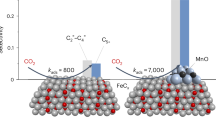
Abstract
Iron carbide catalysts, particularly the Fe7C3 phase, hold significant potential for efficient CO2 hydrogenation to olefins, yet stabilizing this phase under reactive conditions remains a major challenge. Herein, we report a robust and efficient synthesis of nearly phase-pure Fe7C3 catalysts derived from Prussian blue analogues, whose stability is significantly enhanced by strategically incorporating K and Mg promoters. Comprehensive characterization reveals that K accelerates the carbonization process and markedly enhances olefin selectivity, whereas Mg effectively suppresses water-induced oxidation, preserving the structural integrity of the Fe7C3 phase. Under optimized reaction conditions (340 °C, 2 MPa, H2/CO2 = 3), the Fe7C3-KMg catalyst achieves a high CO2 conversion of 41.5% and an olefin selectivity of 67.1%, maintaining exceptional catalytic stability for over 1000 hours. These findings offer valuable new insights into the rational design of robust iron carbide catalysts for sustainable and efficient CO2 conversion into high-value chemicals.
Similar content being viewed by others
Introduction
Hydrogenation of CO2 into hydrocarbons has attracted intense interest as a route to produce value-added fuels1,2 and chemicals3,4 while mitigating CO2 emissions5,6,7,8,9. Modified iron-based Fischer–Tropsch synthesis (FTS) catalysts are particularly appealing for this reaction because of their activity, affordability, and inherent promotion of the water–gas shift reaction (WGS)10,11,12. Under typical CO2 hydrogenation conditions (300 – 360 °C, 1 – 5 MPa), iron catalysts tend to evolve into a mixture of iron carbide and iron oxide phases13. In fact, it is commonly observed in operando studies that iron initially carburizes to form carbides (e.g. χ-Fe5C2)11,14, but the H2O byproduct of CO2 conversion concurrently re-oxidizes some iron into magnetite (Fe3O4)13. Thus, the catalyst reaches a dynamic steady state comprising iron carbide and oxide phases, which synergistically couple FTS and reverse WGS. Consistent with this understanding, the vast majority of literature reports on Fe-based CO2 hydrogenation catalysts have identified Hägg iron carbide (χ-Fe5C2) as the predominant active phase, with magnetite (Fe3O4) playing a secondary but important role in maintaining activity11,14.
Emerging evidence underscores the catalytic importance of Fe7C3. Zhao et al. showed that all major iron carbides, including Fe7C3 are catalytically active in FTS15,16, and Chang et al. reported that Fe7C3 can exhibit the highest intrinsic turnover frequency among the iron carbides under typical FTS conditions17. These findings suggest that Fe7C3 could offer performance advantages over the traditional iron carbide phases, surpassing even χ-Fe5C2 in intrinsic activity, and thus merits special attention in catalyst design. In contrast, the role of the Fe7C3 phase in CO2 hydrogenation has remained largely unexplored18, primarily due to a historical research focus on χ-Fe5C2 and Fe3O4, as well as the practical challenge of obtaining Fe7C3 as a stable active phase under reaction conditions. Recent studies have begun to probe Fe7C3-based catalysts for CO2 hydrogenation, though with mixed19. Pasupulety et al. examined a K and Zn promoted iron catalyst supported on ZrO2 synthesized via a citric acid method with the aim of facilitating Fe7C3 formation. They reported that a CO/H2 pretreatment could indeed produce the Fe7C3 phase in this Fe–Zn–K/ZrO2 system; however, the activated catalyst was far from phase-pure, as a significant fraction of magnetite remained present alongside Fe7C3. The persistence of substantial Fe3O4 as well as the dominant ZrO2 support phase corresponded with suboptimal catalytic performance. The underwhelming results from such attempts highlight the need for new approaches to generate a purer and more stable Fe7C3 phase under CO2 hydrogenation conditions.
The scarcity of stable Fe7C3 in working catalysts arises from several intertwined factors. First, the thermodynamic and kinetic constraints governing phase formation often favor more common iron carbides or metallic iron under typical reaction temperatures and CO/H2 ratios20,21. Second, the complexity of Fe7C3’s formation from precursor materials often necessitates stringent pretreatment conditions that are not easily translated to large-scale or continuous operations17. Third, even when Fe7C3 is formed, maintaining its phase purity and stability under highly dynamic reaction environments, such as high partial pressures of water and oxidizing conditions inherent in CO2 hydrogenation, poses additional hurdles13,22.
In light of the gaps in prior research, we focus on Fe7C3 as a target active phase for CO2 hydrogenation and seeks to overcome the aforementioned stabilization challenges21. In contrast to previous works, we demonstrate that an in-situ carburization strategy can produce a nearly phase-pure Fe7C3 catalyst that remains stable under reaction conditions for prolonged periods. Notably, the Fe7C3-rich phase achieved in our work exhibits enhanced catalytic activity, including higher CO2 conversion and C2+= olefin productivity compared to conventional Fe3O4/χ-Fe5C2-based catalysts. These findings represent the first clear evidence that Fe7C3 can serve as a durable and highly active phase for CO2 hydrogenation to hydrocarbons. Accordingly, the motivation of this study is to fill the knowledge gap regarding Fe7C3 by exploring its formation and function during CO2 hydrogenation. In this work, we aim to highlight a new pathway for designing iron-based CO2 hydrogenation catalysts beyond the traditional Fe3O4/χ-Fe5C2 paradigm, thereby advancing the development of more efficient CO2-to-fuels technologies.
