conditions of chloroazidation of alkene (0.1 mmol, 1.0 equiv.), TMSN3 (2.0 equiv.), Fe(OAc)2 (10 mol%), terpyridine (10 mol%), NCS (1.5 equiv.) and DCM (0.1 M), 24 h, RT, 427 nm Kessil LED (25%). Reaction conditions of bromoazidation of alkene (0.1 mmol, 1.0 equiv.), TMSN3 (2.0 equiv.), Fe(acac)2 (10 mol%), terpyridine (10 mol%), NBS (1.5 equiv.) and DCE (0.1 M), 24 h, RT, 427 nm Kessil LED (50%).a 48 h. b Determined by NMR using CH2Br2 as internal standard.
Scalability, late-stage application, and mechanistic studies
Bolstered by the extensive scope of our hydroazidation and haloazidation protocol, we further sought to demonstrate the scalability and late-stage application of the alkyl azide products. First, large scale hydroazidation was performed, giving corresponding product in good yield. We then sought to test if our protocol could provide entry to a simple late-stage transformation. Following facile chloroazidation, we performed a simple work-up (no isolation required) to remove the iron salt and directly employed the crude chloroazidation product for subsequent Huisgen cyclization, affording synthetically useful yield of chloro-substituted triazole (Fig. 4A). Next, we sought to gain preliminary insight into the mechanism of this reaction. First, including 1 equivalent of 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO), a common radical scavenger, in our optimized conditions for alkene hydroazidation resulted in complete suppression of reactivity and almost full recovery of alkene, indicating a radical process is likely (Fig. 4B). With this result in hand, we applied radical clock substrates in both hydroazidation and chloroazidation to further provide support for a radical pathway. As expected, 5-exo-trig ring-closing products are obtained in both entries, which suggests the subsequent HAT or XAT step is slower than the rate constate of 2 × 105 (approx. for 5-exo-trig) and further supports the system should proceed via a radical mechanism (Fig. 4C). To provide more kinetic details of this cooperative system, we then interrogated the kinetic isotope effect of our hydroazidation69, where a moderate primary KIE value of 2.0 was indicated, suggesting the HAT could have rate determining character in this cooperative system (Fig. 4D) as observed in alkene hydrofluoroalkylation63. To investigate whether the ligand could affect the spectroscopic character of the key photoactive species, we first performed UV–Vis studies of the combination of iron salts and ligand. Unligated iron-azide shows minimal absorption under 400 nm and almost no absorption above 400 nm, consistent with the low reactivity observed using iron catalyst alone. By contrast, the combination of Fe(III) and terpyridine ligand offers a bathochromic shift, which correlates with our observation that ligated iron demonstrates higher reactivity when using wavelengths above 400 nm. Time-resolved experiments demonstrate gradual attenuation of the charge transfer peak of iron(III)-azide, followed by emergence of absorption at 550 nm, which we postulate to correspond to the characteristic absorption of the bis(terpyridine) iron(II) complex (for more details, see supporting information). Based on these results and pioneering work from Zuo and coworkers64, we reason that ligand enhances the reactivity by improving the bathochromic absorption overall, facilitating a more productive light-induced homolysis process.
In summary, we have demonstrated a general photocatalytic protocol for anti-Markovnikov hydroazidation of alkenes enabled by cooperative LMCT and HAT. Broad scope, mild visible light conditions, and late-stage modification of pharmaceuticals/natural products derivatives were illustrated, addressing substrate limitations in previous anti-Markovnikov hydroazidation methods via radical chain pathways or stoichiometric metal-photochemical pathways. Critical to this transformation is coordination of earth-abundant iron with tridentate ligand, facilitating azidyl radical generation under visible light irradiation, and compatibility with organic thiol cocatalyst in efficient HAT to access diverse alkyl azides and some representative deuterated analogues in high anti-Markovnikov regioselectivity. With judicious choice of halogenating reagents, we were able to develop a complementary tandem LMCT/XAT reaction manifold to access redox-neutral, regioselective haloazidation of a broad range of olefins, addressing previous challenges of regioselectivity control in the absence of directing groups and specific alkene classes. We expect these cooperative strategies can serve as a powerful tool in developing redox-neutral hydro- and difunctionalization of feedstock chemicals and future studies based on this dual catalytic manifold are ongoing in our laboratory.