Results
Structural characterizations
Following our previously reported method for synthesizing Prussian blue analogue (PBA) precursors23, a series of PBA-based catalysts were synthesized and activated under an NH3 atmosphere. This NH3 atmosphere not only promotes the thermal decomposition of the PB analogues but also facilitates the formation of Fe2N as the primary phase in the fresh catalysts, as confirmed by X-ray diffraction (XRD) (Supplementary Fig. 1 and Fig. 2). Once subjected to CO2 hydrogenation, Fe2N rapidly undergoes an in situ transformation into iron oxide and iron carbide24, as illustrated in Fig. 1a. Specifically, the Fe and FeMg catalysts both display characteristic Fe3O4 peaks, indicating extensive oxidation of iron under the reaction conditions. Introducing potassium (FeK) induces partial carbide formation, and the subsequent addition of magnesium (FeKMg) leads to the clear emergence of a distinct Fe7C3 phase. Mössbauer spectroscopy and fitting analyses (Fig. 1b, Supplementary Fig. 3, Supplementary Table 1) corroborate the presence of Fe7C3 and reveal the composition of each spent catalyst25.
XRD patterns of the spent Fe, FeMg, FeK, and FeKMg (a). Mössbauer spectrum of the spent FeKMg (b). Fe K-edge X-ray absorption near-edge structure (XANES) spectra (c). Fourier transform extended X-ray absorption fine structure (FT EXAFS) at the Fe K-edge (d) for the Fe, FeMg, FeK, and FeKMg. Particle size distribution (e) and HRTEM image (f) of the FeKMg catalyst. Fe 2p XPS profiles for the Fe, FeMg, FeK, and FeKMg catalysts (g). STEM-EDS elemental mapping (h) and line scanning analysis (i) of the FeKMg catalyst. All spent catalysts (Fe, FeMg, FeK, FeKMg) were collected after reaching steady-state catalytic performance under standard conditions (340 °C, 2 MPa, H2/CO2 = 3, GHSV = 6 L·gcat−1·h−1).
To gain deeper insights into the electronic structure, we employed X-ray absorption spectroscopy (XAS) and X-ray photoelectron spectroscopy (XPS). Fe K-edge XANES spectra (Fig. 1c) reveal that the spent Fe and FeMg catalysts have a pre-edge feature indicative of iron oxides. Introducing K shifts the absorption edge between oxidized and metallic states, while subsequent Mg addition drives a more pronounced shift toward lower energy, closely matching that of Fe7C3. This suggests that, under reaction conditions, Fe predominantly exists as the iron carbide in FeKMg11,26. The XAFS fitting results show that Fe and FeMg catalysts have the similar short-range structure with a Fe-O scattering path at 3.5 Å, whereas the presence of FeKMg maintains a resembling coordination number structure closer to Fe7C3 (Fig. 1d, Supplementary Fig. 4, Supplementary Table 2). Consistent with these findings, XPS results (Fig. 1g) show that Fe and FeMg retain oxidized surfaces, whereas FeK exhibits partial metallic character and FeKMg is dominated by metallic Fe signals27. Catalyst compositions (Supplementary Table 3) indicate that all catalysts possess similar Fe contents.
High-resolution transmission electron microscopy (HRTEM) shows the average crystallite size of the FeKMg catalyst is about 71.2 nm (Fig. 1e) and a lattice spacing of ~2.1 Å corresponding to the (102) plane of Fe7C3 (Fig. 1f). STEM-EDS mapping and line (Fig. 1h–i) scans indicate uniform element distribution. For comparison, TEM images of the spent Fe catalyst (Supplementary Fig. 5) reveal a uniform distribution of Fe and O (average particle size ~47.06 nm). Similarly, STEM-EDS mapping of FeK (Supplementary Fig. 6) and FeMg (Supplementary Fig. 7) show homogeneous elemental distributions. However, FeMg remains mostly Fe3O4, whereas FeK is a mixture of Fe7C3 and Fe3O4.
Figure 2 illustrates the structural evolution of the catalysts during CO2 hydrogenation. The initial Fe2N phase in Fe and FeMg catalysts (Fig. 2a and b) rapidly oxidizes to iron oxides within 8 hours. In contrast, FeK (Fig. 2c) initially undergoes carbonization to form iron carbides, but subsequently experiences partial re-oxidation, resulting in a mixed oxide–carbide phase. Notably, only FeKMg (Fig. 2d) maintains a stable carbide structure throughout the reaction, predominantly featuring the rarely reported Fe7C3 phase. Mössbauer quantification (Fig. 2e, Supplementary Fig. 8, Supplementary Table 4) confirms that potassium significantly promotes the carbonization process, while magnesium plays a key role in stabilizing the Fe7C3 phase. Figure 2f schematically summarizes the distinct roles of K and Mg during phase evolution. In our system, a small amount of Fe7C3 pre-exists in the fresh catalyst and acts as a nucleation base for further carburization. The metastable Fe2N gradually converts into Fe7C3 under the assistance of potassium. Meanwhile, magnesium plays a crucial role in maintaining the structural and chemical stability of the catalyst during CO2 hydrogenation. The thermodynamic phase diagram for iron carbide formation was simulated through theoretical calculations. Under low carbon chemical potential, the formation energies of different iron carbides are very close, suggesting a flat potential energy surface that facilitates facile phase transformations among them. The presence of Fe7C3 under these conditions indicates its thermodynamic viability and the possibility of stabilization even in carbon-lean environments (Supplementary Fig. 9).
Previous catalyst systems predominantly rely on the coexistence and dynamic equilibrium between χ-Fe5C2 and Fe3O4 phases, identifying χ-Fe5C2 as the primary active component9. In clear contrast, our FeKMg catalyst achieves nearly phase-pure Fe7C3 stabilization under realistic CO2 hydrogenation conditions. Comprehensive characterization methods explicitly confirm Fe7C3 as the dominant and stable catalytic phase, providing deeper mechanistic insights into the active site characteristics of iron carbide catalysts. Additionally, surface-sensitive XPS analysis detects minor dispersed Fe3+ oxide species (Fig. 1g) that remain undetectable in bulk analyses, suggesting these trace oxide species could beneficially contribute RWGS activity by forming highly dispersed and advantageous FexO sites on Fe7C3 surface.
Catalytic performance
Beyond their structural differences, these catalysts exhibited notable disparities in catalytic behavior. To clearly demonstrate these differences, we compared their catalytic performance over the first 24 hours of time on stream (TOS) under identical reaction conditions. Table 1 summarizes the corresponding results, while Fig. 3a,b and Supplementary Fig. 10 showed the associated product distributions. The Fe and FeMg catalysts displayed similar behavior, each yielding a high fraction ( ~ 50%) of low-value products (CH4 and CO). Upon the introduction of potassium (FeK), olefin selectivity improved markedly, with high-value olefins reaching approximately 49.2%. Notably, despite initially high CO2 conversion across all four catalysts (Supplementary Fig. 11), catalysts without Mg gradually deactivated, concomitant with increased CO formation. This observation, together with structural analyses, indicates that the depletion of iron carbides, particularly Fe7C3, is a critical factor behind the decreasing FTS activity in later stages. In contrast, FeKMg consistently delivered higher CO2 conversion and lower selectivity to C1 by-products, ultimately achieving around 67.1% selectivity to high-value olefins.
Product distributions from CO2 hydrogenation over Fe-based catalysts: a FeK, and b FeKMg. Bar charts show hydrocarbon selectivity versus carbon number. c CO2 conversion and product selectivity for Fe7C3-K with varying Mg levels. d Comparison of the catalytic performance of Fe7C3 with that of other previously reported catalysts (These catalysts cited from 1 to 23 are shown in supplementary Tables 8, high-value olefins: olefins of all carbon numbers). e Stability test of the Fe7C3-KMg catalyst. (Reaction conditions: 0.10 g of catalyst, 340 °C, 2.0 MPa, H2/CO2 = 3, GHSV = 6 L·gcat−1·h−1).
We next systematically explored the influence of reaction conditions and Mg loading on catalytic performance. Varying the Mg/Fe ratio led to an initial increase in CO2 conversion, followed by a decrease (Fig. 3c, Supplementary Table 5). The optimum Mg/Fe ratio of 0.04 yielded both the highest conversion and relatively low C1 by-product selectivity. XRD analyses (Supplementary Figs. 12 and 13, Supplementary Table 6) showed no significant change in catalyst phase with different Mg contents. Fe7C3 persisted as the main active phase, although MgCO3 appeared at higher Mg loadings. An elevated reaction temperature enhanced CO2 conversion but also boosted C1 by-product formation, while increasing the space velocity diminished conversion and concurrently raised the C1 fraction (Supplementary Fig. 14, Supplementary Tables 7 and 8). The Fe7C3-KMg catalyst exhibited stable performance across varying H2/CO2 ratios, and post-reaction XRD confirmed that Fe7C3 remained the dominant phase without notable Fe3O4 formation (Supplementary Fig. 15). Compared with previously reported catalysts (Fig. 3d, Supplementary Table 9), our Fe7C3-KMg catalyst demonstrates significantly higher olefin selectivity and minimal production of low-value by-products such as CH4 and CO. Under extended operation (340 °C, 2.0 MPa, H2/CO2 = 3, GHSV = 6 L·gcat−1·h−1), Fe7C3-KMg maintained exceptional stability over 1000 hours (Fig. 3e), exhibiting negligible changes in conversion and product distribution. Specifically, relative to recently reported alkali and alkaline-earth metal promoted iron catalysts12,19, our catalyst achieves comparable CO2 conversion ( ~ 41.5%) at notably milder conditions (2 MPa vs. 3 MPa) with superior olefin selectivity (67.1%) and enhanced long-term stability. This outstanding performance primarily results from the high phase purity of Fe7C3, distinctly superior to commonly reported χ-Fe5C2/Fe3O4 mixtures9, and from the synergistic promoting effects of potassium and magnesium. These characteristics underscore its robust potential for practical CO2 hydrogenation applications.