Method
General procedure of photocatalytic hydro-, deuteroazidation of alkenes
Fe salt (10 mol%, 0.1 equiv.) and terpyridine (10 mol%, 0.1 equiv.) were added in an oven-dried 8-mL test vial containing a Teflon®-coated magnetic stir bar. The vial was evacuated and backfilled with N2 (repeated for 4 times), followed by addition of alkenes (0.1 mmol, 1.0 equiv.), TMSN3 (0.40 mmol, 4.0 equiv.), 4-F-thiolphenol (10 mol%, 0.1 equiv.) in HCCl3/H2O (19:1, 0.1 M in regard to alkenes) via syringe under N2. For deuteroazidation of alkenes, HCCl3/D2O is used (19:1, 0.1 M in regard to alkenes). The reaction mixture was placed under 427 nm Kessil® light after sealing the punctured holes of the vial cap with vacuum grease and electric tape/parafilm for better air-tight protection and allowed to react at room temperature for 36–96 h. Following this, the reaction mixture was filtered through a pad of celite, which was subsequently rinsed with DCM. The filtrate was concentrated, and the residue was then purified by flash column chromatography or preparatory thin-layer chromatography to give the corresponding hydroazidated products. The details of hydro- and deuteroazidation of alkenes (the types and equivalents of iron salt, light intensity, and time in each protocol) are demonstrated in the Supplemental Information 1.3.
General procedure of photocatalytic haloazidation of alkenes
Fe salt (10 mol%, 0.1 equiv.) and terpyridine (10 mol%, 0.1 equiv.) were added in an oven-dried 8-mL test vial containing a Teflon®-coated magnetic stir bar. The vial was evacuated and backfilled with N2 (repeated for 4 times), followed by addition of alkenes (0.1 mmol, 1.0 equiv.), TMSN3 (0.20 mmol, 2.0 equiv.), NCS or NBS (0.15 mmol, 1.5 equiv.) in DCM (for chloroazidation) or DCE (for bromoazidation) (0.1 M in regard to alkenes) via syringe under N2. The reaction mixture was placed under 427 nm Kessil® light after sealing the punctured holes of the vial cap with vacuum grease and electric tape/parafilm for better air-tight protection and allowed to react at room temperature for 24 h. Following this, the reaction mixture was filtered through a pad of celite, which was subsequently rinsed with DCM. The filtrate was concentrated, and the residue was then purified by flash column chromatography or preparatory thin-layer chromatography to give the corresponding haloazidated products. The details of haloazidation of alkenes (the types and equivalents of iron salt, light intensity, and time in each protocol) are demonstrated in the Supplemental Information 1.3.
Data availability
The authors declare that all the data supporting the findings of this research are available within the article and its supplementary information. Data supporting the findings of this manuscript are also available from the corresponding author upon request.
References
Sivaguru, P., Ning, Y. & Bi, X. New strategies for the synthesis of aliphatic azides. Chem. Rev. 121, 4253–4307 (2021).
Shee, M. & Singh, N. D. P. Chemical versatility of azide radical: journey from a transient species to synthetic accessibility in organic transformations. Chem. Soc. Rev. 51, 2255–2312 (2022).
Rostovtsev, V. V., Green, L. G., Fokin, V. V. & Sharpless, K. B. A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 41, 2596–2599 (2002).
Speers, A. E., Adam, G. C. & Cravatt, B. F. Activity-based protein profiling in vivo using a copper(I)-catalyzed azide-alkyne [3 + 2] cycloaddition. J. Am. Chem. Soc. 125, 4686–4687 (2003).
Sletten, E. M. & Bertozzi, C. R. Bioorthogonal chemistry: fishing for selectivity in a sea of functionality. Angew. Chem. Int. Ed. 48, 6974–6998 (2009).
Nicolaou, K. C., Bunnage, M. E. & Koide, K. Total synthesis of balanol. J. Am. Chem. Soc. 116, 8402–8403 (1994).
Diethelm, S., Schindler, C. S. & Carreira, E. M. Synthesis of microcin SF608 through nucleophilic opening of an oxabicyclo[2.2.1]heptane. Org. Lett. 12, 3950–3953 (2010).
Xu, Z., Wang, Q. & Zhu, J. Enantioselective total syntheses of leuconolam–leuconoxine–mersicarpine group monoterpene indole alkaloids. J. Am. Chem. Soc. 135, 19127–19130 (2013).
Yang, Y., Bai, Y., Sun, S. & Dai, M. Biosynthetically inspired divergent approach to monoterpene indole alkaloids: total synthesis of mersicarpine, leuconodines B and D, leuconoxine, melodinine E, leuconolam, and rhazinilam. Org. Lett. 16, 6216–6219 (2014).