In summary, our results provide clear evidence that nearly phase-pure Fe7C3 catalysts derived from PBAs can efficiently drive CO2 hydrogenation to olefins with outstanding selectivity and long-term stability. These performance differences among catalysts underscore the critical role of phase purity and promoter interactions. The underlying structural mechanisms driving these catalytic outcomes, especially the stabilization effect of Mg and promotion effect of K, will be discussed in depth in subsequent sections.
Structural information and mechanism of the magnesium promoter
To elucidate the structure-performance relationship in magnesium-promoted Fe7C3 catalysts, a systematic investigation of the magnesium species’ structure and spatial distribution within the catalytic system is critically needed. XPS quantification reveals that magnesium is predominantly localized on the catalyst surface, exhibiting a notably higher Mg/Fe atomic ratio compared to the bulk (Supplementary Fig. 16). Further XPS analyses confirm that Mg and K promoters primarily exist as surface MgCO3 and K2O species, respectively (Supplementary Fig. 17–18). Specifically, the characteristic K 2p peak around 292.7 eV clearly indicates the presence of surface-bound potassium oxide (K2O) rather than metallic potassium or lattice-incorporated potassium species, aligning well with literature consensus on potassium-promoted iron carbide catalysts28,29. Additionally, combined XPS analyses and DFT calculations indicate the absence of significant electronic interactions between the potassium and magnesium promoters (Supplementary Fig. 19).
Considering the critical influence of water formed during CO2 hydrogenation on catalyst stability, we specifically examined the impact of water exposure on catalyst performance (Fig. 4a and Supplementary Fig. 20). Whereas introducing H2O significantly decreased the activity of FeK, the FeKMg catalyst exhibited only minor performance fluctuations, indicating that Mg incorporation critically enhances catalyst resistance to water-induced deactivation. Even under high water partial pressures, FeKMg exhibited exceptional structural and catalytic stability. Raman spectra (Fig. 4b) corroborate this finding: although the FeK catalyst progressively lost surface carbon species and displayed emerging Fe–O vibrational peaks, FeKMg showed no notable oxidation-related signals.
a CO2 hydrogenation activity under dry conditions and with an additional 10% gas-phase water (Reaction conditions: 0.10 g catalyst, 340 °C, 2.0 MPa, H2/CO2 = 3, GHSV = 6 L·gcat−1·h−1). b Corresponding Raman spectra recorded after each reaction stage (spectra 1–6 represent sequential stages of reaction).
To further elucidate the interactions between H2O and the catalyst, we performed D2O-TPD experiments (Supplementary Fig. 21) and transient kinetic analyses (Supplementary Fig. 22). TPD measurements revealed that both FeK and FeKMg catalysts exhibit similar D2O adsorption capacities; however, FeK produced a noticeably higher signal for dissociated D2. This suggests that the addition of Mg inhibit the dissociation of D2O. Moreover, we carried out transient kinetic experiments to quantify the catalyst’s capacity for D2O dissociation. By monitoring the D2 signal generated from the reaction between CO and surface-dissociated D2O, we were able to determine the amount of dissociated D2O on the catalyst surface. The FeKMg catalyst showed a lower quantity of dissociated D2O, further confirming that Mg suppresses D2O dissociation. This reduction in water splitting is a key factor in enhancing the stability of the FeKMg catalyst under reaction conditions, as it protects the active iron carbide phase from oxidation.
Both FeK and FeKMg catalysts exhibited remarkably high olefin selectivity. Previous studies have demonstrated that alkali metal promoters, particularly potassium, effectively enhance olefin selectivity by suppressing secondary olefin hydrogenation30,31. Our pulse experiment using propylene hydrogenation (Supplementary Fig. 23) explicitly confirmed this effect, clearly showing that potassium significantly inhibits olefin hydrogenation. In contrast, magnesium had minimal influence on this reaction. Thus, our findings indicate that the superior olefin selectivity observed is primarily attributed to the potassium promoter’s ability to effectively limit secondary hydrogenation of olefins.
To clarify the atomic-scale mechanism underlying magnesium’s stabilizing effect, we performed DFT calculations to investigate water dissociation behavior on various catalyst surfaces. The results for Fe7C3 and Fe7C3-Mg are presented in Supplementary Fig. 24, while Fig. 5a shows the calculations for Fe7C3-K and Fe7C3-KMg. Adsorption energies of water on all four models are comparable, averaging approximately -0.80 eV, indicating similar water-binding strengths. Calculated energy barriers for the first O–H bond dissociation were 0.29 eV on Fe7C3-K and 0.22 eV on Fe7C3-KMg, indicating that initial dissociation occurs readily under reaction conditions. However, the subsequent dissociation of the second O–H bond is significantly more challenging, especially on Fe7C3-KMg, with an energy barrier increasing to 0.84 eV compared to 0.66 eV on Fe7C3-K. These calculations clearly illustrate magnesium’s substantial inhibitory effect on O–H bond cleavage.
Moreover, introducing magnesium significantly hampers oxygen adsorption, as evidenced by calculated reaction energies for water dissociation: −2.14 eV on Fe7C3-K and −1.73 eV on Fe7C3-KMg. Crystal orbital Hamilton population (COHP) analysis, which quantitatively measures bond strength through integrated COHP (-ICOHP) values (higher values indicate stronger bonding), further corroborates this observation. As depicted in Fig. 5b, the -ICOHP values for Fe–O bonds decrease from 6.96 on Fe7C3-K to 6.88 on Fe7C3-KMg, aligning with the reaction energy trends. Supplementary Fig. 25 shows analogous results for Fe7C3 and Fe7C3-Mg, with -ICOHP values of 7.03 and 6.95, respectively. These results collectively suggest that magnesium markedly reduces the oxygen adsorption capacity on Fe7C3 surfaces. Charge density difference analyses (Supplementary Fig. 26) further reveal electronic interactions: electrons transfer from K2O to Fe7C3 in Fe7C3-K, creating an electron-rich Fe7C3 surface favorable for oxygen adsorption and subsequent oxidation. Conversely, MgCO3 in Fe7C3-Mg acts as an electron acceptor, resulting in a charge-deficient Fe7C3 surface. Bader charge analysis quantifies this electron depletion, showing Fe7C3 loses approximately 1.03 electrons upon magnesium incorporation, thereby significantly mitigating oxygen adsorption and subsequent surface oxidation.
Integrating these findings, we propose the following catalytic mechanism (Fig. 5c): CO2 initially undergoes the RWGS reaction, facilitated by highly dispersed FexO species on the Fe7C3 catalyst surface, producing CO and water. Magnesium critically inhibits dissociation of the generated water, effectively preventing oxidation and subsequent deactivation of the active Fe7C3 phase. Consequently, in the presence of potassium promotion, the stable Fe7C3 surface efficiently catalyzes the conversion of CO and H2 into high-value olefins. This synergistic interplay between magnesium stabilization and potassium promotion underlies the outstanding selectivity and long-term stability of the Fe7C3-KMg catalyst. Nevertheless, despite the intrinsic oxidation resistance provided by Mg, accumulation of water under prolonged industrial operation could still pose challenges to catalyst stability. Therefore, complementary reactor-level engineering strategies may be required for industrial applications. Specifically, strategies such as membrane-assisted in-situ water removal, downstream condensation coupled with recycle loops, and structured or hydrophobic reactor designs to reduce water retention have been successfully applied in catalytic processes. Integrating these reactor-level approaches with the intrinsic material-level oxidation resistance provided by Mg could yield synergistic improvements, significantly enhancing the practical viability of Fe7C3-based catalysts under industrially relevant conditions.
Discussion
In summary, our study introduces a novel synthesis strategy for the Fe7C3 phase that leverages the unique roles of K and Mg promoters. By extracting Fe7C3 seeds from Prussian blue and employing K to enhance carbonization while using Mg to mitigate water-induced oxidation, we have successfully stabilized the Fe7C3 phase under demanding CO2 hydrogenation conditions. Our Fe7C3-KMg catalyst exhibits catalytic performance that far exceeds that of conventional iron catalysts and opens up new avenues for the innovative design of CO2 hydrogenation catalysts. Moreover, we have not only developed an efficient method to synthesize single-phase Fe7C3 but also elucidated the intrinsic link between the structure of Fe7C3 and its catalytic performance, thereby providing a solid foundation for further catalyst optimization. Future work will focus on optimizing this strategy further and exploring its applicability across a broader range of reaction conditions, paving the way for more sustainable and efficient CO2 utilization technologies.
Methods
Catalyst preparation
All catalysts containing different promoters were synthesized by a coprecipitation method, using Fe(NO3)3·9H2O as the iron source, and either (NH4)4Fe(CN)6 or K4[Fe(CN)6] as the precipitating agent. A suitable amount of polyvinylpyrrolidone K30 (average molecular weight ~40,000; TCI, Shanghai) was dissolved in 200 g of deionized water to ensure uniform dispersion and controlled nucleation of the PBA precursors. Subsequently, a solution of either (NH4)4Fe(CN)6 (Honeywell Specialty Chemicals Seelze GmbH) or K4[Fe(CN)6] (solution A) was prepared. Separately, Fe(NO3)3·9H2O (AR, Sinopharm Chemical Reagent Co., Ltd.) was dissolved in 200 g of deionized water (solution B). Solution B was then slowly added into solution A under vigorous stirring to form the catalyst precursor. Finally, different amounts of Mg(NO3)2·4H2O were introduced via incipient wetness impregnation. The final Fe/Mg ratio of each sample was determined by inductively coupled plasma-optical emission spectrometry (ICP-OES, Perkin Elmer). Catalyst samples are named based on the promoters employed: Fe refers to the iron catalyst without any promoter; FeMg refers to the catalyst modified with magnesium only; FeK denotes the catalyst promoted solely with potassium; and FeKMg indicates the catalyst co-promoted with both potassium and magnesium.