Pritchett, B. P., Donckele, E. J. & Stoltz, B. M. Enantioselective catalysis coupled with stereodivergent cyclization strategies enables rapid syntheses of (+)-limaspermidine and (+)-kopsihainanine A. Angew. Chem. Int. Ed. 56, 12624–12627 (2017).
Hassner, A., Fibiger, R. & Andisik, D. Synthetic methods. 19. Lewis acid catalyzed conversion of alkenes and alcohols to azides. J. Org. Chem. 49, 4237–4244 (1984).
Breton, G. W., Daus, K. A. & Kropp, P. J. Surface-mediated reactions. 2. Addition of hydrazoic acid to alkenes. J. Org. Chem. 57, 6646–6649 (1992).
Waser, J., Nambu, H. & Carreira, E. M. Cobalt-catalyzed hydroazidation of olefins: convenient access to alkyl azides. J. Am. Chem. Soc. 127, 8294–8295 (2005).
Waser, J., Gaspar, B., Nambu, H. & Carreira, E. M. Hydrazines and azides via the metal-catalyzed hydrohydrazination and hydroazidation of olefins. J. Am. Chem. Soc. 128, 11693–11712 (2006).
Leggans, E. K., Barker, T. J., Duncan, K. K. & Boger, D. L. Iron(III)/NaBH4-mediated additions to unactivated alkenes: synthesis of novel 20′-vinblastine analogues. Org. Lett. 14, 1428–1431 (2012).
Kapat, A., König, A., Montermini, F. & Renaud, P. A radical procedure for the anti-Markovnikov hydroazidation of alkenes. J. Am. Chem. Soc. 133, 13890–13893 (2011).
Meyer, D. & Renaud, P. Enantioselective hydroazidation of trisubstituted non-activated alkenes. Angew. Chem. Int. Ed. 56, 10858–10861 (2017).
Lonca, G. H. et al. Anti-Markovnikov hydrofunctionalization of alkenes: use of a benzyl group as a traceless redox-active hydrogen donor. Angew. Chem. Int. Ed. 56, 11440–11444 (2017).
Wang, J.-J. & Yu, W. Anti-Markovnikov hydroazidation of alkenes by visible-light photoredox catalysis. Chem. – A Eur. J. 25, 3510–3514 (2019).
Li, H., Shen, S.-J., Zhu, C.-L. & Xu, H. Direct intermolecular anti-Markovnikov hydroazidation of unactivated olefins. J. Am. Chem. Soc. 141, 9415–9421 (2019).
Li, X., Chen, P. & Liu, G. Iodine(III) reagent (ABX—N3)-induced intermolecular anti-Markovnikov hydroazidation of unactivated alkenes. Sci. China Chem. 62, 1537–1541 (2019).
Pellerite, M. J., Jackson, R. L. & Brauman, J. I. Proton affinity of the gaseous azide ion. The nitrogen-hydrogen bond dissociation energy in hydrazoic acid. J. Phys. Chem. 85, 1624–1626 (1981).
Valiulin, R. A., Mamidyala, S. & Finn, M. G. Taming chlorine azide: access to 1,2-Azidochlorides from Alkenes. J. Org. Chem. 80, 2740–2755 (2015).
Cantillo, D., Gutmann, B. & Oliver Kappe, C. Safe generation and use of bromine azide under continuous flow conditions – selective 1,2-bromoazidation of olefins. Org. Biomol. Chem. 14, 853–857 (2016).
Zhou, P. et al. Iron-catalyzed asymmetric haloazidation of α,β-unsaturated ketones: construction of organic azides with two vicinal stereocenters. J. Am. Chem. Soc. 139, 13414–13419 (2017).
Seidl, F. J., Min, C., Lopez, J. A. & Burns, N. Z. Catalytic regio- and enantioselective haloazidation of allylic alcohols. J. Am. Chem. Soc. 140, 15646–15650 (2018).
Zhdankin, V. V. et al. Preparation, X-ray crystal structure, and chemistry of stable azidoiodinanes derivatives of benziodoxole. J. Am. Chem. Soc. 118, 5192–5197 (1996).
Wang, Y. et al. Epimerization of tertiary carbon centers via reversible radical cleavage of unactivated C(sp3)–H bonds. J. Am. Chem. Soc. 140, 9678–9684 (2018).
Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013).
Xuan, J. & Xiao, W.-J. Visible-light photoredox catalysis. Angew. Chem. Int. Ed. 51, 6828–6838 (2012).