Specifically, the general designation “FeKMg” is used throughout the manuscript to describe the co-promoted catalyst. The notation “Fe7C3-KMg” is explicitly employed only when the presence of the Fe7C3 carbide phase has been confirmed through structural characterization.
Catalyst characterizations
XRD patterns were acquired using a Bruker D8 powder diffractometer located in Karlsruhe, Germany, with Co Kα radiation (λ = 0.179 nm). The instrument operated at a voltage of 35 kV and a current of 40 mA. A continuous scan mode was employed with a step size of 0.04° and a dwell time of 0.4 seconds, covering a 2θ range from 40° to 75°.
TEM analysis was performed using an FEI Talos F200A electron microscope operating at 200 kV. The samples were sonicated in ethanol, then deposited onto a copper grid with a porous carbon film. Before testing, the samples were irradiated with an infrared lamp for 15 minutes to remove any residual solvents.
HAADF-STEM was used to capture STEM images (2048 × 2048 pixels) with a camera length of 260 mm and a spot diameter of 0.5 nm.
XPS spectra were recorded using a Thermo Scientific K-alpha system with Al Kα radiation (hν = 1486.6 eV) as the X-ray source. To prevent oxidation, the samples were prepared in a glove box. The C 1 s peak (284.6 eV) was used as a reference for calibration.
PTH experiments for C3H6 were conducted using an AMI-300 apparatus equipped with a mass spectrometer. The catalysts were activated under ammonia gas, then switched to a 10% H2/Ar flow (50 mL/min), with the temperature set to 340 °C. C3H6 was pulsed into the system to complete the PTH. The effluent was monitored for C3H6 (m/z = 42) and C3H8 (m/z = 44) using a PFEIFFER Omnistar mass spectrometer.
XAFS data were collected at the BL14W1 beamline of the Shanghai Synchrotron Radiation Facility (SSRF), China, operating at 3.5 GeV with a maximum current of 260 mA. Energy calibration was performed using the absorption edge of pure Fe foil, and XAFS data were acquired in fluorescence mode.
Mössbauer experiments were conducted using an MR-351 constant acceleration transmission spectrometer at 10 K with 25 mCi 57Co in a Rh matrix. Phase composition was identified based on isomer shift (IS), quadrupole splitting (QS), and magnetic hyperfine field (Hhf) parameters. The content of each phase was determined from the absorption peak areas, assuming the same recoil-free factor for all types of iron nuclei in the catalyst.
Theoretical calculations
For calculation models, a periodical (1×1) of h-Fe7C3(211) slab was truncated from the optimized bulk phase, which contains 56 Fe and 23 C atoms. During the structural optimization, the bottom 27 Fe and 11 C atoms were fixed in the equilibrium positions as in the bulk phase while the others were allowed to relax. We selected the (211) facet for DFT modeling based on prior studies showing this to be a relatively low-index, catalytically active surface with moderate surface energy32,33. Additionally, the (211) plane exposes coordinatively unsaturated Fe atoms, suitable for CO2/H2 adsorption and promoter interaction modeling. A MgCO3 cluster was loaded on the h-Fe7C3(211) facet to represent the Mg-promoted catalyst. All spin-polarized calculations were carried out with VASP code34,35. The frozen-core projector-augmented wave (PAW)36 pseudo-potential with a cutoff energy of 450 eV was selected for the plane-wave expansion. The generalized gradient approximation in the Perdew-Burke-Ernzerhof (GGA-PBE)37 with van deer Waals correction (D3)38 was employed to describe the exchange-correlation energy. The convergence criteria for the force and electronic self-consistent iteration were set to 0.03 eV/Å and 10−5 eV, respectively. Gamma-centered (2×2×1) k-point was used for sampling of Brillouin zone. In all calculations, adsorption energies (Eads) were calculated based on Eads = Ex/slab – [Eslab + Ex], where Ex/slab is the total energy of the slab with adsorbents after full relaxation, Eslab is the total energy of the bare slab, and Ex is the total energy of the free adsorbents in the gas phase. Therefore, the more negative the Eads, the stronger the adsorption. Reaction energies (ΔE) were defined as ΔE = Efinal - Einitial, where Efinal and Einitial represent the final state energy and initial state energy, respectively. Therefore, a negative ΔE represents an exothermic process. All transition states were calculated using the climbing image nudged elastic band method (CI-NEB)39, with the stretching frequencies analyzed in order to characterize whether a stationary point is a transition state with only one imaginary frequency.