Abderrazak, Y., Bhattacharyya, A. & Reiser, O. Visible-light-induced homolysis of earth-abundant metal-substrate complexes: a complementary activation strategy in photoredox catalysis. Angew. Chem. Int. Ed. 60, 21100–21115 (2021).
Juliá, F. Ligand-to-metal charge transfer (LMCT) photochemistry at 3d-metal complexes: an emerging tool for sustainable organic synthesis. ChemCatChem 14, e202200916 (2022).
Kochi, J. K. Photolyses of metal compounds: cupric chloride in organic media. J. Am. Chem. Soc. 84, 2121–2127 (1962).
Hossain, A., Bhattacharyya, A. & Reiser, O. Copper’s rapid ascent in visible-light photoredox catalysis. Science 364, eaav9713 (2019).
Lian, P., Long, W., Li, J., Zheng, Y. & Wan, X. Visible-light-induced vicinal dichlorination of alkenes through LMCT excitation of CuCl2. Angew. Chem. Int. Ed. 59, 23603–23608 (2020).
Treacy, S. M. & Rovis, T. Copper catalyzed C(sp3)–H bond alkylation via photoinduced ligand-to-metal charge transfer. J. Am. Chem. Soc. 143, 2729–2735 (2021).
Heitz, D. R., Tellis, J. C. & Molander, G. A. Photochemical nickel-catalyzed C–H arylation: synthetic scope and mechanistic investigations. J. Am. Chem. Soc. 138, 12715–12718 (2016).
Shields, B. J. & Doyle, A. G. Direct C(sp3)–H cross coupling enabled by catalytic generation of chlorine radicals. J. Am. Chem. Soc. 138, 12719–12722 (2016).
Giedyk, M., Goliszewska, K. & Gryko, D. Vitamin B12 catalysed reactions. Chem. Soc. Rev. 44, 3391–3404 (2015).
Shul’pin, G. B., Druzhinina, A. N. & Shul’pina, L. S. Photo-oxidation of cyclohexane by air in acetonitrile, catalysed by π-complexes of iron. Pet. Chem. 33, 321–325 (1993).
Li, Z., Wang, X., Xia, S. & Jin, J. Ligand-accelerated iron photocatalysis enabling decarboxylative alkylation of heteroarenes. Org. Lett. 21, 4259–4265 (2019).
Jin, Y. et al. Convenient C(sp3)–H bond functionalisation of light alkanes and other compounds by iron photocatalysis. Green. Chem. 23, 6984–6989 (2021).
Jin, Y. et al. Photo-induced direct alkynylation of methane and other light alkanes by iron catalysis. Green. Chem. 23, 9406–9411 (2021).
Kang, Y. C., Treacy, S. M. & Rovis, T. Iron-catalyzed photoinduced LMCT: A 1 °C–H abstraction enables skeletal rearrangements and C(sp3)–H alkylation. ACS Catal. 11, 7442–7449 (2021).
Dai, Z.-Y., Zhang, S.-Q., Hong, X., Wang, P.-S. & Gong, L.-Z. A practical FeCl3/HCl photocatalyst for versatile aliphatic C–H functionalization. Chem. Catal. 2, 1211–1222 (2022).
Zhang, Q. et al. Iron-catalyzed photoredox functionalization of methane and heavier gaseous alkanes: scope, kinetics, and computational studies. Org. Lett. 24, 1901–1906 (2022).
Tu, J.-L., Hu, A.-M., Guo, L. & Xia, W. Iron-catalyzed C(Sp3)–H borylation, thiolation, and sulfinylation enabled by photoinduced ligand-to-metal charge transfer. J. Am. Chem. Soc. 145, 7600–7611 (2023).
Zhang, Y. et al. Bifunctional iron-catalyzed alkyne Z-selective hydroalkylation and tandem Z-E inversion via radical molding and flipping. Nat. Commun. 15, 8619 (2024).
Crocker, M. S. et al. Transformative ligand effects in Fe-photocatalyzed Giese-type additions. Chem. Catal. 4, 101131 (2024).
Wang, M., Wen, J., Huang, Y. & Hu, P. Selective degradation of styrene-related plastics catalyzed by iron under visible light. ChemSusChem 14, 5049–5056 (2021).
Oh, S. & Stache, E. E. Chemical upcycling of commercial polystyrene via catalyst-controlled photooxidation. J. Am. Chem. Soc. 144, 5745–5749 (2022).
Zhang, Z. et al. Controllable C–H Alkylation of polyethers via iron photocatalysis. J. Am. Chem. Soc. 145, 7612–7620 (2023).
Gygi, D. et al. Capturing the complete reaction profile of a C–H bond activation. J. Am. Chem. Soc. 143, 6060–6064 (2021).
Chábera, P. et al. A low-spin Fe(iii) complex with 100-ps ligand-to-metal charge transfer photoluminescence. Nature 543, 695–699 (2017).
de Groot, L. H. M., Ilic, A., Schwarz, J. & Wärnmark, K. Iron photoredox catalysis–past, present, and future. J. Am. Chem. Soc. 145, 9369–9388 (2023).
Hu, A., Guo, J.-J., Pan, H. & Zuo, Z. Selective functionalization of methane, ethane, and higher alkanes by cerium photocatalysis. Science 361, 668–672 (2018).
Bian, K.-J., Kao, S.-C., Nemoto, D., Chen, X.-W. & West, J. G. Photochemical diazidation of alkenes enabled by ligand-to-metal charge transfer and radical ligand transfer. Nat. Commun. 13, 7881 (2022).
Zhang, M., Zhang, J., Li, Q. & Shi, Y. Iron-mediated ligand-to-metal charge transfer enables 1,2-diazidation of alkenes. Nat. Commun. 13, 7880 (2022).
Bian, K.-J. et al. Photocatalytic, modular difunctionalization of alkenes enabled by ligand-to-metal charge transfer and radical ligand transfer. Chem. Sci. 15, 124–133 (2024).
Lindner, H., Amberg, W. M. & Carreira, E. M. Iron-mediated photochemical anti-Markovnikov hydroazidation of unactivated olefins. J. Am. Chem. Soc. 145, 22347–22353 (2023).
Luo, Y.-R. Comprehensive Handbook of Chemical Bond Energies, 1st ed. (CRC Press, 2007).
Lu, Y.-C. & West, J. G. Chemoselective decarboxylative protonation enabled by cooperative earth-abundant element catalysis. Angew. Chem. Int. Ed. 62, e202213055 (2023).
Bian, K.-J. et al. Photocatalytic hydrofluoroalkylation of alkenes with carboxylic acids. Nat. Chem. 15, 1683–1692 (2023).
Pan, H. et al. Iron-catalyzed aerobic carbonylation of methane via ligand-to-metal charge transfer excitation. J. Am. Chem. Soc. 147, 1440–1447 (2025).
Börgel, J. & Ritter, T. Late-stage functionalization. Chem. 6, 1877–1887 (2020).
Borges dos Santos, R. M. et al. S−H bond dissociation enthalpies in thiophenols: a time-resolved photoacoustic calorimetry and quantum chemistry study. J. Phys. Chem. A 106, 9883–9889 (2002).
Soulard, V., Villa, G., Vollmar, D. P. & Renaud, P. Radical deuteration with D2O: catalysis and mechanistic insights. J. Am. Chem. Soc. 140, 155–158 (2018).
Shi, Q. et al. Visible-light mediated catalytic asymmetric radical deuteration at non-benzylic positions. Nat. Commun. 13, 4453 (2022).
Simmons, E. M. & Hartwig, J. F. On the interpretation of deuterium kinetic isotope effects in C–H bond functionalizations by transition-metal complexes. Angew. Chem. Int. Ed. 51, 3066–3072 (2012).
Acknowledgements
We acknowledge financial support from NIH (R35GM142738), the Welch Foundation (C-2085), RCSA (CS-CSA-2023-007), and ACS-PRF (62397-DNI1). Dr. Yohannes H. Rezenom (TAMU/LBMS), Dr. Ian M Riddington (UT Austin Mass Spectrometry Facility), and Dr. Christopher L. Pennington (Rice University Mass Spectrometry Facility) are acknowledged for assistance with mass spectrometry analysis.
Author information
Authors and Affiliations
Contributions
K.-J.B. and J.G.W. designed the project. K.-J.B., S.Y., Y.C., Q.L., X.-W.C., D.N.Jr, and S.-C.K. performed the experiments. K.-J.B. and J.G.W. wrote the manuscript. J.G.W. directed the project. K.-J.B., S.Y., Y.C., Q.L., X.-W.C., D.N.Jr, S.-C.K., A.A.M., and J.G.W. interpreted the results in the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Yunhe Jin, Hua-Dong Xu, and the other, anonymous, reviewer for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bian, KJ., Yu, S., Chen, Y. et al. Photocatalytic anti-Markovnikov hydro- and haloazidation of alkenes. Nat Commun 16, 7906 (2025). https://doi.org/10.1038/s41467-025-63203-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-63203-w