Catalyst testing
The catalytic efficiency of synthesized materials was systematically assessed using a quad-channel fixed-bed microreactor system. For each test run, 100 mg of catalyst was uniformly mixed with 200 mg quartz sand and packed into a quartz reaction chamber (10 mm ID) equipped with a temperature-monitoring stainless-steel sleeve. Standard evaluation parameters were maintained at 340 °C, 2.0 MPa, H2/CO2 = 3, and 6000 mL·gcat−1·h−1 unless otherwise specified. Post-reaction products underwent phase separation through sequential hot (160 °C) and cold (0 °C) trapping systems, enabling collection of solid waxes, liquid hydrocarbons, and aqueous phases for offline analysis via HP-PONA 19091s-001 chromatography.
Continuous gas monitoring was achieved through Agilent 7890B GC with specialized detection modules: Gaspro-FID assemblies resolved C1-C4 hydrocarbons, while a PONA-FID system characterized C4-C7 compounds. Gas composition of H2/CO2/CO/Ar was determined using coupled PLOT/Q, 5 Å molecular sieve, and Haysep Q columns interfaced with TCD detection. Carbon-based mass balance calculations confirmed experimental accuracy within ± 5% deviation through systematic comparison of inlet/outlet carbon fluxes.
The CO2 conversion (XCO2), product selectivity (Si), reaction rate (R) and C2+ olefin selectivity (\({{{\rm{S}}}}_{\,{{{\rm{C}}}}_{2+}^{=}}\)) were calculated by the following equations:
where CO2 in/out denote molar flows of carbon dioxide at reactor feed and effluent streams respectively, Ar in/out refer to molar flows of argon at the reactor inlet and outlet. Si represents carbon-specific selectivity for product i, Ni indicates molar fraction, ni corresponds to carbon count per molecule and Si= is the specifically quantifies olefinic selectivity for i-carbon unsaturated hydrocarbons. GHSV corresponds to gas hourly space velocity. This analytical framework ensures comprehensive characterization of catalytic behavior while maintaining strict adherence to standardized evaluation protocols in heterogeneous catalysis research.
Data availability
The data that support the findings of this study are available within the paper and its Supplementary Information, and all data are also available from the corresponding authors upon request. Source data are provided with this paper.
References
Wei, J. et al. Directly converting CO2 into a gasoline fuel. Nat. Commun. 8, 15174 (2017).
Wang, M. et al. Spinel Nanostructures for the Hydrogenation of CO2 to Methanol and Hydrocarbon Chemicals. J. Am. Chem. Soc. 146, 14528–14538 (2024).
Yang, H. et al. Tuning the selectivity of CO2 hydrogenation to alcohols by crystal structure engineering. Chem 10, 2245–2265 (2024).
Tian, G. et al. Upgrading CO2 to sustainable aromatics via perovskite-mediated tandem catalysis. Nat. Commun. 15, 3037 (2024).
Gao, W. et al. Industrial carbon dioxide capture and utilization: state of the art and future challenges. Chem. Soc. Rev. 49, 8584–8686 (2020).
Feldman, D. R. et al. Observational determination of surface radiative forcing by CO2 from 2000 to 2010. Nature 519, 339–343 (2015).
Wang, L. et al. Cobalt–Nickel Catalysts for Selective Hydrogenation of Carbon Dioxide into Ethanol. ACS Catal. 9, 11335–11340 (2019).
Hu, J. et al. Sulfur vacancy-rich MoS2 as a catalyst for the hydrogenation of CO2 to methanol. Nat. Catal. 4, 242–250 (2021).
Ye, J. et al. Hydrogenation of CO2 for sustainable fuel and chemical production. Science 387, eadn9388 (2025).
Li, Z. et al. Ambient-pressure hydrogenation of CO2 into long-chain olefins. Nat. Commun. 13, 2396 (2022).
Wang, Y. et al. Carbon-Based Electron Buffer Layer on ZnOx-Fe5C2-Fe3O4 Boosts Ethanol Synthesis from CO2 Hydrogenation. Angew. Chem. Int. Ed. 62, e202311786 (2023).
Orege, J. I. et al. Highly stable Sr and Na co-decorated Fe catalyst for high-valued olefin synthesis from CO2 hydrogenation. Appl. Catal. B 316, 121640 (2022).
Zhu, J. et al. Dynamic structural evolution of iron catalysts involving competitive oxidation and carburization during CO2 hydrogenation. Sci. Adv. 8, eabm3629 (2022).
Han, J. et al. Highly Efficient CO2 Hydrogenation to Linear α-Olefins on FeZnK Catalysts with Balanced Zn–O–Fe Interfaces and Fe5C2 Species. ACS Catal. 15, 3940–3954 (2025).
Liu, X., Lin, T., Liu, P. & Zhong, L. Hydrophobic interfaces regulate iron carbide phases and catalytic performance of FeZnOx nanoparticles for Fischer-Tropsch to olefins. Appl. Catal. B 331, 122697 (2023).
Zhao, H. et al. Synthesis of Iron-Carbide Nanoparticles: Identification of the Active Phase and Mechanism of Fe-Based Fischer–Tropsch Synthesis. CCS Chem. 3, 2712–2724 (2021).
Chang, Q. et al. Relationship between Iron Carbide Phases (ε-Fe2C, Fe7C3, and χ-Fe5C2) and Catalytic Performances of Fe/SiO2 Fischer–Tropsch Catalysts. ACS Catal. 8, 3304–3316 (2018).
Xu, M. et al. Regulating iron species compositions by Fe-Al interaction in CO2 hydrogenation. J. Catal. 413, 331–341 (2022).
Pasupulety, N., Alzahrani, A. A., Daous, M. A. & Alhumade, H. CO2-FT activity of Fe7C3 in FeZnK/ZrO2 catalysts synthesized by using citric acid: Effect of pretreatment gas. Fuel 360, 130596 (2024).
Ghosh, P. S., Ali, K., Vineet, A., Voleti, A. & Arya, A. Study of structural, mechanical and thermal properties of θ-Fe3C, o-Fe7C3 and h-Fe7C3 phases using molecular dynamics simulations. J. Alloy. Compd. 726, 989–1002 (2017).
de Smit, E. et al. Stability and reactivity of ε-chi-theta iron carbide catalyst phases in Fischer-Tropsch synthesis: controlling μC. J. Am. Chem. Soc. 132, 14928–14941 (2010).
Zhang, C. et al. Unraveling the Role of Zinc on Bimetallic Fe5C2–ZnO Catalysts for Highly Selective Carbon Dioxide Hydrogenation to High Carbon α-Olefins. ACS Catal. 11, 2121–2133 (2021).
Qian, F. et al. Stabilized epsilon-Fe2C catalyst with Mn tuning to suppress C1 byproduct selectivity for high-temperature olefin synthesis. Nat. Commun. 15, 5128 (2024).
Zhao, B. et al. Unveiling the Activity Origin of Iron Nitride as Catalytic Material for Efficient Hydrogenation of CO2 to C2+ Hydrocarbons. Angew. Chem. Int. Ed. 60, 4496–4500 (2021).
Liu, X. W. et al. Mossbauer Spectroscopy of Iron Carbides: From Prediction to Experimental Confirmation. Sci. Rep. 6, 26184 (2016).
Lu, F., Chen, X., Lei, Z., Wen, L. & Zhang, Y. Revealing the activity of different iron carbides for Fischer-Tropsch synthesis. Appl. Catal. B 281, 119521 (2021).
Liu, Y. et al. Effects of initial crystal structure of Fe2O3 and Mn promoter on effective active phase for syngas to light olefins. Appl. Catal. B 261, 118219 (2020).
Torres Galvis, H. M. et al. Supported iron nanoparticles as catalysts for sustainable production of lower olefins. Science 335, 835–838 (2012).
Xu, D., Ding, M., Hong, X. & Liu, G. Mechanistic aspects of the role of K promotion on Cu-Fe-based catalysts for higher alcohol synthesis from CO2 hydrogenation. ACS Catal. 10, 14516–14526 (2020).
Guo, L. et al. Directly converting carbon dioxide to linear α-olefins on bio-promoted catalysts. Commun. Chem. 1, 11 (2018).
Liu, Y. et al. Stable three-dimensional macroporous iron-foam catalyst for direct conversion of CO2 to olefins. ACS Catal. 14, 12425–12436 (2024).
Zhang, M., Ren, J. & Yu, Y. Investigating the CO activation mechanism on hcp-Fe7C3 (211) via density functional theory. Mol. Catal. 505, 111506 (2021).
Ren, J., Ai, N. & Yu, Y. Insight into the Fischer-Tropsch mechanism on hcp-Fe7C3 (211) by density functional theory: the roles of surface carbon and vacancies. RSC Adv. 11, 34533–34543 (2021).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Com. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Blochl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Acknowledgements
The authors acknowledge financial support from the National Science Fund for Distinguished Young Scholars (22225206 to X.W. and 22025804 to Y.Y.), National Natural Science Foundation of China (22172183 to X.L., 22202224 to J.L. and 22178367 to G.L.), National Key R&D Program of China (2022YFA1604100 to X.W. and 2022YFB4101200 to Y.Y.), Beijing Natural Science Foundation (L255001 to X.L.), CAS Project for Young Scientists in Basic Research (YSBR-005 to X.W.), Key Research Program of Frontier Sciences CAS (ZDBS-LY-7007 to X.W.), National Natural Science Foundation Major Research Plan (92045303 to X.W.), CAS Informatization Plan (CAS-WX2021SF0110 to X.W.), Ordos Key R&D Program (YF20232316 to X.L. and YF20232317 to X.W.), and the funding support from Synfuels China, Co. Ltd.
Author information
Authors and Affiliations
Contributions
Y.Y., X.W. and X.L. designed the study. F.Q. performed most of the reactions and sample characterization. M.W. carried out the X-ray structure characterization and analysis. Y.C. performed the electron microscopy characterization. J.L. performed the DFT calculations. F.Q., X.L., J.L. Z.W., Z.S., R.L., M.Q., H.W., and G.L. wrote and revised the paper. All the authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Ping Lu, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qian, F., Wang, M., Wei, Z. et al. Stabilized Fe7C3 catalyst with K–Mg dual promotion for robust CO2 hydrogenation to high-value olefins. Nat Commun 16, 8044 (2025). https://doi.org/10.1038/s41467-025-63218-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-63218-3